Fresenius medical care 2008T User Manual
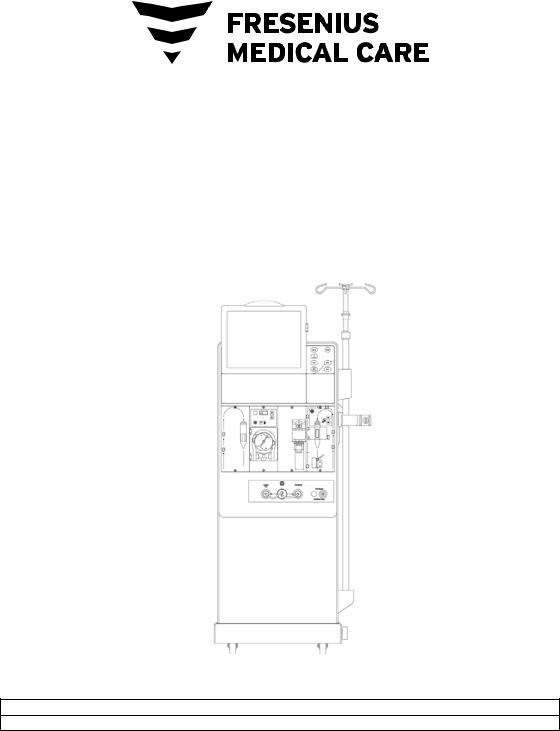
2008T Hemodialysis Machine
Operator’s Manual
Caution: Federal (US) law restricts this device to sale only by or on the order of a physician. Note: The most recent version of this manual can be accessed at fmcna.com/frtmanuals.
490122 Rev Q
2008T Hemodialysis Machine Operator’s Manual
© Copyright 2008 - 2018 Fresenius USA, Inc.—All Rights Reserved
This document contains proprietary information of Fresenius Medical Care Renal Therapies Group, LLC and its affiliates (“Fresenius Medical Care”). The contents of this document may not be disclosed to third parties, copied, or duplicated in any form, in whole or in part, without the prior written permission of Fresenius Medical Care.
Fresenius Medical Care, the triangle logo, 2008, BlueStar, Clinical Data Exchange, Combiset, Twister, NaturaLyte, GranuFlo, bibag, Crit-Line, CLiC, Optiflux, DIAFIX, and DIASAFE are trademarks of Fresenius Medical Care Holdings, Inc., or its affiliated companies. Citrasate is a registered trademark of Advanced Renal Technologies, Inc. in the United States and used under license from Advanced Renal Technologies, Inc. All other trademarks are the property of their respective owners.
The 2008T hemodialysis machine is manufactured by: Fresenius USA, Inc.
4040 Nelson Avenue
Concord, CA 94520 (800) 227-2572
Installation, maintenance, calibration and other technical information may be found in the 2008T Technician’s Manual, P/N 490130.
Contact Fresenius Medical Care Technical Support for applicable Field Service Bulletins. The spare parts manual for the model 2008T and other information may be found on our website at www.fmcna.com
Caution: Federal (US) law restricts this device to sale only by or on the order of a physician.
Caution: Frequency, duration, and parameters of treatment are to be determined by the prescribing physician.
Note: Not all features are available in all regions.
Indications for Use:
2008T BlueStar Hemodialysis Machine: The 2008T BlueStar Hemodialysis Machine is indicated for acute and chronic dialysis therapy in a healthcare facility.
Additional therapy options for patients receiving hemodialysis include: Isolated Ultrafiltration, Sustained Low Efficiency Dialysis (SLED), and low volume hemodialysis (patients weighing ≥ 20kg and ≤ 40 kg). This machine accommodates the use of both low flux and high flux dialyzers. The SLED therapy option is not to be used for patients weighing ≤ 40 kg. The 2008T BlueStar Hemodialysis Machine is not to be used for plasma replacement therapies, for patients weighing less than 20 kg, or for renal therapies using substitution fluid.
bibag System (Optional): The bibag system is used with three stream proportioning hemodialysis systems equipped with the bibag module such as the 2008T BlueStar Hemodialysis Machine and is intended for use in bicarbonate hemodialysis for acute and chronic renal failure. The bibag is intended for extracorporeal bicarbonate hemodialysis according to a physician’s prescription.
Crit-Line Clip Monitor (CLiC) (Optional): The Crit-Line Clip Monitor is used with the 2008T BlueStar Hemodialysis Machine to non-invasively measure hematocrit, oxygen saturation and percent change in blood volume. The CLiC device measures hematocrit, percent change in blood volume and oxygen saturation in real time for application in the treatment of dialysis patients with the intended purpose of providing a more effective treatment for both the dialysis patient and the clinician. Based on the data that the monitor provides, the clinician/nurse, under physician direction, intervenes (i.e., increases or decreases the rate at which fluid is removed from the blood) in order to remove the maximum amount of fluid from the dialysis patient without the patient experiencing the common complications of dialysis which include nausea, cramping and vomiting.
2 |
2008T Machine Operator’s Manual P/N 490122 Rev Q |
Indications for Use:
2008T Hemodialysis Machine: The 2008T hemodialysis machine is indicated for acute and chronic dialysis therapy in a healthcare facility.
bibag System (Optional): The bibag system is used with three stream proportioning hemodialysis systems equipped with the bibag module such as the 2008T Hemodialysis Machine and is intended for use in bicarbonate hemodialysis for acute and chronic renal failure. The bibag is intended for extracorporeal bicarbonate hemodialysis according to a physician’s prescription.
Crit-Line Clip Monitor (CLiC) (Optional): The Crit-Line Clip Monitor is used with the 2008T Hemodialysis Machine to non-invasively measure hematocrit, oxygen saturation and percent change in blood volume. The CLiC device measures hematocrit, percent change in blood volume and oxygen saturation in real time for application in the treatment of dialysis patients with the intended purpose of providing a more effective treatment for both the dialysis patient and the clinician. Based on the data that the monitor provides, the clinician/nurse, under physician direction, intervenes (i.e., increases or decreases the rate at which fluid is removed from the blood) in order to remove the maximum amount of fluid from the dialysis patient without the patient experiencing the common complications of dialysis which include nausea, cramping and vomiting.
2008T Machine Operator’s Manual P/N 490122 Rev Q |
3 |
Contents
|
About this manual…........................................................................................................................ |
9 |
|
Requirements................................................................................................................................. |
10 |
|
Related Reading............................................................................................................................. |
10 |
|
Conventions................................................................................................................................... |
11 |
|
About Hemodialysis…...................................................................................................................14 |
|
|
General Warnings.......................................................................................................................... |
16 |
|
Using a Central Venous Catheter................................................................................................... |
21 |
CHAPTER 1 |
|
|
|
Overview................................................................................................................................................. |
23 |
|
Function of the 2008T Hemodialysis Machine.............................................................................. |
23 |
|
Organization of the 2008T Hemodialysis Machine....................................................................... |
23 |
|
The Control Panel.......................................................................................................................... |
26 |
|
Control Panel Keypad.................................................................................................................... |
27 |
|
The Back Panel.............................................................................................................................. |
35 |
|
PatientCard Reader (Optional)....................................................................................................... |
37 |
|
Modules ......................................................................................................................................... |
38 |
|
The Dialysate Path......................................................................................................................... |
44 |
|
IV Pole and Dialyzer Holder.......................................................................................................... |
48 |
|
Moving the Machine...................................................................................................................... |
49 |
CHAPTER 2 |
|
|
|
Daily Preparation for Treatment............................................................................................................. |
51 |
|
Starting Point ................................................................................................................................. |
51 |
|
Preparing the Dialysis Delivery System........................................................................................ |
52 |
|
Manual Machine Setup.................................................................................................................. |
52 |
|
Auto Start Machine Setup.............................................................................................................. |
56 |
|
Preparing the Extracorporeal Blood Circuit................................................................................... |
58 |
|
Connecting the Extracorporeal Blood Circuit................................................................................ |
59 |
|
Priming the Blood Circuit.............................................................................................................. |
61 |
|
Testing the 2008T Hemodialysis Machine .................................................................................... |
70 |
|
Recirculation and Final Set-Up Procedure..................................................................................... |
73 |
CHAPTER 3 |
|
|
|
Setting Treatment Parameters................................................................................................................. |
75 |
|
Recommended Order for Screen-by-Screen Entry ........................................................................ |
76 |
4 |
2008T Machine Operator’s Manual P/N 490122 Rev Q |
|
New Treatment Key....................................................................................................................... |
77 |
Entering a Treatment Parameter .................................................................................................... |
78 |
Dialysate Screen Settings............................................................................................................... |
80 |
Home Screen Settings.................................................................................................................... |
91 |
Ultrafiltration................................................................................................................................. |
96 |
Isolated Ultrafiltration.................................................................................................................... |
99 |
Sodium Variation System............................................................................................................ |
101 |
Heparin Screen Settings............................................................................................................... |
105 |
Test & Options Screen Settings................................................................................................... |
110 |
Low Volume Dialysis.................................................................................................................. |
113 |
Blood Pressure Screen Settings ................................................................................................... |
114 |
Using the Default Parameters Screen........................................................................................... |
117 |
Using the PatientCard.................................................................................................................. |
124 |
Starting Dialysis........................................................................................................................... |
133 |
CHAPTER 4 |
|
Monitoring the Treatment..................................................................................................................... |
135 |
Home Screen Monitoring............................................................................................................. |
136 |
Trends Screen Monitoring........................................................................................................... |
141 |
Kt/V & Access Flow Monitoring................................................................................................. |
144 |
Blood Temperature Monitor / Blood Volume Monitor Screen.................................................... |
152 |
Crit-Line Screen........................................................................................................................... |
155 |
Blood Pressure Screen Monitoring.............................................................................................. |
161 |
During Treatment......................................................................................................................... |
162 |
Blood Recirculation Procedure.................................................................................................... |
164 |
Power Failure during Dialysis ..................................................................................................... |
165 |
Completion of Dialysis................................................................................................................ |
168 |
Returning Blood to the Patient (Standard Method) ..................................................................... |
169 |
Returning Blood to the Patient Using Assisted Reinfusion......................................................... |
170 |
CHAPTER 5 |
|
Disinfection and Maintenance .............................................................................................................. |
176 |
Cleaning and Disinfection............................................................................................................ |
176 |
Cleaning the Exterior Surface...................................................................................................... |
180 |
Rinse Program.............................................................................................................................. |
183 |
Acid Clean Program..................................................................................................................... |
184 |
Heat Disinfection Program .......................................................................................................... |
185 |
Chemical/Rinse Program............................................................................................................. |
186 |
Chemical/Dwell Program............................................................................................................. |
188 |
Acid & Heat Disin (Disinfect) Program ...................................................................................... |
190 |
2008T Machine Operator’s Manual P/N 490122 Rev Q |
5 |
|
Disinfect Log (Optional).............................................................................................................. |
193 |
CHAPTER 6 |
|
|
|
Alarms and Troubleshooting................................................................................................................. |
194 |
|
Operational Status........................................................................................................................ |
194 |
|
Troubleshooting........................................................................................................................... |
198 |
|
Replacing the DIASAFE®plusUS Filter ........................................................................................ |
267 |
|
Replacing the 9-Volt Battery....................................................................................................... |
268 |
APPENDIX A |
|
|
|
Single Needle Dialysis (Optional) ........................................................................................................ |
270 |
APPENDIX B |
|
|
|
Sustained Low Efficiency Dialysis (SLED) (Optional)........................................................................ |
283 |
|
Preparation................................................................................................................................... |
283 |
|
Treatment Monitoring.................................................................................................................. |
285 |
APPENDIX C |
|
|
|
The CDX System (Optional)................................................................................................................. |
287 |
|
Machine Connections................................................................................................................... |
289 |
|
Instructions for Clinic Information Systems Personnel............................................................... |
291 |
|
CDX PC Specifications for the Intel Atom D525 Processor....................................................... |
293 |
|
CDX PC Specifications for the Intel Atom N270 Processor....................................................... |
294 |
APPENDIX D |
|
|
|
Concentrate Types................................................................................................................................. |
298 |
|
Estimated bibag disposable run times (minutes).......................................................................... |
301 |
APPENDIX E |
|
|
|
Service Mode........................................................................................................................................ |
302 |
|
Treatment Options Screen............................................................................................................ |
303 |
|
Hardware Options Screen............................................................................................................ |
308 |
|
Default Settings Screen................................................................................................................ |
311 |
|
Auto Heat Disinfect (functional software version 2.69 or earlier)............................................... |
314 |
|
Enter Conc Screen: Selecting and Adding Concentrates............................................................. |
316 |
|
UF Profile Screen: Creating Custom UF Profiles........................................................................ |
321 |
|
Module Options Screen................................................................................................................ |
322 |
|
Scheduler Screen (functional software version 2.70 or later)...................................................... |
324 |
|
Auto Heat Disinfect (functional software version 2.70 or later).................................................. |
326 |
|
Auto Start (functional software version 2.70 or later)................................................................. |
328 |
|
CDX Auto On (functional software version 2.70 or later)........................................................... |
330 |
|
PM Reminder............................................................................................................................... |
331 |
6 |
2008T Machine Operator’s Manual P/N 490122 Rev Q |
|
Diasafe Reminder ........................................................................................................................ |
334 |
Testing the Dialysate.................................................................................................................... |
337 |
Equipment Storage and Maintenance .......................................................................................... |
339 |
Machine Specifications................................................................................................................ |
340 |
Essential Performance.................................................................................................................. |
350 |
Manufacturer’s EMC Declaration................................................................................................ |
352 |
Product Improvement Policy ....................................................................................................... |
356 |
Warranty ...................................................................................................................................... |
356 |
Patents.......................................................................................................................................... |
357 |
Glossary ................................................................................................................................................ |
358 |
Index ..................................................................................................................................................... |
364 |
2008T Machine Operator’s Manual P/N 490122 Rev Q |
7 |
This page intentionally blank
8 |
2008T Machine Operator’s Manual P/N 490122 Rev Q |
About this manual…
About this manual…
The purpose of the 2008T Hemodialysis Machine Operator’s Manual is to instruct qualified patient-care staff in the function, operation, and maintenance of the 2008T hemodialysis machine. It is not intended as a guide for performing hemodialysis, a medical treatment that should only be performed under the supervision of a licensed physician.
This manual is organized to systematically guide a patient-care specialist through the set up, operation, and clean up of the 2008T hemodialysis machine in daily use. The book begins with an overview that introduces the operator to the major components and describes how they are organized on the machine. Next, the operator is guided through a daily set-up procedure. Once the machine has been prepared for daily use, a step-by-step guide to preparing the machine for a patient-specific treatment is provided. The operator is then provided a tour of the various treatment screen functions useful in monitoring the treatment, followed by instruction in terminating treatment and post-treatment clean up. Also included are sections on troubleshooting, maintenance, and treatment options.
The organization of the 2008T Hemodialysis Machine Operator’s Manual is as follows:
Preface
Identifies the intended audience, and describes how the manual is organized. It addresses various issues regarding the performance of hemodialysis and product liability, and provides information for contacting Fresenius USA, Inc.
Chapter 1—Overview
Introduces the operator to the 2008T hemodialysis machine, its features, their functions, and how they are organized on the machine through pictures and descriptions.
Chapter 2—Daily Preparation for Treatment
Provides instructions on the recommended methods of preparing the 2008T hemodialysis machine for daily, standard-dialysis operation.
Chapter 3—Setting Treatment Parameters
Describes how to enter treatment data, and guides the operator through the relevant, treatment screens to enter patient-specific, treatment parameters in their recommended order. The chapter also covers the procedure for beginning dialysis treatment.
Chapter 4—Monitoring and the Completion of Treatment
Guides the user through the screens used to monitor the dialysis treatment. It explains the features of each screen and describes the information displayed. The screens that provide a general overview of the treatment status are provided first, followed by the screens providing more in-depth data that are narrower in scope. It concludes with a description of the recommended, end-of-treatment procedure.
Chapter 5—Cleaning and Disinfection
Recommendations for scheduled cleaning and disinfection, as well as maintenance procedures that should be performed by the operator are found here.
Chapter 6—Alarms and Troubleshooting
This chapter is indexed by alarm messages to provide the operator a quick-reference guide for determining the cause and remedies for alarm situations.
2008T Machine Operator’s Manual P/N 490122 Rev Q |
9 |
About this manual…
Appendices
In addition, this manual includes several appendices covering optional hemodialysis treatments, such as single-needle hemodialysis and Sustained Low Efficiency Dialysis (SLED), and provides information on the setup, customizing, storage and specifications of the 2008T hemodialysis machine.
Glossary
A glossary of terms is included
Index
An index to aid the operator in referencing information is included
Requirements
Operators of the 2008T hemodialysis machine must be trained to administer hemodialysis at the direction of a physician. In addition, the operator should be:
Knowledgeable of hemodialysis methodology and relevant physiology.
Proficient in healthcare procedures regarding aseptic techniques.
Thoroughly familiar with the contents of this manual.
Fully trained and qualified to operate this machine, and able to distinguish between normal and abnormal operation.
Related Reading
The following documents contain information related to the 2008T hemodialysis machine:
2008T Hemodialysis Machine bibag System Operator’s Instructions (P/N 508213)
2008T Hemodialysis Machine with CLiC User’s Guide (P/N 490206)
2008T Technicians Manual (P/N 490130)
2008T Calibration Procedures Manual (P/N 508032)
2008T Preventive Maintenance Procedures Manual (P/N 508033)
2008T Troubleshooting Guide (P/N 102297-01)
2008T Spare Parts Manual (P/N 490124)
2008T Installation Checklist (P/N 490129)
2008T Installation Checklist Instructions (P/N 508035)
2008T Field Service Bulletins may be obtained from the Fresenius Medical Care North America (FMCNA) website: www.FMCNA.com or contact your clinic for more information.
Comments are available concerning the expected increased recirculation of blood in the extracorporeal circuit during single needle treatment when using the recommended administration sets, dialyzers, catheters, and fistula needles.
The test procedures by which the effectiveness of disinfection has been verified are available on request.
10 |
2008T Machine Operator’s Manual P/N 490122 Rev Q |

|
|
About this manual… |
Conventions |
|
|
|
|
|
|
Symbol |
Description |
|
|
Warning! A warning is a statement that identifies conditions or actions |
|
and |
that could result in personal injury or loss of life. Warnings found in this |
|
|
manual outside of this section are designated with the warning symbol. |
|
|
Shock Hazard: A shock hazard warning refers to a risk of a possibly |
|
and |
severe electrical shock due to improper use or handling of the equipment. |
|
|
|
|
|
Corrosive Substance Hazard: A corrosive substance hazard warning |
|
|
refers to a risk of injury or machine damage due to improper use or |
|
|
handling of the equipment. |
|
|
Hot Surface, Fluid, or Vapors Hazard: A hot surface, fluid, or vapors |
|
|
hazard warning refers to risk of burn injury due to improper use or |
|
|
handling of the equipment. |
|
|
Tip Hazard: A tip hazard warning refers to a risk of injury or machine |
|
|
damage due to improper handling of the equipment. |
|
|
|
|
|
No Pushing: A no pushing warning refers to a risk of injury or machine |
|
|
damage due to leaning or pushing against the equipment. |
|
|
|
|
|
Caution: A caution is a statement that identifies conditions or actions that |
|
|
could result in damage to the machine. |
|
|
|
|
|
Mandatory Action: A command describing required action to maintain |
|
|
safety. |
|
|
|
|
|
Consult Accompanying Documents: This symbol is located on the |
|
|
2008T hemodialysis machine. It means, refer to the 2008T Operator's |
|
|
Manual for additional information. |
|
|
Note: Notes are advisory comments or recommendations regarding |
|
|
practices or procedures. |
|
|
|
|
|
Do not reuse |
|
|
|
|
|
ON: This symbol, at the top of the switches on the back of your machine, |
|
|
means the switch is in the ON position. |
|
|
|
|
|
OFF: This symbol, at the bottom of the switches on the back of your |
|
|
machine, means the switch is in the OFF position. |
|
|
|
|
|
Degree of protection against electric shock: Type B |
|
|
|
|
|
Degree of protection against electric shock: Type CF – Blood |
|
|
Pressure Cuff only |
|
|
|
2008T Machine Operator’s Manual P/N 490122 Rev Q |
11 |

About this manual…
Symbol |
Description |
|||||||
|
|
|
|
|
|
|
|
MR Unsafe: An item which poses unacceptable risks to the patient, |
|
|
|
|
|
|
|
|
medical staff or other persons within the MR (Magnetic Resonance) |
|
|
|
|
|
|
|
|
environment. |
IPX1 |
Vertical drip-proof level of protection from liquid drips, leaks and spills |
|||||||
|
|
|
|
|
|
|
|
Protective ground terminal |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equipotentiality—this symbol may appear on older machines |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RF transmitter: Intentional Radio Frequency (RF) transmissions for |
|
|
|
|
|
|
|
|
wireless communications (see The CDX System, Appendix B) |
|
|
|
|
|
|
|
|
|
12 |
2008T Machine Operator’s Manual P/N 490122 Rev Q |
|
About this manual… |
|
|
Name |
Description |
Button |
A button refers to specific fields located in the treatment screens that are |
|
used to set treatment parameters or perform an action when selected. |
|
|
Control Panel |
The control panel is located at the top third of the machine and contains |
|
the display screen and panel keys used in controlling the treatment. |
|
|
Display |
The area located at the top of the control console that displays the |
Screen |
treatment screens. |
Key |
A key is a pressure-sensitive, raised pad found on the control panel |
|
outside of the treatment screen that is used to enter a value, make a |
|
selection, or initiate an action or process. |
|
|
Keyboard |
The keyboard is located below the display screen. It flips down for data |
|
entry and can be closed again when not in use. |
|
|
Screen |
The graphic image displayed inside the display screen. There are eight |
|
main screens all of which are accessible from any of the other screens. |
|
|
Subscreen |
A smaller screen that can be opened from inside a particular main screen. |
|
Subscreens are not accessible from all main screens. |
|
|
Touchpad |
A flip-down panel on the right side of the control panel that reacts to |
|
fingertip pressure. The Touchpad controls an on-screen cursor (arrow). |
|
|
Touchscreen |
Optional data input device that overlays the display screen. The |
|
Touchscreen reacts to fingertip pressure. |
|
|
2008T Machine Operator’s Manual P/N 490122 Rev Q |
13 |
About Hemodialysis…
About Hemodialysis…
Indications
Hemodialysis is prescribed by physicians for patients with acute or chronic renal failure, when conservative therapy is judged inadequate. Dialysis therapy may be intermittent or continuous.
Contraindications
There are no absolute contraindications to hemodialysis, but the passing of a patient’s blood through an extracorporeal circuit may require anticoagulation to prevent blood clotting. In addition, the parameters of dialysis should be optimized to avoid discomfort to the patient.
Many patients are taking medicinal therapy prescribed by their physicians. Due to the dialysis treatment, some of the medication may be removed from the patient’s blood thereby lowering the therapeutic level in the blood. In other cases, medications may not be excreted as quickly as expected with patients with renal insufficiency and the level may be higher than expected. Therefore, the prescribing physician should determine the appropriate dosage of the medicine to obtain the desired medicinal response in the patient.
Some Side Effects of Hemodialysis
Dialysis therapy occasionally causes hypovolemia, hypervolemia, hypertension, hypotension and related symptoms, headache, nausea, cramping or other muscular discomfort in some patients. Hypothermia, hyperthermia, itching, anxiety, convulsions, seizure, and other neurologic symptoms associated with dialysis dementia may also be manifested by the patient. These symptoms are thought to occur if the patient’s blood volume or electrolyte balance is not maintained within acceptable limits. Other, more serious, complications arising from dialysis, such as hemorrhage, air embolism, or hemolysis, can cause serious patient injury or death. The prescribing physician must understand that prescribing insufficient bicarbonate may contribute to metabolic acidosis; excessive bicarbonate may contribute to metabolic alkalosis. Both conditions are associated with poor patient outcomes, including increased risk of mortality. Proper control of all elements of dialysis may prevent or control these physiological reactions or complications.
Pyrogenic reactions may occur which can result in patient injury. Generally it is thought that these may be controlled by maintaining the dialysate solution within the chemical and bacteriologic limits (see Water Quality on page 341 of the “Machine Specifications” section for more information). Failure to use these standards for water can also lead to accumulated toxic effects. A regular program for disinfection and testing of the water treatment system, piping, inlet lines, filters, concentrate feed containers or system, and the dialysate delivery machine must be established and followed. This program will vary from facility to facility.
Infections or pyrogen reactions may also result from contamination of the extracorporeal circuit or inadequate procedures used to reuse dialyzers.
14 |
2008T Machine Operator’s Manual P/N 490122 Rev Q |
About Hemodialysis…
Allergic reactions to chemical disinfectants may occur if insufficient procedures are used to remove or maintain the residual disinfectant at acceptable levels. Chemical disinfectants are used for dialyzer disinfection, machine disinfection, or for disinfection of water treatment and distribution systems.
All blood connections must be made using aseptic technique.
All tubes and connections must be secured and closely monitored to prevent loss of blood or entry of air into the extracorporeal circuit or errors in the ultrafiltration control system. The patient may require blood transfusion or other medical intervention to prevent respiratory or cardiac disorders if these occur.
The patient’s blood pressure and general physical status must be closely monitored during dialysis in order to initiate appropriate remedial measures or therapy. Of particular importance is the control of the patient’s serum potassium level to prevent cardiac dysrhythmia and the patient’s blood clotting time to prevent clotting disorders.
These instructions are for the 2008T hemodialysis machine. The machine must only be operated in accordance with these instructions. All operators of this machine must be thoroughly trained and have read this entire manual and any applicable appendices before using the machine. Improper care/use of this device may result in serious patient injury or death.
Blood Pressure Module Contraindications
The 2008T blood pressure monitoring subsystem is not intended for neonatal use. The following are generally accepted contraindications for using a timed automatic blood pressure instrument utilizing the oscillometric principle:
Use of a heart lung machine
Peripheral circulation problems
Severe arrhythmia
Ectopic beats
Convulsions
Spasms
Tremors
Tachycardia
Use of incorrectly sized blood pressure cuffs may result in inaccurate blood pressure readings.
This is a guideline only. Final determination of the suitability of any medical instrument for use with any patient, including pregnant or pre-eclamptic patients, is the responsibility of the treating physician.
2008T Machine Operator’s Manual P/N 490122 Rev Q |
15 |
General Warnings
General Warnings
This section contains general warnings statements regarding the use and maintenance of the 2008T hemodialysis machine. It is not a complete summary, and additional warning statements specific to pertinent topics can be found within this manual.
Water
Warning! Connect water inlet according to the specifications for the machine. For further information, see “Machine Specifications” on page 340. The correct ionic concentration and bacterial quality can generally be achieved in the dialysate only with treated water that meets water quality standards (see Water Quality and Dialysate Quality on page 341 of the “Machine Specifications” section for more information). Be sure that all specifications are satisfied. The water source must be monitored periodically to detect fluctuations in water composition and quality that could have an adverse effect on the patient or dialysate delivery machine. Particular attention must be taken for chemicals such as aluminum, chlorine, and chloramine, as these chemicals can cause complications in dialysis patients.
Warning! Comply with all local regulations in respect of separation of devices in the water supply in case of back siphonage; an air gap must be created between the machine’s drain line and its drain.
Concentrates
Warning! The specific acid and bicarbonate concentrates, including the sodium, bicarbonate, and electrolyte compositions, must be prescribed by a physician.
Warning! Many concentrate types are available for use in dialysate delivery machines. Concentrates contain various amounts of dextrose, potassium, calcium, sodium, chloride, magnesium, and other components. Most concentrates are designed as a two-part system of acid and bicarbonate solutions which are mixed in the machine with water. Even within the subgroup of bicarbonate type concentrates, there are at least four methods of compounding the solutions. Each of these methods requires special calibrations or setups. Certain methods are not supported. It is mandatory that the acid and bicarbonate types be matched to each other. Be sure to use compatible solutions, labeling, and setups. These setups include machine calibration, special adapters for certain concentrate types, correct setting of concentrate option, and labeling. Failure to use the properly matched solutions and machine calibrations may allow improper dialysate to be delivered to the patient, resulting in patient injury or death. Verify composition, conductivity, and pH after converting to a different type of concentrate.
Warning: Acid concentrate, bicarbonate concentrate, and water must be of the appropriate quality to ensure safety and performance of the final dialysate are met (see Water Quality, Dialysate Quality, and Concentrate Quality on page 341 of the “Machine Specifications” section for more information).
Warning: The dissolved bibag bicarbonate concentrate must be used within 24 hours of connecting to the dialysis machine. Do not refill the bibag container.
16 |
2008T Machine Operator’s Manual P/N 490122 Rev Q |
General Warnings
Warning! Connection to a central acid or bicarbonate feed system requires the installation of certain mechanical parts. Contact Fresenius USA, Inc. for more information.
Warning! Bicarbonate and acid concentrates intended for other dialysate delivery machines will deliver safe dialysate solution only if the machine is set up for them. The selection of other dialysate concentrate types must be done by a qualified, authorized person. The 2008T hemodialysis machine can be set up for various concentrate types. Use Table 40 in Appendix C to ensure that you have compatible concentrates and configurations.
Warning! Acid concentrate products are used as one component in mixing dialysate bath. These acid products contain chemical compounds that, after mixing, yield acetate (and citrate in certain products) in the dialysate. (Please refer to the acid concentrate product labeling for specific acetate/citrate amounts.) After diffusion across the dialyzer membrane, acetate (and citrate when present) is metabolized by the liver to serum bicarbonate and adds to the serum bicarbonate that separately results from the diffusion of dialysate bicarbonate across the dialyzer membrane. During dialysis, the dynamic of diffusion and concentration gradients prevent serum bicarbonate concentration from exceeding the dialysate bicarbonate concentration. The bicarbonate concentration of the dialysate is the “bicarbonate” setting on the dialysis machine, and is the bicarbonate dose prescribed by the physician. On the 2008 series hemodialysis machines, the bicarbonate dose may be set in a range between 20 and 40 milliequivalents per liter, but may be set in different ranges in other machines.
When the dialysis session terminates, acetate (and citrate when present) that has not yet metabolized may remain in the blood and will be converted to serum bicarbonate after diffusion ceases, without possibility of diffusion out of the blood. The post dialysis metabolism of acetate (and citrate when present) could thus briefly increase serum bicarbonate concentration above the prescribed bicarbonate concentration of the dialysate. Physicians should consider this possibility in prescribing bicarbonate dose.
Prescription of insufficient bicarbonate may contribute to metabolic acidosis; excessive bicarbonate may contribute to metabolic alkalosis. Both conditions are associated with poor patient outcomes, including increased mortality risk.
Warning! Incorrect composition will result if the acid concentrate nozzle is not connected to the appropriate acid concentrate or the bicarbonate concentrate nozzle is not connected to the appropriate bicarbonate solution. The acid and bicarbonate concentrates must match those selected in the “Dialysate” screen. Patient injury or death may occur if incorrect dialysate solution is used. Fresenius USA, Inc. recommends the operator use the concentrate containers provided with the machine. These containers, being of different size and shape, help to reduce the chances of mismatching the acid and bicarbonate concentrates.
Warning! Always verify the conductivity and approximate pH of the dialysate through independent means before beginning treatment. Independent means could be by using an external conductivity meter, pH meter, pH paper or by using the machine’s independent conductivity test. Verify also when changing concentrates during treatment and when switching from the bibag system to liquid bicarbonate*. The wrong conductivity or pH may cause serious injury or death.
*Note: If alternative liquid bicarbonate concentrate sources are used (jugs or central delivery) the end user must ensure the bicarbonate is of appropriate quality and is prepared per manufacturer’s instructions.
2008T Machine Operator’s Manual P/N 490122 Rev Q |
17 |
General Warnings
Warning! The machine must be labeled to indicate the type of concentrate for which it is configured. Check the composition (i.e., Na, Cl, K, Ca, Mg, HCO3) and pH of the dialysate solution after the machine is installed or after the machine is modified for different concentrate types. Verify the conductivity and approximate pH of the dialysate solution through independent means before initiating dialysis. Independent means could be by using an external conductivity meter, pH meter, pH paper or by using the machine’s independent conductivity test. Improper conductivity or pH could result in patient injury or death.
Machine
Warning! Failure to install, operate, and maintain this equipment according to the manufacturer’s instructions may cause patient injury or death. If this equipment is modified, appropriate inspection and testing must be conducted to ensure continued safe use of the equipment. Substitution of a component different from that supplied may result in measurement errors.
Warning! Use of this equipment adjacent to or stacked with other equipment should be avoided because it could result in improper operation. If such use is necessary, this equipment and the other equipment should be observed to verify that they are operating normally.
Warning! Use of accessories, transducers and cables other than those specified or provided by the manufacturer of this equipment could result in increased electromagnetic emissions or decreased electromagnetic immunity of this equipment and result in improper operation.
Warning! Proper functioning of the machine must be verified prior to initiating treatment. Unidentified malfunctions or alarm failure could potentially expose a patient to a serious health risk. Alarm limits for the arterial pressure monitor, venous pressure monitor, and transmembrane pressure (TMP) monitor are automatically set and delayed for pressure stabilization. Alarm limits for temperature and conductivity are calculated for the dialysate composition and may be somewhat adjusted by the operator. These must be maintained within safe physiological limits as specified by the prescribing physician.
Warning! Never perform maintenance when a patient is connected to the machine. If possible, remove the machine from the treatment area when it is being serviced. Label the machine to ensure it is not accidentally returned to clinical use before the service work is completed. Disinfect the machine and test the dialysate for acceptable conductivity and pH values before returning the machine to clinical use. Always test the machine when maintenance is completed.
Warning! To avoid damaging the equipment or personal injury, internal adjustments to the blood pressure module should only be made by a qualified technician.
Warning! The electrical source must be single phase, three-conductor type provided with a hospital grade receptacle with protective earth and a ground fault interrupter at 120 volts, 60 Hz. The proper polarity and ground integrity must be initially checked and maintained.
Failure to do so may result in electrical shock or burn to the operator or patient. The machine must be plugged directly into the electrical outlet; extension cords and power strips are prohibited.
Warning! Shock hazard. Do not remove covers. Refer servicing to qualified personnel. Replace fuses only with the same type and rating.
18 |
2008T Machine Operator’s Manual P/N 490122 Rev Q |
General Warnings
Warning! Do not install the 9-Volt battery backwards in the machine, as it will damage the “No Power” alarm.
Warning! Do not use devices emitting strong electromagnetic radiation such as portable phones, radio equipment (walkie-talkies, etc.), radio transmitters, and like equipment near your machine. Improper operation may result.
Cellular phones and WiFi connected devices may be conditionally allowed. However, if any interference is noted, such as false pressure readings that disappear when the external signal is removed, it is recommended to move the cellular phone at least ten feet away from the 2008T hemodialysis machine when making or receiving phone calls. If a WiFi-connected device (e.g. laptop computers, tablet devices, smartphones) is found to cause interference, it is recommended to use that device at least four feet away from the 2008T hemodialysis machine.
Portable RF (radio frequency) communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of the hemodialysis machine, including cables specified by the manufacturer.
Otherwise, degradation of the performance of this equipment could result.
For exact separation distance recommendation, please refer to the Manufacturer’s EMC Declaration statement on page 352.
Warning! While controls have been implemented throughout the development of the 2008T to protect against cybersecurity threats, it is the responsibility of the customer to ensure security controls such as WiFi encryption, firewalls, antivirus, access controls (including physical controls), vulnerability management and other security controls are in place and managed appropriately. Please consult your organization’s information security/risk management group to ensure the proper controls are implemented to meet the company’s policies and risk profile. Failure to properly implement protection could put prescription data sent to the machine at risk.
Warning! Transducer protectors should be used between pressure ports and each pressure monitor line of the extracorporeal system to prevent the internal transducer protectors from getting wet. Wet transducer protectors must be replaced, as they will cause inaccurate pressure readings. If the external transducer protector and the internal transducer protector become contaminated with blood, the transducer protectors must be replaced and the transducer, pressure ports, internal tubing and valve must be disinfected or replaced.
Warning! A new, sterile transducer protector should be placed on all the air connections from the drip chambers to the machine pressure monitor ports. This will prevent contamination of the machine and filters air that enters the chambers through the monitor lines. If the transducer protector should get wet and air is not able to pass, replace the transducer protector and clear the monitor line.
Warning! The machine is compatible with a number of venous lines. The Level Detector module must be calibrated for the model venous line being used. In addition, verify that the venous line clamp is capable of fully occluding the model of bloodline that your facility uses. Improper functioning of the level detector may be caused by a clot of blood.
Warning! Possible Explosion Hazard if used in the presence of flammable anesthetics.
Warning! Check all bloodlines for leaks after the treatment has started. Keep access sites uncovered and monitored. Improper bloodline connections or needle dislodgements can result in excessive blood loss, serious injury, and death. Machine alarms may not occur in every blood loss situation.
2008T Machine Operator’s Manual P/N 490122 Rev Q |
19 |
General Warnings
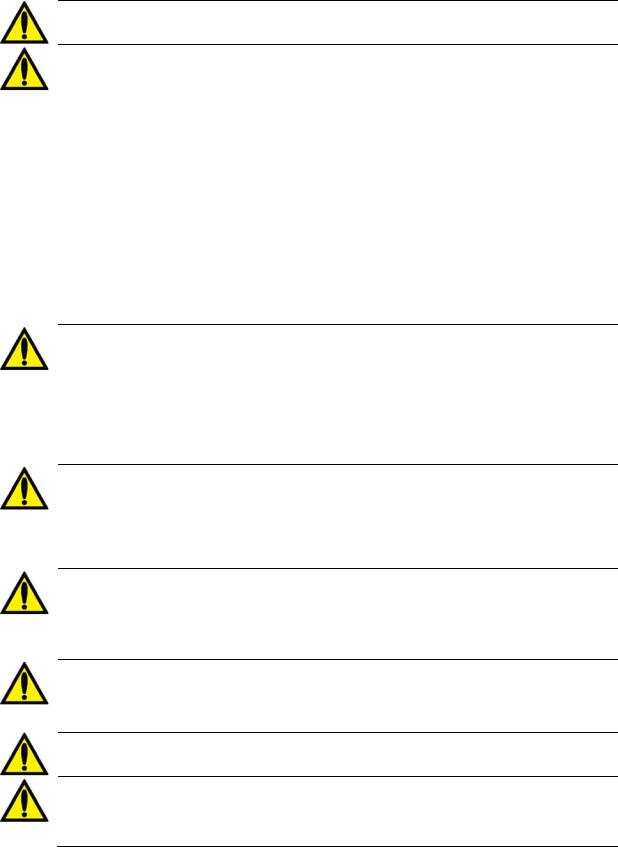
Warning! Air may enter into the extracorporeal circuit at connection points downstream of the air detector, if pressures are negative. This can occur in cases such as single needle applications or central venous catheter applications.
Warning! The dialysate path is a closed fluidics system. Discontinue use immediately if a fluid leak is detected. Do not attempt to administer or continue dialysis treatment with a machine which has a fluid leak, this could result in excessive fluid removal from the patient leading to serious injury or death. System leaks may also pose a slip-and-fall hazard. Clean up spills immediately.
Warning! Replace a leaking bibag disposable immediately. Spills can cause damage to carpeting and other surfaces. To contain such spills, the machine should be on a spilltolerant surface. Spills can cause slips and falls; clean up spills immediately.
Warning! When using the bibag system, the acid and bicarbonate pressures must not exceed 10 psi when using a Central Delivery System. It may be necessary to use pressure regulators in order to reach proper conductivity. When not using the bibag system, the maximum supplied pressure is 2 psi.
Warning! High dose hydroxocobalamin (or any form of Vitamin B-12) causes discoloration of the spent dialysate. This discoloration may cause a false blood leak alarm, stopping the blood pump and preventing treatment unless the operator performs an override of the alarm. The blood leak alarm can be reset and overridden for up to three minutes repeatedly by following the blood leak alarm troubleshooting instructions in the operator’s manual in cases where a blood leak test is negative for blood in the dialysate.
Discontinuation of the hemodialysis treatment could result in persistence or worsening of acidosis, hyperkalemia, and volume overload which can lead to serious injury or death.
Caution: Only the bags manufactured by Fresenius Medical Care may be used in the bibag connector.
Caution: System leaks may occur. Unattended operation of the machine (for example, during disinfection at night) may result in flooding and can cause property damage. Clean up spills immediately.
Caution: Be careful not to tip the machine when rolling over uneven surfaces. Push the machine from the middle when moving it.
Caution: Do not squeeze the blood pressure cuff when deflating it. Squeezing the blood pressure cuff may damage the machine's internal blood pressure module.
Note: The DIASAFE®plusUS filter is required when the bibag system is in use.
Note: A smoke detector should be properly installed in the room used for dialysis. Follow the manufacturer’s instructions. The alarm should be tested according to the manufacturer’s instructions. Replace the battery as specified.
Note: You must follow all environmental regulations regarding waste disposal and eventual machine disposal. Contact your clinic for more information. Prior to the disposal of your machine, any possible risk of infection from blood borne pathogens must also be eliminated by appropriate disinfection.
20 |
2008T Machine Operator’s Manual P/N 490122 Rev Q |
General Warnings
Note: The temperature of the bloodline and the durometer of the tubing affect the ability of the bloodline/blood pump system to prime during setup. Cold tubing may not prime as readily as warm tubing.
Fresenius Medical Care manufactures bloodlines for use with the model 2008T hemodialysis machine. The performance of bloodlines not manufactured by Fresenius Medical Care cannot be guaranteed by Fresenius Medical Care and are therefore the responsibility of the prescribing physician.
Note: The following materials come into contact with purified water, dialysate, or dialysate concentrate:
Dyflor (PVDF)
Ethylene-propylene terpolymer (EPDM) Foraflon (PVDF)
Glass Lupolen (PE) Makrolon (PC)
Noryl (PPE & PS) Polyethersulfone (PES) Polyphenylene oxide (PPO)
Polyphenylene oxide 20% glass fiber (PPOGF20)
Polyphenylsulfone (PPSU)
Polypropylene (PP)
Polypropylene 20% glass fiber (PP-GF20) Radel 10 & 20% glass fiber (PES) Stainless steel (types 300 & 316) Silicone (Si)
Teflon (PTFE) Thermocomp (PES) Titanium – TiAl 4 V6 Ultem (PEI) Ultradur+ (PBT) Victrex (PEEK)
Vinyl chloride polymer (PVC)
Using a Central Venous Catheter
Shock Hazard: Ensure that no conductive electrical devices connected to or near the patient, including water and concentrate central delivery systems connected to the machine, have leakage currents above the maximum CF applied parts limit of 10 μA DC and 50 μA DC in a single fault condition. Failure to follow these precautions may result in serious injury or death.
2008T Machine Operator’s Manual P/N 490122 Rev Q |
21 |
General Warnings
Maintenance
Assembly, installation, adjustment, or repair is to be performed only by persons authorized by the facility medical director or by Fresenius USA, Inc.
Questions?
For further information regarding the operation, repair, parts, or maintenance of the 2008T hemodialysis machine, please contact:
Fresenius USA, Inc. |
(800) 227-2572 |
Attention: Service Department 4040 Nelson Avenue Concord, CA 94520 www.FMCNA.com
Additionally, updates to this operator’s manual are available for download here: www.fmcna.com/product-support-documentation/
22 |
2008T Machine Operator’s Manual P/N 490122 Rev Q |
Chapter 1
Overview
The 2008T hemodialysis machine is designed to perform hemodialysis in hospitals and dialysis clinics. It can be used for patients suffering chronic or acute renal failure.
Function of the 2008T Hemodialysis Machine
The 2008T hemodialysis machine is designed to provide hemodialysis treatment by controlling and monitoring both the dialysate and extracorporeal blood circuits.
In the extracorporeal blood circuit, the blood is continuously circulated from the patient through a dialyzer, where toxins are filtered out through a semi-permeable membrane, before being returned to the patient. During this process, the extracorporeal blood circuit is monitored for venous and arterial blood pressures, and for the presence of air and blood. The 2008T hemodialysis machine can also administer heparin evenly throughout the treatment.
In the dialysate circuit, the dialysate concentrates are mixed with purified water, heated, degassed, and delivered to the dialyzer. Balancing chambers ensure that the incoming flow of the dialysate is volumetrically equal to the outgoing flow in order to control ultrafiltration from the patient.
Organization of the 2008T Hemodialysis Machine
The 2008T hemodialysis machine is designed for functional efficiency. The back of the machine houses the utility connections such as water source, drain, and electrical connections. By mounting them to the back, the water lines and power cord remain out of the way during treatment.
The front of the machine contains all of the controls the operator needs access to during hemodialysis. It can be broken down into three main sections. The top section contains the control panel and houses the computer that runs the treatment program. The middle section contains the modules used for the safe transmission of the blood to and from the dialyzer. Dialysate is the primary concern of the bottom section of the 2008T hemodialysis machine. Here the concentrates used to make up the dialysate are mixed and pumped to the dialyzer.
The following pages contain front and rear views of the 2008T hemodialysis machine and a brief description of the machine’s features. You should familiarize yourself with the location and purpose of these features.
2008T Machine Operator’s Manual P/N 490122 Rev Q |
23 |
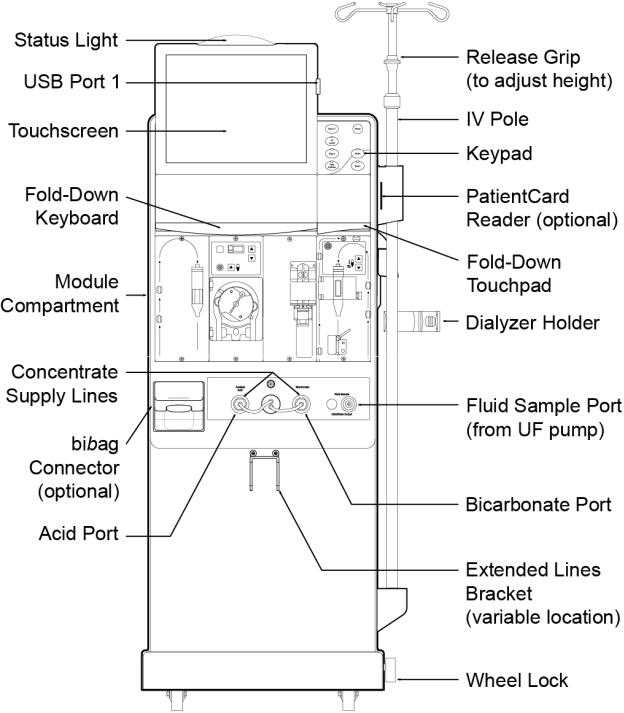
Chapter 1—Overview
Figure 1 – 2008T Hemodialysis Machine—Front View
24 |
2008T Machine Operator’s Manual P/N 490122 Rev Q |
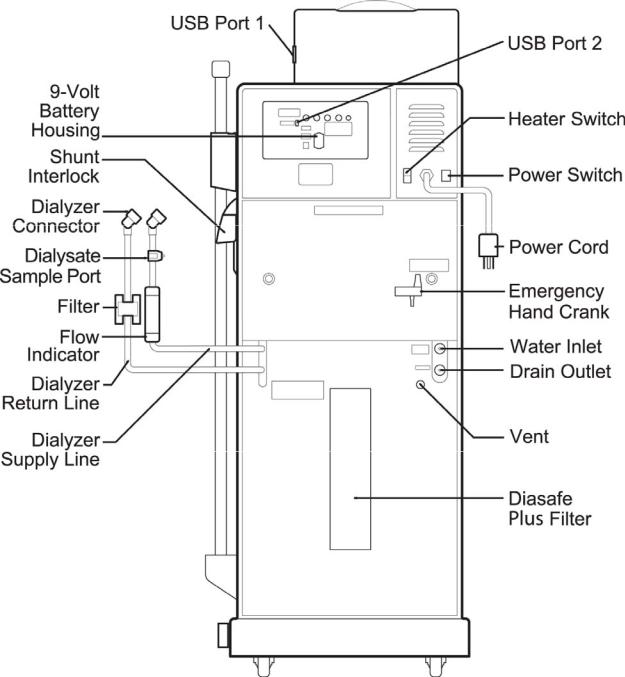
Chapter 1—Overview
Figure 2 – 2008T Hemodialysis Machine—Rear View
2008T Machine Operator’s Manual P/N 490122 Rev Q |
25 |
Chapter 1—Overview
The Control Panel
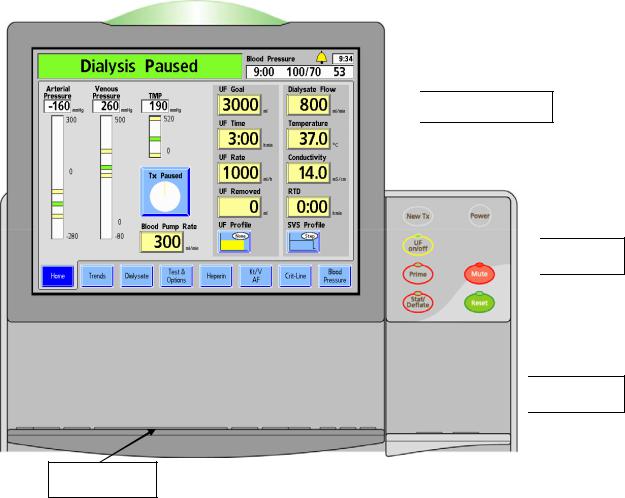
The control panel (see Figure 3) is located at the top, front of the 2008T hemodialysis machine and contains keys that allow the user to control the operation of the 2008T hemodialysis machine. Located at the top of the control panel is a display screen that can show a variety of treatment screens which the operator uses to set treatment parameters and monitor the treatment.
The treatment display screen provides a means of setting the treatment parameters and monitoring the treatment and patient status during dialysis. The operator can access treatment screens, select the Tx Clock, and set treatment parameters by selecting specific, identified sites (buttons) on the screen by using the Touchpad cursor or by touching them directly with the Touchscreen. Changes to settings and parameters selected on the screen must then be confirmed by pressing the CONFIRM key on the control panel.
 Touchscreen
Touchscreen
 Keypad
Keypad
 Touchpad
Touchpad
Keyboard
Figure 3 – The Components of the Control Panel
26 |
2008T Machine Operator’s Manual P/N 490122 Rev Q |
Chapter 1—Overview
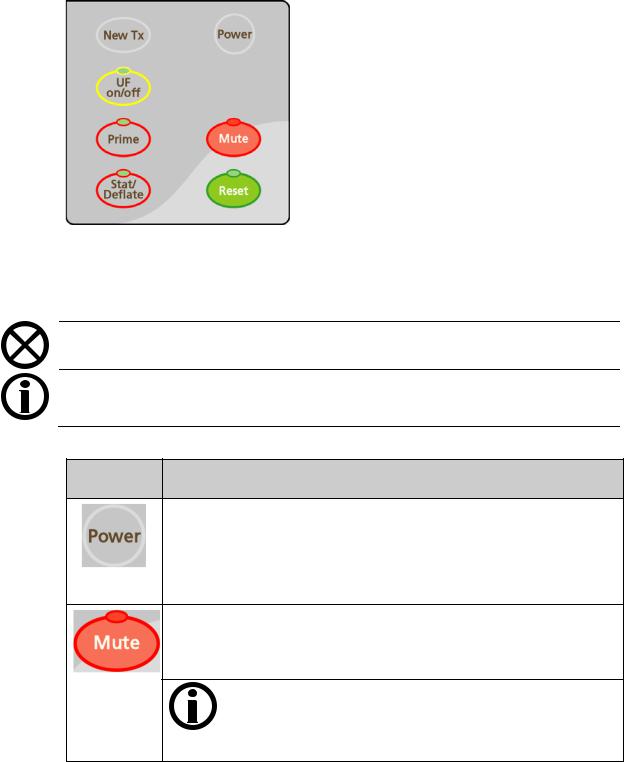
Control Panel Keypad
Figure 4 – Control Panel Keypad
The Control Panel Keypad contains seven keys associated with starting or stopping the basic power and alarm aspects of any dialysis treatment. The table below lists each key and its function.
Caution: Use a finger to press the keys and the touchscreen. Use of objects to press the keys or touchscreen may result in damage or premature failure.
Note: Pressing any control panel key (except for the Power key) while displaying CDX or in Low Power Mode will switch the machine back to full power Dialysis Mode. See page 287 for more information on CDX and page 324 for information about Low Power Mode.
Table 1 – Control Panel Keypad Keys
Press … To …
Turn the machine on. Hold for one second to turn the power off and, if blood is sensed, the machine will power down with an audible alarm.
If the CDX Auto On feature is running for the day, pressing the Power key will only cause the machine to power down for two minutes, after which it will restart. See page 330 for more information about the CDX Auto On feature.
Silence an alarm for two minutes or until another alarm occurs. The red light above the key is on if an alarm is muted.
Functional software version 2.70 or later: A muted alarm is also indicated in the Dialogue Box, see page 30 for more information.
Note: The following alarms are muted for an extra four minutes (for a total of six minutes) when using a bibag disposable for the bicarbonate source: Conductivity Low, Conductivity High, bibag: Cond Low, Bicarb Cond 2 Low, Bicarb Cond 2 High, Low Temperature, and High Temperature.
2008T Machine Operator’s Manual P/N 490122 Rev Q |
27 |
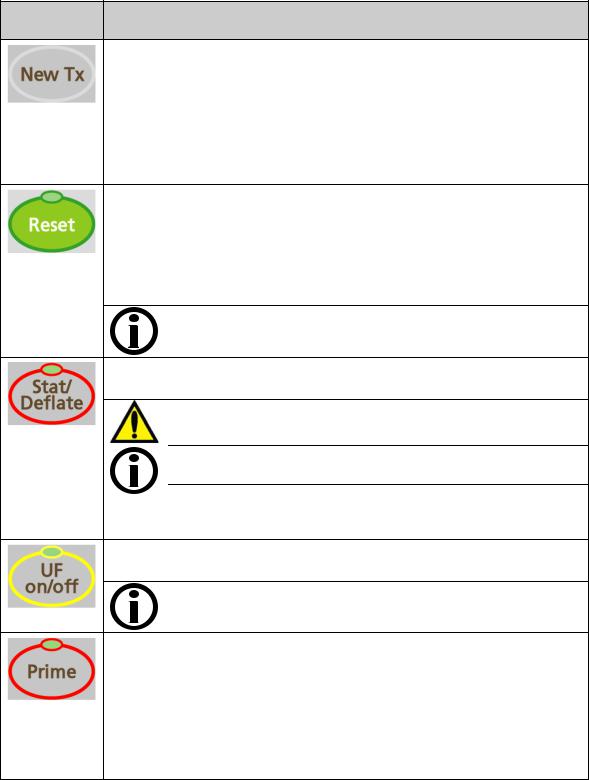
Chapter 1—Overview
Press … To …
(New Treatment) Erase the current treatment information and move the summary information to the previous record in the “Trends” screen.
Press the CONFIRM key on the Touchpad or Enter on the Keyboard to complete the action. To cancel, press the Escape key on the Touchpad or the Esc key on the Keyboard.
If the Service Mode ‘Default Rx Screen’ option is set to ‘Yes’ and no PatientCard is used, pressing and confirming the New Tx key will instead display the “Default Parameters” screen. See page 117 for more information.
Reset the machine after an alarm.
Press and hold for two seconds to spread the alarm window by 300 mmHg for arterial and venous pressures and fully open the transmembrane (TMP) pressure window for 30 seconds. The light above the Reset key will not be on.
During a blood leak alarm, press and hold for three seconds to override the alarm and keep the blood pump running for three minutes. The light above the Reset key will be on during an override.
Note: The Reset key is only used to reset alarms; it does not reset or cancel changes to a parameter.
Start an unscheduled, manual blood pressure measurement when the cuff is deflated, or instantly deflate the inflated blood pressure cuff.
Warning! Too frequent measurements can cause injury to the patient due to blood flow interference.
Note: Certain versions of the blood pressure module require a 30 second delay between blood pressure measurements.
Note: Pressing the Stat/Deflate key while displaying CDX will only exit CDX. The operator must then press the Stat/Deflate key again in order to take a blood pressure measurement.
Turn the ultrafiltration pump on or off. During ultrafiltration, the green light is illuminated. This light will flash when ultrafiltration is interrupted.
Note: When the UF pump is turned off, there is no “minimum” ultrafiltration occurring.
Prime the extracorporeal blood circuit. Pressing Prime will keep the blood pump running when air is sensed in the venous blood chamber and an air detector alarm is present (as is the case during initial set up when the blood circuit tubing is empty). The pump will run for:
Two minutes, or
Until an adequate fluid level is detected by the ultrasonic sensors in the level detector module, or
Until the volume set in Service Mode is reached.
28 |
2008T Machine Operator’s Manual P/N 490122 Rev Q |
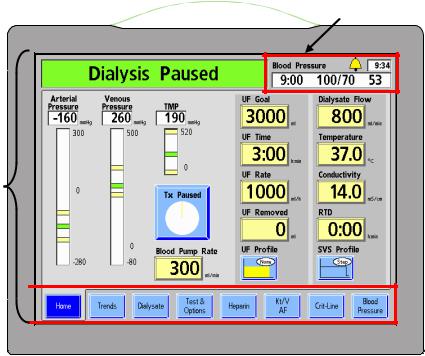
Chapter 1—Overview
Treatment Display Section
Status Light Dialogue Box
Dialogue Box
Status Box
Touchscreen
Screen-Buttons
Figure 5 – Control Panel – Treatment Display Section
The Treatment Display section is used to display information and access and set all treatment parameters.
At the top of the Treatment Display Section is the Status Light. The Status Light indicates the machine’s status with an illuminated dome. Its color matches the Status Box (see Figure 5). The lights (red, green, or yellow) are used to display status information. This allows clinic personnel to monitor the status of each 2008T hemodialysis machine from a distance during treatment. There are several selections for the meaning of the lights described in the ‘Beacon’ option on page 309. When the Status Light flashes green with the display screen off, the machine is in Low Power Mode. To turn the display back on, simply touch the Touchscreen, Keyboard, or Touchpad. For more information about Low Power Mode, see page 324.
The Status Box appears at the top left corner of every treatment screen. During normal operation it displays the operational mode of the machine—Dialysis or SLED. During alarm situations, it displays an informational message. It may also prompt the operator for a specific action in situations when the treatment parameters are being set.
2008T Machine Operator’s Manual P/N 490122 Rev Q |
29 |
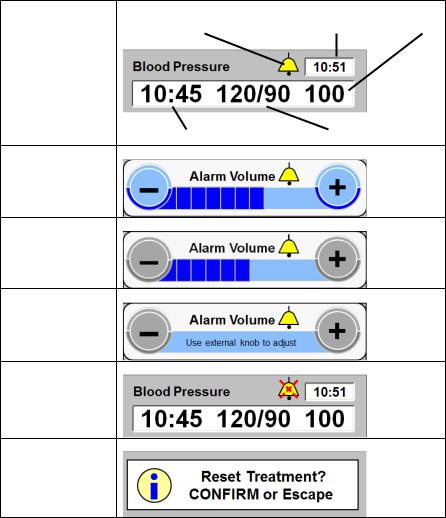
Chapter 1—Overview
To the right of the Status Box, is the Dialogue Box. During normal treatment, the Dialogue Box displays the current time, the time of the last blood pressure reading and the patient’s blood pressure and pulse rate at that time. Starting in functional software version 2.70, the Dialogue Box also displays the Bell button (see Figure 6). Selecting the Bell button displays a pop-up window allowing the operator to adjust the alarm volume (plus) or ▬ (minus) buttons. If an alarm is muted, the Bell button displays a red X over it.
The Dialogue Box also displays advisory messages when an action is required by the operator (for example, to correct a treatment parameter that is outside the range of allowable limits) or when more information about a situation is available. For a listing of advisory messages, see the “Troubleshooting” section on page 198.
Dialogue Box |
Bell button |
Current Time |
Pulse |
Time of last BP reading |
Blood Pressure |
Adjusting
Alarm Volume
Alarm Volume Locked at ‘Medium’
Alarm Volume
Adjusted by
External Knob
Alarm Muted
Dialogue Box
Advisory
Message
Figure 6 – Dialogue Box Features (functional software version 2.70 with BlueStarTM Premium)
The treatment display screen, or Touchscreen, contains the area for viewing and entering the various treatment settings. Adjustments to treatment parameters and options are made using buttons. For a description of the various types of buttons and their states, see Figure 7 on the next page.
30 |
2008T Machine Operator’s Manual P/N 490122 Rev Q |
 Loading...
Loading...