Nihon Koden TEC-7731, TEC-7721, TEC-7631, TEC-7621 Service Manual

SERVICE MANUAL
TEC-7621C
TEC-7631C
TEC-7621E
TEC-7631E
TEC-7621K
TEC-7631K
TEC-7721E
TEC-7731E
TEC-7721K
TEC-7731K
DEFIBRILLATOR
TEC-7600/TEC-7700
0634-002039B
|
|
CONTENTS |
|
Contents |
|
|
GENERAL HANDLING PRECAUTIONS ......................................................................... |
i |
|
WARRANTY POLICY .................................................................................................... |
ii |
|
Conventions Used in this Manual and Instrument ........................................................ |
iv |
|
Dangers, Warnings, Cautions and Notes ............................................................ |
iv |
|
Explanations of the Symbols in this Manual and Instrument ............................... |
v |
Section 1 |
General .................................................................................. |
1C.1 |
|
Introduction .......................................................................................................................... |
1.1 |
|
Models and Functions ................................................................................................ |
1.1 |
|
General Information on Servicing ......................................................................................... |
1.2 |
|
Service Policy, Service Parts and Patient Safety Checks .................................................... |
1.4 |
|
Service Policy ............................................................................................................ |
1.4 |
|
Service Parts ............................................................................................................. |
1.4 |
|
Patient Safety Checks ............................................................................................... |
1.5 |
|
Maintenance Equipments and Tools ........................................................................... |
1.5 |
|
Important Safety Information ............................................................................................... |
1.6 |
|
Specifications .................................................................................................................... |
1.23 |
|
Panel Description ............................................................................................................... |
1.29 |
|
Front Panel ............................................................................................................... |
1.29 |
|
Top Panel (TEC-7631/7731 Series Only) .................................................................. |
1.30 |
|
External Paddles ...................................................................................................... |
1.31 |
|
Left Side Panel ......................................................................................................... |
1.31 |
|
Rear Panel ............................................................................................................... |
1.32 |
|
Bottom Panel ........................................................................................................... |
1.32 |
|
Composition ....................................................................................................................... |
1.33 |
|
Standard Components .................................................................................... |
1.33 |
|
Options .......................................................................................................... |
1.37 |
|
Board/Unit Location ............................................................................................................ |
1.40 |
|
TEC-7600 Series Defibrillator ................................................................................... |
1.40 |
|
TEC-7700 Series Defibrillator ................................................................................... |
1.41 |
|
Block Diagram .................................................................................................................... |
1.42 |
|
TEC-7600 Series Defibrillator ................................................................................... |
1.42 |
|
TEC-7700 Series Defibrillator ................................................................................... |
1.43 |
Section 2 |
Troubleshooting ................................................................... |
2C.1 |
|
How to Troubleshoot ............................................................................................................. |
2.1 |
|
Error Code ............................................................................................................................ |
2.2 |
|
Defibrillation ............................................................................................................... |
2.3 |
|
Operation Panel .......................................................................................................... |
2.5 |
|
Communication .......................................................................................................... |
2.6 |
|
Data Error................................................................................................................... |
2.7 |
|
Pacing (TEC-7631/7731 Series Only) ......................................................................... |
2.7 |
Service Manual TEC-7600/7700 |
1 |
CONTENTS |
|
12 Lead ECG Meaurement ......................................................................................... |
2.8 |
Message............................................................................................................................... |
2.9 |
Instrument and SpO2/CO2 Mesurement ....................................................................... |
2.9 |
NIBP Mesurement .................................................................................................... |
2.11 |
12 Lead ECG Mesurement ....................................................................................... |
2.11 |
Troubleshooting .................................................................................................................. |
2.12 |
General ..................................................................................................................... |
2.12 |
Defibrillation ............................................................................................................. |
2.13 |
Monitoring ................................................................................................................ |
2.13 |
Recording ................................................................................................................. |
2.18 |
Battery ..................................................................................................................... |
2.18 |
Pacing (TEC-7631/7731 Series Only) ....................................................................... |
2.19 |
Section 3 |
Disassembly ......................................................................... |
3C.1 |
|
TEC-7621/7631 Series |
|
|
Before You Begin ............................................................................................................... |
3.1.1 |
|
Warnings, Cautions and Notes ................................................................................ |
3.1.1 |
|
Required Tools ......................................................................................................... |
3.1.1 |
|
Connection Diagram (TEC-7621/7631 Series) ................................................................... |
3.1.2 |
|
Removing the Lower Casing .............................................................................................. |
3.1.4 |
|
Removing the Battery Pack ................................................................................... |
3.1.4 |
|
Removing the Paddles ............................................................................................ |
3.1.4 |
|
Removing the Lower Casing .................................................................................... |
3.1.5 |
|
Removing the AC/DC Unit ................................................................................................. |
3.1.6 |
|
Removing the Main Board ................................................................................................. |
3.1.7 |
|
Removing the Main Chassis ................................................................................... |
3.1.7 |
|
Removing the Main Board ....................................................................................... |
3.1.8 |
|
Removing the HV Inductor ................................................................................................ |
3.1.9 |
|
Removing the HV Capacitor and Relay Unit .................................................................... |
3.1.10 |
|
Cable Connections of the High voltage Unit .......................................................... |
3.1.11 |
|
Removing the Test Load Board ........................................................................................ |
3.1.12 |
|
Removing the Speaker .................................................................................................... |
3.1.13 |
|
Removing the Pacer Board (TEC-7631 Series Only) ....................................................... |
3.1.14 |
|
Removing the LCD Unit ................................................................................................... |
3.1.15 |
|
Removing the Main Key Board and Key Board ................................................................ |
3.1.16 |
|
Removing the Recorder Unit ............................................................................................ |
3.1.17 |
|
Removing the Paddle Locks ............................................................................................ |
3.1.18 |
|
Removing the Battery Connector ..................................................................................... |
3.1.19 |
|
TEC-7721/7731 Series |
|
|
Before You Begin ............................................................................................................... |
3.2.1 |
|
Warnings, Cautions and Notes ................................................................................ |
3.2.1 |
|
Required Tools ......................................................................................................... |
3.2.1 |
|
How to Troubleshoot .......................................................................................................... |
3.2.1 |
|
Connection Diagram (TEC-7721/7731 Series Defibrillator) ................................................. |
3.2.2 |
|
Removing the Battery Pack ................................................................................... |
3.2.4 |
|
Removing the Lower Casing .............................................................................................. |
3.2.4 |
|
Removing the Paddles ............................................................................................ |
3.2.4 |
C.2 |
Service Manual TEC-7600/7700 |
|
|
CONTENTS |
|
Removing the Lower Casing .................................................................................... |
3.2.5 |
|
Removing the AC/DC Unit ................................................................................................. |
3.2.6 |
|
Removing the Main Board ................................................................................................. |
3.2.7 |
|
Removing the Main Chassis ................................................................................... |
3.2.7 |
|
Removing the Main Board and Terminal Bracket ..................................................... |
3.2.9 |
|
Removing the HV Capacitor and Biphasic HV Unit ......................................................... |
3.2.10 |
|
Removing the Test Load Board ........................................................................................ |
3.2.11 |
|
Removing the Speaker .................................................................................................... |
3.2.12 |
|
Removing the Pacer Board (TEC-7731 Series Only) ....................................................... |
3.2.13 |
|
Removing the LCD Unit ................................................................................................... |
3.2.14 |
|
Removing the Main Key Board and Key Board ................................................................ |
3.2.15 |
|
Removing the Recorder Unit ............................................................................................ |
3.2.16 |
|
Removing the Paddle Locks ............................................................................................ |
3.2.17 |
|
Removing the Battery Connector ..................................................................................... |
3.2.18 |
|
Installing the Optional Unit |
|
|
General .............................................................................................................................. |
3.3.1 |
|
Installation Procedure ........................................................................................................ |
3.3.2 |
|
Installing the VP-761V/VC/VE Voice Prompt Board ................................................. |
3.3.3 |
|
Operation Check ........................................................................................... |
3.3.3 |
|
Installing the QI-762V DSI Interface Board or QI-763V DSI/AUX OUT |
|
|
Interface Board ....................................................................................................... |
3.3.4 |
|
Operation Check ........................................................................................... |
3.3.4 |
|
Installing the AC-761VA/VC/VE/VK/VI 12 Lead ECG Unit ...................................... |
3.3.5 |
|
Operation Check ........................................................................................... |
3.3.6 |
|
Installing the SG-761VC/VE/VK NIBP Unit ............................................................. |
3.3.6 |
|
Operation Check ........................................................................................... |
3.3.7 |
|
Installing the QI-761V ZB Interface Unit ................................................................. |
3.3.8 |
|
Operation Check ......................................................................................... |
3.3.10 |
Section 4 |
Maintenance ......................................................................... |
4C.1 |
|
General ................................................................................................................................. |
4.1 |
|
Daily Checks .................................................................................................... |
4.1 |
|
Monthly Checks ............................................................................................... |
4.1 |
|
System Maintenance Screen ............................................................................................... |
4.2 |
|
Calling Up the System Maintenance Screen .............................................................. |
4.2 |
|
About the Menu Items ................................................................................................ |
4.3 |
|
System Maintenance Screen Flowchart ........................................................... |
4.4 |
|
Default Settings ............................................................................................... |
4.5 |
|
Flash Save Procedure ................................................................................................ |
4.6 |
|
Configuration Screen .................................................................................................. |
4.7 |
|
Adjust AD Screen ...................................................................................................... |
4.8 |
|
Adjust ECG/AD Screen .................................................................................... |
4.8 |
|
Adjust HV AD Screen .................................................................................... |
4.10 |
|
TEC-7621/7631 Series Defibrillator .......................................................... |
4.10 |
|
TEC-7721/7731 Series Defibrillator .......................................................... |
4.12 |
|
Adjust Battery AD Screen .............................................................................. |
4.14 |
|
Check Hardware Screen ........................................................................................... |
4.15 |
Service Manual TEC-7600/7700 |
3 |
CONTENTS |
|
Check Key Screen ......................................................................................... |
4.15 |
Check LED Screen ........................................................................................ |
4.17 |
Check LCD Screen ........................................................................................ |
4.17 |
Check Recorder Screen ................................................................................. |
4.17 |
Check Time Constant Screen ........................................................................ |
4.18 |
Check Buzzer Screen .................................................................................... |
4.19 |
Check Memory Screen ................................................................................... |
4.19 |
Check Voice Screen ....................................................................................... |
4.20 |
Check NIBP Screen ....................................................................................... |
4.21 |
Check 12 Lead Screen ................................................................................... |
4.21 |
A/D View Screen ...................................................................................................... |
4.32 |
Operation Time Screen ............................................................................................. |
4.32 |
Version Up Screen ................................................................................................... |
4.33 |
Debug Mode Screen ................................................................................................. |
4.33 |
Check String Screen ...................................................................................... |
4.34 |
Memory Dump Screen ................................................................................... |
4.34 |
Protocol Analysis Screen ............................................................................... |
4.35 |
Card Attribute Screen ..................................................................................... |
4.35 |
Periodic Replacement Schedule ......................................................................................... |
4.36 |
Maintenance Check Sheet ................................................................................................. |
4.37 |
Section 5 Replaceable Parts List......................................................... |
5C.1 |
TEC-7621/7631 Series Defibrillator ....................................................................................... |
5.2 |
TEC-7721/7731 Series Defibrillator ....................................................................................... |
5.6 |
KD-022A Cart ..................................................................................................................... |
5.10 |
C.4 |
Service Manual TEC-7600/7700 |
GENERAL HANDLING PRECAUTIONS
This device is intended for use only by qualified medical personnel.
Use only Nihon Kohden approved products with this device. Use of non-approved products or in a non-approved manner may affect the performance specifications of the device. This includes, but is not limited to, batteries, recording paper, pens, extension cables, electrode leads, input boxes and AC power.
Please read these precautions thoroughly before attempting to operate the instrument.
1.To safely and effectively use the instrument, its operation must be fully understood.
2.When installing or storing the instrument, take the following precautions:
(1)Avoid moisture or contact with water, extreme atmospheric pressure, excessive humidity and temperatures, poorly ventilated areas, and dust, saline or sulphuric air.
(2)Place the instrument on an even, level floor. Avoid vibration and mechanical shock, even during transport.
(3)Avoid placing in an area where chemicals are stored or where there is danger of gas leakage.
(4)The power line source to be applied to the instrument must correspond in frequency and voltage to product specifications, and have sufficient current capacity.
(5)Choose a room where a proper grounding facility is available.
3.Before Operation
(1)Check that the instrument is in perfect operating order.
(2)Check that the instrument is grounded properly.
(3)Check that all cords are connected properly.
(4)Pay extra attention when the instrument is in combination with other instruments to avoid misdiagnosis or other problems.
(5)All circuitry used for direct patient connection must be doubly checked.
(6)Check that battery level is acceptable and battery condition is good when using battery-operated models.
4.During Operation
(1)Both the instrument and the patient must receive continual, careful attention.
(2)Turn power off or remove electrodes and/or transducers when necessary to assure the patient’s safety.
(3)Avoid direct contact between the instrument housing and the patient.
5.To Shutdown After Use
(1)Turn power off with all controls returned to their original positions.
(2)Remove the cords gently; do not use force to remove them.
(3)Clean the instrument together with all accessories for their next use.
6.The instrument must receive expert, professional attention for maintenance and repairs. When the instrument is not functioning properly, it should be clearly marked to avoid operation while it is out of order.
7.The instrument must not be altered or modified in any way.
8.Maintenance and Inspection:
(1)The instrument and parts must undergo regular maintenance inspection at least every 6 months.
(2)If stored for extended periods without being used, make sure prior to operation that the instrument is in perfect operating condition.
Service Manual TEC-7600/7700 |
i |

(3)Technical information such as parts list, descriptions, calibration instructions or other information is available for qualified user technical personnel upon request from your Nihon Kohden distributor.
9.When the instrument is used with an electrosurgical instrument, pay careful attention to the application and/or location of electrodes and/or transducers to avoid possible burn to the patient.
WARRANTY POLICY
Nihon Kohden Corporation (NKC) shall warrant its products against all defects in materials and workmanship for one year from the date of delivery. However, consumable materials such as recording paper, ink, stylus and battery are excluded from the warranty.
NKC or its authorized agents will repair or replace any products which prove to be defective during the warranty period, provided these products are used as prescribed by the operating instructions given in the operator’s and service manuals.
No other party is authorized to make any warranty or assume liability for NKC’s products. NKC will not recognize any other warranty, either implied or in writing. In addition, service, technical modification or any other product change performed by someone other than NKC or its authorized agents without prior consent of NKC may be cause for voiding this warranty.
Defective products or parts must be returned to NKC or its authorized agents, along with an explanation of the failure. Shipping costs must be pre-paid.
This warranty does not apply to products that have been modified, disassembled, reinstalled or repaired without Nihon Kohden approval or which have been subjected to neglect or accident, damage due to accident, fire, lightning, vandalism, water or other casualty, improper installation or application, or on which the original identification marks have been removed.
In the USA and Canada other warranty policies may apply.
CAUTION
United States law restricts this device to sale by or on the order of a physician.
ii |
Service Manual TEC-7600/7700 |

EMC RELATED CAUTION
This equipment and/or system complies with the International Standard IEC60601-1-2 for electromagnetic compatibility for medical electrical equipment and/or system. However, an electromagnetic environment that exceeds the limits or levels stipulated in the IEC60601-1-2, can cause harmful interference to the equipment and/or system or cause the equipment and/or system to fail to perform its intended function or degrade its intended performance. Therefore, during the operation of the equipment and/or system, if there is any undesired deviation from its intended operational performance, you must avoid, identify and resolve the adverse electromagnetic effect before continuing to use the equipment and/or system.
The following describes some common interference sources and remedial actions:
1.Strong electromagnetic interference from a nearby emitter source such as an authorized radio station or cellular phone:
Install the equipment and/or system at another location if it is interfered with by an emitter source such as an authorized radio station. Keep the emitter source such as cellular phone away from the equipment and/or system.
2.Radio-frequency interference from other equipment through the AC power supply of the equipment and/or system:
Identify the cause of this interference and if possible remove this interference source. If this is not possible, use a different power supply.
3.Effect of direct or indirect electrostatic discharge:
Make sure all users and patients in contact with the equipment and/or system are free from direct or indirect electrostatic energy before using it. A humid room can help lessen this problem.
4.Electromagnetic interference with any radio wave receiver such as radio or television:
If the equipment and/or system interferes with any radio wave receiver, locate the equipment and/or system as far as possible from the radio wave receiver.
If the above suggested remedial actions do not solve the problem, consult your Nihon Kohden Corporation subsidiary or distributor for additional suggestions.
In IEC 60601-1-2 Medical Electronic Equipment, Part 1: General Requirements for Safety, 2. Collateral Standard: Electromagnetic compatibility-Requirements and test. Section 36. 202. 2 Radiated radio-frequency electromagnetic fields, PATIENT COUPLED EQUIPMENT and/or SYSTEMS applicable IMMUNITY test methods are under consideration at SC62A/WG13. The 3 V/m IMMUNITY level may be inappropriate especially when measuring SpO2 because physiological signals can be much smaller than those induced by a 3 V/m electromagnetic field.
When measuring SpO2, various interference may produce false waveforms which look like pulse waveforms. SpO2 value and pulse rate may be measured from these false waveforms, causing the alarm to function improperly.
When installing the monitor, avoid locations where the monitor may receive strong electromagnetic interference such as radio or TV stations, cellular phone or mobile two-way radios.
The CE mark is a protected conformity mark of the European Community. The products herewith comply with the requirements of the Medical Device Directive 93/42/EEC.
Service Manual TEC-7600/7700 |
iii |

Conventions Used in this Manual and Instrument
Dangers, Warnings, Cautions and Notes
Dangers, Warnings, cautions and notes are used in this manual to alert or signal the reader to specific information.
DANGER
A danger is used to alert the user to a hazardous situation which will cause death or serious injury.
WARNING
A warning alerts the user to the possible injury or death associated with the use or misuse of the instrument.
CAUTION
A caution alerts the user to possible injury or problems with the instrument associated with its use or misuse such as instrument malfunction, instrument failure, damage to the instrument, or damage to other property.
NOTE
A note provides specific information, in the form of recommendations, prerequirements, alternative methods or supplemental information.
iv |
Service Manual TEC-7600/7700 |

Explanations of the Symbols in this Manual and Instrument
The following symbols found in this manual/instrument bear the respective descriptions as given.
On main unit
Symbol |
Description |
Symbol |
Description |
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Alternating current |
|
|
|
|
|
|
Defibrillation-proof type BF |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
applied part |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Charging |
|
|
|
|
|
|
Equipotential |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Charged (Battery charging is |
|
|
|
|
|
|
Dangerous voltage |
|
|
|
|
|
|
|
|
|
|
finished) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ECG lead |
|
|
|
|
|
|
Input |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ECG sensitivity |
|
|
|
|
|
|
ECG |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Alarm off |
|
|
|
|
|
|
Pacing start |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Check alarms |
|
|
|
|
|
|
Pacing stop |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Real time/delayed recording |
|
|
|
|
|
|
Pacing intensity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Event recording |
|
|
|
|
|
|
Attention, consult operator’s |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
manual |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Inserting or removing the memory |
|
|
|
|
|
|
Defibrillation-proof type CF |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
card |
|
|
|
|
|
|
applied part |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Output |
IPX1 |
Complying with IEC60529 IPX1 |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
IPX4 |
Complying with IEC60529 IPX4 |
IPX7 |
Complying with IEC60529 IPX7 |
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The CE mark is a protected |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
conformity mark of the European |
|
|
|
|
|
|
|
|
|
|
Biphasic defibrillation |
|
|
|
|
|
|
Community. The products |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
herewith comply with the |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
requirements of the Medical |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Device Directive 93/42/EEC. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biphasic defibrillation: TEC-7700 series only
Service Manual TEC-7600/7700 |
v |

On LCD
Symbol |
Description |
Symbol |
Description |
||||
|
|
|
|
|
|
|
|
|
Full battery charge remains |
|
|
|
|
|
Add Z-fold recording paper |
|
|
|
|
|
|
||
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
More than 2/3 battery charge
QRS sync mark
remains
Battery discharged
The point of implanted pacemaker pulse output
|
|
|
Battery power for one 360 J |
Alternating current (AC power |
|
1 |
3 |
charging remains |
operation) |
|
|
|
||
|
|
|
|
|
|
|
|
Battery operation not available |
|
0 |
|
|
||
|
|
|
vi |
Service Manual TEC-7600/7700 |
Section 1 General
Introduction ......................................................................................................................... |
1.1 |
Models and Functions ............................................................................................... |
1.1 |
General Information on Servicing ........................................................................................ |
1.2 |
Service Policy, Service Parts and Patient Safety Checks ................................................... |
1.4 |
Service Policy ........................................................................................................... |
1.4 |
Service Parts ............................................................................................................ |
1.4 |
Patient Safety Checks .............................................................................................. |
1.5 |
Maintenance Equipments and Tools .......................................................................... |
1.5 |
Important Safety Information .............................................................................................. |
1.6 |
Specifications ................................................................................................................... |
1.23 |
Panel Description .............................................................................................................. |
1.29 |
Front Panel .............................................................................................................. |
1.29 |
Top Panel (TEC-7631/7731 Series Only) ................................................................. |
1.30 |
External Paddles ..................................................................................................... |
1.31 |
Left Side Panel ........................................................................................................ |
1.31 |
Rear Panel .............................................................................................................. |
1.32 |
Bottom Panel .......................................................................................................... |
1.32 |
Composition ...................................................................................................................... |
1.33 |
Standard Components ................................................................................... |
1.33 |
Options ......................................................................................................... |
1.37 |
Board/Unit Location ........................................................................................................... |
1.40 |
TEC-7600 Series Defibrillator .................................................................................. |
1.40 |
TEC-7700 Series Defibrillator .................................................................................. |
1.41 |
Block Diagram ................................................................................................................... |
1.42 |
TEC-7600 Series Defibrillator .................................................................................. |
1.42 |
TEC-7700 Series Defibrillator .................................................................................. |
1.43 |
Service Manual TEC-7600/7700 |
1C.1 |
This page is intentionally left blank.
1C.2 |
Service Manual TEC-7600/7700 |
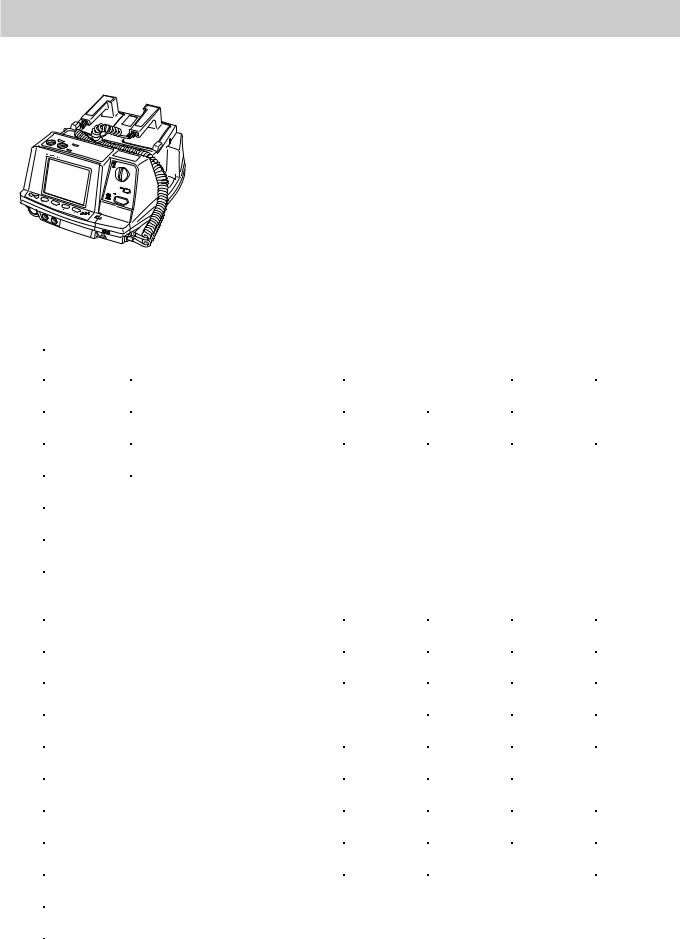
Introduction
CardioLife
1. GENERAL
This service manual provides useful information to qualified service personnel to understand, troubleshoot, service, maintain and repair this TEC-7600 and TEC-7700 series defibrillator (referred to as “instrument” in this service manual).
The information in the operator’s manual is primarily for the user. However, it is important for service personnel to thoroughly read the operator’s manual and service manual before starting to troubleshoot, service, maintain or repair this instrument. This is because service personnel needs to understand the operation of the instrument in order to effectively use the information in the service manual.
Models and Functions
|
Functions |
TEC-7621 |
TEC-7631 |
TEC-7721 |
TEC-7731 |
|
|
|
|
|
|
|
|
Defibrillation |
External paddles |
Standard |
Standard |
Standard |
Standard |
|
and |
|
|
|
|
|
|
|
|
|
|
|
||
synchronized |
Internal paddles |
Option |
Option |
Option |
Option |
|
cardioversion |
|
|
|
|
|
|
Disposable pads |
Option |
Standard |
Option |
Standard |
||
|
||||||
|
|
|
|
|
|
|
|
Pediatric electrode assy 44 mm dia. |
Option |
Option |
Option |
Option |
|
|
|
|
|
|
|
|
3 lead ECG |
|
Standard |
Standard |
Standard |
Standard |
|
|
|
|
|
|
|
|
AED function |
|
Standard |
Standard |
Standard |
Standard |
|
|
|
|
|
|
|
|
Noninvasive pacing |
Not |
Standard |
Not |
Standard |
||
available |
available |
|||||
|
|
|
|
|||
|
|
|
|
|
||
SpO2 measurement |
Option |
Option |
Option |
Option |
||
|
|
|
|
|
||
CO2 measurement |
Option |
Option |
Option |
Option |
||
|
|
|
|
|
|
|
Voice prompt |
|
Option |
Option |
Option |
Option |
|
|
|
|
|
|
|
|
5 lead ECG |
|
Option |
Option |
Option |
Option |
|
|
|
|
|
|
||
External ECG input |
Option |
Option |
Option |
Option |
||
|
|
|
|
|
||
External ECG output |
Option |
Option |
Option |
Option |
||
|
|
|
|
|
||
Memory card slot |
Standard |
Standard |
Standard |
Standard |
||
|
|
|
|
|
||
NIBP measurement |
Option |
Option |
Option |
Option |
||
|
|
|
|
|
||
12 lead ECG measurement |
Option |
Option |
Option |
Option |
||
|
|
|
|
|
|
|
Transmitter |
|
Option |
Option |
Option |
Option |
|
|
|
|
|
|
|
|
Service Manual TEC-7600/7700 |
1.1 |

1. GENERAL
General Information on Servicing
Note the following information when servicing the instrument.
CAUTIONS
Safety
•There is the possibility that the outside surface of the instrument, such as the operation keys, could be contaminated by contagious germs, so disinfect and clean the instrument before servicing it. When servicing the instrument, wear rubber gloves to protect yourself from infection.
•There is the possibility that when the lithium battery is broken, a solvent inside the lithium battery could flow out or a toxic substance inside it could come out. If the solvent or toxic substance touches your skin or gets into your eye or mouth, immediately wash it with a lot of water and see a physician.
Liquid ingress
The instrument is not waterproof, so do not install the instrument where water or liquid can get into or fall on the instrument. If liquid accidentally gets into the instrument or the instrument accidentally drops into liquid, disassemble the instrument, clean it with clean water and dry it completely. After reassembling, verify that there is nothing wrong with the patient safety checks and function/ performance checks. If there is something wrong with the instrument, contact your Nihon Kohden representative for repair.
Environmental Safeguards
Depending on the local laws in your community, it may be illegal to dispose of the lithium battery in the regular waste collection. Check with your local officials for proper disposal procedures.
Disinfection and cleaning
To disinfect the outside surface of the instrument, wipe it with a nonabrasive cloth moistened with any of the disinfectants listed below. Do not use any other disinfectants or ultraviolet rays to disinfect the
instrument. |
|
- Chlorohexidine gluconate solution: |
0.5% |
- Benzethonium chloride solution: |
0.2% |
- Glutaraldehyde solution: |
2.0% |
- Benzalkonium chloride: |
0.2% |
- Hydrochloric alkyl diaminoethylglycine: |
0.5% |
1.2 |
Service Manual TEC-7600/7700 |

1. GENERAL
Caution - continued
Transport
•Use the specified shipment container and packing material to transport the instrument. If necessary, double pack the instrument. Also, put the instrument into the shipment container after packing so that the buffer material does not get into the inside of the instrument.
•When transporting a board or unit of the instrument, be sure to use a conductive bag on. Never use an aluminum bag when transporting a board or unit on which a lithium battery is mounted. Also, never use a styrene foam or plastic bag which generates static electricity to wrap the board or unit of the instrument.
Handling the instrument
•Because the outside surface of the instrument is made of resin, the outside surface of the instrument is easily damaged. So when handling the instrument, remove clutter from around the instrument and be careful to not damage the instrument or get it dirty.
•Because most of the boards in the instrument are multilayer boards with surface mounted electrical devices (SMD), when removing and soldering the electrical devices, a special tool is required. To avoid damaging other electrical components, do not remove and solder SMD components yourself.
Measuring and Test Equipment
Maintain the accuracy of the measuring and test equipment by checking and calibrating it according to the check and calibration procedures.
Service Manual TEC-7600/7700 |
1.3 |

1. GENERAL
Service Policy, Service Parts and Patient Safety Checks
Service Policy
Our technical service policy for this instrument is to replace the faulty unit, board or part or damaged mechanical part with a new one. Do not perform electrical device or component level repair of the multilayer board or unit. We do not support component level repair outside the factory for the following reasons:
•Most of the boards are multilayer boards with surface mounted electrical devices, so the mounting density of the board is too high.
•A special tool or high degree of repair skill is required to repair the multilayer boards with surface mounted electrical devices.
Only disassemble the instrument or replace a board or unit in an environment where the instrument is protected against static electricity.
As background knowledge for repair, pay special attention to the following:
•You can reduce the repair time by considering the problem before starting repair.
•You can clarify the source of most of the troubles using the information from the troubleshooting tables. Refer to “Troubleshooting“ of this manual.
Service Parts
Refer to “Replaceable Parts List” of this manual for the service parts for technical service that we provide.
NOTE
When ordering parts or accessories from your Nihon Kohden representative, please quote the NK code number and part name which is listed in this service manual, and the name or model of the unit in which the required part is located. This will help us to promptly attend to your needs. Always use parts and accessories recommended or supplied by Nihon Kohden Corporation to assure maximum performance from your instrument.
1.4 |
Service Manual TEC-7600/7700 |
Patient Safety Checks
Maintenance Equipments
and Tools
1. GENERAL
Periodic maintenance procedures and diagnostic check procedures are provided in this manual to ensure that the instrument is operating in accordance with its design and production specifications. To verify that the instrument is working in a safe manner with regard to patient safety, patient safety checks should be performed on the instrument before it is first installed, periodically after installation, and after any repair is made on the instrument.
For patient safety checks, perform the following checks as described in the IEC60601-1 “Medical electrical equipment - Part 1: General requirements for safety”:
•Protective earth resistance check
•Earth leakage current check
•Enclosure leakage current check
•Patient leakage current check
•Withstanding voltage check
Test equipment
When repairing or calibrating the instrument, the following test equipment is required.
•Oscilloscope: 2 channels or more for input signal, 50 mV to 5 V input range, 1/ 10 attenuating probe and 100 MHz or more frequency response characteristic must be provided.
•Power supply
•Oscillator: standard type
•Digital voltmeter: standard type (An oscilloscope can be used instead of the digital voltmeter.)
Service Manual TEC-7600/7700 |
1.5 |

1. GENERAL
Important Safety Information
General
DANGER
•Never use the defibrillator in a flammable atmosphere (i.e. areas with flammable anesthetics, concentrated oxygen, hyperbaric oxygen) or in an environment in which an electrical arc could ignite an explosion. Otherwise, the defibrillator will explode or fire.
•Never use the defibrillator in a high-pressure oxygen medical care tank. Otherwise, the defibrillator will explode or fire.
WARNING
•The defibrillator generates high voltage. The defibrillator must only be operated by trained and qualified medical personnel.
•Radiofrequency or Electromagnetic Field
Do not use any kind of non-essential non-patient care device within a radius of 1 meter around the defibrillator. The use of non-essential non-patient care devices that emit radiofrequency or electromagnetic fields may interfere with the operation of the defibrillator by causing noise on the ECG waveform or error messages. If a non-essential non-patient care device is accidentally placed near the defibrillator, quickly remove it.
•MRI examination
-Do not install this defibrillator in an MRI examination room. The defibrillator may not operate properly due to high-frequency magnetic noise from the MRI.
-When performing MRI tests, remove all electrodes and transducers from the patient which are connected to this defibrillator. Failure to follow this warning may cause serious electrical burn on the patient due to local heating caused by dielectric electromotive force. For details, refer to the instruction manual for the MRI.
•Using with ESU
-When using this defibrillator with an ESU, the ESU return plate and the electrodes for monitoring must be firmly attached to the patient. If the return plate is not attached correctly, it may burn the patient’s skin where the electrodes are attached. Refer to the instruction manual for the ESU.
-When using an ESU, use this defibrillator only in the MONITOR mode and use the ECG electrodes for monitoring. Do not monitor ECG with disposable pads, external paddles or internal paddles. Otherwise, high frequency energy from the ESU causes abnormal current to flow in the patient and unexpected discharge. This causes serious electrical burn, shock, or other injury and damages the defibrillator.
1.6 |
Service Manual TEC-7600/7700 |

1. GENERAL
WARNING continued
•Surrounding Conditions
Fluids such as Ringer’s saline solution and blood are excellent electrical conductors; to avoid creating potentially dangerous electrical paths, keep the defibrillator and the immediate area clean and dry at all times.
CAUTION
•Install the defibrillator and ESU appropriately and perform equipotential grounding. Otherwise, noise from the ESU may be falsely recognized as QRS and ECG monitoring may not be performed properly.
•Use only Nihon Kohden products and specified parts and accessories. When other products, parts or accessories are used, the defibrillator heats up and breaks down, and monitoring stops.
•Do not reuse disposable products.
Installation |
WARNING |
• Connect only the specified instrument to the defibrillator by following |
|
the specified procedure. Otherwise, electrical leakage current may |
|
harm the patient and operator. |
|
• Connect only the specified instruments to the connector or sockets |
|
marked with |
by following the specified procedure. Otherwise, |
electrical leakage current may harm the patient and operator.
• Only use the provided power cord. Using other power cords may result in electrical shock or other injury to the patient and operator. When the provided power cord cannot be used, operate the defibrillator on battery power.
• For patient safety, equipotential grounding of all instruments must be performed. Consult with a qualified biomedical engineer.
• Do not connect several grounding leads directly to the equipotential terminal because the grounding lead may be disconnected from the terminal.
CAUTION
•The defibrillator should only be connected to external equipment which complies with the CISPR 11 Second Edition 1990-09, Group 1 and Class B standard.
•Use only the KD-022A cart for this defibrillator. If another cart is used, the cart may tip over or the defibrillator may fall off.
Service Manual TEC-7600/7700 |
1.7 |

1. GENERAL
Battery
DANGER
•Keep the battery pack away from fire. Do not heat the battery pack. Otherwise, the electrolyte comes out and the battery pack explodes.
•Never short-circuit the + and – terminals on the battery pack with a wire. Never store or carry the battery pack with metal such as necklace or hair pins. The battery pack short-circuits and a large current flows, causing leakage of the substance inside the battery and battery explosion.
•Never disassemble or modify the battery pack. Never damage or directly solder the sheath tube. The battery pack short-circuits, the electrolyte comes out and the battery pack explodes.
•Do not subject the battery pack to a strong mechanical shock. The battery leaks and explodes.
•Do not use a battery which is damaged, such as from falling. There is a gas discharge valve inside the battery and if this valve is damaged, the gas cannot be discharged, causing the battery to explode.
•If the battery pack is damaged and the substance inside the battery (alkaline liquid) contacts the eyes or skin, wash immediately and thoroughly with water and see your physician. Never rub your eyes, otherwise you may lose your eyesight.
•The battery pack has + and – polarity. Make sure that the battery is installed with the correct polarity direction. Otherwise, the substance inside the battery leaks and the battery pack explodes.
•Do not charge the battery pack with an instrument other than this defibrillator. With another instrument, abnormal current flows and the substance inside the battery leaks and the battery explodes.
•Do not connect the battery pack to an AC outlet or lighter socket in a car. The substance inside the battery leaks and the battery pack explodes.
WARNING
•Check the battery performance once a month.
•When you start using a new battery pack, write down the date of battery first use on the label on the battery pack.
•Replace the battery pack every one year.
•During the battery test, the defibrillator cannot perform defibrillation or cardioversion with battery power. Use the defibrillator on AC operation or use another defibrillator. If the battery is deteriorated or is not charged enough, defibrillation or cardioversion cannot be performed.
•Do not immerse the battery pack in water or seawater. The battery heats up and rusts and the substance inside the battery leaks.
•Never use a battery pack which is damaged, discolored or has leakage. A damaged battery explodes if used.WARNING continued
•Do not leave the battery unused for more than one year. The battery may leak.
1.8 |
Service Manual TEC-7600/7700 |

1. GENERAL
CAUTION
•When inserting or removing the battery, disconnect the power cord from the defibrillator. Otherwise, the operator may get electrical shock.
•To keep the battery fully charged, always keep the power cord connected to the AC outlet even when the defibrillator is not used.
•Do not expose the battery pack to direct sunlight or leave in a high temperature place. The lifetime of the battery pack may be shortened, the performance of the battery may be degraded and the substance inside the battery may leak.
•The battery pack must be inserted by a qualified service personnel.
•Keep the battery pack away from children.
•Before disposing of the battery, check with your local solid waste officials for details in your area for recycling options or proper disposal. The battery is recyclable. At the end of its useful life, under various state and local laws, it may be illegal to dispose of this battery into the municipal waste stream.
Service Manual TEC-7600/7700 |
1.9 |

1. GENERAL
Disposable Pads
WARNING
•Failure to comply with the following warnings may cause serious skin burn or insufficient energy discharge and pacing current to the heart.
-Do not reuse disposable pads. The pads are disposable.
-If the pad package is broken, dispose of the pads and do not use them.
-Do not use the pads if they are past the expiration date on the package.
-Use the disposable pads as soon as possible after removing them from the package. Do not use a pad which is left for a long period of time after being removed from the package.
-Do not use the disposable pads if the gel has become dry, or the gel breaks down and releases water.
-Do not use the disposable pads if the color of the gel changes to dark brown and dark brown gel is on the protective liner.
•If any pad or connector gets wet, replace it with a new one. If a wet pad or connector is used, it may cause electrical shock.
•Replace the disposable pads after 1 hour pacing.
CAUTION
•When using the disposable pads for long term ECG monitoring, replace them every 24 hours. Failure to follow this caution may cause insufficient pacing current and insufficient energy discharge to the heart.
•Do not attach a disposable pad over another pad. Failure to follow this caution may cause serious skin burn.
•Do not put heavy objects on the disposable pads or bend the pads. Otherwise the pads get damaged and deteriorated, resulting in skin burn on the patient.
1.10 |
Service Manual TEC-7600/7700 |

1. GENERAL
Defibrillation, |
General |
|||||
Cardioversion and AED |
|
|
|
|
|
|
|
|
|
|
|
||
WARNING |
||||||
|
||||||
|
• Before defibrillation and cardioversion, make sure that no one is in |
|||||
|
contact with either the patient or any metal part of any equipment or |
|||||
|
cables which supports or is connected to the patient. Failure to follow |
|||||
|
this warning causes serious electrical shock or injury. |
|||||
|
• Before defibrillation and cardioversion, remove all electrodes, probes |
|||||
|
and transducers connected to a connector without a “ ” or “ |
|
|
|
” |
|
|
|
|
||||
|
mark from the patient. Otherwise the operator may get electrical |
|||||
|
shock and the connected instrument may be damaged. |
|||||
|
• Before defibrillation and cardioversion, move all electrodes and |
|||||
|
medicine on the patient’s chest to positions where the defibrillator |
|||||
|
paddle or disposable pad will not touch. If the defibrillator paddle or |
|||||
|
disposable pad directly touches electrodes or medicine, it causes skin |
|||||
|
burn on the electrode or medicine attachment site. |
|||||
|
• Do not carry or move the defibrillator when the charged energy |
|||||
|
remains in the defibrillator. If the defibrillator falls, it discharges |
|||||
|
energy and can cause electrical shock. |
|||||
|
• For this defibrillator, the CONTACT lamp on the STERNUM paddle |
|||||
|
indicates skin-paddle contact impedance. If the yellow or orange lamp |
|||||
|
lights, the defibrillator may cause serious electric burn on the |
|||||
|
patient’s skin and poor energy discharge to the patient. In case of an |
|||||
|
emergency, medical personnel should decide whether to execute |
|||||
|
discharge immediately, regardless of the CONTACT lamp display, or |
|||||
|
take action to make good contact before discharge. |
|||||
|
• Pay careful attention to the energy selection when using the pediatric |
|||||
|
electrode plates. Applying high energy with the pediatric electrode |
|||||
|
plates can cause serious electrical burn because the electrode plates |
|||||
|
are small. |
|||||
|
• Use the ECG monitoring electrodes (disposable electrodes) to monitor |
|||||
|
the ECG waveforms. Stable ECG cannot be acquired with the PADDLE |
|||||
|
lead because it is difficult to hold the paddles stable. ECG acquired |
|||||
|
from external paddles, internal paddles or disposable pads is unstable |
|||||
|
after discharge because of high polarization voltage. |
|||||
|
• Do not perform defibrillation or cardioversion in a wet place. Before |
|||||
|
defibrillation or cardioversion, move the patient and defibrillator to a |
|||||
|
dry place. Otherwise the operator may get electrical shock. |
|||||
|
• Do not discharge near a person or object other than the patient or test |
|||||
|
electrode plate or energy checker. It may cause electrical shock to the |
|||||
|
person or object. |
|||||
|
• Confirm that there is no artifact on the ECG. If there is artifact on the |
|||||
|
ECG, signals other than ECG are misrecognized to be QRS and |
|||||
|
accidental discharge may occur which is not synchronized with the |
|||||
|
patient’s QRS wave. |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Service Manual TEC-7600/7700 |
1.11 |

1. GENERAL
WARNING continued
•Do not perform synchronized cardioversion with the PADDLE lead unless it is absolutely necessary. In synchronized cardioversion with the PADDLE lead, artifact may be misrecognized as QRS and accidental discharge may occur which is not synchronized with the patient’s QRS wave.
•Never select “TEST” for the ECG lead. “TEST” is for maintenance and the waveform displayed on the screen is not the patient’s ECG. If synchronized cardioversion is performed with the TEST lead, accidental discharge occurs which is not synchronized with the patient’s QRS wave and it may cause ventricular fibrillation.
•For safe synchronized cardioversion, a defibrillator discharge must occur within 60 ms of the peak of the ECG’s R wave. The TEC-7600 detects the peak of the R wave and effects the discharge within 25 ms of the detected peak of R wave. When an external instrument is used to provide the ECG signal, it must amplify the ECG signal from the patient 500-1000 times and output the amplified ECG signal to the TEC-7600 within 35 ms of the peak of the R wave so that the overall delay from the peak of the R wave to discharge does not exceed 60 ms. If these conditions are not met, the cardioversion may be ineffective or may cause ventricular fibrillation.
•The apex-posterior placement is not suitable for ECG monitoring or AED analysis.
•The anterior-posterior placement is not suitable for defibrillation, cardioversion, ECG monitoring or AED analysis. Use this placement only for pacing.
CAUTION
When performing synchronized cardioversion, confirm that the SYNC lamp is lit before every discharge. If “Sync mode after CV” is set to Defib on the System Setup-Configuration screen, the defibrillator automatically turns to the asynchronous defibrillation mode.
With External Paddles
WARNING
•Apply contact gel only to the electrode plates of the external paddles. If contact gel gets on any other part of the defibrillator, it may cause electrical shock to the operator.
•Do not apply contact gel by hand. Failure to follow this warning may cause serious electrical burn, shock, or other injury.
1.12 |
Service Manual TEC-7600/7700 |

1. GENERAL
WARNING continued
•Do not grasp the paddle handles with the wet hand or the hand with contact gel attached. Failure to follow this warning may cause electrical shock to the operator.
•Apply contact gel to the electrode plates of the external paddles. Failure to apply contact gel causes serious skin burn.
•Do not touch the electrode plate or edge of the paddle. Failure to follow this warning may cause serious electrical burn, shock, or other injury.
•When charging or discharging, do not touch anything other than the handles. Failure to follow this warning causes electrical shock to the operator.
•Before discharging, confirm that the paddles are firmly pressed against the chest wall. Failure to follow this warning causes serious skin burn or poor energy discharge to the heart.
•Do not perform open discharge into the air. This may cause electrical shock to the operator or damage the defibrillator.
•Do not discharge the energy if the paddles are shorted to each other by contact gel. Failure to follow this warning causes serious electrical burn and poor energy discharge to the heart.
CAUTION
•If the patient’s body is wet, thoroughly wipe the moisture off the skin so that the paddles do not short to each other.
•Do not discharge when the paddles touch each other. This may damage the defibrillator.
With Disposable Pads
WARNING
•Do not attach pads on the papilla, electrodes or medicine on the patient’s body. Failure to follow this warning causes serious skin burn.
•Fit the pad closely to the body surface so that current flows uniformly through the pad. Failure to follow this warning causes serious skin burn or insufficient energy discharge to the heart.
•During charging or discharging, do not touch the pads or connectors. Failure to follow this warning causes electrical shock to the operator.
•Before discharging, confirm that the pads are firmly applied to the chest wall. Failure to follow this warning causes serious skin burn or poor energy discharge to the heart.
Service Manual TEC-7600/7700 |
1.13 |

1. GENERAL
WARNING continued
•Do not discharge if the pads overlap each other or if the pads are shorted to each other by anything conductive such as contact gel. Failure to follow this warning causes serious electrical burn and poor energy discharge to the heart.
CAUTION
•When connecting the pad adaptor to the paddle connector, do not bend or damage the connector pin. Otherwise energy cannot be discharged to the pads.
•If the patient’s body is wet, thoroughly wipe the moisture off the skin so that the pads do not short to each other.
With Internal Paddles
WARNING
•Always sterilize the internal paddles before use. Failure to follow this warning may cause serious infection.
•Pay careful attention to the energy selection when using internal paddles. Applying high energy to the heart may cause cardiac muscle necrosis. Low energy is recommended.
•During charging and discharging, grip the internal paddles between the guard at the top of the handle and the cable. If you grip the handle between the electrode and the guard, you may get an electrical shock.
•Before discharging, confirm that the paddles are firmly positioned against the heart. Failure to follow this warning causes serious skin burn or poor energy discharge to the heart.
•Do not perform open discharge into the air. This may cause electrical shock to the operator or damage the defibrillator.
CAUTION
•Do not twist the internal paddle holding the electrode part or give strong impact to the paddle. It damages the electrode part.
•When connecting the internal paddles to the paddle connector, do not bend or damage the connector pin. Otherwise energy cannot be discharged to the paddles.
•Do not discharge when the paddles touch each other. This may damage the defibrillator.
1.14 |
Service Manual TEC-7600/7700 |

1. GENERAL
AED
WARNING
•Do not attach pads on the papilla, electrodes or medicine on the patient’s body. Failure to follow this warning causes serious skin burn.
•Fit the pad closely to the body surface so that current flows uniformly through the pad. Failure to follow this warning causes serious skin burn or insufficient energy discharge to the heart.
•When you perform defibrillation in an ambulance, stop the car.
•During charging or discharging, do not touch the pads or connectors. Failure to follow this warning cause electrical shock to the operator.
•Before discharging, confirm that the pads are firmly applied to the chest wall. Failure to follow this warning causes serious skin burn or poor energy discharge to the heart.
•Do not discharge if the pads overlap each other or if the pads are shorted to each other by anything conductive such as contact gel. Failure to follow this warning causes serious electrical burn and poor energy discharge to the heart.
CAUTION
•Before AED analysis or defibrillation, confirm that the patient is unconscious and has no respiration and no pulse.
•The ECG of a child or a patient with a implanted pacemaker cannot be analyzed correctly. For these patients, follow the physician’s instruction.
•During AED analysis, do not touch or move the patient, pad adaptor and disposable pad cable. Stop the life saving treatment such as CPR. Otherwise, correct analysis result cannot be obtained. If the ECG baseline is wandering because of surrounding conditions, measurement conditions or electrode conditions, remove the causes before performing AED analysis.
•When connecting the pad adaptor to the paddle connector, do not bend or damage the connector pin. Otherwise energy cannot be discharged to the pads.
•If the patient’s body is wet, thoroughly wipe the moisture off the skin so that the pads do not short to each other.
•Do not discharge when the paddles touch each other. This may damage the defibrillator.
Service Manual TEC-7600/7700 |
1.15 |

1. GENERAL
Pacing
WARNING
•Do not perform pacing while using an ESU. Before using an ESU, turn the defibrillator power off and remove disposable pads from the patient. Otherwise, high frequency energy from the ESU causes abnormal current to flow in the patient and causes serious electrical burn, shock, or other injury. It also damages the defibrillator.
•Always monitor the ECG waveform with the ECG connection cable and ECG electrodes.
•Confirm that there is no artifact on the ECG. If there is artifact on the ECG, signals other than ECG are misrecognized to be QRS and correct pacing cannot be performed.
•Do not touch the patient during pacing. Failure to follow this warning may cause electrical shock.
•During pacing, do not touch the pads or connectors. Failure to follow this warning causes electrical shock to the operator.
•The pacing rate must be determined by qualified medical personnel based on the heart rate of the patient in a normal state.
•The pacing current must only be increased by qualified medical personnel decision.
•Keep the current intensity as low as possible to minimize pain and discomfort to the patient.
•Failure to follow the following warnings causes serious skin burn.
-Do not attach the pads over ECG electrode.
-Do not attach pads on the papilla or medicine on the patient’s body.
-Fit the pad closely to the body surface so that current flows uniformly through the pad. This reduces the required pacing current and pain and discomfort to the patient.
•The apex-posterior placement is not suitable for ECG monitoring or AED analysis.
•The anterior-posterior placement is not suitable for defibrillation, cardioversion, ECG monitoring or AED analysis. Use this placement only for pacing.
•Never select “TEST”. “TEST” is for maintenance and the waveform displayed on the screen is not the patient’s ECG. Failure to follow this warning causes accidental pacing which is not synchronized with the patient’s QRS wave.
•Do not change the sensitivity or ECG lead setting after pacing is started. If one of these settings is changed, the pacing stops for 3 seconds. Failure to follow this warning may cause serious heart attack.
•For 300 ms after the pacing pulse is output, no signal can be detected as a QRS wave.
1.16 |
Service Manual TEC-7600/7700 |

1. GENERAL
CAUTION
•Check that the pacing pulse is effectively working by observing ECG on the screen.
•When connecting the pad adaptor to the paddle connector, do not bend or damage the connector pin. Otherwise energy cannot be discharged to the pads.
•If the patient’s body is wet, thoroughly wipe the moisture off the skin so that the pads do not short to each other.
ECG Monitoring
WARNING
•When using a defibrillator together with the monitor, use Ag/AgCl electrodes. Other types of electrodes, stainless steel in particular, will adversely affect the ECG waveform by slowing the baseline recovery on the monitor and result in no monitoring immediately following defibrillation.
•False low heart rate indicators may occur with certain pacemakers because of electrical overshoots.
•Keep pacemaker patients under close observation. The pacemaker rate may be counted during cardiac arrest and certain arrhythmias. Do not rely only on heart rate alarms and the displayed heart rate.
•The apex-posterior placement is not suitable for ECG monitoring or AED analysis.
•The anterior-posterior placement is not suitable for defibrillation, cardioversion, ECG monitoring or AED analysis. Use this placement only for pacing.
•With the pacing pulse rejection ON, narrow width QRS of a premature baby or infant cannot be detected correctly and the defibrillator may miscount QRS. In this case, set the pacing pulse rejection to OFF.
•Turn the pacing pulse rejection to OFF when monitoring a child. Otherwise child’s QRS may not be recognized.
CAUTION
•When the “Check ECG Electrodes” message is displayed, ECG cannot be monitored and the ECG alarm does not function. Check the electrode, ECG connection cable, electrode leads and connection cable and if necessary, replace it with a new one.
•Turn the pacing pulse rejection to ON when monitoring a pacemaker patient. Otherwise QRS and pacemaker spike may not be distinguished and pacemaker failure may not be recognized.
Service Manual TEC-7600/7700 |
1.17 |
 Loading...
Loading...