Pelton Crane DeltaQ User manual

USE & CARE
Model AE (8")
Model AF (10")

Dear Customer
Congratulations on the purchase of your new Delta-Q Autoclave. The technical documentation provided is designed as a part of
this product. Always keep this documentation handy. The Use & Care Manual describes the 8” and 10” models. Please read the
instructions and get to know the autoclave. Please carryout maintenance according to relevent instructions.
Pelton & Crane
TABLE OF CONTENTS
Important Safety Instructions ..................................................................................................................................................... 3
Familiarization ............................................................................................................................................................................. 6
Operating Features ............................................................................................................................................................. 6
Safety Features .................................................................................................................................................................. 6
Visual Displays ................................................................................................................................................................... 4
Switch Functions ................................................................................................................................................................ 6
Audible Signals ................................................................................................................................................................... 8
Program Parameters ........................................................................................................................................................... 8
Installation .................................................................................................................................................................................... 9
Programming ............................................................................................................................................................................. 10
Operation ................................................................................................................................................................................... 11
Important Sterilization Practices ........................................................................................................................................ 11
Preparation and Loading ................................................................................................................................................... 12
Operating Steps ................................................................................................................................................................ 13
Maintenance ............................................................................................................................................................................... 14
Maintenance and Performance Schedule .......................................................................................................................... 14
Cleaning Procedure .................................................................................................................................................... 15 -16
Tr o ub l e Shooting Guide ..................................................................................................................................................... 17
Self-Diagnostic Check ...................................................................................................................................................... 19
Operating Alarms .............................................................................................................................................................. 20
Options ....................................................................................................................................................................................... 21
Installation and Servicing Checklist ......................................................................................................................................... 22
-2-
! IMPORTANT SAFETY INSTRUCTIONS !
Caution! Personal Safety
•To avoid electrical shock, never insert plug into outlet with
wet hands.
•Do not attempt to open door unless pressure gauge reads
“0” or injury may result.
•Do not operate Autoclave in area containing combustible
gases.
•Do not place Autoclave within 6 feet of patient.
•Routinely inspect power cord for cuts and abrasions.
Discontinue use and have authorized service representative replace cord if damaged.
•Protect your hands from contact with soiled instruments to
prevent serious infections. Wear heavy rubber gloves when
handling instruments.
Warning! T o A v o i d S e r i o us B u r n s:
Do not open door during sterilization cycle.
Stand to one side when opening door after
sterilization cycle and do not place hands or
face over door.
-Use a tool or hot pad to remove trays and avoid
touching chamber walls.
-Stand away from door after the sterilization cycle is
completed. Liquids may still be in the chamber and
can cause serious burns.
-Ensure unit is properly leveled. Follow the Installa-
tion Instructions on page 9.
-When performing safety valve maintenance (pg. 14)
stand clear of discharge area (lower right corner at
rear of unit).
Caution! Check weekly for proper
door switch operation (see pg. 14).
Product Safety
•This unit requires a dedicated circuit (separate branch circuit
only).
•Always use distilled quality water. Tap water will cause
corrosion of chamber and clog valves and filters with mineral
deposits.
•Never operate unit outside the specified voltage range (see
data plate on back of unit).
•Do not use steel wool or steel brushes on stainless steel.
Metal pads will damage chamber.
•Use only manufacturer’s replacement parts/accessories.
Failure to do so may cause poor performanc e.
•Refer servicing to authorized service representative.
•Do not position the unit so that it is difficult to reach the
circuit breaker on the power plug.
WARNING: If unit is operating in high altitude,
adjustments to time, temperature or pressure
may be required. Also, leaking of safety valve
may indicate need for adjustment.
Do not remove cover: Electrical shock
hazard. Refer servicing to authorized
service representative . Disconnect power
before servicing.
DANGER: Do Not attempt to open door with
pressure in the chamber. Avoid direct contact with hot chamber walls or sterilized load.
Use metal handle (8” model only) and gloves.
Warning! —To avoid serious burns —
If used for liquid sterilization, the liquid
must be allowed to cool or the liquid may
boil when exposed to atmospheric pressure. Pelton & Crane does not recommend use of
this device for liquid sterilization.
Product Disposal
Caution!
Contact your local authorized
dealer for proper disposal of the
device or the components of the
device to ensure compliance with
your local environmental regulations.
CAUTION: ANY LIQUIDS THAT ARE STERILIZED
IN THIS UNIT ARE FOR LABORATORY USE
ONLY AND NOT FOR USE IN DIRECT PATIENT
CONTACT.
Interference with electromedical devices:
To g u a r a nt e e t h e o p e r a t io n a l s a f e ty o f e l ec t r o m e d ic a l d e v i c e s,
it is recommended that the operation of mobile radio telephones in the medical practice or hospital be prohibited.
Strong EMI sources such as electro surgery units or x-ray
units may effect performance. If performance problems occur,
move the unit to another electrical circuit or physical location.
-3-
Power
Ground
Mode
Printer Connection
PRODUCT INFORMATION
Table of Sym b ols
Program
Hot Surface
Low Water
Dry
Printer On/Off
Arrows
Attention: Printer Connection Only
Clear/Start
The conformity of the quality management system is certified with Certificate No. 369CE, dated April 8, 1999 by:
AMTAC Certification Services, LTD
Norman Road, Broadheath, Altrincham
Cheshire WA 14 4EP, United Kingdom
CAUTION. Failure to carefully follow the
described procedure may result in damage
to the equipment.
WAR NING . Fail ure to care fully fo llow the
described procedure may result in damage
to the equipment and the operator.
Risk of electrical shock present. Make sure
power is disconnected before attempting this
procedure.
Ready
Sterilize
-4-
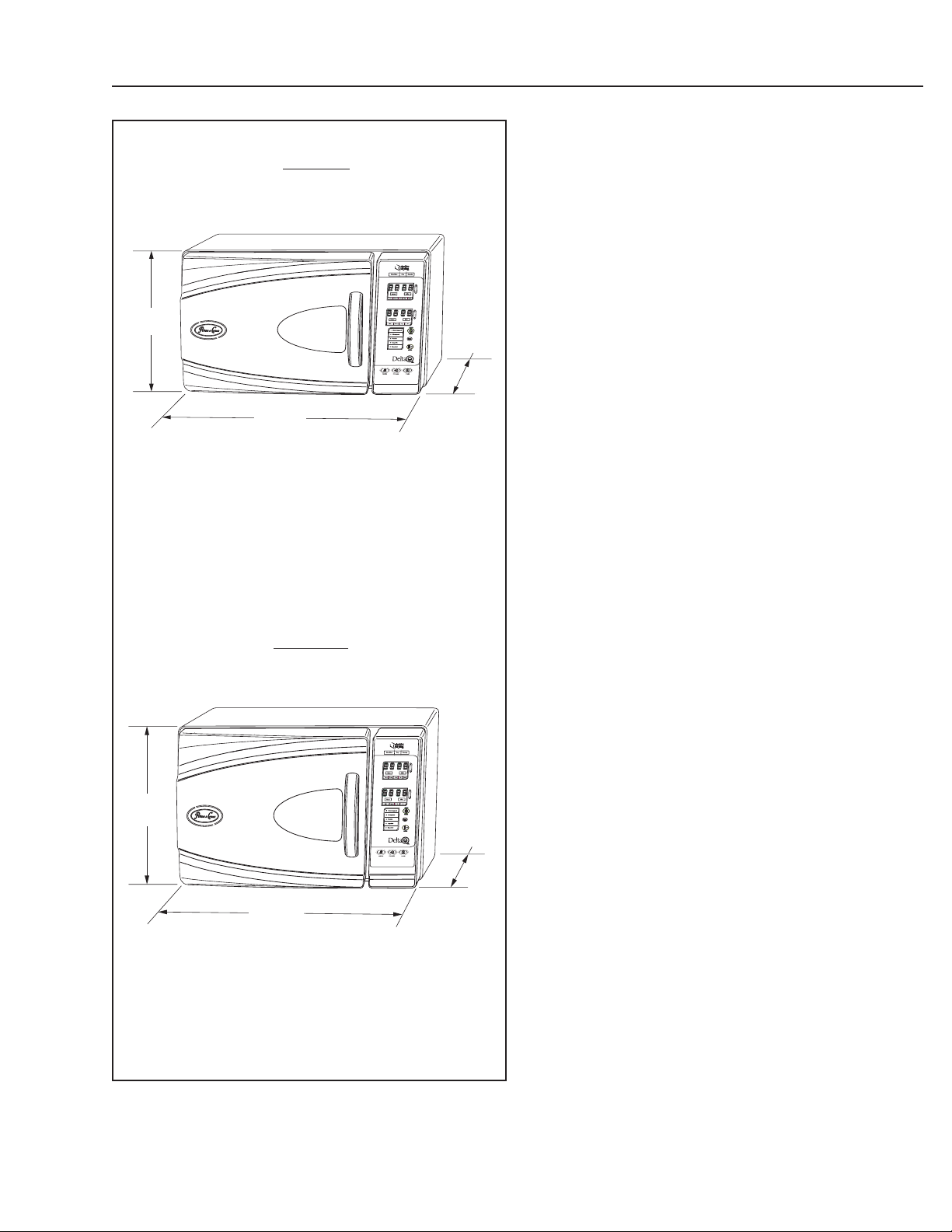
12 1/4”
(31.1cm)
14”
(35.6cm)
8” Model
17 ½”
(44.5cm)
10” Model
19 1/4”
(48.9cm)
PRODUCT INFORMATION
Specifications
Exterior Dimensions
8” Model12 1/4” high x 20 1/4” deep x 17 1/2” wide
(31.1 cm high x 51.4 cm deep x 44.5 cm wide)
10” Model14” high x 24 5/8” deep x 19 1/4” wide
(35.6 cm high x 62.5 cm deep x 48.9 cm wide)
Chamber Dimensions
8” Model8 7/32” inside diameter x 14” useable depth
(21 cm inside diameter x 35.6 cm deep)
20 1/4”
(44.5cm)
24 5/8”
(62.5cm)
10” Model9 7/8” inside diameter x 17 7/16” useable depth
(25.1 cm inside diameter x 44.3 cm deep)
Weight Without Water in Reservoir
8” Model61 lbs. (28 kg)
10” Model84 lbs. (38 kg)
Measurement Accuracy
Pressure: +/- 8 kPa (1.16 PSI or .08 bars)
Te m p e ra t u r e : + / - 3 . 6 ° F ( 2 ° C )
Time: +/- 1 second
Power Supply
Both Models110 - 120 Volts, 50/60 Hz or
220 - 240 Volts, 50/60 Hz
Nominal Current Consumption
8” Model12 Amperes @115 Volts
6 Amperes @230 Volts
10” Model10 Amperes @115 Volts
8 Amperes @230 Volts
Environmental and Storage Limitations
Both ModelsOptimum Operating Temperature Range:
50°F to 104°F
(10°C to 40°C)
Relative Humidity Range:
30% to 75%
Unit is designed for normal dental/medical office environ-
ment.
NOTE: If printer is used, its operating temperature is 41°F
to 95°F (5°C to 35°C).
Mode of Operation:
Both Models- Continuous
NOTICE: Manu facture r wi ll m ake available all information
which will assist the authorized service representative to
repair equipment. Calibration of the power board is to be
done only at the factory.
-5-
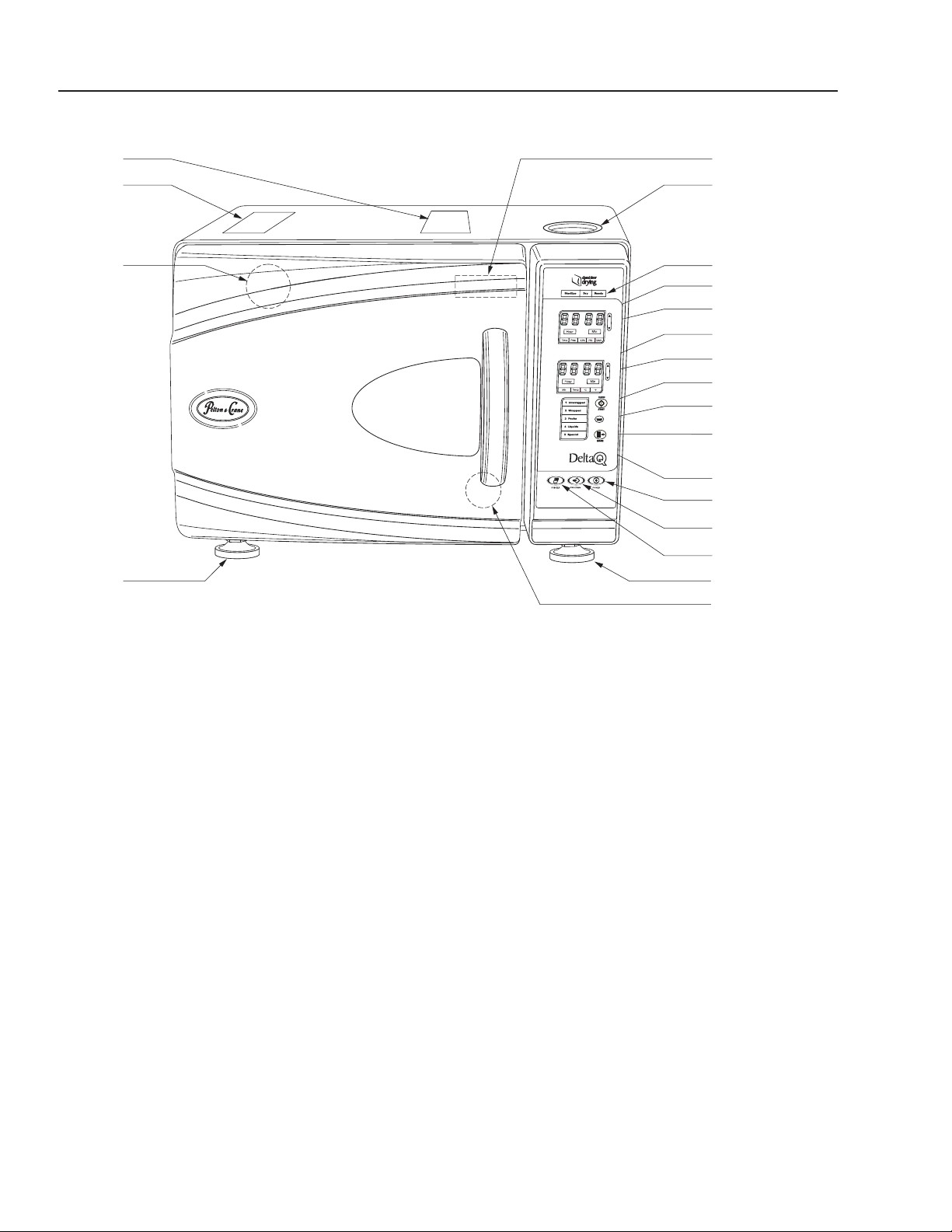
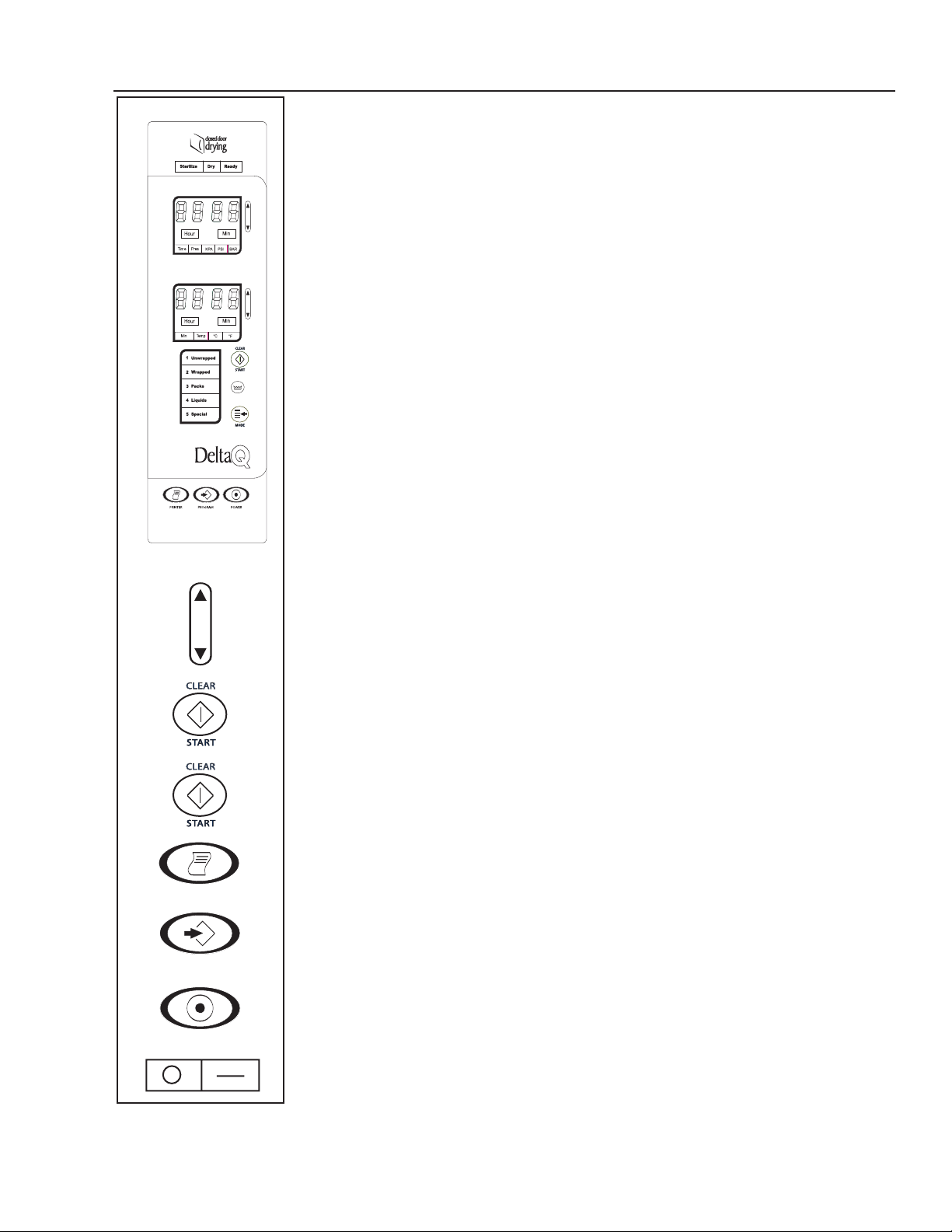
FAMILI ARIZATION
19
18
17
15
20
2
3
4
5
6
5
7
8
9
10
11
12
13
15
14
OPERATING FEATURES
1. Power Switch/Circuit Breaker (rear of unit)
2. Reservoir Fill
3. Operation Indicator Light
4. Display Window (Pressure) kPa
5. Arrow Switches
6. Display Window (Temp/Time) C/F / Minutes
7. Clear/Start Switch
8. Low Water Light
9. Mode Selection Switch
10. Mode/Program Display
11. Power On Switch
12. Programming Switch
13. Printer On/Off Switch
14. Quick Drain Connection (inside door)
15. Leveling Feet
16. Door Lock
17. Safety Valve (rear corner of unit)
18. Operating Instructions Label
19. Caution Label
20. Serial Number Plate (inside door)
.
SAFETY FEATURES
The design of the autoclave has these safety features for
your protection:
Door Lock
Door can be opened only when internal pressure is at
atmospheric pressure.
Vent Valve
The vent valve will open and the P-2 alarm will display should
the chamber pressure exceed 240 kPa.
Safety Valve
The safety valve opens as backup protection should the
chamber pressure exceed 262 kPa.
Overheat Protection
Chamber temperature is protected with a surface sensor so
the temperature will not exceed 159°C. It has additional
overheat protection should the temperature of the heating
elements reach 180°C.
Electrical Power Interruption
In case of a power failure during the sterilization cycle,
pressure in the chamber is automatically vented to the
atmosphere and display is blank
-6-
FAMILI ARIZATION
VISUAL DISPLAYS
Indicator Lights
“Sterilize” light illuminates to indicate sterilization cycle in progress.
“Dry” light indicates the heater and pump are on for the drying cycle.
“Ready” light illuminates when instruments may be removed from chamber.
Upper Window
Displays Time, Pressure, Clock and Year.
Lower Window
Displays Temperature, Date, Operational Timer, Failure Codes, Power colon “:” (
power button is not activated when main power is ON) and End.
Mode/Program
See Program Parameters, pg. 10.
Low Water
When the water level in the reservoir is too low, the “Low Water” light illuminates.
SWITCH FUNCTIONS
Arrows
Increases or decreases values of digits flashing when programming the system
parameter.
Clear/Start
Controls the start of a sterilizing cycle when unit is in stand-by. Also, used to clear
a cycle and returns unit to stand-by. If depressed with “Power” switch, self-diagnostic check is performed.
Mode Selection
Press to select one of the five sterilization mode programs. (See page 14 for
special mode)
Printer On/Off
Use to switch the printer on or off.
Program
Sets the minutes, hour, day, month and year. Chooses units of temperature and
pressure. Changes the drying time. Also, chooses the Special mode to the parameter desired. It initiates selected display mode during sterilization.
Power
Powers on operating contro ls. LCD will be visible. If depres sed wi th “Clea r/Star t”
switch, self-diagnostic check is performed.
Main Power (back of unit)
Depress “I” side of switch to turn unit on. A colon “:” illuminates in the lower
display indicating power is on. Leave switch in the On position.
-7-
FAMI LIAR IZAT ION
AUDIBLE SIGNALS
Switches
One beep occurs when depressing switches, except when depressing Power Switch and Arrow switches. When pressing
Arrow or Power Switch, no beep occurs.
Sterilization/Dry Cycle
Five beeps indicate the Sterilization or Drying cycles are complete.
Operational Alarm
Sixty beeps indicates an operational error or alarm. Depress Clear/Start to put unit in standby mode.
Door Open
Continuous beeping indicates the door has been opened during or prior to start of cycle.
PROGRAM PARAMETERS*
Program/Temp**, Pres,
Time**
1Unwrapped/
134°C, 216 kPa for 3
minutes
2Wrapped/
134°C, 216 kPa for 12
minutes
3Packs/
121°C, 115 kPa for 30
minutes
4Liquids/
121°C, 115 kPa for 30
minutes
Items to be Sterilized
Instruments loose on a tray. Open glass or metal canisters. Heat-resistant rubber tubing
which will not be used in surgical procedure. Any items where 134°C-137°C for 3 minutes
is appropriate.
Loosely wrapped individual instruments.
Wrapped dental handpieces***. Multiple layers of instruments separated by fabric. Instruments in pouches. Wrapped tray of loose instruments. Heat-resistant rubber tubing. Any
items where 134°C-137°C for 12 minutes is appropriate.
Common groups of surgical instruments in commercially prepared packs. Surgical instruments subject to prolonged storage. Any items, other than liquids, where 121°C for 30
minutes is appropriate.
Liquids or gels that could boil or spill out of container. At end of sterilizing cycle, venting is
slowed to allow heat in liquid to dissipate
slowly and eliminate boilovers. Venting occurs at 20 kPa to complete the cycle. There is
NO drying cycle in the “Liquids” mode.
!CAUTION: ANY LIQUIDS THAT ARE STERILIZED IN THIS UNIT ARE FOR
LABORATORY USE ONLY AND NOT FOR USE IN DIRECT PATIENT CONTACT.
Warning! —To avoid serious burns —
If used for liquid sterilization, the liquid must be allowed to cool or the liquid
may boil when exposed to atmospheric pressure. Pelton & Crane does not
recommend use of this device for liquid sterilization.
5Special/
Programmable to
101°C to 135°C, for 1
to 90 minutes.
* For mixed loads, use the longer or lower temperature program (i.e., for loose instruments and surgical dressings in packs, use
“3 Packs”).
** Time and temperatures are minimums.
*** When sterilizing handpieces, check handpiece manufacturer’s recommendations for appropriate sterilization conditions. Use “2
Wrapped” program only if handpieces are able to withstand 134°C-137°C temperature.
The names of the various modes of operation are general categories.
When selecting the mode of operation, take into consideration the density of the individual load and the ability of the steam to
circulate and penetrate wraps. Then determine the correct programmed values to assure sterilization.
Dependent upon parameters user has programmed. Operator is responsible for correct
time and temperature settings for load.
-8-
 Loading...
Loading...