Page 1

KERN & Sohn GmbH
Ziegelei 1
D-72336 Balingen
E-Mail: info@kern-sohn.com
Tel: +49-[0]7433- 9933-0
Fax: +49-[0]7433-9933-149
Internet: www.kern-sohn.com
Operating Instructions
Density Determination Set for
Analytical Balance KERN ABT
KERN ABT-A01
Version 1.0
02/2007
GB
ABT-BA-e-0710
Page 2
GB
KERN ABT-A01
Version 1.0 2/2007
Operating Manual
Density Determination Set for Analytical Balance KERN ABT
Contents:
1 INTRODUCTION ............................................................................................................................ 4
1.1 SCOPE OF DELIVERY ................................................................................................................. 5
2 INSTALLING THE DENSITY DETERMINATION SET.................................................................. 7
3 PRINCIPLE OF DENSITY DETERMINATION............................................................................... 9
3.1 INFLUENCING MAGNITUDES AND ERROR SOURCES .................................................................... 10
4 DENSITY DETERMINATION OF SOLIDS................................................................................... 11
4.1 ACTIVATE FUNCTION............................................................................................................... 12
4.2 ENTERING DENSITY FOR FLUID................................................................................................ 13
4.3 CARRYING OUT MEASUREMENT.............................................................................................. 14
5 DETERMINING DENSITY OF LIQUIDS...................................................................................... 14
5.1 ACTIVATE FUNCTION............................................................................................................... 14
5.2 ENTERING DENSITY OF GLASS PLUMMET................................................................................. 16
5.3 CARRYING OUT MEASUREMENT.............................................................................................. 17
6 PRECONDITIONS FOR PRECISE MEASUREMENTS............................................................... 18
6.1 CALCULATION OF RESULTS..................................................................................................... 18
6.2 INFLUENCE FACTORS FOR MEASUREMENT ERRORS ................................................................. 19
6.2.1 Air bubbles....................................................................................................................... 19
6.2.2 Solid Specimen................................................................................................................ 19
6.2.3 Liquids.............................................................................................................................. 19
6.2.4 Surface............................................................................................................................. 19
6.2.5 Glass Plummet for Measuring Fluids............................................................................... 20
6.3 GENERAL INFORMATION.......................................................................................................... 20
6.3.1 Density / Relative Density................................................................................................ 20
6.3.2 Drift of Balance Display ................................................................................................... 20
7 DENSITY TABLE FOR FLUIDS................................................................................................... 21
8 UNCERTAINTY OF MEASUREMENT FOR DENSITY DETERMINATION OF SOLIDS............22
9 USER INSTRUCTIONS................................................................................................................ 23
ABT-A01-BA-e-0710 3
Page 3

1 Introduction
Safety instructions:
In order to guarantee a safe and smooth operation of this instrument, you have to
comply with the precautions below.
1. Carefully read the operating instructions.
2. Handle this set and the balance with care as they are precision instruments.
This set contains parts made of glass. Protect all parts against shocks and
impacts.
3. Do not disassemble this set nor the balance.
KERN ABT-A01 Density determination set for analytical balances of the
KERN ABT series (readability d = 0.1 mg).
This set is designed for the efficient determination of the density of solids by means
of an analytical balance.
In addition the density of fluids may be determined by means of an additional
plummet.
These operating instructions only describe the operation of the density determination
set. For further information on how to operate your balance please refer to the
operating instructions supplied with each balance.
4 ABT-A01-BA-e-0710
Page 4
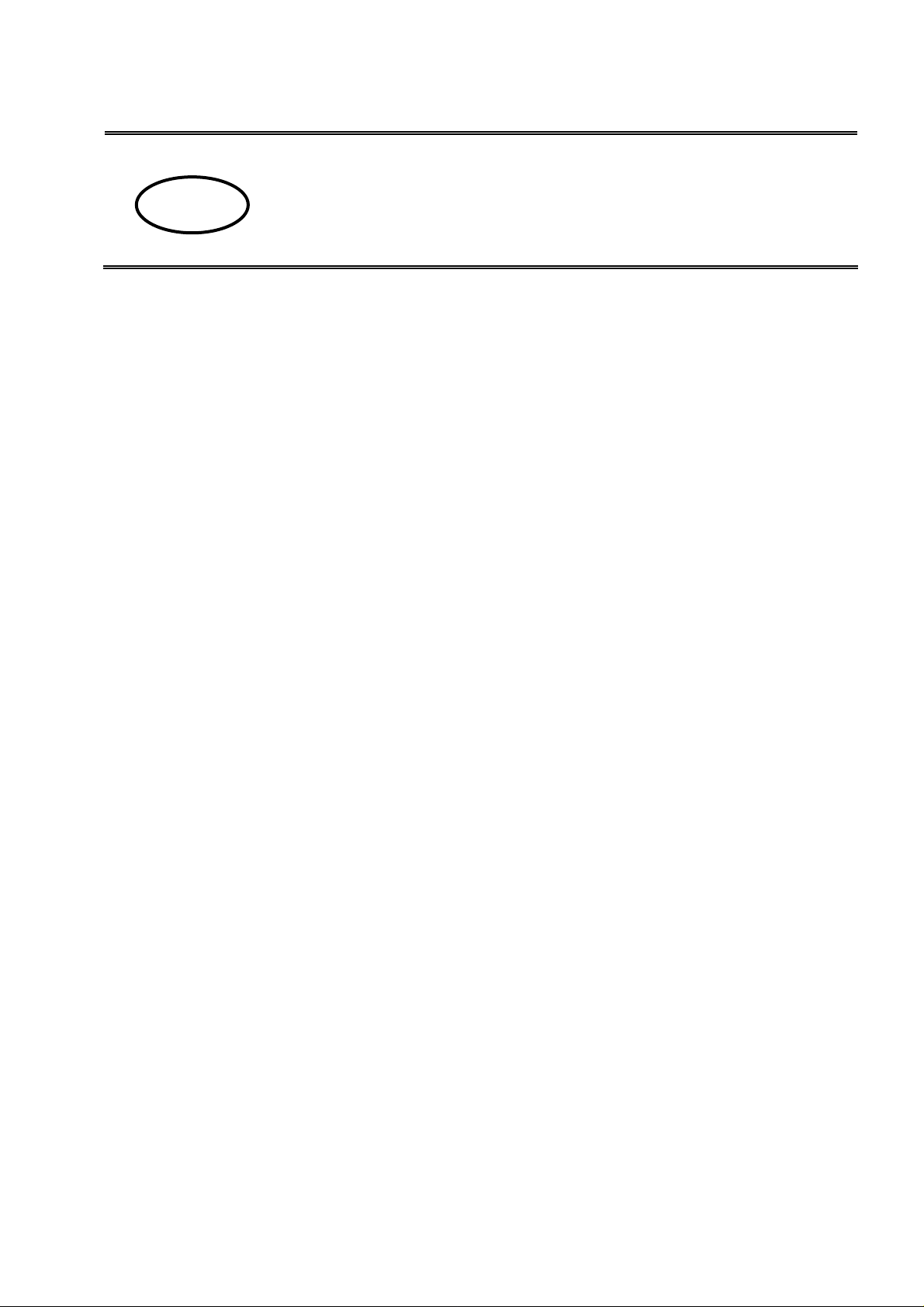
1.1 Scope of deliver y
SAMPLE DISH
FILTER BOWL
SAMPLE DISH
FILTER BOWL
ABT-A01-BA-e-0710 5
Page 5

MONTAGE THERMOMETER:
No. Designation
1 Platform assembly (sample dish and sifting bowl), 2 x
2 Weighing platform with rack
3 Beaker
4 Platform for glass beaker
5 Thermometer
6 Holder for thermometer
7 -8 Glass plummet
6 ABT-A01-BA-e-0710
Page 6

2 Installing the density determination set
1. Turn off and disconnect the power supply for the balance
2. Open the lateral glass doors of the weighing space of the balance and remove the
grading ring, the weighing plate and the support of the weighing plate.
3. Carefully insert the weighing platform with rack onto the floor of the weighing
space.
4. Place the platform for the glass beaker above it without touching the weighing
platform, as shown.
ABT-A01-BA-e-0710 7
Page 7

5. Attach the platform assembly (sample dish and sifting bowl) to the rack of the
weighing platform. In doing so, bear in mind that the centering of the upper sample
dish matches the notch in the upper part of the weighing platform.
6. Close the glass doors and connect the power supply for the balance. Wait for the
auto test of the balance and the display message "oFF“. Some models additionally
carry out an automatic adjustment before displaying “oFF“. (Connect the balance to
the platform assembly but without any fluid in the glass beaker. )
7. Turn on the balance by pressing the [ON/OFF] key, so that gram appears on the
display.
8. Attach the holder with thermometer to the glass beaker. Fill the glass beaker with
known fluid (for density determination of solids) or test fluid (for density determination
of fluids).
9. To be able to place the glass beaker in the centre of the platform, the platform
assembly must first be removed from the rack.
10. Reattach the platform assembly to the rack and ensure that the sifting bowl does
not touch the glass beaker.
11. Observe the waiting time until the test fluid, the known fluid, the instruments or
the plummet have the same temperature. For the balance also observe the required
warm-up time. (For details please refer to the operating instructions for the balance)
Attention:
• The platform for the beaker must not touch the frame!
• When the density set is installed, correct adjustment is not possible. To
achieve correct adjustment reinsert the weighing plate.
8 ABT-A01-BA-e-0710
Page 8

3 Principle of Density Determination
A
A-B
g
g
Three physical magnitudes are the volume and the mass of bodies as well as the
density of matter. In density mass and volume are related.
Density [ ρ ] is the relation of mass [ m ] to volume [ V ].
ρ =
SI-unit of density is kilogram divided by cubic meter (kg/m³). 1 kg/m³ equals the
density of a homogenous body that, for a mass of 1 kg, has the volume of 1 m³.
Additional frequently applied units include:
The application of this density determination set in combination with the KERN ABT
balance provides fast and safe determination of solids and fluids. Our set uses the
"Principle of Archimedes" to determine density:
m
V
,
kg
1
m
1
3
,
l
1
cm
3
BUOYANCY IS A FORCE. IT AFFECTS A BODY THAT IS IMMERSED INTO A FLUID. THE
BUOYANCY OF THE BODY EQUALS THE WEIGHT FORCE OF THE DISPLACED FLUID. THE
FORCE OF BUOYANCY ACTS VERTICALLY UPWARDS.
Thus, density is calculated according to the formulae below:
Density determination of solids:
This balance enables weighing of solids in air [ A ] as well as water [ B ]. If the
density of the buoyancy medium is known [ ρo ] the density of the solid [ ρ ] is
calculated as follows:
ρ =
ρ
o
ρ = density of sample
A = weight of the sample in air
B = weight of sample in measuring fluid
ρ
= density of measuring fluid
o
ABT-A01-BA-e-0710 9
Page 9

Determining density of liquids:
G
A-B
The density of a fluid is determined with the help of a plummet providing a known
volume [ V ]. The plummet is weighed in air [ A ] as well as in the sample fluid [ B ].
According to the Archimedes’ Principle a body immersed in a fluid experiences a
force of buoyancy [ G ]. This force equals the weight force of the fluid displaced by
the volume of the body.
The volume [ V ] of the immersed body equals the volume of the displaced fluid.
ρ =
ρ =
V
V
+
ρ
L
G = buoyancy of plummet
Buoyancy of plummet =
Weight of the sinker in air [ A ] - weight sinker in sample liquid [ B ]
From this follows:
ρ = density of sample fluid
A = weight of plummet in air
B = weight of plummet in sample fluid
V = volume of plummet
= air density (0.0012 g/cm³)
ρ
L
3.1 Influencing magnitudes and error sources
Ö Air pressure
Ö Temperature
Ö Volume deviance of the sinker (± 0.005 cm3)
Ö Surface tension of the liquid
Ö Air bubbles
Ö Immersion depth of the sample dish of sinker
Ö Porosity of the solid
10 ABT-A01-BA-e-0710
Page 10

4 Density determination of solids
Prepare balance as described in chapter 2 "Installation of density determination set".
Ö Install holder for the thermometer on beaker rim.
Ö Suspend thermometer
Ö Fill your measuring liquid, whose density ρo is known, into the beaker.
Filling height should be approx. ¾ of the capacity.
Ö Place beaker in the centre of the platform
Ö Suspend sample dish from the centre of the frame
Ö Heat measuring liquid until temperature is constant.
ABT-A01-BA-e-0710 11
Page 11
4.1 Activate function
Press several times the [UNIT] key to change the display between activated units,
piece counting, percentage and density determination mode.
This does not require additional software.
Settings have to be activated in the menu:
(Example)
Repeatedly press the [CAL] key until "FUnC.SEL" appears.
Press [TARE] key
Repeatedly press the [CAL] key until "Unit.SEL" appears.
Press [TARE] key
Use the [CAL] key, to select the settings below:
„ U- ,d“ („ ,“ this is an upside down triangle)
Current settings are indicated by the standstill display ( ).
Confirm your selection by pressing the [TARE] key.
The [TARE] key is also used to deactivate a unit or function if
the corresponding setting with standstill display is shown in the
display.
Repeatedly press the [ON/OFF] key. This takes you back to the
12 ABT-A01-BA-e-0710
menu/weighing mode.
Page 12

4.2 Entering Densit y for Fluid
Press the [CAL] key repeatedly until "SettinG" appears.
Press the [TARE] key.
Repeatedly actuate the [CAL] key until "LSG SEt" appears
Press the [TARE] key. The currently set density for the liquid to be
(Example)
(Example)
measured appears. In the upper part of the display panel, the
symbol and the # symbol appear in order to indicate
numerical input status. The leftmost digit blinks.
Enter density for the liquid to be measured. When the [UNIT] key
is pressed, the numerical of the blinking digit increases by 1 at a
time. You can determine the value of the flashing digit, or shift the
flashing digit by one position to the right, by pressing the [PRINT]
key. Confirm your setting by pressing the [TARE] key.
Repeatedly press the [ON/OFF] key until the balance is in
weighing mode.
ABT-A01-BA-e-0710 13
Page 13

4.3 Carr ying Out Measurement
Repeatedly press the [UNIT] key until the balance is in density
determination mode for solids ",d". Note that “g” also appears
during weight measurement in air.
Press the [TARE] key. Place the object to be measured on the
sample dish.
When standstill control is complete, press the [CAL] key.
Place the item to be measured on the immersed sifting bowl. The
display is showing the density of the measured item. “dSP oL” may
be displayed when nothing is on the weighing pan, which is
normal.
To start next measurement, press the [CAL] and the [ TARE ] key
and place the object to be measured on the weighing tray.
5 Determining density of liquids
Prepare balance as described in chapter 2 "Installation of density determination set".
Ö Install holder for the thermometer on beaker rim.
Ö Suspend thermometer
Ö Fill your measuring liquid into the beaker. Filling height should be approx. ¾ of the
capacity.
Ö Heat measuring liquid until temperature is constant.
Ö Prepare glass sinker
5.1 Activate function
Press several times the [UNIT] key to change the display between activated units,
piece counting, percentage and density determination mode.
This does not require additional software.
14 ABT-A01-BA-e-0710
Page 14

Settings have to be activated in the menu:
(Example)
Repeatedly press the [CAL] key until "FUnC.SEL" appears.
Press [TARE] key
Repeatedly press the [CAL] key until "Unit.SEL" appears.
Press [TARE] key
Use the [CAL] key, to select the settings below:
„ U- d“
Current settings are indicated by the standstill display ( ).
Confirm your selection by pressing the [TARE] key.
The [TARE] key is also used to deactivate a unit or function if
the corresponding setting with standstill display is shown in the
display.
Repeatedly press the [ON/OFF] key. This takes you back to the
menu/weighing mode.
ABT-A01-BA-e-0710 15
Page 15

5.2 Entering Densit y of Glass Plummet
Press the [CAL] key repeatedly until "SettinG" appears.
Press the [TARE] key.
Repeatedly actuate the [CAL] key until "Sv SEt" appears
Press the [TARE] key. The currently set density for the body to be
(Example)
(Example)
immersed appears. In the upper part of the display panel, the
symbol and the # symbol appear in order to indicate
numerical input status. The leftmost digit blinks.
Enter density for your body to be immersed. When the [UNIT] key
is pressed, the numerical of the blinking digit increases by 1 at a
time. You can determine the value of the flashing digit, or shift the
flashing digit by one position to the right, by pressing the [PRINT]
key. Confirm your setting by pressing the [TARE] key.
Repeatedly press the [ON/OFF] key until the balance is in
weighing mode.
16 ABT-A01-BA-e-0710
Page 16

5.3 Carr ying Out Measurement
Remove the platform assembly and the glass beaker from the platform.
Repeatedly press the [UNIT] key until the balance is in density
determination mode for liquids "d". Note that “g” also appears
during weight measurement in air.
Press the [TARE] key. To carry out a measurement in air, attach
the glass plummet to the rack.
When standstill control is complete, press the [CAL] key
To start the next measurement, press the [CAL] key and [TARE] key and reattach
the glass plummet to the rack without the glass beaker, in order to carry out a
measurement in air.
Ensure that the glass beaker is dry and clean each time you fill it with test fluid.
The same applies to the glass plummet.
Remove the glass plummet.
Place the glass beaker containing the test fluid on the platform for
the glass beaker.
Reattach the glass plummet to the rack and immerse it completely
in the fluid without producing bubbles.
The display is showing the density of the sample. If no glass
plummets are available the message “dSP oL” might appear which
is normal.
ABT-A01-BA-e-0710 17
Page 17

A
A-B
6 Preconditions for Precise Measurements
There are numerous error possibilities during density determination.
Accurate knowledge and caution are required to achieve precise results when
applying this density set in combination with the balance.
6.1 Calculation of Results
The balance displays results for density determination by giving four decimal places.
However, this does not mean that the results are accurate down to the last decimal
place as this would be the case for a calculated value. Therefore all weighing results
used for calculations have to be examined closely.
Example for density determination of solids:
To ensure high-grade results, numerators as well as common denominators of the
formula below must show the desired accuracy. If either of them is instable or flawed,
the result, too, will be instable or flawed.
ρ = density of sample
A = weight of the sample in air
B = weight of sample in measuring fluid
ρ
= density of measuring fluid
o
The use of a heavy specimen contributes to the accuracy of a result because this
increases the numerical value. The use of a light-weight specimen, too, contributes to
the accuracy of a result because this increases buoyancy (A-B). As a consequence,
the result of the common denominator increases. Bear also in mind that the accuracy
of the density of the measuring fluid ρo enters into the common denominator and,
thus, has considerable influence on the accuracy of the result.
The result for the density of the specimen cannot be more accurate than the least
accurate of the aforementioned individual entities.
This fact applies equally to the determination of density for fluids and glass plummet
adjustment.
ρ =
ρ
o
18 ABT-A01-BA-e-0710
Page 18

6.2 Influence Factors for Measurement Errors
6.2.1 Air bubbles
A small bubble of, for example, 1mm3 will have a considerable influence on the
measurement if the specimen is small. Buoyancy will be increased by approximately
1mg resulting immediately in an error of 2 digits. Hence, it has to be ensured that no
air bubbles cling to the solid immersed in the fluid. The same applies to the glass
plummet that is immersed in the test fluid.
Take great care when removing air bubbles by swirling, to prevent the fluid from
spurting out and splashing onto the sifting bowl. Moisture on the suspension bracket
of the sifting bowl results in increased weight.
Do not touch the solid sample or glass plummet with bare fingers. An oily surface
causes air bubbles when immersing the specimen in fluids.
Do not place solid specimens (in particular flat objects) in the sifting bowls as this
would result in air bubbles when immersed together. For this reason examine the
bottom of the sifting bowl for air bubbles after the specimen had been immersed in
fluid.
6.2.2 Solid Specimen
A specimen possessing too great a volume that is immersed in fluid will result in an
increase in fluid level inside the glass beaker. As a result, part of the suspension
bracket of the sifting bowl will also be immersed causing buoyancy to increase. As a
consequence the weight of the specimen in the fluid will drop.
Specimens that change the volume or assimilate fluid are unsuitable for
measurement.
6.2.3 Liquids
Water temperature is another factor to be taken into consideration. The density of
water changes by c. 0.01% per degree Celsius. A temperature measurement
showing an error of 1 degree Celsius results in an inaccurate fourth decimal place.
If the specimen absorbs the fluid or dissolves during the measurement in the fluid, a
different fluid should be chosen. Additionally take into consideration that the fluid
might evaporate.
6.2.4 Surface
The suspension bracket of the sifting bowl penetrates the surface of the fluid. This
state undergoes continuous change. If the specimen or the glass plummet is
relatively small, the surface tension will impair repeatability. The addition of a small
amount of detergent makes the surface tension negligible and increases
repeatability.
ABT-A01-BA-e-0710 19
Page 19

6.2.5 Glass Plummet for Measuring Fluids
To save test fluids used for density determination of fluids, use a small glass beaker
and an accordingly sized glass plummet. However, it needs to be pointed that a large
glass plummet achieves higher accuracy.
It is desirable that the buoyancy and the volume of the glass plummet are determined
as accurately as possible. For the determination of fluid density these results are
applied to the common denominator as well as the numerator of the formula.
6.3 General information
6.3.1 Density / Relative Density
Relative density follows from the weight of a specimen divided by the weight of water
(at 4° Celsius) of the same volume. For this reason relative density does not have a
unit. Density equals mass divided by volume.
The application of the relative density instead of the density of a fluid in a formula
produces an incorrect result. In the case of fluids only their density is
physically meaningful.
6.3.2 Drift of Balance Display
The drifting of a balance does not influence the final result of the density
determination although the shown weight of weighing in air is affected. Accurate
values are merely required if the density of fluids is determined by means of a glass
plummet.
For this purpose some of the models carry out auto-adjustment.
20 ABT-A01-BA-e-0710
Page 20

7 Density Table for Fluids
Density p [g/cm3] Temperature
[°C]
10 0.9997 0.7978 0.8009
11 0.9996 0.7969 0.8000
12 0.9995 0.7961 0.7991
13 0.9994 0.7953 0.7982
14 0.9993 0.7944 0.7972
15 0.9991 0.7935 0.7963
16 0.9990 0.7927 0.7954
17 0.9988 0.7918 0.7945
18 0.9986 0.7909 0.7935
19 0.9984 0.7901 0.7926
20 0.9982 0.7893 0.7917
21 0.9980 0.7884 0.7907
22 0.9978 0.7876 0.7898
23 0.9976 0.7867 0.7880
24 0.9973 0.7859 0.7870
25 0.9971 0.7851 0.7870
26 0.9968 0.7842 0.7861
27 0.9965 0.7833 0.7852
28 0.9963 0.7824 0.7842
29 0.9960 0.7816 0.7833
30 0.9957 0.7808 0.7824
31 0.9954 0.7800 0.7814
32 0.9951 0.7791 0.7805
33 0.9947 0.7783 0.7896
34 0.9944 0.7774 0.7886
35 0.9941 0.7766 0.7877
Water Ethyl alcohol Methyl alcohol
ABT-A01-BA-e-0710 21
Page 21

8 Uncertainty of Measurement for Density
Determination of Solids
This table shows the approximate readability of the balance in connection with the
density set. In doing so take into consideration that these theoretically calculated
values may vary according to ambient conditions.
In addition observe chapter 6.
Example to table below:
Test of a solid weight of 5 gram and a density of 3 g/cm3.
The minimum display value for density is 0.0004 g/cm3. Thus the last display digit of
the display (readability of 0.0001) is not relevant for this measurement.
Approximative readability at the density determination
(when using the 0.1mg range)
Weight (g) of the
specimen
Density of
the specimen
(g/cm3)
1 0.001 0.0001 0.0001 0.0001 0.0001 0.0001
3 0.002 0.0004
5 0.003 0.001 0.0004 0.0002 0.0002 0.0002
8 0.004 0.001 0.0006 0.0003 0.0003 0.0003
10 0.005 0.001 0.0008 0.0004 0.0003 0.0003
12 0.006 0.002 0.001 0.0004 0.0004 0.0004
20 0.01 0.003 0.002 0.001 0.001 0.001
1 5 10 100 200 300
0.0003 0.0001 0.0001 0.0001
22 ABT-A01-BA-e-0710
Page 22

9 User Instructions
• To form a reproducible mean value several density measurement are necessary
• Remove fat from solvent-resistant sample / /glass sinker /beaker.
• Regularly clean sample dishes/glass sinker/beaker, do not touch immersed part
with your hands
• Dry sample/glass sinker/pincers after each measurement.
• Adjust sample size to sample dish (ideal sample size > 5 g).
• Only use distilled water.
• When immersing for the first time, lightly shake sample dishes and sinker, in order
to Dissolve air bubbles.
• Always ensure that, when re-immersing into the liquid no additional bubbles
adhere; it is better to use pincers to place the sample.
• Remove firmly adherent air bubbles with a fine brush or a similar tool.
• To avoid adherent air bubbles smoothen samples with rough surface.
• Take care that no water drips onto the upper sample dish when weighing with the
help of tweezers.
• In order to reduce the surface tension of water and the friction of the liquid on the
wire, add three drops of a common detergent (washing-up liquid) to the
measuring liquid (density modification of dest. water occurring due to the addition
of tensides can be ignored).
• Oval samples can be held more easily with pincers when you cut grooves into
them.
• The density of porous solids may only be determined approximately. Buoyancy
errors occur when not all the air is eliminated from the pores during immersion in
the measuring fluid.
• To avoid great vibrations of the balance, place sample carefully.
• Avoid static charge, e. g. dry glass plummet with cotton cloth only.
• If the density of your solid only deviates slightly from that of distilled water,
ethanol may be used as measuring fluid. However, check beforehand whether the
sample is solvent-proof. In addition you must observe the applicable safety
regulations when working with ethanol.
• Handle glass sinker with care
(no warranty claims in case of damage).
ABT-A01-BA-e-0710 23
 Loading...
Loading...