Olympus HX-110QR, HX-610-090, HX-110UR, HX-610-135, HX-610-090L Instructions Manual
...
INSTRUCTIONS
Rotatable Clip Fixing Device
HX-110LR HX-110QR HX-110UR
Clip
HX-610-090 HX-610-135
Long Clip
HX-610-090L
Short Clip
HX-610-090S HX-610-135S
Colored Short Clip
HX-610-090SC
AUTOCLAVABLE


Contents
Contents
Symbols.................................................................. 1
Important Information – Please Read Before Use 2
Intended Use ..................................................................... 2
Instruction Manual ............................................................. 3
User Qualifications............................................................. 3
Instrument Compatibility .................................................... 3
Reprocessing and Storage ................................................ 4
Repair and Modification ..................................................... 4
Signal Words ..................................................................... 5
Warnings, Cautions and Notes .......................................... 5
Chapter 1 Checking the Package Contents..... 7
1.1 Checking the Package Contents .............................. 7
Chapter 2 Instrument Nomenclature and
Specifications................................... 9
2.1 Nomenclature and Functions.................................... 9
2.2 Specifications............................................................ 10
Chapter 3 Preparation,
Inspection and Operation ................ 16
3.1 Preparation ............................................................... 18
3.2 Inspection ................................................................. 19
3.3 Operation .................................................................. 24
Chapter 4 Emergency Treatment...................... 41
4.1 Emergency Treatment .............................................. 41
Chapter 5 Reprocessing.................................... 45
5.1 General Policy .......................................................... 45
5.2 Required Reprocessing Equipment .......................... 49
5.3 Cleaning.................................................................... 51
5.4 Lubrication ................................................................ 53
5.5 Sterilization ............................................................... 54
Rotatable Clip Fixing Device
i

Contents
Chapter 6 Storage .............................................. 57
6.1 Inspection Before Storage ........................................ 57
6.2 Storage ..................................................................... 58
ii
Rotatable Clip Fixing Device
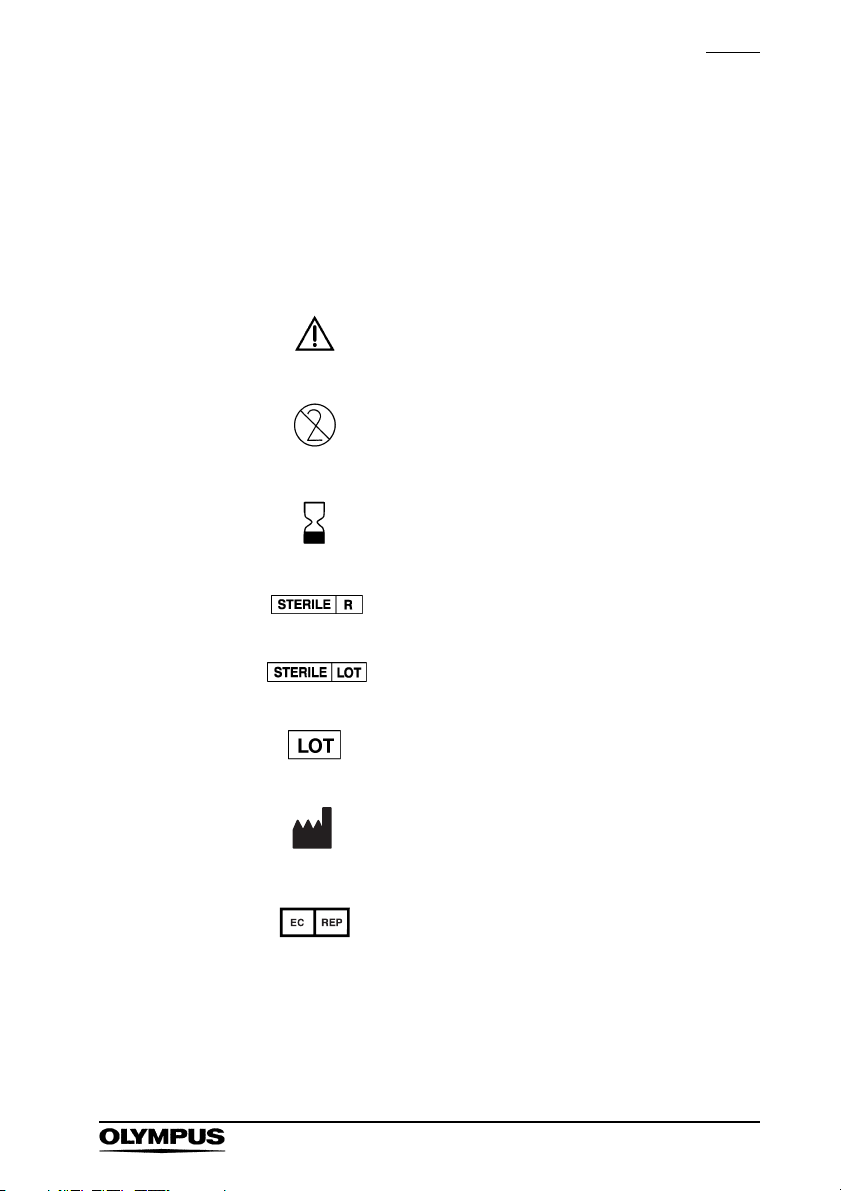
Symbols
Symbols
The meaning(s) of the symbol(s) shown on the package,
the back cover of this instruction manual and/or this
instrument are as follows:
Refer to instructions.
Single use only
Use by (expiration date)
Sterilized using irradiation
Sterilization lot number
Lot number
Manufacturer
Authorised representative in
the European Community
Rotatable Clip Fixing Device
1
Important Information – Please Read Before Use
Important Information – Please
Read Before Use
Intended Use
This instrument has been designed to be used with an
Olympus endoscope for endoscopic clip placement
within the gastrointestinal (GI) tract for the purpose of
(1) endoscopic marking,
(2) hemostasis for
(a) mucosal/sub-mucosal defects < 3 cm,
(b) bleeding ulcers,
(c) arteries < 2 mm,
(d) polyps < 1.5 cm in diameter,
(e) diverticula in the colon,
(3) as a supplementary method, closure of GI tract
luminal perforations < 20 mm that can be treated
conservatively.
Do not use this instrument for any purpose other than its
intended uses.
Due to the range of procedures, the
indications for use of this instrument
should be evaluated by the physician,
taking into account factors such as the
anatomical site, histology, lesion type
and the patient’s condition. In addition,
the cautions and notes contained in
“Warnings, Cautions and Notes” on
page 5 should be thoroughly reviewed
before starting the procedure.
2
Rotatable Clip Fixing Device

Instruction Manual
This instruction manual contains essential information on
using this instrument safely and effectively. Before use,
thoroughly review this manual and the manuals of all
equipment which will be used during the procedure and
use the instruments as instructed.
Keep this and all related instruction manuals in a safe,
accessible location.
If you have any questions or comments about any
information in this manual, please contact Olympus.
User Qualifications
The operator of this instrument must be a physician or
medical personnel under the supervision of a physician
and must have received sufficient training in clinical
endoscopic technique. This manual, therefore, does not
explain or discuss clinical endoscopic procedures.
Important Information – Please Read Before Use
Instrument Compatibility
Refer to the tables in Section 2.2, “Specifications” to
confirm that this instrument is compatible with the
ancillary equipment being used. Using incompatible
equipment can result in patient injury or equipment
damage.
Rotatable Clip Fixing Device
3

Important Information – Please Read Before Use
Reprocessing and Storage
This instrument was not sterilized before shipment.
Before using this instrument for the first time, reprocess it
according to the instructions given in Chapter 5,
“Reprocessing”.
After using this instrument, reprocess and store it
according to the instructions given in Chapter 5,
“Reprocessing” and Chapter 6, “Storage”. Improper and/
or incomplete reprocessing or storage can present an
infection control risk, cause equipment damage or
reduce performance.
Clips are shipped in a sterile condition. Store them
following the instructions given in Chapter 6, “Storage”.
Improper storage can present an infection control risk,
cause equipment damage or reduce performance.
All clips are single-use, disposable items that are not to
be reprocessed after use. Do not reuse or attempt to
sterilize them after use.
Repair and Modification
This instrument and clip do not contain any
user-serviceable parts. Do not disassemble, modify or
attempt to repair them; patient or user injury and/or
equipment damage can result.
4
Rotatable Clip Fixing Device
Signal Words
The following signal words are used throughout this
manual:
Important Information – Please Read Before Use
Indicates a potentially hazardous
situation which, if not avoided, could
result in death or serious injury.
Indicates a potentially hazardous
situation which, if not avoided, may result
in minor or moderate injury. It may also
be used to alert against unsafe practices
or potential equipment damage.
Indicates additional helpful information.
Warnings, Cautions and Notes
Follow the warning, cautions and notes described below
when handling this instrument and clip. This information
is to be supplemented by the warnings, cautions and
notes described in each chapter.
• Operation of this instrument is based on
the assumption that open surgery is
possible as an emergency measure if the
clip cannot be detached from the
instrument or if any other unexpected
circumstance takes place. In this case,
refer to Chapter 4, “Emergency
Treatment”.
Rotatable Clip Fixing Device
5
Important Information – Please Read Before Use
• It might be impossible to stop bleeding
• Re-bleeding may occur on the clipping
• Do not use this instrument when
• Do not perform MRI procedures on
depending on the hemorrhage situation
because the clip performance for
hemostasis is limited. Prepare more than
one hemostatic device and select
appropriate hemostatic device or use it
together to respond to different
hemorrhage situations appropriately.
Choose a surgical hemostasis if
necessary.
site, depending on the local condition.
Check the patient for any re-bleeding
after the operation as appropriate.
hemostasis cannot be verified visually
within the endoscopic field of view after
application.
patients who have clips placed within
their gastrointestinal tracts. This could be
harmful to the patient.
• Limited studies indicate that lesions
located in the esophagus and the lesser
curvature of the stomach may be difficult
to treat with a forward-viewing
endoscope.
• Limited studies indicate that the
treatment of esophageal varices may
require clipping in combination with a
sclerosing agent.
• Limited studies indicate that clipping hard
or severely fibrotic lesions to achieve
hemostasis may be more difficult.
6
Rotatable Clip Fixing Device

Important Information – Please Read Before Use
• Limited studies have shown that the
number of clips required for hemostasis
may vary depending upon the anatomical
site, histology, lesion type and patient
condition and history. A sufficient
quantity of clips should be prepared in
consideration of all of these factors prior
to the procedure.
• Limited studies indicate that the
hemostasis clips remain in place for an
average of 9.4 days; re-bleeding may
occur if the clips detach within 24 hours.
• Limited studies indicate that the use of
clips in the presence of bacterial
contamination may potentiate or prolong
infection.
Rotatable Clip Fixing Device
7
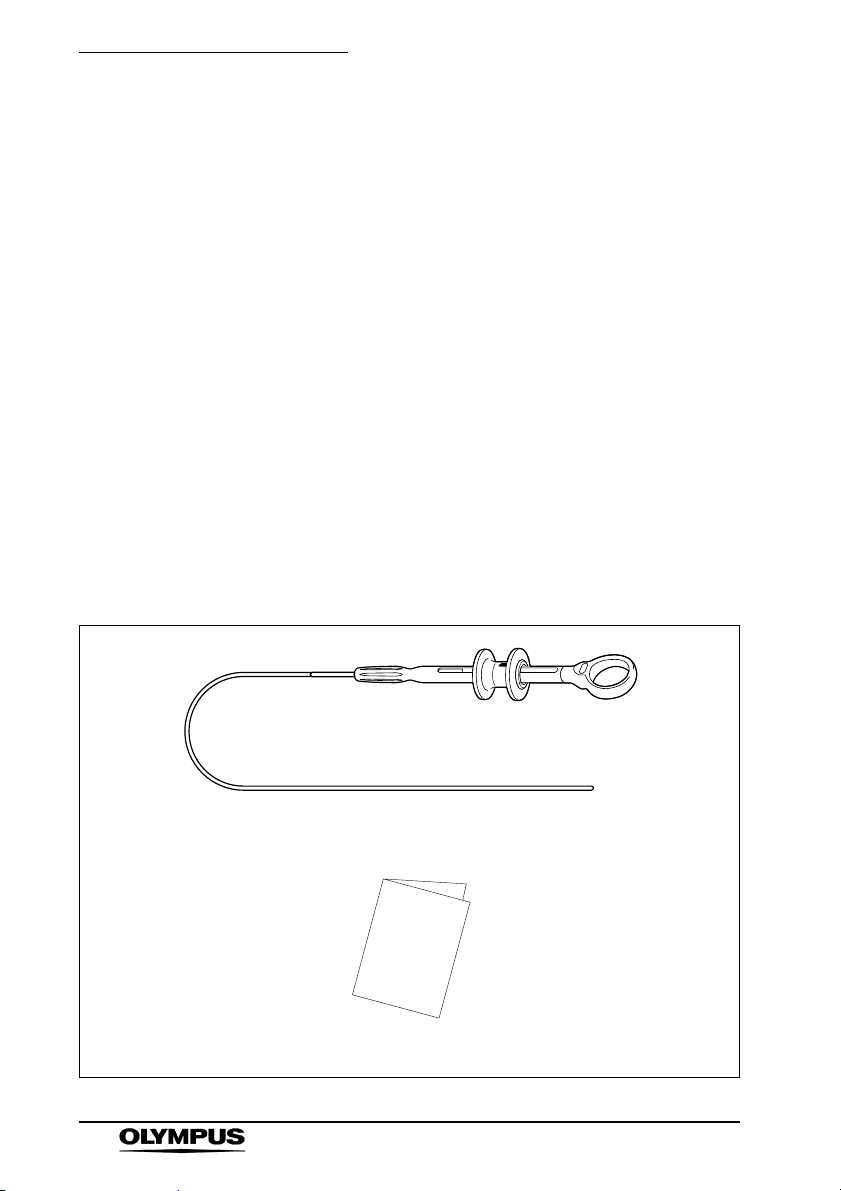
Chapter 1 Checking the Package Contents
Chapter 1 Checking the Package
Contents
1.1 Checking the Package Contents
Match all items in the package with the components
shown below. Inspect each item for damage. If the
instrument or clip is damaged, a component is missing or
you have any questions, do not use the instrument or
clip; immediately contact Olympus.
This instrument was not sterilized before shipment.
Before using this instrument for the first time, reprocess it
according to the instructions in Chapter 5,
“Reprocessing”.
Rotatable Clip Fixing Device
Rotatable clip fixing device (Reusable)
Instruction manual
8
Rotatable Clip Fixing Device
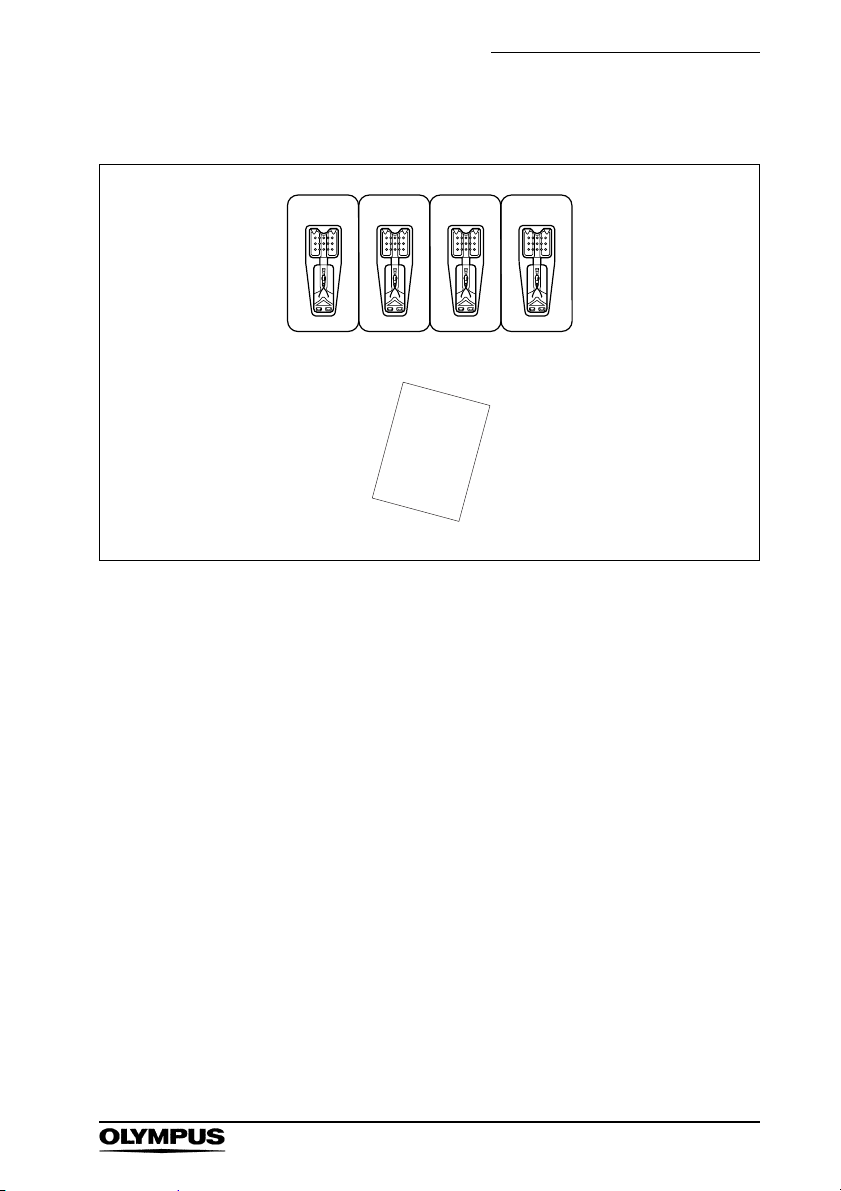
Chapter 1 Checking the Package Contents
Clip (HX-610-090 or HX-610-135)
Clip (Sterile, single use only, 40 pieces)
Access information sheet
Long Clip (HX-610-090L)
Long clip (Sterile, single use only, 40 pieces)
Access information sheet
Short Clip (HX-610-090S or
HX-610-135S)
Short clip (Sterile, single use only, 40 pieces)
Access information sheet
Colored Short Clip (HX-610-090SC)
Colored short clip (Sterile, single use only, 24 pieces)
Access information sheet
Rotatable Clip Fixing Device
9
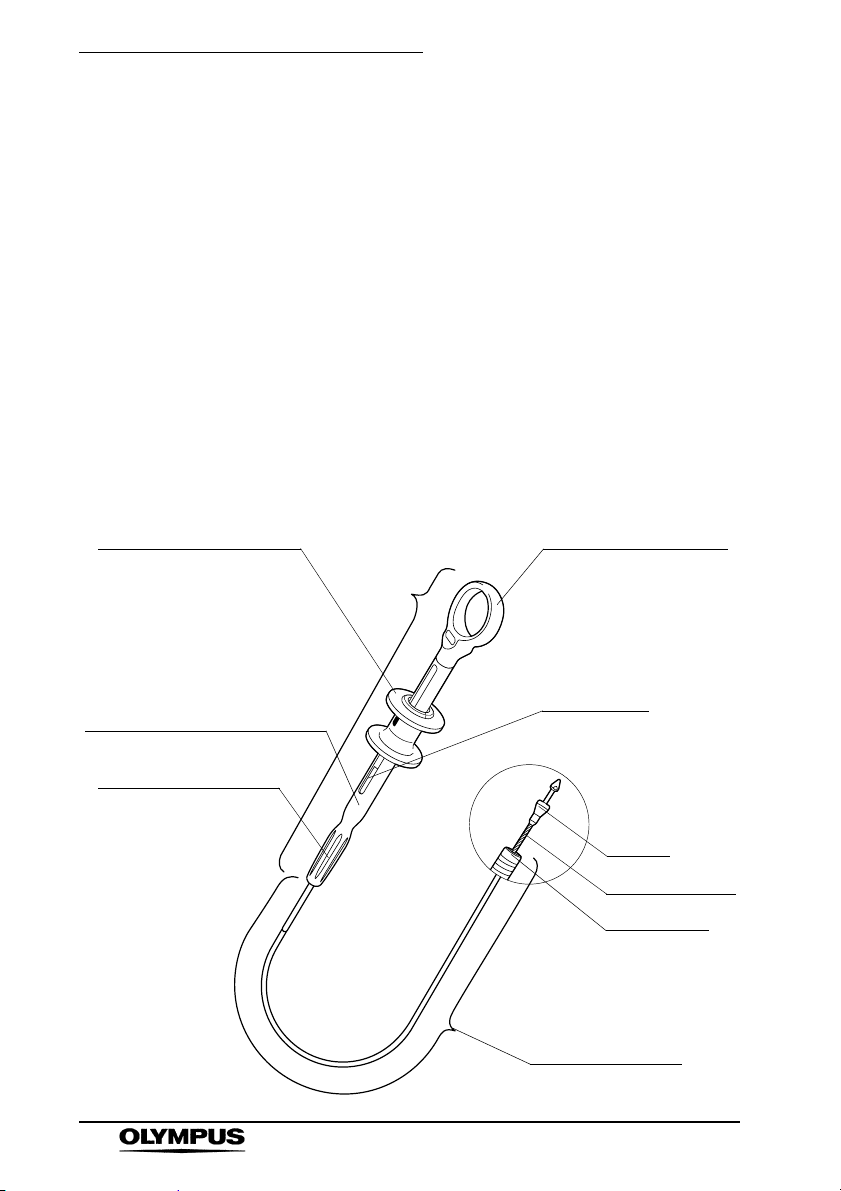
Chapter 2 Instrument Nomenclature and Specifications
Chapter 2 Instrument
Nomenclature and
Specifications
2.1 Nomenclature and Functions
This instrument must be used in combination with the
clip.
Rotatable Clip Fixing Device (Reusable)
Slider
Extends the hook from the
distal end of the coil sheath
when pushed. Retracts the
hook into the coil sheath when
pulled. The green color of this
slider indicates that the
instrument is autoclavable.
Model reference label
Indicates the product number.
Rotation grip
Rotating the rotation grip
will rotate the clip.
Handle
Ring (Yellow)
The color of the ring
indicates the minimum
instrument channel
diameter required for the
endoscope to be
compatible.
Lot number
Distal portion
Hook
Operation wire
Coil sheath
Insertion portion /
Working length
10
Rotatable Clip Fixing Device
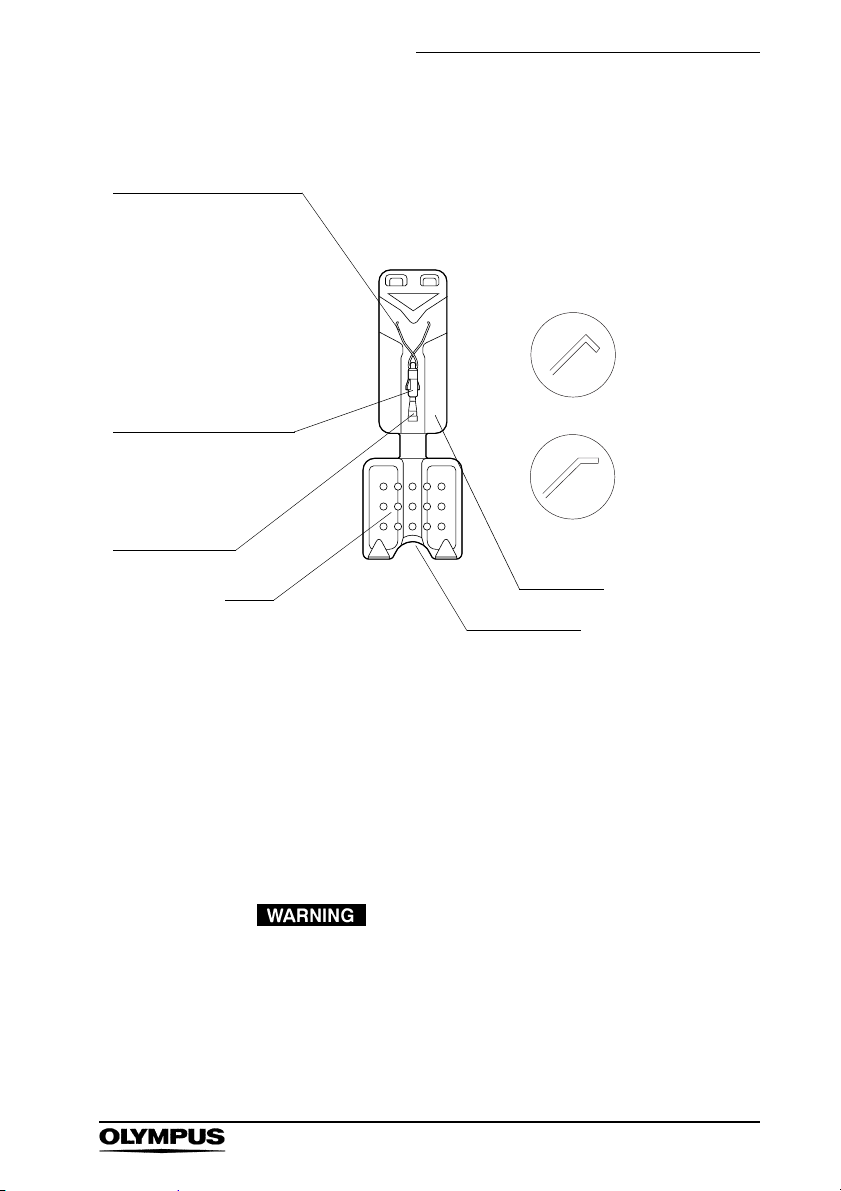
Clip (Single use only)
Clip
The clip of the
HX-610-090SC is coated
to minimize thermal injury
when electrosurgical
accessories are used
after clipping. The
HX-610-090SC is not
guaranteed to be
insulated.
Clip pipe
This part of the
HX-610-090SC will have
a red, yellow or white
coating.
Clip connector
Chapter 2 Instrument Nomenclature and Specifications
Right angle
HX-610-090
HX-610-090L
HX-610-090S
HX-610-090SC
Obtuse angle
HX-610-135
HX-610-135S
Grip
2.2 Specifications
The compatible Olympus endoscopes are listed in the
tables on the following pages. New endoscopes released
after the introduction of this instrument and clip may also
be compatible for use in combination with this instrument
and clip. For further details, contact Olympus.
Rotatable Clip Fixing Device
Cartridge
Insertion port
Use this instrument and clip only in
combination with products recommended
by Olympus. If combined with products
not recommended by Olympus, patient
or operator injury, malfunction or
equipment damage may result.
11

Chapter 2 Instrument Nomenclature and Specifications
Operating Environment
Ambient Temperature 10 to 40°C (50 to 104°F)
Relative Humidity 30 to 85%
Air Pressure 700 to 1060 hPa
Specifications
Model HX-110LR HX-110QR
Shape of the
distal end of
the insertion
portion
Maximum
insertion
portion
diameter
(mm)
Working
length (mm)
Compatible
Olympus
endoscopes
(All of these
parameters
should be met.)
Length and
model
Channel inner
diameter
(mm)
(Color code)
(0.71 to 1.08 kgf/cm
(10.1 to 15.4 psia)
ø 2.75
1650 1950
Working
length
less than 1200
mm;
EF, GIF, OGF,
CF (Exclude I-,
L-length), OSF
ø 2.8, ø 3.2
(Yellow);
ø 3.7, ø 6
(Orange)
2
)
Working
length
less than 1500
mm;
EF, GIF, OGF,
CF (Exclude
L-length),
PCF (I-length
only), OSF
ø 2.8, ø 3.2
(Yellow);
ø 3.7, ø 4.2,
ø 6 (Orange)
12
Rotatable Clip Fixing Device

Chapter 2 Instrument Nomenclature and Specifications
Model HX-110UR
Shape of the
distal end of
the insertion
portion
Maximum
insertion
portion
diameter
(mm)
Working
length (mm)
Compatible
Olympus
endoscopes
(All of these
parameters
should be met.)
Length and
model
Channel inner
diameter
(mm)
(Color code)
EF, GIF, OGF, CF, PCF,
ø 3.7, ø 4.2, ø 6 (Orange)
ø 2.75
2300
Working length
less than 1850 mm;
SIF (SIF-10 only)
ø 2.8, ø 3.2 (Yellow);
Model HX-610-090 HX-610-135
Shape of the clip
Color of the packages Yellow Pink
Clip arm length Standard
General application Hemostasis
Rotatable Clip Fixing Device
13

Chapter 2 Instrument Nomenclature and Specifications
Model HX-610-090L
Shape of the clip
Color of the packages Blue
Clip arm length Long
General application Hemostasis (for large tissue
Model HX-610-135S
Shape of the clip
Color of the packages Green
Clip arm length Short
General application Hemostasis (for smaller tissue
retention)
retention)
14
Rotatable Clip Fixing Device

Chapter 2 Instrument Nomenclature and Specifications
Model HX-610-090SC HX-610-090S
Shape of the clip
Color of the packages Red
White
Yell ow
Clip arm length Short
General application Marking (for smaller tissue
retention)
White
Medical Device
Directive
This device complies with
the requirements of Directive
93/42/EEC concerning
medical devices.
Classification: Class II a
Rotatable Clip Fixing Device
15

Chapter 3 Preparation, Inspection and Operation
Chapter 3 Preparation,
Inspection and
Operation
The clips were shipped in a sterile condition.
• Do not use the clips after the expiration
date displayed on the sterile package.
Doing so may pose an infection control
risk or cause tissue irritation.
• Before each case, prepare and inspect
the instrument and clip as instructed
below. Inspect other equipment to be
used with the instrument and clip as
instructed in their respective instruction
manuals. Should the slightest irregularity
be suspected, do not use the instrument
or clip; contact Olympus.
Damage or irregularity may compromise
patient or user safety by, for example:
posing an infection control risk, causing
tissue irritation, perforation, bleeding or
mucous membrane damage. It may also
result in more severe equipment
damage.
• The instrument was not sterilized before
shipment. Before using the instrument for
the first time, reprocess it according to
the instructions in Chapter 5,
“Reprocessing”.
Do not use an instrument that has not
been cleaned and sterilized. This poses
an infection control risk or can cause
tissue irritation.
16
Rotatable Clip Fixing Device
 Loading...
Loading...