Penlon AV-S User manual
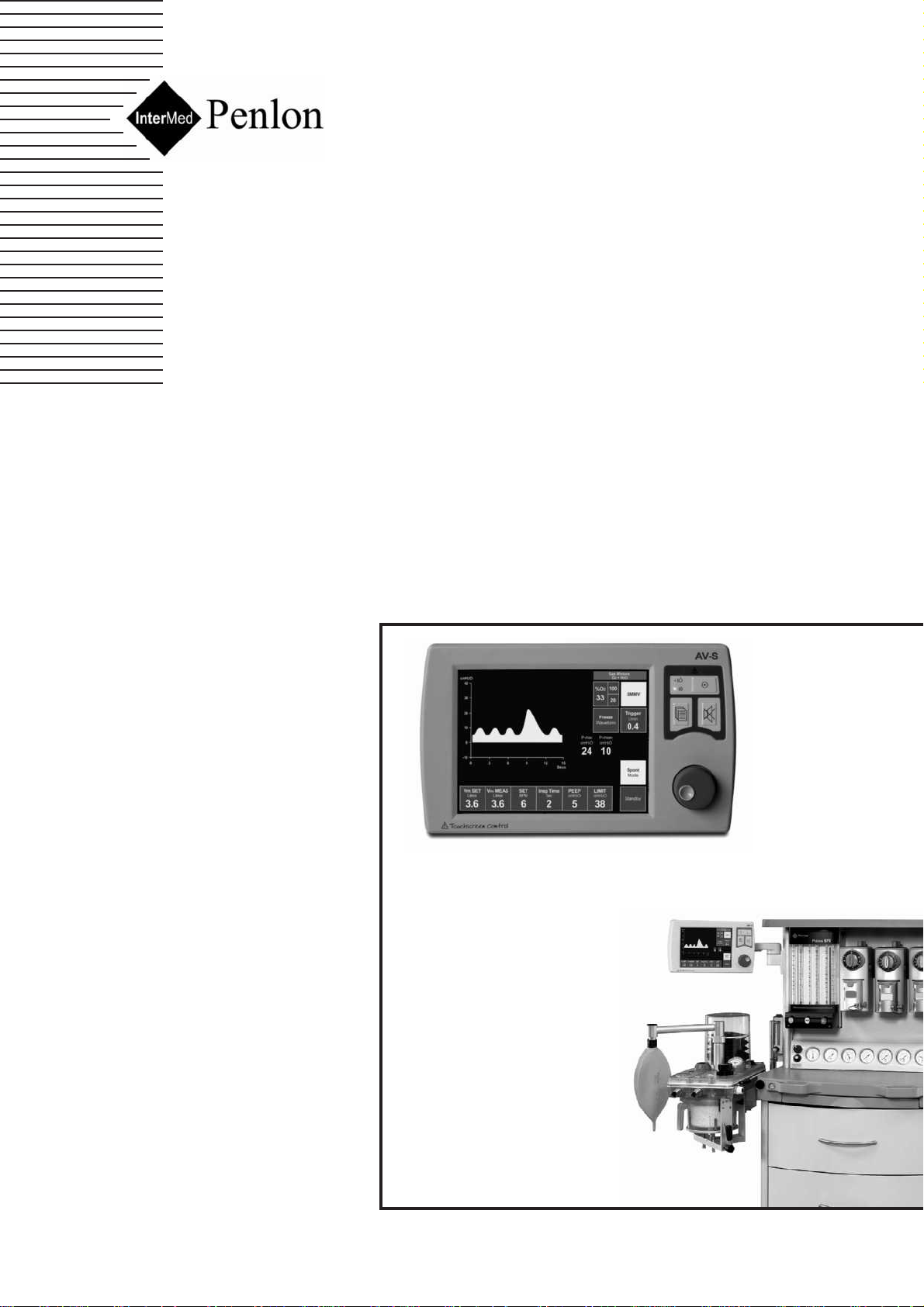
AV-S Ventilator
User Manual
Partnership for Life
www.ardusmedical.com


IMPORTANT
(i)
Servicing and Repairs
In order to ensure the full operational life of this
ventilator, servicing by an engineer trained by
the manufacturer should be undertaken
periodically.
The ventilator must be serviced to the following
schedule:
(a) Six monthly service - inspection and
function testing.
(b) Annual / two year / four year service -
inspection and function testing, and
component replacement.
Details of these operations are given in the
Service Manual for the AV-S, available only for
engineers trained by the manufacturer.
For any enquiry regarding the servicing or
repair of this product, contact Penlon Inc.
Penlon Inc.
11515 K-Tel Drive
Minnetonka
MN 55434
Always give as much of the following
information as possible:
1. Type of equipment
2. Product name
3. Serial number
4. Approximate date of purchase
5. Apparent fault

This manual has been produced to provide
authorised personnel with information on the
function, routine performance and
maintenance checks applicable to the AV-S
Anaesthesia Ventilator.
Information contained in this manual is
correct at the date of publication.
The policy of the manufacturer is one of
continued improvement to its products.
Because of this policy, the manufacturer
reserves the right to make any changes
which may affect instructions in this manual,
without giving prior notice.
Personnel must make themselves familiar
with the contents of this manual and the
machine’s function before using the
apparatus.
FOREWORD
(ii)
The Importance of
Patient Monitoring
WARNING
Anaesthetic systems have the capability to
deliver mixtures of gases and vapours to the
patient which could cause injury or death
unless controlled by a qualified anaesthetist.
There can be considerable variation in the
effect of anaesthetic drugs on individual
patients so that the setting and observation of
control levels on the anaesthesia systems
does not in itself ensure total patient safety.
Anaesthesia system monitors and patient
monitors are very desirable aids for the
anaesthetist but are not true clinical monitors
as the condition of the patient is also
dependent on his respiration and the
functioning of his cardio-vascular system.
IT IS ESSENTIAL THAT THESE ELEMENTS
ARE MONITORED FREQUENTLY AND
REGULARLY AND THAT ANY OBSERVATIONS
ARE GIVEN PRECEDENCE OVER MACHINE
CONTROL PARAMETERS IN JUDGING THE
STATE OF A CLINICAL PROCEDURE.
Before using any monitoring system or
device, the user must check that it conforms
to the relevant standard, as listed in the table
below.
Parameter / Device Relevant Standard
Pressure Measuring ISO 8835-2
Pressure Limitation Device EN 60601-2-13:2006 - 51.101.1
Exhaled Volume Monitor EN 60601-2-13:2006 - 51.101.4
Breathing System Integrity Alarm System EN 60601-2-13:2006 - 51.101.5
Continuing Pressure Alarm EN 60601-2-13:2006 - 51.101.6
Oxygen Monitor ISO 7767
Carbon Dioxide Monitor ISO 9918
Breathing Circuit ISO 8835-2
Agent Monitor ISO 11196
Gas Scavenging ISO 8835-3
For information on installing and connection of any of these systems or devices, please refer to the relevant manufacturer’s instructions.

Page No.
USER RESPONSIBILITY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
1. WARNINGS AND CAUTIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
2. PURPOSE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
3. DESCRIPTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
3.1 General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
3.2 Ventilation cycle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
3.3 Pneumatic system . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
3.4 Electrical system . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . .. 14
3.5 Control panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
3.5.1 Touchscreen operation and navigator wheel / push-button . . . . . . . . . . . . 15
3.5.2 Additional screen functions and displays . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
3.5.3 Start-up screens . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . 17
3.5.4 Selecting functions and parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
3.5.5 User-adjustable parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
3.5.6 Parameter display identification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
3.6 Operational capability . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
3.7 Output compensation functions . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . 20
3.7.1 Fresh gas compensation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
3.7.2 Fresh gas mixture compensation - models with spirometry . . . . . . . . . . . . 20
3.7.3 Compliance compensation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
3.7.4 Altitude compensation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
3.8 Interface with anaesthetic machine and A200SP absorber . . . . . . . . . . . . 22
3.9 Ventilation Modes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
3.9.1 Standby Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
3.9.2 Volume Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
3.9.3 Pressure Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
3.9.4 Spontaneous Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. 28
3.9.5 Advanced Spontaneous Breathing Modes . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
3.9.5.1 SIMV (Synchronised Intermittent Mandatory Ventilation) . . . . . . . . . . . . . . 30
3.9.5.2 SMMV (Synchronised Mandatory Minute Ventilation) . . . . . . . . . . . . . . . 31
3.9.5.3 PSV (Pressure Supported Ventilation) . . . . . . . . . . . . . . . . . .. . . . . . . 32
3.9.5.4 PEEP ( Positive End Expiratory Pressure) . . . . . . . . . . . . . . . . . . . . . . . . . 33
3.10 On-screen Menus . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
3.11 Spirometry . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
3.12 Display Waveforms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
3.13 Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
3.14 Oxygen Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
3.14.1 System operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
3.14.2 The Oxygen Sensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
3.14.3 Menus . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
3.14.4 Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40
3.14.5 Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40
3.14.6 Alarm mute. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . 40
4. SPECIFICATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41
Ventilator . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41
Oxygen Monitor . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45
CONTENTS
(iii)

5. PRE-OPERATION PROCEDURES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46
5.1 Ventilator Set-up . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46
5.1.1 Mounting the Ventilator . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46
5.1.2 Electrical Power Connections . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 47
5.1.3 Ventilator gas supply . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 47
5.1.4 Breathing system schematic . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48
5.1.5 Bellows drive gas connection . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . 50
5.1.6 Anaesthetic gas scavenging system . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . 50
5.1.7 Remote screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
5.1.8 Printer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
5.1.9 Breathing system connection and filters. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
5.1.10 Spirometer connections and Zero Flow Calibration . . . . . . . . . . . . . . . . . . . . . 52
5.1.11 Pressure Monitor Connections . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . 54
5.1.12 Leak test / Compliance value calculation . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
5.1.13 Bellows assembly - adult and paediatric . . . . . . . .. . . . . . . . . . . . . . . . . . . . 57
5.2 Start-up screens . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 58
5.3 Pre-use Checklists . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 59
5.3.1 Daily Checklist . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 59
5.3.1.1 Alarm System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 59
5.3.1.2 Ventilator Internal Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 59
5.3.1.3 Function Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 59
5.3.2 Weekly Checklist . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 60
5.4 Oxygen Monitor Set-up . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . .. . . . . . . 61
5.4.1 Installation . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61
5.4.2 Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61
5.4.3 Sensor Low Indication . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 63
5.4.4 Setting the High and Low O
2 Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 63
6. MAINTENANCE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64
6.1 Service Schedule . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64
6.2 Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64
6.2.1 Outside Surfaces . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64
6.2.2 Bellows Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64
6.2.3 Spirometer Sensors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64
6.2.4 Oxygen Monitor Sensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64
6.2.5 Control Unit Patient Connector Block . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
6.3 Sterilisation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 66
6.4 Oxygen Monitor Sensor Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . 66
7. APPENDIX . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 67
1. Back-up battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 67
2. Menu system . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 68
3. Ventilator spirometry system . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 71
4. Ventilator disposal after use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 73
5. Approved accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 73
(iv)

This anaesthesia ventilator has been built to
conform with the specification and operating
procedures stated in this manual and/or
accompanying labels and notices when
checked, assembled, operated, maintained
and serviced in accordance with these
instructions.
To ensure the safety of this device it must be
checked and serviced to at least the
minimum standards laid out in this manual.
A defective, or suspected defective, product
must not under any circumstances be used.
The user must accept responsibility for any
malfunction which results from noncompliance with the servicing requirements
detailed in this manual.
Additionally, the user must accept
responsibility for any malfunction which may
result from misuse of any kind or noncompliance with other requirements detailed
in this manual.
Worn, broken, distorted, contaminated or
missing components must be replaced
immediately. Should such a repair become
necessary it is recommended that a request
for service advice be made to Penlon Inc.
This device and any of its constituent parts
must be repaired only in accordance with
written instructions issued by the
manufacturer and must not be altered or
modified in any way without the written
approval of the manufacturer. The user of
this equipment shall have the sole
responsibility for any malfunction which
results from improper use, maintenance,
repair, damage or alteration by anyone other
than the manufacturer or Penlon Inc.
USA and Canada:
Federal Law restricts the sale and use of this
device to, or on the order of, a licensed
practitioner.
Statements in this manual preceded by the
following words are of special significance:
WARNING means there is a
possibility of injury to the
user or others.
CAUTION means there is a possibility
of damage to the apparatus
or other property.
NOTE indicates points of
particular interest for more
efficient and convenient
operation.
Always take particular notice of the
warnings, cautions and notes provided
throughout this manual.
USER RESPONSIBILITY
1

1. WARNINGS AND CAUTIONS
The following WARNINGS and CAUTIONS
must be read and understood before using
this ventilator.
WARNINGS
General Information
1. Personnel must make themselves familiar with
the contents of this manual and the machine’s
function before using the ventilator.
Before Using the Ventilator
2. Before the AV-S ventilator is used clinically for
the first time a Calibration Check and Output
Check must be successfully completed.
Calibration and output checks must be carried
out by a Penlon-trained technician, following
the procedure in Appendix 6 in the AV-S Service
Manual.
3. Before the ventilator is used clinically for the
first time, verify that the hospital engineering
department has carried out an earth continuity
test.
If the integrity of the protective conductor is in
doubt, the ventilator must not be used.
4. Excessive electronic noise caused by other
poorly regulated devices, such as an
electrocautery unit, may adversely interfere with
the proper functioning of the ventilator.
To avoid this problem, do not connect the
ventilator’s power cord into the same electrical
wall outlet or adaptor strip into which an
electrocautery unit is connected.
5. If used with a mains extension cord, the unit
may be subject to electro-magnetic
interference.
6. The driving gas supply must be clean and dry to
prevent ventilator malfunction.
7. This ventilator is designed to be driven by
oxygen or medical air only. The drive gas is set
during manufacture and the ventilator is
calibrated for that gas.
Before the ventilator is used clinically for the
first time, the commissioning engineer must
confirm that the air/oxygen selection is set
correctly for the drive gas that is to be used.
The use of any other gas will cause inaccurate
operation and may damage the ventilator,
resulting in potential injury to the patient.
8. The driving gas is discharged through the
opening in the back of the ventilator control unit.
The discharged gas may contaminate the
environment, and should therefore be extracted
using a gas scavenging system.
9. The bellows can only support approximately 1
kPa (10 cmH
2O) differential positive pressure,
above which it may be dislodged from the
mounting ring, resulting in dangerous
malfunction of the ventilator.
Do not connect a positive end expiratory
pressure (PEEP) valve or other restrictive device
to the exhaust port on the bellows base.
This would increase the pressure inside the
bellows and the bellows could detach from the
base, causing serious malfunction.
10. Breathing System
The breathing system which conveys gases
from the anaesthetic machine to the patient, and
disposes of expired gases, must conform to the
requirements of ISO 8835-2.
Because breathing systems require frequent
cleaning and disinfection they are not a
permanent part of the anaesthetic ventilator and
therefore cannot be directly under the control of
the anaesthetic ventilator manufacturer.
However, we strongly recommend that only
breathing systems which have been approved
and authorised by the manufacturer for use with
AV-S should be employed.
Do not use conductive breathing system hoses.
When mechanical ventilation is employed the
patient breathing system must be connected
directly to a pressure relief valve to prevent the
possibility of barotrauma.
11. The spirometer sensors are mounted within the
A200SP absorber. Do not fit a spirometer sensor
to any other location.
The device will not measure exhaled volumes in
any other position.
12. The operation of each alarm function should be
verified daily.
Periodically check the alarms at clinically
suitable intervals. If the audible alarm or the
visual indicator of any alarm function fails to
activate during any alarm condition or fails to
reset after the alarm has been cleared, refer the
unit to an authorised service technician.
2

13. Before using the ventilator check that all
connections are correct, and verify that there
are no leaks.
Patient circuit disconnects are a hazard to the
patient. Extreme care should be taken to
prevent such occurrences.
It is recommended that Safelock fittings are
used throughout the breathing circuit.
14. Check that the cable between the control unit
and remote display screen unit is connected
before use.
Always use a cable type recommended by the
manufacturer.
Using the Ventilator
15. The AV-S ventilator is not intended for use in
intensive care applications.
16. This apparatus must not be used with, or in
close proximity to, flammable anaesthetic
agents.
There is a possible fire or explosion hazard.
17. Anaesthesia apparatus must be connected to an
anaesthetic gas scavenging system (AGSS) to
dispose of waste gas and prevent possible
health hazards to operating room staff. This
requirement must be observed during test
procedures as well as during use with a patient.
The scavenging transfer and receiver system
must conform to ISO 8835-3.
Any problem arising from an improperly
functioning scavenging system is solely the
user’s responsibility.
Do not use a scavenging system that restricts
drive gas flow when negative pressure is
exerted on it.
18. When the ventilator is connected to a patient, it
is recommended that a qualified practitioner is
in attendance at all times to react to an alarm or
other indication of a problem.
19. In compliance with good anaesthesia practice,
an alternative means of ventilation must be
available whenever the ventilator is in use.
20. It is recommended that the patient oxygen
concentration should be monitored
continuously.
WARNINGS AND CAUTIONS
3
21. If the drive gas supply pressure drops below a
nominal 241 kPa (35 psi), the LOW DRIVE GAS
SUPPLY alarm will activate both audibly and
visually. Patient minute volume may be reduced
due to lowered flow rates
22. An audible alarm indicates an anomalous
condition and should never go unheeded.
23. The characteristics of the breathing circuit
connected between the ventilator and the
patient can modify or change patient ventilation.
To assist the maintenance of the delivered
patient tidal volume, the ventilator control
system software includes:
A) a compliance compensation algorithm,
B) a fresh gas compensation algorithm.
However, patient ventilation must be monitored
independently from the ventilator.
It is the responsibility of the user to monitor
patient ventilation.
24. Care must be taken to ensure that the flow
sensors are connected correctly to the
inspiratory and expiratory ports of the
absorber.
25. The Vent Inop (ventilator inoperative) alarm
indicates that one of the following conditions
has occurred:
a) The drive gas solenoid has failed.
b) The flow control valve has failed.
c) Internal electronic fault.
d) Internal electrical fault.
e) Software error.
Note that if a ventilator error is detected,
‘Ventilator Inoperative’ will be displayed on the
front control panel display.
26. The High and Low Airway Pressure Alarms are
important for patient care.
It is important that the sensor is properly
located in the expiratory limb of the circuit refer to section 5.1.11.
27. The patient must be continuously attended and
monitored when Advanced Breathing Modes are
in use.

User Maintenance
28. User maintenance is restricted to cleaning
the outside surfaces of the ventilator, see
section 6.
Other procedures detailed in this manual
must be carried out by trained technicians.
Service and repair operations must only be
carried out by an engineer trained by the
manufacturer.
The warranty for this product is void if the
product is not maintained in accordance
with the service schedule detailed in
section 6.1, and the procedures published
in the Service Manual for this product.
Control Unit
29. Opening the control unit by unauthorised
personnel automatically voids all warranties
and specifications.
Prevention of tampering with the control unit is
exclusively the user’s responsibility. If the
control unit seal is broken, the manufacturer
assumes no liability for any malfunction or
failure of the ventilator.
30. For continued protection against fire hazards,
any replacement fuses must be the identical
type and rating as the original components.
Replacement must be carried out by trained
technician.
See section 4 for fuse rating.
31. If the internal battery is fully discharged, the
ventilator will not function in the event of mains
power failure. The battery must be recharged
before the ventilator is used clinically, otherwise
backup cannot be guaranteed.
See Appendix for battery maintenance.
See also CAUTION No. 7.
Used or defective batteries must be disposed of
according to hospital, local, state, and federal
regulations.
32. No oil, grease or other flammable lubricant or
sealant must be used on any part of the
ventilator in close proximity to medical gas
distribution components.
There is a risk of fire or explosion.
33. Exterior panels must not be removed by
unauthorised personnel and the apparatus must
not be operated with such panels missing.
There is a possible electric shock hazard.
Bellows Assembly
34. The valve seat on the patient gas exhalation
diaphragm valve in the base of the bellows
assembly must be cleaned regularly. Note that
the bellows assembly is built into the A200SP
Absorber - please refer to User Manual for this
product.
Failure to keep the valve seat clean could result
in the diaphragm sticking, thus preventing
exhalation.
Great care must be taken not to damage the
precision surface of the valve seat on the
patient gas exhalation diaphragm valve in the
base of the bellows assembly.
Never use any hard object or abrasive detergent
to clean the valve seat; use only a soft cloth.
If the valve seat is damaged, the valve will leak
and may cause serious ventilator malfunction.
WARNINGS AND CAUTIONS
4

CAUTIONS
1. Do not sterilise the ventilator control unit.
The patient block assembly must be removed from
the control unit before sterilisation ( see section
6.2.5).
All other internal components are not compatible
with sterilisation techniques and damage may
result.
2. For ventilator components which require
sterilisation, peak sterilisation temperatures should
not exceed 134oC (275oF) to prevent possible
damage. (See section 6).
3. Care must be taken not to let any liquid run into the
control unit; serious damage may result.
4. The exhalation valve located in the bellows base
assembly and the paediatric bellows adaptor must
be cleaned and sterilised separately. Note that the
bellows assembly is built into the A200SP Absorber
- please refer to User Manual for this product.
5. Always check for correct fitment, and carry out a full
function test before clinical use, if the bellows has
been removed and refitted for any reason. Note that
the bellows assembly is built into the A200SP
Absorber - please refer to User Manual for this
product.
6. Always check for correct fitment, and carry out a full
function test before clinical use, if the bellows has
been removed and refitted for any reason. See
section 6.
7. Damage will occur to the battery if it is allowed to
remain in a discharged state.
Check the battery frequently if the ventilator is in
storage (see Appendix 1).
8. Fresh gas compensation is disabled if :
a) The spirometry system is turned OFF through the
menu system, or
b) The spirometry system is not functioning correctly.
9. Fresh gas mixture compensation is disabled if :
a) The spirometry system is turned OFF through the
menu system, or
b) The spirometry system is not functioning correctly.
c) The O2 monitor is switched OFF.
10. Circuit compliance is not activated until Fresh Gas
Compensation is switched OFF.
NOTES
1. The term ‘cycle’ is used to designate the transition
to the exhalation phase.
2. The term ‘trigger’ is used to indicate the transition
to the inhalation phase.
WARNINGS AND CAUTIONS
5

Oxygen Monitor
Note that the sensor for the oxygen monitor is
built into the A200SP Absorber - for additional
information, please refer to the A200SP User
Manual.
WARNINGS
1. We recommend a calibration check of the
oxygen monitor every time the system is turned
on, as a safety precaution.
2. Do not attempt to open the fuel cell.
The sensor contains small quantities of :
a) electrolyte, classified as a harmful irritant
which is potentially hazardous, and
b) lead.
Used or defective cells must be disposed of
according to hospital, local, state, and federal
regulations.
3. ALWAYS check the integrity of the sensor
assembly before use.
4. Once exhausted, the sensor must be disposed
of according to hospital, local, state and federal
regulations.
5. The sensor measures oxygen partial pressure,
and its output will rise and fall due to pressure
change.
An increase in pressure of 10% at the sensor
inlet will produce a 10% increase in sensor
output.
6. The oxygen sensor is not suitable for
sterilisation.
If contamination is suspected, fit a new sensor
(see section 6.4) and dispose of the
contaminated unit according to hospital, local,
state and federal regulations.
6
CAUTIONS
1. Do not sterilise any oxygen monitor component.
2. Do not autoclave or expose the sensor to high
temperatures.
3. If the sensor shows signs of being affected by
condensation, dry the sensor with soft tissue.
Do not use heat to dry the sensor.
NOTES
1. The O2 SENSOR FAULT alarm indicates that one
of the following conditions has occurred.
a) Internal electrical fault
b) Software/electronics fault
c) Oxygen sensor fault.
2. The concentration read-out may, in certain
conditions of excess pressure, show a value above
100%.
To accommodate these conditions it is possible to
set the high alarm value up to 105% (see section 5).
3. To maintain maximum sensor life:
i) always switch off the anaesthetic machine after
use, to ensure that the basal flow ceases.
ii) disconnect the breathing circuit after use.
4. The accuracy of flow and volume measurements
may be reduced if the oxygen monitor is not in use.
5. Fresh gas mixture compensation is disabled if the
oxygen monitor is switched OFF.
WARNINGS AND CAUTIONS - Oxygen Monitor

The AV-S Ventilator is a pneumatically
driven, software controlled, multi-mode
ventilator, designed for mechanical
ventilation of adult and paediatric patients
under general anaesthesia.
In addition, in spontaneous mode, it can be
used to monitor spontaneously breathing
patients
It is designed for use in closed-circuit
anaesthesia.
Indications for use of the device:
The AV-S Ventilator is intended to provide
continuous mechanical ventilatory support
during anaesthesia. The ventilator is a
restricted medical device intended for use by
qualified trained personnel under the
direction of a physician. Specifically the
ventilator is applicable for adult and
paediatric patients.
The ventilator is intended for use by health
care providers, i.e. Physicians and Nurses
with patients during general anaesthesia.
The AV-S ventilator is not intended for use in
intensive care applications.
Oxygen Monitor
The Oxygen Monitor is intended to
continuously measure and display the
concentration of oxygen in breathing gas
mixtures used in anaesthesia, and is
intended for adult and paediatric patients.
The oxygen monitor is an integral part of the
ventilator.
The oxygen monitor is intended for use by
health care providers, i.e. Physicians and
Nurses for use with patients during general
anaesthesia.
2. PURPOSE
7
3.1 General Description
The AV-S Ventilator is a pneumatically driven, software
controlled, multi-mode ventilator.
The ventilator is time-cycled, volume/pressure
controlled, and pressure limited.
The ventilator has compliance compensation and
fresh gas compensation.
User-selectable gas mixture compensation is a
standard feature, plus a user-selectable variable
inspiratory pause and sigh option.
Ventilation Modes
Volume Mode - continuous mandatory ventilation
Pressure Mode - pressure controlled ventilation
Spontaneous, with advanced patient support -
SIMV, SMMV, PSV, PEEP
Patient Monitoring
Airway pressure, measured from the expiratory limb of
the breathing circuit.
Tidal Volume and Minute Volume measurement is
provided by a dual spirometry system
An integral oxygen monitor system measures oxygen
concentration in the breathing circuit inspiratory limb.
The print function provides a permanent record of
function activity for up to eight hours during a
procedure, or can be used to record waveforms.
Screen
210 mm (8.4 inch) high definition, colour TFT screen,
with single/dual waveform display.
Mounting:
Remote, arm-mounted as illustrated (1) or optional
combined control unit / screen (see section 5.1.1).
Bellows unit
The bellows unit (2) is built into the A200SP absorber.
A paediatric bellows assembly is available as an
option
Drive gas supply
The drive gas supply can be oxygen or air.
The supply must be at 310 to 689 kPa (45 to 100 psi).
Note that the drive gas is specified by the customer,
and set during manufacture. Conversion from one
drive gas to another must only be carried out by an
authorised service engineer trained by the
manufacturer.
3. DESCRIPTION
8
1
2
Spontaneous Mode Patient Support
SIMV - Synchronised Intermittent Mandatory Ventilation
SMMV - Synchronised Mandatory Minute Ventilation
PSV - Pressure Supported Ventilation
PEEP - Positive End Expiratory Pressure
9
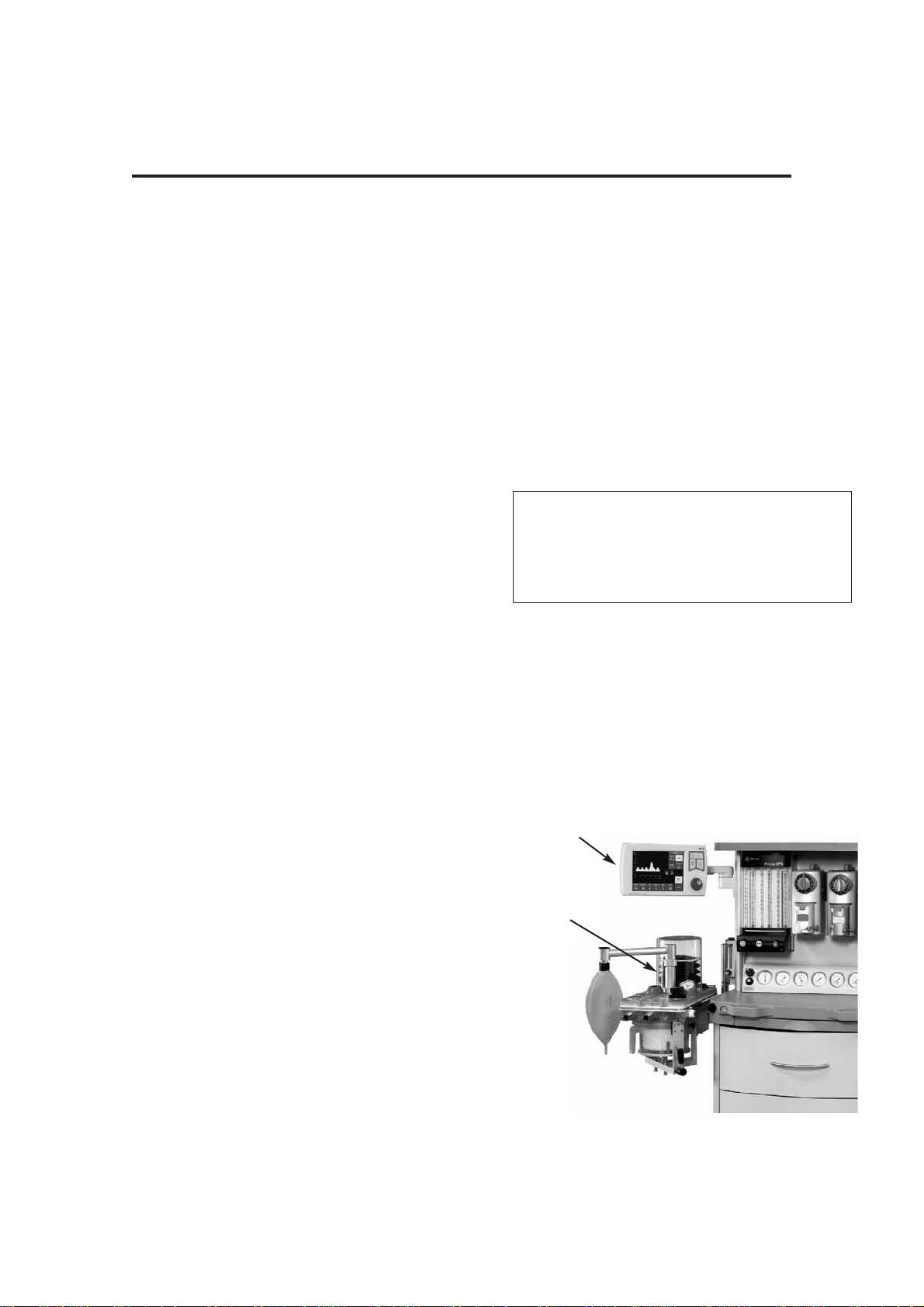
Control Unit
Rear Panel
Interface and Parameter inputs
5. A200SP Absorber Bag/Vent
switch interface, and
Spirometer connector
6. Anaesthetic machine interface
connector - (primary on/off
switch)
7. Pressure Monitor Port
8. Input socket - Oxygen monitor
sensor
Data and Printer Ports
9. Data Output
10. Output to remote display
11. Ethernet
12. USB
13. VGA
14. Printer port
15. RS232 (manufacturer’s use only)
NOTE
USB port is for access only by engineers
trained by the manufacturer.
All other data ports are read only.
For further information, please contact
your distributor’s service department, or
the manufacturer.
Gas Connections
1. Ventilator drive gas inlet
- connect to anaesthetic machine
auxiliary gas outlet
2. Bellows Drive Gas Output
- connect to bellows via A200SP
absorber - see section 5.1.5
3. Outlet - Exhaust Valve
- connect to scavenge system - see
section 5.1.6
Electrical Connection
4. Electrical mains input and fuse unit
DESCRIPTION
2
7
3
13 14
15
12
1110
9
8
6
5
4
1
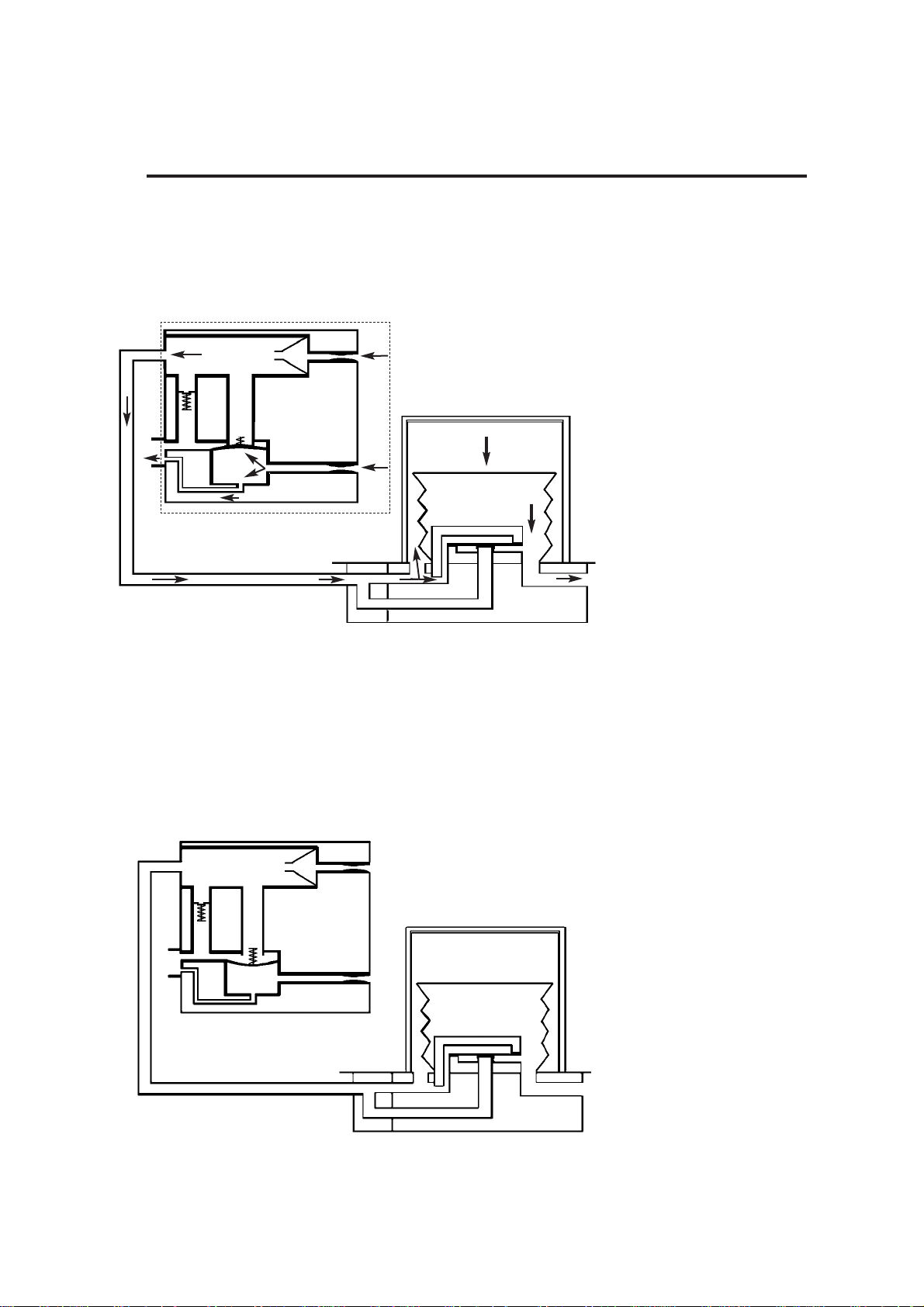
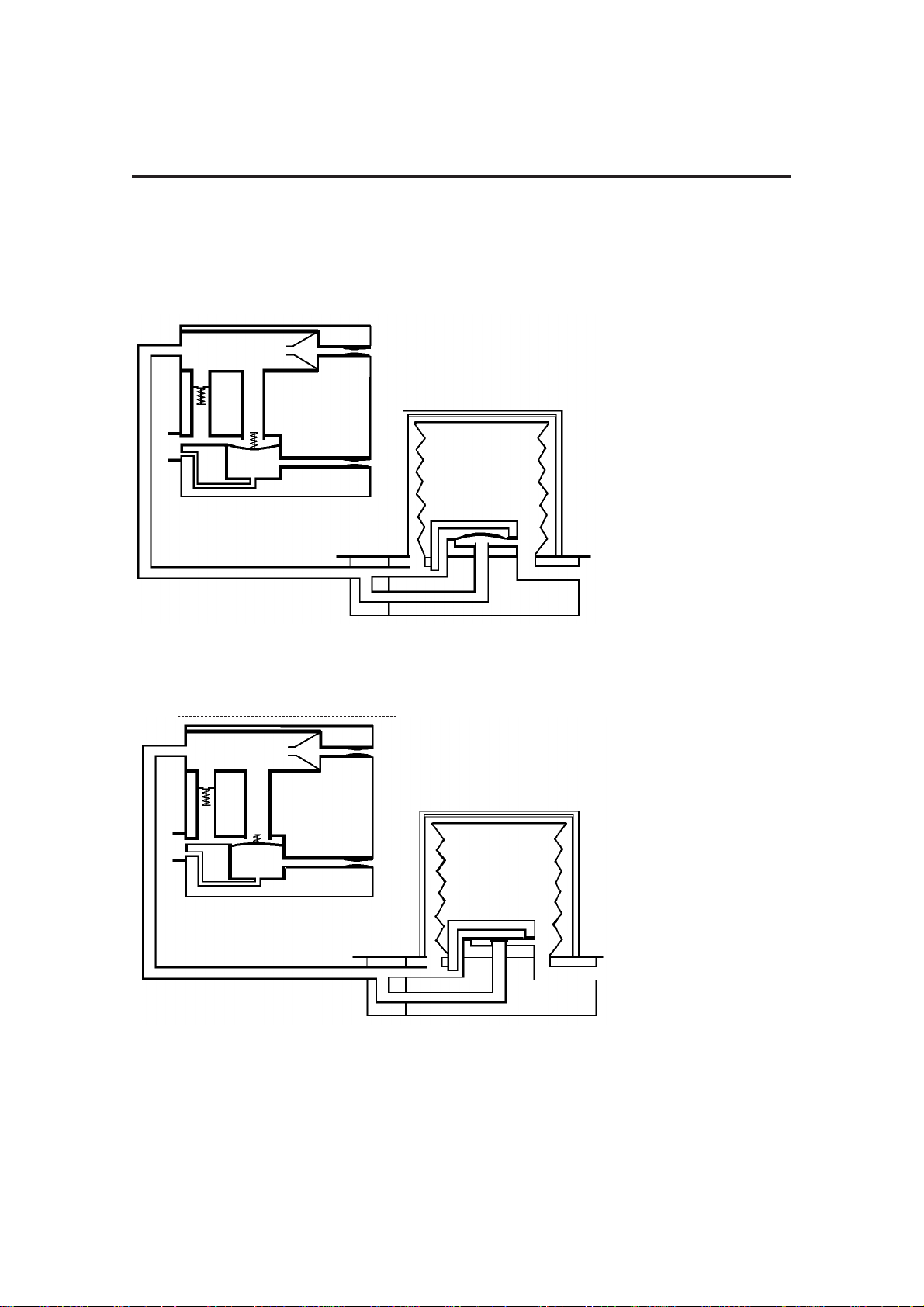
3.2 Ventilation Cycle
This section provides a simplified description of the ventilation cycle.
1. Inspiratory Phase
The drive gas proportional
valve (1) in the control unit
opens.
Drive gas is delivered to the
bellows housing (2).
The patient proportional
valve (3) opens, and gas
flows through the bleed
valve. The back pressure
ensures that the exhaust
valve (4) is kept closed.
Drive gas pressure builds
up above the bellows (5),
which starts to move down.
The diaphragm (6) in the
bellows assembly base is
held closed, and patient gas
is forced out of the bellows
base (7) into the breathing
system.
2. Beginning of
Expiratory Phase
The drive gas proportional
valve (1) closes.
The patient proportional
valve (3) closes.
The exhaust valve (4) opens.
Patient gas returns to the
bellows (5).
As the bellows rises,
redundant drive gas is
pushed out through the
exhaust valve.
DESCRIPTION
10
1
4
4
6
7
5
5
2
3
3
1
3
1
4
DESCRIPTION
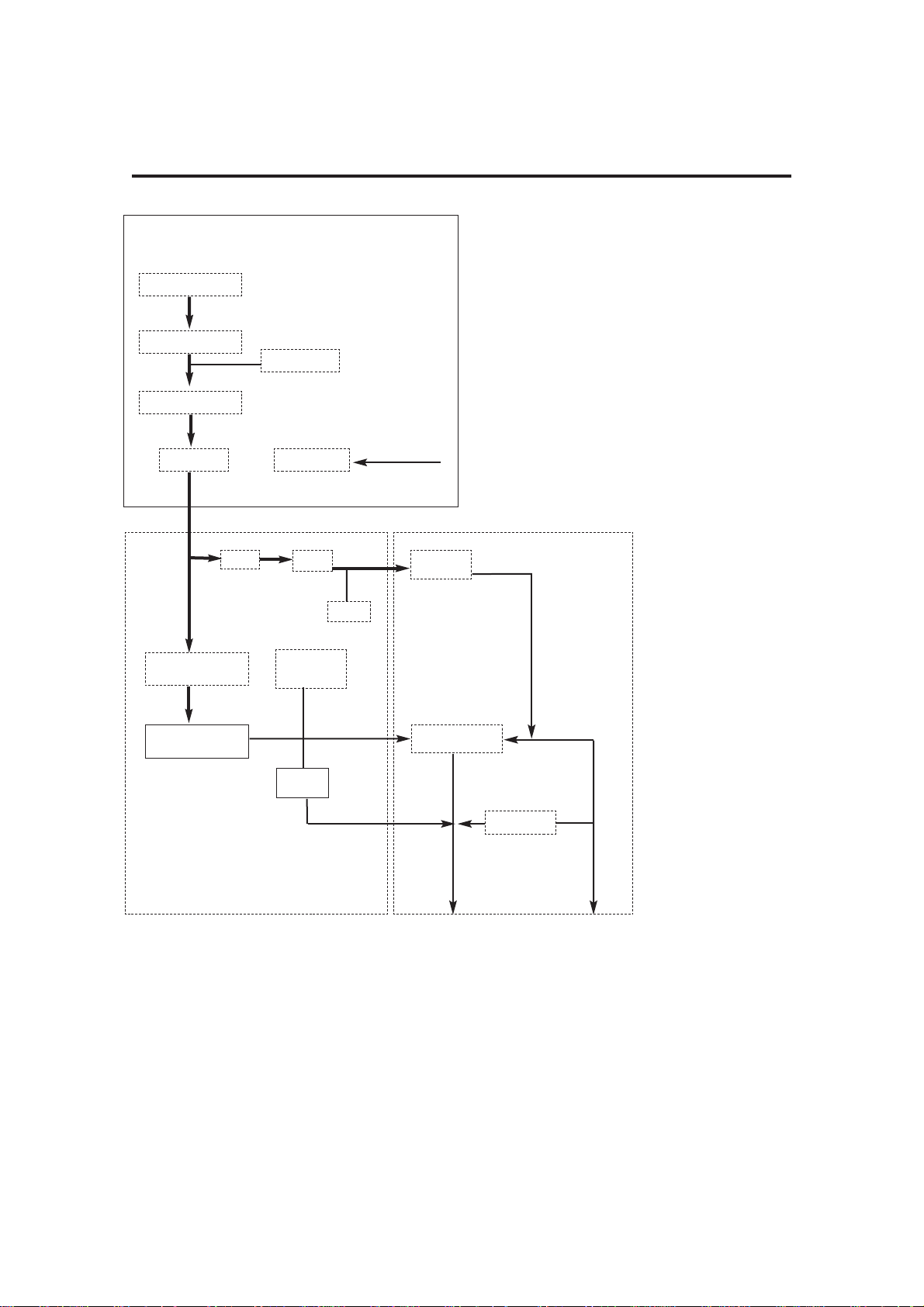
3. End of
Expiratory Phase
With the bellows at the top
of its housing fresh gas
continues to flow.
To prevent a high pressure
build up the exhalation
diaphragm (6) lifts and
allows gas to exit through
the exhaust valve (4).
4. PEEP
Positive End
Expiratory
Pressure
(user selectable)
The patient proportional
valve (3) applies PEEP
pressure plus 20 cmH2O to
the exhaust valve, which
remains closed at this stage.
As fresh gas flows in the
patient circuit, any pressure
increase above PEEP
pressure in the bellows (5)
will cause gas to bleed past
the exhaust valve (4).
If there is a fall in pressure in
the breathing circuit, the
continuous flow from the
drive gas proportional valve
(1) helps maintain the set
PEEP pressure.
11
6
4
5
5
12
DESCRIPTION
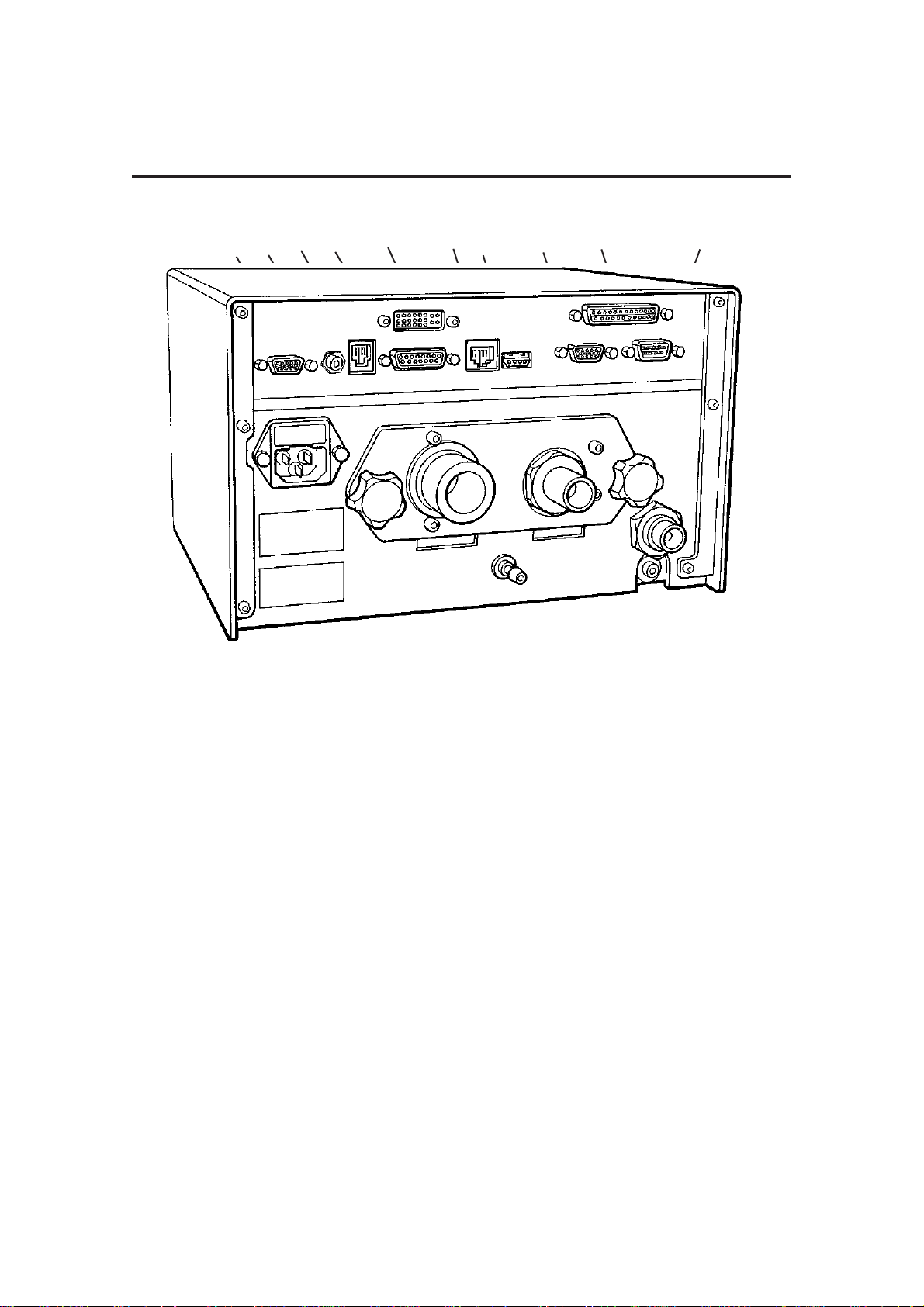
A
Pneumatic Flow
Diagram
C
1817
5
8
14
9
12
6
16
13
15
11
7
1
2
10
4
3
B
0 - 80 cmH2O
100 cmH2O
0 - 90 cmH2O
241 kPa (35 psi)
3 to 7 bar

3.3 Pneumatic System
Refer to the pneumatic system diagram on the previous page.
A) Gas inlet manifold block
The AV-S Ventilator is designed to operate on a 310 - 689 kPa (45 -100 psi)
drive gas supply (oxygen or air - to customer’s requirement).
1. Drive Gas Inlet Connector
The gas source is connected to the DRIVE GAS SUPPLY fitting on the
rear of the ventilator control unit.
The gas supply should be capable of a flow rate of 80 L/min while
maintaining a minimum pressure in excess of 310 kPa (45 psi).
2. Filter
The drive gas is filtered with a 40-micron Input Gas Filter which protects
the pneumatic components from incoming particulate matter.
3. The Low Supply Pressure Detector
The pressure switch is set at a predetermined level to detect a loss or
reduction of the input gas source pressure.
When the pressure falls below 235 kPa (35 psi ± 1 psi), the LOW
SUPPLY PRESSURE indicator will be displayed and the high priority
audible alarm will activate.
4. Input Pressure Regulator
Regulates the input drive gas to 260 kPa ± 21 kPa (38 psi ± 3 psi).
5. Cut-off Valve
The valve isolates the gas supply :
a) when the ventilator is switched off
b) when a fault condition occurs.
6. Airway Pressure Sensor
Connected to expiratory limb of breathing circuit.
B) Pneumatic Control Manifold Block
7. Drive Gas Proportional Valve
8. Drive Gas Flow Sensor
9. Drive Gas Pressure Sensor
10. Low Pressure Regulator
11. Patient Proportional Valve
12. PEEP pressure sensor
13. Restrictor
The restrictor allows a flow of up to 2 L/min (<2 L/min bleeding)
C) Exhaust Manifold Block
14. Check Valve
15. Diaphragm Valve
16. Pressure Relief valve - Set to 100 cmH
2O
17. Exhaust Port ( to AGSS)
18. Bellows drive gas outlet (to bellows assembly)
DESCRIPTION
13

3.4 Electrical System
Mains Supply
The mains supply inlet is designed for
connection to the following mains voltage
supplies:
100 to 120 VAC, 50 to 60 Hz
200 to 240 VAC, 50 to 60 Hz
Note that the ventilator adjusts automatically to
the supply voltage range.
The connector is a standard IEC type.
Back-up Battery
In the event of mains electrical failure, the backup battery cuts in automatically.
Standard battery:
A fully charged battery will power the ventilator
for approximately 30 minutes (depending on
ventilator settings).
High-power battery (option):
A fully charged battery will power the ventilator
for approximately one hour (depending on
ventilator settings).
See Appendix 1 for battery care procedures.
DESCRIPTION
14
DESCRIPTION
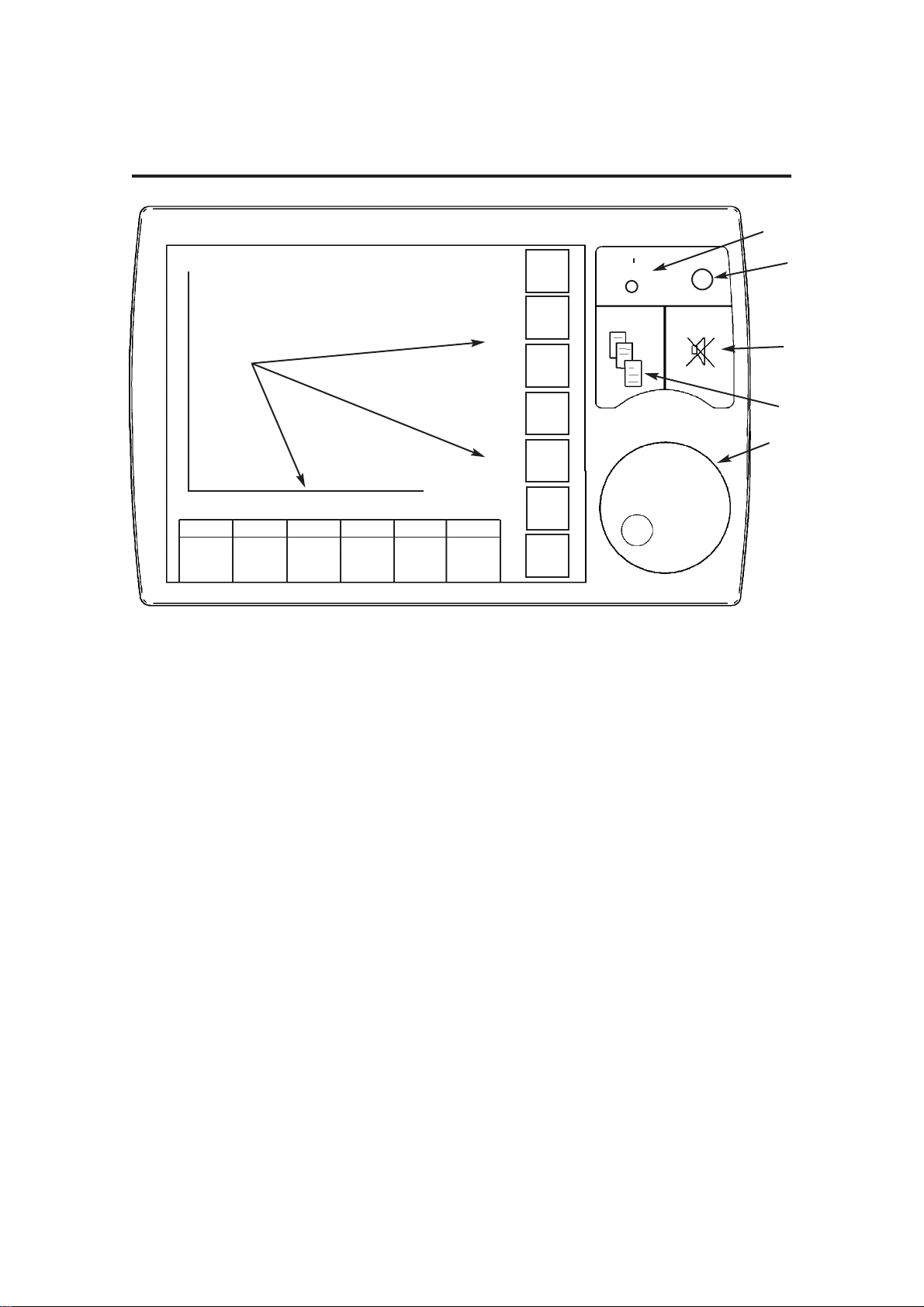
3.5 Control Panel
3.5.1 Touchscreen and Navigator Wheel / Push Button
1. On/Off control
Switch On: Short internal test sequence
Switch Off: Power down sequence with progress indicator
2. Status indicators for electrical power (mains/battery supply)
Yellow indicator - illuminated whenever power is applied to the unit and
internal battery is being charged
Green indicator - illuminates when the unit is switched on
3. Menu switch
The menu function provides access to user and service pages,
including alarm settings.
4. Alarm mute switch
30 second or 120 second alarm silence, depending on alarm status.
Note also that some alarms are not mutable (see 3.13).
5. Navigator Wheel and Press Button
Turn the wheel to select a function or parameter, or to alter the value
of an active parameter (see 3.5.4 and 3.5.5).
Press to confirm the setting.
6. Active Tabs
Touch the screen at the appropriate tab area to activate the required
function/parameter (see 3.5.4 and 3.5.5).
15
VT SETmLTPS INT
cmH2O
SET
BPM
SET
I:E
PEEP
cmH2O
LIMIT
cmH2O
VOLUME
SPONT
STANDBY
PSV
SIMV
SMMV
PRESS
AV-S
1
4
3
5
6
2
.
.
IO
o
o
16
DESCRIPTION
VT SETmLTPS INT
cmH2O
SET
BPM
SET
I:E
PEEP
cmH2O
LIMIT
cmH2O
VOLUME
SPONT
STANDBY
PSV
SIMV
SMMV
PRESS
AV-S
.
.
IO
o
o
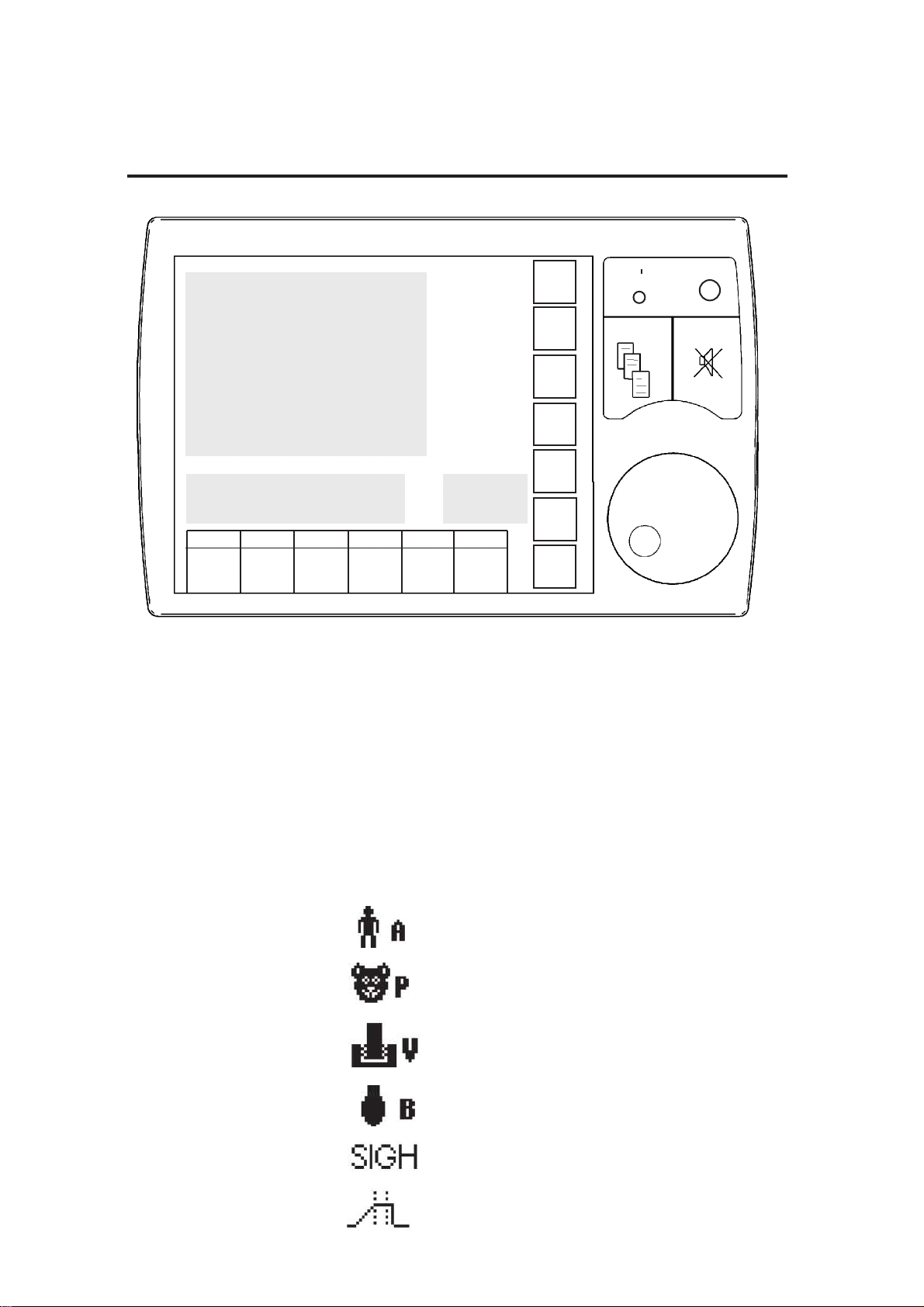
3.5.2 Additional Screen Functions and Displays
Area A Menu and sub-menu window
Waveform display, plus waveform pause and print symbols
Area B Alarm values window
Area C Gas mixture values window
Oxygen monitor values window
Area D Additional breathing mode information symbols:
(1) Adult mode
(2) Paediatric
mode
(3) Ventilator
mode
(4) Bag mode
(5) Sigh
(6) Inspiratory
pause
Area B
Area C
Area D
Area A
17
DESCRIPTION
3.5.3 Start-up Screens
1. Start-up
At start-up, the introduction
screen allows the user to
select one of three default
settings:
ADULT DEFAULTS
PAEDIATRIC DEFAULTS
SITE DEFAULTS
NOTE
a) The user must select one
of the above default groups
before the ventilator will
switch to standby in that
default mode
b) SITE DEFAULT is editable
in standby mode (see below)
c) Settings can be saved via
the service menu to create a
new site default
2. Default Settings
Selection
The user can select ADULT,
or PAEDIATRIC, or SITE, and
view the default parameter
settings.
The options will remain, even
after the ventilator is turned
off.
Site Default Settings
(typical values shown)
Adjust the parameter values
from within the Service menu
(SITE DEFAULTS)
Press to confirm the new
settings for site defaults.
3. Screen Calibration
Adjust the brightness of the
screen back lighting.
<Paediatric Defaults<
VT Set : 150 mL
VM Set : 2.2 Litres
T+PS INT : 10 cmH
2O
Set BPM : 15
I : E : 1: 2.0
PEEP : OFF
Limit : 38 cmH
2O
Trigger : 1.0 L/min
Apnoea Alarm Limit : 15 secs
Volume Type: Tidal
CALIBRATE TOUCH SCREEN
SITE DEFAULTS
VIEW
ADULT DEFAULTS
VIEW
PAEDIATRIC DEFAULTS
PRESS TO CONFIRM
<Site Defaults<
VT Set : 800 mL
VM Set : 6.0 Litres
T+PS INT : 10 cmH2O
Set BPM : 10
I : E : 1: 2.0
PEEP : OFF
Limit : 38 cmH
2O
Trigger : 1.0 L/min
Apnoea Alarm Limit : 15 secs
Volume Type: Tidal
CALIBRATE TOUCH SCREEN
SITE DEFAULTS
PRESS TO CONFIRM
ADULT DEFAULTS
VIEW
PAEDIATRIC DEFAULTS
VIEW
<Adult Defaults<
VT Set : 600 mL
VM Set : 6.0 Litres
T+PS INT : 10 cmH
2O
Set BPM : 10
I : E : 1: 2.0
PEEP : OFF
Limit : 38 cmH
2O
Trigger : 1.0 L/min
Apnoea Alarm Limit : 15 secs
Volume Type: Tidal
CALIBRATE TOUCH SCREEN
SITE DEFAULTS
VIEW
ADULT DEFAULTS
PRESS TO CONFIRM
PAEDIATRIC DEFAULTS
VIEW

18
DESCRIPTION
3.5.4 Selecting Functions and Parameters
The functions/parameters shown on the screen can be activated as
follows:
a) touch the screen at the appropriate tab area, or
b) rotate the navigator wheel and press it when the indicator arrow
is on the required parameter tab
Note that unless Site Defaults are selected (see above) parameters
default to factory-set values for Adult or Paediatric patients when
the ventilator is switched on from OFF, and no further user selection
is made.
3.5.5 User Adjustable Parameters
Variable parameters can be altered by rotating the navigator
wheel.
When the required value is displayed, press the active tab, or
the wheel, to confirm the setting.
Tidal Volume Range 20-1600 ml
Rate 4-100 bpm
I:E Ratio 1:0.3 to 1:8
PEEP 4-20 cmH2O
Can be set to OFF
Pressure Limit
Volume mode: 10-80 cmH2O
Pressure mode: 10-50 cmH2O
Alarm limits (user adjustable alarms only - see 3.13)
3.5.6 Parameter Display Identification
1. Active Parameters
Active parameters that can be set for use in the current
mode are displayed as:
White Text on Blue
2. Inactive Parameters
Inactive parameters that can be set for any non-current
mode are displayed as:
White Text on Blue Label
White values on Black
3. Measured Parameters
Yellow values on Black
4. T+PS INIT (target and pressure support initial value)
The initial pressure value can be changed so that when
entering either PRESSURE or PSV modes the TARGET
value or PSUPP value are pre-selected.
Note that changing either of these limits in their active modes will
maintain the value when changing between PSV, PRESSURE, and
STANDBY modes.
 Loading...
Loading...