Page 1

DOCUMENT NUMBER
Uncontrolled Copy
This document is current on the FCI Website or the FCI intranet Quality Document Control program.
li
ic
SUBJECT
Quality Manual
PREPARED BY:
allp7
CAGE CODE
64818
1
1
1
01
7
LTI
DATE
10-8-2012
QUALITY MANUAL
SECTION
.11
07QA070003
PAGE
CURRENT
REVISION
L
Burt Tanaka
Quality Assurance Manager
Ronald E. Ogle
Director of Administr n & Quality
APPROVED BY:
Signature on File
President
Page 2

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
ii
CURRENT
REVISION
L
COMMITMENT TO QUALITY
FLUID COMPONENTS INTERNATIONAL LLC will hire experienced personnel, continually
train them, and clearly define their responsibilities in order to provide the highest level of quality
products and services to the wide variety of markets and industries that we support. All
employees will be responsible for quality and are expected to participate in continuous quality
improvement functions so that industry standards will be met or exceeded. Employees will also
be expected to take initiative in continuously improving processes and in their own selfdevelopment skills. Employees will be provided the tools, instructions, and authority to act in
the best interest of the company with regard to the quality of our products and or service. The
Quality Assurance and Quality Control organizations will function as a catalyst to establish
informational quality needs, compliance standards, mutual improvement targets, process
changes, and overall quality focus. These organizations will demonstrate continuous
communications and collaboration with all operational departments.
The Quality Assurance organization is chartered with defining and enforcing the standards that
are specific to the various industries that we service. Quality Assurance shall either integrate or
isolate those requirements so that we efficiently meet all industry specific quality expectations.
The Quality Manual that follows shall be used as an internal baseline for quality processes. The
Manual will serve as a standard for which we will compare our performance. The Manual will
be a living document and will be regularly updated with improvements and changes that are
necessary to meet the evolving business environment. The Manual will additionally reflect our
commitment of quality to our customer base and will be readily available for review and
recommendations.
The President
Page 3

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
iii
CURRENT
REVISION
L
TABLE OF CONTENTS
DESCRIPTION
SECTION
PAGE
Title Page
-
i
Commitment to Quality Statement
-
ii
Table of Contents
-
iii
Revision Page
-
iv, v,
Quality Management System
-
vi-viii
Appendix A
-
ix
Appendix B
-
x-xii
Appendix C
-
xiii
Appendix D
-
xiv-xviii
Section One
1
1 – 19
Section Two
2
1 – 32
Section Three
3
1 -- 21
Page 4
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
iv
CURRENT
REVISION
L
REVISION PAGE
Location
REV
DESCRIPTION
APPROVALS
DATE
QA
CO.MGMT.
N/A A COMPLETELY REVISED from QA Manual 8003 Rev. K.
Changed to new numbering system. Initial release at new number
is Rev. A
7-1-97
SM
DMQ
DMF
B Revised to incorporate process changes required as a result of
installing a new business system
2-03-98
SM
DMQ
DF
C Completely revised to incorporate AS9100 and ISO9001
requirements. Made changes to what is now “Section Three”,
(previous “Control Manual in its entirety).
8-24-00
REO
DMQ
REO
2 of
Section 2
D
Added the second paragraph in “1.0 Scope”
10-9-00
REO
DMQ
REO
All, (four
places)
Changed “AS9100” to include “AS9000”, i.e., “AS9000/AS9100”.
2-03-98
SM
DMQ
DF
28 of
Section 2
Added “customers and” (regulatory authorities …) to the last
sentence in 4.16.
8-24-00
REO
DMQ
REO
All E Renumbered pages as necessary
7-31-02
REO
DMQ
REO
v Added (customer and Field Services)
viii Added and corrected “NQA-1” references
ix Updated Org Chart
x Reformatted, deleted 04QA704027, added several procedures.
2 Added second paragraph to 1.0
17 of
Section 1
Added “Notification” paragraph to 4.14.3
12 of
Section 3
Changed the reference from 04QA704027 to 01DM000064 in
paragraphs 5.3 & 5.4
14 of
Section 3
Deleted sentence pertaining to superscript on Op –Sheet and
changed reference from 04QA704027 to 01DM000064 in
paragraph 5.9.
16 of
Section 3
Added sentence pertaining to ASME Certificate to paragraph 7.1.1
17 of
Section 3
Added reference to Quality Assurance Procedure 04QA704032 to
paragraph 7.3
F
Completely revised
8-19-03
REO
DMQ
Page 5
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
v
CURRENT
REVISION
L
Location
REV
DESCRIPTION
APPROVALS
DATE
QA
CO.MGMT.
vi, x, xi,
section 2
pages 2,
10, 15, 16,
20, 22, &
23
G
Made references to 8100.7 in Appendix B and where applicable in
Section 2 of the manual per FAA request.
09/30/05
REO
DMQ
REO
ii Updated company‟s name
vi
Added “Industrial” to Controlled projects paragraph
ix
Change “ATEX” to “EX” in Appendix A
x
Deleted QAP 04QA704025, procedure was inactivated.
Corrected procedure # “07QA04044” to “04QA704044”
Corrected procedure # “04QA404062” to “04QA704062”
Renumbered index
xi
Corrected manual # “007QA070003” to “07QA070003”
Deleted “20” from the 8100.7 reference column for procedure
04QA704091. (No such paragraph exists)
Renumbered index
Updated company‟s name
xii
Updated procedure number reference to correlate to renumbering
of index on pages xi and xii
Added note to explain the number references
Sect 1;2
H
Revised the end of the first paragraph in 1.0 – Scope from “quality
system as it relates to all projects not identified as Controlled” to
“minimum quality system requirements”
09/30/05
REO
DMQ
REO
Sect 1;2
Included reference to “Ex” representative in paragraph 1.0 – Scope
Sect 1; 18
& 19
Included reference to “Ex” representative and 10 year record
retention requirement in 8.3 – Notification
Sect 3;2
Included the “B” in “Appendix B” in paragraph 1.1
Corrected “Appendix B of the manual” to Appendix D of the
manual” in paragraph 1.1
Corrected “Appendix B of the manual” to “Appendix A of the
manual” in paragraph 1.1
Sect 3;2
Updated company‟s name in paragraphs 1.1, 2.1 and 2.2
Sect 3;3
Updated company‟s name in paragraph 2.2
Sect 3; 4
Updated company‟s name in paragraphs 2.6 and 3.1
Sect 3;5
Updated company‟s name in paragraph 3.5
Sect 3;6
Updated company‟s name in paragraph 4.1
Sect 3;12
Updated company‟s name in paragraph 5.1
Sect 3;14
Updated company‟s name in paragraph 9.1
Sect 3;21
Updated company‟s name in paragraph 18.3
Sect. 7.1.
revised the end of paragraphs “(a)” & (b) to add
“Planning”
APX B
J
Made references in Appendix B and where applicable
per UL AS9100/ISO Audit request 2006
9/11/06
REO
REO
Sec 3
7.1.1
EDITED 7.1.1 APPROVED VENDORS LIST
requirements of ANSI/ISO/ISE 17025
Page 6

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
vi
CURRENT
REVISION
L
Location
REV
DESCRIPTION
APPROVALS
DATE
QA
CO.MGMT.
APXD
K
updated table of contents, added new revision page, added “EX
Representative” to Appendix D, deleted “pr” , Sec. 2 8.3 and
“DQS100” from 8.3, Changed all ISO9000 references from Rev.
A to “current rev.” Section 2, 3.0 added “Note” Removed
“Conformal Coat” from section 9.1(section 3) as a special
process. Removed personnel qualifications for Conformal Coat
from section 9.2
Revised Section 1 and 2 to ISO9001 2008 and AS9100C
respectively. Added new FAA regulations 21.137, etc. to Section
2.
Revised the org chart Appendix A and updated Appendix B.
Updated Appendix C.
Revised pages vi, vii, xv, and xviii
11/10/10
BC
REO
REO
Header
vii
APNX A
APNX B
APNX C
APNX D
Section 1
8.3
Section 2
8.2.3c
Section 2
8.2.4
Section 3
1.1
Section 3
2.2
Section 3
2.3
L
Changed Date of Origin to Date
Updated to current specification list. Removed reference to MILI-45208.
Organization Chart Revised.
Added Risk Management & Return to Service
Revised “Validation of Processes for Production and Service
Provision/ Special Procedure” to “Special Processes”
Revised Interrelationship Diagram per 2011 AS9100 Findings
Revised President responsibility for acting as QA Manager.
Added Director of Administration & Quality
Added Administering the ESD material to QA Staff.
Added Identifying Critical… to Nuclear Qualification Engineer
Added DMIR and Return-to-Service.
Removed Quality Engineer/Supervisory position
Corrected Production Test to Test and reporting to Engineering.
Changed 8.3 for EN13980:2002 Section 8.3 b & c
Duplicate information removed “c) Identify and control the
nonconforming product” and 8.2.3.e.
Revised “reorganized” to “recognized”, typographical error.
Removed reference to a specific revision 2000 for ISO and NQA-
1.
Removed reference to Rev A & B of the Manual.
Changed Yearly to Annual.
10/8/12
BT
REO
Page 7
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
vii
CURRENT
REVISION
L
Location
REV
DESCRIPTION
APPROVALS
DATE
QA
CO.MGMT.
Section 3
2.4
Section 3
2.6
Section 3
3.4
Section 3
3.5
Section 3
4.1
Section 3
5.1
Section 3
5.2
Section
5.3
Section 3
5.4
Section 3
Section 3
6.3.1
Section 3
6.3.2
Section 3
7.1
Section 3
7.1.1
Section 3
7.1.2
Section 3
7.2
L
Added “as required by contract.”
Aded “Manual Section 3.”
Added reference to procedure 04QA704091
Removed sentence “The same individual…verification and
analysis.”
Replaced “Fluid Components International LLC”, with “the
Company‟s.”
Revised first three sentences.
Removed requirement to send drawings and certified shippers.
Reworded redirection to paragraph 5.2
Removed the word “some”.
Added “A Process Sheet…the Operation Sheet.”
Removed reference to Process Manual.
Removed Process Manuals Replaced with the document control
system.
Replaced reference to “FCI” to “the Company”
Revised Document Change Notice to Engineering Change
Notice and DCN to ECN.
Replaced DCN with ECN.
Corrected reference to QR, Test Reports…
Replaced The Quality Department…
Added “Audits”
Complete revision.
Added “& Approved Vendor List(AVL)”
Added “Vendor performance…” and reference to listing
08QA080002.
Corrected procedure 04QA704046.
Removed “items as described in”
Replaced „purchased on “Commercial” Purchase Order‟
Replaced “transferred to “re-evaluated”
10/8/12
BT
REO
Page 8

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
viii
CURRENT
REVISION
L
Location
REV
DESCRIPTION
APPROVALS
DATE
QA
CO.MGMT.
Section 3
7.3
Section 3
7.4
Section 3
8.1
Section 3
8.2
Section 3
9.1
Section 3
10.2
Section 3
12.2
Section 3
16.2
Section 3
16.4
Section 3
17
Section 3
18.3
L
Removed third paragraph on Certified Shipper.
Replaced reference the obsolete table to the current database.
Removed See QAP 04QA704025 for more details on Parts List.
Removed explanation of Limited Life…
Corrected the Serialization system to show the current Business
System generated
Removed “utilized by FCI…”
Corrected the “When additional processes…”
Corrected “Currently Welding and Soldering…”
Corrected “according” to “accordance”
Corrected wording in the first sentence.
Added the option for a CPAR.
Removed DR are mostly…
Replaced 04QA704013 with Quality system Database; Quality
Records.
Added Critical Safety Characteristics Evaluation.
10/8/12
BT
REO
Date of effectivity for Revision L is April 8th, 2014
Burt Tanaka
2014.04.29
16:43:54 -07'00'
Page 9
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
ix
CURRENT
REVISION
L
Scope of Work
FCI manufactures Flow, Liquid Level, Temperature and Pressure Instrumentation, and Flow
Conditioners.
Quality Management System
This Quality Manual provides an overview of FCI‟s quality system and identifies the processes
used to ensure that our products and servicing (customer and Field Services) meet specified
requirements. The processes described in this Manual are aimed at achieving customer
satisfaction by preventing nonconformities at all stages of design, product realization, service, and
delivery.
The Quality Manual shall be in English and is available in the database system.
FCI has developed and implemented a quality management system, based on the ISO 9001 and
AS9100 (current revision), the Quality System Requirements of 10CFR50 Appendix B, 14CFR
Part 21.137 (a)-(n) and the basic Quality System Requirements of ANSI NQA-1-2000 to support
our quality policy. This Manual defines the quality management system. Procedures and work
instructions provide additional detail. Procedures address the “what, when and where” and
include responsibilities, objectives, and activities for each applicable function in the company.
Those procedures referring to particular revisions of the above standards shall be viewed as
complying with the current standards and not limited to the revisions that are referenced. Work
instructions provide step-by-step details on performing specific tasks, and include criteria for
determining compliance.
Customer specific requirements, which are not addressed by the current quality system, are
considered on an individual project/contract/order basis, and communicated throughout FCI as
required.
The Quality Management system at FCI has been developed to accommodate three levels of
quality management. The level of quality management to be applied to a specific
project/contract/order is set at Contract Review and is suitably identified thereafter. The three
levels are defined as follows:
Industrial “Controlled” Projects: These projects include (but are not limited to) Nuclear Safety.
These types of projects/contracts/orders adhere to all the requirements of ISO 9001as defined in
Section One of this manual, the Quality System Requirements of 10CFR50 Appendix B, and the
basic Quality System Requirements of ANSI NQA-1as defined in Section Three of this manual.
These projects/contracts/orders and all associated data and documentation have the unique
identification of “Controlled”.
Aerospace Projects: These projects include all Aerospace identified projects. These types of
projects/contracts/orders adhere to all the requirements of ISO 9001, AS9100, and the Quality
System Requirements of 14CFR Part 21.137 (a)-(n) as defined in Section Two of this manual.
Page 10

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
x
CURRENT
REVISION
L
These projects/contracts/orders and all associated data and documentation are also identified as
“Controlled”.
All Other Projects:These projects include (but are not limited to) Commercial projects. These
types of projects/contracts/orders adhere to all the requirements of ISO 9001 as defined in section
one of this manual. Any and all projects/contracts/orders, data, and/or documentation not
identified as “Controlled” is deemed to fall within this level of quality management.
Hierarchy of Quality System Documents
Quality Manual: Level 1 document that provides a general overview of the quality
system and defines the quality policy.
Quality System Procedures: Level 2 documents that provide more detailed
explanation of the quality system elements and describe the structure of the quality
system.
Work Instructions: Level 3 documents that provide step-by-step instructions for
executing activities.
Quality Records: Level 4 documents or data that contain the data, charts, checklists,
or other records which demonstrate conformance to specified requirements and the
effective operation of the quality system.
Page 11

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
xi
CURRENT
REVISION
L
Level 1
Level 2
Level 3
Level 4
Management definition of POLICIES
that support its quality system
Processes that define WHAT is done
to meet quality system requirements
Documents that describe HOW processes
are performed (more detail than Level 2)
Forms, reports or data that demonstrate
compliance to quality system requirements
Quality Manual
Work
Instructions
Procedures
Quality Records
Quality System Documentation Structure
Page 12
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
xii
CURRENT
REVISION
L
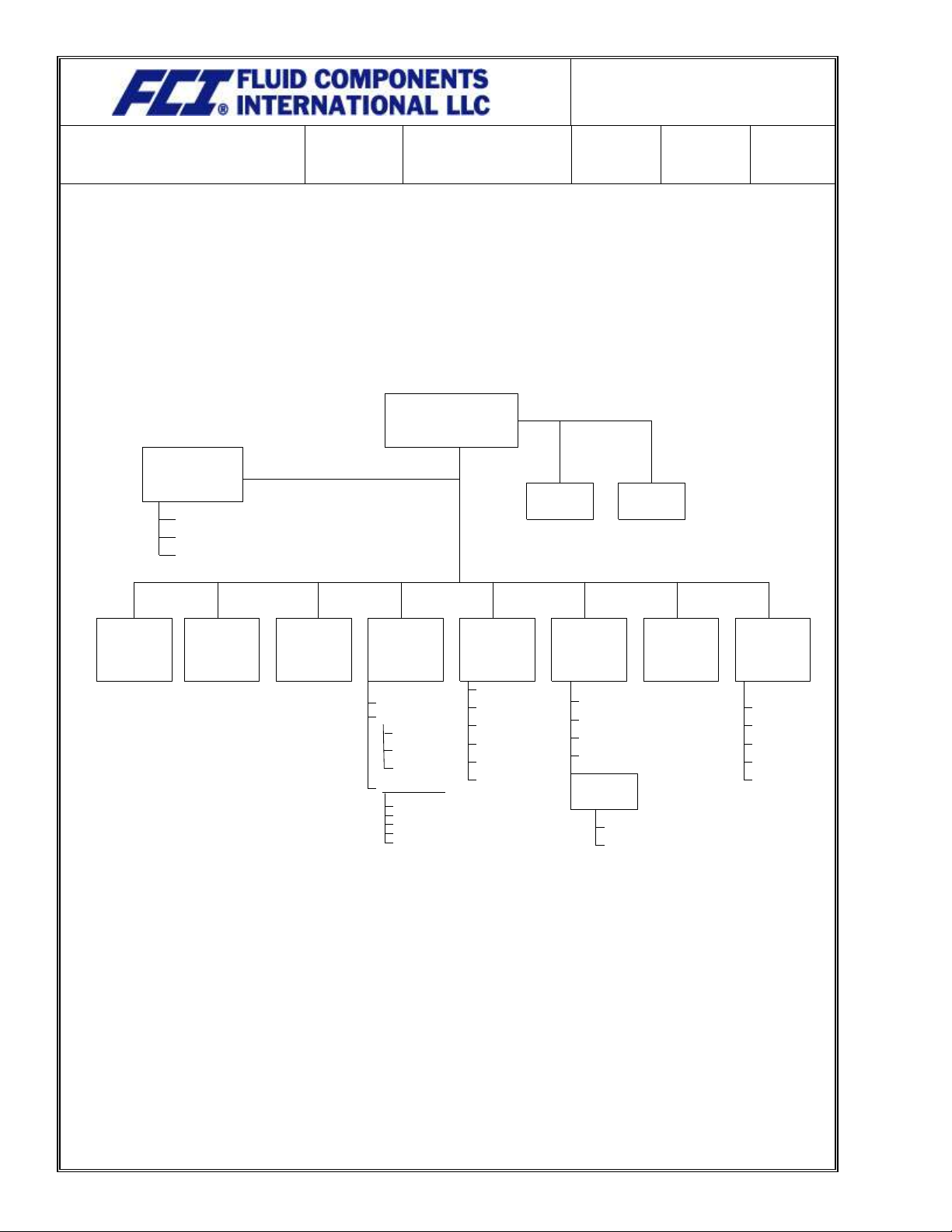
Appendix A: FCI Organization Chart
President & CEO
Executive
Assistant
General Manager
Aerospace Division
Manager
Marketing
Director
Human
Resources
Director
Manufacturing
Aero Production
Aero Engineering
Aero Sales & Service
Industrial Production
Manager
OEM Business
Development
Director
Of
Engineering
Director
Administration
&
Quality
Director
Finance
Director
Sales & Customer
Service
Document
Management
Engineering
Test
Vortab Engineer
Nuclear Qualification
Engineering
Quality Control
Information Systems
Legal
Facilities
Sales
Contract Mgmt
Cust. Service
Order Entry
Metrology
EX Representative
Management Rep
Planning
Material Control Mgr
Purchasing
Assembly
Circuit Board
Fabrication
Welding
Manufacturing Engr
DMIR
Return to
Service
QA Manager
Machining
Material Control
Page 13

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
xiii
CURRENT
REVISION
L
Appendix B: Interrelation of Quality System and ISO 9001/AS9100; FAR Clauses; and NQA-1.
Manual Sections
Level 1 & 2 Documents
Section 1 ISO9001
Section 2 AS9100
Section 3 NQA-1
AS / ISO
FAR Clauses
NQA-1 &
10CFR50
Appendix B
Quality Manual
Quality Management System
4.1
Quality Manual
Documentation Requirements
4.2.1
07QA070003, Quality Manual
Quality Manual
4.2.2 1
01DM000064/01DM000025, Engineering Document
Control
Control of Documents
4.2.3
21.137(b)
6
04QA704007, 04QA704102, Quality System
Control of Documents
4.2.3 6.2
04QA704013, Control of Quality Records
Control of Records
4.2.4
21.137(k)
17
04QA704052, Project Notebooks
Control of Records
4.2.4 17
04QA704078, Back-Up Procedure
Control of Records
4.2.4 17
Quality Manual
Management Responsibility
5.1 - 5.5.3
04QA704062, Management Review
Management Review
5.6.1 - 5.6.3
2.2
Quality Manual
Resource Management
6.1
04QA704034,Competence, Awareness, and Training
Human Resources
6.2.1- 6.2.2
2.6
04QA704097, Preventive Maintenance of the Building
Infrastructure
6.3 --
04QA704086, Preventive Maintenance of Process
Infrastructure
6.3 --
Quality Manual
Work Environment
6.4
Quality Manual
Planning
7.1-7.1.4
04QA704103, Risk Management
Risk Management
7.1.2,8.5.
3
04QA704002, Order Entry Procedure
Customer - Related Processes Identification of Product Related
7.2.1 3.1
04QA704005, Contract Review
Review of Product Requirements
7.2.2 3.1
Quality Manual
Customer Communication
7.2.3
04QA704091, Design and/Or Development
Design / Development
7.3.1 - 7.3.6
3
01DM000010, Design & Development Changes / ECO
Design and Development Changes
7.3.7
21.137(a)
3.5
04QA704044, Vendor Surveys
Purchasing Process
7.4.1
21.137(c)
7.1
04QA704077, Evaluation of Subcontractors
Purchasing Process
7.4.1 7.1.2
04QA704077, 04QA704046, Evaluation of
Purchasing Process
7.4.1 7.1
04QA704003, Purchasing Information
Purchasing Information
7.4.2 4
04QA704094, Signature Authorization
Purchasing Information
7.4.2 4
04QA704058, Plastic Coated Sensor Head Testing
Verification of Purchased Product
7.4.3
04QA704032, Testing Wetted Surfaces
Verification of Purchased Product
7.4.3 7.3
04QA704064, Hardness Testing
Verification of Purchased Product
7.4.3 7.3
04QA704001, Receiving Inspection
Verification of Purchased Product
7.4.3 8.1, 10
Page 14

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
xiv
CURRENT
REVISION
L
Manual Sections
Level 1 & 2 Documents
Section 1 ISO9001
Section 2 AS9100
Section 3 NQ-A-1
AS / ISO
FAR 21.137
NQA-1
04QA704026, In-Process Inspection
Verification of Purchased
Product/Processing
7.4.3 8.1, 10
04QA704020, Job Orders
Production – Control
7.5.1
21.137(d)
5.2
04QA704048, Foreign Object Prevention
Production – Control
7.5.1 --
04QA704050, Return of Controlled Items for
Repair/Rework
Production – Control
7.5.1 10
04QA704074, Aerospace Return Items
Production – Control
7.5.1 --
04QA704079, Field Service/Installation Procedure
Production – Control
7.5.1 --
04QA704080, Repair Procedure
Commercial/Non-Safety
Production – Control
7.5.1 --
04QA704082, Production and Service Provision /
Process Control Procedure: Non-Controlled Jobs
Production – Control
7.5.1 --
04QA704084, Tooling Procedure
Production – Control
7.5.1 --
04QA704049, First Article
Production Validation
7.5.1,8.2.4
-
04QA704026, In-Process Inspection
Production - Validation
7.5.2 10
04QA704039, Welder Qualification
Production - Validation
7.5.2 9.2
04QA704057, Workmanship Standard
Production - Validation
7.5.2 9.2
04QA704088, Special Processes
Production - Validation
7.5.2 9.1
04QA704024, Issuing Stamps
Id & Traceability
7.5.3
45.15
14.3
04QA704067, Issuing Serial Numbers to Mil/Aero
Units
Id & Traceability
7.5.3 --
04QA704085, Product Id
Id & Traceability
7.5.3 --
04QA704055, Customer Property
Customer Property
7.5.4 7.6
04QA704018, Standard Handling, Packaging &
Shipping
Product Preservation
7.5.5
21.137(j)
13.3
04QA704019, Final Cleaning
Product Preservation
7.5.5 13.2
04QA704048, Foreign Object Prevention
Product Preservation
7.5.5 --
04QA704053, Dated Coded Materials
Product Preservation
7.5.5 7.4, 8.1
04QA704081, Storage
Product Preservation
7.5.5 13.1
04QA704010, Special Handling, Storage &
Shipping
Product Preservation
7.5.5 13.3.1
04QA704006, Calibration Program
Control of Monitoring / Measuring
7.6 12.1
04QA704029, Evaluation of Measuring & Test
Control of Monitoring / Measuring
7.6
21.137(f)
12.3
04QA704063, Inspection History
Measurement, Analysis & Improvement
8.1 --
04QA704076, On Time Delivery
Measurement, Analysis & Improvement
8.1 --
Page 15

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
xv
CURRENT
REVISION
L
Manual Sections
Level 1 & 2 Documents
Section 1 ISO9001
Section 2 AS9100
Section 3 NQ-A-1
AS / ISO
FAR 21.137
NQA-1
04QA704098, Customer Satisfaction
Customer Satisfaction
8.2.1 --
04QA704008, Audits
Audits
8.2.2
21.137(l)
18.1, 18.2
04QA704054, Auditor Qualification
Audits
8.2.2 303 & /2S-3
Quality Manual
Monitoring & Measurement Processes
8.2.3
04QA704026, In-Process Inspection
Monitoring & Measurement Product
8.2.4
21.137(g)
21.137(n)
&
21.137(e)
8.1, 10.1
04QA704038, Final Inspection
Monitoring & Measurement Product
8.2.4 8.1, 10.1
04QA704058, Plastic Coated Sensor Head Testing
Monitoring & Measurement Product
8.2.4 8.1, 10.1
04QA704105, Return to Service Aero PMA Parts
Monitor & Measurement of Product
8.2.4 --
04QA704004, Control of Nonconforming Product
Control of Nonconforming Product
8.3
21.137(h)
15
04QA704011, 10 CFR 21.3 Reporting
Control of Nonconforming Product
8.3 15.3
04QA704075, Reporting to the FAA
Control of Nonconforming Product
8.3
21.137(n)
--
Quality Manual
Analysis of Data
8.4 & 8.1
21.137(n)
Quality Manual
Continual Improvement
8.5.1
04QA704070, Vendor Corrective Action
Corrective Action
8.5.2 16.1
04QA704083, Corrective and Preventive Action
Corrective Action
8.5.2 - 8.5.3
21.137(i),
21.137(m)
and
21.137(n)
16
04QA704070, Vendor Corrective Action
Preventive Action
8.5.3
04QA704093, Customer Response
Preventive Action
8.5.3 16
04QA704071, Commercial Grade Item Testing
-- 7.5
06QA020013, Qualification Verification Analysis
-- 3.4
06QA020014, Nuclear Item Dedication Plan
-- 7.5
*Paragraphs 425 & 612 are met by the procedures referenced, no cross-reference to the manual
paragraphs. Paragraph 612 is also met by manual paragraph 7.4.
Note: all “04QA” number sequences listed in this manual reflect Fluid Components International LLC‟s current
numbering system. The core number of the document is 704xxx, which remains the same regardless of the
prefix added. Some of the quality assurance procedures listed in this manual have not been revised since
the implementation of the 04QA system; therefore this note serves as notice that the document with the
core number 704xxx, regardless of the prefix represents the same document.
Page 16
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
xvi
CURRENT
REVISION
L
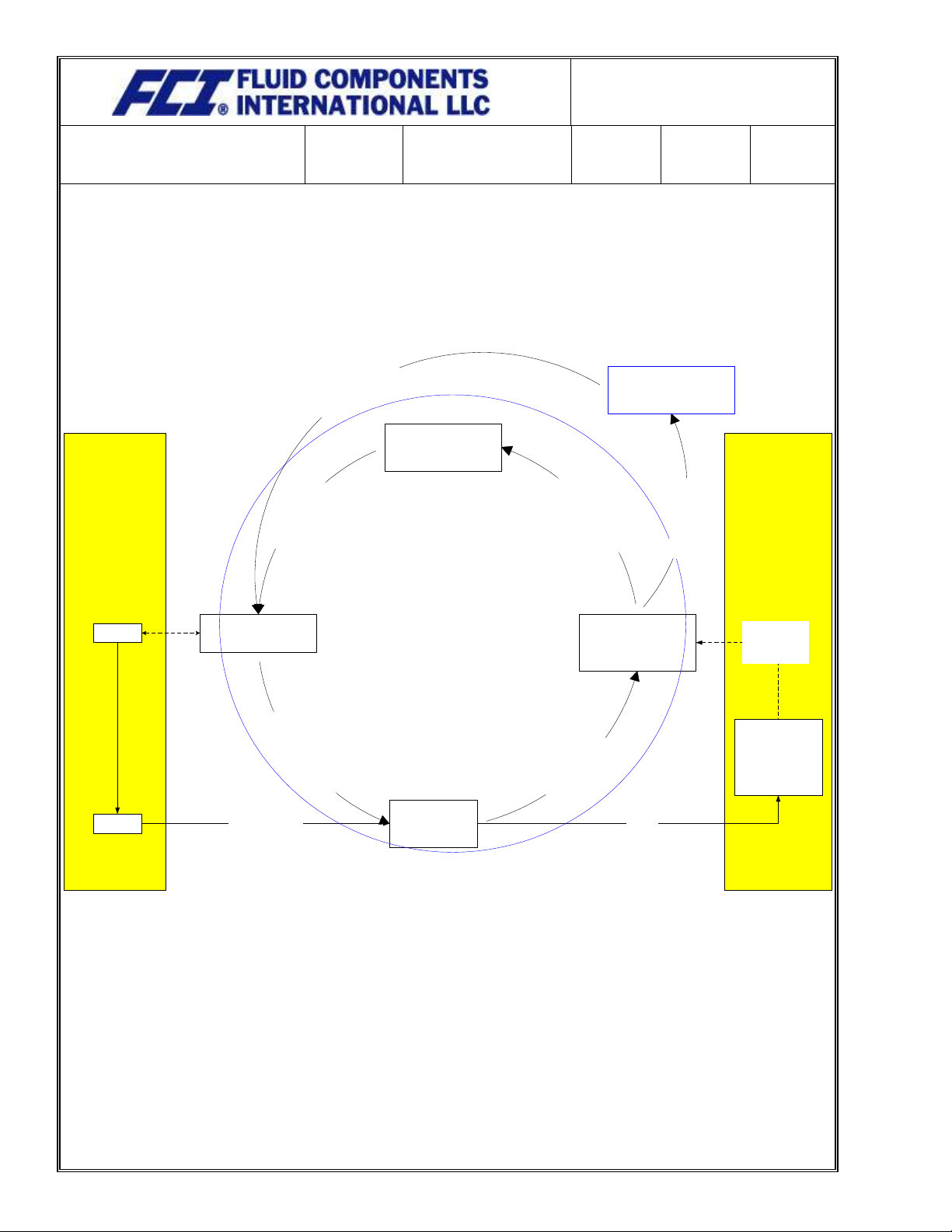
Appendix C: Interrelationships Diagram
Management
Responsibility
(23)
QMS Continuous Improvement
(10, 23, 30)
Continuous Improvement
Process Improvement
Teams
Risk Analysis 04QA704103
Resource Management
QM 07QA070003 Sec
6.1
Management
Responsibility
QM 07QA070003 Sec
5.5
Measurement,
Analysis,
& Improvement
QM 07QA070003 8.1,
8.4
Product
Realization
QM 07QA070003
Sec 7 & 8
Customers
Order
Acknowledgement
Contract Review
Customers
Product
Delivery Reports
CPARs
Customer
Reports
Satisfaction
Management Review
04QA704062 &
QM 07QA070003 Sec 5.6
Discrepancy Reports
Supplier Reports
Reports to Management Review
Reports to
Improvement Teams
Improvement Requests
Objectives & Policies
Improvements to the Process
Quotatio
n
Contrac
t
A description of the interaction between the processes of the quality management system:
Page 17

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
xvii
CURRENT
REVISION
L
Appendix D: Structure, Responsibility, and Authority of the Quality Management System
President
The President is responsible, through the Director of Administration & Quality and the
Quality Assurance Manager, for the authorization and implementation of the Quality
Management System throughout the company, including:
The overall responsibility for the definition of, and adherence to, the quality policy.
Establishing quality goals and monitoring progress to ensure continued suitability and
effectiveness of the quality management system.
Providing the necessary resources to maintain the quality management system.
Conducting management reviews of the quality management system.
Monitor the audit team for timely completion of the annual Internal Quality Assurance
Program audit.
The President shall resolve matters regarding quality that the Director of Administration &
Quality and the Quality Assurance Manager determines necessary to bring to the attention of
executive management or cannot be resolved to the satisfaction of the Quality Assurance
Manager by any other means.
Director of Administration & Quality
In support of the President, the Director of Administration & Quality is responsible for:
Overseeing the Quality, Maintenance and Information Technology departments.
Providing counsel to the President when requested, and in support of the Company.
The Director of Administration & Quality shall act as the Quality Assurance Manager in
the Quality Assurance Manager‟s absence.
Quality Assurance Manager
In support of the President and Director of Administration & Quality, the Quality Assurance
Manager is responsible for:
Administering the Quality Management System and defining, measuring, and
maintaining the overall effectiveness and enforcement of the Program.
Identifying resources to maintain the quality management system.
Reporting to the President on a yearly basis as to the effectiveness of the Quality
Management System.
Reviewing organizational requirements and providing recommendations for changes.
Directing and auditing quality-related activities; and, reporting to and advising the
President and executive staff on quality matters.
Page 18

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
xviii
CURRENT
REVISION
L
Ensuring the quality management system is maintained through appropriate audits, tests,
inspections, and surveys.
Leading and initiating actions to prevent the occurrence of any nonconformities relating
to product, process, and quality management system.
Reporting quality and nonconformance data and trends.
The Quality Assurance Manager delegates quality responsibilities to persons and
organizations within the company. (References to the Quality Assurance Manager
throughout this manual and the supplementary Quality Assurance Procedures shall mean the
Quality Assurance Manager or the Quality Assurance Manager's Representative.)
To liaise with the notified body responsible for the assessment of the quality system in case
of changes to the quality system, together with the EX representative
Managers and Supervisors
Actively support those responsible for implementing and improving the quality management
system.
Ensure the quality policy is fully supported, understood, implemented, and maintained at
appropriate levels of their organizations.
Ensure appropriate supporting procedures are documented and followed throughout their
respective departments.
Ensure adequate resources and prioritization; assign trained personnel to perform work and
verification activities including internal audits, and work affecting product quality.
When appointing a designee to act on their behalf for the purposes of any element of this
quality system, ensure the person appointed is adequately trained and given sufficient
organizational freedom and authority to execute the responsibility.
Initiate “stop shipment” as appropriate to prevent nonconformance, and then,
Initiate a documented corrective action procedure.
Maintain the “stop” until receipt of authorization and associated data and documentation to
release the “stop”.
Employees
Understand and support the quality policy and the appropriate elements of the quality
management system for their areas of work
Dedicate their efforts to the reduction, elimination, and prevention of quality deficiencies
Initiate action to prevent the occurrence of nonconformities related to product, process, and
quality system.
Page 19

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
xix
CURRENT
REVISION
L
Specific Task performed throughout the organization
Quality Assurance Staff (Reports directly to the Quality Assurance Manager) has
responsibility for the following:
Reviewing, approving, and generating instructions, procedures, & forms,
Reviewing, approving internal and customer Purchase Orders,
Compiling project record notebooks,
Administering the Discrepancy Report program,
Auditing, and
Training and certifying personnel performing activities affecting quality.
Administering the ESD program.
Metrology Department (Reports to the Inspection Supervisor)
All in-house calibration of Measuring and Test Equipment shall be performed under the
direction of the Metrology Department. The Metrology Department is responsible for all
Measuring and Test Equipment and shall assure that the equipment is maintained in
accordance with Quality Assurance Procedure 04QA704006, “Calibration program”.
The Metrology Department is also responsible for generating calibration procedures for
Measuring and Test Equipment calibrated in house.
Contract Manager has authority and responsibility for the following:
Assuring that all contractual items, terms, and conditions are identified complied with
and/or met.
Participating as a Material Review Board member,
Acting as a drawing checker, and reviewing and approving Operation Sheets, Test
Procedures, and Test Reports for conformance with contractual requirements and FCI
practices.
Act as the primary liaison between the customer and FCI once a contract has been
accepted and obtaining customer response on all documents and drawings sent to the
customer for approval.
Director Of Engineering/Engineering Manager Staff
Ultimately responsible for design control.
Responsible for incorporating customer specifications, codes, standards, and
requirements into all approval and sub-assembly drawings to be used by the Production
and Quality Assurance/Control Departments.
Responsible for the generation of Operations Sheets, Process Sheets, Process Manuals,
and other such instructions and procedures required by the Production Department to
Page 20

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
xx
CURRENT
REVISION
L
consistently build quality products.
Has the responsibility of controlling copies of the above referenced documents and
ensuring only current or specified revisions are used during the manufacturing process.
Engineering shall also ensure that obsolete or expired documents have been removed
from the factory floor.
Has the authority to participate as a member of the Material Review Board Committee.
Under the direction of Quality Control Metrology and Engineering, the Engineering Test
Group may be responsible for the calibration and maintenance of Flow Stands and
Measuring & Test Equipment used to calibrate product.
Nuclear Qualification Engineering (QE)
Responsible for maintaining qualification of Safety-Related Class 1E product through the
use of Similarity Analyses, Testing, and other appropriate methods.
Responsible for overseeing qualification test programs.
Participating in drawing reviews and the Material Review Board to address qualification
related issues.
Identifying critical characteristics for Commercial Grade Dedication of Components and
services.
Directors Of Manufacturing/Manufacturing Manager
Operations Management is responsible for the activities of the Planning, Purchasing,
Material Control, and Production.
Planning
Planners have the responsibility to schedule projects through the factory areas, obtain
controlled documents, requisition items, and issue Job Orders needed for each contract.
Purchasing
Purchasing is responsible for purchasing items, services, and equipment in accordance
with specific instructions and requirements.
Material Control
Material Control is responsible for all inventories; for providing items as required to fill
orders and maintaining lot control of items so identified. Material Control also has the
responsibility for receiving parts and outside processed goods as well as all aspects of
shipping product.
Page 21
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
xxi
CURRENT
REVISION
L
Production
The Production groups perform the fabrication and assembly of shippable product in
accordance with applicable instructions, procedures, and drawings. Production is also
responsible to record the work and processing performed as required by these documents.
Test
Test shall perform all calibrations of instruments supplied to the customer. All
calibrations and tests shall be in accordance with the applicable contract requirements and
approved procedures. Test shall also perform such other tests as requested by the
Engineering, Production, or Quality Assurance Departments.
EX Representative
The EX Representative shall have the following responsibilities and authority:
Effective monitoring, dissemination, review and implementation of the latest applicable
technical knowledge
Effective coordination of activities with respect to products intended for use in
potentially explosive atmospheres
To liaise with the notified bodies in case of changes to the design in the EC-Type
Examination Certificate and the technical documentation
To liaise with the notified body responsible for the assessment of the quality system in
case of changes to the quality system, together with the Quality Assurance representative.
Authorize the initial approval and changes to related drawings
Authorize concessions if it does not take the product outside the design as defined in
the EC-Type Examination Certificate
Communicate information to the customer of special conditions or limitations
Participate in the Management Review Meeting
Page 22

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
DATE
10-8-2012
SECTION
-
PAGE
xxii
CURRENT
REVISION
L
Designated Manufacturing Inspection Representative (DMIR)
The DMIR shall be responsible to verify conformity by inspecting parts to be released per
the Production Approval Holder (FCI). The release is to conform to various sections of
14 CFR 183.31 as authorized and limited to the Official Certificate of Authority. The
authorization is limited to the Function Codes 01, 03, 05, 06, and 07.
Return-to-Service Inspector
The Return-to-Service Inspector shall be responsible for returning Aerospace PMA parts
used for aircraft and has been rebuilt or altered under the FAA Part 43, section 43.3(j).
Authorization is based on FCI‟s Production Certificate and FAA-Parts Manufacturing
Approval. Return-to-Service Aerospace PMA parts follow the procedure 04QA704105.
Personnel authorized to Return-to-Service under this provision are limited to the
“Personnel Authorized for Return –to-Service” attached to this manual.
Page 23

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
1
CURRENT
REVISION
L
SECTION ONE
DESCRIPTION
PAGE
1.0 Scope
2
2.0 Reference Documents
2
3.0 References
2
4.0 Quality Management System
2
5.0 Management Responsibility
4
6.0 Resource Management
7
7.0 Product Realization
9
8.0 Measurement, Analysis, and Improvement
16
Page 24

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
2
CURRENT
REVISION
L
1.0 SCOPE
The requirements described in this section, Section One, of the Quality Manual
provide an overview of FCI‟s minimum quality system requirements.
Issuance and changes to the manual shall be performed in accordance with
“Document and Data Control, paragraph 4.2.3 of this section of the manual.
Changes to this Manual that substantially effect the Quality System e.g. Change
of Quality Assurance Manger or EX Representative (ATEX, IECEx, etc.),
Certifications i.e. ISO, etc., shall be submitted to a Notified Body (ATEX, IECEx,
etc.) by the appropriate Representative (EX Representative, Management
Representative, Quality Assurance Manager, etc.) if required by the standard.
In addition, FCI shall know and understand the monitoring requirements and
applicable performance criteria for AMS and the impact of these requirements on
instrument design, manufacturing and certification pursuant to EN15267.
2.0 REFERENCED DOCUMENTS
This manual is supplemented by numerous procedures as identified in Appendix
“B” and referenced throughout this manual. Procedures other than those
referenced may also be used to implement the Quality Management system.
3.0 REFERENCES
ISO 9001 (current revision), Quality management systems - Requirements
4.0 QUALITY MANAGEMENT SYSTEM
4.1 GENERAL
FCI has established, documented, implemented, and maintains and continually
improves a quality management system in accordance with the requirements of
ISO 9001.
To implement the quality management system FCI will:
a) Determine the processes needed for the quality management system and their
application throughout the organization,
b) Determine the sequence and interaction of these processes,
c) Determine criteria and methods needed to ensure that both the operation and
control of these processes are effective,
d) Ensure the availability of resources and information necessary to support the
operation and monitoring of these processes,
Page 25

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
3
CURRENT
REVISION
L
e) Measure, monitor and analyze these processes, and
f) Implement action necessary to achieve planned results and continual
improvement of these processes.
FCI manages these processes in accordance with the requirements of the ISO
9001International Standard.
Where FCI chooses to outsource any process that affects product conformity with
requirements, the organization ensures control over such processes. The type and
extent of control to be applied to these outsourced processes shall be defined
within the quality management system.
4.2 DOCUMENTATION REQUIREMENTS
4.2.1 GENERAL
Documentation for the quality management system includes:
a) Documented statements of a quality policy and quality objectives,
b) A quality manual,
c) Documented procedures and records required by ISO 9001,
d) Documents including records determined by FCI to be necessary to ensure
the effective planning, operation and control of its processes.
4.2.2 QUALITY MANUAL
A quality manual is established and maintained that includes the following:
a) The scope of the quality management system, including details of, and
justification for, any exclusions,
b) The documented procedures established for the quality management system,
or reference to them (See Appendix B), and
c) A description of the interaction between the processes of the quality
management system (See Appendix C).
4.2.3 CONTROL OF DOCUMENTS
Documents required for the quality management system are controlled.
A documented procedure is established to define the controls needed:
a) To approve documents for adequacy prior to issue,
b) To review, update as necessary, and re-approve documents,
Page 26

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
4
CURRENT
REVISION
L
c) To ensure that changes and the current revision status of documents are
identified,
d) To ensure that relevant versions of applicable documents are available at
points of use,
e) To ensure that documents remain legible and readily identifiable,
f) To ensure that documents of external origin determined by FCI to be
necessary for the planning and operation of the Quality Management System
are identified and their distribution controlled,
g) To prevent the unintended use of obsolete documents, and to apply suitable
identification to them if they are retained for any purpose.
4.2.4 CONTROL OF QUALITY RECORDS
Records are established to provide evidence of conformity to requirements and of
the effective operation of the quality management system. Records will remain
legible, readily identifiable, and retrievable. FCI shall establish a documented
procedure to define the controls needed for the identification, storage, protection,
retrieval, retention time, and disposition of records.
5.0 MANAGEMENT RESPONSIBILITY
5.1 MANAGEMENT COMMITMENT
Top management provides evidence of its commitment to the development and
implementation of the quality management system and continually improving its
effectiveness by:
a) Communicating to the organization via email, presentations, postings,
and/or a newsletter the importance of meeting customer as well as statutory,
and regulatory requirements,
b) Establishing the quality policy,
c) Ensuring that quality objectives are established,
d) Conducting management reviews, and
e) Ensuring the availability of resources.
5.2 CUSTOMER FOCUS
Top management ensures that customer requirements are determined and are met
with the aim of enhancing customer satisfaction. (See section 7.2.1, and 8.2.1)
Page 27

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
5
CURRENT
REVISION
L
5.3 QUALITY POLICY
Top management ensures that the quality policy:
a) Is appropriate to the purpose of the organization,
b) Includes a commitment to meeting requirements and to continually improve
the effectiveness of the quality management system,
c) Provides a framework for establishing and reviewing quality objectives,
d) Is communicated and understood within the organization, and
e) Is reviewed for continuing suitability.
FCI‟s quality policy is as follows:
“To continually meet and exceed customer expectations and requirements for
products and services and to continually improve the effectiveness of the quality
management system.”
5.4 PLANNING
5.4.1 QUALITY OBJECTIVES
Top management ensures that quality objectives, including those needed to meet
requirements for product, are established at relevant functions and levels within
the organization. The quality objectives are measurable and consistent with the
quality policy.
5.4.2 QUALITY PLANNING
Top management ensures that:
a) The planning of the quality management system is carried out in order to
meet the requirements given in 4.1, as well as the quality objectives, and
b) The integrity of the quality management system is maintained when changes
to the quality management system are planned and implemented.
Page 28
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
6
CURRENT
REVISION
L
5.5 RESPONSIBILITIES, AUTHORITY, AND COMMUNICATION
5.5.1 RESPONSIBILITY AND AUTHORITY
Top Management ensures that responsibilities and authorities are defined and
communicated within the organization through this Quality Manual (also see
Appendix A and D) and individual organizational charts at the departmental level.
5.5.2 MANAGEMENT RESPONSIBILITY
Top Management has appointed (by virtue of this document) the Quality
Assurance Manager as the Management Representative. This individual is a
member of the management structure of FCI. Irrespective of other responsibilities
the Management Representative has the responsibility and authority that includes:
a) Ensuring that processes needed for the quality management system are
established, implemented and maintained,
b) Reporting to top management on the performance of the quality
management system and any need for improvement, and
c) Ensuring the promotion of awareness of customer requirements throughout
the organization.
5.5.3 INTERNAL COMMUNICATION
Top management ensures that appropriate communication processes are
established within the organization and that communication takes place regarding
the effectiveness of the quality management system. This includes the posting of
Quality Objective data to communicate information to the employee population
regarding the effectiveness of the quality management system.
5.6 MANAGEMENT REVIEW
5.6.1 GENERAL
Top management reviews the quality management system, at planned intervals, to
ensure its continuing suitability, adequacy, and effectiveness. This review
includes assessing opportunities for improvement and the need for changes to the
quality management system, including the quality policy and quality objectives.
Records from management reviews are maintained.
5.6.2 REVIEW INPUT
The input to management review includes information on:
Page 29

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
7
CURRENT
REVISION
L
a) Results of audits,
b) Customer feedback,
c) Process performance and product conformity,
d) Status of preventive and corrective actions,
e) Follow-up actions from previous management reviews,
f) Changes that could affect the quality management system, and
g) Recommendations for improvement.
5.6.3 REVIEW OUTPUT
The outputs from the management review include any decisions and actions
related to:
a) Improvement of the effectiveness of the quality management system and its
processes,
b) Improvement of product related to customer requirements, and
c) Resource needs.
6.0 RESOURCE MANAGEMENT
6.1 PROVISION OF RESOURCES
FCI determines and provides the resources needed;
a) To implement and maintain the quality management system and continually
improve its effectiveness, and
b) To enhance customer satisfaction by meeting customer requirements.
On an annual basis, the Directors submit all proposed resource needs for the
upcoming fiscal year to the Chief Financial Officer (CFO). These proposed
resource needs include, the infrastructure and resources needed to:
a. Achieve conformity to product requirements,
b. Implement and maintain the quality management system and continually
improve its effectiveness, and
c. Enhance customer satisfaction by meeting customer requirements.
The President, with input from other corporate entities, determines the
departmental budgets. The approved departmental budgets document the
determination of the infrastructure and resources needed.
Page 30

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
8
CURRENT
REVISION
L
The individual Directors then provide the needed resources based on the approved
annual departmental budgets.
The CFO maintains the individual departmental budgets on the corporate server
by date for a minimum of two years.
6.2 HUMAN RESOURCES
6.2.1 GENERAL
Personnel performing work affecting product quality are competent on the basis
of appropriate education, training, skills, and experience.
6.2.2 COMPETENCY, AWARENESS, AND TRAINING
FCI will:
a) Determine the necessary competence for personnel performing work
affecting conformity to product requirements,
b) Where applicable, provide training or take other actions to satisfy the
necessary competencies,
c) Evaluate the effectiveness of the actions taken,
d) Ensure that personnel are aware of the relevance and importance of their
activities and how they contribute to the achievement of the quality
objectives, and
e) Maintain appropriate records of education, training, skills, and experience.
6.3 INFRASTRUCTURE
FCI determines, provides, and maintains the infrastructure needed to achieve
conformity to product requirements. Infrastructure includes, as applicable:
a) Buildings, workspace and associated facilities;
b) Process equipment (both hardware and software), and
c) Supporting services (such as transport or communication).
6.4 WORK ENVIRONMENT
It is each departmental manager/supervisor‟s responsibility to determine and
manage the work environment needed to achieve conformity to product
requirements.
Page 31

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
9
CURRENT
REVISION
L
7.0 PRODUCT REALIZATION
7.1 PLANNING OF PRODUCT REALIZATION
FCI plans and develops the processes needed for product realization. Planning of
product realization is consistent with the requirements of the other processes of
the quality management system.
The planning of the processes for realization to determine the following occurs as
appropriate:
a) Quality objectives and requirements for the product during the Design and
Planning Phase;
b) The need to establish processes, documents, and provide resources specific
to the product during the Design and Planning Phase;
c) Required verification, validation, monitoring, inspection and test activities
specific to the product and the criteria for product acceptance during the
Design and/or Planning Phase;
d) Records needed to provide evidence that the realization processes and
resulting product meet requirements.
Output from this planning is in a form suitable for FCI‟s method of operations and
may be (but is not limited to) the following:
Quality System Procedures and/or Documentation
MRP Data
Delivery Method Information
Quality Objectives
Organizational Infrastructure
Department Specific Documentation
Product Realization Documentation
7.2 CUSTOMER RELATED PROCESSES
7.2.1 IDENTIFICATION OF PRODUCT RELATED REQUIREMENTS
FCI determines:
a) Requirements specified by the customer, including the requirements for
delivery and post delivery activities,
b) Requirements not stated by the customer but necessary for intended or
Page 32
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
10
CURRENT
REVISION
L
specified use, where known,
c) Statutory and regulatory requirements related to the product, and
d) Any additional requirements determined by the organization.
7.2.2 REVIEW OF PRODUCT REQUIREMENTS
FCI reviews the requirements related to the product. This review is conducted
prior to FCI‟s commitment to supply a product to the customer and ensures that:
a) Product requirements are defined,
b) Contract or order requirements differing from those previously expressed
are resolved, and
c) The organization has the ability to meet the defined requirements.
Records of the results of the review and actions arising from the review are
maintained.
Where the customer provides no documented statement of requirements, the
customer requirements are confirmed by FCI before acceptance.
Where product requirements are changed, FCI ensures that relevant documents
are amended and that relevant personnel are made aware of the changed
requirements.
7.2.3 CUSTOMER COMMUNICATION
FCI determines and implements effective arrangements for communicating with
customers in relation to:
a) Product information,
b) Enquiries, contracts or order handling, including amendments, and
c) Customer feedback, including customer complaints.
Effective arrangements include (but may not be limited to) the following:
a) Pre-Sales: All customer communication is forwarded to the Sales
Department, and
b) Post-Sales: All customer communication is forwarded to the Customer
Service Department.
Page 33

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
11
CURRENT
REVISION
L
7.3 DESIGN AND/OR DEVELOPMENT
7.3.1 DESIGN AND/OR DEVELOPMENT PLANNING
FCI plans and controls design and/or development of the product.
Design and/or development planning determines:
a) Stages of design and/or development,
b) Review, verification and validation activities appropriate to each design
and/or development stage, and
c) Responsibilities and authorities for design and/or development.
Interfaces between different groups involved in design and/or development are
managed to ensure effective communication and clear assignment of
responsibility.
Planning output is updated, as appropriate, as the design and/or development
progresses.
7.3.2 DESIGN AND/OR DEVELOPMENT INPUTS
Inputs relating to product requirements are determined and records maintained.
These include:
a) Functional and performance requirements,
b) Applicable regulatory and legal requirements,
c) Applicable information derived from previous similar designs, and
d) Any other requirements essential for design and/or development.
These inputs are reviewed for adequacy. Incomplete, ambiguous, or conflicting
requirements are resolved.
7.3.3 DESIGN AND/OR DEVELOPMENT OUTPUTS
The outputs of the design and/or development shall be in a form that is suitable
for verification against the design and/or development inputs and shall be
approved prior to release.
Design and/or development output:
a) Meets the design and/or development input requirements,
b) Provides appropriate information for purchasing, production, and service
provisions,
c) Contains or references product acceptance criteria,
Page 34
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
12
CURRENT
REVISION
L
d) Specifies the characteristics of the product that are essential to its safe and
proper use.
7.3.4 DESIGN AND/OR DEVELOPMENT REVIEW
At suitable stages, systematic reviews of design and/or development is performed
in accordance with planned arrangements to:
a) Evaluate the ability of the results of design and development to meet
requirements, and
b) Identify problems and propose necessary actions.
Participants in such reviews include representatives of functions concerned with
the design and/or development stage(s) being reviewed. The results of the
reviews and any necessary actions are maintained.
7.3.5 DESIGN AND/OR DEVELOPMENT VERIFICATION
Verification is performed in accordance with planned arrangements to ensure the
design and development outputs have met the design and/or development input
requirements. The results of the verification and any necessary actions are
maintained.
7.3.6 DESIGN AND/OR DEVELOPMENT VALIDATION
Design and/or development validation is performed in accordance with planned
arrangements to ensure that resulting product is capable of meeting the
requirements for the specified application or intended use, where known.
Wherever practicable, validation is completed prior to the delivery or
implementation of the product. Records of the results of the validation and any
necessary actions are maintained.
7.3.7 CONTROL OF DESIGN AND/OR DEVELOPMENT CHANGES
Design and/or development changes are identified and records maintained. The
changes are reviewed, verified and validated, as appropriate, and approved before
implementation. The review of design and development changes includes
evaluation of the effect of the changes on constituent parts and product already
delivered.
Records of the results of the review of changes and any necessary actions are
maintained.
Page 35

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
13
CURRENT
REVISION
L
7.4 PURCHASING
7.4.1 PURCHASING PROCESS
FCI ensures that purchased product conforms to specified purchase requirements.
The type and extent of control applied to the supplier and the purchased product is
dependent upon the effect of the purchased product on subsequent product
realization or the final product.
FCI evaluates and selects suppliers based on their ability to supply product in
accordance with the organization‟s requirements. Criteria for selection,
evaluation, and re-evaluation are established. Records of the results of
evaluations and any necessary actions arising from the evaluation are maintained.
7.4.2 PURCHASING INFORMATION
Purchasing information describes the product to be purchased, including where
appropriate:
a) Requirements for approval of product, procedures, processes, and
equipment,
b) Requirements for qualification of personnel, and
c) Quality management system requirements
FCI ensures the adequacy of specified purchase requirements prior to their
communication to the supplier.
7.4.3 VERIFICATION OF PURCHASED PRODUCT
FCI establishes and implements the inspection or other activities necessary for
ensuring that purchased product meets specified purchase requirements.
Where FCI or its customer intends to perform verification at the supplier‟s
premises, the organization will state the intended verification arrangements and
method of product release in the purchasing information.
7.5 PRODUCTION AND SERVICE PROVISION
7.5.1 CONTROL OF PRODUCTION AND SERVICE PROVISION
FCI plans and carries out production and service provisions under controlled
conditions. Controlled conditions include, as applicable:
a) The availability of information that describes the characteristics of the
product,
Page 36

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
14
CURRENT
REVISION
L
b) The availability of work instructions, as necessary,
c) The use of suitable equipment,
d) The availability and use of measuring and monitoring devices,
e) The implementation of monitoring and measurement,
f) The implementation of product release, delivery, and post-delivery
activities.
7.5.2 VALIDATION OF PROCESSES
FCI validates any processes for production and service where the resulting output
cannot be verified by subsequent measurement or monitoring; and as a
consequence, deficiencies become apparent only after the product is in use or the
service has been delivered (aka Special Processes).
Validation demonstrates the ability of the processes to achieve planned results.
FCI establishes arrangements for these processes including, as applicable:
a) Defined criteria for review and approval of the processes,
b) Approval of equipment and qualification of personnel,
c) Use of specific methods and procedures,
d) Requirements for records, and
e) Revalidation.
7.5.3 IDENTIFICATION AND TRACEABILTY
Where appropriate, FCI identifies the product by part number throughout product
realization.
FCI identifies the status of the product with respect to measurements and
monitoring requirements on the associated Operation Sheet or in the Business
System.
Where traceability is a requirement, FCI controls and records the unique
identification of the product via the Receiving Inspection, In Process Inspection,
Job Order, and Final Inspection procedures.
7.5.4 CUSTOMER PROPERTY
FCI exercises care with customer property (including intellectual property and
personal data) while it is under the organization‟s control or being used by the
Page 37

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
15
CURRENT
REVISION
L
organization. FCI identifies, verifies, protects, and safeguards customer property
provided for use or incorporation into the product. Occurrence of any customer
property that is lost, damaged, or otherwise found to be unsuitable for use is
recorded and reported to the customer and records maintained.
7.5.5 PRESERVATION OF PRODUCT
FCI preserves the product during internal processing and delivery to the intended
destination in order to maintain conformity to requirements. As applicable, this
preservation includes identification, handling, packaging, storage, and protection.
Preservation also applies to constituent parts of a product.
7.6 CONTROL OF MEASURING AND MONITORING DEVICES
FCI determines the monitoring and measurements to be undertaken and the
measuring and monitoring equipment needed to provide evidence of conformity
of product to determined requirements.
FCI establishes processes to ensure the monitoring and measurement can be
carried out and are carried out in a manner that is consistent with the monitoring
and measurement requirements.
Where necessary to ensure valid results, measuring equipment is:
a) Calibrated or verified at specified intervals, or prior to use, against
measurement standards traceable to international or national standards;
where no such standards exist, the basis used for calibration or verification
is recorded,
b) Be adjusted or re-adjusted as necessary,
c) Be identified in order that the calibration status is determined,
d) Be safeguarded from adjustments that would invalidate the measurement
result, and
e) Be protected from damage and deterioration during handling, maintenance,
and storage.
In addition, FCI assesses and records the validity of the previous measuring
results when the equipment is found not to conform to requirements. FCI takes
appropriate action on the equipment and any product affected. Records of the
results of calibration and verification are maintained.
When used in the monitoring and measurement of specified requirements, the
ability of computer software to satisfy the intended application is confirmed. This
is undertaken prior to initial use and reconfirmed as necessary.
Page 38

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
16
CURRENT
REVISION
L
8.0 MEASUREMENT, ANALYSIS AND IMPROVEMENT
8.1 GENERAL
FCI plans and implements the monitoring, measurement, analysis, and
improvement processes needed:
a) To demonstrate conformity to product requirements,
b) To ensure conformity of the quality management system, and
c) To continually improve the effectiveness of the quality management system.
This includes determination of applicable methods, including statistical
techniques, and the extent of their use.
8.2 MEASUREMENT AND MONITORING
8.2.1 CUSTOMER SATISFACTION
As one of the measurements of the performance of the quality management
system, FCI monitors information relating to customer‟s perception as to whether
FCI has met customer requirements. The methods for obtaining and using this
information are determined.
8.2.2 INTERNAL AUDIT
FCI conducts internal audits at planned intervals to determine whether the quality
management system:
a) Conforms to the planned arrangements, to the requirements of the ISO 9001
International Standard, and to the quality management system requirements
established by FCI,
b) Is effectively implemented and maintained.
The audit program is planned, taking into consideration the status and importance
of the processes and areas to be audited, as well as the results of previous audits.
The audit criteria, scope, frequency, and methods are defined. Selection of
auditors and conduct of audits will ensure objectivity and impartiality of the audit
process. Auditors will not audit their own work.
A documented procedure shall be established to define the responsibilities and
requirements for planning and conducting audits, establishing records and
reporting results.
The management responsible for the area being audited ensures that any
necessary corrections and corrective actions are taken without undue delay to
Page 39
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
17
CURRENT
REVISION
L
eliminate detected nonconformities and their causes. Follow-up activities shall
include the verification of the actions taken and the reporting of verification
results.
8.2.3 MEASUREMENT AND MONITORING OF PROCESSES
FCI applies suitable methods for monitoring and, where applicable, measurement
of the quality management system processes. These methods shall demonstrate
the ability of the processes to achieve planned results. When planned results are
not achieved, correction and corrective action is taken, as appropriate.
8.2.4 MEASUREMENT AND MONITORING OF PRODUCT
FCI measures and monitors the characteristics of the product to verify that
product requirements have been met. This is carried out at appropriate stages of
the product realization process in accordance with planned arrangements.
Evidence of conformity with the acceptance criteria is maintained. Records
indicate the person(s) authorizing release of product for delivery to the customer.
The release of product and delivery of service to the customer shall not proceed
until the planned arrangements have been satisfactorily completed, unless
otherwise approved by a relevant authority and, where applicable, by the
customer.
8.3 CONTROL OF NONCONFORMING PRODUCT
FCI ensures that product which does not conform to requirements is identified and
controlled to prevent unintended use or delivery. A documented procedure shall
be established to define the controls and related responsibilities and authorities for
dealing with nonconforming product.
Where applicable, FCI deals with nonconforming product by one or more of the
following ways:
a) By taking action to eliminate the detected nonconformity,
b) By authorizing its use, release or acceptance under concession by a relevant
authority and, where applicable, by the customer,
c) By taking action to preclude its original intended use or application.
d) By taking action appropriate to the effects, or potential effects, of the
nonconformity when nonconforming product is detected after delivery or
use has started;
Page 40

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
18
CURRENT
REVISION
L
Records of the nature of nonconformities and any subsequent actions taken,
including concessions obtained, shall be maintained.
When nonconforming product is corrected it is subject to re-verification to
demonstrate conformity to the requirements.
When nonconforming product is detected after delivery or use has started, FCI
will take action appropriate to the effects, or potential effects, of the
nonconformity.
NOTIFICATION EN 13980:2002 and IECEx/OD005, Version 2
FCI shall take action appropriate to the degree of risk, where non-conforming
product has been supplied to a customer.
FCI‟s Contract Manager, Quality Assurance Manager, or designation
representative (i.e., EX Representative – “ATEX”, “IECEx”, etc.) will liase
with the notified body responsible for the EC Type certification.
When nonconforming product is deemed unsafe, FCI will inform, by e-mail,
the customer and the notified body responsible for the quality system
notification.
FCI shall maintain records of the above notification for ten years.
8.4 ANALYSIS OF DATA
FCI determines, collects, and analyzes appropriate data to demonstrate the
suitability and effectiveness of the quality management system and to evaluate
where continual improvements of the quality management system can be made.
This includes data generated by measuring and monitoring activities and other
relevant sources.
FCI analyses this data to provide information on:
a) Customer satisfaction,
b) Conformity to product requirements,
c) Characteristics and trends of processes and product including opportunities
for preventive action, and
d) Suppliers.
Page 41
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
Date
10-8-2012
SECTION
1
PAGE
19
CURRENT
REVISION
L
8.5 IMPROVEMENT
8.5.1 CONTINUAL IMPROVEMENT
FCI shall continually improve the effectiveness of the quality management system
through the use of the quality policy, quality objectives, audit results, analysis of
data, corrective and preventive action, and management review.
8.5.2 CORRECTIVE ACTION
FCI takes corrective action to eliminate the cause of nonconformities in order to
prevent recurrence. Corrective action is appropriate to the effects of the
nonconformities encountered.
The documented procedure for corrective action is established to define
requirements for:
a) Reviewing nonconformities (including customer complaints),
b) Determining the causes of nonconformity,
c) Evaluating the need for action to ensure that nonconformities do not recur,
d) Determining and implementing the action needed,
e) Records of the results of action taken, and
f) Reviewing the effectiveness of the corrective action taken.
8.5.3 PREVENTIVE ACTION
FCI determines action to eliminate the causes of potential nonconformities in
order to prevent their occurrence. Preventive actions taken are appropriate to the
effects of the potential problems.
The documented procedure for preventive action defines requirements for:
a) Determining potential nonconformities and their causes,
b) Evaluating the need for action to prevent occurrence of nonconformities,
c) Determining and implementing action needed,
d) Recording results of action taken, and
e) Reviewing preventive action taken.
Page 42

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
1
CURRENT
REVISION
L
SECTION TWO
DESCRIPTION
PAGE
1.0 Scope
4
2.0 Reference Documents
4
3.0 References
4
4.0 Quality Management System
5
5.0 Management Responsibility
8
6.0 Resource Management
11
7.0 Product Realization
12
8.0 Measurement, Analysis, and Improvement
26
Page 43
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
2
CURRENT
REVISION
L
FAA COMMITMENTS
FCI shall comply with FAA Part 21 regulations as follows:
Location of or change to manufacturing facilities:
FCI will immediately notify the FAA, in writing, of any change to the
manufacturing facilities that may affect the inspection, conformity, or
airworthiness of its PMA articles.
Inspections and Tests:
(a) FCI will allow the FAA to inspect its quality system, facilities,
technical data, and any manufactured articles and witness any
tests, including any inspections or tests at a supplier facility,
necessary to determine compliance with Paragraph 21.310.
(b) Unless otherwise authorized by the FAA, FCI –
(1) May not present any article to the FAA for an
inspection or test unless compliance with Chapter
21.303(b)(2) through (4) has been shown for that
article; and
(2) May not make any change to an article between the
time that compliance with Chapter 21.303(b)(2)
through (4) is shown for that article and the time
that the article is presented to the FAA for the
inspection or test.
FCI will also–
(a) Amend the Manual as required by Chapter 21.305 as necessary to reflect changes in the
(b) Maintain the quality system in compliance with the data and procedures approved for the
(c) Ensure that each PMA article conforms to its approved design and is in a condition for
(d) Mark the PMA article for which an approval has been issued. Marking will be in
(e) Identify any portion of the PMA article (e.g., sub-assemblies, component parts, or
(f) Have access to design data necessary to determine conformity and airworthiness for each
organization and provide these amendments to the FAA;
PMA;
safe operation;
accordance with part 45 of this chapter, including any critical parts;
replacement articles) that leave the manufacturer‟s facility as FAA approved with the
manufacturer‟s part number and name, trademark, symbol, or other FAA approved
manufacturer‟s identification;
Page 44

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
3
CURRENT
REVISION
L
article produced under the PMA;
(g) Retain each document granting PMA and make it available to the FAA upon request; and
(h) Make available to the FAA information regarding all delegation of authority to suppliers.
Design changes initiated by FCI:
(a) Classification of design changes for PMA parts:
(1) A “minor change” to the design of an article produced under a PMA is one
that has no appreciable effect on the approval basis and has no effect on fit,
form or function.
(2) A “major change” to the design of an article produced under a PMA is any
change that is not minor.
(b) Approval of design changes:
(1) Minor changes to the basic design of a PMA may be approved using a method
acceptable to the FAA.
(2) FCI will obtain FAA approval of any major change before including it in the
design of an article produced under a PMA.
After the issuance of a PMA –
(a) Changes to the quality system are subject to review by the FAA; and
(b) FCI will immediately notify the FAA, in writing, of any change that may affect
inspection, conformity, or airworthiness of its article.
FCI, as a holder of a PMA part, will report any failure, malfunction, or defect in any product
or article manufactured by it that it determines has resulted in any of the occurrences listed in
Paragraph C of Section 21.3. FCI as a holder of a PMA part, will also report any defect in
any product or article manufactured by it that has left its quality system and that it determines
could result in any of the occurrences listed in Paragraph C of Section 21.3 (see
04QA704075).
Page 45

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
4
CURRENT
REVISION
L
1.0 SCOPE
The requirements described in this section, Section Two, of the Quality Manual
provide an overview of FCI‟s quality system as it relates to all projects identified
as “Controlled for Aerospace”.
In accordance with the provisions of 14 CFR, Part 21, the Federal Aviation
Administration has found that the design data submitted by FCI meets the
airworthiness requirements of the Federal Aviation Administration applicable to
the product on which the parts are to be installed. Additionally, it has been
determined that FCI has established the quality system required by 14CFR Part
21.137 (a) – (n) and is granted Parts Manufacturing Approval for production of
the replacement parts or modification parts, as applicable, listed in the
Supplements to the Parts Manufacturer Approval Letters in conformity with the
Federal Aviation Administration-approved data. The terms and conditions
applicable to this approval are documented in the Federal Aviation Administration
– Parts Manufacturer Approval Letter.
If the physical location of FCI changes, or if additional locations of FCI are
included under this program, the Federal Aviation Administration (FAA) shall be
notified immediately in writing of the relocation of any manufacturing facilities or
addition in accordance with terms 2 and 7 of the Parts Manufacturing Approval
(PMA) Letter.
This Manual, in its entirety, unless identified otherwise, shall be issued as a
controlled document to FCI‟s customers having contractual obligations to this
section of the manual.
2.0 REFERENCED DOCUMENTS
This manual is supplemented by numerous procedures as identified in Appendix
“B” and referenced throughout this manual. Procedures other than those
referenced may also be used to implement the quality management system.
3.0 REFERENCES
AS9100 (current revision) Quality Systems – Aerospace- Model for Quality
Assurance in Design, Development, Production, Installation, and Servicing
Note: All references to AS9100 in either FCI procedures or this QAM shall be
deemed to refer to the latest revision of the standard regardless of the revision
stated at the time of conception.
Mil-I-45208A – Military Specification, Inspection System Requirements
14CFR21.137 - Aeronautics and Space, Code of Federal Regulations, Approval of
Materials, Parts, Processes, and Appliance - Replacement and Modification Parts
Page 46

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
5
CURRENT
REVISION
L
4.0 QUALITY SYSTEM REQUIREMENTS
4.1 GENERAL
FCI has established, documented, implemented, and maintains, and continually
improves a quality management system in accordance with the requirements of
AS9100.
The organization‟s quality management system shall also address customer and
applicable statutory and regulatory quality management system requirements.
To implement the quality management system FCI will:
a) Determine the processes needed for the quality management system and their
application throughout the organization,
b) Determine the sequence and interaction of these processes,
c) Determine criteria and methods needed to ensure that both the operation and
control of these processes are effective,
d) Ensure the availability of resources and information necessary to support the
operation and monitoring of these processes,
e) Measure, monitor and analyze these processes, and
f) Implement action necessary to achieve planned results and the continual
improvement of these processes.
FCI manages these processes in accordance with the requirements of the AS9100,
International Standard.
Where FCI chooses to outsource any process that affects product conformity with
requirements, the organization ensures control over such processes. The type and
extent of control to be applied to these outsourced processes shall be defined within
the FCI quality management system.
Page 47

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
6
CURRENT
REVISION
L
4.2 DOCUMENTATION REQUIREMENTS
4.2.1 GENERAL
Documentation of the quality management system includes:
a) Documented statements of a quality policy and quality objectives,
b) A quality manual,
c) Documented procedures and records required by AS9100, International
Standard,
d) Documents, including records, determined by FCI to be necessary to
ensure the effective planning, operation and control of its processes
FCI:
Ensures access of documentation of the quality management system to its
personnel and customers and/or regulatory authorities representatives, and
Ensures that personnel are aware of relevant procedures.
4.2.2 QUALITY MANUAL
A quality manual is established and maintained that includes the following:
a) The scope of the quality management system, including details of, and
justification for, any exclusions,
b) The documented procedures established for the quality management system,
or reference to them which is shown in Appendix B, and
c) A description of the interaction between the processes of the quality
management system (See Appendix C).
Page 48
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
7
CURRENT
REVISION
L
4.2.3 CONTROL OF DOCUMENTS
Documents required for the quality management system are controlled.
A documented procedure is established to define the controls needed:
a) To approve documents for adequacy prior to issue,
b) To review, update as necessary, and re-approve documents,
c) To ensure that changes and the current revision status of documents are
identified,
d) To ensure that relevant versions of applicable documents are available at
points of use,
e) To ensure that documents remain legible and readily identifiable,
f) To ensure that documents of external origin determined by FCI to be
necessary for the planning and operation of the quality management system
are identified and their distribution controlled, and
g) To prevent the unintended use of obsolete documents, and to apply suitable
identification to them if they are retained for any purpose.
As a regulatory requirement, any revision to this section, section two, of the
Manual must be submitted to the FAA for approval prior to implementation in
accordance with term 13 of the PMA Letter.
4.2.4 CONTROL OF QUALITY RECORDS
Records established to provide evidence of conformity to requirements and of the
effective operation of the quality management system shall be controlled and
retained for at least five (5) years.
FCI shall establish a documented procedure to define the controls needed for the
identification, storage, protection, retrieval, retention and disposition of records.
The documented procedure defines the method for controlling records that are
created by and/or retained by suppliers.
Records shall remain legible, readily identifiable and retrievable.
Page 49

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
8
CURRENT
REVISION
L
5.0 MANAGEMENT RESPONSIBILITY
5.1 MANAGEMENT COMMITMENT
Top management provides evidence of its commitment to the development and
implementation of the quality management system and continually improving its
effectiveness by:
a) Communicating to the organization via email, presentations, postings,
and/or a newsletter the importance of meeting customer as well as statutory,
and regulatory requirements,
b) Establishing the quality policy,
c) Ensuring that quality objectives are established,
d) Conducting management reviews, and
e) Ensuring the availability of resources.
5.2 CUSTOMER FOCUS
Top management ensures that customer requirements are determined and are met
with the aim of enhancing customer satisfaction. (See section 7.2.1, and 8.2.1)
Top management shall ensure that product conformity and on-time delivery
performance are measured and that appropriate action is taken if planned results
are not, or will not be, achieved.
5.3 QUALITY POLICY
Top management ensures that the quality policy:
a) Is appropriate to the purpose of the organization,
b) Includes a commitment to meeting requirements and to continually improve
the effectiveness of the quality management system,
c) Provides a framework for establishing and reviewing quality objectives,
d) Is communicated and understood within the organization, and
e) Is reviewed for continuing suitability.
FCI‟s quality policy is as follows:
“To continually meet and exceed customer expectations and
requirements for products and services and to continually improve
the effectiveness of the quality management system.”
Page 50

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
9
CURRENT
REVISION
L
5.4 PLANNING
5.4.1 QUALITY OBJECTIVES
Top management ensures that quality objectives, including those needed to meet
requirements for product, are established at relevant functions and levels within
the organization. The quality objectives are measurable and consistent with the
quality policy.
5.4.2 QUALITY PLANNING
Top management ensures that:
a) The planning of the quality management system is carried out in order to
meet the requirements given in 4.1, as well as the quality objectives, and
b) The integrity of the quality management system is maintained when
changes to the quality management system are planned and implemented.
5.5 RESPONSIBILITY, AUTHORITY, AND COMMUNICATION
5.5.1 RESPONSIBILITY AND AUTHORITY
Top Management ensures that responsibilities and authorities are defined and
communicated within the organization through this Quality Manual (also see
Appendix A & D) and individual organizational charts at the departmental level.
5.5.2 MANAGEMENT REPRESENTATIVE
Top Management has appointed (by virtue of this document) the Quality
Assurance Manager as the Management Representative. This individual is a
member of the management structure of the organization. Irrespective of other
responsibilities the Management Representative has the responsibility and
authority that includes:
a) Ensuring that processes needed for the quality management system are
established, implemented and maintained,
b) Report to top management on the performance of the quality management
system and any need for improvement,
c) Ensure the promotion of awareness of customer requirements throughout
the organization, and
d) The organizational freedom and unrestricted access to top management to
resolve quality management issues.
Page 51

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
10
CURRENT
REVISION
L
5.5.3 INTERNAL COMMUNICATION
Top management ensures that appropriate communication processes are
established within the organization and that communication takes place regarding
the effectiveness of the quality management system. This includes the posting of
Quality Objective data to communicate information to the employee population
regarding the effectiveness of the quality management system.
5.6 MANAGEMENT REVIEW
5.6.1 GENERAL
Top management reviews the quality management system, at planned intervals, to
ensure its continuing suitability, adequacy, and effectiveness. This review
includes assessing opportunities for improvement and the need for changes to the
quality management system, including the quality policy and quality objectives.
Records from management reviews are maintained.
5.6.2 REVIEW INPUT
The input to management review includes information on:
a) Results of audits,
b) Customer feedback,
c) Process performance and product conformity,
d) Status of preventive and corrective actions,
e) Follow-up actions from previous management reviews,
f) Changes that could affect the quality management system, and
g) Recommendations for improvement.
5.6.3 REVIEW OUTPUT
The outputs from the management review include any decisions and actions
related to:
a) Improvement of the effectiveness of the quality management system and
its processes,
b) Improvement of product related to customer requirements, and
c) Resource needs.
Page 52
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
11
CURRENT
REVISION
L
6.0 RESOURCE MANAGEMENT
6.1 PROVISION OF RESOURCES
FCI determines and provides the resources needed;
a) To implement and maintain the quality management system and continually
improve its effectiveness, and
b) To enhance customer satisfaction by meeting customer requirements.
On an annual basis, the Directors submit all proposed resource needs for the
upcoming fiscal year to the Chief Financial Officer (CFO). These proposed
resource needs include, the infrastructure and resources needed to:
a) Achieve conformity to product requirements,
b) Implement and maintain the quality management system and continually
improve its effectiveness, and
c) Enhance customer satisfaction by meeting customer requirements.
The President, with input from other corporate entities, determines the
departmental budgets. The approved departmental budgets document the
determination of the infrastructure and resources needed.
The individual Directors then provide the needed resources based on the approved
annual departmental budgets.
The CFO maintains the individual departmental budgets on the corporate server
by date for a minimum of two years.
6.2 HUMAN RESOURCES
6.2.1 GENERAL
Personnel performing work affecting product quality are competent on the basis
of appropriate education, training, skills, and experience.
6.2.2 COMPETENCY AWARENESS AND TRAINING
FCI will:
a) Determine the necessary competence for personnel performing work
affecting, conformity to product requirements.
b) Where applicable, provide training or take other actions to satisfy the
necessary competencies,
c) Evaluate the effectiveness of the actions taken,
d) Ensure that personnel are aware of the relevance and importance of their
Page 53

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
12
CURRENT
REVISION
L
activities and how they contribute to the achievement of the quality
objectives, and
e) Maintain appropriate records of education, training, skills and experience.
6.3 INFRASTRUCTURE
FCI determines, provides, and maintains the infrastructure needed to achieve
conformity to product requirements. Infrastructure includes, as applicable:
a) Buildings, workspace and associated facilities;
b) Process equipment (both hardware and software), and
c) Supporting services (such as transport or communication).
6.4 WORK ENVIRONMENT
It is each departmental manager/supervisor‟s responsibility to determine and
manage the work environment needed to achieve conformity to product
requirements.
7.0 PRODUCT REALIZATION
7.1 PLANNING OF PRODUCT REALIZATION
FCI plans and develops the processes needed for product realization. Planning of
product realization is consistent with the requirements of the other processes of
the quality management system.
Upon the identification of the need to make a product available to our customers,
the planning of the processes for realization occurs to determine the following as
appropriate:
a) Quality objectives and requirements for the product during the Design and
Planning Phase;
b) The need to establish processes, *documents, and provide resources specific
to the product during the Design and Planning Phase;
c) Required verification, validation, monitoring, inspection and test activities
specific to the product and the criteria for product acceptance during the
Design and/or Planning Phase;
d) Records needed to provide evidence that the realization processes and
resulting product meet requirements;
e) Configuration management appropriate to the product; at the design phase
Page 54

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
13
CURRENT
REVISION
L
e) Resources to support the use and maintenance of the product during the
design phase.
Output from this planning is in a form suitable for FCI‟s method of
operations and may be (but is not limited to) the following:
Quality System Procedures and/or Documentation
MRP Data
Delivery Method Information
Quality Objectives
Organizational Infrastructure
Department Specific Documentation
Product Realization Documentation
7.1.1 PROJECT MANAGEMENT
As appropriate to FCI and the product, FCI shall plan and manage product
realization in a structured and controlled manner to meet requirements at
acceptable risk, within resource and schedule constraints.
7.1.2 RISK MANAGEMENT
FCI shall establish, implement and maintain a process for managing risk to the
achievement of applicable requirements, that includes as appropriate to the
organization and the product
a) Assignment of responsibilities for risk management,
b) Definition of risk criteria (e.g. , likelihood, consequences, risk
acceptance),
c) Identification, assessment and communication of risks throughout product
realization,
d) Identification, implementation and management of actions to mitigate
risks that exceed the defined risk acceptance criteria, and
e) Acceptance of risks remaining after implementation of mitigating actions.
7.1.3 CONFIGURATION MANAGEMENT
The organization shall establish, implement and maintain a configuration
management process that includes, as appropriate to the product
Page 55
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
14
CURRENT
REVISION
L
a) Configuration management planning,
b) Configuration identification,
c) Change control,
d) Configuration status accounting and
e) Configuration audit
7.1.4 CONTROL OF WORK TRANSFERS
FCI shall establish, implement and maintain a process to plan and control the
temporary or permanent transfer of work and to verify the conformity of the work
to requirements.
7.2 CUSTOMER RELATED PROCESSES
7.2.1 IDENTIFICATION OF PRODUCT RELATED REQUIREMENTS
FCI determines:
a) Requirements specified by the customer, including the requirements for
delivery and post delivery activities,
b) Requirements not stated by the customer but necessary for intended or
specified use, where known,
c) Statutory and regulatory requirements related to the product, and
d) Any additional requirements determined by the organization.
7.2.2 REVIEW OF PRODUCT REQUIREMENTS
FCI reviews the requirements related to the product. This review is conducted
prior to the organization‟s commitment to supply a product to the customer and
ensures that:
a) Product requirements are defined,
b) Contract or order requirements differing from those previously expressed are
resolved,
c) The organization has the ability to meet the defined requirements,
d) Special requirements of the product are determined, and
e) Risks (e.g., new technology, short delivery time scale) have been identified.
The results of the review and actions arising from the review are maintained.
Page 56

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
15
CURRENT
REVISION
L
Where the customer provides no documented statement of requirements, the
customer requirements are confirmed by FCI before acceptance.
Where product requirements are changed, the organization ensures that relevant
documents are amended and that relevant personnel are made aware of the
changed requirements.
7.2.3 CUSTOMER COMMUNICATION
FCI determines and implements effective arrangements for communicating with
its customers and sales channels in relation to:
a) Product information,
b) Inquiries, contracts or order handling, including amendments, and
c) Customer feedback, including customer complaints.
Effective arrangements include (but may not be limited to) the following:
Pre-Sales: All customer communication is forwarded to the Sales Department,
and
Post-Sales: All customer communication is forwarded to the Customer Service
Department.
7.3 DESIGN AND/OR DEVELOPMENT
7.3.1 DESIGN AND/OR DEVELOPMENT PLANNING
FCI plans and controls design and/or development of the product.
Design and/or development planning determines:
a) Stages of design and/or development,
Review, verification and validation activities appropriate to each design
and/or development stage, and
b) Responsibilities and authorities for design and/or development.
Where appropriate, FCI shall divide the design and development effort into
distinct activities and, for each activity, defines the tasks, necessary resources,
responsibilities, design content, input and output data and planning constraints.
The different design and development tasks to be carried out shall be based on the
safety and functional objectives of the product in accordance with customer,
statutory and regulatory requirements.
Design and development planning shall consider the ability to produce, inspect,
test and maintain the product.
Interfaces between different groups involved in design and/or development are
Page 57

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
16
CURRENT
REVISION
L
managed to ensure effective communication and clear assignment of
responsibility.
Planning output is updated, as appropriate, as the design and/or development
progresses.
7.3.2 DESIGN AND/OR DEVELOPMENT INPUTS
Inputs relating to product requirements are determined and records maintained.
These include:
a) Functional and performance requirements,
b) Applicable regulatory and legal requirements,
c) Applicable information derived from previous similar designs, and
d) Any other requirements essential for design and/or development.
These inputs are reviewed for adequacy. Incomplete, ambiguous, or conflicting
requirements are resolved.
7.3.3 DESIGN AND/OR DEVELOPMENT OUTPUTS
The outputs of design and/or development shall be in a form suitable for
verification against the design and development input and shall be approved prior
to release.
Design and/or development output:
a) Meets the design and/or development input requirements,
b) Provides appropriate information for purchasing, production, and service
provisions,
c) Contains or references product acceptance criteria,
d) Specifies the characteristics of the product that are essential to its safe and
proper use, and
e) Specify, as applicable, any critical items, including any key characteristics,
and specific actions to be taken for these items.
FCI shall define the data required to allow the product to be identified,
manufactured, inspected, used and maintained.
Examples may be:
Drawings, parts lists and specifications necessary to define the
configuration and the design features of the product, and
Page 58

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
17
CURRENT
REVISION
L
Information on the material, process, manufacturing and assembly of the
product needed to ensure conformity of the product.
7.3.4 DESIGN AND/OR DEVELOPMENT REVIEW
At suitable stages, systematic reviews of design and/or development is performed
in accordance with planned arrangements to:
a) Evaluate the ability of the results of design and/or development to meet
requirements,
b) Identify problems and propose necessary actions, and
c) Authorize progression to the next stage.
Participants in such reviews include representatives of functions concerned with
the design and/or development stage(s) being reviewed. The results of the
reviews and any necessary actions are maintained.
7.3.5 DESIGN AND/OR DEVELOPMENT VERIFICATION
Verification is performed in accordance with planned arrangements to ensure the
design and/or development outputs have met the design and/or development input
requirements. The results of the verification and any necessary actions are
maintained.
7.3.6 DESIGN AND/OR DEVELOPMENT VALIDATION
Design and/or development validation is performed in accordance with planned
arrangements to ensure that resulting product is capable of meeting the
requirements for the specified application or intended use, where known.
Wherever practicable, validation is completed prior to the delivery or
implementation of the product. Records of the results of the validation and any
necessary actions are maintained.
7.3.6.1 DESIGN AND/OR DEVELOPMENT VERIFICATION OR VALIDATION
TESTING
Where tests are necessary for verification and validation, these tests are planned,
controlled, reviewed, and documented to ensure and prove the following:
a) Test plans or specification identify the product being tested and the
resources being used, define test objectives and conditions, parameters to
be recorded, and relevant acceptance criteria;
Page 59
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
18
CURRENT
REVISION
L
b) Test procedures describe the method of operation, the performance of the
test, and the recording of the results;
c) The correct configuration standard of the product is submitted for the test;
d) The requirements of the test plan and the test procedures are observed;
e) The acceptance criteria are met.
7.3.6.2 DOCUMENTATION OF DESIGN AND/OR DEVELOPMENT
At the completion of design and/or development, FCI ensures that reports,
calculations, test results, etc., demonstrate that the product definition meets the
specification requirements for all identified operational conditions.
7.3.7 CONTROL OF DESIGN AND/OR DEVELOPMENT CHANGES
Design and/or development changes are identified and records maintained. The
changes are reviewed, verified and validated, as appropriate, and approved before
implementation. The review of design and development changes includes
evaluation of the effect of the changes on constituent parts and product already
delivered.
Objective evidence that design changes necessary to ensure only current, correct
and approved changes are used. Records of the results of the review of changes
and any necessary actions are maintained.
Design and/or development changes shall be controlled in accordance with the
configuration management process (see 7.1.3)
7.4 PURCHASING
7.4.1 PURCHASING PROCESS
FCI ensures that purchased product conforms to specified purchase requirements.
The type and extent of control applied to the supplier and the purchased product is
dependent upon the effect of the purchased product on subsequent product
realization or the final product.
FCI shall be responsible for the conformity of all products purchased from
suppliers, including product from sources defined by the customer.
FCI evaluates and selects suppliers based on their ability to supply product in
accordance with the organization‟s requirements. Criteria for selection,
evaluation, and re-evaluation are established. Records of the results of
evaluations and any necessary actions arising from the evaluation are maintained.
Page 60

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
19
CURRENT
REVISION
L
FCI will:
a) Maintain a register of approved suppliers that includes the status of the
approval;
b) Periodically reviews supplier performance; results of these reviews are used
as a basis for establishing the level of controls to be implemented;
c) Define the necessary actions to take when dealing with suppliers that do not
meet requirements;
d) Ensure where required that both the organization and all suppliers use
customer-approved special process sources;
e) Define the process, responsibilities and authority for the approval status
decision, changes of the approval status and conditions for a controlled use of
suppliers depending on the supplier‟s approval status;
f) Determine and manage the risk when selecting and using suppliers;
g) Suppliers shall report to FCI if their product has been released from that
supplier and subsequently found not to conform to the applicable design data.
7.4.2 PURCHASING INFORMATION
Purchasing information describes the product to be purchased, including where
appropriate:
a) Requirements for approval of product, procedures, processes, and
equipment,
b) Requirements for qualification of personnel,
c) Quality management system requirements,
d) The identification and revision status of specifications, drawings, process
requirements, inspection/verification instructions and other relevant
technical data,
e) Requirements for design, test, inspection, verification (including
production process verification), use of statistical techniques for product
acceptance, and related instructions for acceptance by the organization,
and as applicable critical items including key characteristics,
f) Requirements for test specimens (e.g. production method, number, storage
conditions) for design approval, inspection, verification, investigation or
auditing,
g) Requirements regarding the need for the supplier to:
Page 61

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
20
CURRENT
REVISION
L
- Notify the organization of nonconforming product,
- Obtain organization approval for nonconforming product
disposition,
- Notify the organization of changes in product and/or process,
changes of suppliers, changes of manufacturing facility location
and, where required, obtain organization approval, and
- Flow down to the supply chain the applicable requirements
including customer requirements.
h) Records retention requirements, and
i) Right of access by FCI, their customer and regulatory authorities to the
applicable areas of all facilities, at any level of the supply chain, involved
in the order and to all applicable records.
FCI ensures the adequacy of specified purchase requirements prior to their
communication to the supplier.
7.4.3 VERIFICATION OF PURCHASED PRODUCT
FCI establishes and implements the inspection or other activities necessary for
ensuring that purchased product meets specified purchase requirements.
Verification activities may include:
a) Obtaining objective evidence of the conformity of the product from
suppliers,
b) Inspection and audit at supplier‟s premises,
c) Review of the required documentation,
d) Inspection of products upon receipt, and
e) Delegation of verification to the supplier, or supplier certification.
Where purchased product is released for production use pending completion of all
required verification activities, it shall be identified and recorded to allow recall
and replacement if it is subsequently found that the product does not meet
requirements.
Where the organization delegates verification activities to the supplier, the
requirements for delegation shall be defined and a register of delegations
maintained.
Where FCI or its customer intends to perform verification at the supplier‟s
premises, FCI will state the intended verification arrangements and method of
product release in the purchasing information.
Page 62
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
21
CURRENT
REVISION
L
7.5 PRODUCTION AND SERVICE PROVISION
7.5.1 CONTROL OF PRODUCTION AND SERVICE PROVISION
FCI plans and carries out production and service provisions under controlled
conditions using shop orders. Controlled conditions include, as applicable:
a) The availability of information that describes the characteristics of the
product,
b) The availability of work instructions and shop orders, as necessary,
c) The use of suitable equipment,
d) The availability and use of measuring and monitoring devices,
e) The implementation of monitoring and measurement,
f) The implementation of product release, delivery and post-delivery activities,
g) Accountability for all product during production (e.g.; parts quantities, split
orders, nonconforming product),
h) Evidence that all manufacturing and inspection/verification operations have
been completed as planned, or as otherwise documented and authorized using
shop orders,
i) Provision for the prevention, detection, and removal of foreign objects,
j) Monitoring and control of utilities and suppliers such as water, compressed
air, electricity and chemical products to the extent they affect conformity to
product requirements, and
k) Criteria for workmanship, is specified in the clearest practical way (e.g.;
written standard, representative samples or illustrations).
Planning shall consider, as appropriate
- Establishing, implementing and maintaining appropriate processes to manage
critical items, including process controls where key characteristics have been
identified,
- designing, manufacturing and using tooling to measure variable data,
- identifying in-process inspection/verification points when adequate
verification of conformance cannot be performed at later stages of
realization, and
- special processes (see 7.5.2).
Page 63

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
22
CURRENT
REVISION
L
7.5.1.1 PRODUCTION PROCESS VERIFICATION
FCI shall use a representative item from the first production run of a new part
or assembly to verify that the production processes, production
documentation and tooling are capable of producing parts and assemblies that
meet requirements. This process shall be repeated when changes occur that
invalidate the original results (e.g., engineering changes, manufacturing
process changes, tooling changes).
NOTE: This activity is often referred to as first article inspection.
7.5.1.2 CONTROL OF PRODUCTION PROCESS CHANGES
Personnel authorized to approve changes to production processes shall be
identified.
FCI shall control and document changes affecting processes, production
equipment, tools or software programs.
The results of changes to production processes shall be assessed to confirm
that the desired effect has been achieved without adverse effects to product
conformity.
7.5.1.3 CONTROL OF PRODUCTION EQUIPMENT, TOOLS AND
SOFTWARE PROGRAMS
Production equipment, tools and software programs used to automate and
control/monitor product realization processes, shall be validated prior to
release for production and shall be maintained.
Storage requirements, including periodic preservation/condition checks, are
established for production equipment and tooling in storage.
7.5.1.4 POST-DELIVERY SUPPORT
Post-delivery support shall provide as applicable for the:
a) Collection and analysis of in-service data, through use of FAR‟s (failure
analysis reports) and repair tracking programs (not applicable to PMA parts)
in the FCI database.
b) Actions to be taken, including investigation and reporting, when problems
are detected after delivery,
c) Control and updating of technical documentation,
d) Approval, control and use of repair schemes (not applicable to PMA parts),
Page 64

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
23
CURRENT
REVISION
L
and
e) Controls required for off-site work (e.g., organization‟s work undertaken at
the customer‟s facilities).
7.5.2 VALIDATION OF PROCESSES, PRODUCTION AND SERVICE
PROVISION
FCI validates any processes for production and service where the resulting output
cannot be verified by subsequent measurement or monitoring, and as a
consequence, deficiencies become apparent only after the product is in use or the
service has been delivered (a.k.a.; Special Processes).
Validation demonstrates the ability of the processes to achieve planned results.
FCI establishes arrangements for these processes including, as applicable:
a) Defined criteria for review and approval of the processes,
b) Approval of equipment and qualification of personnel,
c) Use of specific methods and procedures,
d) Requirements for records, and
e) Re-validation.
7.5.3 IDENTIFICATION AND TRACEABILITY
Where appropriate, FCI identifies the product by part number throughout product
realization.
FCI maintains the identification of the configuration of the product in order to
identify any differences between the actual configuration and the agreed
configuration.
FCI identifies the status of the product with respect to measurements and
monitoring requirements on the associated Operation Sheet or in the Business
System.
When acceptance authority media are used (e.g.; stamps, electronic signatures,
passwords), FCI establishes and documents controls for the media.
Where traceability is a requirement, FCI controls and records the unique
identification of the product via the Receiving Inspection, In Process Inspection,
Job Order, and Final Inspection procedures.
Traceability requirements can include:
Page 65

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
24
CURRENT
REVISION
L
a) Identification throughout the product life;
b) The ability to trace all products manufactured from the same batch of raw
material, or from the same manufacturing batch, to the destination (e.g.,
delivery, scrap),
c) For an assembly, the ability to trace its components to the assembly and then
to the next higher assembly, and
d) For a product, a sequential record of its production (manufacture, assembly,
inspection/verification) to be retrievable.
FAA-PMA IDENTIFICATION
When the Federal Aviation Administration (FAA) has granted Parts
Manufacturing Approval (PMA) for production of modification or replacement
parts, the parts shall be marked in accordance with 14CFR45.15 with the
following:
a) “FAA-PMA”
b) The name, trademark, or symbol of PMA holder
c) The part number
Note: If the item is too small to record all of the above information, and approval
is obtained from the FAA, the information may be placed on a tag attached to the
item or attached to the individual container for the item.
7.5.4 CUSTOMER PROPERTY
FCI exercises care with customer property (including intellectual property and
personal data while it is under the organization‟s control or being used by the
organization. FCI identifies, verifies, protects, and safeguards customer property
provided for use or incorporation into the product. Occurrence of any customer
property that is lost, damaged, or otherwise found to be unsuitable for use is
recorded and reported to the customer and records maintained.
7.5.5 PRESERVATION OF PRODUCT
FCI preserves the product during internal processing and delivery to the intended
destination in order to maintain conformity to requirements. As applicable, this
preservation includes identification, handling, packaging, storage, and protection.
Preservation also applies to constituent parts of a product.
Preservation of product also includes, where applicable in accordance with
product specifications and/or applicable regulations, provisions for:
Page 66
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
25
CURRENT
REVISION
L
a) Cleaning;
b) Prevention, detection and removal of foreign objects;
c) Special handling for sensitive products, such as caps on connectors;
d) Marking and labeling including safety warnings;
e) Shelf life control and stock rotation;
f) Special handling for hazardous materials.
7.6 CONTROL OF MEASURING AND MONITORING DEVICES
FCI determines the monitoring and measurements to be undertaken and the
measuring and monitoring equipment needed to provide evidence of conformity
of product to determined requirements.
FCI maintains a register of these monitoring and measuring equipment, and
defines the process employed for their calibration/verification including details of
equipment type, unique identification, location, frequency of checks, check
method and acceptance criteria.
The organization establishes processes to ensure the monitoring and measurement
can be carried out and are carried out in a manner that is consistent with the
monitoring and measurement requirements.
FCI ensures that environmental conditions are suitable for the calibrations,
inspections, measurements, and tests being carried out.
Where necessary to ensure valid results, measuring equipment is:
a) Calibrated or verified at specified intervals, or prior to use, against
measurement standards traceable to international or national standards;
where no such standards exist, the basis used for calibration or verification
is recorded,
b) Be adjusted or re-adjusted as necessary,
c) Be identified in order that the calibration status is determined,
d) Be safeguarded from adjustments that would invalidate the measurement
result,
e) Be protected from damage and deterioration during handling, maintenance
and storage,
FCI shall establish, implement and maintain a process for the recall of monitoring
and measuring equipment requiring calibration or verification.
In addition, the organization assesses and records the validity of the previous
measuring results when the equipment is found not to conform to requirements.
Page 67
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
26
CURRENT
REVISION
L
The organization takes appropriate action on the equipment and any product
affected. Records of the results of calibration and verification are maintained.
When used in the monitoring and measurement of specified requirements, the
ability of computer software to satisfy the intended application is confirmed. This
is undertaken prior to initial use and reconfirmed as necessary.
8.0 MEASUREMENT, ANALYSIS AND IMPROVEMENT
8.1 GENERAL
FCI plans and implements the monitoring, measurement, analysis, and
improvement processes needed:
a) To demonstrate conformity to product requirements,
b) To ensure conformity of the quality management system, and
c) To continually improve the effectiveness of the quality management system.
This includes determination of applicable methods, including statistical
techniques, and the extent of their use.
8.2 MEASUREMENT AND MONITORING
8.2.1 CUSTOMER SATISFACTION
As one of the measurements of the performance of the quality management
system, the organization monitors information relating to customer‟s perception
as to whether the organization has met customer requirements. The methods for
obtaining and using this information are determined.
Information to be monitored and used for the evaluation of customer satisfaction
shall include, but is not limited to, product conformity, on-time delivery
performance, customer complaints and corrective action requests. Organizations
shall develop and implement plans for customer satisfaction improvement that
address deficiencies identified by these evaluations, and assess the effectiveness
of the results.
NOTE: Monitoring customer perception can include obtaining input from
sources such as customer satisfaction surveys, customer data on delivered product
quality, user opinion surveys, lost business analysis, compliments, warranty
claims and manufacturing representatives and distributor reports.
Page 68

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
27
CURRENT
REVISION
L
8.2.2 INTERNAL AUDIT
FCI conducts internal audits at planned intervals to determine whether the quality
management system:
Is effectively implemented and maintained.
The audit program is planned, taking into consideration the status and importance
of the processes and areas to be audited, as well as the results of previous audits.
The audit criteria, scope, frequency, and methods are defined. Selection of
auditors and conduct of audits will ensure objectivity and impartiality of the audit
process.
Auditors will not audit their own work.
A documented procedure shall be established to define the responsibilities and
requirements for planning and conducting audits, establishing records and
reporting results.
Records of the audits and their results shall be maintained.
The management responsible for the area being audited shall ensure that any
necessary corrections and corrective actions are taken without undue delay to
eliminate detected nonconformities and their causes. Follow-up activities shall
include the verification of the actions taken and the reporting of verification
results.
The President of FCI shall be notified of the findings and assure that the
corrective actions are implemented.
8.2.3 MEASUREMENT AND MONITORING OF PROCESSES
FCI applies suitable methods for monitoring and where applicable, measurement
of the quality management system processes. These methods demonstrate the
ability of the processes to achieve planned results. When planned results are not
achieved, correction and corrective action is taken, as appropriate. In the event of
process nonconformity, the organization will:
a) Take appropriate action to correct the nonconforming process,
b) Evaluate whether the process nonconformity has resulted in product
nonconformity, and
c) Determine if the process nonconformity is limited to a specific case or whether
it could have affected other processes or products, and
d) Identify and control any nonconforming product (see 8.3).
Page 69

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
28
CURRENT
REVISION
L
8.2.4 MEASUREMENT AND MONITORING OF PRODUCT
FCI measures and monitors the characteristics of the product to verify that
product requirements have been met. This is carried out at appropriate stages of
the product realization process in accordance with planned arrangements.
Evidence of conformity with the acceptance criteria shall be maintained, through
the use of Lot Packages, Discrepancy Reports, Operation Sheets and Final
Acceptance Test Data Sheets.
Measurement requirements for product acceptance shall be documented and shall
include:
a) Criteria for acceptance and/or rejection,
b) Where in the sequence measurement and testing operations are to be
performed,
c) Required records of the measurement results (at minimum, indication of
acceptance or rejection), and
d) Any specific measurement instrument required and any specific instructions
associated with their use.
When critical items, including key characteristics, have been identified the
organization shall ensure they are controlled and monitored in accordance with
the established processes.
When FCI uses sampling inspection as a means of product acceptance, the plan
shall be justified on the basis of recognized statistical principles and appropriate
for use (i.e., matching the sampling plan to the criticality of the product and to the
process capability).
Where product is released for production use pending completion of all required
measurement and monitoring activities, it shall be identified and recorded to allow
recall and replacement if it is subsequently found that the product does not meet
requirements.
Records shall indicate the person(s) authorizing release of product for delivery to
the customer.
Where required to demonstrate product qualification, the organization shall ensure
that records provide evidence that the product meets the defined requirements.
The release of product and delivery of service to the customer shall not proceed
until the planned arrangements have been satisfactorily completed, unless
otherwise approved by a relevant authority and, where applicable, by the
customer.
FCI shall ensure that all documents required to accompany the product are present
at delivery.
Page 70
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
29
CURRENT
REVISION
L
8.3 CONTROL OF NONCONFORMING MATERIAL
FCI ensures that product which does not conform to requirements is identified and
controlled to prevent unintended use or delivery. A documented procedure shall
be established to define the controls and related responsibilities and authorities for
dealing with nonconforming product.
FCI‟s documented procedure defines the responsibility for review and authority
for the disposition of nonconforming product and the process for approving
personnel making these decisions.
Where applicable, FCI deals with nonconforming product by one or more of the
following ways:
a) By taking action to eliminate the detected nonconformity,
b) By authorizing its use, release or acceptance under concession by a relevant
authority and, where applicable, by the customer,
c) By taking action to preclude its original intended use or application.
d) By taking action appropriate to the effects, or potential effects, of the
nonconformity when nonconforming product is detected after delivery or
use has started:
- The organizations nonconforming product control process shall
provide for timely reporting of delivered nonconforming product;
e) By taking actions necessary to contain the effect of the nonconformity on
other processes or products.
Disposition of use-as-is or repair (not applicable to PMA parts) shall only be used
after approval by an authorized representative of the organization responsible for
design.
The organization shall not use dispositions of use-as-is or repair (not applicable to
PMA parts), unless specifically authorized by the customer, if the nonconformity
results in a departure from the contract requirements.
Product dispositioned for scrap shall be conspicuously and permanently marked,
or positively controlled, until physically rendered unusable.
When nonconforming product is corrected, it shall be subject to re-verification to
demonstrate conformity to the requirements.
Records of the nature of nonconformities and any subsequent actions taken,
including concessions obtained, shall be maintained.
Page 71

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
30
CURRENT
REVISION
L
14CFR21.3 REPORTING OF FAILURES, MALFUNCTIONS, AND DEFECTS:
Failures, malfunctions, or defects found in any product, part, process, or article
manufactured by FCI, including those that have been delivered to customers, that
are determined to have resulted in any of the conditions identified in QAP
04QA704075 shall be reported to the Aircraft Certification Office within 24 hours
after FCI has determined that the failure, malfunction, or defect has occurred.
(Reports due on weekends or holidays shall be delivered the following workday
after the weekend or holiday.)
8.4 ANALYSIS OF DATA
FCI determines, collects, and analyzes appropriate data to demonstrate the
suitability and effectiveness of the quality management system and to evaluate
where continual improvements of the quality management system can be made.
This includes data generated by measuring and monitoring activities and other
relevant sources.
The organization analyses this data to provide information on:
a) Customer satisfaction,
b) Conformity to product requirements,
c) Characteristics and trends of processes and product including opportunities
for preventive action, and
d) Suppliers.
8.5 IMPROVEMENT
8.5.1 CONTINUAL IMPROVEMENT
FCI shall continually improve the effectiveness of the quality management system
through the use of the quality policy, quality objectives, audit results, analysis of
data, corrective and preventive action, and management review.
The organization shall monitor the implementation of improvement activities and
evaluate the effectiveness of the results.
8.5.2 CORRECTIVE ACTION
FCI takes corrective action to eliminate the cause of nonconformities in order to
prevent recurrence. Corrective action is appropriate to the effects of the
nonconformities encountered.
Page 72
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
31
CURRENT
REVISION
L
The documented procedure for corrective action is established to define
requirements for:
a) Reviewing nonconformities (including customer complaints),
b) Determining the causes of nonconformity,
c) Evaluating the need for action to ensure that nonconformities do not recur,
d) Determining and implementing the action needed,
e) Records of the results of action taken,
f) Reviewing the effectiveness of the corrective action taken,
g) Flow down of the corrective action requirement to a supplier, when it is
determined that the supplier is responsible for the nonconformity
h) Specific actions where timely and/or effective corrective actions are not
achieved,
i) Determining if additional nonconforming product exists based on the causes
of the nonconformities and taking further action when required,
j) Addressing any in-service problem involving design changes,
k) Determining if any changes to the instructions for Continued Airworthiness
are necessary, and
l) Initiating corrective action for products or articles that have been released
and do not conform to the applicable design data or quality system
requirements.
For FAA-PMA parts FCI utilizes Failure Analysis Reports (FAR's) for each
returned part. Completed FAR's are analyzed to determine if there are any trends
in part defects, design defects, malfunctions, failures or conformance to
requirements. Corrective Action is then initiated using Discrepancy Reports,
Corrective and Preventative Action Reports or other tools. As part of the
corrective action, Continued Airworthiness is considered.
8.5.3 PREVENTIVE ACTION
FCI determines action to eliminate the causes of potential nonconformities in
order to prevent their occurrence. Preventive actions taken are appropriate to the
effects of the potential problems.
The documented procedure for preventive action defines requirements for:
a) Determining potential nonconformities and their causes,
b) Evaluating the need for action to prevent occurrence of nonconformities,
Page 73

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
2
PAGE
32
CURRENT
REVISION
L
c) Determining and implementing action needed,
d) Recording results of action taken, and
e) Reviewing preventive action taken.
Page 74
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
1
CURRENT
REVISION
L
SECTION THREE
DESCRIPTION
PAGE
Organization
1
2
Quality Assurance Program
2
2
Design Control
3
4
Procurement Document Control
4
6
Instructions, Procedures, and Drawings
5
6
Document Control
6
9
Control of Purchased Material, Equipment and Services
7
10
Identification and Control of Material, Parts, and Components
8
12
Control of Special Processes
9
13
Inspection
10
14
Test Control
11
15
Control of Measuring and Test Equipment
12
16
Handling, Storage, and Shipping
13
17
Inspection, Test, and Operating Status
14
17
Non-Conforming Materials, Parts, or Components
15
18
Corrective Action
16
19
Quality Assurance Records
17
20
Audits
18
20
Page 75

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
2
CURRENT
REVISION
L
1 ORGANIZATION
1.1 Scope
This section, Section Three, of the Manual sets forth the Quality Assurance Program
and the methods used to achieve implementation and documentation of the
“Controlled” program. This program complies with the Requirements of ISO 9001;
the Quality System requirements of 10CFR50 Appendix B; and the basic Quality
System Requirements of ANSI NQA-1.
This Manual, in its entirety, unless identified otherwise, shall be issued as a controlled
document to Fluid Components International LLC‟s customers having contractual
obligation to this section of the manual.
Appendix D to this manual lists job descriptions to show the activities that affect
quality and how they interface with the Quality Assurance Program. For their
relationship to the Organization, see Appendix A, Company Organization Chart.
2 QUALITY ASSURANCE PROGRAM
2.1 Referenced Documents
Fluid Components International LLC‟s Quality Manual 07QA070003 is
supplemented by numerous implementing procedures listed in Appendix B and
referenced throughout this section of the Manual. Procedures other than those
referenced in this section may also be used to implement the Quality Assurance
Program. When determined necessary, any of the procedures listed may be deleted
from the program; however, the Quality Assurance Manager shall ensure that if a
procedure is deleted the requirements stated in this manual are not compromised.
2.1.1 Engineering Procedures
2.2 Review and Approval by Management
A review of this manual shall be performed every calendar year at the direction of the
01DM000064/01DM000025 Engineering Document Control
Quality Assurance Manager. The purpose of this review is to determine the adequacy
of the Quality Assurance Program in meeting Quality System Requirements outlined
in the “Scope” in this section of this Manual. The review shall also consider other
codes or regulations as they apply to Fluid Components International LLC. The
Internal Audit Team shall typically accomplish this review during the annual internal
audit. Additional reviews may be performed when determined necessary by the
Quality Assurance Manager. Reviews of the Quality Manual shall be documented.
Page 76

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
3
CURRENT
REVISION
L
Changes found during the review that are necessary to improve the clarity or
effectiveness of the Quality Assurance Program shall be incorporated.
The President hereinafter shall approve changes or revisions to the manual.
2.3 Quality Assurance Program Implementation
Organizations participating in the Quality Assurance Program shall be reviewed on a
annual basis. This review shall substantiate the effectiveness of implementation of
the portion of the program for which each organization has responsibility. This
review shall be accomplished during the internal audit of the Quality Assurance
Program.
2.4 Quality Plans
In some instances there are customers with unique contractual quality requirements
that are not specifically addressed in this manual or the supporting Quality Assurance
Procedures. Some of these requirements may even come in conflict with stated
policies in this Manual. To ensure unique contractual requirements relating to quality
are incorporated, Quality Plans may be used to document and accomplish these
requirements.
Quality Plans will state when and for whom they are applicable. They will also state
that Quality Manual 07QA070003 will be used as a basis for the quality system. All
additional or conflicting requirements will be addressed. Quality Plans shall take
precedence over the Quality Manual. Quality Plans shall be generated by the Quality
Assurance Manager and submitted to the customer for approval prior to
implementation as required by contract.
2.5 Indoctrination and Training
To the degree necessary, as determined by the Quality Assurance Manager, personnel
performing quality functions and activities affecting quality shall be properly trained
and indoctrinated in their respective areas of responsibility. Learning tools, such as
on-the-job training, seminars, classes, testing, and the like, shall be used to
accomplish these tasks. The documentation of indoctrination and training of Quality
personnel and other personnel performing activities affecting quality shall be in
accordance with Quality Assurance Procedure 04QA704034.
Page 77

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
4
CURRENT
REVISION
L
2.6 Qualification of Personnel Performing Verification and Auditing Activities
Personnel performing verification and auditing activities shall have the experience
and training commensurate with the activity being performed. The capabilities of
personnel performing these activities shall be determined initially by a review of their
education, experience, training, and test results or capability demonstration.
Performance shall be re-evaluated periodically to assure continued satisfactory
performance. Qualification requirements are more thoroughly described in Quality
Assurance Procedure 04QA704034 for verification personnel and 04QA704054 for
Auditing personnel.
NOTE: Fluid Components International LLC does not employ Nondestructive
Examination personnel; this is considered a ”Special Process” and is covered in
paragraph 10 of this Manual, Section 3.
3 DESIGN CONTROL
3.1 Contract Review
Upon receipt of all new Customer Contracts, the customer‟s Purchase Order and
accompanying documents (i.e., specifications and drawings) shall be assigned to a
Contract Manager. The Contract Manager, Quality Assurance Manager and when
necessary an assigned Engineer; shall perform a Contract Review. At the option of
the Contract Manager, other personnel from disciplines such as Qualification
Engineering, Design Engineering, Production, and Purchasing may participate in this
review. This review identifies design, regulatory and specification requirements,
suitable materials and processes, and applicable codes and standards that shall be
incorporated into drawings, procedures, and instructions. The review shall also
include assignments for contract related design tasks. The review and assignments
shall be documented on Contract Review forms described in Quality Assurance
Procedure 04QA704005.
3.2 Design Document Review
Documents created to support a customer contract such as Outline Drawings,
Acceptance Test Procedures, etc. shall be reviewed by Engineering, and Quality
Assurance at a minimum to assure contractual requirements have been incorporated.
Additional parties may participate in the review of these documents when specifically
requested by the Contract Manager.
Page 78

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
5
CURRENT
REVISION
L
3.3 New Designs
New product requiring qualification to specific requirements shall have a
Qualification Test Procedure developed to accomplish the testing. When required
this document shall be submitted to the customer for approval.
The results of completed qualification testing shall be documented in the
Qualification Test Report.
3.4 Qualification Design Verification
Design applications based on previously qualified designs and requiring traceability
to an existing qualification test report shall be reviewed against the original design to
ascertain any possible design changes or deviations made necessary by the customer's
application and/or qualification envelope. Written documentation of the verification
shall be required for all domestic Nuclear customers, and if imposed by the customer,
for all other industries and regions.
Qualified personnel not responsible for initiating the original design for all nuclear
applications shall perform this design verification and analysis.
This verification shall incorporate as appropriate, suitable testing programs, alternate
or simplified calculations, design reviews, similarity analysis, or other approved
methods. The verification shall be documented in accordance with Quality Assurance
Procedure 06QA020013, Qualification Verification Analysis”.
3.5 Design Changes
Changes to or deviations from existing engineering design documents (i.e., revisions,
Any of the above-described design changes that affect the customer‟s specifications
temporary “Deviations” and "Use As Is" or "Repair" dispositions of nonconforming
items) shall be properly documented and controlled. Design changes or deviations to
engineering documents shall be reviewed, approved, and recorded in accordance with
Engineering Procedure 01DM000064/01DM000025 and the nonconforming material
procedure 04QA704004.
or the Company‟s documents approved by the Customer, shall require customer
participation in the formal approval cycle. Customer approval shall be documented.
Page 79

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
6
CURRENT
REVISION
L
4 PROCUREMENT DOCUMENT CONTROL
4.1 Fluid Components International LLC Purchase Order Review
Quality shall ensure that controlled item Purchase Orders incorporate the applicable
quality requirements. When applicable, the Purchase Order shall require vendors to
impose the Company‟s Customer Quality Assurance and contractual requirements on
sub-tiers. The Purchase Orders shall identify and be reviewed for inclusion of
applicable information such as Drawings, and Drawing Revisions. As appropriate,
provisions for compliance to contract and federal regulations shall be included.
When appropriate, provisions shall be included for access to the vendor's plant to
perform audits, surveys, or source inspection. After review and approval, an
authorized Quality Assurance Representative shall sign each Purchase Order. The
signing of the Purchase Order by Quality Assurance acts as the official issuance of
the contract.
4.2 Purchase Order Procedure
For detailed instructions on completing, reviewing, correcting, or changing
Controlled Purchase Orders and related documents, see Quality Assurance Procedure
04QA704003.
5 INSTRUCTIONS, PROCEDURES, AND DRAWINGS
Activities affecting quality shall be prescribed by and performed in accordance with
documented instructions, procedures, or drawings of a type appropriate to the
circumstances. The documents described in this section of the Manual shall provide
the means by which these activities are accomplished. The purpose for these
documents is to supply Inspectors, Production and Test personnel, and vendors with
adequate information to satisfactorily perform their respective activities and functions
affecting quality.
5.1 Customer Orders and Order Verification Packages
All contracts subject to the requirements of this manual shall be processed in
accordance with Quality Assurance Procedure 07QA704002.
For reorders or long term-scheduled purchases, parts may be built for “Stock” and
placed in finished goods inventories. Finished goods may be pulled directly from
stock and shipped using only the Order Verification package, no cross- referenced
Job Orders are required, see Paragraph 5.2 for further details.
Page 80

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
7
CURRENT
REVISION
L
5.2 Job Orders
Job Orders subject to the requirements of this Manual shall be described and
processed in accordance with Quality Assurance Procedure 04QA704020. In
addition to basic information, Job Orders can list Bills of Materials, Special
Requirements, and Procedures required for completing the item being built or created.
The Job Order may also act as a substitute for the Parts List as part of the Lot Number
Data Package when the Engineering Parts List is on the face of the drawing and not a
separate page.
5.3 Process Sheets
A Process Sheet is identified by the Operation Sheet. A Process Sheet outlines an
individual, specific process contained within a workstation and designates specific
tools and instructions necessary to accomplish a particular process. Process Sheets
are controlled in accordance with Document Management‟s Procedure
01DM000064/01DM000025.
5.4 Final Acceptance Test Procedures
Final Acceptance Test Procedures (FATP) is typically model specific documents
(although a few generic procedures exist). These documents delineate all necessary
functional and performance testing instructions, both electrical and mechanical.
FATP's also include inspections necessary for final acceptance by the customer, the
government, and or the Company. The purpose of these procedures is to assure that
each instrument is within the scope of the intended application and FCI established
acceptance criteria. To carry out this objective, the procedure contains sequential
instructions, acceptance criteria, and requirements for recording the test equipment
used and objective evidence of the results of the tests and inspections.
5.5 Quality Assurance Procedures
Quality Assurance Procedures outline detailed functions or activities directly related
5.6 Departmental Procedures
to or affecting quality. This may include, but not be limited to, functions such as
Purchasing, Inspections, Calibration of Measuring & Test Equipment, Internal
Audits, Discrepancy Reports, and Quality Assurance Contract Review. The Quality
Assurance Procedures assure that all applicable criteria, specifications, and
requirements are met each time an activity is performed, and assure proper record
keeping of the activity or function.
Page 81
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
8
CURRENT
REVISION
L
Various departments may generate other procedures to provide consistency in tasks
performed. These procedures shall be controlled in accordance with Paragraph 6,
Document Control, of this section of the manual when they are determined by the
Quality Assurance Manager to be related to quality activities.
5.7 Drawings
Drawings shall be created to ensure that parts, equipment, and services are
manufactured, assembled, and/or performed identically each time an item is
processed. Each drawing shall have an identifying number assigned which shall also
be the Part Number for the item depicted. As appropriate, customer requirements,
quantitative criteria such as dimensions, tolerances, angles, and surface finishes,
fabrication instructions, installation instructions, material requirements, and the like
may be delineated in drawings. Drawings are controlled in accordance with
Document Management‟s Procedure 01DM000064/01DM000025.
5.8 Operation Sheets
All assembly drawings shall have an Operation Sheet (Op Sheet) detailing the step-
by-step processes used to manufacture, assemble, fabricate, and test the item depicted
on the drawing. Op sheets shall carry the same number and revision letter as the
drawing for which they are written. Assembly Kits and assemblies fabricated solely
by outside vendors do not require an accompanying Op Sheet. Operation Sheets are
controlled in accordance with Document Management‟s Procedure
01DM000064/01DM000025.
6 DOCUMENT CONTROL
6.1 Identification
Documents such as Drawings, Operation Sheets, Quality Assurance Procedures,
Forms, Test Procedures, Test Reports, Purchase Orders, and other quality related
documents shall be controlled to assure that correct documents are being employed.
Such documents, including changes thereto, shall be reviewed for adequacy and
approved by authorized personnel only.
Page 82

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
9
CURRENT
REVISION
L
6.2 Methods and Distribution
Documents released for production shall be stamped or marked as controlled copies.
Approvals, responsibility, and distributions, and controls shall be in accordance with
Quality Assurance Procedure 04QA704007, for Quality Assurance Procedures and
Manuals and in accordance with procedure number 01DM000064/01DM000025 for
Engineering Documents. These procedures assure that only current revisions are
used, unless a specific revision is required, and that obsolete documents are removed
from the shop floor.
6.3 Change Control
Changes to documents affecting quality shall be recorded and approved using the
following methods to ensure changes have been approved by all applicable
organizations.
6.3.1 Engineering Change Notice
Changes to Drawings, Operation Sheets, and Process Sheets, shall be recorded on an
Engineering Change Notice (ECN). For more information please refer to Engineering
Procedure 01DM000064/01DM000025.
6.3.2 Revision Page
A Revision Page can be used in lieu of an ECN for book type documents such as
Qualification Reports, Test Reports or Instruction Manuals. The revision page shall
identify the changed pages contain a brief description of the change.
6.3.3 Cover Sheet
Documents such as Departmental Procedures that have a cover page containing new
approval signatures at each revision may be revised without use of an ECN or
Revision Page. Copies of each preceding revision shall be kept as change history for
that document.
6.3.4 Use of Initials
Other controlled documents such as FCI Purchase Orders, Order Verification
documents, and Job Orders shall have changes initialed as approved by those
departments or personnel generating and approving the original document.
Page 83

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
10
CURRENT
REVISION
L
7 CONTROL OF PURCHASED MATERIAL, EQUIPMENT, AND SERVICES
7.1 Vendor Control
Quality shall perform Vendor Surveys, Audits and Receiving Inspection activities as
appropriate to assure that purchased items and services conform to procurement
documents and that the items are of required quality. Additionally, requests for
certifications of tests, inspections, or contract compliance may be made.
7.1.1 Quality Approved Vendors (QAV)
Quality Approved Vendors document 08QA080004 shall be maintained as a
controlled document for calibration services, test laboratories, special process and
10CFR50 Appendix B items.
All QAV providing nuclear services shall receive an Evaluation of the Critical Safety
Characteristics of their service (04QA704044). All QAV providing 10CFR21 part B
items shall get an audit (04QA704008).
All services shall be procured for nuclear product using vendors that have been
surveyed or audited.
(ASME NPT Certificate of Authorization may be accepted in lieu of a vendor survey
for Nuclear Grade items for “non-domestic” orders.)
Suppliers of items or services having independent nuclear qualification are not required
to be on the QAV when the purchase of these items has been at the direction of the
customer for use on the customer‟s contract.
Vendors that are certified to 17025 shall be evaluated monthly to assure continued
accreditation (04QA704044). The accreditation shall cover the scope of the contracted
services.
For ISO 17025 accredited Calibration Service providers that have a third party
accreditation approval from a source recognized by the NRC, a survey may be waived (
04QA704044).
For calibration services, the Purchase Order shall require that the supplier provide a report
with the as-found data of items found out-of-tolerance; and, referencing the calibration
procedure and equipment/standards used. The report shall include the vendor‟s name, the
calibration date, and a statement that the calibration is traceable to NIST. The Purchase Order
Number shall appear on the Certificate of Calibration. (From NUPIC audit)
The vendors on QAV 08080004 are reflected on the Approved Vendor List (AVL)
08QA080002, including the vendor‟s approval status.
Page 84

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
11
CURRENT
REVISION
L
7.1.2 Vendor Performance Record & Approved Vendor List (AVL)
Vendor performance is evaluated per 04QA704077. A Vendor Performance Record
(VPR) shall be maintained. The record shall be used to document the receipts of
goods or services of vendors contained on the AVL (08QA080002). A review of
these records shall be conducted periodically and a determination made to either keep
or delete each vendor from the Approved Vendors List. A review may be conducted
on any particular vendor at any time deemed necessary by the Quality Assurance
Manager. Reviews shall be conducted in accordance with Quality Assurance
Procedure 04QA704044.
7.2 Purchasing Method
Purchased items with special requirements such as Shelf Life, Special Processing, or
Material Certifications where traceability to those requirements is essential shall be
purchased as “Controlled.” See Paragraph 4, “Procurement Document Control”, of
this section of the manual.
Standard items that can be verified by inspection to engineering documents may be
transferred to Controlled items. When those commercially purchased items are to be
used for Controlled production they shall be re-evaluated to controlled stock by being
submitted to inspection and re-inspected in accordance with Quality Assurance
Procedures 04QA704001 or 04QA704026.
Non-Conforming items shall be handled in accordance with paragraph 15, “Non-
7.3 Certificates
A Certified Material Test Report (CMTR) shall accompany parts used for pressure
When a Purchase Order requests a Certificate of Conformance/Compliance, the
Conforming Items”, of this section of the manual.
boundary/wetted surfaces or surfaces exposed to the process media. The Receiving
Inspection checklist, 05QA000104, shall be used as a guideline to assure that all
required information is listed on the CMTR. When material to be used on wetted
surfaces, etc. can only be purchased with "typical" test reports or a certificate of
conformance, an independent chemical analysis shall be performed to verify
compliance to the stated specification. Quality Assurance Procedure 04QA704032
gives more detail on the process used for Testing Wetted Surface/or Pressure
Boundary Materials.
certificate shall be reviewed to assure that all information required by the Purchase
Order and applicable Engineering Drawing has been met. The Receiving Inspection
Page 85

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
12
CURRENT
REVISION
L
checklist, 05QA000104, shall be used as a guideline to assure that all required
information is listed on the Certificate.
7.4 Age Sensitive Materials
All age sensitive materials are listed in the Quality System Database; Date Coded
Material, are required to be purchased with the date of manufacture and/or an
expiration date. If codes are used for either date, an interpretation of the code must be
provided. Rotation practices, date code extensions, and internal shelf life policies are
documented in Quality Assurance Procedure 04QA704053.
7.5 Commercial Grade Item (CGI) Dedication
Special consideration must be addressed for Commercial Grade Items intended for
use in Safety-Related, Class 1E product. These include how the product will be used,
critical characteristics for the environment and application, and qualification
maintenance. These issues are addressed in Quality Assurance Procedure
06QA020014 and supported by numerous documents classified by FCI as “Technical
Evaluations”.
Testing methods of Commercial Grade Items are defined in Quality Assurance
Procedure 04QA704071.
7.6 Government or Purchaser Supplied Items
Government or purchaser supplied items are discussed in Quality Assurance
Procedure 04QA704055.
8 IDENTIFICATION AND CONTROL OF ITEMS
8.1 Lot Number Control
Lot Numbers shall be used as traceability and identification methods. These Lot
Numbers establish the means whereby the traceability to processes performed,
material certifications, and receipt inspection is assured. All “Controlled” items shall
be identified by a Lot Number as described in Quality Assurance Procedures
04QA704001, 04QA704026, and 04QA704038 for Receiving, In Process, and Final
Inspections. Labels, Tags, and Decals are exempt from this requirement. When
items require additional processing, the initial Lot Number(s) for each item will be
Page 86

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
13
CURRENT
REVISION
L
identified on a Parts List of the item being processed. The Lot Number shall be
marked on the item by metal stamping, vibro peening, scribing, electro-chem-etching,
or by marking with permanent ink. When the marking of each piece is not practical
or is impossible, other methods such as bagging and tagging shall be used. Care
should be used in choosing a marking method so as not to compromise the integrity
of item(s) being marked. All records associated with an inspection shall be identified
with the inspection Lot Number.
8.2 Serialization
Items submitted to Final Inspection, including complete instruments and all
calibrateable subassemblies intended as spare parts shall be identified by a unique
Serial Number. This Serial Number provides a link between units in the field and
production history documentation at the factory. It also provides a means of
identifying specific calibration and Final Acceptance Test records for each unit
shipped.
Serial Numbers are issued by the FCI Business System.
9 CONTROL OF SPECIAL PROCESSES
9.1 Types of Processes
Special processes include Welding, Coating, Soldering, Non-Destructive Examination
(NDE), Painting, Plating, Passivation, and Brazing. When special processes are
brought in-house, a review shall be conducted to determine applicable specification
compliance as well as training and certification requirements.
Welding and Soldering are performed in-house. Measuring & Test Equipment used
to control or verify quality of special processes shall be in accordance with Paragraph
12, “Control of Measuring & Test Equipment”, of this section of the manual.
Weld filler metal used for special processes shall be controlled to assure only
accepted and correct items are used, and to assure that the filler metal is kept clean
and free of contamination. Incoming Weld Fillers shall be processed through
Receiving Inspection in accordance with Quality Assurance Procedure 04QA704021,
Weld Filler Metal Control”, and certified as directed in Paragraph 7, “Control of
Purchased Material, Equipment, and Services”, of this section of the manual.
Special processes performed by outside vendors (NDE, Painting, Plating, Passivation,
and Brazing) are performed in accordance with applicable specifications documented
on the Purchase Order or applicable drawings. Special Processes shall be processed
on Controlled Purchased Orders in accordance with Quality Assurance Procedure
04QA704003. Items sent out for special processing shall pass through Receiving
Page 87

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
14
CURRENT
REVISION
L
Inspection where the item and the processing certification shall be reviewed for
conformance to the required specifications. The Receiving Inspection Checklist,
05QA000104, shall be used as a guideline to assure that all required information is
contained on the Certification.
9.2 Personnel Qualifications
Vendors performing NDE shall have personnel qualified to SNT-TC-1A perform the
work.
Welders and Welding Procedures shall be qualified and their records shall be
maintained in accordance with Quality Assurance Procedure 04QA704039.
Soldering personnel shall be certified to FCI Workmanship Standards Manual,
04QA704057 or J Standard, as a minimum requirement. Personnel shall be trained
and certified to other contractually specified requirements as necessary.
10 INSPECTION
10.1 Areas of Inspection
FCI‟s inspection activities are divided into three categories: Receiving, In-Process,
and Final Inspection. Receiving Inspection shall be performed in accordance with
Quality Assurance Procedure 04QA704001, Quality Assurance Procedure
04QA704026 shall be used for In-Process Inspection, and Quality Assurance
Procedure 04QA704038 for Final Inspection. These procedures contain inspection
methods, and acceptance and rejection criteria necessary to verify and document
conformance to Quality, Engineering, and Customer requirements.
10.2 Inspection Plan
Receiving Inspections shall be performed by using sampling plans in accordance with
10.3 Inspection Personnel
Properly trained Inspectors shall perform inspections. Training and qualification
Quality Assurance Procedure 04QA704001. Depending on the complexity of the
items being inspected, In-Process Inspections are performed per a sampling plan or
performed 100%. Final Inspections shall be performed 100%.
records for Inspection personnel shall be kept on file per Quality Assurance
Procedure 04QA704034.
Page 88

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
15
CURRENT
REVISION
L
Personnel other than those who performed the activity being inspected shall perform
inspection activities.
10.4 Inspection Hold Points
Mandatory hold or witness points required by the customer shall be listed on the
Order Verification document and Job Order as noted in Paragraph 5 “Instructions,
Procedures, and Drawings”, of this section of the manual. Work shall not proceed
past individual hold points unless authorized and initialed by the assigned Contract
Manager.
11 TEST CONTROL
Tests performed on items during fabrication, final assembly and inspection are
controlled through documents such as Operation Sheets, Process Sheets, Test
Procedures, and Final Acceptance Test Procedures. These documents describe the
test equipment to be used, environmental constraints, and instructions for properly
conducting the tests. These documents may also include instructions for documenting
test results, and the calibration control numbers of the actual test equipment used to
perform the test. They may also include acceptance and or rejection criteria. When
acceptance or rejection criteria are not identified specifically in the test document,
this information shall be obtained from the Job Order or project Order Verification
package. A more complete description of these documents can be found in Paragraph
5, “Instructions, Procedures and Drawings” of this section of the manual. The above
listed documents shall be reviewed and approved by the Quality Assurance
Department before implementation, as required by Paragraph 6, “Document Control”
of this section of the manual.
12 CONTROL OF MEASURING AND TEST EQUIPMENT
12.1 Calibration Traceability
Measuring and Test Equipment used to determine product acceptance shall be
12.2 Personnel Qualification
controlled and calibrated in accordance with Quality Assurance Procedure
04QA704006. Calibrations performed in-house or by outside vendors shall be
traceable to the National Institute of Standards and Technology (NIST) or other
recognized standard. All equipment entered into the calibration program shall be
assigned a unique Calibration Control Number (CCN).
Page 89
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
16
CURRENT
REVISION
L
Calibrations of Measuring & Test Equipment shall be performed at the Company by
qualified personnel. Records of the qualifications of calibration personnel shall be
kept on file by the Quality Assurance Department in accordance with Quality
Assurance Procedure 04QA704034.
12.3 Equipment Calibration Status
Measuring & Test Equipment shall have the current calibration status marked on the
instrument. Where the marking of the instrument is impractical, a container shall be
provided and marked with the instrument's calibration status. Whenever the
instrument is not in use, it shall be stored in a marked container.
“Out of Service” and “Out of Calibration” Measuring & Test Equipment shall be so
marked or segregated from regularly available equipment. Items found to be past due
for calibration or damaged during service shall be immediately removed from service,
identified as to its condition, and returned to the Metrology Department.
Measuring & Test Equipment used to perform Final Acceptance Tests and Final
Inspections found out of tolerance shall cause the test and inspection results to be
reevaluated to determine if retest is necessary. If any other Measuring & Test
Equipment was calibrated by the out of tolerance Measuring & Test Equipment, it
shall also be reevaluated to determine if re-calibration is necessary. For more details,
please refer to Quality Assurance Procedure 04QA704029.
13 HANDLING, STORAGE, AND SHIPPING
13.1 In-Process Handling and Storage
Items shall be handled and stored using good commercial practices to protect them
13.2 Final Cleaning
Unless otherwise specified by contract, items to be supplied to the customer shall be
13.3 Packaging
from contamination, damage, or loss of identification and traceability.
cleaned prior to packaging in accordance with Quality Assurance Procedure
04QA704019.
Page 90
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
17
CURRENT
REVISION
L
Unless otherwise specified by contract, Quality Assurance Procedure 04QA704018
shall be used.
Special packaging and shipping requirements or requirements in addition to those
listed above shall be identified on the Order Verification package specific to the
contract.
13.3.1 Special Packaging
When ANSI N45.2.2 compliance is required by contract, Quality Assurance
Procedure 04QA704010 shall be used as a guideline.
13.3.1.a Inspection
Prior to shipment, Inspection personnel shall review the packaged equipment to
assure that all quality and contractual requirements have been met. The Inspector
shall stamp the appropriate area on the Inspection Checklist to signify acceptance of
the packaging and preparation for shipment.
14 INSPECTION, TEST, AND OPERATING STATUS
14.1 Test Status Identification
Inspection, test, and operating status are identified through the use of stampings and
data recorded on Job Orders, Test Reports, Inspection Records, and the like.
14.2 Non-Conforming Items
Non-conforming items are immediately removed from regular production, as
described in Paragraph 15, "Non-Conforming Items" of this section of the manual.
14.3 Stamp Control
Weld, Production, Test, and Inspection personnel are provided unique stamps that are
used to identify records where they have entered information, test data, inspection
data or where they have performed a specific assembly activity. The types of stamps
and how they are controlled is explained in Quality Assurance Procedure
04QA704024.
15 NON-CONFORMING ITEMS
Page 91
DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
18
CURRENT
REVISION
L
Items removed from regular production flow due to a nonconformance or discrepancy
shall have the problem outlined on a Discrepancy Report (DR) as described below
and in Quality Assurance Procedure 04QA704004.
15.1 Discrepancy Report Review
The initial review of the discrepant item shall be made by the Inspector to determine
what corrective action is to be taken or if further review is necessary. Additional
review may be accomplished through submittal of the Discrepancy Report to the
Quality Assurance Manager, or to the Material Review Board.
Discrepant items shall be dispositioned per Quality Assurance Procedure
04QA704004.
Technical justification for the acceptability of items dispositioned "Repair" or "Use
As Is" shall be documented.
15.2 Customer Notification
Non-Conformances dispositioned "Repair" or "Use As Is" which result in a deviation
from the customer's specification or from customer approved documents shall be
submitted by the Contract Manager to the customer for review and approval prior to
implementation of the corrective action.
15.3 10 CFR 21 Compliant
When imposed by contract, non-conformances determined to be subject to 10 CFR 21
16 CORRECTIVE ACTION
16.1 Adverse Conditions
When conditions adverse to quality are discovered (i.e., failures, deficiencies,
shall be processed in accordance with Quality Assurance Procedure 04QA704011. A
copy of 10CFR21, Section 206 of the Energy Reorganization Act of 1974 and Quality
Assurance Procedure 04QA704011 shall be posted in a conspicuous location on the
premises.
deviations, defective material or equipment), steps shall be taken to correct the
condition. Correction may be accomplished through, but is not limited to,
Discrepancy Reports, Material Review Board action, Internal Audit Reports, and the
Corrective and Preventive Action procedure.
Page 92

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
19
CURRENT
REVISION
L
16.2 Significant Conditions
Significant conditions adverse to quality as determined by the Quality Assurance
Manager shall be addressed in a memo to the President, a CPAR per 04QA 704083 or
if discovered during an internal audit, on an Audit Finding Report, with the following
information included:
A description and/or cause of the condition.
Recommended corrective action to be taken to eliminate the condition and
preclude repetition.
Proposed follow-up action to verify implementation of the corrective action.
When determined necessary by the Quality Assurance Manager, copies of the memo
shall be distributed to management personnel responsible for the area affected.
16.3 Document Non-Conformances
Document Change Requests (DCR's) are used to initiate changes to drawings and
other documents found necessary during Production or Inspection activities. They
may be used in conjunction with a Discrepancy Report as a vehicle to notify
Engineering that a document change is needed as part of a discrepancy corrective
action. DCR's may be generated by anyone discovering a document-related error.
16.4 Product Non-Conformances
Non-conformances related to product are best documented on Discrepancy Reports as
described in Paragraph 15, Non-Conforming Items”, of this section of the Manual.
The Corrective Action section is used to initiate actions that will preclude the product
nonconformance from recurring.
17 QUALITY ASSURANCE RECORDS
Quality records are maintained to demonstrate conformance to specified requirements
and the effective operation of the quality system.
Documented procedures describe the identification, collection, indexing, accessing,
filing, storage, maintenance, and disposition of quality records.
Quality records are stored to minimize deterioration and damage and prevent loss;
they are legible, identifiable and readily retrievable.
The Quality Assurance Department shall process and maintain Quality records per
Quality Assurance Procedures 04QA704007 and the Quality System Database;
Quality Records.
Page 93

DOCUMENT NUMBER
07QA070003
SUBJECT
Quality Manual
CAGE CODE
64818
RELEASE DATE
10-8-2012
SECTION
3
PAGE
20
CURRENT
REVISION
L
18 AUDITS
18.1 Internal Audits
An internal audit of the FCI Quality Assurance Program shall be performed per
Quality Assurance Procedure 04QA704008. This audit shall cover all sections of the
Quality Manual and applicable addendums. An Audit Plan shall be issued for each
Internal Audit. The Audit Plan will describe the audit team, audit timetable, and audit
assignments.
A report to Management (including the President) shall be issued at the end of the
audit describing the audit results. The report shall include copies of all Audit
Findings and discuss the status, adequacy, and effectiveness of the Quality System.
See Quality Assurance Procedure 04QA704008 for detailed instructions.
18.2 External Audits
FCI shall perform audits of vendors providing nuclear qualified items. The scope and
content of the audit shall be in accordance with Quality Assurance Procedure
04QA704008.
18.3 Vendor Surveys
The Company shall perform surveys of vendors providing calibration services and
testing services. Surveys of special process vendors shall be performed when
determined necessary by the Quality Assurance Manager. These surveys shall be
performed to determine the vendor‟s capability in complying with FCI contractual
requirements or determining continued compliance to contractual requirements. The
scope and content of the survey shall be in accordance with Quality Assurance
Procedure 04QA704044. Vendors surveyed shall also be evaluated for Critical Safety
Characteristics of their service.
18.4 Auditor Qualification
The audit or survey shall be conducted by a qualified Lead Auditor, and can be
assisted by other qualified Auditors. Auditors shall not have direct responsibility for
the activities being audited.
Auditor qualifications shall be documented and shall conform to the requirements of
Quality Assurance Procedure 04QA704054.
Page 94

FLUID COMPONENTS
®
Company Headquarters: 1755 La Costa Meadows Drive, San Marcos, CA 92078-5115
INTERNATIONAL LLC
DOCUMENT NUMBER
04QA704105
SUBJECT
Return to Service for Aerospace
CAGE CODE
64818
DATE
5/7/2013
SECTION
PAGE
6
REVISION
NC
PMA Parts
Appendix A
Table of Persons Authorized for Return to Service
Personnel Authorized for Return to Service
The personnel listed below are authorized to perform Return to Service functions in accordance
with FLUID COMPONENTS INTERNATIONAL LLC Return to Service for Aerospace PMA
Parts 04QA704105. This authority also extends to the issuance of FAA Form 8130-3, and
signatory authority for Blocks 19 through 23 of that form for Products, Parts and Appliances
manufactured by FLUID COMPONENTS INTERNATIONAL LLC in accordance with 14 CFR
Part 21 § 21.303 and rebuilt in accordance with 14 CFR Part 43 § 43.3 (j) and FLUID
COMPONENTS INTERNATIONAL LLC, Return to Service for Aerospace PMA Parts,
04QA704105.
1. Dana Wollter, Inspecto
ignature,
Date
A Copy of this Roster with Original Signatures will be maintained with the Quality Manual.
This document is considered to be "UNCONTROLLED" when printed!
 Loading...
Loading...