Page 1
First Edition:
Printed:
OPERATOR’S MANUAL
TRANSMITTER
ZS-910PA
0614-008543
Page 2
TRANSMITTER
ZS-910PA
0614-008543
Page 3
Model: ZS-910PA
Manual code no.: 0614-008543
Reader Comment Card
We welcome your comments about this manual. Your comments and suggestions
help us improve our manuals. Please circle the number for each of the following
statements corresponding to your evaluation and add comments in the space
provided.
Fax or send your completed comment card to:
Fax: +81 (3) 5996-8100
International Div ., Sales Promotion Section, Nihon Kohden Corp., 1-31-4, Nishiochiai
Shinjuku-ku, Tokyo 161-8560, Japan
Strongly Agree 1 Disagree 4
cutting line
Agree 2 Strongly Disagree 5
Nuetral 3
This manual is organized. 1 2345
I can find the information I want. 1 2345
The information is accurate. 1 2345
I can understand the instructions. 1 2345
The illustrations are appropriate and helpful. 1 2345
The manual length is appropriate. 1 2345
Comments:
Thank you for your cooperation. We appreciate it very much.
Name:
Occupation/Position:
Hospital/Company:
Address:
Phone:
Page 4

Contents
GENERAL HANDLING PRECAUTIONS.........................................i
WARRANTY POLICY....................................................................iii
EMC RELATED CAUTION .............................................................v
Conventions Used in this Manual and Instrument ....................... vii
Warnings, Cautions and Notes...............................................vii
Explanations of the Symbols in this Manual and Instrument viii
Introduction ......................................................................................... 1
Panel Description................................................................................ 2
Top Panel....................................................................................... 2
Front Panel .................................................................................... 3
Rear Panel .................................................................................... 4
Important Safety Information .............................................................. 5
General.......................................................................................... 5
Battery........................................................................................... 6
For Patients Using Implantable Pacemaker .................................. 7
Output Signal ................................................................................ 7
Electrodes and Electrode Leads ................................................... 8
Maintenance.................................................................................. 8
Preparation ......................................................................................... 9
Installing (Replacing) a Battery ..................................................... 9
Procedure .............................................................................. 10
Situations Requiring Battery Replacement ................................. 10
WARNING and CAUTION for Battery Handling .......................... 11
Attaching a Strap to the Transmitter ............................................ 12
Turning On/Off the Transmitter .................................................... 13
Turning on the power ............................................................. 13
Turning off the power............................................................. 13
Operator's Manual ZS-910PA i
Page 5
Check Items Before Use ............................................................. 14
Appearance ........................................................................... 14
Battery ................................................................................... 14
Channel Setting ..................................................................... 14
Check Items After the Power On ................................................. 14
Power on................................................................................ 14
Basic Operation ..................................................................... 14
Monitoring ......................................................................................... 15
ECG Monitoring.......................................................................16
Measurement Procedure...............................................................17
Selecting Electrode Lead and Disposable Electrode......................17
Standard Accessory................................................................17
Option .....................................................................................17
Connecting the Electrode Lead to the Transmitter..........................18
Selecting the Electrode Position ....................................................19
Three Electrodes .....................................................................19
Two electrodes ........................................................................21
Electrode Position for Respiration Monitoring.................................22
Electrode Position Examples ...................................................22
Connecting the Electrode Lead and Disposable Electrodes ..........24
Prepare the patient skin...........................................................24
Attaching Electrodes to the Patient ................................................24
Using Electrode Lead Tube......................................................25
Detection and Display of Measurement Condition .........................26
Checking Electrodes ...............................................................26
Condition List by Sound ......................................................................27
Troubleshooting...................................................................................28
Check After Use .................................................................................30
Storage....................................................................................30
Lifetime and Disposal..........................................................................31
Disposing of Used Batteries..........................................................31
Replacement ...........................................................................31
Disposal ..................................................................................31
Disposing of Disposable Electrodes..............................................31
ii Operator's Manual ZS-910P A
Page 6
Lifetime....................................................................................31
Disposal ..................................................................................31
Cleaning, Disinfection and Sterilization................................................32
Transmitter and Electrode Lead .....................................................32
Cleaning ..................................................................................32
Disinfection..............................................................................32
Specifications .....................................................................................34
ECG measurement..................................................................34
Transmitter ..............................................................................34
Safety standards .....................................................................34
Water resistance .....................................................................35
Power requirements.................................................................35
Environment ............................................................................36
Dimension and Weight .............................................................36
Standard Accessories ........................................................................37
Options ...............................................................................................38
Operator's Manual ZS-910PA iii
Page 7

GENERAL HANDLING PRECAUTIONS
This device is intended for use only by qualified medical personnel.
Use only Nihon Kohden approved products with this device. Use of
non-approved products or in a non-approved manner may affect the
performance specifications of the device. This includes, but is not
limited to, batteries, recording paper, pens, extension cables,
electrode leads, input boxes and AC power.
Please read these precautions thoroughly before attempting to operate
the instrument.
1. To safely and effectively use the instrument, its operation must be
fully understood.
2. When installing or storing the instrument, take the following
precautions:
(1) Avoid moisture or contact with water, extreme atmospheric
pressure, excessive humidity and temperatures, poorly ventilated
areas, and dust, saline or sulphuric air.
(2) Place the instrument on an even, level floor. Avoid vibration and
mechanical shock, even during transport.
(3) Avoid placing in an area where chemicals are stored or where there
is danger of gas leakage.
(4) The power line source to be applied to the instrument must
correspond in frequency and voltage to product specifications, and
have sufficient current capacity.
(5) Choose a room where a proper grounding facility is available.
3. Before Operation
(1) Check that the instrument is in perfect operating order.
(2) Check that the instrument is grounded properly.
(3) Check that all cords are connected properly.
(4) Pay extra attention when the instrument is in combination with
other instruments to avoid misdiagnosis or other problems.
Operator's Manual ZS-910PA i
Page 8

(5) All circuitry used for direct patient connection must be doubly
checked.
(6) Check that battery level is acceptable and battery condition is good
when using battery-operated models.
4. During Operation
(1) Both the instrument and the patient must receive continual, careful
attention.
(2) Turn power off or remove electrodes and/or transducers when
necessary to assure the patient’s safety.
(3) Avoid direct contact between the instrument housing and the
patient.
5. To Shutdown After Use
(1) Turn power off with all controls returned to their original positions.
(2) Remove the cords gently; do not use force to remove them.
(3) Clean the instrument together with all accessories for their next
use.
6. The instrument must receive expert, professional attention for
maintenance and repairs. When the instrument is not functioning
properly, it should be clearly marked to avoid operation while it is
out of order.
7. The instrument must not be altered or modified in any way.
8. Maintenance and Inspection:
(1) The instrument and parts must undergo regular maintenance
inspection at least every 6 months.
(2) If stored for extended periods without being used, make sure prior
to operation that the instrument is in perfect operating condition.
(3) Technical information such as parts list, descriptions, calibration
instructions or other information is available for qualified user
technical personnel upon request from your Nihon Kohden
distributor.
ii Operator's Manual ZS-910P A
Page 9

9. When the instrument is used with an electrosurgical instrument,
pay careful attention to the application and/or location of electrodes
and/or transducers to avoid possible burn to the patient.
10. When the instrument is used with a defibrillator, make sure that the
instrument is protected against defibrillator discharge. If not,
remove patient cables and/or transducers from the instrument to
avoid possible damage.
WARRANTY POLICY
Nihon Kohden Corporation (NKC) shall warrant its products against all
defects in materials and workmanship for one year from the date of delivery.
However, consumable materials such as recording paper, ink, stylus and
battery are excluded from the warranty.
NKC or its authorized agents will repair or replace any products which
prove to be defective during the warranty period, provided these products
are used as prescribed by the operating instructions given in the operator’s
and service manuals.
No other party is authorized to make any warranty or assume liability for
NKC’s products. NKC will not recognize any other warranty, either implied
or in writing. In addition, service, technical modification or any other
product change performed by someone other than NKC or its authorized
agents without prior consent of NKC may be cause for voiding this
warranty.
Defective products or parts must be returned to NKC or its authorized
agents, along with an explanation of the failure. Shipping costs must be prepaid.
This warranty does not apply to products that have been modified,
disassembled, reinstalled or repaired without Nihon Kohden approval or
which have been subjected to neglect or accident, damage due to accident,
Operator's Manual ZS-910PA iii
Page 10
fire, lightning, vandalism, water or other casualty, improper installation or
application, or on which the original identification marks have been
removed.
In the USA and Canada other warranty policies may apply.
CAUTION
United States law restricts this device to sale by or on the order
of a physician.
Equipment Authorization Requirement
Operation of this equipment requires the prior coordination with a frequency
coordinator designated by the FCC for the Wireless Medical Telemetry
Service.
iv Operator's Manual ZS-910P A
Page 11
EMC RELA TED CAUTION
This equipment and/or system complies with the International
Standard IEC60601-1-2 for electromagnetic compatibility for
medical electrical equipment and/or system. However, an
electromagnetic environment that exceeds the limits or levels
stipulated in the IEC60601-1-2, can cause harmful interference to
the equipment and/or system or cause the equipment and/or
system to fail to perform its intended function or degrade its
intended performance. Therefore, during the operation of the
equipment and/or system, if there is any undesired deviation
from its intended operational performance, you must avoid,
identify and resolve the adverse electromagnetic effect before
continuing to use the equipment and/or system.
The following describes some common interference sources and
remedial actions:
1.Strong electromagnetic interference from a nearby emitter
source such as an authorized radio station or cellular phone:
Install the equipment and/or system at another location if it is
interfered with by an emitter source such as an authorized
radio station. Keep the emitter source such as cellular phone
away from the equipment and/or system.
2.Effect of direct or indirect electrostatic discharge:
Make sure all users and patients in contact with the equipment
and/or system are free from direct or indirect electrostatic
energy before using it. A humid room can help lessen this
problem.
3.Electromagnetic interference with any radio wave receiver
such as radio or television:
If the equipment and/or system interferes with any radio wave
receiver, locate the equipment and/or system as far as
possible from the radio wave receiver.
Operator's Manual ZS-910PA v
Page 12
If the above suggested remedial actions do not solve the
problem, consult your Nihon Kohden Corporation subsidiary or
distributor for additional suggestions.
Conventions Used in this Manual and Instrument
Warnings, Cautions and Notes
Warnings, cautions and notes are used in this manual to alert or signal the
reader to specific information.
WARNING
A warning alerts the user to possible injury or death associated
with the use or misuse of the instrument.
CAUTION
A caution alerts the user to possible injury or problems with the
instrument associated with its use or misuse such as instrument
malfunction, instrument failure, damage to the instrument, or
damage to other property.
NOTE
A note provides specific information, in the form of
recommendations, prerequirements, alternative methods or
supplemental information.
vi Operator's Manual ZS-910P A
Page 13
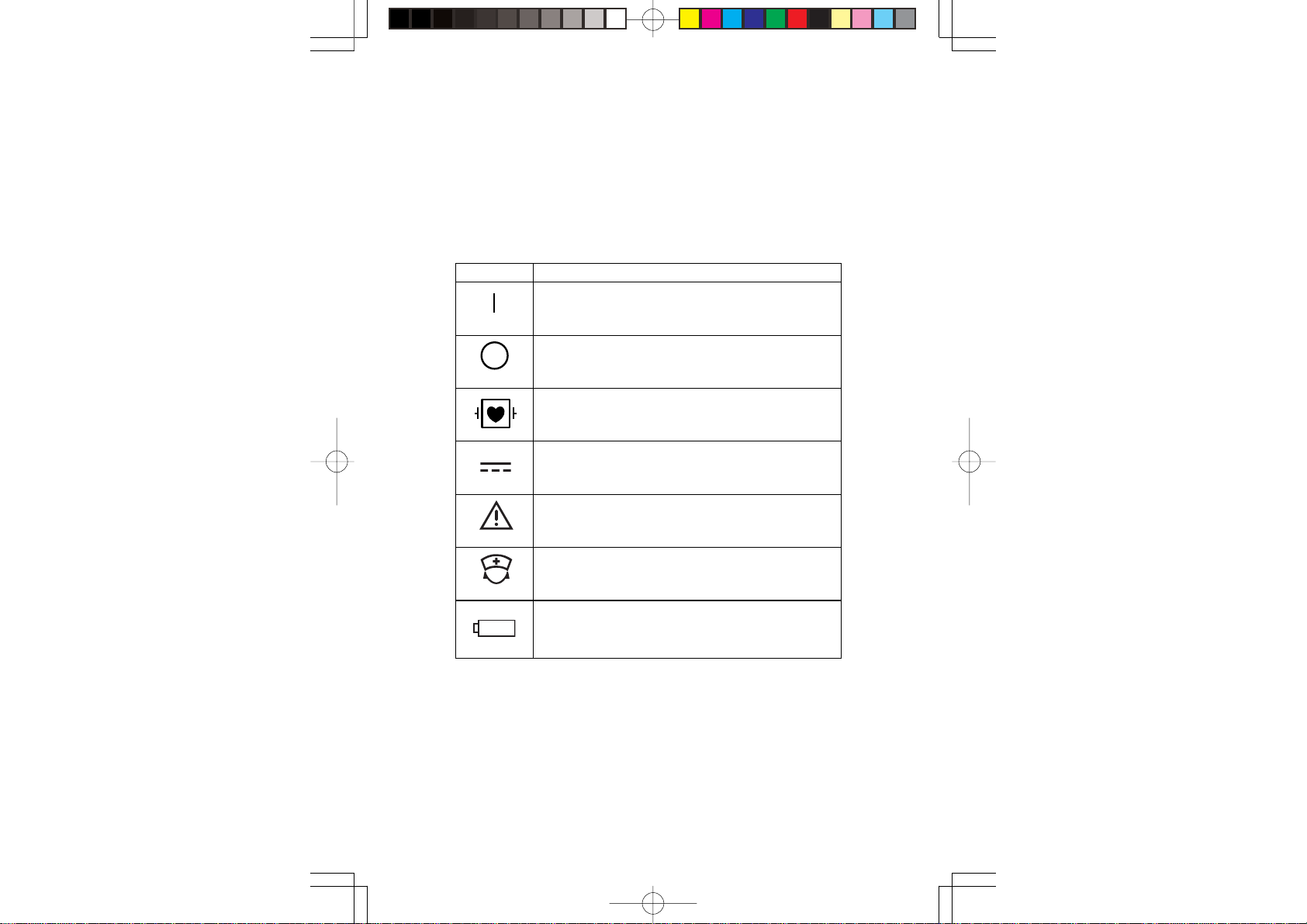
Explanations of the Symbols in this Manual and Instrument
The following symbols found in this manual/instrument bear the
respective descriptions as given.
On Main Unit
Symbol Description
Power ON
Power Off
Defibrillation proof type CF applied part
DC
Attention, consult operator’s manual
Nurse call
+
Operator's Manual ZS-910PA vii
Page 14
Introduction
The ZS-910PA transmits ECG from a patient to a Nihon Kohden monitor.
The transmitter can change channels when connected to the QI-901PK
channel writer. Read the operator’s manual for the monitor together with this
manual before operation.
CAUTION
••
• Do not use the same channel for different patients. Otherwise,
••
two patients’ data will be lost due to mutual modulation
interference, or another patient’s data may appear on the
receiving monitor screen.
••
• Do not use transmitters of adjacent channels in a hospital.
••
Otherwise, radio waves from one transmitter affects the receiver
of the adjacent channel’s transmitter and there may be
interference.
NOTE
••
• To prevent interference between channels, assign a channel
••
administrator in the hospital and only he or she should manage
channel assignment.
••
• Use Nihon Kohden parts and accessories to assure maximum
••
performance from your instrument.
••
• It is recommended to use a diversity antenna system on the
••
receiving monitor for stable signal reception. Otherwise, spike
noise from transient fading of electric field strength (for example,
people moving) may interfere with the transmitter signal and may
be mistaken as an arrhythmia on the receiving monitor.
Operator's Manual ZS-910PA 1
Page 15
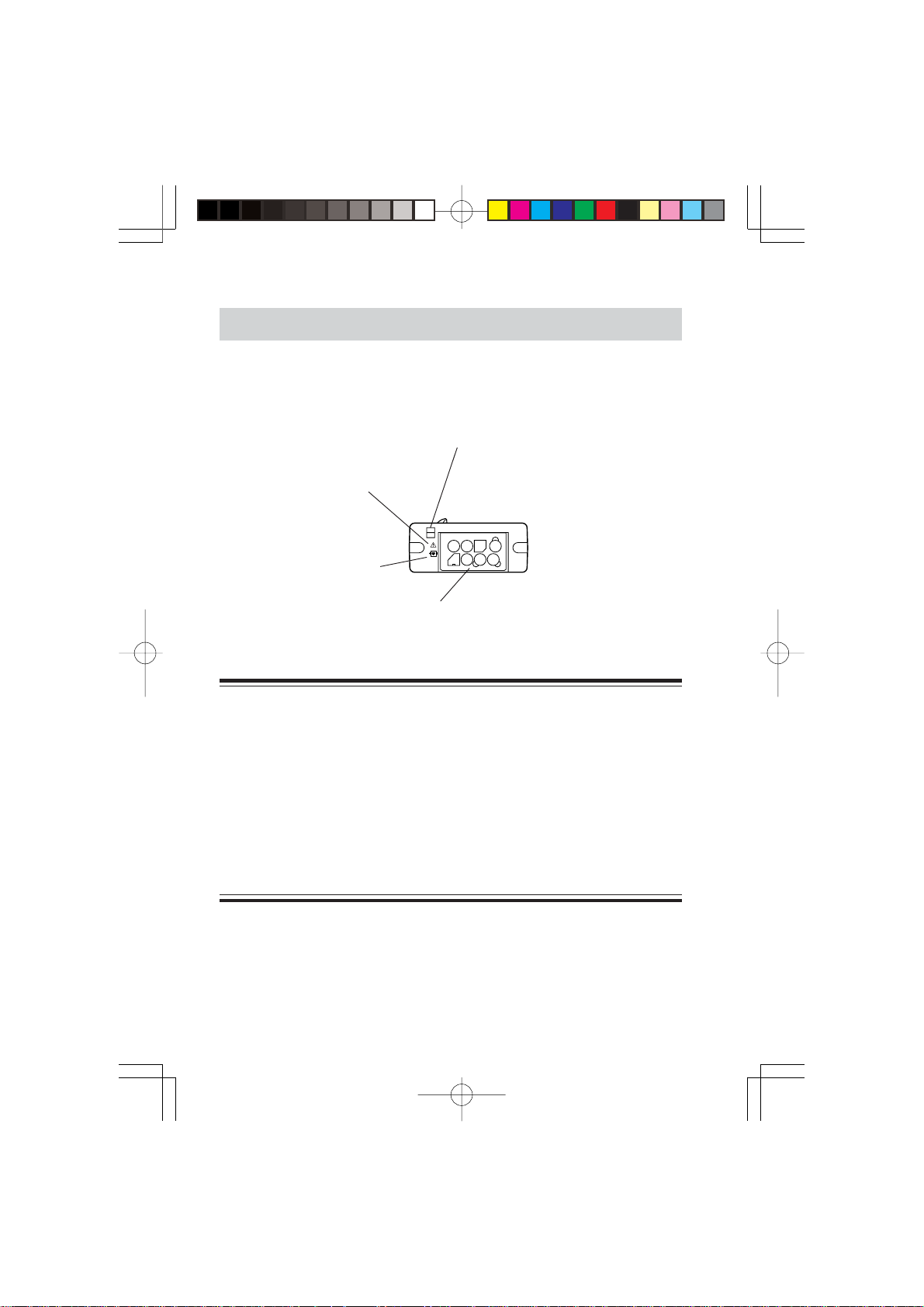
Panel Description
T op Panel
Attach a strap
Refer to the WARNING below.
Defibrillation proof type CF
applied part
Input socket
Connects the electrode lead.
WARNING
••
• Before performing defibrillation, check that the electrode leads
••
attached to the patient are properly connected to the transmitter.
Touching the metal parts of disconnected leads and probes
causes serious electrical shock or injury by discharged energy.
••
• When performing defibrillation, all persons must keep clear of the
••
bed and must not touch the patient, any equipment connected to
the patient or the metal parts of leads connected to the patient.
Failure to follow this warning may result in serious electrical burn,
shock or other injury.
2 Operator's Manual ZS-910P A
Page 16

Front Panel
Battery case
CALL key:
When this key is pressed,
a “peep” sounds at the
transmitter, and a
Channel number label:
Indicates the channel
number of the
transmitter. Attach the
accessory channel
number label to the panel
of the monitor.
CAUTION
Only use your finger to press the CALL key. Do not press the key
with a sharp object. Otherwise the key may be broken.
“CALL” message
appears on the monitor.
Depending on the
settings on the monitor,
an ECG waveform is
recorded when this key is
pressed.
Operator's Manual ZS-910PA 3
Page 17
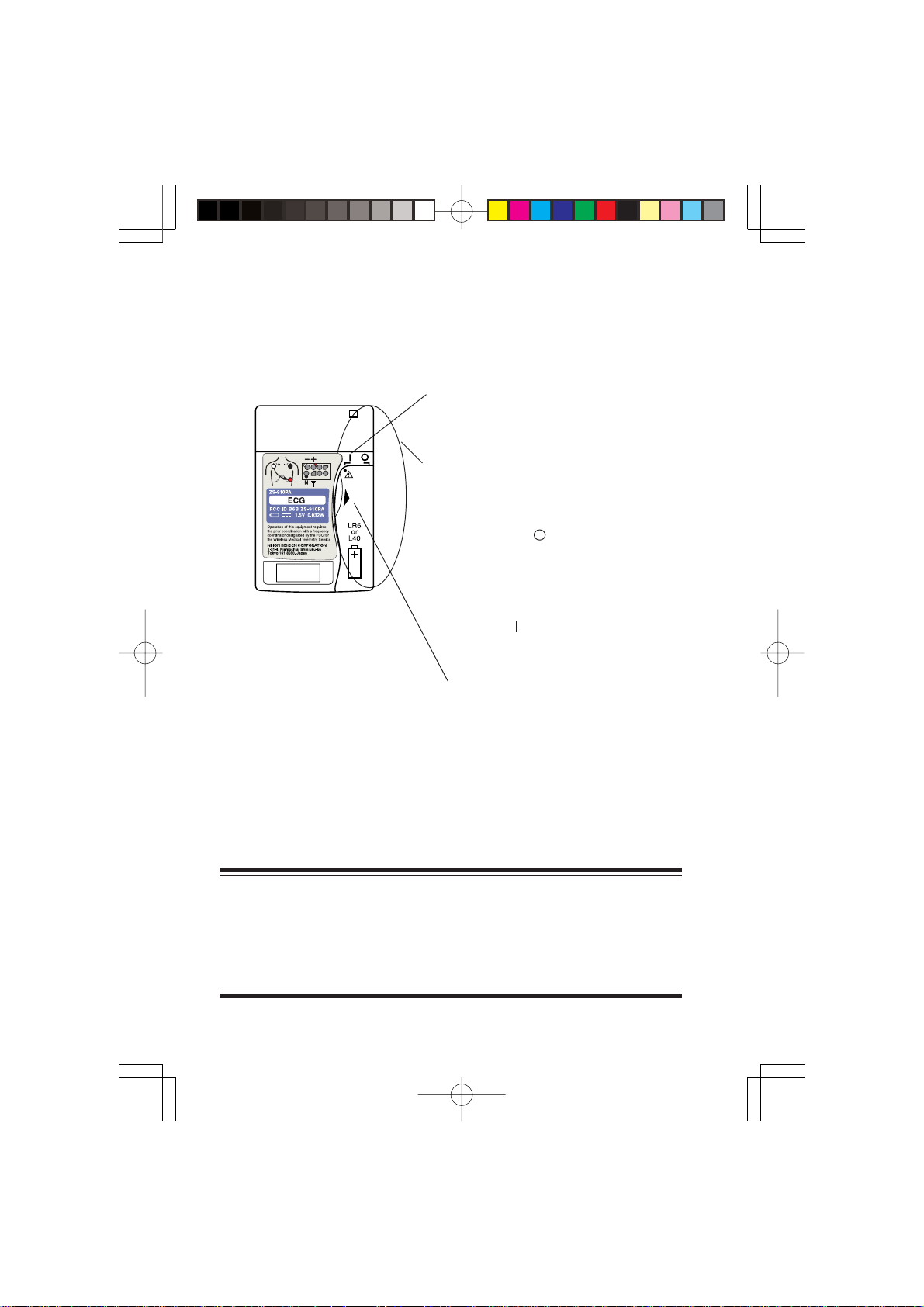
Rear Panel
<ZS-910PA>
Refer to the
CAUTION below.
Battery case/Power switch
Contains a battery.
Opening/closing the battery case cover turns
the instrument power off/on.
The cover opens out in two steps.
Open to one step ( position):
Turns the power off. The battery
cannot come out.
Open to two steps:
The battery can be replaced.
Cover closed ( position):
Turns on the power
Tab for opening and closing the
battery case cover
CAUTION
Battery replacement must be performed by medical staff. When
replacing the battery of the transmitter currently used for a patient,
disconnect electrode leads from the transmitter before replacing the
battery or do not touch the patient during replacement.
4 Operator's Manual ZS-910P A
Page 18

Important Safety Information
General
WARNING
••
• Never use this instrument in the presence of any flammable
••
anesthetic gas or high concentration oxygen atmosphere. Failure
to follow this warning may cause explosion or fire.
••
• Never use this instrument in a high-pressure oxygen medical care
••
tank. Failure to follow this warning may cause explosion or fire.
••
• Do not take this transmitter into the MRI test room. This
••
transmitter is not designed to be used during MRI tests.
••
• Before performing defibrillation, check that the electrode leads
••
attached to the patient are properly connected to the transmitter.
Touching the metal parts of disconnected leads causes serious
electrical shock or injury by discharged energy.
••
• When performing defibrillation, all persons must keep clear of the
••
bed and must not touch the patient, any equipment connected to
the patient or the metal parts of leads connected to the patient.
Failure to follow this warning may result in serious electrical
burn, shock or other injury.
••
• Before performing defibrillation, remove all electrodes and gel
••
from the chest of the patient. If the defibrillator touches
electrodes or gel, the discharged energy may burn the patient’s
skin.
••
• When using this instrument with an ESU, refer to the instruction
••
manual for the ESU. Before measurement, check that the return
plate is correctly attached to the patient and check that the
instrument operates correctly when using with the ESU. If the
return plate is not attached correctly, it may burn the patient’s
skin where the electrodes are attached.
Operator's Manual ZS-910PA 5
Page 19

CAUTION
••
• Attach a strap to the transmitter to prevent it from falling.
••
••
• Turn off the power of cellular telephones, small wireless devices
••
and other devices which produce strong electromagnetic
interference around a patient (except for PHS telephones allowed
by the hospital administrator). Otherwise, radio waves from
devices such as cellular telephones or small wireless devices
may be mistaken as respiration waves and the displayed data may
be incorrect.
••
• Do not use the same channel for different patients. Otherwise,
••
two patients’ data will be lost due to mutual modulation
interference, or another patient’s data may appear on the
receiving monitor screen.
••
• Do not use transmitters of adjacent channels in a hospital.
••
Otherwise, radio waves from one transmitter affect the receiver of
the adjacent channel’s transmitter and there may be interference.
Battery
WARNING
••
• Do not dispose of the battery in fire, or it may explode.
••
••
• Do not disassemble the battery. The contents of the battery are
••
harmful and the battery may catch fire.
••
• Never short-circuit the
••
overheat and catch fire.
••
• Take care that the patient does not swallow batteries.
••
6 Operator's Manual ZS-910P A
+ +
−−
+ and
− terminals. The battery may
+ +
−−
Page 20

CAUTION
Battery replacement must be performed by medical staff. When
replacing the battery of the transmitter currently used for a patient,
disconnect electrode leads from the transmitter before replacing the
battery or do not touch the patient during replacement.
For Patients With an Implantable Pacemaker
WARNING
Interaction Between Minute Ventilation Rate-Adaptive Pacemakers
and Cardiac monitoring and Diagnostic Equipment
The bioelectric impedance measurement sensor of a minute
ventilation rate-adaptive implantable pacemaker may be affected by
the transmitter which is connected to the same patient. If this
occurs, the pacemaker may pace at its maximum rate and the
transmitter may give incorrect data to the monitor. If this occurs,
disconnect the electrode leads from the patient or change the
setting on the pacemaker by referring to the pacemaker’s manual.
For more details, contact your pacemaker distributor or Nihon
Kohden distributor.
Output Signal
CAUTION
Do not use the output signal from the receiving monitor as the
synchronization signal for other equipment such as IABP, MRI,
echocardiography or defibrillation because there may be time delay
between the monitor and the other equipment caused by waveform
transmission delay and spike noise may interfere on the output
signal and be mistaken as a trigger.
Operator's Manual ZS-910PA 7
Page 21

Electrodes and Electrode Leads
CAUTION
••
• Use Nihon Kohden specified electrodes and electrode leads. With
••
electrodes and electrode leads other than specified ones, the
message indicating checking electrodes appears and monitoring
may stop.
••
• Do not reuse disposable products.
••
••
• Do not shake or swing the transmitter holding the leads/cables
••
connected to the transmitter. The transmitter may come off and
injure a person or damage surrounding instruments.
••
• When the message indicating checking electrodes is displayed on
••
the receiving monitor, check electrodes and electrode leads and
remove the cause.
While the message is displayed, there is no ECG monitoring and
no alarms.
Maintenance
WARNING
If detergents or dirty liquid get on the transmitter, clean it and dry it
completely before use. If a wet transmitter is used, the patient or
anyone in contact with the transmitter may receive an electric
shock.
CAUTION
Do not disassemble the transmitter when performing maintenance
and inspection. Do not repair the transmitter. When there is any
problem with the transmitter after maintenance and inspection,
contact your Nihon Kohden distributor.
8 Operator's Manual ZS-910P A
Page 22

Preparation
Installing (Replacing) a Battery
Use one AA type alkaline dry cell battery. Manganese dry cell battery, NiCd
rechargeable battery or NiMH battery can also be used.
With a new alkaline battery, the ZS-910PA transmitter can continuously
measure ECG for approximately 5 days.
CAUTION
Battery replacement must be performed by medical staff. When
replacing the battery of the transmitter currently used for a patient,
disconnect electrode leads from the transmitter before replacing the
battery or do not touch the patient during replacement.
NOTE
••
• T ell the patient not to open or close the battery case cover.
••
••
• Insert the battery with the correct polarity (+ and
••
If electrode leads are attached to the patient and a person replacing the
battery touches the patient during battery replacement, patient leakage current
over the amount allowed for the defibrillation proof type CF applied part may
flow.
−−
−).
−−
Operator's Manual ZS-910PA 9
Page 23

Procedure
1. Open the battery case cover by pulling the tab
until it clicks twice and until the cover stops.
The cover opens two steps.
When replacing the battery, take out the old
battery.
2. Insert one alkaline dry cell battery (LR6) into the
battery case observing the correct polarity.
NOTE
Do not use the transmitter if the battery case
cover is lost.
3. Close the cover. Confirm that there is a “peep”
sound for about one second.
Situations Requiring Battery Replacement
Replace the batteries when any of the following occurs:
• With the power ON, the transmitter generates a constant alarm (continuous
high-pitched sound).
• The receiving monitor displays the battery replacement message on the
screen.
• The transmitter does not generate a “peep” sound when the power is turned
on (when the battery case cover is closed).
10 Operator's Manual ZS-910P A
Page 24

WARNING and CAUTION for Battery Handling
WARNING
••
• Do not dispose of the battery in fire, or it may explode.
••
••
• Do not disassemble the battery. The contents of the battery are
••
harmful and the battery may catch fire.
••
• Never short-circuit the
••
overheat and catch fire.
••
• Take care that the patient does not swallow batteries.
••
When the transmitter is not in use, remove the battery or turn the
power OFF. With the power ON, battery power is consumed even
if measurement is not performed.
Especially, when NiCd or NiMH batteries remain in the transmitter
when the transmitter is not in use, the battery may become
unusable from overdischarge and leak liquid which will damage
the transmitter.
••
• Remove the battery before disposing of the transmitter.
••
••
• The capacity of manganese, NiCd and NiMH batteries is less than
••
that of alkaline batteries and the battery lifetime is shorter.
+ +
−−
+ and
− terminals. The battery may
+ +
−−
CAUTION
NOTE
Type Lifetime
Manganese About 1/2 of alkaline batteries
NiCd About 1/3 of alkaline batteries
NiMH About 1/2 of alkaline batteries
••
• When using rechargeable NiCd batteries or NiMH batteries, shallow
••
charging/discharging shortens battery capacity . For details, refer to
the battery operator’s manual.
Operator's Manual ZS-910PA 11
(when fully charged)
(when fully charged)
Page 25

Attaching a Strap to the Transmitter
CAUTION
Attach a strap to the transmitter to prevent the transmitter from
falling.
NOTE
Do not attach the clip to hard objects such as thick cloth or a zipper.
It will break the clip.
Attach a strap to the transmitter and fasten the clip to the patient clothes’s or bed
sheets.
NOTE
••
• If the transmitter falls off, it may become damaged.
••
••
• If the transmitter falls on the patient foot, it will injure the patient.
••
••
• When the transmitter falls into water or a toilet, clean and
••
disinfect the transmitter.
To open the clip, firmly pull out the
tab in the direction of the arrow.
12 Operator's Manual ZS-910P A
Page 26

Turning the Transmitter On/Off
The transmitter power is turned on or off by closing or opening the battery
case cover.
Turning on the power
To turn on the power, close the battery case cover. After about a one second
“peep” sound, the power is turned on and transmission starts.
Turning off the power
There are two ways of turning off the power.
Normal power off
1. Open the battery case cover until it clicks twice and until the cover stops.
2. Take out the battery and close the cover. When not using the transmitter,
remove the battery and store the transmitter.
Temporary power off
Open the battery case cover until it clicks once. At this position, the battery
does not fall out if the transmitter is upside down.
Operator's Manual ZS-910PA 13
Page 27

Check Items Before Use
Before turning on the transmitter power switch, check the following to
confirm that the transmitter can be used in normal and safe condition.
Appearance
• There are no damaged or dirty points on the outside of the transmitter and
the CALL key.
• The battery case cover is not lost.
• The transmitter is completely dry.
• The electrode lead is not broken.
• There are no damaged or dirty points on the disposable electrodes.
Battery
• The battery polarity is correct.
• The battery case spring is firmly fixed and the battery is not loose.
Channel Setting
• The transmitter channel corresponds to that of the receiving monitor.
• No other transmitter in the surrounding area has the same channel.
Check Items After the Power On
After turning on the power, check the following.
Power on
• There are no broken points on the battery case cover.
• The transmitter generates about a one second “peep” sound.
• The transmitter does not generate a continuous high-pitched sound.
• The transmitter does not produce excessive heat.
• The transmitter does not interfere with the operation of medical instruments
used near it.
Basic Operation
• The “signal loss” message is not displayed on the monitor when the
transmitter is inside the receiving range of the monitor.
• A “peep” sounds at the transmitter and a “CALL” message appears at the
receiving monitor when the CALL key is pressed and the transmitter is
inside the receiving range of the monitor.
• The battery replacement message is not displayed on the monitor.
14 Operator's Manual ZS-910P A
Page 28

Monitoring
WARNING
••
• Interaction Between Minute Ventilation Rate-Adaptive Pacemakers
••
and Cardiac monitoring and Diagnostic Equipment
The bioelectric impedance measurement sensor of a minute
ventilation rate-adaptive implantable pacemaker may be affected
by the transmitter which is connected to the same patient. If this
occurs, the pacemaker may pace at its maximum rate and the
transmitter may give incorrect data to the monitor. If this occurs,
disconnect the electrode leads from the patient or change the
setting on the pacemaker by referring to the pacemaker’s manual.
For more details, contact your pacemaker distributor or Nihon
Kohden distributor.
••
• When using this instrument with an ESU, refer to the instruction
••
manual for the ESU. Before measurement, check that the return
plate is correctly attached to the patient and check that the
instrument operates correctly when using with the ESU. If the
return plate is not attached correctly, it may burn the patient’s skin
where the electrodes are attached.
CAUTION
Turn off the power of cellular telephones, small wireless devices
and other devices which produce strong electromagnetic
interference around a patient (except for PHS telephones allowed
by the hospital administrator). Otherwise, radio waves from
devices such as cellular telephones or small wireless devices
may be mistaken as respiration waves and the displayed data may
be incorrect.
Operator's Manual ZS-910PA 15
Page 29

NOTE
••
• Noise overlaps when the transmitter is used with the ESU, but it
••
does not cause instrument trouble.
••
• If an electric blanket is used and incorrect heart rate is displayed
••
on the receiving monitor, turn off the pacing pulse detection on
the monitor.
ECG Monitoring
This transmitter sends the ECG waveform detected between the R/RA electrode
and F/LL electrode to the monitor. The monitor displays the ECG waveform
and measures heart rate, etc.
Refer to the operator’s manual of the monitor for details.
16 Operator's Manual ZS-910P A
Page 30
Measurement Procedure
1. Select the type of electrode lead and disposable electrode according to the
purpose.
2. Connect the electrode lead to the transmitter.
3. Connect disposable electrodes to the electrode lead and attach electrodes to
the patient.
After steps 1 to 3 are finished, monitoring automatically starts.
Selecting Electrode Lead and Disposable Electrode
CAUTION
Use Nihon Kohden specified electrodes and electrode leads. With
electrodes and electrode leads other than specified ones, the
message indicating checking electrodes appears and monitoring
may stop.
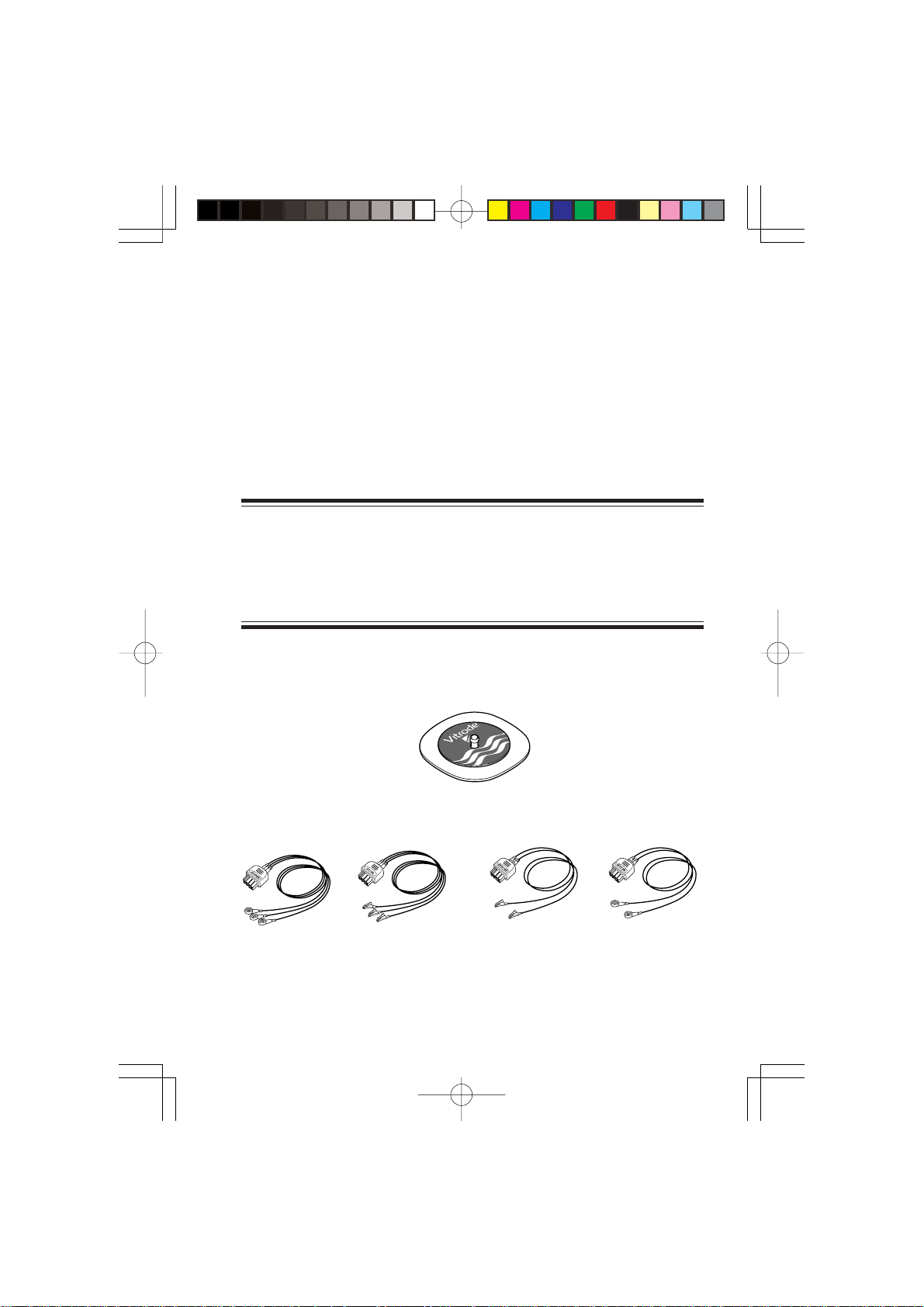
Option
Disposable electrode
Vitrode
Electrode lead
BR-913PA BR-903PA BR-902PA BR-912PA
3 electrodes 3 electrodes, 2 electodes, 2 electrodes,
snap type clip type clip type snap type
Operator's Manual ZS-910PA 17
Page 31

Connecting the Electrode Lead to the Transmitter
Connect the electrode lead to the input socket on the transmitter.
CAUTION
••
• Do not shake or swing the transmitter holding the leads/cables
••
connected to the transmitter. The transmitter may come off and
injure a person or damage surrounding instruments.
••
• Hold the connector of the electrode lead when connecting/
••
disconnecting the electrode lead. If you disconnect the electrode
lead holding the lead, it damages the electrode lead.
18 Operator's Manual ZS-910P A
Page 32

Selecting the Electrode Position
Follow the physician’s instructions for electrode placement when available.
For ECG monitoring, electrodes are attached only on the chest to allow patient
movement and obtain continuous stable ECG. Following leads are examples.
When also monitoring respiration, refer to “Electrode Position for Respiration
Monitoring”.
NOTE
The optimum electrode positions for ECG measurement of a patient
are not always optimum for respiration measurement of the patient.
Select positions suitable for both ECG and respiration
measurements, or positions which have priority for one
measurement.
Three Electrodes
• Lead MII, which is similar to standard lead II, used when ECG measurement
has priority
Symbol Lead ColorElectrode Position
AHA IEC AHA IEC
Left infraclavicular fossa LA L Black Yellow
Right infraclavicular fossa RA R White Red
Below lowest rib on the left
LL F Red Green
anterior axillary line
Operator's Manual ZS-910PA 19
Page 33

• Lead MI, which is similar to standard lead I
Change F/LL and L/LA of the lead MII.
• Lead MIII, which is similar to standard lead III.
Change R/RA and L/LA of the lead MII.
If the electrode position shown above is not available due to chest surgery,
attach the electrodes to the root of the limbs or below the clavicles for stable
ECG monitoring.
20 Operator's Manual ZS-910P A
Page 34

Two electrodes
With the optional BR-912P and BR-902P electrode leads, measurement with
two electrodes is available. The L/LA electrode is not used.
This is effective for a neonate or a patient whose body area is small and difficult
to attach three electrodes.
(ex.) Lead MII, which is similar to standard lead II
Symbol Lead ColorElectrode Position
AHA IEC AHA IEC
Right infraclavicular fossa RA R White Red
Below lowest rib on the left
LL F Red Green
anterior axillary line
Difference between measurement with two electrodes and three electrodes
Measurement with two electrodes is less stable than measurement with three
electrodes because of hum overlapping and body movement. Pay sufficient
attention to this point. If ECG of necessary quality cannot be obtained, measure
with three electrodes.
Operator's Manual ZS-910PA 21
Page 35

Connecting the Electrode Lead and Disposable
Electrodes
Prepare the patient skin
Shave off excessive body hair.
To reduce skin impedance, clean the electrode site with cream or with a gauze
moistened with alcohol. Thoroughly dry the skin with a clean cotton pad.
NOTE
••
• For a patient with frequent body movement, rub the sites with
••
Skinpure skin preparation gel. However, do not use Skinpure skin
preparation gel for sensitive skin.
••
• Do not place electrodes on a wound or on an inflamed, wrinkled or
••
uneven skin surface.
Attaching Electrodes to the Patient
CAUTION
Do not reuse disposable products.
NOTE
••
• To maintain good contact between the electrode and skin, check
••
that the paste of the disposable electrode is not dry.
••
• When contact between the disposable electrode and skin
••
becomes poor, replace electrodes with new ones immediately.
Otherwise, contact impedance between the skin and the electrode
increases and the correct ECG cannot be obtained.
Refer to the electrode operator’s manual for details.
22 Operator's Manual ZS-910P A
Page 36

(ex. Attaching Vitrode C disposable electrode)
1. Connect the electrode lead to the electrode.
2. Carefully remove the backing paper from the
electrode. Avoid touching the adhesive
surface.
3. Place the electrode on the previously cleaned
skin. Pay attention to the electrode lead color
and symbol.
4. Fasten the electrode lead wire with surgical
tape with an extra length of wire between the
tape and the electrode. This lessens the
movement of electrode leads by body
movement and helps stable monitoring.
Using Electrode Lead T ube (Option)
The electrode lead tube can prevent the electrode
leads from getting tangled during monitoring.
During monitoring, slide the tube toward the
transmitter. When not using the transmitter, slide
the tube toward the lead clips or snaps.
electrode lead tube
If the tube comes off the electrode leads easily, bind
the tube with tape to firmly attach it to the
electrode leads.
Operator's Manual ZS-910PA 23
Page 37

Set one electrode lead into one opening of the
tube.
Detection and Display of Measurement Condition
Checking Electrodes
The message indicating checking electrodes is displayed on the screen of the
monitor when:
• An electrode is detached from skin.
• An electrode lead is disconnected.
• Polarization voltage between an electrode and skin is excessively high.
In these cases, check the cause and if necessary, replace electrodes with new
ones.
CAUTION
When the message indicating checking electrodes is displayed on
the receiving monitor, check electrodes and electrode leads and
remove the cause.
While the message is being displayed, there is no ECG monitoring
and no alarms.
NOTE
When the L/LA electrode is detached from the skin or L/LA
electrode lead is disconnected, the message indicating checking
electrodes does not appear on the receiving monitor. If hum
overlaps or waveform is unstable for body movement, check the
L/LA electrode and electrode lead condition.
24 Operator's Manual ZS-910P A
Page 38

Condition List by Sound
Single “peep” sound The CALL key is pressed.
Continuous “peep” sound Battery is completely
One second “peep” sound The power is turned on. ---
Sound Cause Countermeasure
The sound lasts while the
key is pressed.
discharged.
---
Replace the battery
with a new one.
To stop the sound,
turn off the power.
Changing the T ransmitter Channel
The transmitter channel can be changed when the transmitter is connected to
the QI-901PK channel writer. Refer to the operator’s manual of the QI-901PK
channel writer for details.
WARNING
The following action must be taken to properly receive the transmitter
signal of the correct patient on the receiving monitor. Otherwise,
there may be signal loss or signals may mix. This causes a serious
accident, such as monitoring a different patient.
••
• Assign a channel administrator in the hospital and only he or she
••
should manage channel assignment on his or her responsibility .
••
• The channel administrator must manage the channels in the facility
••
so that there is no signal interference.
••
• When the transmitter channel is changed, the channel administrator
••
must check that the channel on the receiving monitor is also
changed and that the signal is properly received.
••
• The channel administrator must replace the channel number label
••
on the transmitter with the new one after changing the channel.
Operator's Manual ZS-910PA 25
Page 39

T roubleshooting
If the problem still remains after checking the following, contact your Nihon
Kohden distributor.
Problem Cause Countermeasure
The power cannot be
turned on.
Nothing is displayed
on the monitor after
turning the transmitter
power on.
Signal receiving
condition is poor.
Batteries are not
installed correctly.
The battery polarity is
wrong.
Batteries are completely
discharged.
The channel of the
transmitter and monitor
does not match.
Electrode lead is not
connected to the
transmitter.
Another transmitter of
the same channel is
used nearby.
Signals are mixing. Follow the instruction of
Transmitter radio wave
is temporarily cut by
people and objects
moving.
The transmitter is
broken.
Install the batteries correctly.
Replace the batteries with
new ones.
Set the correct channel on
the monitor.
Connect the electrode lead
to the transmitter.
Turn the transmitte r power
off. If the monitor still
receives a signal, there is a
high probability that another
transmitter of the same
channel is used nearby.
Follow the instruction of
your channel administrator
and use another tra nsmitter
of a different channel.
your channel administrator
and use another tr a ns m i t ter
of a different channel.
It is recommended to use a
diversity antenna system.
Contact your Nihon Kohden
distributor.
26 Operator's Manual ZS-910P A
Page 40

Problem Cause Countermeasure
ECG baseline is thick.
(Hum is overlapping)
The gel on the elec trode
is dried out.
Replace the electrode with a
new one.
The gel on the elec trode
is coming off.
Electric blanket is used. Cover the blanket with a
shield cover.
Hum filter is set to OFF
Set the filter to ON.
on the monitor
The heart rate of a
patient using an
electric blanket is
The pacing pulse
detection is ON on the
monitor.
Turn off the pacing pulse
detection
incorrect on the
receiving monitor.
The transmitter is
dropped in water or a
toilet
--- Clean and disinfect the
transmitter.
Refer to “Cleaning and
Disinfection” in this manual.
Use the strap to prevent
falling.
Operator's Manual ZS-910PA 27
Page 41
Check After Use
To use the instrument in safe and optimum condition, perform inspection after
use.
CAUTION
Do not disassemble the transmitter when performing maintenance
and inspection. Do not repair the transmitter. When there is any
problem with the transmitter after maintenance and inspection,
contact your Nihon Kohden distributor.
Storage
• ECG electrode leads are cleaned and disinfected.
• When the transmitter gets wet, liquid is wiped off and the transmitter is
thoroughly dried.
• There are enough consumables, such as disposable electrodes.
• The power is turned off.
• The batteries are removed from the transmitter when it will not be used for
a long time.
• Dead batteries are disposed of properly.
28 Operator's Manual ZS-910P A
Page 42

Lifetime and Disposal
Disposing of Used Batteries
Replacement
When the battery replacement message is displayed on the receiving monitor,
the battery are running out.
Replace the battery with a new one. When using a rechargeable battery,
recharge it.
Disposal
NOTE
Remove the battery before disposing of the transmitter.
Before disposing of batteries, check with your local solid waste officials for
details in your area for proper disposal. It may be illegal to dispose of these
batteries in the municipal waste stream.
Disposing of Disposable Electrodes
Lifetime
Replace the disposable electrodes with new ones 48 hours after the start of
usage. Otherwise, the gel on the electrode gets dry and adhesive property
decreases. This increases skin electrode contact impedance and causes incorrect
measurement.
Replace the electrodes with new ones even before 48 hours if the contact
between skin and electrode becomes poor.
Disposal
Follow your local laws for disposing of medical waste.
Operator's Manual ZS-910PA 29
Page 43

Cleaning, Disinfection and Sterilization
Transmitter and Electrode Lead
WARNING
If detergents or dirty liquid get on the transmitter, clean it and dry it
completely before use. If a wet transmitter is used, the patient or
anyone in contact with the transmitter may receive an electric
shock.
CAUTION
••
• Before cleaning or disinfecting the transmitter, remove the battery.
••
••
• The transmitter cannot be sterilized.
••
Cleaning
Wipe the transmitter and electrode leads with a soft cloth moistened with
disinfecting alcohol or neutral detergent diluted with water. If the surface is
very dirty, wash with running water. After cleaning, dry them completely.
Disinfection
CAUTION
••
• Do not immerse the electrode lead connector in liquid.
••
••
• Do not disinfect with hypochlorous acid.
••
••
• Use the recommended concentration.
••
Wipe the outside surface of the transmitter and electrode lead with a nonabrasive cloth moistened with any of the disinfectants listed on the next page.
Use the recommended concentration.
30 Operator's Manual ZS-910P A
Page 44

Disinfectant Concentration (%)
Glutaraldehyde solution 2.0
Hydrochloric alkyl diaminoethylglycine 0.5
Benzalkonium chloride 0.2
Benzethonium chloride solution 0.2
Chlorohexidine gluconate solution 0.5
Operator's Manual ZS-910PA 31
Page 45

Specifications
ECG measurement
Channels: 1
Input range: ±5mV or more
DC offset: ±500mV or more
Input impedance: 5 MΩ or more (5Hz)
Pacing pulse detection: ANSI/AAMI EC13
Based upon pacemaker pulse rejection
capability
Transmitter
FCC regulation: FCC part 95 Subpart-H
Wireless Medical Telemetry Service
(WMTS)
Field strength limits: <200 mV/m(at 3 m)
Undesired emission: below 960 MHz: 200µV/m (at 3 m)
above 960 MHz: 500µV/m (at 3 m)
Antenna: ECG electrode lead
Transmission channel: indicated on the transmitter
Transmission frequency range: 608.0125 to 613.9875 MHz
Channel spacing: 25kHz (12.5 kHz when interleave)
Type of emission: F1D
Occupied bandwidth: <8.5 kHz
Effective radiated power: 1.0 mW (conducted)
Safety standards
Safety standard: CSA C22.2 No.601-1 M90 (1994)
IEC 60601-1 (1988)
IEC 60601-1 Amendment1 (1991)
IEC 60601-1 Amendment2 (1995)
IEC 60601-1-2 (1993)
IEC 60601-2-27 (1994)
32 Operator's Manual ZS-910P A
Page 46

According to the type of protection
against electrical shock: INTERNALLY POWERED
EQUIPMENT
According to the degree of protection
against electrical shock: DEFIBRILLATION-PROOF
TYPE CF APPLIED PART
According to the degree of protection
against harmful ingress of water: Ordinary equipment
According to the degree of safety of
application in the presence of a
FLAMMABLE ANAESTHETIC
MIXTURE WITH AIR, OR WITH
OXYGEN OR NITROUS OXIDE: Equipment not suitable for use in the
presence of FLAMMABLE
ANAESTHETIC MIXTURE WITH AIR,
OR WITH OXYGEN OR NITROUS
OXIDE
According to the mode of operation: CONTINUOUS OPERATION
Water resistance
Water does not get inside the transmitter except for the battery case when
immersed in water up to 30 cm deep for 3 minutes.
Power requirements
Battery type: One AA type alkaline dry cell battery recommended,
One manganese dry cell battery,
One NiCd rechargeable battery,
One NiMH battery
Battery lifetime: ZS-910PA approximately 5 days
(with an alkaline battery)
Operator's Manual ZS-910PA 33
Page 47

Environment
Operating environment
Operating temperature: 5 to 40°C, 41 to 104°F
Operating humidity: 30 to 85% (non-condensing)
Operating atmospheric pressure: 70 to 106 kPa
Operating voltage: 0.9V to 1.6V
Storage environment
Storage temperature: −20 to 65°C, −4 to 149°F
Storage humidity: 15 to 95% (non-condensing)
Storage atmospheric pressure: 70 to 106 kPa
Dimension and Weight
Dimension: 54 W × 85 H × 22 D (mm)
Weight: about 85 g (without battery)
34 Operator's Manual ZS-910P A
Page 48

Operator's Manual ZS-910PA 35
Page 49

Standard Accessories
Attach the channel number label to the monitor, too.
No Name Model Q’ty Suppl y code
1strap --- 1 Y233
36 Operator's Manual ZS-910P A
Page 50
Options
CAUTION
Use only Nihon Kohden electrodes and electrode leads. Otherwise,
the message indicating checking electrodes appears and
monitoring may stop.
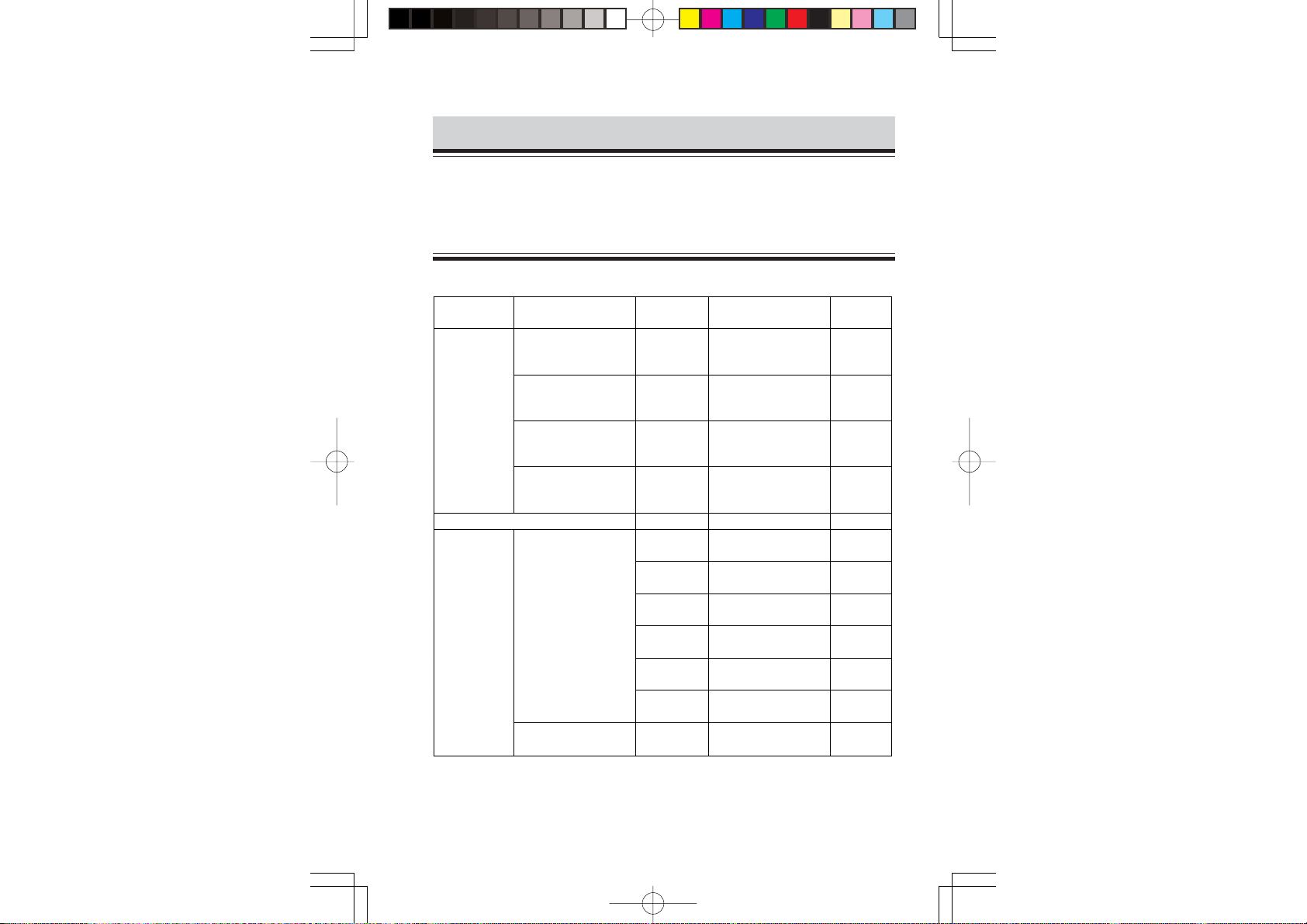
Name Application Model Q’ty Supply
code
Electrode
lead
Electrode lead tube --- 1 K120
Vitrode,
disposable
electrode
3 electrodes,
snap type,
lead length 80 cm
3 electrodes,
clip type,
lead length 80 cm
2 electrodes,
clip type,
lead length 80 cm
2 electrodes,
snap type,
lead length 80 cm
General
Neonate
Premature baby
BR-913P 1 K910A
BR-903P 1 K911
BR-902P 1 K907A
BR-912P 1 K908A
Bs-150 1 box
(30 × 5 package)
C-150 1 box
(30 × 5 package)
D-90 1 box
(3 × 30 package)
F-150M 1 box
(3 × 50 package)
G-600 1 box
(30 × 20 package)
J-150 1 box
(30 × 5 package)
F-150S 1 box
(3 × 50 package)
G201
G204
G217
G210D
G221
G250
G210C
Operator's Manual ZS-910PA 37
 Loading...
Loading...