Page 1
Multiparameter Simulator
Users Manual
PN 2631808
April 2006, Rev. 1, 12/07
© 2006, 2007 Fluke Corporation, All rights reserved. Specifications are subject to ochange without notice. Printed in USA
All product names are trademarks of their respective companies.
PS420
Page 2

Warranty and Product Support
Fluke Biomedical warrants this instrument against defects in materials and workmanship for one full year from the date of
original purchase. During the warranty period, we will repair or, at our option, replace at no charge a product that proves to
be defective, provided you return the product, shipping prepaid, to Fluke Biomedical. This warranty does not apply if the
product has been damaged by accident or misuse or as the result of service or modification by other than Fluke Biomedical.
IN NO EVENT SHALL FLUKE BIOMEDICAL BE LIABLE FOR CONSEQUENTIAL DAMAGES.
Only serialized products and their accessory items (those products and items bearing a distinct serial number tag) are covered under this one–year warranty. PHYSICAL DAMAGE CAUSED BY MISUSE OR PHYSICAL ABUSE IS NOT COVERED
UNDER THE WARRANTY. Items such as cables and nonserialized modules are not covered under this warranty.
Recalibration of instruments is not covered under the warranty.
This warranty gives you specific legal rights, and you may also have other rights which vary from state to state, province to
province, or country to country. This warranty is limited to repairing the instrument to Fluke Biomedical’s specifications.
Warranty Disclaimer
Should you elect to have your instrument serviced and/or calibrated by someone other than Fluke Biomedical, please be
advised that the original warranty covering your product becomes void when the tamper-resistant Quality Seal is removed or
broken without proper factory authorization. We strongly recommend, therefore, that you send your instrument to Fluke Biomedical for factory service and calibration, especially during the original warranty period.
Page 3
Notices
All Rights Reserved
© Copyright 2006, Fluke Biomedical. No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system, or translated into
any language without the written permission of Fluke Biomedical.
Copyright Release
Fluke Biomedical agrees to a limited copyright release that allows you to reproduce manuals and other printed materials for use in service training programs
and other technical publications. If you would like other reproductions or distributions, submit a written request to Fluke Biomedical.
Unpacking and Inspection
Follow standard receiving practices upon receipt of the instrument. Check the shipping carton for damage. If damage is found, stop unpacking the instrument.
Notify the carrier and ask for an agent to be present while the instrument is unpacked. There are no special unpacking instructions, but be careful not to damage the instrument when unpacking it. Inspect the instrument for physical damage such as bent or broken parts, dents, or scratches.
Technical Support
For application support or answers to technical questions, either email techservices@flukebiomedical.com or call 1-800- 648-7952 or 1-425-446-6945.
Claims
Our routine method of shipment is via common carrier, FOB origin. Upon delivery, if physical damage is found, retain all packing materials in their original
condition and contact the carrier immediately to file a claim. If the instrument is delivered in good physical condition but does not operate within specifications, or if there are any other problems not caused by shipping damage, please contact Fluke Biomedical or your local sales representative.
Page 4

Standard Terms and Conditions
Refunds and Credits
Please note that only serialized products and their accessory items (i.e., products and items bearing a distinct serial number tag) are eligible for
partial refund and/or credit. Nonserialized parts and accessory items (e.g., cables, carrying cases, auxiliary modules, etc.) are not eligible for return or refund. Only products returned within 90 days from the date of original purchase are eligible for refund/credit. In order to receive a partial refund/credit of a product purchase price on a serialized product, the product must not have been damaged by the customer or by the carrier chosen by the customer to return the goods, and the product must be returned complete (meaning with all manuals, cables, accessories, etc.) and in “as new” and resalable condition. Products not returned within 90 days of purchase, or products which are not in “as new” and resalable condition, are not eligible for credit return and
will be returned to the customer. The Return Procedure (see below) must be followed to assure prompt refund/credit.
Restocking Charges
Products returned within 30 days of original purchase are subject to a minimum restocking fee of 15 %. Products returned in excess of 30 days after purchase, but prior to 90 days, are subject to a minimum restocking fee of 20 %. Additional charges for damage and/or missing parts and accessories will be applied to all returns.
Return Procedure
All items being returned (including all warranty-claim shipments) must be sent freight-prepaid to our factory location. When you return an instrument to
Fluke Biomedical, we recommend using United Parcel Service, Federal Express, or Air Parcel Post. We also recommend that you insure your shipment for its
actual replacement cost. Fluke Biomedical will not be responsible for lost shipments or instruments that are received in damaged condition due to improper
packaging or handling.
Use the original carton and packaging material for shipment. If they are not available, we recommend the following guide for repackaging:
Use a double–walled carton of sufficient strength for the weight being shipped.
Use heavy paper or cardboard to protect all instrument surfaces. Use nonabrasive material around all projecting parts.
Use at least four inches of tightly packed, industry-approved, shock-absorbent material around the instrument.
Page 5
Returns for partial refund/credit:
Every product returned for refund/credit must be accompanied by a Return Material Authorization (RMA) number, obtained from our Order Entry Group at
1-800-648-7952 or 1-425-446-6945.
Repair and calibration:
To find the nearest service center, go to www.flukebiomedical.com/service
In the U.S.A.:
Cleveland Calibration Lab
Tel: 1-800-850-4606
Email: globalcal@flukebiomedical.com
Everett Calibration Lab
Tel: 1-800-850-4606
Email: service.status@fluke.com
In Europe, Middle East, and Africa:
Eindhoven Calibration Lab
Tel: +31-402-675300
Email: ServiceDesk@fluke.com
In Asia:
Everett Calibration Lab
Tel: +425-446-6945
Email: service.international@fluke.com
or
Certification
This instrument was thoroughly tested and inspected. It was found to meet Fluke Biomedical’s manufacturing specifications when it was shipped from the
factory. Calibration measurements are traceable to the National Institute of Standards and Technology (NIST). Devices for which there are no NIST calibration standards are measured against in-house performance standards using accepted test procedures.
WARNING
Unauthorized user modifications or application beyond the published specifications may result in electrical shock hazards or improper operation. Fluke Biomedical will not be responsible for any injuries sustained due to unauthorized equipment modifications.
Page 6

Restrictions and Liabilities
Information in this document is subject to change and does not represent a commitment by Fluke Biomedical. Changes made to the information in
this document will be incorporated in new editions of the publication. No responsibility is assumed by Fluke Biomedical for the use or reliability
of software or equipment that is not supplied by Fluke Biomedical, or by its affiliated dealers.
Manufacturing Location
The PS420 Multiparameter Simulator is manufactured in Everett, WA, USA.
Page 7

Table of Contents
Title Page
Introduction .................................................................................................................... 1
Safety............................................................................................................................. 1
Specifications ................................................................................................................. 3
General...................................................................................................................... 3
Accessories ............................................................................................................... 4
ECG........................................................................................................................... 4
Pacemaker Selections............................................................................................... 5
Arrhythmia Selections................................................................................................ 6
Blood Pressure.......................................................................................................... 7
Cardiac Output Option............................................................................................... 8
Respiration ................................................................................................................ 8
Temperature.............................................................................................................. 8
Controls and Terminals .................................................................................................. 9
Powering the Simulator .................................................................................................. 13
Operating the Simulator ................................................................................................. 13
Simulating Functions...................................................................................................... 16
Temperature.............................................................................................................. 16
i
Page 8

PS420
Users Manual
Respiration................................................................................................................ 16
Respiration Rate................................................................................................... 16
Baseline Impedance............................................................................................. 17
Impedance Variations........................................................................................... 18
Apnea................................................................................................................... 18
Blood Pressure ......................................................................................................... 19
Transducer Sensitivity (All Channels)................................................................... 19
Waveform (All Channels) ..................................................................................... 19
Blood Pressure Artifact (All Channels) ................................................................. 19
BP Static Levels (All Channels)............................................................................ 19
Channel BP-1....................................................................................................... 20
Channel BP-2....................................................................................................... 20
ECG/Arrhythmia........................................................................................................ 21
Adult and Pediatric NSR QRS.............................................................................. 21
NSR...................................................................................................................... 21
Arrhythmias: Premature Beats ............................................................................. 22
Arrhythmias: Ventricular....................................................................................... 23
Arrhythmias: Atrial................................................................................................ 23
Arrhythmias: Conduction Defects......................................................................... 24
ECG Waveform .................................................................................................... 25
ECG Rate............................................................................................................. 25
Superimposed Artifacts ........................................................................................ 26
Pacemaker ........................................................................................................... 26
Pacemaker Pulse Amplitudes, Lead II.................................................................. 27
Pacemaker Pulse Width....................................................................................... 27
Cardiac Output.......................................................................................................... 28
ECG Performance Testing ................................................................................... 30
Cleaning......................................................................................................................... 31
ii
Page 9

List of Tables
Table Title Page
1. Symbols................................................................................................................................. 2
2. Controls and Terminals ......................................................................................................... 10
3. Temperature Settings............................................................................................................ 15
4. Current Settings .................................................................................................................... 15
List of Figures
Figure Title Page
1. Controls and Terminals ......................................................................................................... 9
iii
Page 10

PS420
Users Manual
iv
Page 11

PS420 Multiparameter Simulator
Introduction
The PS420 Multiparameter Simulator (hereafter called the
Simulator) is a compact, lightweight, high-performance
simulator for use by trained service technicians for patient
monitor testing. Through settings that you manipulate, it
simulates various electrocardiogram, respiration, blood
pressure, temperature, and cardiac output conditions.
The Simulator offers two-channel simulation.
Safety
WXWarning. Read before using the
Simulator.
To avoid personal injury, follow these
guidelines:
• Do not use in any manner not specified in the
Users Manual. Otherwise, the protection
provided by this product may be impaired.
• Always press power off on the Simulator and
unplug the Battery Eliminator before
cleaning the outer surface.
• Inspect the product. If the Simulator appears
damaged or appears to operate in a manner
not specified in the manual, DO NOT
CONTINUE USE. Return for service.
• Avoid spilling liquids on the Simulator; fluid
seepage into internal components creates
corrosion and a potential shock hazard. Do
not operate the instrument if internal
components are exposed to fluid.
Do not open this product. There are no user
replaceable parts.
1
Page 12
PS420
Users Manual
WCaution
The Simulator should be calibrated annually.
Only qualified technical personnel should
perform troubleshooting and service
procedures on the Simulator.
Do not expose the Simulator to temperature
extremes. Ambient operating temperatures
should remain between 15 and 35 °C.
Simulator performance may be adversely
affected if temperatures fluctuate above or
below this range.
Refer to Table 1 for descriptions of symbols found on the
Simulator.
Table 1. Symbols
Symbol Description
W
X
P
…
~
See Users Manual.
Caution risk of electric shock
Manufacturer’s declaration of
product compliance with applicable
EU directives
Battery Eliminator Port
Do not dispose of this product as
unsorted municipal waste. Go to
Fluke’s website for recycling
information.
2
Page 13

Multiparameter Simulator
Specifications
Specifications
General
Display/Control......................................................16 alphanumeric display keys; two switches for Respiratory Leads LL/LA and Power
Interface .................................................................RS232 bi-directional interface. Baud rate: 9600
ECG Output Connectors.......................................10 AHA/IEC color-coded connectors accepting ECG snaps and pins.
Power......................................................................9 V alkaline battery or battery eliminator
Case........................................................................High impact plastic
Weight (w/o battery) ..............................................0.343 kg / 12.1 oz.
Environmental .......................................................Indoor use
Temperature, Operating........................................15 to 35
Temperature, Storage ...........................................0 to 50
Maximum Humidity, Operating ...........................80 % relative humidity up to 31
Maximum Humidity, Storage ............................... 95 %
Altitude ...................................................................Up to 2000m
Dimensions
Height ..................................................................16.0 cm (6.3 in.)
Width ..................................................................10.7 cm (4.2 in.)
Depth................................................................... 3.4 cm (1.4 in.)
Part No....................................................................PS420 Multiparameter Simulator (PN 2631290)
ON/OFF
°C (59 to 95 °F)
°C (32 to 122 °F)
relative humidity at 40
°C (88 °F), decreasing linearly to 50 %
°C (104 °F).
3
Page 14

PS420
Users Manual
Accessories
Item Part Number
Standard Accessories
Users Manual CD-ROM 2631721
Users Manual (printed) 2631808
9 VDC Battery Eliminator 2647372
Optional Accessories
Cardiac Output Adapter Box 2462200
Temperature Cable *
Blood Pressure Cable *
Cardiac Output Cable *
* Contact your local Fluke Biomedical Sales Agent for further details
ECG
12 Lead ECG with nine independent outputs referenced to RL
Baseline Impedances.......................................... 500, 1000, 1500 or 2000 Ohms for Leads I, II, and III
High Level Output ...............................................1000 x (Lead II)
Rates................................................................... 30, 40, 60, 80, 100, 120, 140, 160, 180, 200, 220, 240, 260, 280, and 300 BPM
Default Rate ........................................................80 BPM.
Rate Accuracy..................................................... ± 1% of selection
Adult or Pediatric Waveform
ECG Amplitudes..................................................0.5, 1.0, 1.5, and 2.0 mV
Amplitude Accuracy ............................................± 2 %. (Lead II)
Superimposed Artifact......................................... 50 and 60 Hz, muscle, baseline wander, and respiration
4
Page 15

Multiparameter Simulator
Specifications
ECG Performance
Square Wave.......................................................0.125 and 2.0 Hz
Pulse ...................................................................30, 60, and 120 BPM 60 ms pulse width
Sine Wave ............................................................5, 5, 10, 40, 50, 60, and 100 Hz
Triangle Wave .....................................................2.0 and 2.5 Hz
ST Segment Analysis
Elevated or Depressed........................................-.8 mV to +.8 mV in .1 mV steps
Pacemaker Selections
Pacer Spike Amplitude (2, 4, 6, 8, and 10 mV in Lead II)
Pacer Spike Duration (0.1, 0.5, 1.0, 1.5, and 2.0 ms)
Asynchronous Pacemaker
Pacer Non-Function
Pacer Non-Capture
Demand Occasional Sinus
Demand Frequent Sinus
AV Sequential
5
Page 16

PS420
Users Manual
Arrhythmia Selections
Base Rate of 80 BPM
Sinus Arrhythmia PVCs 24 / minute
Atrial (PAC) * Frequent Multifocal PVCs
Missed Beat * Bigeminy
Atrial Tachycardia Trigeminy
Atrial Flutter Pair PVCs *
Nodal (PNC) * Run 5 PVCs *
Nodal Rhythm Run 11 PVCs *
Supraventricular Tachycardia Ventricular Tachycardia
PVC1 Left Ventricular Focus * Ventricular Fibrillation (Coarse)
PVC1 Early, LV Focus * Ventricular Fibrillation (Fine)
PVC1 R on T, LV Focus * Asystole
PVC2 Right Ventricular Focus * Conduction Defects
PVC2 Early, RV Focus * First Degree
PVC2 R on T, RV Focus * Second Degree
Multifocal PVCs * Third Degree
Atrial Fibrillation Coarse/Fine Right Bundle Branch Block
PVCs 6 / minute Left Bundle Branch Block
PVCs 12 / minute
* Will go to NSR ECG @ 80 BPM after completion
6
Page 17

Multiparameter Simulator
Specifications
Blood Pressure
Input/Output Impedance .......................................350 Ohms
Exciter Input Limit .................................................± 10 V
Exciter Input Frequency Range ...........................DC to 4000 Hz
Transducer Sensitivity..........................................5 or 40 µV/V/mmHg
Level Accuracy ......................................................± 1% ± 1 mmHg
Static Levels
BP 1.....................................................................-10, 0, 80, 160, 240, 320, and 400 mmHg
BP 2.....................................................................-10, 0, 50, 100, 150, 200, and 240 mmHg
Channel Selections
Arterial 120/80 .....................................................Channels 1 and 2
Radial Artery 120/80............................................Channels 1 and 2
Left Ventricle 120/00 ...........................................Channels 1 and 2
Right Ventricle 25/00 ...........................................Channels 1 and 2
Central Venous 15/10..........................................Channel 2
Pulmonary Artery 25/10.......................................Channel 2
Pulmonary Wedge 10/2.......................................Channel 2
Left Atrium 14/4 ...................................................Channel 2
Automatic Swan/Ganz......................................... Every 20 seconds
Manual Swan/Ganz ............................................ Changes every time entry is selected
Synchronized with all normal sinus rates
Physiologically tracks all arrhythmia selections
7
Page 18

PS420
Users Manual
Cardiac Output Option
Catheter Type ........................................................Baxter-Edwards, 10 cc
Blood Temperature ...............................................37 °C (98.6 °F) and 36 °C (95.9 °F)
CO for 2 Degrees C ...............................................3, 5, and 7 L/Min
CO for 20 Degrees C .............................................3, 5, and 7 L/Min
Cal Pulse ................................................................ Of 1 degree C for 1 second; of delta 402 Ohms for 4 seconds
Accuracy................................................................± 5 %
Computational Constant
2 Degrees C ........................................................ 0.561
20 Degrees C ...................................................... 0.608
Left to Right Shunt * .............................................2 and 20 Degrees C
Faulty Injectate *.................................................... 2 and 20 Degrees C
*Note: These four CO simulations are examples of defective (uncalibrated) curves.
Respiration
Baseline Impedances............................................ 500, 1000, 1500, 2000 Ohms (Leads I - III)
Lead Selection.......................................................LL or LA
Impedance Variations ........................................... 0.2, 0.5, 1.0, and 3.0 Ohms
Impedance Accuracy ............................................± 5 %
Rates ......................................................................0 (apnea), 15, 20, 30, 40, 60, 80, 100, 120 BPM
Apnea .....................................................................12 sec, 22 sec, 32 sec, and continuous
Rate Accuracy .......................................................± 2 %
Temperature
30 °C (86 °F), 35 °C (95 °F), 37 °C (98.6 °F), 40 °C (104 °F), 42 °C (107.6 °F), compatible with YSI 400/700 series
Accuracy................................................................± 0.25 Degree C
8
Page 19

Multiparameter Simulator
Controls and Terminals
Controls and Terminals
Refer to Figure 1 and Table 2 for descriptions of Simulator controls and terminals.
Front Panel
MULTIPARAMETER SIMULATOR
PS420
SCROLL SCROLL
STATUS
CHANGE
TEMP
1
456
789
RATE
0
RESPIRATION
2
RATE
BLOOD PRESSURE
WAVEFORM
ECG
SIZE
CLEAR ENTER
3
BASELINE
ZEROSTATIC
NSR
4
5
6
5
ebt001f.eps
Top Panel
Left Panel
8
1
2
7
9
10
Right Panel
3
RA
R
LA
L
RL
N
LL
F
V1
7
C1
V2
C2
V3
C3
V4
C4
V5
C5
V6
C6
Figure 1. Controls and Terminals
9
Page 20

PS420
Users Manual
Table 2. Controls and Terminals
Item Name Description
A Battery Eliminator For use in operating the Simulator from any standard electrical outlet. To ensure safe
operation, use only the Fluke Biomedical Battery Eliminator (PN 2647372).
WXWarning
Caution risk of electric shock. Use only the Battery Eliminator specified in this
manual or the protection provided may be impaired.
B Power Switch Switches the power on and off.
C LA - LL Slide
Switch
Selects the reference lead, either LA (left arm) or LL (left leg). The position of the switch
must correspond to the type of patient monitor in use.
D LCD Display 15 mm x 60 mm (.58 in. x 2.37 in.) window displaying up to two lines of 20-point font.
E
10
Control Keys
ENTER Enters the selected code line value into memory.
CLEAR Clears the code line value from the LCD window.
SCROLL Causes the code line number to increase or decrease. The display arrows indicate which
SCROLL to use. The right SCROLL
SCROLL
decreases the code lines by 1.
increases the code lines by 1, while the left
Page 21

Multiparameter Simulator
Controls and Terminals
Table 2. Controls and Terminals (cont.)
Item Name Description
E CHANGE These keys are functional when the top line in the LCD window displays the up/down
arrows
CHANGE arrow
decreases the preset codes by 1.
. They allow you to increment or decrement the current setting. The up
increases the preset codes by 1, while the down CHANGE arrow
F
Keypad Soft Keys These keys have two functions: numeric and one-step selection of Simulator functions.
0 / STATUS Enters a numeric 0 to code line. Displays current parameter settings.
1 / TEMP Enters a numeric 1 to code line. Changes temperature.
2 / RATE Enters a numeric 2 to code line. Changes respiration rate.
3 / BASELINE Enters a numeric 3 to code line. Changes baseline resistance.
4 / STATIC Enters a numeric 4 to code line. Sets BP channels static levels.
5 / WAVEFORM Enters a numeric 5 to code line. Sets BP channels to BP waveforms.
6 / ZERO Enters a numeric 6 to code line. Sets BP channels to zero level.
7 / RATE Enters a numeric 7 to code line. Changes ECG rate for NSR.
8 / SIZE Enters a numeric 8 to code line. Changes ECG amplitude (lead II).
9 / NSR Enters a numeric 9 to code line. Selects Normal Sinus Rhythm (80 BPM).
11
Page 22

PS420
Users Manual
Table 2. Controls and Terminals (cont.)
Item Name Description
G ECG Connectors
H CO / Temp 1& 2
Ten snap and multi-banana connectors for ECG output, allowing for connection to any
twelve-lead ECG. These terminals are labeled and on the top panel. The labels are
AHA/IEC color-coded to aid in matching them to corresponding patient leads. Labels and
their definitions are as follows:
Label Definition
RA / R Right Arm
LA / L Left Arm
RL / N Right Leg (Reference or grounded)
LL / F Left Leg
V1 / C1 to V6 / C6 V leads (US and Canada). Also referred to as pericardial,
precardial or unipolar chest leads, and chest leads (IEC)
8-pin mini-DIN plug connector for the Cardiac Output and Temperature cables. These
cables are available separately from your Fluke Biomedical Sales Agent.
I BP 1 8-pin mini-DIN connector for BP cable plugs.
J BP 2 / RS-232 8-pin mini-DIN plug connector for BP cable plugs, as above, and for connecting an RS-232.
12
Page 23

Multiparameter Simulator
Powering the Simulator
Powering the Simulator
The Simulator uses a 9 V alkaline battery (Duracell®
MN1604 or equivalent). It is designed to use as much of
the battery as possible. When it detects less than about
5.6 volts, it goes into a shutdown mode, sounds a
continuous tone alarm, and displays the following
message:
The battery is in the base of the instrument. Use a 9-volt
alkaline battery (Duracell® MN1604 or equivalent). Do not
use mercury, air, or carbon-zinc batteries.
W Warning
The 9-volt alkaline battery provided with the
Simulator may explode or leak if recharged,
inserted improperly, disposed of in a fire, or
mixed with different battery types. Dispose of
the battery in accordance with any applicable
state or local regulations.
As an alternative to a battery, you can power the
Simulator with the Fluke Biomedical Battery Eliminator,
PN 2647372.
WXWarning
Caution risk of electric shock. Use only the
Battery Eliminator specified in this manual or
the protection provided may be impaired.
Note
Remove the battery and disconnect the Battery
Eliminator if you do not intend to use the
Simulator for an extended period of time.
Operating the Simulator
Connect the Simulator to the device under test. Use the
Simulator’s alphanumeric keypad to enter the code
presets. The Simulator then transmits these values to the
device. The following steps walk you through a sample
procedure.
1. Switch the Simulator ON. The LCD window displays
the program version for about two seconds.
13
Page 24

PS420
Users Manual
The window then displays the code entry display.
The display arrows indicate which SCROLL to use.
The right SCROLL increases the preset codes by
1, while the left SCROLL
decreases the preset
codes by 1. For example, pressing the right SCROLL
once will display the first preset, “0=VIEW”.
2. Key in the required preset code. For example, to
simulate a 30 °C (86 °F) temperature (code line 185),
press menu keys 1 + 8 + 5. Then, press the ENTER
key. This now becomes the preset temperature.
Press CLEAR to return to the default code entry
display.
14
3. To adjust some settings you can use the keypad’s
two-function alphanumeric keys. For temperature, as
an example, press 1, then ENTER. The LCD displays
the current preset.
Note the two additional up and down arrows. These
indicate which way to increase or decrease the
preset. Use the menu CHANGE arrows for scrolling
through the presets. The up CHANGE arrow
increases the preset codes by 1, while the down
CHANGE arrow
decreases the preset codes by
1. You can then advance through the temperature
settings shown in Table 3.
Page 25

Multiparameter Simulator
Operating the Simulator
Table 3. Temperature Settings
Code Temperature
189 42 °C (107.6 °F)
188 40 °C (104 °F)
187 37 °C (98.6 °F)
186 35 °C (95 °F)
185 30 °C (86 °F)
After reaching the required preset, press ENTER.
This now becomes the active temperature. Press
CLEAR to return to the default code entry display.
Note
Only use the ENTER key when scrolling through
the current settings. Using a SCROLL or
CHANGE key interrupts the scrolling operation.
At any point, you can view the Simulator’s current
parameter settings simply by pressing STATUS (0).
The Simulator then displays “0=VIEW”.
Press ENTER. The first parameter setting
(“ECG=NSR”) is then displayed. Thereafter, each
time you press ENTER, the Simulator displays
current settings in the sequence shown in Table 4.
Table 4. Current Settings
Display Description
ECG NSR Normal sinus rhythm in BPM.
NSR QRS Adult or pediatric waveform.
ECG AMPL ECG amplitude in mV.
RESP RATE Respiration rate in RPM.
R DELTA Impedance variation in ohms.
BASELINE Baseline impedance in ohms.
TEMPERATURE Temperature in °C.
BP SENS Transducer sensitivity in µV.
BP1, BP2 Blood pressure channel settings in
mmHg.
15
Page 26

PS420
Users Manual
Simulating Functions
This section describes simulation procedures by function.
If you are unfamiliar with basic Simulator operation, refer
to Operating the Simulator.
Temperature
The Simulator replicates normal, hypothermic, and
hyperthermic conditions with five temperature presets. All
temperature outputs are compatible with YSI 400/700
series probes. Temperature can be set through direct
code entry, as below, by keying the preset code and
pressing ENTER. You can also use the SCROLL keys
to cycle through the other presets before pressing
ENTER.
In addition, you can select and adjust settings by first
keying 1 = TEMPERAT. or
ENTER and use the CHANGE keys to cycle through
the presets. Then press ENTER to set the temperature. If
you key 190, press ENTER and use the SCROLL keys
to cycle through the other presets. Then press ENTER
to set the temperature.
190. If you key 1, press
Code Display Selects temperature of:
185 TEMP 30C 30 °C (86 °F)
186 TEMP 35C 35 °C (95 °F)
187 TEMP 37C 37 °C (98.6 °F)
188 TEMP 40C 40 °C (104 °F)
189 TEMP 42C 42 °C (107.6 °F)
Respiration
Respiration Rate
The Simulator replicates nine rate settings. These can be
set through direct code entry, as below, by keying the
preset code and pressing ENTER. You can also use the
SCROLL keys
before pressing ENTER. In addition, you can select and
adjust settings by first keying 2 RATE. If you key 2, press
ENTER and use the CHANGE keys
the other presets. Then press ENTER to set the rate.
to cycle through the other presets
to cycle through
16
Page 27
Multiparameter Simulator
Simulating Functions
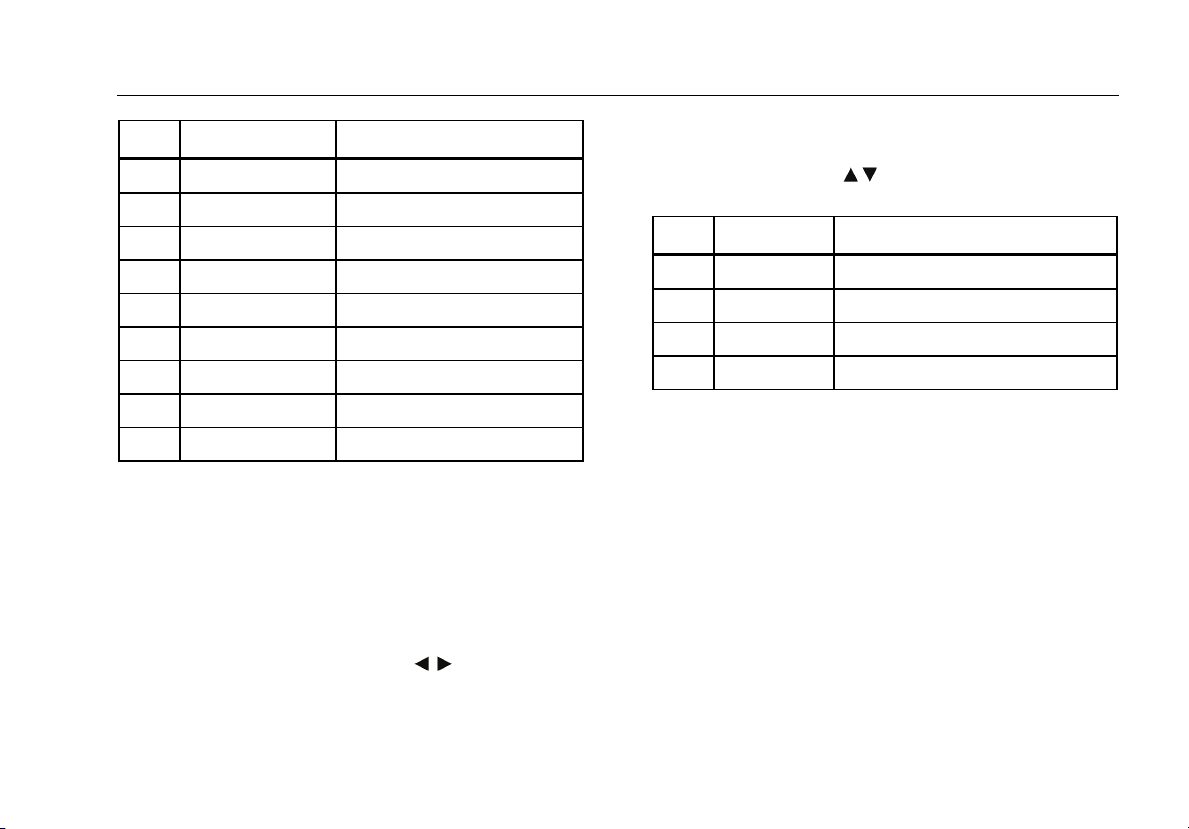
Code Display Selects respiration rate of:
152 RESPPM 0 0 RPM
153 RESPPM 15 15 RPM
154 RESPPM 20 20 RPM
155 RESPPM 30 30 RPM
156 RESPPM 40 40 RPM
157 RESPPM 60 60 RPM
158 RESPPM 80 80 RPM
159 RESPPM 100 100 RPM
160 RESPPM 120 120 RPM
Baseline Impedance
The baseline resistance selection of 500, 1000, 1500, and
2000 Ohms is between leads LA, RL, RA, and LL. The
resistance for the V leads is 1020 Ohms between any V
leads (V1-V6).
The impedance can be set through direct code entry, as
below, by keying the preset code and pressing ENTER.
You can also use the SCROLL keys
through the other presets before pressing ENTER.
to cycle
In addition, you can select and adjust settings by first
keying 3 = BASELINE. If you key 3, press ENTER and
use the CHANGE keys
presets. Then press ENTER to set the impedance.
Code Display Selects baseline resistance of:
166 BASEL 500 500 Ohms
167 BASEL 1000 1000 Ohms
168 BASEL 1500 1500 Ohms
169 BASEL 2000 2000 Ohms
When you switch the Simulator on, the baseline
impedance is set at 1000 Ohms. If changed,
some monitors check lead impedance and, if too
high (e.g., 2000 Ohms), may revert to their
inoperative modes.
to cycle through the other
Note
17
Page 28

PS420
Users Manual
Impedance Variations
The Simulator replicates impedance variations of 0.2, 0.5,
1.0, 2.0, and 3.0 and 2000 delta Ohms. Use the LA/LL
switch on the top panel to select which lead will have the
respiration signal on it. This will not affect the baseline
operation.
The variation can be set through direct code entry, as
below, by keying the preset code and pressing ENTER.
You can also use the SCROLL keys to cycle
through the other presets before pressing ENTER.
Code Display Selects respiration delta Ohms:
161 R DELTA .2 0.2 Ohms
162 R DELTA .5 0.5 Ohms
163 R DELTA 1 1.0 Ohms
164 R DELTA 2 2.0 Ohms
165 R DELTA 3 3.0 Ohms
Apnea
The Simulator replicates the general apnea types by
duration only: continuous; and 12, 22, and 32 seconds.
Apnea can be set through direct code entry, as below, by
keying the preset code and pressing ENTER. You can
use the SCROLL keys
presets before pressing ENTER.
Code Display Selects respiration apnea:
144 APNEA ON ON. Respiration rate of 0 RPM.
145 APNEA OFF OFF. Respiration reverts to
146 12 SEC APN For 12 seconds.
147 22 SEC APN For 22 seconds.
148 32 SEC APN For 32 seconds.
to cycle through the other
previously selected respiration
rate.
18
Page 29

Multiparameter Simulator
Simulating Functions
Blood Pressure
The Simulator synchronizes dynamic blood pressure
waveforms with all NSR rates and tracks all arrhythmia
selections. Both blood pressure channels (BP-1 and 2)
can be controlled. Each channel can act independently or
can be set together for universal settings.
The Simulator will set the blood pressure channels to a
zero level on power up. This is to allow you to set up the
monitoring equipment under test.
Transducer Sensitivity (All Channels)
Before simulation can begin, blood pressure transducer
sensitivity must be set to correlate with the monitor
manufacturer’s specifications: 40 µV/V/mmHg (code 76)
or 5 µV/V/mmHg (code 77). Simulator accuracy is ± 1%,
± 1 mmHg.
The sensitivity is set through direct code entry by keying
the preset code and pressing ENTER.
Waveform (All Channels)
The Simulator can set a single waveform for all blood
pressure channels. This is set by first selecting 5 =
WAVEFORM. After selecting 5, press ENTER. The LCD
will display BP CHANNELS SET. The waveforms will
appear only during ECG waveforms where blood
pressure waveforms occur (e.g., during the asystole
selection, all pressure channels drop to 0 level).
Blood Pressure Artifact (All Channels)
You can insert a respiratory artifact, as required, into any
waveform over all channels. Insert artifacts by keying 84,
then pressing ENTER. Stop insertion by keying 85, and
then pressing ENTER.
BP Static Levels (All Channels)
The blood pressure static level can be set through direct
code entry, as below, or selected and adjusted through
first selecting 4 = STATIC. After selecting 4, press
ENTER, and then use the CHANGE keys
the available presets.
to scroll to
BP Static Levels, Channels 1 and 2
BP Static Levels, Channels 1 and 2 can be set through
direct code entry, as below, or selected and adjusted
through first selecting 94 = P1,2 STAT. After selecting 94,
press ENTER, and then use the CHANGE keys
scroll to the available presets. Note that this will affect all
pressure channels, and the Simulator will not display the
levels for BP channels 1 and 2.
to
19
Page 30

PS420
Users Manual
Code Display Sets
207 BP STAT L0 BP channel to static level 0.P1= -
10 P2= -10 P3= -5 P4= -5
208 BP STAT L1 BP channel to static level 0.P1=0
P2=0 P3=0 P4=0
209 BP STAT L2 BP channel to static level
0.P1=80 P2=50 P3=20 P4=20
210 BP STAT L3 BP channel to static level 0.
P1=160 P2=100 P3=40 P4=40
211 BP STAT L4 BP channel to static level 0.
P1=240 P2=150 P3=60 P4=60
212 BP STAT L5 BP channel to static level 0.
P1=320 P2=200 P3=80 P4=80
213 BP STAT L6 BP channel to static level 0.
P1=400 P2=240 P3=100
P4=100
Channel BP-1
Code Display Selects:
78 P1 ARTERIA Arterial waveform, 120/80.
79 P1 RADIAL Radial waveform, 120/80.
80 P1 LVNT Left ventricle waveform, 120/00.
81 P1 RVNT Right ventricle waveform, 25/00
Channel BP-2
Code Display Selects:
86 P2 ARTERIA Arterial waveform, 120/80.
87 P2 RADIAL Radial waveform, 120/80.
88 P2 LVENT Left ventricle waveform, 120/00.
89 P2 RVNT Right ventricle waveform, 25/00.
90 P2 PULAR Pulmonary arterial waveform,
25/10.
91 P2 PULWDG Pulmonary wedge waveform,
10/2.
92 P2 L ATRIU Left atrium waveform, 14/4.
93 P2 CVP Central venous waveform (right
atrium), 15/10.
20
Page 31

Multiparameter Simulator
Simulating Functions
ECG/Arrhythmia
The Simulator replicates 37 different types of arrhythmias,
from inconsequential types of PNCs to asystole. In
addition, the Simulator can send waveforms to test any
electrocardiograph, and can accommodate twelve-lead
configurations with independent outputs for each signal
lead referenced to the right leg (RL).
Adult and Pediatric NSR QRS
An adult NSR with a QRS width of 80 ms, or a pediatric
NSR with a QRS width of 40 ms, can be set. These will
remain in effect throughout ECG and arrhythmia
selections until changed by reentering the below codes
and pressing ENTER.
Code Display Selects:
11 PEDIATRIC Pediatric NSR with QRS width of
40 ms.
12 ADULT Adult NSR with QRS width of 80
ms.
NSR
The Simulator replicates fifteen normal sinus rhythms, or
NSRs. These NSRs can be set through direct code entry,
as below, or selected and adjusted through first selecting
9 = NSR. After selecting 9, press ENTER, and then use
the CHANGE keys
Code Display Selects NSR rate of:
170 NSR 30BPM 30 BPM
171 NSR 40BPM 40 BPM
172 NSR 60BPM 60 BPM
173 NSR 80BPM 80 BPM
174 NSR 100BPM 100 BPM
175 NSR 120BPM 120 BPM
176 NSR 140BPM 140 BPM
177 NSR 160BPM 160 BPM
178 NSR 180BPM 180 BPM
179 NSR 200BPM 200 BPM
180 NSR 220BPM 220 BPM
181 NSR 240BPM 240 BPM
182 NSR 260BPM 260 BPM
183 NSR 280BPM 280 BPM
184 NSR 300BPM 300 BPM
to scroll to the available presets.
21
Page 32

PS420
Users Manual
Arrhythmias: Premature Beats
Code Display Selects:
13 PVC1 LVF PVC with left ventricle
focus. The Simulator then
assumes NSR at 80 BPM.
14 PVC1 E LVF Early PVC with left ventricle
focus. The Simulator then
assumes NSR at 80 BPM.
15 R ON T LVF R on T PVC with left
ventricle focus. The
Simulator then assumes
NSR at 80 BPM.
16 PVC2 RVF PVC with right ventricle
focus. The Simulator then
assumes NSR at 80 BPM.
17 PVC2 E RVF Early PVC with right
ventricle focus. The
Simulator then assumes
NSR at 80 BPM.
Code Display Selects:
18 R ON T RVF R on T PVC with right
ventricle focus. The
Simulator then assumes
NSR at 80 BPM.
19 MULTIFOCAL Multifocal PVCs. The
Simulator then assumes
NSR at 80 BPM.
20 ATRIAL PAC Atrial premature contraction.
The Simulator then
assumes NSR at 80 BPM.
21 NODAL PNC Nodal premature
contraction. The Simulator
then assumes NSR at 80
BPM.
22
Page 33

Multiparameter Simulator
Simulating Functions
Arrhythmias: Ventricular
Code Display Selects:
24 PAIR PVCS Pair of PVCs. The Simulator
then assumes NSR at 80 BPM.
25 RUN 5
PVCS
26 RUN 11 PVC Run of 11 PVCs. The Simulator
27 BIGEMINY Bigeminy rhythm.
28 TRIGEMINY Trigeminy rhythm.
29 PVCS 6/M 6 PVCs per minute.
30 PVCS 12/M 12 PVCs per minute.
31 PVCS 24/M 24 PVCs per minute.
32 FREQ
MULTI
33 VENT
TACHY
34 VENT FIB 1 Ventricular fibrillation.
35 VENT FIB 2 Ventricular fibrillation at 1/2.
36 ASYSTOLE Asystole. No ECG.
Run of 5 PVCs. The Simulator
then assumes NSR at 80 BPM.
then assumes NSR at 80 BPM.
Frequent multifocal rhythm.
Ventricular tachycardia.
Arrhythmias: Atrial
Code Display Selects:
38 ATRL FIB1 Atrial fibrillation.
39 ATRL FIB2 Atrial fibrillation 1/2 value.
40 ATRIAL FLT Atrial flutter.
41 SINUS ARRH NSR with irregular rate.
42 MISSED Missed beat. The Simulator
then assumes NSR at 80 BPM.
43 ATRL TACHY Atrial tachycardia.
44 NODAL RHYT Nodal rhythm.
45 SUPRAVENT Supraventricular tachycardia.
23
Page 34

PS420
Users Manual
Arrhythmias: Conduction
Code Display Selects:
47 RT BNDL BB Right bundle branch block
48 LT BNDL BB Left bundle branch block
49 1ST DEG BL 1st degree heart block rhythm.
50 2ND DEG BL 2nd degree heart block rhythm.
51 3RD DEG BL 3rd degree heart block rhythm.
Defects
rhythm.
rhythm.
ST Elevation and Depression Waves
These can be set through direct code entry, as below, or
adjusted and set through first selecting 52 = ST WAVES
or 142 = ST WAVES. After selecting 52 or 142, press
ENTER, and then use the CHANGE keys
the available presets.
to scroll to
Code Display Selects ST wave of:
236 ST +.8 mV +.8 mV elevation
237 ST +.7 mV +.7 mV elevation
238 ST +.6 mV +.6 mV elevation
239 ST +.5 mV +.5 mV elevation
240 ST +.4 mV +.4 mV elevation
241 ST +.3 mV +.3 mV elevation
242 ST +.2 mV +.2 mV elevation
243 ST +.1 mV +.1 mV elevation
244 ST - .1 mV -.1 mV depression
245 ST - .2 mV -.2 mV depression
246 ST - .3 mV -.3 mV depression
247 ST - .4 mV -.4 mV depression
248 ST - .5 mV -.5 mV depression
249 ST - .6 mV -.6 mV depression
250 ST - .7 mV -.7 mV depression
251 ST - .8 mV -.8 mV depression
24
Page 35

Multiparameter Simulator
Simulating Functions
ECG Waveform
The Simulator replicates four ECG waveform amplitudes,
with a ± 2% accuracy of selection (Lead II). The Simulator
uses them as references only during arrhythmia
simulations. They are set through direct code entry, as
below, or selected and adjusted through first selecting 8 =
ECG AMPL. After selecting 8, press ENTER, and then
use the CHANGE keys
presets. Then, press ENTER to set the amplitude. This
setting remains in effect until changed, or until you switch
the Simulator off.
Code Display Selects ECG amplitude of:
191 ECGSEN .5 0.5 mV
192 ECGSEN 1 1.0 mV
193 ECGSEN 1.5 1.5 mV
194 ECGSEN 2 2.0 mV
to scroll to the available
ECG Rate
The Simulator replicates fifteen heart rates, with a ± 1%
accuracy of selection. These are set by selecting 7 =
RATE. After selecting 7 and pressing ENTER, use the
CHANGE keys
Then press ENTER to set the rate.
to scroll to the available presets.
Code Display Selects ECG rate of:
170 ECG = 30BPM 30 BPM
171 ECG = 40BPM 40 BPM
172 ECG = 60BPM 60 BPM
173 ECG = 80BPM 80 BPM
174 ECG = 100BPM 100 BPM
175 ECG = 120BPM 120 BPM
176 ECG = 140BPM 140 BPM
177 ECG = 160BPM 160 BPM
178 ECG = 180BPM 180 BPM
179 ECG = 200BPM 200 BPM
180 ECG = 220BPM 220 BPM
181 ECG = 240BPM 240 BPM
182 ECG = 260BPM 260 BPM
183 ECG = 280BPM 280 BPM
184 ECG = 300BPM 300 BPM
25
Page 36

PS420
Users Manual
Superimposed Artifacts
The Simulator replicates five different artifacts. Their
purpose is to evaluate the effect of these type artifacts on
ECG accuracy. After selecting the artifact, press ENTER
to transmit it to the ECG. The Simulator deactivates the
artifact when you make another ECG or arrhythmia
selection.
Code Display Selects:
53 50 HZ ARTI 50 Hz artifact (European
lines).
54 60 HZ ARTI 60 Hz artifact (US lines).
55 MUSCLE ART Muscle artifact.
56 BASE WANDR Baseline wandering.
57 RESP ARTIF Respiration artifact.
Pacemaker
The Simulator replicates six paced rhythms/signals. After
selecting the required rhythm, press ENTER. Use the
CHANGE keys to scroll through the available
rhythms.
Code Display Selects:
58 ASYNCHRONO Asynchronous pacemaker
rhythm.
59 DEMND FSIN Pacemaker rhythm with
frequent sinus beats.
60 DEMND OSIN Pacemaker rhythm with
occasional sinus beats.
61 AV SEQUENT Atrial ventricular pacemaker
rhythm.
62 NONCAPTURE Noncapture event. The
Simulator then assumes
asynchronous pacemaker.
63 NON FUNCT Non-function pacemaker
rhythm.
26
Page 37

Multiparameter Simulator
Simulating Functions
Pacemaker Pulse Amplitudes, Lead II
When you select any pacemaker waveform, you can
adjust and set pulse amplitudes in Lead II. These can be
set through direct code entry, as below, or selected and
adjusted through first selecting 64 = PACE AMP. After
selecting 64, press ENTER, and then use the CHANGE
to scroll to the available presets.
keys
Code Display Sets pacemaker pulse
amplitude (Lead II) of:
224 PACE 2 mV 2 mV
225 PACE 4 mV 4 mV
226 PACE 6 mV 6 mV
227 PACE 8 mV 8 mV
228 PACE 10 mV 10 mV
Pacemaker Pulse Width
When you select any pacemaker waveform, you can
adjust and set pulse width. These can be set through
direct code entry, as below, or selected and adjusted
through first selecting 65 = PACE WIDTH. After selecting
65 press ENTER, and then use the CHANGE keys
to scroll to the available presets.
Code Display Sets pacemaker pulse
width of:
229 PACE 0.1mS 0.1 ms
230 PACE 0.5mS 0.5 ms
231 PACE 1 mS 1.0 ms
232 PACE 1.5mS 1.5 ms
233 PACE 2 mS 2.0 ms
27
Page 38

PS420
Users Manual
Cardiac Output
The software detects the cardiac output option when you
connect the Simulator to the patient monitor’s cardiacoutput computer using the available accessories. Refer to
your local Fluke Biomedical representative regarding the
available optional Cardiac Output Adapter Box and
cables.
To set up the cardiac output procedure while using the
Cardiac Output Adapter Box, you must match the
computational constant for the injectate temperature
required. You must then adjust the injectate temperature
pot to the required value for the model used. Use the
following procedure:
1. Set the computational constant on your monitor
under test to 0.561 for 2 °C, or 0.608 for 20 °C
injectate temperature.
2. Connect the cable from the Cardiac Output Adapter
Box to the CO/Temp1&2 port on the right side of the
Simulator.
3. Connect the cable for the injectate temperature into
the INJEC. TEMP. port on the Cardiac Output
Adapter Box.
4. Connect the cable for the blood temperature into the
BLOOD TEMP. port on the Cardiac Output Adapter
Box.
5. You may also connect other temperature probes
through the TEMP. 1&2 port on the Cardiac Output
Adapter Box.
6. Rotate the INJEC. TEMP. dial on the face of the
Cardiac Output Adapter Box until the monitor under
test indicates the proper injectate temperature. This
will be either 2 °C or 20 °C.
Note
The number value on the INJEC. TEMP. dial is a
relative setting for obtaining the same value on
identical monitors. It does not indicate a
numerical temperature value.
Once you have the injectate temperature set to either
2 °C or 20 °C, you are ready to run the appropriate
simulation from the Simulator. Select the appropriate test
code and then press ENTER.
28
Page 39

Multiparameter Simulator
Simulating Functions
Code Display Selects:
107 3L/M @ 2C Cardiac output wave of 3
L/min for 2 degrees.
Computational constant is
0.561.
108 5L/M @ 2C Cardiac output wave of 5
L/min for 2 degrees.
Computational constant is
0.561.
109 7L/M @ 2C Cardiac output wave of 7
L/min for 2 degrees.
Computational constant is
0.561.
110 3L/M @ 20C Cardiac output wave of 3
L/min for 20 degrees.
Computational constant is
0.608.
111 5L/M @ 20C Cardiac output wave of 5
L/min for 20 degrees.
Computational constant is
0.608.
Code Display Selects:
112 7L/M @ 20C Cardiac output wave of 3
L/min for 20 degrees.
Computational constant is
0.608.
113 FLT INJ 2 Faulty injection cardiac output
wave for 2 degrees.
Computational constant is
0.561.
114 L- T SHT 2 Left to right shunt cardiac
output wave for 2 degrees.
Computational constant is
0.561.
115 FLT INJ 20 Faulty injection cardiac output
wave for 20 degrees.
Computational constant is
0.608.
116 L- R SHT 20 Left to right shunt cardiac
output wave for 20 degrees.
Computational constant is
0.608.
29
Page 40

PS420
Users Manual
Code Display Selects:
117 CAL WAVES Calibrated cardiac output
waves.
118 NONCAL
WVE
119 CAL 1 SEC Calibration pulse of 1 degree
120 CAL 4 SEC Calibration pulse of a delta of
121 SET BT 37C 37 °C (98.6 °F)
122 SET BT 36C 36 °C (95.9 °F)
When the monitor under test indicates that it is ready to
perform a Cardiac Output calculation, press ENTER on
the Simulator to generate a test. The 4-second calibration
pulse provides a delta change of 402 Ohms. This
provides a standard for testing cardiac output units.
Uncalibrated cardiac output
waves. Gives 4 different
values per 3, 5, and 7 L/min
waves.
for 1 second.
402 Ohms for 4 seconds.
If you select any cardiac output waveform key a second
time while a waveform is proceeding, the selected
waveform will stop. The blood temperature will return to
37 °C (98.6 °F.)
ECG Performance Testing
Square Wave
Code Display Selects:
128 2 Hz SQR 2.0 Hz square waveform.
129 .125 Hz SQ 0.125 Hz square waveform.
Triangle Wave
Code Display Selects:
130 2 Hz TRIAN 2.0 Hz triangle waveform.
131 2.5 Hz TRI 2.5 Hz triangle waveform
Pulse Wave
Code Display Selects:
132 PULSE 30 Pulse of 30 BPM, width of 60 ms.
133 PULSE 60 Pulse of 60 BPM, width of 60 ms.
30
Page 41

Multiparameter Simulator
Cleaning
Sine Wave
Code Display Selects:
135 SINE .5 Hz 0.5 Hz sine wave.
136 SINE 5 Hz 5.0 Hz sine wave.
137 SINE 10 Hz 10.0 Hz sine wave.
138 SINE 40 Hz 40.0 Hz sine wave.
139 SINE 50 Hz 50.0 Hz sine wave.
140 SINE 60 Hz 60.0 Hz sine wave.
141 SINE 100Hz 100.0 Hz sine wave.
Cleaning
Clean only with a damp, lint-free cloth, using mild
detergent, and wipe down gently.
W Caution
Do not pour fluid onto the Simulator surface;
fluid seepage into the electrical circuitry may
cause Simulator failure.
W Caution
Do not use spray cleaners on the Simulator;
such action may force cleaning fluid into the
Simulator and damage electronic
components.
31
Page 42

PS420
Users Manual
32
 Loading...
Loading...