
ProTime
Microcoagulation System
For in vitro Diagnostic Use
Operator’s Manual
TABLE OF CONTENTS
®
0123
INTENDED USE.............................................................................. 2
SUMMARY AND EXPLANATION.................................................... 2
SYSTEM FEATURES...................................................................... 3
IMPORTANT LABELS AND SYMBOLS ..........................................4
PRINCIPLES OF OPERATION .......................................................5
SAFETY FEATURES AND QUALITY CONTROL ........................... 5
INSTRUMENT SPECIFICATIONS .................................................. 7
SERVICE AND MAINTENANCE ..................................................... 7
PREPARING THE INSTRUMENT...................................................9
TEST PROCEDURE .....................................................................10
TROUBLESHOOTING ..................................................................14
MAIN MENU OPTIONS................................................................. 16
SET UP .........................................................................................22
FOR PROFESSIONALS ONLY - PROGRAM MODE.................... 27
PERFORMANCE CHARACTERISTICS ........................................ 31
TECHNICAL ASSISTANCE........................................................... 34
SUGGESTED READING............................................................... 35
SAFETY STANDARDS .................................................................36
INDEX ...........................................................................................39
This manual is published by International Technidyne Corporation (ITC) for use with the ProTime
Microcoagulation System and Tenderlett
Questions or comments regarding the contents of this manual can be directed to the address at the back of
this manual or to your ITC representative.
Please read the instructions before use.
© 2000, 2001, 2002, 2003, 2004, 2005. This document is the copyright of ITC and must not be copied or
reproduced in any form without prior consent. ITC reserves the right to make technical improvements to
equipment and documentation without prior notice as part of a continuous program of product
development.
®
Plus fingerstick blood collection device.
®

INTENDED USE
The ProTime Microcoagulation System is a portable, battery-operated instrument with a disposable cuvette
for quantitative determination of prothrombin time from fingerstick whole blood or anticoagulant-free
venous whole blood. The product is intended for use in the management of patients treated with oral
anticoagulants by a healthcare professional and for patient self-testing. Additional information for medical
professionals to monitor patients is located at the end of this instruction manual. Materials are available
through ITC for professionals to train patient self-testers using the ProTime Microcoagulation System.
ProTime instruments intended for patient self-testing are available in the U.S. by prescription only. These
instruments include patient-specific product instructions.
For in vitro Diagnostic Use.
SUMMARY AND EXPLANATION
What Does The ProTime Microcoagulation System Do?
The ProTime Microcoagulation System is designed for testing prothrombin time (PT) and International
Normalized Ratio (INR). The reagents for a prothrombin time test are in the cuvette. Whole blood clotting
time is converted to INR, than the result is calculated for plasma equivalent PT seconds. This test is done to
check the status of patients receiving oral anticoagulation therapy.
What is INR?
The International Normalized Ratio (INR) was developed to help the doctor compare an individual’s
prothrombin time results from one lab to another. An advantage of reporting an INR is to allow for
normalization of comparisons from one lab or instrument to another. The precision of the INR is improved
when a reagent with a lower ISI is used.
Note: ISI stands for International Sensitivity Index. ProTime uses this number to calculate PT
seconds from INR.
Blood Coagulation Test Methodology
Traditional coagulation tests measure the time required for the formation of a fibrin clot following the
addition of a coagulation-activating reagent. Laboratory assays typically use plasma recovered from
anticoagulated (citrated) whole blood samples. The clotting time is a measure of the functionality of the
patient’s hemostatic system. Specific coagulation activating reagents are employed in various clotting tests to
measure specific components of the hemostasis system. Clotting times are prolonged in the case of either
decreased procoagulant activity or increased anticoagulant activity.
Summary of the Blood Coagulation System
The events leading to the formation of a fibrin clot are simplified in coagulation theory into two coagulation
pathways: the intrinsic and the extrinsic pathways. There are twelve clotting factors or proteins involved in
this cascade scheme, numbered I through XIII (excluding VI). The prothrombin time test measures the
extrinsic coagulation pathway and is sensitive to coagulation Factors VII, X, V, II and Fibrinogen (I). With the
exception of Factor V, vitamin K is a required co-factor for biosynthesis of these factors in the liver. The
prothrombin time (PT) test uses thromboplastin as the active reagent to initiate the extrinsic pathway. The
prothrombin time test will be prolonged in patients with liver disease or vitamin K deficiency. The test is
widely used to monitor oral anticoagulant therapy that suppresses the synthesis of vitamin K-dependent
clotting factors.
2

SYSTEM FEATURES
1) Tenderlett 2) ProTime Cuvette
7) AC/DC Power Module
3) Display Panel
4) MENU/SCROLL
Button
5) START/SELECT
Button
6) Cuvette Entry Port
8) Input DC Port
9) Output Data Port
1.
Tenderlett Plus/Tenderlett Plus LV. The finger incision and blood collection device used with
ProTime and ProTime cuvettes.
2.
ProTime/ProTime3 Cuvette. The cuvette performs the PT test.
3.
Display Panel. The display panel prompts you through the test procedure, displays results and error
messages.
4.
MENU/SCROLL Button. The button is used to move through menu screens and to scroll through
results.
5.
START/SELECT Button. The button is pressed to turn ProTime on and off, to start the test and to
select menu items.
6.
Cuvette Entry Port. The cuvette entry port accepts only ProTime and ProTime3 cuvettes.
7.
AC/DC Power Module. The AC/DC power module is used to connect the ProTime instrument to the
AC power cord to recharge the battery.
8.
Input DC Port. Plug the DC power cord from the AC/DC power module into this port when
connecting the ProTime instrument to the AC/DC power module.
9.
Output Data Port. Use this port to download records in the ProTime memory.
3

IMPORTANT LABELS AND SYMBOLS
Before using the ProTime Microcoagulation System, it is essential that the contents of this Operator’s
Manual and any instructions accompanying the ProTime cuvettes and Tenderlett Plus incision devices are
read and understood by the operator. These materials make reference to various symbols that are
explained below:
Start/Select
Menu/Scroll
Expiration Date of Cuvettes
Serial Number of Device
Lot Number of Cuvettes/Tenderlett Plus
ITC Catalogue Number of Device
Do Not Reuse – Single Use Only
Upper and Lower Temperature Limitations (For Storage or Use)
For in vitro Diagnostic Use
Attention - Read Accompanying Documentation or Instructions
Consult Instructions for Use
Class II Protection Against Electrical Shock
Input Port for DC Power Cord from AC/DC Power Module - Polarity, VDC
and A Input
Output Port for Data Transfer
Name and Address of Manufacturer
4

PRINCIPLES OF OPERATION
The ProTime Microcoagulation System measures the PT using fibrin clot formation and detection. The
ProTime cuvette is a self-contained, micro volume reaction cell constructed of precision-molded plastic.
There are two user options within the ProTime Microcoagulation System: the standard ProTime cuvette and
the ProTime3 cuvette. These cuvettes differ from each other in the amount of blood that needs to be
collected and tested.
The standard ProTime cuvette has five micro-channels, which contain the dried reagents required to
perform triplicate testing of the PT assay and two levels of controls. The ProTime3 cuvette has three
functional micro-channels. Two micro-channels perform the controls, and one micro-channel performs the
PT test. The standard ProTime uses the Tenderlett Plus device for performing the fingerstick, and it is
designed to hold 65 µL of blood (approximately 3 drops) needed to fill all five micro-channels. The
ProTime3 uses the Tenderlett Plus LV (low volume) device for performing the fingerstick, and it collects 27
µL of blood (approximately 1 large drop) needed to fill the three micro-channels of the ProTime3 cuvette.
The instrument draws the precise volume of blood into the micro-channels of each cuvette, which contain
thromboplastin and other reagents. An array of LEDs detects the motion of sample/reagent mixtures as they
move through a precision restriction in each channel. The blood is pumped back and forth until a clot
forms, obstructing the channel and slowing the flow of blood. The instrument detects the clot when the
blood movement decreases below a predetermined rate.
SAFETY FEATURES AND QUALITY CONTROL
Each ProTime and ProTime3 cuvette has two channels for performing the two levels of control which work
simultaneous with the channel(s) used for testing the PT assay. The built-in safety features of the
instrument and integral reagent controls work together to ensure that the instrument and reagent systems
are working properly and that the test procedure was performed correctly. Two levels of quality control are
performed with each and every test.
Calibration
The ProTime instrument and cuvettes are pre-calibrated. No additional calibration is required.
Reagents
ProTime cuvettes are pre-loaded with dried thromboplastin, stabilizers and buffers. The thromboplastin
has a high sensitivity, measured as an ISI near 1.0. Each cuvette performs the prothrombin time test and,
in addition, has one channel for a Level I control and one channel for a Level II control. The controls
consist of purified plasma-extracted coagulation factors and anticoagulants.
5
Operating Precautions
•
For in vitro Diagnostic Use.
•
The ProTime instrument is designed for use only with ProTime cuvettes.
•
The ProTime instrument will not perform a test if the cuvette is past its expiration date or has been
improperly stored.
•
The ProTime instrument is designed to be used for testing in a stationary position. DO NOT perform
testing while carrying or holding the instrument.
•
In order to charge the ProTime instrument, the AC power cord should be plugged into an electrical
service outlet and the AC/DC power module while the DC power cord from the AC/DC power module is
plugged into the DC port in the back of the ProTime instrument.
•
DO NOT expose the ProTime instrument to extreme temperature (above 35°C, 95°F). Such exposure
could affect the performance of any type of electronic instrumentation.
•
DO NOT drop the ProTime instrument, and do not use the results if the instrument is dropped during
a test.
•
DO NOT attempt to open the ProTime instrument other than for battery replacement, as there are no
user-serviceable parts.
•
DO NOT remove the AC/DC power module from the ProTime instrument by pulling on the cord.
Patient specimens and used cuvettes are potentially infectious. The cuvettes include materials that have
been prepared from human plasma or serum that has been tested using US FDA recognized methods and
found to be non-reactive for HIV antibody and for hepatitis B surface antigen. Handle with appropriate care and
dispose of cuvettes and blood collection materials in accordance with standard methods of biohazard control.
The use of accessory equipment (e.g., printers, etc.) not identified in this manual either in the patient
vicinity, or that does not comply with either the equivalent safety requirements of this equipment or
UL/IEC 60601-1 or IEC 60601-1-2, may lead to a reduced level of safety with the resulting system.
Limitations
•
The ProTime instrument uses only fresh capillary or venous whole blood. Plasma or serum cannot be
used. Glass tubes or syringes must not be used to collect venous samples. Use only plastic syringes
without anticoagulants to collect venous samples. Poor fingerstick blood collection technique may
affect results.
•
In clinical trials, no significant difference was observed between fingerstick and venous specimens run
on ProTime. During those trials, ProTime measured patients with an INR range of 0.8 to 7.0.
ProTime is programmed to calculate and report INR results as follows:
If the calculated INR is: Then ProTime displays:
< 0.6 INR LOW
0.6 - 0.79 INR < 0.8
0.8 - 7.0 Result
7.1 - 9.9 Result - followed by "*"
10.0 - 12.0 INR > 9.9
> 12.0 INR HIGH
•
Results may be affected in patients receiving heparin or who have an abnormal response to heparin.
•
Correlation of results reported by the ProTime instrument to laboratory results depends on the precision
of the laboratory method and on the ISI value of the laboratory reagent and instrument system.
•
Do not disturb instrument while test is in progress.
As with all diagnostic tests, ProTime Microcoagulation System test results should be scrutinized in light of a
specific patient’s condition and anticoagulant therapy. Any results exhibiting inconsistency with the
patient’s clinical status should be repeated or supplemented with additional test data.
Follow doctor’s instructions if you have difficulty performing the test or receive a result outside of the
prescribed therapeutic range.
6

Reagent Preparation and Storage
•
Bring the foil-pouched cuvette to room temperature prior to use.
•
ProTime cuvettes are ready-to-use. No additional preparation is required.
•
Store the foil-pouched cuvettes in a refrigerator (2°C to 8°C, 36°F to 46°F).
•
An unopened cuvette pouch is stable when stored at 2°C to 8°C until the date printed on the pouch.
Unopened cuvette pouches may be stored at room temperature for 60 days. Once the pouch has been
opened, the cuvette must be used within 16 hours.
Cuvette or Tenderlett Disposal
The ProTime cuvette and Tenderlett Plus are for single use only and are not to be reused. After use they
contain human blood and should be disposed of in accordance with local regulations for human blood
contaminated waste.
INSTRUMENT SPECIFICATIONS
Size
Weight
Operating Temperature
PT Test Temperature
Battery Type
Operating Time On Battery
2.77 (h) x 4.5 (w) x 8.75 (l) inches
1.6 pounds
Room temperature (15°C to 30°C, 59°F to 86°F)
37±1.0°C
Nickel Metal Hydride (NiMH)
Approximately 2 hours (constant run), or 10 complete test
cycles per charge. Tests may also be run while ProTime is
plugged into AC/DC power module.
Anticipated Battery Life
100 - 240 VAC Power
Supply/Charger
500 charges
Input: 100 – 240 volts AC, 47 - 63 Hz
Output: 15 volts DC, 2.0 A
SERVICE AND MAINTENANCE
Routine Maintenance and Cleaning
DO NOT immerse the ProTime instrument or allow fluid to enter the cuvette housing. Inspect and clean
the outside of the cuvette slot as required. Remove residual dried blood or other foreign matter on the
outside of the instrument using gauze dampened with a 10% dilution of household bleach in water or with
gauze dampened with isopropyl alcohol or other disinfectant. DO NOT use other solvents or strong cleaning
solutions as they may damage the plastic components of the instrument.
Servicing The ProTime Instrument
Other then replacement of the rechargeable battery as described in this manual, the ProTime instrument is
not user serviceable. Should service be required, please contact Technical Support at 1-732-548-5700,
1-800-631-5945, or e-mail us at techsupport@itcmed.com. If you are advised to return the instrument to
ITC for service or repair, prior to shipping please clean the instrument, as described above.
Instrument Disposal
If instrument disposal is required, follow local regulations for the disposal of electronic devices. For battery
disposal, see the Battery Replacement section.
Battery Information
The ProTime Microcoagulation System is designed to run either on AC power supplied by the AC/DC power
module or on the rechargeable battery supplied within the unit.
7

Rechargeable Battery Facts
•
Batteries discharge naturally over time (approximately 5% per month).
•
Battery capacity (the amount of charge the battery will hold) is lower at low temperatures.
•
Batteries that are charged to their maximum capacity will generate heat if they continue to be charged.
•
The ProTime instrument uses a rechargeable NiMH (Nickel Metal Hydride) type battery. The maximum
capacity of any rechargeable battery will gradually decrease over time. To ensure maximum life of the
rechargeable battery, read and follow the information in Care of the ProTime Battery for Maximum
Life section.
Care of the ProTime Battery for Maximum Life
A new instrument, an instrument that is used infrequently, or an instrument with a new replacement
battery, should be plugged in for at least 8 hours before use to ensure that the battery is completely charged.
The instrument screen will show CHARGING BATTERY when the AC/DC power module is connected to the
AC power cord and the ProTime instrument. The screen will show CHARGE COMPLETE when the battery is
fully charged. The AC/DC power module should be disconnected after the CHARGE COMPLETE message is
seen. The AC/DC power module that has been supplied by ITC has been selected specifically for use with
your ProTime Microcoagulation System. Do not use any other AC/DC power module.
When the battery indicator on the screen shows less than 25% charge remaining, please refer to the
Charging the Battery section for instructions. To maximize battery life, allow your ProTime instrument to
discharge completely before re-charging. Avoid charging the ProTime instrument for frequent, short
periods of time (such as charging for a few minutes, removing from the AC/DC power module, and then
recharging again). Avoid storing and charging the ProTime instrument at extreme temperatures.
Built-in ProTime Battery Features
The ProTime instrument will shut off after 5 minutes if left unattended in order to preserve the battery
charge. If the instrument shuts off automatically, press the
button to re-start the instrument. Before the
start of each test, the ProTime instrument checks the amount of battery charge. If there is not enough
charge in the battery to run a test, the ProTime screen will display PLEASE CHARGE IT. If this occurs, follow
the charging instructions located in the Charging the Battery section. Whether or not the batteries are
charged, you may continue to run tests once the ProTime instrument is plugged into its AC/DC power
module.
Battery Replacement
Refer to the instructions provided with the replacement battery. The used battery should be disposed of in
accordance with local regulations for NiMH batteries.
8
SPECIMEN COLLECTION
Fingerstick whole blood is the recommended specimen. The Tenderlett Plus device is to be used with the
ProTime cuvette, and the Tenderlett Plus LV (low volume) device is to be used with the ProTime3 cuvette.
The Tenderlett Plus device will collect approximately 65 µL of blood (approximately 3 drops), while the
Tenderlett Plus LV device will collect approximately 27 µL of blood (approximately 1 large drop). Samples
should be analyzed immediately after collection. No additional sample preparation is required.
For venous samples, collect venous whole blood into an anticoagulant-free plastic syringe in place of
fingerstick sampling steps 3 and 4 of the Test Procedure section. Immediately dispense venous sample
into the Tenderlett Plus collection cup, filling the Tenderlett Plus collection cup. Follow instructions from
step 5 of the Test Procedure section.
Note: Serum, plasma or whole blood collected with any anticoagulants is NOT suitable
samples.
PREPARING THE INSTRUMENT
Unpacking and Inspection
1.
Remove any protective packaging that may be present around the instrument.
2.
Examine the packaging material to be sure that the AC/DC power module, AC power cord (see
note below), connecting cables, or other components have been removed. The materials that are
provided are listed below.
Note: Inspect each component for damage when unpacking. If damage is observed, contact
your ProTime representative.
Materials Provided
Article Quantity
ProTime Microcoagulation Instrument 1
ProTime Microcoagulation System Information and Training CD
(PROTIMEPRO only)
AC/DC power module – ITC Part No. IR5226
1
1
ProTime Microcoagulation Operator’s Manual 1
Note: An AC power cord is provided in the United States and Canada only. For use outside the
US and Canada, the customer must obtain a power supply cord that is internationally
harmonized and marked "<HAR>", 2-conductor, 0.75 mm
2
minimum wire, rated 300 V, with
a PVC insulated jacket. The cord and plug cap must be suitable for medical use. The cord must
have a molded on plug cap rated 250 V, 2.5 A.
Materials Needed But Not Supplied
•
ProTime cuvettes
•
Tenderlett Plus incision device
Optional Materials
Article
Personal Computer Interface Cable – ITC No. PROCABLE (ITC Part No. IR5313X)
Printer/Label Maker – ITC No. LBLKIT
Replacement Printer/Label Maker Interface Cable – ITC No. LBLCABLE (ITC Part No. IR5314X)
Replacement Battery – ITC No. PROBATTERY
9
Charging the Battery
The battery in the instrument needs to be charged before the first use.
1.
Connect the AC/DC power module to an electrical service outlet, using the AC power cord.
2.
Connect the DC power cord from the AC/DC power module into the DC port on the back of the
instrument. The instrument screen will show CHARGING BATTERY when the AC/DC power
module is connected to the AC power cord and the ProTime instrument. The screen will show
CHARGE COMPLETE when the battery is fully charged.
3.
Allow the battery to charge for at least eight hours.
TEST PROCEDURE
1.
Turn on the ProTime Instrument
Press the
button to start. The ProTime instrument performs a self-check procedure that may
take up to 60 seconds. ProTime will prompt you through the test. Watch the screen and follow
the prompts.
2.
Insert a Cuvette
Make sure the ProTime cuvette is brought to room temperature before use. Wait for the prompt.
Insert the cuvette into the slot with the printing face up and the bar-code down.
The WARMING screen will appear as follows:
10
3.
Prepare for Finger Incision
While the cuvette is warming, prepare the finger. Wait for the prompt before incising the finger
and collecting blood.
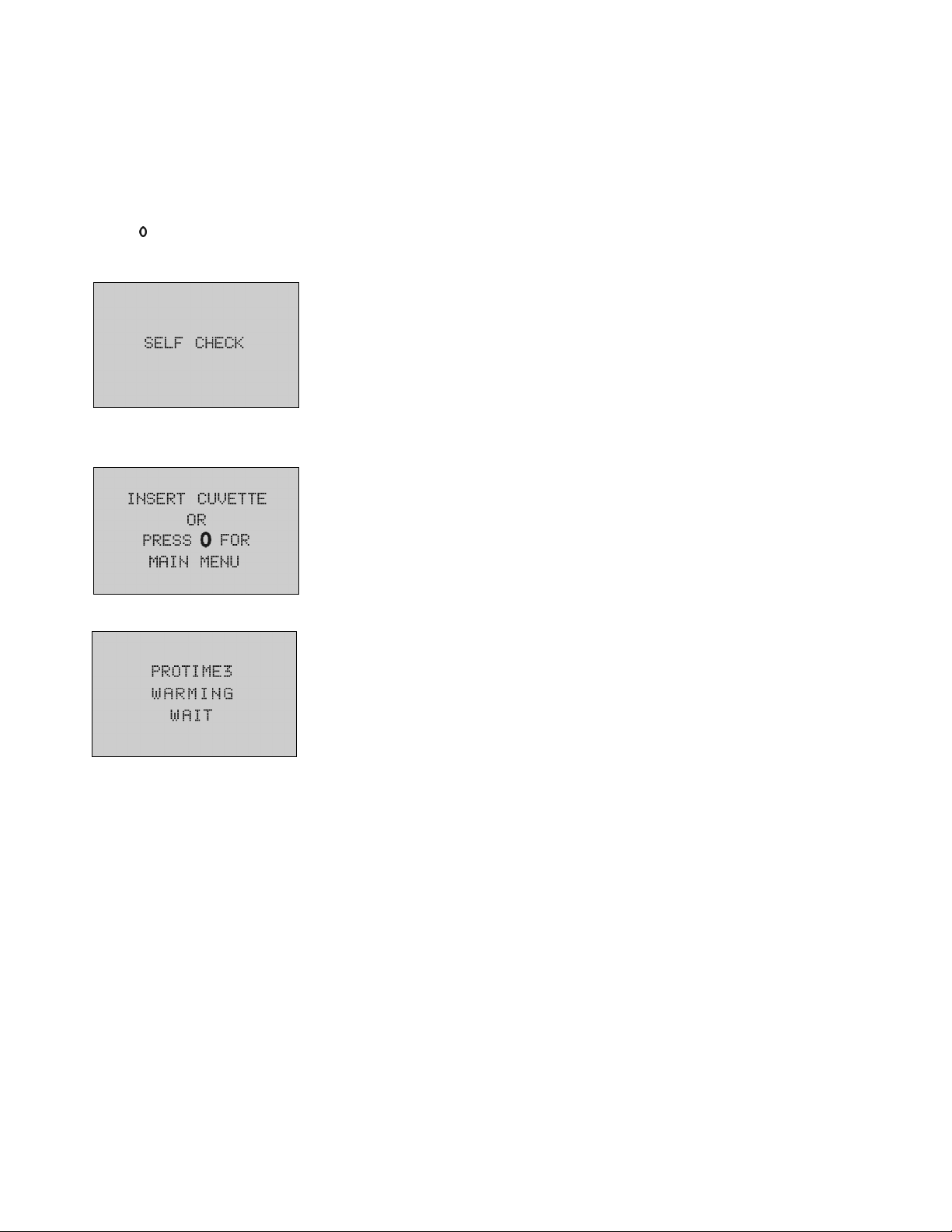
It is easier to collect blood if the hands are warm. Follow these steps to ensure a good sample:
•
Wash the hands in warm water or rub hands together to stimulate blood flow.
•
Apply firm pressure to the palm and finger. Massage the hand and push blood into the
fingertips.
•
Cleanse middle or ring finger and dry. To prevent contamination, do not touch the site after
cleansing.
4.
Blood Collection
When this screen appears, it is time for the finger incision.
CAUTION: Blood collection must be finished within 2:10 minutes to prevent early
clotting of the sample. ProTime will keep time. If the 2:10 minute interval has expired
and the
button has not been pressed during this interval, a TIME OUT error message
is displayed. To run another test, repeat procedure from Step 2.
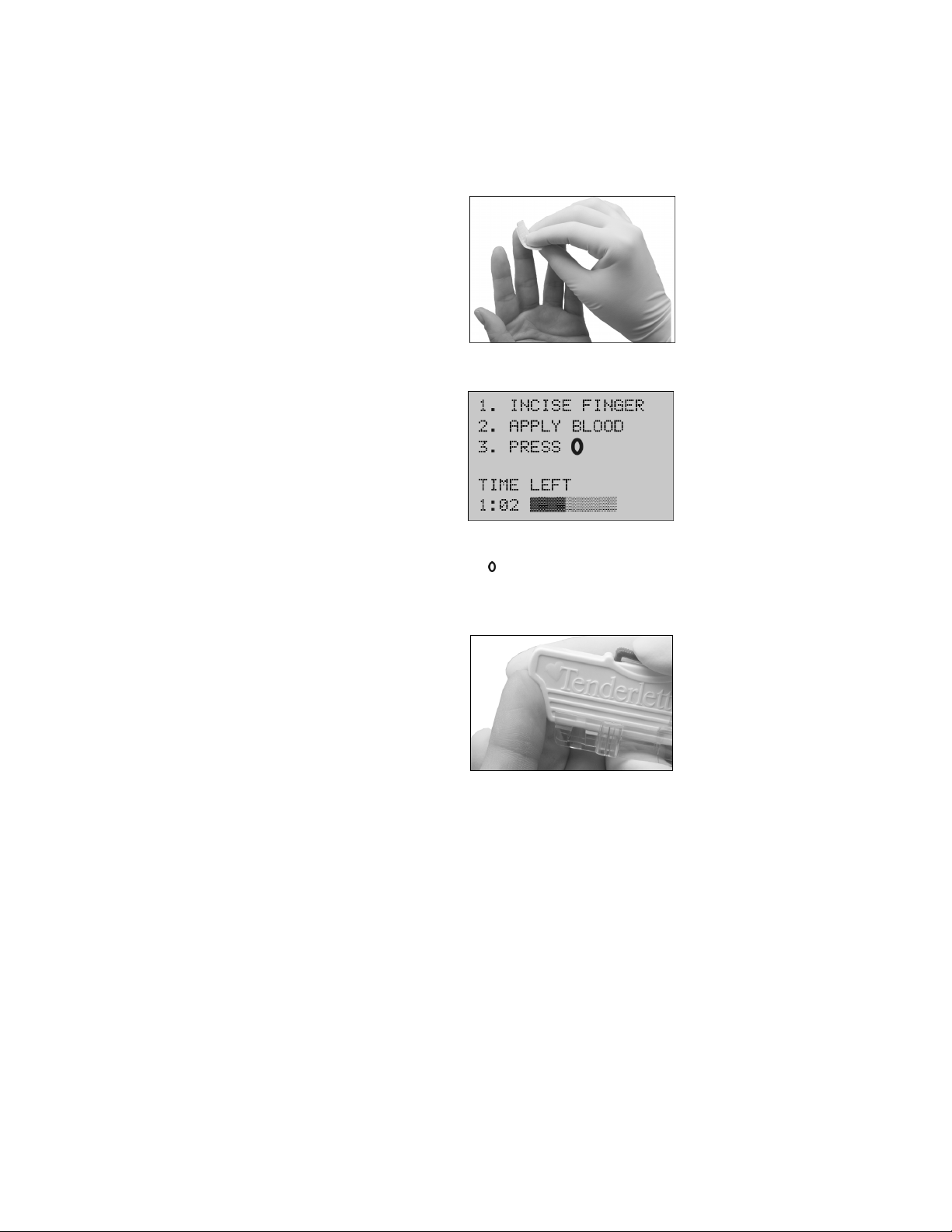
•
Place the Tenderlett Plus device firmly against the side of the finger. Place thumb on top of
the device as shown, and press the red trigger firmly using the other thumb.
•
Wipe away the first trace of blood.
•
Gently massage from the base of the finger to force blood to the tip so that a large drop of
blood forms.
11
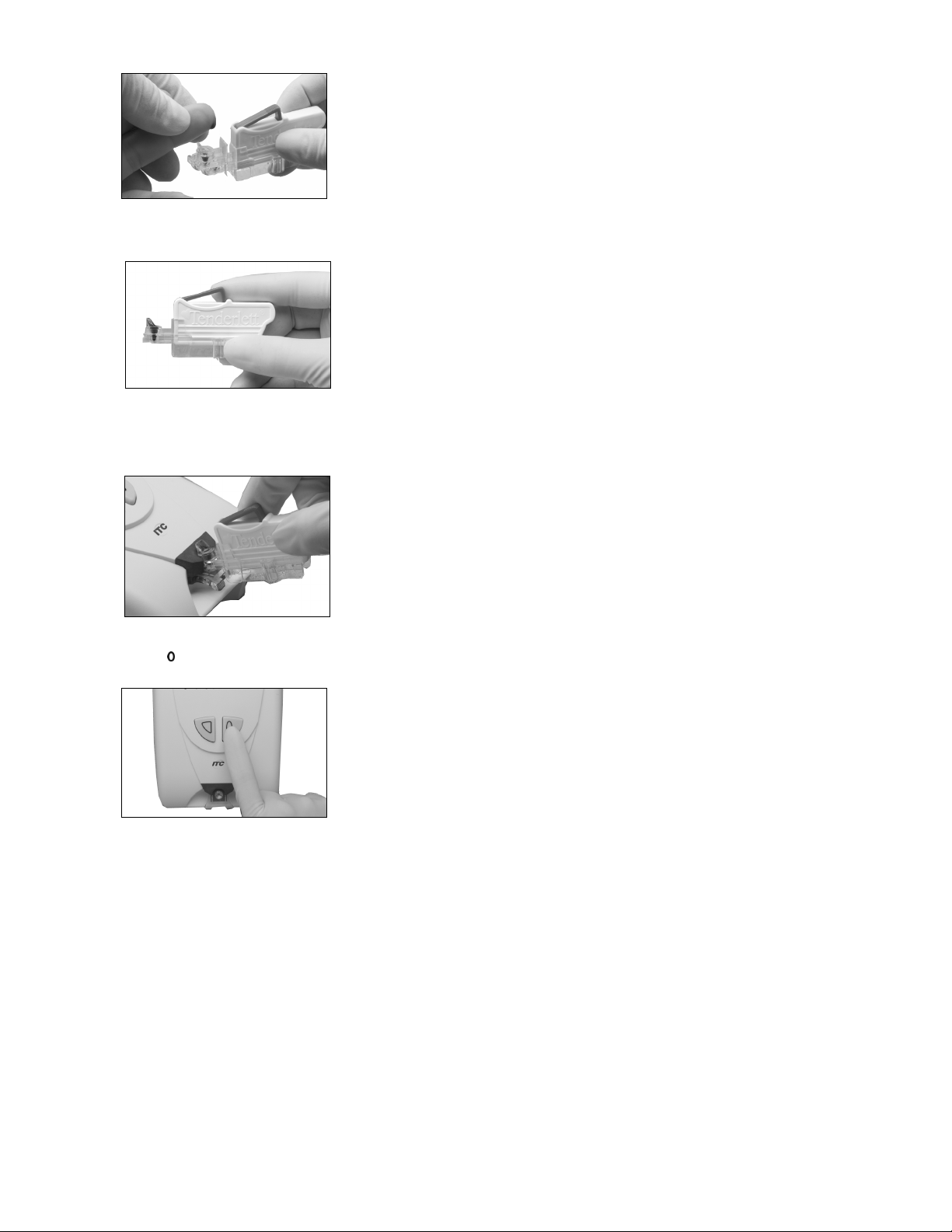
•
Touch the large drop of blood to the collection cup. Keep adding blood until the blood level
fills the cup above the line.
•
For Tenderlett Plus LV, ensure the cup is filled completely. Ensure the blood extends all
the way to the bottom of the cup. Add another drop if you are not sure you have
enough.
5. Snap Tenderlett Plus to ProTime
•
Hold the device at an angle and place the front end of the device into the slot in the
instrument.
•
Press down to click the Tenderlett Plus in place. You should hear a soft click.
6. Start the Test
•
Press the button to start the test. This signals ProTime to draw the sample into the
cuvette.
Note: Proper engagement of the
Tenderlett Plus to the cuvette is
critical to prevent a sample error.
12

•
It takes only a few seconds for ProTime to draw the blood into the cuvette. Watch the
screen for the next prompt.
7. Remove Tenderlett Plus
Remove Tenderlett Plus immediately when prompted to do so.
CAUTION: Failure to do so will result in an error message. ProTime allows you six
seconds.
•
Do not press the button after the Tenderlett Plus is removed from the ProTime
instrument while you are testing your INR. This will interrupt the test procedure, and you
will have to start over with a new blood sample.
•
The instrument then progresses to the test and displays the TESTING screen.
13

8. Read the Result
In a few minutes, the result is ready.
•
Press the button to turn off.
•
Press the button to go to the MAIN MENU if you want to run another test, review the data
in memory, print results, transfer results to a computer, or perform set up functions.
Notes:
•
The result remains displayed for 5 minutes or until the button or the button is pressed.
•
If the cuvette has not been removed and the instrument is left unattended for 5 minutes, the
instrument will display the following messages before shutting down and powering OFF:
What Does the Result Mean?
The result indicates the clotting activity of blood. When ProTime is used as a self-testing instrument, the
healthcare provider may program ProTime with the upper and lower limits that are right for the patient. In
this case, ProTime will display OTR if results are outside of the therapeutic range. The OTR will not display
if results are within the limits. If no limits are set, ProTime will display only the result.
Note: If OTR (Out of Therapeutic Range) appears after the INR result, the result is out of the
therapeutic range (TR) limits that have been preset by the professional (see the For
Professionals Only – Program Mode section).
TROUBLESHOOTING
Most often, an error message indicates a problem with blood collection or a mistake in the test procedure. If
an error message appears, read the instructions again and repeat the test with a new cuvette. The
Troubleshooting Guide section gives the messages for the most common errors with possible causes and
solutions.
As with all diagnostic tests, the ProTime Microcoagulation System test results should be scrutinized in light
of a specific patient’s condition and anticoagulant therapy. Any results exhibiting inconsistency with the
patient’s clinical status should be repeated or supplemented with additional test data.
If you get a persistent error, write down the error message and call ITC Technical Support at
1-732-548-5700, 1-800-631-5945, or e-mail us at techsupport@itcmed.com.
Please have the ProTime serial number and cuvette lot number ready when you contact ITC Technical
Support.
14

Troubleshooting Guidelines
•
Become skilled at obtaining blood with the fingerstick technique. This will help avoid sample errors.
•
Follow all of the directions on the screen for optimal operation.
•
Do not try to hasten the test process by performing the fingerstick while the WARMING screen is
displayed, as blood may clot before it is drawn into the cuvette and an error may occur. FOLLOW THE
INSTRUCTIONS ON THE SCREEN.
Troubleshooting Guide
Screen Display Source of Error Method of Control
INR HIGH
REPEAT TEST
IF SAME, CALL MD
INR LOW
REPEAT TEST
IF SAME, CALL MD
ON-BOARD QC
OUT OF RANGE
TRY AGAIN
NO CLOT DETECTED
REPEAT TEST
IF SAME CALL MD
Patient’s PT result is too
high (INR >12.0)
Patient’s PT result is too low
(INR < 0.6)
Level I or II control is too
high or too low
At least one channel did not
clot
Repeat the test. If it occurs again,
patient should be referred to their
physician IMMEDIATELY and re-tested
at the lab.
Repeat the test. If it occurs again,
patient should be referred to their
physician IMMEDIATELY and re-tested
at the lab.
Repeat the test. Check for adequate
storage of cuvettes or sampling
technique. Confirm results with the
lab.
Repeat the test. If it occurs again,
patient should be referred to their
physician IMMEDIATELY and re-tested
at the lab.
Test again with new Tenderlett Plus
TIME OUT
TRY AGAIN
Time ran out before starting
test
and a fresh fingerstick. You may reuse
the cuvette if no blood was in contact
with the cuvette.
TEST INTERRUPTED
TRY AGAIN
SAMPLE NOT SEEN
TURN OFF
TRY AGAIN
SAMPLE TOO LARGE
TURN OFF
TRY AGAIN
SAMPLE TOO SMALL
TURN OFF
TRY AGAIN
SAMPLE ERROR
TURN OFF
TRY AGAIN
Cuvette removed or operator
interrupted test
Can be caused by small
samples, clots, air bubbles,
or a seal problem in one or
more channels
Review correct procedure. Repeat test.
Check for proper collection technique.
Verify cup is completely filled. Use
another cuvette.
Sample oversize Review directions and repeat the test.
Sample undersize
Incorrect fluid movement
Air bubble detected in one
or more of the channels
Repeat test. Exceed the fill line on the
cup to ensure sample size.
Turn off and on again and repeat test
with new cuvette and fresh fingerstick.
15

Screen Display Source of Error Method of Control
Check to make sure cuvettes have not
CUVETTE EXPIRED
BARCODE ERROR
REMOVE CUVETTE
TRY AGAIN
Expired cuvette
Instrument cannot read
barcode accurately
expired. The expiration date is located
on the packaging and alongside the
barcode on the cuvette.
Visually inspect barcode. If scratched,
discard cuvette. If dirty, wipe clean. If
barcode is good, review correct
procedure and repeat test.
BATTERY ERROR
TURN OFF
TRY AGAIN
INSTRUMENT ERROR
TURN OFF
TRY AGAIN
CHARGE ERROR
TURN OFF
TRY AGAIN
TEMP ERROR
TURN OFF
TRY AGAIN
PHOTO ERROR
TURN OFF
TRY AGAIN
MAIN MENU OPTIONS
The options presented in the MAIN MENU are:
Power supply battery error
Instrument set up, data log
or communication error
Power supply error
Temperature not in range
LED blocked or other photo
system error
Repeat the test. If it occurs again,
replace the battery. If problem
persists, call ITC Technical Support.
Repeat the test. If problem persists,
call ITC Technical Support.
Check the AC/DC power module,
repeat the test. If problem persists,
call ITC Technical Support.
Check for proper operating
temperature. Repeat the test. If
problem persists, call ITC Technical
Support.
Repeat the test. If problem persists,
call ITC Technical Support.
Each of these options will lead to sub menus within the selection. The following buttons are used to navigate
the menu:
•
The button is used to move the highlight bar to select the option.
•
The button is used to select the option that is highlighted.
16

Run Test
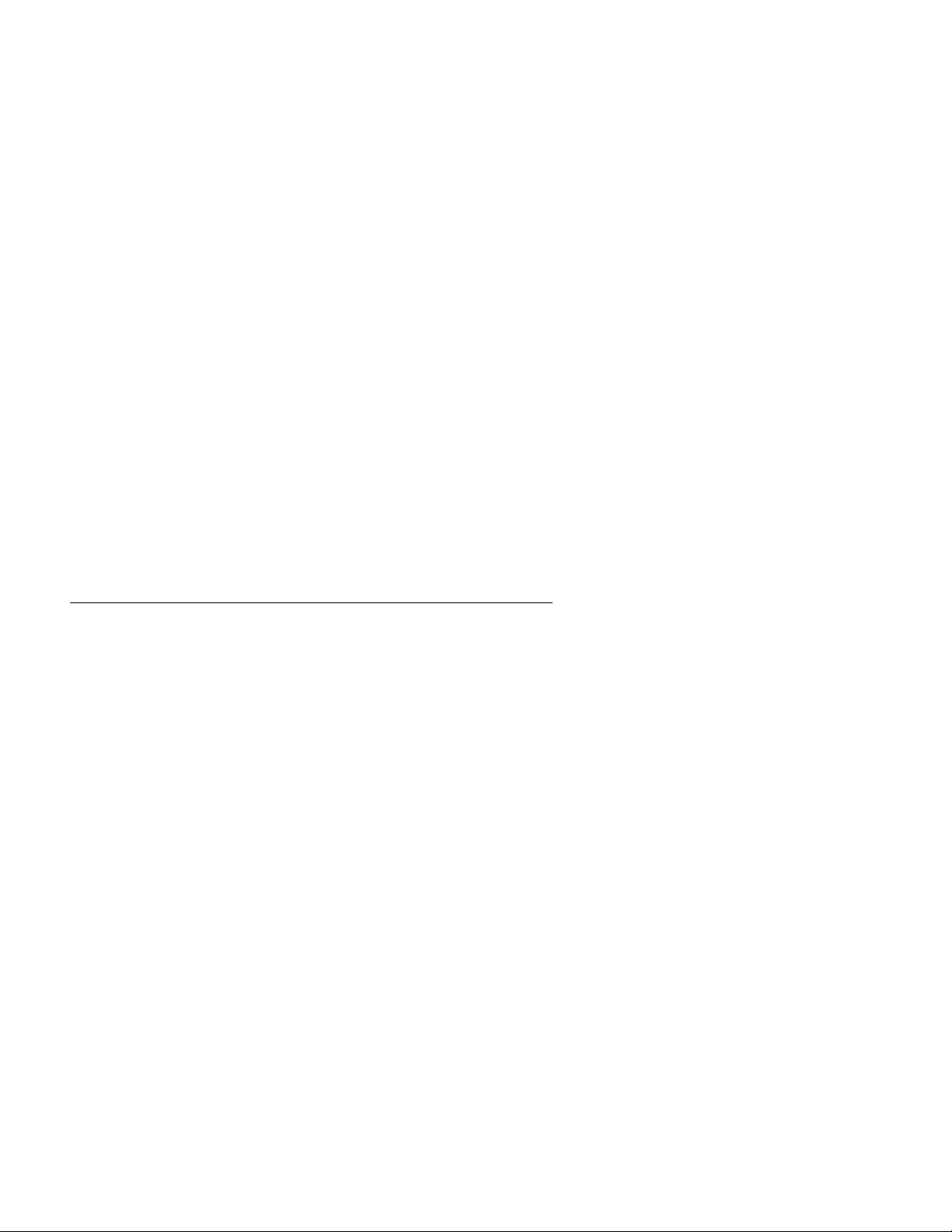
To run the test, select the RUN TEST menu item. The instrument does a SELF CHECK procedure, which
may take up to 60 seconds. The following screen is displayed for this period of time:
The test sequence continues as described in the Test Procedure section.
When the test completes, the result will appear as one of the following screens, depending on PID/OID
selections (see SET UP below).
Note: “X” is used for illustrative purposes only in the following examples.
If PID is ON and OID is ON: If PID is OFF and OID is ON:
If PID is ON and OID is OFF: If PID is OFF and OID is OFF:
17

RUN LQC
When RUN LQC is selected from the MAIN MENU, the following screen is displayed and the user can select
NOR/ABN (NORMAL/ABNORMAL).
The instrument performs SELF CHECK procedure which may take up to 60 seconds. The testing proceeds as
described above except that no prompt for PID will be shown for a QC test, whether PID is ON or OFF.
When the test completes, the result will be displayed as follows, depending on OID ON/OFF and LQC
NOR/ABN selection:
NOTE: The result and ID information will be stored in the database as a QC record.
For LQC Normal with OID on: For LQC Normal with OID off:
For LQC Abnormal with OID on: For LQC Abnormal with OID off:
18

SHOW RESULTS
Press the
Press the
button to move the highlight bar to the SHOW RESULTS line on the MAIN MENU.
button to view the SHOW RESULTS menu and observe the following screen:
DATA HISTORY
Press the
holds 50 results. Pressing the
memory from the most recent to the oldest. Press the
PATIENT RECORDS
The result and ID information will be stored in the database as one of the following screens depending upon
the PID/OID ON/OFF selections:
If PID is ON and OID is OFF: If PID is OFF and OID is OFF:
button to view the most recent result in the DATA HISTORY memory. The instrument memory
button will scroll through individual results. The results are stored in
button to return to the MAIN MENU.
Note: “MM/DD/YYYY” and “HH:MM” are used for illustrative purposes only in the following
examples of date and time screens.
If PID is ON and OID is ON: If PID is OFF and OID is ON:
19

LQC RECORDS
The result and ID information will be stored in the database as a QC record. QC result will be displayed as
follows, depending upon the OID ON/OFF selections and the LQC NOR/ABN selections.
For LQC Normal with OID on: For LQC Normal with OID off:
For LQC Abnormal with OID on: For LQC Abnormal with OID off:
PRINT DATA OR SEND DATA
Press the
the
button to move the highlight bar to the SHOW RESULTS line on the MAIN MENU. Then press
button to view the SHOW RESULTS menu and observe the following screen:
20

The instrument auto send feature allows transmitting of test results directly to a serial printer or to a
computer by using the PROCABLE. Contact ITC Customer Service to order the PROCABLE.
To use the AUTO SEND feature, press the
SEND is highlighted. Press the
button and, depending on the current settings in the instrument, one of
button on the PRINT DATA or SEND DATA display until AUTO
the following displays will appear.
Example: Printing Data
Press the
•
•
•
•
button to set the feature to ON or OFF and then press the button to save the setting.
Highlight the SHOW RESULTS line in the MAIN MENU.
Press the button to view the SHOW RESULTS menu.
Press the button to move the highlight bar to the PRINT DATA or SEND DATA line.
Press the button to access the PRINT DATA or SEND DATA option and select THIS RESULT or ALL
RESULTS option in the PRINT DATA or SEND DATA screen.
Upon selecting THIS RESULT, the last recorded test is sent from the instrument memory to a printer or to a
computer. If ALL RESULTS is selected, all results (up to 50) are printed/sent from the instrument memory
to a printer or to a computer. If MAIN MENU selection is made, the user is returned to the main menu.
Note: If the AUTO SEND feature is on, results will be automatically sent if the PROCABLE is
connected prior to the start of a test. To use a laser printer, the data must first be transferred to
an IBM-compatible personal computer and the results can be printed from that computer. The
following screen displays until the PRINT DATA or SEND DATA process is complete:
21

SET UP
Go to the MAIN MENU screen and choose the SET UP option by using the
button to select SET UP. The following screen appears:
button to scroll and the
SET LANGUAGE
Press the
button to select SET LANGUAGE. The following options are displayed when SET LANGUAGE is
selected:
Highlight a language selection and press the button to set it.
Note: English will be highlighted as the default language in a new instrument. After changing
the language, the new language becomes the default. The instrument will automatically turn
off after changing the language selection.
SET TIME / DAY
Select SET TIME/DAY from the SET UP menu. The following screen is displayed:
12 HOUR – MM/DD will be highlighted as a default selection. Highlight your TIME-DAY preference, then
press the
button.
Note: In the case of a 24 HOUR selection, AM/PM acronyms will not appear on the screens.
22

Changing the Hour
The time is pre-set to Eastern Standard Time (EST). For example, the time is 1:25 PM. Change the hour by
pressing the
is 10 AM. To change the hour, press the
changes to AM at midnight. Once the correct hour appears, press the
procedure will then advance to the MINUTES screen.
button until the correct hour appears in the highlight bar. In this example, the correct hour
button until 10 AM appears in the highlight bar. The PM
button to set the hour. The
Changing the Minutes
The hour on the top line has changed to the time just set and PM has changed to AM.
Change the minute by pressing the button until the correct minute appears.
Once the correct minute appears, press the
automatically advance to the DAY screen.
button to set the minutes. The procedure will then
Changing the Day
Only the day can be changed. The month and year can be changed only in PROGRAM MODE. Change the
day by pressing the
to set the day.
button until the correct day appears. Once the correct day appears, press the button
23

SET PID/OID
The PID/OID selection is indicated only by the location of the highlight (text on the screen is unchanged).
•
Use the button to select PID/OID options:
•
Selecting PID/OID ON enables both a patient ID and operator ID to be entered.
•
Selecting PID ON enables only a patient ID to be entered.
•
Selecting OID ON enables only an operator ID to be entered.
•
Selecting OFF disables both a patient ID and operator ID.
Note: The selection is highlighted and remains the default until changed again by the user.
Entering and Changing Numerical Information (PID/OID, PASSWORD)
By using a combination of the
numeric information can be entered into the instrument for operator identification number (OID), patient
identification number (PID) and PASSWORD fields. When entering numeric information into a field,
pressing a button has the following actions:
•
CHANGE - Pressing the button once will increment the digit in the current cursor position by
one, starting at 0 and going through 9 and back to 0 again.
•
OK - Pressing the button briefly enters the digit displayed in the current cursor position and then
moves the cursor right, to the next field position. Pressing the
the complete numeric field.
button and button and the triangular cursor on the display screen,
button for 2 seconds or longer saves
EXAMPLE: Entering a PID number:
With the PID feature enabled, the following screen appears after a cuvette is inserted into the instrument:
24

To enter a PID, the first position in the PID field (starting from the left) is indicated by a triangular cursor.
•
Press the button until the desired digit is displayed.
•
Press the button briefly to enter the digit in that position of the field. The cursor will automatically
move to the next field position.
•
Repeat these steps until the desired field length is entered.
•
To save the entered PID value press and hold the button for approximately 2 seconds until the
second audio beep is heard.
Note: These audio beeps will be heard regardless of BEEP setting (ON/OFF).
After the cuvette is inserted, the user has the option to set an operator ID (OID) and/or a patient ID (PID) if
the OID and/or PID have been enabled in the SET UP section of the MAIN MENU. The PID may contain up
to twelve digits, the OID can be a value with up to six digits.
Note: In the different scenarios related to SET PID/OID ON/OFF settings, some of the following
four screens are not presented to the user. For example, none of these four screens will appear
if both PID and OID are set to OFF value. The default numerical PID and OID values are zero.
If OID is set to the ON position, the following screen will appear:
Use the and buttons to move the cursor and to enter the numeric ID.
The CONFIRM OID confirmation screen will follow the ENTER OID screen:
If button is pressed, the user will return to the previous screen, otherwise (if the button is pressed and
PID is set to the ON position) the program will proceed to the following ENTER PID screen:
Use the and buttons to move the cursor and to enter the numeric ID.
25

The CONFIRM PID confirmation screen will follow the ENTER PID screen:
After setting of both requested PID and/or OID, the WARMING screen will appear in the center of the
display:
The test sequence then continues as described in TEST PROCEDURE.
SET BEEP
If SET BEEP is selected, the following screen appears:
To turn the BEEP SOUND ON, press the CHANGE button. The SET BEEP ON screen appears:
Then press the button to turn the beep sound ON.
Note: Regardless of BEEP setting, the beep will sound under the following conditions: power on,
power off, and following a key hold.
26

FOR PROFESSIONALS ONLY - PROGRAM MODE
Note: The Program Mode feature is only available to professional users. The following
information is not contained in the Operator’s Manual for patients performing self-testing.
For PROTIMEPRO, to access the Professionals Only section of the training CD, enter the following
code when prompted by the software: 54321 (the training CD is not provided with PROTIMEINT).
If the PROGRAM MODE option is selected from the SET UP Menu, the following screen appears:
Note: The PROGRAM MODE option is password protected. The default password in a newly
manufactured instrument is 000000, which the user can then change to a password of their
own choosing (see the Change Password section). If you forget or lose your password, an
emergency password is available from ITC Technical Support by calling 1-732-548-5700,
1-800-631-5945, or e-mail us at techsupport@itcmed.com.
If the entered password matches the password stored in the instrument, the instrument will enter the
PROGRAM MODE. If the entered password is incorrect, the user will be given two more chances to enter the
correct password in the ENTER PASSWORD screen. After three unsuccessful attempts to enter the correct
password, the program will return to the MAIN MENU and the user should contact ITC Technical Support.
After performing all programming functions the user will return to the MAIN MENU.
After the correct password has been entered the PROGRAM MODE menu screen appears:
SET MM/YYYY
Selecting SET MM/YYYY (set month and year) will guide you through the following sequence of screens:
Change the month by pressing the
button until the correct month appears.
27

Once the correct month appears, press the button to set the month. The procedure will then advance to
changing the year screen.
Change the year by pressing the
button until the correct year appears.
Once the correct year appears, press the button to set the year. The procedure will then advance to MAIN
MENU.
SET TR LIMITS
To set therapeutic range (TR) limits, select the SET TR LIMITS from PROGRAM MODE menu screen. The
following screens appear for selection of the upper and lower TR limits.
Set upper limit by pressing the button until the desired upper limit is displayed. Select the limit using
button. The upper limit can be set to one of eight values ranging from 2.0 to 5.5 with an incremental
the
step of 0.5 or to the value 9.9. Once the upper limit is set, the lower limit will be available for setting.
Set lower limit by pressing the
button. The lower limit can be set to 0.0, 1.2, or to one of six values ranging from 1.5 to 4.0 with an
incremental step of 0.5.
button until the desired lower limit is displayed. Select the limit using the
If limits are not changed, the instrument will use its default limits 9.9 and 0.0. Once the limits are set, the
instrument will return to the MAIN MENU.
28

SET ISI
If SET ISI is selected from the PROGRAM MODE, the following screen is displayed:
To change the ISI used in the calculation, access the PROGRAM MODE from the SET UP on MAIN MENU and
enter the ISI of the local laboratory reagent
Displayed plasma equivalent values are indicative of the results obtained using a laboratory reagent with an
ISI of 1.0. If the ISI of the reagent used in the local facility is significantly different from 1.0, the operator
may wish to select an ISI more closely aligned with their lab reagent. Change the pre-programmed ISI by
pressing the
normal control population change as the theoretical ISI is changed. The normal control population values
are determined from clinical data.
REPORTABLE RANGE
In clinical trials, no significant difference was observed between fingerstick and venous specimens run on
ProTime. During those trials, ProTime measured patients with an INR range of 0.8 to 7.0. ProTime is
programmed to calculate and report INR results as follows:
If the calculated INR is: Then ProTime displays:
button until the desired ISI is displayed. Press the button to set. The values used for the
< 0.6 INR LOW
0.6 – 0.79 INR < 0.8
0.8 – 7.0 Result
7.1 – 9.9 Result - followed by “*”
10.0 – 12.0 INR > 9.9
> 12.0 INR HIGH
29

CHANGE PASSWORD
If CHANGE PASSWORD option is selected from the PROGRAM MODE menu, the user must enter the
password (up to 6 digits) as a left justified number without any blanks. The rules for entering a numerical
PID or OID are also applicable for entering the 6 digit numerical password (see page 24).
After password has been entered, the CONFIRMATION OF PASSWORD screen appears:
If the button is pressed, the newly entered password is accepted by the instrument. If the button is
pressed, the user will repeat the change password procedure by returning to the previous CHANGE
PASSWORD screen.
Note: Upon manufacturing, the default password is set to 000000.
CLEAR MEMORY
Upon this selection from the PROGRAM MODE menu all results will be erased from the instrument
memory. To prevent accidental loss of data, the following confirmation screen will precede the data
removal from the instrument memory:
Selecting NO will return the instrument to the MAIN MENU, and no results will be erased from the
instrument memory.
30

PERFORMANCE CHARACTERISTICS
Reporting ProTime Results
The ProTime Microcoagulation System reports a result as International Normalized Ratio (INR) and in PT
seconds. The ProTime system calculates the INR directly from whole blood clotting time based on a
conversion equation that was established in clinical trials. The result in plasma equivalent seconds is then
calculated from the INR result.
Historically, laboratories have reported prothrombin time results in seconds, specifically the time it takes
for the plasma sample to clot following the addition of the reagents. Reporting the results in seconds is
problematic since the clotting time depends on the sensitivity of the reagent used. Results cannot be
compared from one laboratory to another. The INR system was introduced by the World Health
Organization (WHO) to standardize PT reporting such that patient results could be uniform across different
laboratories. INR is calculated using the formula:
ISI
)
INR = (PT/PT
where PT is the time for the patient plasma sample to clot, PT
n
is the mean clotting time of a non-
n
anticoagulated population, and ISI is the international sensitivity index that is assigned by the manufacturer
of the reagent according to a standard protocol developed by the WHO. The ISI value ranges from 1.0 to
approximately 3.0. A low ISI indicates that the reagent has the highest sensitivity to vitamin K-dependent
clotting factors. Typically, the variable PT
is generated by the laboratory with each new reagent lot by
n
running 20 samples and calculating the mean.
The ProTime system enhances clinical utility for the physician and patient by providing results in both
formats, so that anticoagulant dosing can be managed using a familiar format. Since results reported in PT
seconds depend on the sensitivity (ISI) of the reagent employed, the physician has the option of changing
the ISI value in ProTime so that the ProTime results reported in PT seconds closely match the results
reported by the hospital laboratory. To change the ISI used in the calculation, the physician simply accesses
the programming screens and enters the ISI of the historical laboratory reagent. (Please see the For
Professionals Only – Program Mode section).
The default parameters used to calculate PT seconds are ISI = 1.0, which is the sensitivity of the
thromboplastin reagent used in the ProTime cuvette, and PT
clinical trials. The PT
value used in the calculation is automatically adjusted when the reporting ISI is
n
= 13.1 seconds, which was established in
n
changed.
Table 1. Options for programming ISI and associated PT
n
ISI 1.0 1.2 1.4 1.6 1.8 2.0 2.2 2.4 2.6
PT
13.1 12.9 12.8 12.6 12.5 12.3 12.2 12.0 11.9
n
Table 2. Relationship of INR to PT seconds with varied ISI
PT seconds vs. ISI
INR
ISI = 1.0 ISI = 1.6 ISI = 2.0 ISI = 2.4
1.0 13.1 12.6 12.3 12.0
1.5 19.6 16.2 15.1 14.2
2.0 26.1 19.4 17.4 16.0
2.5 32.6 22.3 19.5 17.6
3.0 39.2 25.0 21.3 19.0
3.5 45.7 27.6 23.0 20.2
4.0 52.2 30.0 24.6 21.4
4.5 58.7 32.3 26.1 22.5
31

Table 2 shows examples of how the calculation of PT seconds is affected by changing the ISI. As the ISI
increases, the plasma equivalent PT decreases at any given INR. For example, a patient with INR = 3.00
would have PT seconds = 39.2 if the ISI is the default ISI = 1.0 or PT seconds =21.3 if the ISI is reset to
ISI = 2.0.
Expected Values
ProTime measures both normal and therapeutic prothrombin times in fresh whole blood. Results are
displayed in plasma equivalent seconds and INR. Expected values for patients taking oral anticoagulants
depend on the patient’s disease state and the target values established by the physician.
Sample Condition INR PT Seconds (ISI = 1.0)
Normal 0.8-1.2 10.4-15.7 sec
Low anticoagulation 1.5-2.0 19.6-26.1 sec
Moderate anticoagulation 2.0-3.0 26.1-39.2 sec
High anticoagulation 2.5-4.0 32.6-52.2 sec
Note: The ProTime instrument converts whole blood clotting time to INR, then calculates PT
seconds. The default ISI used in this calculation is 1.0. Please see the For Professionals Only
– Program Mode section if you wish to select an ISI that is more closely aligned with the local
lab reagent.
The ProTime instrument has been tested extensively by doctors, nurses and patients. Eighty-four people,
ages 7 to 81, participated in the first home-use trial. The trial was conducted to see how well ProTime
results compare to the lab. Comparisons of this type are described by correlation values and a correlation
value near 1.0 means the comparison is good. The home tests compared to tests run at the clinic using
ProTime with venous samples (421 samples) had a correlation of 0.92. The home test results compared to
a reference laboratory results (368 samples) had a correlation of 0.87. These correlation values mean that
home testing is comparable to lab testing.
Precision
Precision testing was conducted with two levels of standard control plasma substrate preparations (Level I
and Level II).
A. Standard ProTime Cuvette
Control Precision n Mean SD
Level I
Level II
B. ProTime3 Cuvette
Control Precision n mean SD
Level I
Level II
Within day 17 0.9 0.06
Day-to-day (5 days) 4/day 1.0 0.08
Within day 19 3.2 0.19
Day-to-day (5 days) 4/day 3.2 0.12
Within day 18 0.9 0.07
Day-to-day (5 days) 4/day 0.9 0.12
Within day 20 4.0 0.19
Day-to-day (5 days) 4/day 4.2 0.22
32

Accuracy
INR results generated by the ProTime and ProTime3 cuvettes using venous and fingerstick whole blood
samples were compared to INR values obtained using standard laboratory plasma PT methods with samples
collected in 3.2% sodium citrate tubes. The following accuracy data was obtained.
A. Standard ProTime Cuvette vs. Lab (Plasma)
Sample Type Regression Equation r n
Fingerstick Y = 0.94x + 0.38 0.95 229
Venous Y = 0.91x + 0.44 0.94 232
B. ProTime3 Cuvette vs. Lab (Plasma)
Sample Type Regression Equation r n
Fingerstick y = 1.05x + 0.07 0.95 229
Venous y = 0.97x + 0.19 0.95 219
Clinical Performance Comparison
A. ProTime3 vs. ProTime
Linear regression containing clinical fingerstick results from three sites yielded a regression equation
as below:
y = 1.02x - 0.14 r = 0.94 n = 229
B. Patient Self-Testing
In a trial of patient self-testing (PST) in the home vs. professional testing in the clinical and reference
lab, equivalent ProTime results were obtained.
ProTime Professional vs. Patient Self-Testing
y = .94x + 0.13 r = 0.92 n = 421
ProTime Patient Self-Testing vs. Reference Lab
y = .77x + 0.38 r = 0.87 n = 368
Sensitivity
The ProTime instrument is sensitive to deficiencies in vitamin K-dependent coagulation factors known to
influence the PT test (i.e., Factors II, VII and X.)
Hematocrit levels between 20% and 60% do not significantly affect test results.
Quality Control
The ProTime instrument has been designed with multiple systems to ensure proper instrument function.
The instrument self-check at startup checks temperature and timing functions, battery level, and optical,
electrical and mechanical functions. The instrument does not require further calibration. Each ProTime
cuvette has two integral reagent controls that ensure assay reliability and performance. Both levels of
control produce quantifiable clotting endpoints that are compared to pre-set acceptance limits programmed
in the instrument.
Other in-process instrument QC features and the integral reagent controls function together to ensure
correct sample size and collection technique, correct test procedure, instrument functionality and reagent
integrity. A fault message is displayed instead of PT results when any instrument or reagent quality criterion
is not met. When a fault message is displayed, the user should review the product instructions and repeat
the test.
33

Additional external control materials may be used to check instrument function, reagent integrity and user
technique. Each institution should establish a quality assurance program for prothrombin time testing that
complies with existing local, state and federal regulations as applicable.
As with all diagnostic tests, the ProTime Microcoagulation System test results should be scrutinized in light
of a specific patient’s condition and anticoagulant therapy. Any results exhibiting inconsistency with the
patient’s clinical status should be repeated or supplemented with additional test data.
TECHNICAL ASSISTANCE
For further information and technical assistance, contact ITC Technical Support at 1-732-548-5700,
1-800-631-5945, or e-mail us at techsupport@itcmed.com.
34

SUGGESTED READING
Adcock DM, Kressin DC, Marlar RA. Effect of 3.2% vs 3.8% Sodium Citrate Concentration on Routine
Coagulation Testing. Am J Clin Pathol 1997;107:105-10.
Brien WF, Baskerville JC, Taberner DA, Crawford L. Calculation vs. Calibration Curve for INR Determination:
Results of an Interlaboratory Proficiency Scheme. Am J Clin Pathol 1999;111:193-201.
Eckman MH, Levine HJ, Pauker SG. Effect of Laboratory Variation in the Prothrombin-Time Ratio on the
Results of Oral Anticoagulant Therapy. N Engl J Med 1993;329:696-702.
Fairweather RB, Ansell J, van den Besselaar AMHP, Brandt JT, Bussey HI, Poller L, et al. College of American
Pathologists Conference XXXI on Laboratory Monitoring of Anticoagulant Therapy. Arch Pathol Lab Med
1998;122:768-81.
Gosselin R, Owings JT, White RH, Hutchinson R, Branch J, Mahackian K, et al. A Comparison of Point-ofCare Instruments Designed for Monitoring Oral Anticoagulation with Standard Laboratory Methods. Thromb
Haemost 2000;83:698-703.
Hirsh J. Antithrombotic Therapy in Deep Vein Thrombosis and Pulmonary Embolism. Am Heart J
1992;123:1115-22.
Hirsh J, Poller L. The International Normalized Ratio. A Guide to Understanding and Correcting its
Problems. Arch Intern Med. 1994 Feb 14;154(3):282-8.
Hubbard AR, Margetts SM, Weller LJ, Macnab J, Barrowcliffe TW. An International Collaborative Study on the
INR Calibration of Freeze-Dried Reference Plasmas. Br J Haematol. 1999 Mar;104(3):455-60.
Levine M HJ, Landefeld, Raskob G. Hemorrhagic Complications of Anticoagulant Treatment. CHEST
1992;102:352s-63s.
Technical Assistance
For further information and technical assistance, call ITC Technical Support at 1-732-548-5700,
1-800-631
-
5945, or e-mail us at techsupport@itcmed.com.
Ordering Information
For further information on ordering and supplies, contact your ProTime distributor.
35

SAFETY STANDARDS
The ProTime instrument complies with the following safety standard requirements and directives:
CSA C22.2. 601.1. Medical Electrical Equipment –General Requirements for Safety
EN 60601-1/
UL/IEC 60601-1
EN 60601-1-2 /
IEC 60601-1-2
Medical Electrical Equipment – General Requirements for Safety
Medical Electrical Equipment – Part 1-2 – General Requirements
for Safety – Collateral Standard: Electromagnetic Compatibility –
Requirements and Tests
Directives: 89/336/EEC and as amended by 91/263/EEC, 92/31/EEC, 93/68/EEC and 98/13/EC, 98/79/EC
Equipment Classifications As Defined Per UL 60601-1:2003/ IEC60601-1 2nd Edition
•
Protection against electrical shock: Class II, Internally Powered Equipment with no applied parts
•
Protection against ingress of liquids: Ordinary (no protection as defined by IEC 529)
•
Product cleaning and disinfection: Only according to recommendations of the manufacturer’s
accompanying documentation
•
Mode of operation of equipment: Continuous
•
Degree of safety of application in the presence of flammable anesthetic mixture with air, oxygen or
nitrous oxide: Not Suitable
NOTE: As defined in the above standards, the classification of “Not Suitable” DOES NOT MEAN
that the instrument is not suitable for use in an Operating Room (OR) environment. Rather, it is
intended to indicate that the instrument is not suitable for use in the direct presence of a
flammable anesthetic mixture with air, oxygen or nitrous oxide.
All relevant documentation is kept on file at ITC.
36

Guidance and Manufacturer’s Declaration – Electromagnetic Emissions
The ProTime® Microcoagulation System is intended for use in the electromagnetic environment specified
below. The customer or the user of the ProTime® Microcoagulation System should assure that it is used in
such an environment.
Emissions Test Compliance Electromagnetic Environment - Guidance
RF Emissions
CISPR 11
Group 1 The ProTime
its internal function. Therefore, its RF emissions are very low
and are not likely to cause any interference in nearby electronic
®
Microcoagulation System uses RF energy only for
equipment.
RF Emissions
CISPR 11
Harmonic Emissions
Class B
Class A
The ProTime
establishments, including domestic establishments and those
directly connected to the public low-voltage power supply
network that supplies buildings used for domestic purposes.
®
Microcoagulation System is suitable for use in all
IEC 61000-3-2
Voltage Fluctuations/
Complies
Flicker Emissions
IEC 61000-3-3
37

Guidance and Manufacturer’s Declaration – Electromagnetic Immunity
The ProTime® Microcoagulation System is intended for use in the electromagnetic environment specified
below. The customer or the user of the ProTime® Microcoagulation System should assure that it is used in
such an environment.
Immunity Test
Electrostatic
Discharge (ESD)
IEC 61000-4-2
Electrical fast
transient / Burst
IEC 61000-4-4
Surge
IEC 6100-4-5
Voltage dips,
short
interruptions,
and voltage
variations on
power supply
input lines
IEC 61000-4-11
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
IEC 60601 Test
Level
+
6 kV contact
8 kV air
+
+
2 kV for
power supply
lines
1 kV for
+
input/output
lines
+1 kV
differential mode
2 kV common
+
mode
<5 % U
T
(>95 % dip in
for 0.5 cycle)
U
T
40 % U
T
(60 % dip in U
for 5 cycles
70 % U
T
(30 % dip in U
for 25 cycles
<5 % U
T
(>95 % dip in
U
) for 5 sec
T
3 A/m 3 A/m Power frequency magnetic fields should
Compliance
Level
+
6 kV contact
8 kV air
+
2 kV for
+
power supply
lines
1 kV for
+
input/output
lines
1 kV
+
differential mode
2 kV common
+
mode
<5 % UT
(>95 % dip in
for 0.5 cycle)
U
T
40 % U
(60 % dip in U
)
T
for 5 cycles
70 % U
(30 % dip in U
)
T
for 25 cycles
<5 % U
(>95 % dip in
U
) for 5 sec
T
Electromagnetic Environment Guidance
Floors should be wood, concrete or
ceramic tile. If floors are covered with
synthetic material, the relative humidity
should be at least 30 %.
Mains power quality should be that of a
typical domestic, commercial or hospital
environment.
Mains power quality should be that of a
typical domestic, commercial or hospital
environment.
Mains power quality should be that of a
typical domestic, commercial or hospital
environment.
T
T
T
If the user of the ProTime
Microcoagulation System requires
)
T
continued operations during power mains
interruptions, it is recommended that the
®
ProTime
powered from an uninterruptible power
)
T
supply or battery.
Microcoagulation System be
®
be at levels characteristic of a typical
location in a typical domestic, commercial
or hospital environment.
NOTE: UT is the AC mains voltage prior to application of the test level.
38

INDEX
attention label ...................................................4
battery care........................................................8
battery information ...........................................8
charging .....................................................10
battery replacement ..........................................8
blood coagulation systems ................................2
blood collection...............................................11
data history......................................................19
LQC records ...............................................20
patient records ...........................................19
print/send ..................................................21
finger incision preparation..............................11
insert a cuvette ................................................11
intended use......................................................2
introduction ......................................................2
ISI
setting.........................................................29
main menu .....................................................16
memory
clear ...........................................................30
month
setting.........................................................27
OID
entering and changing ...............................24
setting.........................................................25
password
changing.....................................................30
entering and changing ...............................24
performance characteristics
accuracy .....................................................33
comparisons ..............................................33
patient self-testing......................................33
precision ....................................................32
sensitivity ...................................................33
PID
entering and changing ...............................24
setting.........................................................25
preparation
contents .......................................................9
unpacking and inspection............................9
professional guidelines
reporting results.........................................31
programming mode ........................................27
quality control .................................................33
reporting..........................................................31
expected values ..........................................32
reportable range.........................................29
safety features
calibration....................................................5
cuvette disposal............................................7
limitations....................................................6
precautions ..................................................6
reagent preparation......................................7
reagents........................................................5
storage..........................................................7
service and maintenance
battery facts..................................................7
instrument disposal.....................................7
routine maintenance ...................................7
servicing .......................................................7
set up options..................................................22
beep ...........................................................26
day..............................................................23
hour ...........................................................23
language.....................................................22
minutes......................................................23
OID.............................................................24
password ....................................................24
PID .............................................................24
specifications.....................................................7
specimen collection ..........................................9
technical assistance.............................14, 34, 35
test methodology ...............................................2
test procedures................................................10
therapeutic range
setting.........................................................28
TR limits
setting.........................................................28
troubleshooting ...............................................14
year
setting.........................................................27
39

IR5246 1/08
 Loading...
Loading...