Page 1

User manual
Vacuklav®23 B+
Vacuklav®31 B+
Steam sterilizer
From software version 5.15
Dear Dr
We should like to extend our thanks for the expression of trust in our company which you have displayed through the
purchase of this MELAG device.
As an owner-run and operated family concern founded in 1951, we have a long history of successful specialization in
hygiene products for practice-based use. Our focus on quality, highest standards of operational reliability and innovation
has established MELAG as the world’s leading manufacturer in the area instrument treatment and hygiene.
You, our customer are justified in your demand for the best products, quality and reliability. Providing "competence in
hygiene" and "quality - made in Germany", we guarantee that these demands will be met. Our certified quality
management systems is subject to close monitoring: one instrument to this end is our annual multi-day audit conducted
in accordance with ISO 13485 and ISO 9001. This guarantees that all MELAG products are manufactured and tested in
accordance with strict quality criteria.
The MELAG management and team.
Page 2
General notes
Symbol
Explanation
Indicates a dangerous situation, which if not avoided, could entail slight to lifethreatening injuries.
Draws your attention to a situation, which if not avoided, could result in damage to
the instruments, the practice fittings or the device.
Draws your attention to important information.
Symbol
Explanation
Universal
Program
Words or phrases appearing on the display of the sterilizer are marked as software
citations.
Chapter 6 -
Logging
Reference to another text section within these instructions.
Figure 1/5
Reference to a detail in a figure – in the example, to part no. 5 in Figure 1.
General notes
Please read this user manual carefully before commissioning the device. The manual includes important safety
information. The functionality and value-retention of this sterilizer depends on the care accorded to it.
Please store this user manual carefully and in close proximity to your sterilizer. It represents a component of the product.
User group
This manual is addressed to doctors, their assistants and service departments.
Validity
This manual is valid for the steam sterilizer Vacuklav 23 B+ und Vacuklav 31 B+.
About this manual
Symbols used
Formatting rules
Page 3
Symbols on the device
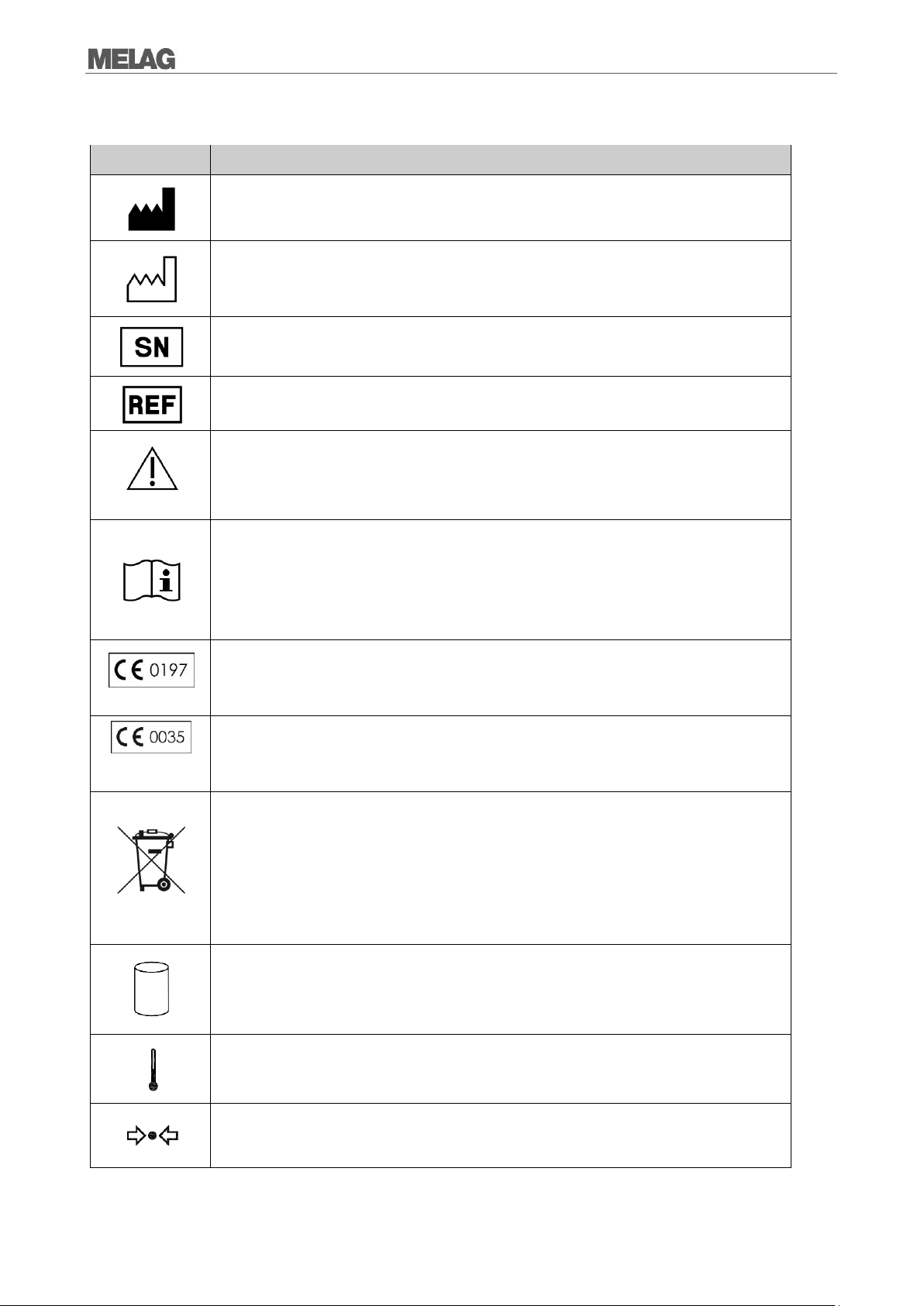
Symbol
Erklärung
Manufacturer of the medical device
Date of manufacture of the medical device
Serial number of the medical device by the manufacturer
Article number of the medical device
This User Manual contains important safety information.
Failure to comply of the safety instructions could result in human and material damage.
Please read this user manual carefully before commissioning the device. The manual
includes important safety information. The functionality and value-retention of this sterilizer
depends on the care accorded to it. Please store this user manual carefully and in close
proximity to your sterilizer. It represents a component of the product.
In affixing this CE mark, the manufacturer declares that this medical product fulfils the basic
requirements of the medical device directive. The four-digit number confirms that this is
monitored by an approved certification agency.
In affixing this CE mark, the manufacturer declares that this medical product fulfils the basic
requirements of the pressure device directive. The four-digit number confirms that this is
monitored by an approved certification agency.
The symbol of the struck out waste bin identifies a device that may not be disposed in the
domestic waste. The vendor is responsible for appropriate disposal of the device - it must be
delivered to the vendor to be disposed of. With the designation of an apparatus with this
symbol, the manufacturer furthermore declares that he satisfies all requirements of the law
concerning the release, redemption and environmentally sound disposal of electric and
electronic appliances.
MELAG devices are synonymous for long-term quality. When you eventually need to
decommission your MELAG device, we offer a special disposal service. Simply contact your
stockist.
Indication of the scale of the chamber volume
Operating temperature of the device
Operating pressure of the device
General notes
Page 4

General notes
Scope of delivery
Standard scope of delivery
Vacuklav 23 B+ or Vacuklav 31 B+
User manual
Technical manual
Guarantee
Manufacturer's inspection report
Declaration of conformity medical products directive
Declaration of conformity pressure device directive
Installation / set-up protocol
Mounts for trays and cassettes
Tray jack
Hose for emptying the interior water storage tank
TORX key for removing the carrying strap
Lever for emergency opening of the door
Key for the filter inside the chamber
2 Replacement device fuses on the door interior of the sterilizer
Optionally
Trays
Standard tray cassettes and jack
Additional mounts
Page 5

Table of contents
Chapter 1 – Device description ....................... 8
Intended Use ............................................................... 8
Views of the device ...................................................... 9
Operating panel ..........................................................10
Mountings for the load ................................................10
Chapter 2 – Installation ................................. 11
Electrical connection ...................................................11
Feed water supply ......................................................11
Waste water connection .............................................11
Record of installation and set-up ................................11
Chapter 3 – Initial start-up ............................. 12
Switching on the sterilizer ...........................................12
Opening and closing the door .....................................12
Providing feed water ...................................................12
Setting the date and time ............................................13
Chapter 4 - Sterilizing ................................... 14
Preparing the sterilization material .............................14
Loading the sterilizer...................................................15
Selecting the program.................................................18
Selecting automatic pre-heating .................................19
Starting the program ...................................................19
Selecting additional drying ..........................................20
Program run ................................................................20
Sterilization phase is ended ........................................20
Drying phase ..............................................................21
Program end ...............................................................21
Manual program abort ................................................21
Displaying the daily batch counter ..............................23
Displaying the total batch counter ...............................23
Removing the sterilized equipment .............................24
Storing sterile equipment ............................................24
Chapter 5 - Logging ...................................... 25
Batch documentation ..................................................25
Output media ..............................................................25
Outputting logs immediately and automatically ...........28
Subsequent log output ................................................29
Displaying the log memory .........................................30
Deleting logs in the internal log memory .....................30
Reading logs correctly ................................................31
Table of contents
Batch-related checks .................................................. 33
Vacuum test ............................................................... 33
Bowie & Dick test ....................................................... 34
Checking the quality of the feed water ....................... 35
Check pre-heating temperature of the chamber ......... 35
Chapter 7 - Maintenance .............................. 36
Checks and cleaning .................................................. 36
Avoiding staining ........................................................ 37
Replacing the door seal ............................................. 37
Aligning the door seal sealing lip ................................ 38
Replacing or sterilzing the sterile filter ....................... 39
Cleaning the filter in the chamber............................... 40
Maintenance .............................................................. 41
Chapter 8 – Operating Pauses ..................... 41
Sterilization times ....................................................... 41
Operating pauses ....................................................... 41
Decommissioning ....................................................... 41
Recommissioning after relocation .............................. 41
Chapter 9 – Description of function ............... 42
The sterilization procedure ......................................... 42
Type of the feed water supply .................................... 42
Internal process monitoring ........................................ 42
Program ..................................................................... 42
Chapter 10 – Malfunctions ............................ 46
Before you call customer service ............................... 46
Opening the Emergency door during a power failure . 53
Changing the device fuses ......................................... 54
Glossary ....................................................... 55
Technical Data ............................................. 57
Accessories .................................................. 58
Chapter 6 – Functional Checks ..................... 33
Automatic functional checks .......................................33
Manual functional checks ...........................................33
Page 6

Safety Instructions
When operating the sterilizer, please observe the following safety instructions as well as those contained
in subsequent chapters.
Use the device only for the purpose named in the user manual.
Power cable and mains socket
Set-up installation and commissioning
Safety Instructions
Never use this sterilizer to sterilize any fluids.
Never damage or alter the plug or power cable.
Never operate the sterilizer if the plug or power cable are damaged.
Never unplug by pulling on the power cable. Always take a grip on the plug.
The sterilizer should only be set-up, installed and commissioned by MELAG authorized persons.
The connections for electrical provision and water supply and discharge must be set-up by trained
personnel.
In accordance with current VDE specifications, the sterilizer is unsuitable for operation in areas
exposed to the danger of explosion.
The sterilizer is conceived for use outside the patient area. The device should be located a
minimum of 1.5 m radius away from the treatment area.
Observe all the information contained in the technical manual during commissioning.
Documentation media (computer, CF card reader, etc.) must be placed in such a way that they
cannot come into contact with liquids.
Failure to comply with the set-up conditions can result in malfunctions or damage to the sterilizer
and/or human injury.
Preparation and sterilization
Follow the manufacturer instructions of your textile articles and instruments regarding their
treatment and sterilization.
Observe the relevant standards and directives applicable to the treatment and sterilization of
textiles and instruments e.g. from the RKI, and DGSV.
Only ever use packaging material and systems which have been cleared by their manufacturer for
steam sterilization (consult the manufacturer’s instructions).
Only ever operate the steam sterilizer with a sterile filter inserted.
Program abort
Please observe that depending on the time of the program abort, opening the door following a
program abort can lead to hot steam leaving the chamber.
Depending on the time of the program abort, it is possible that the load is unsterile. Observe the
clear instructions on the sterilizer display. It may be necessary to re-pack and re-sterilize the
sterilization material.
Removing the sterilized equipment
Never use force to open the door.
Use a tray jack to remove the tray. Never touch the sterilized equipment, the chamber or the door
with bare hands. The components are hot.
Check the packaging on the sterilized equipment for damage when removing it from the sterilizer.
Should the packaging be damaged, re-pack the sterilization material and re-sterilize it.
6
Page 7

Maintenance
Maintenance should only be performed by authorized personnel.
Carrying the sterilizer
The sterilizer should always be carried by two people.
Use the correct carrying strap to carry the sterilizer.
Malfunctions
Upon the incidence of repeated malfunction messages in the sterilizer, turn off the sterilizer and if
necessary, inform your stockist.
The sterilizer may only be serviced by authorized personnel.
The sterile filter must be sterilized or replaced following a power outage suffered in over-pressure
or following the incidence of the malfunction message Malfunction 32.
Safety Instructions
7
Page 8

Chapter 1 – Device description
Chapter 1 – Device description
Intended Use
The sterilizer is designed for application in a medical context, e.g. clinics and medical and dental practices.
According to DIN EN 13060, this sterilizer is a Class-B-sterilizer. As a universal sterilizer, it is suited to
highly-demanding sterilization tasks. It can be used to sterilize instruments with a low inner diameter and
transfer instruments - both wrapped or unwrapped - and large quantities of textiles.
DANGER
The sterilization of fluids can result in a delay in boiling, which could result in damage to
the sterilizer and burns.
Never use this sterilizer to sterilize any fluids. It is not licensed for the sterilization of fluids.
WARNING
Failure to observe these provisions can result in damage or can compromise safety.
Only ever use the sterilizer for the applications as foreseen in the technical documentation
and only in connection with the devices and components as recommended by MELAG.
As with the preceding instrument treatment and in accordance with §2 MPBetreibV, the
sterilization of instruments and textiles using this sterilizer may only be carried out by
competent personnel.
When conducting sterilization procedures, only use instruments, packaging and textiles which
the manufacturer has cleared for steam sterilization.
8
Page 9
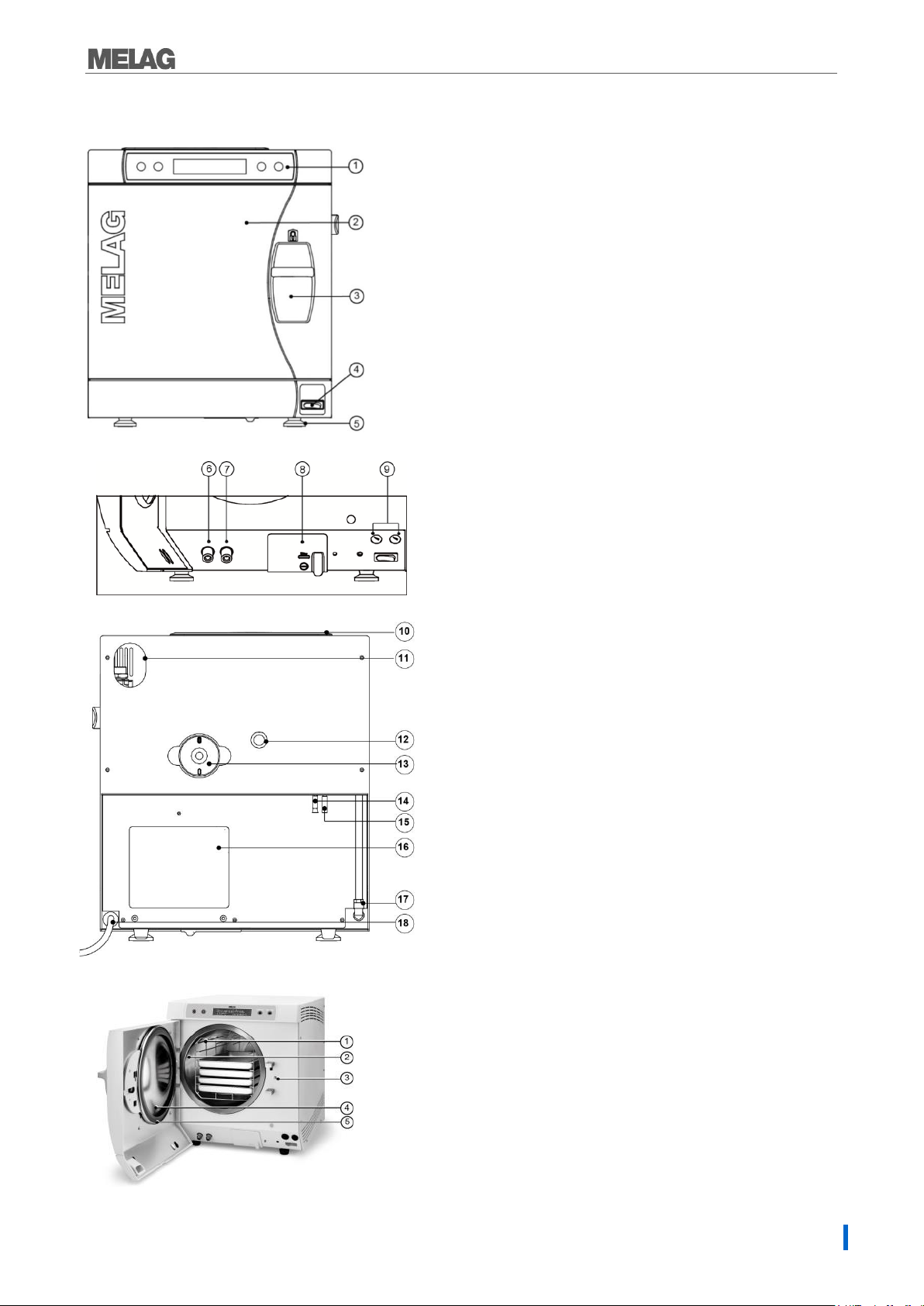
1. Operating and display panel
2. Door, pivots to the left
3. Sliding closure grip
4. Power switch
5. Front device foot (adjustable)
6. Connection for emptying the
storage tank waste water
7. Connection for emptying the
storage tank feed water
8. Serial data and printer connection (RS232)*
9. Fuses – 2x 16A/gRL
*hidden behind the white cover
10. Tank lid
11. Slot for optional upgrade with the
safety combination EN 1717
12. Spring safety valve
13. Sterile filter
14. One-way discharge (optional)
15. Emergency overflow hose
16. Cooler
17. Purified feed water inlet for
water treatment unit
18. Power supply cord
Fig. 2: Device view rear panel
1. Mounting to hold trays/cassettes
2. Chamber
3. Door locking pin
4. Round blank
5. Door seal
Views of the device
Chapter 1 – Device description
Fig. 1: Device view front side
Fig. 3: View of the interior
9
Page 10
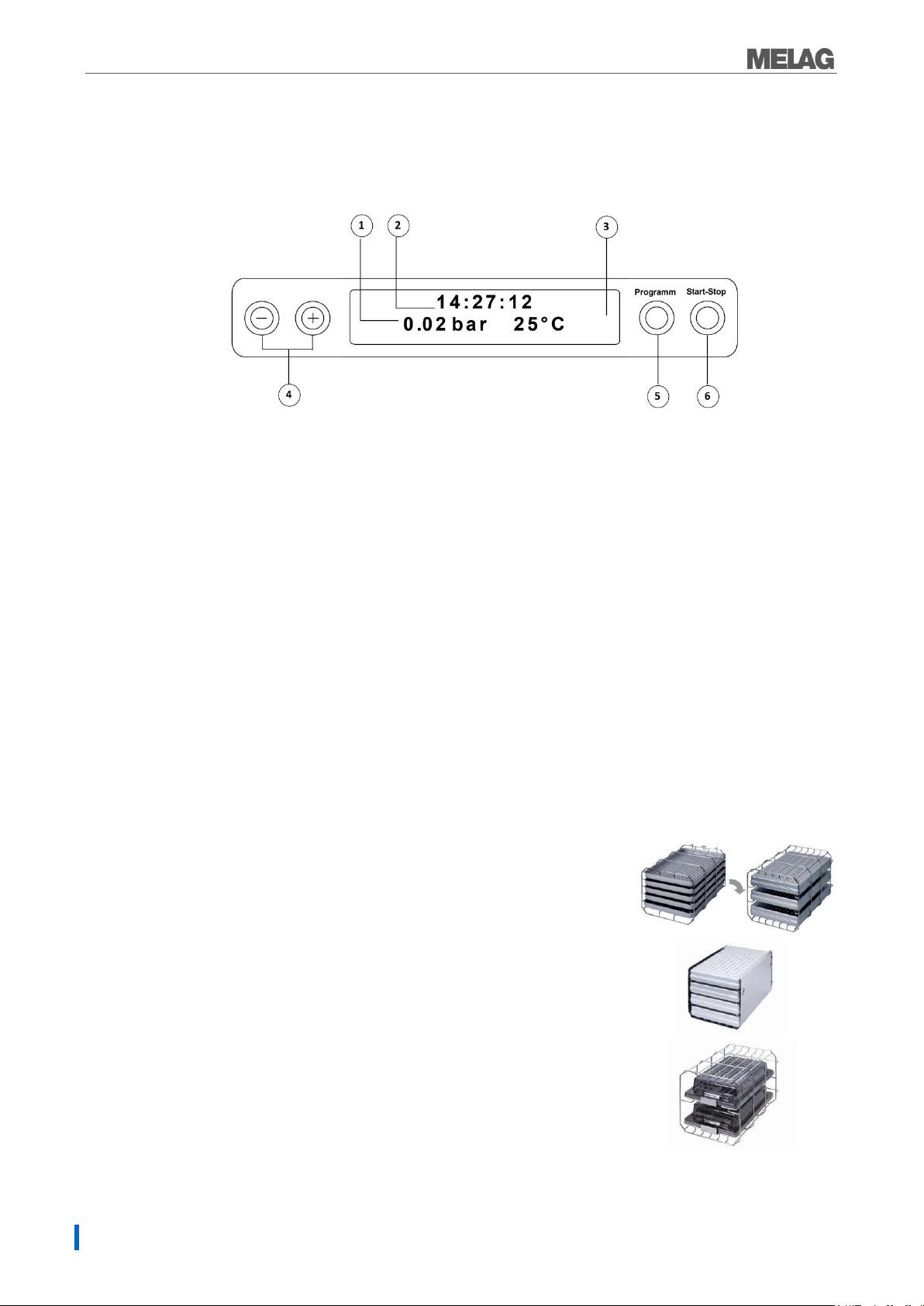
Chapter 1 – Device description
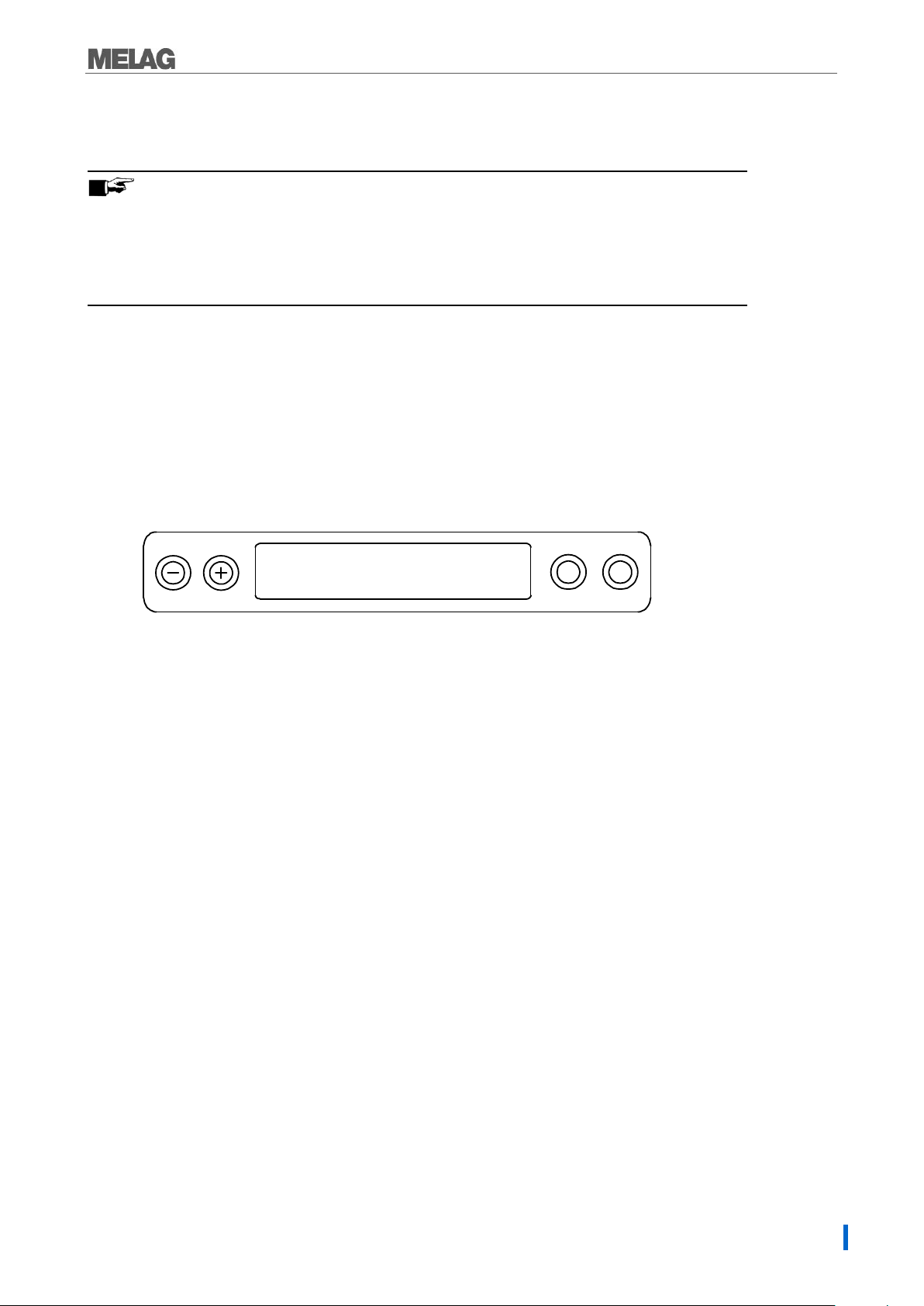
1. 2-line LC display
for program status display and parameter display.
2. Time (h:min:s)
3. Chamber pressure (bar) and (steam)- temperature (°C)
4. Function key (-) and (+)
to select, set and display special functions:
print, date/time, preheating, total batches, conductivity, acknowledge error, key (+) for unlocking the door.
5. Program selection key (P)
to select the sterilization programs/test programs and select or set options (submenus) of the
special functions.
6. Start – Stop key (S)
to start programs, terminate programs/drying as well as control of the special functions.
Mount A
The sterilizer is always delivered with a mount for holding trays or
cartridges.
The mounting (A) is standard and can hold either five trays or three
standard tray cassettes rotated by 90°.
Mount B
The mounting (B) can hold four standard tray cassettes or four trays.
Mount D
The mounting (D) can hold two high cassettes
(e.g. for implant cassettes) or four trays rotated by 90°.
Operating panel
The operating panel consists of a two-row alphanumerical LED display and four membrane keys.
Initial state
The display switches to the initial state after every activation of the device. This displays the current time
and chamber pressure in bar and the (steam) temperature in °C.
Mountings for the load
10
Page 11

Chapter 2 – Installation
Chapter 2 – Installation
PLEASE NOTE
The autoclave should only be set-up, installed and commissioned by MELAG authorized persons.
Please observe the technical manual regarding installation. This contains all building-side
requirements.
Electrical connection
DANGER
Incorrectly performed electrical connections can result in a short-circuit, fire, water damage and/or
an electric shock.
This could result in serious injury.
The connections for electrical provision and water supply and discharge must be set-up by
trained personnel.
Observe the information regarding the installation and commissioning provided in the
technical manual.
Observe the following safety measures when dealing with the mains cable and plug:
Never splice or change the power cable.
Never bend or twist the power cable.
Never pull on the mains cable to take the power plug out of the socket.
Never place any heavy objects on the power cable.
Ensure that the power cable does not become jammed (e.g. between the doors or windows)
Never lead the cable along a source of heat.
Never use any nails, paper fasteners or similar objects to fix the cable.
Should the cable or plug become damaged, switch off the sterilizer. The power cable and plug should
only be replaced by authorized personnel.
Failure to observe these provisions can result in damage to the cable or plug and/or a fire or an
electric shock. This could result in serious injury.
Feed water supply
Steam sterilization requires distilled or demineralized/de-ionized water. Use only demineralized or distilled
water according to DIN EN 13060, Appendix C. The feed water supply is provided either by an external
water storage tank or with a water treatment unit see Chapter 3 – Initial start-up. Detailed information
regarding the connection to a water treatment unit is provided in the technical manual.
Waste water connection
The waste water can either be collected in an internal storage tank (left side) and manually emptied or
automatically drained via the one-way drain. An upgrade set for the tank drain is available for connecting
the sterilizer to the effluent. Detailed information regarding the connection to the effluent is provided in the
technical manual.
Record of installation and set-up
The record of installation and set-up is to be completed by the responsible person and a copy be sent to
both MELAG and the stockist as proof of correct set-up, installation and commissioning. This is a
constituent part of any guarantee claim.
11
Page 12

Chapter 3 – Initial start-up
When feed water is supplied via the internal storage tank,
this needs to be filled manually from time to time. The
sterilizer will issue a maintenance message at the relevant
time.
The internal storage tank holds max. 5 liters. This volume
of feed water in the circulation system is sufficient for up to
7 sterilization runs.
To fill the storage tank with fresh feed water, remove the
lid and fill the right-hand chamber of the storage tank with
fresh feed water up to the MAX mark.
Chapter 3 – Initial start-up
Switching on the sterilizer
Turn the power switch on to power the sterilizer (page 9(4).
After switching on the sterilizer with the power switch, the display shows in alternation to the initial state
the message: Unlocking door with key (+), if the door is closed.
PLEASE NOTE
The trays and all accessories must be removed from the chamber directly after the sterilizer
having been switched on for the first time and before commissioning.
Opening and closing the door
The door can only be opened when the display shows: Acknowledge with '+'/ Unlock door
with '+' key.
1. Press the (+) key. You can open the door after hearing an audible click.
2. Close the door with light pressure against the chamber flange and simultaneously press down the
sliding-closure grip.
Providing feed water
Using the internal storage tank
Setting the feed water supply on the sterilizer
The INTERN function must be set in order to enable feed water supply via the internal storage tank. The
EXTERN function must be set in order to enable feed water supply via a water treatment unit.
1. Press the (+) and (-) keys simultaneously to select the set-up menu Function.
The display shows the menu Function: Last batch no.
2. Navigate using the (+) or (-) keys until the display shows: Function: Feed water test:
3. Press the (P) key. The display shows the option currently set, e.g. pre-heating yes.
4. Press the (P) key again to change to the desired setting (INTERN/EXTERN).
12
5. Press the (S) key to save the setting and to leave the menu.
Repeated pressing of the (S) key enables you to leave the menu entirely and return to the display basic
state.
Page 13
Chapter 3 – Initial start-up
Programm
Start-Stop
Function
Date / time
Using a water treatment unit
Observe the specifications in the technical manual when using a water treatment unit.
PLEASE NOTE
Should you wish to use a water treatment unit from another manufacturer, please consult
MELAG.
Failure to comply with these provisions can result in damage to the sterilizer and/or human
injury.
Setting the date and time
Correct batch documentation requires the correct date and time setting on the sterilizer. Ensure that you
take into account the clock change in autumn and summer, as this is not adjusted automatically. Set the
date and time as follows:
1. Press the (+) and (-) keys simultaneously to select the set-up menu Function.
The display shows the menu Function: Last batch no.
2. Navigate in the Function menu using the (+) or (-) keys until the display shows:
3. Press the (P) key to confirm. The current hour is displayed.
4. Choose one of the following setting possibilities using the (+) or (-) keys: Hours, minute, second,
day, month, year.
5. To adjust the Hours parameter, press the (P) key to confirm.
The current value flashes on the display.
6. You can increase or reduce the value using the (+) and (-) keys.
7. To save the value, confirm with the (P) key.
The current value set no longer flashes on the display.
To alter the other parameters, proceed in a similar fashion.
8. After ending the settings, press the (S) key to leave the menu.
The display shows the menu Function: Date / time.
9. Repeated pressing of the (S) key enables you to leave the menu and the display returns to its
basic state.
13
Page 14
Chapter 4 - Sterilizing
Chapter 4 - Sterilizing
Preparing the sterilization material
A significant prerequisite for safe disinfection and sterilization of sterilizing materials is the appropriate
preparation, i.e. cleaning and maintenance of the sterilizing materials according to the manufacturer's
instructions. Furthermore the materials, cleaning agents and processing procedure employed are of
significance.
Only ever operate the steam sterilizer with a sterile filter inserted.
PLEASE NOTE
Wherever possible, please ensure the separate sterilization of textiles and instruments in
separate sterilization containers or sterilization packaging. This leads to better drying results.
WARNING
Treating instruments
Please ensure the following when treating used and brand-new instruments:
Follow both the instrument manufacturer’s instructions regarding treatment and sterilization and
comply with the relevant standards and directives e.g. from the BGV A1, RKI and DGSV.
Clean the instruments exceptionally thoroughly e.g. using a washer-disinfector.
Rinse the instruments after washing and disinfecting, where possible with de-mineralized or distilled
water and then dry the instruments thoroughly with a clean, non-fuzzing cloth.
Use only those care agents suitable for steam sterilization. Consult the manufacturer of the care
agents.
DANGER
The incorrect treatment of instruments could result in any dirt residue being loosened during
sterilization. The presence of residual disinfection and cleaning fluids results in corrosion.
The use of unsuitable care agents e.g. water repellent agents or oils impermeable to steam
could result in unsterile instruments. This represents a danger to the health of both
patients and yourself.
This could result in increased maintenance requirements and a restriction of the sterilizer
function.
Comply with the treatment instructions contained in these instructions.
When using ultra-sound devices, care equipment for hand pieces and washing and disinfection devices,
please observe the manufacturer’s treatment instructions.
14
Page 15

Chapter 4 - Sterilizing
Treating textiles
Please observe the following points when treating textiles and putting the textiles in sterilization containers:
Observe and comply with both the manufacturer's instructions of the textiles regarding treatment and
sterilization as well as the relevant standards and directives e.g. from the RKI, and DGSV.
Arrange the folds in the textiles parallel to each other.
Stack textiles vertically wherever possible and not too closely together in the sterilization chamber.
This enables the development of flow channels.
Retain the vertical stacking system when packing textiles in the sterilization container.
If textile packages do not remain together, wrap the textiles in sterilization paper.
Only ever sterilize dry textiles.
The textiles must not be permitted to come into direct contact with the floor or walls of the sterilization
chamber; otherwise they will become saturated with condensate.
DANGER
Steam penetration of the textile package can be restricted and/or will produce poor drying results.
The textiles could not be sterilized.
This could endanger the health of patient and practice team.
Comply with the treatment instructions contained in these instructions.
Loading the sterilizer
Only when correctly loaded is effective sterilization and good drying possible.
Ensure the following during loading:
Insert trays or cassettes in the chamber only with their appropriate mount.
Use perforated trays such as those from MELAG. Only in this way can the condensate drain off. The
use of a non-perforated base or half-shell to accept the sterilization material can result in poor drying
results.
The use of paper tray inserts can result in poor drying results.
Packaging
Only ever use packaging materials and systems (sterilization barrier systems) corresponding to the
standard DIN EN IS0 11607-1.
The correct use of suitable packaging is important in achieving successful sterilization results.
You can use re-usable rigid packaging systems such as e.g. standard tray cassettes or soft packaging
such as transparent sterilization packaging, paper bags, sterilization paper, textiles or fleece.
Closed sterilization containers
Please observe the following when using closed sterilization containers for sterilization material:
Use aluminium sterilization containers. Aluminium retains and conducts heat and thus improves
drying.
Closed sterilization containers must be either perforated or have a valve on at least one side -
optimally the bottom.
Wherever possible, please ensure that sterilization containers are stacked on top of those of identical
size, so that the condensate can run down their sides.
Our TIP: MELAG sterilization containers fulfil the requirements of DIN EN 868-8 for
successful sterilization and drying. They have a perforated lid and are fitted with
single-use paper filters.
15
Page 16
Chapter 4 - Sterilizing
WARNING
The use of unsuitable sterilization containers results in insufficient steam penetration and even
failure of the sterilization. This can also prevent condensate drain-off.
This produces poor drying results. This can result in unsterile instruments and thus
endanger the health of patient and practice team.
Closed sterilization containers must be either perforated on at least one location - optimally
the bottom - or be equipped with a valve.
WARNING
Incorrect stacking of the sterilization containers can result in the dripping condensate being
unable to drain off to the chamber floor. This would then saturate the sterilization material directly
underneath it.
This produces poor drying results. This can result in unsterile instruments and thus
endanger the health of patient and practice team.
Do not cover the perforations when stacking the sterilization containers.
Soft sterilization packaging
Soft sterilization packaging can be used in both sterilization containers and on trays. Please observe the
following when using soft sterilization packaging e.g. MELAfol:
Arrange soft sterilization packaging in a perpendicular position and at narrow intervals.
Do not place multiple soft sterilization packages flat on top of each other on a tray or in a container.
If the seam seal tears during sterilization, this could be caused by the choice of undersized packaging.
Should this not be the case, re-pack the instruments and sterilize them again.
Should the seam seal rip during sterilization, extend the sealing pulse on the sealing device or make a
double seam.
Multiple wrapping
The sterilizer functions on the fractionated pre-vacuum method. This permits the use of multiple wrapping.
16
Page 17
Chapter 4 - Sterilizing
Loading variations*
Vacuklav 23 B+
Vacuklav 31 B+
Instruments
Textiles
Instruments
Textiles
Max no. per single piece
2 kg
1.8 kg
2 kg
1.8 kg
Maximum total
5 kg
1.8 kg
5 kg
1.8 kg
Loading variant
mounting A
max. 5 trays, depth 420 mm max. 6
sterilization containers 15 K
max. 3 sterilization containers 15M
max. 2 sterilization containers 15G
max. 6 sterilization containers 17K
max. 3 sterilization containers 17M
max. 1 sterilization containers 17G
max. 3 swab drums 17R
max. 1 sterilization containers 23G
max. 2 sterilization containers 23M
max. 2 swab drums 23R
max. 2 sterilization containers 28M
max. 1 sterilization containers 28G
max. 3 standard tray cassettes
max. 5 trays, depth 290 mm
max. 3 sterilization containers 15 K
max. 3 sterilization containers 17K
max. 3 swab drums 17R
max. 2 swab drums 23R
max. 2 sterilization containers 28M
max. 1 sterilization containers 28G
max. 3 standard tray cassettes
*MELAG mount, trays and sterilization containers. See appendix A – accessories
Mixed loads
Please observe the following when using mixed loads:
Always place textiles at the top.
Place the sterilization containers at the bottom.
Place unwrapped instruments at the bottom.
Place transparent sterilization packaging and paper bags at the top - except in combination with
textiles. In this case, place them at the bottom.
Place heavy loads at the bottom.
Transparent sterilization packaging should be loaded on their edges so that the paper side and film
side are alternating in contact. If this is not possible, the paper side should face downwards.
Load patterns designed especially for the dental sector are available from the download area of the
MELAG website: www.melag.de.
17
Page 18
Chapter 4 - Sterilizing
Universal-
Program
Quick-
Program B:
Quick-
Program S
Gentle-
Program
Prion-
Program
Sterilization
temperature
134 ˚C
134 ˚C
134 ˚C
121 ˚C
134 ˚C
Sterilization pressure
2 bar
2 bar
2 bar
1 bar
2 bar
Sterilization time
5.5 min.
5.5 min.
3.5 min.
20.5 min.
20.5 min.
Operating times
Operating time*
30min.
30 min.
15 min.
45 min.
45 min.
Drying
20 min.
10 min.
5 min.
20 min.
20 min.
Program
Packaging/suitability
Load amount*
Universal-Program
Single and multiple wrapped mixed loads;
hollow-bodied articles, long articles with a low
diameter; instruments with narrow lumen
(hollow body A) andsimple hollow items
(hollow body B)
5 kg instruments
1.8 kg textiles
Quick-Program B
single wrapped and unwrapped (no textiles)
Transfer instruments, long, instruments with narrow
lumen (hollow body A) andsimple hollow items
(hollow body B)
single wrapped
1.5 kg or
unwrapped 5 kg
Quick-Program S
Only unwrapped (no textiles)
Simple solid instruments,simple hollow items(hollow
body B)
5 kg unwrapped
instruments
Gentle-Program
single and multiple wrapping Larger quantities of
textiles, thermo-instable goods (e.g. plastic, rubber
articles); Mixed loads; instruments with narrow lumen
(hollow body A) andsimple hollow items(hollow body
B)
textiles 1.8 kg or
Thermo-unstable
items 5 kg
Prion-Program
Single and multiple wrapped instruments under
suspicion of carrying the danger of infection through
abnormally altered proteins (e.g. Creutzfeld-Jacob,
BSE); instruments with narrow lumen (hollow body A)
andsimple hollow items(hollow body B)
5 kg instruments
1.8 kg textiles
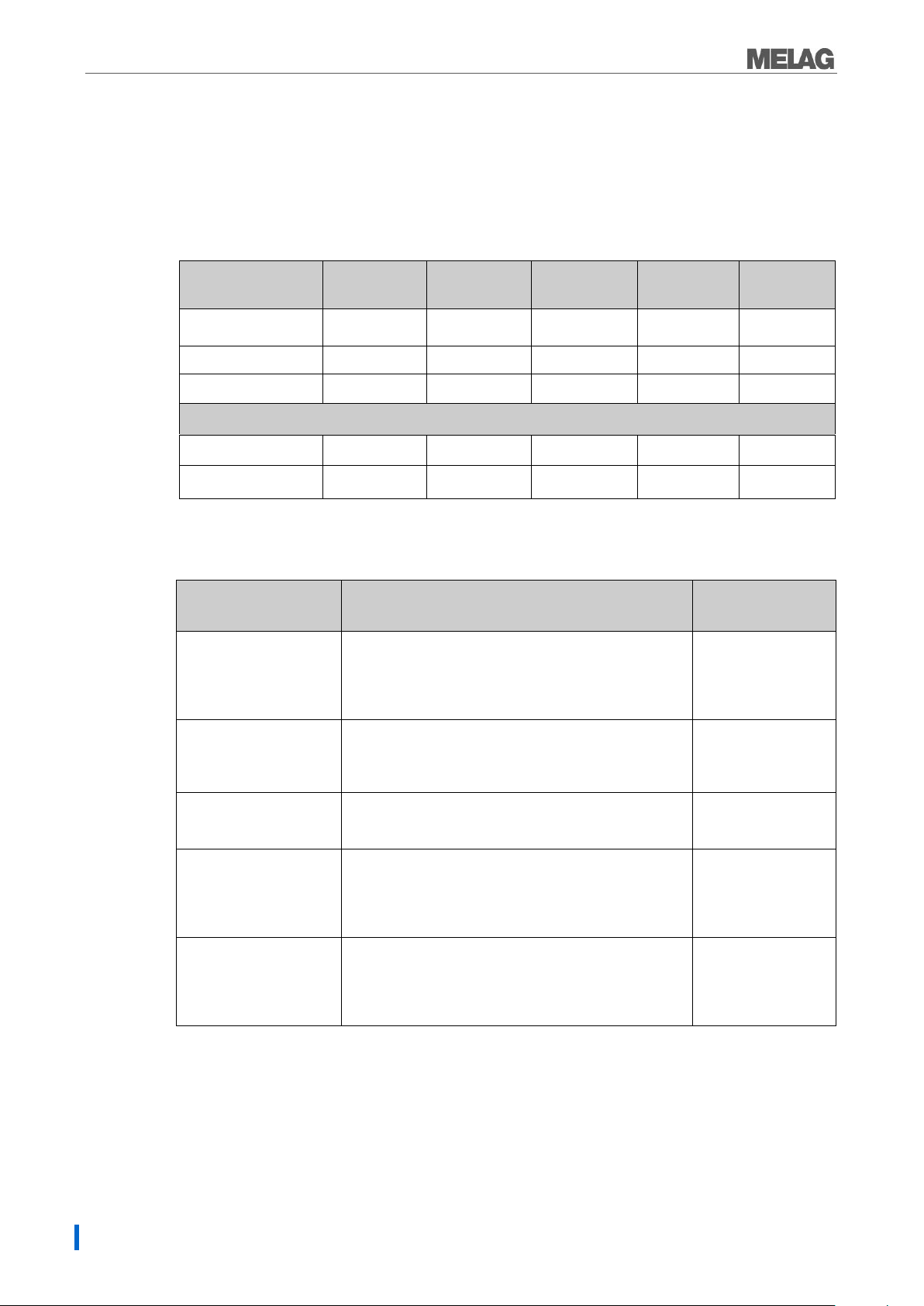
Selecting the program
You can switch between the initial state and the desired program using the program selection switch.Now
select the sterilization program according to how and whether the sterilization material is packed. It is also
necessary to take into account the temperature resistance of the sterilization material. The following tables
show which program is to be selected for which sterilization material.
Table 1: Overview of the Sterilization programs
*without drying (full load for Vacuclav 23 B+ and Vacuklav 31 B+: 5 kg) and depending on loading and
installation conditions, eg. mains voltage.
Table 2: Overview of the use of the respective sterilization programs
*valid for Vacuklav 23 B+ and Vacuklav 31 B+
18
Page 19

Chapter 4 - Sterilizing
Programm
Start-Stop
Programm
Start-Stop
Function:
autom. preheating
Universal-Program
134°C wrapped
Selecting automatic pre-heating
Automatic pre-heating is activated as standard. The automatic pre-heating function heats the sterilizer
chamber to a program-specific pre-heated temperature before the program start, or holds this temperature
between two program runs. This will shorten the cycle times.
PLEASE NOTE
The sterilizer remains switched on continuously for automatic pre-heating!
To alter this setting proceed as follows:
1. Press the (+) and (-) keys simultaneously to select the set-up menu Function: Last batch
number.
Navigate in the Function menu using the (+) or (-) keys until the display shows:
2. Press the (P) key to confirm. The display shows the option currently set, e.g. pre-heating yes.
3. Pressing the (P) key again makes the dispaly switch to Pre-heating no. The pre-heating
function has been deactivated.
4. In order to end the menu Function: autom. Pre-heating and return to the initial state, press the (S)
key twice.
PLEASE NOTE
MELAG recommends activating the function automatic pre-heating.
Starting the program
WARNING
Unsupervised operation of electrical devices, including this sterilizer at the operator’s risk. MELAG
accepts no liability what so ever for any damage resulting from unsupervised operation.
After selecting a program using the program keys, in addition to the program selected, the display also
indicates the temperature and holding time. You will also see whether the program is suitable for wrapped
or unwrapped sterilization material.
1. Press the (S) key to start the program.
The sterilizer checks the feed water supply and its conductivity.
19
Page 20

Chapter 4 - Sterilizing
Programm
Start-Stop
Programm
Start-Stop
Programm
Start-Stop
1. Fractionation
-0.085 bar 22°C
Sterilization
2 min 12 s
Additional drying
selected
Selecting additional drying
The function additional drying extends the drying time by 50%. This is suitable for difficult drying
tasks.
To do so, proceed as follows:
Press the keys (S) and (+) simultaneously upon starting the program. The display shows the menu
Function:
The program run will now begin.
PLEASE NOTE
If the Quick-Program S has been started, the warning Warning, only unwrapped
instruments appears on the display.
If a load consists entirely of unwrapped instruments, press the (S) key again to confirm and to
start the program.
Program run
After starting the program, you can follow the program run in the display. It shows the chamber
temperature and pressure as well as the time until the end of sterilization and the drying time which has
passed.
Sterilization phase is ended
The display enables you to see whether the sterilization phase has already been completed successfully.
The time left in the sterilization phase is shown in the display in alternation with the pressure and
temperature.
20
Page 21

Chapter 4 - Sterilizing
Programm
Start-Stop
Programm
Start-Stop
Universal-Program
successfully ended
Vacuum drying
since 2` -0.12 bar 60°C
Drying phase
The regular drying time for the Quick-Program S: 5 minutes. For the Quick-Program B: 10 minutes and for
all other programs: 20 minutes. The display will show the corresponding message during the drying phase.
The sterilizer provides excellent drying of the sterilization material. If difficult-to-dry items require better
drying, you can undertake the following steps to improve drying:
Load the sterilizer properly. e.g. stand the transparent and paper sterilization packaging upright.
Observe the contents of the section Loading the sterilizer Loading the sterilizer on page 15. Use a film
bracket if necessary.
Activate additional drying. Observe the contents of the section Selecting additional drying
Loading the sterilizer on page 15.
Program end
The respective program has ended successfully. The display shows the Function menu:
Working in the "Settings" menu under Function if immediate output after program end is activated, the
log of the completed program will be outputted to the activated output medium after opening the door
(see 25, page Chapter 5 - Logging).
Manual program abort
You can abort a current program in all phases. If you end the program before drying begins, the
sterilization material remains unsterile. The program will not be classified as successfully completed.
WARNING
Aborting a current program by switching off the power switch can result in the egress of
hot steam from the sterile filter. This will contaminate the sterile filter.
Never abort a program by switching off at the power switch.
DANGER
The sterilization chamber, door and the sterilized equipment are hot. Moreover, depending on the
time of the program abort, opening the door following a program abort can lead to the egress of
hot steam.
Danger of burns from hot steam.
Only remove the trays with a tray jack .
Never touch the sterilized equipment, the sterilization chamber or the door inside with bare
hands.
21
Page 22

Chapter 4 - Sterilizing
Programm
Start-Stop
Programm
Start-Stop
Immediate removal
press 'STOP'
Last batch no. 46
Quit with button '+'
Manual abort during drying
You can abort the program during the drying phase via the (S) key without the sterilizer registering a fault.
You then need to expect insufficient drying, especially in the case of wrapped sterilized equipment. Sterile
storage requires sufficient drying. To ensure this, please allow programs with wrapped sterilized
equipment to continue to the end of the drying phase.
Unwrapped instruments sterilized in a Quick-Program dry after being removed from their own warmth.
The drying time completed thus far is indicated on the display during the drying phase. This is performed
via a change on the display.
A program abort requires the following steps:
1. Press the (S) key.
2. Confirm the following safety question Immediate removal`Stop’ by pressing the (S) key
The display confirms the abort with Drying interrupted.
After ventilation of the chamber, the display will show: Universal-Program completed
successfully altering with:
repeatedly.
PLEASE NOTE
The safety question will be shown on the display for approx. 5 seconds. If the key is not pressed
repeatedly, the program will continue with the usual program run.
If a printer or other output media is connected to the sterilizer, and the option Immediate output is set to
Yes, the warning Drying interrupted is outputted on the log.
Manual abort before drying begins
If you end the program before drying begins, the sterilization material remains unsterile. The program will
not be classified as successfully completed.
A program abort requires the following steps:
1. Press the (S) key.
2. Confirm the following safety question Abort program? By pressing the (S) key repeatedly.
PLEASE NOTE
The safety question will be shown on the display for approx. 5 seconds. If the (S) key is not
pressed repeatedly, the program will continue with the usual program run.
22
Page 23

Chapter 4 - Sterilizing
Programm
Start-Stop
Programm
Start-Stop
Last batch no.
10
Total batch
22
Depending on the time of abandonment occurs a pressure relief or venting of the device. A corresponding
display text appears on the display.
After the ventilation of the chamber follows the request to quit the Program abort.
The display will alternate between Abort end and Clear with ’–’ key.
3. Press the (-) key.
The display alternates between displaying the message Unlock door with ’+’ key and the program
previously selected.
4. You can open the door after pressing the (+) key.
The log records the note “program aborted / load not sterilized.”
Displaying the daily batch counter
The last batch number of the day is shown on the display after every program run.
You can also arrange for the batch number to be displayed. To do so:
1. Press the (+) and (-) keys simultaneously to select the set-up menu Function. The display shows
the menu Function: Last batch no.
2. Press the (P) key to display the current daily batch number.
To return to the basic state, press the (S) key twice.
Displaying the total batch counter
You can arrange the display of the number of the batches previously recorded.
Press the (+) and (-) keys simultaneously to select the set-up menu Function.
The display shows the Function menu Last batch no.
Navigate in the Function menu using the (+) or (-) keys until the display shows:
1. Press the (P) key.
The display shows the current total number of batches.
2. To return to the basic state, press the (S) key twice.
23
Page 24

Chapter 4 - Sterilizing
Removing the sterilized equipment
You must observe the following specifications whilst removing the sterilized equipment upon a program
end:
Never use force to open the door. This could damage the sterilizer and / or result in the emission of
hot steam.
Use a tray jack to remove the tray.
Never touch the sterilized equipment, the chamber or the inside of the door with bare hands.
The components are hot.
Check the packaging on the sterilized equipment for damage when removing it from the sterilizer.
Should the packaging be damaged, re-pack the sterilization material and re-sterilize it.
If you remove the sterilized equipment from the sterilizer directly after the end of the program, it is possible
that the instruments can be partially damp.
According to the “Arbeitskreis für Instrumentenaufbereitung” (AKI; Red Broschure; 10 Edition; S.57): "In
practice, residual moisture in the form of a few drops of water capable of evaporating within 15 minutes is
tolerated, but actual pools of water are not acceptable."
DANGER
Danger of burns
Metal parts and load are hot after the program end. Hot steam egress is possible.
Comply with the instructions regarding removal of the sterilized equipment.
DANGER
If packaging is damaged or split during a program run, the instruments may not be sterile.
This can endanger the health of your patients and practice team.
Damaged or split packaging must be repackaged and re-sterilized.
24
Storing sterile equipment
Use only standard-conform packaging for the sterilized equipment. Do not store the sterilized instruments
in the treatment room. Observe the provisions of DIN 58953, part 8 and the following criteria when storing
sterilized equipment:
Observe the following criteria when selecting the storage location and duration of the sterilized equipment:
Protected against dust e.g. in a closed instrument cupboard.
Protected from damage to their shiny surfaces.
Protected from significant temperature differences.
Protected from moisture (e.g. from alcohol, disinfection fluids).
The possible length of storage depends on the type of packaging.
The maximum storage time is dependent on the packaging and the storage conditions. For standard-
conform packaged sterilized equipment (protected from dust) it can amount up to six months.
Page 25

Chapter 5 - Logging
Chapter 5 - Logging
Batch documentation
The batch documentation acts as proof of the successful conclusion of the sterilization process and
represents an obligatory part of quality control. The sterilizer internal log memory saves such data as the
program type, batch and process parameters of the programme completed.
To obtain the batch documentation, you can read out the internal log memory and transfer its data to
various output media. This can be performed immediately at the end of every program or at a later point,
such as at the end of the day.
Capacity of the internal log memory
The capacity of the internal log memory is sufficient for 40 logs.
If the internal log memory is full, the oldest log will be overwritten automatically at the beginning of the next
program.
If a log printer is connected and the option Immediate output "No“ is set (see also page 28, Outputting logs
immediately), a safety question will be displayed before the log is overwritten. For further information about
connecting the printer, consult the operating manual of the respective device.
Output media
You are able to output and archive the logs of the completed programs on the following output media.
Please observe the user manual of the respective device.
Log printer MELAprint 42
MELAflash CF card printer on a CF card
Connecting the devices to the MELAnet Box
Computer, e.g. with the software MELAtrace/MELAview*
*From the Device Software 5.11 at least the software MELAview/trace is required.
In its state of delivery, an option for log output is not set on the sterilizer.
NOTICE
For further information of the protocoll printer (for example for the duration of for he log prinouts)
please refer to the respective operating instructions.
Using a computer as an output medium (without a network connection)
The following example shows how to use the computer as an output medium.
You can connect the sterilizer to a computer if the following conditions are fulfilled:
The computer is either fitted with a serial interface or a USB serial adapter is connected.
The software MELAview/MELAtrace is installed on your computer.
PLEASE NOTE
The MELAnet Box and the software MELAtrace/MELAview is required for integration in the
practice network.
In order to be able to use a computer as an output medium, the computer must be connected to the
sterilizer via the serial interface. Connect the computer to the sterilizer as follows:
1. Open the white cover of the serial data- and printer connection from the sterilizer.
2. Turn a coin by a quarter-revolution inserted in the locking slot (Fig. 4/1) on the white cover.
3. Take off the white cover.
25
Page 26

Chapter 5 - Logging
Fig. 4: Connection to the sterilizer
Programm
Start-Stop
Output medium
Computer
1
2
If the computer is continually connected to the sterilizer, the data connection cable of the computer is laid
in the cable ducts (Fig. 4/2), the metal casing retracted and the cover is closed again.
Reading logs on to the computer
You can use the software MELAview or MELAsoft to read out the logs.
The following sterilizer settings are required to enable registration of the computer on the sterilizer:
4. Press the metal casing somewhat downwards until it engages and fold the interior metal casing to
the left (Fig. 4/2).
5. Connect the sterilizer to the RS232 connection with a compatible data connection cable to the
computer.
1. Switch on the sterilizer.
2. Wait until the display shows the state menu.
3. Press the (+) and (-) keys simultaneously to select the set-up menu Function. The display shows
the Function menu Last batch no.
4. Navigate in the Function menu using the (+) or (-) keys until the display shows
Function:Log output.
5. Press the (P) key to select the sub-menu Log issue – output medium.
6. Press the (P) key again. The display shows Log issue – no outut medium, if a printer has not
been selected.
7. Navigate in the Function menu using the (+) or (-) keys until the display shows:
After repeated pressing of the (S) key, the display returns to its initial state.
26
8. Press the (P) key to confirm. The display returns to the menu Log issue – output medium.
9. Press the (S) key to return to the set-up menu Function: log issue.
Page 27

Chapter 5 - Logging
Opening text logs with a computer
You can open and print all text logs using a text editor of every operating system or with a word processing
or table calculation program.
NOTICE
Graphic logs can only be displayed with the documentation software MELAview
(as of MELAview 3)/MELAtrace.
To ensure that the operating system at your computer will automatically open the text logs with a text
editor, you need to connect the text logs (e.g. PRO, STL, ML etc.) to the text editor. For the meanings of
the log endings please see page 31, Reading logs correctly.
The following example of the Windows editor shows how you can link other Windows programs with a
particular ending.
1. Double click in Windows Explorer on the log file.
2. Windows 7 displays the adjacent message.
3. Select Select program from a list of installed programs and confirm with OK.
4. Select the editor from a list of programs in the opening window. Tick the option Always use the
selected program to open this kind of file and confirm with OK.
You can then open text logs (e.g. PRO, STL, ML etc.) via a double-click in Windows Editor.
Alternatively, all text logs can be opened with the documentation software MELAview
(as of MELAview 3)/MELAtrace.
27
Page 28

Chapter 5 - Logging
Programm
Start-Stop
Immediate output
YES
Outputting logs immediately and automatically
Text log
The following requirements must be fulfilled in order to issue logs immediately after the end of a program.
If you want to output the associated text and graphic logs automatically after the end of a program on an
output medium, use the function Immediate output - yes. This is not set on the sterilizer in its state
of delivery.
The options for immediate log issue upon program end are to be set in the following way:
Working in the Setup menu Function: log output Immediate output is set to YES.
At least one output medium must be selected (computer, log printer MELAprint 42).
The activated output medium must be connected and initialized.
1. Switch on the sterilizer at the power switch.
2. Press the (+) and (-) keys simultaneously to select the set-up menu Function.
The display shows the Function menu Last batch no.
3. Navigate in the Function menu using the (+) or (-) keys until the display shows: Function: log
issue and then press the (P) key.
4. Navigate in the Function menu using the (+) or (-) keys until the display shows:
5. Press the (P) key, to switch between Immediate issue no / yes..
To issue logs immediately, Immediate issue yes must be set.
6. Press the (S) key to save the settings and to leave the menu.
The display shows the menu Function: log issue.
Pressing the (S) key once again enables you to leave the menu and return to the display initial state.
PLEASE NOTE
If automatic logging is unable to issue a log, for example, because the output medium activated is
not connected, a warning will appear. MELAG recommends using the immediate log output
function.
Graphic logs (optional)
The following requirements must be fulfilled in order to issue logs immediately after the end of a program.
Working in the Setup menu Function: Log issue the MELAnet+graphic data is selected as
the output medium.
The computer or another medium must be connected and initialized.
28
Page 29

Chapter 5 - Logging
Programm
Start-Stop
Programm
Start-Stop
Last cycle
output: 25
Output
stored cycles
Subsequent log output
It is possible to issue logs subsequently and independently of the time of the end of the program. You can
choose whether all or only the saved logs (up to 40) are to be printed. Use the output media connected for
this task e.g. the log printer.
Printing selected logs
To print the subsequently selected logs of a particular program proceed as follows:
1. Press the (+) and (-) keys simultaneously to select the set-up menu Function.
The display shows the menu Function: Last batch no.
2. Navigate in the Function menu using the (+) or (-) keys until the display shows: Function: log
issue and then press the (P) key.
The menu Log issue – output medium is displayed.
3. Navigate in the Function menu using the (+) or (-) keys until the display shows: Last cycle
output No. 40 (as example no. 40).
4. Press the (+) key. The current log number flashes.
5. To issue a log or another cycle, navigate to the desired number using the (+) or (-) keys until you
have reached the following number eg. In this case, no. 25.
6. Press the (P) key in order to start the selected program. The display shows the
Function menu.
After a successful output, the display returns to its previous setting Output last cycle:
Repeat the last three steps in order to issue further logs.
7. Press the (S) key to leave the sub-menu without outputting the log.
8. Press the (S) key to leave the menu after having outputted the log. The display shows the menu
Function: log issue.
Repeated pressing of the (S) key enables you to leave the menu entirely and return to the display basic
state.
Printing all saved logs
Proceed as follows to issue all the saved logs subsequently:
1. Press the (+) and (-) keys simultaneously to select the set-up menu Function.
The display shows the Function menu Last batch no.
2. Navigate in the Function menu using the (+) or (-) keys until the display shows: log issue and
then press the (P) key.
3. Navigate with the (+) or (-) key until the display shows: output stored cycles.
4. Press the (P) key in order to start the selected program. Once the issue has been performed, the
display will show:
Press the (S) key to leave the sub-menu without issuing the log.
29
Page 30

Chapter 5 - Logging
Programm
Start-Stop
Programm
Start-Stop
Delete
all cycles
Allocated: 26
Free: 14
Repeated pressing of the (S) key enables you to leave the menu entirely and return to the display basic
state.
Displaying the log memory
If a printer or other output medium is connected and initialized, you can check how many logs have
already been saved in the sterilizer log memory.
Proceed as follows:
PLEASE NOTE
A termination during the output on the log printer is only possible by disconnecting the instrument
at the mains switch or interrupting the power supply of the printer.
5. Press the (S) key to leave the menu. The display shows the set-up menu
Function: log issue.
1. Press the (+) and (-) keys simultaneously to select the set-up menu Function.
The display shows the Function menu Last batch no.
2. Navigate in the Function menu using the (+) or (-) keys until the display shows: log issue and
then press the (P) key.
3. Navigate in the Function menu using the (+) or (-) keys until the display shows:
Press the (S) key twice to leave the menu.
Deleting logs in the internal log memory
Delete the saved logs manually to suppress warning messages, e.g. Log memory full with the option
Immediate issue set. The following example shows how to delete all the logs saved.
1. Press the (+) and (-) keys simultaneously to select the set-up menu Function.
The display shows the Function menu Last batch no.
2. Navigate in the Function menu using the (+) or (-) keys until the display shows: log issue and
then press the (P) key.
Navigate in the Function menu using the (+) or (-) keys until the display shows:
3. Press the (P) key to delete all logs.
4. To cancel the set-up menu without deleting, press the (S) key.
5. Press the (P) key to leave the menu after having deleted it. The display shows the menu
Function: log issue.
Repeated pressing of the (S) key enables you to leave the menu entirely and return to the display basic
state.
30
Page 31

Chapter 5 - Logging
Log type
File ending
Explanation
text protocol
.PRO
Log of a successfully completed program.
Malfunction log
.STR
Log of a successfully completed program.
Graphic log
.GPD
Program run displayed as a graphic curve.
Standby log
.STB
Log for faults in standby.
Demo log
.DEM
Protocols of a simulated program.
No real sterilization will be performed!
Demo graphic log
.DEG
Simulated program run displayed as a graphic curve.
No real sterilization will be performed!
Reading logs correctly
Log head
The head of the program log comprises the general basic information regarding the program run. This
includes date, the program selected, the daily batch number and the sterilizer type.
Program step values
The phases of the program run are recorded whilst it runs and the values for steam pressure, temperature
and time (related to the program start) are recorded.
Summary
The summary indicates whether the program has been completed successful. The values of the
sterilization time recorded, the sterilization temperature and the pressure (including the maximum
deviation) are also displayed.
31
Page 32

Chapter 5 - Logging
-----------------------------------------MELAG Vacuklav 31-B
------------------------------------------
Sterilizer type
Program: Universal-Program
134°C wrapped
Program started
Date: 24/03/2015
Time: 09:14:19 (Start)
Batch no.: 2
SN: 201531-B1541
Current day
Time of program start
Daily batch number
Serial number
Pre-heating 127.5 °C
AIN6: Conductivity 15 µS/cm
Pre-heating temperature
Feed water Conductivity
Program step Pressure Temperat. Time
bar °C min
Start 0.00 77.0 00:01
1.Fractionating
Evacuation -0.92 58.2 02:23
Steam inlet 1.00 108.7 04:53
2.Fractionating
Evacuation -0.82 71.3 06:45
Steam inlet 1.00 109.2 08:33
3.Fractionating
Evacuation -0.82 66.7 10:35
Steam inlet 0.41 109.3 12:24
Pressure build-up 2.05 134.0 14:40
Steril. Begin 2.05 134.0 14:40
Steril. End 2.19 135.9 20:10
Pressure reduc. 0.14 105.2 20:55
Vacuum-drying
Drying pump -0.31 94.4 21:03
Drying pressure -0.91 75.1 23:01
+49 -0.91 85.9 25 01 99
+49 -0.92 84.3 27 01 99
+49 -0.93 81.4 29 01 99
+49 -0.93 79.2 31 01 99
+49 -0.93 77.6 33 01 99
+49 -0.94 76.3 35 01 99
+49 -0.94 75.4 37 01 99
+49 -0.94 74.5 39 01 99
+49 -0.94 73.9 41 01 99
Drying end -0.86 73.8 41:03
+49 -0.29 77.3 41 12 99
End 0.00 79.2 41:24
The phases of the program run are recorded
whilst it runs and the values for steam pressure,
temperature and time (related to the program
start) are recorded.
Program stage phases with the associated values
for pressure, temperature and time (relative to the
program start).
------------------------------------------------------------------
Summary
The summary indicates whether the program has
been completed successfuly. The values of the
sterilization time recorded, the sterilization
temperature and the pressure (including the
maximum deviation) are also displayed.
PROGRAM SUCCESSFULLY COMPLETED
Control message
Temperature 135.6 +0.4 /-0.3 °C
Pressure: 2.17 +0.03/-0.03 bar
Sterilization time: 5 min 30 s
Time: 09:55:43 (end)
32 201501541 5.15 5.05
Median sterilization temperature with max.
deviations
Median sterilization pressure with max. deviations
Sterilization time maintained
Time upon program end
Information with total batch counter, factory
number and device software number version no.
Table 3: Example for a text log of a successfully completed program
32
Page 33

Chapter 6 – Functional Checks
Chapter 6 – Functional Checks
Automatic functional checks
The electronic parameter control subjects the interaction of the sterilization-relevant parameters pressure,
temperature and time to constant automatic monitoring. The sterilizer process evaluation system compares
the process parameters during the program with each other and monitors them in terms of their threshold
values. The sterilizer monitoring system checks the device components for their functionality and their
plausible interaction. Should the parameters exceed pre-set threshold values, the sterilizer emits warning
messages or malfunction messages. If necessary, it interrupts the program with appropriate information.
When the program has ended successfully, the corresponding message will be issued on the display.
Manual functional checks
You can follow the program run on the display via the values displayed there. You can also use the logs
recorded for every program to determine the success of a program (see Chapter 5 - Logging).
Batch-related checks
Helix test body system MELAcontrol / MELAcontrol PRO
The Helix test body system is an indicator and batch control system fulfilling the requirements of
DIN EN 867-5. It consists of a test body, the Helix and an indicator strip.
If sterilizing category "critical B“ instruments, you should add the MELAcontrol/PRO test body to every
sterilization cycle as a batch control.
Regardless of this, you can perform a steam penetration test at any time using MELAcontrol/
MELAcontrol PRO in the Universal-Program.
Intended use of the Helix test body can result in the colouration of the plastic surface. This colouration
exercises no influence on the functionality of the Helix test body.
Vacuum test
The test serves to determine leaks in the sterilizer. The leakage rate is determined in the process.
Conduct a vacuum test in the following situations:
Once weekly in routine operations.
During commissioning.
Following longer operating pauses.
Following a malfunction (e.g. in the vacuum system).
Perform the vacuum test with the sterilizer in a cold and dry state as follows:
1. Switch on the device at the mains switch. The display switches to its initial state.
2. Press the (P) key until the display shows Vakuum-test.
3. Close the door.
4. Press the (S) key to start the vacuum-program.
The evacuation pressure and the equilibration time or measuring times are shown on the display. The
chamber will be ventilated after the end of the measuring time (corresponding message on the display).
Then the message will be shown on the display with an indication of the leakage rate. Should the leakage
rate be too high e.g. over 1.3 mbar, a corresponding message will be issued on the display. Following a
successful test program, the current daily batch number is displayed, alternating with the message Clear
with ’+’. You can open the door after pressing the (+) key.
33
Page 34

Chapter 6 – Functional Checks
Programm
Start-Stop
Bowie & Dick test
134°C 2.2 bar 3.5`
PLEASE NOTE
If a log printer or another output medium is connected and the setting immediate output is set, a
log printout will be issued at the same time.
Bowie & Dick test
The Bowie & Dick test serves as proof of the steam penetration of porous materials such as textiles.
Specialist stockists provide various test systems for the Bowie & Dick test . Perform the test according to
the test -system manufacturer information.
How to start the Bowie & Dick test program:
1. Switch on the device at the power switch.
2. Select the Bowie & Dick test using the (P) key.
3. Press the (S) key to start the Bowie & Dick test .
Following a successful test program, the current daily batch number is displayed, alternating with the
message Clear with ’+’. You can open the door after pressing the (+) key.
PLEASE NOTE
If a log printer or another output medium is connected and the setting immediate output is set, a
log printout will be issued at the same time.
PLEASE NOTE
Treatment indicator strips often exhibit differing intensities in the colour change indicating a
different length of storage of the manufacturer batches or other influences. Of crucial importance
for evaluating the Bowie & Dick test is not the strength of contrast in the colour change on the test
sheet, but its even nature.
If the treatment strips/treatment indicator sheet indicates an equal distribution of colour change,
the air-removal of the sterilization chamber is without fault.
If the treatment indicator strips or the treatment indicator sheets are uncoloured or exhibit less
colour in the centre of the star in comparison to the end, air-removal was insufficient. In such a
case, please consult the stockist customer services / MELAG customer services.
34
Page 35

Chapter 6 – Functional Checks
Programm
Start-Stop
Programm
Start-Stop
AIN6: Conductivity
15 µS/cm
AIN4: Temp. preheat.
120°C
Checking the quality of the feed water
You can access the water quality on the display at any time during a current program when the sterilizer is
switched on.
To do so, hold the (-) key depressed until the display shows the conductivity. The conductivity is displayed
in µS/cm.
As soon as you have released the (-) key, the display returns to its previous state (e.g. initial state).
Check pre-heating temperature of the chamber
If pre-heating is activated, the sterilizer will warm the cold chamber or will maintain the temperature
between two sterilization runs. This reduces program times and reduces the accretion of condensation,
thus improving drying results.
After having pressed the (-) key shortly twice, hold depressed the second time. Instead of displaying the
conductivity, you will see the chamber pre-heating temperature.
35
Page 36

Chapter 7 - Maintenance
Chapter 7 - Maintenance
Checks and cleaning
Door seal, chamber, chamber sealing face, mount, trays
Check the chamber, including the door seal and chamber sealing face and the load mount once a week for
impurities, deposits or damage. If you find any impurities, remove the trays or cartridges from the chamber
from the front. Clean the soiled components.
When cleaning the chamber, load brackets and chamber seal face, please observe the following:
Switch off the sterilizer before cleaning and remove the plug from the socket.
Ensure that the chamber is not hot.
Use a soft, non-fuzzing cloth.
Use a chlorine- and vinegar-free cleaning fluid.
First soak the cloth with the cleaning alcohol or spirit and attempt to remove the impurities with this
method.
Only if the chamber, mount or chamber seal face has persistent soiling should you use a mild
stainless steel cleaning agent, with a pH value between 5 and 8.
To clean the door seal, use a neutral liquid cleaning agent.
You should not allow cleaning fluid to enter the piping coming from the sterilizer chamber.
Do not use any hard objects such as metal saucepan cleaner or a steel brush.
WARNING
Inappropriately performed cleaning can lead to the scratching of and damage of surfaces
and the development of leaks in sealing surfaces. This creates conditions favourable to
dirt deposits and corrosion in the sterilization chamber.
Comply with all information regarding cleaning of the part affected.
Internal storage tank
PLEASE NOTE
Ensure that all soiling is removed from the chamber using a cloth. Do not leave any residue. If
soiling particles are loosened but not removed, they can enter the dirt particle filter (integrated in
the drainage hose) when the waste water tank is emptied.
Failure to comply could impair the life-expectancy of the dirt particle filter and necessitate
short-term replacement.
Should you decide upon manual supply of the feed water via the internal storage tank, check the feed
water side (the right-hand side) for soiling whilst refilling. If necessary, use a cloth and fresh feed water to
clean the storage tank before filling.
Clean the waste water side (left chamber) of the internal storage tank every two weeks.
Empty both chambers of the storage tank as follows:
1. Connect the effluent hose on a quick coupling (left: waste water tank, right: feed water tank) until
this snaps in.
2. Discharge the water into a container with min. volume of 5 litres.
3. Repeat the procedure for the other chamber if necessary.
Press the grey unlocking key on the quick coupling to remove the effluent hose. The hose will free itself
from the coupling on its own.
36
Page 37

Chapter 7 - Maintenance
WARNING
When removing the quick coupling, please observe:
To empty the reservoir, stand in front of the connection to one side.
Hold the hose with one hand whilst pressing the grey unlocking key on the quick coupling with the
other. This dampens the spring force of the seal.
Failure to observe these provisions can result in injury.
Avoiding staining
Only after cleaning instruments properly prior to sterilization is it possible to avoid residue from the load or
the instrument treatment from being released during sterilization. Loosened dirt residue (e.g. from
disinfectants) can clog the sterilizer filter, nozzles and valves and deposit themselves on the instruments
and chamber as deposits and stains (see page 14, Preparing the sterilization load).
All steam-conducting parts of the sterilizer consist of non-rusting material. This rules out the possibility of
stain or rust development being caused by the sterilizer. The development of rust is always extraneous
rust.
Incorrect instrument treatment can result in the accretion of rust even on stainless steel instruments of
leading manufacturers. Often, an instrument which drops rust can suffice to cause the development of rust
on another instrument or in the sterilizer.
Remove foreign rust from the instruments using chlorine-free stainless steel cleaning fluid
(see page 36, cleaning) or send the damaged instrument to the manufacturer.
Replacing the door seal
The door seal may not be greased or oiled. It should be kept clean and dry. If the door seal becomes worn
and looses form, it must be replaced. Otherwise, this could result in leaks which will enable steam egress,
or too high a leakage rate in the vacuum test.
Proceed as follows to replace the door seal:
1. Open the sterilizer door and remove the old door seal. The door seal is now inserted in the
groove of the round blank (page 9, Fig. 3/5). Insert the new door seal in the groove in such a way
that the wider seal face points towards the chamber side.
IMPORTANT!
Ensure you observe the different breadths of the seal faces. The door can only be shut correctly
and the chamber sealed, if the door seal sits correctly in the groove.
37
Page 38

Chapter 7 - Maintenance
Aligning the door seal sealing lip
Long periods of storage with the door closed can result in the sealing lips of the door seal becoming stuck.
Align the sealing lips to prevent leaks.
Proceed as follows:
1. Remove the door seal.
2. Press your thumb between the two sealing lips and separate the sealing lips once around with
your thumb.
PLEASE NOTICE
Note the differences in the widths of the sealing surfaces when inserting the door seal. The door
can only be shut correctly and the chamber sealed, if the door seal sits correctly in the groove.
3. Insert the door seal into the groove. The wide sealing surface points towards the chamber.
38
Page 39

Chapter 7 - Maintenance
Replacing or sterilizing the sterile filter
The sterile filter must be replaced regularly within the scope of the maintenance. Given the incidence of a
malfunction and the malfunction message "Malfunction 32: power outage/sterilize sterile filter, the sterile
filter should either be replaced or sterilized.
WARNING
Only ever operate the steam sterilizer with a sterile filter inserted.
Changing the sterile filter
1. Remove the sterile filter by turning and pulling it from the holding sockets simultaneously.
2. Replace the sterile filter or sterilize the current sterile filter as described under the point
"Sterilizing the sterile filter".
3. Exert a little pressure on the sterile filter and turn to insert it into the holding sockets.
Sterilizing the sterile filter
1. Remove the sterile filter by turning and pulling it from the holding sockets simultaneously.
2. Slide a perforated tray into the steam sterilizer and place the sterile filter vertically on the tray.
Ensure that the sterile filter does not fall over, otherwise the condensate will not be able to drain
away correctly.
3. Start the Gentle-Program.
4. Remove the sterile filter from the device after the program end and allow it to cool for min.
15 minutes.
5. Exert a little pressure on the sterile filter and turn to insert it into the holding sockets.
39
Page 40

Chapter 7 - Maintenance
a
b
c
Fig. 5 view of the interior
Fig. 6 unscrew the filter of the chamber
Cleaning the filter in the chamber
1. Unscrew and remove the filter (anti-clockwise) from the opening to check and clean it.
2. Rinse the filter with water to clean.
3. Screw in the filter into the opening in a clockwise direction.
Unscrew the chamber filter (fig. 6/c) using the chamber filter wrench included in the scope of delivery.
(a) Condensate reflux filter .
(b) Chamber filter.
(c) Wrench for the chamber filter.
Maintenance
WARNING
Continuing operation despite maintenance messages can result in malfunctions in the
sterilizer.
Maintenance should only be performed by trained customer services technicians, or stockist
technicians. Consult your stockist or the nearest MELAG customer services point.
Maintain the specified servicing intervals.
Regular maintenance is vital to ensure reliable operation and value retention of the sterilizer.
All function and safety-relevant components and electrical units are checked during maintenance and
replaced where necessary. Maintenance should be performed after every 1000 program cycles or 2 years.
The sterilizer will issue a maintenance message at the relevant time.
A NOTE CONCERNING THE ORDINANCE OF INDUSTRIAL SAFETY & HEALTH
According to BetrSichV §15, the operators of pressure devices such as sterilizers are obliged to
arrange for regular checks of their devices for their correct state. Our homepage contains
guidelines to download. These contain recommendations as to the intervals at which you should
check each component.
40
Page 41

Chapter 8 – Operating Pauses
Duration of the operating pause
Measure
between two sterilizations, longer then one hour
Switch off sterilizer (saves energy).
overnight or on the weekend
Switch off sterilizer.
Leave the door ajar to prevent a sticking of the
door seal.
Close, if available, the water feed of the water
treatment unit.
Longer than two weeks
Perform vacuum test.
Then perform an empty sterilization with the
Quick Program (see page 33, Chapter 6 –
Functional Checks).
Chapter 8 – Operating Pauses
Sterilization times
Pause times between individual programs are not necessary. After the end or abort of the drying time and
removal of the sterilized equipment, you can load the sterilizer again and start the sterilizer afresh.
Operating pauses
Depending on the duration of the operating pauses, the following measures must be maintained:
After pauses, perform the checks described in chapter 6 – functional checks depending on the length of
pause.
Decommissioning
When decommissioning the sterilizer for a long pause (e.g. due to holiday or planned transport), proceed
as follows:
1. Switch off the sterilizer at the power switch.
2. Remove the plug from the socket.
3. Empty both chambers of the storage tank.
4. Close the water inflow if you are using a water treatment unit.
PLEASE NOTE
Please comply with the technical manual. This contains all building-side requirements.
Recommissioning after relocation
When recommissioning after a move, proceed as with the first commissioning (see page 12, Chapter 3 –
First commissioning).
41
Page 42

Chapter 9 – Description of function
Program phase
Description
1. Air-removal phase
During the air-removal phase, air is removed repeatedly until a programindependent pressure has been reached. This is performed in alternation with
steam injection until a low over-pressure has been reached.
Depending on the program selected and the current chamber temperature
upon program start, further fractionations can also follow.
2. Heating phase
The heating phase follows the ventilation phase. The continued steam
admittance into the chamber leads to an increase in pressure and temperature
which continues until the sterilization parameters have been reached.
3. Sterilization phase
After the sterilization parameters pressure and temperature have been met,
the sterilization phase begins.
4. Drying phase
The drying phase begins after the pressure release. Chamber ventilation and
simultaneous pressure equalization is performed at the end of drying.
5. Ventilation
Once the program has come to an end, the chamber pressure is adapted to
the ambient pressure. The corresponding display message "ventilation" is
displayed.
Chapter 9 – Description of function
The sterilization procedure
The sterilizer sterilizes on the basis of the fractionated vacuum procedure. This guarantees the complete
and effective wetting / penetration of the sterilization material with saturated steam. This option enables
the sterilization of loads common to a doctor's practice or clinic.
The sterilizer uses a separate steam generator to generate the sterilization steam. Steam is generated
upon program start and led into the sterilization chamber. This establishes a pre-defined pressure and
temperature.
The sterilization material is dried using a vacuum (vacuum drying). This brings the best drying results even
when using wrapped sterilization material.
Type of the feed water supply
The sterilizer works with a feed water one-way system. This means that it uses fresh feed water for each
sterilization procedure. The quality of the feed water is subject to permanent monitoring via an integrated
conductivity sensor.
Internal process monitoring
The sterilizer electronics has an integrated process evaluation system. It compares the process
parameters (such as temperature, time and pressure) during a program run. This means that the door
cannot be opened during excess pressure in the sterilization chamber. The sterilization chamber is
protected against overheating and the total operating time of a program is optimized in dependence on the
load.
It monitors the parameters in terms of their threshold values during control and regulation and guarantees
safe and successful sterilization. If one or more parameters depart from the threshold values determined,
the sterilizer issues warning or malfunction messages and if necessary, aborts the program.
Program
Program type
Regular sterilization program
42
Page 43

Program phase
Description
1. Evacuation
The chamber will be evacuated until the pressure for the vacuum test has
been reached.
2. Equilibration time
An equilibration time of five minutes will follow.
3. Measuring time
The measurement time amounts to ten minutes. The pressure increase within
the chamber is measured within the measurement time. The evacuation
pressure and the equilibration time or measuring times are shown on the
display.
3. Ventilation
The chamber is ventilated after the end of the measuring time. Then the
message will be shown on the display with an indication of the leakage rate.
Should the leakage rate be too high (i.e. over 1.3 mbar), this will also be
indicated on the display.
4. Test end
Following a successful test program, the current daily batch number is
displayed, alternating with the message Clear with ’+’. You can open the door
after pressing the (+) key.
Type tests
UniversalProgram
QuickProgram B
QuickProgram S
GentleProgram
PrionProgram
Program type in accordance
with DIN EN 13060
Type B
Type B
Type S
Type B
Type B
Dynamic pressure test of the
sterilization chamber
X X X X X
Air leakage
X X X X X
Empty chamber test
X X X X X
Solid load X X X X
X
Porous partial load
X
--
-- X X
Porous partial load
X
--
-- X X
simple hollow items(hollow
body B)
--
-- X --
--
Instruments with narrow
lumen (hollow body A)
X X -- X X
Single wrapping
X X -- X X
Mltiple wrapping
X -- X X
Drying massive load
X X X X X
Drying, porous load
X
--
-- X X
Vacuum test
Chapter 9 – Description of function
Overview of the Sterilization programs
The results in this table show which inspections were performed on the sterilizer. Fields marked show
compliance with all applicable sections of the standard DIN EN 13060.
43
Page 44

Chapter 9 – Description of function
PRESS KEY (S) and (+) simultaneously
KEY „Start/Stop“ and terminate a program
KEY „Program“: "Enter/Confirm/Input
Manually terminate
during drying
Program sequence
Function
hh:mm:ss
0.00 bar 89°C
Initial state
P
Universal-Program
134°C wrapped
Quick-Program S
134°C unwrapped
Gentle-Program
121°C wrapped
Prion-Program
134°C wrapped 20’
Bowie & Dick test
134°C 2.2 bar 3.5’
Vacuum test
P
P
P
P
P
Release
AIN4: Temp.preheat.
120°C
Release
1st time Press
START
S
Supplement drying
selected
S+
Stop program
key ‚Stop’
S
Immed. removal
press 'STOP’
Drying stopped
Quick-Program B
run successfully
Program runs
Last batch number. x
Quit with key ’+’
see next page
+
Stop program?
key `Stop’
Program
stopped
S
pressure release
1.52 bar 112°C
Stop/ End
0.02 bar 88°C
Acknowledge
with key ’-`
Manually terminate
before drying
S
–
h
2nd time Press
–
h
AIN6: Conductivity
10 µS/cm
Quick-Program B
134°C wrapped
P P P
Press (+) and (-) simultaneously to select the
SETUP menu
Select by keep pressing the key (-)
Unlocking door with key (+)
h
–
Unlocking door
with key ’+`
S
P
S+
Overview of programs
MAIN menu
44
Page 45

Water system
One-way
Water system
circlulatory flow
Preheating
No
Preheating
Yes
Press both keys
simultaneously
Function:
autom. Pre-
heating
hh:mm:ss
0.00 bar 89°C
SETUP menu
Function:
+
Initial state
S
Function:
Feed water supply*
3)
Function:
Feed water-
supply*
2)
+
Main and submenus can be selected by key (+) „next“ and key
(-) “previous” and you can always leave them with key (S).
+
Feed water supply
internal
Feed water supply
External
*2) Only valid for: Vacuklav®23/31 B+ ; Euroklav®S+/ VS+
*3) Only valid for: Euroklav®S+/ VS+
S
KEYS (+) and (+): (next / previous) in the menu
KEY “Program”: "Enter/Confirm/Input"
KEY “Start/Stop”: " Terminate/Escape/Leave"
KEY Start/Stop: Escape/Leave without saving
S
P
–
+
S
P
P
Function:
Total batch
S
P
Total batch
xx
Function:
Last batch
number
S
Last batch number
xx
P
P
+
–
S
Date/ Time
Month: xx
Date/ Time
Minute: xx
Date/ Time
Second: xx
Date/ Time
Day: xx
Date/ Time
Year: xx
Function
Date/Time
= see above
= see above
= see above
= see above
= see above
Date/ Time
Hour: xx
Allocated: xx
Open: xx
Immediate output
Yes/ No
Function:
Batch output
P P
+
–
S
P
Output medium*1)
P
+
–
S
P
Last cycle output:
No xx
S
Stored cycles
output
P
S
All cycles
delete
P
S
P
Test output
+
*1) No output medium
MELAprint
MELAflash
MELAnet+graphic data
Computer
Modem
P S P
S P S P S P S
+
P
P
Chapter 9 – Description of function
Program overview: SETUP menu: Function
45
Page 46

Chapter 10 – Malfunctions
Chapter 10 – Malfunctions
Warnings
Warning messages are not malfunction messages. They help to ensure malfunction-free operation and to
recognize undesirable situations. Observe these warnings early in order to avoid malfunctions.
Malfunction message
Warnings and malfunction messages are issued on the display with an event number. This number serves
identification purposes.
Malfunction messages are issued when it is not possible to ensure safe operationor safety of sterilization.
These can appear on the display shortly after switching on the sterilizer or while a program is running.
If a malfunction occurs during a program run, the program will be aborted.
DANGER
Aborting a program before the drying phase means that the load is unsterile.
This endangers the health of your patients and practice team.
If necessary, repack the load and repeat the sterilization for the sterilization material affected.
Before you call customer service
Ensure that you have complied with all instructions relating to a warning or malfunction message issued by
the display of the sterilizer. The following table contains a summary of the most important events. The
events contain possible causes and the corresponding operator information.
Should you be unable to find the relevant event, or your efforts do not redress the problem, you can
contact your nearest stockist or authorized MELAG customer service provider. To enable us to give the
best possible service, please have your sterilizer serial number and a detailed description of the fault
contained in the malfunction message to hand.
46
Page 47

Incident
Possible cause
What you can do:
Empty display
No current
(2 points.)
Check the power plug for its correct
position in the socket.
Check the electricity supply on the socket
Change the device fuses on the lower
sterilizer front if necessary
(see page 9/10 ). To do so, follow the
instructions in the technical manual under
device fuse.
You cannot open the
door
The door seal sticks to the seal
face.
Switch on the sterilizer, confirm with the (+)
key and pull strongly on the door.
Too high feed water
consumption
The sterilizer is loaded
incorrectly.
The sterilizer is not set-up
correctly.
Condensate reflux is prevented.
Comply with the prescribed load quantity.
(Page 15, Loading the sterilizer).
Check for the correct set-up of the
sterilizer. If necessary, increase the slope
of the device feet by unscrewing them by
max. two revolutions.
Remove any instruments, filter paper or
other objects which have fallen onto the
chamber floor.
Bad drying results
The sterilizer is loaded
incorrectly.
The sterilizer is not set-up
correctly.
Condensate reflux is prevented
or blocked.
Comply with the prescribed load quantity
(see page15, loading the sterilizer). The
textiles may not have direct contact with
the chamber wall and floor.
Check for the correct set-up of the
sterilizer. If necessary, increase the slope
of the device feet by unscrewing them by
max. two revolutions.
Remove any instruments, filter paper or
other objects which have fallen onto the
chamber floor.
Check the chamber filter and condensate
reflux filter for blockage.
Activate the pre-heating (see page
19,Select automatic pre-heating).
Activate additional drying
(see page 20, additional drying).
General event
Chapter 10 – Malfunctions
47
Page 48

Chapter 10 – Malfunctions
Warning
Possible cause
What you can do
Warning, door open and
Start not possible
Door contact is not closed
upon start.
Press down the slide locking grip
downwards to its fullest extent.
Warning no feed water /
refill feed water - start not
possible
Only with feed water supply
from an internal storage tank:
Insufficient feed water in the
internal storage tank.
Check the fill level of the feed water in the
internal storage tank; if necessary, fill the
feed water up to the MAX mark.
Attention no feed water/
check the feed water
inflow
The warning will be
displayed after a program
start. The installed flow
monitor does not close.
Feed water via the internal storage tank
Should this message appear repeatedly,
arrange for an inspection by MELAG
customer service.
Feed water supply from the MELdem 40
Check the water treatment unit; open the
inflow to the unit if necessary.
Should this message appear repeatedly,
arrange for an inspection by MELAG
customer service.
Feed water supply from the MELdem 47
Check the water treatment unit; open the
inflow to the unit if necessary. Should this
message be repeatedly issued with an
empty pressure accumulator after c. 1
hour new start, arrange for an inspection
of the water treatment unit by MELAG
customer service.
Please note! This message can be issued
following commissioning/recommissioning,
as the pipe system is still empty. Repeat
the start.
Poor feed water/replace
the cartridge or module
Feed water conductivity too
high.
Conductivity 40 µS/cm.
Start through repeated depressing of
the (S) key still possible
Feed water supply from:
Mixed-bed resin exhausted.
MELAdem 40:
Change the mixed-bed resin, see
operating manual for the water treatment
unit MELAdem 40.
Mixed-bed resin in
subsequent ion exchanger
(3. cartridge) exhausted.
MELAdem 47:
Change the mixed-bed resin, see
operating manual for the water treatment
unit MELAdem 47 and check the treatment
unit.
On repeated occurrence, arrange for
maintenance to be performed by MELAG
customer service / the customer service of
your stockist. The pre-filter and active coal
filter may need to be changed.
Warnings
48
Page 49

Chapter 10 – Malfunctions
Warning
Possible cause
What you can do
Poor feed water/replace
the cartridge or module
Mixed-bed resin in reverseosmosis unit exhausted.
Other water treatment unit:
Change the module / resin cartridge
according to the manufacturer’s operating
manual.
Upon repeated occurrence.
PLEASE NOTE! Perform a program start
after finishing the work outlined above.
This warning can be issued upon the initial
start after maintenance of the water
treatment unit, as the inflow hose has not
rinsed the measurement cell fully with
fresh water.
Insufficient quality of feed
water / start not possible
Feed water conductivity too
high.
Conductivity 65 µS/cm.
Start no longer possible:
See warning message "Poor feed
water/replace the cartridge or module."
Please wait
The chamber is warming
This display appears during
the program start phase. The
sterilizer has not yet reached
the starting temperature.
The sterilizer starts automatically after the
starting temperature has been reached.
Warning / change sterile
filter
Min./Max. pressure is
exceeded / undercut during
air-drying.
Sterile filter soiled or torn.
Replace the sterile filter.
PLEASE NOTE The message comes at
the end of the program and in the last line
of the log print-out.
Output medium is not
ready
The sterilizer is operating
without an output medium,
but one has been registered.
In the menu log issue, set the option no
output medium.
The output medium has not
been connected properly
Check the correct connection of the data
cable to the sterilizer and the output
medium.
The electricity supply to the
printer has been interrupted.
The printer is “offline.”
Check the electricity supply. The red LED
“P” on the log printer MELAprint 42 must
be illuminated.
Set the printer to “online“ (press the “SEL“
key on the MELAprint 42, the “SEL“ LED
must illuminate green).
49
Page 50

Chapter 10 – Malfunctions
Warning
Possible cause
What you can do
Log memory full
The device-internal log
memory is full (max. 40 logs
possible).
The message is displayed upon program
start.
Repeated pressing of the (S) key removes
the message and the program starts. The
oldest log will be deleted in the process.
An output medium has been
registered and the option
Immediate issue – no
has been set in the Log issue
menu.
Set sterilizer to Immediate output yes
(see page 28,
Outputting logs immediately and
automatically).
Delete the printer memory (see page,
deleting the printer memory (see page 30,
Deleting logs in the internal log memory
Delete the logs in the internal log
memory.Deleting logs in the internal log
memory,see page 29), If necessary,
output all the saved logs beforehand (see
page 29, Printing all saved logs Printing all
saved logs
Unregister the output medium in the Log
issue menu and set the option No
output medium.
Carry out maintenance
The maintenance message
has been activated and the
device has reached the preset number of charges.
This message is displayed upon every
program start.
Repeated pressing of the (S) key removes
the message and the program starts.
Press the (S) key twice.
Arrange for maintenance to be performed
by the MELAG customer services / your
specialist stockist customer services
PLEASE NOTE The maintenance counter
is to be reset by customer services
Test not successful
Rate of leakage: 3.2
The leakage rate determined
during the vacuum test lies
over the maximum
permissible value of 1.3
mbar.
Door seal, chamber flange
soiled.
Check that the door seal and chamber
flange are clean and clean if necessary.
Control the door seal for wear, change if
necessary (see page 37 Replacing the
door seal).
Repeat the vacuum test with an entirely
cold device.
Door seal set incorrectly.
Check the door seal for its correct position.
Repeat the vacuum test with an entirely
cold device.
Warning Battery empty
Monitoring of the internal
battery voltage has returned
too low a value.
The battery is to be changed by MELAG
customer services / your specialist stockist
customer services.
50
Page 51

Malfunction message
Possible cause
What you can do
Fault 1: Vacuum
system
Door seal, seal face on the
chamber soiled or defective.
Check the door seal and seal face on the
chamber for soiling and clean.
Check the door seal for wear, change if
necessary (see page 37 Replacing the
door seal).
Door seal set incorrectly.
Check the door seal for its correct
position.
Check that the sterilizer is set up correctly.
Check the sterilizer for instruments, filter
papers or other objects with have fallen
onto the chamber bottom.
The chamber filter is blocked.
Check the chamber filter for soiling and
clean if necessary. To do so, use the
chamber filter wrench (see page 37
Replacing the door seal).
Fault 2: Steam
generator
Sterilizer is overloaded.
Reduced heat production, as
the mains voltage is too low.
Ensure that the sterilizer is loaded
correctly (see page 15, Loading the
sterilizer).
Check the on-site electrical connection.
Try operating the device on a different
electrical circuit.
Upon repeated occurrence, inform your
stockist.
Fault 4: Pressure
release
Pressure release filter is soiled.
Check whether the pressure-release filter
is clogged (in chamber bottom in the rear
area). Unscrew the filter beforehand.
Upon repeated occurrence, inform your
stockist.
Fault 8:
The maximum difference
between the program run time
and the internal computer clock
has been exceeded.
Upon repeated occurrence, inform your
stockist.
Fault 9: Door open
The locking sliding handle was
pushed upwards during a
running program.
Push the sliding closure grip downwards
until the stop. Correct display: Door
closed.
Upon repeated occurrence, inform your
stockist.
Fault 10: Overheated
Steam generator
Overheat Steam generator.
This malfunction message can be
generated following a program abort and
direct re-start. Repeat after a two minute
pause.
Upon repeated occurrence, inform your
stockist.
Fault 12: Door lock
Locking pin of the door is stiff.
Check the door locking pin for free
movement. To do so, press in the door
locking pin (see fig. 3/3).
Upon repeated occurrence, inform your
stockist.See also Fault 35.
Fault 14: No feed water
This warning will be displayed
after a program start.
See warning text Warning no feed
water.
Fault 21: Pre-heating
The monitoring time between
activation of the pre-heating
On repeated occurrence select option
Automatic pre-heating No
Fault messages
Chapter 10 – Malfunctions
51
Page 52

Chapter 10 – Malfunctions
Malfunction message
Possible cause
What you can do
and the temperature being
reached has been exceeded.
The pre-heating temperature
was exceeded.
(see page 19 Selecting automatic preheating) and notify specialist dealers.
Fault 22: Overheated
pre-heating
The maximum pre-heating
temperature has been
exceeded.
On repeated occurrence select option
Automatic pre-heating No (see page
23, Select automatic pre-heating) and
your stockist 19
Selecting automatic pre-heating) and
notify the stockist.
Fault 31:System leak
During the program vacuum
test the permitted pressure
maximum was exceeded (very
large leak) after achieving the
evacuation pressure.
Repeat vacuum test,
if renewed error message, notify specialist
dealers.
Fault 32: Power outage
Sterilize the sterile filter
The operating voltage outed
after a program start.
The malfunction message is
issued after the operating
voltage is restored.
A power outage during a
program already started in
over-pressure will lead to a
further instruction to sterilize the
sterile filter as it has become
damp and possibly even
affected by germs.
Check all on-site installations. If you are
unable to locate a fault, inform MELAG
customer services.
Remove the sterile filter from the rear of
the steam sterilizer and change or sterilize
it (see page 38, Replacing or sterilizing
the sterile filter).
Switching off the sterilizer
during a current program.
A running program should only be aborted
with the (S) key. (see also page 21,
Manual program abort).
Fault 34: Sterilization
TU1
Minimum permissible
sterilization temperature has
been undercut (temperature
sensor 1).
Operate device with smaller load, possibly
carry out vacuum test. Check door seal for
wear. Upon repeated occurrence, inform
your stockist.
Fault 35: Sterilization
TO1
Maximum permissible
sterilization temperature has
been exceeded (temperature
sensor 1).
Perform vacuum test.
Upon repeated occurrence, inform your
stockist.
Fault 36: Sterilization
PU
Minimum sterilization pressure
has been undercut.
Operate device with smaller load, possibly
carry out Vacuum test.
Check door seal for wear.
Upon repeated occurrence, inform your
stockist.
Fault 51: Sterilization
TU2
Minimum permissible
sterilization temperature has
been undercut (temperature
sensor 2).
Operate device with smaller load, possibly
carry out Vacuum test.
Check door seal for wear.
Upon repeated occurrence, inform your
stockist.
Fault 52: Sterilization
TÜ2
Maximum permissible
sterilization temperature has
been exceeded (temperature
sensor 2).
Perform vacuum test.
Upon repeated occurrence, inform your
stockist.
52
Page 53

Chapter 10 – Malfunctions
Display with door unlocked
Opening the Emergency door during a power failure
DANGER
Non compliance can lead to severe burning and injuries.
Be absolutely sure that the sterilizer is completely relieved from pressure:
No steam may be permitted to escape between the sterile filter and the reverse side of the
sterilizer.
The sliding closure grip must be easy to manipulate.
It must be possible to push back the door about 2 mm with only slight pressure.
Ensure to allow the sterilizer to cool down. Metal parts such as door and chamber can be hot.
If the door cannot be opened, for instance due to a power failure, comply with the safety instructions
outlined above and proceed as follows:
1. Switch the sterilizer off at the power switch and pull the power plug from the wall socket.
Fig. 7 emergency unlocking of the door
2. If the lever is in the guide, pull it forwards with your right hand. Push the slide locking grip
upwards with your other hand.
3. Open the door.
Fig.8 opening the door
53
Page 54

Chapter 10 – Malfunctions
Changing the device fuses
If the device fuses have been tripped, (see p. 9/10), proceed as follows to change:
1. Switch off the sterilizer at the power switch and remove the plug from the socket.
2. Open the door manually in accordance with the section “
3.
4. Opening the Emergency door during a power failure”. Unscrew both screw caps of the fuse holder
(S. 9/10) at the lower front of the sterilizers with a screwdriver or a coin.
Two replacement fuses are mounted on the door interior (see marking).
Fig. 9 Replacement fuses on the door interior
5. Remove the defective device fuses and insert the new fuses securely in the holder.
Fig. 10 Fore view, below right
6. Screw the cap of the fuse holder to the lower sterilizer front.
7. Reconnect the sterilizer plug to the socket and switch on the sterilizer at the power switch.
Should this trigger repeatedly, please inform MELAG customer service/the customer service of your
stockist.
54
Page 55

Glossary
Glossary
Aqua dem
→Demineralized water
Aqua dest
→Distilled water
Heat-up phase
The time required after the sterilizer has been switched
on / after the start of a sterilization program, to heat the
double jacket steam generator before the sterilization
procedure starts. The duration is dependent on
temperature at which sterilization takes place.
Authorized persons
Depot technicians or MELAG-specified customer
services trained by MELAG.
BGV A1
Specifications from professional associations – the
principles of prevention.
Bowie & Dick test
Steam penetration test with a standard test package;
described in DIN EN 285; the test is usually recognized
in the large-scale sterilization industry.
CF card
Compact Flash-Card; a memory card for digital data.
Batch
Collection of sterilization material which has been
processed together in the same sterilization program.
Delay in boiling
Refers to the phenomenon that it is possible under
certain circumstances to heat a fluid beyond its boiling
point without them boiling. This represents an unstable
state; even low-level agitation can produce a large
bubble within the shortest period, which expands
explosively.
Demineralized water
Water without the minerals usually found in normal
spring or tap water; is produced through ion exchange of
normal tap water. Used here as feed water.
Feed water
Is required for the creation of water steam for the
sterilization; typical values for the water quality according
to DIN EN 285 or DIN EN 13060 – Appendix C.
Distilled water
From the Latin aqua destillata; also referred to as aqua
dest; water which to a great extent is free from salts,
organic material and micro-organisms, is produced from
normal tap water or pre-cleaned water through the
process of distillation (evaporation and subsequent condensation). Used here as feed water.
DGSV
Deutsche Gesellschaft für Sterilgutverordnung (German
Association for the Sterilized Equipment Ordinance). The
DSGV training centres are specified in DIN 58946, part 6
as "Requirements of personnel".
DIN 58953
Standard – sterilisation, sterile equipment supply.
DIN EN 867-5
Standard – non-biological systems for use in sterilizers –
part 5: The determination of indicator systems and test
bodies for the performance test of small sterilizers of the
type B and type S.
DIN EN 868-8
Standard – packaging materials and systems for medical
products requiring sterilization.
DIN EN ISO 11140-1
Standard – the sterilization of products for use in medical
treatment – chemical indicators – part 1: General
requirements.
DIN EN ISO 11607-1
Standard – materials requirements, Sterile barrier
systems and packaging systems; this standard
represents the result of the harmonization of EN 868 part
1 and the international standard DIN EN ISO 11607.
DIN EN 13060
Standard – Small steam sterilizers.
DIN EN 285
Standard – Sterilization – Steam sterilizers – Large
sterilizers.
Dynamic pressure test of the sterilization chamber
Serves to verify that the rate of the change of pressure
occurring in the sterilization chamber during a
sterilization cycle does not exceed a certain value, which
could lead to damage of the wrapping material
[DIN EN 13060].
Dynamic pressure test of the sterilization chamber
Serves to prove that the rate of pressure variations
during a sterilization cycle does not exceed a particular
value which could result in the damage of the packaging
material. [DIN EN 285].
Single wrapping
Wrapped once e.g. instruments sealed in foil – in
opposition to: Multiple wrapping.
Evacuation
Creation of a vacuum in a vessel.
Fractionated vacuum procedure
Technical procedure in steam sterilization;
the repeated evacuation of the sterilization chamber in
alternation with steam injection.
FTP
(File Transfer Protocol) is a data transmission procedure
serving to transport data from the internet. This data can
include programs, files or even information. Special FTP
programs (FTP clients) serve to load the data onto a
server (upload).
Instruments with narrow lumen
An article open on one side to which the following
applies:
1 ≤ L/D ≤ 750 and L ≤ 1500 mm or an article with an
opening on both sides which is:
2 ≤ L/D ≤ 1500 and L ≤ 3000 mm and which does not
correspond to a hollow body article B
L…length of hollow body article
D…Diameter of hollow body article [DIN EN 13060]
Mixed loads
Wrapped and unwrapped sterilization material within a
single load.
Hollow body A
→Instruments with narrow lumen
Hollow body B
→Simple hollow instruments
55
Page 56

Glossary
Initialization
Creating a specific starting situation of the software upon
starting.
Condensate
Fluid (e.g. water) produced by the cooling of and
resultant separation from the vaporous state.
Corrosion
The chemical alteration or destruction of metal materials
by water and chemicals.
Contamination
Here: the impurification of the sterilizer load through
undesirable or damaging materials.
Empty chamber test
Test run without a load, performed to assess the
performance of a sterilizer without the influence of a
load; facilitating verification of the temperatures
maintained in comparison to the temperatures set
[DIN EN 285].
Conductivity
Is the reciprocal value of electrical resistance; measured
in micro-Siemens / centimetre (µS/cm); the greater the
amount of dissolute matter in the water, the better it can
conduct electrical current and thus the higher its
conductivity.
Conductivity measurement
Conductivity measurement. Measurement of the
conductivity.
Air leakage- verification of the air leakage
Verification of the leakage serves to prove that the
volume of air ingress in the sterilization chamber during
the vacuum phase does not exceed a value which would
prevent steam penetration of the sterilizer load and that
the air leakage does not cause the possible
contamination of the sterilizer load during the drying
phase.
Solid
Without hollows or gaps, solid, compact, closed.
Massive load – verification of a massive load
Serves to prove that the necessary sterilization
conditions have been reached within the entire load with
the values set in the control. The load must represent the
largest weight of massive instruments designed for
sterilization in a sterilizer in accordance with
DIN EN 285.
Multiple wrapping
E.g. wrapped instruments sealed in a double layer of film
or wrapped in film and placed in an additional container
or a container wrapped in textiles.
MPBetrieib V
MPBetreibV regulation covering the installation,
operation, application and maintenance of medical
products according to § 3 of the Medical Devices
Directive with the exception of medical products for
clinical evaluation or performance evaluation.
Standard conform
Satisfies all relevant standards.
Porous
Permeable for fluids and air e.g. textiles.
Porous small components
Made of materials which are able to absorb fluids.
Porous partial load – test of porous partial load
Serves to prove that the values set on the control allow
steam to enter the pre-determined test package quickly
and equally [DIN EN 13060].
Porous full load – test of porous full load
Serves to prove that the values set on the control satisfy
the necessary sterilization conditions in porous loads
with a maximum mass for which the sterilizer is designed
in accordance with DIN EN 285 .
Process evaluation system
Also known as the self-monitoring system – observes
itself, compares the various sensors during a current
program.
Self monitoring system
Process evaluation system.
Separate steam production
The steam generator is located outside the sterilization
chamber. The sterilization chamber is protected from
overheating in this way.
Simple hollow items
An article open on one side to which the following
applies:
1 ≤ L/D ≤ 5 and D ≥ 5 mm or an article with an opening
on both sides which is:
2 ≤ L/D ≤ 10 and D ≥ 5 L…length of hollow article
D…diameter of hollow article [DIN EN 13060
Sterile barrier system
Steriebarrier system: a closed minimum packaging which
prevents the entrance of microorganisms e.g. through
sealing bags, sealed and re-usable containers and
folded sterilization towels etc.
Sterilized equipment
Also referred to as a batch: a load which has already
been sterilized, i.e. is sterile.
Sterilization chamber
The interior of a sterilizer, accommodates the sterilizing
material.
Sterilization material
Unsterile, sterilizable material which is still to be
sterilized.
TCP
Transmission control protocol: refers to a standard
protocol for connecting computers and networks.
Vacuum
In common parlance, an area devoid of all material in the
technical sense: volumes with a reduced gas pressure
(at least air pressure).
Vacuum drying
Gentle drying: the drying load is subject to underpressure. This reduces the boiling point and thus leads
to evaporation even at low temperatures.
VDE
Verband der Elektrotechnik, Elektronik und
Informationstechnik e.V. (German: The Association of
Electrotechnology, Electronics and Information
Technology).
Soft sterilization packaging
E.g. a paper bag or transparent sterilization packaging.
56
Page 57

Model name
Vacuklav 23 B+
Vacuklav 31 B+
Device dimensions (HxWxD)
49 x 42.5 x 75.5 cm
49 x 42.5 x 61,5 cm
Sterilization chamber (diam x depth)
Ø 25 cm x 45 cm
Ø 25 cm x 35 cm
Volume of the sterilization chamber
22 litres
17 litres
Volume of the storage tank
Feed water (right chamber) 5 litres (c.7 cycles);
waste water side (left chamber): 3 litres
Weight (empty)
50 kg
45 kg
Electrical power
2100 W
Electrical connection
A 220 -240V circuit (max. voltage range 207-253V) and
50/60 Hz building-side recommended: separate circuit with 16 A
fuse, an additional FI switch 30 mA
Noise emission
Sound pressure level @1m space, 65db (A)
Waste heat (with max. solid load)
0.9 kWh
Ambient temperature
5-40 °C (recommended max. 25 °C)
Relative humidity
80% at 31 °C, decreasing in a linear fashion up to a relative
humidity of 50% at 40 °C
Max. altitute
2000 m
Length of power cable
1.35 m
Feed water quality
Distilled or demineralized feed water in accordance with
DIN EN 13060, Appendix C (with central demineralization
system max. conductivity 5 µ
CE mark
CE 0197, CE 0035
Degree of protection
(following IEC 60529)
IP20
Technical Data
Technical Data
57
Page 58

Accessories
Article
Order no.*
Vacuklav 23 B+
Vacuklav 31 B+
Tray mounts
A for 5 trays or 3 standard-tray cassette
40244
40233
Bracket >B< for 4 standard tray cassettes
40224
40234
D for two tall cassettes of 4 trays
46840
Sterilization
container with a
single-use paper
filter in accordance
with DIN EN 868-8
15K depth / width / height in cm: 18/ 12/4.5
01151
15M depth / width / height in cm: 35/ 12/4.5
01152
15G depth / width / height in cm: 35/ 12/8
01153
17K depth / width / height in cm: 20/ 14/5
01171
17M depth / width / height in cm: 41/ 14/ 5
01172
15M depth / width / height in cm: 14/ 14/ 9
01173
23M depth / width / height in cm: 42/ 16/ 6
01231
23G depth / width / height in cm: 42/ 16/ 12
01232
28M depth / width / height in cm: 32/ 16/ 6
01284
28G depth / width / height in cm: 32/ 16/12
01285
Swab drums with
filter cloth
17R diameter/ height in cm: 13/ 10.5
00174
23R diameter/height in mm: 18/ 14
00233
Package holder
For chamber 25 cm x 45 or 35 cm
22420
22410
Standard tray
cassettes
depth / width / height in mm: 29/ 19/ 4
with filter cloth
00289
without filter cloth
00286
Trays
Tray
00230
00280
Test body system
MELAcontrol consisting of a Helix test body
and 250 indicator strips
01080
MELAcontrol PRO consisting of a Helix test
body and 40 indicator strips
01075
Water treatment
unit
MELAdem 40; floor unit mounting
01049
MELAdem 47; floor unit mounting
01047
Upgrade set for the tank drain
26695
For documentation:
MELAflash CF-Card printer with CF card
and card reader
01039
MELAprint 42 log printer
01042
MELAnet Box
40296
Other
Water stop
01056
Device fuses 16A /gRL
57592
Door seal
58512
Sterile filter
20160
Accessories
*All articles listed are available via your specialist stockist
58
Page 59

Page 60

BA_EN_23B+_31B+_v13.docx Rev.:13– 16/1284
MELAG Medizintechnik oHG
Geneststraße 6-10
10829 Berlin
Germany
e-mail: info@melag.de
Web: www.melag.de
Responsible for content: MELAG Medizintechnik oHG
Subject to technical alterations
Date of update: 30.06.2016
 Loading...
Loading...