Page 1
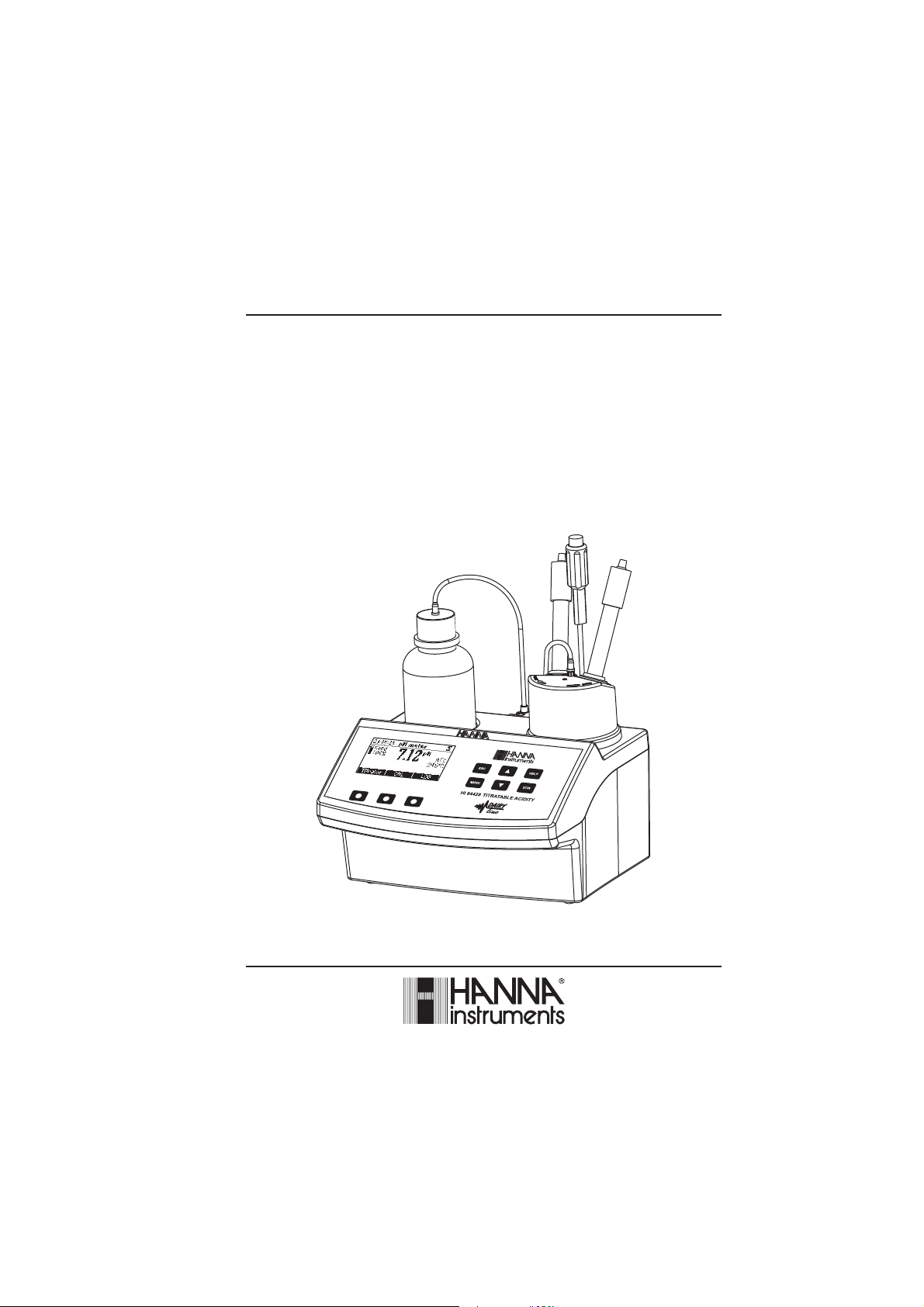
Instruction Manual
HI 84429
TITRATABLE ACIDITY
MINITITRATOR & pH METER
for Dairy Products
www.hannainst.com
1
Page 2

Dear Customer,
Thank you for choosing a Hanna product. This manual will provide you with the necessary
information for the correct use of the instrument. Please read it carefully before using the meter.
If you need additional technical information, do not hesitate to e-mail us at tech@hannainst.com.
TABLE OF CONTENTS
PRELIMINARY EXAMINATION................................................................................................. 3
GENERAL DESCRIPTION ........................................................................................................ 4
SPECIFICATIONS ................................................................................................................... 6
PRINCIPLE OF OPERATION .................................................................................................... 8
FUNCTIONAL DESCRIPTION................................................................................................... 9
TITRATOR STARTUP ........................................................................................................... 12
SETUP CONFIGURATION MENU .......................................................................................... 13
ELECTRODE PREPARATION .................................................................................................. 18
ELECTRODE CALIBRATION PROCEDURE................................................................................. 19
pH BUFFER TEMPERATURE DEPENDENCE ............................................................................ 23
PUMP TUBE INSTALLATION................................................................................................. 24
PURGE .............................................................................................................................. 24
PUMP CALIBRATION PROCEDURE ........................................................................................ 26
TITRATION PROCEDURE ..................................................................................................... 29
pH MEASUREMENT ............................................................................................................ 34
TEMPERATURE CALIBRATION PROCEDURE (for technical personnel only) ............................... 38
TROUBLESHOOTING GUIDE ................................................................................................ 40
ELECTRODE CONDITIONING & MAINTENANCE ...................................................................... 41
ACCESSORIES .....................................................................................................................42
WARRANTY........................................................................................................................43
All rights are reserved. Reproduction in whole or in part is prohibited without the written consent
of the copyright owner, Hanna Instruments Inc., Woonsocket, Rhode Island, 02895 , USA.
2
Page 3

PRELIMINARY EXAMINATION
Please examine this product carefully. Make sure that the instrument is not damaged. If any
damage occured during shipment, please notify your Dealer.
Each HI 84429 minititrator is supplied complete with:
• FC 260B pH electrode
• HI 5315 Reference electrode
• HI 7072 Filling solution (30 mL)
• HI 7662-M Temperature probe
• HI 84429-50 Titrant (100 mL)
• HI 84429-55 Standard (500 mL)
• HI 700640 Cleaning solution for milk deposits (2x20mL)
• pH 4.01 buffer solution (230 mL)
• pH 6.00 buffer solution (230 mL)
• pH 8.30 buffer solution (230 mL)
• Two 50 mL beakers
• Two 20 mL beakers
• Tube set with cap
• Stir bars (2 large)
• 12 Vdc power adapter
• One 1 mL syringe
• One capillary dropper pipette
• Instruction manual
Note: Save all packing material until you are sure that the instrument works correctly.
Any defective item must be returned in its original packing.
3
Page 4

GENERAL DESCRIPTION
The HI 84429 is an easy to use microprocessor-based automatic minititrator and pH meter designed
for the rapid and accurate analysis of Total Titratable Acidity in milk. By eliminating subjective
factors including color indicators, errors in mathematical calculations or erratic titrant additions from
the measurement, the HI 84429 makes Total Titratable acidity analysis precisely and totally
impartially. This will quickly become a valuable analysis tool in your dairy lab.
The instrument benefits from Hanna’s many years of experience as manufacturer of quality analytical
instruments. A clear and well-designed user interface makes the instrument intuitive and simple to
use. A dedicated HELP key aids in set-up, calibration, status and troubleshooting.
By simply pressing the Start key, the HI 84429 automatically starts pump operation and titrates the
sample to the end point. The HI 84429 has a simple and accurate peristaltic pump to ensure the best
accuracy and repeatability. By performing pump calibration with the Hanna standard provided, the
instrument accuracy is assured.
The instrument employs a powerful and effective built-in algorithm to analyze the pH response to
determine the exact pH endpoint, then uses this to make the necessary calculations. The Titratable
Acidity Determination is instantaneously display in user selected measurement units on the large dot
matrix display. The instrument is ready for the next analysis immediately!
Other features:
• Log on demand up to 100 samples (50 for pH measurement; 50 for titration results)
• GLP feature, to view last calibration data for pH electrode and pump
MEASUREMENT SIGNIFICANCE
Both pH and titratable acidity are used to measure milk acidity. A decrease in pH or increase in
titratable acidity indicates lactic fermentation has occurred, most likely due to bacterial activity. pH
and titratable acidity measurements, together with other tests available to the analyst, provide a
mechanism to ensure quality and freshness of the milk products.
The pH of milk (or other dairy products) is a measurement of the actual acidity of the milk at the time
of measurement. The measurement uses a pH electrode and pH meter that reads out directly in units
of pH (after calibrating the electrode and meter together using pH buffers). The pH of fresh milk is
slightly acidic typically falling between 6.50 to 6.70 pH at 25°C.
4
Page 5
Titratable acidity measures the total titratable acidity and differs from pH as it also includes the
buffering capacity of the milk constituents. Titratable acidity in dairy products, is determined by
titrating a sample with sodium hydroxide to a fixed endpoint pH (pH 7.00 or a phenolphtalein
endpoint of pH 8.30). The actual neutralization of milk occurs at an endpoint of pH 7.00, however,
standard methods utilize the phenolphtalein endpoint value. The results will differ depending upon
which endpoint is utilized. The endpoint can be determined visually using color change produced by
phenolphtalein indicator or less subjectively, using a pH electrode as the indicator in a potentiometric
acid-base titration.
Titratable acidity can be expressed in various units values:
Soxhlet Henkel degrees (°SH) - mostly used in Central Europe
This value is obtained by titrating 100 mL of milk with 0.25N NaOH, using phenolphtalein as
indicator.
Thorner degrees (°Th) - mostly used in Sweden and the CIS
This value is obtained by titrating 100 mL of milk, diluted with 2 parts of distilled water, with 0.1N
NaOH, using phenolphtalein as indicator.
Dornic degrees (°D
This value is obtained by titrating 100 mL of milk, diluted with 2 parts of distilled water, with N/9
NaOH, using phenolphtalein as indicator.
Percent lactic acid (%l.a.
This value is obtained in the same way as °D, dividing the result by 100.
))
) - mostly used in Netherlands and France
))
))
) - frequently used in the UK, USA, Canada, Australia and New Zeeland
))
The result can be easily converted into any of the other units as shown in the table below:
HS°hT°D°.a.l%
52.01.0111.01110.0
15.252.25220.0
4.019.0900.0
9/49/011 10.0
5
Page 6

SPECIFICATIONS
Titrator Range Titratable acidity (low range)
°SH: 0.0 to 15.0 °SH
°Th: 0 to 40 °Th
°D: 0 to 35 °D
%I.a.: 0.00 to 0.35 %I.a.
Titratable acidity (high range)
°SH: 10.0 to 75.0 °SH
°Th: 20 to 200 °Th
°D: 20 to 175 °D
%I.a.: 0.0 to 2.0 %I.a.
Resolution Titratable acidity (low range):
0.1 °SH
1 °Th
1 °D
0.01 %I.a.
Titratable acidity (high range):
0.5 °SH
1 °Th
1 °D
0.1 %I.a.
Accuracy 5% of reading
Titration method Acid-base titration
Principle End point titration, 8.30 pH
Pump debit 0.5 ml/min
Stirring speed 800 rpm
Log data Up to 50 samples
pH meter pH meter -2.0 to 16.0 pH / -2.00 to 16.00 pH
pH Resolution: 0.1 pH / 0.01 pH
pH Accuracy: ± 0.01 pH
pH Calibration: 1, 2 or 3 point calibration;
3 available buffers (4.01, 6.00, 8.30)
Temperature manual or automatic from
compensation: -20 to 120 °C (-4 to 248 °F)
Log data Up to 50 samples
6
Page 7

Temperature Range -20.0 to 120.0 °C (-4.0 to 248.0 °F)
Resolution 0.1 °C
Accuracy ±0.4 °C without probe error
Electrode FC 260B pH electrode (included)
HI 5315 reference electrode (included)
Temperature Probe HI 7662-M (included)
Environment 0 to 50 °C (32 to 122 °F); max 95% RH non-condensing
Power supply 12 Vdc power adapter
Dimensions 208 × 214 × 163 mm (8.2×8.4×6.4”) (with beaker)
Weight 2200 g (77 oz.)
REQUIRED REAGENTS
Code Description Quantity/Test
HI 84429-50 Titrant 0.5 mL
HI 84429-55 Standard 50 mL
7
Page 8

PRINCIPLE OF OPERATION
A neutralization titration is one where an acid and base react to form a neutral pH solution.
H++OH¯→H2O
A potentiometric acid-base titration employs an indicator electrode (such as a pH sensor) to establish
the equivalence point of the titration.
The accepted methodology for Titratable Acidity in dairy products employs a neutralization titration.
Acids (such as lactic acid) in the milk products react with a base (the titrant sodium hydroxide) to
produce water. When manually performed, the exact sample volume, titrant volume and titrant
concentration must be known. Additionally, the endpoint determination can be subjective when a
visual indicator (phenolphthalein) is utilized in an opaque or colored sample. Calculations are then
required. The entire procedure can be quite time consuming.
Titratable Acidity in dairy products, as performed on the HI 84429 minititrator, utilizes a simple
sample preparation, a high quality peristaltic dosing pump for titrant, potentiometric endpoint
detector and instantaneaous computations. To maintain the high precision of the titrator, a simple
pump calibration procedure is required. The calibration involves the analysis of a known volume of
a known solution (standard provided) and compensates for changes in pump dosing that may occur
due to many factors including tube stretching or aging. This procedure should be performed regularly.
8
Page 9
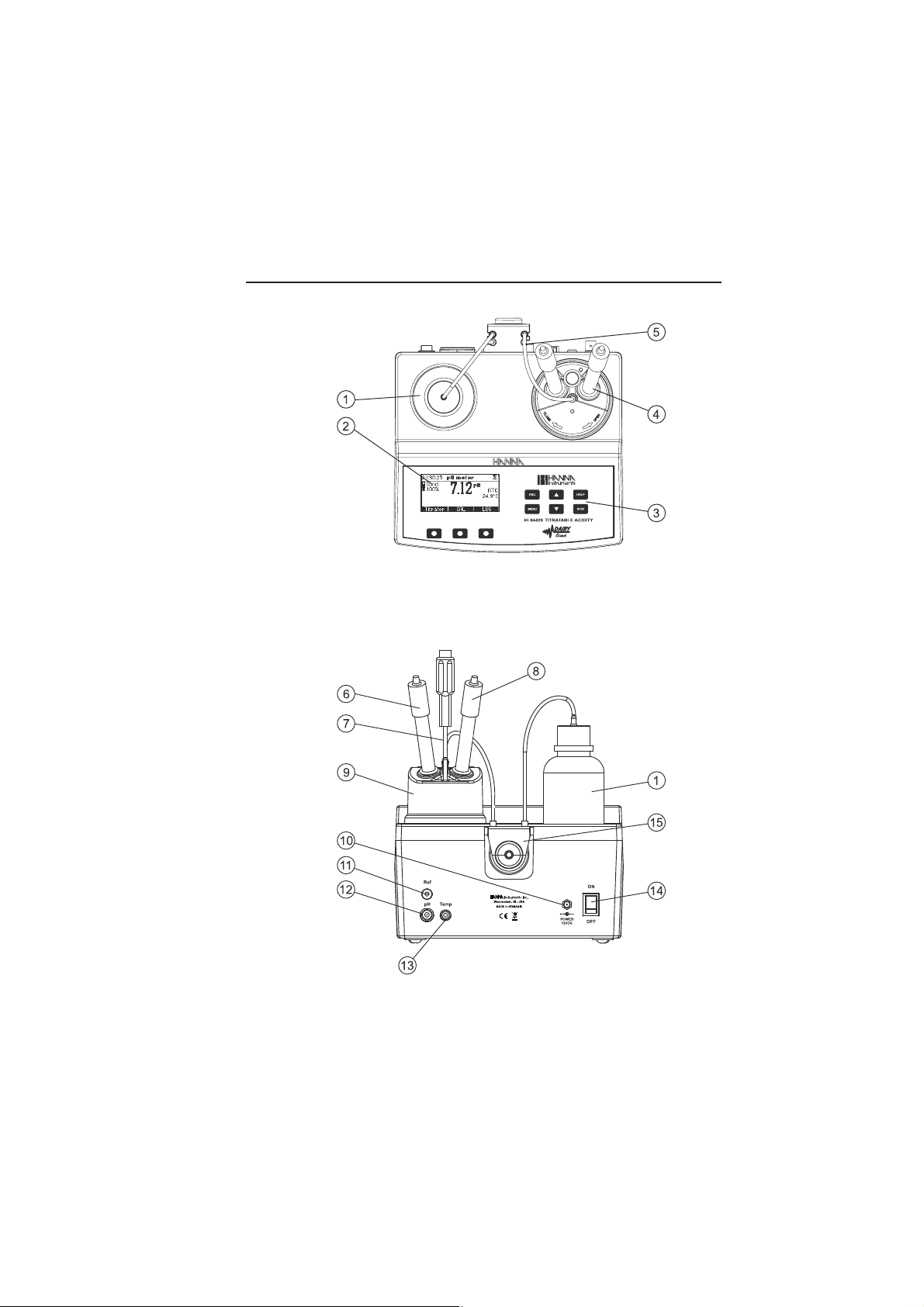
FUNCTIONAL DESCRIPTION
FRONT PANEL
1. Titrant bottle 3. Keypad 5. Peristaltic pump tubes
2. Graphic LCD 4. Electrode holder
REAR PANEL
6. pH Electrode 10. Power adapter connector 14. Power switch
7. Temperature probe 11. Reference electrode socket 15. Peristaltic pump
8. Reference electrode 12. BNC electrode connector
9. Beaker 13. Temperature probe socket
9
Page 10
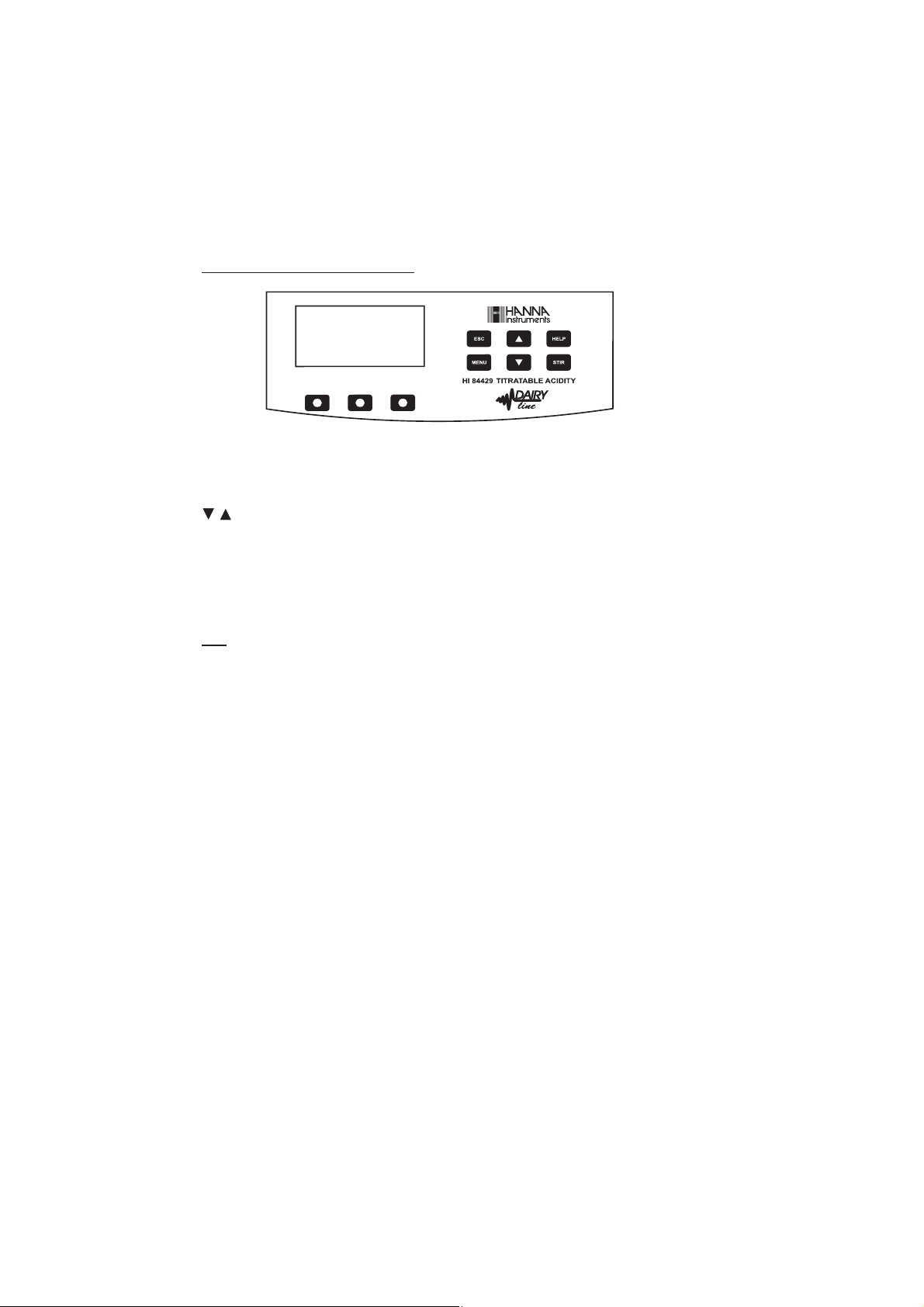
KEYPAD FUNCTION AND INDICATORS
ESC - used to leave the current screen and to return either to the previous screen or to the
main screen, depending on the context, while pressed in SETUP the new value of
the set parameter is not changed.
/
- used to modify a parameter’s value, to scroll the information displayed while
viewing a help or to move between the options from the instrument’s SETUP
HELP - used to access/leave the instrument’s contextual help
MENU - used to enter Setup, Recall or GLP selection menu, while instrument is in pH or
Titration main screen
STIR - used to start/stop the stirrer.
Note: The stirrer starts automatically during pump calibration and titration and cannot be
stopped by pressing STIR key.
10
Page 11

GUIDE TO INCDICATORS
During the instrument’s operation a set of information are displayed on the LCD.
Displayed icons:
Unstable reading.
Stirrer on.
1
2
3
4
5
Pump activity.
Parameter can be changed.
1. Current time and instrument mode information (pH meter or Titrator)
2. pH electrode condition information
3. Main reading information
4. Instrument status information
5. Functional key area
6. Indicates that the displayed value can be changed using ARROW keys
7. Temperature reading display
8. pH temperature compensation mode (manual or Automatic)
9. Stirrer and reading status area
9
8
7
6
11
Page 12

PERISTALTIC PUMP
Peristaltic pumps are self priming. Liquid never contacts the pump components. The titrant tubing
is pressed along the rotating rollers of the pump. The rollers compress the tubing drawing titrant
along and out the titrator tip.
TITRATOR STARTUP
• Place the instrument on a flat table. Do not place the instrument in direct sun light.
• Connect the power adapter to the instrument.
• Turn the instrument ON using the power switch from the rear panel of the instrument.
• Set up the instrument. See the “Setup Configuration Menu” section for details.
• Prepare the electrodes and attach pH sensor and reference electrode to the instrument.
• Calibrate the pH electrode. At least a single point calibration in 8.30 pH buffer is necessary
for titration.
• Place the peristaltic pump tube on the pump. See the “Pump Tube Replacement” section for
the procedure.
• Remove the reagent bottle cap and replace the bottle cap with the tubes. Place the reagent
bottle in the appropriate place on the titrator top.
• Connect the tubes with the peristaltic pump (inlet tube is connected with the reagent bottle,
outlet tube is connected with the dosing tip).
• Purge line.
• Calibrate the pump.
• Select Low Range in “Acidity Titration” setup parameter.
• Prepare the sample.
• Run a titration and log sample results.
12
Page 13

SETUP CONFIGURATION MENU
The titrator’s setup configuration menu may be accessed from the pH or titration screens by
pressing the MENU key, then Setup.
A list of setup parameters will be displayed with currently configured setting.
While in the setup menu it is possible to modify the instrument’s operation parameters. The
ARROW keys permit the user to scroll the setup parameters.
Press HELP to view the contextual help.
Press ESC to return to the main screen.
Concentration unit
Option: °SH, °Th, °D, % l.a.
Press the corresponding function key to change the
option.
Acidity titration
Option: Low Range, High Range.
From your knowledge of expected concentrations,
use the table below to determine which settings are
appropriate.
Press the corresponding function key to select the
new option.
Note: The milk sample size will change with these settings:
TINUegnaRwoL
HS°0.51ot0.00.57ot0.01
HT°04ot0002ot02
D°53ot0571ot02
.a.l
%53.0ot00.00.2ot0.0
)elpmasklimLm05(
egnaRhgiH
)elpmasklimLm01(
Select Low Range for dairy products in the 0 to 15 °SH range. Select High Range for dairy
product in the 10 to 75 °SH range. The titrant solution remains the same for either selection.
13
Page 14
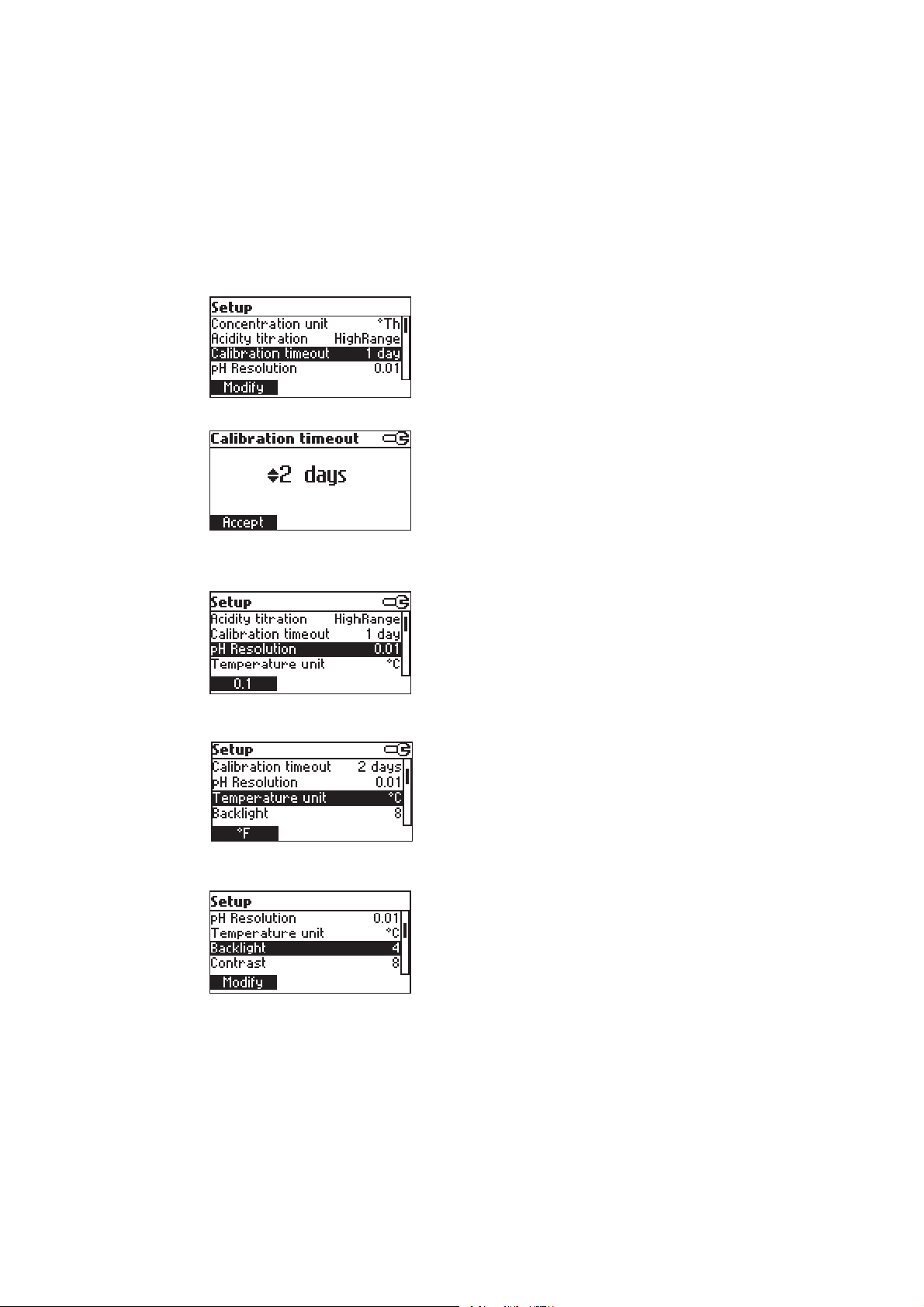
Calibration timeout
pH resolution
Option: Disabled or 1 to 7 days.
This option is used to set the number of days
before the pH calibration expired warning message
is flagged.
Press Modify to access the calibration timeout
value modify parameter.
Use the ARROW keys in order to increase/decrease
the value.
Press Accept to confirm or ESC to return to the
setup menu without saving the new value.
Option: 0.1 or 0.01.
Press the displayed function key in order to change
the option.
Temperature unit
Backlight
Option: °C or °F.
Press the function key in order to change the option.
Option: 0 to 8.
Press Modify to access the backlight value.
14
Page 15

Contrast
Use the ARROW keys or / in order to
increase/decrease the displayed constrast.
Press Accept to confirm or ESC to return to the
setup menu.
Option: 0 to 20.
This option is used to set the display’s contrast.
Press Modify to change the display’s contrast.
Date / Time
Use the ARROW keys or
increase/decrease the value.
Press Accept to confirm the value or ESC to return
to the setup menu.
This option is used to set the instrument’s date and
time.
Press Modify to change the date/time.
Press / to highlight the value to be modified
(year, month, day, hour, minute or second). Use
the ARROW keys to change the value.
Press Accept to confirm the new value or ESC to
return to the setup.
/ in order to
15
Page 16

Time format
Date format
Language
Option: AM/PM or 24 hours.
Press the functional key to select the new value.
Press Modify to change the Date Format.
Use the ARROW keys to select the desired format.
Press Accept to confirm the value or ESC to return
to the setup menu.
Press the corresponding function key to change the
option.
If the new selected language cannot be loaded, the
previously selected language will be reloaded.
If no language can be loaded the instrument will work in the “safe mode”. In “safe mode” all
the messages are displayed in English and tutorial and help information are not available.
Tutorial
This option is used to enable/disable tutorial mode.
If enabled this option will provide the user short
guides on the screen.
Press the function key to select this option.
16
Page 17

Beep On
Meter information
Press the function key to select the new option.
When enabled, a short beep is heard every time a
key is pressed or when the calibration can be
confirmed.
A long beep alert sounds when the pressed key is
not active or a wrong condition is detected while in
calibration.
Press Select to view the firmware version, language
version, mV factory calibration date and time and
temperature factory calibration date and time.
Press ESC to return to the Setup mode.
17
Page 18

ELECTRODE PREPARATION
PREPARATION PROCEDURE
Remove the electrode protective cap.
DO NOT BE ALARMED IF ANY SALT DEPOSITS ARE PRESENT. This is normal with electrodes and
they will disappear when rinsed with water.
During transport tiny bubbles of air may have formed inside the glass bulb. The electrode cannot
function properly under these conditions. These bubbles can be removed by "shaking down" the
electrode as you would do with a glass thermometer.
If the bulb is dry, soak the electrode in HI 70300 Storage Solution for at least one hour.
Preparation of reference electrode:
• Unwrap ParafilmTM seal found over ceramic junction
on inner stern of the reference electrod and discard.
This is only used for shipping.
• Rinse inner stern with deionized water making cer-
tain to wet o-ring found on the inner stern.
• Reassemble reference electrode by gently pushing
the inner assembly into the outer body, sliding spring
down cable, and screwing cap into place.
• Remove fill hole cover and o-ring or fill hole spout.
• Using the dropper pipette provided, add a few drops
of HI 7072 filling solution to the reference electrode,
wetting the o-ring and rinsing out the fill solution
chamber.
• Holding the body of the electrode depress the black cap with your thumb. This permits the
fill solution to drain out of the body. Verify if the electrode returns to its original position.
(You may need to gently assist for this to occur).
• Tighten the electrode cap onto the body and fill electrode body with HI 7072 filling solution
until fill solution volume is just below fill hole.
Note: During measurement always operate reference electrode with the fill hole open.
MEASUREMENT
Place pH electrode and reference electrode into electrode holder and connect the Cable
Connectors to the instrument.
Rinse the pH and reference electrodes tip with distilled or deionized water. Immerse the pH and
reference electrodes (bottom 4 cm /1½”) in the sample and stir gently for a few seconds.
For a faster response and to avoid cross-contamination of the samples, rinse the electrode tip
with a few drops of the solution to be tested, before taking measurements.
18
Page 19

ELECTRODE CALIBRATION PROCEDURE
It is recommended to calibrate the instrument frequently, especially if high accuracy is required.
The pH range should be recalibrated:
a) Whenever the pH electrode is replaced
b) At least once a week
c) After testing aggresive chemicals and after electrode is cleaned
d) When extreme accuracy is required
e) If the pH calibration expired warning is displayed during measurement. Every time
you calibrate the instrument use fresh buffers and perform an electrode Cleaning Procedure (see
page 41).
PROCEDURE
A single, two or three-point calibration can be performed, using the 3 buffers 4.01, 6.00 and
8.30 pH. For a single point calibration any of the three buffers may be used, but using 8.30 pH
is advised.
Note: The HI 84429 will not accept other pH buffers for calibration.
• Pour small quantities of selected buffer solutions into clean beakers. For accurate calibration
use two beakers for each buffer solution, the first one for rinsing the electrode and the second
one for calibration.
• Put a magnetic stir bar in each beaker with the calibration buffer solution.
• Remove the protective cap and rinse the electrodes with some of the buffer solution to be
used for the first calibration point.
• Put the first beaker with calibration buffer in the beaker holder.
• Place the electrode holder on the top of the beaker and secure it by turning clockwise.
• Immerse the pH and reference electrodes and the temperature probe approximately 2 cm
(0.8”) into the buffer paying attention not to touch the stir bar.
To select Electrode calibration screen follow the next steps:
• From pH meter screen press CAL function key then Electrode.
• From Titrator screen press CAL function key then Electrode.
19
Page 20

THREE POINT CALIBRATION
• From the pH meter/titrator calibration menu screen select the Electrode calibration option.
The electrode calibration screen will be displayed.
• The 6.00 buffer will be selected by default. If necessary press the ARROW keys in order to
select a different buffer value.
• The
• When the reading is stable and close to the selected buffer, the
• Press CFM to confirm the calibration.
• The calibrated value will be shown on the display and the second expected buffer value will
(unstable measurement) symbol will be shown on the display until the reading
becomes stable.
(unstable measurement)
symbol will disappear and the CFM key will become active.
be displayed.
• After the first calibration point has been confirmed, press STIR to stop stirring.
• Remove the electrode holder with electrodes from the top of the beaker.
• Place the second buffer into beaker and place in beaker holder. Rinse the electrodes in a
beaker containing the second buffer rinsing solution.
• Place the electrode holder (with electrodes) on the top of the beaker, lock cap by turning and
press STIR.
• If necessary press the ARROW keys in order to select a different buffer value.
• The
(unstable measurement) symbol will be shown on the display until the reading
becomes stable.
20
Page 21

• When the reading is stable and close to the selected buffer, the
symbol will disappear and the CFM key will become active.
• Press CFM to confirm the calibration.
• The calibrated value will be shown on the display and the third expected buffer value will be
automatically selected.
• After the second calibration point has been confirmed, press STIR to stop stirring.
• Remove the electrode holder with electrodes from the top of the beaker.
• Place the third buffer solution in a beaker and place in beaker holder. Rinse the probes in a
beaker with third buffer rinsing solution.
• Place the electrode holder (with electrodes) in the beaker with third buffer and secure top by
locking. Press STIR.
• If necessary press the ARROW keys in order to select correct buffer value.
• The
• When the reading is stable and close to the selected buffer, the
• Press CFM to confirm the calibration. The instrument stores the calibration value and returns
Notes:
• A buffer confirmed during the calibration process is removed from the list of calibration buffers
• If the value measured by the instrument is not close to the selected buffer a “Wrong Buffer”
(unstable measurement) symbol will be shown on the display until the reading
becomes stable.
symbol will disappear and the CFM key will become active.
to pH meter/titrator calibration menu, where the date and time for the last pH will be
updated.
available for further calibration points.
error message will be shown on the display.
(unstable measurement)
(unstable measurement)
Check if the correct buffer has been used or regenerate the pH electrodes by following the
Cleaning Procedure (see page 41). If necessary change the buffer or the electrode or pop the
reference junction.
• If the measured offset isn’t within the preset limits (±45mV) the meter will display the
message “Buffer Contaminated” alternatively with ”Electrode Dirty/Broken”.
21
Page 22

• If the computed slope isn’t within the preset limits the meter will display the message
“Wrong Slope”. If the slope is too high the symbol
too low the symbol
• If the “Wrong Old Slope” error message is displayed, an inconsistency exists between the
current and the previous (old) calibration. Clear the calibration parameters by pressing Clear
and proceed with calibration from the current calibration point. The instrument will keep all
the confirmed values during the current calibration point.
• If the temperature reading is out of the defined temperature range of the buffer (0÷45 °C)
the “Wrong Buffer Temperature” error message will be displayed, and the symbol °C will
blink on the dsplay. Calibration cannot be confirmed in this situation.
will be displayed.
will be displayed. If the slope is
22
Page 23

Notes: • To clear a previous calibration and to return to the default value, press Clear at any
time after entering calibration mode. The “Calibration cleared” message will be
shown for a few seconds on the display. If Clear is invoked during the first
calibration point the instrument returns to the measurement mode.
• The Clear key is displayed only if a previous calibration exists.
pH BUFFER TEMPERATURE DEPENDENCE
The temperature has an effect on pH. The calibration buffer solutions are affected by temperature
changes to a lesser degree than normal solutions. During calibration the instrument will
automatically calibrate to the pH value corresponding to the measured or set temperature.
PMETSREFFUBHp
CºFº10.400.603.8
023 10.421.684.8
514 00.490.644.8
0105 00.460.614.8
5195 00.440.673.8
0286 00.420.633.8
5277 10.400.603.8
368 20.499.572.8
0
5359 30.489.542.8
04401 40.479.512.8
During calibration the instrument will display the pH buffer value at 25 ºC.
23
Page 24

PUMP TUBE INSTALLATION
To mount the new peristaltic pump tube follow next steps:
• Position one peristaltic pump fixing ring on its location.
• Stretch the tube over the peristaltic pump cylinders.
• Fix the second pump fixing ring on its location.
• Attach the tube to the reagent bottle and to the dosing
tip.
Note: Purge the peristaltic pump until drops of reagent appears on the dosing tip by pressing the
Purge key from the titrator main screen.
To remove the tube of the peristaltic pump follow next steps:
Caution: Purge line with water to remove NaOH from tube.
• Detach the old tube system from the reagent bottle and from the dosing tip.
• Grasp one fixing ring of the peristaltic pump tube.
• Pull the tube until the fixing rings are taken out from their location.
• Remove the other side of the tube.
PURGE
Purging should be performed whenever the tube of the peristaltic pump is replaced or before
starting a pump calibration or a titration.
In order to start purging press the Purge key from the titrator main screen. The purging stops
automatically after 5 minutes.
To access the Purge key follow the next steps.
• From the instrument main screen (pH meter screen) press Titrator function key.
The instrument will display the next screen if any of the following conditions exist:
- the meter hasn’t been calibrated in 8.30 pH buffer
- the pH calibration has expired
- a pump calibration hasn’t been performed or more than 3 days have passed since the last
pump calibration
24
Page 25

Press CAL to access the titration calibration menu where electrode and pump calibration may
be accessed.
Press HELP to view the contextual help.
• Press Continue or ESC to skip the message and enter Titrator main screen.
• Press Purge to begin a purge cycle.
The purging stops automatically after 5 minutes.
To stop purging at any time and return to the main screen press ESC or Stop.
During a purge, the remaining time until the purge process will be completed is shown on the
lower right side of the display.
Press Pause to interrupt the purge process. on the lower right side of the display
Press Pause or Stop (by pressing the corresponding function key in the purge screen)
• after the first drops of fresh titrant appear at the dosing tip
• in case of error conditions (empty titrant, bottle, tubes or dosing tip disconected, pump error)
• if you want to resume at a later time
If Pause is pressed the next screen is displayed:
Press Resume to continue purging.
25
Page 26

After the 5 minutes purging interval has elapsed the “Completed” message is displayed.
Another purge period can be initiated by pressing Restart.
PUMP CALIBRATION PROCEDURE
The calibration of the pump must be performed each time the pump tube, the reagent bottle or
the pH electrode is changed. It is recommended to perform the pump calibration before each set
of measurements.
Verify: The electrode has been calibrated in 8.30 pH buffer.
• Sample preparation: Fill a beaker up to the 50 mL mark
with HI 84429-55 Standard. Place the stir bar into the
beaker and then place the beaker in the appropiate place on
the instrument top.
• Place the electrode holder on the top of the beaker and secure
it by turning clockwise.
• Immerse the pH, reference and the temperature electrodes
approximatively 2 cm (0.8”) into the sample to be tested
paying attention not to touch the stir bar.
• Insert the dosing tip in the appropriate holder place. Do not
immerse tip into solution.
26
Page 27

• From the titrator main screen press CAL.
The instrument displays the date and time of the last electrode calibration, and the date and
time of the last pump calibration, or calibration expired messages.
• Press Pump.
The next screen will be displayed.
• Press Start.
• After the pump calibration is started, on the upper right side of the display two animations
will be shown in order to indicate that the pump and the stirrer are working. On the lower
right side of the display is shown the amount of time that has passed since the begining of
the calibration.
27
Page 28

• After the pump calibration is complete a confirmation message is displayed for a few seconds,
then the instrument will return to the titrator calibration menu and will display the new time
and date for the last pump calibration.
Notes: • The calibration of the pump is independent of the selected range and concentration unit.
• If an erroneous situation is encountered during the calibration, an error message is
displayed and the calibration can be restarted by pressing Restart.
• If the calibration doesn’t complete within 6 minutes the error message “Too much
standard” will be displayed and the calibration can be restarted by pressing Restart
after a new sample is prepared.
28
Page 29
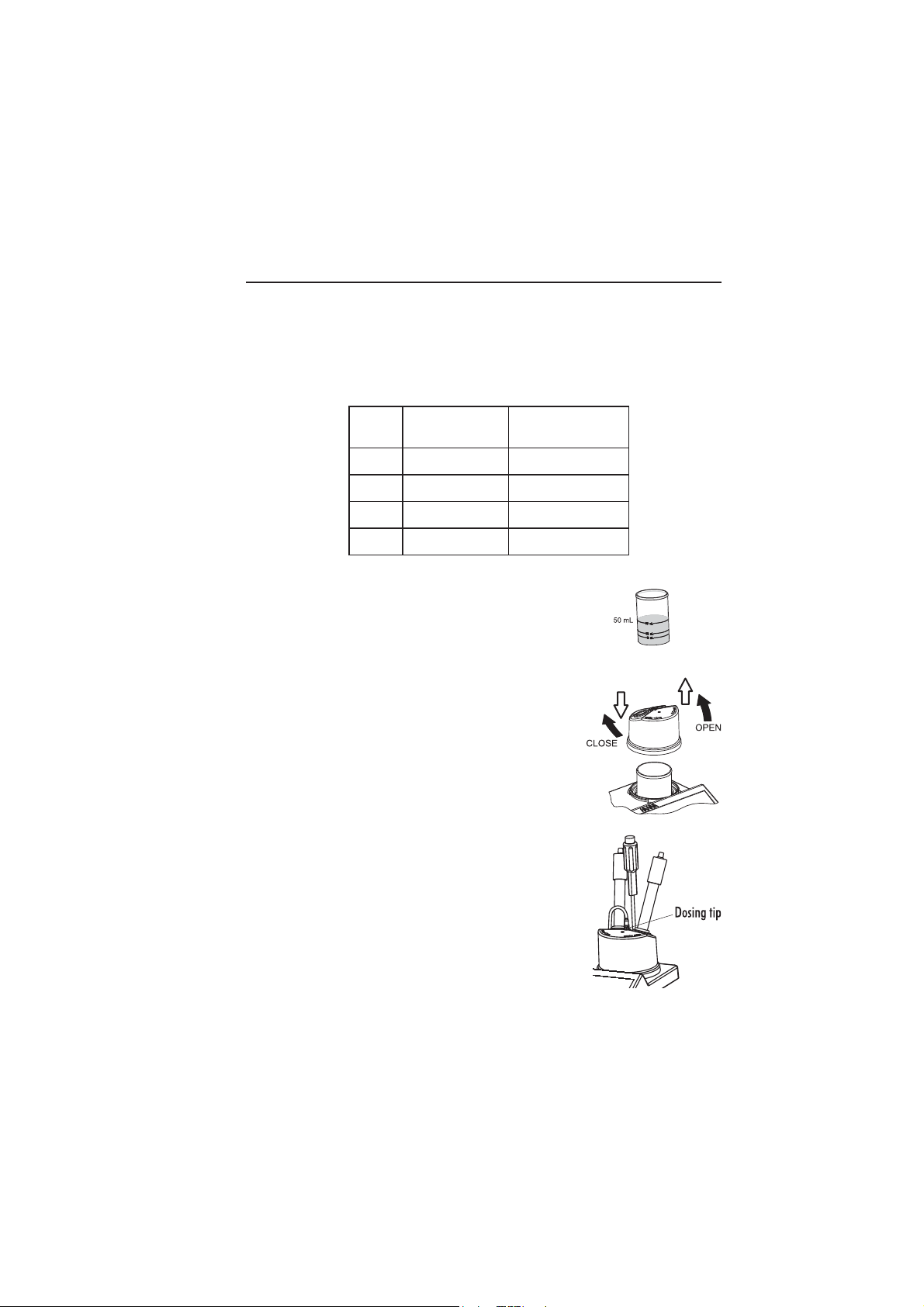
TITRATION PROCEDURE
Verify: The instrument has been calibrated (pH and pump) before performing a titration. An
electrode calibration in 8.30 pH buffer is recommended.
• Refer to Setup Configuration Menu (see page 13) to set up instrument for your measurement.
• Select the corresponding Low Range or High Range according to the table bellow.
TINUegnaRwoL
HS°0.51ot0.00.57ot0.01
HT°04ot0002ot02
D°53ot0571ot02
%53.0ot00.00.2ot0.0
.a.l
)elpmasklimLm05(
• Sample preparation: For Low Range measurement fill a
beaker up to the 50 mL mark with sample. For High Range
measurement use the 20 mL beaker to measure 10 mL of
sample. Put the sample in the 50 mL beaker. Fill the beaker
up to 50 mL with deionized water. Place the stir bar into the
beaker and then place the beaker in the appropiate place on
the instrument top.
• Place the electrode holder on the top of the beaker and secure
it by turning clockwise.
• Immerse the pH, reference and the temperature electrodes
approximatively 2 cm (0.8”) into the sample to be tested
paying attention not to touch the stir bar. Use O-Rings
provided to secure electrodes in holder.
egnaRhgiH
)elpmasklimLm01(
• Insert the dosing tip in the appropriate holder place. Do not
immerse tip into solution.
29
Page 30
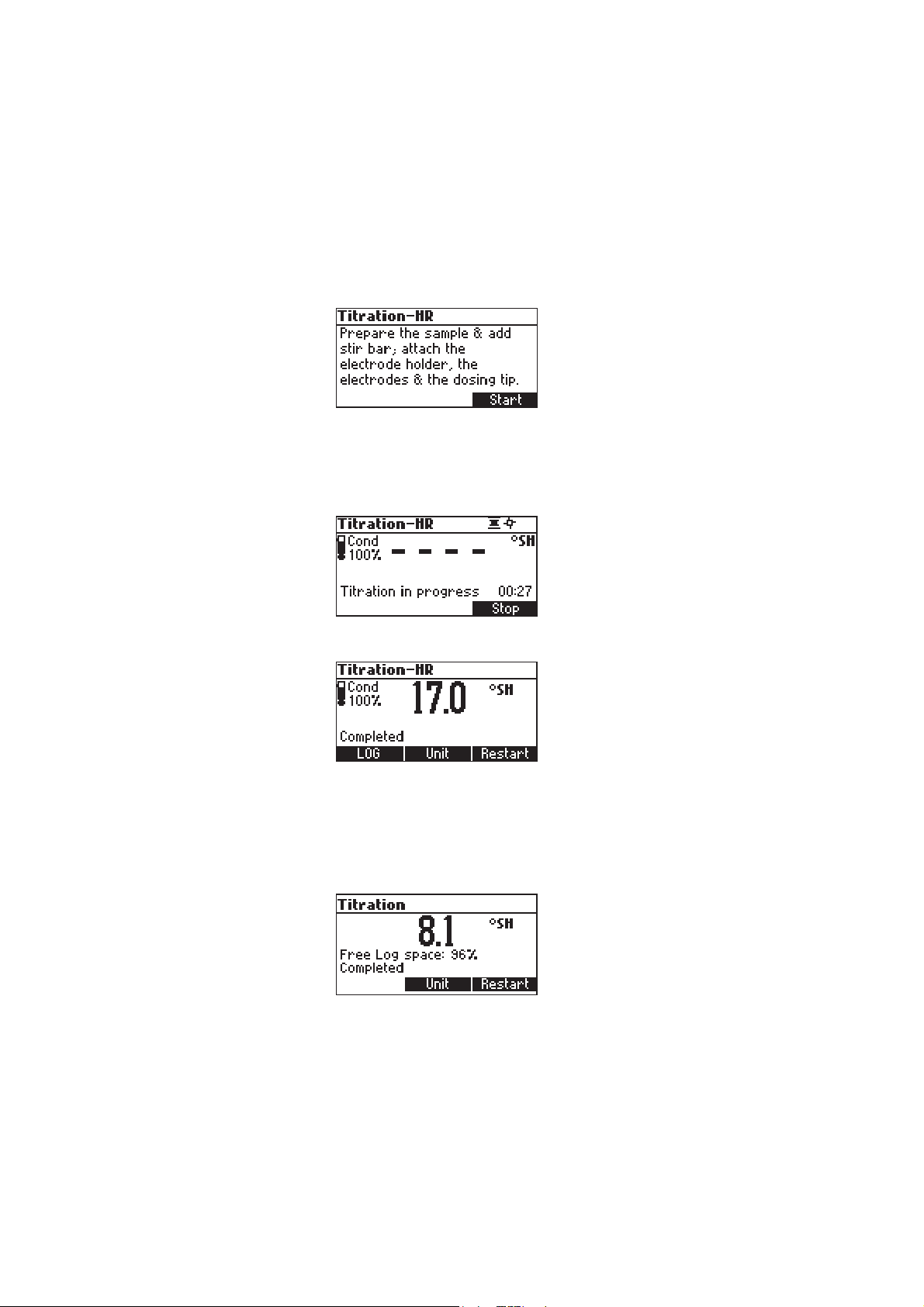
• From the titrator main screen press Titration. To enter titrator main screen from pH meter
mode press Titrator and then Continue.
• Press Start to begin the titration process.
• After the titration is started on the upper right side of the display two animations will be
shown in order to indicate that the pump and the stirrer are running. On the lower right side
of the display is shown the period of time since the titration has been started.
• After the titration is complete, the concentration value is displayed in the selected unit.
• Press Unit to change the display unit.
• Press LOG to record the concentration value into the instrument’s memory.
A message will be dislayed for a few seconds indicating the amount of the free log space. Up
to 50 log samples can be recorded in the instrument’s memory. When the free titrator log
space is under 12% the message will be shown permanently.
30
Page 31

• If the concentration is out of limits (15 SH for Low Range, 10 or 75 SH for High Range - see
page 6 for range limits) the exceeded range limit will be displayed blinking and the
message “Out of range” will be shown. Another titration can be initiated by pressing Restart.
Note: If the end-point is not reached or it is not recognized or the input reading is out of 3.00
to 8.30 pH range, an error message will be displayed. The titration can be restarted after
a new sample is prepared by pressing Restart.
TIPS FOR AN ACCURATE MEASUREMENT
• Calibrate the instrument in 8.30 pH buffer solution at least once a day, before you start to
perfom measurements.
• Purge the peristaltic pump to have the fresh titrant when starting a new analysis or
calibration.
• Calibrate the peristaltic pump daily before performing an analysis.
• Clean the electrode and the reference in order to remove the possible coating from bulb.
VIEW/DELETE MEMORIZED SAMPLES
Press MENU key while in Titrator main screen.
Press Recall to access the titrator memorized data.
31
Page 32

The instrument will display a list of all the titration records stored in the titration log.
Use the ARROW keys to scroll the stored records list.
If the saved concentration was out of range the “!” symbol is displayed in front of the reading.
Press Delete to enter record deleting mode.
Press Delete All to enter all records deleting mode.
Press More to view more information.
Press Unit to convert the result to other unit.
Use the ARROW keys when
Press ESC to return to the previous screen.
If Delete was pressed the instrument will ask for confirmation.
is displayed to scroll between the log records.
Use the ARROW keys to focus on the record to be deleted.
Press CFM to delete the record or ESC to return to the previous screen.
Deleting a record will reorganise the list of records.
If Delete All was pressed the instrument will ask for confirmation.
Press CFM to delete all the records or ESC to return to the previous screen.
32
Page 33

If the titrator log is empty the message “No Records!” will be displayed.
TITRATOR GLP INFORMATION
Press MENU while in Titration mode and then GLP.
From this screen it is possible to select between viewing the electrode GLP or the pump GLP.
Press GLP elec. to view the electrode’s last calibration parameters and date.
Press GLP pump to view the pump’s last calibration time and date.
If GLP elec. is pressed one of the next screens will be displayed.
GLP contains a set of information regarding electrode calibration. The following items are
included in electrode GLP: the time and date of the last calibration, offset, slope, electrode
condition, calibration timeout and the calibration buffers. The buffers displayed in video inverse
mode are from the previous calibration.
If a calibration hasn’t been performed the message “Not Calibrated” will be displayed.
33
Page 34

If GLP pump is pressed, one of the next screens is displayed.
The pump GLP displays the Time and Date of the last pump calibration.
If a calibration hasn’t been performed the message “Not Calibrated” will be displayed.
pH MEASUREMENT
The HI 84429 may be used as a pH meter for direct measurements.
Verify that the instrument has been calibrated before taking pH measurements. Set the
instrument to pH meter. At power up the instrument enters pH meter mode. From titrator mode
press ESC until pH units are displayed.
If an electrode calibration hasn’t been performed, or the number of days since the last pH
calibration is greater than the number of days after wich a calibration time-out warning is
displayed the message “CAL DUE” will blink on the left side of the display (see Calibration
timeout option in Setup for details).
If CAL DUE is displayed perform an electrode calibration.
Press MENU to access the instrument’s menu.
Press HELP to view the contextual help, every time you need suplimentary information. The help
is customized for every situation that can appear during instrument usage.
Press STIR to start/stop the stirrer.
Press Titrator to enter titrator mode.
Press CAL to access the calibration menu.
34
Page 35

Press LOG to memorize the current reading. A message indicating the free log space will be
displayed for a few seconds.
Up to 50 log samples can be saved into the instrument’s memory. When the free pH log space
is under 12% a message will be permanently shown on the display, indicating the remaining
free log space.
In order to take pH measurements follow the next steps:
• Submerge the tip of the electrode (4 cm/1 1/2’’) and the temperature probe into the sample
to be tested and stir gently. Allow time for the electrode to stabilize. When the reading
becomes stable the
• If the pH reading is out of range (-2.00 to 16.00 pH) the closest full-scale value (-2.00 pH
or 16.00 pH) will be displayed blinking.
If measurements are taken successively in different samples, it is recommended to rinse the
electrodes thoroughly with deionized water or tap water and then with some of the next sample
to prevent cross-contamination.
The pH reading is affected by temperature. In order to have accurate pH measurements, the
temperature effect must be compensated for. To use the Automatic Temperature Compensation
(ATC) feature, connect and submerge the HI 7662-M temperature probe into the sample as
close as possible to the electrode and wait for a few seconds. The “ATC” message will be shown
on the display. Automatic Temperature Compensation will provide pH corrected values at the
temperature of measurements. If Manual Temperature Compensation (MTC) is desired, the
temperature probe must be disconnected from the instrument. The default temperature of 25 °C
(77 °F) or the last temperature reading will be displayed preceded by the symbol
“MTC” message.
(unstable measurement) symbol will disappear.
and the
The manually set temperature can now be adjusted with the ARROW keys (from -20.0 to 120.0 °C).
35
Page 36

VIEW/DELETE pH MEMORIZED SAMPLES
Press MENU key while in pH meter screen.
Press Recall to access the pH recall.
A list of records is stored in the pH log.
Use the ARROW keys to scroll the list of records.
Press More to see detailed information about focused record.
Press Delete to enter record deleting mode.
Press Delete All to enter all records deleting mode.
If More is pressed a complete set of data is displayed.
Use ARROW keys when
If Delete was pressed the instrument will ask for confirmation.
Use the ARROW keys to focus on the record to be deleted.
Press CFM to delete the record or ESC to return to the previous screen without deleting.
Deleting a record will reorganize the list of records.
is displayed to scroll between the records.
36
Page 37

If Delete All was pressed the instrument will ask for confirmation.
Press CFM to delete all records or ESC to return to the previous screen without deleting.
If the pH log is empty the message “No Records!” will be displayed.
pH METER GLP INFORMATION
The pH meter GLP information refers to the pH calibration date.
To view this information press MENU key while in pH meter mode then GLP.
A set of information regarding electrode calibration is displayed.
The following items are included in electrode GLP: the time and date of the last calibration,
offset, slope, electrode condition, calibration timeout and the calibration buffers. The buffers
displayed in video inverse mode are from the previous calibration.
If a calibration hasn’t been performed the message “Not Calibrated” will be displayed.
37
Page 38

TEMPERATURE CALIBRATION PROCEDURE (for technical personnel only)
All the instruments are factory calibrated for temperature.
HANNA’s temperature probes are interchangeable and no temperature calibration is needed when
they are replaced.
If the temperature measurements are inaccurate, temperature recalibration should be performed.
For an accurate recalibration, contact your dealer or the nearest HANNA Customer Service Center,
or follow the instructions bellow.
• Prepare a vessel containing ice and water and another one containing hot water (at a
temperature of around 50 °C). Place insulation material around the vessels to minimize
temperature changes.
• Use a calibrated thermometer with a resolution of 0.1 °C as a reference.
• To enter user calibration screen press and hold down the ARROW keys simultaneously, then
power on the instrument. After a few seconds the User calibration screen is displayed.
• Press Temp function key to enter temperature calibration.
• Immerse the temperature probe in the vessel with ice and water as near as possible to the
reference thermometer. Allow a few seconds for the probe to stabilize.
• Use the ARROW keys to set the calibration point value to that of the ice and water measured
by the reference thermometer.
• The
• When the reading is stable and close to the selected calibration point, the
(unstable measurement) symbol will be shown on the display until the reading
becomes stable.
(unstable
measurement) symbol will disappear and the CFM key will become active.
• Press CFM to confirm the calibration point.
• The meter will be automatically move to the second calibration point, and will display 50 °C
for the buffer value.
38
Page 39

• Immerse the temperature probe in the second vessel as near as possible to the reference
thermometer. Allow a few seconds for the probe to stabilize.
• Use the ARROW keys to set the calibration point value to that of the hot water, measured by
the reference thermometer.
• The
• When the reading is stable and close to the selected calibration point, the
• Press CFM to confirm the calibration point. The instrument will return to the pH meter/titrator
Note: If the reading is not close to the selected calibration point, the “Wrong” message will be
(unstable measurement) symbol will be shown on the display until the reading
becomes stable.
(unstable
measurement) symbol will disappear and the CFM key will become active.
main screen.
displayed. Change the temperature probe and restart the calibration.
If the temperature probe is disconnected or the measured temperature is out of the - 20 to120 °C
range the instrument will display “----”. The calibration point value can be changed using the
ARROW keys.
39
Page 40

TROUBLESHOOTING GUIDE
SMOTPMYSSMOTPMYS
SMOTPMYSSMOTPMYSMELBORPMELBORP
SMOTPMYS
.tfird
culfgnidaeR
Hp
.gniknilb
acpmupehT
egnaR tnemurtsnieht
.gniknilb
egnaR tnemurtsnieht
etceles
evissecxe/esnopserwolS
pusetaut
.)esion(nwoddna
gnidaerHpnielihW
00.61ro00.2-,edom
deyalpsidsi
tonseodretemehT
reffubHpehttpecca
.noitarbilacrofnoitulos
noitarbil
demrofrepebt'nac
eborperutarepmetehT
ehttub,detcennocsi
."CTM"syalpsidretem
ninoitartitaretfA woL
roHS°51syalpsid
roD°53roHT°04
gnidrocca(.a.I%53.0
detcelesehthtiw
)tinu
ninoitartitaretfA hgiH
roHS°01syalpsid
D°02roHT°02
ehthtiwgnidrocca(
.gniknilb)tinud
.)ylno
.eborp
.egnar
.egnar
MELBORPMELBORPNOITULOSNOITULOS
MELBORP
1607IH ro 1608IH
.edortcele
.edortcele
.elpmas
.elpmas
ecnereferroHpytriD
.noitcnujytrid/deggolC
leveletylortcelewoL
sedortceleHpelballifer(
.egnarfotuognidaeR
ecnereferroHpnekorB
(
.dradnatsgnorW
.sedortcelenekorB
erutarepmetnekorB
.sedortcelenekorB
tontnemurtsnI
gnorW.detarbilac
fotuonoitartnecnoC
.sedortcelenekorB
tontnemurtsnI
gnorW.detarbilac
fotuonoitartnecnoC
alpeR
.eborp
pmas
NOITULOSNOITULOS
NOITULOS
pitedortceleehtkaoS
m03rofnoitulos
setuni
gninaelcehtwollofdna
ehtpoP.erudecorp
dnaedortceleecnerefer
.etylortcelehtiwllifer
dortceleehtnaelC
dnase
htiwllifeR.ecnerefereht
.noitulosllifhserf
ehtfoytilauqehtkcehC
ehtnaelC.elpmas
ehtdnasedortcele
htiwllifeR.ecnerefer
noitulosllifhserf
.
edortceleehtwolloF
fI.erudecorpgninaelc
ecalperstsisreprorreeht
.rodneveht
sedortceleeht
.)nekorb/ytrid
rbilaceht
tcatnocroedortceleeht
rodradnatsehtkcehC
ehtegnahc/naelC
.yrasseccenfisedortcele
rehtonaeraperP
evahotegrup,dradnats
tratserdnatnartithserf
.noita
erutarepmetec
ehtnaelc/kcehC
etarbilaceR.sedortcele
pmup(tnemurtsnieht
hgiHtceleS.)Hpdna
taeracekaT.egnaR
.noitaraperpel
ehtnaelc/kcehC
eta
rbilaceR.sedortcele
pmup(tnemurtsnieht
woLtceleS.)Hpdna
taeracekaT.egnaR
.noitaraperpelpmas
40
Page 41

SMOTPMYSSMOTPMYS
SMOTPMYSSMOTPMYSMELBORPMELBORP
SMOTPMYS
MELBORPMELBORPNOITULOSNOITULOS
MELBORP
NOITULOSNOITULOS
NOITULOS
egnaR tnemurtsnieht
.gniknilb
A
xrorrE"
ninoitartitaretfA hgiH
roHS°57syalpsid
roD°57roHT°002
gnidrocca(.a.I%0.2
elesehthtiw
.deyalpsid
)tinudetc
retemehtputratst
ANNAHehtsyalpsid
.yltnenamrepogol
siegassem"x
.elpmas
.egnar
.dekcolb
.sedortcelenekorB
tontnemurtsnI
gnorW.detarbilac
fotuonoitartnecnoC
sisyekehtfoenO
.rorrelanretnI
aelc/kcehC
ehtn
etarbilaceR.sedortcele
pmup(tnemurtsnieht
taeracekaT.)Hpdna
.noitaraperpelpmas
rodraobyekehtkcehC
.rodnevehttcatnoc
dnaretemehtfforewoP
.niaganotirewopneht
tnoc
,stsisreprorreehtfI
.rodnevehttca
ELECTRODE CONDITIONING AND MAINTENANCE
STORAGE PROCEDURE
To assure a quick response time, the glass bulb should be kept moist and not allowed to dry out.
Replace the solution in the protective cap with a few drops of HI 70300 or HI 80300 Storage
Solution. The HI 5313 Reference may be stored with its black cap and Fill hole covered. Rinse
and refill before using. Follow the Preparation Procedure on page 18 before taking measurements.
Note:NEVER STORE THE pH ELECTRODE IN DISTILLED OR DEIONIZED WATER.
PERIODIC MAINTENANCE
Inspect the electrodes and the cables. The cable used for connection to the instrument must be
intact and there must be no points of broken insulation on the cable or cracks on the electrode
stem or bulb. Connectors must be perfectly clean and dry. If any scratches or cracks are present,
replace the electrode. Rinse off any salt deposits with water.
pH CLEANING PROCEDURE
•
General
•
Milk deposits
•
Protein
IMPORTANT: After performing any of the cleaning procedures, rinse the electrode thoroughly
with distilled or deionized water and soak the electrode in HI 70300 or HI 80300 Storage
Solution for at least 1 hour before taking measurements.
Soak in Hanna HI 7061 or HI 8061 General Cleaning Solution for
approximately ½ hour.
Soak in Hanna HI 700640 Cleaning Solution for milk deposits for
approximately ½ hour (pH half cell only).
Soak in Hanna HI 7073 or HI 8073 Protein Cleaning Solution for 15 minutes.
41
Page 42

REFERENCE ELECTRODE CLEANING
• Drain the old filling solution, rinse with an adequate HI 7072 solution, drain, then refill with HI 7072 solution.
• Do not use an electrode if crystalized salts are visible inside the electrode. Drain electrode, dissasamble
and rinse internal body with deionized water. Reassemble and refill with fresh fill solution.
• The internal chamber of this electrode is gell filled. If the electrode has been left dry for long periods of
time the gell may be dehydrated and stable measurements may not be obtenable. Dissasamble
electrode and soak internal assembly in HI 7072 filling solution. Verify the ceramic is wetted by the fill
solution. Warming the solution slightly (50 °C) before soaking will hasten this process. Permit the
electrode to cool completely while immersed in this solution.
ACCESORIES
REAGENTS
HI 84429-50 Titrant solution 100 ml
HI 84429-55 Pump Calibration Standard (500 mL)
pH CALIBRATION SOLUTIONS
HI 84429-65 pH 4.01 Buffer Solution (6 pcs×230 mL)
HI 84429-70 pH 6.00 Buffer Solution (6 pcs×230 mL)
HI 84429-60 pH 8.30 Buffer Solution (6 pcs×230 mL)
ELECTRODES
FC 260B pH electrode
HI 5315 Reference electrode
HI 7662-M Temperature probe
ELECTRODE FILL SOLUTION
HI 7072 Reference electrode filling solution
ELECTRODE STORAGE SOLUTION
HI 70300L Storage Solution, 500 mL bottle
CLEANING SOLUTIONS
HI 70640L Cleaning solution for remaining milk deposits (500 mL)
HI 70641L Cleaning and desinfecting for dairy products (500 mL)
HI 70642L Cleaning solution for remaining cheese deposits (500 mL)
OTHER ACCESSORIES
HI 70483T Tube set with cap for titrant bottle and tip
HI 731316 Stir bar 12x5 mm (5 pcs)
HI 731319 Stir bar 25x7 mm (10 pcs)
HI 740036P 50 mL plastic beaker (10 pcs)
HI 740037P 20 mL plastic beaker (10 pcs)
HI 740143 Syringe 1 mL (6 pcs)
HI 740144 Pipette tip 1 mL (6 pcs)
42
Page 43
WARRANTY
HI 84429 is warranted for two years against defects in workmanship and materials when used for its
intended purpose and maintained according to the instructions.
This warranty is limited to repair or replacement free of charge.
Damage due to accident, misuse, tampering or lack of prescribed maintenance is not covered.
If service is required, contact your dealer. If under warranty, report the model number, date of purchase,
serial number and the nature of the failure. If the repair is not covered by the warranty, you will be
notified of the charges incurred.
If the instrument is to be returned to Hanna Instruments, first obtain a Returned Goods Authorization
Number from the Customer Service Department and then send it with shipment costs prepaid. When
shipping any instrument, make sure it is properly packaged for complete protection.
To validate your warranty, fill out and return the enclosed warranty card within 14 days from the date of
purchase.
RECOMMENDATION FOR USERS
Before using this product, make sure that it is entirely suitable for your specific application and for the
environment in which it is used.
Operation of this instrument may cause unacceptable interferences to other electronic equipments, this
requiring the operator to take all necessary steps to correct interferences.
Any variation introduced by the user to the supplied equipment may degrade the instrument EMC
performance.
To avoid damages or burns, do not put the instrument in microwave ovens. For yours and the instrument
safety do not use or store the instrument in hazardous environments.
Hanna Instruments reserves the right to modify the design, construction and appearance of its products
without advance notice.
43
Page 44

Hanna Instruments Inc.
Highland Industrial Park
584 Park East Drive
Woonsocket, RI 02895 USA
Technical Support for Customers
Tel. (800) 426 6287
Fax (401) 765 7575
E-mail tech@hannainst.com
www.hannainst.com
Local Sales and Customer Service Office
Printed in EUROPE
(ROMANIA)
MAN84429 11/09
44
 Loading...
Loading...