Page 1
®
AOBS
Leica TCS SP5: The Broadband Confocal
High Sensitivity for High-Quality Results
Page 2
Thousands of applications
The applications of fluorescence microscopy have enormously increased during the last five decades. The growing variety of different
fluorochromes featuring different excitation and emission properties
caused an increasing demand of new fluorescence filters and dichroics. The benefits of the numerous stains call for successful missions,
but the numberless filters are rather inconvenient for researchers, too.
Here, a broadband confocal microscope – covering all requirements
by a single device – means a true relief.
Light – a precious matter
Sensitivity tips the scales
Efficiency is the key to success. Not only in science and economy –
where it turns to profitability, but as well in research and routine laboratories, where productivity has always been a strong requirement.
Efficiency strongly depends on the suitability and the capabilities of the
instrumentation. What does this mean in terms of confocal fluorescence
microscopy? Here, first and foremost, efficiency is determined by the
sensitivity of the detection device. Of course, speed, flexibility and the
ergonomics are important parameters, though but the principle purpose
of the device is to guide the maximum number of photons, emitted by the
fluorochromes in the sample, safely to the detector.
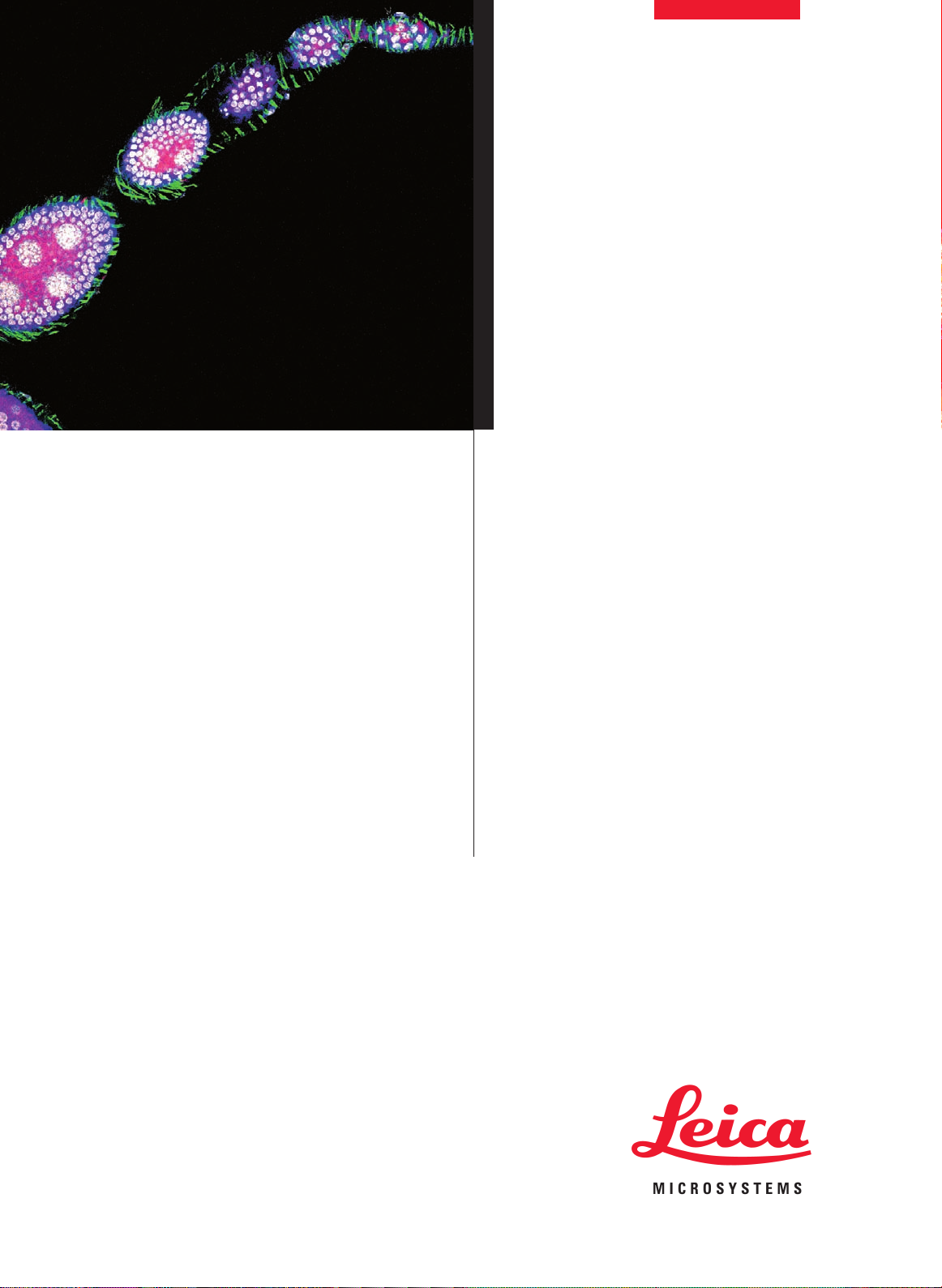
Drosophila melanogaster larvae (eye-disc)
Green: RNA binding protein (nuclei) Alexa 488
Red: Axons, Cy 3
Blue: Axon endings of MJ94-positive neurons, Cy 5
Courtesy of Dr. Christoph Melcher, Research Center Karlsruhe,
Institute for Toxikology and Genetics, Eggenstein-Leopoldshafen, Germany
Page 3

The technical task
In fluorescence, it has become a standard to illuminate the sample and
detect the image from the same side. A technique called „incident light
microscopy“, therefore, uses a device in the beam path, which decides
which light goes where, very much like a three-way valve. For a singly
FITC stained sample, blue light has to reach the sample and green light
has to reach the detector. Classically, this is achieved by a color-discriminating mirror: in the FITC-case, it is a mirror for blue light, but a
window for green light. For other fluorochromes, one has to insert other
dichroics, meeting the different color requirements. So far, this is manageable – although not efficiently.
It becomes truly confusing, when multiple stainings from the same
sample need to be recorded. You can easily calculate: if e.g. eight different excitations are needed, all possible permutations make 255 different
dichroics.
A severe problem is the low transmission of dichroic mirrors, which is
even worse for mirrors serving multiple stains (the so-called double and
triple dichroics). Moreover, to insert them in the beam path, one needs
a turret or slider: a quite slow solution and prone to misalignment and
failure. Not to speak about the moment, when you need to exchange one
of the mirrors for new dyes that you employ – a costly field service call
is necessary in most cases.
Left: conventional beam splitting by dichroic mirrors requires many
optical elements with fixed properties.
Right: the AOBS®is electronically adaptable to all tasks.
Our Solution
Acousto Optical Beam Splitter AOBS
To make the researcher’s life easier, Leica has introduced a revolutionary technology in confocal microscopy, which overcomes all drawbacks
of dichroic mirrors described above: the acousto optical beam splitter,
AOBS®. In brief, the new device is not a specially coated mirror, but a
switching valve for light, which is tunable to channel any laser line
onto the sample and simultaneously transmit very efficiently the emitted
light to the detector. It consists of an acousto optical crystal, known as
tunable deflection device. The clever bit: we operate the crystal
in reverse mode. For details, you may want to consult the suggested
readings.
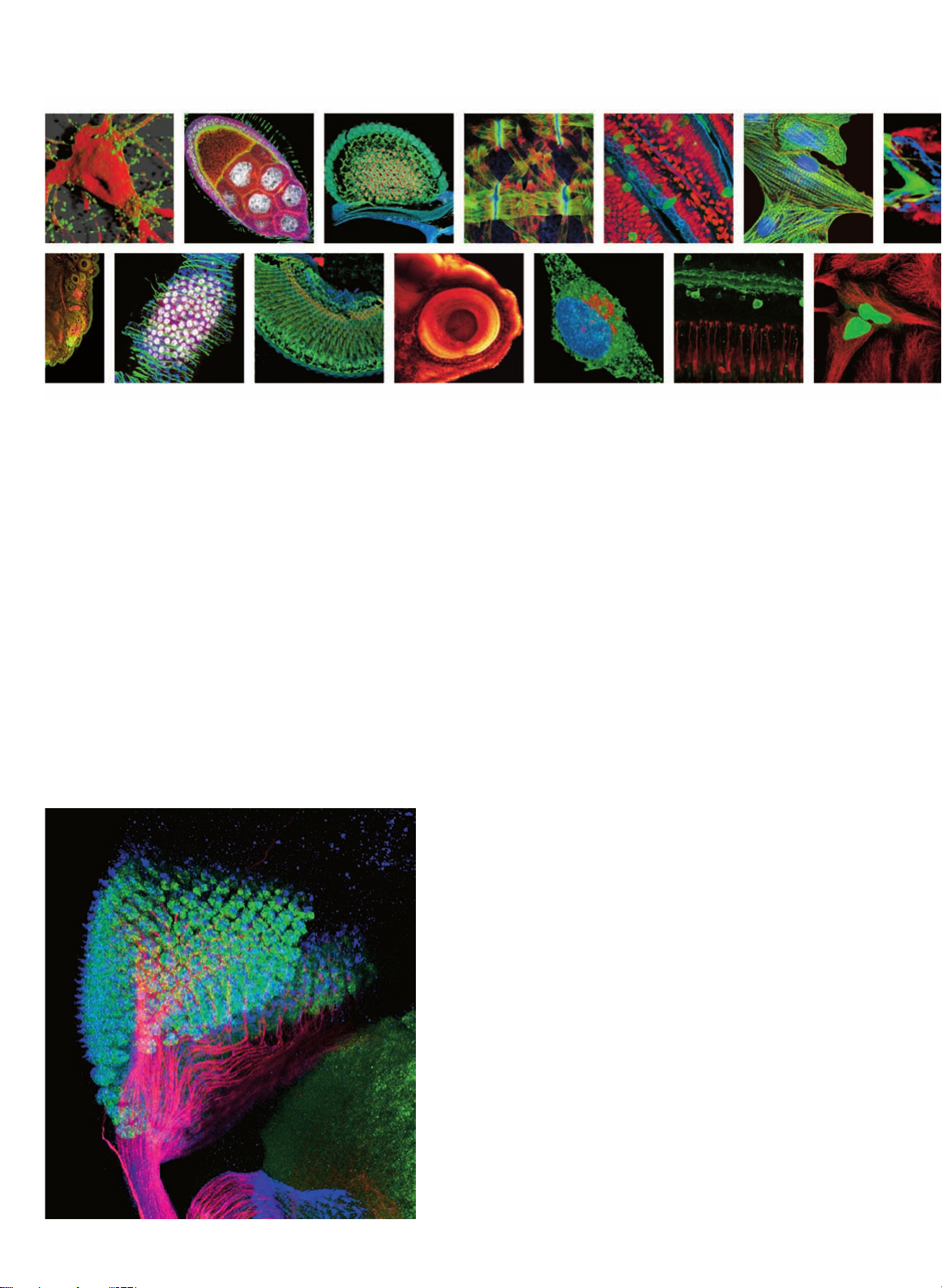
Green: Feb211 positive neurons and their axons, Alexa 488
Red: Fibrous part of the cns (i.e. all axons), Cy3
®
Drosophila melanogaster
Blue: Nuclei, DAPI
Grey: Nuclei of neurons, Alexa 594
Page 4

The benefits of AOBS
®
How does the AOBS®improve your scientific work?
Here is a convincing list of beneficial features:
1. Clear, low noise imaging needs high transmission. The sample
bleaching results from high numbers of averaging. The transmission
of the AOBS®is superior to most dichroic mirrors over the full visible
spectrum. Consequently, less averaging is necessary. The sample
will live much longer.
2. Bright and crisp images require wide emission bands as provided
by the AOBS®. This is important to channel as much photons as
possible from the sample to the detector – again improving the
image quality.
3. Low bleaching during image acquisition is important to protect
the sample from fading and to protect living specimen from toxic
chemicals that accumulate on photolysis of fluorochromes. The
AOBS®has very steep slopes allowing collecting emission very
closely to the excitation band.
4. Any visible-range dye can be excited, as the position of the reflection-pins can be tuned individually.
5. Multiparameter fluorescence is solved: up to eight laser lines programmable, leaving still sufficient space for emission collection –
and the frequencies are tunable!
6. Ratio dyes, like excitation ratio metabolite-probes, e.g. for Ca2+, membrane potential, pH or chloride expect fast switching in sequential
scanning. The AOBS®has switch times of only few microseconds.
7. Reflected light imaging as another option. The very strong suppression of the excitation can be reduced individually, if necessary for
reflection imaging.
8. ROI-scanning is improved as well: different excitation patterns are
possible for different regions during a single scan.
9. Large 3D volume recording, in sequential mode will benefit as well
from fast switching devices, as speed improves dramatically the
system efficiency.
10. Fluorescence correlation spectroscopy (FCS) requires very low
background and stray-light. Only the AOBS®sufficiently blocks close
co-emitted lines, e.g. from Ar-lasers.
11. Spectral recording (lambda scan) supply correct spectra, as the
transmission of the AOBS®is “white”, which means, that is does not
alter the emission spectra – a common problem, if spectral scanning
is done in a dichroic-mirror system.
12. True confocal optical sectioning requires point-shaped illumination
and emission. The AOBS®fits to point-scanning confocal devices.
13. Multiphoton and UV-imaging can be done in parallel without any
drawbacks or restrictions. The AOBS does not alter the excitation of
non-visible lasers, and the emission is not modified.
100
90
80
70
60
50
40
30
20
10
0
350 400 450 500 550 600 650 700 750 800
Transmission curves
Blue: triple dichroic, blue, green, red
Red: AOBS®tuned to 488, 543,594, 633 nm
Higher transmission, wider bands and steeper slopes with AOBS
Cyprinus carpio (retina)
Green: Amacrincells, FITC
Red: red and green cones, Cy3
Courtesy of Dr. Konrad Schultz, Carl-von-Ossietzky University
Oldenburg, Neurobiology, Oldenburg, Germany
®
Page 5

14. No maloperation is possible, as the AOBS®is directly controlled
together with the excitation control via AOTF. If an excitation line is
selected, the AOBS®is programmed accordingly. No decision has to
be taken by the operator – it is always correct and automatic.
15. No misalignment is introduced by mechanical turrets or sliders, as
there are no moving parts. The crystal is firmly mounted and the programming is purely electronics.
16. No expensive accessories like filter-cubes, dichroic-sliders etc. are
necessary. And will consequently save expensive field service calls
for mounting new planar optical parts.
No doubt: a true broadband confocal needs an AOBS®to meet all the
expectations from future-oriented research in the biomedical field. And
it is a must in multi-user environments, the most challenging being imaging facilities in large institutions.
Perfect Fit
Acousto-Optical Beam Splitter
l
Adaptable to any new dye
l
8 lines simultaneously
l
Reflected light imaging
l
High transmission
l
Truly confocal – real optical
sectioning
l
Fast switching
l
Freely tunable
l
FCS with multi-line lasers
How the AOBS fits the future
The AOBS®is only one out of three ingenious improvements Leica
invented for confocal microscopes. The Leica SP®overcomes the
restrictions of classical filter cascades for emission splitting. It is a series
of tunable elements at very high transmission, allowing selecting any
wavelength band for emission collection. Up to five such bands simultaneously! Tunable emission bands fit perfectly to tunable dichroics:
the combination of these two technologies will not leave open any application requirement.
Suggested reading:
1. V. Seyfried, H. Birk, R. Storz and H. Ulrich: Advances in multispectral confocal imaging. Progress in Biomedical optics and imaging. Vol 5139, 22-23 June, pp 146 ... 157
2. R. Borlinghaus: The AOBS: Acousto Optical Beam Splitter – colorful brightness in confocal microscopy. Imaging and Microscopy
3/2002, pp 10 ... 12.
Page 6

Fax +49 621/70 28 10 28 LEICA and the Leica Logo are registered trademarks of Leica IR GmbH.
•
Tel. +49 621/70 28 0
•
D-68165 Mannheim, Germany
•
Am Friedensplatz 3
•
August 2005
•
Leica Microsystems CMS GmbH
©
Copyright
Order no: 1593102110
www.confocal-microscopy.com
@
 Loading...
Loading...