Page 1
Adult Ventilation Bag, 1600 ml 87 01 50
PHT
DEHP
51 01 12
Patient Valve
56 02 00
ID15 mm
87 52 00
87 02 20
OD 23 mm
ID 23 mm
54 01 03
OD 22 mm
54 01 05
ID 22 mm
Optional Equipment/Accessories
Masks
85 15 00 Silicone Mask No. 00
85 16 00 Silicone Mask No. 0/1
85 17 00 Silicone Mask No.2
86 02 20 Silicone Child Mask 3-4
w/Multi-Function Mask Cover
87 02 20 Silicone Adult Mask 4-5+
w/Multi-Function Mask Cover
86 52 00 Multi-Function Mask cover 3-4
87 52 00 Multi-Function Mask cover 4-5
Patient Valves
56 02 00 Patient Valve
54 01 03 Lip Valve
54 01 05 Disk Membranes, pkg. 10
85 12 50 Patient Valve w/ Pressure Relief Valve
85 12 52 Pressure Relief Valve (35 cm H2O)
Storage
For storage in small space, the Adult and Paediatric
(but not the Preterm) ventilation bags may be folded
as shown in fig. 1-2-3. Fold the Paediatric in same fashion as the Adult.
Long Term Storage
Laerdal Silicone Resuscitators and/or spare parts may
be placed in long term storage. They should be periodically inspected and tested (at least yearly) according
to the Function Testing section in this manual.
Storage Pouch (Not illustrated)
The Adult, Paediatric or Preterm resuscitator
can be stored dust proof in the transparent
resealable pouch. A loop through a metal eyelet permits hanging the pouch.
Compact Case
The Adult, Paediatric or Preterm resuscitator
and accessories can be stored as illustrated.
The Compact Case can also be wall mounted.
Display Case (Not illustrated)
A fully assembled Adult, Paediatric or Preterm resuscitator can be stored for immediate use.
The Display Case may be hung on optional
Wall Mount.
Wall Bracket
Separate Wall Bracket is available
Hanging Loop
The resuscitator can be hung ready for immediate use.
For mounting Hanging Loop on the resuscitator, see
separate instructions enclosed with the Hanging Loop.
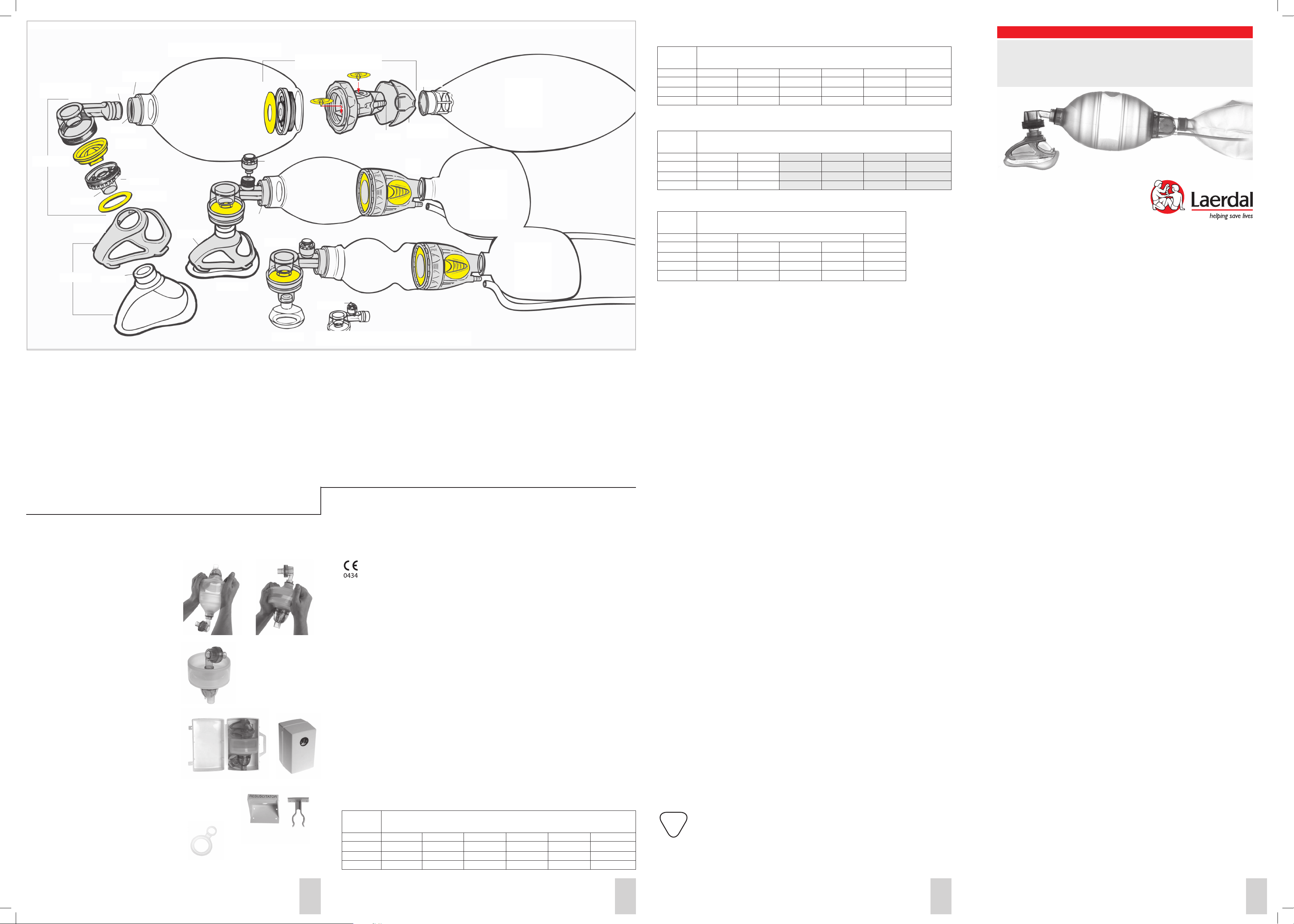
Laerdal Silicone Resuscitators Parts/Assembly Illustration
Intake/Reservoir Valve 87 54 00
51 04 04
Cap, pkg 3
51 01 03
Patient Valve
w/Press
Relief Valve
85 12 50
86 52 20
86 02 20
85 13 50 Patient Valve w/Press
Relief Valve and Lock Clip
85 11 03 Lock Clips, pkg. 10
Ventilation Bags/Intake Valve
85 01 50 Preterm Bag, 240 ml
86 01 50 Paediatric Bag, 500 ml
87 01 50 Adult Bag, 1600 ml
51 01 12 O-rings, pkg. 10
87 54 00 Intake Valve
87 19 50 Flap Valves, pkg 3
51 04 04 Intake membranes, pkg. 10
51 01 03 Cap, pkg. 3
1 2
3
Adult
85 12 52
85 12 50
85 16 00
OD 6 mm
Paediatric Ventilation Bag, 500 ml 86 01 50
Preterm Ventilation Bag, 240 ml 85 01 50
85 11 03
Patient Valve w/Lock Clip 85 13 50
Reservoirs
53 19 01 O2 Reservoir 2.6 litres
55 19 01 O2 Reservoir, 0.6 litre
Containers
85 07 00 Display Case cpl., Preterm
86 03 00 Display Case cpl., Paediatric
87 06 00 Display Case cpl., Adult
86 04 10 Compact Case cpl. Preterm/Paediatric
86 04 20 Compact Case cpl. Adult
Technical Specifications
Performance data may vary a great deal with
the conditions under which they were obtained.
Consequently, the findings in one test are not
directly comparable with data found in another test
unless test conditions were identical.
The product is in compliance with the
essential requirements of Council Directive
93/42/EEC as amended by Council
Directive 2007/47/EC
Product meets the following Product Standards:
- EN/ISO 10651-4:2002, Lung ventilators-
Particular requirements for operator powered resuscitators
- ISO 8382: 1988 Resuscitators intended for
Use With Humans.
- ASTM F 920 - 93, Standard Specification for
Minimum Performance and Safety
Requierments for Resuscitators Intended for
Use With Humans.
- AS 2488-1995 Resuscitators intended for
Use With Humans.
Operating environmental limits:
Operating condition: -18¡C to 60¡C.
(-0,4˚F to 140˚F)
15% to 95 %
Feasible oxygen concentration
The feasible O2 concentrations are approximated values and depend on the O2 concentration
delivered.
ADULT: Ventilation bag volume: 1600 ml
Reservoir bag volume: 2600 ml
Delivered O2 concentrations under various test conditions:
O2-flow Tidal vol. (ml) x bag cycling rate per min.O2-concentrations (%)
lpm using reservoir (without reservoir).
400x12 400x24 600x12 600x24 1000x12 1000x24
3 74 (38) 51 (39) 58 (34) 40 (34) 44 (33) 33 (30)
8 100 (44) 100 (44) 100 (40) 68 (40) 78 (38) 51 (34)
15 100 (51) 100 (50) 100 (47) 100 (47) 100 (42) 75 (36)
ID 25 mm
Reusable
Oxygen
Reservoir
Bag
600 ml
55 19 01
Reusable
Oxygen
Reservoir
Bag
2600 ml
53 19 01
Reusable
Oxygen
Reservoir
Bag
600 ml
55 19 01
Optional Equipment/Accessories
85 05 00 Expiration Diverter (OD 30 mm)
87 10 00 Silicone Extension Tube (28 cm)
85 09 00 Manometer Connector
87 04 00 Laerdal Head Strap w/Attachment Ring
87 13 00 Attachment Ring f/Standard Head Strap
87 01 20 Hanging Loop
51 17 00 Wall Bracket
52 11 00 Wall Mount, Paediatric/Preterm displ.
case
57 20 00 Wall Mount, ad. displ. case
87 05 50 Wall Poster reassembly guide
87 09 50 Directions for Use
53 19 07 Intake Valve Outer Part (23mm OD)
53 04 00 Airways, set of 4
Relative Humidity
Storage environmental limits:
Storage : -40¡C to 70¡C.
(-40˚F to 158˚F)
40% to 95 %
Relative Humidity
Dead space of Patient Valve:
Approx. 7,0 ml for all models
Expiratory resistance:
Approx. 2,6 cmH2O
Measured with airflow of 50 lpm
Inspiratory resistance:
w/reservoir approx. 4,2 cmH2O
w/o reservoir approx. 3,1 cmH2O
Measured with airflow of 50 lpm
Attainable delivery volume
Adult: Approx. 800ml
Paediatric: Approx. 320ml
Preterm: Approx. 150ml
Test conditions: Compliance 0,02 l/cm H2O,
Resistance 20 cm H2O/l/s
No leakage;
Pressure Relief Valve overridden.
PAEDIATRIC: Ventilation bag volume: 500 ml
Reservoir bag volume: 600 ml
O2-flow Tidal vol. (ml) x bag cycling rate per min.O2-concentrations (%)
lpm using reservoir (without reservoir).
20x40 20x60 150x20 150x30 300x12 300x24
3 100 (97) 100 (97) 98 (56) 78 (57) 85 (48) 56 (46)
8 100 (100) 100 (100) 100 (70) 100 (70) 100 (58) 100 (57)
15 100 (100) 100 (100) 100 (82) 100 (83) 100 (71) 100 (70)
PRETERM: Ventilation bag volume: 240 ml
Reservoir bag volume: 600 ml
O2-flow Tidal vol. (ml) x bag cycling rate per min.O2-concentrations (%)
lpm using reservoir (without reservoir).
20x40 20x60
3 100 (98) 100 (97)
8 100 (100) 100 (100)
15 100 (100) 100 (100)
Spontaneous breathing patient
O2-flow Tidal vol. (ml) x bag cycling rate per min.O2-concentrations (%)
lpm using reservoir (without reservoir).
Adult Paediatric Preterm
600x20 300x20 150x25 20x60 20x60
3 44 (39) 66 (49) 99 (62) 100 (99) 100 (99)
8 81 (54) 98 (62) 99 (75) 100 (100) 100 (100)
15 96 (74) 98 (79) 99 (87) 100 (100) 100 (100)
Useful Life
Laerdal Silicone Resuscitator products, accessories and parts are carefully engineered and produced using
materials that are suitable for the purpose. Care in accordance with these Directions for Use will help
ensure that each product has a long and useful lifetime. (Tested in 100 cycle decontamination study)
Material Chart
Article Component Part Material
Storage Pouch Polyethylene PE
Compact Case Case Polypropylene PP
Partition wall Acryl nitrilbutadiene styrene ABS
Display Case Case Polypropylene PP
Window Styrene acrylonitril SAN
Tray Acryl nitrilbutadiene styrene ABS
Lock Polyamide PA
Bag Bag Silicone rubber SI
Valve Connector Polysulfone PSU
O-Ring Fluorelastomer VITON
Patient Valve Upper Housing Polysulfone PSU
Patient side Housing Polysulfone PSU
Lip Valve Silicone rubber SI
Disk Membrane Silicone rubber SI
Pressure Relief Valve Stem Polysulfone PSU
Housing Polysulfone PSU
Spring Stainless steel
Bush Silicone rubber SI
Intake Valve Outer part Polysulfone PSU
Inner part Polysulfone PSU
Cap Polysulfone PSU
Flap Valve Silicone rubber SI
Intake Membrane Silicone rubber SI
O2 Reservoir Bag Reservoir Bag Polyvinyl chloride PVC
Coupling for bag Polysulfone PSU
Masks No.00-0/1-2 Silicone rubber SI
No.3-4, 4-5+ Silicone rubber SI
No.0-1-2 Silicone rubber SI
Optional Equipment
Mask Cover Polysulfone PSU
Lock Clip Stainless steel
Head Strap w/Ring Strap Polyvinyl chloride PVC
Attachement Ring Polycarbonate PC
Expiration Diverter Housing Polysulfone PSU
Center gasket Silicone rubber SI
External gasket Silicone rubber SI
Extension Tube Tube Silicone rubber SI
Coupling Polysulfone PSU
Manometer Connector Polysulfone PSU
Hanging Loop Silicone rubber SI
Wall Mount Acryl nitrilbutadiene styrene ABS
Wall Bracket Acetal POM
Shipping weights and dimensions
Cat. Nos. Weights Dimensions
850050 340g 12 oz 25 x 14,5 x 13 cm 10 x 5.7 x 5.1 in
850051 380g 13 oz 25 x 14,5 x 13 cm 10 x 5.7 x 5.1 in
850053 830g 1 lb 13 oz 24 x 15 x 16 cm 9.4 x 5.9 x 6.3 in
850055 1640g 3 lb 10 oz 37 x 33 x 12 cm 14.6 x 13 x 4.7 in
860050 370g 13 oz 25 x 14,5 x 13 cm 10 x 5.7 x 5.1 in
860051 510g 1 lb 2 oz 25 x 14,5 x 13 cm 10 x 5.7 x 5.1 in
860052 440g 16 oz 25 x 14,5 x 13 cm 10 x 5.7 x 5.1 in
860053 920g 2 lb 24 x 15 x 16 cm 9.4 x 5.9 x 6.3 in
860055 1760g 3 lb 14 oz 37 x 33 x 12 cm 14.6 x 13 x 4.7 in
860056 390g 14 oz 25 x 14,5 x 13 cm 10 x 5.7 x 5.1 in
870050 520g 1 lb 2 oz 25 x 14,5 x 13 cm 10 x 5.7 x 5.1 in
870051 700g 1 lb 9 oz 25 x 14,5 x 13 cm 10 x 5.7 x 5.1 in
870052 625g 1 lb 6 oz 25 x 14,5 x 13 cm 10 x 5.7 x 5.1 in
870053 1060g 2 lb 5 oz 24 x 15 x 16 cm 9.4 x 5.9 x 6.3 in
870055 2090g 4 lb 10 oz 37 x 33 x 15 cm 14.6 x 13 x 4.7 in
The Laerdal oxygen reservoir bag used with this product contains DEHP.
The product is only intended for short-term/transient patient application and it is not directly, or
through a liquid, in contact with the patient.
When cleaned and used according to these Directions for Use, there is no added risk involved
when treating children or pregnant or nursing women.
ENGLISH Directions for Use
Laerdal Silicone Resuscitators
www.laerdal.com
© 2010 Laerdal Medical AS, All Rights Reserved.
4492 rev F Printed in Norway
Scope
This manual provides the information required to fully utilise the Laerdal¨ Silicone Resuscitator,
and to help assure safe and trouble free operation over a maximum period of time. The Laerdal
Silicone Resuscitator is available in three sizes: Adult, Paediatric and Preterm. The 3 sizes have many
features and functions in common and are therefore described jointly whenever possible.
The detailed description of design and function should be read carefully by every user of the Laerdal
Silicone Resuscitators.
It is mandatory that anyone who uses a manual resuscitator receive adequate instruction.
It is also helpful if the student practices bag-valve-mask ventilation on realistic training manikins,
such as the Laerdal manikins.
Cautions and warnings
Read these Directions for Use carefully and become thoroughly familiar with the
operation and maintenance of the Laerdal Silicone Resuscitator before using it.
- Resuscitators should only be used by persons who have received adequate training.
- Federal law (US) restricts this device to sale by or on the order of a physician.
- Resuscitators should not be used with supplemental oxygen where smoking
is permitted or when fire, flame, oil or grease is in close proximity.
- Resuscitators should not be used in toxic or hazardous atmospheres.
- Before first time use of the resuscitator parts and its accessories, decontamination
is necessary.
- The use of third party products and oxygen delivery devices (e.g. filters and demand
valves) with the Laerdal Silicone Resuscitator may have an affect on LSR performance.
Please consult with the manufacturer of the third party device to verify compatability
with the LSR and obtain information on possible LSR performance changes.
- Do not use parts other than genuine Laerdal parts. Use of non-Laerdal parts may
affect safety and/or performance.
- Laerdal strongly discourages the use of rinsing and drying agents. Such agents may not
be compatible with the materials used in the Laerdal Silicone Resuscitator and may
affect the material and/or performance.
Service
Laerdal Silicone Resuscitators are designed and engineered for utility and economy.
All components, parts and assemblies listed in the parts list may be replaced, when
necessary, by the operator who is directed to carefully inspect them during decontamination
procedures. Under normal conditions of use, no regularly scheduled factory service should be
necessary. However, whenever a question arises we hope you will contact Laerdal Medical AS
or an authorised Laerdal distributor.
Product specifications are subject to change without notice.
Limited warranty
Please refer to the Global Warranty statement for additional terms and conditions (www.laerdal.
com)
Manufactured by:
LAERDAL MEDICAL AS
P.O. Box 377, N-4002 Stavanger
Tel. +47 51 51 17 00, Fax +47 51 52 35 57
E-mail: laerdal.norway@laerdal.no
Distributed in USA by:
LAERDAL MEDICAL CORPORATION
167 Myers Corners Road, P.O. Box 1840
Wappingers Falls, New York 12590-8840
Tel. (800) 431-1055, +1 (845) 297-7770
Fax (800) 227-1143, +1 (845) 298-4545
E-mail: customerservice@laerdal.com
Distributed in Canada by:
LAERDAL MEDICAL CANADA LTD.
151 Nashdene Rd., Unit #45
Toronto, ON, Canada, M1V 4C3
Tel. +1 (416) 298-9600
Fax +1 (416) 298-8016
E-mail: savelives@laerdal.ca
1876
Page 2
Indications for Use
The Laerdal Silicone Resuscitator is a self-inflating manual resuscitator that is intended for patients
requiring total or intermittent ventilatory support. The Laerdal Silicone Resuscitator provides positive
pressure ventilation and allows spontaneous breathing either with a 22 mm ID (inner diameter) facemask port, through an artificial airway or with a facemask that has a 15 mm OD (outer diameter)
connection. The Preterm model is intended for patients below 2,5 kg (5,5 lb), the Paediatric model
is intended for patients from 2,5 (5,5 lb) to 25 kg (55 lb), and the Adult model is intended for patients over 25 kg (55 lb).
Ventilation with ambient air
Resuscitator ventilation without supplemental
oxygen is possible.
Ventilation with oxygen
The Laerdal Silicone Resuscitator can be connected to an
O2 source via the oxygen nipple. Concentrations delivered
to the patient depend on O2 flow rate, use (or non-use)
of a Reservoir Bag and ventilation technique, e.g. tidal
volume, ventilation frequency, time relations during
O
2
compression-release cycles. See Technical Specifications.
Inhalation of supplemental oxygen
A patient who breathes spontaneously can inhale
O2 through the resuscitator with minimal resistance.
Attachment of the reservoir increases O2 concentration.
See Technical Specifications.
The mask can be hand held or strapped to the face.
Safety when using oxygen
1. Build-up and transfer of high pressure to the patient is
O
2
prevented since excess O2 is vented to atmosphere over
the outlet membrane of the Intake Valve.
2. When O2 supply is insufficient, adequate ventilation
volume is ensured by intake of ambient air over
the intake membrane of the Intake Valve.
3. A Reservoir Bag that stays flat during the whole
ventilation cycle is a visual indication that no,
or little supplemental O2 is being provided.
Pressure Relief Valve
The Preterm and Paediatric resuscitators feature a Patient
Valve with a pressure limiting device mounted on the upper
valve housing. If patient airway pressure exceeds 35 cm
H2O, the device opens to reduce
the risk of stomach distention and barotrauma. A hissing
sound can be heard when the device opens.
When higher airway pressures are necessary, the operator
can keep the Pressure Relief Valve closed with the tip of
index finger while squeezing the bag.
A Lock Clip (optional) can be used as an alternative
to finger pressure.
Accessories
Masks
The Laerdal Silicone Resuscitator can be combined
with the following mask types and sizes:
a) Circular Infant Masks 00, 0/1, 2
b) Laerdal Child Mask 3-4 and Laerdal Adult Mask 4-5+
For difficult facial anatomies the Multi-Function Mask
Cover is used to assist in getting a better mask seal.
All masks are transparent to enable the user to
observe the patient’s face and lip colour and
the temporary fogging caused by exhalations.
Mask connection
The Patient Valve has a standard 15 (ID)/22(OD) mm
patient port which connects to all standard masks or
tube adapters. The Laerdal Masks 4-5+ and 3-4, plus
the mask size 2, fit outside the patient valve connector.
All other infant sizes fit inside, to reduce deadspace.
To use the Laerdal Head Strap
b)
a) 00
0/1 2
4-5+
3-4
For Laerdal Child Mask 3-4 and Laerdal Adult
Mask 4-5+, place the correct size Multi/Function Mask
Cover over the mask connector. Fasten end of strap
into the hooks on the cover. Tighten just enough to
provide an airtight seal between mask and face.
For the Infant Mask 2, use the Attachment Ring
supplied with the Laerdal Head Strap.
Expiration Diverter
An Expiration Diverter with two silicone gaskets can be
snapped onto the Patient Valve.
The diverter provides an airtight seal to the valve housing
but does not prevent the swivel function (possibility of
horizontally rotating the bag without interfering with the
position of mask or tube) of the valve connector.
The diverter will provide an airtight seal when expired air
is free flowing. The use of the Expiration Diverter with
a restriction device (e.g. PEEP Valve) may cause some
air leakage around the silicone gasket of the Expiration
Diverter.
Equipment for measuring, scavenging or monitoring
expired gases, can be attached to the standard
(30 mm OD) outlet port of the diverter.
Manometer Connector
If used insert the Manometer Connector between the
Patient Valve and the mask or tube adapter.
Attach a manometer via tubing to the connector nipple
(OD 6 mm) to monitor both inspiratory
and expiratory pressures.
Extension Tube
The flexible Silicone Extension Tube may be used
between ventilation bag and the Patient Valve.
This extension tube makes it easier to ventilate when
the patient is being transported.
It also permits an operator to squeeze the bag against
a bed, strecher or themselves.
Practical Operation
a) When used in accordance with ISO 10651-4 the following resuscitator size recommendation
applies: Adult for patients over 20 kg (44 lb), Paediatric for patients from 2.5 (5,5 lb) to 20 kg
(44 lb) and Preterm for patients below 2,5 kg (5,5 lb).
When used to deliver tidal volumes as recommended by the AHA/ILCOR Guidelines 2000, the
following applies. Adult for patients over 25 kg (5,5 lb), Paediatric for patients from 2,5 kg (5,5 lb)
to 25 kg (55 lb) and Preterm for patients below 2,5 kg.
b) Either connect the Patient Valve directly to the patient«s tube, or choose the appro-
priate size mask and attach it to the Patient Valve. Mask seal on difficult anatomies may be improved by using the Multi Function Mask Cover (Mask size 3-4 and 4-5+ only).
c) Ventilate the patient by rhythmically compressing the bag for inspiration, allowing ample time
between inspirations for patient’s passive exhalation and bag re-expansion.
d) Follow local guidelines for resuscitation.
e) If the Patient Valve becomes contaminated with vomitus during ventilation, disconnect the
resuscitator from the patient and clear the Patient Valve as follows.
- Tap the Patient Valve with the patient port against your gloved hand to shake free any
contaminant and squeeze the silicone bag to deliver several sharp breaths through
the Patient Valve to expel the contaminant.
- If contaminant does not clear; disassemble the Patient Valve and rinse.
Caution:
Visually inspect and test valve function to ensure proper operation of the Laerdal Silicone
Resuscitator prior to patient use. Improper assembly of the flap valves, intake membrane,
disk membrane and lip valve may affect performance. Misassembly of two lip valves may cause
inadvertant EEP (End Expiratory Pressure) or prevent proper patient exhalation.
Decontamination
Thorough decontamination of the resuscitator components and accessories is necessary after
each use. To reduce risk of cross contamination, follow steps below.
1. Washing and Rinsing
Washing and rinsing is always the first step in the decontamination process.
A Disassembly
- Disassemble the LSR into individual parts as shown in the Parts Illustration in Directions for Use,
to make surfaces accessible to cleaning
- Separate Expiration Diverter (if used) into its three parts
- Separate Patient Valve into its four main parts
- For Preterm and Paediatric models, unscrew top of Pressure Relief Valve, but do not
disassemble this part any further.
- Separate Intake Reservoir Valve into its six parts
CAUTION: Leave connectors in the necks of Ventilation Bags, Extension Tube, and Reservoir
Bags during the entire decontamination procedure.
Laerdal strongly discourages the use of rinsing and drying agents. Such agents may not be compatible
with the materials used in the Laerdal Silicone Resuscitator.
The use of non-validated cleaning, disinfecting or sterilisation methods may have adverse effects on
the LSR material and/or performance.
Chose either the manual (I) or automatic (II & III) method below for cleaning the product.
I. Manual Cleaning
B Rinse parts in a sink under cold running water from a tap. Submerge parts in warm tap water
(30-40¼C / 86-104¼F) ensuring that all surfaces are in contact with the warm water for at least
2 minutes before exposure to detergent.
C Immerse all parts in hot tap water (60-70¼C / 140-158¼F) containing a Dish Washing
detergent 3.
Thoroughly clean all surfaces using a brush as necessary.
D Rinse all components free of detergent 4 in warm tap water (30-40¼C / 86-104¼F).
Dry the components thoroughly
5
E Inspect all components to confirm that they are CLEAN and DRY.
II. Automatic Cleaning by Washer/Disinfector
Place parts in wire baskets. Cycle 1 : 90-95¼C (194-203¼F) for more than 12 seconds.
Total process time: approx. 52 min. 2 Use a Non-enzymatic alkaline detergent containing 2-5%
NaOH3.
III. Automatic Cleaning by Pasteurmatic Compact. 6 30 min wash cycle at 32-43¼C (90-110¼F)
CAUTION: Thorough cleaning and rinsing are the first and most important steps in the reprocessing of any reusable
medical device. Without thorough cleaning and rinsing, it might not be possible to achieve high-level disinfection or
sterilisation of the device.
1. The cycle has been validated on a Getinge Model A8666 validated to HTM2030. Ninhydrin protein detection test was
used to qualify the process (to determine if any soil remained on the parts). Use of alternative washer/disinfector must
be validated.
2. Includes pre rinse, main wash, rinse, final rinse and drying.
3. The Washing Detergent used in the validation – Olympic Chemicals Sprayclean 2000 (Non-enzymatic alkaline detergent
containing 2-5% NaOH). Alternative detergents must be validated to show cleaning efficacy and material compatibility.
Method has been validated using a common available tenside based Dish Washing Detergent (Zalo Ultra manufactured
by Lilleborg AS). A pH neutral detergent solutions or hydrogen peroxide-based formulations may also be used for
manual cleaning but must be validated to show they effectively clean the components.
4. CAUTION: If detergent or disinfectant residuals are allowed to dry on the resuscitator parts, surfaces may become
sticky, which may cause valve malfunction
5. Drying; the most effective method of drying is a fan assisted hot air cabinet, 50-70¡C (122-158¼F) for at least 30
minutes. Other drying methods may be used but must be validated to show they effectively dry the components. The
Reservoir Bag must be dried by blowing air into the Reservoir Bag opening.
6. The cycle has been validated on a Pasteurmatic Compact from Olympic Medical.
2. Disinfection/Sterilisation
To obtain high-level disinfection/sterilisation of the resuscitator, the following 5 methods
(I to V) have been validated and are recommended.
The sterilisation methods apply to all parts except reservoir bags, Head Straps, Wall Bracket, Storage
Pouch and Containers. High-level disinfection methods apply to all parts.
Pasteurization applies to all parts except Wall Bracket, Storage Pouch and Containers
Method
Process parameters Post-treatment
Parameters/Concentration Exposure time
Sterilisation
I. Steam Autoclaving Autoclave at 132-137¼C 15min. 00s
(gravity- displacement) (270 - 279˚F) (+ 30s)
Allow parts to cool
and dry.
II. Steam Autoclaving Autoclave at 134-137¼C 3min. 00s
(prevacuum - pulse) (273-279¼F) (+ 30s)
High-level disinfection
III. Cidex OPA Conc.: 0,55% 60 minutes
(orthophtalaldehyde) Ambient temperature
IV. Sodium Hypochlorite Conc.: 0,5% 20 minutes
Ambient temperature
Remove traces of
disinfectant by rinsing in
warm tap water
(30-40¼C / 86-104¼F)
for minimum 2 minutes.
Dry the components
thoroughly
Dry the components
V. Pasteurization Pasteurization cycle 30 minutes
thoroughly
70-75¼C (158-167¼F)
3. Inspection
Carefully inspect all parts for signs of wear or damage. Worn or damaged components must be
discarded and replaced with new components.
4. Reassembly
Reassemble resuscitator as shown in Parts/Assembly Illustration, in this Directions for Use.
Caution: Patient Valve reassembly
Make sure that only one Lip Valve
Cat.No. 54 01 03 is installed.
If the valve housing does not tighten completely
during reassembly, it may indicate that two lip valves
have been mounted instead of one. Also, be sure not
to mix the Disk Membrane for the Patient Valve
with the Intake Membrane meant for the Intake Valve
assembly. Test functions as described in Function Testing.
Intake Valve reassembly
Reassembly as shown right.
Upper part of valve housing
Lip valve
Valve housing, patient side
Disk membrane
Function Testing
Test valve functions to ensure proper operation of
the resuscitator after each disassemblyreassembly. An O2 Reservoir Bag is needed to
complete the test procedures described below:
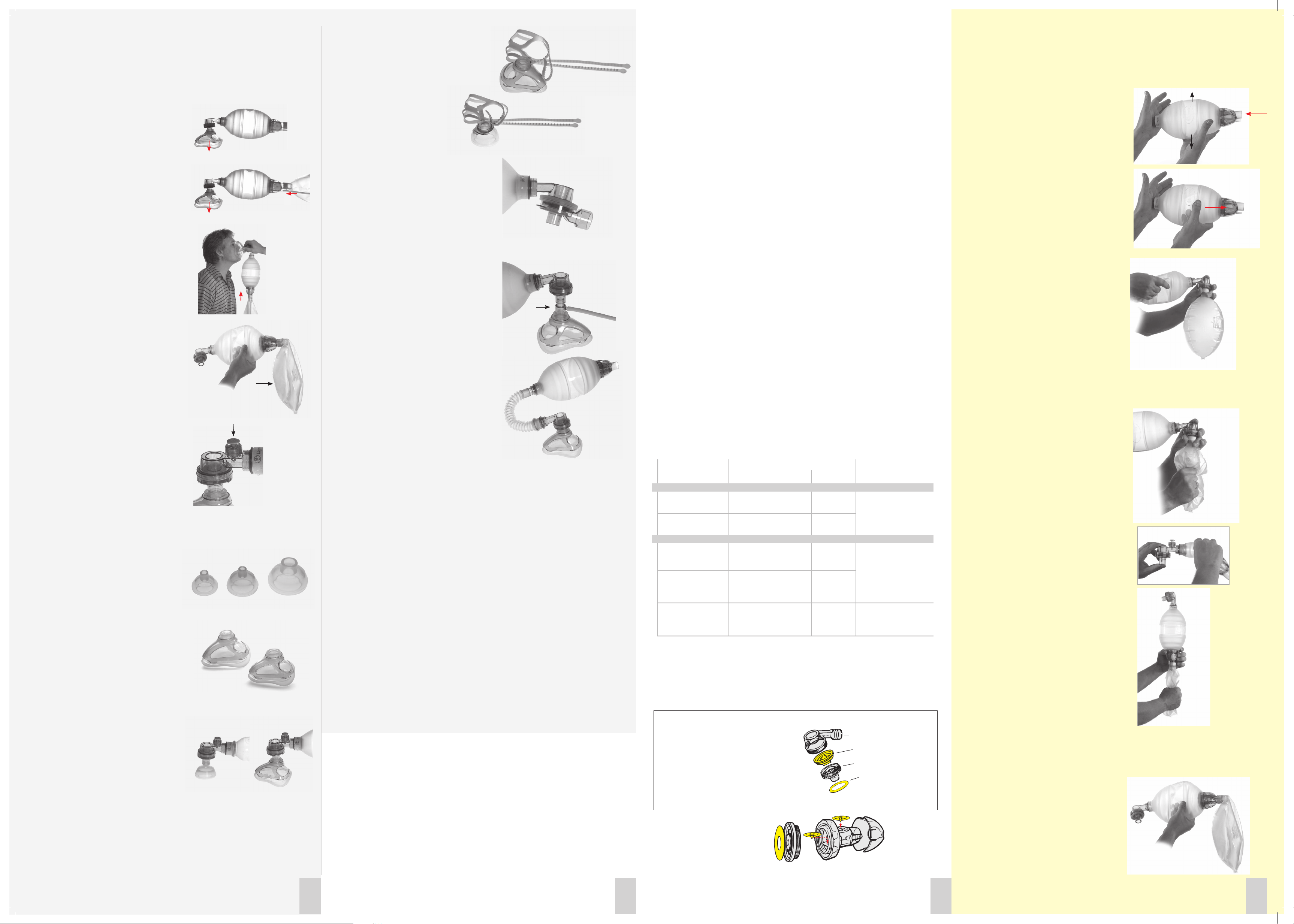
1. Intake/Reservoir Valve
a) Compress the ventilation bag with one hand
and close its neck opening with your other hand.
Release the grip on the bag. Rapid bag reexpansion
confirms efficient air intake.
b) Close the neck opening and try to compress
the bag. If the bag cannot be compressed with rea-
sonable force, or if bag compression forces the air
out between your hand and neck of the bag, the
valve efficiently prevents backward leakage of air.
2.1 Patient Valve
a) Assure that a (single) Lip Valve has been installed
in the Patient Valve. Attach the Patient Valve to
the bag. Hold a Reservoir Bag over the patient
port connector pressing with your thumb on the
reservoir bag connector.
Ensure tight seal between the patient port and
Reservoir Bag.
Compress the bag with your other hand
several times. Inspect that the Lip Valve opens
during compression.
Filling of the Reservoir Bag in this set-up
confirms that the Patient Valve efficiently
directs air to the patient.
b) With the filled Reservoir Bag held firmly to the
valve connector, compress the Reservoir Bag while
watching the external Disk Membrane.
Lifting of the Disk Membrane from its seat
confirms that air is correctly directed to
atmosphere instead of being returned to the
ventilation bag.
2.2 Patient Valve with Pressure Relief Valve
Close patient port connector with your thumb
while compressing the bag several times. Visual
and audible opening of the relief valve confirms its
operation.
3. Reservoir Flap Valves
(located in the Intake Valve assembly.)
a) Do as described and shown in 2.1a above in
order to fill the Reservoir Bag with ambient air.
Attach reservoir to the Intake Valve and press on
Reservoir Bag.
Compression of the Reservoir Bag and visual rise
of the outlet Flap Valve confirms that the Reservoir
Valve efficiently vents excessive gas to atmosphere.
b) Do as described and shown in 2.1a above in
order to fill a Reservoir Bag with ambient air.
Attach reservoir to the Intake Valve. With the
Patient Valve in place and the reservoir
attached to the Intake Valve, perform several
compression-release cycles on the ventilation bag
until the Reservoir Bag is flat and empty.
Rapid reexpansion of the ventilation bag after
flattening of the Reservoir Bag confirms that the
Reservoir Valve efficiently lets in ambient air.
2 3 4 5
 Loading...
Loading...