Page 1
Instruction Manual
HI 38022
Total
Chlorine
High Range
Test Kit
SPECIFICATIONS
Range 0 to 4.0 mg/L (ppm) as Chlorine
0 to 20.0 mg/L (ppm) as Chlorine
Smallest Increment 0.2 mg/L in the 0-4.0 range
1.0 mg/L in the 0-20.0 range
Analysis Method Drop count titration
Sample Size 10 mL and 50 mL
Number of Tests 100
Case Dimensions 235x175x115 mm (9.2x6.9x4.5")
Shipping Weight 561 g (19.8 oz.)
SIGNIFICANCE AND USE
2- Add 5 drops of Potassium Iodide Solution and swirl
gently to mix.
3- Add 1 packet of Sulfamic Reagent. Use the spoon to mix
and dissolve.
fate Reagent used to turn the solution colorless.
drops x 1 = mg/L Total Chlorine
8- If the result is lower than 4 ppm, the precision of test
can be improved as follows.
9- Fill the large (50 mL) plastic vessel with water sample
up to the 50 mL mark; use the 3 mL pipette to adjust
the sample level so that the meniscus formed on the
walls of the vessel is exactly on the 50 mL mark.
50 mL
www.hannainst.com
Dear Customer,
Thank you for choosing a Hanna Product.
Please read the instruction sheet carefully before using the
test kit. It will provide you with the necessary information
for correct use of the kit. If you need additional information,
do not hesitate to e-mail us at tech@hannainst.com.
Remove the chemical test kit from the packing material and
examine it carefully to make sure that no damage has
occurred during shipping. If there is any noticeable damage, notify your Dealer or the nearest Hanna office
immediately.
Each kit is supplied with:
Potassium Iodide Solution, 1 bottle with dropper (30 mL);
•
Sulfamic Reagent, packets (100 pcs);
•
• Starch Indicator, 1 bottle with dropper (25 mL);
Thiosulfate Reagent, 1 bottle (100 mL);
•
• 1 calibrated plastic vessel (50 mL)
• 1 calibrated plastic vessel (20 mL)
• 1 plastic pipette (3 mL);
• 1 plastic pipette (1 mL);
• 1 spoon.
Note: Any damaged or defective item must be returned in
its original packing materials.
;
;
The chlorination of water supplies and polluted waters is
used mainly to destroy or deactivate disease-producing
microorganisms. It also serves to improve the quality of
drinking waters, as chlorine reacts with ammonia, iron,
manganese, sulfide and some organic substances.
Nevertheless high amounts of chlorine will produce adverse
effects, like formation of compounds which are potentially
carcinogenic (e.g. chloroform) or harmful to aquatic life
(e.g. chloramines). Thus it is essential to control that the
proper amount of chlorine has been added in order to
fulfill the primary purpose of disinfecting and to minimize
any adverse effect.
Note: mg/L is equivalent to ppm (parts per million).
CHEMICAL REACTION
An iodometric titration method is used. The water sample is
treated with potassium iodide and strongly acidified with
acid. The amount of iodine generated is equivalent to the
chlorine in the sample; the concentration of iodine is
calculated by titration with thiosulfate ions that reduce
iodine back to iodide ions.
INSTRUCTIONS
READ THE ENTIRE INSTRUCTIONS BEFORE USING THE KIT
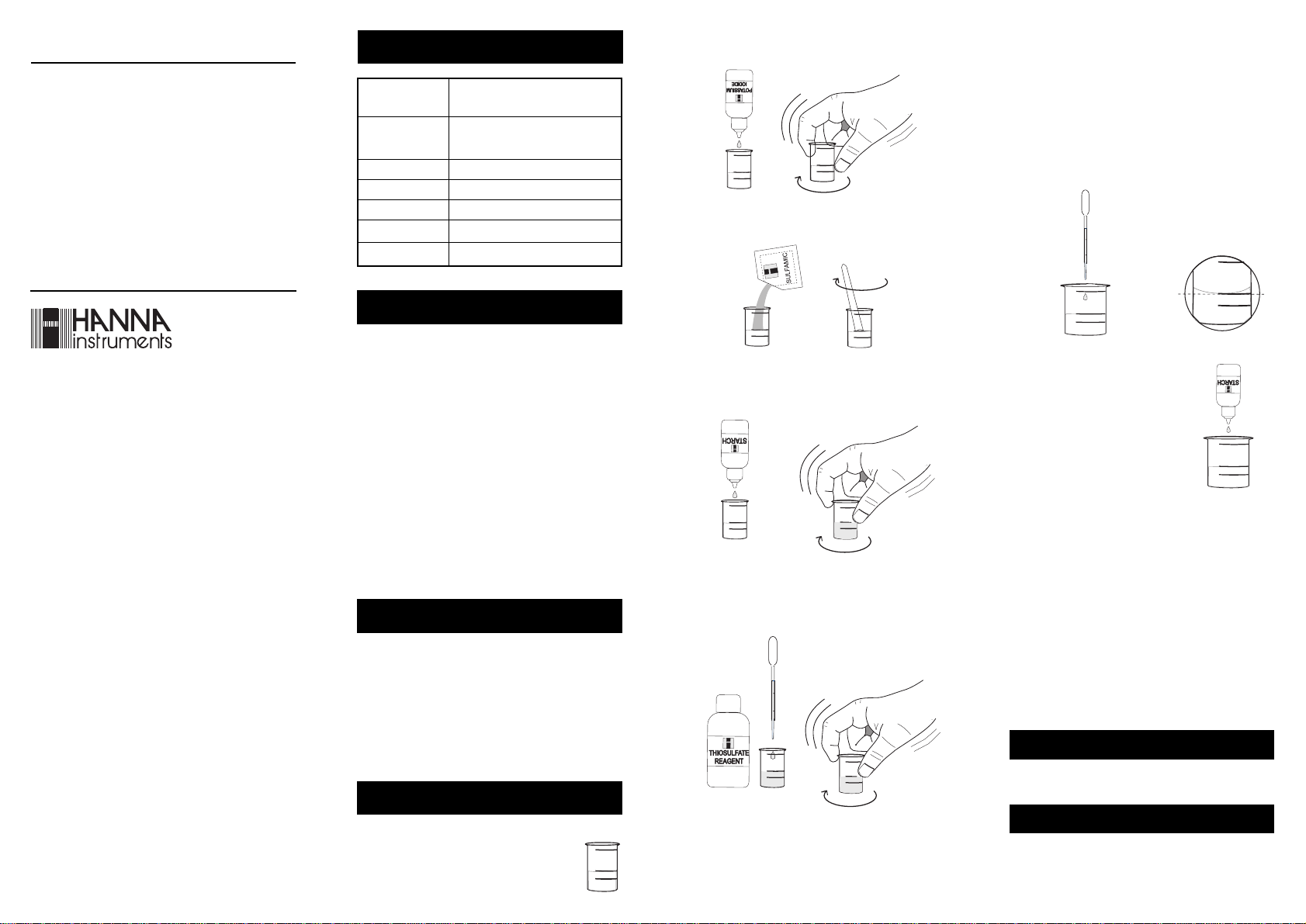
1- Using the 3 mL plastic pipette, fill
the small (20 mL) vessel with water
ISTR38022 12/00 PRINTED IN ITALY
sample up to the 10 mL mark.
10 mL
4- Add 1 drop of Starch Indicator and swirl gently to mix.
If chlorine is present, the solution will turn a blue color.
5- Using the 1 mL plastic pipette, add Thiosulfate Reagent
drop by drop, swirling after each drop, while keeping
an accurate count of the drops being added to the
solution.
6- Continue adding Thiosulfate Reagent until the solution
changes from blue to colorless.
7- The concentration in mg/L (or ppm) of total chlorine in
your sample is equal to the number of drops of Thiosul-
10- Add 5 drops of Potassium Iodide Solution and swirl
gently to mix.
11-Add 1 packet of Sulfamic Acid Re-
agent and use the spoon to mix and
dissolve.
12-Add 4 drops of Starch Indicator and
swirl gently to mix. If chlorine is present,
the solution will turn a blue color.
13-Using the 1 mL plastic pipette, add Thiosulfate Reagent
drop by drop, swirling after each drop, while keeping
an accurate count of the drops being added to the
solution.
14-Continue adding Thiosulfate Reagent until the solution
changes from blue to colorless.
15-To obtain the concentration in mg/L (or ppm) of total
chlorine in your sample, multiply by 0.2 the number of
drops of Thiosulfate Reagent used to turn the solution
from blue to colorless.
drops x 0.2 = mg/L Total Chlorine
REFERENCES
Standard methods for the Examination of Water and Wastewater, 20th Ed., 1998, APHA-AWWA-WEF
HEALTH AND SAFETY
The chemicals contained in this kit may be hazardous if
improperly handled. Read the relevant Health and Safety
Data Sheet before performing this test.
Page 2

 Loading...
Loading...