Page 1
Instruction Manual
HI 83741
IRON
ISM
for wine analysis
www.hannainst.com
MAN83741R3 04/06
www.hannainst.com
This Instrument is in
Compliance with the CE Directives
Page 2
Dear Customer,
Thank you for choosing a Hanna product. This manual will provide you with the necessary
information for the correct use of the instrument. Please read it carefully before using the meter. If
you need additional technical information, do not hesitate to e-mail us at tech@hannainst.com.
This instrument is in compliance with
directives.
TABLE OF CONTENTS
PRELIMINARY EXAMINATION ............................................................................................... 3
GENERAL DESCRIPTION ...................................................................................................... 4
SPECIFICATIONS ................................................................................................................ 5
PRECISION AND ACCURACY ................................................................................................. 5
PRINCIPLE OF OPERATION ..................................................................................................6
ABBREVIATIONS ................................................................................................................ 7
FUNCTIONAL DESCRIPTION .................................................................................................8
GUIDE TO DISPLAY CODES .................................................................................................. 9
GENERAL TIPS FOR AN ACCURATE MEASUREMENT ............................................................. 11
MEASUREMENT PROCEDURE.............................................................................................13
BATTERIES REPLACEMENT ................................................................................................ 16
ACCESSORIES ................................................................................................................. 16
CE DECLARATION OF CONFORMITY .................................................................................... 17
WARRANTY ................................................................................................................. 17
HANNA LITERATURE .........................................................................................................18
USER NOTES ................................................................................................................. 19
USER NOTES
Date Iron Value (mg/L)
Notes
All rights are reserved. Reproduction in whole or in part is prohibited without the written consent
of the copyright owner, Hanna Instruments Inc., Woonsocket, Rhode Island, 02895 , USA.
2
19
Page 3
HANNA LITERATURE
PRELIMINARY EXAMINATION
Hanna publishes a wide range of catalogs and handbooks for an equally wide range of applications.
The reference literature currently covers areas such as:
• Water Treatment
• Process
• Swimming Pools
• Agriculture
• Food
• Laboratory
and many others. New reference material is constantly being added to the library.
For these and other catalogs, handbooks and leaflets contact your dealer or the Hanna Customer
Service Center nearest to you. To find the Hanna Office in your vicinity, check our home page at
www.hannainst.com.
Please examine this product carefully. Make sure that the instrument is not damaged. If any
damage occured during shipment, please notify your Dealer.
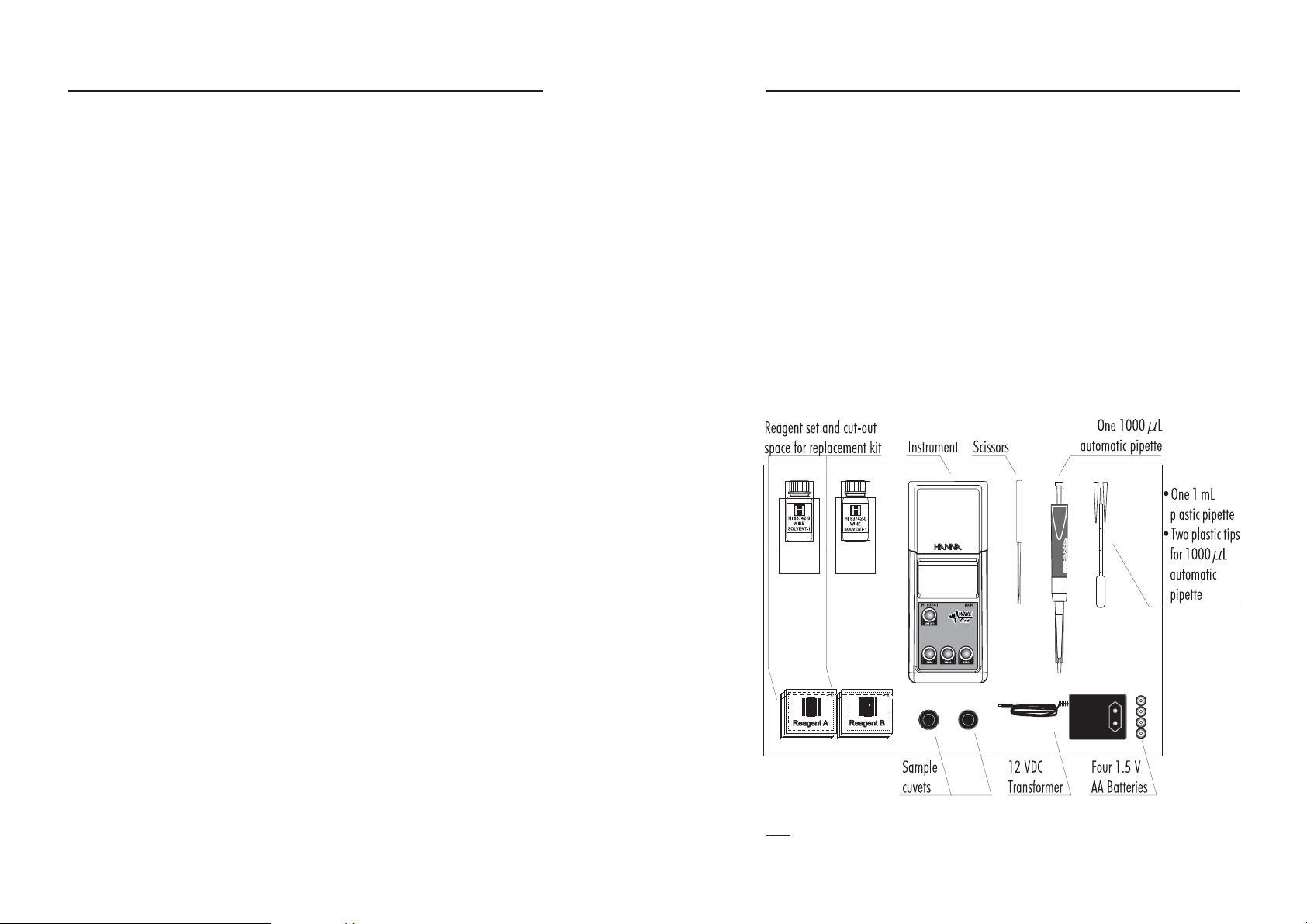
Each HI 83741 Ion Selective Meter is supplied complete with:
• Two sample cuvets and caps
• Reagents for 5 tests (HI 83741A-O, HI 83741B-O, HI 83742-O)
• Scissors
• One 1000 µL automatic pipette with Instruction Sheet
• Two plastic tips for 1000 µL automatic pipette
• One 1 mL plastic pipette
• 12 VDC transformer (
• Four 1,5V AA batteries
• Tissue for wiping cuvets
• Instruction manual
• Instrument Quality Certificate
• Rigid carrying case
HI 710005 HI 710005
HI 710005 or
HI 710005 HI 710005
HI 710006HI 710006
HI 710006)
HI 710006HI 710006
18
Note:save all packing material until you are sure that the instrument works correctly.
Any defective item must be returned in its original packing.
3
Page 4

GENERAL DESCRIPTION
CE DECLARATION OF CONFORMITY
The HI 83741 is an auto-diagnostic portable microprocessor meter that benefits from Hanna’s
years of experience as a manufacturer of analytical instruments. It has an advanced optical system
based on a special tungsten lamp and a narrow band interference filter that allows most accurate
and repeatable readings. All instruments are factory calibrated.
The auto-diagnostic feature of this meter ensures always optimal measurement conditions to
ensure most precise readings. The light level is automatically adjusted each time a zero-measurement
is made, and the temperature of the lamp is controlled to avoid overheating.
SIGNIFICANCE OF USE
Trace iron concentrations in wine are beneficial for enzyme activity, as stabilizer, and as a functional
component for proteins.
At higher concentrations it alters the redox potential, in favouring oxidation, affecting sensory
characteristics and participating in the formation of complexes with tannin and phosphates resulting
in instabilities (casse). The most common iron case is ‘white casse’ (iron phosphate), it is initially seen
as milky white cloud and later as a precipitate. The ‘blue casse’ (ferric tannate) that occours less often
can be observed in white wines for example after tannic acid additions.
Most of the iron present in wine is present in the ferrous Fe(II) state. The ratio of the Fe(II)/Fe(III)
depends on the oxidation state of the wine. If Fe(III) is formed, it can bind with phosphates that are
normally present in wine.
Since iron strongly binds with several organic acids, some wine makers add citric acid to the wine to
complex free iron if the concentration exceeds 5 mg/L.
If no contatamination occurs the normal iron concentrations in must range from 1 to 5 ppm. The most
important source of iron in wine is contact with iron containing alloys during processing. During
fermentation a part of the iron is absorbed by yeast and thus removed from the wine during filtration.
Casse formation depends on: iron concentration, pH, ORP, phosphate, content and the type of wine.
white casse formationwhite casse formation
white casse formation
white casse formationwhite casse formation
iron concentration > 7 ppm iron concentration < 5 ppm
high redox potential (Fe3+ present) clarification with bentonite
pH 2.9-3.6 citric acid addition 12-24 g/hL
white casse inhibitionwhite casse inhibition
white casse inhibition
white casse inhibitionwhite casse inhibition
Recommendations for Users
Before using these products, make sure that
they are entirely suitable for your specific
application and for the environment in which
they are used.
Operation of these instruments may cause
unacceptable interferences to other electronic
equipments, this requiring the operator to take
all necessary steps to correct interferences.
Any variation introduced by the user to the
supplied equipment may degrade the
instruments' EMC performance.
To avoid damages or burns, do not put the
instrument in microwave ovens. For yours and
the instrument safety do not use or store the
instrument in hazardous environments.
WARRANTY
HI 83741 is warranted for two years against defects in workmanship and materials when used for
its intended purpose and maintained according to the instructions.
This warranty is limited to repair or replacement free of charge.
Damages due to accident, misuse, tampering or lack of prescribed maintenance are not covered.
If service is required, contact your dealer. If under warranty, report the model number, date of
purchase, serial number and the nature of the failure. If the repair is not covered by the warranty,
you will be notified of the charges incurred.
If the instrument is to be returned to Hanna Instruments, first obtain a Returned Goods Authorization
Number from the Customer Service Department and then send it with shipment costs prepaid.
When shipping any instrument, make sure it is properly packaged for complete protection.
To validate your warranty, fill out and return the enclosed warranty card within 14 days from the
date of purchase.
Hanna Instruments reserves the right to modify the design, construction and appearance of its products
without advance notice.
4
17
Page 5

BATTERIES REPLACEMENT
SPECIFICATIONS
Battery replacement must only take place in a nonhazardous area.
The blinking “ ” will appear when the batteries power
gets low.
When batteries are completely discharged, “0% bAtt”
will appear and after two seconds the instrument is
switched off.
Remove the battery cover from the bottom of the
instrument and change the old batteries with 4 fresh
1.5V batteries, paying attention to the correct polarity.
Replace the cover.
ACCESSORIES
REAGENT SETS
HI 83741-20 Iron reagents set for wine (20 tests)
OTHER ACCESSORIES
HI 740027P 1.5V AA batteries (10 pcs)
HI 731318 Tissue for wiping cuvets (4 pcs)
HI 731321 Glass cuvets (4 pcs)
HI 731325W Caps for cuvets (4 pcs)
HI 93703-50 Cuvets cleaning solution (230 mL)
HI 731341 1000 µL automatic pipette
HI 731351 Plastic tips for 1000 µL automatic pipette (25 pcs)
Range 0.0-15.0 mg/L
Resolution 0.1 mg/L
Precision SD ±0.4 mg/L @ 4.0 mg/L
Light Source Tungsten lamp with narrow band interference filter @ 560 nm
Light Detector Silicon Photocell
Method The reaction between Iron and the reagents causes a purple tint in
the sample.
Environment 0 to 50°C (32 to 122°F); max 95% RH non-condensing
Battery Type 4 x 1,5 volt AA batteries / 12 to 20 VDC through voltage adapter
Auto-Shut off
Dimensions 225 x 85 x 80 mm (8.7 x 3.3 x 3.1")
Weight 500 g (17,6 oz.)
REQUIRED REAGENTS
Code Description Quantity/test
HI 83741A-0 Iron Reagent A 1 packet
HI 83741B-0 Iron Reagent B 1 packet
HI 83742-0 Wine solvent-1 9 mL
After 15' of non-use in measurement mode.
PRECISION AND ACCURACY
Precision is how closely repeated measurements agree
with each other. Precision is usually expressed as
standard deviation (SD). Accuracy is defined as the
nearness of a test result to the true value.
Although good precision suggests good accuracy,
precise results can be inaccurate. The figure explains
these definitions.
In a laboratory using a standard solution of 4.0
mg/L iron and a representative lot of reagent, an
operator obtained with a single instrument a standard
deviation of
0.4 mg/L.
16
5
Page 6

PRINCIPLE OF OPERATION
Absorption of Light is a typical phenomenon of interaction between electromagnetic radiation and
matter. When a light beam crosses a substance, some of the radiation may be absorbed by atoms,
molecules or crystal lattices.
If pure absorption occurs, the fraction of light absorbed depends both on the optical path length
through the matter and on the
Lambert-Beer Law:
Where:
-log I/I
= Absorbance (A)
o
Io= intensity of incident light beam
I = intensity of light beam after absorption
ε
= molar extinction coefficient at wavelength λ
λ
c = molar concentration of the substance
d = optical path through the substance
Therefore, the concentration "c" can be calculated from the absorbance of the substance as the
other factors are known.
Photometric chemical analysis is based on the possibility to develop an absorbing compound from
a specific chemical reaction between sample and reagents. Given that the absorption of a
compound strictly depends on the wavelength of the incident light beam, a narrow spectral
bandwidth should be selected as well as a proper central wavelength to optimize measurements.
The optical system of Hanna's HI 83000 series colorimeters is based on special subminiature
tungsten lamps and narrow-band interference filters to guarantee both high performance and
reliable results.
physical-chemical characteristics of the substance according to the
-log I/Io = ε
λ
c d
or
A = ε
c d
λ
• Reinsert the cuvet into the instrument and
close the lid.
• Press TIMER and the instrument will show the
countdown or, alternatively, wait for 2 minutes.
At the end an acoustic signal alerts the user
that the countdown has finished.
• Press READ and the display will show “----”
during measurement.
• The instrument directly displays concentration
in mg/L (ppm) of iron on the Liquid Crystal
Display.
Note
If the iron concentration exceeds 15.0 ppm or
if the sample is very turbid, it is recommended
to dilute the sample 5 times (see “General
tips for an accurate measurement”, page 11)
and repeat the measurement procedure. In
this case the displayed value needs to be
multiplied by 5 to compensate for dilution.
Block diagram (optical layout)
6
15
Page 7

• Place the cuvet into the holder and close
the lid.
• Press TIMER and the instrument will show the
countdown or, alternatively, wait for 2 minutes.
The instrument gives an acoustic signal to
alert the user that the countdown is finished.
• Press ZERO and “----” will blink on the display.
• After a few seconds the display will show
“-0.0-”. The meter is now zeroed and ready
for measurement
Note: If the “L Lo” (Low Light) message
appears, the sample must be diluted. See
“General tips for an accurate measurement”
(page 11).
A microprocessor controlled special tungsten lamp emits radiation which is first optically conditioned
and beamed to the sample contained in the cuvet. The optical path is fixed by the diameter of the
cuvet. Then the light is spectrally filtered to a narrow spectral bandwidth, to obtain a light beam of
intensity I
or I.
o
The photoelectric cell collects the radiation I that is not absorbed by the sample and converts it
into an electric current, producing a potential in the mV range.
The microprocessor uses this potential to convert the incoming value into the desired measuring
unit and to display it on the LCD.
The measurement process is carried out in two phases: first the meter is zeroed and then the actual
measurement is performed.
The cuvet has a very important role because it is an optical element and thus requires particular
attention. It is important that both the measurement and the calibration (zeroing) cuvets are
optically identical to provide the same measurement conditions. Whenever possible use the same
cuvet for both. It is necessary that the surface of the cuvet is clean and not scratched. This to avoid
measurement interference due to unwanted reflection and absorption of light. It is recommended
not to touch the cuvet walls with hands.
Furthermore, in order to maintain the same conditions during the zeroing and the measuring
phases, it is necessary to close the cuvet to prevent any contamination.
ABBREVIATIONS
• Remove the cuvet from the instrument and
open the cap.
• Add the content of one powder packet of
HI 83741B-0 reagent B. Replace the cap and
shake gently for 1 minute to dissolve the
reagent.
14
#2
US Environmental Protection Agency
EPA:
degree Celsius
°C:
degree Fahrenheit
°F:
milligrams per liter. mg/L is equivalent to ppm (part per million)
mg/L:
milliliter
mL:
Liquid Crystal Display
LCD:
7
Page 8

FUNCTIONAL DESCRIPTION
INSTRUMENT DESCRIPTION
FRONT
REAR
1) Lid
2) Cuvet Holder
3) Liquid Crystal Display (LCD)
4) ON/OFF key, to turn the meter on and off
5) ZERO key, to zero the meter
6) TIMER key, to activate a countdown
7) READ key, to perform measurement
8) Power Socket 12V to 20V DC 2.5 Watt
MEASUREMENT PROCEDURE
• Turn the instrument on by pressing ON/OFF.
• When the LCD displays “---”, it is ready.
• Use the 1000 µL automatic pipette to add
exactly 1 mL of wine sample to an empty
cuvet.
For a correct use of the automatic pipette
please follow the related Instruction Sheet.
• Use the plastic dropper pipette to fill the cuvet
up to the 10 mL mark with wine solvent-1
(HI83742-0).
DISPLAY ELEMENTS DESCRIPTION
1) Four digit main display.
2) Battery icon: appears when the battery voltage is getting low.
3) The hourglass icon: appears during the countdown.
4) Status information.
5) Measurement unit.
6) Lamp status indicator.
7) Four digit secondary display.
8
• Add the content of one powder packet of
HI 83741A-0 reagent A. Replace the cap and
shake gently for 1 minute to dissolve the
reagent.
13
Page 9

• Whenever the cuvet is placed into the measurement
cell, it must be dry outside, and completely free of
fingerprints, oil or dirt. Wipe it thoroughly with
HI 731318 (tissue for wiping cuvets, see chapter
ACCESSORIES) or a lint-free cloth prior to insertion.
• Do not let the reacted sample stand too long after
reaction, or accuracy will be lost.
GUIDE TO DISPLAY CODES
This prompt appears for a few seconds each time the instrument is
turned ON.
• After the reading it is important to discard immediately
the sample, otherwise the glass might become
permanently stained.
• All the reaction times reported in this manual are
referred to 20°C (68°F). As a general rule of thumb,
they should be doubled at 10°C (50°F) and halved at
30°C (86°F).
These prompts indicate the type of power supply: “Line” (if the
external power supply is used) or the battery level.
Indicates that the instrument is in a ready state and waiting for the
next command (Timer or Zero).
After Timer is pressed, a blinking hourglass icon appears and the
display shows a 2 minutes coundown. Also the Zero tag might
blink if no zero measurement has been made before. At the end an
acoustic signal alerts the user that the countdown has finished.
Indicates that the meter is performing a zero measurement. The
light intensity is automatically re-adjusted (auto-calibration features)
if necessary.
The instrument is zeroed and a measurement can be made.
12
Indicates that the meter is making a measurement.
Batteries voltage is getting low and the batteries need to be
replaced.
9
Page 10

Indicates that the batteries are dead and must be replaced. After this
message appears, the instrument is switched off. Change the batteries
and restart the meter.
GENERAL TIPS FOR AN ACCURATE MEASUREMENT
The instructions listed below should be carefully followed during testing to ensure best accuracy.
ERROR MESSAGES
The meter has lost its configuration. Contact your dealer or the nearest
Hanna Customer Service Center.
a) on zero reading:a) on zero reading:
a) on zero reading:
a) on zero reading:a) on zero reading:
“Light high”: there is too much light to perform a measurement.
Please check the preparation of the zero cuvet.
“Light low”: there is not enough light to perform a measurement.
Please dilute the sample five times (see “General tips for an accurate
measurement”, page 12).
“No Light”: the lamp is not working because of a malfunction. Contact
your dealer or the nearest Hanna Customer Service Center.
b) on sample reading:b) on sample reading:
b) on sample reading:
b) on sample reading:b) on sample reading:
“Inverted”: the sample and the zero cuvet are inverted.
• For a correct filling of the cuvet: the liquid in the
cuvet forms a convexity on the top; the bottom of
this convexity must be at the same level of the 10
mL mark.
• For dosing the wine sample, we recommend to
use the supplied Hanna HI 731341 automatic
pipette.
For a correct use of the Hanna automatic pipette,
please follow the related Instruction Sheet.
• Proper use of the powder reagent packet:
(a) use scissors to open the powder packet;
(b) push the edges of the packet to form a spout;
(c) pour out the content of the packet.
(a) (b)
• In order to avoid reagent leaking and to obtain
more accurate measurements, it is recommended
to close the cuvet first with the supplied HDPE
plastic stopper
cap.
and then with the black
10 mL
Hanna
automatic pipette
(c)
The sample absorbs less light than the zero reference. Check the procedure and make sure you use the same cuvet for reference (zero)
and measurement.
A flashing value of the maximum concentration indicates an over
range condition. The concentration of the sample is beyond the
programmed range: dilute the sample and measure again.
10
•
Diluting procedure: Use the 1000 µL automatic
pipette to add twice exactly 1 mL of sample to an
empty cuvet. Then fill the cuvet up to the
mark with iron-free deionized water. Close the
cap and invert the cuvet several times. This is the
diluted sample. Follow the measurement procedure.
The final reading must be multiplied by 5 to
compensate for dilution.
x2
11
 Loading...
Loading...