Page 1

Instruction Manual
HI 83215
Grow Master Basic for Nutrient
Analyses
www.hannainst.com
1
Page 2

Dear Customer,
Thank you for choosing a Hanna product. Please read this instruction manual carefully before using the
instrument. This manual will provide you with the necessary information for the correct use of the
instrument. If you need additional technical information, do not hesitate to e-mail us at tech@hannainst.com.
TABLE OF CONTENTS
PRELIMINARY EXAMINATION .............................................................................................................................................. 3
GENERAL DESCRIPTION ......................................................................................................................................................3
ABBREVIATIONS ................................................................................................................................................................4
SPECIFICATIONS ............................................................................................................................................................... 4
PRECISION AND ACCURACY ................................................................................................................................................4
PRINCIPLE OF OPERATION ................................................................................................................................................. 5
FUNCTIONAL DESCRIPTION ................................................................................................................................................ 6
TIPS FOR AN ACCURATE MEASUREMENT ............................................................................................................................7
HEALTH & SAFETY ..........................................................................................................................................................10
METHOD REFERENCE TABLE ............................................................................................................................................. 11
OPERATIONAL GUIDE ....................................................................................................................................................... 11
SETUP ........................................................................................................................................................................... 13
HELP MODE ................................................................................................................................................................... 15
INTRODUCTION ............................................................................................................................................................... 16
SAMPLE PREPARATION .................................................................................................................................................... 19
AMMONIA HR ................................................................................................................................................................ 23
AMMONIA MR ................................................................................................................................................................ 25
AMMONIA LR ................................................................................................................................................................. 27
NITRATE HR .................................................................................................................................................................... 29
NITRATE MR ................................................................................................................................................................... 31
NITRATE LR .................................................................................................................................................................... 33
PHOSPHORUS HR .......................................................................................................................................................... 35
PHOSPHORUS MR .......................................................................................................................................................... 37
PHOSPHORUS LR ........................................................................................................................................................... 39
POTASSIUM HR .............................................................................................................................................................. 41
POTASSIUM MR .............................................................................................................................................................. 44
POTASSIUM LR ............................................................................................................................................................... 46
ERRORS AND WARNINGS ................................................................................................................................................. 48
DATA MANAGEMENT ........................................................................................................................................................ 49
STANDARD METHODS ..................................................................................................................................................... 49
ACCESSORIES ................................................................................................................................................................ 50
WARRANTY ...............................................................................................................................
HANNA LITERATURE ........................................................................................................................................................ 51
..................................... 51
All rights are reserved. Reproduction in whole or in part is prohibited without the written consent of the copyright owner, Hanna
Instruments Inc., Woonsocket, Rhode Island, 02895 , USA.
2
Page 3
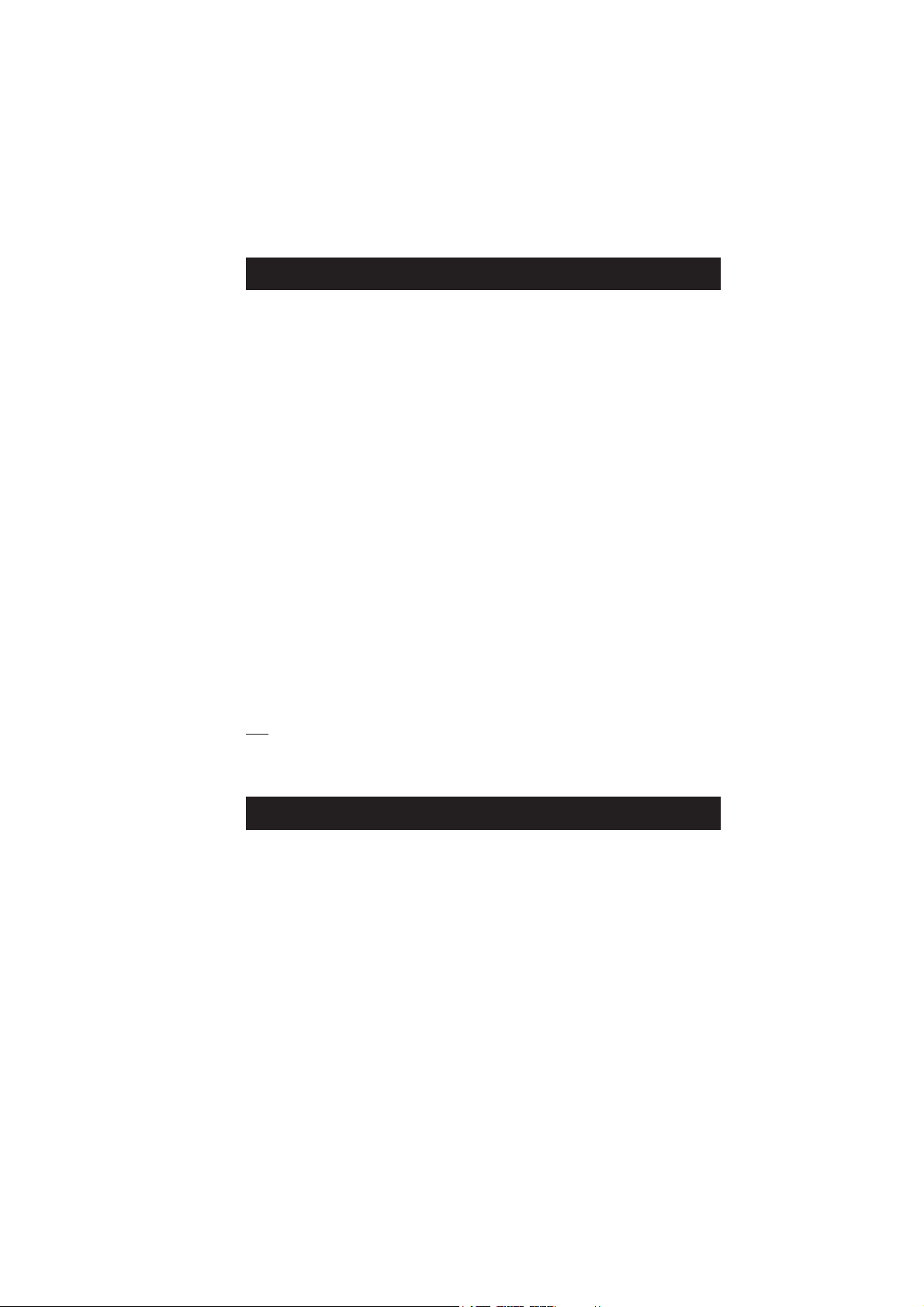
PRELIMINARY EXAMINATION
Please examine this product carefully. Make sure that the instrument is not damaged. If any damage
occurred during shipment, please notify your local Hanna Office.
Each Meter is supplied complete with:
• Four Sample Cuvettes and Caps
• Sample Preparation Kit (see page 19)
• Cloth for wiping cuvettes (1 pcs)
• Scissors
• AC/DC Power Adapter
• Instruction Manual
The sample preparation kit contains:
• 4 cuvettes (10 mL) with caps
• 2 plastic beakers (100 and 170 mL)
• 1 graduated cylinder (100 mL)
• 1 syringe with screw rim (60 mL)
• 1 syringe (5 mL)
• 1 funnel
• 25 filter discs
• 1 spoon
• 2 pipettes
• Carbon powder packets (50 pcs)
• 1 Demineralizer Bottle with filter cap for about 12 liters of deionized water (depending on the
hardness level of water to be treated)
Note: Save all packing material until you are sure that the instrument works correctly. Any defective item
must be returned in its original packing with the supplied accessories.
GENERAL DESCRIPTION
HI 83215 is a multiparameter bench photometer dedicated for Nutrient analyses. It can measure 12
different methods using specific liquid or powder reagents. The amount of reagent is precisely dosed to
ensure maximum reproducibility.
HI 83215 bench photometer can be connected to a PC via an USB cable. The optional HI 92000
Windows® Compatible Software helps users manage all their results.
HI 83215 has a powerful interactive user support that assists the user during the analysis process.
Each step in the measurement process is help supported. A tutorial mode is available in the Setup Menu.
3
Page 4
ABBREVIATIONS
°C: degree Celsius
°F: degree Fahrenheit
μg/L: micrograms per liter (ppb)
mg/L: milligrams per liter (ppm)
g/L: grams per liter (ppt)
mL: milliliter
HR: high range
MR: medium range
LR: low range
SPECIFICATIONS
Light Life Life of the instrument
Light Detector Silicon Photocell
Environment 0 to 50°C (32 to 122°F);
max 95% RH non-condensing
Power Supply external 12 Vdc power adapter
built-in rechargeable battery
Dimensions 235 x 200 x 110 mm (9.2 x 7.87 x 4.33")
Weight 0.9 Kg
For specifications related to each method (e.g. range, resolution, etc.) refer to the related measurement
section.
PRECISION AND ACCURACY
Precision is how closely repeated measurements agree
with each other. Precision is usually expressed as
standard deviation (SD).
Accuracy is defined as the nearness of a test result to
the true value.
Although good precision suggests good accuracy, precise
results can be inaccurate. The figure explains these
definitions.
For each method, the precision is expressed in the
related measurement section.
4
Page 5

PRINCIPLE OF OPERATION
Absorption of light is a typical phenomenon of interaction between electromagnetic radiation and matter.
When a light beam crosses a substance, some of the radiation may be absorbed by atoms, molecules or
crystal lattices.
If pure absorption occurs, the fraction of light absorbed depends both on the optical path length through
the matter and on the physical-chemical characteristics of substance according to the Lambert-Beer Law:
-log I/Io = ε
A = ε
Where:
-log I/I
= Absorbance (A)
o
Io= intensity of incident light beam
I = intensity of light beam after absorption
ε
= molar extinction coefficient at wavelength λ
λ
c = molar concentration of the substance
d = optical path through the substance
Therefore, the concentration "c" can be calculated from the absorbance of the substance as the other factors
are known.
Photometric chemical analysis is based on the possibility to develop an absorbing compound from a specific
chemical reaction between sample and reagents.
Given that the absorption of a compound strictly depends on the wavelength of the incident light beam,
a narrow spectral bandwidth should be selected as well as a proper central wavelength to optimize
measurements.
The optical system of HI 83215 is based on special subminiature tungsten lamps and narrow-band
interference filters to guarantee both high performance and reliable results.
Three measuring channels allow a wide range of tests.
c d
λ
or
c d
λ
Instrument block diagram (optical layout)
A microprocessor controlled special tungsten lamp emits radiation which is first optically conditioned and
beamed through the sample contained in the cuvette. The optical path is fixed by the diameter of the
cuvette. Then the light is spectrally filtered to a narrow spectral bandwidth, to obtain a light beam of
intensity Io or I.
The photoelectric cell collects the radiation I that is not absorbed by the sample and converts it into an
electric current, producing a potential in the mV range.
The microprocessor uses this potential to convert the incoming value into the desired measuring unit and to
display it on the LCD.
5
Page 6
The measurement process is carried out in two phases: first the meter is zeroed and then the actual
measurement is performed.
The cuvette has a very important role because it is an optical element and thus requires particular
attention. It is important that both the measurement and the calibration (zeroing) cuvette are optically
identical to provide the same measurement conditions. Most methods use the same cuvette for both, so it
is important that measurements are taken at the same optical point. The instrument and the cuvette cap
have special marks that must be aligned in order to obtain better reproducibility.
The surface of the cuvette must be clean and not scratched. This is to avoid measurement interference due
to unwanted reflection and absorption of light. It is recommended not to touch the cuvette walls with
hands.
Furthermore, in order to maintain the same conditions during the zeroing and the measurement phases,
it is necessary to cap the cuvette to prevent any contamination.
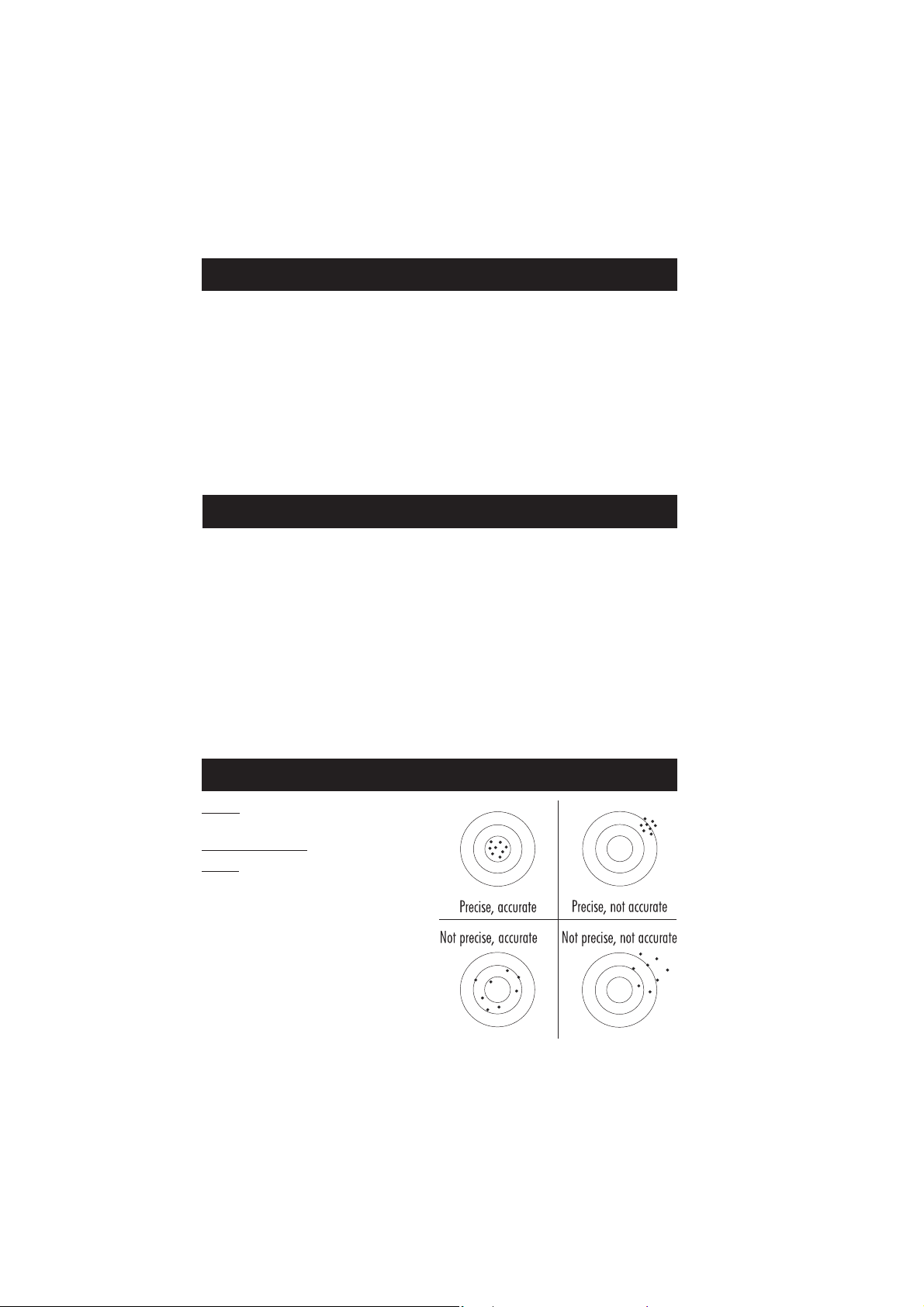
FUNCTIONAL DESCRIPTION
INSTRUMENT DESCRIPTION
6
Page 7

1) Open Cuvette Lid
2) Indexing mark
3) Cuvette point
4) Liquid Crystal Display (LCD)
5) Splash proof keypad
6) ON/OFF power switch
7) Power input connector
8) USB connector
KEYPAD DESCRIPTION
The keypad contains 8 direct keys and 3 functional keys with the following functions:
Press to perform the function displayed above it on the LCD.
Press to exit the current screen.
Press to access the select method menu.
Press to move up in a menu or a help screen, to increment a set value, to access second level
functions.
Press to move down in a menu or a help screen, to decrement a set value, to access second
level functions.
Press to log the current reading.
Press to recall the log.
Press to display the help screen.
Press to access the setup screen.
TIPS FOR AN ACCURATE MEASUREMENT
The instructions listed below should be carefully followed during testing to ensure most accurate results.
• Color or suspended matter in large amounts may cause interference, and should be removed by
treatment with active carbon and filtration: refere to the SAMPLE PREPARATION Chapter (see page 19)
• Ensure the cuvette is filled correctly: the liquid in the cuvette forms a convexity on the top; the bottom
of this convexity must be at the same level as the 10 mL mark.
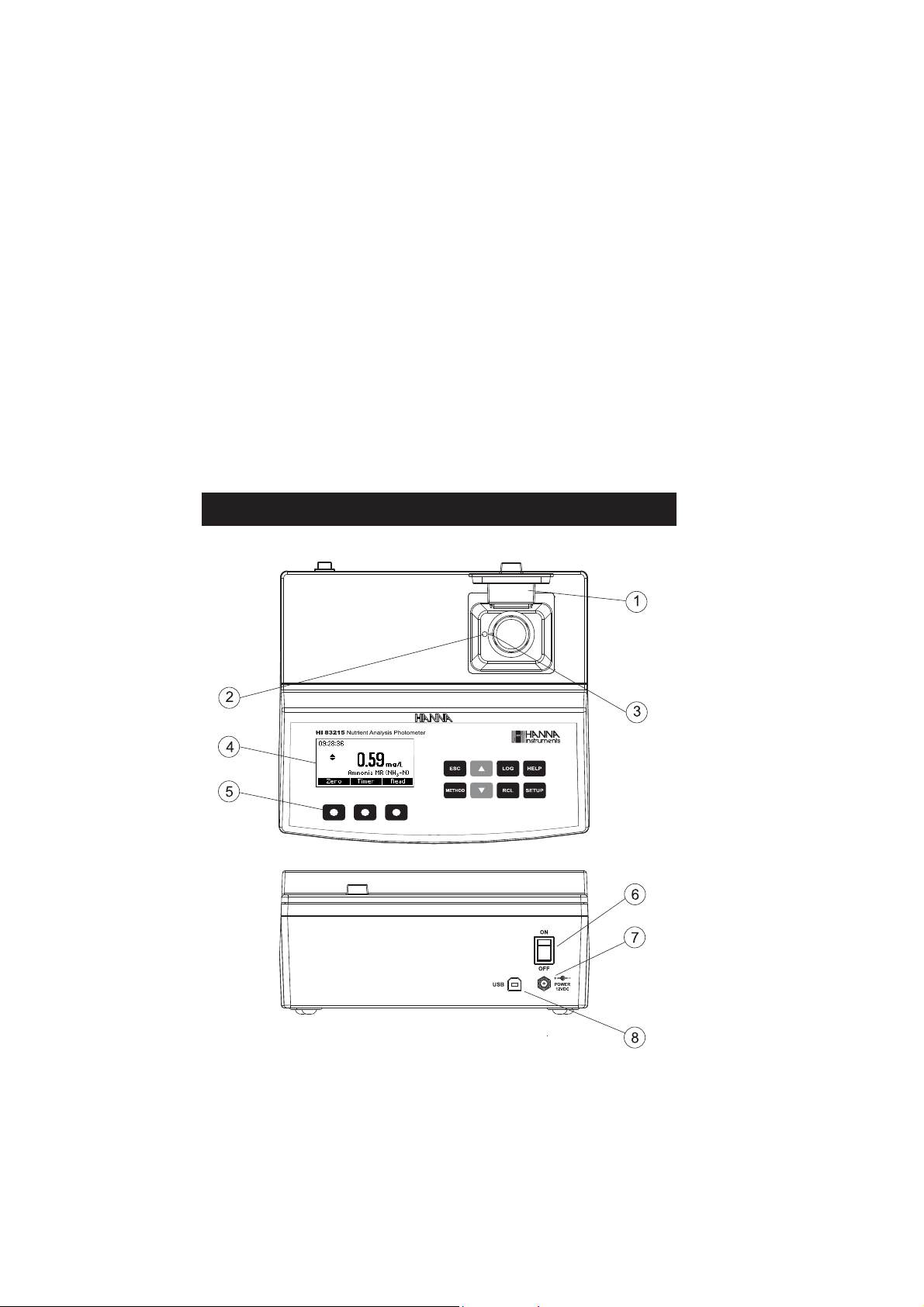
COLLECTING AND MEASURING SAMPLES
• In order to measure exactly 0.5 mL of reagent with the 1 mL syringe:
(a) push the plunger completely into the syringe and insert the tip into the solution.
7
Page 8
(b) pull the plunger up until the lower edge of the seal is exactly on the 0.0 mL mark.
(c) take out the syringe and clean the outside of the syringe tip. Be sure that no drops are hanging
on the tip of the syringe, if so eliminate them. Then, keeping the syringe in vertical position above
the cuvette, push the plunger down into the syringe until the lower edge of the seal is exactly on
the 0.5 mL mark. Now the exact amount of 0.5 mL has been added to the cuvette, even if the
tip still contains some solution.
USING LIQUID AND POWDER REAGENTS
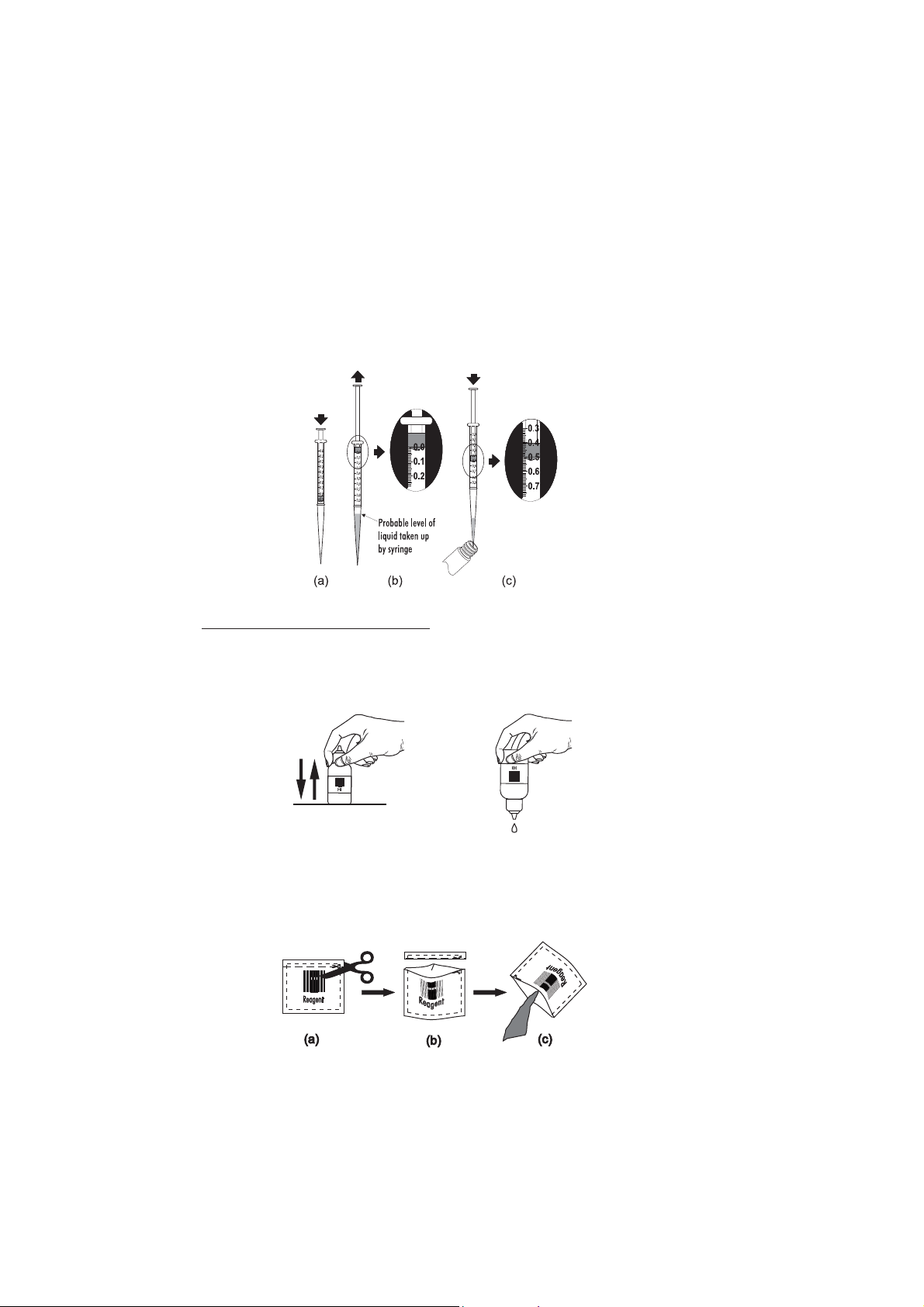
• Proper use of the dropper:
(a) for reproducible results, tap the dropper on the table for several times and wipe the outside of the
dropper tip with a cloth.
(b) always keep the dropper bottle in a vertical position while dosing the reagent.
(a) (b)
• Proper use of the powder reagent packet:
(a) use scissors to open the powder packet;
(b) push the edges of the packet to form a spout;
(c) pour out the content of the packet.
8
Page 9
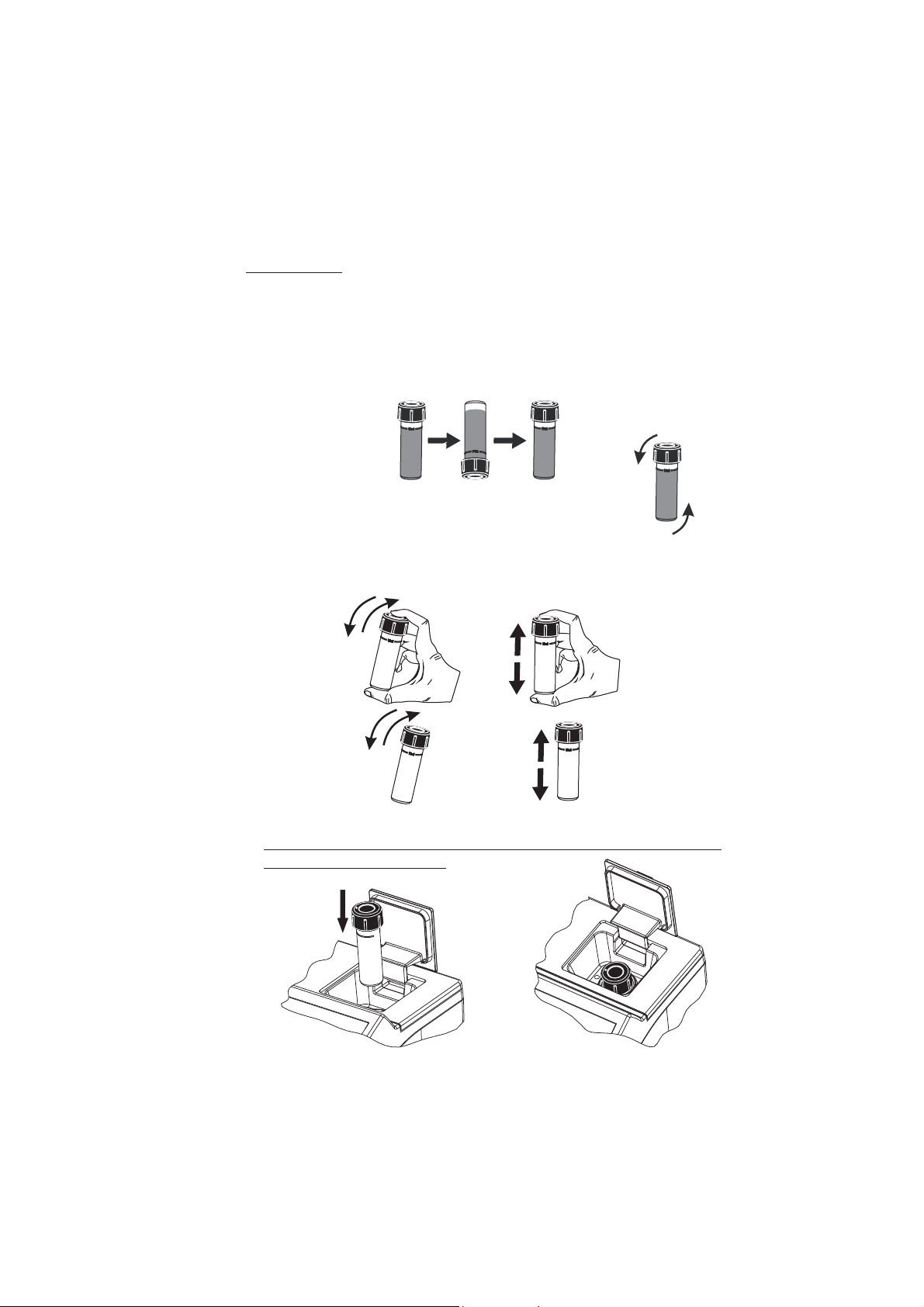
USING CUVETTES
• Proper mixing is very important for reproducibility of the measurements. The right way of mixing a
cuvette is specified for each method in the related chapter.
(a) invert the cuvette a couple of times or for a specified time: hold the cuvette in the vertical position. Turn
the cuvette upside-down and wait for all of the solution to flow to the cap end, then return the cuvette
to the upright vertical position and wait for all of the solution to flow to the cuvette bottom. This is one
inversion. The correct speed for this mixing technique is 10-15 complete inversions in 30 seconds.
This mixing technique is indicated with “invert to mix” and the following icon:
(b) shaking the cuvette, moving the cuvette up and down. The movement may be gentle or vigorous.
This mixing method is indicated with “shake gently” or “shake vigorously”, and one of the following
icons:
shake gently shake vigorously
• Pay attention to push the cuvette completely down in the holder and to align the white point on the
cap to the indexing mark on the meter.
9
Page 10
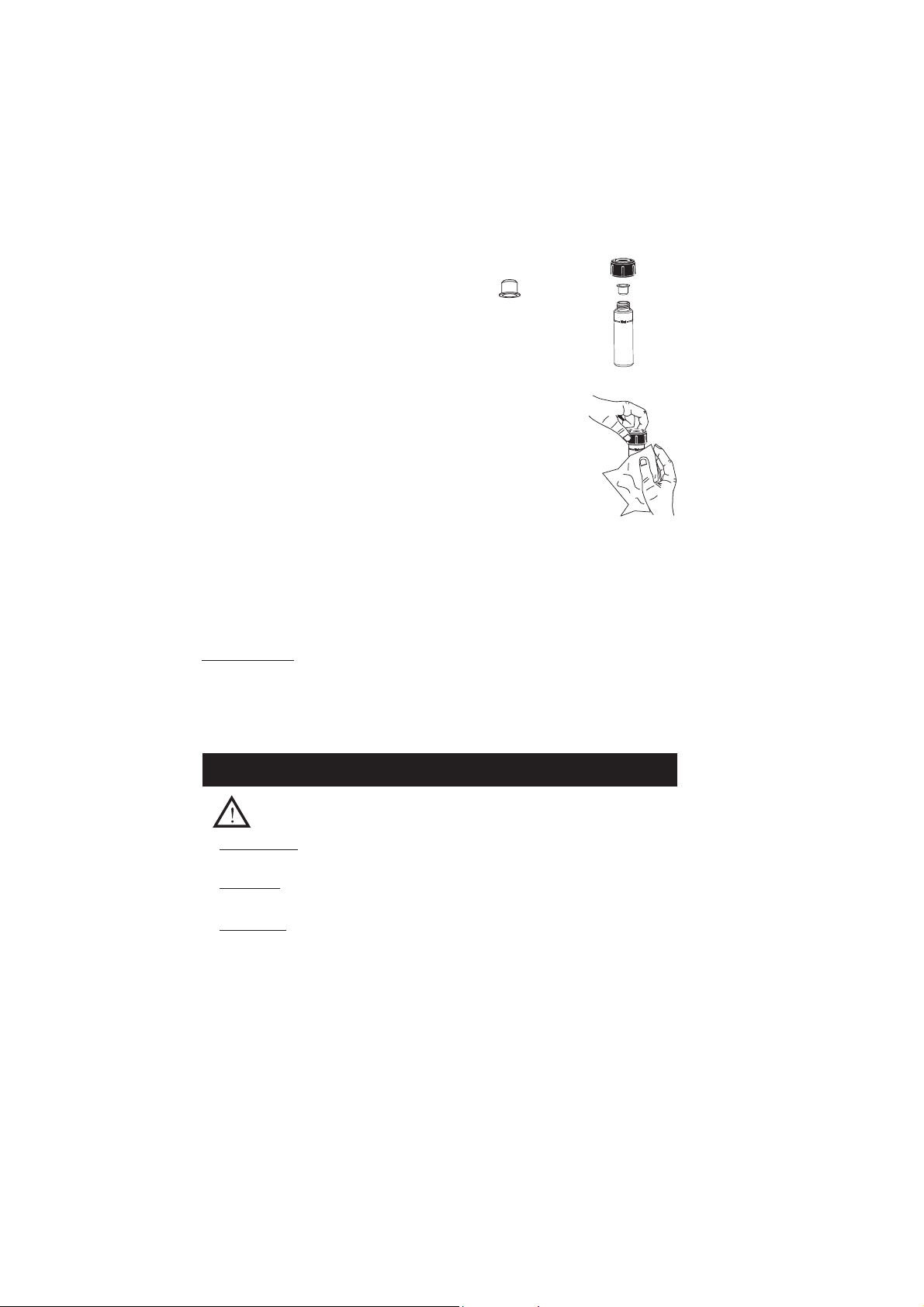
• In order to avoid reagent leaking and to obtain more accurate measurements,
close the cuvette first with the supplied HDPE plastic stopper and then
the black cap.
• Whenever the cuvette is placed into the measurement cell, it must be dry
outside, and free of fingerprints, oil or dirt. Wipe it thoroughly with HI 731318
or a lint-free cloth prior to insertion.
• Shaking the cuvette can generate bubbles in the sample, causing higher
readings. To obtain accurate measurements, remove such bubbles by swirling
or by gently tapping the cuvette.
• Do not let the reacted sample stand too long after reagent is added. For best
accuracy, respect the timings described in each specific method.
• It is possible to take multiple readings in a row, but it is recommended to take
a new zero reading for each sample and to use the same cuvette for zeroing
and measurement when possible (for most precise results follow the measurement
procedures carefully).
• Discard the sample immediately after the reading is taken, or the glass might become permanently
stained.
• All the reaction times reported in this manual are at 25 °C (77 °F). In general, the reaction time
should be increased for temperatures lower than 20 °C (68 °F), and decreased for temperatures higher
than 25 °C (77 °F).
INTERFERENCES
• In the method measurement section the most common interferences that may be present in an average
sample matrix have been reported. It may be that for a particular treatment process other compounds
do interfere with the method of analysis.
HEALTH & SAFETY
• The chemicals contained in the reagent kits may be hazardous if improperly handled.
• Read the Material Safety Data Sheet (MSDS) before performing tests.
• Safety equipment: Wear suitable eye protection and clothing when required, and follow instructions
carefully.
• Reagent spills: If a reagent spill occurs, wipe up immediately and rinse with plenty of water.
If reagent contacts skin, rinse the affected area thoroughly with water. Avoid breathing released vapors.
• Waste disposal: for proper disposal of reagent kits and reacted samples, refer to the Material Safety
Data Sheet (MSDS).
10
Page 11

METHOD REFERENCE TABLE
Method Method Page
description
1 Ammonia HR 23
2 Ammonia MR 25
3 Ammonia LR 27
4 Nitrate HR 29
5 Nitrate MR 31
6 Nitrate LR 33
Method Method Page
description
7 Phosphorus HR 35
8 Phosphorus MR 37
9 Phosphorus LR 39
10 Potassium HR 41
11 Potassium MR 44
12 Potassium LR 46
OPERATIONAL GUIDE
POWER CONNECTION AND BATTERY MANAGEMENT
The meter can be powered from an AC/DC adapter (included) or from the built-in rechargeable battery.
Note: Always turn the meter off before unplugging it to ensure no data is lost.
When the meter switches ON, it verifies if the power supply adapter is connected. The battery icon on the
LCD will indicate the battery status:
- battery is charging from external adapter - battery fully charged (meter connected to AC/DC adapter)
- battery capacity (no external adapter) - battery Low (no external adapter)
- battery Dead (no external adapter)
11
Page 12

METHOD SELECTION
• Turn the instrument ON via the ON/OFF power switch.
• The meter will perform an autodiagnostic test. During this test, the Hanna Instrument logo will appear
on the LCD. After 5 seconds, if the test was successful, the last method used will appear on the display.
• In order to select the desired method press the METHOD key and a screen with the available methods
will appear.
• Press the keys to highlight the desired method. Press Select.
• After the desired method is selected, follow the measurement described in the related section.
• Before performing a test you should read all the instructions carefully.
DATA MANAGEMENT
The instrument features a data log function to help you keep track of all your analysis. The data log can
hold 200 individual measurements. Storing, viewing and deleting the data is possible using the LOG
and
RCL keys
Storing data
stored with date and time stamps.
..
.
..
: You can store only a valid measurement. Press LOG
and the last valid measurement will be
Viewing and deleting
the last saved measurement. Additionally, you can delete the data records all at once.
: You can view and delete the data log by pressing the RCL key. You can only delete
12
Page 13
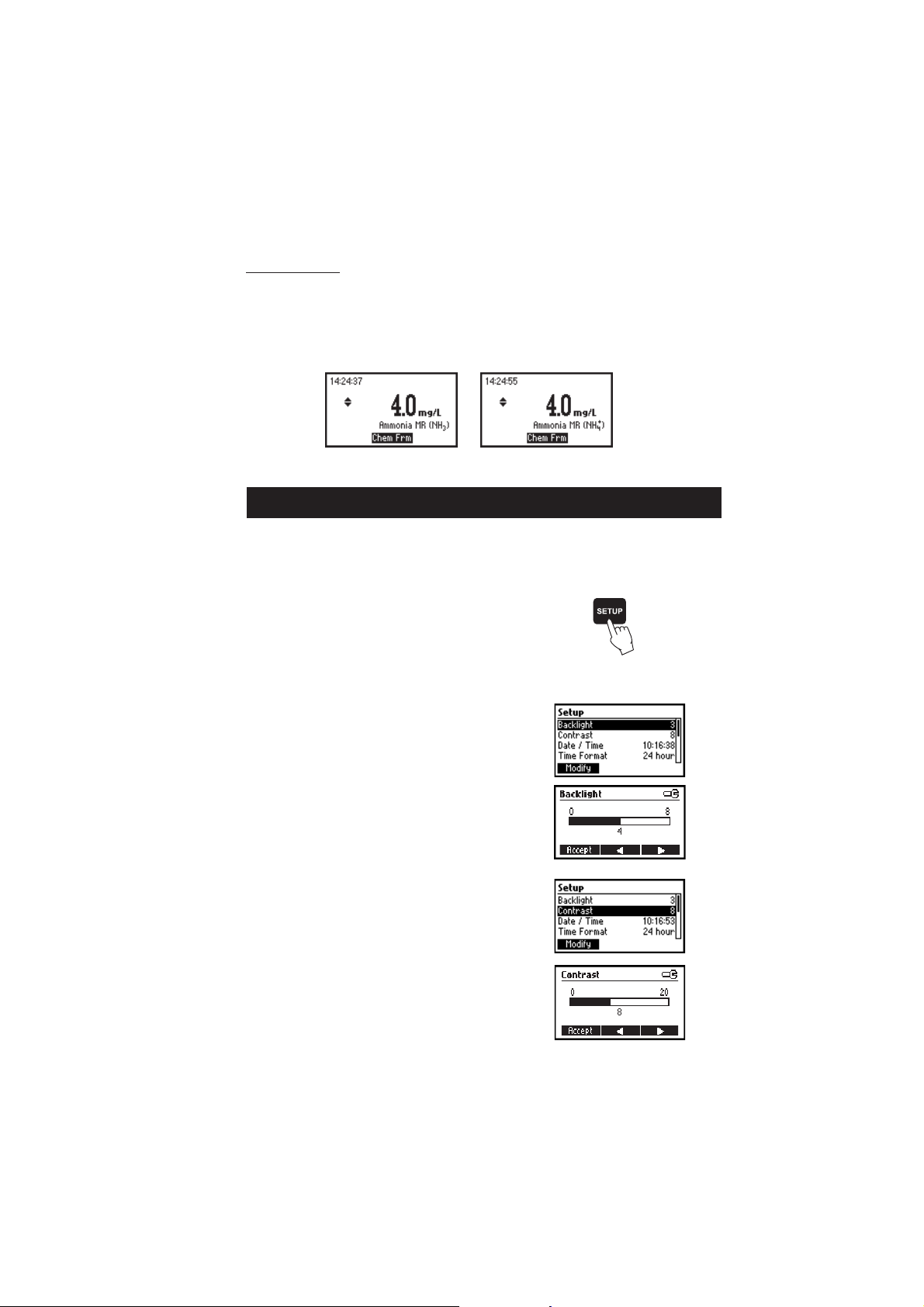
CHEMICAL FORM
Chemical form conversion factors are pre-programmed into the instrument and are method specific. In order
to view the displayed result in the desired chemical form press or to access the second level functions
and then press the Chem Frm key to toggle between the available chemical forms for the selected method.
SETUPSETUP
SETUP
SETUPSETUP
In the Setup mode the instrument’s parameters can be changed. Some parameters affect the measuring
sequence and others are general parameters that change the behavior or appearance of the instrument.
Press SETUP to enter the setup mode.
Press ESC or SETUP to return to the main screen.
A list of setup parameters will be displayed with currently
configured settings. Press HELP for additional information.
Press the keys to select a parameter and change the
value as follows:
Backlight
Values: 0 to 8.
Press the Modify key to access the backlight value.
Use the W X functional keys or the keys to increase or
decrease the value.
Press the Accept key to confirm or ESC to return to the setup
menu without saving the new value.
Contrast
Values: 0 to 20.
This option is used to set the display’s contrast.
Press the Modify key to change the display’s contrast.
Use the W X functional keys or the keys to increase or
decrease the value.
Press the Accept key to confirm the value or ESC to return to the
setup menu without saving the new value.
13
Page 14
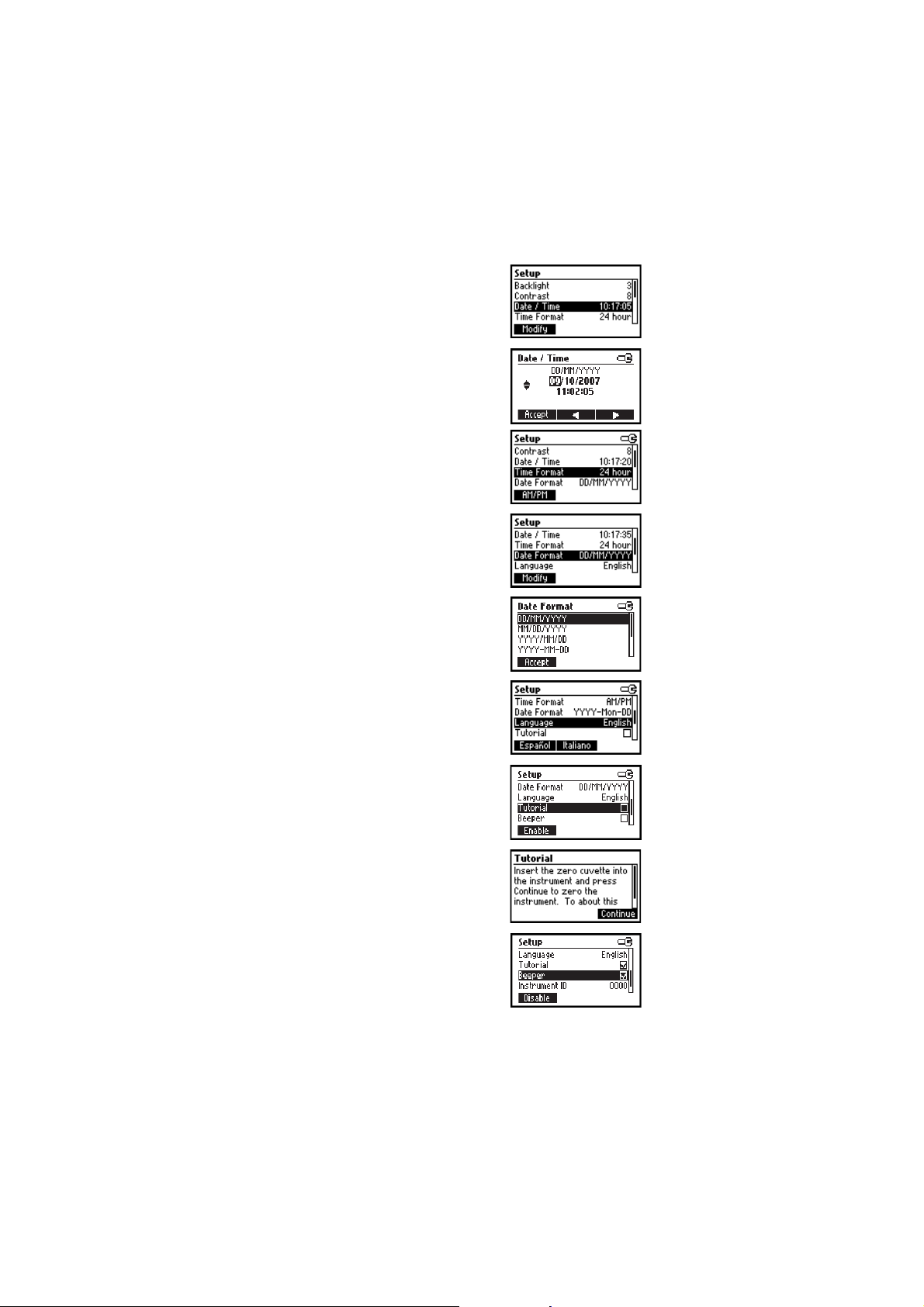
Date / Time
This option is used to set the instrument’s date and time.
Press the Modify key to change the date/time.
Press the W X functional keys to highlight the value to be
modified (year, month, day, hour, minute or second). Use the
keys to change the value.
Press the Accept key to confirm or ESC to return to the setup
without saving the new date or time.
Time format
Option: AM/PM or 24 hour.
Press the functional key to select the desired time format.
Date format
Press the Modify key to change the Date Format.
Use the keys to select the desired format.
Press the Accept key to confirm or ESC to return to the setup
menu without saving the new format.
Language
Press the corresponding key to change the language.
If the new language cannot be loaded, the previously selected
language will be reloaded.
Tutorial
Option: Enable or Disable.
If enabled this option will provide the user short guide related to
the current screen.
Press the functional key to enable/disable the tutorial mode.
Beeper
Option: Enable or Disable.
When enabled, a short beep is heard every time a key is pressed.
A long beep alert sounds when the pressed key is not active or an
error condition is detected.
Press the functional key to enable/disable the beeper.
14
Page 15
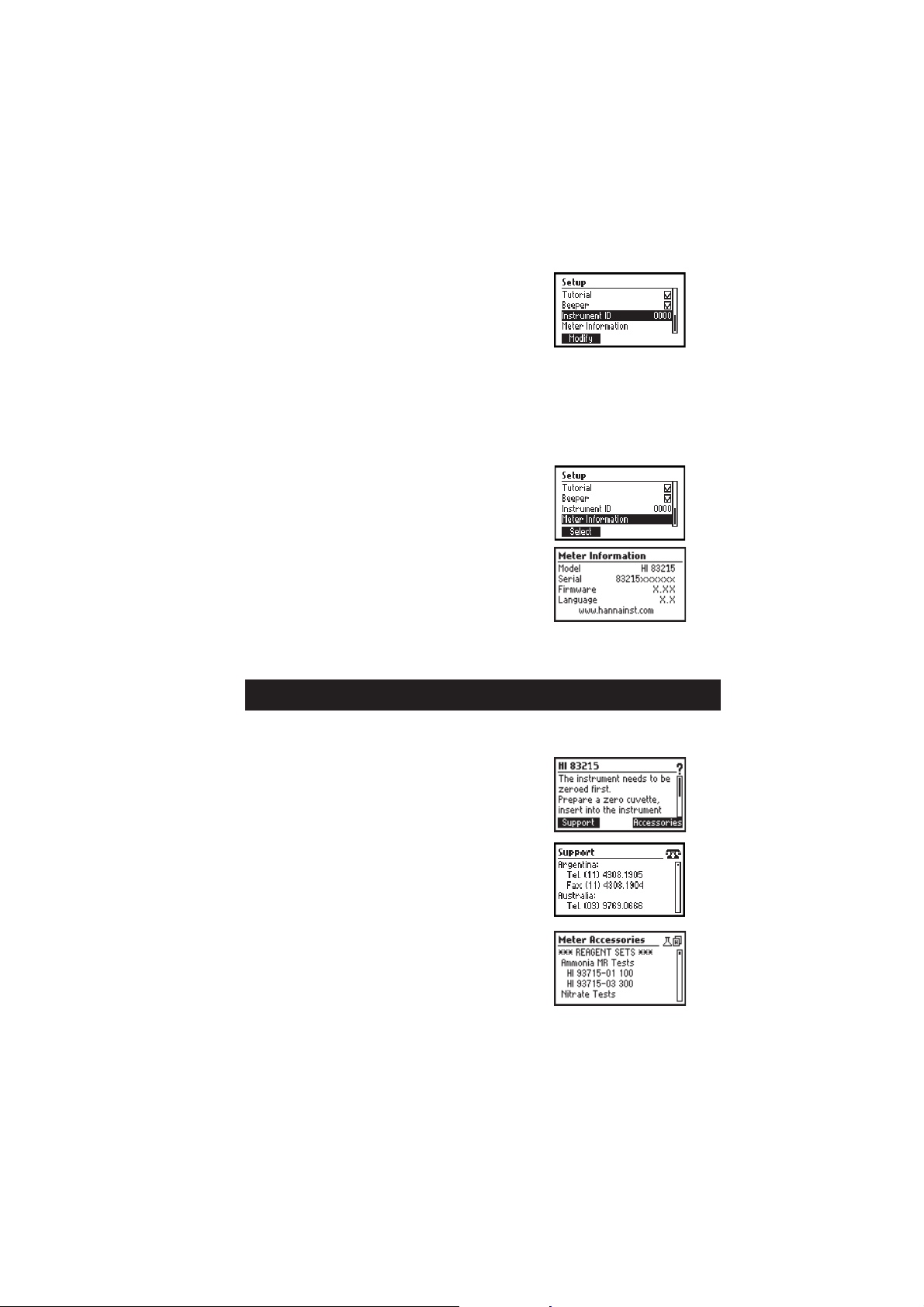
Instrument ID
Option: 0 to 9999.
This option is used to set the instrument’s ID (identification
number). The instrument ID is used while exchanging data with
a PC.
Press the Modify key to access the instrument ID screen. Press
the keys in order to set the desired value.
Press the Accept key to confirm the value or ESC to return to the
setup menu without saving the new value.
Meter information
Press the Select key to view the instrument model, firmware
version, language version and instrument serial number.
Press ESC to return to the Setup mode.
HELP MODEHELP MODE
HELP MODE
HELP MODEHELP MODE
HELP MODE
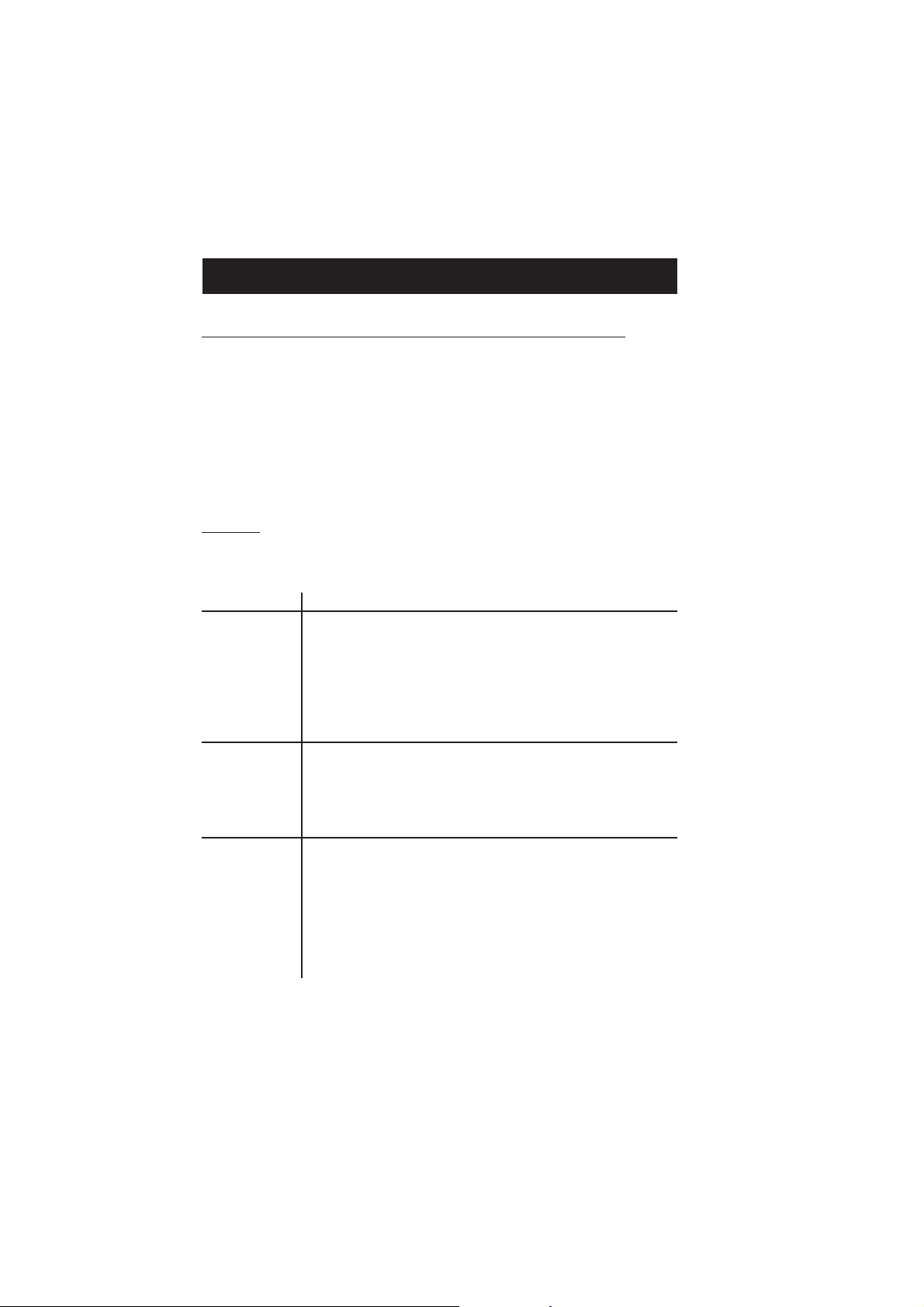
HI 83215 offers an interactive contextual help mode that assists the user at any time.
To access the help screens press HELP.
The instrument will display additional information related to the
current screen. To read all the available information, scroll the
text using the keys.
Press the Support key to access a screen with Hanna service
centers and their contact details.
Press the Accessories key to access a list of instrument reagents
and accessories.
To exit support or accessories screens press ESC and the
instrument will return to the previous help screen.
To exit help mode press the HELP or ESC key again and the
meter will return to the previously selected screen.
15
Page 16
INTRODUCTIONINTRODUCTION
INTRODUCTION
INTRODUCTIONINTRODUCTION
THE ROLE OF NUTRIENTS IN THE GROWTH AND PRODUCTION OF PLANTS
The three elements that are mostly needed by the plants are nitrogen (N), phosphorus (P) and potassium
(K). They are called the macronutrients while other elements, needed by plants in smaller amounts, are
called microelements. In hydroponics, plants need a balanced nutritive solution, composed of macro and
microelements.
Shortage or excess of even only one nutritive element may cause an imbalance in plant physiology and in
the absorption of the other nutrients. Nutrients shortages may result in irregular plant growth, low
resistance to diseases, scarce production both in quantity and quality, while nutrients excess may cause
waste of fertilizer, pollution of the groundwater and the possible accumulation of dangerous substances in
the crops produced.
NITROGEN
Nitrogen (N) is mostly absorbed by plants as nitrates (NO3¯) and, in smaller amount, in the form of
ammonium (NH
solutions.
PRESENT IN
ACTION
SHORTAGE
EFFECTS
EXCESS EFFECTS
+
). In hydroponics, an adequate ratio between the two forms is generally used in nutritive
4
proteins, enzymes, chlorophyll, hormones, vitamins, DNA and RNA
• is fundamental for plants in phase of growth
• promotes the lengthening of trunks and sprouts
• increases the production of foliage
• helps to absorb other nutrients
(in particular phosphorus)
• assists a bigger production for both size and
number of fruits
• slower growth
• smaller leaves
• yellowing of leaves
• smaller fruits
• premature ripening
• reduction in resistance to diseases and atmospheric agents
• increase of water demand (caused by an excessive production of leaves)
• bad quality of fruits
• delayed ripening
• reduction in potassium absorption
16
Page 17

PHOSPHORUS
Phosphorus (P) has an important role in many fundamental biochemical and physiological processes. Plants
take up phosphorus in the form of phosphate ion (PO
PRESENT IN
ACTION
SHORTAGE
EFFECTS
EXCESS EFFECTS
DNA and RNA, ATP, ADP
• stimulates the roots growth
• stimulates blooming
• stimulates fecundation and ripeness
• strengthens the plant tissues
• is necessary in the formation of seeds
• delayed ripening
• slower growth
• small leaves
• decrease of production (smaller fruits and difficult seeds formation)
• reduction of root system
• premature ripening
• excess of fruit-setting
• negative effects on the absorption of some microelements such as iron, zinc, boron and copper
3
¯).
4
POTASSIUM
Potassium (K) is essential in proteic synthesis. The problem of lack of potassium is quite frequent in
calcareous soils.
Potassium is absorbed as K+.
PRESENT IN
ACTION
SHORTAGE
EFFECTS
tissues responsible for the growth of plants (primary and secondary meristems), embryos and cell
vacuole
• improves the quality of fruits and flowers
• gives more resistance both to frost and to diseases caused by fungi (increases the cuticular thickness)
• regulates the cellular turgidity (helps to regulate the osmotic processes and increases the
resistance to dryness)
• regulates the stomatic opening and closing (it means a strong influence on transpiration and
photosynthesis)
• slower growth
• smaller fruits, less colored and less preserved
• increase of transpiration
• less resistance to the cold
EXCESS EFFECTS
• reduced absorption of calcium and magnesium
• increase of water consumption
• increase of the substrate salinity
17
Page 18

IRRIGATION WATER (LOW RANGE)
In agricultural areas it is quite common to find altered values in the chemical composition of irrigation waters.
The problem concerns mostly the high nitrate concentration, usually determined by excessive fertilization or
irrational liquid manure spreading. The analysis of irrigation waters allows us to find out which are the
substances present in major or minor quantity and to organize an advantageous fertilization plan.
For example, if the quantity of water utilized for crop cultivation is 250 mm/ha (= 2500000 L/ha) and
the nitrate (NO3¯) concentration is 150 mg/L (34 mg/L as nitrate-nitrogen NO3-N), soil receives 85 kg/ha
of nitrogen. In choosing type and quantity of fertilizer to be used, it is important to consider this
information, in order not to waste fertilizer nor to induce soil pollution.
NUTRIENTS SOLUTIONS (MEDIUM AND HIGH RANGE)
The nutrients requirements of the plants are determined by the type of plant, its age and the
environmental conditions. The control of chemical composition of nutrients solutions given to the plants is an
operation that allows a correct preparation of the fertilizer. In analyzing the solution, it is possible to choose
between medium range and high range values, depending on the concentration of substances.
Medium range usually covers the analysis of residual solutions in recycling systems. The nutritive elements
are differently absorbed by the plants, hence the nutrient solution loses substances, becomes impoverished
and must be enriched.
High range normally corresponds to the typical values of nutrients solutions. It is therefore possible to verify
that the solution given to the plants contains the correct quantities of nutritive substances.
18
Page 19

SAMPLE PREPARATION
SAMPLE PREPARATION PROCEDURE
Note: If the water sample to be analyzed is very turbid, let it stand in a beaker for a while until most
of the solid particles have settled. Then, use the pipette to transfer the supernatant solution to the
other beaker and prepare the samples as described below. To prevent the displacement of the settled
solids at the bottom of the beaker, do not induce air bubbles into the solution.
IRRIGATION WATER (LR):
• Measure 100 mL of sample with the graduated cylinder.
• If the solution contains some turbidity or color, pour it in the large 170 mL beaker and add a powder
packet of active carbon.
• Mix well using the spoon and then wait for 5 minutes.
19
Page 20

• Fold a filter disc twice as shown in the figure. Separate one side from the other three to form a cone.
Insert the folded filter disc in the funnel.
• Filter the treated sample into an empty beaker.
The sample is now ready.
Note: Filter at least 40 mL of solution if all the four methods will be tested. If the solution is still turbid
or colored, treat it again with a packet of active carbon. After use, throw the filter disc away and wash
the syringe and the filter assembly well. Always use a new disc for another sample.
NUTRIENTS SOLUTION (MR):
• Use the graduated cylinder to measure exactly 20 mL of sample.
• Remove the cap and fill the Demineralizer Bottle with tap water.
20
Page 21

• Replace the cap and shake gently for at least 2 minutes.
• Open the upper part of the Demineralizer Bottle cap and gently squirt
the demineralized water into the cylinder, up to the 100 mL mark.
Note: The ion exchange resin contained in the Demineralizer Bottle is
provided with an indicator substance. The indicator will change from
green to blue when the resin has been exhausted and needs to be
replaced.
• Pour the solution in the large 170 mL beaker, replace the cap and
invert several times to mix.
• If the solution contains some turbidity or color, add a powder packet of active carbon and follow the
procedure described for Irrigation Water (LR).
NUTRIENTS SOLUTION (HR):
• Add 10 mL of sample to the graduated cylinder using the 5 mL syringe
(twice).
21
Page 22

Note: To measure exactly 5 mL of sample with the syringe, push the plunger completely into the
syringe and insert the tip into the sample. Pull the plunger out until the lower edge of the seal is on
the 5 mL mark of the syringe.
• Remove the cap and fill the Demineralizer Bottle with tap water.
• Replace the cap and shake gently for at least 2 minutes.
• Open the upper part of the Demineralizer Bottle cap and squirt
gently the demineralized water into the cylinder, up to the 100 mL
mark.
• Pour the solution in the large 170 mL beaker, replace the cap and
invert several times to mix.
• If the solution contains some turbidity or color, add a powder packet of active carbon and follow the
procedure described for Irrigation Water (LR).
22
Page 23

AMMONIA HIGH RANGE
SPECIFICATIONS
Range 0 to 100 mg/L
Resolution 1 mg/L
Accuracy ±1 mg/L ±4% of reading at 25 °C
Typical EMC ±1 mg/L
Deviation
Light Source Tungsten lamp with narrow band interference filter @ 420 nm
Method Adaptation of the
Nessler method. The reaction between ammonia and reagents causes a yellow tint in
the sample.
REQUIRED REAGENTS
Code Description Quantity
HI 93715A-0 First Reagent 4 drops (6 drops for seawater)
HI 93715B-0 Second Reagent 4 drops (10 drops for seawater)
REAGENT SETS
HI 93715-01 Reagents for 100 tests
HI 93715-03 Reagents for 300 tests
For other accessories see page 50.
MEASUREMENT PROCEDURE
Note: for sample preparation follow the NUTRIENTS SOLUTION (HR)
procedure at page 21.
ASTM Manual of Water and Environmental Technology, D1426-92
,
• Select the
• Fill the cuvette with 10 mL of unreacted sample (up to the mark)
• Place the cuvette into the holder and close the lid.
• Press the Zero key. The display will show “-0.0-” when the meter is zeroed and ready for
Ammonia HR
the
Method Selection
and replace the cap.
measurement.
method using the procedure described in
section (see page 12).
23
10 mL
Ammonia HR
Page 24

• Remove the cuvette.
• Add 4 drops of HI 93715A-0 First Reagent (6 drops for
seawater analysis). Replace the cap and mix the solution by
inverting the cuvette a couple of times.
• Add 4 drops of HI 93715B-0 Second Reagent (10 drops for
seawater analysis). Replace the cap and mix the solution by
inverting the cuvette a couple of times.
• Reinsert the cuvette into the instrument.
• Press Timer and the display will show the countdown prior to
the measurement or, alternatively, wait for 3 minutes and 30
seconds and press Read. When the timer ends the meter will
perform the reading. The instrument displays the results in
mg/L of ammonia nitrogen (NH3-N).
• Press or to access the second level functions.
• Press the Chem Frm key to convert the result in mg/L of ammonia (NH3) and ammonium (NH
+
4
• Press or to return to the measurement screen.
INTERFERENCES
Interference may be caused by: acetone, alcohols, aldehydes, glycine, hardness above 1 g/L, iron, organic
chloramines, sulfide, various aliphatic and aromatic amines.
Ammonia HR
24
).
Page 25

AMMONIA MEDIUM RANGE
SPECIFICATIONS
Range 0.0 to 50.0 mg/L
Resolution 0.5 mg/L
Accuracy ±0.5 mg/L ±4% of reading at 25 °C
Typical EMC ±0.5 mg/L
Deviation
Light Source Tungsten lamp with narrow band interference filter @ 420 nm
Method Adaptation of the
Nessler method. The reaction between ammonia and reagents causes a yellow tint in
the sample.
REQUIRED REAGENTS
Code Description Quantity
HI 93715A-0 First Reagent 4 drops (6 drops for seawater)
HI 93715B-0 Second Reagent 4 drops (10 drops for seawater)
REAGENT SETS
HI 93715-01 Reagents for 100 tests
HI 93715-03 Reagents for 300 tests
For other accessories see page 50.
MEASUREMENT PROCEDURE
Note: for sample preparation follow the NUTRIENTS SOLUTION (MR)
procedure at page 20.
ASTM Manual of Water and Environmental Technology, D1426-92
,
10 mL
• Select the
• Fill the cuvette with 10 mL of unreacted sample (up to the mark)
• Place the cuvette into the holder and close the lid.
• Press the Zero key. The display will show “-0.0-” when the meter is zeroed and ready for
Ammonia MR
the
Method Selection
and replace the cap.
measurement.
method using the procedure described in
section (see page 12).
25
Ammonia MR
Page 26

• Remove the cuvette.
• Add 4 drops of HI 93715A-0 First Reagent (6 drops for
seawater analysis). Replace the cap and mix the solution by
inverting the cuvette a couple of times.
• Add 4 drops of HI 93715B-0 Second Reagent (10 drops for
seawater analysis). Replace the cap and mix the solution by
inverting the cuvette a couple of times.
• Reinsert the cuvette into the instrument.
• Press Timer and the display will show the countdown prior to
the measurement or, alternatively, wait for 3 minutes and 30
seconds and press Read. When the timer ends the meter will
perform the reading. The instrument displays the results in
mg/L of ammonia nitrogen (NH3-N).
• Press or to access the second level functions.
• Press the Chem Frm key to convert the result in mg/L of ammonia (NH3) and ammonium (NH
+
4
• Press or to return to the measurement screen.
INTERFERENCES
Interference may be caused by:
acetone, alcohols, aldehydes, glycine, hardness above 1 g/L, iron, organic chloramines, sulfide, various
aliphatic and aromatic amines.
Ammonia MR
26
).
Page 27

AMMONIA LOW RANGE
SPECIFICATIONS
Range 0.0 to 10.0 mg/L
Resolution 0.1 mg/L
Accuracy ±0.1 mg/L ±4% of reading at 25 °C
Typical EMC ±0.1 mg/L
Deviation
Light Source Tungsten lamp with narrow band interference filter @ 420 nm
Method Adaptation of the
Nessler method. The reaction between ammonia and reagents causes a yellow tint in
the sample.
REQUIRED REAGENTS
Code Description Quantity
HI 93715A-0 First Reagent 4 drops (6 drops for seawater)
HI 93715B-0 Second Reagent 4 drops (10 drops for seawater)
REAGENT SETS
HI 93715-01 Reagents for 100 tests
HI 93715-03 Reagents for 300 tests
For other accessories see page 50.
MEASUREMENT PROCEDURE
Note: for sample preparation follow the IRRIGATION WATER (LR)
procedure at page 19.
• Select the
the
Ammonia LR
Method Selection
method using the procedure described in
section (see page 12).
ASTM Manual of Water and Environmental Technology, D1426-92
,
10 mL
• Fill the cuvette with 10 mL of unreacted sample (up to the mark)
and replace the cap.
• Place the cuvette into the holder and close the lid.
• Press the Zero key. The display will show “-0.0-” when the meter is zeroed and ready for
measurement.
27
Ammonia LR
Page 28

• Remove the cuvette.
• Add 4 drops of HI 93715A-0 First Reagent (6 drops for
seawater analysis). Replace the cap and mix the solution by
inverting the cuvette a couple of times.
• Add 4 drops of HI 93715B-0 Second Reagent (10 drops for
seawater analysis). Replace the cap and mix the solution by
inverting the cuvette a couple of times.
• Reinsert the cuvette into the instrument.
• Press Timer and the display will show the countdown prior to the
measurement or, alternatively, wait for 3 minutes and 30 seconds
and press Read. When the timer ends the meter will perform the
reading. The instrument displays the results in mg/L of ammonia
nitrogen (NH3-N).
• Press or to access the second level functions.
• Press the Chem Frm key to convert the result in mg/L of ammonia (NH3) and ammonium (NH
+
4
• Press or to return to the measurement screen.
INTERFERENCES
Interference may be caused by: acetone, alcohols, aldehydes, glycine, hardness above 1 g/L, iron, organic
chloramines, sulfide, various aliphatic and aromatic amines.
Ammonia LR
28
).
Page 29

NITRATE HIGH RANGE
SPECIFICATIONS
Range 0 to 300 mg/L
Resolution 5 mg/L
Accuracy ±10 mg/L ±8% of reading at 25 °C
Typical EMC ±5 mg/L
Deviation
Light Source Tungsten lamp with narrow band interference filter @ 525 nm
Method Adaptation of the cadmium reduction method. The reaction between nitrate-nitrogen
and the reagent causes an amber tint in the sample.
REQUIRED REAGENTS
Code Description Quantity
HI 93728-0 Powder reagent 1 packet
REAGENT SETS
HI 93728-01 Reagents for 100 tests
HI 93728-03 Reagents for 300 tests
For other accessories see page 50.
MEASUREMENT PROCEDURE
Note: for sample preparation follow the NUTRIENTS SOLUTION (HR) procedure at page 21.
• Select the
described in the
• Using the pipette, fill the cuvette with 6 ml of sample,
up to half of its height, and replace the cap.
• Place the cuvette into the holder and close the lid.
• Press the Zero key. The display will show “-0.0-” when the meter is zeroed and ready for measurement.
• Remove the cuvette and add the content of one packet of
HI 93728-0 reagent.
Nitrate HR
Method Selection
method using the procedure
section (see page 12).
29
6 mL
Nitrate HR
Page 30

• Replace the cap and immediately shake vigorously up
and down for exactly 10 seconds. Continue to mix by
inverting the cuvette gently for 50 seconds, while
taking care not to induce air bubbles. Powder will not
completely dissolve. Time and way of shaking could
sensitively affect the measurement.
• Reinsert the cuvette into the instrument, taking care
not to shake it.
• Press Timer and the display will show the countdown
prior to the measurement or, alternatively, wait for
4 minutes and 30 seconds and press Read. When
the timer ends the meter will perform the reading.
The instrument displays the results in mg/L of
nitrate-nitrogen.
• Press or to access the second level functions.
• Press the Chem Frm key to convert the result in mg/L of nitrate (NO3¯).
• Press or to return to the measurement screen.
INTERFERENCES
Interference may be caused by:
Ammonia and amines, as urea and primary aliphatic amines
Chloride above 100 ppm
Chlorine above 2 ppm
Copper
Iron(III)
Strong oxidizing and reducing substances
Sulfide must be absent
Nitrate HR
30
Page 31

NITRATE MEDIUM RANGE
SPECIFICATIONS
Range 0 to 150 mg/L
Resolution 2.5 mg/L
Accuracy ±5 mg/L ±8% of reading at 25 °C
Typical EMC ±2.5 mg/L
Deviation
Light Source Tungsten lamp with narrow band interference filter @ 525 nm
Method Adaptation of the cadmium reduction method. The reaction between nitrate-nitrogen
and the reagent causes an amber tint in the sample.
REQUIRED REAGENTS
Code Description Quantity
HI 93728-0 Powder reagent 1 packet
REAGENT SETS
HI 93728-01 Reagents for 100 tests
HI 93728-03 Reagents for 300 tests
For other accessories see page 50.
MEASUREMENT PROCEDURE
Note: for sample preparation follow the NUTRIENTS SOLUTION (MR) procedure at page 20.
• Select the
described in the
• Using the pipette, fill the cuvette with 6 ml of
sample, up to half of its height, and replace the cap.
• Place the cuvette into the holder and close the lid.
• Press the Zero key. The display will show “-0.0-” when the meter is zeroed and ready for measurement.
• Remove the cuvette and add the content of one packet of
HI 93728-0 reagent.
Nitrate MR
Method Selection
method using the procedure
section (see page 12).
31
6 mL
Nitrate MR
Page 32

• Replace the cap and immediately shake vigorously up
and down for exactly 10 seconds. Continue to mix by
inverting the cuvette gently for 50 seconds, while
taking care not to induce air bubbles. Powder will not
completely dissolve. Time and way of shaking could
sensitively affect the measurement.
• Reinsert the cuvette into the instrument, taking care
not to shake it.
• Press Timer and the display will show the countdown
prior to the measurement or, alternatively, wait for
4 minutes and 30 seconds and press Read. When
the timer ends the meter will perform the reading.
The instrument displays the results in mg/L of
nitrate-nitrogen.
• Press or to access the second level functions.
• Press the Chem Frm key to convert the result in mg/L of nitrate (NO3¯).
• Press or to return to the measurement screen.
INTERFERENCES
Interference may be caused by:
Ammonia and amines, as urea and primary aliphatic amines
Chloride above 100 ppm
Chlorine above 2 ppm
Copper
Iron(III)
Strong oxidizing and reducing substances
Sulfide must be absent
Nitrate MR
32
Page 33

NITRATE LOW RANGE
SPECIFICATIONS
Range 0.0 to 30.0 mg/L
Resolution 0.5 mg/L
Accuracy ±1.0 mg/L ±8% of reading at 25 °C
Typical EMC ±0.5 mg/L
Deviation
Light Source Tungsten lamp with narrow band interference filter @ 525 nm
Method Adaptation of the cadmium reduction method. The reaction between nitrate-nitrogen
and the reagent causes an amber tint in the sample.
REQUIRED REAGENTS
Code Description Quantity
HI 93728-0 Powder reagent 1 packet
REAGENT SETS
HI 93728-01 Reagents for 100 tests
HI 93728-03 Reagents for 300 tests
For other accessories see page 50.
MEASUREMENT PROCEDURE
Note: for sample preparation follow the IRRIGATION WATER (LR) procedure at page 19.
• Select the
described in the
• Using the pipette, fill the cuvette with 6 ml of sample,
up to half of its height, and replace the cap.
• Place the cuvette into the holder and close the lid.
• Press the Zero key. The display will show “-0.0-” when the meter is zeroed and ready for measurement.
• Remove the cuvette and add the content of one packet of
HI 93728-0 reagent.
Nitrate LR
Method Selection
method using the procedure
section (see page 12).
33
6 mL
Nitrate LR
Page 34

• Replace the cap and immediately shake vigorously up
and down for exactly 10 seconds. Continue to mix by
inverting the cuvette gently for 50 seconds, while
taking care not to induce air bubbles. Powder will not
completely dissolve. Time and way of shaking could
sensitively affect the measurement.
• Reinsert the cuvette into the instrument, taking care
not to shake it.
• Press Timer and the display will show the countdown
prior to the measurement or, alternatively, wait for
4 minutes and 30 seconds and press Read. When
the timer ends the meter will perform the reading.
The instrument displays the results in mg/L of
nitrate-nitrogen.
• Press or to access the second level functions.
• Press the Chem Frm key to convert the result in mg/L of nitrate (NO3¯).
• Press or to return to the measurement screen.
INTERFERENCES
Interference may be caused by:
Ammonia and amines, as urea and primary aliphatic amines
Chloride above 100 ppm
Chlorine above 2 ppm
Copper
Iron(III)
Strong oxidizing and reducing substances
Sulfide must be absent
Nitrate LR
34
Page 35

PHOSPHORUS HIGH RANGE
SPECIFICATIONS
Range 0 to 100 mg/L
Resolution 1 mg/L
Accuracy ±5 mg/L ±4% of reading at 25 °C
Typical EMC Dev. ±1 mg/L
Light Source Tungsten lamp with narrow band interference filter @ 525 nm
Method Adaptation of the
18th edition,
causes a blue tint in the sample.
REQUIRED REAGENTS
Code Description Quantity
HI 93706A-0 Molybdate 10 drops
HI 93706B-0 Amino Acid Powder 1 packet
REAGENT SETS
HI 93706-01 Reagents for 100 tests
HI 93706-03 Reagents for 300 tests
For other accessories see page 50.
MEASUREMENT PROCEDURE
Note: for sample preparation follow the NUTRIENTS SOLUTION (HR) procedure at page 21.
Standard Methods for the Examination of Water and Wastewater,
Amino Acid method. The reaction between phosphorus and reagents
• Select the
• Fill the cuvette with 10 mL of unreacted sample (up to the
• Place the cuvette into the holder and close the lid.
• Press the Zero key. The display will show “-0.0-” when the meter is zeroed and ready for measurement.
• Remove the cuvette.
• Add 10 drops of HI 937O6A-0 Molybdate reagent.
Phosphorus HR
in the
Method Selection
mark) and replace the cap.
method using the procedure described
section (see page 12).
10 mL
Phosphorus HR
35
Page 36

• Add the content of one packet of HI 93706B-0 Phosphorus
Reagent B (Amino Acid) to the cuvette. Replace the cap
and shake gently until completely dissolved.
• Reinsert the cuvette into the instrument.
• Press Timer and the display will show the countdown prior to the measurement or, alternatively, wait
for 5 minutes and press Read. When the timer ends the meter will perform the reading. The instrument
displays the results in mg/L of phosphorus (P).
• Press or to access the second level functions.
• Press the Chem Frm key to convert the result in mg/L of phosphate (PO
3
¯) and phosphorus pentoxide
4
(P2O5).
• Press or to return to the measurement screen.
INTERFERENCES
Interference may be caused by:
Sulfide
Chloride above 150000 mg/L
Calcium above 10000 mg/L as CaCO
Magnesium above 40000 mg/L as CaCO
Ferrous iron above 100 mg/L
Phosphorus HR
3
3
36
Page 37

PHOSPHORUS MEDIUM RANGE
SPECIFICATIONS
Range 0.0 to 50.0 mg/L
Resolution 0.5 mg/L
Accuracy ±2.5 mg/L ±4% of reading at 25 °C
Typical EMC Dev. ±0.5 mg/L
Light Source Tungsten lamp with narrow band interference filter @ 525 nm
Method Adaptation of the
18th edition,
causes a blue tint in the sample.
REQUIRED REAGENTS
Code Description Quantity
HI 93706A-0 Molybdate 10 drops
HI 93706B-0 Amino Acid Powder 1 packet
REAGENT SETS
HI 93706-01 Reagents for 100 tests
HI 93706-03 Reagents for 300 tests
For other accessories see page 50.
MEASUREMENT PROCEDURE
Note: for sample preparation follow the NUTRIENTS SOLUTION (MR) procedure at page 20.
Standard Methods for the Examination of Water and Wastewater,
Amino Acid method. The reaction between phosphorus and reagents
• Select the
described in the
• Fill the cuvette with 10 mL of unreacted sample (up to
the mark) and replace the cap.
• Place the cuvette into the holder and close the lid.
• Press the Zero key. The display will show “-0.0-” when the meter is zeroed and ready for measurement.
• Remove the cuvette.
• Add 10 drops of HI 937O6A-0 Molybdate reagent.
Phosphorus
Method Selection
method using the procedure
section (see page 12).
37
10 mL
Phosphorus MR
Page 38

• Add the content of one packet of HI 93706B-0 Phosphorus
Reagent B (Amino Acid) to the cuvette. Replace the cap
and shake gently until completely dissolved.
• Reinsert the cuvette into the instrument.
• Press Timer and the display will show the countdown prior to the measurement or, alternatively, wait
for 5 minutes and press Read. When the timer ends the meter will perform the reading. The instrument
displays the results in mg/L of phosphorus (P).
• Press or to access the second level functions.
• Press the Chem Frm key to convert the result in mg/L of phosphate (PO
3
¯) and phosphorus pentoxide
4
(P2O5).
• Press or to return to the measurement screen.
INTERFERENCES
Interference may be caused by:
Sulfide
Chloride above 150000 mg/L
Calcium above 10000 mg/L as CaCO
Magnesium above 40000 mg/L as CaCO
Ferrous iron above 100 mg/L
Phosphorus MR
3
3
38
Page 39

PHOSPHORUS LOW RANGE
SPECIFICATIONS
Range 0.0 to 10.0 mg/L
Resolution 0.1 mg/L
Accuracy ±0.5 mg/L ±4% of reading at 25 °C
Typical EMC Dev. ±0.1 mg/L
Light Source Tungsten lamp with narrow band interference filter @ 525 nm
Method Adaptation of the
18th edition,
causes a blue tint in the sample.
REQUIRED REAGENTS
Code Description Quantity
HI 93706A-0 Molybdate 10 drops
HI 93706B-0 Amino Acid Powder 1 packet
REAGENT SETS
HI 93706-01 Reagents for 100 tests
HI 93706-03 Reagents for 300 tests
For other accessories see page 50.
MEASUREMENT PROCEDURE
Note: for sample preparation follow the IRRIGATION WATER (LR) procedure at page 19.
Standard Methods for the Examination of Water and Wastewater,
Amino Acid method. The reaction between phosphorus and reagents
• Select the
described in the
• Fill the cuvette with 10 mL of unreacted sample (up to
the mark) and replace the cap.
• Place the cuvette into the holder and close the lid.
• Press the Zero key. The display will show “-0.0-” when the meter is zeroed and ready for measurement.
• Remove the cuvette.
• Add 10 drops of HI 937O6A-0 Molybdate reagent.
Phosphorus
Method Selection
method using the procedure
section (see page 12).
39
10 mL
Phosphorus LR
Page 40

• Add the content of one packet of HI 93706B-0 Phosphorus
Reagent B (Amino Acid) to the cuvette. Replace the cap
and shake gently until completely dissolved.
• Reinsert the cuvette into the instrument.
• Press Timer and the display will show the countdown prior to the measurement or, alternatively, wait
for 5 minutes and press Read. When the timer ends the meter will perform the reading. The instrument
displays the results in mg/L of phosphorus (P).
• Press or to access the second level functions.
• Press the Chem Frm key to convert the result in mg/L of phosphate (PO
3
¯) and phosphorus pentoxide
4
(P2O5).
• Press or to return to the measurement screen.
INTERFERENCES
Interference may be caused by:
Sulfide
Chloride above 150000 mg/L
Calcium above 10000 mg/L as CaCO
Magnesium above 40000 mg/L as CaCO
Ferrous iron above 100 mg/L
Phosphorus LR
3
3
40
Page 41

POTASSIUM HIGH RANGE
SPECIFICATIONS
Range 20 to 200 mg/L
Resolution 5 mg/L
Accuracy ±30 mg/L ±7% of reading at 25 °C
Typical EMC ±5 mg/L
Deviation
Light Source Tungsten lamp with narrow band interference filter @ 610 nm
Method Adaptation of the Turbidimetric Tetraphenylborate method. The reaction between
Potassium and reagents causes turbidity in the sample.
REQUIRED REAGENTS
Code Description Quantity
HI 93750A-0 Potassium Reagent 6 drops
HI 93750B-0 Powder Reagent 1 packet
REAGENT SETS
HI 93750-01 Reagents for 100 tests
HI 93750-03 Reagents for 300 tests
For other accessories see page 50.
MEASUREMENT PROCEDURE
Note: for sample preparation follow the NUTRIENT SOLUTIONS (HR) procedure at page 21.
• Select the
described in the
• Fill the cuvette with 10 mL of sample, up to the mark.
• Add six drops of HI 93750A-0, replace the cap and swirl
the solution.
• Place the cuvette into the holder and close the lid.
Potassium HR
Method Selection
method using the procedure
section (see page 12).
41
10 mL
Potassium HR
Page 42

• Press the Zero key. The display will show “-0.0-” when the meter is zeroed and ready for
measurement.
• Remove the cuvette and add the content of one packet of
HI 93750B-0 reagent. Replace the cap and gently mix for
one minute by slowly turning the cuvette upside down.
• Reinsert the cuvette into the instrument.
• Press Timer and the display will show the countdown prior to the measurement or, alternatively, wait
for 2 minutes and press Read. When the timer ends the meter will perform the reading. The instrument
displays the results in mg/L (ppm) of potasium (K).
• Press or to access the second level functions.
• Press the Chem Frm key to convert the result in mg/L of potassium oxide (K2O)
• Press or to return to the measurement screen.
• For ULTRA HIGH RANGE samples: follow the procedure described at page 43.
Potassium HR
42
Page 43

INTERFERENCES
Interferences may be caused by:
Ammonium above 10 ppm
Calcium above 10000 ppm as CaCO
3
Chloride above 12000 ppm
Magnesium above 8000 ppm as CaCO
3
Sodium above 8000 ppm
POTASSIUM ULTRA HIGH RANGE
For samples containing more than 200 ppm of Potassium: follow the sample preparation procedure
described at page 21 for NUTRIENTS SOLUTION (HR). Then add to the graduated cylinder 20 mL of the
prepared sample (for HR) and fill the cylinder with demineralized water from the Demineralizer Bottle up
to the 100 mL mark.
Follow the MEASUREMENT PROCEDURE at page 41.
Read the result in mg/L of Potassium on the display and multiply the reading by 5 to obtain the actual
concentration of Potassium.
43
Potassium HR
Page 44

POTASSIUM MEDIUM RANGE
SPECIFICATIONS
Range 10 to 100 mg/L
Resolution 2.5 mg/L
Accuracy ±15 mg/L ±7% of reading at 25 °C
Typical EMC ±2.5 mg/L
Deviation
Light Source Tungsten lamp with narrow band interference filter @ 610 nm
Method Adaptation of the Turbidimetric Tetraphenylborate method. The reaction between
Potassium and reagents causes turbidity in the sample.
REQUIRED REAGENTS
Code Description Quantity
HI 93750A-0 Potassium Reagent 6 drops
HI 93750B-0 Powder Reagent 1 packet
REAGENT SETS
HI 93750-01 Reagents for 100 tests
HI 93750-03 Reagents for 300 tests
For other accessories see page 50.
MEASUREMENT PROCEDURE
Note: for sample preparation follow the NUTRIENTS SOLUTION (MR) procedure at page 20.
• Select the
described in the
• Fill the cuvette with 10 mL of sample, up to the mark.
• Add six drops of HI 93750A-0, replace the cap and swirl
the solution.
• Place the cuvette into the holder and close the lid.
Potassium MR
Potassium MR
Method Selection
method using the procedure
section (see page 12).
44
10 mL
Page 45

• Press the Zero key. The display will show “-0.0-” when the meter is zeroed and ready for measurement.
• Remove the cuvette and add the content of one packet
of HI 93750B-0 reagent. Replace the cap and gently mix
for one minute by slowly turning the cuvette upside down.
• Reinsert the cuvette into the instrument.
• Press Timer and the display will show the countdown prior
to the measurement or, alternatively, wait for 2 minutes
and press Read. When the timer ends the meter will
perform the reading. The instrument displays the results in
mg/L (ppm) of potassium (K).
• Press or to access the second level functions.
• Press the Chem Frm key to convert the result in mg/L of potassium oxide (K2O).
• Press or to return to the measurement screen.
INTERFERENCES
Interferences may be caused by:
Ammonium above 10 ppm
Calcium above 10000 ppm as CaCO
Chloride above 12000 ppm
Magnesium above 8000 ppm as CaCO
Sodium above 8000 ppm
3
3
45
Potassium MR
Page 46

POTASSIUM LOW RANGE
SPECIFICATIONS
Range 0.0 to 20.0 mg/L
Resolution 0.5 mg/L
Accuracy ±3.0 mg/L ±7% of reading at 25 °C
Typical EMC ±0.5 mg/L
Deviation
Light Source Tungsten lamp with narrow band interference filter @ 610 nm
Method Adaptation of the Turbidimetric Tetraphenylborate method. The reaction between
Potassium and reagents causes turbidity in the sample.
REQUIRED REAGENTS
Code Description Quantity
HI 93750A-0 Potassium Reagent 6 drops
HI 93750B-0 Powder Reagent 1 packet
REAGENT SETS
HI 93750-01 Reagents for 100 tests
HI 93750-03 Reagents for 300 tests
For other accessories see page 50.
MEASUREMENT PROCEDURE
Note: for sample preparation follow the IRRIGATION WATER (LR) procedure at page 19.
• Select the
described in the
• Fill the cuvette with 10 mL of sample, up to the mark.
• Add 6 drops of HI 93750A-0 Potassium Reagent,
replace the cap and swirl the solution.
• Place the cuvette into the holder and close the lid.
Potassium LR
Potassium LR
Method Selection
method using the procedure
section (see page 12).
46
10 mL
Page 47

• Press the Zero key. The display will show “-0.0-” when the meter is zeroed and ready for measurement.
• Remove the cuvette and add the content of one packet
of HI 93750B-0 reagent. Replace the cap and gently
mix for one minute by slowly turning the cuvette upside
down.
• Reinsert the cuvette into the instrument.
• Press Timer and the display will show the countdown prior to
the measurement or, alternatively, wait for 2 minutes and
press Read. When the timer ends the meter will perform the
reading. The instrument displays the results in mg/L (ppm)
of potassium (K).
• Press or to access the second level functions.
• Press the Chem Frm key to convert the result in mg/L of potassium oxide (K2O).
• Press or to return to the measurement screen.
INTERFERENCES
Interferences may be caused by:
Ammonium above 10 ppm
Calcium above 10000 ppm as CaCO
Chloride above 12000 ppm
Magnesium above 8000 ppm as CaCO
Sodium above 8000 ppm
3
3
47
Potassium LR
Page 48

ERRORS AND WARNINGS
The instrument shows clear warning messages when erroneous conditions appear and when measured values are
outside the expected range. These messages are described bellow.
No Light: The light source is not functioning properly.
Light Leak: There is an excess amount of ambient light reaching the
detector.
Inverted cuvettes: The sample and the zero cuvettes are inverted.
Battery Low: The battery capacity is lower than 10%.
Light Low: The instrument cannot adjust the light level. Please check
that the sample does not contain any debris.
Light High: There is too much light to perform a measurement. Please
check the preparation of the zero cuvette.
48
Page 49

DATA MANAGEMENT
The analyzed data can be managed using Hanna’s product HI92000, Windows® Compatible Software.
STANDARD METHODS
Description Range Method
Ammonia HR 0 to 100 mg/L Nessler
Ammonia MR 0.0 to 50.0 mg/L Nessler
Ammonia LR 0.0 to 10.0 mg/L Nessler
Nitrate HR 0 to 300 mg/L Cadmium Reduction
Nitrate MR 0 to 150 mg/L Cadmium Reduction
Nitrate LR 0.0 to 30.0 mg/L Cadmium Reduction
Phosphorus HR 0 to 100 mg/L Amino Acid
Phosphorus MR 0.0 to 50.0 mg/L Amino Acid
Phosphorus LR 0.0 to 10.0 mg/L Amino Acid
Potassium HR 20 to 200 mg/L Turbidimetric
Potassium MR 10 to 100 mg/L Turbidimetric
Potassium LR 0.0 to 20.0 mg/L Turbidimetric
Windows® is registered Trademark of "Microsoft Co."
49
Page 50

ACCESSORIES
REAGENT SETS
HI 93706-01 100 phosphorus tests
HI 93706-03 300 phosphorus tests
HI 93715-01 100 ammonia tests
HI 93715-03 300 ammonia tests
HI 93728-01 100 nitrate tests
HI 93728-03 300 nitrate tests
HI 93750-01 100 potassium tests
HI 93750-03 300 potassium tests
OTHER ACCESSORIES
HI 731318 cloth for wiping cuvettes (4 pcs)
HI 731321 glass cuvettes (4 pcs)
HI 731325W new cap for cuvette (4 pcs)
HI 740034 cap for 100 mL beaker (6 pcs)
HI 740036 100 mL plastic beaker (6 pcs)
HI 740157 plastic refilling pipette (20 pcs)
HI 740223 170 mL plastic beaker
HI 740224 170 mL plastic beakers (12 pcs)
HI 740225 60 mL graduated syringe
HI 740226 5 mL graduated syringe
HI 740227 filter assembly
HI 740228 filter discs (25 pcs)
HI 740229 100 mL graduated cylinder
HI 740230 230 mL demineralized water
HI 92000 Windows compatible software
HI 920013 PC connection cable
HI 93703-50 cuvette cleaning solution (230 mL)
HI 93703-54 dried resin (100 g)
HI 93703-55 activated carbon (50 pcs)
50
Page 51

WARRANTY
All Hanna Instruments meters are warranted for two years against defects in workmanship and materials
when used for its intended purpose and maintained according to the instructions.
This warranty is limited to repair or replacement free of charge.
Damages due to accident, misuse, tampering or lack of prescribed maintenance are not covered.
If service is required, contact your dealer. If under warranty, report the model number, date of purchase,
serial number and the nature of the failure. If the repair is not covered by the warranty, you will be notified
of the charges incurred.
If the instrument is to be returned to Hanna Instruments, first obtain a Returned Goods Authorization Number
from the Customer Service Department and then send it with shipment costs prepaid. When shipping any
instrument, make sure it is properly packaged for complete protection.
To validate your warranty, fill out and return the enclosed warranty card within 14 days from the date of
purchase.
Recommendations for Users
Before using these products, make sure that they are entirely suitable for your specific application and for the environment in which they are used.
Operation of these instruments may cause unacceptable interferences to other electronic equipments, this requiring the operator to take all necessary steps to
correct interferences.
Any variation introduced by the user to the supplied equipment may degrade the instruments' EMC performance.
To avoid damages or burns, do not put the instrument in microwave ovens. For yours and the instrument safety do not use or store the instrument in hazardous
environments.
Hanna Instruments reserves the right to modify the design, construction and appearance of its products
without advance notice.
HANNA LITERATURE
Hanna publishes a wide range of catalogs and handbooks for an equally wide range of applications. The
reference literature currently covers areas such as:
• Water Treatment
• Process
• Swimming Pools
• Agriculture
• Food
• Laboratory
and many others. New reference material is constantly being added to the library.
For these and other catalogs, handbooks and leaflets contact your dealer or the Hanna Customer Service
Center nearest to you. To find the Hanna Office in your vicinity, check our home page at www.hannainst.com.
51
Page 52

Hanna Instruments Inc.
Highland Industrial Park
584 Park East Drive
Woonsocket, RI 02895 USA
Technical Support for Customers
Tel. (800) 426 6287
Fax (401) 765 7575
E-mail tech@hannainst.com
www.hannainst.com
Local Sales and Customer Service Office
Printed in EUROPE
(ROMANIA)
MAN83215 04/10
52
 Loading...
Loading...