
Leica TCS STED CW
The Fast Track to Superresolution

• Freedom to choose – popular fl uorescent dyes and proteins
• Observe what´s inside – with confocal superresolution
• Nanoscale imaging – devoid of mathematical artifacts
• Upgrading, to STED – quick and affordable
• Watch live cell dynamics – at the nanoscale!
2
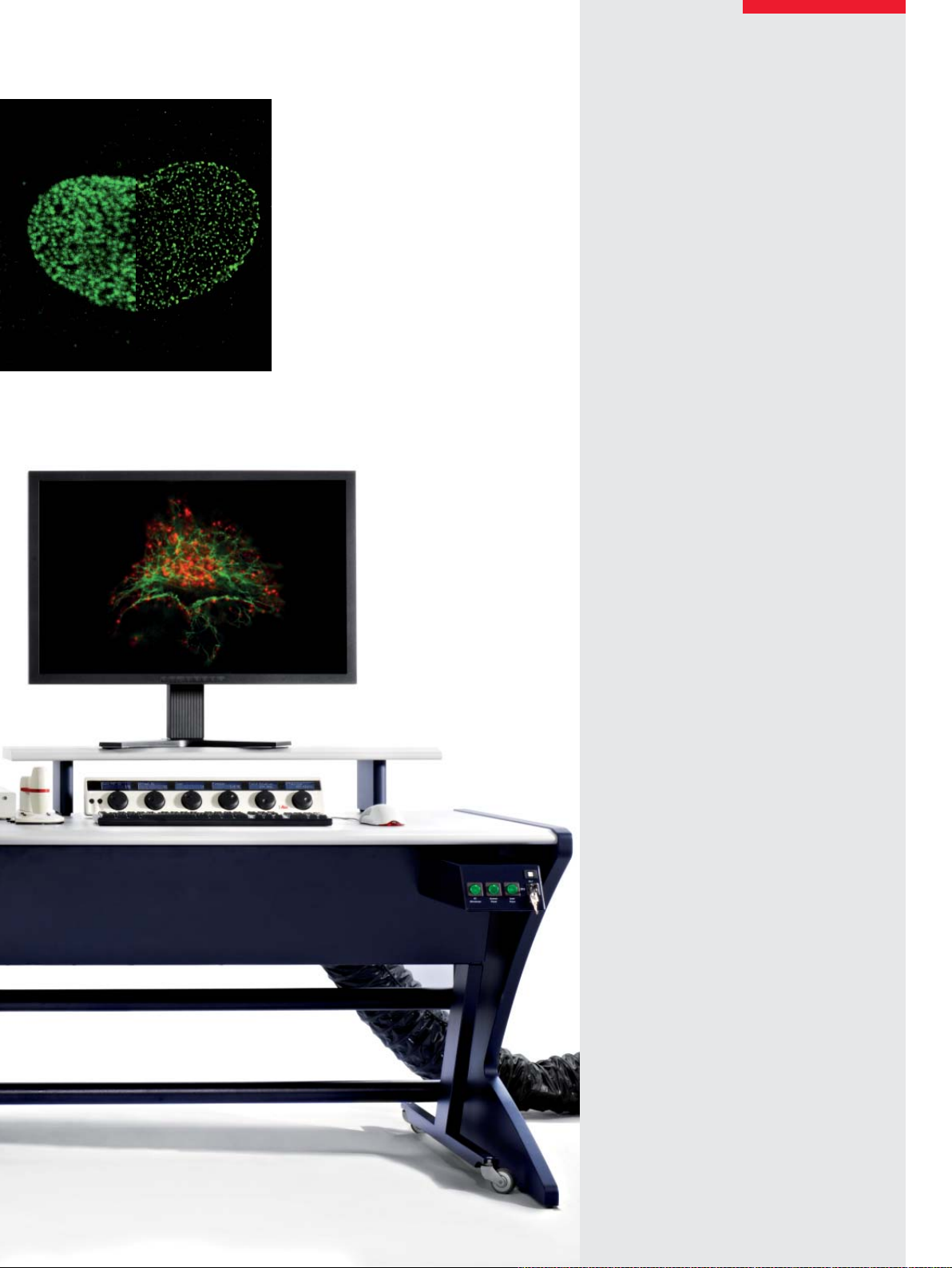
Superresolution light microscopy is revolutionizing life science research with increasing speed.
The charm of direct visual results from an intact
specimen on the nanometer scale attracts scientists from all fi elds of biomedical research.
In its early days, superresolution was only for
biophysicists and optical specialists. Nowadays, it has become an indispensable method
in many life science research institutes working
with light microscopes. Structural details of synapses, ensembles of small vesicles or receptor
arrangements have now become accessible for
fl uorescence microscopy.
Leica TCS STED CW
The Fast Track to Superresolution
Subdiffraction microscopy needs to meet the
requirements of daily research. But many superresolution imaging tasks require special labeling
or restricted user environments. So far it was
diffi cult to get super-resolved images with standard fl uorophores, fl uorescent proteins, and from
living specimens.
However, the access to these data means a crucial improvement of results for any researcher.
Leica Microsystems, the fi rst provider of integrated superresolution technology, fi lls this gap
by extending its STED portfolio with the new
Leica TCS STED CW.
It is a stunningly simple solution which combines
the high-end confocal TCS SP5 with purely optical and patented superresolution technology. It
opens the door to the nanoworld – easy, highly
affordable and as an upgrade for already installed systems! K. Willig, B. Harke, R. Medda,
S.W. Hell, Nature Meth. 4, 915 (2007)
3
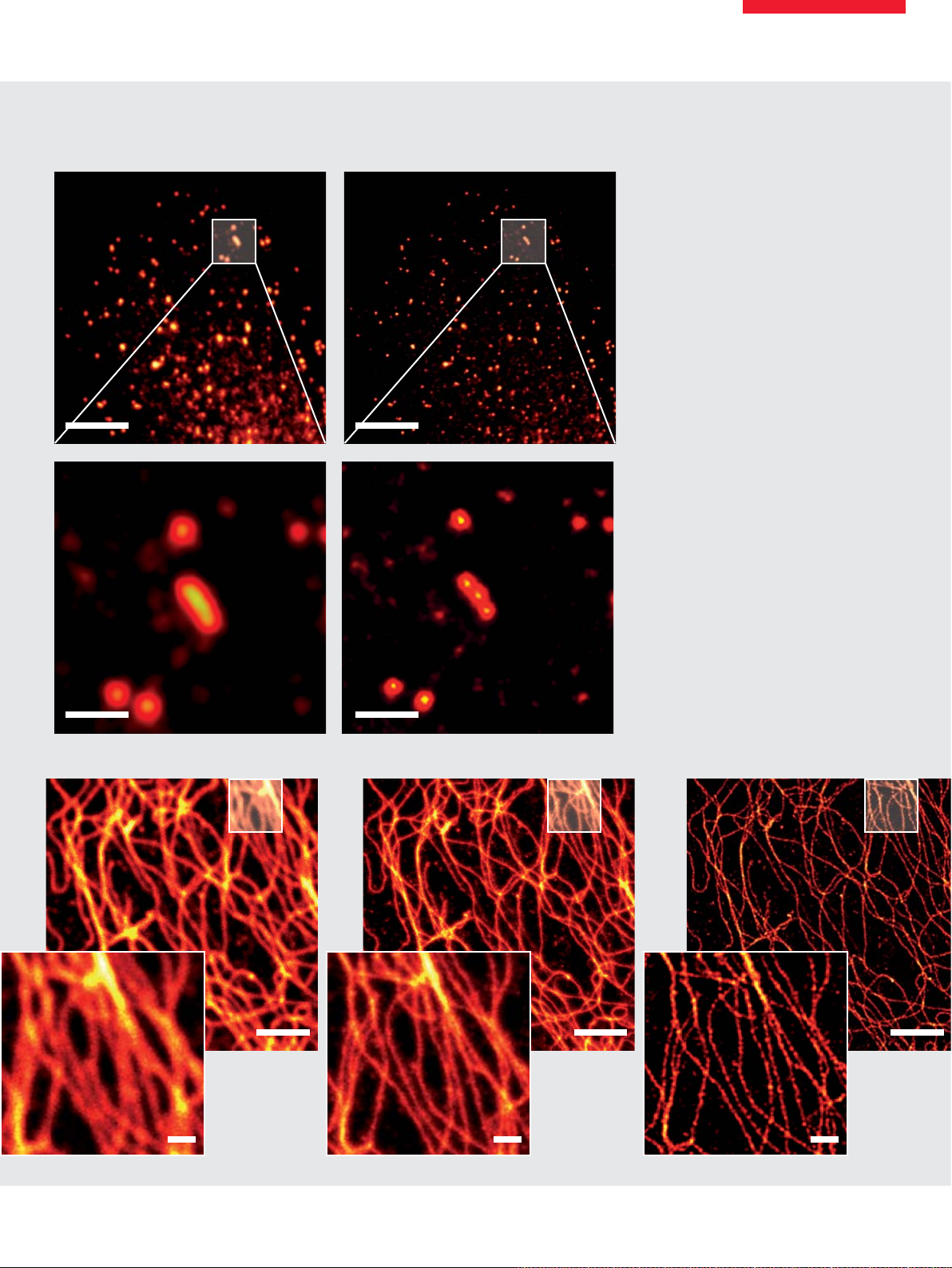
New Horizons in Neuroscience
1
Res
Brp
Confocal STED
Structural studies of the nervous system have been identifi ed as one of the most promising fi elds for superresolution microscopy. With the new Leica TCS STED CW
investigation of the neuromuscular junction with subdiffraction resolution has become possible – not only in fi xed specimens (as shown above) but also in 3D, 10 µm
inside the living larvae, in time lapse recordings.
Immunofl uorescent staining of neuromuscular junctions of a drosophila larvae. Labels: Bruchpilot (Chromeo 488, red), Res (green, Cy3). Courtesy of Stephan Sigrist and
Wernher Fouquet, Freie Universitaet Berlin, Germany.
2 µm
1
500 nm 500 nm
2
2
4
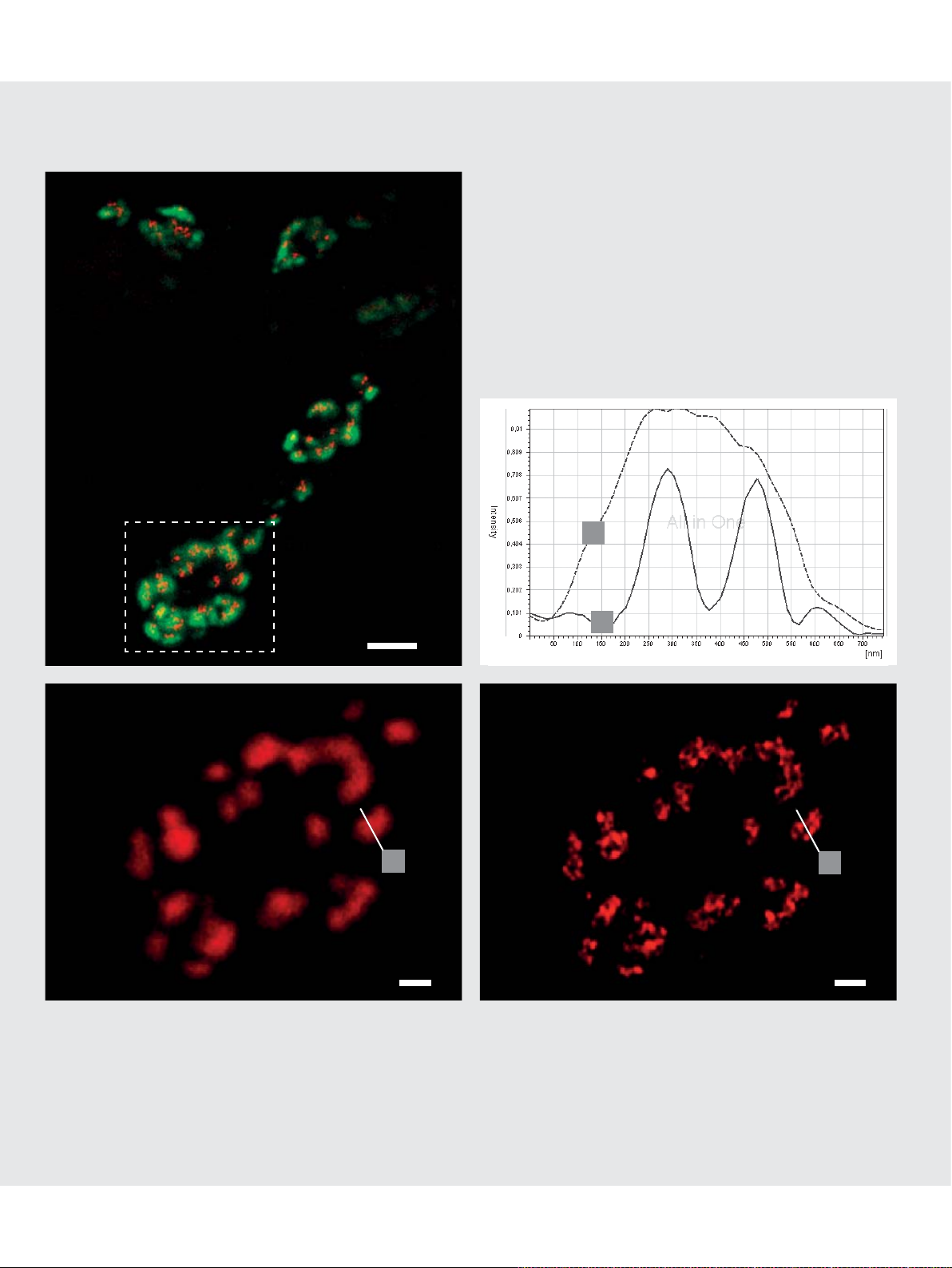
Standard Dyes for STED Microscopy
Confocal
2 µm
Confocal
STED
2 µm
STED
Alexa 488
500 nm 500 nm
Oregon Green
Confocal
2 µm
500 nm
Intermediate fi lamentous protein Vimentin in Vero cells. Fluorescence label: Oregon Green.
STED
500 nm
Distribution of Clathrin vesicles in HeLa cells.
Fluorescent marker: Alexa 488, immunolabeling.
STED & Deconvolution
2 µm
2 µm
500 nm
5
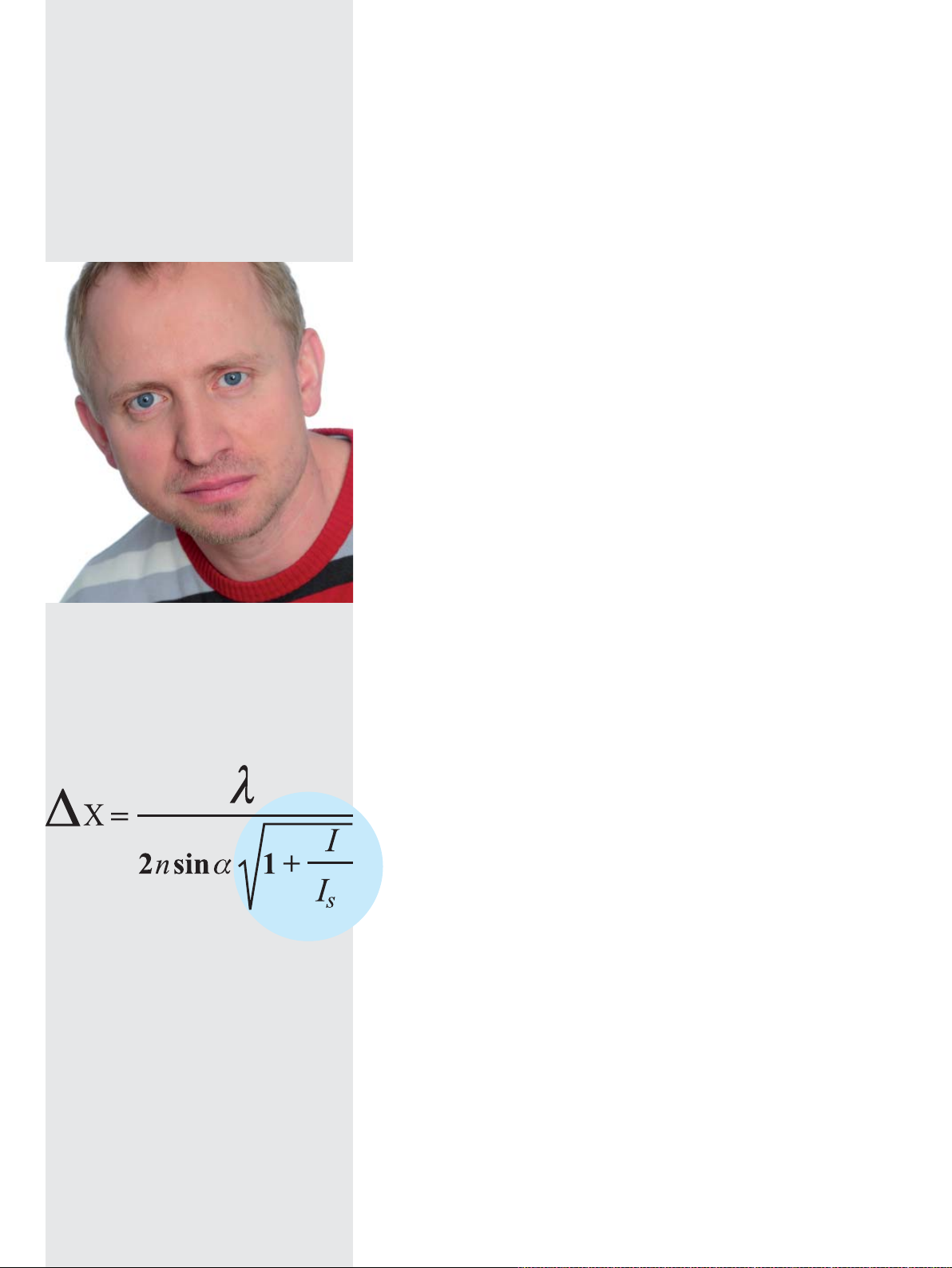
Prof. Dr. Stephan Sigrist, Charité, Berlin
“STED means for me:
Seeing the Essential Details!”
Superresolution at High Speed
STED with continuous wave laser beams
Continuous Wave (CW) STED is a stunningly simple way to overcome the diffraction resolution limit in light microscopy. The fundamental difference to the proven STED realization with paired
pulsed lasers is the continuous excitation of fl uorophores resulting in non-stop signal delivery. The benefi t for the user: superresolution without speed limits and increased fl uorophore fl exibility!
The basic concept of pulsed STED and CW STED is the same. The
spot where fl uorescence is generated is scaled down to subdiffraction size by switching off the ability of the dye to fl uorescence
in the periphery of the excitation spot. This means genuine superresolution pixel by pixel.
The physical process behind it, stimulated emission, is well-known
as being the functional principle of lasers. In addition to the excitation laser used in standard confocal microscopy, STED adds
a second laser with a longer wavelength and adjustable output
power. This laser keeps fl uorophores at the excitation spot periphery dark by driving excited dye molecules back to the electronic
ground state before they can emit fl uorescence. It is necessary to
restrict this fl uorescence deactivation process to the periphery
of the focal spot in order to make it usable for resolution improvement. The shape of the STED laser beam is modifi ed to create a
ring. This is accomplished by helical phase masks which provide
an optimal laser energy distribution for STED.
Pulsed lasers for maximum STED effi ciency
Pulsed lasers, such as Ti:Sa infrared lasers, known from two-photon microscopy, deliver high peak power intensities connected to
a maximized resolution improvement. The 12 ns interpulse period
exceeds the average fl uorescence lifetime of a dye. This minimizes potential bleaching but limits the generation of fl uorescence
signal.
The Abbe equation decribes the achievable optical
resolution. Stefan Hell extended this equation by a –
superresolution – term, breaking Abbe’s diffraction
barrier.
6
Continuous wave lasers for persistent fl uorescence emission
The new, powerful CW lasers increase STED recording speeds
substantially, without losing superresolution power. The great advantage of CW STED is the abolition of dark interpulse periods. A
continuous generation – and readout – of fl uorescence signals
become possible and result in approximately three times faster
recordings!

Nanoscopy with STED – the Principle
Fine optics and dye photophysics break the diffraction barrier
Resolution enhancement in STED microscopy requires two different lasers. One for fl uorophore excitation (CW STED: Argon-gas laser with 488 & 514 nm) and one red
shifted laser (CW: 592 nm fi ber laser) to annihilate excitation by stimulated emission. This applies for pulsed STED (red and green lines in the drawing) but also for STED
with continuous wave lasers (red and green faint solid areas). Both laser beams are focused through the objective onto the sample and moved, perfectly aligned, by
scanning mirrors (beam scanning). The intensity distribution of the STED beam features a ring shape with zero intensity in the center. Thus, no excitation annihilation
occurs in the inside of the STED doughnut. This ring shape is generated by a highly effi cient helical vortex phase fi lter so that fl uorescence spot is minimized.
S
1
hν
exc.
S
0
Excitation and fluorescence emission
hν
detected
em.
center
STED area
S
1
hν
exc.
S
0
hν
STED
filtered out
Excitation and stimulated emission
The involved photophysical processes are confi ned to different areas of the STED scanning spot. The conventional excitation of the fl uorophores that is followed by
spontaneous emission of photons with different energies (= wavelength) dominates inside the ring, where the STED intensity is close to zero. The STED laser depopulates
the excited electronic state S1 by inducing stimulated emission in the periphery. The released photons are indistinguishable from the STED laser photons and spectrally
fi ltered out. The process is not related to bleaching and can be repeated many thousand times.
t
t
Excitation laser
Excitation laser
STED laser
Depletion laser
t
t
Excitation laser
Excitation laser
STED laser
Depletion laser
Pulsed CW (Continuous Wave)
Both, excitation and STED laser, are permanently active in CW STED. Thus, there is a constant competition between fl uorescence emission and stimulated emission
inside the doughnut. While STED with pulsed laser sources delivers pulse trains with up to 80 MHz (A), CW STED utilizes constantly emitting lasers (B). This results in a
persistent delivery of fl uorescence signals allowing even higher recording speeds than already possible with pulsed STED.
7

Freedom to Choose – Fluorescent
Proteins & Standard Dyes for STED
Fluorescent Dyes for CW STED:
Chromeo 488 ✓
Alexa 488 ✓
FITC ✓
Oregon Green ✓
ATTO 488 ✓
and many more ✓
Usable Fluorescent Proteins
for the New TCS STED CW:
eYFP ✓
Citrin ✓
Venus ✓
Standard fl uorophores – for fi xed and living cell investigations
All researchers are attracted by the potential of superresolution
microscopy. Still, they want to rely on their well proven standard
procedures and labeling strategies.
The expansion of the STED concept into the green range of the
spectrum takes this requirement into account and opens up a
wealth of new opportunities. Well known dyes such as Alexa 488,
FITC and Oregon Green allow the appliance of established immunocytochemical protocols for highest resolution imaging. There is
no need to get used to new, especially photo switchable markers
or time-consuming statistical methods.
This saves time and money and makes the Leica TCS STED CW
an integrated part of the daily imaging workfl ow. Superresolution
experiments do not require any planning. They are simply done by
activating the STED mode and turning on the depletion laser.
STED and STED CW are proven, purely physical methods covering
all of the sample. This is a benefi t for reliability in your research.
Auto-fl uorescent proteins for superresolution microscopy
The development of fl uorescent proteins as genetically encoded
markers, established by Roger Tsien, has marked a milestone for
light microscopy.
Nowadays, the use of these proteins as endogenous, highly specifi c markers has become a standard tool in light microscopy. The
expression of a fusion protein allows the selective labeling of distinct structures without the need to permeabilize the cell and to
incubate it in a dye containing solution. This reduces the effort of
fl uorescence labeling and offers the most direct way for live cell
imaging. By offering excitation lines such as 488 nm and 514 nm
in combination with the depletion line at 592 nm, proteins such
as eYFP and Citrin can be imaged with the Leica TCS STED CW.
This enables researchers to record in order to follow structural
changes on the nanoscale – live!
8

Living Cell Imaging using Fluorescent Proteins
Vesicle movement
Confocal STED
3 µm 3 µm
Confocal
t = 0 sec
STED
t = 36 sec
Time lapse experiment: movement of large dense core vesicles labeled with the fl uorescent protein Venus inside of living PC12 cells.
1 µm 1 µm
STED
t = 0 sec
STED
t = 54 sec
STED
t = 18 sec
STED
t = 72 sec
9

Observe What’s Inside –
with Confocal Superresolution
STED CW Features
Flexible STED-excitation:•
Ar Laser (488 & 514 nm)
STED: Fiber laser 592 nm;
intensity modulated by AOTF
XY-resolution (FWHM) < 80 nm (measured •
on Chromeo 488 nano-beads), depending
on sample, embedding and staining
Integrated linear deconvolution•
Z-resolution: confocal•
Auto beam alignment of excitation and •
STED beam for long term stability
Vortex phase fi lter for maximum•
resolution
Available in combination with AOBS•
and dichroic systems
Simultaneous line sequential recording •
of STED and confocal possible
Life is three-dimensional – and many important events which scientists are interested in happen under the surface. Thus, there is
no chance to get insights with imaging technologies which are
limited to the area in direct contact with the coverslip.
The superior optical sectioning of the true point scanning system
TCS SP5 provides superresolution where you need it: deep in the
sample. STED images of a 12 m thick drosophila larvae featuring a
thick cuticula – no problem. Complete 3D stacks can be recorded.
This is the edge of confocal superresolution!
Purely Optical
The well-known saying “seeing is believing” expresses it best:
The success story of light microscopy is directly connected to its
direct delivery of information to the researcher about the investigated specimen. Breaking the diffraction resolution barrier by
STED is the logical consequence of this very fact. STED is based
on a well-thought-out interplay of fi ne optics and well understood
photophysical processes of the fl uorophore. And it is currently the
method to achieve superresolution in a purely optical way.
Recording speeds of > 20 frames per •
seconds with < 80 nm lateral resolution
Full range of SP5 features supported, •
exclusive 405/UV
10
The Leica TCS STED CW microscope delivers superresolution pixel
by pixel – independent of recording speed or the dye being used.
Plenty of time can be saved since time-consuming data processing steps and complex algorithms are obsolete. STED microscopy
requires one single frame to generate one superresolution image –
in contrast to other localization or interference based concepts.
This makes STED extremely robust against interframe-drifts and
facilitates data handling due to their size. In addition, the experienced user can improve his data by applying image deconvolution
on top of the STED recording. The processing steps are uncomplicated and the workfl ow is embedded into the Leica confocal
software LAS AF. The results are immediately visible and give additional substantial improvement of image quality depending on
the imaged sample.

Superresolution Deep Inside the Sample –
Without Compromises
STEDConfocal
3 µm 3 µm
Cytoskeletal Vimentin (Chromeo 488) in HeLa cells. Top left: STED maximum projection, top right: confocal
maximum projection. Below: individual optical section from a STED xyz recording (z position: 0.9 µm; 1.8 µm;
3.6 µm; 5.4 µm). Stack size (xyz): 22 x 22 x 6 µm. Sample: courtesy of Max Planck Institute for Biophysical
Chemistry, Dept. Nanobiophotonics, Goettingen, Germany.
11

Green Light for
Highest Resolution
Software Workfl ow
Intuitive ✓
Easy to operate ✓
Fully fl exible ✓
Feedback on correct settings ✓
Pure Optics
Reliable results ✓
Immediate imaging ✓
Resolution is a key issue for scientists researching biologically
and medically relevant problems. The user interface of the Leica
TCS STED CW takes this into account by giving access to superresolution data to all kind of users and providing maximum fl exibility for image acquisition settings such as selection of laser lines
and scan parameters.
Leica Microsystems has created the perfect synthesis of highly
developed STED technology and proven user interface. The benefi t: a substantial gain in resolution that is not compromised by
an increase in complexity. The established concept of “one-click
usability” as known for the Leica TCS STED has been retained and
adjusted.
There is no fi xed pairing of excitation and STED laser in the Leica
TCS STED CW. The software continuously reports the suitability of
the selected settings while leaving all decisions to the researcher.
With the help of a comprehensive traffi c light concept parameters
like selection of laser lines, scan format and others are checked
and reported.
The balancing act between maximum fl exibility and optimal
user guidance has been achieved on the basis of the approved LAS AF software interface.
12

Auto-Alignment
Continuous wave STED makes temporal synchronization of the
lasers obsolete. As in the TCS STED with pulsed laser beams,
spatial overlay accuracy of excitation laser and STED “doughnut”
remains crucial to get the best results. This is granted by the patented and software controlled alignment routine which adjusts
the laser beams automatically, activated by a single mouse click
and completed within a minute. The entire calibration routine
takes place inside the scanner chassis, not on the sample being
investigated. The specimen is not illuminated during that process
and the experiments can be continued immediately afterwards,
since all recording parameters are restored.
With the help of the newly developed Vortex phase mask it has
become possible to increase the effi ciency of the STED process
and to reduce the time required for these alignments. The system
is ready for more, exciting experiments.
Deconvolution
Results can be improved by applying the integrated deconvolution
in simple steps:
1. Generate a point spread function based on the image
to be processed with one mouse click.
2. Select the image, the according psf and defi ne the
sharpness of the deconvolution – and preview the result.
3. Apply the selected settings to generate the result image.
Auto-Alignment
Fully automated:•
calibrates by a single mouseclick
Convenient:•
just once every 1-2 hours during work
Time saving:•
duration less than 1 minute
No disturbance of ongoing experiments: •
settings restored after completion, no
light on the sample (alignment inside the
scanner)
3
1
2
13

Flexible Adaptation to a
Broad Range of Applications
Neuroscience Nanotechnology Cell Biology Membrane Biology Virology Oncology
Cell-cell interactions ••••
Assembly of structures •••••
Material science ••
Quantifi cation ••••••
Vesicle transport and movement •••
Cytoskeletal organization ••••
Confocal STED
4 µm
1 µm
The capability to use conventional fl uorescent proteins for labeling reduces preparational efforts substantially. Already well-examined structures reveal new details.
YFP labeled keratin fi laments in SW-13 cells. Courtesy of Reiner Windoffer. RWTH Aachen University, Institute of Molecular and Cellular Anatomy, Aachen, Germany
14
YFP

Upgrading
Ready for the future: Leica Microsystems’ upgrading concept
You own a Leica TCS SP5 already and you would like to enter the
fl uorescence nanoworld? No problem. The modular STED concept in combination with our highly educated service teams make
it possible to upgrade installed Leica TC SP5 systems to STED – on
site! This saves money and precious time that you can invest into
your research. Contact your local Leica sales representative and
discuss a tailor-made STED upgrade confi guration.
Not sure yet? The STED module is fully compatible with AOBS and
with dichroic based SP5 systems. Leica grants upgradability for
years, protecting your investment. You can expand the capabilities of your current system – whenever you want.
Upgrade to STED CW
All TCS SP5 systems can be upgraded•
Dichroic and AOBS based•
System less then two years old•
can be upgraded on site
The STED technology is intregrated into an ultracompact module to ensure highest
long term stability.
All electronics and optics for operating the 592 nm STED
depletion laser and maximizing the incoupling effi ciency
are integrated into a stable and compact rack.
15

AOBS
Left: conventional beam splitting by dichroic mirrors
requires many optical elements with fi xed properties.
Right: the AOBS® is electronically adaptable to all tasks.
Confocal Superresolution:
the Best of Both Worlds
The Leica TCS SP5 is not simply the platform for excellent STED
superresolution experiments. It features plenty of elements that
encourage outstanding results, not only in combination with STED,
but also in conventional confocal mode.
Defi ne your requirements and confi gure the TCS STED CW to
match your needs – starting from a dedicated superresolution
system with excellent dichroic and a minimum of two spectral detectors and ending with a fully versatile workhorse for all kinds of
research, e.g. in imaging facilities.
Resonant scanner
The true confocal point scanning as realized in the SP5 delivers
the best optical resolution. However, to monitor dynamic events
it is sometimes necessary to record with highest possible speed.
Leica offers the resonant scanner that combines true confocal imaging and fast frame recordings with up to 25 frames per second
for a 512 x 512 pixel image. It allows fast events to be recorded and
xt scans with up to 200 lines per second.
Due to the outstanding positioning accuracy of the resonant
scanner it is even possible to use it for STED experiments – although not only one, but two laser beams have to be moved fast
and perfectly aligned. This does not compromise the achievable
spatial resolution of < 80 nm. Furthermore, the resonant scanner
is a versatile tool for STED especially when performing live cell
experiments. The reduced dwell time per pixel reduces potential
bleaching – ideal for time lapse experiments.
High fl exibility in detection
Leica Microsystems has developed spectral multiband detectors
that offer the detection of several variable emission bands without any gaps and at the same time. The SP detector resembles a
multiband spectrophotometer, based on a prism and mirror sliders. This allows optimal spectral separation of signals to do multichannel recordings and to optimize for any kind of emission-band
adjustment. The dynamic range of 6 orders of magnitude in combination with fast signal recordings make them the perfect choice
to record detailed images – even in combination with the resonant
scanner.
16

Acousto-Optical Beam Splitter (AOBS)
A critical element of incident light fl uorescence microscopy is the
beam splitter. Leica Microsystems has set the standard with the
introduction of the AOBS. This optical device is a programmable
defl ection crystal, which very specifi cally directs narrow excitation lines onto the sample while passing the full emission onto the
detection module. The effi ciency is in the range of 95% transmission. As the excitation lines are computer-controlled, the system
can switch the excitation regimes of various laser lines in a matter
of a few microseconds.
Leica TCS SP5 Features
Precise optical sectioning with SuperZ •
Galvo stage
Femtosecond and picosecond IR lasers•
Up to 64 Megapixels/image, fi eld rotation •
200°, also for resonant scan
Maximum transmission with prism-based •
Leica SP detector
Avalanche Photodetectors
Avalanche Photodetectors (APDs) are established tools for single
molecule detection methods like fl uorescence correlation spectroscopy (FCS) because of their substantially higher quantum effi ciency compared to conventional internal photodetectors. This
increase in sensitivity makes them a good choice when working with weakly fl uorescent samples and the ideal complement
for STED microscopy. Particularly for CW STED it is possible to
achieve a signifi cant resolution increase, depending on sample
and recording settings.
5 spectral confocal channels (max)•
Extreme sensitivity with Leica AOBS•
8 non-descanned channels (max)*
APD (Avalanche Photo Diode) detection •
for ultimate sensitivity*
Very fast beam path confi guration•
Most effective channel separation•
*optional
®
Tandem Scanner.
By means of a motorized and computer controlled high precision device, a conventional
and a resonant gavanometrically driven scan mirror are moved into the proper position
for scanning, while the scan-electronics are switched simultaneously.
17

Configure Your STED System –
According to Your Science!
Applications/technical features Live cell
imaging
AOBS •• ••
Resonant scanner •••
APD ••
5 PMT ••
IR laser •••
Transmitted & refl ected light detectors ••
Figure legends:
Page 2-3 (from left to right)
1. Immunofl uorescent staining of Bruchpilot in neuromuscular junctions of a drosophila larvae. Fluorescent Marker: Chromeo 488
Courtesy of Stephan Sigrist and Wernher Fouquet, Freie Universitaet Berlin.
2. ß-Tubulin in fi broblast. Immunolabeling. Marker: Chromeo 488
3. Nuclear protein in HeLa cells. Marker: Alexa 488
Various
fl uorophores
Multiphoton
imaging
Multiple
users
Deep tissue
imaging
Page 4:
Immunofl uorescent staining of neuromuscular junctions of a drosophila larvae. Labels: Bruchpilot (Chromeo 488, red), Res (green, Cy3).
Courtesy of Stephan Sigrist and Wernher Fouquet, Freie Universitaet Berlin.
Page 5:
Top: Clathrin vesicles in HeLa cells. Marker: Alexa 488.
Bottom: Vimentin in Vero cells. Marker: Oregon Green.
Page 9:
Large density core vesicles inside living PC12 cells. Marker: fl uorescent protein Venus.
Page 11:
Vimentin in HeLa cells. Marker: Chromeo 488.
Courtesy of Max Planck Institute for Biophysical Chemistry, Dept. Nanobiophotonics, Goettingen, Germany.
Page 14:
YFP labeled keratin fi laments in SW-13 cells.
Sample: courtesy of Reiner Windoffer, RWTH Aachen University, Institute of Molecular and Cellular Anatomy, Aachen, Germany.
18

System Components
APD
2
19
20
18
1
A
P
D
17
16
15
13
14
11
V
IS-
L
a
se
r
12
IR- La
se
r
4
10
10
10
10
10
6
5
9
7
3
21
8
1 Housing for 592 nm STED laser, AOTF, electronics
2 Fiber
3 Helical vortex phase fi lter
4 Incoupling STED dichroic
5 Tandem Scanner
6 Field rotation optics
7 Quarter wave plate
8 Transmitted light detector
9 Refl ected light detectors
10 Photomultipliers
11 Multi-function port
12 IR EOM
LEI
CA
STE
D
CW
1
13 Visible range AOTF
14 AOBS
15 Confocal detection pinhole
16 Filter- and polarizer wheel incl. notch fi lters
17 X1 emission port
18 APD fi lter cubes
19 Avalanche photodetectors
20 Spectrophotometer prism
21 STED objective lens
2
visible and ultraviolet
radiation:
infrared radiation:
19

“With the user, for the user”
Leica Microsystems
Leica Microsystems operates globally in four divi sions,
where we rank with the market leaders.
Life Science Division
•
The Leica Microsystems Life Science Division supports the
imaging needs of the scientifi c community with advanced
innovation and technical expertise for the visualization,
measurement, and analysis of microstructures. Our strong
focus on understanding scientifi c applications puts Leica
Microsystems’ customers at the leading edge of science.
Industry Division
•
The Leica Microsystems Industry Division’s focus is to
support customers’ pursuit of the highest quality end result.
Leica Microsystems provide the best and most innovative
imaging systems to see, measure, and analyze the microstructures in routine and research industrial applications,
materials science, quality control, forensic science investigation, and educational applications.
Biosystems Division
•
The Leica Microsystems Biosystems Division brings histopathology labs and researchers the highest-quality,
most comprehensive product range. From patient to pathologist, the range includes the ideal product for each
histology step and high-productivity workfl ow solutions
for the entire lab. With complete histology systems featuring innovative automation and Novocastra™ reagents,
Leica Microsystems creates better patient care through
rapid turnaround, diagnostic confi dence, and close customer collaboration.
Surgical Division
•
The Leica Microsystems Surgical Division’s focus is to
partner with and support surgeons and their care of patients with the highest-quality, most innovative surgi cal
microscope technology today and into the future.
The statement by Ernst Leitz in 1907, “with the user, for the user,” describes the fruitful collaboration
with end users and driving force of innovation at Leica Microsystems. We have developed fi ve
brand values to live up to this tradition: Pioneering, High-end Quality, Team Spirit, Dedication to
Science, and Continuous Improvement. For us, living up to these values means: Living up to Life.
Active worldwide
Australia: North Ryde Tel. +61 2 8870 3500 Fax +61 2 9878 1055
Austria: Vienna Tel. +43 1 486 80 50 0 Fax +43 1 486 80 50 30
Belgium: Groot Bijgaarden Tel. +32 2 790 98 50 Fax +32 2 790 98 68
Canada: Richmond Hill/Ontario Tel. +1 905 762 2000 Fax +1 905 762 8937
Denmark: Herlev Tel. +45 4454 0101 Fax +45 4454 0111
France: Nanterre Cedex Tel. +33 811 000 664 Fax +33 1 56 05 23 23
Germany: Wetzlar Tel. +49 64 41 29 40 00 Fax +49 64 41 29 41 55
Italy: Milan Tel. +39 02 574 861 Fax +39 02 574 03392
Japan: Tokyo Tel. +81 3 5421 2800 Fax +81 3 5421 2896
Korea: Seoul Tel. +82 2 514 65 43 Fax +82 2 514 65 48
Netherlands: Rijswijk Tel. +31 70 4132 100 Fax +31 70 4132 109
People’s Rep. of China: Hong Kong Tel. +852 2564 6699 Fax +852 2564 4163
Portugal: Lisbon Tel. +351 21 388 9112 Fax +351 21 385 4668
Singapore Tel. +65 6779 7823 Fax +65 6773 0628
Spain: Barcelona Tel. +34 93 494 95 30 Fax +34 93 494 95 32
Sweden: Kista Tel. +46 8 625 45 45 Fax +46 8 625 45 10
Switzerland: Heerbrugg Tel. +41 71 726 34 34 Fax +41 71 726 34 44
United Kingdom: Milton Keynes Tel. +44 1908 246 246 Fax +44 1908 609 992
USA: Bannockburn/lllinois Tel. +1 847 405 0123 Fax +1 847 405 0164
and representatives in more than 100 countries
LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.
Order no.: English 1593002008 • 12/09/???/????
www.leica-microsystems.com
 Loading...
Loading...