Page 1

INSTRUCTIONS
EVIS EXERA II ULTRASOUND GASTROVIDEOSCOPE
OLYMPUS GF TYPE UCT180
CAUTION : Balloons Used with This Product Contain Natural Rubber Latex, Which May Cause
Allergic Reactions.
USA: CAUTION: Federal law restricts this device to sale by or on the order of a
physician.
Page 2

Page 3

Contents
Contents
Symbols......................................................................................... 1
Important Information — Please Read Before Use.................... 3
Intended use ............................................................................................ 3
Applicability of endoscopy and endoscopic treatment ............................. 3
Instruction manual..................................................................................... 4
User qualifications .................................................................................... 4
Instrument compatibility ............................................................................ 5
Reprocessing before the first use/reprocessing and storage after use .... 5
Spare equipment ...................................................................................... 5
Maintenance management........................................................................ 6
Prohibition of improper repair and modification ........................................ 6
Signal words ............................................................................................. 6
Warnings and cautions.............................................................................. 7
Examples of inappropriate handling ......................................................... 11
Chapter 1 Checking the Package Contents............................ 13
1.1 Standard components..................................................................... 13
1.2 Ultrasonic cable .............................................................................. 15
1.3 Optional components ...................................................................... 15
Chapter 2 Instrument Nomenclature and Specifications ...... 16
2.1 Nomenclature.................................................................................. 16
2.2 Endoscope functions....................................................................... 18
2.3 Specifications.................................................................................. 21
2.4 Attaching the chain for water-resistant cap (MAJ-1739) ................. 25
Chapter 3 Preparation and Inspection .................................... 28
3.1 Preparation of the equipment.......................................................... 29
3.2 Inspection of the endoscope ........................................................... 30
3.3 Preparation and inspection of accessories ..................................... 34
3.4 Attaching accessories to the endoscope ........................................ 38
3.5 Inspection and connection of ancillary equipment .......................... 40
3.6 Inspection of the endoscopic system .............................................. 46
3.7 Preparation and inspection of the balloon....................................... 52
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
i
Page 4

Contents
Chapter 4 Operation.................................................................. 57
4.1 Insertion .......................................................................................... 59
4.2 Observation of the ultrasound image .............................................. 64
4.3 Using EndoTherapy accessories .................................................... 66
4.4 Withdrawal of the endoscope.......................................................... 73
4.5 Removal of the balloon ................................................................... 74
4.6 Transportation of the endoscope .................................................... 75
Chapter 5 Reprocessing: General Policy................................ 76
5.1 Instructions...................................................................................... 76
5.2 Importance of cleaning, disinfection, and sterilization..................... 76
5.3 Precautions ..................................................................................... 77
5.4 Reprocessing before the first use/reprocessing
and storage after use ...................................................................... 80
Chapter 6 Compatible Reprocessing Methods and
Chemical Agents ..................................................... 81
6.1 Compatibility summary.................................................................... 81
6.2 Detergent solution........................................................................... 83
6.3 Disinfectant solution ........................................................................ 84
6.4 Rinse water ..................................................................................... 84
6.5 Ethylene oxide gas sterilization....................................................... 85
6.6 Steam sterilization (autoclaving) of accessories ............................. 87
Chapter 7 Cleaning, Disinfection, and Sterilization
Procedures .............................................................. 88
7.1 Required reprocessing equipment .................................................. 88
7.2 Cleaning, disinfection, and sterilization
procedures for the endoscope ........................................................ 102
7.3 Precleaning ..................................................................................... 103
7.4 Leakage testing............................................................................... 108
7.5 Manual cleaning .............................................................................. 113
7.6 High-level disinfection ..................................................................... 132
7.7 Rinsing after high-level disinfection................................................. 135
7.8 Sterilization ..................................................................................... 138
7.9 Cleaning, disinfection, and sterilization procedures for reusable
parts and reprocessing equipment.................................................. 139
7.10 Care of the ultrasonic cable (MAJ-1597)......................................... 147
ii
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 5

Contents
Chapter 8 Cleaning and Disinfection Equipment................... 148
Chapter 9 Storage and Disposal.............................................. 151
9.1 Storage of the endoscope............................................................... 152
9.2 Storage of the balloon..................................................................... 152
9.3 Storage of reusable parts and reprocessing equipment ................. 152
9.4 Storage of the ultrasonic cable ....................................................... 153
9.5 Disposal .......................................................................................... 153
Chapter 10 Troubleshooting ...................................................... 154
10.1 Troubleshooting guide .................................................................... 154
10.2 Withdrawal of the endoscope with an irregularity ........................... 159
10.3 Returning the endoscope for repair ................................................ 161
Appendix A: System Chart .......................................................... 163
Appendix B: Inspection of the endoscope after cleaning,
disinfection or sterilization in accordance with
IEC 60601-2-37 ......................................................... 170
Appendix C: EMC Information..................................................... 172
Appendix D: Acoustic Output Information in Accordance
with the FDA Guidance:
“Information for Manufacturers Seeking
Marketing Clearance of Diagnostic Ultrasound
Systems and Transducers” .................................... 177
Symbol key ............................................................................................... 177
Acoustic output table with ALOKA diagnostic ultrasound system ............. 179
Acoustic output table when combined with Olympus universal
endoscopic ultrasound center EU-ME1 .................................................... 182
Clinical measurement accuracy with ALOKA diagnostic
ultrasound system ..................................................................................... 183
Clinical measurement accuracy when combined with Olympus
universal endoscopic ultrasound center EU-ME1 .................................... 183
Appendix E: Acoustic Output Information Accordance with
IEC 60601-2-37 ......................................................... 184
Acoustic output table with ALOKA diagnostic ultrasound system ............ 184
Acoustic output table with Olympus universal endoscopic ultrasound
center EU-ME1 ......................................................................................... 195
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
iii
Page 6

Contents
iv
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 7
Symbols
Symbols
The meaning(s) of the symbol(s) shown on the package, the back cover of this
instruction manual and/or this instrument are as follows:
Refer to instructions.
Caution
TYPE BF applied part
Serial number
IPX7 Ingress protection rating (except for connectors)
Lock the ultrasound connector
Release the ultrasound connector
Ultrasonic endoscope
Single use only
Do not resterilize
Use by (expiration date)
Sterilized using ethylene oxide
Sterilization lot number
Lot number
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
1
Page 8

Symbols
Nonsterile
Keep away from sunlight
Keep dry
Do not use if package is damaged
Contains or Presence of Natural Rubber Latex
Date of manufacture
Manufacturer
Authorized representative in the European Community
2
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 9

Important Information — Please Read Before Use
Important Information — Please Read
Before Use
Intended use
This instrument has been designed to be used with an Olympus universal
endoscopic ultrasound center or a diagnostic ultrasound system (ALOKA CO.,
LTD), video system center, light source, documentation equipment, monitor,
EndoTherapy accessories and other ancillary equipment.
This instrument is designed for endoscopic real-time ultrasound imaging,
ultrasound guided needle aspiration and other endoscopic procedures within the
upper gastrointestinal tract and surrounding organs.
Do not use this instrument for any purpose other than its intended use.
Applicability of endoscopy and endoscopic treatment
If there is an official standard on the applicability of endoscopy and endoscopic
treatment that is defined by the hospital’s administration or other official
institutions such as academic societies on endoscopy, follow that standard.
Before starting endoscopy and endoscopic treatment, thoroughly evaluate its
properties, purposes, effects, and possible risks (their nature, extent, and
probability). Perform endoscopy and endoscopic treatment only when its
potential benefits are greater than its risks.
Fully explain to the patient the potential benefits and risks of the endoscopy and
endoscopic treatment as well as any examination/treatment methods that can be
performed in its place, and perform the endoscopy and endoscopic treatment
only after obtaining the consent of the patient.
Even after starting the endoscopy and endoscopic treatment, continue to
evaluate the potential benefits and risks, and immediately stop the
endoscopy/treatment and take proper measures if the risks to the patient
become greater than the potential benefits.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
3
Page 10

Important Information — Please Read Before Use
Instruction manual
This instruction manual contains essential information for using this instrument
safely and effectively. Before use, thoroughly review this manual and the
manuals of all equipment that will be used during the procedure. Then use the
equipment as instructed.
Keep this and all related instruction manuals in a safe and accessible location. If
you have any questions or comments about any information in this manual,
please contact Olympus.
Terms used in this manual
NBI (Narrow Band Imaging) observation mode:
This is an observation mode using narrow band observation light.
Normal light observation (or WLI (White Light Imaging) observation
mode):
This is an observation mode using the standard white light illumination.
Elastography:
Mode for displaying the relative elasticity information of a tissue using
color images.
For more details, refer to the instruction manual for the ultrasound
instrument for which elastography is available.
User qualifications
If there are official standards for user qualifications for performing endoscopy
and endoscopic treatment that are defined by the hospital’s medical
administrators or other official institutions, such as academic societies on
endoscopy, follow those standards. If there are no official qualification standards,
the operator of this instrument must be a physician approved by the medical
safety manager of the hospital or person in charge of the department
(department of internal medicine, etc.).
The physician should be capable of safely performing the planned endoscopy
and endoscopic treatment following guidelines set by the academic societies on
endoscopy, etc., and considering the difficulty of endoscopy and endoscopic
treatment. This manual does not explain or discuss endoscopic procedures.
4
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 11

Important Information — Please Read Before Use
Instrument compatibility
Refer to the “System chart” in the Appendix to confirm that this instrument is
compatible with the ancillary equipment being used. Using incompatible
equipment can result in patient or operator injury and/or equipment damage.
This instrument complies with EMC standard for medical electrical equipment,
edition 3 (IEC 60601-1-2: 2007). However, when connected with an instrument
that complies with EMC standard for medical electrical equipment, edition 1
(IEC 60601-1-2: 1993), the whole system complies with edition 1.
Reprocessing before the first use/reprocessing and storage after use
This instrument was not cleaned, disinfected, or sterilized before shipment.
Before using this instrument for the first time, reprocess it according to the
instructions given in Chapter 7, “Cleaning, Disinfection, and Sterilization
Procedures”.
After using this instrument, reprocess and store it according to the instructions
given in the endoscope’s companion reprocessing manual. Improper and/or
incomplete reprocessing or storage can present an infection control risk, cause
equipment damage, or reduce performance.
The balloons are disposable, and are intended for a single use only; a new one
must be used for each patient. Do not attempt to reuse or resterilize a balloon.
Spare equipment
Be sure to prepare another endoscope to avoid interruption of the examination
due to equipment failure or malfunction.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
5
Page 12

Important Information — Please Read Before Use
Maintenance management
The probability of failure of the endoscope and ancillary equipment increases as
the number of procedures performed and/or the total operating hours increase.
In addition to the inspection before each procedure, the person in charge of
medical equipment maintenance in each hospital should inspect the items
specified in this manual periodically. An endoscope with an observed irregularity
should not be used, but should be inspected by following Section 10.1,
“Troubleshooting guide” on page 154. If the irregularity is still observed after
inspection, contact Olympus.
Prohibition of improper repair and modification
This instrument does not contain any user-serviceable parts. Do not
disassemble, modify, or attempt to repair it; patient or operator injury and/or
equipment damage can result. Equipment that has been disassembled,
repaired, altered, changed, or modified by persons other than Olympus’ own
authorized service personnel is excluded from Olympus’ limited warranty and is
not warranted by Olympus in any manner.
Signal words
The following signal words are used throughout this manual:
Indicates a potentially hazardous situation which, if not
avoided, could result in death or serious injury.
Indicates a potentially hazardous situation which, if not
avoided, may result in minor or moderate injury. It may also
be used to alert against unsafe practices or potential
equipment damage.
Indicates additional helpful information.
6
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 13

Warnings and cautions
Follow the warnings and cautions given below when handling this instrument.
This information is to be supplemented by the warnings and cautions given in
each chapter.
• After using this instrument, reprocess and store it according
• Before endoscopy, remove any metallic objects (watch,
Important Information — Please Read Before Use
to the instructions given in Chapter 7, “Cleaning, Disinfection,
and Sterilization Procedures”. Using improperly or
incompletely reprocessed or stored instruments may cause
patient cross-contamination and/or infection.
glasses, necklace, etc.) from the patient. Performing
high-frequency cauterization treatment while the patient is
wearing metallic objects may cause burns on the patient in
areas around the metallic objects.
• Move the elevator control lever slowly in the opposite
direction of the “ U” direction until it stops and visually
confirm that the portion of the elevator wire extending from
the distal end of the insertion section is not broken or bent. If
the elevator wire is broken or bent, patient injury, bleeding,
and/or perforation could result.
• Do not strike, hit, or drop the endoscope’s distal end,
insertion tube, bending section, control section, universal
cord, or endoscope connector. Also, do not bend, pull, or
twist the endoscope’s distal end, insertion tube, bending
section, control section, universal cord, or endoscope
connector with excessive force. The endoscope may be
damaged and could cause patient injury, burns, bleeding,
and/or perforations. It could also cause parts of the
endoscope to fall off inside the patient.
• Never perform angulation control forcibly or abruptly. Never
forcefully pull, twist, or rotate the angulated bending section.
Patient injury, bleeding, and/or perforation may result. It may
also become impossible to straighten the bending section
during an examination.
• Never insert or withdraw the endoscope’s insertion section
while the bending section is locked in position. Patient injury,
bleeding, and/or perforation may result.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
7
Page 14

Important Information — Please Read Before Use
• Never operate the bending section, feed air, perform suction,
• Never operate the bending section, feed air, perform suction,
• Never insert or withdraw the insertion section abruptly or with
• Do not touch the light guide of the endoscope connector
• That before each use or after a change of viewing
insert or withdraw the endoscope’s insertion section, or use
EndoTherapy accessories without viewing the endoscopic
image. Patient injury, bleeding, and/or perforation may result.
insert or withdraw the endoscope’s insertion section, or use
EndoTherapy accessories while the image is frozen. Patient
injury, bleeding, and/or perforation may result.
excessive force. Patient injury, bleeding, and/or perforation
may result.
immediately after removing it from the light source because it
is extremely hot. Operator or patient burns can result.
modes/settings, check to ensure the view observed through
the endoscope provides a live image (rather than a stored
one) and has the correct image orientation. Patient injury,
bleeding, and/or perforation could result.
• When the endoscopic image does not appear on the monitor,
the CCD may have been damaged. Turn the video system
center OFF immediately. Continued power supply in such a
case will cause the distal end to become hot and could cause
operator and/or patient burns.
• Turn ON the diagnostic ultrasound system only when the
ultrasonic cable is connected to both the diagnostic
ultrasound system and the ultrasonic cable connector on the
endoscope. In particular, confirm that the diagnostic
ultrasound system is OFF before connecting or
disconnecting the ultrasonic cable from the ultrasonic cable
connector on the endoscope. Operator injury may result
and/or equipment damage may result.
• Do not rely on the NBI observation mode alone for primary
detection of lesions or to make a decision regarding any
potential diagnostic or therapeutic intervention.
• Never withdraw the endoscope while the balloon is still
inflated. Otherwise, the balloon may burst or detach from the
distal end of the endoscope. If the balloon cannot be
deflated, insert the channel cleaning brush (BW-7L) into the
balloon channel. Using slow, short strokes, carefully feed the
brush to remove debris.
8
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 15

Important Information — Please Read Before Use
• When withdrawing the endoscope, make sure that the
balloon is completely deflated, using the ultrasound image
and endoscopic field of view. Withdrawing the endoscope
while the balloon is inflated could result in patient injury.
• If it is difficult to insert the endoscope, do not forcibly insert
the endoscope; stop the endoscopy. Forcible insertion can
result in patient injury, bleeding, and/or perforation.
• If any irregularity in the ultrasound image is observed, turn
the ultrasound center OFF immediately. Continued
ultrasound radiation will cause the distal end to become hot
and could cause operator and/or patient burns.
• Elastography
*1
uses the pulsation of a living body.
Intentional pressurization is not necessary. Compression
onto the tissue by operating the bending section, inserting or
withdrawing the endoscope may cause tissue damage,
bleeding or perforation.
1 Elastography is not available with the diagnostic
ultrasound system (Hitachi Aloka Medical, Ltd.) in
Canada.
• After using the endoscope reprocess it according to the
instructions given in Chapter 7, “Cleaning, Disinfection, and
Sterilization Procedures”. Using improperly or incompletely
reprocessed, the endoscope’s distal end damage may result.
• Do not pull the universal cord during an examination. The
endoscope connector will be pulled out from the output
socket of the light source and the endoscopic image will not
be visible.
• Do not coil the insertion tube or universal cord with a
diameter of less than 12 cm. Equipment damage can result.
• Do not attempt to bend the endoscope’s insertion section
with excessive force. Otherwise, the insertion section may be
damaged.
• Do not touch the electrical contacts inside the videoscope
cable connector. CCD damage may result.
• Do not apply shock to the distal end of the insertion section,
particularly the ultrasound transducer and the objective lens
surface at the distal end. Visual abnormalities may result.
• Do not hold the ultrasonic transducer when holding the
insertion tube. The ultrasonic transducer damage can result
and/or the ultrasonic image will be abnormal.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
9
Page 16

Important Information — Please Read Before Use
• Do not twist or bend the bending section with your hands.
• Do not squeeze the bending section forcefully. The covering
• Turn the video system center ON only when the videoscope
• The endoscope’s remote switches cannot be removed from
Equipment damage may result.
of the bending section may stretch or break and cause water
leaks.
cable is connected to both the video system center and the
videoscope cable connector on the endoscope. In particular,
confirm that the video system center is OFF before
connecting or disconnecting the videoscope cable from the
electrical connector on the endoscope. Failure to do so can
result in equipment damage, including destruction of the
CCD.
the control section. Pressing, pulling, or twisting them with
excessive force can break the switches and/or cause water
leaks.
• If remote switch 1 does not return to the OFF position after
being pressed strongly from the side, gently pull the switch
upwards to return it to the OFF position.
• Do not hit or bend the electrical contacts on the endoscope
connector. The connection to the light source may be
impaired and faulty contact can result.
• Do not touch the electrical contacts in the ultrasonic cable
connector. Equipment damage can result.
• Do not pull, twist or tightly coil the ultrasonic cable. Noise can
develop in the ultrasound image.
• Electromagnetic interference may occur on this instrument
near equipment marked with the following symbol or other
portable and mobile RF (radio frequency) communications
equipment such as cellular phones. If electromagnetic
interference occurs, mitigation measures may be necessary,
such as reorienting or relocating this instrument, or shielding
the location.
10
• To check the electromagnetic interference from other
equipment (any equipment other than this instrument or the
components that constitute this system), the system should
be observed to verify its normal operation in the configuration
in which it will be used.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 17
Important Information — Please Read Before Use
• To prevent unnecessary patient exposure to ultrasound
radiation, follow the ‘as-low-as-reasonably achievable’
(ALARA) principle when using ultrasound equipment. Freeze
the image whenever you are not actively viewing the “live”
ultrasound image. When the equipment is in the FREEZE
mode, no ultrasound energy is emitted.
• It is highly recommended that a backup ultrasonic cable be
available to continue clinical procedures in case of a
malfunction.
• This endoscope contains a memory chip that stores
information about the endoscope and communicates this
information to the video system center CV-160 and CV-180.
Examples of inappropriate handling
Details on clinical endoscopic technique are the responsibility of trained
specialists. Patient safety in endoscopic examinations and endoscopic treatment
can be ensured through appropriate handling by the physician and the medical
facility. Examples of inappropriate handling are described below.
• Over-insufflating the lumen may cause patient pain, injury, bleeding,
and/or perforation.
• Applying suction with the distal end in prolonged contact with the
mucosal surface, with higher suction pressure than required, or with
prolonged suction time may cause bleeding and/or lesions.
• The endoscope has not been designed for use in retroflexed
observation in parts of the body other than the stomach. Performing
retroflexed observation in a narrow lumen may make it impossible to
straighten the angle of the bending section and/or withdraw the
endoscope from the patient. Retroflexed observation in parts of the body
other than the stomach should be performed only when the usefulness
of doing so is determined to be greater than the risk that is posed to the
patient.
• Inserting, withdrawing, and using EndoTherapy accessories without a
clear endoscopic image may cause patient injury, burns, bleeding,
and/or perforation.
• Inserting or withdrawing the endoscope, feeding air, applying suction, or
operating the bending section without a clear endoscopic image may
cause patient injury, bleeding, and/or perforation.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
11
Page 18

Important Information — Please Read Before Use
• For reasons described below, do not rely on the NBI
alone for primary detection of lesions or to make a decision regarding
any potential diagnostic or therapeutic intervention.
NBI has not been demonstrated to increase the yield or
sensitivity of finding any specific mucosal lesion including
colonic polyps or Barrett’s esophagus.
NBI has not been demonstrated to aid in differentiating
establishing the presence or absence of dysplasia or
neoplastic changes within mucosa or mucosal lesions.
1 NBI stands for Narrow Band Imaging. For more details,
refer to the instruction manual for the video system
center CV-180.
Natural rubber latex medical alert
Balloons used with this instrument contain natural rubber
latex that may cause allergic reactions.
Do not use the balloon on a latex-sensitive patient. Instead,
perform the procedure using “The sterile deaerated water
immersion method” described in Section 4.2, “Observation of
the ultrasound image” on page 64, in Chapter 4, “Operation”.
1
observation mode
12
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 19

Chapter 1 Checking the Package Contents
Chapter 1 Checking the Package
Contents
1.1 Standard components
Match all items in the package with the components shown below. Inspect each
item for damage. If the instrument is damaged, a component is missing, or you
have any questions, do not use the instrument; immediately contact Olympus.
This instrument was not disinfected or sterilized before shipment. Before using
this instrument for the first time, reprocess it according to the instructions given
in Chapter 7, “Cleaning, Disinfection, and Sterilization Procedures”.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
13
Page 20
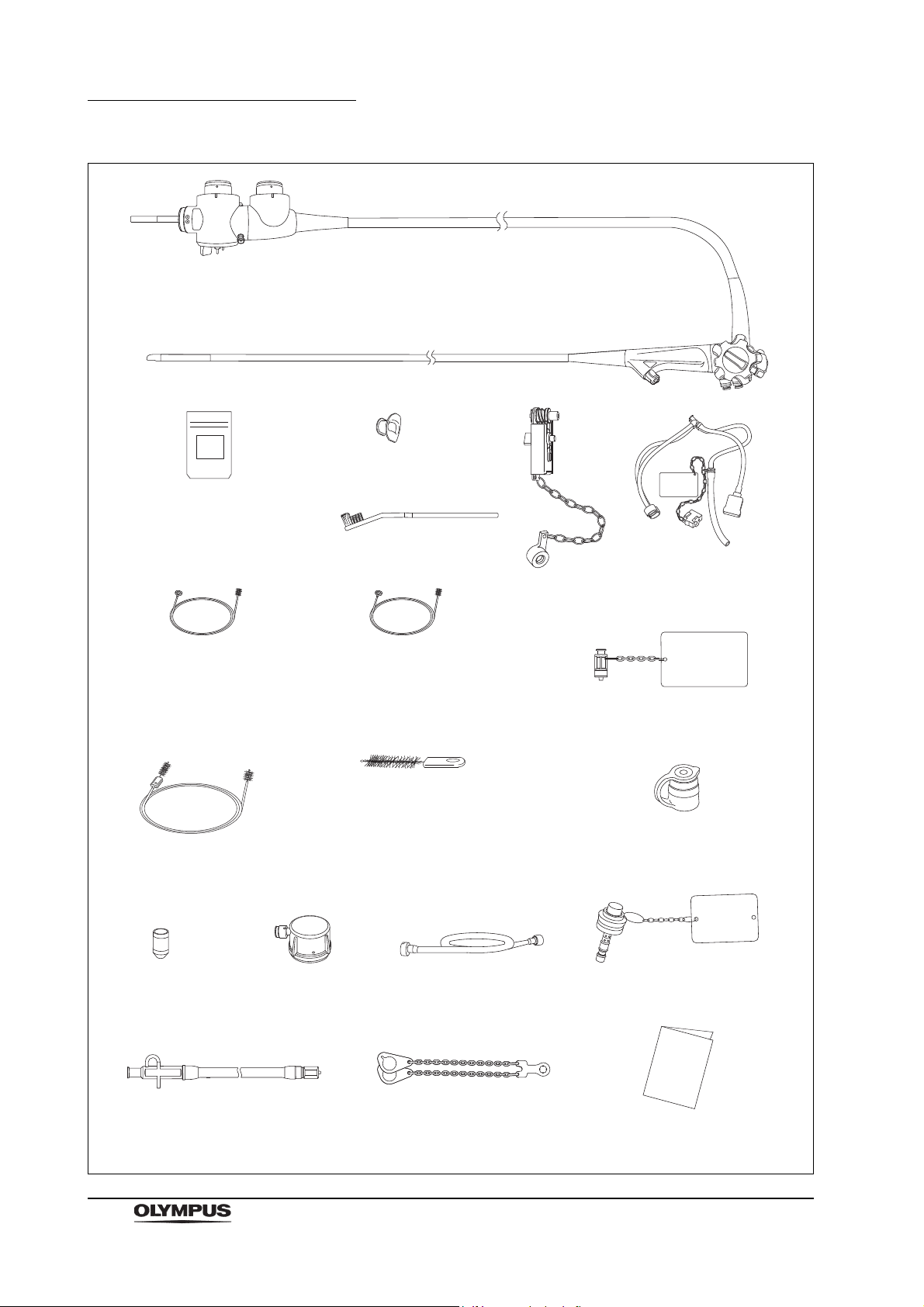
Chapter 1 Checking the Package Contents
Ultrasound endoscope
Balloon 3 for the USA
(MAJ-249 sterile, 20 pcs.)
Balloon for countries other
than the USA (MAJ-213,
nonsterile, 20 pcs.)
Mouthpiece
(MB-142, 2 pcs.)
Single use channel cleaning
brush for the USA
(BW-201T, 3 pcs.
1
)
Channel cleaning brush for
countries other than the USA
(BW-20T, 1 pcs.)
Channel cleaning brush
(BW-7L, 2 pcs.
1
)
Single use single-ended
cleaning brush
(BW-400L, 3 pcs.
1
)
Water-resistant cap
(MH-553, 2 pcs.)
Washing tube
(MH-974)
Chain for water-resistant cap
(MAJ-1739)
Air/water channel cleaning adapter
(MAJ-629)
Instruction manual
Suction cleaning adapter
(MH-856)
Single use channel-opening cleaning
brush for the USA
(MAJ-1339, 3 pcs.
1
)
Channel-opening cleaning brush for
countries other than the USA
(MH-507, 1 pcs.)
Balloon applicator
(MAJ-675)
Biopsy valve
(MAJ-853, nonsterile, 10 pcs.)
Cleaning adapter for instrument
channel port (MAJ-350)
Injection tube
(MH-946)
Channel plug
(MAJ-621)
Cleaning brush
(MAJ-1534)
1 These products may not be available in some areas.
Single use combination
cleaning brush
(BW-412T, 3 pcs.
1
)
14
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 21
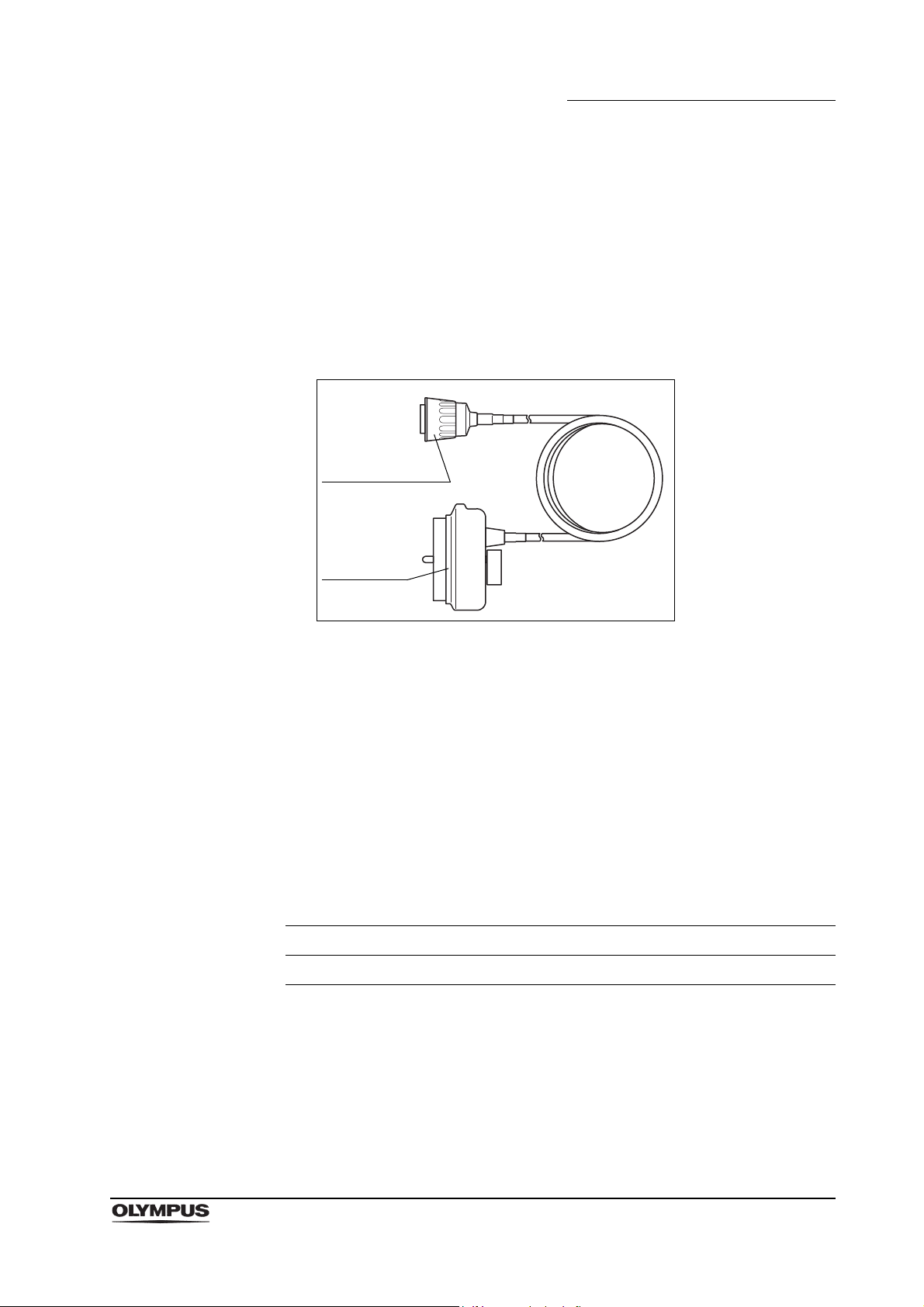
1.2 Ultrasonic cable
Ultrasonic cable
(MAJ-1597)
Endoscope-side
connector
Ultrasound
connector
The ultrasonic cable (MAJ-1597) is necessary to use this endoscope
(GF-UCT180), but it must be purchased separately (optional) from Olympus.
For Olympus universal endoscopic ultrasound center
EU-ME1 and the diagnostic ultrasound system (ALOKA
CO., LTD)
Chapter 1 Checking the Package Contents
1.3 Optional components
The item listed below is optional for countries other than the USA, and may be
purchased from Olympus.
Balloon 3
Balloon 3 is shipped sterile in sets of 20 pieces, enclosed in a resealable
package. The correct model to be used with this endoscope is listed in Table 1.1
below.
Endoscope Balloon 3
GF-UCT180 MAJ-249
Table 1.1
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
15
Page 22
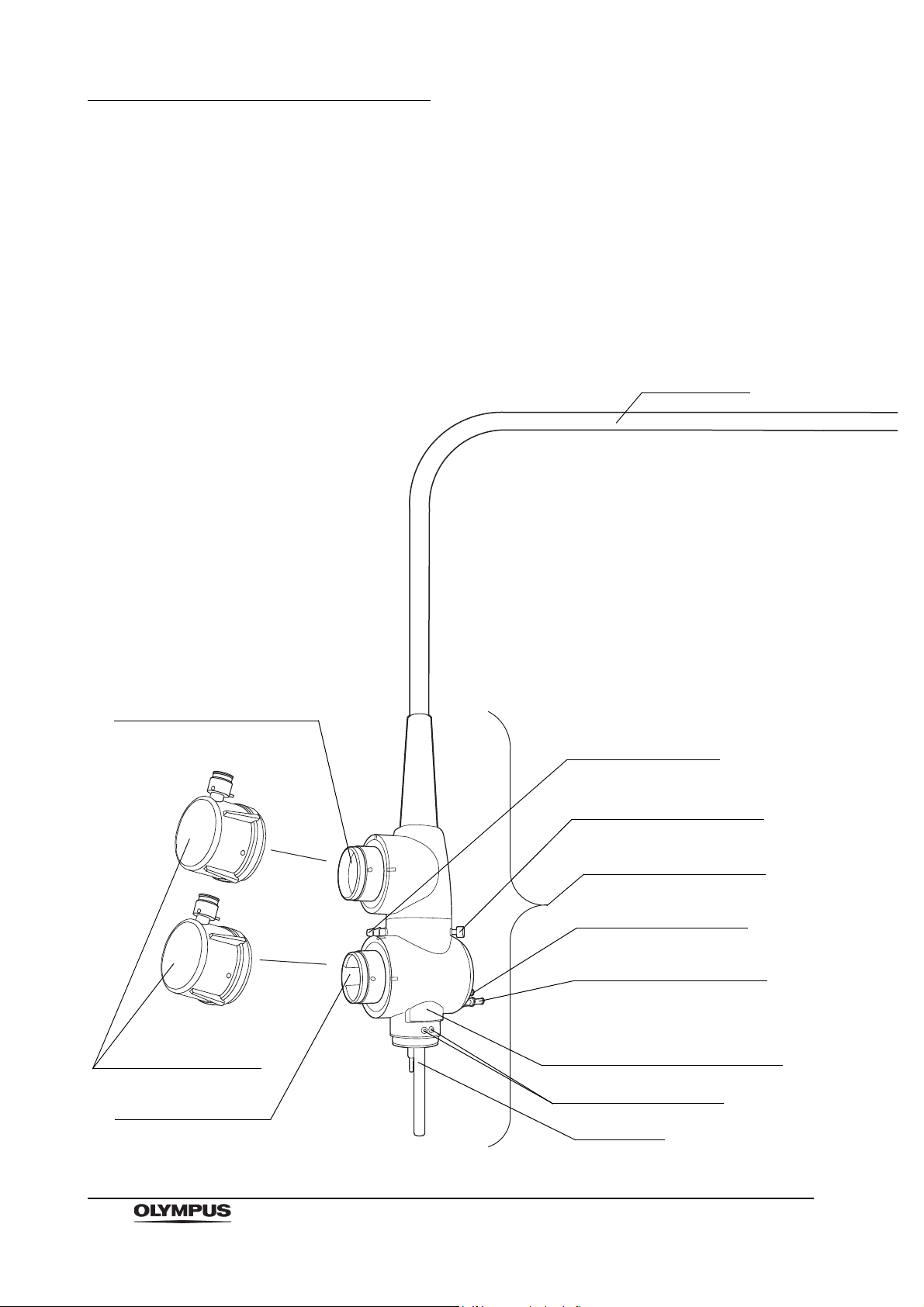
Chapter 2 Instrument Nomenclature and Specifications
Universal cord
Product number and serial number
Electrical contact points
4. Endoscope connector
Light guide
1. Ultrasonic cable connector
6. Videoscope cable
connector
Water-resistant cap
(MH-553)
2. Suction connector
3. S-cord connector mount
5. Air supply connector
5. Water supply connector
Chapter 2 Instrument Nomenclature
and Specifications
2.1 Nomenclature
16
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 23
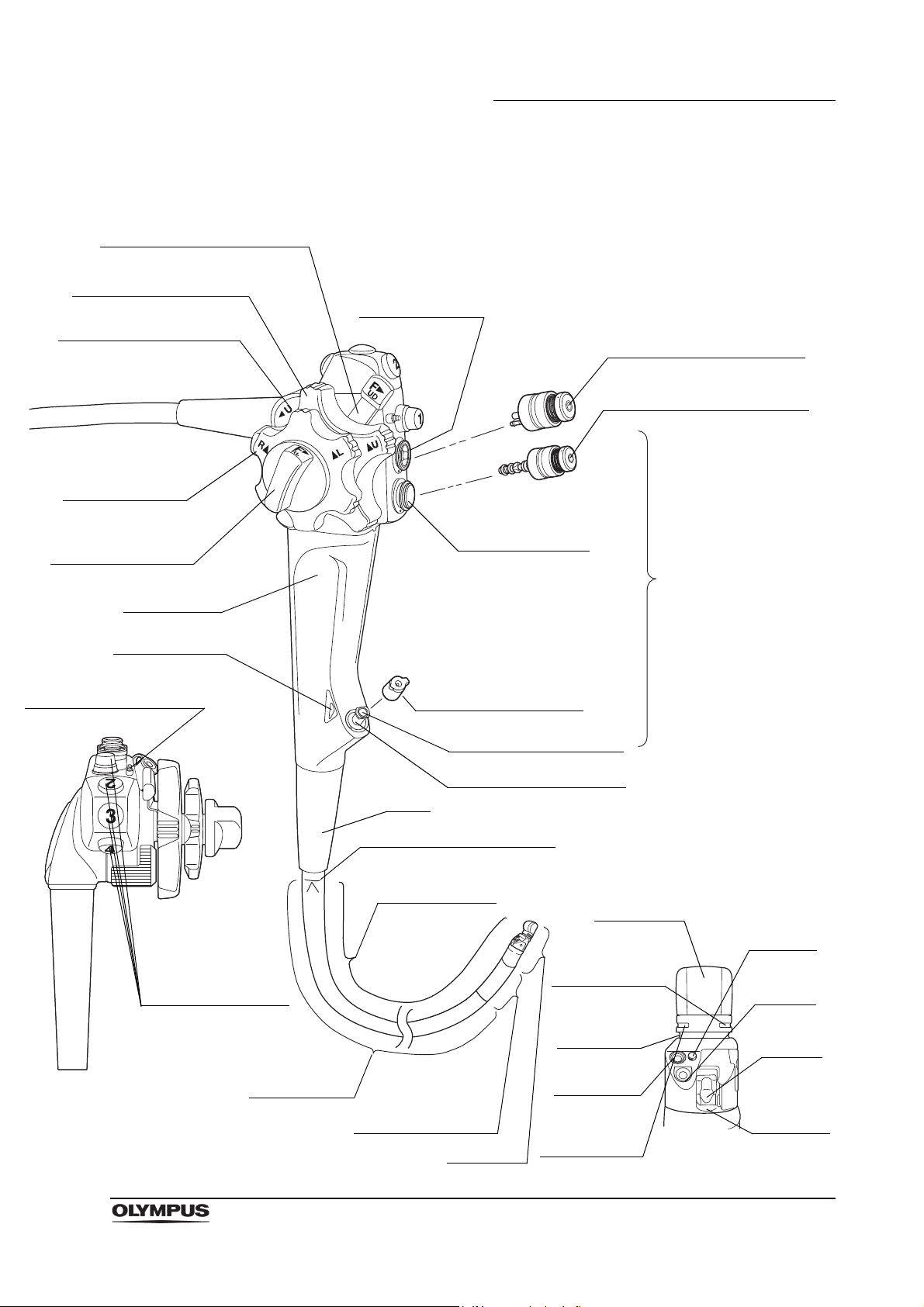
Control
section
Top view
Suction cylinder
11. Air/water valve (MAJ-1444)
9. UP/DOWN angulation lock
7. Elevator control lever
8. UP/DOWN angulation
control knob
18. Color code
17. Elevator channel plug
20. RIGHT/LEFT
angulation
control knob
19. RIGHT/LEFT
angulation lock
Grip section
Boot
Insertion section
Insertion tube
Distal end
14. Bending section
Instrument channel port
12. Instrument channel
Air/water cylinder
Biopsy valve (MAJ-853)
Insertion section limit mark
16. Remote
switches 1 to 4
10. Suction valve (MAJ-1443)
Air/water
nozzle
Detail of distal end
Objective
lens
Forceps
elevator
Instrument
channel
outlet
Light guide
lens
Balloon
attachment
groove
Balloon water
supply port is in
this groove
Ultrasound
transducer
Balloon water
suction port is
in this groove
Chapter 2 Instrument Nomenclature and Specifications
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
17
Page 24

Chapter 2 Instrument Nomenclature and Specifications
2.2 Endoscope functions
1. Ultrasonic cable connector
Connects the ultrasonic cable of the ultrasound diagnostic equipment to the
endoscope.
2. Suction connector
Connects the endoscope to the suction tube of the suction pump.
3. S-cord connector mount
Connects the endoscope with the Olympus electrosurgical unit via the
S-cord. The S-cord conducts leakage current from the endoscope to the
electrosurgical unit. To connect the S-cord, refer to the instruction manual
for the electrosurgical unit. Connect the fitting part of the chain for
water-resistant cap to this mount, as required (see Section 2.4, “Attaching
the chain for water-resistant cap (MAJ-1739)” on page 25).
4. Endoscope connector
Connects the endoscope to the output socket of the light source and
transmits light from the light source to the endoscope.
5. Water supply connector and air supply connector
Connects the endoscope to the water container via the water container tube
to supply water to the distal end of the endoscope.
6. Videoscope cable connector
Connects the endoscope to the video system center via the videoscope
cable. The endoscope contains a memory chip that stores information about
the endoscope and communicates this information to the video system
center CV-160, CV-180. For more details, refer to the instruction manual for
the CV-160, CV-180.
7. Elevator control lever
When this lever is moved in the “ U” direction, the forceps elevator is
raised; when the lever is moved in the opposite direction, the forceps
elevator is lowered.
8. UP/DOWN angulation control knob
When this knob is turned in the “ U” direction, the bending section moves
UP; when the knob is turned in the “D ” direction, the bending section
moves DOWN.
9. UP/DOWN angulation lock
Moving this lock in the “F ” direction frees angulation. Moving the lock in
the opposite direction locks the bending section at any desired position.
18
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 25

Chapter 2 Instrument Nomenclature and Specifications
10. Suction valve (MAJ-1443)
The suction valve is depressed to the first stage to activate suction. The
valve is also used to remove any fluid or debris adhering to the objective
lens.
The suction valve is depressed completely to activate suction of sterile
water from the balloon through the balloon channel.
11. Air/water valve (MAJ-1444)
The hole in the air/water valve is covered to insufflate air and the valve is
depressed to the first stage to feed water for lens washing. It also can be
used to feed air to remove any fluid or debris adhering to the objective lens.
The valve is depressed completely to fill the balloon with sterile water
through the balloon channel.
12. Instrument channel
The instrument channel functions as:
channel for the insertion of EndoTherapy accessories
suction channel
Fluid feed channel (from a syringe via the biopsy valve)
13. Insertion section limit mark
This mark shows the maximum point to which the endoscope may be
inserted into the patient’s body.
14. Bending section
This section moves the distal end of the endoscope when the UP/DOWN
and RIGHT/LEFT angulation control knobs are operated.
15. Forceps elevator
The elevator moves EndoTherapy accessories when the elevator control
lever is operated.
16. Remote switches 1 to 4
The functions of remote switches 1 to 4 can be selected on the video system
center. Refer to the instruction manual for the video system center when
setting these functions.
17. Elevator channel plug
This plug is used for connection of the washing tube to clean and disinfect
the elevator channel.
18. Color code (orange)
This code is used to quickly determine the compatibility of EndoTherapy
accessories. The endoscope can be used with EndoTherapy accessories
that have the same color code. For more information on combining the
endoscope with particular EndoTherapy accessories, refer to the “System
chart” in the Appendix and the instruction manuals for the compatible
accessories.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
19
Page 26

Chapter 2 Instrument Nomenclature and Specifications
19. RIGHT/LEFT angulation lock
Turning this lock in the “F ” direction frees angulation. Turning the lock in
the opposite direction locks the bending section at any desired position.
20. RIGHT/LEFT angulation control knob
When this knob is turned in the “R ” direction, the bending section moves
RIGHT; when the knob is turned in the “ L” direction, the bending section
moves LEFT.
20
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 27

2.3 Specifications
Environment
Chapter 2 Instrument Nomenclature and Specifications
Operating
environment
Transportation
and storage
environment
Ambient temperature 10 – 40C (50 – 104F)
Relative humidity 30 – 85%
Atmospheric
pressure
Ambient temperature –47 to 70C (–52.6 to 158F)
Relative humidity 10 – 95%
Atmospheric
pressure
700 – 1060 hPa
(0.7 – 1.1 kgf/cm
(10.2 – 15.4 psia)
700 – 1060 hPa
(0.7 – 1.1 kgf/cm
(10.2 – 15.4 psia)
2
)
2
)
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
21
Page 28
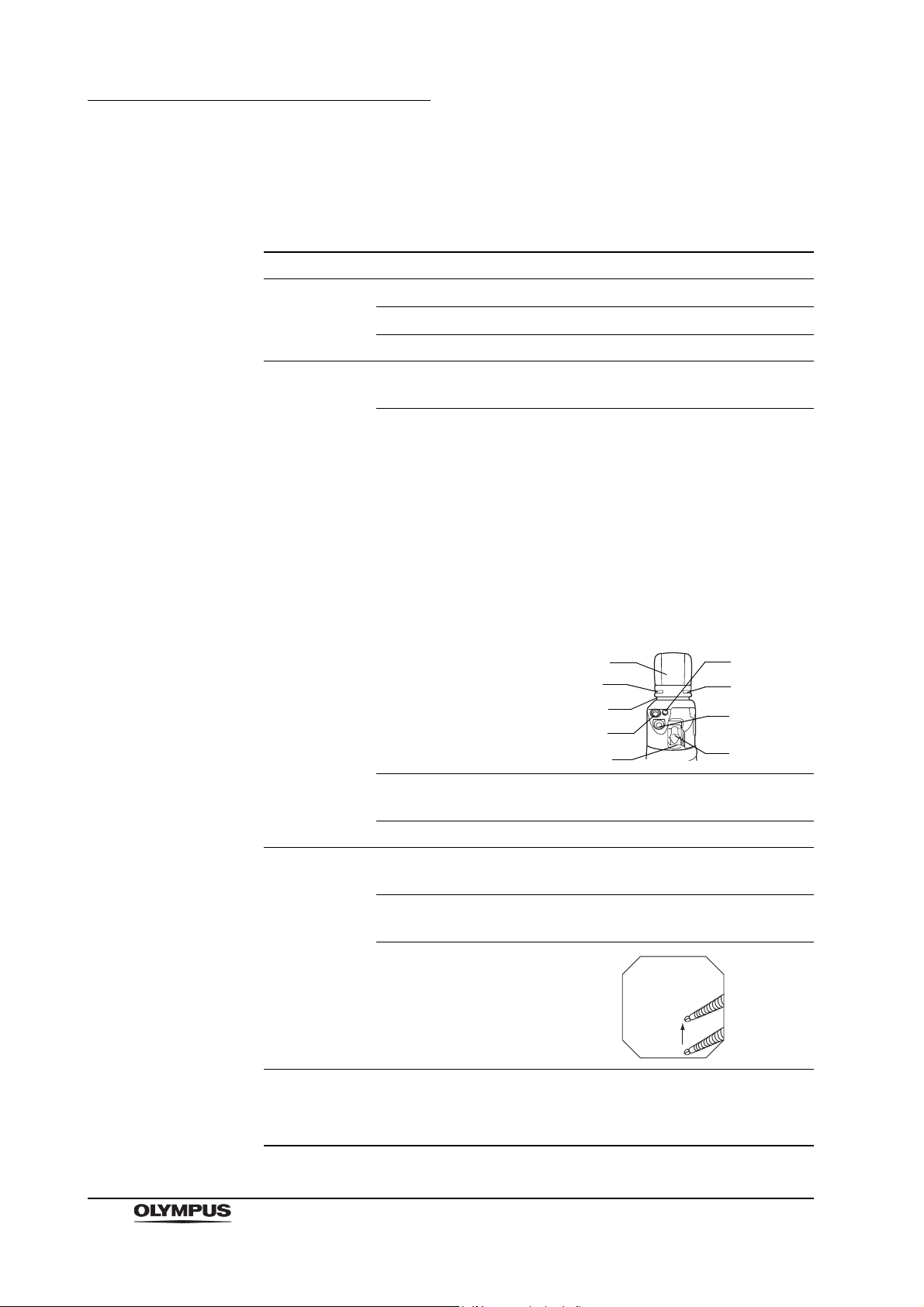
Chapter 2 Instrument Nomenclature and Specifications
6.
7.
9.
3.
2.
4.
5.
1.
8.
Specifications
Endoscope functions
Model GF-UCT180
Optical system Field of view
100
Direction of view
Depth of field 3 – 100 mm
Insertion tube Distal end outer
diameter
Distal end enlarged 1. Air/water nozzle
55
forward oblique
ø 14.6 mm
2. Objective lens
3. Light guide lens
4. Forceps elevator
5. Instrument channel outlet
6. Ultrasonic transducer
7. Balloon water supply port
8. Balloon suction port
9. Balloon attachment groove
Insertion tube outer
diameter
Working length 1250 mm
Instrument
channel
Airflow rate
22
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Channel inner
diameter
Minimum visible
distance
Direction from which
EndoTherapy
accessories enter
and exit the
endoscopic image
6 mm from the objective lens
Note: Standard when CLV-180 (high air
pressure) is used.
ø 12.6 mm
ø3.7mm
3
20 cm
/s
Page 29

Chapter 2 Instrument Nomenclature and Specifications
Bending
section
Total l e ngth 1555 mm
NBI observation mode
Angulation range
1
UP 130
, DOWN 90,
RIGHT 90
Available
, LEFT 90
1 For more details, refer to the instruction manual for the CV-180.
Ultrasound function with ALOKA diagnostic ultrasound
system SSD-10/ProSound 71/ProSound F75
Operation mode B-mode, M-mode, D-mode, Bflow mode, Powerflow mode
Scanning method Electronic curved linear array
Scanning direction Parallel to the insertion direction
Receiving frequency
Scanning range 180
Contacting method Balloon method, Direct contact method
Transducer surface
max. temperature
5, 6, 7.5, 10 MHz (SSD-10/ProSound F75
4, 6.67, 10, 13.3 MHz (ProSound 7)
43C >
1
1
)
1
1 These products may not be available in some areas.
Ultrasound function with Olympus universal endoscopic
ultrasound center EU-ME1
Operation mode B mode, color flow mode, power flow mode
Scanning method Electronic curved linear array
Scanning direction Parallel to the insertion direction
Receiving frequency 5, 6, 7.5, 10, 12 MHz
Scanning range 180
Contacting method Balloon method, Direct contact method
Transducer surface
max. temperature
43C >
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
23
Page 30
Chapter 2 Instrument Nomenclature and Specifications
1912345
General safety
standard for medical
electrical equipment
Medical Devices
Directive
EMC Applied standards;
Year of manufacture
IEC 60601-1: 2005 Whole of this instrument is possible
IEC 60601-1-2: 2007
IEC 60601-2-37: 2007
to contact live bodies of operators or
patients.
This device complies with the
requirements of Directive 93/42/EEC
concerning medical devices.
Classification: Class II a
This instrument complies with the
standards listed in the left column.
CISPR 11 of emission:
Group 1, Class B
This instrument complies with the
EMC standard for medical electrical
equipment, edition 3 (IEC 60601-1-2:
2007). However, when connecting to
an instrument that complies with the
EMC standard for medical electrical
equipment, edition 1 (IEC 60601-1-2:
1993), the whole system complies
with edition 1.
The last digit of the year of
manufacture is given in the second
digit of the serial number.
24
Degree of protection
against electric
shock
Ingress protection
rating
IPX7 This instrument complies with the
TYPE BF applied part
standards for medical electrical
equipment: IEC 60601-1: 2005
IEC 60601-2-37: 2007.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 31

Chapter 2 Instrument Nomenclature and Specifications
2.4 Attaching the chain for water-resistant cap (MAJ-1739)
• Do not lift the endoscope by the chain for water-resistant cap.
Doing so may result in the fitting part of the chain detaching
from the S-cord connector mount, causing the endoscope to
fall. This could cause operator or patient injury and/or
equipment damage.
• Connect the fitting part only to the S-cord connector mount.
Connecting the fitting part to the suction connector may
impair the connection of the suction tube to the suction
connector. It may also cause the suction tube to become
disconnected from the endoscope and allow patient debris to
spray.
• The chain for water-resistant cap and water-resistant cap
itself cannot be ultrasonically cleaned; doing so could
damage them. The water-resistant cap with the chain can
only be ultrasonically cleaned if connected to endoscopes
that are being cleaned in an endoscope reprocessor (such as
1
OER
• Connecting the fitting part to the suction connector may
impair the connection of the suction tube to the suction
connector. It may also cause the suction tube to become
disconnected from the endoscope and allow patient debris to
spray.
• When attaching the water-resistant cap to the connector of
the endoscope, do not pinch the chain for water-resistant cap
between the connector of the endoscope and the
water-resistant cap. Otherwise, equipment damage may
result.
• The chain for water-resistant cap and water-resistant cap
cannot be steam sterilized (autoclaved); doing so can
damage them severely.
, OER-A1) with an ultrasonic cleaning phase.
1 These products may not be available in some areas.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
25
Page 32

Chapter 2 Instrument Nomenclature and Specifications
Notch
Chain part
Fitting part
Connecting plate
Hole
Connecting plate
Notch
Pin
Venting connector
Water-resistant cap
Figure 2.1
To ensure that you do not forget to attach the water-resistant
cap, it is recommended that you connect the chain for
water-resistant cap to the endoscope’s S-cord connector
mount.
1. Confirm that the chain for water-resistant cap is free from cracks, flaws,
wear, deformation, or other damages (see Figure 2.1).
2. Align the notch on the connecting plate with the pin on the venting connector
of the water-resistant cap (MH-553, see Figure 2.2).
3. Place the connecting plate over the venting connector (see Figure 2.2).
4. Confirm that the connecting plate is securely attached to the foot of the
venting connector and can be smoothly rotated.
5. Place the hole on the fitting part over the endoscope’s S-cord connector
mount.
26
Figure 2.2
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 33

Chapter 2 Instrument Nomenclature and Specifications
The instructions on the remaining pages of this manual are
given under the assumption that the chain for water-resistant
cap is detached from the endoscope.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
27
Page 34

Chapter 3 Preparation and Inspection
Chapter 3 Preparation and Inspection
Before each procedure, prepare and inspect this instrument as instructed below.
Inspect other equipment to be used with this instrument as described in their
respective instruction manuals. If this instrument malfunctions, do not use it.
Return it to Olympus for repair as described in Section 10.3, “Returning the
endoscope for repair” on page 161. If any irregularities are suspected after
inspection, follow the instructions given in Chapter 10, “Troubleshooting”.
• This instrument was not cleaned, disinfected, or sterilized
before shipment. Before using this instrument for the first
time, reprocess it according to the instructions given in
Chapter 7, “Cleaning, Disinfection, and Sterilization
Procedures”.
• Using an instrument that is not functioning properly may
compromise patient or operator safety and may result in
more severe equipment damage.
Do not pull the universal cord or the ultrasonic cable with
excessive force when the endoscope is connected to the
other equipment. Doing so could cause equipment damage.
28
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 35

3.1 Preparation of the equipment
mouthpiece
Monitor
Video system center
Light source
Water container
Suction pump
Ultrasound endoscope
Olympus universal endoscopic
ultrasound center EU-ME1 or
ALOKA diagnostic ultrasound
system
Ultrasonic cable
EndoTherapy accessories
Prepare the equipment shown in Figure 3.1 (for compatibility, see the “System
chart” in the Appendix) and personal protective equipment, such as eyewear,
face mask, moisture-resistant clothing, and chemical-resistant gloves that fit
properly and are long enough so that your skin is not exposed. Refer to the
respective instruction manuals for each piece of equipment.
Chapter 3 Preparation and Inspection
• Paper towels • Trays • Lint-free cloths • Personal protective equipment
• 3-way stopcock • Syringe • Extension tube • Sterile deaerated water
• Medical-grade, water-soluble lubricant
Figure 3.1
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
29
Page 36

Chapter 3 Preparation and Inspection
3.2 Inspection of the endoscope
Clean and disinfect or sterilize the endoscope as described in Chapter 7,
“Cleaning, Disinfection, and Sterilization Procedures” and Chapter 8, “Cleaning
and Disinfection Equipment” of this manual. Then remove the water-resistant
cap from the endoscope connector.
Inspection of the endoscope
1.
Inspect the control section and the endoscope connector for excessive
scratching, deformation, loose parts, or other irregularities.
2. Inspect the boot and the insertion section near the boot for bends, twists, or
other irregularities.
3. Inspect the external surface of the entire insertion section including the
bending section and the distal end for dents, bulges, swelling, scratches,
holes, sagging, transformation, bends, adhesion of foreign bodies, missing
parts, protruding objects, or other irregularities.
4. Gently holding the insertion section with one hand, carefully run your other
hand back and forth over the entire length of the insertion section (see
Figure 3.2). Confirm that no objects or metallic wire protrude from the
insertion section. Also, confirm that the insertion tube is not abnormally rigid.
Figure 3.2
30
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 37

Chapter 3 Preparation and Inspection
5. Using both hands, bend the insertion tube of the endoscope into a
semicircle. Then, moving your hands as shown by the arrows in Figure 3.3,
confirm that the entire insertion tube can be smoothly bent to form a
semicircle and that the insertion tube is pliable.
Figure 3.3
6. Gently hold the midpoint of the bending section and a point 20 cm from the
distal end. Push and pull gently to confirm that the junction between the
bending section and the insertion tube is not loose.
7. Inspect the objective lens and light guide lens at the distal end of the
endoscope’s insertion section for scratches, cracks, stains, or other
irregularities.
8. Inspect the air/water nozzle at the distal end of the endoscope’s insertion
section for abnormal swelling, bulges, dents, or other irregularities.
9. Inspect the ultrasound transducer surface at the distal end of the
endoscope’s insertion tube for scratching, cracks, bulges, dents or other
irregularities.
Inspection of the bending mechanisms
Perform the following inspections while the bending section is straight.
If the movement of the UP/DOWN angulation lock,
RIGHT/LEFT angulation lock, and the angulation control
knobs are loose and/or not smooth, or the bending section
does not angulate smoothly, the bending mechanism may be
abnormal. In this case, do not use the endoscope because it
may be impossible to straighten the bending section during
an examination.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
31
Page 38

Chapter 3 Preparation and Inspection
Inspection for smooth operation
1. Confirm that both the UP/DOWN and RIGHT/LEFT angulation locks move
all the way in the “F ” direction.
2. Turn the UP/DOWN and RIGHT/LEFT angulation control knobs slowly in
each direction until they stop, and return them to their respective neutral
positions. Confirm that the bending section angulates smoothly and
correctly, that maximum angulation can be achieved, and that the bending
section returns to its neutral position.
3. When the UP/DOWN and RIGHT/LEFT angulation control knobs are turned
to their respective neutral positions as shown in Figure 3.4, confirm that the
bending section returns smoothly to an approximately straight condition.
Figure 3.4
Inspection of the UP/DOWN angulation mechanism
1. Move the UP/DOWN angulation lock all the way in the opposite direction of
the “F ” mark. Then turn the UP/DOWN angulation control knob in the
“ U” or the “D ” direction until it stops.
2. Confirm that the angle of the bending section is roughly stabilized when the
UP/DOWN angulation control knob is released.
3. Confirm that the bending section straightens out when the UP/DOWN
angulation lock is moved all the way in the “F ” direction and the
UP/DOWN angulation control knob is released.
32
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 39

Chapter 3 Preparation and Inspection
Inspection of the RIGHT/LEFT angulation mechanism
1. Turn the RIGHT/LEFT angulation lock all the way in the opposite direction of
the “F ” mark. Then turn the RIGHT/LEFT angulation control knob in the
“R ” or the “ L” direction until it stops.
2. Confirm that the angle of the bending section is roughly stabilized when the
RIGHT/LEFT angulation control knob is released.
3. Confirm that the bending section straightens out when the RIGHT/LEFT
angulation lock is turned in the “F ” direction and the RIGHT/LEFT
angulation control knob is released.
Inspection of the forceps elevator mechanism
Perform the following inspections while the bending section is straight.
Move the elevator control lever slowly in the opposite
direction of the “ U” direction until it stops and visually
confirm that the portion of the elevator wire extending from
the distal end of the insertion section is not broken or bent. If
the elevator wire is broken or bent, patient injury, bleeding,
and/or perforation could result.
Inspection for smooth operation
1. Move the elevator control lever slowly all the way in the opposite direction of
the “ U” direction. Visually confirm that the portion of the elevator wire
extending from the distal end of the insertion section is not broken or bent
(see Figure 3.5).
2. While observing the forceps elevator at the distal end of the insertion
section, slowly move the elevator control lever all the way in the “ U”
direction. Confirm that the lever can be operated smoothly and that the
forceps elevator is raised smoothly. Also confirm that the forceps elevator
remains stationary when pushed from behind while holding the elevator
control lever stationary (see Figure 3.5).
3. Move the elevator control lever slowly all the way in the opposite direction of
the “ U” direction. Confirm that the lever can be operated smoothly and
that the forceps elevator is lowered smoothly (see Figure 3.5).
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
33
Page 40

Chapter 3 Preparation and Inspection
Forceps elevator
Elevator control lever
Figure 3.5
3.3 Preparation and inspection of accessories
Clean and disinfect or sterilize the air/water valve, suction valve, biopsy valve as
described in Chapter 7, “Cleaning, Disinfection, and Sterilization Procedures”.
Inspection of the air/water and suction valves
Confirm that the top hole of the air/water valve is not blocked
(see Figure 3.6). If the hole is blocked, air is fed continuously
and patient pain, bleeding, and/or perforation can result.
1. Confirm that the holes of the valves are not blocked (see Figures 3.6 and
3.7).
2. Confirm that the valves are not deformed or cracked (see Figures 3.6 and
3.7).
3. Check for excessive scratching or tears in the air/water valve’s seals (see
Figure 3.6).
34
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 41

Figure 3.6
Air/water valve (MAJ-1444)
Skirt
Seal
Packings
Spring
Cap
Top view
Hole
Suction valve (MAJ-1443)
Skirt
Packing
Hole
Spring
Cap
Chapter 3 Preparation and Inspection
Figure 3.7
The air/water and suction valves are consumables. If the
inspection of the air/water or suction valve reveals any
irregularity, use new valves.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
35
Page 42

Chapter 3 Preparation and Inspection
Normal Abnormal
Slit
Hole
Main body
Cap
Splits, cracks
Discoloration
Slit
Main body
Cap
Inspection of the biopsy valve
1. Confirm that the slit and hole on the biopsy valve have no splits, cracks,
deformations, discoloration, or other damage (see Figure 3.8).
The biopsy valve is a consumable that should be inspected
as follows before each use. Replace it with a new one if any
irregularity is observed during the inspection. An irregular,
abnormal, or damaged valve can reduce the efficacy of the
endoscope’s suction system, and may leak or spray patient
debris or fluids, posing an infection control risk.
Figure 3.8
2. Attach the cap to the main body (see Figure 3.9).
Figure 3.9
36
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 43

Inspection of the mouthpiece
Opening
Outer flange
Main body
Do not use a mouthpiece that is damaged, deformed, or
reveals other irregularities. Doing so may cause patient injury
and/or equipment damage.
Placing the mouthpiece in the patient’s mouth before the
procedure prevents the patient from biting and/or damaging
the endoscope’s insertion section.
1. Confirm that the mouthpiece is free from cracks, deformations, or
discoloration (see Figure 3.10).
2. Using your fingers, check all surfaces of the mouthpiece for scratches,
cracks, or other irregularities (see Figure 3.10).
Chapter 3 Preparation and Inspection
Figure 3.10
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
37
Page 44

Chapter 3 Preparation and Inspection
Suction valve
Suction cylinder
Skirt
Moving surface
3.4 Attaching accessories to the endoscope
The air/water valve and the suction valve do not require
lubrication. Lubricants can cause swelling of the valves’
seals, which will impair valve function.
Attaching the suction valve
Attach the suction valve (MAJ-1443) to the suction cylinder of the endoscope
(see Figure 3.11). Confirm that valve is fitted properly without any bulging of the
skirt.
Figure 3.11
The suction valve will make a whistling noise when it is dry;
this does not indicate a malfunction.
38
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 45

Attaching the air/water valve
Skirt
Air/water cylinder
Air/water valve
Biopsy valve
Instrument
channel port
Attach the air/water valve (MAJ-1444) to the air/water cylinder of the endoscope
(see Figure 3.12). Confirm that valve is fitted properly without any bulging of the
skirt.
Chapter 3 Preparation and Inspection
Figure 3.12
The air/water valve may stick at first, but it should operate
smoothly after it is depressed a few times.
Attaching the biopsy valve
If a biopsy valve is not properly connected to the instrument
channel port, it can reduce the efficacy of the endoscope’s
suction system, and leak or spray patient debris, posing an
infection control risk.
Attach the biopsy valve to the instrument channel port of the endoscope (see
Figure 3.13). Confirm that the biopsy valve fits properly.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Figure 3.13
39
Page 46

Chapter 3 Preparation and Inspection
3.5 Inspection and connection of ancillary equipment
Inspection of ancillary equipment
• Attach the water container to the specified receptacle on the
trolley (cart) or the light source. If the water container is
attached anywhere else, water may drip from the water
container’s water supply tube, and equipment malfunction
can result.
• Take care not to spill water from the water container’s
connection adapter when detaching the connection adapter
from the endoscope. Spilled water could splash on the
equipment, and may cause equipment malfunction.
1. Prepare and inspect the light source, video system center, ultrasound
diagnostic equipment, monitor, water container, suction pump, and
EndoTherapy accessories as described in their respective instruction
manuals.
2. Confirm that there are no scratches, cracks, excessive wear, or deformation
of the ultrasonic cable.
Connection of the ultrasonic cable (MAJ-1597) and Olympus universal endoscopic ultrasound center
Insert the ultrasonic connector properly into the transducer port of the Olympus
universal endoscopic ultrasound center. Rotate the connector’s lock handle 1/4
turn clockwise (see Figure 3.14).
40
Figure 3.14
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 47

Chapter 3 Preparation and Inspection
Connection of the ultrasonic cable (MAJ-1597) and ALOKA diagnostic ultrasound system
Insert the ultrasound connector properly into the transducer port of the
diagnostic ultrasound system. Rotate the connector’s lock handle 1/4 turn
clockwise (see Figure 3.15).
Connect the Ultrasound connector to one of the probe
connectors on the lower side (PROBE 3 or 4) of the
ProSound F75 (see Figure 3.15).
If the Ultrasound connector is connected to one of the probe
connectors on the upper side (PROBE 1 or 2) , the operation
panel of the ProSound F75 may hit the Ultrasound connector,
which may result in the equipment damage. When adjusting
the height, a horizontal and / or vertical position of the
operation panel of the ProSound F75 with the Ultrasound
connector connected to the PROBE 1 or 2, move slowly the
operation panel with visually confirming the position of the
bottom of the operation panel.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
41
Page 48

Lock handle
Ultrasound connector
Transducer port
SSD-10
ProSound 7
1
1 These products may not be available in some areas.
Lock handle
Ultrasonic connector
ProSound F75
1
PROBE 1
PROBE 2
PROBE 4
Lock handle
Ultrasound connector
PROBE 3
Chapter 3 Preparation and Inspection
Figure 3.15
42
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 49

Chapter 3 Preparation and Inspection
Connection of the endoscope and ancillary equipment
Firmly connect the suction tube from the suction pump to the
suction connector on the endoscope connector. If the suction
tube is not attached properly, debris may drip from the tube
and can pose an infection control risk, cause equipment
damage, and/or reduce suction capability.
The electrical contacts inside the ultrasound connector may
be damaged by handling. Do not touch the electrical
contacts. If electrical contacts are dirty, wipe the contacts with
a soft and lint-free cloth.
1. If any ancillary equipment is ON, turn it OFF.
2. Insert the endoscope connector completely into the output socket of the light
source.
3. Make sure that the inside of the videoscope cable connector is dry and free
of debris.
4. Place the water container’s water supply channel onto the water supply
connector on the endoscope connector at an angle of 90 and push it in until
it stops (see Figure 3.16 (1)).
5. Turn the water container’s connection adapter 90 clockwise to align the air
supply channel with the air supply connector of the endoscope connector
(see Figure 3.16 (2)).
6. Push the water container’s connection adapter again until it stops (see
Figure 3.16 (3)).
7. Confirm that the water container’s connection adapter fits properly and that
it cannot be rotated (see Figure 3.16 (4)).
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
43
Page 50

Chapter 3 Preparation and Inspection
(1) (2) (3) (4)
Water supply connector
Air supply connector
Water container’s connection adapter
Endoscope connector
Air supply channel
Water supply channel
Metal tip
Mark (gray)
Mark 1 (gray)
Mark 2 (gray)
Figure 3.16
8. Align the mark on the videoscope cable with mark 1 on the endoscope
connector and push it in until it stops (see Figure 3.17).
Figure 3.17
44
9. Turn the connector of the videoscope cable towards mark 2 until it stops
(see Figure 3.17).
10. Confirm that the mark on the videoscope cable is aligned with mark 2 on the
endoscope connector.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 51

Chapter 3 Preparation and Inspection
Suction pump
Suction connector
Suction tube
11. Connect the suction tube from the suction pump to the suction connector on
the endoscope connector (see Figure 3.18).
Figure 3.18
Connection of the endoscope and ultrasonic cable
The electrical contacts inside the ultrasound connector have
sharp tips and may be damaged by handling. Do not touch
the electrical contacts.
For more information on combining the endoscope with the
ultrasonic cable, refer to the “System chart” in the Appendix.
When connect the endoscope-side connector of the
ultrasonic cable to the ultrasonic cable connector, do not
entwine the universal cord with the ultrasonic cable.
Equipment damage can result.
1. Make sure that the inside of the ultrasonic cable connector is dry and free of
debris.
2. Align the mark on the ultrasonic cable with mark A on the ultrasound
connector and push it until it stops (see Figure 3.19).
3. Turn the endoscope-side connector of the ultrasonic cable clockwise until it
stops (see Figure 3.19).
4. Confirm that the mark on the ultrasonic cable is aligned with mark B on the
endoscope connector (see Figure 3.19).
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
45
Page 52

Chapter 3 Preparation and Inspection
Mark A (red)
Mark B (red)
Mark (red)
Mark A (red)
Mark B (red)
Mark (red)
Endoscope-side connector of the ultrasonic cable
Figure 3.19
3.6 Inspection of the endoscopic system
Inspection of the endoscopic image
Do not stare directly into the distal end of the endoscope
while the examination light is ON. Otherwise, eye injury may
result.
1. Turn the video system center, light source, and monitor ON and inspect the
WLI and NBI endoscopic image as described in their respective instruction
manuals.
2. Confirm that light is output from the endoscope’s distal end.
3. While observing the palm of your hand, confirm that the WLI and NBI
endoscopic image is free from noise, blur, fog, or other irregularities.
4. Angulate the endoscope and confirm that the WLI and NBI endoscopic
image does not momentarily disappear or display any other irregularities.
46
If the object cannot be seen clearly, wipe the objective lens
using a clean, lint-free cloth moistened with 70% ethyl or
isopropyl alcohol.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 53

Inspection of the remote switches
All remote switches should be checked prior to the
examination, even when they are not expected to be used.
The endoscopic image may freeze, or other irregularities
may occur during examination and may cause patient injury,
bleeding, and/or perforation.
Depress every remote switch and confirm that the specified functions work
normally.
Inspection of the air-feeding function
1.
Set the airflow regulator on the light source to “High”, as described in the
light source’s instruction manual.
2. Immerse the distal end of the insertion section in sterile water to a depth of
10 cm and confirm that no air bubbles are emitted when the air/water valve
is not operated.
Chapter 3 Preparation and Inspection
3. Cover the hole in the air/water valve with your finger and confirm that air
bubbles are continuously emitted from the air/water nozzle.
4. Uncover the hole in the air/water valve and confirm that no air bubbles are
emitted from the air/water nozzle.
If a stream of air bubbles is emitted from the air/water nozzle
even though the air/water valve is not being operated and the
distal end of the insertion section is 10 cm or more below the
surface of the sterile water, there may be an irregularity in the
air-feeding function. If the endoscope is used while air is
continuously fed, over-insufflation and patient injury may
result. If air bubbles are emitted from the air/water nozzle,
remove and reattach the air/water valve correctly, or replace
it with a new one. If this fails to stop air bubbles from being
emitted, do not use the endoscope because there may be a
malfunction. Contact Olympus.
When the distal end of the insertion section is immersed less
than 10 cm below the surface of the sterile water, a small
amount of air bubbles may be emitted from the air/water
nozzle even when the air/water valve is not operated. This
does not indicate a malfunction.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
47
Page 54

Chapter 3 Preparation and Inspection
Inspection of the objective lens cleaning function
1. Keep the air/water valve’s hole covered with your finger and depress the
valve to the first stage. Observe the endoscopic image and confirm that
water flows on the entire objective lens.
Use sterile water only. Nonsterile water may cause patient
cross-contamination and/or infection.
• When the air/water valve is depressed for the first time, it
may take a few seconds before water is emitted.
• If the air/water valve returns to its original position slowly after
water feeding, remove the air/water valve and moisten the
seals with sterile water.
• During the inspection, place the distal end of the endoscope
in a beaker or other container so that the floor does not get
wet.
2. Release the air/water valve. While observing the endoscopic image, confirm
that the emission of water stops and that the valve returns smoothly to its
original position.
3. While observing the endoscopic image, feed air after feeding water by
covering the hole in the air/water valve with your finger. Confirm that the
emitted air removes the remaining water from the objective lens and clears
the endoscopic image.
Inspection of the water feeding function into the balloon
Cover the air/water valve’s hole and completely depress the valve. Confirm that
water is emitted through the balloon water supply port. It may take a few seconds
until water is emitted when the air/water valve is depressed for the first time.
48
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 55

Inspection of the suction function
• If the suction valve does not operate smoothly, detach it and
reattach it, or replace it with a new one. If the endoscope is
used while the suction valve is not working properly, it may
be impossible to stop suction, which could cause patient
injury. If the reattached or replaced suction valve fails to
operate smoothly, the endoscope may be malfunctioning;
stop using it and contact Olympus.
• If the biopsy valve leaks, replace it with a new one. A leaking
biopsy valve can reduce the efficacy of the endoscope’s
suction system and may leak or spray patient debris or fluids,
posing an infection control risk.
1. Place the container of sterile water and the endoscope at the same height.
For the inspection, adjust the suction pressure to the same level as it will be
during the procedure.
Chapter 3 Preparation and Inspection
2. Immerse the distal end of the insertion section in sterile water with the
endoscope’s instrument channel port at the same height as the water level
in the water container. Press the suction valve and confirm that water is
continuously aspirated into the suction bottle of the suction pump.
3. Release the suction valve. Confirm that suction stops and the valve returns
to its original position.
4. Depress the suction valve to the first stage and aspirate water for one
second. Then, release the suction valve for one second. Repeat this several
times and confirm that no water leaks from the biopsy valve.
5. Remove the distal end of the endoscope from the water. Depress the
suction valve and aspirate air for a few seconds to remove any water from
the instrument and suction channels.
Inspection of aspiration from the balloon water suction port
1.
Immerse the distal end of the insertion tube in sterile water and completely
depress the suction valve. Confirm that water is continuously aspirated.
2. Release the suction valve. Confirm that suction stops and the suction valve
returns to its original position.
3. Remove the distal end from the water.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
49
Page 56

Chapter 3 Preparation and Inspection
Inspection of the instrument channel and forceps elevator
1. Confirm that the forceps elevator is lowered, then insert the EndoTherapy
accessory through the biopsy valve. Confirm that the EndoTherapy
accessory extends smoothly from the distal end, and that a foreign object
does not come out.
2. Extend the EndoTherapy accessory approximately 3 cm from the distal end.
Move the elevator control lever in the “ U” direction and confirm that the
forceps elevator is raised smoothly.
3. Move the elevator control lever in the opposite direction of the “ U”
direction and confirm that the forceps elevator is lowered.
Keep your eyes away from the distal end when inserting
EndoTherapy accessories. Extending the EndoTherapy
accessory from the distal end could cause eye injury.
4. Confirm that the EndoTherapy accessory can be withdrawn smoothly from
the biopsy valve.
Inspection of the ultrasound image with the Olympus universal endoscopic ultrasound center EU-ME1
1.
Turn on Olympus universal endoscopic ultrasound center EU-ME1.
A progress bar is displayed after connecting the ultrasound
endoscope (see Figure 3.20). During displaying the progress
bar, the information on the connected ultrasonic endoscope
has been updated to the ultrasound center. Do not turn OFF
the ultrasound center while the progress bar is displayed.
50
Figure 3.20
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 57

Chapter 3 Preparation and Inspection
Ultrasound image (B-mode)
2. Inspect the endoscopic ultrasound center as described in its instruction
manual.
3. When setting the frequency, choose the setting value CLA2, and then the
frequencies available with the keyboard (see Figure 3.21). Refer to
EU-ME1’s instruction manual for instructions on how to set the frequency.
Figure 3.21
4. Press the “FREEZE” switch on the diagnostic ultrasound system to change
the ultrasound image to the REAL-TIME mode.
5. Confirm that the ultrasound image is visible on the endoscopic ultrasound
center (see Figure 3.22).
Figure 3.22
6. Press the “FREEZE” switch to change the ultrasound image to the FREEZE
mode.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
51
Page 58

Chapter 3 Preparation and Inspection
Ultrasound image (B-mode)
Inspection of the ultrasound image with the ALOKA diagnostic ultrasound system
1.
Inspect the diagnostic ultrasound system as described in its instruction
manual.
2. Turn the diagnostic ultrasound system ON.
3. Confirm that the ultrasound image is visible on the diagnostic ultrasound
system (see Figure 3.23).
Figure 3.23
4. Press the “FREEZE” switch to change the ultrasound image to the FREEZE
mode.
3.7 Preparation and inspection of the balloon
• Balloons used with this instrument contain natural rubber
latex that may cause allergic reactions in some individuals.
Do not use the balloon on a latex-sensitive patient. Instead,
perform the procedure using “The sterile deaerated water
immersion method” described in Section 4.2, “Observation of
the ultrasound image” on page 64.
• When using the sterile balloon (MAJ-249), do not use an
instrument after the expiration date displayed on the sterile
package. Doing so may pose an infection control risk.
• The balloons are disposable, and are intended for a
single-use only. A new one must be used for each patient. Do
not attempt to reuse the balloon. This could pose an
infection-control risk or cause equipment damage.
52
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 59

Chapter 3 Preparation and Inspection
• Confirm that the balloon applicator (MAJ-675) and cotton
thread that is used to tie the balloon have been properly
reprocessed as described in Chapters 5, “Reprocessing:
General Policy” through 7, “Cleaning, Disinfection, and
Sterilization Procedures”.
• If you find that a sterile balloon’s peel pack is open,
damaged, or soiled, do not use that balloon.
• When using the sterile balloon (MAJ-249), inspect the sterile
package for tears, inadequate sealing, or water damage. If
the sterile package shows any irregularity, the sterile
condition of the instrument may have been compromised.
• The balloons and the balloon applicator to be used with this
endoscope are listed below. Balloon
1
is provided clean and
should be ETO gas sterilized before use. Balloon 3 is
shipped sterile.
Balloon (nonsterile) MAJ-213
Balloon 3 (sterile) MAJ-249
Balloon applicator MAJ-675
1 This item is not available in the USA.
• The balloon can easily tear, so beware of sharp objects.
• Store the balloon in a cool environment with low humidity.
• The resealable package containing the balloons also
contains a deoxidizer to maintain a deoxygenated condition
until the sterile peel pack is opened. After the resealable
package is opened, the balloons will gradually deteriorate. To
minimize deterioration, always keep the package sealed.
• The balloon applicator is reusable and supplied nonsterile.
Prepare the balloon and the balloon applicator.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
53
Page 60

Chapter 3 Preparation and Inspection
Balloon
Rear band
Rear end
Balloon applicator
Groove
Installation side
Front end
Balloon attachment
groove
Distal end of
the endoscope
Attaching the balloon
1.
Inspect the balloon and confirm that there are no holes, swelling, color
changes or any other irregularities. If an irregularity is detected, do not use
the balloon; use a spare instead, inspecting it thoroughly before use.
2. Insert the front end of the balloon into the installation side of the balloon
applicator. Slide the rear band of the balloon into the groove of the balloon
applicator (see Figure 3.24).
Figure 3.24
3. Insert the distal end of the endoscope into the balloon applicator until it
contacts the front end of the balloon.
4. Remove the rear band from the balloon applicator and attach it to the
endoscope’s balloon attachment groove (see Figure 3.25).
Figure 3.25
54
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
5. Remove the balloon applicator from the endoscope.
Do not apply excessive force to the distal end of the
endoscope or bend it. Instrument damage may occur.
Page 61

Chapter 3 Preparation and Inspection
Inspection of the balloon and expelling air
• Do not feed water into the balloon when the water level in the
water container is too low. The balloon may not be inflated
properly. If this occurs, refer to Section 10.1,
“Troubleshooting guide” on page 154.
• Disconnecting the water container from the endoscope just
after removing the air bubbles from the balloon may cause
them to reenter the balloon when inflating the balloon again.
Therefore, confirm that there are no air bubbles in the balloon
according to the following procedure.
1. Check the water level in the water container. If the water level is low, fill the
water container with sterile deaerated water. After adding water to the water
container or removing the water container from the endoscope, cover the
hole in the air/water valve and depress it to the first stage for about
15 seconds. Turn the light source and suction pump ON.
2. Cover the hole in the air/water valve and completely depress the valve to fill
the balloon with deaerated water.
3. Fill the balloon with deaerated water to a diameter of approximately 3 cm.
Remove your finger from the air/water valve. If the balloon is eccentrically
shaped after inflation, gently hold the balloon and turn it around. Confirm
that there are no holes or other irregularities in the balloon.
4. Confirm that there are no air bubbles in the balloon.
5. If the balloon has no air bubbles in it, completely depress the suction valve
until all the water has been aspirated from the balloon.
6. If air bubbles are observed in the balloon, lower the distal end of the
endoscope and repeat Steps 2 through 5 until they are completely removed
from the balloon. After confirming that all air bubbles have been removed,
repeat Steps 2 through 5 two times to make sure they are completely
removed.
7. Completely depress the suction valve until all the water has been aspirated
from the balloon.
8. Tie the rear end of the balloon tightly with a sterile cotton thread.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
55
Page 62

Chapter 3 Preparation and Inspection
Balloon water supply port
Balloon attachment groove
Do not obstruct the balloon water supply port with the cotton
thread. If the balloon water supply port is obstructed, the
balloon cannot be inflated or deflated (see Figure 3.26).
Figure 3.26
56
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 63

Chapter 4 Operation
This manual does not explain or discuss clinical endoscopic procedures. It only
describes basic operation and precautions related to the operation of this
instrument. Therefore, the operator of this instrument must be a physician or
medical personnel under the supervision of a physician and must have received
sufficient training in clinical endoscopic technique.
• To guard against dangerous chemicals and potentially
infectious material during the procedure, wear personal
protective equipment, such as eyewear, face mask,
moisture-resistant clothing, and chemical-resistant gloves
that fit properly and are long enough so that your skin is not
exposed.
Chapter 4 Operation
• The temperature of the distal end of the endoscope may
exceed 41C (106F) and reach 50C (122F) due to intense
endoscopic illumination. Surface temperatures over 41C
(106F) may cause mucosal burns. Always maintain a
suitable distance necessary for adequate viewing while using
the minimum level of illumination for the minimum amount of
time. Do not use close stationary viewing or leave the distal
end of the endoscope close to the mucous membrane for a
long time without necessity.
• Whenever possible, do not leave the endoscope illuminated
before and/or after an examination. Continued illumination
will cause the distal end of the endoscope to become hot and
could cause operator and/or patient burns.
• Turn the video system center ON to operate the light source’s
automatic brightness function. When the video system center
is OFF, it cannot operate the light source’s automatic
brightness function, and the light intensity is set to the
maximum level. In this case, the distal end of the endoscope
can become hot and could cause operator and/or patient
burns, when using the light source CLV-160, CLV-U40.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
57
Page 64

Chapter 4 Operation
• Never insert or withdraw the endoscope under any of the
following conditions. Otherwise, patient injury, bleeding,
and/or perforation can result.
While the EndoTherapy accessory extends from the distal
end of the endoscope.
While the bending section is locked in position.
Insertion or withdrawal with excessive force.
Insertion or withdrawal while the forceps elevator is
raised.
• If any of the following phenomena occur during an
examination, immediately stop the examination and withdraw
the endoscope from the patient as described in Section 10.2,
“Withdrawal of the endoscope with an irregularity” on
page 159.
Should any irregularity be observed with the functionality
of the endoscope.
If the endoscopic image on the monitor disappears or
freezes unexpectedly.
If the angulation control knob is locked.
If the angulation control mechanism is not functioning
properly.
Continued use of the endoscope under these conditions
could result in patient injury, bleeding, and/or perforation.
• If an abnormal endoscopic image or function occurs, but
quickly corrects itself, the endoscope may have
malfunctioned. In this case, stop using the endoscope
because the irregularity can occur again and the endoscope
may not return to its normal condition. Therefore, stop the
examination immediately and slowly withdraw the endoscope
while viewing the endoscopic image. Otherwise, patient
injury, bleeding, and/or perforation can result.
• The endoscopic image may be disturbed while switching
between WLI observation mode and NBI observation mode.
Therefore, do not perform an endoscopic operation or
treatment while switching between WLI observation mode
and NBI observation mode. Otherwise, injury in the body
cavity may result.
58
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 65

Chapter 4 Operation
• Set the brightness of the light source to the minimum level
necessary to perform the procedure safely. If the endoscope
is used for a prolonged period at or near maximum light
intensity, vapor may be observed in the endoscopic image.
This is caused by the evaporation of organic material (blood,
moisture in stool, etc.) due to heat generated by the light
guide near the light guide lens. If this vapor continues to
interfere with the examination, remove the endoscope, wipe
the distal end with a lint-free cloth moistened with 70% ethyl
or isopropyl alcohol, reinsert the endoscope, and continue
the examination.
• The color tone and brightness of NBI observation mode is
different from WLI observation mode. Use NBI observation
mode only when fully understanding its features.
4.1 Insertion
Holding and manipulating the endoscope
The control section of the endoscope is designed to be held in the left hand. The
air/water and suction valves can be operated using the left index finger. The
UP/DOWN angulation control knob and the elevator control lever can be
operated using the left thumb. The right hand is free to manipulate the insertion
section and the RIGHT/LEFT angulation control knob (see Figure 4.1).
Figure 4.1
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
59
Page 66

Chapter 4 Operation
> 10 cm
Insertion of the endoscope
Keep the elevator control lever moved all the way in the
opposite direction of the “ U” direction while inserting or
withdrawing the endoscope into or from the patient. If the
elevator control lever is moved all the way in the “ U”
direction and the forceps elevator is raised while inserting or
withdrawing the endoscope into or from the patient, this may
cause patient injury.
• To prevent the patient from accidentally biting the insertion
section during an examination, it is strongly recommended
that a mouthpiece be placed in the patient’s mouth before
inserting the endoscope.
• To prevent the patient from accidentally loosing dental
prosthesis, make sure that the patient removes it before the
examination.
Figure 4.2
• Do not apply olive oil or products containing petroleum-based
lubricants (e.g., Vaseline
products may cause stretching and deterioration of the
bending section’s covering.
• Do not allow the insertion section to be bent within a distance
of 10 cm or less from the junction of the boot. Insertion
section damage can occur (see Figure 4.2).
®
) to the endoscope. These
60
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 67

1. Move the elevator control lever in the opposite direction of the “ U” until it
stops.
2. If necessary, apply a medical-grade, water-soluble lubricant to the insertion
section.
3. Place the mouthpiece between the patient’s teeth or gums, with the outer
flange on the outside of the patient’s mouth.
Angulation of the distal end
Avoid forcible or excessive angulation as this imposes load
on the wire controlling the bending section. This may cause
stretching or tearing of the wire, which could impair the
movement of the bending section.
Chapter 4 Operation
1. Operate the angulation control knobs as necessary to guide the distal end
for insertion and observation.
2. The endoscope’s angulation locks are used to hold the angulated distal end
in position.
• When passing an EndoTherapy accessory through the
instrument channel while the angulation is locked, the angle
of the distal end may change. When it is necessary to keep
the angulation stationary, hold the angulation control knobs in
place with your hand.
• When operating the UP/DOWN or RIGHT/LEFT angulation
lock, hold the angulation control knob stationary with your
finger. If this is not done, the angulation will change.
Air/water feeding and suction
• If the sterile water level in the water container is too low, then
air, not water, will be supplied. In this case, turn the airflow
regulator on the light source OFF and add sterile water to the
water container until it reaches the specified water level.
• If air/water feeding does not stop, turn the airflow regulator
on the light source OFF and replace the air/water valve with a
new one.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
61
Page 68

Chapter 4 Operation
Suction valve
Air/water valve
• Before using a syringe to inject liquid through the biopsy
valve, detach the valve’s cap from the main body. Then insert
the syringe straight into the valve and inject the liquid. If the
cap is not detached and/or the syringe is not inserted
straight, the biopsy valve could be damaged, which could
reduce the efficacy of the endoscope’s suction system, and
may leak or spray patient debris or fluids, posing an infection
control risk.
• If the biopsy valve is left uncapped during the procedure,
debris or fluids could leak or spray from it, posing an infection
control risk. When the valve is uncapped, place a piece of
sterile gauze over it to prevent leakage.
If the endoscope is cold, condensation may form on the
surface of the objective lens and the endoscopic image may
appear cloudy. In this case, increase the temperature of the
sterile water in the water container to between 40 – 50C
(104 – 122F) and then use the endoscope.
Air/water feeding
1. Cover the air/water valve’s hole to feed air from the air/water nozzle at the
distal end (see Figure 4.3).
2. Depress the air/water valve to the first stage to feed water onto the objective
lens (see Figure 4.3).
62
Figure 4.3
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 69

Suction
Chapter 4 Operation
• Avoid aspirating solid matter or thick fluids; instrument
channel, suction channel, or suction valve clogging can
occur. If the suction valve clogs and suction cannot be
stopped, disconnect the suction tube from the suction
connector on the endoscope connector. Turn the suction
pump OFF, detach the suction valve and remove solid matter
or thick fluids.
• When aspirating, maintain the suction pressure at the lowest
level necessary to perform the procedure. Excessive suction
pressure could cause aspiration of and/or injury to the
mucous membrane. In addition, patient fluids could leak or
spray from the biopsy valve, posing an infection control risk.
• When aspirating, attach the cap to the main body of the
biopsy valve. An uncapped biopsy valve can reduce the
efficacy of the endoscope’s suction system, and may leak or
spray patient debris or fluids, posing an infection control risk.
During the procedure, make sure that the suction bottle does
not fill completely. Aspirating fluids into a full bottle may
cause the suction pump to malfunction.
Depress the suction valve to the first stage to aspirate excess fluid or other
debris obscuring the endoscopic image (see Figure 4.3).
Performing both air feeding and suction at the same time
sometimes makes it easier to remove water droplets from the
objective lens surface.
Observation of the endoscopic image
Do not rely only on the NBI observation mode for primary
detection of lesions or to make a decision regarding any
potential diagnostic or therapeutic intervention.
Refer to the light source’s instruction manual for instructions on how to adjust the
brightness.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
63
Page 70

Chapter 4 Operation
4.2 Observation of the ultrasound image
The sterile deaerated water immersion method
• Completely insert the water feeding valve (MD-744) of the
water supply unit (UWS-1) into the biopsy valve (MAJ-853).
Improper connection may cause water to leak.
• If no balloon is attached, do not depress the suction valve
completely. The balloon channel may be clogged.
1. Position the distal end of the endoscope near the target site and push the
“FREEZE” switch on the ultrasound system to change the ultrasound image
to the REAL-TIME mode.
2. Connect the water supply unit’s water-feeding valve to the biopsy valve of
the endoscope.
3. Depress the water supply unit’s water feeding valve as described in its
instruction manual and supply the necessary amount of sterile deaerated
water.
The balloon method
• Do not inflate the balloon to a diameter of more than 3 cm.
• Do not feed water into the balloon when the water level in the
water container is too low. The balloon may not be inflated
properly.
If the water container is removed from the endoscope while
inflating the balloon, cover the hole in the air/water valve and
depress the valve to the first stage for about 15 seconds. By
doing so, air bubbles can be prevented from entering the
balloon.
64
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 71

1. Position the distal end of the endoscope near the target site and press the
2. Cover the small hole in the air/water valve of the endoscope and completely
3. While viewing the ultrasound image, inflate the balloon to the desired size.
4. Use the endoscope’s angulation mechanism to bring the balloon into full
Observation
Chapter 4 Operation
“FREEZE” switch on the ultrasound system to change the ultrasound image
to the REAL-TIME mode.
depress the valve to inflate the balloon.
Do not inflate the balloon to a diameter greater than 3 cm.
contact with the intestinal wall. Move the distal end to the target site.
Keep the ultrasound system in the FREEZE mode, except
during ultrasound observation.
When combined with another electrical instrument, noise
may be displayed on the video monitor.
When the ultrasound image of the target appears, adjust the ultrasound system
as described in its instruction manual to obtain a suitable image.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
65
Page 72

Chapter 4 Operation
4.3 Using EndoTherapy accessories
For more information on combining the endoscope with particular EndoTherapy
accessories, refer to the “System chart” in the Appendix and the instruction
manuals for the accessories.
• When using EndoTherapy accessories, keep the distance
between the distal end of the endoscope and the mucous
membrane greater than the endoscope’s minimum visible
distance so that the EndoTherapy accessory remains visible
in the endoscopic image. If the distal end of the endoscope is
placed closer than its own minimum visible distance, the
position of the accessory cannot be seen in the endoscopic
image, which could cause serious patient injury and/or
equipment damage. The minimum visible distance depends
on the type of endoscope being used. Refer to Section 2.3,
“Specifications” on page 21.
• When inserting or withdrawing an EndoTherapy accessory,
confirm that its distal end is closed or completely retracted
into the sheath. Slowly insert or withdraw the EndoTherapy
accessory straight into/from the slit of the biopsy valve.
Otherwise, the biopsy valve may be damaged and pieces of it
could fall off.
• If the insertion or withdrawal of EndoTherapy accessories is
difficult, straighten the bending section as much as possible
without losing the endoscopic image. Inserting or
withdrawing EndoTherapy accessories with excessive force
may damage the instrument channel or EndoTherapy
accessories and could cause some parts to fall off and/or
cause patient injury.
• Do not switch between WLI observation mode and NBI
observation mode while using an EndoTherapy accessory.
The endoscopic image may be disturbed while switching
between WLI observation mode and NBI observation mode.
This could cause patient injury, bleeding, and/or perforation.
• If the distal end of an EndoTherapy accessory is not visible in
the endoscopic image, do not open the distal end or extend
the needle of the instrument. This could cause patient injury,
bleeding, perforation, and/or equipment damage.
66
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 73

Chapter 4 Operation
• Do not insert EndoTherapy accessories without the forceps
elevator being raised. If they are inserted without the forceps
elevator being raised, the accessory cannot be observed in
the endoscopic image and it may cause patient injury.
• While raising the forceps elevator, do not insert or withdraw
the EndoTherapy accessory with excessive force, open or
close the distal end of the EndoTherapy accessory, or extend
the needle of the instrument. This could damage the
instrument channel and/or the EndoTherapy accessory and
could cause patient injury, bleeding, and/or perforation. If the
EndoTherapy accessory cannot be inserted or withdrawn,
the distal end of the EndoTherapy accessory cannot be
opened or closed, or the needle of the instrument cannot be
extended, move the elevator control lever in the opposite
direction of the “ U” direction to lower the forceps elevator.
• If the forceps elevator cannot be lowered while using an
EndoTherapy accessory, stop the procedure immediately
and take appropriate measures.
• Do not inflate air or a nonflammable gas excessively into the
patient. This could cause gas embolism.
• EUS guided FNA should only be performed when the needle
is visible in the ultrasound image.
• If the ultrasound image disappears while using the needle,
stop the procedure immediately and withdraw the needle
from the tissue.
• When using a biopsy forceps with a needle, confirm that the
needle is not excessively bent. A bent needle could protrude
from the closed cups of the biopsy forceps. Using biopsy
forceps with a protruding needle could damage the
instrument channel and/or cause patient injury.
• When using an injector, be sure not to extend or retract the
needle from the catheter of the injector until the injector is
extended from the distal end of the endoscope. The needle
could damage the instrument channel if extended inside the
channel, or if the injector is inserted or withdrawn while the
needle is extended.
• Although the color code is orange as with GF-UCT180, do
not use EndoTherapy accessories designed for ø 4.2 mm
channel, otherwise the endoscope and/or the EndoTherapy
accessories may be damaged.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
67
Page 74

Chapter 4 Operation
Insertion of EndoTherapy accessories into the endoscope
• Do not insert EndoTherapy accessories forcibly or abruptly.
Otherwise, the EndoTherapy accessory may extend from the
distal end of the endoscope abruptly, which could cause
patient injury, bleeding, and/or perforation.
• When the biopsy valve’s cap is detached from the main body,
it is easier to insert an EndoTherapy accessory into the
instrument channel port (see Figure 3.9 on page 36).
However, the open biopsy valve, after withdrawing an
EndoTherapy accessory, can reduce the efficacy of the
endoscope’s suction system, and it may leak or spray patient
debris or fluids, posing an infection control risk. When not
using an EndoTherapy accessory, attach the cap to the main
body of the biopsy valve.
• When the biopsy valve’s cap is detached from the main body,
it may cause patient debris or fluids to leak or spray from the
endoscope, posing an infection control risk. When the biopsy
valve’s cap has to be detached, place a piece of sterile gauze
over it to prevent leakage.
• Do not let the EndoTherapy accessory hang down from the
biopsy valve. Doing so can create a space between the
accessory and the valve’s slit or hole. This can cause
damage to the valve that can reduce the efficacy of the
endoscope’s suction system, and may leak or spray patient
debris or fluids, posing an infection control risk.
• When inserting an EndoTherapy accessory, hold it close to
the biopsy valve and insert it slowly and straight into the
biopsy valve. Otherwise, the EndoTherapy accessory and/or
biopsy valve could be damaged. This can reduce the efficacy
of the endoscope’s suction system, and may leak or spray
patient debris or fluids, posing an infection control risk.
When using the aspiration needle (NA series), refer to the
instruction manuals for the aspiration needle (NA series).
Otherwise, the instrument channel and/or the EndoTherapy
accessory may become damaged.
68
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 75

Chapter 4 Operation
1. Select EndoTherapy accessories compatible with the instrument from the
“System chart” in the Appendix. Refer to the accessories’ instruction
manuals for operating instructions.
2. Move the elevator control lever all the way in the “ U” direction.
3. Hold the UP/DOWN and RIGHT/LEFT angulation knobs stationary.
4. Confirm that the tip of the EndoTherapy accessory is closed or retracted into
its sheath and insert the EndoTherapy accessory slowly and straight into the
slit of the biopsy valve.
Do not open the tip of the EndoTherapy accessory or extend
the tip of the EndoTherapy accessory from its sheath while
the accessory is in the instrument channel. The instrument
channel and/or the EndoTherapy accessory may become
damaged.
5. Hold the EndoTherapy accessory approximately 4 cm from the biopsy valve
and advance it slowly and straight into the biopsy valve using short strokes
while observing the endoscopic image.
When the tip of the EndoTherapy accessory extends
approximately 6 mm from the distal end of the endoscope,
the accessory will appear in the endoscopic image, and/or
ultrasound image.
6. Hold the EndoTherapy accessory approximately 4 cm from the biopsy valve
and advance it slowly and straight into the biopsy valve using short strokes
while observing the endoscopic image. Confirm that the tip of the
EndoTherapy accessory contacts the forceps elevator.
7. Move the elevator control lever in the opposite direction of the “ U”
direction to lower the forceps elevator. Advance the EndoTherapy
accessory slightly and move the elevator control lever in the “ U” direction
confirm that the accessory appears in the endoscopic image.
8. Manipulate the elevator control lever to adjust the height of the elevator (see
Figures 4.4 and 4.5).
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
69
Page 76

Chapter 4 Operation
Figure 4.4
Figure 4.5
70
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 77

Operation of EndoTherapy accessories
Operate the EndoTherapy accessory according to the directions given in its
instruction manual.
Withdrawal of EndoTherapy accessories
• Patient debris might spray when EndoTherapy accessories
are withdrawn from the biopsy valve. To prevent this, hold a
piece of gauze around the accessory and the biopsy valve
during withdrawal.
• Do not withdraw the EndoTherapy accessory if the tip is open
or extended from its sheath; patient injury, bleeding,
perforation, and/or instrument damage may occur.
• Withdraw the EndoTherapy accessory slowly and straight out
of the biopsy valve. Otherwise, the valve’s slit and/or hole
could be damaged. This can reduce the efficacy of the
endoscope’s suction system, and may leak or spray patient
debris or fluids, posing an infection control risk.
Chapter 4 Operation
• If the EndoTherapy accessory cannot be withdrawn from the
endoscope, close the EndoTherapy accessory and/or retract
it into its sheath, then carefully withdraw both the endoscope
and the EndoTherapy accessory together under endoscopic
observation. Take care not to cause tissue trauma.
Withdraw the EndoTherapy accessory slowly while the tip of the EndoTherapy
accessory is closed and/or retracted into its sheath.
1. Close the tip of the EndoTherapy accessory and/or retract it into its sheath.
2. While lowering the forceps elevator gradually, slowly withdraw the
EndoTherapy accessory.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
71
Page 78

Chapter 4 Operation
High-frequency cauterization treatment
• Performing treatment while the intestines are filled with a
flammable gas could result in an explosion, fire, and/or
serious patient injury. If the intestines contain a flammable
gas, replace it with air or a nonflammable gas such as CO
before performing high-frequency treatment.
• Not all parts of the endoscope are electrically insulated.
When applying high-frequency current, there is a danger of
unintentional diathermy burns. Always wear electrically
insulating, chemical-resistant gloves.
• Never emit high-frequency current before confirming that the
distal end of the high-frequency EndoTherapy accessory is in
the endoscope’s field of view. Also, confirm that the electrode
section and the mucous membrane in the vicinity of the target
area are at an appropriate distance from the distal end of the
endoscope. If the high-frequency current is emitted while the
distal end of the EndoTherapy accessory is not visible or too
close to the distal end of the endoscope, patient injury,
bleeding, and/or perforation as well as equipment damage
can result.
2
Prepare, inspect, and connect the electrosurgical unit and electrosurgical
accessories as described in their instruction manuals.
The application of high-frequency current may interfere with
the endoscopic image. This does not indicate a malfunction.
72
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 79

4.4 Withdrawal of the endoscope
If blood unexpectedly adheres to the surface of the insertion
section of the withdrawn endoscope, carefully check the
condition of the patient.
1. When using the balloon method, aspirate water from the balloon by
depressing the suction valve while covering the hole in the valve.
2. Freeze the ultrasound image.
3. Aspirate the accumulated air, blood, mucous or other debris by depressing
the suction valve to the first stage while covering the hole in the valve.
• Observe the ultrasound image on the video monitor and
confirm that the balloon deflates.
Chapter 4 Operation
• If the balloon does not deflate even when the suction valve is
completely depressed, turn OFF the switch of the airflow
regulator and remove the air/water valve from the
endoscope. In most cases, the balloon will be automatically
deflated.
• If the balloon does not deflate even when the air/water valve
is removed from the endoscope, tear and deflect the balloon
with the EndoTherapy accessory (for example, biopsy
forceps).
4. Turn the UP/DOWN and RIGHT/LEFT angulation locks to the “F ”
direction to release them.
5. Carefully withdraw the endoscope while observing the endoscopic image.
6. Remove the mouthpiece from the patient’s mouth.
7. If using the balloon method, detach the balloon.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
73
Page 80

Chapter 4 Operation
4.5 Removal of the balloon
• Do not hold the ultrasound transducer when holding the
insertion section. The ultrasonic transducer can be damaged,
resulting in an abnormal ultrasonic image.
• Do not squeeze the ultrasound transducer forcefully. The
ultrasonic transducer can be damaged, resulting in an
abnormal ultrasonic image.
• Do not remove the balloon with equipment such as forceps,
needle holder, or hemostat that may scratch the surface of
the ultrasound transducer. The ultrasonic transducer can be
damaged, resulting in an abnormal ultrasonic image.
• Do not pinch the ultrasound transducer surface and balloon
together when removing the balloon. The ultrasonic
transducer can be damaged, resulting in an abnormal
ultrasonic image.
1. Use a clean, lint-free cloth to gently wipe and dry the balloon surface.
2. Roll up the rear end of the balloon with your fingers (see Figure 4.6).
3. After the removal of the balloon, confirm that the surface of the ultrasound
transducer is free from scratches. In case the ultrasound transducer surface
is scratched, stop using the endoscope and contact Olympus.
Figure 4.6
74
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 81

4.6 Transportation of the endoscope
Transporting within the hospital
When carrying the endoscope by hand, loop the universal cord, hold the
endoscope connector with the control section in one hand and hold the distal
end of the insertion tube securely, but gently without squeezing, in the other
hand (see Figure 4.7).
Chapter 4 Operation
Figure 4.7
Transporting outside the hospital
Transport the endoscope in the carrying case.
Always clean, disinfect, or sterilize the endoscope after
removing it from the carrying case. If the endoscope is not
cleaned, disinfected, or sterilized, it could pose an infection
control risk.
• The carrying case cannot be cleaned, disinfected, or
sterilized. Clean and disinfect or sterilize the endoscope
before placing it in the carrying case.
• To avoid damage to the endoscope caused by changes in air
pressure, do not attach the water-resistant cap when
transporting the endoscope.
• Before putting the endoscope in the carrying case, always
make sure that the forceps elevator is not raised. Putting the
endoscope in the carrying case while the forceps elevator is
raised could damage the endoscope.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
75
Page 82

Chapter 5 Reprocessing: General Policy
Chapter 5 Reprocessing: General
Policy
5.1 Instructions
• Chapters 5, “Reprocessing: General Policy” through 8, “Cleaning and
Disinfection Equipment” describe recommended procedures and
equipment for cleaning and disinfecting or sterilizing this instrument.
• This instruction manual contains essential information on reprocessing
this instrument safely and effectively.
• Before reprocessing, thoroughly review the manuals of the reprocessing
chemicals and all equipment that will be used, and reprocess the
equipment as instructed.
• Keep this and all related instruction manuals in a safe, accessible
location.
• If you have any questions or comments about any information in this
manual, or if a problem that cannot be solved occurs while reprocessing,
contact Olympus.
5.2 Importance of cleaning, disinfection, and sterilization
The medical literature reports incidents of cross-contamination resulting from
improper cleaning, disinfection, or sterilization. It is strongly recommended that
all individuals engaged in reprocessing closely observe all instructions given in
this manual and the manuals of all ancillary equipment, and have a thorough
understanding of the following items:
• Professional health and safety criteria of your hospital
• Individual cleaning, disinfection, and sterilization protocols
• Structure and handling of endoscopic equipment
76
• Handling of pertinent chemicals
For the types and conditions of the means of cleaning, disinfection, and
sterilization to be adopted, please make judgments from your professional
viewpoints.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 83

5.3 Precautions
Chapter 5 Reprocessing: General Policy
• Some endoscope reprocessors are not designed to
reprocess an elevator wire channel. If the elevator wire
channel cannot be reprocessed by the endoscope
reprocessor, clean, disinfect, and sterilize the endoscope
according to procedures described in Chapter 7, “Cleaning,
Disinfection, and Sterilization Procedures”.
• Failure to properly clean and high-level disinfect or sterilize
endoscope equipment after each procedure can compromise
patient safety. To minimize the risk of transmitting infectious
agents from one patient to another, after each procedure the
endoscope and the equipment must undergo thorough
manual cleaning followed by high-level disinfection or
sterilization, as described in Chapter 7, “Cleaning,
Disinfection, and Sterilization Procedures”. Reprocess not
only the external surface of the endoscope but also all
channels.
• ALL channels of the endoscope, including the elevator wire
channel and balloon channel, MUST be cleaned and
high-level disinfected or sterilized during EVERY
reprocessing cycle, even if the channels were not used
during the previous patient procedure. Otherwise, insufficient
cleaning and disinfection or sterilization of the endoscope
may pose an infection control risk to the patient and/or
operators performing the next procedure with the endoscope.
• After thoroughly brushing or wiping all external surfaces and
the distal end of the endoscope and around the forceps
elevator with a soft brush or lint-free cloth, always visually
confirm that the elevator wire is not broken. If the elevator
wire is broken, patient and/or operator injury could result.
• If the endoscope is not cleaned meticulously, effective
disinfection or sterilization may not be possible. Clean the
endoscope and accessories thoroughly before disinfection or
sterilization to remove microorganisms and organic material
that could reduce the efficacy of disinfection or sterilization.
• Olympus only confirms validation of the endoscope
reprocessors it recommends. When using an endoscope
reprocessor that is not recommended by Olympus, the
manufacturer of the endoscope reprocessor is responsible
for validating compatibility of the reprocessor with the
endoscope models listed in its instruction manual.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
77
Page 84

Chapter 5 Reprocessing: General Policy
• Before using an endoscope reprocessor, confirm that it is
capable of reprocessing the endoscope including all
channels. If you are uncertain as to the ability of your
endoscope reprocessor to clean and high-level disinfect the
endoscope including all channels, contact the endoscope
reprocessor’s manufacturer for specific instructions and/or
information on connectors. Insufficient cleaning and
disinfection or sterilization of the endoscope may pose an
infection control risk to the patient and/or operators
performing the next procedure with the endoscope.
• Patient debris and reprocessing chemicals are hazardous.
Wear personal protective equipment to guard against
dangerous chemicals and potentially infectious material.
During cleaning and disinfection or sterilization, wear
appropriate personal protective equipment, such as eyewear,
face mask, moisture-resistant clothing, and
chemical-resistant gloves that fit properly and are long
enough so that your skin is not exposed. Always remove
contaminated personal protective equipment before leaving
the reprocessing area.
• Thoroughly rinse off the disinfectant solution. Rinse the
external surfaces of the endoscope, channels, and cleaning
equipment thoroughly with clean water to remove any
disinfectant solution residue.
• The disinfection/sterilization room must be adequately
ventilated. Adequate ventilation protects against the buildup
of toxic chemical fumes.
• Store alcohol in an airtight container. Alcohol stored in an
open container is a fire hazard and will lose its efficacy due to
evaporation.
• Be sure to perform a leakage test on the endoscope prior to
manual cleaning, and do not use the endoscope if a leak is
detected. Use of an endoscope with a leak may cause a
sudden loss of the endoscopic image, damage to the
bending mechanism or other malfunctions.
• Prior to each procedure, confirm that the endoscope has
undergone proper cleaning, disinfection, and sterilization. If it
is determined that the endoscope has not been properly
reprocessed, reprocess it again following the instructions
given in this manual.
78
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 85

Chapter 5 Reprocessing: General Policy
• With the cleaning, disinfection, and sterilization methods
stated in this instruction manual, prions, which are
considered to be the pathogenic substance of the
Creutzfeldt-Jakob disease (CJD) cannot be destroyed or
inactivated. When using this instrument on a patient with CJD
or variant Creutzfeldt-Jakob disease (vCJD), be sure to use
this product for such patient only and/or immediately dispose
of this product after use in an appropriate manner. For
methods to handle CJD, please follow the respective
guidelines in your country.
• This instrument is not durable, or does not have sufficient
durability against the respective methods stated in the
guidelines of each country for destroying or inactivating
prions. For information on the durability against each
method, please contact Olympus. If cleaning, disinfection,
and sterilization methods not stated in this instruction manual
are performed, Olympus cannot guarantee the effectiveness,
safety and durability of this instrument. Make sure to confirm
that there is no irregularity before use, and use under
responsibility of a physician. Do not use if any irregularity is
found.
• When aerating or irrigating the endoscope channels, the air
or water pressure must not exceed 0.2 MPa (2 kgf/cm
2
,
29 psig). Higher pressures may cause damage to the
endoscope.
• When reprocessing an endoscope, confirm that the two
water-resistant caps are securely attached to the endoscope
connector before immersion in reprocessing fluids. If two
water-resistant caps are not securely attached, water,
detergent solution and/or disinfectant solution could enter the
endoscope and damage the equipment.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
79
Page 86

Chapter 5 Reprocessing: General Policy
5.4 Reprocessing before the first use/reprocessing and storage after use
This instrument was not cleaned, disinfected, or sterilized before shipment.
Before using this instrument for the first time, reprocess it according to the
instructions given in this manual.
After using this instrument, reprocess and store it according to the instructions
given in this manual. Improper and/or incomplete reprocessing or storage can
present an infection control risk, cause equipment damage, or reduce
performance.
80
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 87

Chapter 6 Compatible Reprocessing Methods and Chemical Agents
Chapter 6 Compatible Reprocessing
Methods and Chemical
Agents
6.1 Compatibility summary
Olympus endoscopic equipment is compatible with several methods of
reprocessing. However, certain components and accessories are not compatible
with some methods, which can cause equipment damage. For appropriate
reprocessing methods, refer to see Table 6.1, the recommendations of your
infection control committee and all national and local hospital guidelines and
policies.
Endoscope
Water-resistant cap
(MH-553)
Chain for water-resistant
cap (MAJ-1739)
Channel cleaning brush
(BW-20T, BW-7L)
Channel-opening cleaning
brush (MH-507)
Air/water valve (MAJ-1444)
Suction valve (MAJ-1443)
Channel plug (MAJ-621)
Injection tube (MH-946)
Air/water channel cleaning
adapter (MAJ-629)
Steam sterilization (autoclaving)
Ethylene oxide gas sterilization (gas mixture 20% ethylene oxide
gas/80% CO
Ethylene oxide gas sterilization (100% ethylene oxide gas)
ACECIDE disinfectant solution
(use OER-A, OER-AW, OER-Pro)
2 – 3.5% glutaraldehyde
70% ethyl or isopropyl alcohol
Detergent solution
Ultrasonic
cleaning
1
2
for countries other than the USA)
2
3
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
81
Page 88

Chapter 6 Compatible Reprocessing Methods and Chemical Agents
Steam sterilization (autoclaving)
Ethylene oxide gas sterilization (gas mixture 20% ethylene oxide
gas/80% CO
Ethylene oxide gas sterilization (100% ethylene oxide gas)
ACECIDE disinfectant solution
(use OER-A, OER-AW, OER-Pro)
2 – 3.5% glutaraldehyde
70% ethyl or isopropyl alcohol
Detergent solution
Ultrasonic
cleaning
Suction cleaning adapter
(MH-856)
Mouthpiece (MB-142)
Washing tube (MH-974)
Cleaning adapter for
instrument channel port
(MAJ-350)
Biopsy valve (MAJ-853)
for countries other than the USA)
2
3
Balloon applicator
(MAJ-675)
Balloon (MAJ-213)
Cleaning brush (MAJ-1534)
Balloon 3 (MAJ-249)
Single use channel
cleaning brush (BW-201T)
Single use channel-opening
cleaning brush (MAJ-1339)
Ultrasonic cable (MAJ-1597)
4
compatible not compatible
Table 6.1
1 The endoscope is compatible with ultrasonic cleaning only when using an
endoscope reprocessor such as OER, OER-A, OER-AW and OER-Pro
(OER, OER-A, OER-AW and OER-Pro may not be available in some
areas).
2 The water-resistant caps and the chain for water-resistant caps can only
be ultrasonically cleaned if connected to the endoscope that is being
cleaned in an endoscope reprocessor with an ultrasonic cleaning phase.
82
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 89

Chapter 6 Compatible Reprocessing Methods and Chemical Agents
3 ACECIDE disinfectant solution is exclusively for an
Olympus-recommended endoscope reprocessor such as OER-A,
OER-AW and OER-Pro (ACECIDE and Acecide-C may not be available in
some areas).
4 This item is not available in the USA.
Alcohol is not a sterilant or high-level disinfectant.
• The endoscope is not compatible with steam sterilization
(autoclaving). Reprocessing using steam sterilization will
result in severe equipment damage.
• The ultrasonic cable is not waterproof. Never immerse it in
disinfectant solution or any other fluids.
• The endoscope is compatible with some endoscope
reprocessors such as the ETD
Olympus. Refer to the respective instruction manual for
details on operation. For any other details, please contact
Olympus.
• EndoTherapy accessories that are marked by the words
“AUTOCLAVE” or “AUTOCLAVABLE”, or accessories with a
green model reference label are compatible with steam
sterilization (autoclaving).
6.2 Detergent solution
Use a medical-grade, low-foaming, neutral pH detergent or enzymatic detergent
and follow the manufacturer’s dilution and temperature recommendations.
Contact Olympus for the names of specific brands that have been tested for
compatibility with the endoscope. Do not reuse detergent solutions.
1
system distributed by
1 This product may not be available in some areas.
Excessive detergent foaming can prevent fluid from
adequately contacting internal lumens (e.g., channels).
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
83
Page 90

Chapter 6 Compatible Reprocessing Methods and Chemical Agents
6.3 Disinfectant solution
In the U.S., agents used to achieve high-level disinfection are defined as liquid
chemical germicides registered with the U.S. Food and Drug Administration as
“sterilant/disinfectants” that are used according to the time, temperature and
dilution recommended by the disinfectant manufacturer for achieving high-level
disinfection. These conditions usually coincide with those recommended by the
disinfectant manufacturer for 100% kill of mycobacterium tuberculosis.
In general, 2.0 – 3.5% glutaraldehyde solutions, when used according to the
manufacturer’s instructions for achieving high-level disinfection, are compatible
with Olympus endoscopes. Contact Olympus for the names of specific brands
that have been tested for compatibility with this endoscope.
If disinfectant solution is reused, routinely check its efficacy according to the
manufacturer’s recommendations. Do not use solutions beyond their expiration
date.
6.4 Rinse water
Once removed from disinfectant solution, the instrument must be thoroughly
rinsed with sterile water to remove any disinfectant residue. If sterile water is not
available, clean, potable tap water or water that has been processed (e.g.,
filtered) to improve its microbiological quality may be used.
When nonsterile water is used after disinfection, wipe the endoscope and flush
the channels with 70% ethyl or isopropyl alcohol, then air-dry all internal
channels to inhibit the growth of residual bacteria. Do not reuse rinse water.
Alcohol is not a sterilant or high-level disinfectant.
84
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 91

Chapter 6 Compatible Reprocessing Methods and Chemical Agents
6.5 Ethylene oxide gas sterilization
This instrument and other accessories listed as compatible with ethylene oxide
gas sterilization in Table 6.1 on page 82 can be sterilized by ethylene oxide gas
and aerated within the parameters given in Tables 6.2 and 6.3. When performing
ethylene oxide gas sterilization, follow the cleaning, disinfection, and sterilization
protocols of your hospital and the instruction manuals of the sterilization
equipment.
• Before sterilization, the instrument must be thoroughly
cleaned and dried. Residual moisture may inhibit sterilization.
• The results of sterilization depend on various factors such as
how the sterilized instrument was packed or the positioning,
method of placing and loading of the instrument in the
sterilization device. Please verify the sterilization effects by
using biological or chemical indicators. Also follow the
guidelines for sterilization issued by medical administrative
authorities, public organizations or the infection management
sections at each medical facility, as well as the instruction
manual for the sterilization device.
• All instruments must be properly aerated following ethylene
oxide gas sterilization to remove toxic ethylene oxide
residuals.
• Exceeding the recommended parameters may cause
equipment damage (see Tables 6.2 and 6.3).
• Disconnect the water-resistant caps from the endoscope
connector before ethylene oxide gas sterilization. If the
water-resistant cap is attached during ethylene oxide gas
sterilization, the air inside the endoscope will expand and
rupture the covering of the bending section and/or damage
the angulation mechanism.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
85
Page 92

Chapter 6 Compatible Reprocessing Methods and Chemical Agents
Parameters for 100% ethylene oxide gas sterilization
cycles
Process phase Parameter Value
Sterilization Temperature
Vac uum
(Absolute pressure)
Relative humidity 50 – 80%
Ethylene oxide gas
concentration
Exposure time 60 minutes
Aeration Minimum aeration
parameters
55
C (130F)
0.05 – 0.07 MPa
(7.25 – 10.15 psia)
0.735 – 0.740 mg/cm
(735 – 740 mg/L)
12 hours in an aeration chamber at
50 – 57
C (122 – 135F) or 7 days at
room temperature
3
Table 6.2
Parameters for 20% ethylene oxide gas/80% CO2 gas
sterilization cycles, for countries other than the USA
Process phase Parameter Value
Sterilization Temperature
Relative pressure 0.1 – 0.17 MPa
Relative humidity 55%
Ethylene oxide gas
concentration
Exposure time 105 minutes
57C (135F)
0.6 – 0.7 mg/cm
(600 – 700 mg/L)
3
86
Aeration Minimum aeration
parameters
12 hours in an aeration chamber at
50 – 57
room temperature
Table 6.3
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
C (122 – 135F) or 7 days at
Page 93

Chapter 6 Compatible Reprocessing Methods and Chemical Agents
6.6 Steam sterilization (autoclaving) of accessories
The accessories listed as compatible with steam sterilization (autoclaving) in
Table 6.1 on page 82 can be sterilized by steam within the parameters given in
Table 6.4. When steam sterilizing, follow the cleaning, disinfection, and
sterilization protocols of your hospital as well as the instructions provided by the
manufacturer of your sterilization equipment.
The results of sterilization depend on various factors such as
how the sterilized instrument was packed or the positioning,
method of placing and loading of the instrument in the
sterilization device. Please verify the sterilization effects by
using biological or chemical indicators. Also follow the
guidelines for sterilization issued by medical administrative
authorities, public organizations or the infection management
sections at each medical facility, as well as the instruction
manual for the sterilization device.
• Do not steam sterilize the endoscope. Steam sterilization
(autoclaving) will severely damage the endoscope.
• Do not exceed a setting temperature of 134C (273F),
equivalent to a maximum temperature of 137C (279F), nor
an exposure time greater than 20 minutes. Otherwise, the
accessories may be damaged.
Process Parameters
Prevacuum Temperature
Exposure time 5 minutes
Table 6.4 Steam sterilization (autoclaving) exposure parameters
132 – 134C
(270 – 274
F)
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
87
Page 94

Chapter 7 Cleaning, Disinfection, and Sterilization Procedures
Chapter 7 Cleaning, Disinfection, and
Sterilization Procedures
ALL channels of the endoscope, including the elevator wire
channel and balloon channel, MUST be cleaned and
high-level disinfected or sterilized during EVERY
reprocessing cycle, even if the channels were not used
during the previous patient procedure. Otherwise, insufficient
cleaning and disinfection or sterilization of the endoscope
may pose an infection control risk to the patient and/or
operators performing the next procedure with the endoscope.
• Do not coil the endoscope’s insertion section or universal
cord into a diameter of less than 12 cm. The endoscope can
be damaged if coiled too tightly.
• For proper reprocessing results, do not coil the insertion
section or universal cord with a diameter of less than 40 cm.
If the diameter is less than 40 cm, it will be difficult to insert
the channel cleaning brushes (BW-20T, BW-7L), single use
single-ended cleaning brush (BW-400L), single use
combination cleaning brush (BW-412T) and/or the single use
channel cleaning brush (BW-201T).
7.1 Required reprocessing equipment
Preparation of the equipment
Prior to cleaning and disinfection or sterilization, prepare the equipment shown in
Figure 7.1.
88
Use basins that are at least 40 cm by 40 cm (16” by 16”) in
size and deep enough to allow the endoscope to be
completely immersed.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 95

Chapter 7 Cleaning, Disinfection, and Sterilization Procedures
Cleaning adapter for
instrument channel
port (MAJ-350)
Channel-opening cleaning
brush (MH-507) or
single use channel-opening
cleaning brush
(MAJ-1339
1
)
Biopsy valve
(MAJ-853)
Water-resistant cap
(MH-553, 2 pcs)
Leakage tester
(MB-155)
Suction cleaning adapter
(MH-856)
Washing tube (MH-974)
Channel cleaning
brush (BW-7L
1
) or
single use
single-ended cleaning
brush (BW-400L
1
)
Channel cleaning
brush (BW-20T) or
single use channel
cleaning brush
(BW-201T
1
)
Injection tube
(MH-946)
Channel plug
(MAJ-621)
Air/water channel cleaning
adapter (MAJ-629)
Cleaning brush
(MAJ-1534)
Suction pump
(KV-4, KV-5, SSU-2)
(sold separately)
Maintenance unit
(MU-1) (sold separately)
Light source
(sold separately)
Single use combination
cleaning brush
(BW-412T
1
)
1 These products may not be available in some areas.
• Detergent solution • Large basin with a
• Clean water • Large basin for rinsing
• 70% ethyl or
isopropyl alcohol
• Small containers • Sterile water • Personal protective
tight-fitting lid for
detergent and
disinfectant solution
and leakage testing
• Disinfectant solution
Figure 7.1
• Large basins for
rinsing
• 500 cm
container
•30cm
equipment
• Clean, lint-free cloths
3
(500 ml)
3
(30 ml) syringe • 5 cm3 (5 ml) syringe
• Sterile, cotton swabs
• Soft-bristled brush
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
89
Page 96

Chapter 7 Cleaning, Disinfection, and Sterilization Procedures
Groove
Seal
Venting connector
Metal tip
Shaft
Brush head
Reprocessing equipment parts and functions
For inspection of other equipment than that mentioned below, refer to the
instruction manual for the equipment being used.
Water-resistant cap (MH-553)
The two water-resistant caps are attached to the videoscope cable connector
and the ultrasonic cable connector on the endoscope to protect the connectors
from water penetration during reprocessing. For leakage testing, the venting
connector on the water-resistant cap must be connected to the leakage tester
(MB-155) (see Figure 7.2).
Figure 7.2
Channel cleaning brush (BW-20T, reusable)
The channel cleaning brush is used to brush the inside of the instrument
channel, suction channel, and the interior and/or openings of the suction valve,
air/water valve, AW channel cleaning adapter, and biopsy valve (see Figure 7.3).
Figure 7.3
90
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 97

Chapter 7 Cleaning, Disinfection, and Sterilization Procedures
Tip
Shaft
Caution sticker (OLYMPUS SINGLE USE ONLY)
Brush head
Metal tip
Shaft
Single use channel cleaning brush (BW-201T)
The single use channel cleaning brush is used to brush the inside of the
instrument channel, suction channel, and the interior and/or openings of the
suction valve, AW channel cleaning adapter, and biopsy valve (see Figure 7.4).
Figure 7.4
Channel cleaning brush (BW-7L/reusable)
/Single use single-ended cleaning brush (BW-400L)
The channel cleaning brush or the single use single-ended cleaning brush is
used to clean the balloon channel (see Figure 7.5).
Figure 7.5
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
91
Page 98

Chapter 7 Cleaning, Disinfection, and Sterilization Procedures
Brush handle
Shaft
Brush head
Handle
Shaft
Brush head
Channel-opening cleaning brush (MH-507, reusable)
The channel-opening cleaning brush is used to brush the external surface of the
distal end of the endoscope, the suction cylinder, the irrigation port, and the
instrument channel port (see Figure 7.6).
Figure 7.6
Single use channel-opening cleaning brush (MAJ-1339)
The single use channel-opening cleaning brush is used to brush the external
surface of the distal end of the endoscope, the suction cylinder, the irrigation
port, and the instrument channel port (see Figure 7.7).
Figure 7.7
92
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Page 99

Chapter 7 Cleaning, Disinfection, and Sterilization Procedures
Long bristle row
Short bristle row
Grip section
Connecting end
Weighted end
Cleaning brush (MAJ-1534)
The cleaning brush is used to brush the external surface of the distal end of the
endoscope (see Figure 7.8).
Figure 7.8
Suction cleaning adapter (MH-856)
The suction cleaning adapter is used to aspirate reprocessing fluids from the
distal end of the endoscope through the instrument channel (see Figure 7.9).
Figure 7.9
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
93
Page 100

Chapter 7 Cleaning, Disinfection, and Sterilization Procedures
Notice card
Pakings
Piston
Suction channel tube
Air pipe port
Air/water channel tube
Connector plug
Notice card
Suction channel port
Air/water channel port
Suction channel port
(including filter mesh)
Air/water channel cleaning adapter (MAJ-629)
During precleaning, the air/water channel cleaning adapter is connected to the
air/water cylinder. When the adapter is depressed, water is fed through the
air/water channel. Air is continuously fed when the adapter is not depressed (see
Figure 7.10).
Figure 7.10
Use the air/water channel cleaning adapter only during
precleaning.
Injection tube (MH-946)
The injection tube is used to inject detergent solution, disinfectant solution, water
and alcohol into the air/water channel, instrument channel and suction channel
and to flush air through the channels to expel fluids (see Figure 7.11).
94
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180
Figure 7.11
 Loading...
Loading...