Page 1
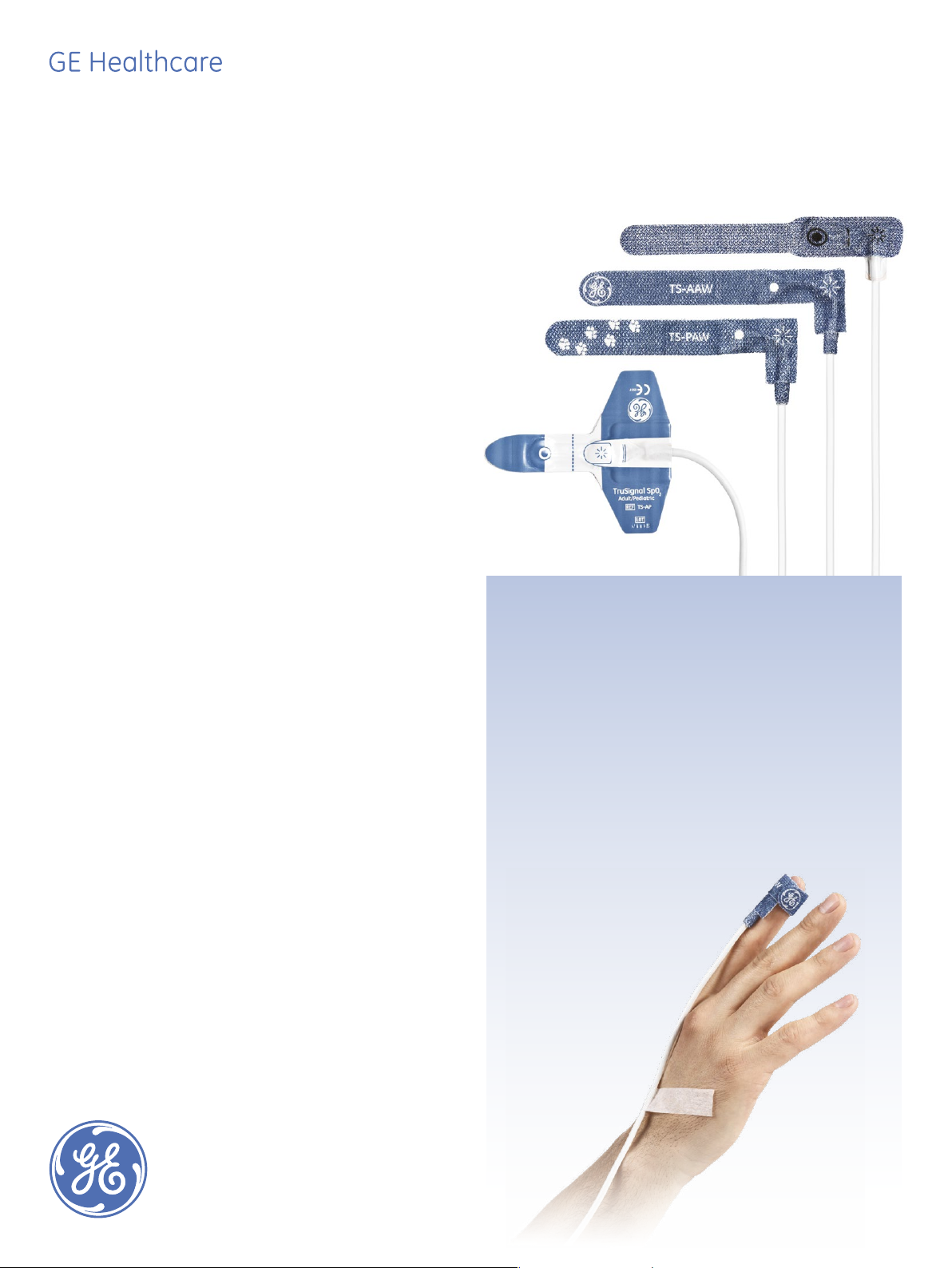
TruSignal adhesive
SpO
sensors
2
Connecting intelligence and care.
Excellent performance in single-patient-use oximetry sensors
TruSignal* adhesive single-patient-use SpO
clinical performance you expect in settings that require elevated
infection control – such as the burn unit, trauma unit , emergency
department, intensive care unit, isolation room and operating room.
Designed for use in continuous non-invasive arterial oxygen saturation
(SpO
) and pulse rate monitoring, the sensors hold securely in place
2
despite patient motion, delivering accurate readings that help clinicians
make appropriate and confident care decisions.
GE Healthcare offers four varieties of adhesive sensors, designed with
patient comfort in mind. All are backward compatible with Datex,
Ohmeda and GE SpO
calibrated to ensure the best achievable accuracy. The sensors are
drip-proof and able to withstand cleaning with 60% isopropyl or 90%
ethyl alcohol if needed.
technologies. Sensors of all types are individually
2
sensors provide the
2
TruSignal SpO2 Adult Adhesive Wrap sensor
(TS-AAW-10, TS-AAW-25)
TruSignal Adult Adhesive Wrap sensors are designed with
a highly flexible sensor body for ease of application to the
measurement site and a high degree of patient comfort.
Suitable for patients 20 kg (44 lb.) and above, they are
applied to a finger or toe. The adhesive is strong enough
to hold the sensor in place yet gentle enough not to hurt
the skin.
The sensor materials do not contain natural latex rubber or known
environmental contaminants. All four sensor models comply with:
• ISO 9919:2009 – Pulse oximeters for medical use – Requirements
• ISO 10993-1:2009 – Biological evaluation of medical devices
Sensors are designed to last two to three days with the same patient;
sensor adhesiveness withstands re-attachment every four hours.
Page 2

TruSignal SpO2 Pediatric Adhesive Wrap sensors
(TS-PAW-10, TS-PAW-25)
The TruSignal Pediatric Adhesive Wrap sensor dimensions are tailored for children’s digits; the adhesive is especially gentle. Suitable
for children from 3 to 20 kg, they are applied to a finger or toe. The
design aims to help ease children’s anxiety at being in the hospital.
Each sensor bears the paw prints of a friendly teddy bear who has
come to the hospital to keep the child company. The sensor pouch
opens to reveal a picture of the teddy bear, suitable for coloring.
TruSignal SpO
Adult/Pediatric sensor
2
(TS-AP-10, TS-AP-25)
TruSignal Adult/Pediatric sensors are designed for patients weighing
20 kg (44 pounds) and up. They are applied to a finger or toe.
TruSignal SpO2 AllFit sensors
(TS-AF-10, TS-AF-25)
TruSignal AllFit sensors are designed for all patients from neonates to
adults. They are applied to a finger, toe, palm of the hand or side of
the foot, depending on patient size.
TruSignal Adult Adhesive Wrap, AllFit and Adult/Pediatric SpO
sensors
2
enable use of the Adequacy of Anesthesia (AoA) concept with Surgical
Pleth Index (SPI) measurement.
1
(1) Surgical Pleth Index (SPI) is to be used for unconscious and fully anesthetized adult
(>18 years old) patients during general anesthesia. SPI monitoring can be performed
with the CARESCAPE modular monitors and GE SpO
technology.
2
GE Healthcare
P.O. Box 900, FIN-00031 GE, Finland
GE Direct United Kingdom: +44 (0)800 0329201
www.gehealthcare.com
Find out more
TruSignal SpO
adhesive single-patient-use sensors come
2
in boxes of 10 or 25. The Adult Adhesive Wrap sensor and
Pediatric Adhesive Wrap sensor boxes easily convert to a
shelf container that lets clinicians visually estimate the
remaining supply.
Learn which TruSignal SpO
sensors are best suited for your
2
care areas and patient population. Contact your local
GE Healthcare representative.
© 2013 General Electric Company – All rights reserved.
General Electric Company reserves the right to make changes in specifications and
features shown herein, or discontinue the product described at any time without notice
or obligation. Contact your GE representative for the most current information.
GE and GE Monogram are trademarks of
General Electric Company.
* Trademarks of General Electric Company.
GE Healthcare, a division of General Electric Company.
GE Healthcare Finland Oy
Kuortaneenkatu 2
00510 Helsinki, Finland
Europe
EMEA DOC1403863 06/13
(Global version DOC1316393 Rev 2)
 Loading...
Loading...