Page 1
EN
NE-C803-E_A_M09_140715.pdf
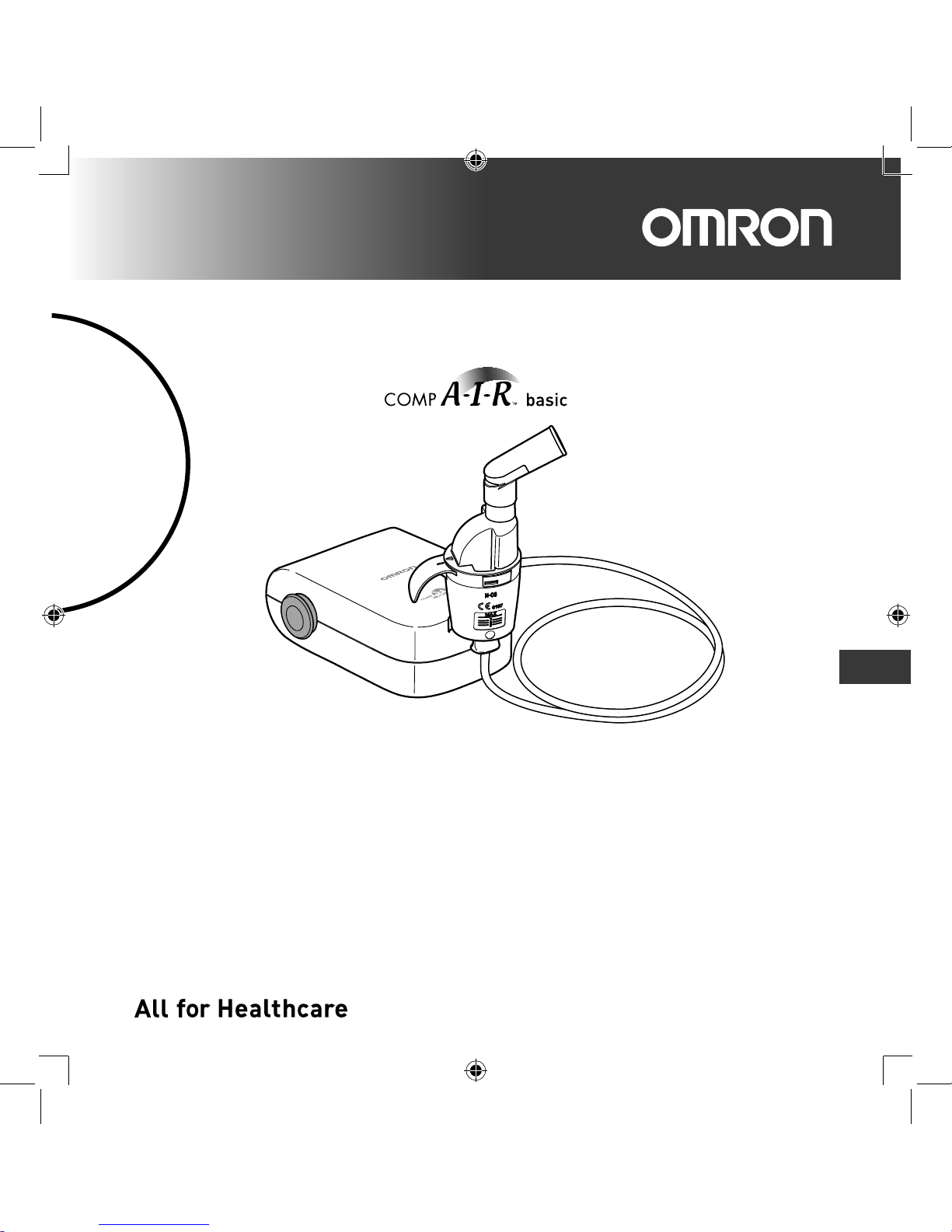
Compressor Nebulizer
NE-C803 (NE-C803-E)
Instruction Manual
IM-NE-C803-E-01-05/2014
8300641-7A
14G1762
Page 2

2
Introduction
Thank you for purchasing the OMRON COMP AIR basic.
This product was developed in conjunction with respiratory therapists for the successful
treatment of asthma, chronic bronchitis, allergies and other respiratory disorders.
This is a medical device. Operate the device only as instructed by your doctor and/or
respiratory therapist.
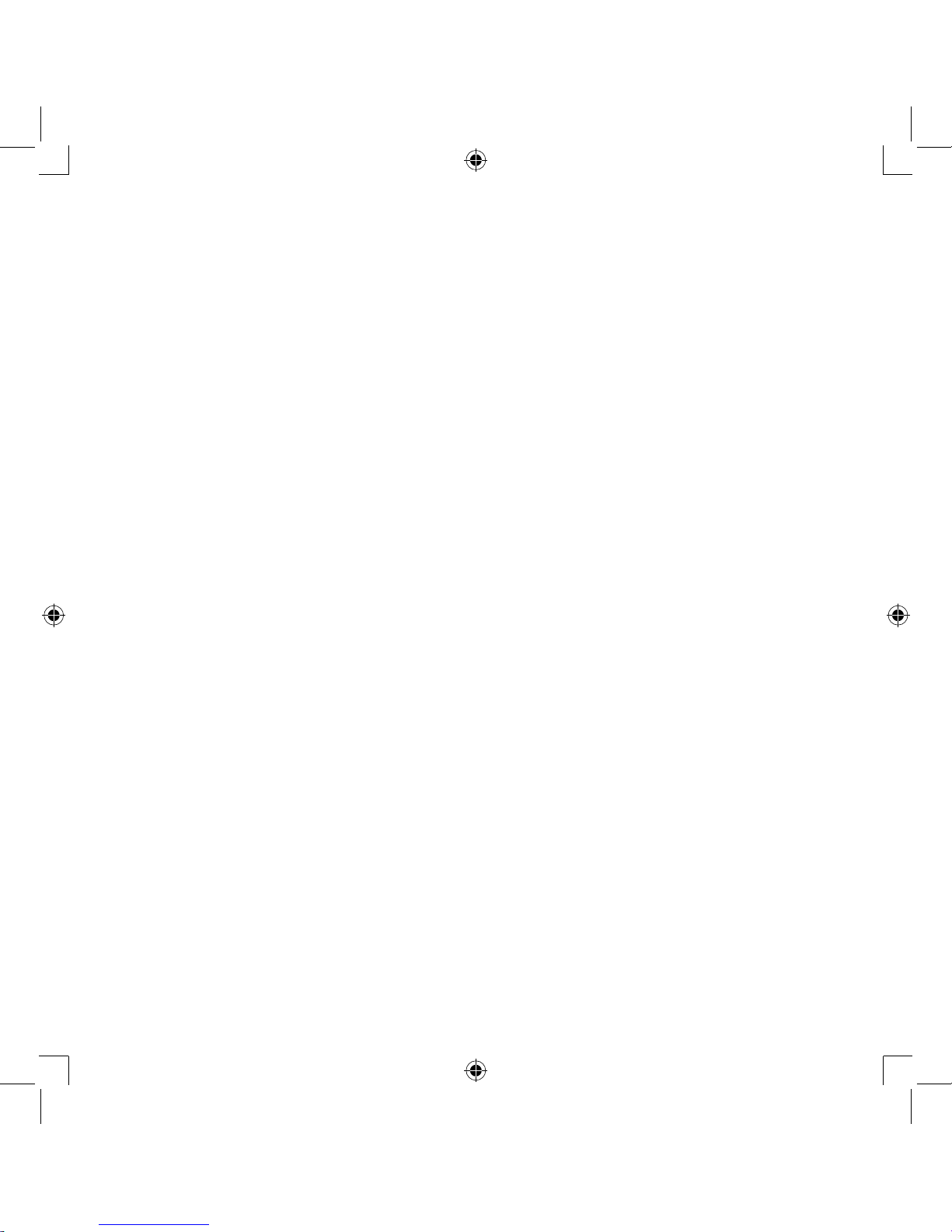
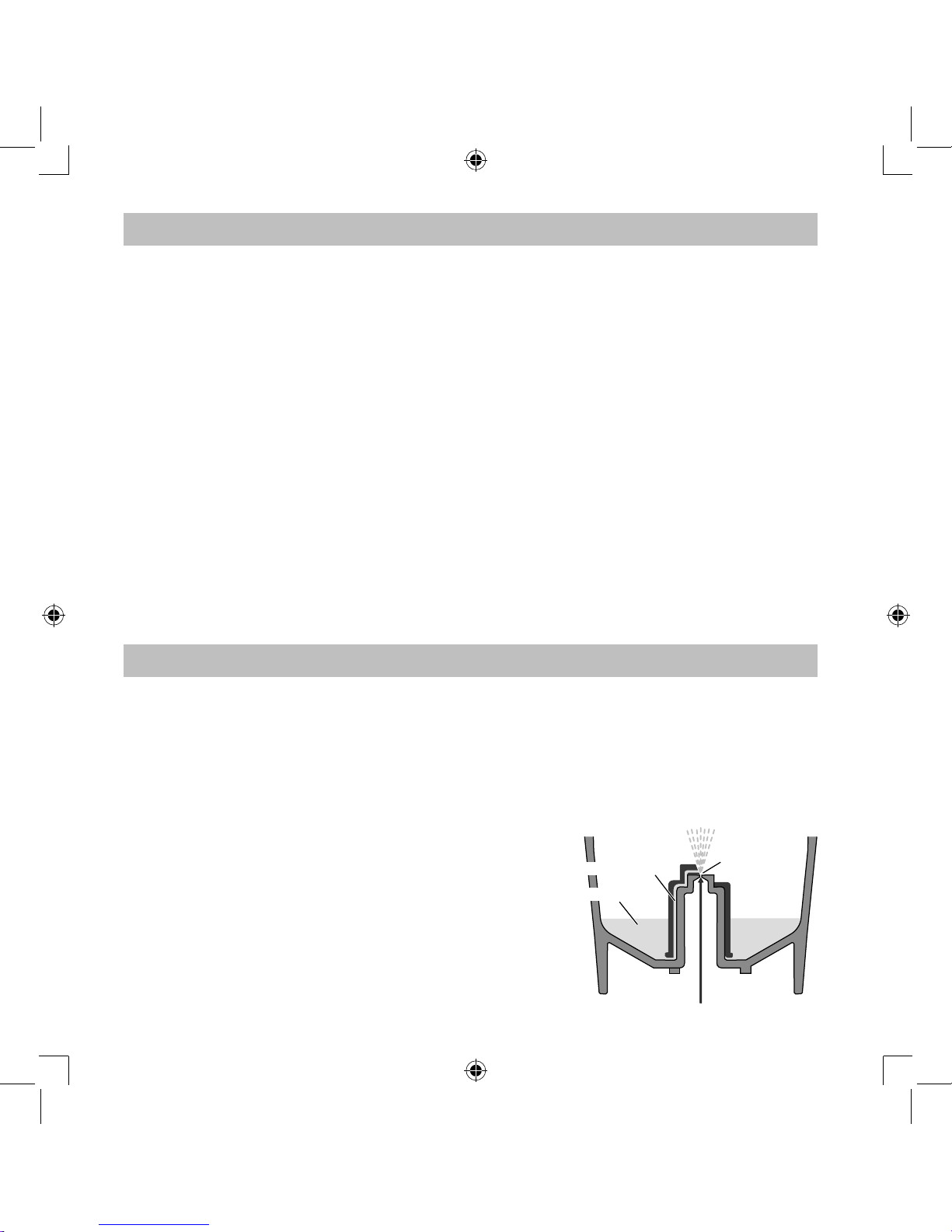
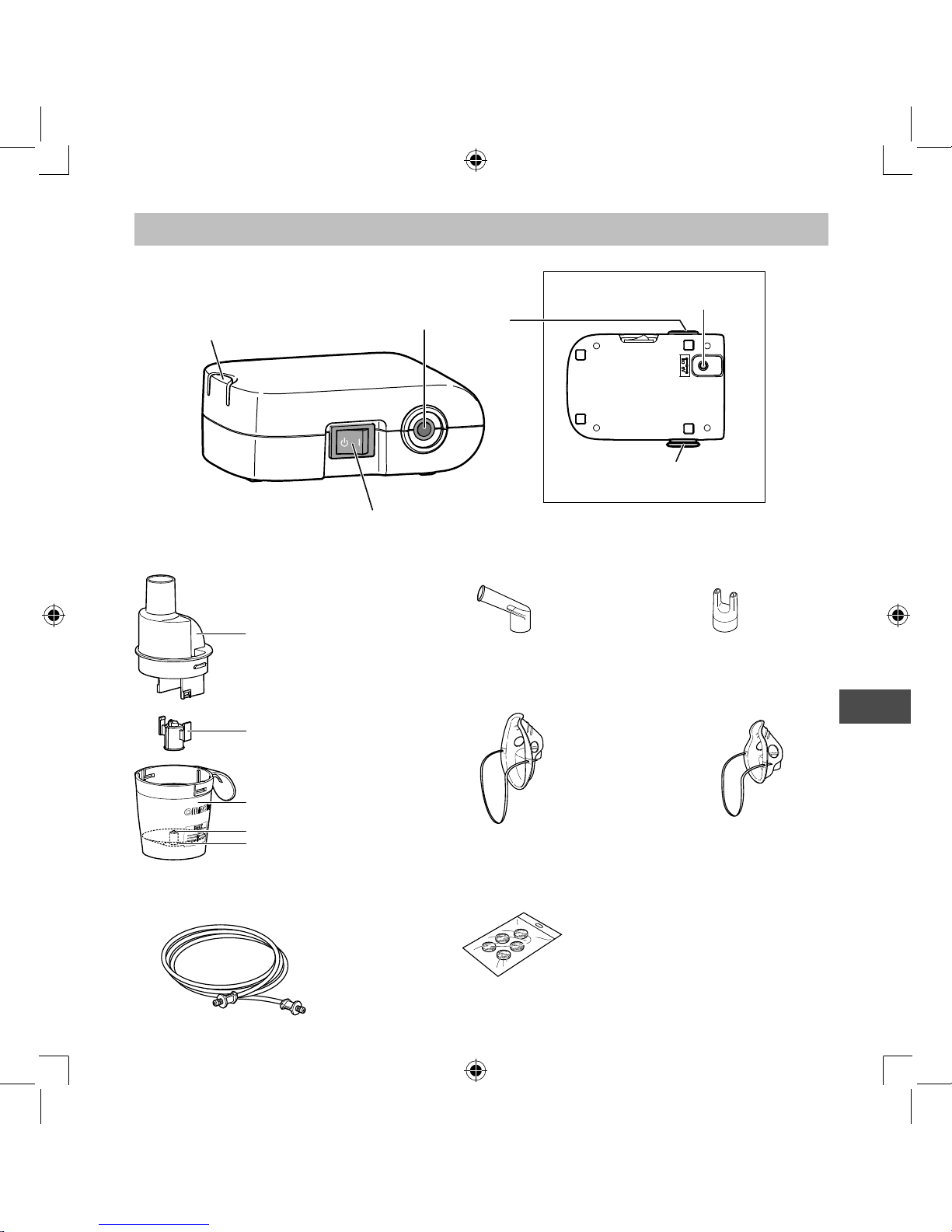
How the nebulizer kit works
The medication that is pumped up through the
medication channel is mixed with compressed air
which is generated by a compressor pump.
The medication is turned into fine particles and
sprayed when in contact with the compressed air.
Aerosol
Nozzle
Compressed Air
Medication Channel
Medication
Before using the device
Contents
Before using the device
Introduction ................................................................................................................. 2
Intended use ............................................................................................................... 3
Important safety instructions ....................................................................................... 4
Know your unit ............................................................................................................ 7
Optional Medical Accessories ..................................................................................... 8
Other Optional/Replacement Parts ............................................................................. 8
Operating instructions
How to use .................................................................................................................. 9
Care and maintenance
Cleaning and disinfecting.......................................................................................... 13
Removing condensation from the air tube ................................................................ 16
Changing the air filter................................................................................................ 16
Troubleshooting ........................................................................................................ 17
Technical data ........................................................................................................... 18
Guarantee ................................................................................................................. 21
Page 3
EN
3
Intended use
Medical Purpose
This product is intended to be used for inhaling medication for respiratory disorders.
Intended User
• Legally certified medical experts, such as doctor, nurse and therapist.
• Caregiver or patient under the guidance of qualified medical experts for home
treatment.
• The user should also be capable of understanding general operation of NE-C803 and
the content of this instruction manual.
Intended Patients
This product should not be used on patients, who are unconscious or are not breathing
spontaneously.
Environment
This product is intended for use in a medical facility, such as Hospital, clinic and doctor’s
office, and in a room of general household.
Durable period
Durable periods are as follows, provided the product is used to nebulize 2 ml of
medication twice a day for 7.5 minutes each time at room temperature (23°C).
Durable period may vary depending on usage environment.
Frequent use of the product may shorten the durable period.
Compressor (Main unit) 5 years
Nebulizer Kit 1 year
Mouthpiece 1 year
Nosepiece 1 year
Air Tube (PVC, 100cm) 1 year
Air Filter 60 days
Mask (PVC) (SEBS) 1 year
Precautions for use
Warnings and cautions described in the instruction manual should be observed.
Page 4
4
Important safety instructions
Read all the information in the instruction manual and any other literature included in the box before
using the device.
Warning:
Indicates a potentially hazardous situation which, if not avoided, could result in serious injury.
(Usage)
• For type, dose, and regime of medication follow the instructions of your doctor or respiratory therapist.
• If you feel anything unusual during use, stop using the device immediately and consult your doctor.
• Do not use only water in the nebulizer for inhaling purposes.
• Keep the device out of reach of unsupervised infants and children. The device may contain small pieces
that can be swallowed.
• Do not store the air tube while there is moisture or medication remaining inside.
• Do not use or store the device where it may be exposed to noxious fumes or volatile substances.
• Do not use the device where it may be exposed to flammable gas.
• Do not cover the compressor with a blanket, towel, or any other type of cover during use.
• Always dispose of any remaining medication after use, and use fresh medication each time.
• Do not use in anaesthetic or ventilator breathing circuits.
• Store the parts in a clean location to avoid infection.
(Risk of electrical shock)
• Never plug or unplug the AC adapter with wet hands.
• The compressor and the AC adapter are not waterproof. Do not spill water or other liquids on these parts.
If liquid does spill on these parts, immediately unplug the AC adapter
and wipe off the liquid with gauze or
other absorbent material.
• Do not immerse the compressor in water or other liquid.
• Do not use or store the device in humid locations.
• Do not operate the device with a damaged power cord or plug.
• Keep the power cord away from heated surfaces.
• Use only original OMRON AC adapter. Use of unsupported AC adapter may damage the device.
(Cleaning and disinfecting)
Observe the rules below when cleaning or disinfecting parts. Failure to observe these rules may result
in damage, inefficient nebulization or infection. For the instructions, refer to “
Cleaning and disinfecting
”
section.
• Clean and disinfect the nebulizer kit, mask, mouthpiece or nosepiece before using them when as
follows:
- the first time after purchase.
- if the device has not been used for a long period of time
- if more than one person uses the same device
• Be sure to wash or wipe the parts after use, and ensure that they are thoroughly disinfected and dried,
and stored in a clean location.
• Do not leave the cleaning solution in the parts.
Page 5
EN
5
Caution:
Indicates a potentially hazardous situation which if not avoided, may result in minor or
moderate injury, or physical damage.
(Usage)
• Provide close supervision when this device is used by, on, or near children or invalids.
• Make sure that the parts are attached correctly.
• Make sure that the air filter is correctly attached.
• Make sure that the air filter is clean. If the air filter has been used for more than 60 days, replace it with
a new one.
• Do not tilt the nebulizer kit at an angle of greater than 45 degrees in all directions or shake it while in use.
• Do not use or store the device while the air tube is creased.
• Use only original nebulizing parts, air tube, air filter and air filter cover.
• Do not add more than 10 ml of medication to the medication tank.
• Do not carry or leave the nebulizer kit while the medication tank contains medication.
• Do not leave the device unattended with infants or persons who cannot express their consent.
• Do not subject the device to any strong shocks such as dropping on the floor.
• Do not jab the nozzle of the medication tank with a pin or any sharp object.
• Do not insert fingers or objects inside the compressor.
• Do not disassemble or attempt to repair the compressor or AC adapter.
• Do not leave the device in extreme hot or cold temperature, or under direct sunlight.
• Do not use the device while sleeping or if drowsy.
• Approved for human use only.
• The compressor may become hot during operation.
• Do not touch the compressor for other than necessary operation such as turning off the power while
nebulizing.
• To avoid the medication residue on the face, be sure to wipe the face after removing the mask.
• To avoid injury to the nose mucosa, do not squeeze the nosepiece into the back of the nose.
• Do not use a damaged nebulizer kit, mouthpiece or nosepiece.
(Risk of electrical shock)
• Always unplug the AC adapter from the power outlet after use and before cleaning.
• Plug the device into the appropriate voltage outlet. Do not overload power outlets or use extension
cords.
• Do not misuse the power cord of AC adapter.
• Do not wind the power cord around the compressor or AC adapter.
• Do not pull the power cord of AC adapter.
• Changes or modifications not approved by OMRON HEALTHCARE will void the user warranty.
• Always remove the AC adapter from the device after use and before cleaning.
(Cleaning and disinfecting)
Observe the rules below when cleaning or disinfecting parts. Failure to observe these rules may result in
damage, inefficient nebulization or infection. For the instructions, refer to “Cleaning and disinfecting” section.
• Do not use a microwave oven, dish dryer or hair dryer to dry the device or the parts.
• Do not use an autoclave, EOG gas sterilizer or low temperature plasma sterilizer.
• When disinfecting parts by boiling, make sure that the container does not boil dry. Otherwise it may also
result in fire.
Page 6
6
General Safety Precautions:
• Inspect the device before using them each time, and check that there are no problems. In particular, be
sure to check the following:
- That the nozzle or air tube are not damaged.
- That the nozzle is not blocked.
- That the compressor operates normally.
• When using this device, there will be some noise and vibration caused by the pump in the compressor.
There will also be some noise caused by the emission of compressed air from the nebulizer kit. This is
normal and does not indicate a malfunction.
• Operate the device only as intended. Do not use the device for any other purpose.
• Do not use the device at temperatures greater than +40°C.
• Make sure that the air tube is securely attached to the compressor and nebulizer kit, and does not come
loose.
• To completely isolate the device from the power source unplug the plug from the power source.
Keep these instructions for future reference.
Page 7
EN
7
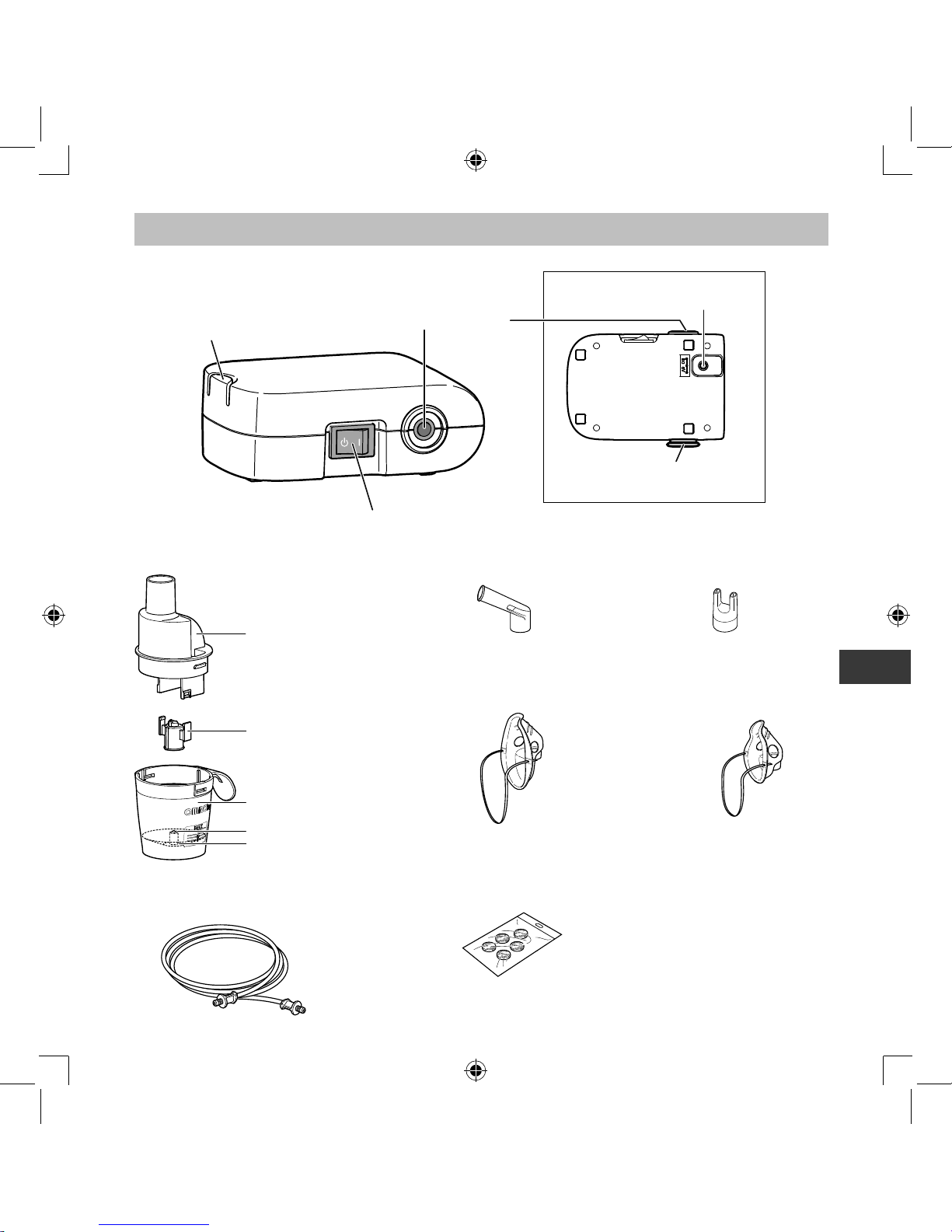
Know your unit
AC Adapter x 1
Storage Bag x 1
Instruction Manual
Rear View
Power Connector
Air Filter Cover
*Air filter inside
Compressor (Main Unit) x 1
Nebulizer Kit Holder
Air Tube Connector
Power Switch
Spare Air Filters x 5Air Tube
(PVC, 100cm) x 1
Mouthpiece x 1
Child Mask (PVC) x 1
Adult Mask (PVC) x 1
Nebulizer Kit x 1
Air Tube Connector
Inhalation Top
Vaporizer Head
Nozzle
Medication Tank
Nosepiece x 1
Page 8
8
Optional Medical Accessories
(within the scope of EC Medical Device Directive 93/42/EEC)
Product description Model
Nebulizer Kit & Mouthpiece Set NEB-NSET3-83E
Nebulizer Kit & Adult Mask (PVC) & Air Tube Set NEB-NSET4-83AP
Nebulizer Kit & Child Mask (PVC) & Air Tube Set NEB-NSET5-83AP
Adult Mask (PVC)
NEB-MSLP-E
Child Mask (PVC)
NEB-MSMP-E
Adult Mask (SEBS)
NEB-MSLS-E
Child Mask (SEBS) Set
NEB-MSSS-E
Mouthpiece
NEB-MP-E
Nosepiece
NEB-NP-E
Air Tube (PVC, 100cm)
NEB-TP-82E
AC Adapter HHP-CF01
Other Optional/Replacement Parts
Product description Model
Air Filters x 5
NEB-AFR-30E
O
Page 9
EN
9
Operating instructions
How to use
Warning:
Clean and disinfect the nebulizer kit, mask, mouthpiece or nosepiece before using them
when as follows:
- the first time after purchase.
- if the device has not been used for a long period of time
- if more than one person uses the same device
For the instructions, refer to “Cleaning and disinfecting” section.
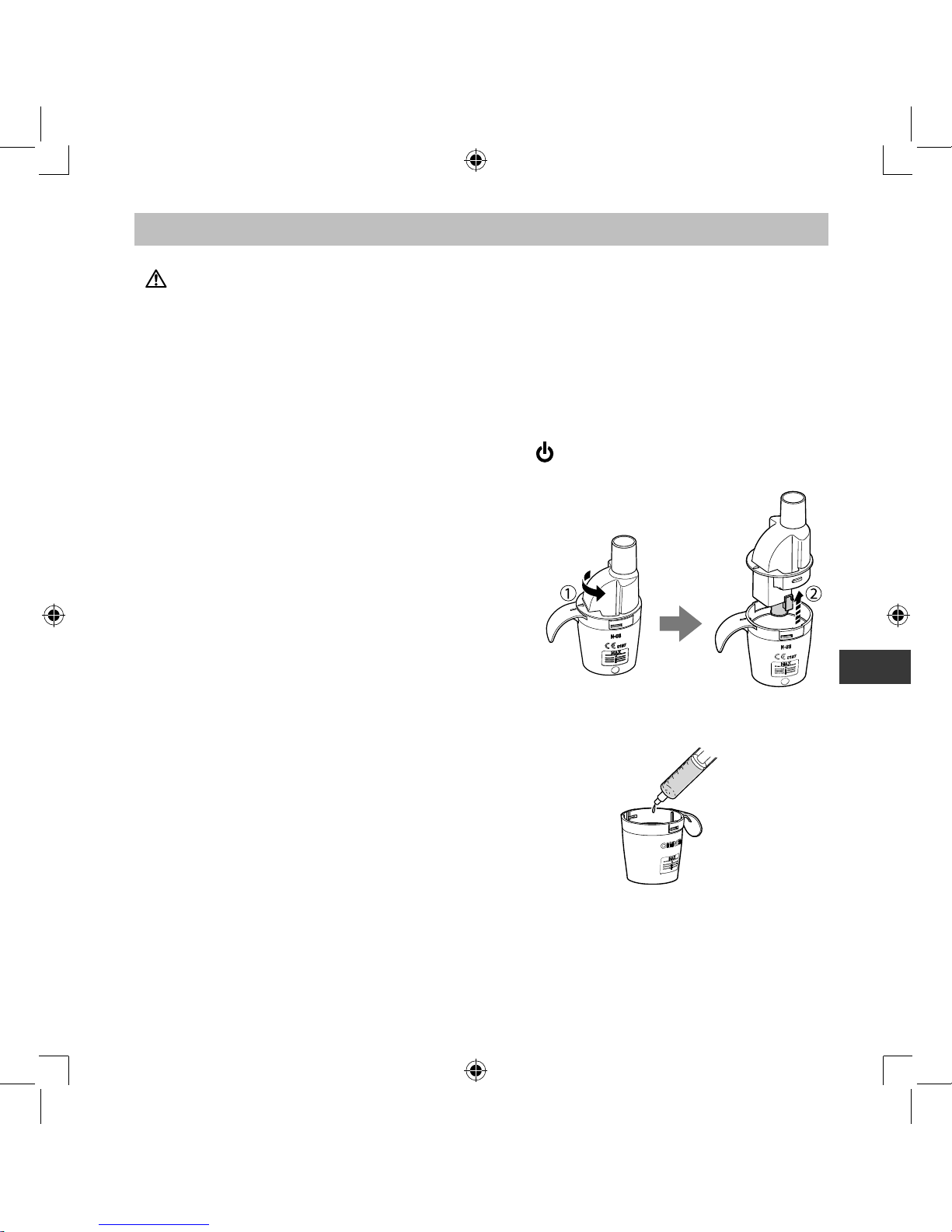
1.
Make sure that the power switch is in the off ( ) position.
2.
Connect the AC adapter to the compressor
and plug the power plug into a power outlet.
3.
Remove the inhalation top together with
vaporizer head from the medication tank.
Note:
If the vaporizer head came off the inhalation
top and fell into the medication tank, remove
it out of the tank.
4.
Add the correct amount of prescribed
medication to the medication tank.
Page 10
10
5.
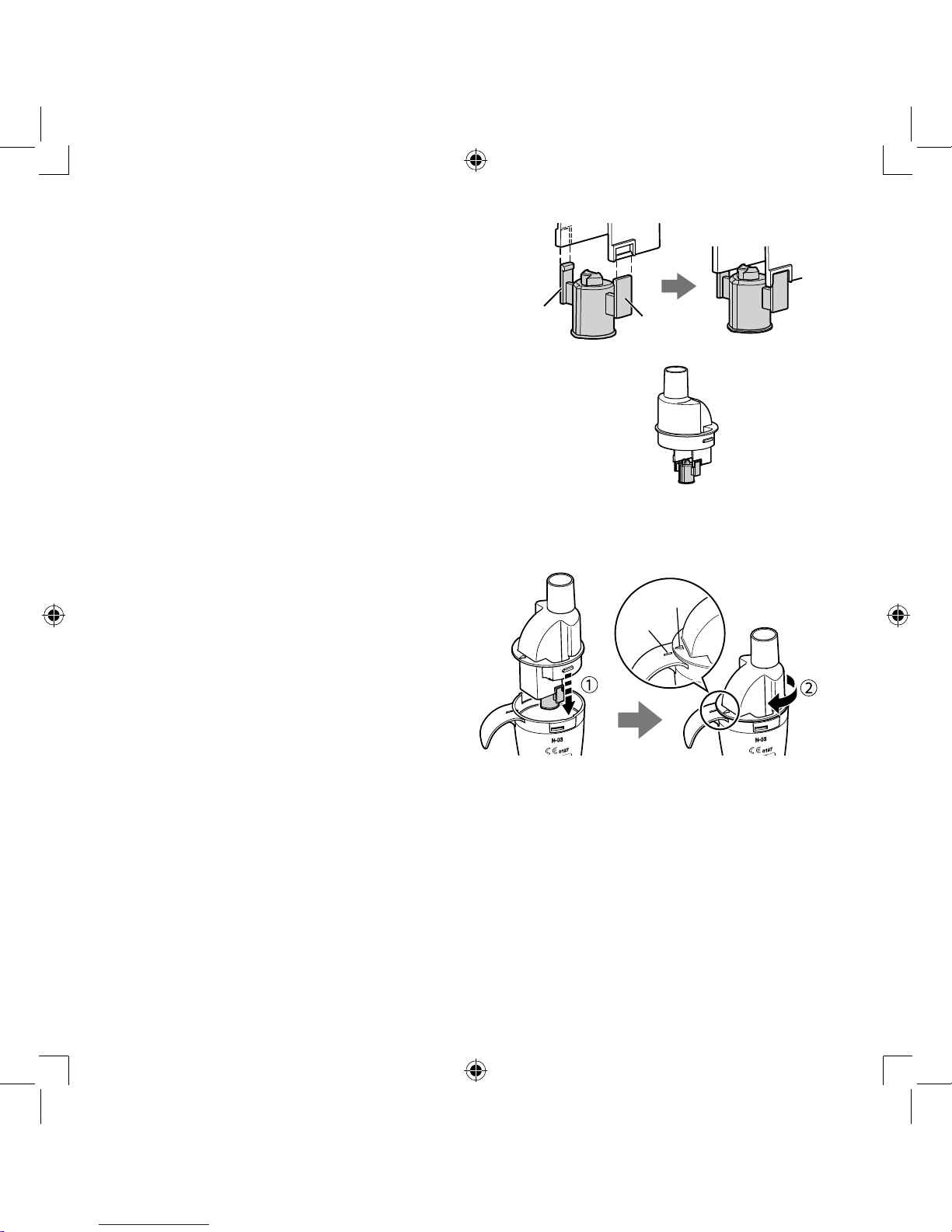
Make sure that the vaporizer head is
securely attached to the inhalation top.
Note:
The sizes of the left- and right-hand
attached parts of the vaporizer head are
not the same. If the vaporizer head is not
attached in the correct orientation, it will not
nebulize correctly.
6.
Put the inhalation top back inside the
medication tank.
1) Align the protrusion on the inhalation
top with the indent on the medication
tank, as shown.
2) Rotate the inhalation top clockwise
until the arrow mark on it and the
alignment mark on the medication
tank are aligned, and they click in
place.
Arrow mark
Alignment
mark
Small
Large
Page 11
EN
11
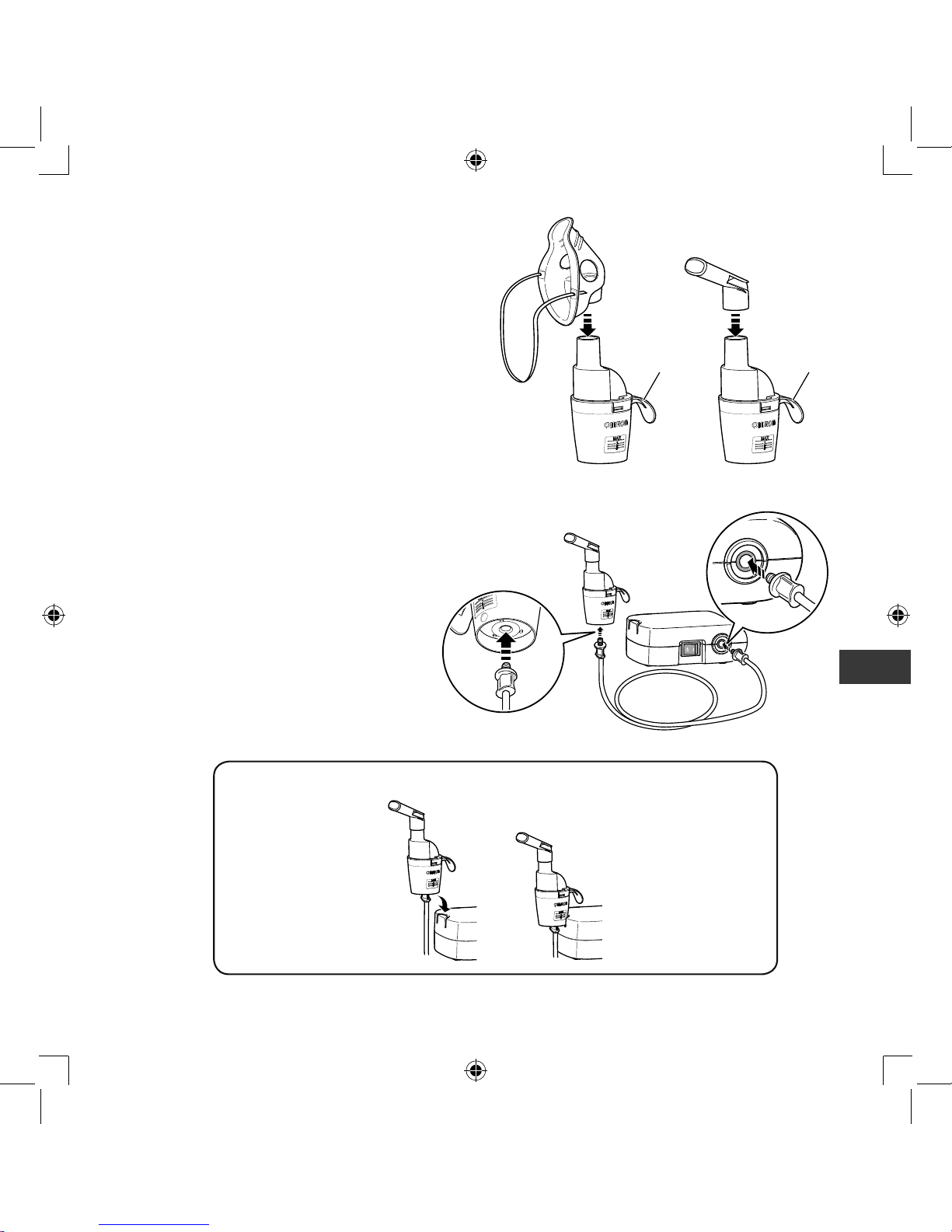
7.
Attach the mask, mouthpiece or
nosepiece to the nebulizer kit tightly.
Note:
Make sure that these attachments are
fitted in such a way that the handle is
positioned away from the patient.
Otherwise, medication may be sprayed
on patient’s skin or clothes.
8.
Attach the air tube.
Push the air tube plugs firmly into the air tube
connectors.
Handle Handle
The nebulizer kit can be fixed on the compressor.
Page 12
12
C
a
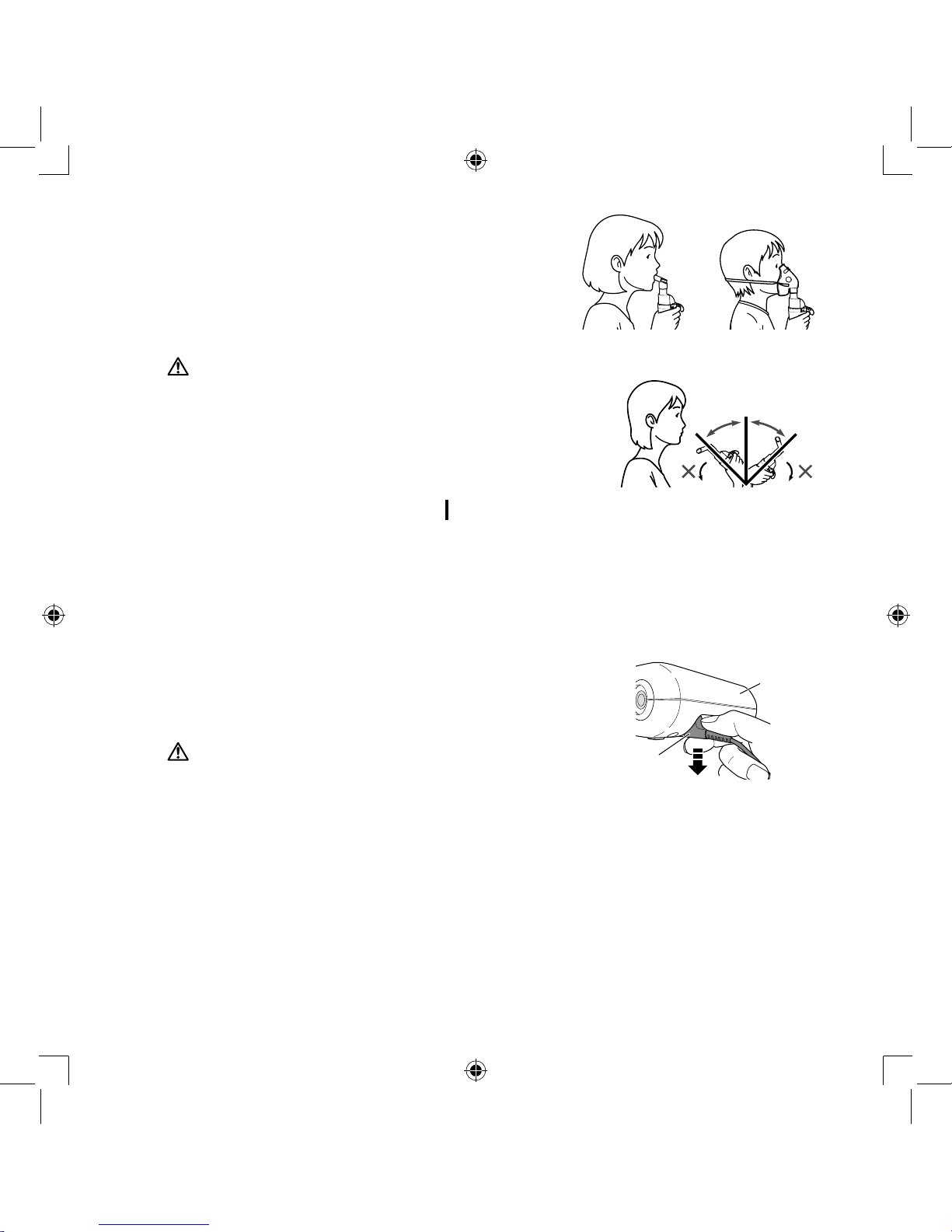
9.
Hold the nebulizer kit as indicated on the right.
Follow the instructions of your doctor or respiratory
therapist. Exhale as normal through mouthpiece,
mask or through the nose.
Caution:
Do not tilt the nebulizer kit at an angle of greater than 45
degrees in all directions. Medication may flow into the
mouth or it may result in not nebulizing effectively.
10.
Press the power switch to the on ( ) position. As the compressor starts,
nebulization begins and aerosol is generated.
Inhale the medication.
11.
When treatment is complete, turn the power off. Unplug the AC adapter from the
power outlet and disconnect it from the compressor.
Note:
Securely hold and press the power plug down
to disconnect the AC adapter.
Caution:
Do not pull the power cord.
Damage may be caused.
Right angle
45°45°
Compressor
Power plug
Page 13
EN
13
Care and maintenance
Cleaning and disinfecting
Caution: Handling the Vaporizer Head
• Always wash the vaporizer head after each use.
• Do not use a brush or pin to clean the vaporizer head.
• When disinfecting the parts by boiling, be sure to boil them in plenty of water.
• Do not boil vaporizer head together with objects other than applicable nebulizer
accessories.
Cleaning
Clean the parts after each use to remove residual medication. This will avoid inefficient
nebulization or infection.
■Washable parts
• Nebulizer Kit (Inhalation top, Medication tank), Mask (PVC)/(SEBS), Mouthpiece,
Nosepiece
Wash them in warm water and mild detergent (neutral detergent). Rinse them
thoroughly with clean hot tap water and allow to air dry in a clean place.
• Vaporizer Head
Wash it with running water.
■Non-washable parts
• Compressor, Air Tube
Firstly, make sure that the power plug is unplugged from the power outlet. Wipe clean
with a soft cloth moistened with water or mild detergent (neutral detergent).
• Air Filter
Do not wash or clean the air filter. If the air filter becomes wet, replace it. Damp air
filters can cause blockages.
Disinfecting
Disinfect the parts once a week. If the parts are heavily stained, replace them with new
ones.
To select a method for disinfection, refer to the table on page 14.
Page 14
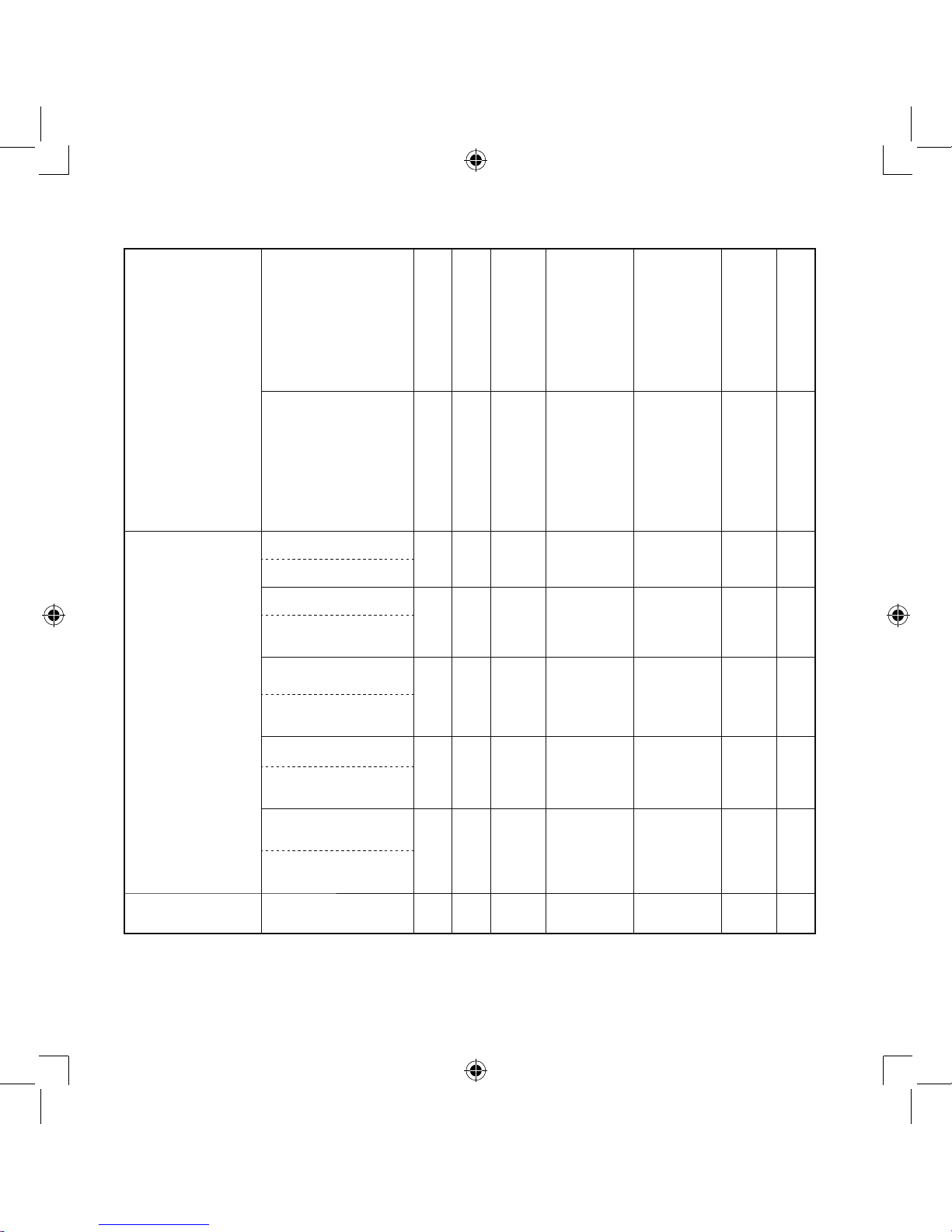
14
o
: applicable X: not applicable
Disinfecting
method
(See the next page)
Parts
Mouthpiece
Nosepiece
Nebulizer Kit
Adult Mask (SEBS)
(optional)
Child Mask (SEBS)
(optional)
Adult Mask (PVC)
Child Mask (PVC)
Air Tube
(PVC, 100cm)
Air Filter
Materials
PP
PP
PP
(Vaporizer Head: PC)
Mask: SEBS
Band: Rubber
(non-latex)
Adapter: PP
Mask: PVC
(Phthalate free)
Band: Rubber
(non-latex)
PVC
(Phthalate free)
Polyester
A
Alcohol
oo o o o X X
Disinfecting ethanol
Sodium hypochlorite
oo o o o X X
Milton*
(0.1%, 15min.)
Quaternary ammonium
oo o o o X X
Osvan*
(0.1%, 10min.)
Chlorhexidine
oo o o o X X
Hibitane*
(0.5%, 30min.)
Amphoteric
Surfactant
oo o o o X X
Tego 51*
(0.2%, 15min.)
B
Boiling
oo o o X X X
Page 15
EN
15
A. Use a commercially available disinfectant. Follow the instructions provided by the
disinfectant manufacturer.
Note: Never clean with benzene or thinner.
B. Parts may be boiled between 15 to 20 minutes.
After boiling, carefully remove the parts, shake off excess
water and allow to air dry in a clean environment.
* an example of commercially available disinfectant. (The concentration and residence time specified in the table
are under the conditions where the service life of the parts is tested with each disinfectant used as described in its
instruction manual. Please note that the testing was not performed for the purpose of ensuring the effectiveness
of the disinfectants. There is no intention of suggesting that such disinfectants be used. Conditions of use and
ingredients of disinfectant vary for manufacturers. Please read the instruction manuals carefully before use and
apply disinfectant to each part in an appropriate way. Please notice that service life of the parts may be shorter
depending on conditions, environments and frequency of use.)
Page 16
16
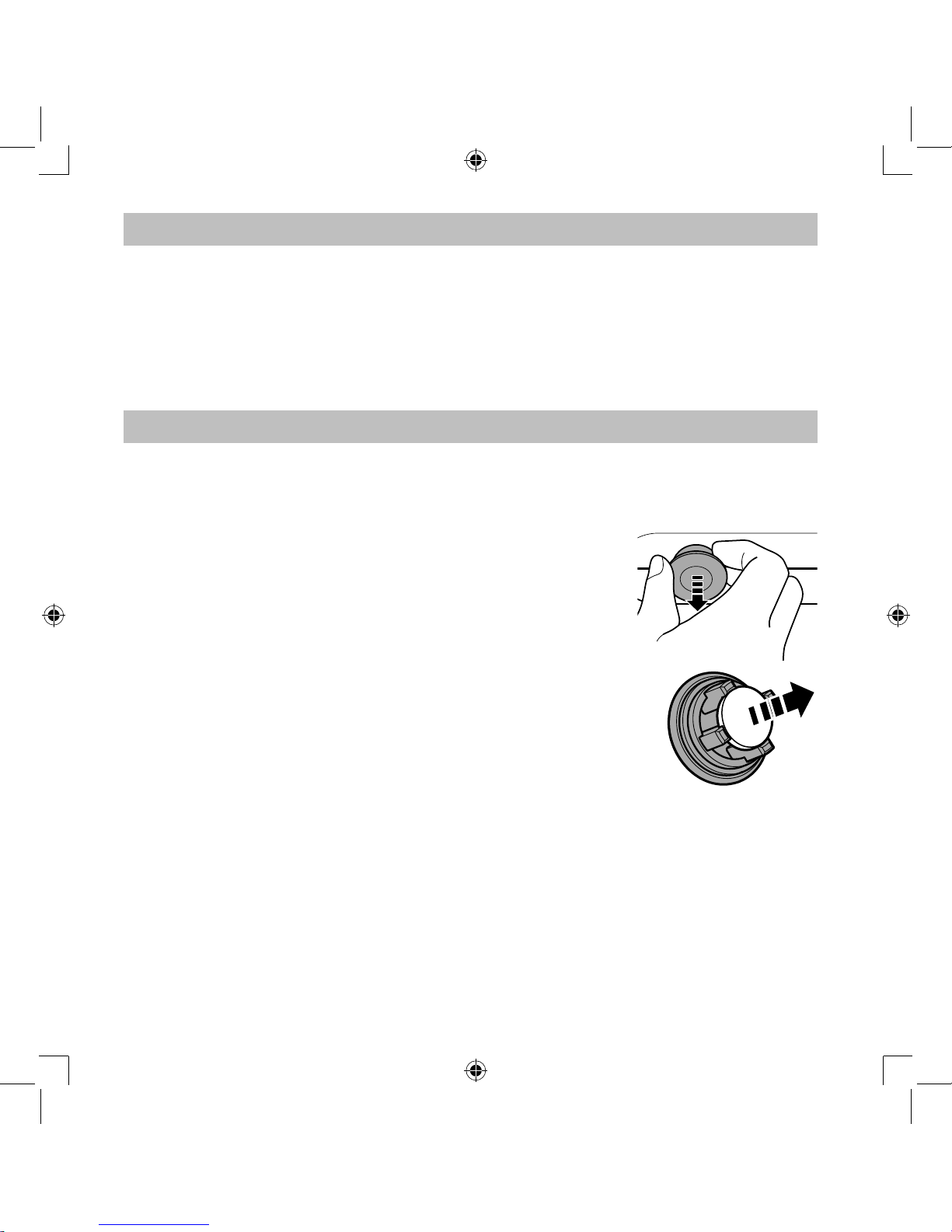
Removing condensation from the air tube
If there is moisture or liquid remaining in the air tube, be sure to follow the procedure
below to remove any moisture from within the air tube.
1) Make sure that the air tube is connected to the air connector on the compressor.
2) Remove the air tube from the nebulizer kit.
3) Turn on the compressor and pump air through the air tube to expel the moisture.
Changing the air filter
If the air filter has been used for more than 60 days, replace it with a new one.
1.
Pull the air filter cover off the compressor.
2.
Change the air filter.
3.
Put the air filter cover back in place.
Make sure it is attached properly.
Notes:
• Use only OMRON air filters designed for this device. Do not operate without air filter.
• Do not wash or clean the air filter. If the air filter becomes wet, replace it. Damp air filters
can cause blockages.
• There is no front or back orientation for the air filters.
• Check that air filters are clean and free of dust before inserting them.
Page 17

EN
17
Troubleshooting
Check the following if the device should fail during operation.
Problem Cause Remedy
Nothing happens
when the power switch
is pressed.
Is the AC adapter connected
correctly to a power outlet and
the compressor?
Check that the AC adapter is connected to
a power outlet and the compressor. Unplug
then reinsert the plug if necessary.
No nebulization or
low nebulization rate,
when the power is on.
Is there medication in the
medication tank?
Add the correct amount of medication to
the medication tank.
Is there too much/little
medication in the medication
tank?
Is the vaporizer head missing
or not assembled correctly?
Attach the vaporizer head correctly.
Is the nebulizer kit assembled
correctly?
Assemble the nebulizer kit correctly.
Is the nozzle blocked? Make sure that the nozzle is free of
blockages.
Is the nebulizer kit tilted at a
sharp angle?
Make sure that the nebulizer kit is not tilted
at an angle of more than 45 degrees.
Is the air tube connected
correctly?
Make sure that the air tube is correctly
connected to the compressor and nebulizer
kit.
Is the air tube folded? Make sure that the air tube is not twisted or
bended.
Is the air tube blocked? Make sure that the air tube is free of
blockages.
Is the air filter dirty? Replace the air filter with a new one.
The compressor is
abnormally loud.
Is the air filter cover attached
correctly?
Attach the air filter cover correctly.
The compressor is
very hot.
Is the compressor being
covered?
Do not cover the compressor during
operation.
Note: If the suggested remedy does not solve the problem, do not try to repair the
device - the device are not user serviceable. Return the device to an authorized
OMRON retail outlet or distributor.
Page 18
18
Technical data
Product Description: Compressor Nebulizer
Model (-Code): NE-C803 (NE-C803-E)
Rating (AC adapter): 100 - 240V ~ 50/60Hz 0.12 - 0.065A
Rating (Compressor Nebulizer): 6V
0.7A
Operating Temperature / Humidity: +10°C to +40°C / 30% to 85%RH
Storage and Transport
Temperature / Humidity /
Air Pressure:
-20°C to +60°C / 10% to 95%RH / 700 - 1060hPa
Weight: Approx. 180g (compressor only)
Dimensions: Approx. 85 (W) × 43 (H) ×115 (D) mm (compressor only)
Contents: Compressor, Nebulizer Kit, Air Tube (PVC, 100cm),
Mouthpiece, Nosepiece, Adult Mask (PVC), Child Mask (PVC),
5 pcs Spare Air Filters, AC Adapter, Storage Bag, Instruction
Manual.
Classifications: Class ll (Protection against electric shock),
Type BF (Applied part)
IP21 (Ingress Protection)
= Class ll equipment
= Type BF
applied part
Read the instruction
manual carefully
= No operation
= Power on
Notes:
• Subject to technical modification without prior notice.
• This OMRON product is produced under the strict quality system of OMRON
HEALTHCARE Co., Ltd., Japan.
• The device may not work if the temperature and voltage conditions are different to
those defined in the specifications.
• The device fulfils the provisions of the EC directive 93/42/EEC (Medical Device
Directive) and the European Standard EN13544-1:2007+A1:2009, Respiratory therapy
equipment - Part1: Nebulizing systems and their components.
• See website of OMRON HEALTHCARE EUROPE to update technical information.
URL: www.omron-healthcare.com
Page 19
EN
19
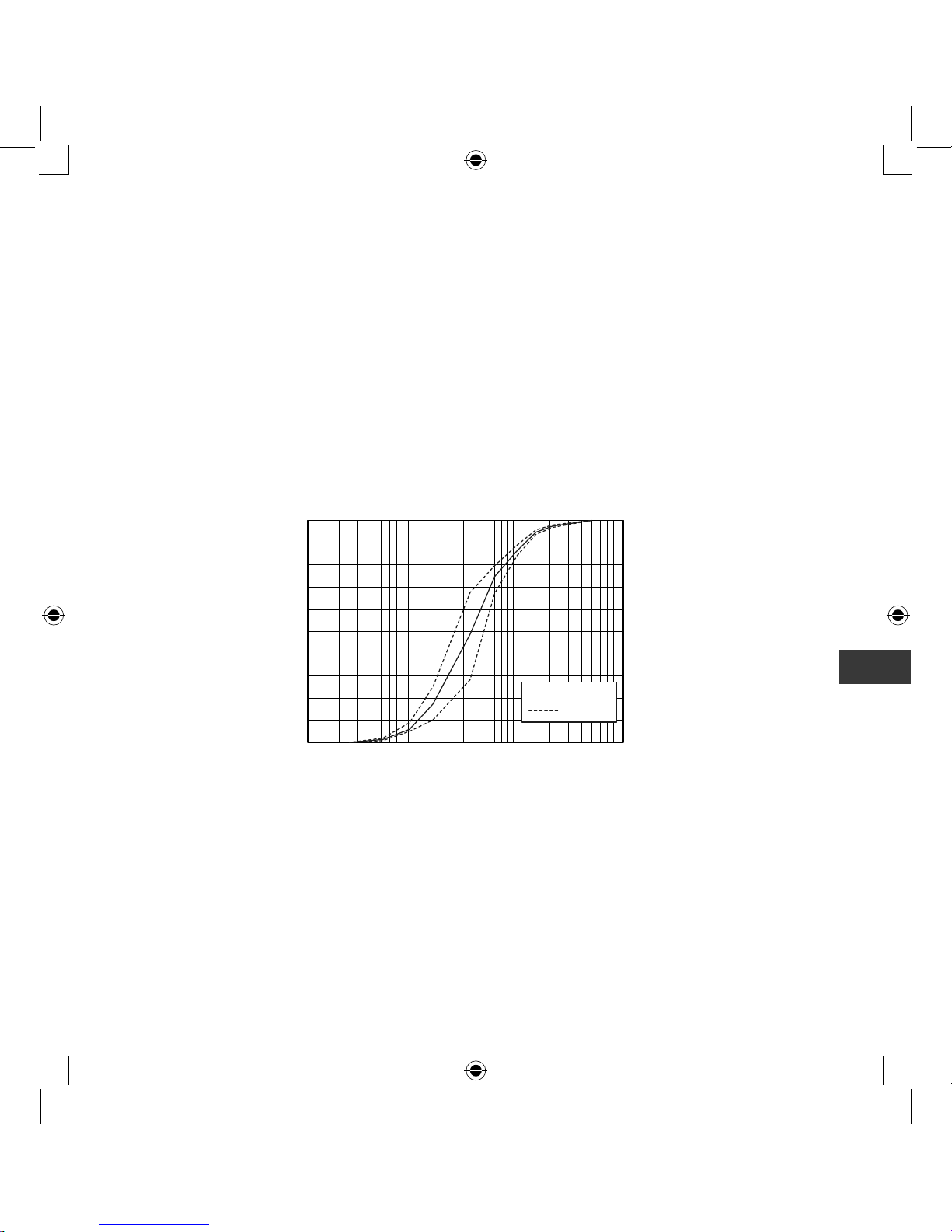
Technical data for the OMRON NE-C803 Compressor with the Nebulizer kit:
Particle Size: **MMAD Approx. 3μm
MMAD = Mass Median Aerodynamic Diameter
Medication Tank Capacity: 10ml maximum
Appropriate Medication Quantities: 2ml minimum - 10ml maximum
Sound: *Less than 45dB
Nebulization Rate: *Approx. 0.30ml/min (by weight loss)
Aerosol Output: ** 0.37ml (2ml, 1%NaF)
Aerosol Output Rate: ** 0.05ml/min (2ml, 1%NaF)
Result of cascade impactor measurements for particle size**
0
10
20
30
40
50
60
70
80
90
100
0.1 1 10 100
Cumulative % particle mass of sodium fluoride under size
Particle Size Dp (μm)
Cumulative Undersize %
Mean
Individual
* Measured by OMRON HEALTHCARE Co., Ltd. Measured with saline at ambient
temperature of 23°C and humidity of 40%. It may vary with the type of medication.
** Independently measured by Prof. Dr. Hiroshi Takano, Therapeutic Systems Research
Center, Doshisha University, Kyoto, Japan, according to EN13544-1:2007+A1:2009.
Note:
Performance may vary with medication such as suspensions or high viscosity. See
medication supplier’s data sheet for further details.
Page 20
20
Important information regarding Electro Magnetic Compatibility (EMC)
With the increased number of electronic devices such as PC’s and mobile (cellular) telephones,
medical devices in use may be susceptible to electromagnetic interference from other devices.
Electromagnetic interference may result in incorrect operation of the medical device and create a
potentially unsafe situation.
Medical devices should also not interfere with other devices.
In order to regulate the requirements for EMC (Electro Magnetic Compatibility) with the aim to
prevent unsafe product situations, the EN60601-1-2:2007 standard has been implemented. This
standard defines the levels of immunity to electromagnetic interferences as well as maximum
levels of electromagnetic emissions for medical devices.
This medical device manufactured by OMRON HEALTHCARE conforms to this EN60601-1-2:2007
standard for both immunity and emissions.
Nevertheless, special precautions need to be observed:
• Do not use mobile (cellular) telephones and other devices, which generate strong electrical or
electromagnetic fields, near the medical device. This may result in incorrect operation of the unit
and create a potentially unsafe situation.
Recommendation is to keep a minimum distance of 7 m. Verify correct operation of the device in
case the distance is shorter.
Further documentation in accordance with EN60601-1-2:2007 is available at OMRON
HEALTHCARE EUROPE at the address mentioned in this instruction manual.
Documentation is also available at www.omron-healthcare.com.
Correct Disposal of This Product
(Waste Electrical & Electronic Equipment)
This marking shown on the product or its literature, indicates that it should not be disposed of, with
other household wastes at the end of its working life. To prevent possible harm to the environment
or human health from uncontrolled waste disposal, please separate this product from other types
of wastes and recycle it responsibly to promote the sustainable reuse of material resources.
Household users should contact either the retailer where they purchased this product, or their
local government office, for details of where and how they can return this item for environmentally
safe recycling.
Business users should contact their supplier and check the terms and conditions of the purchase
contract. This product should not be mixed with other commercial wastes for disposal.
Page 21

EN
21
Guarantee
Thank you for buying an OMRON product. This product is constructed of high quality
materials and great care has been taken in its manufacturing. It is designed to give you
a high level of comfort, provided that it is properly operated and maintained as described
in the instruction manual.
This product is guaranteed by OMRON for a period of 3 years after the date of purchase.
The proper construction, workmanship and materials of this product is guaranteed by
OMRON. During this period of guarantee OMRON will, without charge for labour or
parts, repair or replace the defect product or any defective parts.
The guarantee covers only products purchased in Europe, Russia and other CIS
countries, Middle East and Africa.
The guarantee does not cover any of the following:
a. Transport costs and risks of transport.
b. Costs for repairs and / or defects resulting from repairs done by unauthorised
persons.
c. Periodic check-ups and maintenance.
d. Failure or wear of accessories or other attachments other than the main device itself,
unless explicitly guaranteed above.
e. Costs arising due to non-acceptance of a claim (those will be charged for).
f. Damages of any kind including personal caused accidentally or from misuse.
Should guarantee service be required please apply to the dealer whom the product
was purchased from or an authorised OMRON distributor. For the address refer to the
product packaging / literature or to your specialised retailer. If you have difficulties in
finding OMRON customer services, visit our website (www.omron-healthcare.com) for
contact information.
Repair or replacement under the guarantee does not give rise to any extension or
renewal of the guarantee period.
The guarantee will be granted only if the complete product is returned together with the
original invoice / cashticket issued to the consumer by the retailer. OMRON reserves the
right to refuse the guarantee service if any unclear information has been given.
Page 22
22
Manufacturer
OMRON HEALTHCARE Co., Ltd.
53, Kunotsubo, Terado-cho, Muko, KYOTO, 617-0002 JAPAN
EU-representative
OMRON HEALTHCARE EUROPE B.V.
Scorpius 33, 2132 LR Hoofddorp, THE NETHERLANDS
www.omron-healthcare.com
Production facility
OMRON HEALTHCARE MANUFACTURING VIETNAM CO., LTD.
No.28 VSIP II, Street 2, Vietnam-Singapore Industrial Park II,
Binh Duong Industry-Services-Urban Complex, Hoa Phu Ward,
Thu Dau Mot City, Binh Duong Province, Vietnam
Subsidiaries
OMRON HEALTHCARE UK LTD.
Opal Drive, Fox Milne, Milton Keynes, MK15 0DG, UK
www.omron-healthcare.co.uk
OMRON MEDIZINTECHNIK HANDELSGESELLSCHAFT mbH
Gottlieb-Daimler-Strasse 10, 68165 Mannheim, GERMANY
www.omron-healthcare.de
OMRON SANTÉ FRANCE SAS
14, rue de Lisbonne, 93561 Rosny-sous-Bois Cedex, FRANCE
www.omron-healthcare.fr
Made in Vietnam
Page 23
FR
NE-C803-E_A_M09_140715.pdf
Nébuliseur à compresseur
NE-C803 (NE-C803-E)
Mode d’emploi
Page 24
24
Introduction
Merci d’avoir acheté le système OMRON COMP AIR basic.
Ce produit a été développé en collaboration avec des spécialistes du traitement de
l’asthme, de la bronchite chronique, des allergies et autres troubles respiratoires.
Ce produit est un appareil médical. Utilisez l’appareil selon les instructions de votre
médecin et/ou du thérapeute.
Fonctionnement du kit de nébulisation
Le médicament, qui est pompé par le canal de
médicament, est mélangé à l’air comprimé généré
par une pompe du compresseur.
Le médicament est transformé en fines particules
et vaporisé lorsqu’il entre en contact avec l’air
comprimé.
Aérosol
Canule
Air comprimé
Canal du médicament
Médicament
Avant d’utiliser l’appareil
Table des matières
Avant d’utiliser l’appareil
Introduction ............................................................................................................... 24
Utilisation prévue ...................................................................................................... 25
Instructions de sécurité importantes ......................................................................... 26
Familiarisation avec l’appareil................................................................................... 29
Accessoires médicaux en option .............................................................................. 30
Autres pièces en option/de rechange ....................................................................... 30
Instructions de fonctionnement
Utilisation .................................................................................................................. 31
Entretien et maintenance
Nettoyage et désinfection ......................................................................................... 35
Élimination de la condensation du tube à air ............................................................ 38
Changement du filtre à air ........................................................................................ 38
Dépannage ............................................................................................................... 39
Données techniques ................................................................................................. 40
Garantie .................................................................................................................... 43
Page 25
FR
25
Utilisation prévue
Objectif médical
Ce produit est destiné à être utilisé pour inhaler le médicament prescrit pour le
traitement des troubles respiratoires.
Utilisateur visé
• Personnel médical (médecin, infirmière ou thérapeute).
• Soignant ou patient sous la directive de personnel médical qualifié pour traitement
à domicile.
• L’utilisateur doit également être en mesure de comprendre le fonctionnement général
du NE-C803, ainsi que le contenu de ce mode d’emploi.
Patients visés
Ce produit ne doit pas être utilisé sur des patients non conscients ou ne respirant
pas spontanément.
Environnement
Ce produit est destiné à être utilisé dans un établissement médical (hôpital, clinique et
cabinet médical) ou dans une maison.
Durée de conservation
Les durées de conservation sont les suivantes, sous réserve que le produit soit utilisé
pour nébuliser 2 ml de médicament deux fois par jour pendant 7,5 minutes à chaque
utilisation à température ambiante (23 °C).
La durée de conservation peut varier selon l’environnement d’utilisation.
L’utilisation fréquente du produit peut réduire la durée de conservation.
Compresseur (unité principale) 5 ans
Kit de nébulisation 1 an
Embout buccal 1 an
Embout nasal 1 an
Tube à air (PVC, 100 cm) 1 an
Filtre à air 60 jours
Masque (PVC) (SEBS) 1 an
Précautions d’utilisation
Les avertissements et les mises en garde décrits dans le mode d’emploi doivent
être respectés.
Page 26
26
Instructions de sécurité importantes
Lisez attentivement toutes les informations contenues dans le mode d’emploi et dans la
documentation incluse dans l’emballage avant d’utiliser l’appareil.
Avertissement :
indique une situation potentiellement dangereuse qui, si elle n’est pas évitée,
peut entraîner de graves lésions.
(Utilisation)
• Suivez les instructions de votre médecin ou du personnel soignant pour la nature du médicament, son
dosage et sa posologie.
• En cas de sensations inhabituelles pendant l’utilisation, arrêtez immédiatement d’utiliser l’appareil et
consultez votre médecin.
• N’utilisez pas de l’eau seule dans le nébuliseur pour l’inhalation.
• Gardez l’appareil hors de portée des nourrissons et des enfants laissés sans surveillance. L’appareil est
susceptible de contenir de petites pièces pouvant être avalées.
• Ne rangez pas le tube à air si de l’humidité ou du médicament est resté à l’intérieur.
• N’utilisez ni ne conservez l’appareil dans des endroits où il risquerait d’être exposé à des émanations
nocives ou à des substances volatiles.
• N’utilisez pas l’appareil dans des endroits où il risquerait d’être exposé à des gaz inflammables.
• Ne couvrez pas le compresseur avec une couverture, une serviette ou quoi que ce soit d’autre pendant
l’utilisation.
• Mettez toujours au rebut tout reste de médicament après utilisation et utilisez une nouvelle dose de
médicament à chaque fois.
• Ne l’utilisez pas dans les circuits anesthésiques ou dans les circuits de respiration du ventilateur.
• Conservez les composants dans un endroit propre pour éviter tout risque d’infection.
(Risque de choc électrique)
• Ne branchez ou ne débranchez jamais l’adaptateur secteur avec les mains mouillées.
• Le compresseur et l’adaptateur secteur ne sont pas étanches. Ne renversez pas de l’eau ou d’autres
liquides sur ces composants. Si un liquide est renversé sur ces composants, débranchez immédiatement
l’adaptateur secteur
et essuyez le liquide avec une compresse de gaze ou un autre tissu absorbant.
• N’immergez pas le compresseur dans de l’eau ou dans un autre liquide.
• N’utilisez pas et ne rangez pas l’appareil dans un endroit humide.
• Ne faites pas fonctionner l’appareil lorsque le cordon ou la fiche d’alimentation est endommagé.
• Maintenez le cordon d’alimentation éloigné des surfaces chauffées.
• Utilisez uniquement l’adaptateur secteur OMRON d’origine. L’utilisation d’un adaptateur secteur non pris
en charge risque d’endommager l’appareil.
(Nettoyage et désinfection)
Observez les règles ci-dessous lors du nettoyage ou de la désinfection des composants. Le non-respect
de ces règles peut occasionner des dommages, rendre la nébulisation inefficace ou entraîner une
infection. Pour prendre connaissance des instructions, consultez la section « Nettoyage et désinfection ».
• Nettoyez et désinfectez le kit de nébulisation, le masque, l’embout buccal ou l’embout nasal avant
utilisation dans les cas suivants :
- à la première utilisation après l’achat ;
- si l’appareil n’a pas été utilisé pendant une longue période ;
- si plusieurs personnes utilisent le même appareil.
• Veillez à laver ou à essuyer les composants après l’utilisation et assurez-vous qu’ils sont
minutieusement désinfectés et séchés, et conservés dans un endroit propre.
• Ne laissez pas la solution de nettoyage dans les composants.
Page 27

FR
27
Attention :
Indique une situation potentiellement dangereuse qui, si elle n’est pas évitée, peut
entraîner des blessures mineures ou modérées, ou des dommages physiques.
(Utilisation)
• Il faut être très vigilant lorsque cet appareil est utilisé par, sur ou à proximité d’enfants ou de
personnes infirmes.
• Vérifiez que les composants sont correctement fixés.
• Vérifiez que le filtre à air est correctement fixé.
• Assurez-vous que le filtre à air est propre. Si le filtre à air a été utilisé pendant plus de 60 jours,
remplacez-le par un filtre neuf.
• N’inclinez pas le kit de nébulisation à un angle supérieur à 45 degrés dans tous les sens et ne le secouez
pas pendant qu’il est en fonctionnement.
• N’utilisez pas et ne rangez pas l’appareil alors que le tube à air est plié.
• Utilisez uniquement des composants de nébulisation, un tube à air, un filtre à air et un couvercle de filtre
à air d’origine.
• N’ajoutez pas plus de 10 ml de médicament dans le réservoir de médicaments.
• Ne transportez pas et ne retirez pas le kit de nébulisation lorsque le réservoir de médicaments contient
un médicament.
• Ne laissez pas l’appareil sans surveillance en présence de jeunes enfants ou de personnes ne pouvant
pas exprimer leur consentement.
• Ne soumettez pas l’appareil à des chocs excessifs, par exemple en le faisant tomber sur le sol.
• Ne percez pas la canule du réservoir de médicaments avec une épingle ou un objet pointu.
• N’introduisez pas les doigts ou des objets à l’intérieur du compresseur.
• Ne démontez ni ne tentez de réparer le compresseur ou l’adaptateur secteur.
• Ne laissez pas l’appareil dans un endroit soumis à des températures chaudes ou froides extrêmes
ou sous la lumière directe du soleil.
• N’utilisez jamais l’appareil pendant que vous dormez ou somnolez.
• Autorisé pour un usage humain uniquement.
• Le compresseur peut devenir chaud pendant son fonctionnement.
• Ne touchez pas le compresseur, excepté lorsque cela est nécessaire, par exemple pour éteindre
l’appareil pendant la nébulisation.
• Pour éviter que du médicament ne reste sur le visage, essuyez-vous le visage après avoir retiré le
masque.
• Pour ne pas provoquer de lésions au niveau des muqueuses du nez, ne pressez pas l’embout nasal
dans l’arrière-nez.
• N’utilisez pas un kit de nébulisation, un embout buccal ou un embout nasal endommagé.
(Risque de choc électrique)
• Débranchez toujours l’adaptateur secteur de la prise de courant après utilisation et avant nettoyage.
• Branchez l’appareil sur la prise de la tension appropriée. Ne surchargez pas les prises d’alimentation
et n’utilisez pas de rallonges.
• N’utilisez pas le cordon d’alimentation de l’adaptateur secteur de manière incorrecte.
• N’enroulez pas le cordon d’alimentation autour du compresseur ou de l’adaptateur secteur.
• Ne tirez pas sur le cordon d’alimentation de l’adaptateur secteur.
• Tous les changements ou toutes les modifications n’ayant pas été approuvés par OMRON
HEALTHCARE annuleront la garantie de l’utilisateur.
• Débranchez toujours l’adaptateur secteur de l’appareil après utilisation et avant le nettoyage.
Page 28
28
(Nettoyage et désinfection)
Observez les règles ci-dessous lors du nettoyage ou de la désinfection des composants. Le non-respect
de ces règles peut occasionner des dommages, rendre la nébulisation inefficace ou entraîner une infection.
Pour prendre connaissance des instructions, consultez la section « Nettoyage et désinfection ».
• N’utilisez pas de four à micro-ondes, de séchoir à vaisselle ou de sèche-cheveux pour sécher l’appareil
ou les composants.
• N’utilisez pas d’appareil de stérilisation par autoclave, par oxyde d’éthylène ou par plasma basse
température.
• Lorsque vous désinfectez des composants par ébullition, assurez-vous que le conteneur ne soit pas
chauffé à sec. Cela pourrait provoquer un incendie.
Précautions de sécurité générales :
• Examinez l’appareil avant chaque utilisation et assurez-vous qu’il ne présente aucune anomalie. Vérifiez
tout particulièrement les points suivants :
- La canule et le tuyau à air ne doivent pas être endommagés.
- La canule ne doit pas être bouchée.
- Le compresseur doit fonctionner normalement.
• La pompe du compresseur émet des bruits et des vibrations lorsque cet appareil est utilisé. L’émission
d’air comprimé provenant du kit de nébulisation engendre également du bruit. Ceci est normal et n’est
pas le signe d’un dysfonctionnement.
• Utilisez l’appareil uniquement aux fins attendues. Ne l’utilisez pas pour un usage différent.
• N’utilisez pas l’appareil à des températures supérieures à +40 °C.
• Vérifiez que le tube à air est bien fixé au compresseur et au kit de nébulisation et qu’il ne se détache pas.
• Afin d’isoler totalement l’appareil de la source d’alimentation, débranchez la fiche de la source
d’alimentation.
Gardez soigneusement ces instructions pour vous y référer ultérieurement.
Page 29
FR
29
Familiarisation avec l’appareil
Adaptateur secteur x 1
Étui de rangement x 1
Mode d’emploi
Vue arrière
Connecteur
d’alimentation
Couvercle de filtre à air
*Filtre à air à l’intérieur
Compresseur (unité principale) x 1
Support du kit de
nébulisation
Connecteur du tuyau à air
Interrupteur
Filtres à air de
rechange x 5
Tube à air
(PVC, 100 cm) x 1
Embout buccal x 1
Masque pour enfant
(PVC) x 1
Masque pour adulte
(PVC) x 1
Kit de nébulisation x 1
Connecteur du tuyau à air
Partie supérieure du
système d’inhalation
Tête du vaporisateur
Canule
Réservoir de médicaments
Embout nasal x 1
Page 30
30
Accessoires médicaux en option
(dans le cadre de la Directive européenne 93/42/CEE relative aux dispositifs
médicaux)
Description du produit Modèle
Ensemble du kit de nébulisation et de l’embout buccal NEB-NSET3-83E
Ensemble du kit de nébulisation, du masque pour adulte (PVC)
et du tuyau à air
NEB-NSET4-83AP
Ensemble du kit de nébulisation, du masque pour enfant (PVC)
et du tuyau à air
NEB-NSET5-83AP
Masque pour adulte (PVC) NEB-MSLP-E
Masque pour enfant (PVC) NEB-MSMP-E
Masque pour adulte (SEBS) NEB-MSLS-E
Ensemble du masque pour enfant (SEBS) NEB-MSSS-E
Embout buccal NEB-MP-E
Embout nasal NEB-NP-E
Tuyau à air (PVC, 100 cm) NEB-TP-82E
Adaptateur secteur HHP-CF01
Autres pièces en option/de rechange
Description du produit Modèle
Filtres à air x 5 NEB-AFR-30E
I
n
Page 31

FR
31
Instructions de fonctionnement
Utilisation
Avertissement :
Nettoyez et désinfectez le kit de nébulisation, le masque, l’embout buccal ou l’embout
nasal avant utilisation dans les cas suivants :
- à la première utilisation après l’achat ;
- si l’appareil n’a pas été utilisé pendant une longue période ;
- si plusieurs personnes utilisent le même appareil.
Pour prendre connaissance des instructions, consultez la section « Nettoyage et
désinfection ».
1.
Vérifiez que l’interrupteur est en position Arrêt ( ).
2.
Connectez l’adaptateur secteur au
compresseur et branchez la fiche
d’alimentation sur une prise d’alimentation.
3.
Retirez la partie supérieure du système
d’inhalation avec la tête du vaporisateur du
réservoir de médicaments.
Remarque :
Si la tête du vaporisateur s’est détachée de la
partie supérieure du système d’inhalation et
est tombée dans le réservoir de médicaments,
récupérez-la.
4.
Ajoutez le volume approprié de médicament
prescrit dans le réservoir de médicaments.
Page 32

32
5.
Vérifiez que la tête du vaporisateur est bien
fixée à la partie supérieure du système
d’inhalation.
Remarque :
Le composant fixé sur la partie droite de
la tête du vaporisateur et celui situé sur
sa partie gauche ne sont pas de même
dimension. Si la tête du vaporisateur n’e st
pas fixée dans le bon sens, la nébulisation
ne sera pas optimale.
6.
Remettez la partie supérieure du
système d’inhalation à l’intérieur du
réservoir de médicaments.
1) Alignez la saillie figurant sur la partie
supérieure du système d’inhalation
avec la dentelure du réservoir de
médicaments, comme illustré.
2) Faites pivoter la partie supérieure du
système d’inhalation dans le sens
des aiguilles d’une montre jusqu’à
ce que la flèche figurant sur celle-ci
et le repère d’alignement figurant sur
le réservoir de médicaments soient
alignés. Lorsqu’ils sont bien alignés,
un clic se fait entendre.
Flèche
Repère
d’alignement
Petit
Grand
Page 33

FR
33
7.
Fixez solidement le masque, l’embout
buccal ou l’embout nasal au kit de
nébulisation.
Remarque :
Assurez-vous que ces accessoires sont
installés de façon à ce que la poignée
soit positionnée à distance du patient.
Dans le cas contraire, des particules de
médicament risqueraient d’être projetées
sur la peau et les vêtements du patient.
8.
Fixez le tuyau à air.
Introduisez fermement les fiches du tuyau à air
dans les connecteurs du tuyau à air.
Poignée Poignée
Le kit de nébulisation peut être fixé sur le compresseur.
Page 34

34
E
n
9.
Tenez le kit de nébulisation comme indiqué à
droite.
Suivez les instructions de votre médecin ou du
thérapeute. Expirez normalement par l’embout
buccal, le masque ou le nez.
Attention :
N’inclinez pas le kit de nébulisation à un angle supérieur
à 45 degrés dans tous les sens. Le médicament risque
de s’écouler dans la bouche ou la nébulisation pourrait ne
pas être efficace.
10.
Placez l’interrupteur en position Marche ( ). Lorsque le compresseur démarre, la
nébulisation commence et l’aérosol est généré.
Inhalez le médicament.
11.
Éteignez l’appareil à la fin du traitement. Débranchez l’adaptateur secteur de la
prise d’alimentation et déconnectez-le du compresseur.
Remarque :
Tenez et tirez fermement la fiche
d’alimentation vers le bas pour débrancher
l’adaptateur secteur.
Attention :
Ne tirez pas sur le cordon d’alimentation.
Cela pourrait l’endommager.
Angle droit
45°45°
Compresseur
Fiche
d’alimentation
Page 35

FR
35
Entretien et maintenance
Nettoyage et désinfection
Attention : manipulation de la tête du vaporisateur
• Lavez systématiquement la tête du vaporisateur après chaque utilisation.
• N’utilisez pas de brosse ou d’épingle pour nettoyer la tête du vaporisateur.
• Si vous désinfectez les composants dans de l’eau bouillante, veillez à les faire bouillir
dans de grandes quantités d’eau.
• Ne faites pas bouillir la tête du vaporisateur avec des objets autres que les accessoires
correspondants du système de nébulisation.
Nettoyage
Nettoyez les composants après chaque utilisation pour éliminer les résidus de
médicament. Ceci permettra d’éviter une nébulisation inefficace ou une infection.
■Composants lavables
• Kit de nébulisation (partie supérieure du système d’inhalation, réservoir de
médicaments), masque (PVC)/(SEBS), embout buccal, embout nasal
Lavez-les à l’eau chaude et avec un détergent doux (neutre). Rincez-les
minutieusement à l’eau chaude et pure du robinet et laissez-les sécher à l’air dans un
endroit propre.
• Tête du vaporisateur
Lavez-le à l’eau courante.
■Composants non lavables
• Compresseur, tube à air
Vérifiez tout d’abord que la fiche d’alimentation est bien débranchée de la prise
d’alimentation. Nettoyez avec un chiffon doux humecté avec de l’eau ou un détergent
doux (neutre).
• Filtre à air
Ne lavez pas et ne nettoyez pas le filtre à air. Si le filtre à air est mouillé, remplacez-le.
Les filtres à air humides peuvent provoquer des obstructions.
Désinfection
Désinfectez les composants une fois par semaine. Si les composants sont fort tachés,
remplacez-les par des composants neufs.
Reportez-vous au tableau de la page 36 pour choisir une méthode de désinfection.
Page 36

36
o
: applicable X : non applicable
Méthode de
désinfection
(Voir la page
suivante)
Composants
Embout buccal
Embout nasal
Kit de nébulisation
Masque pour adulte
(SEBS) (facultatif)
Masque pour enfant
(SEBS) (facultatif)
Masque pour adulte
(PVC)
Masque pour enfant
(PVC)
Tube à air
(PVC, 100 cm)
Filtre à air
Matériaux
PP
PP
PP
(Tête du
vaporisateur : PC)
Masque : SEBS
Courroie :
caoutchouc
(sans latex)
Adaptateur : PP
Masque : PVC
(sans phtalates)
Courroie :
Caoutchouc
(sans latex)
PVC
(sans phtalates)
Polyester
A
Alcool
oo o o o X X
Éthanol désinfectant
Hypochlorite de sodium
oo o o o X X
Milton*
(0,1 %, 15 min)
Ammonium quaternaire
oo o o o X X
Osvan*
(0,1 %, 10 min)
Chlorhexidine
oo o o o X X
Hibitane*
(0,5 %, 30 min)
Tensioactif
amphotère
oo o o o X X
Tego 51*
(0,2 %, 15 min)
B
Ébullition
oo o o X X X
Page 37

FR
37
A. Utilisez un désinfectant disponible dans le commerce. Suivez les instructions fournies
par le fabricant du désinfectant.
Remarque : ne nettoyez jamais avec du benzène ou un diluant.
B. Vous pouvez faire bouillir les composants entre 15 et 20 minutes.
Après ébullition, retirez prudemment les composants, ôtez
l’excédent d’eau et laissez sécher à l’air dans un endroit propre.
* exemple de désinfectant disponible dans le commerce. (La concentration et le temps de séjour indiqués dans le
tableau s’entendent dans les conditions de test de la durée de vie des pièces avec chaque désinfectant utilisé
conformément aux indications fournies dans son mode d’emploi. Veuillez noter que les tests n’ont pas été
effectués en vue de garantir l’efficacité des désinfectants. Il n’y a aucune intention de suggérer l’utilisation de ces
désinfectants. Les conditions d’utilisation et les ingrédients du désinfectant varient selon les fabricants. Veuillez
lire attentivement les modes d’emploi avant utilisation et désinfectez chaque pièce de la manière adéquate.
Veuillez noter que la durée de vie des pièces peut diminuer en fonction des conditions, des environnements et de
la fréquence d’utilisation.)
Page 38

38
Élimination de la condensation du tube à air
Si de l’humidité ou du liquide se trouve encore dans le tube à air, procédez comme suit
pour l’en éliminer.
1) Vérifiez que le tube à air est relié à la prise d’air sur le compresseur.
2) Retirez le tube à air du kit de nébulisation.
3) Mettez le compresseur en route et pompez l’air par le tube à air afin d’expulser l’humidité.
Changement du filtre à air
Si le filtre à air a été utilisé pendant plus de 60 jours, remplacez-le par un filtre neuf.
1.
Retirez le couvercle du filtre à air du compresseur.
2.
Changez le filtre à air.
3.
Remettez le couvercle du filtre à air en place.
Assurez-vous qu’il est correctement fixé.
Remarques :
• Utilisez uniquement les filtres à air d’OMRON conçus spécifiquement pour cet appareil.
Ne faites pas fonctionner l’unité sans filtre à air.
• Ne lavez pas et ne nettoyez pas le filtre à air. Si le filtre à air est mouillé, remplacez-le.
Les filtres à air humides peuvent provoquer des obstructions.
• Les filtres à air peuvent être insérés dans n’importe quel sens.
• Vérifiez que les filtres à air sont propres et exempts de poussière avant de les insérer.
Page 39

FR
39
Dépannage
Vérifiez les points suivants en cas de défaillance de l’appareil pendant son
fonctionnement.
Problème Cause Solution
Rien ne se produit
lorsque l’interrupteur
est placé en position
Marche.
L’adaptateur secteur est-il
correctement connecté à
une prise de courant et au
compresseur ?
Vérifiez que l’adaptateur secteur est
connecté à une prise de courant et au
compresseur. Débranchez puis réinsérez la
fiche si nécessaire.
Pas de nébulisation ou
taux de nébulisation
bas lorsque l’appareil
est en marche.
Le réservoir de médicaments
contient-il un médicament ?
Ajoutez le volume approprié de médicament
dans le réservoir de médicaments.
Le réservoir de médicaments
contient-il trop ou pas assez
de médicament ?
La tête du vaporisateur est-elle
manquante ou non montée
correctement ?
Fixez correctement la tête du vaporisateur.
Le kit de nébulisation est-il
monté correctement ?
Montez correctement le kit de nébulisation.
La canule est-elle bouchée ? Vérifiez que la canule ne comporte aucune
obstruction.
Le kit de nébulisation est-il
incliné à un angle trop
prononcé ?
Assurez-vous que le kit de nébulisation
n’est pas incliné à un angle de plus de
45 degrés.
Le tuyau à air est-il connecté
correctement ?
Vérifiez que le tube à air est correctement
fixé au compresseur et au kit de
nébulisation.
Le tuyau à air est-il déformé ? Vérifiez que le tuyau à air n’est pas tordu
ou plié.
Le tuyau à air est-il bouché ? Vérifiez que le tuyau à air ne comporte
aucune obstruction.
Le filtre à air est-il sale ? Remplacez le filtre à air par un filtre neuf.
Le compresseur est
anormalement bruyant.
Le couvercle du filtre à air
est-il correctement fixé ?
Fixez correctement le couvercle du filtre à air.
Le compresseur est
très chaud.
Le compresseur est-il
couvert ?
Ne recouvrez pas la compresseur pendant
l’utilisation.
Remarque : Si la solution suggérée ne permet pas de résoudre le problème, n’essayez
pas de réparer l’appareil, celui-ci n’étant pas réparable par l’utilisateur.
Retournez l’appareil à un point de vente ou un distributeur OMRON agréé.
Page 40

40
Données techniques
Description du produit : Nébuliseur à compresseur
Modèle (-réf.) : NE-C803 (NE-C803-E)
Valeur nominale
(adaptateur secteur) :
100 - 240 V ~ 50/60 Hz 0,12 - 0,065 A
Valeur nominale
(compresseur à nébuliseur) :
6 V
0,7 A
Température/Humidité de
fonctionnement :
+10 °C à +40 °C/30 % à 85 % HR
Température/Humidité/Pression
atmosphérique de stockage et
de transport :
-20 °C à +60 °C/10 % à 95 % HR/700 - 1 060 hPa
Poids : Environ 180 g (compresseur uniquement)
Dimensions : Environ 85 (L) × 43 (H) × 115 (P) mm
(compresseur uniquement)
Contenu : Compresseur, kit de nébulisation, tuyau à air (PVC, 100 cm),
embout buccal, embout nasal, masque pour adulte (PVC),
masque pour enfant (PVC), 5 filtres à air de rechange,
adaptateur secteur, étui de rangement, mode d’emploi.
Classifications : Classe ll (protection contre les chocs électriques),
Type BF (pièce appliquée)
IP21 (protection d’entrée)
= Équipement de
classe II
= Pièce appliquée
de type BF
Lisez attentivement le
mode d’emploi
= Hors tension
= Mise sous tension
Remarques :
• Soumis à des modifications techniques sans préavis.
• Ce produit OMRON a été fabriqué en respectant le système de qualité rigoureux
d’OMRON HEALTHCARE Co., Ltd., Japon.
• L’appareil risque de ne pas fonctionner si les conditions de température et de tension
diffèrent de celles définies dans les spécifications.
• L’appareil respecte les dispositions de la directive de la Communauté européenne
93/42/CEE (Directive relative aux appareils médicaux) et de la norme européenne
EN13544-1:2007+A1:2009, Équipement d’inhalothérapie - Partie 1 : Systèmes de
nébulisation et leurs composants.
• Visitez le site Web d’OMRON HEALTHCARE EUROPE pour mettre à jour les
informations techniques.
Adresse Internet : www.omron-healthcare.com
Page 41

FR
41
Données techniques du compresseur OMRON NE-C803 avec kit
de nébulisation :
Taille des particules : **MMAD Environ 3 μm
MMAD = Diamètre Aérodynamique Médian de Masse
Capacité du réservoir de
médicaments :
10 ml maximum
Volume de médicament approprié : 2 ml minimum - 10 ml maximum
Acoustique : *Inférieure à 45 dB
Taux de nébulisation : *Environ 0,30 ml/min (selon la perte de poids)
Sortie d’aérosol : ** 0,37 ml/ (2 ml, 1 % NaF)
Débit d’aérosol : ** 0,05 ml/min (2 ml, 1 % NaF)
Résultats des mesures de la taille des particules effectuées avec l’impacteur en cascade**
0
10
20
30
40
50
60
70
80
90
100
0,1 1 10 100
Taille insuffisante cumulée en % de la masse
de particule de fluorure de sodium
Taille des particules de diamètre Dp (μm)
Taille insuffisante cumulée en %
Moyenne
Individuelle
* Mesuré par OMRON HEALTHCARE Co., Ltd. Mesurée avec une solution saline à
une température ambiante de 23 °C et un taux d’humidité de 40 %. Peut varier en
fonction du type de médicament.
** Mesuré indépendamment par le professeur et docteur Hiroshi Takano du centre
Therapeutic Systems Research Center, à l’université Doshisha de Kyoto au Japon,
conformément à la norme EN13544-1:2007+A1:2009.
Remarque :
Les performances peuvent varier en fonction des médicaments, comme les suspensions
ou les médicaments à viscosité élevée. Consultez la fiche des données du fournisseur
du médicament pour de plus amples détails.
Page 42

42
Informations importantes sur la compatibilité électromagnétique (CEM)
Avec l’accroissement du nombre d’appareils électroniques, tels que les PC et les téléphones
mobiles (cellulaires), les dispositifs médicaux utilisés peuvent être soumis aux interférences
électromagnétiques dégagées par d’autres appareils. Les interférences électromagnétiques peuvent
perturber le fonctionnement de l’appareil médical et créer une situation potentiellement dangereuse.
Les appareils médicaux ne doivent pas non plus interférer avec d’autres appareils.
Afin de réglementer les exigences relatives à la CEM (compatibilité électromagnétique) dans le but
de prévenir toute situation dangereuse causée par le produit, la norme EN60601-1-2:2007 a été
mise en œuvre. Cette norme définit les niveaux d’immunité aux interférences électromagnétiques
ainsi que les niveaux maximum d’émissions électromagnétiques pour les appareils médicaux.
Cet appareil médical fabriqué par OMRON HEALTHCARE est conforme à cette norme
EN60601-1-2:2007 tant pour l’immunité que pour les émissions.
Il importe toutefois d’observer des précautions spéciales :
• N’utilisez pas des téléphones mobiles (cellulaires) et autres appareils générant des champs
électriques ou électromagnétiques puissants à proximité de l’appareil. Cela risquerait de
perturber le fonctionnement de l’appareil et de créer une situation potentiellement dangereuse.
Il est recommandé de maintenir une distance minimum de 7 m. Vérifiez le bon fonctionnement
de l’appareil si la distance est inférieure.
Une documentation complémentaire conforme à la norme EN60601-1-2:2007 est disponible auprès
d’OMRON HEALTHCARE EUROPE à l’adresse mentionnée dans le présent mode d’emploi.
Une documentation est également disponible sur le site www.omron-healthcare.com.
Comment éliminer ce produit
(déchets d’équipements électriques et électroniques)
Ce symbole figurant sur le produit ou sa documentation indique qu’il ne doit pas être mis au rebut
en fin de vie avec les autres déchets ménagers. L’élimination incontrôlée des déchets pouvant
porter préjudice à l’environnement et à la santé humaine, veuillez séparer ce produit des autres
types de déchets et le recycler de façon responsable afin de favoriser la réutilisation durable des
ressources matérielles.
Les particuliers sont invités à contacter le distributeur leur ayant vendu ce produit ou leur
mairie pour savoir où et comment ils peuvent le renvoyer afin qu’il soit recyclé en respectant
l’environnement.
Les entreprises sont invitées à contacter leurs fournisseurs et à consulter les conditions de leur
contrat de vente. Ce produit ne doit pas être éliminé avec les autres déchets commerciaux.
Page 43

FR
43
Garantie
Merci d’avoir acheté un produit OMRON. Ce produit a été élaboré avec des matériaux
de haute qualité et un grand soin a été apporté à sa fabrication. Il a été conçu pour
vous apporter un confort de haute qualité, à condition de l’utiliser et de l’entretenir
correctement, conformément aux indications du mode d’emploi.
Ce produit est garanti par OMRON pour une période de 3 ans après sa date d’achat.
La construction, la fabrication et les matériaux mêmes de ce produit sont garantis
par OMRON. Pendant cette période de garantie, OMRON s’engage à réparer ou
remplacer tout produit défectueux ou toute pièce défectueuse sans facturer la main
d’œuvre ni les pièces.
Cette garantie ne s’applique qu’à des produits achetés dans les pays suivants :
Europe, Russie et autres pays de la C.E.I., Moyen-Orient et Afrique.
La garantie ne couvre pas les points suivants :
a. Frais et risques liés au transport.
b. Coûts des réparations et/ou des défauts résultant de réparations effectuées par des
personnes non agréées.
c. Contrôles et maintenance périodiques.
d. Panne ou usure des accessoires ou d’autres parties autres que l’unité principale
même, sauf garantie expresse ci-dessus.
e. Coûts résultant de la non-acceptation d’une réclamation (ces coûts seront facturés).
f. Dommages quelconques, y compris dommages personnels d’origine accidentelle ou
résultant d’une utilisation inappropriée.
Si le service de garantie s’avère nécessaire, veuillez contacter le distributeur chez qui le
produit a été acheté ou un distributeur OMRON agréé. Pour obtenir l’adresse, reportezvous à l’emballage/la documentation du produit ou à votre détaillant spécialisé. Si vous
rencontrez des difficultés à trouver des services clientèles OMRON, rendez-vous sur
notre site Web (www.omron-healthcare.com) pour obtenir des informations de contact.
Une réparation ou un remplacement sous garantie ne donne pas lieu à une prolongation
ou au renouvellement de la période de garantie.
La garantie sera accordée uniquement si le produit complet est renvoyé avec la facture/
le ticket de caisse d’origine délivré(e) au client par le détaillant. OMRON se réserve le
droit de refuser toute garantie si les informations données manquent de précision d’une
façon quelconque.
Page 44

44
Fabricant OMRON HEALTHCARE Co., Ltd.
53, Kunotsubo, Terado-cho, Muko, KYOTO, 617-0002 JAPON
Mandataire
dans l’UE
OMRON HEALTHCARE EUROPE B.V.
Scorpius 33, 2132 LR Hoofddorp, PAYS-BAS
www.omron-healthcare.com
Site de production
OMRON HEALTHCARE MANUFACTURING VIETNAM CO., LTD.
No.28 VSIP II, Street 2, Vietnam-Singapore Industrial Park II,
Binh Duong Industry-Services-Urban Complex, Hoa Phu Ward,
Thu Dau Mot City, Binh Duong Province, Vietnam
Succursales
OMRON HEALTHCARE UK LTD.
Opal Drive, Fox Milne, Milton Keynes, MK15 0DG, ROYAUME-UNI
www.omron-healthcare.co.uk
OMRON MEDIZINTECHNIK HANDELSGESELLSCHAFT mbH
Gottlieb-Daimler-Strasse 10, 68165 Mannheim, ALLEMAGNE
www.omron-healthcare.de
OMRON SANTÉ FRANCE SAS
14, rue de Lisbonne, 93561 Rosny-sous-Bois Cedex, FRANCE
Uniquement pour le marché français:
OMRON Service Après Vente
Nº Vert 0 800 91 43 14
www.omron-healthcare.fr
Fabriqué en Vietnam
Page 45

DE
NE-C803-E_A_M09_140715.pdf
Kompressor-Vernebler
NE-C803 (NE-C803-E)
Gebrauchsanweisung
Page 46

46
Einführung
Vielen Dank, dass Sie sich für den OMRON COMP AIR entschieden haben.
Dieses Produkt wurde in Zusammenarbeit mit Atemtherapeuten für die erfolgreiche
Behandlung von Asthma, chronischer Bronchitis, Allergien und anderen
Atemwegserkrankungen entwickelt.
Dies ist ein Medizinprodukt. Bedienen Sie das Gerät nur gemäß den Anweisungen des
Arztes und/oder Atemtherapeuten.
Funktionsweise des Vernebler-Sets
Das Medikament, das durch den
Medikamentenkanal nach oben gepumpt wird,
wird mit Druckluft gemischt, die von einer
Kompressorpumpe erzeugt wird.
Das Medikament wird durch die Druckluft in Form
von feinen Partikeln versprüht.
Aerosol
Düse
Druckluft
Medikamentenkanal
Medikament
Vor der Verwendung des Geräts
Inhalt
Vor der Verwendung des Geräts
Einführung ................................................................................................................ 46
Verwendungszweck .................................................................................................. 47
Wichtige Sicherheitsanweisungen ............................................................................ 48
Übersicht über Ihr Gerät ........................................................................................... 51
Optionales medizinisches Zubehör........................................................................... 52
Andere optionale Teile/Ersatzteile ............................................................................ 52
Gebrauchsanweisung
Verwendung .............................................................................................................. 53
Pflege und Wartung
Reinigung und Desinfektion ...................................................................................... 57
Entfernen von Kondensat aus dem Luftschlauch ..................................................... 60
Auswechseln des Luftfilters ...................................................................................... 60
Fehlersuche und -behebung ..................................................................................... 61
Technische Daten ..................................................................................................... 62
Garantie .................................................................................................................... 65
Page 47

DE
47
Verwendungszweck
Medizinischer Verwendungszweck
Dieses Produkt ist für das Inhalieren von Medikamenten bei Atemwegserkrankungen
vorgesehen.
Vorgesehene Benutzer
• Gesetzlich zertifiziertes medizinisches Fachpersonal, wie z. B. Ärzte, Pflegepersonal
und Therapeuten.
• Pflegepersonal bzw. der Patient selbst unter Anleitung durch qualifiziertes
medizinisches Fachpersonal für Hauskrankenpflege.
• Der Benutzer sollte außerdem in der Lage sein, die allgemeine Betriebsweise des
NE-C803 und den Inhalt der Gebrauchsanweisung zu verstehen.
Vorgesehene Patienten
Dieses Produkt darf nicht bei Patienten verwendet werden, die bewusstlos sind oder
deren Spontanatmung ausgesetzt hat.
Umgebung
Dieses Produkt ist für die Verwendung in einer medizinischen Einrichtung, wie z. B. in
einem Krankenhaus oder einer Arztpraxis bzw. in Privathaushalten vorgesehen.
Haltbarkeit
Die Haltbarkeit ist wie folgt, unter der Annahme, dass das Produkt für die
Vernebelung von 2 ml eines Medikaments zwei Mal täglich für jeweils 7,5 Minuten bei
Zimmertemperatur (23 °C) verwendet wird.
Die Haltbarkeit kann je nach Verwendungsumgebung variieren.
Häufige Verwendung des Produkts kann die Haltbarkeit verkürzen.
Kompressor (Hauptgerät) 5 Jahre
Vernebler-Set 1 Jahr
Mundstück 1 Jahr
Nasenstück 1 Jahr
Luftschlauch (PVC, 100 cm) 1 Jahr
Luftfilter 60 Tage
Maske (PVC) (SEBS) 1 Jahr
Vorsichtsmaßnahmen bei der Verwendung
Bitte beachten Sie die Warn- und Vorsichtshinweise in der Gebrauchsanweisung.
Page 48

48
Wichtige Sicherheitsanweisungen
Lesen Sie alle Informationen in der Gebrauchsanweisung und alle sonstigen Hinweise in der
Verpackung vor der Verwendung des Geräts genau durch.
Warnung:
Zeigt eine möglicherweise gefährliche Situation an, die zu sehr schweren Verletzungen
führen kann.
(Verwendung)
• Um Typ, Dosis und Verabreichung des Medikaments richtig vorzunehmen, befolgen Sie die
Anweisungen Ihres Arztes oder Atemtherapeuten.
• Wenn Sie während der Verwendung etwas Ungewöhnliches bemerken, beenden Sie die Verwendung
des Geräts sofort und wenden Sie sich an Ihren Arzt.
• Verwenden Sie nicht nur Wasser im Vernebler, um ein Aerosol zum Inhalieren zu erzeugen.
• Bewahren Sie das Gerät außerhalb der Reichweite von unbeaufsichtigten Säuglingen und Kindern auf.
Das Gerät kann kleine Teile enthalten, die verschluckt werden könnten.
• Lagern Sie den Luftschlauch erst dann, wenn sich keine Feuchtigkeit bzw. kein Medikament mehr
darin befindet.
• Verwenden und lagern Sie das Gerät nicht in einer Umgebung, in der es schädlichen Gasen oder
flüchtigen Substanzen ausgesetzt ist.
• Verwenden Sie das Gerät nicht in einer Umgebung, in der es entflammbaren Gasen ausgesetzt ist.
• Decken Sie den Kompressor während des Betriebs nicht mit einer Decke, einem Handtuch oder
ähnlichem ab.
• Entsorgen Sie immer nach der Verwendung das verbleibende Medikament und verwenden Sie jedes Mal
frisches Medikament.
• Nicht in Schlauchsystemen für Anästhesie- oder Beatmungsgeräte verwenden.
• Bewahren Sie die Teile an einem sauberen Ort auf, um Infektionen zu vermeiden.
(Stromschlagrisiko)
• Verbinden und trennen Sie das Netzteil nicht mit nassen Händen.
• Der Kompressor und das Netzteil sind nicht wasserfest. Spritzen Sie kein Wasser oder andere Flüssigkeiten
auf diese Teile. Falls Flüssigkeit auf diese Teile gelangt, ziehen Sie unverzüglich das Netzteil ab
und wischen
Sie die Flüssigkeit mit Gaze oder einem anderen Flüssigkeit aufnehmenden Material ab.
• Tauchen Sie den Kompressor nicht in Wasser oder andere Flüssigkeiten.
• Verwenden oder lagern Sie das Gerät nicht an feuchten Orten.
• Verwenden Sie das Gerät nicht, wenn Netzkabel oder Stecker beschädigt sind.
• Achten Sie darauf, dass das Netzkabel weit genug von heißen Oberflächen entfernt ist.
• Verwenden Sie nur das Original-Netzteil von OMRON. Die Verwendung eines nicht unterstützten
Netzteils kann das Gerät beschädigen.
(Reinigung und Desinfektion)
Beachten Sie die unten angeführten Regeln bei der Reinigung und Desinfektion von Teilen. Die
Nichtbeachtung dieser Regeln kann zu Beschädigungen, ineffizienter Vernebelung oder zu Infektionen
führen. Anweisungen dazu finden Sie im Abschnitt „Reinigung und Desinfektion“.
Page 49

DE
49
• Reinigen und desinfizieren Sie Vernebler-Set, Maske, Mundstück oder Nasenstück, bevor sie wie folgt
verwendet werden:
- bei Erstverwendung nach dem Kauf
- wenn das Gerät lange Zeit nicht verwendet wurde
- wenn das Gerät von mehreren Personen verwendet wird
• Achten Sie darauf, die Teile nach der Anwendung zu waschen oder abzuwischen, und vergewissern Sie
sich, dass sie gründlich desinfiziert und getrocknet sind, und lagern Sie sie an einem sauberen Ort.
• Die Reinigungslösung nicht in den Teilen belassen.
Achtung:
Gefahrensituation die, wenn Sie nicht vermieden wird, zu leichten oder mittelschweren
Verletzungen oder Geräteschäden führen kann.
(Verwendung)
• Lassen Sie höchste Aufmerksamkeit walten, wenn dieses Gerät von Kindern oder Behinderten oder in
deren Nähe verwendet wird.
• Vergewissern Sie sich, dass sämtliche Teile richtig angebracht sind.
• Stellen Sie sicher, dass der Luftfilter richtig angebracht ist.
• Stellen Sie sicher, dass der Luftfilter sauber ist. Wenn der Luftfilter länger als 60 Tage verwendet wurde,
ersetzen Sie ihn durch einen neuen.
• Kippen Sie das Vernebler-Set nicht in einen Winkel über 45 Grad in alle Richtungen und schütteln Sie es
nicht während der Anwendung.
• Verwenden oder lagern Sie das Gerät nicht, wenn der Luftschlauch geknickt ist.
• Verwenden Sie nur originale Teile für Vernebler-Set, Luftschlauch, Luftfilter und Luftfilterabdeckung.
• Geben Sie nicht mehr als 10 ml des Medikaments in den Medikamententank.
• Tragen Sie das Vernebler-Set nicht und lassen Sie es nicht unbeaufsichtigt stehen, wenn der
Medikamententank Medikament enthält.
• Lassen Sie höchste Aufmerksamkeit walten, wenn dieses Gerät von Säuglingen und Menschen
verwendet wird, die ihre Wünsche nicht ausdrücken können.
• Setzen Sie das Gerät keinen starken Schlägen aus (lassen Sie es zum Beispiel nicht auf den Boden fallen).
• Stechen Sie nicht mit einer Nadel oder einem anderen spitzen Gegenstand in die Düse des
Medikamententanks.
• Stecken Sie weder Finger noch Gegenstände in den Kompressor.
• Zerlegen Sie weder den Kompressor noch das Netzteil und versuchen Sie nicht, diese zu reparieren.
• Setzen Sie das Gerät nicht extrem hohen oder niedrigen Temperaturen oder direktem Sonnenlicht aus.
• Das Gerät nicht verwenden, wenn Sie müde oder schläfrig sind.
• Nur zur Verwendung für Menschen zugelassen.
• Der Kompressor kann während des Betriebs heiß werden.
• Berühren Sie den Kompressor nur, wenn dies für bestimmte Bedienungshandlungen nötig ist, zum
Beispiel zum Ausschalten des Geräts während des Vernebelns.
• Wischen Sie das Gesicht nach dem Abnehmen der Maske ab, es dürfen keine Medikamentreste auf
dem Gesicht zurückbleiben.
• Führen Sie das Nasenstück vorsichtig und nicht zu weit ein, um eine Verletzung der Nasenschleimhaut
zu vermeiden.
• Verwenden Sie kein beschädigtes Vernebler-Set, Mundstück oder Nasenstück.
Page 50

50
(Stromschlagrisiko)
• Ziehen Sie nach der Verwendung und vor der Reinigung des Geräts stets das Netzteil aus der Steckdose.
• Stecken Sie das Gerät in eine Steckdose mit geeigneter Spannung. Überlasten Sie die Steckdose nicht.
Verwenden Sie keine Verlängerungskabel.
• Achten Sie stets auf einen bestimmungsgemäßen Gebrauch des Netzteilkabels.
• Wickeln Sie das Netzkabel nicht um den Kompressor oder das Netzteil.
• Ziehen Sie nicht am Netzteilkabel.
• Veränderungen, die nicht von OMRON HEALTHCARE genehmigt sind, führen zu einem Erlöschen der Garantie.
• Ziehen Sie nach der Verwendung und vor der Reinigung des Geräts stets das Netzteil aus dem Gerät.
(Reinigung und Desinfektion)
Beachten Sie die unten angeführten Regeln bei der Reinigung und Desinfektion von Teilen. Die
Nichtbeachtung dieser Regeln kann zu Beschädigungen, ineffizienter Vernebelung oder zu Infektionen
führen. Anweisungen dazu finden Sie im Abschnitt „Reinigung und Desinfektion“.
• Zum Trocknen des Geräts oder der Teile weder einen Mikrowellenofen noch einen Geschirrtrockner
oder einen Haartrockner verwenden.
• Verwenden Sie keinen Dampfsterilisator (Autoklav), EOG-Gassterilisator oder NiedertemperaturPlasma-Sterilisator.
• Achten Sie beim Desinfizieren der Teile durch Abkochen darauf, dass nicht das gesamte Wasser
verdampft. Andernfalls kann es zu einem Brand kommen.
Allgemeine Vorsichtsmaßnahmen:
• Überprüfen Sie das Gerät vor jedem Gebrauch und stellen Sie sicher, dass keine Mängel vorliegen.
Überprüfen Sie vor allem folgende Punkte:
- Düse und Luftschlauch sind nicht beschädigt.
- Düse ist nicht blockiert.
- Kompressor arbeitet normal.
• Bei der Verwendung des Geräts treten Geräusche und Vibrationen auf, die von der Pumpe im
Kompressor erzeugt werden. Durch den Austritt der Druckluft aus dem Vernebler-Set treten ebenfalls
Geräusche auf. Dies ist völlig normal und deutet nicht auf eine Störung hin.
• Setzen Sie das Gerät nur zu seinem bestimmungsgemäßen Gebrauch ein. Das Gerät nicht für andere
Zwecke verwenden.
• Gerät nicht bei Temperaturen über +40 °C verwenden.
• Stellen Sie sicher, dass der Luftschlauch fest am Kompressor und am Vernebler-Set angeschlossen ist
und sich nicht lösen kann.
• Zur vollständigen Isolierung von der Stromquelle den Stecker aus der Steckdose abziehen.
Bitte bewahren Sie diese Gebrauchsanweisung zum späteren Nachschlagen auf.
Page 51

DE
51
Übersicht über Ihr Gerät
Netzteil x 1
Aufbewahrungs-
tasche x 1
Gebrauchsanweisung
Rückansicht
Netzteilanschlussbuchse
Luftfilterabdeckung
*Luftfilter innen
Kompressor (Hauptgerät) x 1
Vernebler-Set-Halter
Luftschlauchanschluss
Netzschalter
Ersatzluftfilter (5)Luftschlauch
(PVC, 100 cm) x 1
Mundstück x 1
Kindermaske
(PVC) x 1
Erwachsenenmaske
(PVC) x 1
Vernebler-Set x 1
Luftschlauchanschluss
Inhalationsoberteil
Zerstäuberkopf
Düse
Medikamententank
Nasenstück x 1
Page 52

52
Optionales medizinisches Zubehör
(im Rahmen der Richtlinie 93/42/EWG über Medizinprodukte)
Produktbeschreibung Modell
Vernebler-Set und Mundstück-Set NEB-NSET3-83E
Vernebler-Set, Erwachsenenmaske (PVC) und Luftschlauch-Set NEB-NSET4-83AP
Vernebler-Set, Kindermaske (PVC) sowie Luftschlauch-Set NEB-NSET5-83AP
Erwachsenenmaske (PVC) NEB-MSLP-E
Kindermaske (PVC) NEB-MSMP-E
Erwachsenenmaske (SEBS) NEB-MSLS-E
Kindermaske (SEBS) Set NEB-MSSS-E
Mundstück NEB-MP-E
Nasenstück NEB-NP-E
Luftschlauch (PVC, 100 cm) NEB-TP-82E
Netzteil HHP-CF01
Andere optionale Teile/Ersatzteile
Produktbeschreibung Modell
Luftfilter x 5 NEB-AFR-30E
G
Page 53

DE
53
Gebrauchsanweisung
Verwendung
Warnung:
Reinigen und desinfizieren Sie Vernebler-Set, Maske, Mundstück oder Nasenstück,
bevor sie wie folgt verwendet werden:
- bei Erstverwendung nach dem Kauf
- wenn das Gerät lange Zeit nicht verwendet wurde
- wenn das Gerät von mehreren Personen verwendet wird
Anweisungen dazu finden Sie im Abschnitt „Reinigung und Desinfektion“.
1.
Stellen Sie sicher, dass der Netzschalter in der AUS-Stellung ( ) steht.
2.
Schließen Sie das Netzteil an den
Kompressor an und stecken Sie den
Netzstecker in eine Netzsteckdose.
3.
Entfernen Sie das Inhalationsoberteil
zusammen mit dem Zerstäuberkopf vom
Medikamententank.
Hinweis:
Falls sich der Zerstäuberkopf vom
Inhalationsoberteil löst und dabei in den
Medikamententank fällt, dann entfernen
Sie ihn aus dem Tank.
4.
Geben Sie die richtige Menge des
verschriebenen Medikaments in den
Medikamententank.
Page 54

54
5.
Stellen Sie sicher, dass der Zerstäuberkopf
richtig am Inhalationsoberteil befestigt wurde.
Hinweis:
Die Größe der an der linken und rechten
Seite des Zerstäuberkopfs angebrachten
Teile unterscheiden sich. Wenn der
Zerstäuberkopf nicht in der richtigen
Ausrichtung angebracht ist, funktioniert die
Verneblung nicht ordnungsgemäß.
6.
Bringen Sie das Vernebleroberteil
wieder im Medikamententank an.
1) Richten Sie den überstehenden Teil am
Inhalationsoberteil wie abgebildet mit
der Kerbe am Medikamententank aus.
2) Drehen Sie dazu das
Inhalationsoberteil im
Uhrzeigersinn bis der Pfeil auf
dem Inhalationsoberteil mit der
Ausrichtungsmarkierung auf dem
Medikamententank ausgerichtet ist
und beide Teile einrasten.
Pfeilmarkierung
Ausrichtungsmarkierung
Klein
Groß
Page 55

DE
55
7.
Befestigen Sie die Maske, das Mundstück
oder das Nasenstück fest am Vernebler-
Set.
Hinweis:
Stellen Sie sicher, dass die Teile so
angebracht sind, das der Griff vom
Patienten weg positioniert ist.
Andernfalls kann es sein, dass das
Medikament auf die Haut oder die
Bekleidung des Patienten sprüht.
8.
Bringen Sie den Luftschlauch an.
Drücken Sie die Luftschlauchstecker fest auf die
Luftschlauchanschlüsse.
Griff
Griff
Das Vernebler-Set kann auf dem Kompressor befestigt werden.
Page 56

56
Pfl
9.
Halten Sie das Vernebler-Set wie rechts dargestellt.
Befolgen Sie die Anweisungen Ihres Arztes oder
Atemtherapeuten. Atmen Sie normal durch das
Mundstück, die Maske oder die Nase aus.
Achtung:
Kippen Sie das Vernebler-Set nicht in einen Winkel über
45 Grad in alle Richtungen. Das Medikament könnte in
den Mund laufen oder es könnte zu einer schlechten
Verneblung führen.
10.
Drücken Sie den Netzschalter in die EIN-Stellung ( ). Sobald der Kompressor
startet, beginnt die Vernebelung und Aerosol wird erzeugt.
Inhalieren Sie das Medikament.
11.
Schalten Sie das Gerät nach der Behandlung aus. Ziehen Sie das Netzteil aus der
Steckdose und trennen Sie es vom Kompressor.
Hinweis:
Halten und drücken Sie dabei den Netzstecker
nach unten, um das Netzteil sicher zu trennen.
Achtung:
Ziehen Sie nicht am Netzkabel.
Dies kann zu Beschädigungen führen.
Rechter Winkel
45°45°
Kompressor
Adapterstecker
Page 57

DE
57
Pflege und Wartung
Reinigung und Desinfektion
Achtung: Handhabung des Zerstäuberkopfs
• Spülen Sie den Zerstäuberkopf nach jeder Anwendung.
• Zum Reinigen des Zerstäuberkopfs weder Bürste noch Stecknadeln verwenden.
• Bei der Desinfektion der Teile durch Abkochen stets reichlich Wasser verwenden.
• Kochen Sie den Zerstäuberkopf nicht mit anderen Gegenständen als für den Vernebler
geeignet ab.
Reinigung
Reinigen Sie die Teile nach jedem Gebrauch, um Medikamentenrückstände zu
entfernen. Dadurch wird eine schlechte Verneblung oder eine Infektion verhindert.
■Abwaschbare Teile
• Vernebler-Set (Vernebleroberteil, Medikamententank), Maske (PVC)/(SEBS),
Mundstück, Nasenstück
Waschen Sie die Teile in warmem Wasser mit einem milden Reinigungsmittel (neutrales
Reinigungsmittel). Spülen Sie sie gründlich mit sauberem, heißem Leitungswasser ab
und lassen Sie sie in sauberer Umgebung an der Luft trocknen.
• Zerstäuberkopf
Mit fließendem Wasser spülen.
■Nicht abwaschbare Teile
• Kompressor, Luftschlauch
Stellen Sie zunächst sicher, dass der Netzstecker aus der Steckdose gezogen ist.
Wischen Sie die Teile mit einem weichen Tuch ab, das mit Wasser oder einem milden
Reinigungsmittel (neutrales Reinigungsmittel) angefeuchtet ist.
• Luftfilter
Waschen oder reinigen Sie nicht den Luftfilter. Wenn der Luftfilter feucht wird,
wechseln Sie ihn aus. Feuchte Luftfilter können zur Blockade führen.
Desinfektion
Desinfizieren Sie die Teile einmal in der Woche. Wenn die Teile stark verschmutzt sind,
tauschen Sie sie durch neue aus.
Wählen Sie aus der Tabelle auf Seite 58 eine Desinfektionsmethode aus.
Page 58

58
o
: geeignet X: nicht geeignet
Desinfektions-
methode
(Siehe nächste
Seite)
Teile
Mundstück
Nasenstück
Vernebler-Set
Erwachsenenmaske
(SEBS) (optional)
Kindermaske (SEBS)
(optional)
Erwachsenenmaske
(PVC)
Kindermaske (PVC)
Luftschlauch
(PVC, 100 cm)
Luftfilter
Material
PP
PP
PP
(Zerstäuberkopf: PC)
Maske: SEBS
Band: Gummi
(latexfrei)
Adapter: PP
Maske: PVC
(phthalatfrei)
Band: Gummi
(latex-frei)
PVC
(phthalatfrei)
Polyester
A
Alkohol
oo o o o X X
Desinfizierendes
Ethanol
Natriumhypochlorit
oo o o o X X
Milton*
(0,1 %, 15 min)
Quaternäres
Ammonium
oo o o o X X
Osvan*
(0,1 %, 10 min)
Chlorhexidin
oo o o o X X
Hibitane*
(0,5 %, 30 min)
Amphoteres
Tensid
oo o o o X X
Tego 51*
(0,2 %, 15 min)
B
Kochen
oo o o X X X
Page 59

DE
59
A. Verwenden Sie ein handelsübliches Desinfektionsmittel. Befolgen Sie die
Anweisungen des Herstellers des Desinfektionsmittels.
Hinweis: Verwenden Sie niemals Benzol oder Verdünner zum Reinigen.
B. Die Teile können 15 bis 20 Minuten abgekocht werden.
Entfernen Sie nach dem Kochen vorsichtig die Teile, schütteln
Sie überschüssiges Wasser ab und lassen Sie sie in sauberer
Umgebung an der Luft trocknen.
* Beispiel für im Handel erhältliches Desinfektionsmittel. (Die in der Tabelle angegebene Konzentration und
Verweildauer gelten für die Bedingungen, unter denen die Lebensdauer der Teile getestet wurde, wobei
jedes Desinfektionsmittel gemäß der Beschreibung in seiner jeweiligen Gebrauchsanweisung verwendet
wurde. Beachten Sie, dass die Tests nicht durchgeführt wurden, um die Wirksamkeit der Desinfektionsmittel
nachzuweisen. Es ist nicht beabsichtigt, diese Desinfektionsmittel zu empfehlen. Die Gebrauchsbedingungen und
Inhaltsstoffe der Desinfektionsmittel sind je nach Hersteller unterschiedlich. Lesen Sie die Gebrauchsanweisung
vor der Verwendung aufmerksam durch und wenden Sie das Desinfektionsmittel entsprechend auf die Teile
an. Beachten Sie, dass die Lebensdauer der Teile abhängig von den Bedingungen, der Umgebung und der
Gebrauchshäufigkeit kürzer sein kann.)
Page 60

60
Entfernen von Kondensat aus dem Luftschlauch
Falls Feuchtigkeit oder Flüssigkeit im Luftschlauch verbleibt, achten Sie darauf, wie
unten beschrieben vorzugehen, um die Feuchtigkeit aus dem Luftschlauch zu entfernen.
1) Stellen Sie sicher, dass der Luftschlauch an der Luftschlauchbuchse des
Kompressors eingesteckt ist.
2) Ziehen Sie den Luftschlauch vom Vernebler-Set ab.
3) Schalten Sie den Kompressor ein und pumpen Sie Luft durch den Luftschlauch,
um die Feuchtigkeit herauszublasen.
Auswechseln des Luftfilters
Wenn der Luftfilter länger als 60 Tage verwendet wurde, ersetzen Sie ihn durch einen neuen.
1.
Ziehen Sie die Luftfilterabdeckung vom Kompressor.
2.
Auswechseln des Luftfilters.
3.
Bringen Sie die Luftfilterabdeckung wieder an.
Stellen Sie sicher, dass er richtig befestigt ist.
Hinweise:
• Verwenden Sie nur OMRON-Luftfilter, die für dieses Gerät bestimmt sind. Nicht ohne
Luftfilter verwenden.
• Waschen oder reinigen Sie nicht den Luftfilter. Wenn der Luftfilter feucht wird, wechseln
Sie ihn aus. Feuchte Luftfilter können zur Blockade führen.
• Die Luftfilter haben keine bestimmte Vorder- oder Rückseite.
• Überprüfen Sie, ob die Luftfilter sauber und frei von Staub sind, bevor Sie sie einsetzen.
Page 61

DE
61
Fehlersuche und -behebung
Überprüfen Sie folgende Punkte, falls das Gerät während des Betriebs ausfällt.
Problem Ursache Abhilfe
Es passiert nichts, wenn
der Ein-/Ausschalter
gedrückt wird.
Ist das Netzteil richtig in
die Steckdose und in den
Kompressor eingesteckt?
Stellen Sie sicher, dass das Netzteil in
eine Steckdose und in den Kompressor
eingesteckt ist. Bei Bedarf den Stecker
abziehen und dann erneut einstecken.
Keine Verneblung
oder geringe
Verneblungsrate, wenn
die Stromversorgung
eingeschaltet ist.
Befindet sich das Medikament
im Medikamententank?
Geben Sie die richtige Menge des
Medikaments in den Medikamententank.
Befindet sich zu viel/
zu wenig Medikament im
Medikamententank?
Fehlt der Zerstäuberkopf oder
ist er nicht richtig eingesetzt?
Bringen Sie den Zerstäuberkopf richtig an.
Ist das Vernebler-Set richtig
zusammengebaut?
Bauen Sie das Vernebler-Set richtig
zusammen.
Ist die Düse blockiert? Stellen Sie sicher, dass die Düse frei von
Blockaden ist.
Ist das Vernebler-Set stark
gekippt?
Achten Sie darauf, dass das Vernebler-Set
um nicht mehr als 45 Grad gekippt ist.
Ist der Luftschlauch richtig
angeschlossen?
Stellen Sie sicher, dass der Luftschlauch
richtig am Kompressor und Vernebler-Set
angeschlossen ist.
Ist der Luftschlauch gefaltet? Stellen Sie sicher, dass der Luftschlauch
nicht verdreht oder geknickt ist.
Ist der Luftschlauch blockiert? Stellen Sie sicher, dass der Luftschlauch
frei von Blockaden ist.
Ist der Luftfilter verschmutzt? Den Luftfilter durch einen neuen ersetzen.
Der Kompressor ist
übermäßig laut.
Ist die Luftfilterabdeckung
richtig angebracht?
Bringen Sie die Luftfilterabdeckung
richtig an.
Der Kompressor ist
sehr heiß.
Ist der Kompressor
abgedeckt?
Decken Sie den Kompressor während des
Betriebs nicht ab.
Hinweis: Falls die vorgeschlagene Abhilfe das Problem nicht behebt, versuchen
Sie nicht, das Gerät zu reparieren – das Gerät enthält keine vom Benutzer
reparierbaren Teile. Geben Sie das Gerät an einen autorisierten OMRONHändler oder -Vertreter zurück.
Page 62
62
Technische Daten
Produktbeschreibung: Kompressor-Vernebler
Modell (-Artikelnummer): NE-C803 (NE-C803-E)
Bewertung (Netzteil): 100 - 240 V ~ 50/60 Hz 0,12 - 0,065 A
Bewertung (Kompressor-Vernebler):
6V 0,7 A
Betriebstemperatur /
Luftfeuchtigkeit:
+10 °C bis +40 °C / 30 % bis 85 % relative Luftfeuchtigkeit
Lagerungs- und
Transporttemperatur,
Luftfeuchtigkeit, Luftdruck:
-20 °C bis +60 °C / 10 % bis 95 % relative Luftfeuchtigkeit /
700 – 1060 hPa
Gewicht: Ca. 180 g (nur Kompressor)
Abmessungen: Ca. 85 (B) × 43 (H) × 115 (T) mm (nur Kompressor)
Inhalt: Kompressor, Vernebler-Set, Luftschlauch (PVC, 100 cm),
Mundstück, Nasenstück, Erwachsenenmaske (PVC),
Kindermaske (PVC), 5 Ersatzluftfilter, Netzteil,
Aufbewahrungstasche, Gebrauchsanweisung.
Klassifikationen: Klasse II (Schutz gegen elektrischen Schlag),
Typ BF (Anwendungsteil)
IP21 (Eindringschutz)
= Gerät der
Klasse ll
= Typ BF
Anwendungsteil
Lesen Sie die
Gebrauchsanweisung
sorgfältig durch
= Kein Betrieb
= Eingeschaltet
Hinweise:
• Technische Änderungen ohne Vorankündigung vorbehalten.
• Die Herstellung dieses OMRON-Produkts unterliegt dem strengen Qualitätssystem von
OMRON HEALTHCARE Co., Ltd., Japan.
• Wird das Gerät bei Temperaturen oder bei einer Spannung betrieben, die von den
Angaben in den technischen Daten abweichen, funktioniert es möglicherweise nicht.
• Das Gerät entspricht den Vorschriften der EG-Richtlinie 93/42/EWG über
Medizinprodukte und der europäischen Norm EN13544-1:2007+A1:2009,
Atemtherapiegeräte - Teil 1: Verneblersysteme und deren Bauteile.
• Aktuelle technische Informationen finden Sie auf der Website von
OMRON HEALTHCARE EUROPE.
URL: www.omron-healthcare.com
Page 63
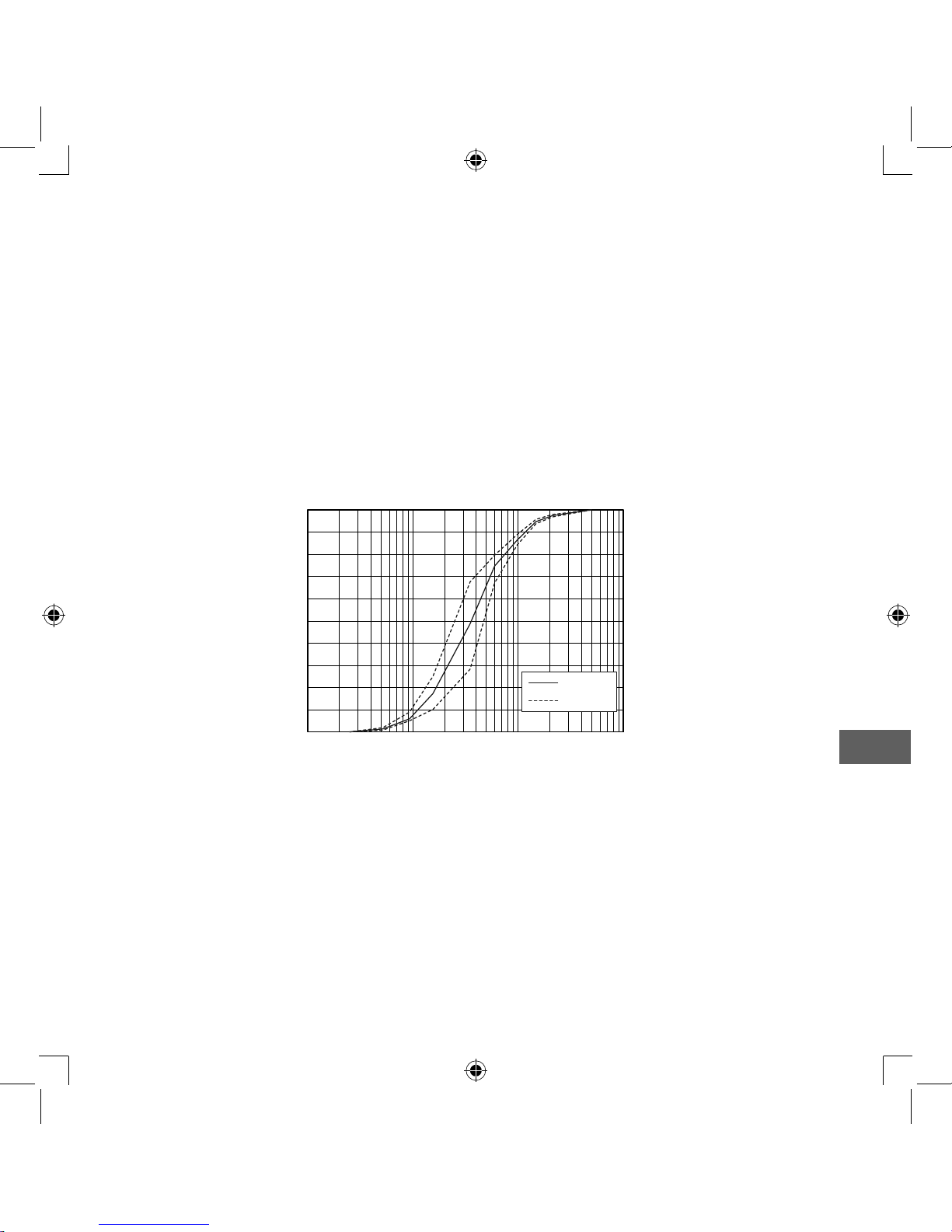
DE
63
Technische Daten für den OMRON NE-C803 Kompressor mit Vernebler-Set:
Teilchengröße: **MMAD ca. 3 μm
MMAD = Massenbezogener medianer aerodynamischer
Durchmesser
Medikamententankkapazität: Max. 10 ml
Geeignete Medikamentenmenge: Min. 2 ml - max. 10 ml
Lautstärke: *Weniger als 45 dB
Verneblerrate: *Ca. 0,30 ml/min (durch Gewichtsverlust)
Aerosolausgabe: **0,37 ml (2 ml, 1 % NaF)
Aerosolausgaberate: **0,05 ml/min (2 ml, 1 % NaF)
Ergebnis der Messungen der Teilchengröße mit einem Kaskadenimpinger**
0
10
20
30
40
50
60
70
80
90
100
0,1 1 10 100
Kumulative % Teilchenmasse von Natriumfluorid in Untergröße
Teilchengröße Dp (μm)
Kumulative Untergröße %
Mittelwert
Individuell
* Messwerte erstellt von OMRON HEALTHCARE Co., Ltd. Gemessen mit einer
Salzlösung bei 23 °C Umgebungstemperatur und einer Luftfeuchtigkeit von 40 %.
Es kann je nach Medikament variieren.
** Messwerte unabhängig erstellt von Prof. Dr. Hiroshi Takano, Therapeutic Systems
Research Center, Doshisha University, Kyoto, Japan, gemäß EN13544-1:2007+A1:2009.
Hinweis:
Die Leistung kann abhängig von den verwendeten Medikamenten, wie zum Beispiel bei
Suspensionen oder Medikamenten mit hoher Viskosität, variieren. Weitere Informationen
finden Sie im Datenblatt des Medikamentenlieferanten.
Page 64

64
Wichtige Informationen zur elektromagnetischen Verträglichkeit (EMV)
Die wachsende Anzahl von elektronischen Geräten wie PCs und Mobiltelefonen kann dazu
führen, dass medizinische Geräte beim Einsatz elektromagnetischen Störungen von anderen
Geräten ausgesetzt sind. Elektromagnetische Störungen können zu Fehlfunktion des
medizinischen Geräts führen und eine potentiell unsichere Situation erzeugen.
Medizinische Geräte sollten auch keine anderen Geräte stören.
Die Norm EN60601-1-2:2007 wurde eingeführt, um die Anforderungen für EMV
(elektromagnetische Verträglichkeit) mit dem Ziel zu regeln, unsichere Produktsituationen zu
vermeiden. Diese Norm definiert die Stufen der Immunität gegenüber elektromagnetischen
Störungen und die maximalen elektromagnetischen Emissionswerte für medizinische Geräte.
Dieses von OMRON HEALTHCARE hergestellte medizinische Gerät erfüllt die Norm EN60601-1-2:2007
sowohl in Bezug auf die Immunität als auch in Bezug auf Emissionen.
Trotzdem sollten besondere Vorsichtsmaßnahmen beachtet werden:
• Verwenden Sie in der Nähe des medizinischen Geräts keine Mobiltelefone und sonstige Geräte,
die starke elektrische oder elektromagnetische Felder erzeugen. Dies könnte zu Fehlfunktion
des medizinischen Geräts führen und eine potentiell unsichere Situation erzeugen.
Es wird ein Mindestabstand von 7 m empfohlen. Überprüfen Sie im Falle eines kürzeren
Abstands, ob das Gerät ordnungsgemäß funktioniert.
Weitere Dokumentationen gemäß EN60601-1-2:2007 können bei OMRON HEALTHCARE
EUROPE (Adresse in der Gebrauchsanweisung) angefordert werden.
Dokumentation steht auch unter www.omron-healthcare.com zur Verfügung.
Korrekte Entsorgung dieses Produkts
(Elektromüll)
Die Kennzeichnung auf dem Produkt bzw. auf der dazugehörigen Literatur gibt an, dass es
nach seiner Lebensdauer nicht zusammen mit dem normalen Haushaltsmüll entsorgt werden
darf. Entsorgen Sie dieses Gerät bitte getrennt von anderen Abfällen, um der Umwelt bzw. der
menschlichen Gesundheit nicht durch unkontrollierte Müllbeseitigung zu schaden. Recyceln Sie
das Gerät, um die nachhaltige Wiederverwertung von stofflichen Ressourcen zu fördern.
Private Nutzer sollten den Händler, bei dem das Produkt gekauft wurde, oder die zuständigen
Behörden kontaktieren, um in Erfahrung zu bringen, wie sie das Gerät auf umweltfreundliche
Weise recyceln können.
Gewerbliche Nutzer sollten sich an ihren Lieferanten wenden und die Bedingungen des Verkaufsvertrags
konsultieren. Dieses Produkt darf nicht zusammen mit anderem Gewerbemüll entsorgt werden.
Page 65

DE
65
Garantie
Vielen Dank, dass Sie sich für ein OMRON Produkt entschieden haben. Dieses Produkt
wurde aus hochwertigen Materialien mit großer Sorgfalt gefertigt. Es wurde entwickelt
für optimalen Gebrauchskomfort, vorausgesetzt es wird ordnungsgemäß verwendet und
gepflegt wie in der Gebrauchsanweisung beschrieben.
OMRON gewährt für dieses Produkt eine Garantie von 3 Jahren ab dem Kaufdatum.
OMRON gibt auf geeignete Konstruktion, Fertigung und Materialien für dieses Produkt
Garantie. Während der Garantiezeit werden das defekte Produkt und defekte Teile durch
OMRON ohne Kosten für Arbeiten oder Teile repariert oder ersetzt.
Die Garantie gilt nur für Produkte, die gekauft wurden in Europa, Russland und
anderen GUS-Ländern, dem Nahen Osten und Afrika.
Von der Garantie ausgeschlossen sind:
a. Transportkosten und -risiken.
b. Kosten für Reparaturen und/oder Defekte, die durch Reparaturen von unautorisierten
Personen entstanden sind.
c. Periodische Überprüfungen und Wartung
d. Fehler oder Verschleiß von Zubehör oder Teilen, die nicht zum Hauptgerät gehören,
sofern nicht ausdrücklich oben garantiert.
e. Durch Abweisung einer Forderung entstandene Kosten (diese werden berechnet).
f. Schäden jeglicher Art, einschließlich Personenschäden, die versehentlich oder durch
unsachgemäßen Gebrauch entstanden sind.
Sollten Garantieleistungen erforderlich sein, wenden Sie sich an den Händler, bei
dem Sie das Produkt gekauft haben, oder an einen autorisierten OMRON-Händler.
Die Adresse finden Sie auf der Produktverpackung/in den Produktunterlagen oder
bei Ihrem Fachhändler. Auf unsere Website (www.omron-healthcare.com) finden Sie
Kontaktinformationen für den OMRON Kundendienst.
Reparaturen oder der Austausch von Ersatzteilen innerhalb der Garantie verlängert
nicht die Garantiezeit.
Die Garantie wird nur gewährt, wenn das komplette Produkt zusammen mit der
Originalrechnung oder dem Kaufbeleg, die bzw. den der Händler dem Kunden
ausgestellt hat, eingereicht wird. OMRON behält sich das Recht vor, die Garantieleistung
zu verweigern, wenn die angegebenen Informationen in irgendeiner Weise unklar sind.
Page 66

66
Hersteller
OMRON HEALTHCARE Co., Ltd.
53, Kunotsubo, Terado-cho, Muko, KYOTO, 617-0002 JAPAN
EU-Repräsentant
OMRON HEALTHCARE EUROPE B.V.
Scorpius 33, 2132 LR Hoofddorp, NIEDERLANDE
www.omron-healthcare.com
Produktionsstätte
OMRON HEALTHCARE MANUFACTURING VIETNAM CO., LTD.
No.28 VSIP II, Street 2, Vietnam-Singapore Industrial Park II,
Binh Duong Industry-Services-Urban Complex, Hoa Phu Ward,
Thu Dau Mot City, Binh Duong Province, Vietnam
Niederlassungen
OMRON HEALTHCARE UK LTD.
Opal Drive, Fox Milne, Milton Keynes, MK15 0DG, UK
www.omron-healthcare.co.uk
OMRON MEDIZINTECHNIK HANDELSGESELLSCHAFT mbH
Gottlieb-Daimler-Strasse 10, 68165 Mannheim, DEUTSCHLAND
www.omron-healthcare.de
OMRON SANTÉ FRANCE SAS
14, rue de Lisbonne, 93561 Rosny-sous-Bois Cedex, FRANKREICH
www.omron-healthcare.fr
Hergestellt in Vietnam
Page 67

IT
NE-C803-E_A_M09_140715.pdf
Nebulizzatore a compressore
NE-C803 (NE-C803-E)
Manuale di istruzioni
Page 68

68
Introduzione
Grazie per aver acquistato l’unità OMRON COMP AIR base.
Questo prodotto è stato sviluppato in collaborazione con esperti del trattamento dei
problemi respiratori, per la cura di asma, bronchite cronica, allergie e altri disturbi
respiratori.
Questo apparecchio è un dispositivo medico. Usare questo dispositivo solo nel modo
indicato dal proprio medico e/o terapista della riabilitazione respiratoria.
Funzionamento dell’ampolla nebulizzatrice
Il farmaco, che viene spinto verso l’alto attraverso
il canale per il farmaco, viene miscelato con
l’aria compressa generata dalla pompa del
compressore.
Il farmaco viene trasformato in particelle
microscopiche e vaporizzato mentre è a contatto
con l’aria compressa.
Aerosol
Ugello
Aria compressa
Canale per il farmaco
Farmaco
Prima di usare il dispositivo
Indice
Prima di usare il dispositivo
Introduzione .............................................................................................................. 68
Destinazione d’uso ................................................................................................... 69
Istruzioni importanti relative alla sicurezza ............................................................... 70
Componenti dell’unità ............................................................................................... 73
Accessori medici opzionali........................................................................................ 74
Altre parti opzionali/di ricambio ................................................................................. 74
Istruzioni operative
Modalità di utilizzo ....................................................................................................75
Cura e manutenzione
Pulizia e disinfezione ................................................................................................ 79
Rimozione della condensa dal tubo dell’aria ............................................................ 82
Sostituzione del filtro dell’aria ................................................................................... 82
Risoluzione dei problemi........................................................................................... 83
Dati tecnici ................................................................................................................ 84
Garanzia ................................................................................................................... 87
Page 69

IT
69
Destinazione d’uso
Finalità mediche
Questo prodotto è destinato ad essere utilizzato per l’inalazione di farmaci per la cura
dei disturbi respiratori.
Utilizzatori ai quali è destinato il prodotto
• Personale medico legalmente abilitato (medici, infermieri e terapisti).
• Per il trattamento in casa, l’assistenza domiciliare o il paziente sotto la guida di
personale medico qualificato.
• L’utilizzatore deve inoltre essere in grado di comprendere in linea generale il
funzionamento del dispositivo NE-C803 e il contenuto del manuale di istruzioni.
Pazienti ai quali è destinato il prodotto
Questo prodotto non deve essere utilizzato su pazienti in stato di incoscienza o che non
respirino spontaneamente.
Ambiente
Questo prodotto è destinato all’utilizzo in una struttura medica (per es. ospedale, clinica
o studio medico) e nei comuni ambienti domestici.
Durata prevista
I periodi di durata sono riportati di seguito e partono dal presupposto che il prodotto
venga utilizzato per nebulizzare 2 ml di farmaco per due volte al giorno, 7,5 minuti per
volta, a una temperatura ambiente di 23 °C.
Il periodo di durata può variare in base all’ambiente di utilizzo.
L’utilizzo frequente del prodotto può ridurre il periodo di durata.
Compressore (Unità principale) 5 anni
Ampolla nebulizzatrice 1 anno
Boccaglio 1 anno
Forcella nasale 1 anno
Tubo dell’aria (PVC, 100 cm) 1 anno
Filtro dell’aria 60 giorni
Maschera (PVC) (SEBS) 1 anno
Precauzioni d’uso
È necessario seguire le avvertenze e le precauzioni riportate nel manuale di istruzioni.
Page 70

70
Istruzioni importanti relative alla sicurezza
Prima di usare il dispositivo, leggere attentamente le informazioni contenute nel manuale di
istruzioni e nella documentazione presente nella confezione.
Avvertenza:
Indica una situazione potenzialmente pericolosa che, se non viene evitata, può
provocare lesioni gravi.
(Utilizzo)
• Attenersi alle indicazioni del proprio medico o terapista della riabilitazione respiratoria per quanto
riguarda il tipo di medicinale, la posologia e le indicazioni di cura.
• Se si dovesse rilevare qualcosa di insolito mentre si usa il dispositivo, interromperne immediatamente
l’utilizzo e consultare il medico curante.
• Non usare esclusivamente l’unità come nebulizzatore d’acqua per inalazioni.
• Conservare il dispositivo al di fuori della portata dei bambini quando non è presente un adulto. Il
dispositivo potrebbe contenere parti di piccole dimensioni che possono essere ingoiate facilmente.
• Non riporre il tubo dell’aria se risulta umido o presenta residui di farmaco al suo interno.
• Non usare né conservare il dispositivo in luoghi in cui potrebbe essere esposto a esalazioni nocive o a
sostanze volatili.
• Non usare il dispositivo in luoghi in cui potrebbe essere esposto a gas infiammabili.
• Non coprire il compressore con coperte, asciugamani o altri oggetti durante l’uso.
• Gettare sempre il farmaco residuo dopo l’uso; usare ogni volta una nuova dose di farmaco.
• Non usare in circuiti anestetici o di ventilazione assistita.
• Conservare i componenti in un luogo pulito al fine di evitare infezioni.
(Rischio di folgorazione)
• Non collegare né scollegare mai l’adattatore CA con le mani bagnate.
• Il compressore e l’adattatore CA non sono impermeabili. Non far cadere acqua o altri liquidi su questi
componenti. Qualora su questi componenti si dovesse rovesciare del liquido, scollegare immediatamente
l’adattatore CA
e asciugare il liquido con una garza o altro materiale assorbente.
• Non immergere il compressore in acqua o altro liquido.
• Non utilizzare né conservare il dispositivo in luoghi umidi.
• Non accendere il dispositivo se il cavo di alimentazione o la spina sono danneggiati.
• Tenere il cavo di alimentazione lontano da superfici calde.
• Utilizzare esclusivamente l’adattatore CA originale OMRON. L’uso di adattatori CA non supportati può
danneggiare il dispositivo.
(Pulizia e disinfezione)
Quando si esegue la pulizia o la disinfezione dei componenti, attenersi alle indicazioni fornite di seguito.
La mancata osservanza delle stesse può causare danni, una nebulizzazione inefficiente o infezioni. Per le
istruzioni, fare riferimento alla sezione “Pulizia e disinfezione”.
• Pulire e disinfettare l’ampolla nebulizzatrice, la maschera, il boccaglio o la forcella nasale prima di
utilizzarli nei casi elencati di seguito:
- la prima volta dopo l’acquisto.
- se il dispositivo non è stato usato per un periodo di tempo prolungato
- se più di una persona usa lo stesso dispositivo
Page 71

IT
71
• Avere sempre cura di lavare o asciugare i componenti dopo l’uso; accertarsi che questi siano
completamente disinfettati e asciutti e siano conservati in un luogo pulito.
• Non lasciare residui di soluzione detergente all’interno dei componenti.
Attenzione:
Indica una situazione potenzialmente pericolosa che, se non viene evitata, può
provocare lesioni di lieve o moderata entità oppure danneggiare il dispositivo.
(Utilizzo)
• Prestare la massima attenzione nel caso in cui il dispositivo sia utilizzato da bambini o invalidi oppure in
loro presenza.
• Accertarsi che i componenti siano montati correttamente.
• Accertarsi che il filtro dell’aria sia montato correttamente.
• Accertarsi che il filtro dell’aria sia pulito. Se il filtro dell’aria è stato utilizzato per più di 60 giorni, sostituirlo
con un nuovo filtro.
• Non inclinare l’ampolla nebulizzatrice ad un’angolazione superiore a 45 gradi in qualsiasi direzione, né
scuoterla durante l’uso.
• Non usare né conservare il dispositivo con il tubo dell’aria piegato.
• Usare esclusivamente ricambi originali per l’ampolla di nebulizzazione, il tubo dell’aria, il filtro dell’aria e
il coperchio del filtro dell’aria.
• Non inserire più di 10 ml di medicinale nel serbatoio del farmaco.
• Non trasportare né lasciare incustodita l’ampolla nebulizzatrice quando il serbatoio del farmaco contiene
del medicinale.
• Non lasciare il dispositivo incustodito in presenza di bambini o persone non in grado di esprimere il
proprio consenso.
• Non sottoporre l’apparecchio a forti urti (ad esempio, evitare di farlo cadere in terra).
• Non pungere l’ugello del serbatoio del farmaco con spilli o altri oggetti affilati.
• Non inserire le dita né alcun oggetto all’interno del compressore.
• Non smontare né tentare di riparare il compressore o l’adattatore CA.
• Non esporre il dispositivo a temperature eccessivamente calde o fredde né alla luce diretta del sole.
• Non usare il dispositivo durante il sonno o se si è assonnati.
• Approvato solo per uso umano.
• Quando si utilizza il dispositivo, il compressore potrebbe scaldarsi.
• Non toccare il compressore se non per le operazioni necessarie, ad esempio per lo spegnimento del
dispositivo durante la nebulizzazione.
• Per evitare residui di farmaco sul viso, avere cura di asciugare il viso dopo aver rimosso la maschera.
• Per evitare lesioni alle mucose nasali, non spingere eccessivamente la forcella nasale verso la parte
posteriore del naso.
• Non utilizzare l’ampolla nebulizzatrice, il boccaglio o la forcella nasale se tali componenti
presentano danni.
(Rischio di folgorazione)
• Scollegare sempre l’adattatore CA dalla presa elettrica dopo ciascun utilizzo e prima della pulizia.
• Collegare il dispositivo a una presa elettrica di tensione appropriata. Non sovraccaricare le prese
elettriche né usare prolunghe.
• Non utilizzare il cavo di alimentazione dell’adattatore CA in modo improprio.
• Non avvolgere il cavo di alimentazione intorno al compressore o all’adattatore CA.
• Non tirare il cavo di alimentazione dell’adattatore CA.
Page 72

72
• Eventuali modifiche o alterazioni non approvate da OMRON HEALTHCARE renderanno nulla la
garanzia.
• Rimuovere sempre l’adattatore CA dal dispositivo dopo ciascun utilizzo e prima della pulizia.
(Pulizia e disinfezione)
Quando si esegue la pulizia o la disinfezione dei componenti, attenersi alle indicazioni fornite di seguito.
La mancata osservanza delle stesse può causare danni, una nebulizzazione inefficiente o infezioni. Per le
istruzioni, fare riferimento alla sezione “Pulizia e disinfezione”.
• Non utilizzare un forno a microonde, un’asciugatrice per stoviglie o un asciugacapelli per asciugare il
dispositivo o i componenti.
• Non utilizzare un’autoclave, uno sterilizzatore a gas EOG o uno sterilizzatore al plasma a bassa
temperatura.
• Se si disinfettano i componenti per ebollizione, accertarsi che l’acqua nel contenitore utilizzato non
evapori completamente. In caso contrario, si possono verificare incendi.
Precauzioni generali relative alla sicurezza:
• Prima di ogni utilizzo ispezionare sempre il dispositivo e verificare che non sussistano problemi. In
particolare, avere cura di verificare quanto segue:
- Assenza di eventuali danni all’ugello o al tubo dell’aria.
- Assenza di ostruzioni nell’ugello.
- Corretto funzionamento del compressore.
• Durante l’uso di questo dispositivo si può notare la presenza di rumore e vibrazioni causate dalla pompa
del compressore. Un altro fattore di rumorosità è costituito dall’emissione di aria compressa dall’ampolla
nebulizzatrice. Si tratta di una situazione normale che non è indice di un guasto.
• Usare il dispositivo solo per gli scopi per i quali è progettato. Non usare il dispositivo per scopi diversi da
quanto indicato.
• Non usare il dispositivo a una temperatura superiore a +40 °C.
• Accertarsi che il tubo dell’aria sia fissato saldamente al compressore e all’ampolla nebulizzatrice e che
non si allenti durante l’uso.
• Per isolare completamente il dispositivo dalla rete elettrica, staccare la spina dalla fonte di
alimentazione.
Conservare queste istruzioni per farvi riferimento in futuro.
Page 73

IT
73
Componenti dell’unità
Adattatore CA x 1
Custodia x 1
Manuale di istruzioni
Vista posteriore
Connettore di
alimentazione
Coperchio del filtro dell’aria
*Filtro dell’aria all’interno
Compressore (Unità principale) x 1
Supporto ampolla
nebulizzatrice
Connettore per tubo dell’aria
Interruttore di alimentazione
Filtri per l’aria di
ricambio x 5
Tubo dell’aria
(PVC, 100 cm) x 1
Boccaglio x 1
Maschera per bambini
(PVC) x 1
Maschera per adulti
(PVC) x 1
Ampolla nebulizzatrice x 1
Connettore per tubo dell’aria
Parte superiore dell’ampolla
Gruppo vaporizzatore
Ugello
Serbatoio del farmaco
Forcella nasale x 1
Page 74

74
Accessori medici opzionali
(nell’ambito di quanto disposto dalla Direttiva CE sui dispositivi medicali
93/42/CEE)
Descrizione del prodotto Modello
Set ampolla nebulizzatrice e boccaglio NEB-NSET3-83E
Set ampolla nebulizzatrice, maschera per adulti (PVC) e tubo dell’aria NEB-NSET4-83AP
Set ampolla nebulizzatrice, maschera per bambini (PVC) e tubo dell’aria NEB-NSET5-83AP
Maschera per adulti (PVC) NEB-MSLP-E
Maschera per bambini (PVC) NEB-MSMP-E
Maschera per adulti (SEBS) NEB-MSLS-E
Set maschera per bambini (SEBS) NEB-MSSS-E
Boccaglio NEB-MP-E
Forcella nasale NEB-NP-E
Tubo dell’aria (PVC, 100 cm) NEB-TP-82E
Adattatore CA HHP-CF01
Altre parti opzionali/di ricambio
Descrizione del prodotto Modello
Filtri per l’aria, 5 pezzi NEB-AFR-30E
Is
Page 75

IT
75
Istruzioni operative
Modalità di utilizzo
Avvertenza:
Pulire e disinfettare l’ampolla nebulizzatrice, la maschera, il boccaglio o la forcella
nasale prima di utilizzarli nei casi elencati di seguito:
- la prima volta dopo l’acquisto.
- se il dispositivo non è stato usato per un periodo di tempo prolungato
- se più di una persona usa lo stesso dispositivo
Per le istruzioni, fare riferimento alla sezione “Pulizia e disinfezione”.
1.
Accertarsi che l’interruttore di alimentazione sia in posizione di spegnimento ( ).
2.
Collegare l’adattatore CA al nebulizzatore e
inserire la spina in una presa elettrica.
3.
Rimuovere dal serbatoio del farmaco la parte
superiore dell’ampolla insieme al gruppo
vaporizzatore.
Nota:
Se il gruppo vaporizzatore dovesse
fuoriuscire dalla parte superiore dell’ampolla e
cadere nel serbatoio del farmaco, rimuoverlo
dal serbatoio stesso.
4.
Aggiungere al serbatoio del farmaco la
quantità di medicinale prescritta.
Page 76

76
5.
Accertarsi che il gruppo vaporizzatore sia
fissato saldamente alla parte superiore
dell’ampolla.
Nota:
Le dimensioni dei componenti presenti a
sinistra e a destra del gruppo vaporizzatore
non sono uguali. Se il gruppo vaporizzatore
non viene montato nel giusto orientamento,
la nebulizzazione non verrà eseguita
correttamente.
6.
Reinserire la parte superiore
dell’ampolla sul serbatoio del farmaco.
1) Allineare la sporgenza presente nella
parte superiore dell’ampolla con
la tacca presente sul serbatoio del
farmaco, come indicato in figura.
2) Far ruotare in senso orario la
parte superiore dell’ampolla fino
ad allineare la freccia presente su
di essa al simbolo di allineamento
presente sul serbatoio del farmaco;
i due elementi si posizioneranno in
sede con uno scatto.
Freccia
Simbolo
di allineamento
Piccolo
Grande
Page 77

IT
77
7.
Montare correttamente la maschera,
il boccaglio o la forcella nasale
sull’ampolla nebulizzatrice.
Nota:
Assicurarsi di fissare questi elementi in
modo che la maniglia sia posizionata
lontano dal paziente.
In caso contrario, il farmaco potrebbe
essere vaporizzato sulla pelle o sugli
abiti del paziente.
8.
Collegare il tubo dell’aria.
Spingere con decisione gli attacchi del tubo
dell’aria nei connettori per il tubo dell’aria.
Maniglia
Maniglia
L’ampolla nebulizzatrice può essere fissata sul compressore.
Page 78
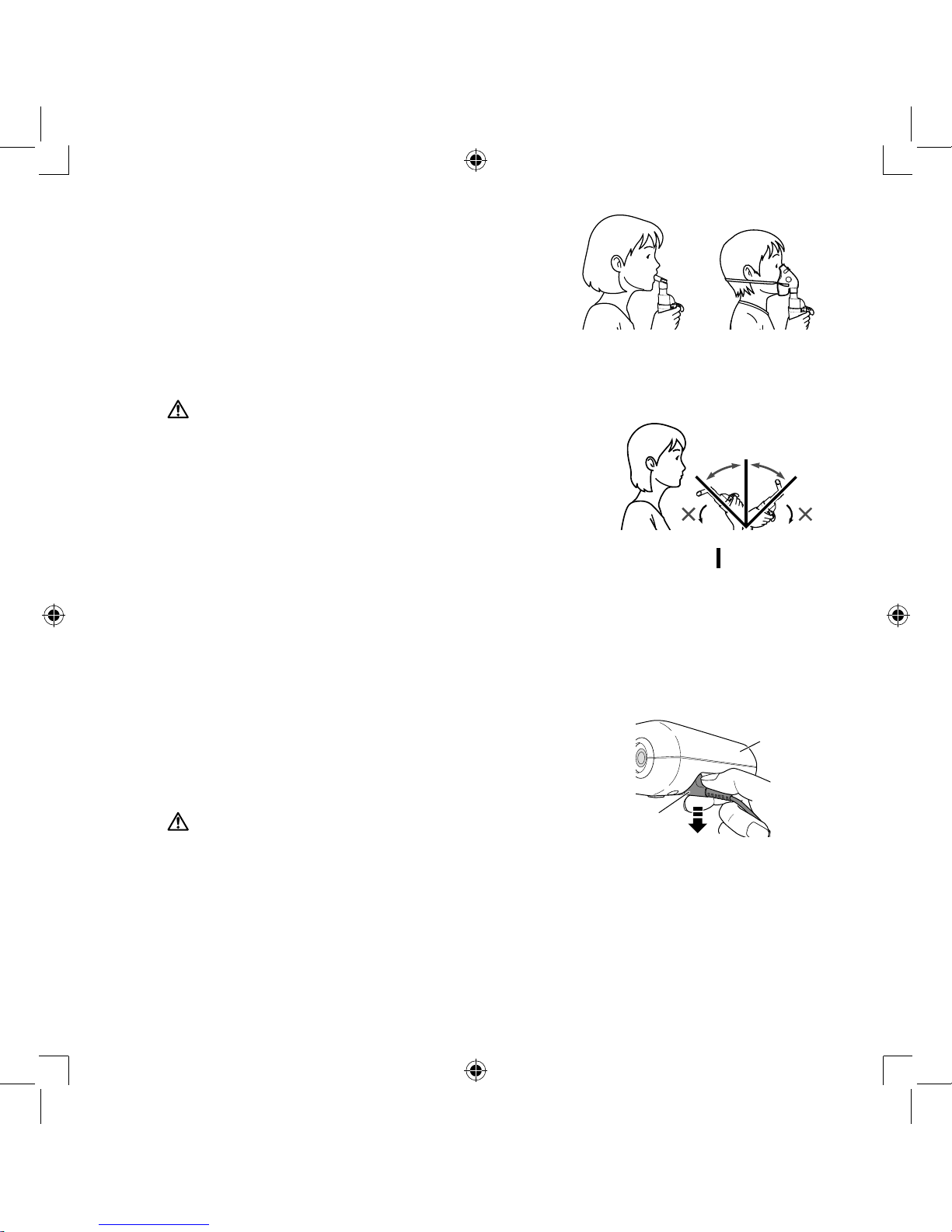
78
C
u
9.
Tenere l’ampolla nebulizzatrice come indicato a
destra.
Attenersi alle indicazioni del proprio medico o
terapista della riabilitazione respiratoria. Espirare
normalmente attraverso il boccaglio o la maschera,
oppure dal naso.
Attenzione:
Non inclinare l’ampolla nebulizzatrice ad un’angolazione
superiore a 45 gradi in qualsiasi direzione. Il farmaco
potrebbe riversarsi in bocca o la nebulizzazione potrebbe
risultare inefficace.
10.
Spostare l’interruttore di alimentazione sulla posizione di accensione ( ). Non
appena il compressore si avvia, ha inizio la nebulizzazione e viene generato l’aerosol.
Inalare il farmaco.
11.
Una volta completato il trattamento, spegnere l’alimentazione. Scollegare l’adattatore
CA dalla presa elettrica e quindi dal compressore.
Nota:
Tenere saldamente la spina del cavo di
alimentazione e premerla verso il basso per
scollegare l’adattatore CA.
Attenzione:
Non tirare il cavo di alimentazione.
Ciò potrebbe comportare danni.
Angolo corretto
45°45°
Sistema di
nebulizzazione
Spina del cavo
di alimentazione
Page 79

IT
79
Cura e manutenzione
Pulizia e disinfezione
Attenzione: Precauzioni d’uso del gruppo vaporizzatore
• Lavare sempre il gruppo vaporizzatore dopo ogni utilizzo.
• Non utilizzare spazzolini né oggetti appuntiti per pulire il gruppo vaporizzatore.
• Se si sterilizzano i componenti per ebollizione, avere cura di immergerli in abbondante
acqua.
• Non far bollire il gruppo vaporizzatore insieme ad oggetti diversi dagli accessori del
nebulizzatore per i quali è indicato tale procedimento.
Pulizia
Pulire i componenti dopo ogni utilizzo per rimuovere i residui di farmaco. Questa
operazione consente di evitare infezioni o problemi nella qualità della nebulizzazione.
■Parti lavabili
• Ampolla nebulizzatrice (parte superiore dell’ampolla, serbatoio del farmaco),
maschera (PVC)/(SEBS), boccaglio, forcella nasale
Lavare con acqua tiepida con un detergente delicato (detergente neutro). Risciacquare
bene con acqua calda corrente pulita e lasciare asciugare all’aria in un ambiente pulito.
• Gruppo vaporizzatore
Lavare con acqua corrente.
■Parti non lavabili
• Compressore, tubo dell’aria
Accertarsi innanzitutto che la spina del cavo di alimentazione sia scollegata dalla
presa. Pulire con un panno morbido inumidito con acqua o detergente delicato
(detergente neutro).
• Filtro dell’aria
Non lavare o pulire il filtro dell’aria. Se il filtro dell’aria si inumidisce, sostituirlo.
La presenza di umidità nei filtri dell’aria può causare ostruzioni.
Disinfezione
Disinfettare i componenti una volta alla settimana. Se i componenti sono
eccessivamente macchiati, sostituirli.
Per scegliere un metodo di disinfezione, fare riferimento alla tabella a pagina 80.
Page 80

80
o
: applicabile X: non applicabile
Metodo di
disinfezione
(Vedere alla pagina
seguente)
Componenti
Boccaglio
Forcella nasale
Ampolla
nebulizzatrice
Maschera per adulti
(SEBS) (opzionale)
Maschera per
bambini (SEBS)
(opzionale)
Maschera per adulti
(PVC)
Maschera per
bambini (PVC)
Tubo dell’aria
(PVC, 100 cm)
Filtro dell’aria
Materiali
PP
PP
PP
(Gruppo
vaporizzatore: PC)
Maschera: SEBS
Fascia: Gomma
(priva di lattice)
Adattatore: PP
Maschera: PVC
(privo di ftalati)
Fascia: gomma
(priva di lattice)
PVC
(privo di ftalati)
Poliestere
A
Alcol
oo o o o X X
Etanolo per
disinfezione
Ipoclorito di sodio
oo o o o X X
Milton*
(0,1%, 15 min.)
Ammonio quaternario
oo o o o X X
Osvan*
(0,1%, 10 min.)
Clorexidina
oo o o o X X
Hibitane*
(0,5%, 30 min.)
Tensioattivo
anfotero
oo o o o X X
Tego 51*
(0,2%, 15 min.)
B
Ebollizione
oo o o X X X
Page 81

IT
81
A. Usare un disinfettante disponibile in commercio. Attenersi alle istruzioni fornite dal
produttore del disinfettante.
Nota: Non pulire mai con benzene o solvente.
B. I componenti possono essere sottoposti a ebollizione (da 15 a 20 minuti).
Dopo l’ebollizione, rimuovere con cura i componenti dal
recipiente, scuoterli per eliminare l’acqua in eccesso e lasciarli
asciugare all’aria in un ambiente pulito.
* esempio di disinfettante disponibile in commercio. (La concentrazione e il tempo di permanenza specificati nella
tabella fanno riferimento a condizioni in cui la durata operativa dei componenti è stata sottoposta a verifica con
ciascuno dei disinfettanti utilizzati come descritto nei rispettivi manuali di istruzioni. Tenere presente che il test
non è stato eseguito allo scopo di garantire l’efficacia dei disinfettanti. Non si intende in alcun modo suggerire che
sia necessario utilizzare i disinfettanti indicati. Le condizioni di utilizzo e i componenti del disinfettante variano in
base al produttore. Prima di utilizzare un disinfettante, leggere attentamente il rispettivo manuale e utilizzare il
prodotto sui vari componenti dell’apparecchio nel modo appropriato. Tenere presente che la durata operativa dei
componenti può risultare inferiore in funzione delle condizioni, dell’ambiente e della frequenza di utilizzo.)
Page 82

82
Rimozione della condensa dal tubo dell’aria
Se il tubo presenta residui di umidità o liquido, accertarsi di procedere come segue per
rimuovere dal tubo dell’aria l’umidità residua.
1) Accertarsi che il tubo dell’aria sia collegato al connettore per il tubo dell’aria posto sul
nebulizzatore.
2) Rimuovere il tubo dell’aria dall’ampolla nebulizzatrice.
3) Accendere il compressore e pompare aria attraverso il tubo dell’aria per eliminare
l’umidità.
Sostituzione del filtro dell’aria
Se il filtro dell’aria è stato utilizzato per più di 60 giorni, sostituirlo con un nuovo filtro.
1.
Estrarre dal nebulizzatore il coperchio del filtro dell’aria.
2.
Sostituire il filtro dell’aria.
3.
Richiudere il coperchio del filtro dell’aria.
Verificare che sia fissato correttamente.
Note:
• Usare esclusivamente filtri dell’aria OMRON del tipo progettato per questo dispositivo.
Non azionare l’apparecchio senza il filtro dell’aria.
• Non lavare o pulire il filtro dell’aria. Se il filtro dell’aria si inumidisce, sostituirlo.
La presenza di umidità nei filtri dell’aria può causare ostruzioni.
• I filtri dell’aria non vanno inseriti con un orientamento specifico.
• Prima di inserire il filtro, verificare che sia pulito e privo di polvere.
Page 83

IT
83
Risoluzione dei problemi
Se il dispositivo dovesse presentare problemi durante il funzionamento, verificare quanto
segue.
Problema Causa Rimedio
Premendo il pulsante
di alimentazione non
accade nulla.
L’adattatore CA è collegato
correttamente a una presa
elettrica e al nebulizzatore?
Controllare che l’adattatore CA sia collegato
a una presa elettrica e al compressore. Se
necessario, scollegare e poi reinserire la
spina.
Quando l’alimentazione
è accesa, la
nebulizzazione non
avviene o risulta debole.
Il serbatoio del farmaco
contiene il medicinale?
Aggiungere al serbatoio del farmaco la
quantità di medicinale corretta.
Il serbatoio del farmaco
contiene troppo medicinale o
troppo poco?
Il gruppo vaporizzatore
manca oppure non è stato
montato correttamente?
Montare il gruppo vaporizzatore nel modo
corretto.
L’ampolla nebulizzatrice è
montata correttamente?
Montare l’ampolla nebulizzatrice nel modo
corretto.
L’ugello è ostruito? Accertarsi che l’ugello non sia ostruito.
L’ampolla nebulizzatrice è
fortemente inclinata?
Accertarsi che l’angolazione dell’ampolla
nebulizzatrice non sia superiore a 45 gradi.
Il tubo dell’aria è collegato
correttamente?
Accertarsi che il tubo dell’aria sia collegato
correttamente al compressore e all’ampolla
nebulizzatrice.
Il tubo dell’aria è piegato? Accertarsi che il tubo dell’aria non sia
attorcigliato o piegato.
Il tubo dell’aria è ostruito?
Accertarsi che il tubo dell’aria non sia
ostruito.
Il filtro dell’aria è sporco?
Sostituire il filtro dell’aria con un nuovo filtro.
Il compressore è più
rumoroso del solito.
Il coperchio del filtro dell’aria
è montato correttamente?
Montare il coperchio del filtro dell’aria nel
modo corretto.
Il compressore è molto
caldo.
Il compressore è stato
coperto?
Non coprire il compressore durante il
funzionamento.
Nota: Se il rimedio suggerito non risolve il problema, non tentare di riparare il
dispositivo, in quanto esso non può essere sottoposto a riparazione da parte
dell’utente. Restituire l’unità a un rivenditore autorizzato o al distributore OMRON.
Page 84

84
Dati tecnici
Descrizione del prodotto: Nebulizzatore a compressore
Modello (-Codice): NE-C803 (NE-C803-E)
Tensione nominale
(adattatore CA):
100 - 240 V ~ 50/60 Hz 0,12 - 0,065 A
Tensione nominale
(nebulizzatore a compressore):
6 V
0,7 A
Temperatura / umidità di
esercizio:
Da +10 °C a +40 °C / Dal 30% all’85% di umidità relativa
Temperatura / Umidità /
Pressione dell’aria
(Conservazione e trasporto):
Da -20 °C a +60 °C / Dal 10% al 95% di umidità relativa /
700 - 1060 hPa
Peso: Circa 180 g (solo compressore)
Dimensioni: Circa 85 (L) × 43 (H) × 115 (P) mm (solo compressore)
Contenuto della confezione: Compressore, ampolla nebulizzatrice, tubo dell’aria (PVC,
100 cm), boccaglio, forcella nasale, maschera per adulti
(PVC), maschera per bambini (PVC), 5 pz. filtri per l’aria di
ricambio, adattatore CA, custodia, manuale di istruzioni.
Classificazioni: Classe ll (Protezione contro le folgorazioni),
Tipo BF (Parte applicata)
IP21 (Protezione IP)
= Apparecchio di
classe ll
= Parte applicata
di tipo BF
Leggere attentamente
il manuale di istruzioni
= Spento
= Acceso
Note:
• Soggetto a modifiche tecniche senza preavviso.
• Questo prodotto OMRON è realizzato in conformità al rigoroso sistema di qualità
adottato da OMRON HEALTHCARE Co., Ltd., Giappone.
• Il dispositivo potrebbe non funzionare in condizioni di temperatura e di tensione
elettrica diverse da quelle definite nelle caratteristiche tecniche.
• Il dispositivo è conforme alle disposizioni della direttiva CE 93/42/CEE (Direttiva sui
dispositivi medici) e alla norma europea EN13544-1:2007+A1:2009, Attrezzatura per
terapia respiratoria - Parte 1: Sistemi di nebulizzazione e loro componenti.
• Per l’aggiornamento delle informazioni tecniche, fare riferimento al sito Web
OMRON HEALTHCARE EUROPE.
URL: www.omron-healthcare.com
Page 85

IT
85
Dati tecnici relativi al Compressore OMRON NE-C803 con ampolla
nebulizzatrice:
Dimensione delle particelle: **MMAD circa 3 μm
MMAD = Diametro aerodinamico mediano di massa
Capacità del serbatoio del farmaco: massimo 10 ml
Quantità adeguata di farmaco: minimo 2 ml - massimo 10 ml
Rumorosità: *Inferiore a 45 dB
Velocità di nebulizzazione: *Circa 0,30 ml/min (per perdita di peso)
Emissione aerosol: ** 0,37 ml (2 ml, 1% NaF)
Velocità emissione aerosol: ** 0,05 ml/min (2 ml, 1% NaF)
Risultato della misura granulometrica mediante impattometro multistadio**
0
10
20
30
40
50
60
70
80
90
100
0,1 1 10 100
% cumulativa di massa delle particelle di fluoruro di
sodio di dimensioni inferiori a
Dimensione delle particelle Dp (μm)
% cumulativa di dimensioni inferiori a
Media
Singolo
* Misurazione eseguita da OMRON HEALTHCARE Co., Ltd. Misurazione eseguita con
soluzione salina a una temperatura ambiente di 23 °C e con umidità pari al 40%. Può
variare in funzione del tipo di farmaco.
** Misurazione indipendente eseguita dal Prof. Dr. Hiroshi Takano, Therapeutic
Systems Research Center, Doshisha University, Kyoto, Giappone, secondo
EN13544-1:2007+A1:2009.
Nota:
Le prestazioni possono variare con particolari tipi di farmaci (ad esempio quelli in
sospensione oppure ad alta viscosità). Per ulteriori informazioni, fare riferimento al
foglietto illustrativo fornito dal produttore del farmaco.
Page 86

86
Informazioni importanti relative alla compatibilità elettromagnetica (EMC)
A causa del numero sempre maggiore di dispositivi elettronici (computer, telefoni cellulari,
ecc.), i dispositivi medici in uso potrebbero essere soggetti a interferenze elettromagnetiche
prodotte da altre apparecchiature. Tali interferenze elettromagnetiche potrebbero determinare il
funzionamento errato del dispositivo medico e creare una situazione potenzialmente non sicura.
I dispositivi medici, inoltre, non devono interferire con altre apparecchiature.
Per la conformità alle normative sulla compatibilità elettromagnetica (EMC) e allo scopo di prevenire
situazioni potenzialmente non sicure nell’utilizzo del prodotto, sono state implementate le norme
contenute in EN60601-1-2:2007. Tali standard definiscono i livelli di immunità alle interferenze
elettromagnetiche, nonché i livelli massimi di emissioni elettromagnetiche per i dispositivi medici.
Questo dispositivo medico prodotto da OMRON HEALTHCARE è conforme agli standard
EN60601-1-2:2007 per quanto concerne sia l’immunità che le emissioni.
È necessario tuttavia osservare le precauzioni indicate di seguito:
• Non usare in prossimità di questo dispositivo medico telefoni mobili (cellulari) o altri dispositivi
che generano forti campi elettrici o elettromagnetici. Ciò potrebbe determinare il funzionamento
errato dell’unità e creare una situazione potenzialmente non sicura.
Si consiglia di mantenere tali apparecchiature a una distanza minima di 7 m. Verificare il corretto
funzionamento del dispositivo se la distanza è inferiore.
Ulteriore documentazione relativa agli standard EN60601-1-2:2007 è disponibile presso
OMRON HEALTHCARE EUROPE, all’indirizzo indicato nel presente manuale di istruzioni.
La documentazione è disponibile inoltre presso il sito web www.omron-healthcare.com.
Corretto smaltimento del prodotto
(rifiuti elettrici ed elettronici)
Il marchio riportato sul prodotto o sulla sua documentazione indica che il prodotto non deve
essere smaltito con altri rifiuti domestici al termine del ciclo di vita. Per evitare eventuali danni
all’ambiente o alla salute causati da uno smaltimento non appropriato dei rifiuti, si invita l’utente a
separare questo prodotto da altri rifiuti e a riciclarlo in maniera responsabile per favorire il riutilizzo
sostenibile delle risorse materiali.
Gli utenti domestici sono invitati a contattare il rivenditore presso il quale è stato acquistato il
prodotto o l’ufficio locale preposto per tutte le informazioni relative alla raccolta differenziata e al
riciclaggio per questo tipo di prodotto.
Gli utenti aziendali sono invitati a contattare il proprio fornitore e verificare i termini e le condizioni
del contratto di acquisto. Questo prodotto non deve essere smaltito unitamente ad altri rifiuti
commerciali.
Page 87

IT
87
Garanzia
Grazie per aver acquistato un prodotto OMRON. Questo prodotto è stato costruito
impiegando materiali di alta qualità ed è stato realizzato con estrema cura. Il dispositivo
è progettato per fornire all’utilizzatore un alto livello di comfort, purché venga usato nel
modo corretto e gestito secondo le indicazioni fornite nel manuale di istruzioni.
Il prodotto è garantito da OMRON per un periodo di 3 anni a partire dalla data di
acquisto. La correttezza di realizzazione, la competenza tecnica e i materiali utilizzati
per questo prodotto sono garantiti da OMRON. Nell’ambito del periodo di garanzia,
OMRON riparerà o sostituirà il prodotto difettoso o eventuali componenti difettosi, senza
alcun costo per la manodopera o i componenti di ricambio.
La garanzia vale solo per i prodotti acquistati nei seguenti territori: Europa,
Russia e altri paesi della CSI, Medio Oriente e Africa.
La garanzia non copre in alcun caso quanto segue:
a. Costi di trasporto e rischi associati al trasporto.
b. Costi relativi a riparazioni e/o difetti derivanti da riparazioni eseguite da persone non
autorizzate.
c. Controlli e manutenzione periodici.
d. Guasti o usura degli accessori o di altre parti diverse dal dispositivo principale
propriamente detto, fatte salve le garanzie esplicitamente summenzionate.
e. Costi derivanti da richieste di intervento in garanzia ingiustificate (tali richieste sono
soggette a pagamento).
f. Danni di qualsiasi tipo, inclusi danni a persone causati accidentalmente o dovuti a
utilizzo errato.
Per le richieste di assistenza in garanzia, rivolgersi al rivenditore presso il quale è stato
acquistato il prodotto oppure a un distributore autorizzato OMRON. Per l’indirizzo, fare
riferimento alla confezione del prodotto o alla documentazione fornita in dotazione
oppure rivolgersi al rivenditore. In caso di problemi nel reperire il servizio assistenza
clienti, visitare il sito web di OMRON (www.omron-healthcare.com) per ottenere
informazioni riguardo i contatti.
La riparazione o la sostituzione in garanzia non comporta in alcun caso l’estensione o il
rinnovo del periodo di garanzia.
La garanzia è valida solo se il prodotto viene restituito nella sua interezza insieme alla
fattura o allo scontrino originale rilasciato dal negoziante al consumatore. OMRON si
riserva il diritto di rifiutare la prestazione di garanzia qualora le informazioni qui riportate
non fossero in qualche modo chiare.
Page 88

88
Produttore OMRON HEALTHCARE Co., Ltd.
53, Kunotsubo, Terado-cho, Muko, KYOTO, 617-0002 GIAPPONE
Rappresentante
per l’UE
OMRON HEALTHCARE EUROPE B.V.
Scorpius 33, 2132 LR Hoofddorp, PAESI BASSI
www.omron-healthcare.com
Stabilimento di
produzione
OMRON HEALTHCARE MANUFACTURING VIETNAM CO., LTD.
No.28 VSIP II, Street 2, Vietnam-Singapore Industrial Park II,
Binh Duong Industry-Services-Urban Complex, Hoa Phu Ward,
Thu Dau Mot City, Binh Duong Province, Vietnam
Consociate
OMRON HEALTHCARE UK LTD.
Opal Drive, Fox Milne, Milton Keynes, MK15 0DG, UK
www.omron-healthcare.co.uk
OMRON MEDIZINTECHNIK HANDELSGESELLSCHAFT mbH
Gottlieb-Daimler-Strasse 10, 68165 Mannheim, GERMANIA
www.omron-healthcare.de
OMRON SANTÉ FRANCE SAS
14, rue de Lisbonne, 93561 Rosny-sous-Bois Cedex, FRANCIA
www.omron-healthcare.fr
Prodotto in Vietnam
Page 89

ES
NE-C803-E_A_M09_140715.pdf
Nebulizador compresor
NE-C803 (NE-C803-E)
Manual de instrucciones
Page 90

90
Introducción
Le agradecemos que haya adquirido el OMRON COMP AIR basic.
Este producto ha sido desarrollado conjuntamente con neumólogos para el tratamiento
con éxito del asma, bronquitis crónica, alergias y otros trastornos respiratorios.
Este es un dispositivo médico. Utilice este dispositivo únicamente si ha sido prescrito
por su médico y/o terapeuta respiratorio.
Funcionamiento del kit nebulizador
La medicación que se introduce a través del canal
de medicación se mezcla con el aire comprimido
que genera la bomba del compresor.
La medicación se transforma en pequeñas
partículas y se pulveriza al entrar en contacto con
el aire comprimido.
Aerosol
Cánula
Aire comprimido
Canal de medicación
Medicación
Antes de utilizar el dispositivo
Contenidos
Antes de utilizar el dispositivo
Introducción .............................................................................................................. 90
Uso previsto .............................................................................................................. 91
Instrucciones importantes sobre la seguridad .......................................................... 92
Conozca su unidad ................................................................................................... 95
Accesorios médicos opcionales................................................................................ 96
Otras piezas de recambio/opcionales....................................................................... 96
Instrucciones de funcionamiento
Instrucciones de uso ................................................................................................. 97
Cuidado y mantenimiento
Limpieza y desinfección.......................................................................................... 101
Eliminación de la condensación del tubo de aire.................................................... 104
Cambio del filtro de aire .......................................................................................... 104
Resolución de problemas ....................................................................................... 105
Datos técnicos ........................................................................................................ 106
Garantía .................................................................................................................. 109
Page 91

ES
91
Uso previsto
Objetivo médico
Este producto está destinado para su uso con medicamentos inhalados indicados en el
tratamiento de trastornos respiratorios.
Usuario al que está destinado
• Profesionales sanitarios legalmente titulados como médicos y enfermeros.
• Cuidadores o pacientes que sigan las instrucciones de médicos cualificados para
prescribir el tratamiento en casa.
• El usuario también deberá tener conocimientos generales sobre el funcionamiento del
NE-C803 y sobre el contenido de este manual de instrucciones.
Pacientes a los que está destinado este producto
Este producto no deberá ser utilizado en pacientes en estado de inconsciencia o que no
puedan respirar de forma espontánea.
Entorno
Este producto está destinado para su uso en instalaciones médicas como un hospital,
una clínica, la consulta del médico o en una habitación en un domicilio particular.
Periodo de duración
Los períodos de duración son los que aparecen a continuación, siempre que el
producto se utilice para la nebulización de 2 ml de medicación dos veces al día durante
7,5 minutos cada vez a temperatura ambiente (23 °C).
El periodo de duración podría variar dependiendo del entorno en el que sea utilizado.
El uso frecuente de este producto podría acortar el periodo de duración.
Compresor (unidad principal) 5 años
Juego nebulizador 1 año
Boquilla 1 año
Pieza nasal 1 año
Tubo de aire (PVC, 100 cm) 1 año
Filtro de aire 60 días
Mascarilla (PVC) (SEBS) 1 año
Precauciones para su uso
Es necesario tener en cuenta los avisos y advertencias descritos en este manual de
instrucciones.
Page 92
92
Instrucciones importantes sobre la seguridad
Lea toda la información contenida en el manual de instrucciones y cualquier otra documentación
que se incluya en la caja antes de utilizar el dispositivo.
Advertencia:
Indica una situación potencialmente peligrosa que, si no se evita, podría provocar
serios daños.
(Uso)
• Para obtener el tipo, la dosis y régimen de medicación, siga las indicaciones de su médico o neumólogo.
• Si nota algo inusual durante su utilización, detenga el funcionamiento del dispositivo de inmediato y
consulte a su médico.
• No utilizar sólo con agua, este dispositivo está pensado para la inhalación de medicamentos.
• Mantenga el dispositivo lejos del alcance de niños y bebés sin vigilancia adulta. El dispositivo puede
contener piezas pequeñas que podrían tragarse.
• No guarde el tubo de aire mientras queden restos de humedad o medicación en su interior.
• No utilice ni guarde el dispositivo donde pudiera verse expuesto a gases nocivos o sustancias volátiles.
• No utilice el dispositivo donde pueda estar expuesto a gas inflamable.
• Durante su utilización, no cubra el compresor con ningún tipo de cubierta, manta o toalla.
• Deshágase siempre de la medicación que sobre tras su uso, utilice medicación nueva con cada uso.
• No utilizar en circuitos anestésicos ni de respiración por ventilación.
• Guarde las distintas piezas en un lugar limpio con el fin de evitar infecciones.
(riesgo de descarga eléctrica)
• Nunca enchufe o desenchufe el adaptador de CA con las manos mojadas.
• El compresor y el adaptador de CA no son resistentes al agua. No vierta agua ni otros líquidos en estas
piezas. Si se vierte líquido en estas piezas, desconecte el adaptador de CA inmediatamente
y seque el
líquido con una gasa u otro material absorbente.
• No sumerja el compresor en agua u otro líquido.
• No utilice ni guarde el dispositivo en un ambiente húmedo.
• No utilice el dispositivo si el cable o el enchufe están dañados.
• Mantenga el cable de alimentación alejado de las superficies calientes.
• Utilice solamente el adaptador de CA OMRON original. Si utiliza un adaptador de CA que no sea
compatible podría dañar el dispositivo.
(Limpieza y desinfección)
Siga las normas descritas a continuación sobre la limpieza o desinfección de las piezas. Si no lo hace,
podrían producirse daños, una nebulización ineficaz o una infección. Para consultar las instrucciones,
lea la sección “Limpieza y desinfección”.
• Limpie y desinfecte el kit nebulizador, la mascarilla, la boquilla o la pieza nasal antes de utilizarlos en
los siguientes casos:
- por primera vez tras la compra
- si el dispositivo no se ha utilizado durante un periodo de tiempo prolongado
- si el mismo dispositivo está siendo utilizado por má s de una persona
• Asegúrese de lavar o limpiar las piezas tras su uso, asegúrese de que se secan y desinfectan
completamente, y guárdelas en un lugar limpio.
• No deje ningún resto de solución de limpieza en las piezas.
Page 93
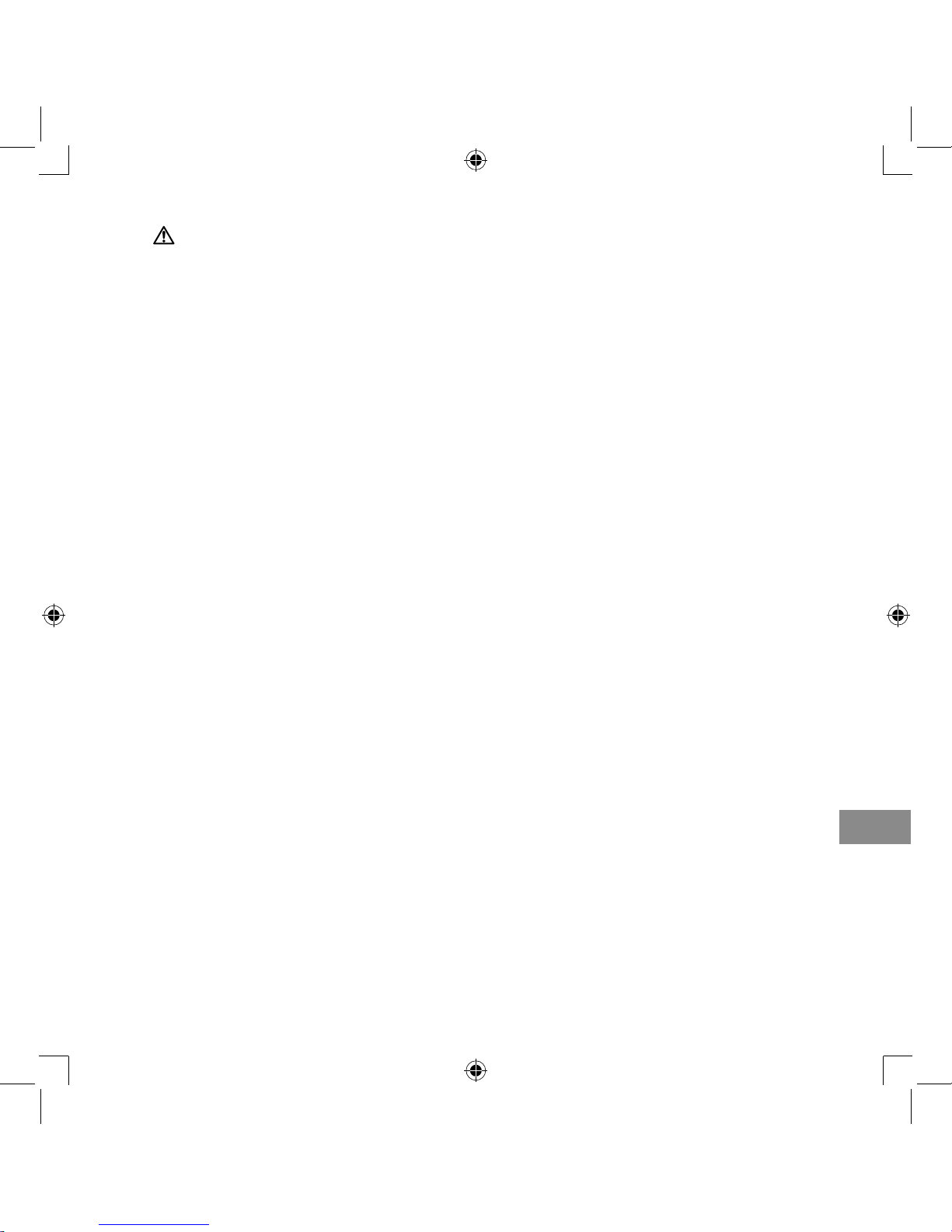
ES
93
Precaución:
Indica una situación potencialmente peligrosa que, si no se evita, podría provocar
daños menores o moderados o daños físicos.
(Uso)
• Cuando este dispositivo se utilice con o junto a niños o inválidos, vigile la situación.
• Asegúrese de que las piezas están conectadas correctamente.
• Asegúrese de que el filtro de aire esté correctamente conectado.
• Asegúrese de que el filtro de aire esté limpio. Si se ha utilizado el filtro de aire durante más de 60 días,
cámbielo por uno nuevo.
• No incline el kit nebulizador con un ángulo superior a 45 grados en cualquier dirección ni lo agite
mientras lo utiliza.
• No utilice ni guarde el dispositivo si el tubo de aire está arrugado.
• Utilice exclusivamente piezas nebulizadoras, tubos de aire, filtros de aire y cubiertas de filtro de
aire originales.
• No añada más de 10 ml de medicación al depósito de medicación.
• No transporte ni guarde el kit nebulizador cuando el depósito de medicación contenga algún tipo
de medicamento.
• No deje el dispositivo desatendido con niños pequeños o personas que no puedan expresar
su consentimiento.
• No deje que el dispositivo sufra golpes fuertes como una caída al suelo.
• No pinche la cánula del depósito de medicación con un pasador u otro objeto puntiagudo.
• No introduzca los dedos ni objetos dentro del compresor.
• No desmonte ni intente reparar el compresor ni el adaptador de CA.
• No deje el dispositivo a temperaturas extremadamente altas o bajas ni directamente a la luz del sol.
• No utilice el dispositivo cuando duerma o si está adormilado.
• Apto sólo para uso humano.
• El compresor podría calentarse durante su funcionamiento.
• No toque el compresor por otras razones que no estén relacionadas con su funcionamiento, como por
ejemplo apagado de la unidad durante la nebulización.
• Para evitar que queden residuos medicamentosos en la cara, asegúrese de limpiarla después de retirar
la mascarilla.
• No presione la pieza nasal contra la parte interior de la nariz, así evitará que se produzcan daños en la
mucosa nasal.
• No utilice un kit nebulizador, boquilla o pieza nasal que estén dañados.
(riesgo de descarga eléctrica)
• Desconecte siempre el adaptador de CA de la toma de corriente tras utilizarlo y antes de limpiarlo.
• Conecte el dispositivo a una toma de corriente con el voltaje adecuado. No sobrecargue las salidas
de corriente ni utilice cables de extensión.
• Manipule con cuidado el cable de alimentación del adaptador de CA.
• No enrolle el cable de alimentación alrededor del compresor ni del adaptador de CA.
• No tire del cable de alimentación del adaptador de CA.
• Los cambios o modificaciones no aprobados por OMRON HEALTHCARE anularán la garantía
de usuario.
• Desconecte siempre el adaptador de CA tras utilizarlo y antes de limpiarlo.
Page 94

94
(Limpieza y desinfección)
Siga las normas descritas a continuación sobre la limpieza o desinfección de las piezas. Si no lo hace,
podrían producirse daños, una nebulización ineficaz o una infección. Para consultar las instrucciones, lea la
sección “Limpieza y desinfección”.
• No utilice un horno microondas, una secadora de platos, ni un secador de pelo para secar el dispositivo
o las piezas.
• No utilice un autoclave, un esterilizador de gas de óxido de etileno (EOG) o un esterilizador de plasma a
baja temperatura.
• Cuando desinfecte las piezas hirviéndolas, asegúrese de que el contenedor nunca se quede sin agua.
Si no lo hace, se podría causar un incendio.
Precauciones de seguridad generales:
• Cada vez que vaya a utilizarlo, revise el dispositivo y compruebe que no existe ningún problema. Sobre
todo, asegúrese de comprobar lo siguiente:
- Que la cánula o el tubo de aire no estén dañados.
- Que la cánula no esté obstruida.
- Que el compresor funciona con normalidad.
• Al utilizar este dispositivo, se producirán ruidos y vibraciones provocados por la bomba del compresor.
Habrá también ruido provocado por la emisión de aire comprimido desde el kit nebulizador. Esta
situación es normal y no indica un fallo de funcionamiento.
• Utilice el dispositivo sólo como se indica. No utilice el dispositivo con ningún otro fin.
• No utilice el dispositivo a temperaturas superiores a los 40 °C.
• Asegúrese de que el tubo de aire está correctamente conectado al compresor y al kit nebulizador,
y que no quede suelto.
• Para aislar completamente el dispositivo de la fuente de alimentación, desconecte el enchufe de
esta fuente.
Guarde estas instrucciones para consultarlas en el futuro.
Page 95

ES
95
Conozca su unidad
Adaptador de CA x 1
Estuche protector x 1
Manual de
instrucciones
Vista posterior
Conector de
alimentación
eléctrica
Cubierta del filtro de aire
*Filtro de aire en el interior
Compresor (unidad principal) x 1
Soporte del kit
nebulizador
Conector del tubo de aire
Interruptor de encendido
5 filtros de aire de
recambio
Tubo de aire
(PVC, 100 cm) x 1
Boquilla x 1
Mascarilla para niños
(PVC) x 1
Mascarilla para adultos
(PVC) x 1
Kit nebulizador x 1
Conector del tubo de aire
Parte superior
de inhalación
Cabezal vaporizador
Cánula
Depósito de medicación
Pieza nasal x 1
Page 96

96
Accesorios médicos opcionales
(recogido en el ámbito de la directiva relativa a los productos sanitarios de la
CE 93/42/CEE)
Descripción del producto Modelo
Juego de kit nebulizador y boquilla NEB-NSET3-83E
Juego de kit nebulizador, mascarilla para adultos (PVC)
y tubo de aire
NEB-NSET4-83AP
Juego de kit nebulizador, mascarilla para niños (PVC)
y tubo de aire
NEB-NSET5-83AP
Mascarilla para adultos (PVC) NEB-MSLP-E
Mascarilla para niños (PVC) NEB-MSMP-E
Mascarilla para adultos (SEBS) NEB-MSLS-E
Mascarilla para niños (SEBS) NEB-MSSS-E
Boquilla NEB-MP-E
Pieza nasal NEB-NP-E
Tubo de aire (PVC, 100 cm) NEB-TP-82E
Adaptador de CA HHP-CF01
Otras piezas de recambio/opcionales
Descripción del producto Modelo
Filtros de aire x 5 NEB-AFR-30E
I
n
Page 97

ES
97
Instrucciones de funcionamiento
Instrucciones de uso
Advertencia:
Limpie y desinfecte el kit nebulizador, la mascarilla, la boquilla o la pieza nasal antes de
utilizarlos en los siguientes casos:
- por primera vez tras la compra
- si el dispositivo no se ha utilizado durante un periodo de tiempo prolongado
- si el mismo dispositivo está siendo utilizado por má s de una persona
Para consultar las instrucciones, lea la sección “Limpieza y desinfección”.
1.
Asegúrese de que el interruptor de encendido está en la posición de apagado ( ).
2.
Conecte el adaptador de CA al compresor e
introduzca el enchufe en la toma de corriente.
3.
Saque la parte superior de inhalación junto
con el cabezal vaporizador del depósito de
medicación.
Nota:
Si el cabezal vaporizador se ha desprendido
de la parte superior de inhalación y ha caído
al depósito de medicación, sáquelo del depósito.
4.
Agregue la cantidad correcta de medicación
prescrita al depósito de medicación.
Page 98

98
5.
Asegúrese de que el cabezal vaporizador
está bien colocado en la parte superior
de inhalación.
Nota:
El tamaño de las piezas montadas a la
izquierda y la derecha del vaporizador no
es igual. Si el cabezal vaporizador no está
montado con la orientación correcta, no
nebulizará correctamente.
6.
Coloque de nuevo la parte superior
de inhalación en el depósito de
medicación.
1) Alinee el saliente de la parte superior
de inhalación con la hendidura del
depósito de medicación tal y como
se muestra en la ilustración.
2) Rote la parte superior de inhalación
hacia la derecha hasta que la flecha
y la marca de alineación del depósito
de medicación estén alineadas y
escuche un clic cuando encajen.
Flecha
Marca de
alineación
Pequeño
Grande
Page 99

ES
99
7.
Acople firmemente la mascarilla,
la boquilla o la pieza nasal al kit
nebulizador.
Nota:
Asegúrese de que estos elementos
están montados de forma que el mango
esté colocado en el lado opuesto al
paciente.
De lo contrario, el medicamento se
podría rociar sobre la piel o la ropa del
paciente.
8.
Coloque el tubo de aire.
Introduzca con firmeza los extremos del tubo de
aire en los conectores del tubo de aire.
Mango Mango
El kit nebulizador puede fijarse al compresor.
Page 100

100
C
u
9.
Sujete el kit nebulizador tal y como se indica a la
derecha.
Siga las indicaciones de su médico o de su
neumólogo. Exhale con normalidad a través de la
boquilla, la mascarilla o la nariz.
Precaución:
No incline el kit nebulizador con un ángulo superior a
45 grados en cualquier dirección. La medicación podría
fluir hacia el interior de la boca o la nebulización podría
no realizarse de forma efectiva.
10.
Pulse el interruptor de encendido para colocarlo en la posición ( ) de encendido.
Una vez que el compresor se encienda, la nebulización comienza y se genera
el aerosol.
Inhale la medicación.
11.
Cuando haya completado el tratamiento, apague el dispositivo. Desenchufe el
adaptador de CA de la toma de corriente y desconéctelo del compresor.
Nota:
Sostenga y presione hacia abajo con firmeza el
enchufe para desconectar el adaptador de CA.
Precaución:
No tire del cable de alimentación.
Podría dañarse.
Ángulo correcto
45°45°
Compresor
Enchufe
 Loading...
Loading...