Page 1
NE-C802-E_A_M06_120420.pdf
Compressor Nebulizer
Instruction Manual
IM-NE-C802-UK-01-08/2012
Intended use
Medical Purpose
This product is intended to be used for inhaling medication for respiratory
disorders.
Intended User
• Legally certified medical experts, such as doctor, nurse and therapist.
• Caregiver or patient under the guidance of qualified medical experts for
home treatment.
• The user should also be capable of understanding general operation of
NE-C802 and the content of instruction manual.
Intended Patients
This product should not be used by patients, who are unconscious or are
not breathing spontaneously.
Environment
This product is intended for use in a medical facility, such as Hospital,
clinic and doctor’s office, and in a room of general household.
Durable period
Durable periods are as follows, provided the product is used to nebulize
2 ml of medication twice a day for 8 minutes each time at room
temperature (23°C).
Durable period may vary depending on usage environment.
Frequent use of the product may shorten the durable period.
Compressor (Main unit) 5 years
Nebulizer Kit 1 year
Mouthpiece 1 year
Nosepiece 1 year
Air Tube (PVC, 100cm) 1 year
Air Filter 60 days
Adult Mask (PVC) 1 year
Child Mask (PVC) 1 year
Precautions for use
Warnings and cautions described in the instruction manual should be
observed.
1675660-6A
EN
Thank you for purchasing the OMRON COMP AIR basic.
This product was developed in conjunction with respiratory therapists
for the successful treatment of asthma, chronic bronchitis, allergies
and other respiratory disorders.
This is a medical device. Operate the device only as instructed by
your doctor and/or respiratory therapist.
Aerosol
How the nebulizer kit works
The medication that is pumped up
through the medication channel is
mixed with compressed air which is
Medication Channel
Medication
Nozzle
generated by a compressor pump.
The medication is turned into fine
particles and sprayed when in contact
with the compressed air.
Compressed Air
Manufacturer OMRON HEALTHCARE Co., Ltd.
EU-representative
Production facility
Subsidiary
53, Kunotsubo, Terado-cho, Muko, Kyoto, 617-0002 JAPAN
OMRON HEALTHCARE EUROPE B.V.
Scorpius 33, 2132 LR Hoofddorp, THE NETHERLANDS
www.omron-healthcare.com
OMRON (DALIAN) CO., LTD.
Dalian, CHINA
OMRON HEALTHCARE UK LTD.
Opal Drive, Fox Milne, Milton Keynes, MK15 0DG, U.K.
Made in China
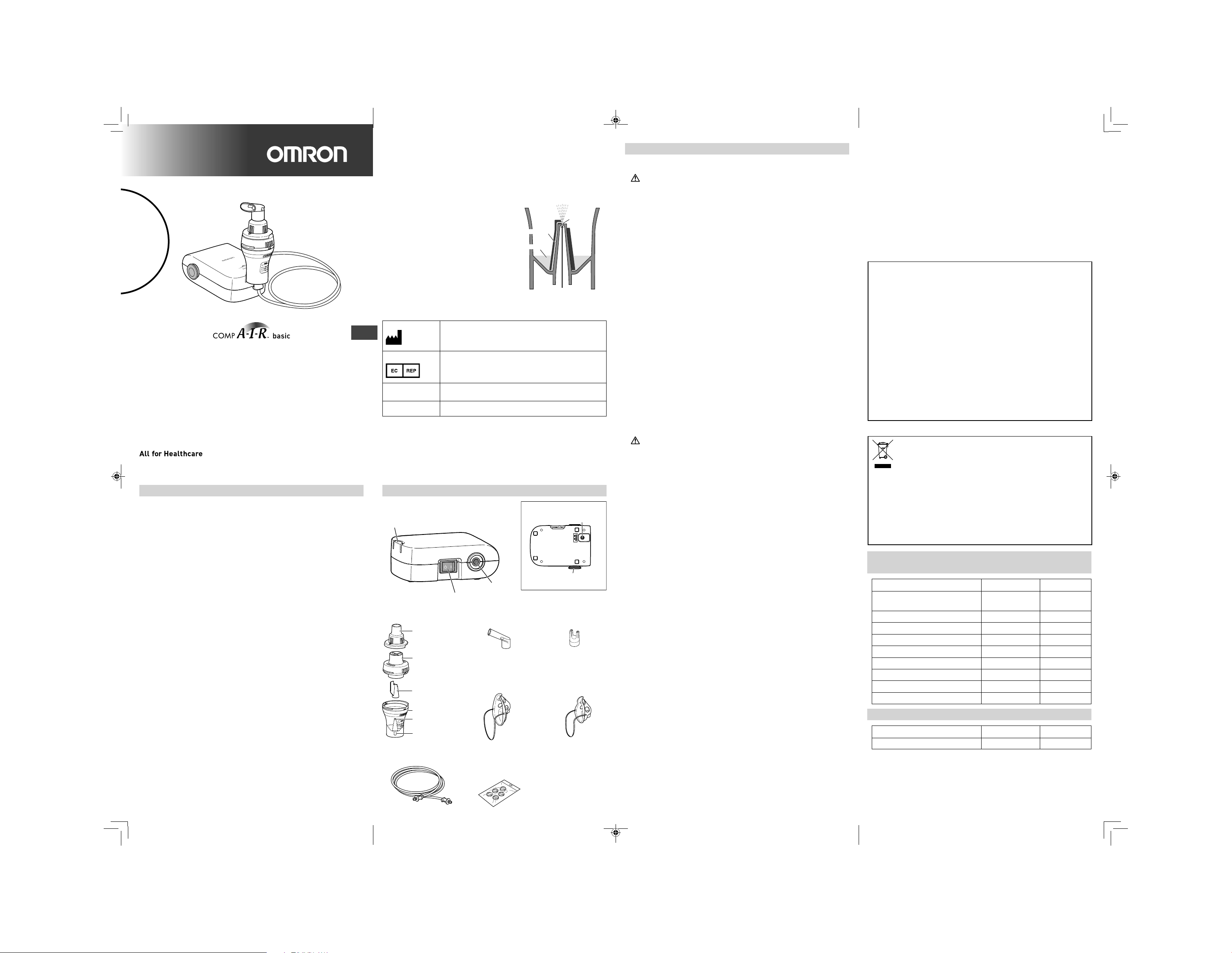
Know your unit
Compressor (Main Unit) x 1
Nebulizer Kit Holder
Air Tube Connector
Power Switch
Nebulizer Kit x 1 Nosepiece x 1Mouthpiece x 1
Accessory Adapter
Inhalation Top
Adult Mask (PVC) x 1
Vaporizer Head
Medication Tank
Nozzle
Air Tube
Connector
Air Tube
(PVC, 100cm) x 1
Spare Air Filters x 5
Bottom view
Power Connector
Air Filter Cover
*Air filter inside
Child Mask (PVC) x 1
AC Adapter x 1
Storage Bag x 1
Instruction Manual
Warranty Card
Important safety instructions
Read all the information in the instruction manual and any other literature included in
the box before using the device.
Warning
(Usage)
• For type, dose, and regime of medication follow the instructions of your doctor or
respiratory therapist.
• If you feel anything unusual during use, stop using the device immediately and
consult your doctor.
• Do not use only water in the nebulizer for inhaling purposes.
• Clean and disinfect the nebulizer kit, mask, mouthpiece or nosepiece before using
them for the first time after purchase.
• If the device has not been used for a long period of time, or if more than one person
uses the same device, clean and disinfect the nebulizer kit, mask, mouthpiece or
nosepiece before using them.
• Be sure to wash the parts after use, and ensure that they are thoroughly disinfected
and dried, and stored in a clean location.
• Keep the device out of the reach of unsupervised infants and children. The device
may contain small pieces that can be swallowed.
• Do not store the air tube while there is moisture or medication remaining inside.
• Do not leave the cleaning solution in the nebulizer parts. Rinse them with clean hot
tap water after disinfecting.
• Do not use or store the device where it may be exposed to noxious fumes or volatile
substances.
• Do not use the device where it may be exposed to flammable gas.
• Do not cover the compressor with a blanket, towel, or any other type of cover during
use.
• Always dispose of any remaining medication after use, and use fresh medication each
time.
• Do not use in anaesthetic or ventilator breathing circuits.
(Risk of electrical shock)
• Never plug or unplug the AC adapter with wet hands.
• The compressor and the AC adapter are not waterproof. Do not spill water or other
liquids on these parts. If liquid does spill on these parts, immediately unplug the AC
adapter
• Do not immerse the compressor in water or other liquid.
• Do not use or store the device in humid locations.
• Do not operate the device with a damaged power cord or plug.
• Keep the power cord away from heated surfaces.
• Use only original OMRON AC adapter. Use of unsupported AC adapter may damage
the device.
Caution:
(Usage)
• Provide close supervision when this device is used by, on, or near children or invalids.
• Make sure that the parts are attached correctly.
• Make sure that the air filter is correctly attached.
• Make sure that the air filter is clean. If the air filter has changed colour, or has been
used on average for more than 60 days, replace it with a new one.
• Do not tilt the nebulizer kit at an angle of greater than 45 degrees in all directions or
shake it while in use.
• Do not use or store the device while the air tube is creased.
• Use only original nebulizing parts, air tube, air filter and air filter cover.
• Do not add more than 10 ml of medication to the medication tank.
• Do not carry or leave the nebulizer kit while the medication tank contains medication.
• Do not leave the device unattended with infants or persons who cannot express their
consent.
• Do not subject the device to any strong shocks such as dropping on the floor.
• Do not jab the nozzle of the medication tank with a pin or any sharp object.
• Do not insert fingers or objects inside the compressor.
• Do not disassemble or attempt to repair the compressor or AC adapter.
• Do not leave the device in extreme hot or cold temperature, or under direct sunlight.
• When disinfecting parts by boiling, make sure that the container does not boil dry.
• Do not use the device while sleeping or if drowsy.
• Do not use a microwave oven, dish dryer or hair dryer to dry the device.
• Approved for human use only.
• The compressor may become hot during operation.
• Do not touch the compressor for other than necessary operation such as turning off
the power while nebulizing.
• To avoid the medication residue on the face, be sure to wipe the face after removing
the mask.
• To avoid injury to the nose mucosa, do not squeeze the nosepiece into the back of
the nose.
• Do not use an autoclave, EOG gas disinfection or low temperature plasma sterilizer
to disinfect the device.
• Do not use a damaged nebulizer kit, mouthpiece or nosepiece.
(Risk of electrical shock)
• Always unplug the AC adapter from the power outlet after use and before cleaning.
• Plug the device into the appropriate voltage outlet. Do not overload power outlets or
use extension cords.
• Do not misuse the power cord of AC adapter.
• Do not wind the power cord around the compressor or AC adapter.
• Do not pull the power cord of AC adapter.
• Changes or modifications not approved by OMRON HEALTHCARE will void the
user warranty.
• Always remove the AC adapter from the device after use and before cleaning.
Indicates a potentially hazardous situation which, if not avoided, could result in
:
serious injury.
and wipe off the liquid with gauze or other absorbent material.
Indicates a potentially hazardous situation which if not avoided, may result in
minor or moderate injury, or physical damage.
General Safety Precautions:
• Inspect the device before using them each time, and check that there are no
problems. In particular, be sure to check the following:
-That the nozzle or air tube are not damaged.
-That the nozzle is not blocked.
-That the compressor operates normally.
• When using this device, there will be some noise and vibration caused by the pump in
the compressor. There will also be some noise caused by the emission of compressed
air from the nebulizer kit. This is normal and does not indicate a malfunction.
• Operate the device only as intended. Do not use the device for any other purpose.
• Do not use the device at temperatures greater than +40°C.
• Make sure that the air tube is securely attached to the compressor and nebulizer kit,
and does not come loose.
• To completely isolate the device from the power source unplug the plug from the
power source.
Keep these instructions for future reference.
Important information regarding Electro Magnetic Compatibility (EMC)
With the increased number of electronic devices such as PC’s and mobile (cellular)
telephones, medical devices in use may be susceptible to electromagnetic interference
from other devices. Electromagnetic interference may result in incorrect operation of the
medical device and create a potentially unsafe situation.
Medical devices should also not interfere with other devices.
In order to regulate the requirements for EMC (Electro Magnetic Compatibility) with
the aim to prevent unsafe product situations, the EN60601-1-2:2007 standard has
been implemented. This standard defines the levels of immunity to electromagnetic
interferences as well as maximum levels of electromagnetic emissions for medical
devices.
This medical device manufactured by OMRON HEALTHCARE conforms to this
EN60601-1-2:2007 standard for both immunity and emissions.
Nevertheless, special precautions need to be observed:
• Do not use mobile (cellular) telephones and other devices, which generate strong
electrical or electromagnetic fields, near the medical device. This may result in
incorrect operation of the unit and create a potentially unsafe situation.
Recommendation is to keep a minimum distance of 7 m. Verify correct operation of
the device in case the distance is shorter.
Further documentation in accordance with EN60601-1-2:2007 is available at OMRON
HEALTHCARE EUROPE at the address mentioned in this instruction manual.
Documentation is also available at www.omron-healthcare.com.
This marking shown on the product or its literature, indicates that it should not be
disposed of, with other household wastes at the end of its working life. To prevent
possible harm to the environment or human health from uncontrolled waste disposal,
please separate this product from other types of wastes and recycle it responsibly to
promote the sustainable reuse of material resources.
Household users should contact either the retailer where they purchased this product,
or their local government office, for details of where and how they can return this item
for environmentally safe recycling.
Business users should contact their supplier and check the terms and conditions of the
purchase contract. This product should not be mixed with other commercial wastes for
disposal.
(Waste Electrical & Electronic Equipment)
Correct Disposal of This Product
Optional Medical Accessories
(within the scope of EC Medical Device Directive 93/42/EEC)
Name Model Order No.
Nebulizer Kit &
Mouthpiece Set
Adult Mask (PVC)
Child Mask (PVC)
Adult Mask (SEBS)
Child Mask (SEBS) Set
Mouthpiece
Nosepiece
Air Tube (PVC, 100cm)
NEB-NSET3-82E 9065690-7
NEB-MSLP-E 9956275-1
NEB-MSMP-E 9956276-0
NEB-MSLS-E 9956312-0
NEB-MSSS-E 9956281-6
NEB-MP-E 9956273-5
NEB-NP-E 9956274-3
NEB-TP-82E 9065693-1
AC Adapter NEB-AC-82UK 9065658-3
Other Optional/Replacement Parts
Name Model Order No.
Air Filters x 5
NEB-AFR-30E 9956636-6
NE-C802-UK_A_M EN.indd 1NE-C802-UK_A_M EN.indd 1 8/21/2012 12:18:32 PM8/21/2012 12:18:32 PM
12G1856
Page 2
How to use
Cleaning and disinfecting
Removing condensation from the air tube
Technical data
1. Make sure that the power switch is in the off (
) position.
2. Connect the AC adapter to the compressor and plug the power plug into
a power outlet.
3. Remove the inhalation top together with
the accessory adapter and vaporizer
head from the medication tank.
1
2
4. Add the correct amount of prescribed
medication to the medication tank.
Reattach the vaporizer head to the
medication tank.
5. Put the inhalation top and accessory
adapter back inside the medication tank.
1
Clicks
2
6. Attach the mask, mouthpiece or
nosepiece to the nebulizer kit tightly.
Note:
Make sure an arrow mark on the nebulizer
kit is facing toward the patient. Otherwise,
medication may be sprayed on patient’s skin
or clothes.
Arrow
7. Attach the air tube.
Push the air tube plugs firmly
into the air tube connectors.
8. Hold the nebulizer kit as
indicated on the right.
Follow the instructions of your
doctor or respiratory therapist.
Exhale as normal through
mouthpiece, mask or through
the nose.
Caution:
Do not tilt the nebulizer kit at an
angle of greater than 45 degrees
in all directions. Medication may
flow into the mouth or it may result
in not nebulizing effectively.
9. Press the power switch to the on (
) position. As the compressor
Right angle
45°45°
starts, nebulization begins and aerosol is generated.
Inhale the medication.
10. When treatment is complete, turn the power off. Unplug the AC adapter
from the power outlet and disconnect it from the compressor.
Note:
Securely hold and press the power plug
down to disconnect the AC adapter.
Caution:
Do not pull the power cord. Damage
may be caused.
Power plug
Compressor
Cleaning
Clean the parts after each use to remove residual medication. This will avoid
inefficient nebulization or infection.
Firstly, make sure that the power plug unplugged from the power outlet.
Nebulizer Kit (Inhalation top, Accessory Adapter, Medication tank),
■
Mask (PVC), Mouthpiece, Nosepiece
Wash them in warm water and mild detergent. Rinse them thoroughly with
clean hot tap water and allow to air dry in a clean place.
Note: Nebulizer kit should be replaced after using for one year. Replace ONLY with
nebulizer kit designed for NE-C802.
Compressor, Air Tube
■
Wipe clean with a soft cloth moistened with water or mild detergent.
Vaporizer Head
■
Wash it with running water.
Disinfecting
Disinfect the parts once a week. If the parts are heavily stained, replace
them with new ones.
Use one of the following methods:
To select the method, refer to the table below.
A. Use a commercially available disinfectant. Follow the instructions
provided by the disinfectant manufacturer.
1) Submerge the parts in the disinfectant for the specified period.
2) Rinse them with clean hot tap water and allow to air dry in a clean place.
Note: Never clean with benzene, thinner or a flammable chemical.
B. Parts may be boiled between 15 to 20 minutes.
After boiling, carefully remove the parts, shake
off excess water and allow to air dry in a clean
environment.
Note: Do not boil Air tube, Mask (PVC), Air filter and Air filter
cover.
Caution: Handling the Vaporizer Head
• Always wash the vaporizer head after each use.
• Do not use a brush or pin to clean the vaporizer head.
• When disinfecting the parts by boiling, be sure to boil them in plenty of water.
• Do not boil vaporizer head together with objects other than applicable nebulizer
accessories.
Use the table below as a guideline to select a method for disinfecting.
O: applicable ×: not applicable
Parts
Mouthpiece
Nosepiece
Nebulizer Kit
Air Filter
Air Filter Cover
Adult Mask (PVC)
Child Mask (PVC)
Air Tube
(PVC, 100cm)
Adult Mask (SEBS)
(optional)
Child Mask (SEBS)
(optional)
Materials
Boiling O O O × × × × O
Alcohol
Disinfecting
ethanol
Sodium
hypochlorite
Milton*
(0.1%)
Quaternary
ammonium
Osvan*
(0.1%)
Chlorhexidine
Hibitane*
(0.5%)
Amphoteric
Surfactant
Tego*
(0.2%)
PP
PP
PP
(Vaporizer Head: PC)
OOO×O O × O
OOO×O O × O
OOO×O O × O
OOO×O O × O
OOO×O O × O
*
an example of commercially available disinfectant.
ABS
Polyester
(non-latex)
Mask: PVC
Band: Rubber
(Phthalate free)
PVC
Mask: SEBS
(Phthalate free)
(non-latex)
Band: Rubber
Adapter: PP
If there is moisture or liquid remaining in the air tube, be sure to follow the
procedure below to remove any moisture from within the air tube.
1) Make sure that the air tube is connected to the air connector on the
compressor.
2) Remove the air tube from the nebulizer kit.
3) Turn on the compressor and pump air through the air tube to expel the
moisture.
Changing the air filter
If the air filter has changed colour, or has been used on average for more
than 60 days, replace it with a new one.
1. Pull the air filter cover off the compressor.
2. Change the air filter.
3. Put the air filter cover back in place.
Notes:
• Use only OMRON air filters designed for this device. Do not operate without air filter.
• Do not attempt to wash or clean the air filter. If the air filter becomes wet, replace it.
Damp air filters can cause blockages.
• There is no front or back orientation for the air filters.
• Check that air filters are clean and free of dust before inserting them.
• Do not boil the air filter cover.
Troubleshooting
Check the following if the device should fail during operation.
Problem Cause Remedy
Nothing happens when the
power switch is pressed.
No nebulization or low
nebulization rate, when the
power is on.
The compressor is
abnormally loud.
The compressor is very hot. Is the compressor covered?
Note:
If the suggested remedy does not solve the problem, do not try to repair the device
- no parts of the device are user serviceable. Return the device to an authorized
OMRON retail outlet or distributor.
Is the AC adapter connected
correctly to a power outlet and
the compressor?
Is there medication in the
medication tank?
Is there too much/little
medication in the medication
tank?
Is the vaporizer head missing
or not assembled correctly?
Is the nebulizer kit assembled
correctly?
Is the nozzle blocked? Make sure that the nozzle is free
Is the nebulizer kit tilted at a
sharp angle?
Is the air tube connected
correctly?
Is the air tube folded or
damaged?
Is the air tube blocked? Make sure that the air tube is free
Is the air filter dirty? Replace the air filter with a new
Is the air filter cover attached
correctly?
Check that the AC adapter is
connected to a power outlet and
the compressor. Unplug then
reinsert the plug if necessary.
Add the correct amount of
medication to the medication tank.
Attach the vaporizer head
correctly.
Assemble the nebulizer kit
correctly.
of blockages.
Make sure that the nebulizer kit is
not tilted at an angle of more than
45 degrees.
Make sure that the air tube
is correctly connected to the
compressor and nebulizer kit.
Make sure that the air tube does
not contain kinks.
of blockages.
one.
Attach the air filter cover correctly.
Do not cover the compressor with
any type of cover during use.
Product Description: Compressor Nebulizer
Model: NE-C802 (NE-C802-UK)
Rating (AC adapter): 100-240V ~ 50/60Hz 15VA
Rating (Compressor
6V
0.6A
Nebulizer):
Operating Temperature/
+10°C to +40°C / 30% to 85%RH
Humidity:
Storage and Transport
Temperature/Humidity/
-20°C to +60°C / 10% to 95%RH /
700 - 1060hPa
Air Pressure:
Weight: Approx. 190g (compressor only)
Dimensions: Approx. 85 (W) × 43 (H) ×115 (D) mm
(compressor only)
Contents:
Compressor, Nebulizer Kit, Air Tube (PVC,
100cm), Mouthpiece, Nosepiece, Adult Mask
(PVC), Child Mask (PVC), 5 pcs Spare Air
Filters, AC Adapter, Storage Bag, Instruction
Manual, Warranty Card.
Classification: Class ll equipment, Type BF applied part
= Class ll
equipment
= No operation
= Type BF
applied part
= Power on
Read the instruction
manual carefully
Technical data for the OMRON NE-C802 Compressor with the Nebulizer kit:
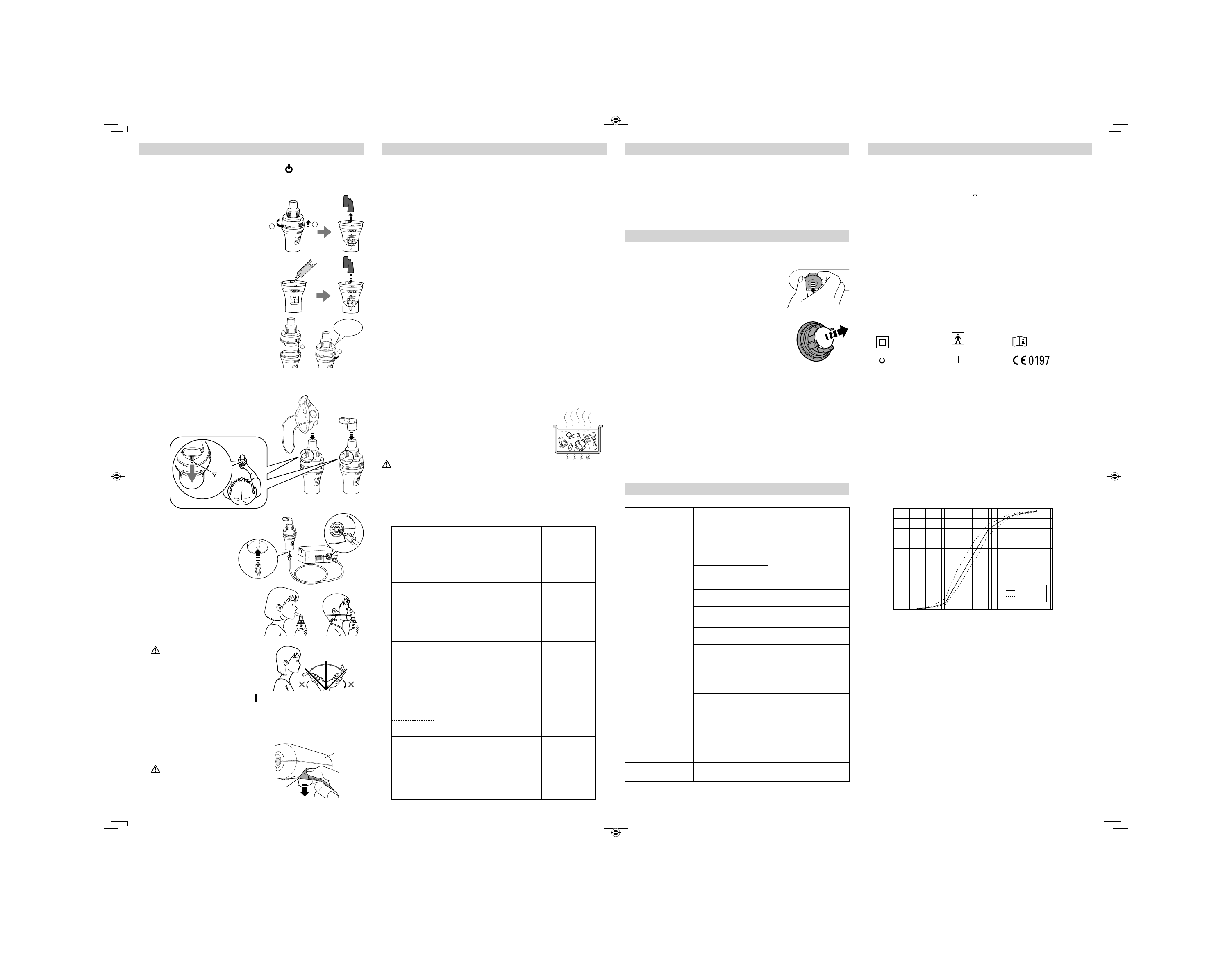
Particle Size: **MMAD Approx. 3m
MMAD = Mass Median Aerodynamic Diameter
Medication Tank Capacity: 10ml maximum
Appropriate Medication
2ml minimum - 10ml maximum
Quantities:
Sound: *Less than 45dB
Nebulization Rate: *Approx. 0.25ml/min (by weight loss)
Aerosol Output: **0.56ml (2ml, 1%NaF)
Aerosol Output Rate: **0.07ml/min (2ml, 1%NaF)
Result of cascade impactor measurements for particle size**
Cumulative % particle mass of sodium fluoride undersize
100
90
80
70
60
50
40
30
Cumulative Undersize%
Mean
Individual
1 10 1000.1
Particle Size (m)
*
Measured by OMRON HEALTHCARE Co., Ltd. Measured with saline at ambient
temperature of 23°C and humidity of 40%. It may vary with the type of medication.
**
Independently measured by Prof. Dr. Hiroshi Takano, Department of Chemical
Engineering and Materials Science, Faculty of Science and Engineering, Doshisha
University, Kyoto, Japan, according to EN 13544-1:2007+A1:2009.
20
10
0
Notes:
• Subject to technical modification without prior notice.
• This OMRON product is produced under the strict quality system of OMRON
HEALTHCARE Co., Ltd., Japan.
• The device may not work if the temperature and voltage conditions are different to
those defined in the specifications.
• The device fulfils the provisions of the EC directive 93/42/EEC (Medical Device
Directive) and the European Standard EN13544-1:2007+A1:2009, Respiratory
therapy equipment - Part1: Nebulizing systems and their components.
• Performance may vary with drugs such as suspensions or high viscosity. See drug
supplier’s data sheet for further details.
• See website of OMRON HEALTHCARE EUROPE to update technical information.
URL: www.omron-healthcare.com
NE-C802-UK_A_M EN.indd 2NE-C802-UK_A_M EN.indd 2 8/21/2012 12:18:44 PM8/21/2012 12:18:44 PM
 Loading...
Loading...