Melag Vacuklav 23B+, Vacuklav 31B+ User manual

User manual
Vacuklav®23 B+
Vacuklav®31 B+
Steam sterilizer
From software version 5.15
Dear Dr
We should like to extend our thanks for the expression of trust in our company which you have displayed through the
purchase of this MELAG device.
As an owner-run and operated family concern founded in 1951, we have a long history of successful specialization in
hygiene products for practice-based use. Our focus on quality, highest standards of operational reliability and innovation
has established MELAG as the world’s leading manufacturer in the area instrument treatment and hygiene.
You, our customer are justified in your demand for the best products, quality and reliability. Providing "competence in
hygiene" and "quality - made in Germany", we guarantee that these demands will be met. Our certified quality
management systems is subject to close monitoring: one instrument to this end is our annual multi-day audit conducted
in accordance with ISO 13485 and ISO 9001. This guarantees that all MELAG products are manufactured and tested in
accordance with strict quality criteria.
The MELAG management and team.
General notes
Symbol
Explanation
Indicates a dangerous situation, which if not avoided, could entail slight to lifethreatening injuries.
Draws your attention to a situation, which if not avoided, could result in damage to
the instruments, the practice fittings or the device.
Draws your attention to important information.
Symbol
Explanation
Universal
Program
Words or phrases appearing on the display of the sterilizer are marked as software
citations.
Chapter 6 -
Logging
Reference to another text section within these instructions.
Figure 1/5
Reference to a detail in a figure – in the example, to part no. 5 in Figure 1.
General notes
Please read this user manual carefully before commissioning the device. The manual includes important safety
information. The functionality and value-retention of this sterilizer depends on the care accorded to it.
Please store this user manual carefully and in close proximity to your sterilizer. It represents a component of the product.
User group
This manual is addressed to doctors, their assistants and service departments.
Validity
This manual is valid for the steam sterilizer Vacuklav 23 B+ und Vacuklav 31 B+.
About this manual
Symbols used
Formatting rules
Symbols on the device
Symbol
Erklärung
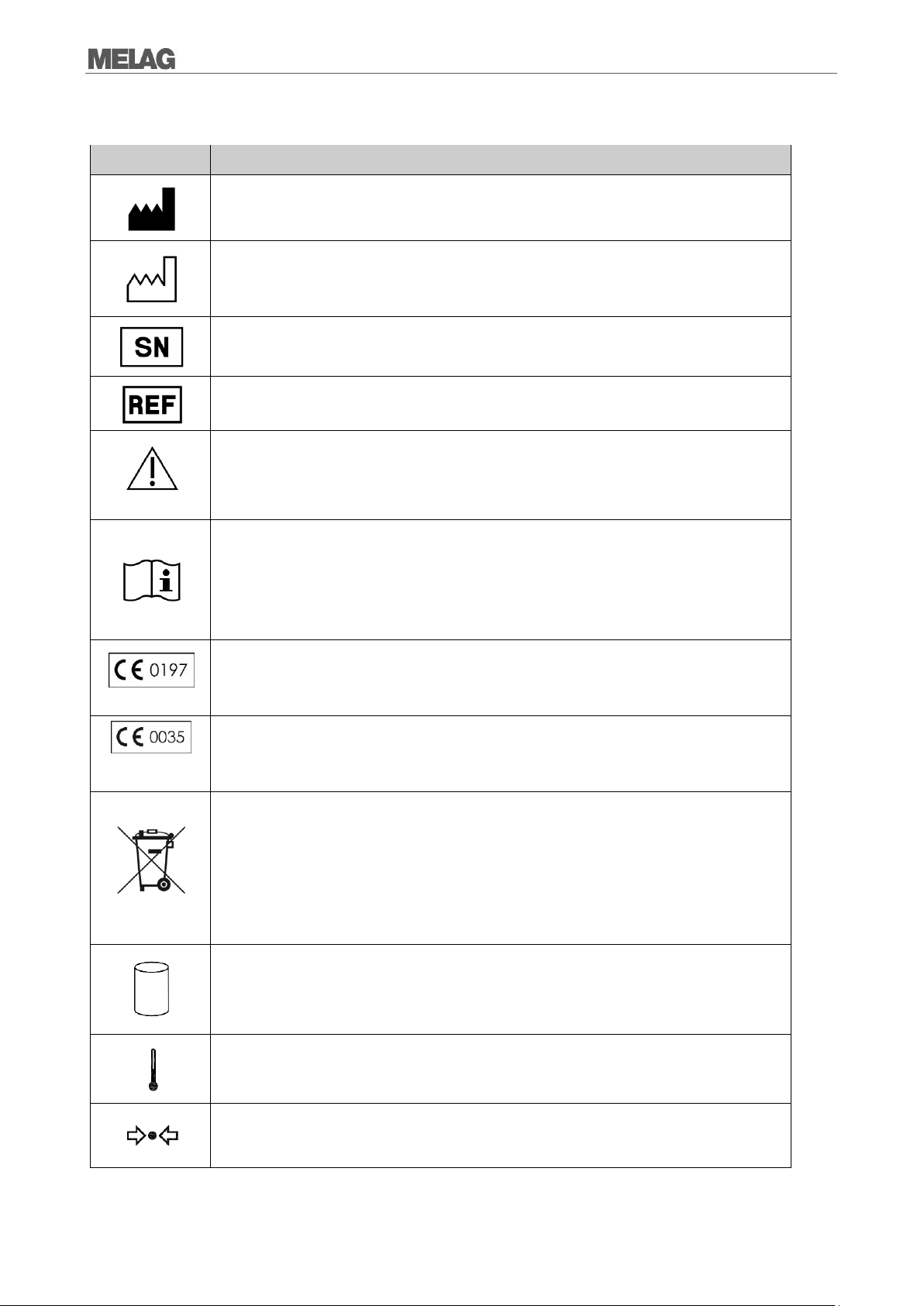
Manufacturer of the medical device
Date of manufacture of the medical device
Serial number of the medical device by the manufacturer
Article number of the medical device
This User Manual contains important safety information.
Failure to comply of the safety instructions could result in human and material damage.
Please read this user manual carefully before commissioning the device. The manual
includes important safety information. The functionality and value-retention of this sterilizer
depends on the care accorded to it. Please store this user manual carefully and in close
proximity to your sterilizer. It represents a component of the product.
In affixing this CE mark, the manufacturer declares that this medical product fulfils the basic
requirements of the medical device directive. The four-digit number confirms that this is
monitored by an approved certification agency.
In affixing this CE mark, the manufacturer declares that this medical product fulfils the basic
requirements of the pressure device directive. The four-digit number confirms that this is
monitored by an approved certification agency.
The symbol of the struck out waste bin identifies a device that may not be disposed in the
domestic waste. The vendor is responsible for appropriate disposal of the device - it must be
delivered to the vendor to be disposed of. With the designation of an apparatus with this
symbol, the manufacturer furthermore declares that he satisfies all requirements of the law
concerning the release, redemption and environmentally sound disposal of electric and
electronic appliances.
MELAG devices are synonymous for long-term quality. When you eventually need to
decommission your MELAG device, we offer a special disposal service. Simply contact your
stockist.
Indication of the scale of the chamber volume
Operating temperature of the device
Operating pressure of the device
General notes

General notes
Scope of delivery
Standard scope of delivery
Vacuklav 23 B+ or Vacuklav 31 B+
User manual
Technical manual
Guarantee
Manufacturer's inspection report
Declaration of conformity medical products directive
Declaration of conformity pressure device directive
Installation / set-up protocol
Mounts for trays and cassettes
Tray jack
Hose for emptying the interior water storage tank
TORX key for removing the carrying strap
Lever for emergency opening of the door
Key for the filter inside the chamber
2 Replacement device fuses on the door interior of the sterilizer
Optionally
Trays
Standard tray cassettes and jack
Additional mounts

Table of contents
Chapter 1 – Device description ....................... 8
Intended Use ............................................................... 8
Views of the device ...................................................... 9
Operating panel ..........................................................10
Mountings for the load ................................................10
Chapter 2 – Installation ................................. 11
Electrical connection ...................................................11
Feed water supply ......................................................11
Waste water connection .............................................11
Record of installation and set-up ................................11
Chapter 3 – Initial start-up ............................. 12
Switching on the sterilizer ...........................................12
Opening and closing the door .....................................12
Providing feed water ...................................................12
Setting the date and time ............................................13
Chapter 4 - Sterilizing ................................... 14
Preparing the sterilization material .............................14
Loading the sterilizer...................................................15
Selecting the program.................................................18
Selecting automatic pre-heating .................................19
Starting the program ...................................................19
Selecting additional drying ..........................................20
Program run ................................................................20
Sterilization phase is ended ........................................20
Drying phase ..............................................................21
Program end ...............................................................21
Manual program abort ................................................21
Displaying the daily batch counter ..............................23
Displaying the total batch counter ...............................23
Removing the sterilized equipment .............................24
Storing sterile equipment ............................................24
Chapter 5 - Logging ...................................... 25
Batch documentation ..................................................25
Output media ..............................................................25
Outputting logs immediately and automatically ...........28
Subsequent log output ................................................29
Displaying the log memory .........................................30
Deleting logs in the internal log memory .....................30
Reading logs correctly ................................................31
Table of contents
Batch-related checks .................................................. 33
Vacuum test ............................................................... 33
Bowie & Dick test ....................................................... 34
Checking the quality of the feed water ....................... 35
Check pre-heating temperature of the chamber ......... 35
Chapter 7 - Maintenance .............................. 36
Checks and cleaning .................................................. 36
Avoiding staining ........................................................ 37
Replacing the door seal ............................................. 37
Aligning the door seal sealing lip ................................ 38
Replacing or sterilzing the sterile filter ....................... 39
Cleaning the filter in the chamber............................... 40
Maintenance .............................................................. 41
Chapter 8 – Operating Pauses ..................... 41
Sterilization times ....................................................... 41
Operating pauses ....................................................... 41
Decommissioning ....................................................... 41
Recommissioning after relocation .............................. 41
Chapter 9 – Description of function ............... 42
The sterilization procedure ......................................... 42
Type of the feed water supply .................................... 42
Internal process monitoring ........................................ 42
Program ..................................................................... 42
Chapter 10 – Malfunctions ............................ 46
Before you call customer service ............................... 46
Opening the Emergency door during a power failure . 53
Changing the device fuses ......................................... 54
Glossary ....................................................... 55
Technical Data ............................................. 57
Accessories .................................................. 58
Chapter 6 – Functional Checks ..................... 33
Automatic functional checks .......................................33
Manual functional checks ...........................................33

Safety Instructions
When operating the sterilizer, please observe the following safety instructions as well as those contained
in subsequent chapters.
Use the device only for the purpose named in the user manual.
Power cable and mains socket
Set-up installation and commissioning
Safety Instructions
Never use this sterilizer to sterilize any fluids.
Never damage or alter the plug or power cable.
Never operate the sterilizer if the plug or power cable are damaged.
Never unplug by pulling on the power cable. Always take a grip on the plug.
The sterilizer should only be set-up, installed and commissioned by MELAG authorized persons.
The connections for electrical provision and water supply and discharge must be set-up by trained
personnel.
In accordance with current VDE specifications, the sterilizer is unsuitable for operation in areas
exposed to the danger of explosion.
The sterilizer is conceived for use outside the patient area. The device should be located a
minimum of 1.5 m radius away from the treatment area.
Observe all the information contained in the technical manual during commissioning.
Documentation media (computer, CF card reader, etc.) must be placed in such a way that they
cannot come into contact with liquids.
Failure to comply with the set-up conditions can result in malfunctions or damage to the sterilizer
and/or human injury.
Preparation and sterilization
Follow the manufacturer instructions of your textile articles and instruments regarding their
treatment and sterilization.
Observe the relevant standards and directives applicable to the treatment and sterilization of
textiles and instruments e.g. from the RKI, and DGSV.
Only ever use packaging material and systems which have been cleared by their manufacturer for
steam sterilization (consult the manufacturer’s instructions).
Only ever operate the steam sterilizer with a sterile filter inserted.
Program abort
Please observe that depending on the time of the program abort, opening the door following a
program abort can lead to hot steam leaving the chamber.
Depending on the time of the program abort, it is possible that the load is unsterile. Observe the
clear instructions on the sterilizer display. It may be necessary to re-pack and re-sterilize the
sterilization material.
Removing the sterilized equipment
Never use force to open the door.
Use a tray jack to remove the tray. Never touch the sterilized equipment, the chamber or the door
with bare hands. The components are hot.
Check the packaging on the sterilized equipment for damage when removing it from the sterilizer.
Should the packaging be damaged, re-pack the sterilization material and re-sterilize it.
6

Maintenance
Maintenance should only be performed by authorized personnel.
Carrying the sterilizer
The sterilizer should always be carried by two people.
Use the correct carrying strap to carry the sterilizer.
Malfunctions
Upon the incidence of repeated malfunction messages in the sterilizer, turn off the sterilizer and if
necessary, inform your stockist.
The sterilizer may only be serviced by authorized personnel.
The sterile filter must be sterilized or replaced following a power outage suffered in over-pressure
or following the incidence of the malfunction message Malfunction 32.
Safety Instructions
7

Chapter 1 – Device description
Chapter 1 – Device description
Intended Use
The sterilizer is designed for application in a medical context, e.g. clinics and medical and dental practices.
According to DIN EN 13060, this sterilizer is a Class-B-sterilizer. As a universal sterilizer, it is suited to
highly-demanding sterilization tasks. It can be used to sterilize instruments with a low inner diameter and
transfer instruments - both wrapped or unwrapped - and large quantities of textiles.
DANGER
The sterilization of fluids can result in a delay in boiling, which could result in damage to
the sterilizer and burns.
Never use this sterilizer to sterilize any fluids. It is not licensed for the sterilization of fluids.
WARNING
Failure to observe these provisions can result in damage or can compromise safety.
Only ever use the sterilizer for the applications as foreseen in the technical documentation
and only in connection with the devices and components as recommended by MELAG.
As with the preceding instrument treatment and in accordance with §2 MPBetreibV, the
sterilization of instruments and textiles using this sterilizer may only be carried out by
competent personnel.
When conducting sterilization procedures, only use instruments, packaging and textiles which
the manufacturer has cleared for steam sterilization.
8
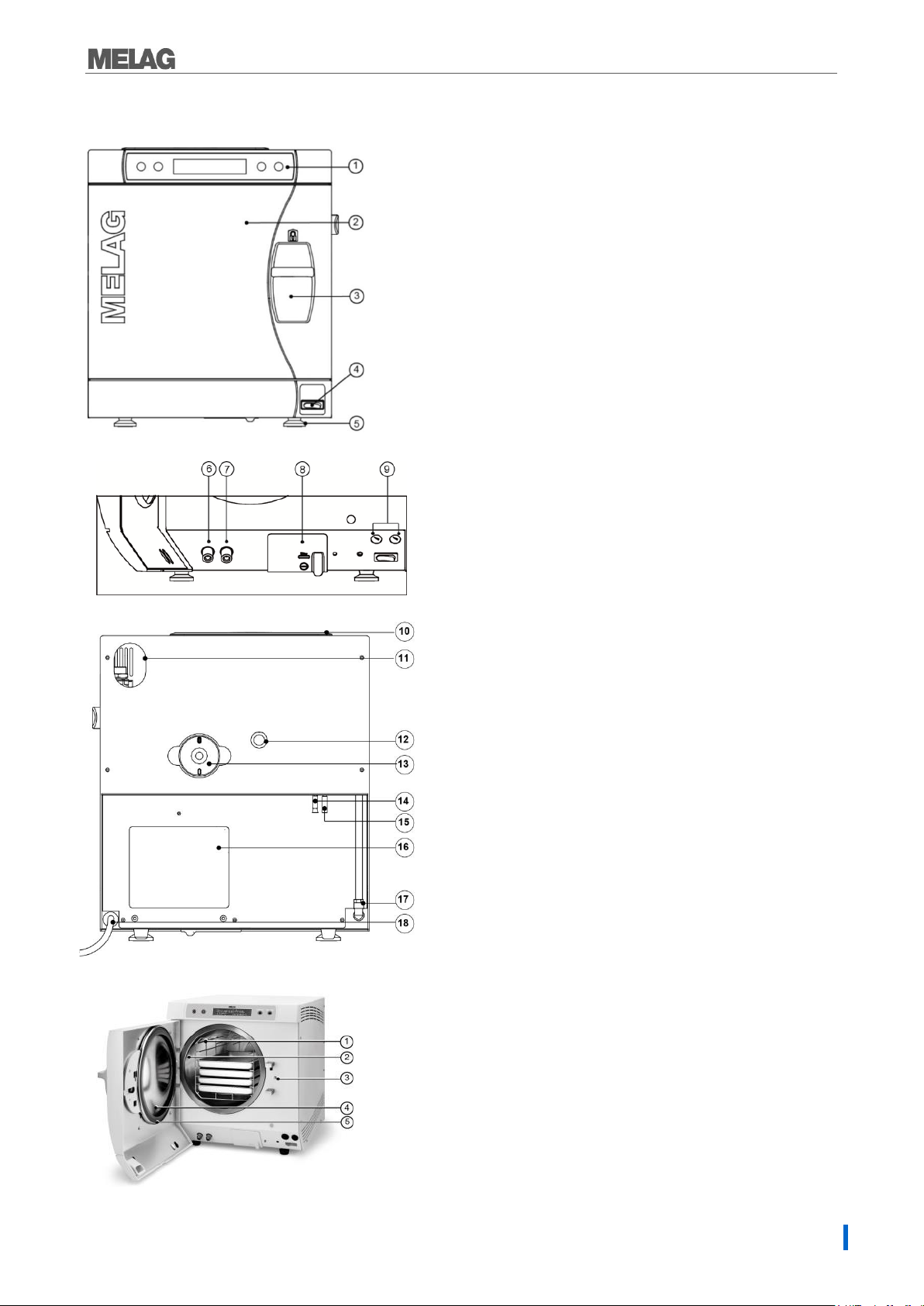
1. Operating and display panel
2. Door, pivots to the left
3. Sliding closure grip
4. Power switch
5. Front device foot (adjustable)
6. Connection for emptying the
storage tank waste water
7. Connection for emptying the
storage tank feed water
8. Serial data and printer connection (RS232)*
9. Fuses – 2x 16A/gRL
*hidden behind the white cover
10. Tank lid
11. Slot for optional upgrade with the
safety combination EN 1717
12. Spring safety valve
13. Sterile filter
14. One-way discharge (optional)
15. Emergency overflow hose
16. Cooler
17. Purified feed water inlet for
water treatment unit
18. Power supply cord
Fig. 2: Device view rear panel
1. Mounting to hold trays/cassettes
2. Chamber
3. Door locking pin
4. Round blank
5. Door seal
Views of the device
Chapter 1 – Device description
Fig. 1: Device view front side
Fig. 3: View of the interior
9
Chapter 1 – Device description
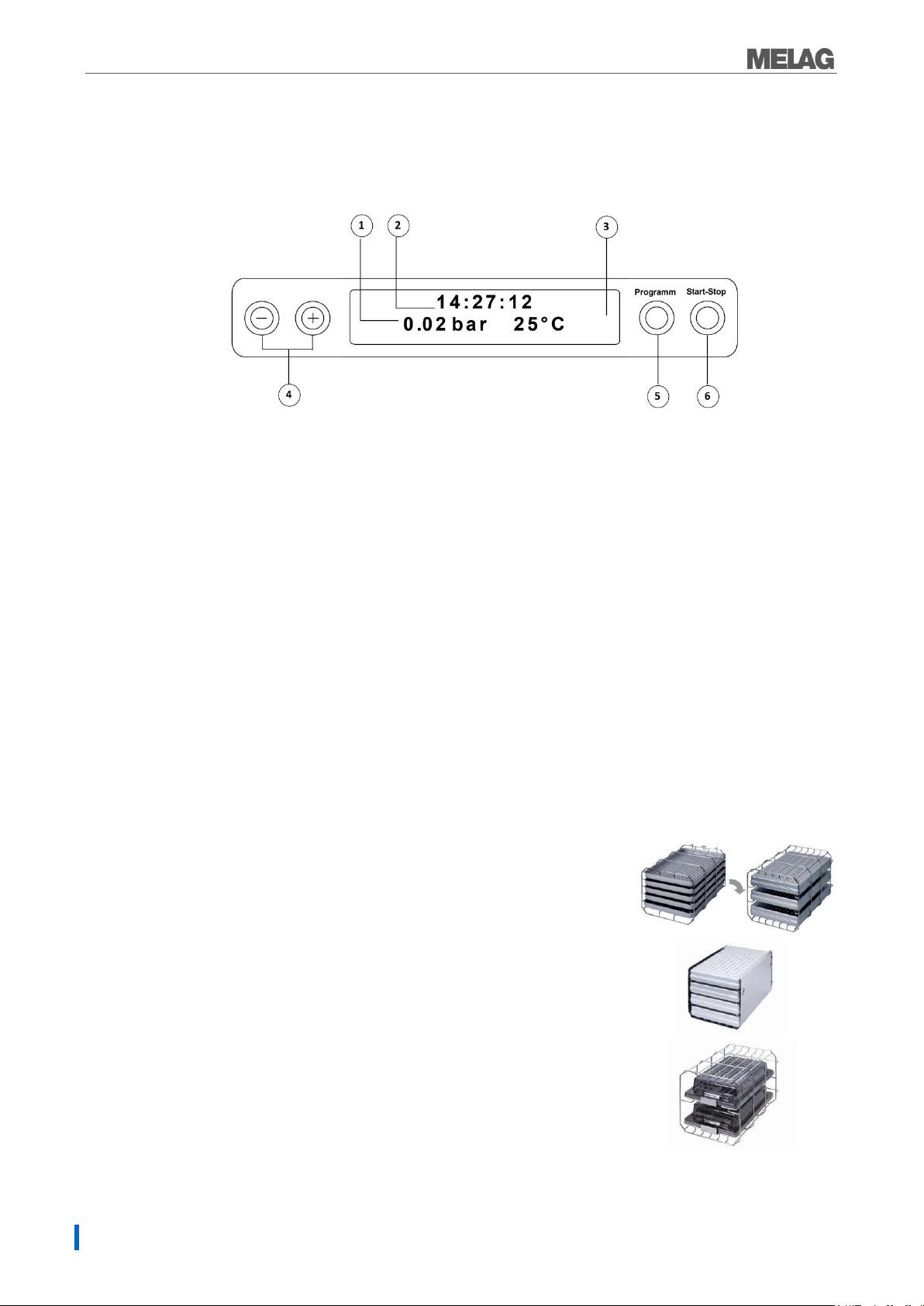
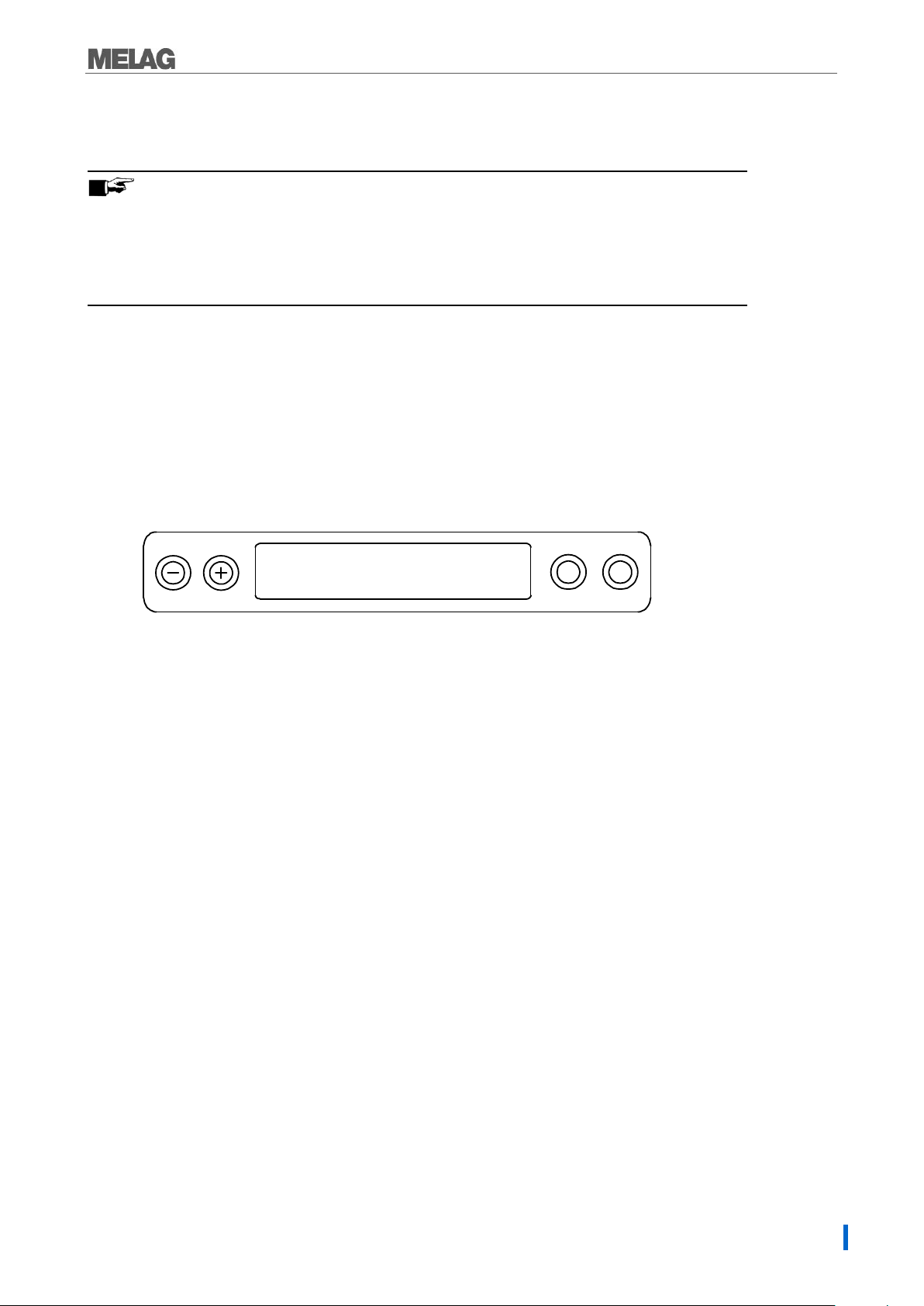
1. 2-line LC display
for program status display and parameter display.
2. Time (h:min:s)
3. Chamber pressure (bar) and (steam)- temperature (°C)
4. Function key (-) and (+)
to select, set and display special functions:
print, date/time, preheating, total batches, conductivity, acknowledge error, key (+) for unlocking the door.
5. Program selection key (P)
to select the sterilization programs/test programs and select or set options (submenus) of the
special functions.
6. Start – Stop key (S)
to start programs, terminate programs/drying as well as control of the special functions.
Mount A
The sterilizer is always delivered with a mount for holding trays or
cartridges.
The mounting (A) is standard and can hold either five trays or three
standard tray cassettes rotated by 90°.
Mount B
The mounting (B) can hold four standard tray cassettes or four trays.
Mount D
The mounting (D) can hold two high cassettes
(e.g. for implant cassettes) or four trays rotated by 90°.
Operating panel
The operating panel consists of a two-row alphanumerical LED display and four membrane keys.
Initial state
The display switches to the initial state after every activation of the device. This displays the current time
and chamber pressure in bar and the (steam) temperature in °C.
Mountings for the load
10

Chapter 2 – Installation
Chapter 2 – Installation
PLEASE NOTE
The autoclave should only be set-up, installed and commissioned by MELAG authorized persons.
Please observe the technical manual regarding installation. This contains all building-side
requirements.
Electrical connection
DANGER
Incorrectly performed electrical connections can result in a short-circuit, fire, water damage and/or
an electric shock.
This could result in serious injury.
The connections for electrical provision and water supply and discharge must be set-up by
trained personnel.
Observe the information regarding the installation and commissioning provided in the
technical manual.
Observe the following safety measures when dealing with the mains cable and plug:
Never splice or change the power cable.
Never bend or twist the power cable.
Never pull on the mains cable to take the power plug out of the socket.
Never place any heavy objects on the power cable.
Ensure that the power cable does not become jammed (e.g. between the doors or windows)
Never lead the cable along a source of heat.
Never use any nails, paper fasteners or similar objects to fix the cable.
Should the cable or plug become damaged, switch off the sterilizer. The power cable and plug should
only be replaced by authorized personnel.
Failure to observe these provisions can result in damage to the cable or plug and/or a fire or an
electric shock. This could result in serious injury.
Feed water supply
Steam sterilization requires distilled or demineralized/de-ionized water. Use only demineralized or distilled
water according to DIN EN 13060, Appendix C. The feed water supply is provided either by an external
water storage tank or with a water treatment unit see Chapter 3 – Initial start-up. Detailed information
regarding the connection to a water treatment unit is provided in the technical manual.
Waste water connection
The waste water can either be collected in an internal storage tank (left side) and manually emptied or
automatically drained via the one-way drain. An upgrade set for the tank drain is available for connecting
the sterilizer to the effluent. Detailed information regarding the connection to the effluent is provided in the
technical manual.
Record of installation and set-up
The record of installation and set-up is to be completed by the responsible person and a copy be sent to
both MELAG and the stockist as proof of correct set-up, installation and commissioning. This is a
constituent part of any guarantee claim.
11

Chapter 3 – Initial start-up
When feed water is supplied via the internal storage tank,
this needs to be filled manually from time to time. The
sterilizer will issue a maintenance message at the relevant
time.
The internal storage tank holds max. 5 liters. This volume
of feed water in the circulation system is sufficient for up to
7 sterilization runs.
To fill the storage tank with fresh feed water, remove the
lid and fill the right-hand chamber of the storage tank with
fresh feed water up to the MAX mark.
Chapter 3 – Initial start-up
Switching on the sterilizer
Turn the power switch on to power the sterilizer (page 9(4).
After switching on the sterilizer with the power switch, the display shows in alternation to the initial state
the message: Unlocking door with key (+), if the door is closed.
PLEASE NOTE
The trays and all accessories must be removed from the chamber directly after the sterilizer
having been switched on for the first time and before commissioning.
Opening and closing the door
The door can only be opened when the display shows: Acknowledge with '+'/ Unlock door
with '+' key.
1. Press the (+) key. You can open the door after hearing an audible click.
2. Close the door with light pressure against the chamber flange and simultaneously press down the
sliding-closure grip.
Providing feed water
Using the internal storage tank
Setting the feed water supply on the sterilizer
The INTERN function must be set in order to enable feed water supply via the internal storage tank. The
EXTERN function must be set in order to enable feed water supply via a water treatment unit.
1. Press the (+) and (-) keys simultaneously to select the set-up menu Function.
The display shows the menu Function: Last batch no.
2. Navigate using the (+) or (-) keys until the display shows: Function: Feed water test:
3. Press the (P) key. The display shows the option currently set, e.g. pre-heating yes.
4. Press the (P) key again to change to the desired setting (INTERN/EXTERN).
12
5. Press the (S) key to save the setting and to leave the menu.
Repeated pressing of the (S) key enables you to leave the menu entirely and return to the display basic
state.
Chapter 3 – Initial start-up
Programm
Start-Stop
Function
Date / time
Using a water treatment unit
Observe the specifications in the technical manual when using a water treatment unit.
PLEASE NOTE
Should you wish to use a water treatment unit from another manufacturer, please consult
MELAG.
Failure to comply with these provisions can result in damage to the sterilizer and/or human
injury.
Setting the date and time
Correct batch documentation requires the correct date and time setting on the sterilizer. Ensure that you
take into account the clock change in autumn and summer, as this is not adjusted automatically. Set the
date and time as follows:
1. Press the (+) and (-) keys simultaneously to select the set-up menu Function.
The display shows the menu Function: Last batch no.
2. Navigate in the Function menu using the (+) or (-) keys until the display shows:
3. Press the (P) key to confirm. The current hour is displayed.
4. Choose one of the following setting possibilities using the (+) or (-) keys: Hours, minute, second,
day, month, year.
5. To adjust the Hours parameter, press the (P) key to confirm.
The current value flashes on the display.
6. You can increase or reduce the value using the (+) and (-) keys.
7. To save the value, confirm with the (P) key.
The current value set no longer flashes on the display.
To alter the other parameters, proceed in a similar fashion.
8. After ending the settings, press the (S) key to leave the menu.
The display shows the menu Function: Date / time.
9. Repeated pressing of the (S) key enables you to leave the menu and the display returns to its
basic state.
13
Chapter 4 - Sterilizing
Chapter 4 - Sterilizing
Preparing the sterilization material
A significant prerequisite for safe disinfection and sterilization of sterilizing materials is the appropriate
preparation, i.e. cleaning and maintenance of the sterilizing materials according to the manufacturer's
instructions. Furthermore the materials, cleaning agents and processing procedure employed are of
significance.
Only ever operate the steam sterilizer with a sterile filter inserted.
PLEASE NOTE
Wherever possible, please ensure the separate sterilization of textiles and instruments in
separate sterilization containers or sterilization packaging. This leads to better drying results.
WARNING
Treating instruments
Please ensure the following when treating used and brand-new instruments:
Follow both the instrument manufacturer’s instructions regarding treatment and sterilization and
comply with the relevant standards and directives e.g. from the BGV A1, RKI and DGSV.
Clean the instruments exceptionally thoroughly e.g. using a washer-disinfector.
Rinse the instruments after washing and disinfecting, where possible with de-mineralized or distilled
water and then dry the instruments thoroughly with a clean, non-fuzzing cloth.
Use only those care agents suitable for steam sterilization. Consult the manufacturer of the care
agents.
DANGER
The incorrect treatment of instruments could result in any dirt residue being loosened during
sterilization. The presence of residual disinfection and cleaning fluids results in corrosion.
The use of unsuitable care agents e.g. water repellent agents or oils impermeable to steam
could result in unsterile instruments. This represents a danger to the health of both
patients and yourself.
This could result in increased maintenance requirements and a restriction of the sterilizer
function.
Comply with the treatment instructions contained in these instructions.
When using ultra-sound devices, care equipment for hand pieces and washing and disinfection devices,
please observe the manufacturer’s treatment instructions.
14

Chapter 4 - Sterilizing
Treating textiles
Please observe the following points when treating textiles and putting the textiles in sterilization containers:
Observe and comply with both the manufacturer's instructions of the textiles regarding treatment and
sterilization as well as the relevant standards and directives e.g. from the RKI, and DGSV.
Arrange the folds in the textiles parallel to each other.
Stack textiles vertically wherever possible and not too closely together in the sterilization chamber.
This enables the development of flow channels.
Retain the vertical stacking system when packing textiles in the sterilization container.
If textile packages do not remain together, wrap the textiles in sterilization paper.
Only ever sterilize dry textiles.
The textiles must not be permitted to come into direct contact with the floor or walls of the sterilization
chamber; otherwise they will become saturated with condensate.
DANGER
Steam penetration of the textile package can be restricted and/or will produce poor drying results.
The textiles could not be sterilized.
This could endanger the health of patient and practice team.
Comply with the treatment instructions contained in these instructions.
Loading the sterilizer
Only when correctly loaded is effective sterilization and good drying possible.
Ensure the following during loading:
Insert trays or cassettes in the chamber only with their appropriate mount.
Use perforated trays such as those from MELAG. Only in this way can the condensate drain off. The
use of a non-perforated base or half-shell to accept the sterilization material can result in poor drying
results.
The use of paper tray inserts can result in poor drying results.
Packaging
Only ever use packaging materials and systems (sterilization barrier systems) corresponding to the
standard DIN EN IS0 11607-1.
The correct use of suitable packaging is important in achieving successful sterilization results.
You can use re-usable rigid packaging systems such as e.g. standard tray cassettes or soft packaging
such as transparent sterilization packaging, paper bags, sterilization paper, textiles or fleece.
Closed sterilization containers
Please observe the following when using closed sterilization containers for sterilization material:
Use aluminium sterilization containers. Aluminium retains and conducts heat and thus improves
drying.
Closed sterilization containers must be either perforated or have a valve on at least one side -
optimally the bottom.
Wherever possible, please ensure that sterilization containers are stacked on top of those of identical
size, so that the condensate can run down their sides.
Our TIP: MELAG sterilization containers fulfil the requirements of DIN EN 868-8 for
successful sterilization and drying. They have a perforated lid and are fitted with
single-use paper filters.
15
Chapter 4 - Sterilizing
WARNING
The use of unsuitable sterilization containers results in insufficient steam penetration and even
failure of the sterilization. This can also prevent condensate drain-off.
This produces poor drying results. This can result in unsterile instruments and thus
endanger the health of patient and practice team.
Closed sterilization containers must be either perforated on at least one location - optimally
the bottom - or be equipped with a valve.
WARNING
Incorrect stacking of the sterilization containers can result in the dripping condensate being
unable to drain off to the chamber floor. This would then saturate the sterilization material directly
underneath it.
This produces poor drying results. This can result in unsterile instruments and thus
endanger the health of patient and practice team.
Do not cover the perforations when stacking the sterilization containers.
Soft sterilization packaging
Soft sterilization packaging can be used in both sterilization containers and on trays. Please observe the
following when using soft sterilization packaging e.g. MELAfol:
Arrange soft sterilization packaging in a perpendicular position and at narrow intervals.
Do not place multiple soft sterilization packages flat on top of each other on a tray or in a container.
If the seam seal tears during sterilization, this could be caused by the choice of undersized packaging.
Should this not be the case, re-pack the instruments and sterilize them again.
Should the seam seal rip during sterilization, extend the sealing pulse on the sealing device or make a
double seam.
Multiple wrapping
The sterilizer functions on the fractionated pre-vacuum method. This permits the use of multiple wrapping.
16
Chapter 4 - Sterilizing
Loading variations*
Vacuklav 23 B+
Vacuklav 31 B+
Instruments
Textiles
Instruments
Textiles
Max no. per single piece
2 kg
1.8 kg
2 kg
1.8 kg
Maximum total
5 kg
1.8 kg
5 kg
1.8 kg
Loading variant
mounting A
max. 5 trays, depth 420 mm max. 6
sterilization containers 15 K
max. 3 sterilization containers 15M
max. 2 sterilization containers 15G
max. 6 sterilization containers 17K
max. 3 sterilization containers 17M
max. 1 sterilization containers 17G
max. 3 swab drums 17R
max. 1 sterilization containers 23G
max. 2 sterilization containers 23M
max. 2 swab drums 23R
max. 2 sterilization containers 28M
max. 1 sterilization containers 28G
max. 3 standard tray cassettes
max. 5 trays, depth 290 mm
max. 3 sterilization containers 15 K
max. 3 sterilization containers 17K
max. 3 swab drums 17R
max. 2 swab drums 23R
max. 2 sterilization containers 28M
max. 1 sterilization containers 28G
max. 3 standard tray cassettes
*MELAG mount, trays and sterilization containers. See appendix A – accessories
Mixed loads
Please observe the following when using mixed loads:
Always place textiles at the top.
Place the sterilization containers at the bottom.
Place unwrapped instruments at the bottom.
Place transparent sterilization packaging and paper bags at the top - except in combination with
textiles. In this case, place them at the bottom.
Place heavy loads at the bottom.
Transparent sterilization packaging should be loaded on their edges so that the paper side and film
side are alternating in contact. If this is not possible, the paper side should face downwards.
Load patterns designed especially for the dental sector are available from the download area of the
MELAG website: www.melag.de.
17
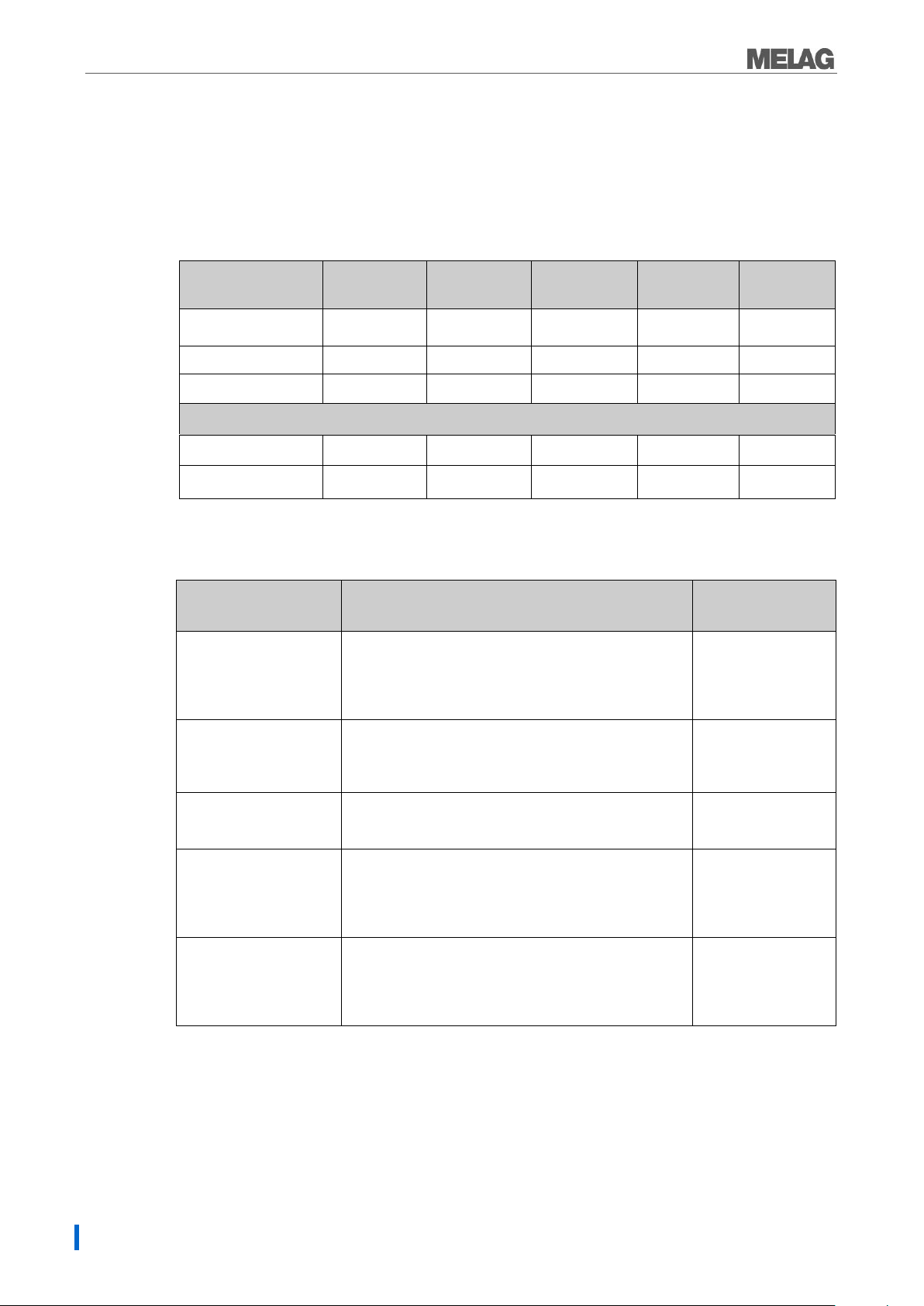
Chapter 4 - Sterilizing
Universal-
Program
Quick-
Program B:
Quick-
Program S
Gentle-
Program
Prion-
Program
Sterilization
temperature
134 ˚C
134 ˚C
134 ˚C
121 ˚C
134 ˚C
Sterilization pressure
2 bar
2 bar
2 bar
1 bar
2 bar
Sterilization time
5.5 min.
5.5 min.
3.5 min.
20.5 min.
20.5 min.
Operating times
Operating time*
30min.
30 min.
15 min.
45 min.
45 min.
Drying
20 min.
10 min.
5 min.
20 min.
20 min.
Program
Packaging/suitability
Load amount*
Universal-Program
Single and multiple wrapped mixed loads;
hollow-bodied articles, long articles with a low
diameter; instruments with narrow lumen
(hollow body A) andsimple hollow items
(hollow body B)
5 kg instruments
1.8 kg textiles
Quick-Program B
single wrapped and unwrapped (no textiles)
Transfer instruments, long, instruments with narrow
lumen (hollow body A) andsimple hollow items
(hollow body B)
single wrapped
1.5 kg or
unwrapped 5 kg
Quick-Program S
Only unwrapped (no textiles)
Simple solid instruments,simple hollow items(hollow
body B)
5 kg unwrapped
instruments
Gentle-Program
single and multiple wrapping Larger quantities of
textiles, thermo-instable goods (e.g. plastic, rubber
articles); Mixed loads; instruments with narrow lumen
(hollow body A) andsimple hollow items(hollow body
B)
textiles 1.8 kg or
Thermo-unstable
items 5 kg
Prion-Program
Single and multiple wrapped instruments under
suspicion of carrying the danger of infection through
abnormally altered proteins (e.g. Creutzfeld-Jacob,
BSE); instruments with narrow lumen (hollow body A)
andsimple hollow items(hollow body B)
5 kg instruments
1.8 kg textiles
Selecting the program
You can switch between the initial state and the desired program using the program selection switch.Now
select the sterilization program according to how and whether the sterilization material is packed. It is also
necessary to take into account the temperature resistance of the sterilization material. The following tables
show which program is to be selected for which sterilization material.
Table 1: Overview of the Sterilization programs
*without drying (full load for Vacuclav 23 B+ and Vacuklav 31 B+: 5 kg) and depending on loading and
installation conditions, eg. mains voltage.
Table 2: Overview of the use of the respective sterilization programs
*valid for Vacuklav 23 B+ and Vacuklav 31 B+
18
 Loading...
Loading...