Page 1
Micro Motion™ Corrosion Guide
H2SO
4
HCl
NaOH
HNO
3
C6H8O
7
CH
4
Cl
2
H3PO
4
C3H8O
Coriolis flow meters, density meters, and viscosity meters
User Guide
GI-00415, Rev I
October 2020
Page 2
Safety and approval information
This Micro Motion product complies with all applicable European directives when properly installed in accordance with the
instructions in this manual. Refer to the EU declaration of conformity for directives that apply to this product. The EU declaration
of conformity, with all applicable European directives, and the complete ATEX Installation Drawings and Instructions are available
on the internet at www.emerson.com or through your local Micro Motion support center.
Information affixed to equipment that complies with the Pressure Equipment Directive, can be found on the internet at
www.emerson.com.
For hazardous installations in Europe, refer to standard EN 60079-14 if national standards do not apply.
Other information
Full product specifications can be found in the product data sheet. Troubleshooting information can be found in the configuration
manual. Product data sheets and manuals are available from the Micro Motion web site at www.emerson.com.
Return policy
Follow Micro Motion procedures when returning equipment. These procedures ensure legal compliance with government
transportation agencies and help provide a safe working environment for Micro Motion employees. Micro Motion will not accept
your returned equipment if you fail to follow Micro Motion procedures.
Return procedures and forms are available on our web support site at www.emerson.com, or by phoning the Micro Motion
Customer Service department.
Emerson Flow customer service
Email:
• Worldwide: flow.support@emerson.com
• Asia-Pacific: APflow.support@emerson.com
Telephone:
North and South America
United States 800-522-6277 U.K. and Ireland 0870 240 1978 Australia 800 158 727
Canada +1 303-527-5200 The Netherlands +31 (0) 70 413
Mexico +52 55 5809 5300 France +33 (0) 800 917
Argentina +54 11 4809 2700 Germany 0800 182 5347 Pakistan 888 550 2682
Brazil +55 15 3413 8000 Italy +39 8008 77334 China +86 21 2892 9000
Chile +56 2 2928 4800 Central & Eastern +41 (0) 41 7686
Peru +51 15190130 Russia/CIS +7 495 995 9559 South Korea +82 2 3438 4600
Europe and Middle East Asia Pacific
New Zealand 099 128 804
6666
India 800 440 1468
901
Japan +81 3 5769 6803
111
Egypt 0800 000 0015 Singapore +65 6 777 8211
Oman 800 70101 Thailand 001 800 441 6426
Qatar 431 0044 Malaysia 800 814 008
Kuwait 663 299 01
South Africa 800 991 390
Saudi Arabia 800 844 9564
UAE 800 0444 0684
2
Page 3

User Guide Contents
GI-00415 October 2020
Contents
Chapter 1 Before you begin........................................................................................................5
Chapter 2 Meters and corrosion................................................................................................. 7
2.1 General corrosion vs. localized corrosion..................................................................................... 7
2.2 Coriolis flow and density meters.................................................................................................. 7
2.3 Fork density and viscosity meters.................................................................................................8
2.4 GDM and SGM density and viscosity meters ................................................................................ 8
Chapter 3 Chemical compositions and meter compatibility........................................................ 9
3.1 Halogens..................................................................................................................................... 9
3.2 pH............................................................................................................................................. 10
3.3 Chemical potential measurements............................................................................................ 11
Chapter 4 Materials that mitigate corrosion.............................................................................13
4.1 Tefzel.........................................................................................................................................13
4.2 Super duplex stainless steel....................................................................................................... 13
Chapter 5 Mixed material in meters......................................................................................... 15
5.1 Reasons to mix materials........................................................................................................... 15
5.2 Meter parts and materials.......................................................................................................... 15
5.3 Condensate............................................................................................................................... 16
5.4 Methane, ethane, propane, and ethylene.................................................................................. 17
5.5 Nitrogen and argon gases.......................................................................................................... 17
5.6 Natural and petroleum gases..................................................................................................... 17
5.7 Produced water......................................................................................................................... 17
5.8 Process water............................................................................................................................ 17
Chapter 6 Typical chemical applications................................................................................... 19
6.1 Hydrochloric acid ...................................................................................................................... 19
6.2 Sodium hydroxide......................................................................................................................19
6.3 Nitric acid.................................................................................................................................. 20
6.4 Sulfuric acid............................................................................................................................... 20
Chapter 7 Material compatibility tables................................................................................... 23
7.1 A chemicals............................................................................................................................... 24
7.2 B chemical tables....................................................................................................................... 36
7.3 C chemical tables.......................................................................................................................40
7.4 D chemical tables.......................................................................................................................47
7.5 E chemical tables....................................................................................................................... 52
7.6 F chemical tables....................................................................................................................... 54
7.7 G chemical tables.......................................................................................................................57
7.8 H chemical tables.......................................................................................................................57
User Guide 3
Page 4

Contents User Guide
October 2020 GI-00415
7.9 I chemical tables........................................................................................................................ 61
7.10 J chemical tables...................................................................................................................... 62
7.11 K chemical tables.....................................................................................................................62
7.12 L chemical tables..................................................................................................................... 63
7.13 M chemical tables.................................................................................................................... 65
7.14 N chemical tables.....................................................................................................................69
7.15 O chemical tables.................................................................................................................... 72
7.16 P chemical tables..................................................................................................................... 73
7.17 Q chemical tables.................................................................................................................... 79
7.18 R chemical tables.....................................................................................................................79
7.19 S chemical tables..................................................................................................................... 80
7.20 T chemical tables..................................................................................................................... 89
7.21 U chemical tables.....................................................................................................................94
7.22 V chemical tables.....................................................................................................................94
7.23 W chemical tables....................................................................................................................96
7.24 X chemical tables.....................................................................................................................97
7.25 Y chemical tables.....................................................................................................................97
7.26 Z chemical tables.....................................................................................................................98
4 Micro Motion Corrosion Guide
Page 5

User Guide Before you begin
GI-00415 October 2020
1 Before you begin
Use this document as a pre sales document to help you select the correct material for Micro Motion meters
that measure corrosive chemicals.
The information in this document assumes that users understand all corporate and government safety
standards and requirements that guard against injuries and death.
The guidelines in this publication are only for information. Minor changes in fluid properties (for example;
temperature, concentration, and impurity levels) can affect the compatibility of wetted parts. Material
compatibility choices are solely the responsibility of the end user.
User Guide 5
Page 6

Before you begin User Guide
October 2020 GI-00415
6 Micro Motion Corrosion Guide
Page 7
User Guide Meters and corrosion
GI-00415 October 2020
2 Meters and corrosion
Choosing the correct meter material requires more consideration than choosing the correct pressurecontaining pipe. Material compatibility for a pressure-containing pipe is covered in any general corrosion
guide.
2.1 General corrosion vs. localized corrosion
General corrosion
General corrosion refers to the uniform loss of material. Material loss due to corrosion is expressed in terms of
inches or millimeters lost per year. These rates are determined by exposing a sample to the environment for a
specific time period. Weight loss or dimensional changes are then used to determine the corrosion rate.
General corrosion tests cannot detect localized corrosion and are inadequate for determining material
compatibility for Micro Motion meters.
Localized corrosion
Localized corrosion consists of pitting, inter-granular attack, stress corrosion cracking, and corrosion fatigue.
Localized corrosion of the flow tube can initiate fatigue cracking. Meter failure occurs when fatigue cracks
propagate rapidly. Avoid the onset of fatigue cracks by selecting the appropriate wetted materials using this
guide.
Material compatibility cannot always be assessed by considering the alloys selected for the remainder of the
piping system. Material compatibility for most piping systems is based upon general corrosion rates alone and
does not account for localized corrosion or cyclic loading. Coriolis meters require vibration of one or two flow
tubes to make a mass flow or density measurement. The cyclic loading condition is inherent to all Coriolis
meters and must be considered in the material selection process.
2.2 Coriolis flow and density meters
Coriolis meters are as reliable in measuring corrosive chemicals as they are in measuring noncorrosive fluids.
This reliability requires that corrosive fluids are compatible with the meter construction material.
In order to provide compatible meter construction for every application, Micro Motion manufactures meters
in the following materials:
• 316L, 304L, and super duplex stainless steels
• 316L stainless steel lined with Tefzel® coating
• Nickel alloy C22
• Titanium
• Tantalum
Environments
Typical 316L stainless steel
Nitric acid 304L stainless steel
Oilfield chlorides and CO
User Guide 7
2
Meter material
Super duplex stainless steels
Page 8
Meters and corrosion User Guide
October 2020 GI-00415
Environments Meter material
Aqueous fluorine 316L stainless steel lined with Tefzel coating
Corrosive process fluids Nickel alloy C22
Chlorides Titanium
Extreme high temperatures, low PH, or high chloride
concentrations
Tantalum
2.3 Fork density and viscosity meters
Micro Motion manufactures fork density and viscosity meters in a variety of wetted materials including 316L
and 304L stainless steels, nickel alloy C22, titanium, and zirconium.
The specific material compatibility recommendations for fork meters can vary from Coriolis meters. Use the
tables specifically for fork meters in the Material compatibility tables.
2.4 GDM and SGM density and viscosity meters
Gas Density Meters (GDM) and Gas Specific Gravity Meters (SGM) are not listed in the material compatibility
tables. For any implementation questions, contact customer support.
Process gases must be dry (above their dew point), clean, and compatible with Ni-Span-C Alloy 902 and 316L
stainless steel. Ideal gases include natural gas, hydrogen, methane, propane, etc. Heat may be applied and/or
a coalescing filter may be installed in some applications to reduce the presence of liquids that can damage the
meters.
SGMs
• Can be used in refinery and fuel gas applications. Fluids with a molecular weight of pentane and higher are
generally in liquid form and will have to be removed from the process stream by equipment that removes
liquid.
• Are not recommended for use with hydrogen sulfide (H2S), except for low concentrations of hydrogen
sulfide in which all of the water and moisture has been removed.
GDMs
The pressure-containing components of GDM meters are NACE compliant. Low concentrations of hydrogen
sulfide (H2S) are permitted (less than 1000 ppm), provided the process gas is clean and dry. Install a
coalescing filter into the GDM's process line.
Hydrogen sulfide wells
Do not use a GDM nor an SGM in sour gas (hydrogen sulfide-containing) wells.
8 Micro Motion Corrosion Guide
Page 9

A
B
SS C22 Ti
Ta
User Guide Chemical compositions and meter compatibility
GI-00415 October 2020
3 Chemical compositions and meter compatibility
The variety of possible meter environments make it difficult to define fluid compatibility for every possible
material combination. Nevertheless, you can choose the best material by comparing alloy limitations based
on the chemical composition of your fluids. The chemical composition of most environments can be
characterized by the following variables:
• Halogen concentration
• pH
• Chemical potential
• Temperature
The following topics show the levels of acceptable performance for 316L stainless steel, nickel alloy C22,
titanium, and tantalum. You can characterize the effect of temperature on meter life by considering its effect
on the other three variables.
3.1 Halogens
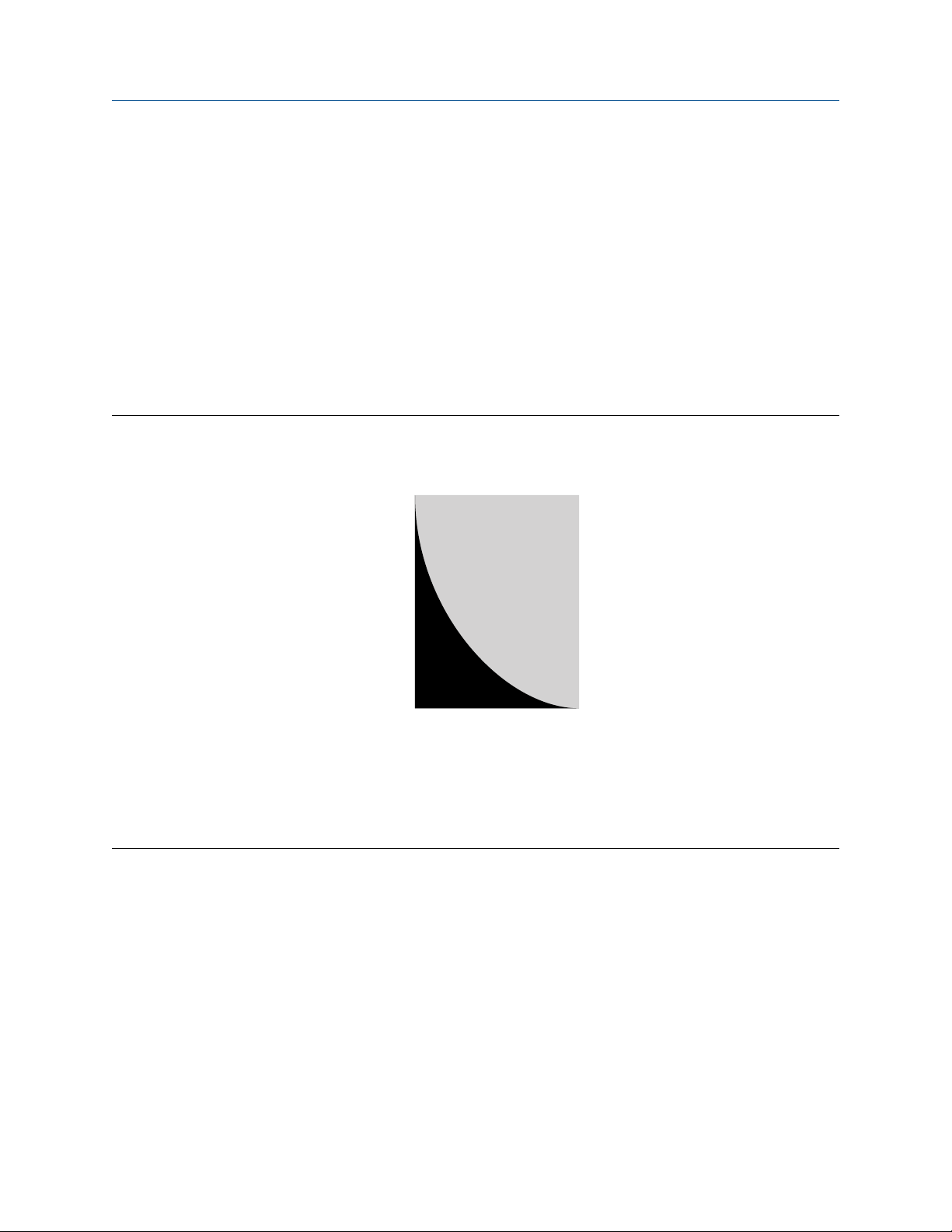
Halogen refers to a group of elements that includes chlorine, fluorine, bromine, and iodine. The most
common halogen is chlorine. The presence of the ionic chloride form, Cl¯, even as a contaminant, can be
detrimental to corrosion resistance. Stainless steels are particularly susceptible in the oxygen-saturated
conditions typically found in chemical processing facilities. Meters constructed of 316L stainless steel have
been reliable in numerous applications where chloride and oxygen concentrations can be maintained at
sufficiently low levels or where free chlorides are absent (see the following figure). Stainless steel can also be
used in organic solutions that contain a chloride component, provided ion formation is avoided. Two factors
that influence dissociation are temperature and moisture.
Figure 3-1: Typical chloride concentration range for meter materials
A. High
B. Low
User Guide 9
Page 10
A
B
C
D
0 40 80 120 160 200
20
30
40
50
60
Chemical compositions and meter compatibility User Guide
October 2020 GI-00415
Abbreviations
• SS = stainless steel
• C22 = nickel alloy C22
• Ti = titanium
• Ta = tantalum
Temperature and moisture need to be kept low to avoid failure. The following figure shows that the
resistance of 316L to free chloride-induced corrosion fatigue is temperature dependent. Low combinations of
temperature and chloride concentration are compatible with 316L stainless steel. Pitting and corrosion
fatigue are possible for higher combinations of temperature and chloride concentrations. Nickel alloy C22
should be used when these conditions exist. If the chloride content is increased further and pH lowered, nickel
alloy C22 may also succumb to localized attack and corrosion fatigue.
Figure 3-2: Chloride ion concentrations and temperature limits for 316L under oxygen-saturated
conditions
A. Temperature °C
B. Chloride (ppm)
C. Use high nickel-based alloy C22
D. Use 316L stainless steel
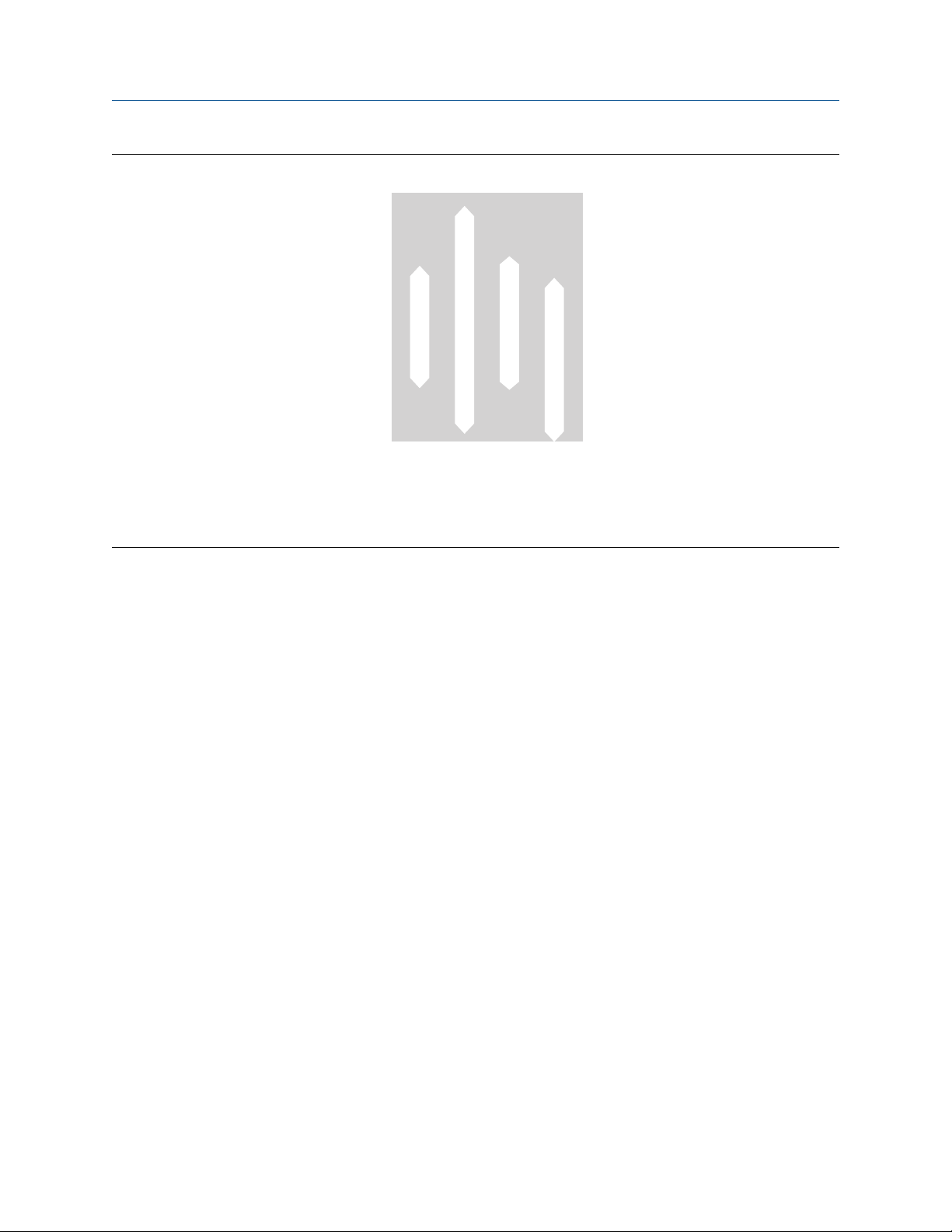
3.2 pH
The pH of a solution can also alter the corrosion of any alloy. In general, solutions that have a neutral pH (near
7) have a slower corrosion rate than strongly acidic (pH < 3) or strongly alkaline (pH > 11) solutions (see the
following figure). Tantalum has superior corrosion resistance to 316L stainless steel and nickel alloy C22 in
neutral and acidic environments. However, high corrosion rates will occur if tantalum is used in alkaline
caustic applications such as sodium hydroxide, even at room temperature. At higher temperatures, stress
corrosion cracking and corrosion fatigue of 316L stainless steel are possible. Under these conditions, nickel
alloy C22 is recommended. Use nickel alloy C22 in all caustic applications when there is a possibility of
chloride contamination.
10 Micro Motion Corrosion Guide
Page 11
A
B
SS C22 Ti
Ta
C
User Guide Chemical compositions and meter compatibility
GI-00415 October 2020
Figure 3-3: Typical pH range for meter material
A. High
B. Neutral
C. Low
Abbreviations
• SS = stainless steel
• C22 = nickel alloy C22
• Ti = titanium
• Ta = tantalum
3.3 Chemical potential measurements
Chemical potential measures the oxidizing or reducing power of a process fluid. Chemical potential,
sometimes referred to as redox potential, is defined relative to the H2 -> 2H+ + 2e– half reaction, which is
assigned a value of zero volts. Any environment that has a chemical potential greater than the reference is
considered oxidizing. Chemical potentials that are equal to or less than the reference are considered
reducing. Chemical potential is important because a minimum amount of oxidizing power is required to
enable the formation of protective surface oxide layers. Optimal life will be realized as long as this layer is
stable. Environments that are too oxidizing or reducing will prevent stable oxide formation. Under such
conditions, failure due to corrosion fatigue or erosion/corrosion is possible.
The corrosion fatigue resistance of a material of construction is related to the range of chemical potentials
over which oxide layer stability is maintained. The broader the range in the following figure, the more
environments where the material will resist corrosion.
User Guide 11
Page 12

A
SS C22 Ti
Ta
B
Chemical compositions and meter compatibility User Guide
October 2020 GI-00415
Figure 3-4: Potential chemical range for meter materials
A. Reducing
B. Oxidizing
Abbreviations
• SS = stainless steel
• C22 = nickel alloy C22
• Ti = titanium
• Ta = tantalum
Tantalum pentoxide (Ta2O5) is stable on the surface of metallic tantalum at low reducing potentials. This
oxide also resists breakdown in all but the most oxidizing environments.
The wide range of chemical potentials over which passivity is maintained make tantalum resistant to most,
but not all, corrosive fluids. Hydrofluoric acid, oleum, and caustics are some of the exceptions that can
corrode tantalum. The second most stable oxide forms on the surface of nickel-based alloys, such as nickel
alloy C22. A high chromium and molybdenum content stabilizes the oxide layer, yielding improved
performance over 316L stainless steel in chloride bearing applications. 316L stainless steel exhibits passivity
over a narrow range, as compared to the other two materials. However, 316L stainless steel is suitable for a
large number of chemical processing applications.
12 Micro Motion Corrosion Guide
Page 13

User Guide Materials that mitigate corrosion
GI-00415 October 2020
4 Materials that mitigate corrosion
4.1 Tefzel
Some applications cause high corrosion to all metallic components. Process fluids containing fluorine will
rapidly corrode any metal. For example, hydrofluoric acid can be a contaminant in low quality grades of
hydrochloric and phosphoric acids. Meters using metallic materials, including 316L stainless steel, nickel alloy
C22, and tantalum, will have short lives in aqueous fluorine applications. You can avoid premature meter
failure by checking the process stream for aqueous fluorine. If low concentrations are unavoidable, you can
use a Coriolis meter lined with Tefzel. Tefzel is similar to Teflon® in both physical properties and corrosion
resistance. The Tefzel lining acts as a barrier that prevents the process fluid from coming in contact with the
underlying metal and causing corrosion cracking. However, Tefzel is not immune from corrosion. Strong acids
and strong bases will make Tefzel brittle. Certain organic chemicals can permeate through the liner over time
and temperatures can influence the mechanical strength of Tefzel. For this reason, Tefzel-lined instruments
are limited to applications where the temperature is less than 248 °F (120 °C). Because the Tefzel lining and
the 316L stainless steel flow tubes have different coefficients of thermal expansion, special temperature
considerations apply. Tefzel-lined meters have a maximum allowable rate of meter temperature change
equal to 30 °F/h (17 °C/h) and should not be exposed to temperatures below 32 °F (0 °C).
4.2 Super duplex stainless steel
For high capacity applications, super duplex stainless steel is an option when a 316L stainless meter is not
compatible. Super duplex combines higher strength and better chloride corrosion resistance than 316L,
making large meters usable for more demanding conditions. Higher strength allows use at higher operating
pressures, and better chloride resistance allows use with higher chloride contents at higher process
temperatures.
The oil and gas industry uses super duplex stainless steel in moderate temperature applications containing
levels of chlorides and CO2 too high for 316L stainless. However, sour conditions with elemental sulfur or a
H2S partial pressure over 3 psia (0.21 bara) can cause corrosion problems. Consider the total process
environment when selecting the best construction materials. For recommendations, contact Micro Motion
with complete process conditions, including fluid temperature, pressure, bubble point, pH, amounts of
chlorides, oxygen, H2S, CO2, bicarbonates, water, and elemental sulfur.
The wetted components of a super duplex meter that contact the process fluid are made from alloys 2507
and CE3MN (2507 equivalent). Both alloys have a two-phase structure of austenite and ferrite, which is the
source of the duplex name. Due to the ferrite content of super duplex, avoid cryogenic applications.
User Guide 13
Page 14

Materials that mitigate corrosion User Guide
October 2020 GI-00415
14 Micro Motion Corrosion Guide
Page 15
User Guide Mixed material in meters
GI-00415 October 2020
5 Mixed material in meters
A meter that consists of two materials is called a bi-metallic meter.
Important
Micro Motion's policy states that when selecting materials for a bi-metallic meter, all materials must meet all
the recommendations in this guide.
All CMF400P orders are referred to the Micro Motion metallurgy department for alloy approval.
5.1 Reasons to mix materials
Typically, bi-metallic meters are used for high-pressure applications.
Example 1
The CMF010P sensor has a higher strength nickel alloy C22 tube for high pressure applications and stainless
steel process connections that are less compatible with aggressive environments. This option should be used
only in environments compatible with stainless steel, the less resilient material.
Example 2
For a CMF400P sensor with a 900# SS process connection for a higher pressure rating, use only in
environments compatible with stainless steel.
For most applications, nickel alloy C22 has better corrosion resistance than stainless steel. One exception is
nitric acid, where 304 stainless steel has better corrosion resistance.
5.2 Meter parts and materials
A meter is comprised of three main components that contact the process fluid, known as wetted components.
Component
Tubes • 316L stainless steel
Manifolds • CF-3M (equivalent to 316L stainless steel)
Process connections and adapters • 316L stainless steel
316L is a common stainless steel alloy with corrosion resistance to a variety of process fluids. C22 is more
resistant to Chloride-induced Stress Corrosion Cracking (CSCC).
Available in the following material
• Nickel, chromium, and molybdenum alloy, such as C22
• CW-2M (equivalent to a nickel, chromium, and molybdenum alloy)
• Nickel, chromium, and molybdenum alloy, such as C22
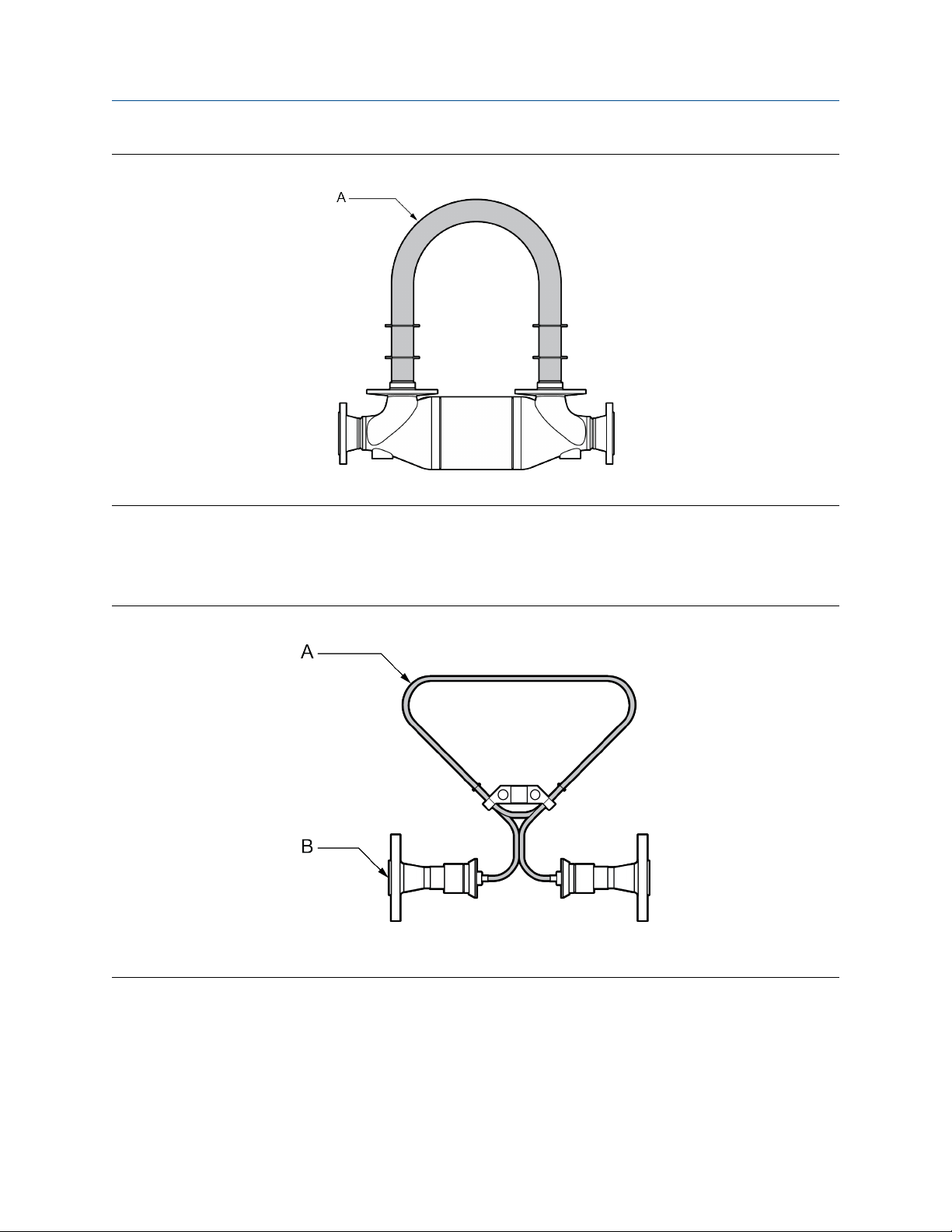
The following figure shows the inside of a CMF400P sensor where the tubes are made from C22 for high
pressure applications. The process connections are made from 900# stainless steel for a higher pressure
rating. Use this option only for environments that are compatible with the process connection material
(316L), the least resilient material.
User Guide 15
Page 16
Mixed material in meters User Guide
October 2020 GI-00415
Figure 5-1: CMF400P with flow tubes highlighted
A. C22 flow tubes
The following figure shows the inside of a CMF010P sensor where the tubes are made from C22 for high
pressure applications. The process connections are made from 900# stainless steel for a higher pressure
rating. This option should be used only for environments that are compatible with the process connection
material (316L), the least resilient material.
Figure 5-2: CMF010P tubes and process connections
A. C22 flow tubes
B. Process connection
5.3 Condensate
Water-free petroleum condensates are not corrosive when temperatures are below the threshold for
hydrocarbon cracking. Hydrocarbon cracking occurs during refining at extreme high temperatures. H2S
contained in these hydrocarbons do not attack steel at < 900 °F (482 °C), as long as water is not present. For
16 Micro Motion Corrosion Guide
Page 17

User Guide Mixed material in meters
GI-00415 October 2020
316L and nickel alloy C22, corrosion from H2S without water does not occur until temperatures exceed 900 °F
(482 °C).
However, when water is present, pH, chloride content, water cut, H2S, CO2, dissolved oxygen content,
pressure, and temperature can cause corrosion, pitting, and stress corrosion cracking (SCC) of 316L. See
Produced water.
5.4 Methane, ethane, propane, and ethylene
These hydrocarbons are non-corrosive to stainless steels and nickel alloys, even when water is present (such
as condensed fresh water).
5.5 Nitrogen and argon gases
Nitrogen and argon gases are non-corrosive to stainless steels and nickel alloys and can be used up to the
temperature limits of the meter.
5.6 Natural and petroleum gases
Liquid Natural Gas (LNG) and Liquid Petroleum Gas (LPG) are non-corrosive. One exception is the fracture
toughness of the CF-3M alloy manifold. Since CF-3M contains ferrite, the fracture toughness at -260 °F
(-162 °C) LNG temperatures may not be adequate. For LNG applications, C22 is the best choice for low
temperature impact toughness.
5.7 Produced water
Produced water contains numerous possible compositions of oil and gas. The composition depends on
reservoir conditions, the formation water chemistry, and the amount of H2S and CO2 in the reservoir.
Moreover, these conditions can change over time due to water or CO2 flooding, or if any enhanced oil
recovery methods are applied.
For oil field operations that are less than 400 °F (204 °C), there are no restrictions or limits for nickel alloy C22,
even in the presence of H2S.
However, carefully consider the chemical environment and process conditions before using a stainless steel or
bi-metallic meter.
5.8 Process water
As with produced water, there are multiple possible compositions of process water. Process water can be sea
water with a high chloride content, from the city tap with a low chloride content, or from distilled water
without chlorides.
User Guide 17
Page 18

Mixed material in meters User Guide
October 2020 GI-00415
18 Micro Motion Corrosion Guide
Page 19

User Guide Typical chemical applications
GI-00415 October 2020
6 Typical chemical applications
6.1 Hydrochloric acid
Hydrochloric acid (HCI) in the 1-37% concentration range acts as a reducing agent.
Hydrochloric acid causes severe corrosion due to strong acids combined with chlorine. Tantalum and
zirconium are the few materials resistant to hydrochloric acid's corrosive nature.
Avoid nickel alloy C22 meters with medium to high acid concentrations and at high temperatures, due to loss
of passivity and corrosion in the active state. Tantalum is a better choice in these circumstances.
A zirconium FDM, which is generally compatible with pure hydrochloric acid and water, is susceptible to
corrosion in certain applications containing oxidizing impurities, such as ferric ions (Fe+3) and cupric ions
(Cu+2). Zirconium can succumb to pitting and intergranular corrosion when these impurities are present in
hydrochloric acid solutions. Oxidizing ions can be in the source acid, or they could enter the stream from
corrosion of other components in the system. The material compatibility tables attempt to address these
concerns where possible, but use care selecting meter material when oxidizing impurities are known to be
present in an application.
6.2 Sodium hydroxide
Sodium hydroxide (NaOH) is used to control pH or as a cleaning compound. Due to advanced production
methods, stainless steel can be an appropriate material for sodium hydroxide. Nevertheless, stress corrosion
cracking can occur at high temperatures. If stress corrosion cracking occurs, corrosion fatigue is also possible
depending upon the stress state resulting from the applied loads. Since sodium hydroxide is often mixed with
water containing chlorine, the chlorine may have a greater affect on meter life than the concentration or
temperature of the sodium hydroxide alone.
Sodium hydroxide with and without C1-
Experiments were conducted using a 50% sodium hydroxide solution that was compared to a 50% sodium
hydroxide solution with an additional 2.5% chloride ion (C1-). Electrochemical and corrosion fatigue data were
collected on 316L samples exposed to these solutions. After 4 months of exposure to the solution without
the chloride ion, there was no failure of stainless steel meters. Metallographic analysis showed no indication
of stress corrosion cracking or localized corrosion. A second group of meters exposed to solutions containing
the chloride ion showed corrosion fatigue after 4 days of exposure. The temperature in all cases was 200 °F
(93 °C). Electrochemical tests in these environments indicated the presence of an oxide layer on 316L
surfaces. The passive current density, which is an inverse measure of oxide layer thickness, was 25 times
higher when the chloride ion was present. The higher current density indicates that the chloride ion will
substantially thin the oxide layer, resulting in a higher susceptibility to mechanical damage. This in turn
explains the dramatically lower life shown in corrosion fatigue tests.
High concentrations of sodium hydroxide and high temperatures
Stress corrosion cracking, or corrosion fatigue, is not expected in stainless steel meters exposed to pure
sodium hydroxide solutions where the concentration is less than 50% by weight and the temperature is 200 °F
(93 °C) or lower. Higher concentrations, and especially higher temperatures, could cause failure. Nickel alloy
C22 is recommended under these conditions. Nickel-based alloys (such as nickel alloy C22) should be
resistant to stress corrosion cracking at all concentrations of sodium hydroxide up to the boiling point of the
solution. The presence of the chloride ion can be detrimental to 316L stainless steel meter life. If the presence
of chlorine is possible, use nickel alloy C22 over stainless steel.
User Guide 19
Page 20

Typical chemical applications User Guide
October 2020 GI-00415
Sodium hydroxide in cleaning compounds
Sodium hydroxide is also used as the alkaline component in many standard clean-in-place (CIP) solutions.
These solutions are typical in food and beverage industries and in the life sciences industry. These solutions
are flushed through the meter for varying periods of time and at elevated temperatures. In general, these
solutions have been designed and successfully used in process streams constructed with stainless steel (316L
or 304L). The use of titanium in the aforementioned industries has raised concerns regarding compatibility. In
many cases, titanium is more corrosion resistant than stainless steel. However, in strong bases where the
protective oxide film has a difficult time regenerating, the titanium can be more susceptible to uniform
attacks involving the entire tube.
Important
Consider all potential process fluids passing through a meter when assessing materials.
6.3 Nitric acid
Since nitric acid (HNO3) is a strong oxidizing acid, use alloys that form stable adhering oxide films. In general,
high chromium-containing alloys and strong passivating metals like tantalum are the most resistant.
The most commonly-used material for nitric acid is 304L stainless steel. The corrosion resistance of 304L is
slightly better than 316L, which contains molybdenum.
Corrosion rates increase with higher temperatures and concentrations. Intergranular corrosion can occur
when stainless steel or nickel alloys are sensitized, which means they contain precipitated carbides. Low
carbon grades like 316L and 304L are normally not susceptible to intergranular corrosion.
However, intergranular corrosion can also occur regardless of heat treatment or composition of the alloy if
hexavalent chromium ions are allowed to accumulate in the acid to some critical concentration. Chromium
ion contamination can be in the source acid, or can enter the process stream from the corrosion of stainless
steel tanks and pipes.
Titanium is not compatible with red fuming nitric acid at any temperature.
6.4 Sulfuric acid
Selecting the best material for sulfuric acid applications can be difficult. Applications that appear to be similar
can have drastically different electrochemical properties. For newer applications, or applications where the
risk of fluid release is to be minimized, Micro Motion ELITE™ meters have excellent turndown characteristics
that you can size to reduce fluid velocity in the sensor.
Micro Motion’s Tefzel-lined meters perform well in sulfuric acid (H2SO4) applications up to 98%
concentrations and at temperatures up to 200 °F (93 °C). However, if the process temperature changes at a
rate greater than 30 °F (17 °C) per hour, the liner integrity may degrade. 316L stainless steel meters are best
suited for low temperatures at both low and high concentrations of sulfuric acid. Use nickel-based alloy
meters for slightly higher temperatures and for broader concentration ranges.
316L stainless steel and nickel-based alloys depend on electrochemical passivity for resistance to corrosion in
sulfuric acid. Electrochemical passivity refers to the state of the material’s protective oxide layer. The
material’s protective oxide layer exists in one of following states:
Passive
Active
20 Micro Motion Corrosion Guide
The oxide layer is highly stable and provides the material’s excellent corrosion resistance.
The oxide layer is not stable or protective. The base metal can be exposed, allowing corrosion
to occur.
Page 21

User Guide Typical chemical applications
GI-00415 October 2020
Transpassive
The transpassive state is similar to the active state in that the oxide layer is less stable.
To maximize meter life, maintain the oxide layer in the passive state. However, exposure to sulfuric acid under
varying conditions can cause the passive or stable oxide layer to become active or less stable.
When making the decision to place a 316L stainless steel or nickel-based alloy meter in a sulfuric acid
application, consider all of the following factors. Each of the following factors can impact the stability of the
protective oxide layer.
Concentration
Sulfuric acid can be oxidizing and not aggressively corrosive at diluted concentrations up to about 10–15%. As
concentration increases into the intermediate range, sulfuric acid becomes reducing and considerably more
aggressive. Micro Motion does not recommend 316L stainless steel in the intermediate concentration ranges
of sulfuric acid. However, nickel alloy C22 is more resistant in mildly reducing environments, and are more
applicable in the intermediate concentration range. High acid concentrations are more oxidizing, and less
likely to attack the protective oxide layer as concentration increases.
Temperature
The temperature of the process stream affects the stability of the oxide layer. As temperature increases, the
margin between an active and passive oxide layer lessens. For any sulfuric acid application, lowering the
temperature enhances the stability of the oxide layer.
Velocity
There are no erosive constituents in most sulfuric acid process streams. Yet, sulfuric acid can still erode pipes.
Sulfuric acid in the intermediate and higher concentration range can cause unexpected oscillations in the
oxide layer from passive to active, and then back to passive (and so on).
When the oxide layer is in the less stable active state, the acid can pull the layer into the process stream before
it can make the transition back to the more stable passive state. This forms a passive layer that becomes
active and then gets stripped. Another passive layer forms and the cycle repeats, causing erosion.
Reducing the fluid velocity can lessen the likelihood of the active oxide layer eroding from the material
surface. The compatibility tables include general guidelines for maximum fluid velocity at different
concentrations and temperatures. The velocity recommendations apply to only 316L stainless steel and C22
nickel. Tantalum is less affected by acid velocity.
Velocity recommendations primarily came from data for 316L stainless steel. However, nickel-based alloy
implementations could also benefit from these recommendations. Based on the corrosiveness of sulfuric acid
in the 75%–90% range, maintain fluid velocity as low as possible.
Stabilizing factors
Aeration of the sulfuric acid solution can stabilize the passive oxide layer in both 316L stainless steel and
nickel-based alloys.
Oxidizing impurities such as Fe+++ (ferric), Cu++ (cupric), Sn++++ (stannic), or Ce++++ (cerric) ions in the
process stream stabilize the passive film. In concentrations of sulfuric acid above 97%, SO3 (sulfite) can also
stabilize the passive film. However,halides in sulfuric acid (such as chlorides) can have a detrimental effect on
the stability of the oxide layer.
User Guide 21
Page 22

Typical chemical applications User Guide
October 2020 GI-00415
22 Micro Motion Corrosion Guide
Page 23

User Guide Material compatibility tables
GI-00415 October 2020
7 Material compatibility tables
Chemicals
Chemicals are listed alphabetically under the appropriate chemical names, not under trade names. Trade
names and other commonly-used names are listed in the synonym tables. Consider all fluids and flow
conditions when selecting material. This includes the primary fluid, contaminants, cleaning, and/or other
chemical solutions.
Temperature and concentration
Consider each chemical's various temperature and concentration combinations when choosing material.
In general, lower chemical temperatures reduce the possibility of localized corrosion.
Both high and low chemical concentrations can cause corrosion. Low chemical concentrations can cause
corrosion due to fluid evaporation. Avoid this situation by keeping the meter full at all times. To empty the
meter, completely flush the meter of any residual corrosive.
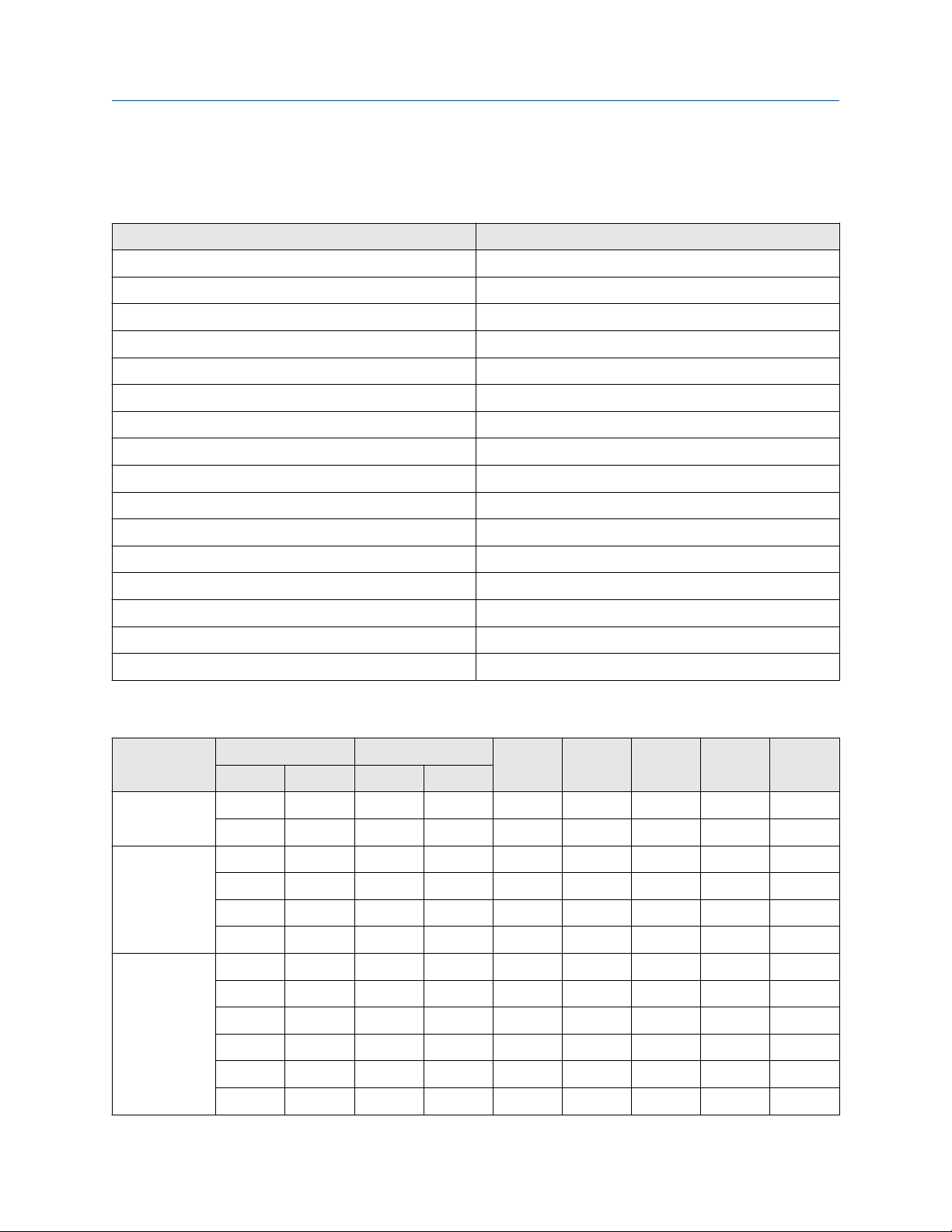
Material compatibility table legend
X
O
—
C
Material is not compatible
Material is compatible
No data is available
Conflicting data
Note
Corrosion data is not always available for the full temperature range of the meter. Materials typically maintain
corrosion resistance at temperatures below the lower limits in the table. Contact Micro Motion if your process
might exceed the maximum temperature limits listed here. Where temperature ranges have been omitted
from the tables, corrosion resistance is believed to be maintained throughout the temperature range of the
meter. For applications that do not appear in this corrosion guide, contact Micro Motion.
Material codes
The material tables have columns that contain the following codes:
304L
316L
C22
SS
304L stainless steel
316L stainless steel
Nickel alloy C22
Stainless steel
Ta
Tantalum
Ti
Titanium (grade 2 for fork meters)
Tz
Tefzel-lined 316L
Zr
Zirconium grade 702
User Guide 23
Page 24
Material compatibility tables User Guide
October 2020 GI-00415
7.1 A chemicals
Table 7-1: Synonyms for A chemicals
Synonym Listed under
Acetic aldehyde Acetaldehyde
Acetic ether Ethyl acetate
Acetic oxide Acetic anhydride
Acetic oxide, acetyl oxide Acetic anhydride
Acetylaldehyde Acetaldehyde
Acetylchloride Acetyl chloride
Acetyl oxide Acetic anhydride
Acryl amide Acrylamide
Actylene tetrachloride Tetrachloroethane
Albone Hydrogen peroxide
Allylic alcohol Allyl alcohol
Amino benzene Aniline
Ammonium hydroxide Ammonia
Ar Argon
Azine Pyridine
Aziotic acid Nitric acid
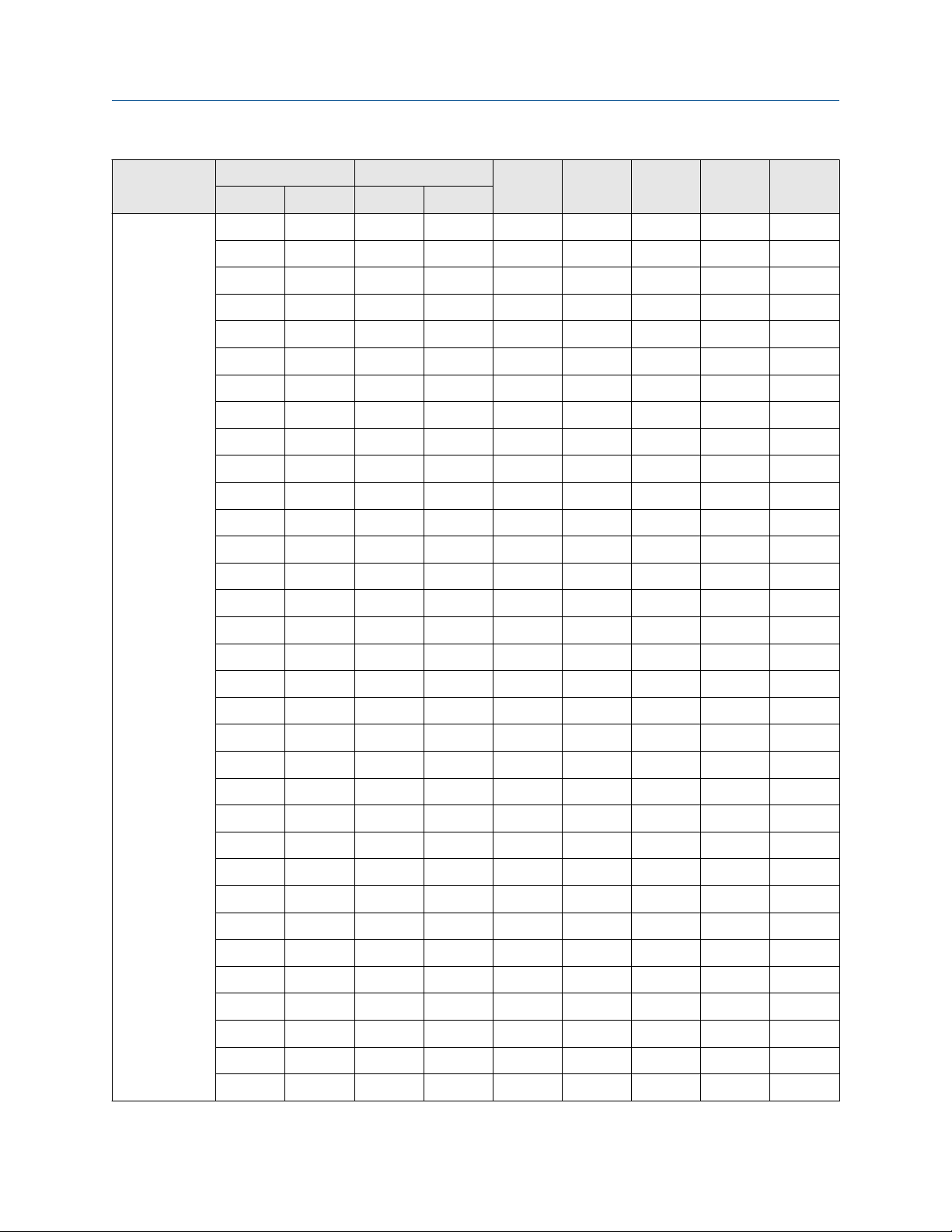
7.1.1 A chemicals with Coriolis meters
Chemical
Temp °C Conc. % wt
Low High Low High
-18 93 0 100 O O O X O
Acetaldehyde
93 149 0 100 — — — — O
-18 52 0 100 O O O — O
52 77 0 100 O O — — O
Acetate
77 100 0 100 O O X — O
100 204 0 100 O O X — O
-18 10 0 50 O O X O O
SS C22 Tz Ta Ti
-18 10 50 80 O O X O O
Acetic acid
24 Micro Motion Corrosion Guide
-18 10 80 95 — O X O O
-18 10 95 100 O O X O O
10 38 0 100 O O X O O
38 71 0 50 O O X O O
Page 25
User Guide Material compatibility tables
GI-00415 October 2020
Chemical
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
38 71 50 80 O O X O O
38 71 80 95 X O X O O
38 66 95 100 O O X O O
66 93 95 100 O O X O O
71 79 0 45 O O X O O
71 79 45 50 C O X O O
71 79 50 80 — O X O O
79 93 0 45 O O X O O
79 93 45 50 C O X O O
79 93 50 55 — O X O O
79 93 55 95 X O X O O
93 99 0 20 O O X O O
93 99 20 50 C O X O O
93 99 50 55 — O X O O
93 99 55 80 X O X O O
93 99 80 95 X O X O —
93 118 95 100 X O X O X
99 104 0 20 O O X O O
99 104 20 50 C O X O O
99 104 50 55 — O X O O
99 104 55 80 X O X O O
99 104 80 95 X O X O —
104 127 0 20 O O X O O
104 127 20 50 C O X O O
104 127 50 55 — O X O O
104 127 50 80 X X X O O
104 127 80 85 X X X O —
104 127 85 95 X X X O X
118 204 95 100 X O X O X
127 135 0 20 O O X O —
127 135 20 50 C X X O —
127 135 50 55 — X X O —
127 135 50 85 X X X O —
User Guide 25
Page 26
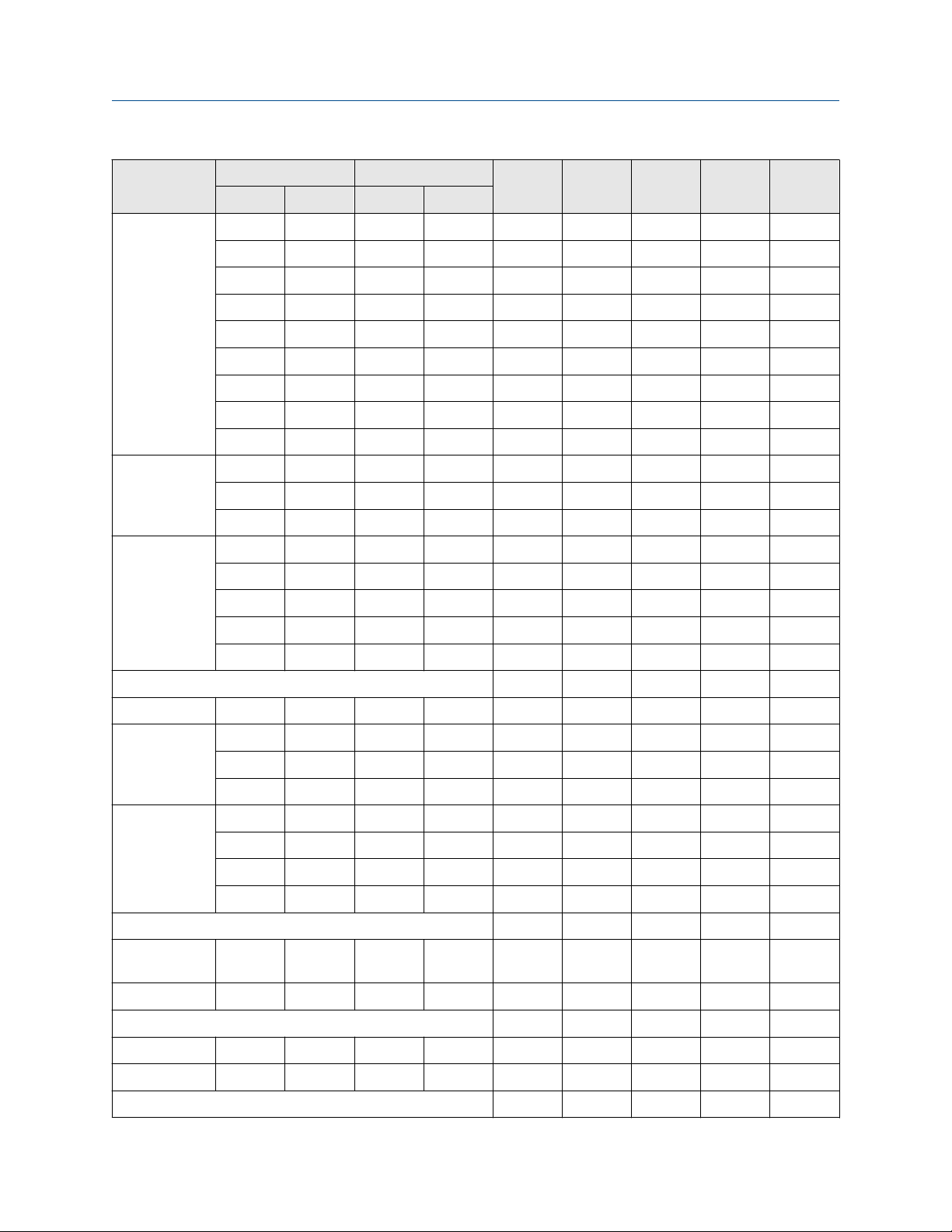
Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Acetic
anhydride
Acetone
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
127 135 85 95 X X X O X
135 149 0 20 O O X O X
135 149 20 50 C X X O X
135 149 50 55 — X X O X
135 149 55 95 X X X O X
149 204 0 20 O — X O X
149 204 20 50 C X X O X
149 204 50 55 — X X O X
149 204 55 95 X X X O X
-18 38 0 100 X O O O O
38 121 0 100 X O O X O
121 143 0 100 X O X X O
-18 60 0 100 O O X O O
60 93 0 100 O O X O O
93 104 0 100 O O X O —
104 149 0 100 O — X O —
149 204 0 100 O — X — —
Acetone cyanohydrin O — — O —
Acetonitrile 0 50 0 100 O X O O X
-18 21 0 100 X O X O —
Acetyl chloride
Acetylene
Acetylene tetrabromide X — O O —
Acetylene
trichloride
Acid pulping 0 80 0 100 X O O O —
Acid soluble oil X X X X X
Acrylamide 0 40 O O — — —
Acrylic acid 0 53 O O — — —
21 37 0 100 X O X — —
37 60 0 100 X — X — —
0 26 0 100 O O O O O
26 37 0 100 O O O — —
37 116 0 100 O — O — —
116 204 0 100 O — — — —
0 106 0 90 X O O O —
Acrylic emulsion O O O O —
26 Micro Motion Corrosion Guide
Page 27
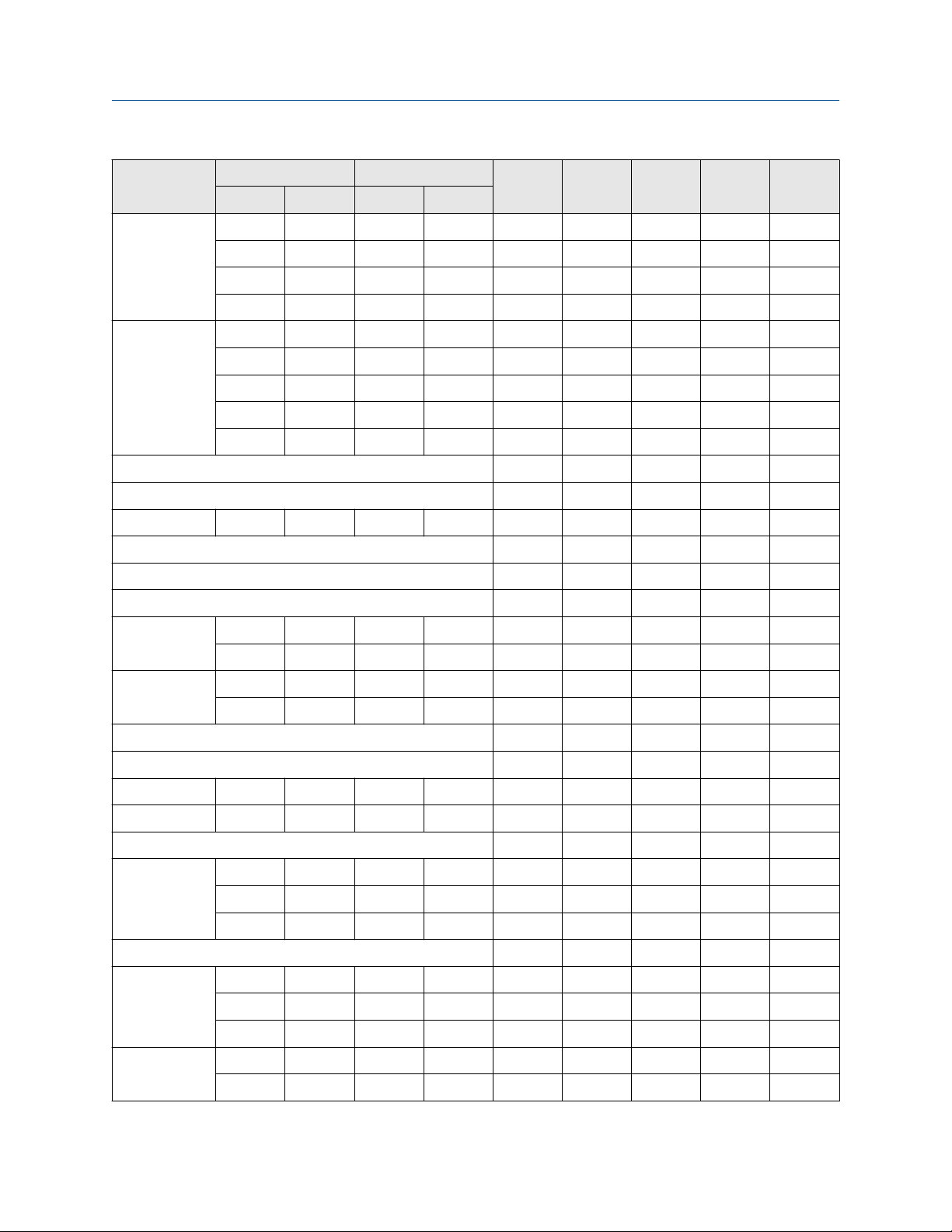
User Guide Material compatibility tables
GI-00415 October 2020
Chemical
SS C22 Tz Ta Ti
Low High Low High
0 60 0 100 O O O O O
60 87 0 100 O O — O O
Acrylonitrile
87 104 0 100 X O — O X
104 130 0 100 — — — O X
0 10 0 100 O O O O O
10 93 0 100 O O O X O
Temp °C Conc. % wt
Adipic acid
93 120 0 100 C C O X O
120 134 0 100 C C X X O
134 220 0 100 X — X — O
Air O O O O O
Alachlor technical; chlorodiethylacetanilide — O — O —
Alcohols 0 100 0 100 O O O O C
Alkylbenzene sulfonic acid X O
(1)
— O X
Alkyldimethyl ammonium chloride X O O O —
Alkylsulfonic acid X O
(1)
— O X
Allyl alcohol
0 93 0 100 O O O X X
93 209 0 100 O X — — —
0 26 0 100 C O O — O
Allyl chloride
26 82 0 100 X X O — O
Allyl chloride phenol X O O O O
Allyl chloroformate (anhydrous) X O — O —
Allyl phenol 0 130 0 100 O — X — —
Allylbenzene 20 60 0 100 O — — — —
alpha-methylstyrene O O O O —
0 30 0 100 O O O X O
Alum
30 98 0 100 — X O — O
98 120 0 100 — — O — —
Alumina O O O O O
Aluminum
chloride
aqueous
Aluminum
chloride dry
0 93 0 10 X O O O O
0 93 10 100 X O O O X
93 120 0 100 X X O — X
0 93 0 100 X O O O X
93 120 0 100 X — O O —
User Guide 27
Page 28
Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Aluminum chlorohydrate X O O O —
Aluminum chlorohydroxide X O O O —
Aluminum
fluorosulfate
Aluminum
nitrate
Aluminum oxide O O O O O
Aluminum silicate — — — — —
Aluminum
sulfate
Amine
Amine oxide O O — — —
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 200 0 15 — O — O —
0 98 0 100 O C O O O
98 120 0 100 X — O O —
0 38 0 100 X O O O O
38 93 0 100 X — X O O
0 100 0 100 C O — O C
100 120 0 100 X X O O —
120 148 0 100 — — X O —
-35 0 0 50 O O X X C
0 30 0 50 O O O X O
Ammonia
Ammonia anhydrous O O O X X
Ammonium
bifluoride
Ammonium
bisulfate
Ammonium
bisulfite
Ammonium
carbamate
Ammonium
carbonate
Ammonium
chloride
30 70 0 30 O O O X X
30 70 30 50 X O O X X
70 130 0 50 X O X X X
10 120 0 100 X X O X X
0 38 0 60 X C O — O
0 60 0 30 X C O — O
80 120 0 65 C C X O X
0 20 0 30 O O O O O
20 93 0 30 O X O O O
93 120 0 30 X — O — —
0 93 0 10 X O O O O
0 82 0 50 X O O O O
82 104 0 50 X — O O O
104 120 0 50 X — O — —
28 Micro Motion Corrosion Guide
Page 29
User Guide Material compatibility tables
GI-00415 October 2020
Chemical
SS C22 Tz Ta Ti
Low High Low High
Ammonium dihydrozene phosphate — O — O —
0 30 0 50 O O O X O
Temp °C Conc. % wt
Ammonium
hydroxide
30 70 0 30 O O O X X
30 70 30 50 X O O X X
70 150 0 50 X O X X X
Ammonium laurate O — — — —
Ammonium laureth sulfate — O — O —
Ammonium
nitrate
Ammonium
oxalate
0 93 0 100 C
93 120 0 100 X
0 24 0 10 X O — O —
(2)
(2)
O O O O
C O — —
0 25 0 5 O O O O O
Ammonium
persulfate
0 25 5 10 O O O — O
0 60 10 100 O — O — O
60 120 10 100 — — O — —
0 60 0 10 O O O O O
0 60 10 100 X O O O O
Ammonium
phosphate
60 104 0 10 X X O O O
60 120 10 100 — — O O —
104 120 0 10 — — O O —
120 148 10 100 — — — O —
Ammonium
saltwater
20 80 0 15 X O O X —
0 104 0 10 X O O O O
0 120 10 100 X X O O O
Ammonium
sulfate
104 120 0 10 X X O O —
120 160 0 10 X X X O —
120 149 10 100 X X X O —
Ammonium
sulfide
0 70 0 100 — O O O —
Ammonium thioglycolate O O — — —
Ammonium thiosulfate — O — — O
User Guide 29
Page 30
Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Amyl chloride
Amyl
mercaptan
Amylphenol 0 200 0 100 — O X O —
Aniline
Animal fat — O O — O
Anodizing solution aluminum — O — O —
Anthracene oil 80 90 0 100 O — — — —
Anthraquinone — — O — —
Antibiotic fermentation media — O — O —
Anti-static agent 743 X O — — —
Antimony
pentachloride
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 60 0 100 O O O O X
60 120 0 100 — — O O —
120 148 0 100 — — X O —
0 160 0 100 — O X O —
0 110 0 100 O O O O O
110 120 0 100 O O O — —
120 265 0 100 O — — — —
0 71 0 50 X O O O —
Apple juice O O O O O
Aqua quinine O O — — —
Aqua regia
Argon O O O O O
Arsenic acid
Asphalt
Atropine 0 60 0 100 — O — — —
(1) Maintain velocity < 10 ft/sec (3 m/sec)
(2) 304L = O
0 20 0 75 X X X O O
20 82 0 75 X X X O —
0 52 0 100 O X O — —
52 120 0 100 X X O — —
0 60 0 100 O O X — O
60 200 0 100 O O X O O
30 Micro Motion Corrosion Guide
Page 31

User Guide Material compatibility tables
GI-00415 October 2020
7.1.2 A chemicals with fork density and viscosity meters
Chemical
Acetaldehyde
Acetate
Temp °C Conc. % wt
Low High Low High
–18 93 0 100 O — O O —
93 120 0 100 — — — O O
–18 52 0 100 O — O O O
52 77 0 100 O — O O O
77 100 0 100 O — O O O
100 204 0 100 O — O O —
–18
–18 10 50 80 O — O O O
–18 10 80 95 — — O O O
–18 10 95 100 O — O O O
10 38 0 100 O C O O O
38 71 0 50 O — O O O
38 71 50 80 O — O O O
38 71 80 95 O — O O O
38 66 95 100 O C O O O
10 0 50 O — O O O
316L 304L C22 Ti Zr
Acetic acid
(1)
66 93 95 100 O C O O O
71 79 0 45 O C O O O
71 79 45 50 C C O O O
71 79 50 80 C C O O O
79 93 0 45 O C O O O
79 93 45 50 C C O O O
79 93 50 55 — C O O O
79 93 55 95 X X O O O
93 99 0 20 O — O O O
93 99 20 50 C C O O O
93 99 50 55 — C O O O
93 99 55 80 X X O O O
93 99 80 95 X X O O O
93 118 95 100 X X O X O
99 104 0 20 O — O O O
99 104 20 50 C C O O O
99 104 50 55 — C O O O
User Guide 31
Page 32

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
99 104 55 80 X X O O O
99 104 80 95 X X O — O
104 127 0 20 O X O O O
104 127 20 50 C X O O O
104 127 50 55 — C O O O
104 127 50 80 X X O O O
104 127 80 85 X X O O O
104 127 85 95 X X O X O
118 204 95 100 O X O X —
127 135 0 20 O X O X O
127 135 20 50 O X X X O
127 135 50 55 O X X X O
127 135 50 85 C X X X O
127 135 85 95 C X X X O
135 149 0 20 O X O X O
135 149 20 50 O X X X O
135 149 50 55 O X X X O
135 149 55 95 C X X X O
149 204 0 20 O X X — —
149 204 20 50 O X X — —
149 204 50 55 O X X — —
149 204 55 95 C X X — —
–18 38 0 100 C — O O O
Acetic anhydride
Acetone
Acetone cyanohydrin O — — — —
Acetonitrile 0 60 0 100 O — X X —
38 121 0 100 C — O O —
121 143 0 100 C — O O —
–18 60 0 100 O — O O O
60 93 0 100 O — O O O
93 104 0 100 O — O O O
104 149 0 100 O — — — —
149 204 0 100 O — — — —
Acetyl chloride
32 Micro Motion Corrosion Guide
–18 21 0 100 X — O — —
Page 33

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
Acetylene
Acetylene tetrabromide X X O — —
Acetylene trichloride (dry) O X O — —
Acid pulping 0 80 0 100 — — O — —
Acrylic acid 0 53 O — O — —
Acrylic emulsion O — O — —
Acrylonitrile
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
21 37 0 100 X — O — —
0 26 0 100 O — O — —
26 37 0 100 O — O — —
37 116 0 100 O — O — —
116 204 0 100 O — O — —
0 60 0 100 O — O O O
60 87 0 100 O — O O O
87 104 0 100 — — O — O
104 130 0 100 X X — X O
0 10 0 100 O — O O —
Adipic acid
Alachlor technical; chlorodiethylacetanilide X X O — —
Alcohols 0 100 0 100 O O O C —
Alkylbenzene sulfonic acid X X O X —
Alkyldimethyl ammonium chloride X X O — —
Allyl alcohol
Allyl chloride
Allyl chloride phenol X X O O —
Allyl chloroformate X X O — —
Allyl phenol 0 130 0 100 O — — — —
Allylbenzene 20 60 0 100 O — — — —
Alphamethylstyrene O — O — —
10 93 0 100 O — O O —
93 120 0 100 — — — O —
120 220 0 100 — — — O —
0 93 0 100 O O O — —
93 209 0 100 O — — — —
0 26 0 100 X X O O —
26 82 0 100 X X O O —
User Guide 33
Page 34

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Alum
Alumina O — O O —
Aluminum chloride
aqueous
Aluminum chloride
dry
Aluminum chlorohydroxide X X O —
Aluminum
fluorosulfate
Aluminum nitrate
Aluminum oxide O — O — —
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
0 30 0 100 O X O O O
30 75 0 100 — X O O O
75 120 0 100 — X — — —
0 93 0 10 X X O O O
0 93 10 100 X X O C C
93 120 0 100 X X C X —
0 21 0 10 O O O X O
21 93 0 100 X X O X —
93 120 10 100 X X — X —
0 200 0 15 — X O X X
0 98 0 100 O X C O —
98 120 0 100 X X — —
Aluminum silicate O — O — —
Aluminum sulfate
Amine
No quaternary
amines for stainless
steel
Ammonia (aqueous)
(ammonia + water)
Ammonia
(anhydrous)
Ammonium
carbonate
0 38 0 100 X X O O O
38 93 0 100 X X — O C
0 100 0 100 C X O — —
100 120 0 100 C X O — —
120 148 0 100 X X O — —
0 30 0 50 O O O O O
30 70 0 30 O O O — O
30 70 30 50 O O O — O
70 150 0 50 — — O — O
100 100 O O O X X
0 20 0 30 O X O O —
20 80 0 30 O X O O —
80 120 0 30 — X — O —
34 Micro Motion Corrosion Guide
Page 35

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
Ammonium chloride
Ammonium dihydrozene phosphate — — O — —
Ammonium laurate O — O — —
Ammonium laureth sulfate O — O — —
Ammonium nitrate
Ammonium oxalate 0 24 0 10 X X O O —
Ammonium
persulfate
Ammonium
phosphate
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
0 93 0 10 X X O O O
0 82 0 50 X X O O O
82 104 0 50 X X O X O
104 120 0 50 X X O X O
0 38 0 100 X O O O O
38 120 0 100 X O X O O
0 25 0 5 O — O O —
0 25 5 10 O — O O —
0 60 10 100 O — — O —
0 60 0 10 O — O O —
0 60 10 100 — — O O —
60 104 0 10 — — — O —
104 120 0 10 — — — O —
Ammonium
saltwater
Ammonium sulfate
Ammonium sulfide
Ammonium thioglycolate O — O — —
Ammonium thiosulfate — — O O —
Amyl chloride
Amyl mercaptan 0 160 0 100 O O O — —
Amylphenol 0 200 0 100 O O O — —
20 80 0 15 X X O — —
0 104 0 10 X X O O O
0 120 10 100 X X X O O
104 120 0 10 X X O — —
120 160 0 10 X X O — —
120 149 10 100 X X X — —
0 70 0 100 X X O — —
40 60 0 100 X X O — —
0 60 0 100 O X O X X
60 120 0 100 — X O X X
120 148 0 100 — X — X X
User Guide 35
Page 36

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Aniline
Animal fat — — O O —
Anthracene oil 80 90 0 100 O — — — —
Anthraquinone — — — — —
Antibiotic fermentation media — O — —
Anti-static agent 743 X X O — —
Antimony
pentachloride
Apple juice O — O O —
Aqua quinine O — O — —
Aqua regia
Argon O — O O —
Arsenic acid
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
0 110 0 100 O — O C —
110 120 0 100 C — O — —
120 265 0 100 C — — — —
0 71 0 50 X O — —
0 20 X X X O X
20 120 X X X X X
0 52 0 100 O — X X —
52 120 0 100 X — X X —
Asphalt
Atropine 0 60 0 100 O — O — —
(1) Consult Micro Motion before using zirconium with acetic acid containing copper ions.
0 60 0 100 O — O O —
60 200 0 100 O — O O —
7.2 B chemical tables
Table 7-2: Synonyms for B chemicals
Synonym Listed under
1, 2 - Benzenedicarboxylic acid anhydride Phthalic anhydride
Baking soda Sodium bicarbonate
Battery acid Sulfuric Acid
Benzene carboxylic acid Benzoic Acid
Benzine Benzene
Benzol Benzene
Boron fluoride Boron trifluoride
Boronic acid Boric acid
36 Micro Motion Corrosion Guide
Page 37

User Guide Material compatibility tables
GI-00415 October 2020
Table 7-2: Synonyms for B chemicals (continued)
Synonym Listed under
Br Bromine
Bromoform Tribromomethane
Bromomethane Methyl bromide
Butanal Butyl aldehyde
Butyl alcohol Butanol
Butylene Butadiene
Butyraldehyde Butyl aldehyde
7.2.1 B chemicals with Coriolis meters
Chemical
Barium
chloride
Barium sulfate
Beef tallow O O — X O
Beer
Beeswax 0 104 0 100 — O — O —
Benzene 0 116 0 100 O O O O O
Benzene
hexachloride
Benzene sulfonic acid X O
Benzoic acid
Benzophenone — O — — —
Temp °C Conc. % wt
Low High Low High
0 60 0 100 X O O — O
0 93 0 100 X O O X O
93 120 0 100 X — O — —
0 37 0 100 O O O O O
37 150 0 100 O — — — O
0 200 0 100 X O — — —
0 82 0 10 X O O O O
0 104 10 100 — — O O O
104 120 10 100 — — O — O
SS C22 Tz Ta Ti
(1)
— O X
Benzoquinine O O — O —
Benzoyl chloride — O O O —
Benzoyl
peroxide
Benzyl chloride
Betaine 0 90 X O — O —
User Guide 37
0 50 0 75 O O O O —
0 50 0 100 X O O C O
0 120 0 100 X X O C —
Page 38

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Black acid 0 210 0 100 X X X O —
Black liquor 20 80 0 120 X O O X X
Bleach X O O O O
Boric acid
Boron sulfate — O — O —
Boron
trifluoride
Boron
trifluoride
etherate
Brine X O O O O
Bromethylbenzene X X O O —
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 30 0 10 O O O O O
0 120 0 10 X O O O O
120 150 0 10 — O X O —
150 250 0 10 — O — — —
0 40 — O — — —
0 57 0 100 — O — — —
0 66 X X O O O
Bromine (wet)
Bromine (dry or
anhydrous)
Bromine water 0 65 X O O O O
Bromonitropropanediol X O O — X
Bromotoluene X X O O —
Butadiene
Butane O O O O O
Butanol O — — O O
Butyl acetate 0 120 0 100 O O O O O
Butyl acrylate 0 40 98 100 O O — — —
Butyl aldehyde O — — O —
Butyl bromide 0 120 0 100 X X O O —
Butylamine O O — — —
66 93 X X O O O
93 120 X X O O —
120 150 0 100 X X X O —
0 66 100 100 X X O O X
0 66 0 100 X X O O X
0 60 0 100 O O O — —
60 120 0 100 — O O — —
Butylene glycol 0 120 O O O O —
38 Micro Motion Corrosion Guide
Page 39

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
Butyllithium in
alkane
(1) Maintain a velocity of < 10 ft/sec (3 m/sec).
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 60 0 50 O O — — —
7.2.2 B chemicals with fork density and viscosity meters
Chemical
Barium chloride 0 60 0 100 X X O — —
Barium sulfate
Beef tallow O — O O —
Beer
Beeswax bleach
solution
Benzene 0 116 0 100 O — — O O
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
0 93 0 100 X X O — —
93 120 0 100 X X O — —
0 37 0 100 O — O — —
37 150 0 100 O — O — —
0 104 0 100 — — O — —
Benzene
hexachloride
Benzene sulfonic acid X X O — —
Benzoic acid
Benzophenone X X O — —
Benzoquinine O — O — —
Benzoyl chloride X X O — —
Benzoyl peroxide O — O — —
Benzyl chloride
Black acid 0 210 0 100 X X X X —
Black liquor 20 90 0 100 C X O — —
Bleach X X O O —
0 200 0 100 X — O — —
0 82 0 10 X X O O O
0 104 10 100 X X — O O
104 120 10 100 X X — — O
0 50 0 100 X X O O —
0 120 0 100 X X X — —
User Guide 39
Page 40

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Boric acid
Boron sulfate — — O — —
Boron trifluoride 0 50 — X O — —
Boron trifluoride
etherate
Brine X X O O —
Bromethylbenzene X X X — —
Bromine (dry or
anhydrous)
Bromine (wet)
Bromine water 0 65 X X O O O
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
0 30 0 10 O X O O O
0 120 0 10 X X O O O
120 150 0 10 X X O — —
150 250 0 10 X X O — —
0 57 0 100 — X O — —
0 66 100 100 X X X X O
0 66 X X X O —
66 93 X X X O —
93 120 X X X X X
Bromonitropropanediol X X O X —
Butadiene
Butane O O O — —
Butanol O O O O —
Butyl acetate 0 120 0 100 O — O O —
Butyl acrylate 0 40 98 100 O — O — —
Butyl aldehyde O — — — —
Butylamine O — O — —
Butyl bromide 0 120 0 100 X X X — —
Butylene glycol O — O — —
0 60 0 100 O — O — —
60 120 0 100 — — O — —
7.3 C chemical tables
Table 7-3: Synonyms for C chemicals
Synonym Listed under
Carbamide Urea
Carbolic acid Phenol
40 Micro Motion Corrosion Guide
Page 41

User Guide Material compatibility tables
GI-00415 October 2020
Table 7-3: Synonyms for C chemicals (continued)
Synonym Listed under
Carbon dichloride Perchloroethylene
Carbon oxychloride Phosgene
Carbonyl chloride Phosgene
Carbonyl diamide Urea
Caustic potash Potassium hydroxide
Caustic soda Sodium hydroxide
Chlorallylene Allyl chloride
Chlorinated cyclic olefin Hexachlorocyclopentadiene
Chlorine gas Chlorine
Chlorine liquid Chlorine
Chlorodiethylacetanilide Alachlor technical
Chloroethylen Vinyl chloride
Chloromethane Methyl chloride
Chloropentane Amyl chloride
CIP See each fluid used, or contact customer support
CNG Compressed natural gas
Crude oil Oil, crude
Cupric sulfate Copper sulfate
Cyanhydrin, cyanohydrin Acetone cyanohydrin
7.3.1 C chemicals with Coriolis meters
Chemical
Calcium
bromide
Calcium carbonate O O O O O
Calcium
chloride
Temp °C Conc. % wt
Low High Low High
0 60 0 50 X O O O —
0 93 0 40 X O O O O
0 93 40 100 X O O O O
93 120 0 40 X X O O O
93 120 40 100 X X O — X
SS C22 Tz Ta Ti
120 200 4 100 X X — — —
Calcium
hydroxide
User Guide 41
0 50 0 50 O O O X O
0 100 0 50 X O O X X
Page 42

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Calcium
hypochlorite
Calcium lignosulphonate — O — — —
Calcium
pyridine
sulfonate
Calcium
stearate
Calcium sulfide 0 47 0 100 X O O O —
Calcium
thiosulfate
Canola oil O O — — —
Carbolite O O O O —
Carbon dioxide
(dry)
Carbon dioxide 0 120 0 100 X C X O —
Carbon
disulfide
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 60 0 15 X O O X O
0 66 0 100 — O X — —
0 60 0 40 O O — — O
0 38 0 30 O O — — O
0 120 0 100 O O O O —
0 43 0 100 O — O O O
43 65 0 100 — — O X O
65 93 0 100 — — — — O
Carbon
tetrachloride
(anhydrous)
Carbon tetrachloride (wet) X O O O O
Carbon tetrafluoride X — — — —
Carbonic acid (wet) X O X O O
Carbonochloric acid X O — O —
Carboxylic acid salts — O — — —
Ceda clean — O — — —
Cement O O O — —
Cerium acetate — O — O —
Cetylpyridinium O O — — —
Cetylpyridinium chloride X O O O —
Chloric acid
Chlorinated hydrocarbons X O O O —
Chlorinated phenol X O O O —
0 60 0 100 O O O O O
60 120 0 100 — — O O O
0 31 0 20 X O O O —
0 70 0 50 X X O O —
42 Micro Motion Corrosion Guide
Page 43

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
SS C22 Tz Ta Ti
Low High Low High
Chlorinated pyridine X O O O —
Chlorinated, fluorinated pyradines X O X O —
Temp °C Conc. % wt
Chlorine
-40 0 0 100 X O X O X
(anhydrous gas
or liquid)
0 104 0 100 X O O O X
Chlorine (wet) 0 120 0 100 X X O O —
Chlorine
dioxide
0 40 0 15 X O O O O
0 120 0 100 X X O O C
Chloro nitro ethane X O — O —
Chloro
trifluoro-
0 49 0 100 — O — O —
ethylene
Chloroacetic acid X O X O C
Chloroacetyl chloride X O — O —
Chlorobenzene 0 38 0 60 X O O O O
Chlorodifluoroethane X O O — —
Chlorodifluoromethane X — O — —
0 21 0 100 O O O O O
Chloroform
21 95 0 100 X X O O O
95 104 0 100 X X O O O
Chloromethylisothiazolinone X O — — —
Chlorophenol 0 60 0 5 X O O — —
Chloropicrin 0 95 0 100 X O — O —
Chlorosilane — O O O —
Chlorosulfonic
acid
0 85 0 100 X O
(1)
X O X
Chlorotetrahydrophthalic anhydride X O — O —
Chocolate O — O — O
Choline chloride X O — O —
Chromic oxide (based on 50% chromic acid) — O — O —
Chromium
trioxide;
0 100 — — — — O
chromic acid
Chromium sulfate O O — O —
0 100 0 50 O O O O O
Citric acid
100 120 0 50 X O O O X
User Guide 43
Page 44

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Coal tar fuel O O X O —
Coal tar pitch O O X O —
Cobalt
hydroxide
Cobalt octoate O O — — —
Cocoa butter O — O O O
Coconut oil O — O O O
Concrete O O O O —
Copper bromide X — O O —
Copper sulfate 0 104 0 100 X O O O O
Corn oil O O O O O
Corn oil and garlic O O O O —
Corn steep liquor O O O O —
Corn syrup O O O O O
Creosote oil X O — — O
Cresol O O O — O
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 200 0 100 X — O X —
Cresylic acid 0 100 0 100 — O X O O
Cupric bromide 0 30 0 100 X X — O —
0 104 0 5 X X O O O
Cupric chloride
Cyanogen bromide X X X O X
Cyanogen
chloride
Cyclohexane
Cyclopropylamine O O — — —
(1) Maintain a velocity of < 10 ft/sec (3 m/sec).
0 21 5 50 X O — O O
21 120 5 50 X X — O O
0 46 0 20 — O — O —
0 93 0 100 O X O X O
93 120 0 100 O X O — O
44 Micro Motion Corrosion Guide
Page 45

User Guide Material compatibility tables
GI-00415 October 2020
7.3.2 C chemicals with fork density and viscosity meters
Chemical
Calcium chloride
Calcium hydroxide
Calcium
hypochlorite
Calcium lignosulphonate — X O — —
Calcium pyridine
sulfonate
Calcium sulfide
Calcium thiosulfate 0 38 0 30 O — O O —
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
0 93 0 40 X X O O O
0 93 40 100 X X O O O
93 120 0 40 X X O O O
93 120 40 100 X X O O O
120 150 0 100 X X O O X
0 50 0 50 O — O O O
0 100 0 50 — — O O O
0 60 0 15 X X O O —
0 66 0 100 — X O — —
0 50 0 10 O — O — —
0 25 0 100 O — O — —
25 100 0 100 — — O — —
Canola oil O — O — —
Carbolite O — O — —
Carbon dioxide 0 120 O — O — —
0 43 0 100 O — O O —
Carbon disulfide
Carbon tetrachloride
Carbon tetrafluoride X X O X —
Carbonic acid X X O — —
Carbonochloric acid X X O — —
Carboxylic acid salts — — O — —
Cement O — O — —
Cerium acetate O — O — —
Cetylpyridinium O — O — —
Cetylpyridinium chloride — — O — —
43 65 0 100 — — O O —
65 93 0 100 — — — O —
0 60 O — O O —
60 120 — — O O —
0 100 0 100 X X O O —
User Guide 45
Page 46

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Chloric acid
Chlorinated hydrocarbons — O — —
Chlorinated phenol X X O — —
Chlorinated pyridine X X O — —
Chlorinated, fluorinated pyradines X X O — —
Chlorine
Chlorine dioxide
Chloro nitro ethane X X O — —
Chloro
trifluoroethylene
Chloroacetic acid X X O — O
Chloroacetyl chloride X X O — —
Chlorobenzene 0 38 0 60 X X O O O
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
0 31 0 20 X X O X —
0 70 0 50 X X — X —
0 104 0 100 C X O X X
0 120 0 100 X X O O X
0 40 0 15 X X O O —
0 120 0 100 X X X — —
0 49 0 100 — O — —
Chlorodifluoroethane — — O — —
Chlorodifluoromethane — — O — —
0 21 0 100 X X O O —
Chloroform
Chlorophenol 0 60 0 5 X X O — —
Chloropicrin 0 95 0 100 X X O O —
Chlorosilane — — O — —
Chlorosulfonic acid 0 85 0 100 X X O X —
Chlorotetrahydrophthalic anhydride X X O — —
Chocolate O — — O —
Choline chloride X X O — —
Chromic oxide X X O — —
Chromium trioxide 0 100 — — — O —
Chromium sulfate — — O — —
Citric acid
21 95 0 100 X X — O —
95 104 0 100 X X — O —
0 100 0 50 O C O O O
100 120 0 50 X X O X O
Coal tar fuel O — O — —
46 Micro Motion Corrosion Guide
Page 47

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
Coal tar pitch O — O — —
Cobalt octoate O — O — —
Cocoa butter O — O O —
Coconut oil O — O — —
Coke gas oil — — O — —
Concrete O — O — —
Copper bromide X X O — —
Copper sulfate 0 104 0 100 — — O O —
Corn oil O — O O —
Corn oil and garlic O — O — —
Corn steep liquor O — O — —
Corn syrup O — O O —
Creosote oil — — O O —
Cresol O — O O —
Cresylic acid 0 100 0 100 — — O O —
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
Cupric bromide 0 30 0 100 X X O — —
Cupric chloride 0 104 0 5 X X O O X
Cupric chloride
Cyanogen chloride 0 46 0 20 — — O — —
Cyclohexane
Cyclopropylamine O — O — —
0 21 5 50 X X O O X
21 120 5 50 X X C O X
0 93 0 100 O — — O O
93 120 0 100 O — — O —
7.4 D chemical tables
Table 7-4: Synonyms for D chemicals
Synonym Listed under
1,3 - Dioxophthalan Phthalic anhydride
Darammon Ammonium chloride
DEAC Dimethylacetimide
Deionized water Water
Dichloroethane Ethylene dichloride
Dichloroethylene Vinylidene chloride
User Guide 47
Page 48

Material compatibility tables User Guide
October 2020 GI-00415
Table 7-4: Synonyms for D chemicals (continued)
Synonym Listed under
Dichloromethane Methylene chloride
Diethylene oxide Tetrahydrofuran
Diethyl ether Ether
Dihydroxyethane Ethylene glycol
Dimethyl benzene Xylene
Dimethyl ketone Acetone
Dipping acid Sulfuric acid
DMAC Dimethylacetimide
DMAPA Dimethylaminopropylamine
Dodecyl bromide Lauryl bromide
7.4.1 D chemicals with Coriolis meters
Chemical
Decane sulfonyl fluoride X — O — —
Diacryl
phthalate
Dibromobenzene
Dibromonitrilopropionamide X O X — X
Dichloroacetyl chloride X — O — —
Dichlorobenzene
Dichlorobutene X O — O —
Dichlorodifluoromethane
Dichlorofluoroethane — O — O O
Dichlorophenol 0 120 0 100 X O O O —
Dichlorotrifluoroethane X — O — —
Diesel fuel
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 15 0 100 O — — O —
0 200 0 100 X — — O —
0 120 X O O — X
0 21 0 100 X O O O O
21 71 0 100 X — O — —
0 38 0 100 O O O — X
38 120 0 100 O O O — —
Diethanolamine
Diethyl aluminum chloride X — — O —
48 Micro Motion Corrosion Guide
0 100 0 100 O O — O O
Page 49

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
Diethyl
disulfide
Diethyl sulfate — O O O —
Diethyl sulfide — O O O —
Diethylamine 0 120 0 100 O X O — X
Diethylene
glycol
Difluorobenzonitrile — — O — —
Difluoromonochlorethane — O — — —
Diisononylphtalate O O — — —
Diisopropyl peroxydicarbonate O O — — —
Dimethyl aminoethyl methacrylate O — — O —
Dimethyl chloride X O — O —
Dimethyl dichloride X O O O —
Dimethyl disulfide O O C O —
Dimethyl formaldehyde O — — O —
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 90 0 100 — O — O —
0 52 0 100 O X O — O
52 76 0 100 O — — — O
Dimethyl hydrazine O O — — —
Dimethyl
malonate
Dimethyl
succinate
Dimethyl sulfate O O O O —
Dimethyl sulfide O O C O —
Dimethyl terephthalate O — X O —
Dimethylacetamide
Dimethylamine
Dimethylaminopropylamine
Dimethylformamide
Dimethylpolysiloxanes O O O O —
Dimethyltin dichloride X O — — —
Dinitrotoluene O O — O —
0 100 0 100 — O — O —
0 100 O O — O —
0 200 0 100 X O — — —
25 180 0 100 O — X O X
0 90 O O — — —
0 60 0 100 X O — — —
User Guide 49
Page 50

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Diphenyl methane diisocyanate (drainable is best) O O — — —
Diphenylamine 0 100 0 100 — O X O —
Dipropyl peroxydicarbonate O O — — —
Dipropylene glycol monomethyl ether O — O O O
Disobutylene O O — O —
Disodium iminodiacetate X — — — —
Divinylbenzene O O — — —
Dodecyl mercaptan O O — O —
Dodecylbenzene sulfonic acid X O
Drilling mud O O — — O
(1) Maintain a velocity of < 10 ft/sec (3 m/sec).
Temp °C Conc. % wt
Low High Low High
SS C22 Tz Ta Ti
(1)
— O —
7.4.2 D chemicals with fork density and viscosity meters
Chemical
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
Decane sulfonyl fluoride X X O X X
Diacryl phthalate 0 15 0 100 O — O — —
Dibromobenzene 0 200 0 100 X X — — —
Dichloroacetyl chloride X X O — —
Dichlorobenzene — X O — —
Dichlorobutene — X O — —
Dichlorodifluoromethane
Dichlorofluoroethane — — O — —
Dichlorophenol 0 120 0 100 X X O — —
Dichlorotrifluoroethane — — O — —
Diesel fuel
Diethanolamine 0 100 0 100 O — O O —
Diethyl aluminum chloride X X O — —
Diethylamine 0 120 0 100 O — — X —
Diethyl disulfide 0 90 0 100 — — O — —
0 21 0 100 C — O — —
21 71 0 100 — — O — —
0 38 0 100 O — O — —
38 120 0 100 O — O — —
50 Micro Motion Corrosion Guide
Page 51

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
Diethylene glycol
Diethyl sulfate — — O — —
Diethyl sulfide — — O — —
Difluorobenzonitrile — X O X —
Difluoromonochlorethane — — O — —
Diisononylphtalate O — O — —
Diisopropyl peroxydicarbonate O — O — —
Dimethylacetamide 0 200 0 100 X O — — —
Dimethylamine 25 180 0 100 O — — X —
Dimethyl aminoethyl methacrylate O — — — —
Dimethyl chloride X X O — —
Dimethyl dichloride X X O — —
Dimethyl formaldehyde O — — — —
Dimethyl hydrazine O O O — —
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
0 52 0 100 O — — O —
52 76 0 100 O — — — —
Dimethyl malonate 0 100 0 100 — — O — —
Dimethylpolysiloxanes O — O — —
Dimethyl succinate 0 100 O — O — —
Dimethyl sulfate O — O — —
Dimethyl sulfide O — O — —
Dimethyl terephthalate O — — — —
Dimethyltin dichloride X X O — —
Dinitrotoluene O — O — —
Diphenylamine 0 100 0 100 — — O — —
Diphenyl methane diiosocyanate O — O — —
Dipropylene glycol monomethyl ether O — O — —
Dipropyl peroxydicarbonate O — O — —
Disobutylene O — O — —
Disodium iminodiacetate X X O — —
Divinylbenzene O — O — —
Dodecylbenzene sulfonic acid X X O X —
Dodecyl mercaptan O — O — —
Drilling mud O — O O —
User Guide 51
Page 52

Material compatibility tables User Guide
October 2020 GI-00415
7.5 E chemical tables
Table 7-5: Synonyms for E chemicals
Synonym Listed under
Epsom salt Magnesium sulfate
Ethanal Acetaldehyde
Ethanoate Acetate
Ethanoic acid Acetic acid
Ethanol Ethyl alcohol
Ethanonitrile Acetonitrile
Ethanoyl chloride Acetyl chloride
Ethenyl benzene Styrene
Ethyl aldehyde Acetaldehyde
Ethylene chloride Ethylene dichloride
Ethyl ethanoate Ethyl acetate
Ethylic acid Acetic acid
Ethyne Acetylene
Ethyrene Butadiene
7.5.1 E chemicals with Coriolis meters
Chemical
Egg slurry O O O O O
Epichlorohydrin (dry)
Epoxy resin O O — — O
Ercimide — O — O —
Ester vinyl ether X O O — —
Ethane (H2S free) O O — — —
Ethanol O O O O C
Ether 20 100 0 100 O X O O O
Temp °C Conc. % wt
Low High Low High
0 60 0 100 O O O O —
SS C22 Tz Ta Ti
Ethyl acetate 0 65 0 100 O O O O C
Ethyl alcohol O O O O C
Ethyl benzene
Ethyl monochloroacetate X O X O —
52 Micro Motion Corrosion Guide
0 60 0 100 O O O — —
60 100 0 100 O O — — —
Page 53

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
Ethylbenzene sulfonyl fluoride — O O — —
Ethylene O O O O —
Ethylene
chlorohydrin
Ethylene
diamine
Ethylene
dichloride
Ethylene glycol
Ethylene
glycol/
bromoform
Ethylene oxide
Ethylhexyl acrylate O O — O —
Ethylpropylacrolein O O — — —
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 100 0 100 X O O — X
0 37 0 100 O X O X O
37 43 0 100 — X O X —
0 93 0 100 X O O O C
0 120 0 100 O O O O X
120 200 0 100 — O — — X
97 X — X O —
0 31 0 100 O O O O O
31 120 0 100 O — O — —
Evaposhine X O X O —
7.5.2 E chemicals with fork density and viscosity meters
Chemical
Egg slurry O — O O —
Epichlorohydrine
(dry)
Epoxy resin O — O O —
Ercimide — — O — —
Ester vinyl ether — — O — —
Ethanol O O O C —
Ether 20 100 0 100 O — — O —
Ethyl acetate 20 65 0 100 O O O O —
Ethyl alcohol O O O C —
Ethyl benzene
Temp °C Conc. % wt
Low High Low High
0 60 100 100 O — O — —
0 60 0 100 O — O — —
60 100 0 100 O — O — —
316L 304L C22 Ti Zr
Ethylbenzene sulfonyl fluoride — — O — —
User Guide 53
Page 54

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Ethylene O — O — —
Ethylene
chlorohydrin
Ethylene diamine
Ethylene dichloride 0 93 0 100 X X O O C
Ethylene glycol
Ethylene glycol,
bromoform
Ethylene oxide
Ethyl monochloroacetate X X O — —
Ethylproplacrolein O — O — —
Evaposhine X X O — —
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
0 100 0 100 — X O — —
0 37 0 100 O — O O —
37 43 0 100 — — O — —
0 120 0 100 O — O O —
120 200 0 100 — — O — —
97 X X X — —
0 31 0 100 O — O — —
31 120 0 100 O — — — —
7.6 F chemical tables
Table 7-6: Synonyms for F chemicals
Synonym Listed under
Formalin Formaldehyde
Freon 10 Carbon tetrachloride
Freon 113 Trichlorotrifluoroethane
Freon 12 Dichlorodifluoromethane
Freon 17 Trichloromonofluoroethane
Freon 22 Monochlorodifluoromethane
Fuel oil Oil, fuel
Fuming sulfuric acid Oleum
54 Micro Motion Corrosion Guide
Page 55

User Guide Material compatibility tables
GI-00415 October 2020
7.6.1 F chemicals with Coriolis meters
Chemical
Fat/Garlic O O O — O
Fatty acid
Ferric chloride
Ferric nitrate
Ferric nitrite O O — O —
Ferric sulfate
Ferrous
chloride
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 120 0 100 C O C O O
120 200 0 100 C O X O —
0 25 0 10 X O O O O
25 93 0 100 X X O O O
0 20 0 100 X O O O O
20 120 0 100 X — O — O
0 60 0 10 O O O O O
0 60 10 30 — O O O O
0 98 30 100 — — O O O
60 98 0 10 — — O O O
60 98 10 30 — — O O O
0 25 0 10 — O — O O
0 120 0 100 X X O O O
Ferrous sulfate 0 120 0 100 X O O O O
Fluorine (dry) 0 100 X O O X O
Fluoroalcohol X — O — —
Fluorobenzene X — O — —
Fluorosilicic
acid
Fluorosulfonic acid X — — X —
Fluorotrichloromethane X — O — —
Food product — O O O —
Formaldehyde O — O — X
0 30 0 10 O O O O O
Formic acid
(aerated)
Formic acid
(non aerated)
Fruit juice O O O O O
0 100 0 5 X O O O O
100 120 0 5 X — O O X
120 153 0 5 X — X O X
0 75 10 100 X O O O X
10 50 X X O X X
User Guide 55
Page 56

Material compatibility tables User Guide
October 2020 GI-00415
7.6.2 F chemicals with fork density and viscosity meters
Chemical
Fat/Garlic O — O O —
Fatty acid
Ferric chloride
Ferric nitrate
Ferric nitrite O — O — —
Ferric sulfate
Ferrous chloride
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
0 120 0 100 C — O — —
120 200 0 100 C — O — —
0 25 0 10 X X O O X
25 93 0 100 X X — O X
0 20 0 100 O X X O —
20 120 0 100 X X X O —
0 60 0 10 O X O O O
0 60 10 30 — X O O O
0 98 30 100 — X — O —
60 98 0 10 — X — O O
60 98 10 30 — X — O O
0 25 0 10 X X O O O
0 120 0 50 X X — — O
0 100 0 30 X X O O O
Ferrous sulfate
Fluoroalcohol X X O X X
Fluorobenzene X X O X X
Fluorosulfonic acid X X — X X
Fluorotrichloromethane X X O X X
Food product — — O — —
Formaldehyde O — — X —
Formic acid
Fruit juice O — O — —
0 100 30 50 X X — O O
0 100 50 100 X X — O —
0 30 0 10 O — O O O
0 100 0 5 O X O C O
0 75 10 100 O C O X O
100 120 0 5 O X O X O
120 153 0 5 X X — X —
56 Micro Motion Corrosion Guide
Page 57

User Guide Material compatibility tables
GI-00415 October 2020
7.7 G chemical tables
Table 7-7: Synonyms for G chemicals
Synonym Listed under
Glycol Ethylene glycol
7.7.1 G chemicals with Coriolis meters
Chemical
Gasoline
Gelatin O — — — O
Geranyl ester O O O O —
Glutaraldehyde 0 100 O O — — —
Glycerine 0 120 0 100 O O O O O
Glycolite O O O O —
Glyoxylic acid 0 50 X O — O —
Green liquor — O O — X
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 43 0 100 O O O O O
43 120 0 100 — O O — —
7.7.2 G chemicals with fork density and viscosity meters
Chemical
Temp °C Conc. % wt
Low High Low High
0 43 0 100 O — O — —
Gasoline
43 120 0 100 — — O — —
316L 304L C22 Ti Zr
Gelatin O — O O —
Geranyl ester O — O — —
Glycerine 0 104 0 100 O O O O —
Glycolite O — O — —
Glyoxylic acid 0 50 X X O — —
7.8 H chemical tables
Table 7-8: Synonyms for H chemicals
Synonym Listed under
Hartshorn Ammonium carbonate
Hexandioic acid, hexanedioic acid Adipic acid
User Guide 57
Page 58

Material compatibility tables User Guide
October 2020 GI-00415
Table 7-8: Synonyms for H chemicals (continued)
Synonym Listed under
Hydoxy benzoic acid Salicylic acid
Hydraulic cylinder oil Oil, hydraulic cylinder
Hypo photographic solution Sodium thiosulfate
7.8.1 H chemicals with Coriolis meters
Chemical
Halogenated alkyl ether X — — O —
Halogenated styrene — O — O —
Helium O O O O O
Heptane
Hexachlorocyclopentadiene; chlorinated cyclic olefin X X — O —
Hexafluoropropene — O — O —
Hexahydrophthalic anhydride O O — — —
Hexamethylenediisocyanate — O — O —
Hexane O O O X O
Hydrazine O O O — —
Hydrobromic acid X X O O X
Hydrochloric
acid
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 60 0 100 O O O O O
60 98 0 100 — O O — O
0 30 0 5 X O O O X
0 120 0 15 X C O O X
0 120 15 38 X X C O X
120 200 0 38 X X X O X
Hydrofluoric
acid (aqueous)
Hydrofluosilicic
acid
Hydrogen
Hydrogen bromide X X — O —
Hydrogen chloride (moist) X — O O X
Hydrogen chloride (anhydrous) O O O O X
58 Micro Motion Corrosion Guide
0 120 0 100 X X O X X
10 50 X X O X X
0 120 0 100 O O
120 200 0 100 O O
(1)
(1)
O X X
X X X
Page 59

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
Hydrogen
cyanide
Hydrogen
fluoride
(anhydrous)
Hydrogen
peroxide
Hydrogen
peroxide (acid
free)
Hydrogen
sulfide
(anhydrous
gas)
Hydrogen
sulfide (moist
gas)
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 31 0 100 O O O — O
31 53 0 100 — O O — —
53 120 0 100 — — O — —
0 43 0 100 X O O X X
0 90 0 5 O O O X X
0 38 0 30 O O O X X
0 48 50 90 X O O X X
0 90 5 50 X O O X X
0 31 0 100 O O O O O
31 82 0 100 O O O O —
82 120 0 100 X — O O —
0 38 0 100 X O O O O
38 120 0 100 X — O O —
Hydrogen sulfide (aqueous solution) X X O O X
Hydroquinone O O O O X
Hydroxymethyl ester O O — — —
Hydroxyethylacrylate
Hydroxyphenylethanone O O — — —
Hydroxypropylmethylcellulose X — — O —
Hypochlorite X O O O —
Hypochlorous acid X O O O O
(1) 6,500 psi (448 bar) maximum
0 38 O O — — —
7.8.2 H chemicals with fork density and viscosity meters
Chemical
Temp °C Conc. % wt
Low High Low High
Halogenated alkyl ether — X O — —
Halogenated styrene — X O — —
Helium O — O O —
316L 304L C22 Ti Zr
User Guide 59
Page 60

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
316L 304L C22 Ti Zr
Low High Low High
0 60 0 100 O — O O —
Heptane
60 98 0 100 — — O O —
Hexachlorocyclopentadiene X X X — —
Hexafluoropropene — X O — —
Hexahydrophthalic anhydride O — O — —
Hexamethylenediisocyanate — — O — —
Hexane O — O — —
Hydrazine — O — — —
Hydrobromic acid X X C X C
0 40 0 5 X X O X O
0 25 0 8 X X O X O
Hydrochloric acid
0 100 0 25 X X X X O
0 40 8 40 X X X X O
Temp °C Conc. % wt
Hydrofluoric acid
(aqueous)
0 21 0 10 X X C X X
21 120 10 100 X X C X X
(1)
(1)
(1)
(1)
Hydrofluosilicic acid 10 50 X X C X X
0 31 0 100 O — O — —
Hydrogen cyanide
31 53 0 100 — — O — —
53 120 0 100 — — — — —
0 90 0 5 O — O — O
0 38 0 30 O O O — —
Hydrogen peroxide
0 90 0 50 X X O X O
0 48 50 90 X X O X —
Hydroquinone O — O X —
Hydroxymethyl ester O — O — —
Hydroxyethylacrylate O — O — —
Hydroxyphenylethanone O — O — —
Hydroxypropylmethylcellulose — — O — —
Hypochlorous acid X X O O —
(1) Consult Micro Motion before using Zr when oxidizing impurities are present, such as ferric ions Fe+3 or cupric ions Cu+2.
60 Micro Motion Corrosion Guide
Page 61

User Guide Material compatibility tables
GI-00415 October 2020
7.9 I chemical tables
Table 7-9: Synonyms for I chemicals
Synonym Listed under
Isopropanol Isopropyl alcohol
7.9.1 I chemicals with Coriolis meters
Chemical SS C22 Tz Ta Ti
Ice cream O O O O O
Igepon surfactant O O — — —
Ink O — — O O
Insulin extract — O — O —
Isobutane O O — O —
Isobutanol O — — O O
Isobutyl acetate O — — O —
Isooctyl alcohol O O — — —
Isopar E O O — — —
Isopentane O O — — —
Isopropyl acetate O O O — —
Isopropyl alcohol O O O O O
Isopropylamine O O — — —
7.9.2 I chemicals with fork density and viscosity meters
Chemical
Ice cream O — O — —
Igepon surfactant O — O — —
Ink O — O — —
Insulin extract — — O — —
Isobutane O — O — —
Isobutanol O — O — —
Isobutyl acetate O — O — —
316L 304L C22 Ti Zr
Isooctyl alcohol O — O — —
Isopar E O — O — —
Isopentane O — O — —
Isopropyl acetate O — O — —
User Guide 61
Page 62

Material compatibility tables User Guide
October 2020 GI-00415
Chemical 316L 304L C22 Ti Zr
Isopropyl alcohol O — O O —
Isopropylamine O — O — —
7.10 J chemical tables
Table 7-10: Synonyms for J chemicals
Synonym Listed under
JP-4, JP-5 Jet fuel
7.10.1 J chemicals with Coriolis meters
Chemical
Jet fuel 0 30 0 100 O O O O O
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
7.10.2 J chemicals with fork density and viscosity meters
Chemical
Jet fuel 0 30 0 100 O — O — —
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
7.11 K chemical tables
Table 7-11: Synonyms for K chemicals
Synonym Listed under
KOH Potassium hydroxide
7.11.1 K chemicals with Coriolis meters
Chemical
Kathon LX X O O O —
SS C22 Tz Ta Ti
Kerosene O O O O O
Ketchup O O O O O
62 Micro Motion Corrosion Guide
Page 63

User Guide Material compatibility tables
GI-00415 October 2020
7.11.2 K chemicals with fork density and viscosity meters
Chemical 316L 304L C22 Ti Zr
Kathon Lx 1.5% biocide X X O — —
Kerosene O — O O —
Ketchup O — O O —
7.12 L chemical tables
Table 7-12: Synonyms for L chemicals
Synonym Listed under
Li Lithium
Lime Limestone
Lime sulfur Calcium sulfide
Liquid chlorine Chlorine
Liquor, waste Black liquor
LPG Liquefied petroleum gas
Lube oil Oil, lube
Lycine Betaine
7.12.1 L chemicals with Coriolis meters
Chemical
Lactic acid
Lacquer thinner; lupranate O O — O O
Lard oil O O O O O
Temp °C Conc. % wt
Low High Low High
0 49 0 10 O O O O C
0 49 10 25 O O O O C
49 104 0 10 X O O O C
49 60 10 25 X O O O C
104 120 0 10 — — O O C
25 100 X C O O C
0 100 0 100 O — — — —
SS C22 Tz Ta Ti
Lasso herbicide X — — O —
Latex 0 60 0 100 O — — — O
Latex emulsion O O O — O
Laurylamine oxide O O — — —
User Guide 63
Page 64

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Lauryl bromide X O O O —
Lead acetate 0 104 0 100 O O O O O
Lime slurry
Limestone
Liquified petroleum gas O O — O O
Lithium bromide X O O O —
Lithium
chloride
(1) Maintain a velocity of < 10 ft/sec (3 m/sec)
(1)
(1)
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 55 0 100 X O — — C
0 49 0 8 C O O O C
0 93 0 60 X O O O O
7.12.2 L chemicals with fork density and viscosity meters
Chemical
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
0 49 0 10 O — O O O
0 49 10 25 O — O — O
Lactic acid
Lactose 0 100 0 100 O — O — —
Lacquer thinner/lupranate O — O O —
Lard oil O — O O —
Lasso herbicide X X O — —
Latex 0 60 0 100 O — O O —
Latex emulsion O — O O —
Laurylamine oxide O — O — —
Lauryl bromide X X O — —
Lead acetate 0 104 0 100 O — O O —
Lime slurry 0 55 0 100 — — O O —
Limestone
Lithium bromide X X O — —
Lithium chloride 0 100 0 60 X X O O —
(1)
49 104 0 10 — — O — O
49 60 10 25 — — O — O
104 120 0 10 — — O — O
0 30 25 100 — — O — O
0 49 0 8 — — O O —
(1) Maintain a velocity of < 10 ft/sec (3 m/sec)
64 Micro Motion Corrosion Guide
Page 65

User Guide Material compatibility tables
GI-00415 October 2020
7.13 M chemical tables
Table 7-13: Synonyms for M chemicals
Synonym Listed under
2-methyllactonitrile Acetone cyanohydrin
MDEA Methyldiethanolamine
Methacrylic acid Acrylic acid
Methanal Formaldehyde
Methanecarboxylic acid Acetic acid
Methanoic acid Formic acid
Methanol Methyl alcohol
Methyl benzene Toluene
Methyl cyanide Acetonitrile
Methyltrichlorosilane Methyldichlorosilane
Morkit Anthraquinone
MTBE Methyl Tert Butyl Ether
Muriatic acid Hydrochloric acid
7.13.1 M chemicals with Coriolis meters
Chemical
Magnesium
chloride
Magnesium
hydroxide
Magnesium
nitrate
Magnesium oxide O O O O O
Magnesium silicate O O O — —
Magnesium
sulfate
Magnetic slurries — O O O X
Temp °C Conc. % wt
Low High Low High
0 120 0 100 X O O O O
120 153 50 100 X O X O —
0 100 0 100 O O O O O
100 120 0 100 — — O — —
0 93 0 100 O
0 93 0 50 — O O O O
SS C22 Tz Ta Ti
(1)
O O O O
Maleic acid
Maleic anhydride O O O O O
Malumar O O — — —
User Guide 65
0 80 0 100 O O O O O
80 120 0 100 X — O — O
Page 66

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
SS C22 Tz Ta Ti
Low High Low High
Manganese cobalt acetate O O — — —
Temp °C Conc. % wt
Manganese
sulfate
0 63 0 100 — O — O O
Mayonnaise O O O O O
Mercaptan O O — O —
Mercapto ethanol O O — — —
Methacrylic
acid
0 100 O O — O —
Methane O O O O O
Methane sulfonic acid X O
(2)
— O X
Methanol 0 100 0 100 O O O O X
Methyl acetate 0 60 0 60 O O — — —
Methyl acrylate O O — O —
Methyl alcohol 0 100 0 100 O O O O X
Methyl benzimidazole zinc salt — O — O —
Methyl
bromide
0 20 0 100 O — O O O
20 120 0 100 — — O — —
Methyl chloride 0 104 0 100 X O O O O
Methyl chloride
(anhydrous)
Methyl ethyl
ketone
0 120 100 100 O O O O O
0 93 0 100 O O O O O
Methyl iodide X — — O X
Methyl methacrylate (DL meter only) O O — O —
Methyl sulfonic acid X O
(2)
— O X
Methyl tert butyl ether O — — — —
Methylamine O — X O X
Methyldichlorosilane X O — O —
Methyldiethan
olamine
0 100 O O — — —
Methylene
chloride
0 59 O O O O X
(anhydrous)
Methylene
chloride
0 30 0 100 X C O O X
0 120 0 100 X X O O X
Methylpyrrolidone O O — — —
66 Micro Motion Corrosion Guide
Page 67

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
Methylstyrene O O O O —
Mineral oil O O O O O
Mineral spirits O O — O —
Molasses C O O O O
Monochlorobenzene X O O O X
Monochlorodifluoromethane O O O O —
Monoethanolamine
hydrochloride
Monoethanolamine
Morpholine O O — X —
Musk concentrate O O — — —
Mustard gas X — O O —
(1) 304L included
(2) Maintain a velocity of < 10 ft/sec (3 m/sec).
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 65 0 100 — O X O —
0 100 0 100 O O O O O
7.13.2 M chemicals with fork density and viscosity meters
Chemical
Magnesium chloride
Magnesium
hydroxide
Magnesium nitrate 0 93 0 100 O O O O —
Magnesium oxide O — O O —
Magnesium silicate O — O — —
Magnesium sulfate
Magnetic slurries — — O — —
Maleic acid
Maleic anhydride O — O O —
Manganese cobalt acetate O — O — —
Temp °C Conc. % wt
Low High Low High
0 120 0 100 X X O O —
120 153 50 100 X X O — —
0 100 0 100 O — O O —
100 120 0 100 — — — — —
0 93 0 50 O O O O O
0 100 50 100 — — O O —
0 80 0 100 O — O O —
80 120 0 100 — — — O —
316L 304L C22 Ti Zr
Manganese sulfate 0 63 0 100 O X O O —
User Guide 67
Page 68

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Mayonnaise O — O O —
Mercaptan O — O — —
Mercapto ethanol O — O — —
Methacrylic acid O — O — —
Methane O — O O —
Methanol 0 100 0 100 O O O X O
Methyl acetate 0 60 0 60 O O O — —
Methyl acrylate O — O — —
Methyl alcohol 0 100 0 100 O O O X O
Methyl benzimidazole zinc salt — — O — —
Methyl bromide
Methyl chloride (dry) 0 59 0 100 O X O X —
Methyl chloride 0 104 0 100 X X O O —
Methyl ethyl ketone 0 93 0 100 O — O O —
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
0 20 0 100 O — O O —
20 120 0 100 — — O — —
Methyl iodide X X O X —
Methyl methacrylate O — O — —
Methylsulfonic acid X X O X —
Methylamine060 O X O X —
Methyldichlorosilane — — O — —
Methylene chloride030 O X O X —
Methylene chloride
Methylpyrolidone O — O — —
Mineral oil O — O O —
Mineral spirits O — O — —
Molasses O — O O —
Monochlorobenzene — — O — —
Monochlorodifluoromethane O — O O —
Monoethanoamine
hydrochloride
Monoethanol amine O — O X —
Monoethanolamine 0 100 0 90 O — O X —
0 30 0 100 — X O X —
0 120 0 100 — X — X —
0 65 0 100 — X O — —
68 Micro Motion Corrosion Guide
Page 69

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
316L 304L C22 Ti Zr
Low High Low High
Morpholine O X C — —
Musk concentrate O — O — —
7.14 N chemical tables
Table 7-14: Synonyms for N chemicals
Synonym Listed under
N Nitrogen
NCI-c56326 Acetaldehyde
Nitrobenzol Nitrobenzene
Nordhausen acid (DOT) Sulfuric acid
7.14.1 N chemicals with Coriolis meters
Temp °C Conc. % wt
Chemical
Nadir methyl anhydride O O — — —
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
Nalco 625 — O — — —
Naphtha O O O O O
Naphthalene 0 120 0 100 O O O O O
Naphthalene
sulfonic acid
0 200 0 100 — O
(1)
X O —
Neopentyl glycol — O — — —
Nickel chloride 0 90 0 100 X O O O O
Nickel slurry O O O — —
Nickel sulfate X O O — —
(2)
(2)
(3)
(2)
(2)
(2)
(2)
(2)
(2)
O C O O
O C O O
X O O O
X O O X
O O O O
X O O O
X O O X
X X O X
X X O X
Nitric acid
-18 10 0 75 O
-18 10 75 100 O
10 24 0 70 O
10 24 70 100 O
24 38 0 20 O
24 38 20 50 O
24 38 50 70 X
24 38 70 90 X
24 38 90 100 X
User Guide 69
Page 70

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Temp °C Conc. % wt
Low High Low High
38 52 0 10 O
38 52 10 40 O
38 52 40 70 X
38 52 70 80 X
38 52 80 100 X
52 66 0 30 O
52 66 30 70 X
52 66 70 100 X
66 80 0 20 O
66 80 20 45 X
66 80 45 55 X
66 80 55 100 X
80 93 0 45 X
80 93 45 100 X
93 163 0 100 X
SS C22 Tz Ta Ti
(2)
(2)
(4)
(4)
(3)
(2)
(4)
(3)
(2)
(4)
(4)
(3)
(3)
(3)
(3)
O O O O
X O O O
X O O X
X X O X
X X O X
X O O X
X O O X
X X O X
X O O X
X O O X
X X O X
X X O X
X O O X
X X O X
X X O X
Nitroaniline X O — O —
Nitrobenzene O O O O O
Nitrochlorobenzene X O — O —
Nitrogen O O O O O
Nitrous oxide O O — — X
Nonanoic acid O O X O —
Nonyl phenol O O — O —
(1) Maintain a velocity of < 10 ft/sec (3 m/sec).
(2) 304L = O
(3) 304L = X
(4) 304L = C
7.14.2 N chemicals with fork density and viscosity meters
Chemical
Temp °C Conc. % wt
Low High Low High
Nadir methyl anhydride O — O — —
Nalco 625 — — O — —
Naphtha O — O — —
316L 304L C22 Ti Zr
Naphthalene 0 120 0 100 O — O — —
70 Micro Motion Corrosion Guide
Page 71

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
Naphthalene
sulfonic acid
Neopentyl glycol — — O — —
Nickel chloride 0 90 0 100 X X O O —
Nickel slurry O — O — —
Temp °C Conc. % wt
Low High Low High
0 200 0 100 X X O X —
–18
–18 10 75 100 O O O X O
10 24 0 70 O O X X O
10 24 70 90 O O X X O
10 24 90 100 O O X X X
24 38 0 20 O O O O O
24 38 20 50 O O X X O
24 38 50 90 X O X X O
24 38 90 100 X C X X X
38 52 0 10 O O O O O
38 52 10 40 O O X X O
10 0 75 O O O O O
316L 304L C22 Ti Zr
38 52 40 80 X O X X O
Nitric acid
Nitroaniline X X O — —
Nitrobenzene O — O O —
Nitrochlorobenzene X X O — —
38 52 80 90 X C X X O
38 52 90 100 X C X X X
52 66 0 30 O O O X O
52 66 30 70 X O O X O
52 66 70 90 X C X X O
52 66 90 100 X C X X X
66 80 0 20 X O X X O
66 80 20 45 X C X X O
66 80 45 55 X C X X O
66 80 55 100 X X X X O
80 93 0 45 X X X X O
80 93 45 100 X X X X O
93 140 0 90 X X X X O
Nitrogen O — O O —
User Guide 71
Page 72

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Nonanoic acid sludge O — O — —
Nonyl phenol O — O — —
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
7.15 O chemical tables
Table 7-15: Synonyms for O chemicals
Synonym Listed under
Oil of mirbane Nitrobenzene
Oil of vitriol Sulfuric acid
Opadry Hydroxypropylmethylcellulose
Oxyacetylene Acetylene
Oxybisethanol Diethylene glycol
7.15.1 O chemicals with Coriolis meters
Chemical
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
Octanol O O — — —
Oil, crude C O O O C
Oil, fuel C O O O C
Oil, hydraulic cylinder O O O O O
Oil, lube O O O O O
Oil, soybean O O O O O
Oil, spindle O O O O O
Oil, turpentine O O O O O
Oil, vegetable
Oleum
Orange juice O O O O O
Oxalic acid 0 104 0 10 X O O O X
Oxygen C C O O X
Ozonated water O — O — O
Ozone O O O — O
0 43 0 100 O O O O O
43 104 0 100 O O O O —
-18 20 100 O
20 60 100 O
(1)
(1)
O X O X
C X X X
(1) 304L = O; 10 ft/sec (3 m/sec) maximum for all SS types
72 Micro Motion Corrosion Guide
Page 73

User Guide Material compatibility tables
GI-00415 October 2020
7.15.2 O chemicals with fork density and viscosity meters
Chemical
Octanol O — O — —
Oil, crude C X O — —
Oil, fuel C X O — —
Oil, hydraulic cylinder O — O O —
Oil, lube O — O O —
Oil, soybean O — O O —
Oil, spindle O — O O —
Oil, transformer O — O — —
Oil, turpentine O — O O —
Oil, vegetable
Oil, waste — — O — —
Oleum 20 50 0 100 O O — — —
Orange juice O — O O —
Oxalic acid 0 104 0 10 — — O X O
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
0 43 0 100 O — O O —
43 104 0 100 O — O — —
Oxygen O O O X —
Ozonated water O — — O —
7.16 P chemical tables
Table 7-16: Synonyms for P chemicals
Synonym Listed under
1, 3 - Phthalandione Phthalic anhydride
2 - Propenoic acid Acrylic acid
Pentanethiol Amyl mercaptan
Perchlorocyclopentadiene Hexachlorocyclopentadiene
Phenyl amine Aniline
Phenyl chloride Chlorobenzene
Phenyl ethylene Styrene
Phthalic acid anhydride Phthalic anhydride
Potash, caustic Potassium hydroxide
Propanoic acid Propionic acid
User Guide 73
Page 74

Material compatibility tables User Guide
October 2020 GI-00415
Table 7-16: Synonyms for P chemicals (continued)
Synonym Listed under
Propanol Propyl alcohol
Propanone Acetone
Propenonitrile Acrylonitrile
Propenyl alcohol Allyl alcohol
P-toluenesulphinic acid, p-toluene sulphonic acid Alkylbenzene sulfonic acid
7.16.1 P chemicals with Coriolis meters
Chemical
Paint O O O O —
Palmitic acid O — O — —
Paraffin O O — O O
Paranitrochlorinebenzene X — X O —
Pentamethyl indan O O — — —
Pentane O O O O O
Peracetic acid 0 38 0 35 O O X X X
Perchloroethylene
(anhydrous)
Perchloroethylene
Perfluorochemical inert liquid X — O — —
Peroxide acid — O — — —
Peroxyacetic
acid
Petroleum gas (liquified) O O — O O
Phenol 0 100 0 100 O
Temp °C Conc. % wt
Low High Low High
0 120 100 100 O O O — —
0 120 0 100 X O O — —
0 40 X O X — —
SS C22 Tz Ta Ti
(1)
O X O X
Phenol
formaldehyde
Phenolsulfonic acid X O
Phenothiazine O O — O —
Phosgene 20 65 0 100 X O O O —
Phosphoric
acid-food
grade
74 Micro Motion Corrosion Guide
0 130 0 100 O O X O X
(2)
0 25 0 80 O O O O X
— O —
Page 75

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
SS C22 Tz Ta Ti
Low High Low High
0 100 0 5 O O O O C
Temp °C Conc. % wt
Phosphoric
acid
0 65 5 95 X O O O X
65 100 5 100 X X O O
(3)
Phosphorus, molten X O X O X
Phosphorous acid X O X O —
Phosphorous oxychloride X — O O C
Phosphorous trichloride X X O O O
Phthalic acid O O O O O
-18 99 98 100 O O C C —
Phthalic
anhydride
99 149 98 100 O O X C —
149 204 98 100 O O X — —
Phthalic anhydride/thermon — O — O —
Picric acid X O O O X
Pitch 100 200 0 100 O — X O O
Pivalic acid O O — — —
X
Pivaloyl
chloride
0 50 X X X O X
Platinum chloride X — O O —
Polyacrylamide O O — — —
Polyaluminum
chloride
0 80 0 50 X O O O X
Polyamine 0 182 0 100 — O X O —
Polybutyl chloride X O — O —
Polydimethylaminetetra-chlorohydrate — O — O —
Polydimethylsiloxane
0 200 100 100 O O — — —
Polyester O O — O —
Polyethylene O O — O —
Polyethylene glycol O O O O O
Polyethylene wax O O O — O
Polyisobutylene O O — — —
Polyol O O — — —
Polyphosphoric
acid
0 100 105 118 O O — O X
Polyphosphorous X O X O —
User Guide 75
Page 76

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Polyvinyl alcohol O O — O —
Potassium acetate — — X — —
Potassium
bisulfite
Potassium
bromide
Potassium
carbonate
Potassium
chloride
Potassium
chromate
Potassium
hydroxide
Potassium iodide C O O O O
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 63 0 100 — O O O —
0 31 0 30 X O O O O
0 104 30 50 X X O — O
0 104 50 100 — — O — O
0 100 0 20 O O O X O
0 100 20 90 X O O X O
0 110 0 99 X O X O O
0 160 0 99 X X X O O
0 24 0 10 X O O O —
0 93 0 40 O O O X X
0 100 40 50 X O O X X
Potassium
nitrate
Potassium
permanganate
Potassium
persulfate
Primary stearyl amine O O — — —
Propane O O O O O
Propionic acid
Propyl alcohol 0 104 0 100 O O O O O
Propylene O O O O O
Propylene glycol O O O O O
Propylene oxide O O O O —
Pyridine O C O O X
Pyridine hydrochloride X C C O C
(1) 304L = X
(2) Maintain a velocity < 10 ft/sec (3 m/sec).
(3) Low fluoride
0 100 X O O O O
0 100 0 50 X O O O O
0 24 0 4 X O O O —
0 50 0 97 O O X O —
50 140 0 97 — O X O —
76 Micro Motion Corrosion Guide
Page 77

User Guide Material compatibility tables
GI-00415 October 2020
7.16.2 P chemicals with fork density and viscosity meters
Chemical
Paint O — O O —
Palmitic acid O X O — —
Paper pulp (chlorine
bleached)
Paraffin O — O O —
Paranitrochlorinebenzene X X — — —
Pentamethyl indan O — O — —
Pentane O — O O —
Perchloroethylene (anhydrous) O — O — —
Perchloroethylene (wet) X X O — —
Perfluorochemical inert liquid — X O — —
Peroxide acid — — O — —
Phenol 0 100 0 95 O X O X O
Phenol
formaldehyde
Phenolsulfonic acid X X O X —
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
0 74 0 15 — — O O —
0 130 0 100 O — O X —
Phenothiazine O — O — —
Phosgene 20 65 0 100 — — O — —
Phosphoric acid
Food grade (thermal
process)
Phosphoric acid
Wet chemical
process acid
Phosphorous — X O — —
Phosphorous acid — — O — —
Phosphorous
oxychloride
Phosphorous trichloride X X O C —
Phthalic acid O — O — —
Phthalic anhydride
Phthalic anhydride/thermon — — O — —
0 25 0 85 O — O — —
0 100 0 5 O X O X O
0 65 5 95 C X O X X
65 100 5 100 X X C X X
0 80 X X O C —
–18 99 98 100 O O O O —
99 149 98 100 O O O O —
149 204 98 100 O — O — —
User Guide 77
Page 78

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Picric acid X X O X —
Pitch 100 200 0 100 O — O O —
Pivalic acid O — O — —
Platinum chloride X X O — —
Polyacrylamide O — O — —
Polyamine 0 182 0 100 — — O — —
Polybutyl chloride X X O — —
Polydimethylaminetetra-chlorohydrate X X O — —
Polyester O — O — —
Polyethylene O — O — —
Polyethylene glycol O — O — —
Polyethylene wax O — O O —
Polyisobutylene O — O — —
Polyol O — O — —
Polyphosphorous X X O — —
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
Polyvinyl alcohol O — O — —
Potassium acetate — — O — —
Potassium bisulfite 0 63 0 100 — — O — —
0 31 0 30 X X O O O
Potassium bromide
Potassium carbonate
Potassium chloride
Potassium chromate 0 24 0 10 — — O O O
Potassium hydroxide
Potassium iodide C X O O O
Potassium nitrate 0 100 0 90 — — O O O
Potassium
permanganate
Potassium persulfate 0 24 0 4 O — O — —
0 104 30 50 X X — O O
0 104 50 100 X X — O —
0 100 0 20 O O O O —
0 100 20 90 — X O O —
0 110 0 99 X X O O O
110 160 0 99 X X C O O
0 93 0 40 O — O C O
0 100 40 50 X X O C O
0 100 0 50 C X C O —
Primary stearyl amine O — O — —
78 Micro Motion Corrosion Guide
Page 79

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
Propane O — O O —
Propionic acid
Propyl alcohol 0 104 0 100 O — O O —
Propylene O — O O —
Propylene glycol O — O O —
Propylene oxide O — O — —
Pyridine 0 100 O O C X —
Pyridine
hydrochloride
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
0 70 0 97 O O O X —
0 140 0 97 X X O X —
0 100 X X C X —
7.17 Q chemical tables
Table 7-17: Synonyms for Q chemicals
Synonym Listed under
Quartz Silicon dioxide
7.17.1 Q chemicals with Coriolis meters
Chemical
Quaternary ammonium
chloride
SS C22 Tz Ta Ti
X O — X —
7.17.2 Q chemicals with fork density and viscosity meters
Chemical
Quaternary ammonium chloride X X O — —
316L 304L C22 Ti Zr
7.18 R chemical tables
7.18.1 R chemicals with Coriolis meters
Chemical
Temp °C Conc. % wt
Low High Low High
Red liquor 0 120 X O — — —
Rhodium O O O O —
Rosin 0 200 0 100 — O X O —
SS C22 Tz Ta Ti
User Guide 79
Page 80

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Roundup herbicide X O — O X
Rubber cement O O O — —
Rubber hydrocarbon O O — — —
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
7.18.2 R chemicals with fork density and viscosity meters
Chemical
Red liquor X X O — —
Rosin 0 200 0 100 — — O — —
Roundup herbicide X X O — —
Rubber cement O — O — —
Rubber hydrocarbon O — O — —
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
7.19 S chemical tables
Table 7-18: Synonyms for S chemicals
Synonym Listed under
Saline solution Sodium chloride
Salmiac Ammonium chloride
Salt, salt brine, salt water Sodium chloride
Sea water Brine
Soda, caustic Sodium hydroxide
Soda ash Sodium carbonate
Spindle oil Oil, spindle
Sugar of lead Lead acetate
Sulfonic acid, sulphonic acid Alkylbenzene sulfonic acid and alkylsulfonic acid
Sulfuric acid, fuming Oleum
Sulfurous acid Sulphurous acid
Sulphuric acid Sulfuric acid
80 Micro Motion Corrosion Guide
Page 81

User Guide Material compatibility tables
GI-00415 October 2020
7.19.1 S chemicals with Coriolis meters
Chemical
Safety-kleen 105 O O O O —
Salicylic acid 0 120 0 100 X O O O O
Scalp oil X O O O O
Seawater X O O O O
Sebacic acid 0 104 0 10 — O — O —
Sentol (liquid acid cleaner) — O — O —
Silane 0 80 100 100 O O — — —
Silica slurry O O O O —
Silicon dioxide O O O O —
Silicon tetrafluoride X — O O —
Silicone O O O O O
Silicone oil O O O O O
Silicontetrachloride slurry O O O O —
Silver nitrate O O O O O
Soap fat 0 200 0 100 — O X O O
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
Soap solution O O O O O
Sodium alkyl glyceryl sulfonate — O O — —
Sodium aluminate O O — — O
Sodium
bicarbonate
Sodium
bisulfate
Sodium
bisulfide
Sodium
bisulfite
Sodium
carbonate
Sodium
chlorate
Sodium
chloride
Sodium
chlorite
0 82 0 20 X O O O C
0 60 0 12 O O — X O
0 93 12 60 X C — X —
0 60 0 30 X O O O O
0 100 0 100 O O O X O
0 150 0 100 X O O O O
0 60 0 100 X O O O O
0 35 0 50 X O — X O
0 20 O O O O O
20 100 — — O O O
User Guide 81
Page 82

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
SS C22 Tz Ta Ti
Low High Low High
Sodium citrate 0 38 O X — O O
Temp °C Conc. % wt
Sodium
cyanide
0 38 0 10 O O O — O
0 120 0 100 X X O — —
Sodium formaldehyde O O — O —
Sodium formaldehyde bisulfate O O — O —
Sodium formaldehyde sulfoxylate — O — O —
Sodium
formate
0 100 0 50 O O — — X
Sodium gluconate O O — — —
Sodium
hydrosulfate
Sodium
hydrosulfide
Sodium
hydrosulfite
0 82 0 20 X O O O O
0 60 0 12 O O — X O
0 93 12 60 X C — X —
0 50 0 40 O O O X O
0 53 0 15 O O O X C
0 53 15 20 O O O X X
Sodium
hydroxide
(1)
0 53 20 50 X O O X X
53 86 0 50 X O O X X
86 120 0 100 X X O X X
Sodium
hypochlorite
Sodium
hypophosphite
Sodium laureth
sulfate
Sodium lauryl
sulfate
0 60 0 16 X O
60 80 16 20 X X O O O
O O O O —
0 38 0 70 O — — — —
0 38 0 70 O O — — —
(2)
O O O
Sodium metabisulfite — O O — —
Sodium metal X O X O O
Sodium
methoxide
Sodium
methylate
0 90 0 30 X O — X X
0 90 0 30 X O — X X
0 100 0 60 O O O O O
Sodium nitrate
0 120 60 100 — — O — O
82 Micro Motion Corrosion Guide
Page 83

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
SS C22 Tz Ta Ti
Low High Low High
Sodium nitrite X O O O O
Sodium omadine X O — — O
Temp °C Conc. % wt
Sodium
perchlorlate
0 65 0 100 — O O O —
Sodium persulfate — O — O —
Sodium
phenolate
Sodium
phosphate
0 120 0 100 — O O O —
0 100 X O O C O
Sodium polyphosphate — O O — —
Sodium silicate O O O O O
Sodium sulfate 0 100 0 20 O O O O O
Sodium sulfide 0 120 0 50 X O O O O
0 60 0 10 O O O O O
Sodium sulfite
60 120 0 10 X O O O O
Sodium
thiosulfate
0 38 0 30 O O — — O
Sodium xylene sulphonate O O — O —
Soy oil O O O O O
Soy protein 0 18 — O O O O
Soy sauce X O O O O
Stannic chloride X O X O O
Stannous
chloride
0 75 0 10 X O O O O
0 120 10 100 X O O O —
Starch syrup O O O O —
Stearic acid O O O O O
Styrene O O O O —
Sucrose 0 93 0 62 O O O O —
Sulfamic acid 0 30 O O O O X
Sulfanilic acid O O — — —
Sulfite liquor X O O X O
Sulfolane O O O O —
Sulfonic acid C
(3)
O — O X
Sulfonylchloride X O — O —
Sulfur, molten 0 160 0 100 O
(4)
(3)
O
X O O
User Guide 83
Page 84

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
SS C22 Tz Ta Ti
Low High Low High
Sulfur dichloride X O O O —
Sulfur dioxide (anhydrous) O O X O O
Sulfur dioxide (dry) X O X O X
Sulfur hexafluoride O O — — —
Sulfur monochloride X — — O —
Sulfur tetrafluoride X X — X X
Sulfur trioxide 0 25 0 100 — O O X X
Temp °C Conc. % wt
-18
0 0 20 O
-18 0 20 65 X
(3)
(3)
(3)
O
(3)
O
X O X
X O X
-18 0 65 75 X X X O X
-18 0 75 85 X
-18 0 85 93 —
-18 0 93 100 O
0 24 0 20 O
0 24 20 65 X
(5)
(3)
(3)
(3)
(5)
(5)
O
(3)
O
(3)
O
(3)
O
(5)
O
X O X
X O X
X O X
O O X
O O X
Sulfuric acid
0 24 65 75 X X O O X
0 24 75 85 X
0 24 85 93 X
0 24 93 98 O
0 24 98 100 O
24 38 0 10 O
24 38 10 40 X
(6)
(5)
(5)
(3)
(3)
(3)
(6)
O
(5)
O
(5)
O
(3)
O
(3)
O
(3)
O
O O X
O O X
O O X
X O X
O O X
O O X
24 38 40 75 X X O O X
24 38 75 85 —
24 38 85 93 X
24 38 93 98 O
24 38 98 100 O
38 52 0 5 O
38 52 5 25 X
(6)
(7)
(6)
(3)
(3)
(3)
(6)
—
(5)
O
(6)
O
(3)
O
(3)
O
(3)
O
O O X
O O X
O O X
X O X
O O X
O O X
38 52 25 75 X X O O X
38 52 75 90 X
38 52 90 98 —
38 52 98 100 —
(7)
(5)
(3)(8)
(7)
—
(5)
O
(3)
C
O O X
O O X
X O X
84 Micro Motion Corrosion Guide
Page 85

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
SS C22 Tz Ta Ti
Low High Low High
Temp °C Conc. % wt
52 54 0 5 X
(3)
(3)
O
O O X
52 54 5 75 X X O O X
52 54 75 98 —
52 54 98 100 —
54 66 0 5 X
(9)
(9)(8)
(3)
(9)
—
(9)
—
(9)
O
O O X
X O X
O O X
54 66 5 98 X X O O X
54 66 98 100 X X X X X
66 93 0 50 X X O O X
66 93 50 98 X X O O X
66 93 98 100 X X X X X
93 204 0 100 X X X X X
Sulfuryl fluoride X — O — —
Sulfuryl chloride X X O O —
Sulphurous acid X O O O —
Syrups (water, flour, starch, and corn) — O O O O
(1) Observe the chloride limits shown in Halogens.
(2) No C22 with hypochlorite generators
(3) Maintain a velocity of < 10 ft/sec (3 m/sec).
(4) Avoid H2O and H2S with 316L
(5) Maintain a velocity of < 5 ft/sec (1.5m/sec).
(6) Maintain a velocity of < 4 ft/sec (1.2 m/sec).
(7) Maintain a velocity of < 3 ft/sec (0.9 m/sec).
(8) 304L = O
(9) Maintain a velocity of < 2 ft/sec (0.6 m/sec).
7.19.2 S chemicals with fork density and viscosity meters
Chemical
Temp °C Conc. % wt
Low High Low High
Safety-kleen 105 O O O — —
Salicylic acid 0 120 0 100 X X O O —
Scalp oil X X O O —
Seawater X X O O O
Sebacic acid 0 104 0 10 — — O O —
Sentol (liquid acid cleaner) — — O — —
Silica slurry O — O — —
316L 304L C22 Ti Zr
Silicon dioxide O — O — —
User Guide 85
Page 86

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
316L 304L C22 Ti Zr
Low High Low High
Silicone O — O O —
Silicone oil O — O O —
Silicontetrachloride slurry O — O — —
Silver nitrate X X O — —
Soap Fat 0 200 0 100 — — O — —
Soap solution O — O O —
Sodium alkyl glyceryl sulfonate — — O — —
Sodium aluminate 0 100 0 25 O — — O —
0 120 0 20 O O — O O
Sodium bicarbonate
0 65 20 100 O O O O —
Sodium bisulfate 0 82 0 20 X X O — —
0 60 0 12 O — O O —
Sodium bisulfide
0 93 12 60 X X C — —
Sodium bisulfite X X O O —
Sodium carbonate 0 100 0 100 O O O O O
Temp °C Conc. % wt
Sodium chlorate
60 150 70 100 X
(1)
X O — —
Sodium chloride 0 60 0 100 C X O O O
Sodium chlorite 0 35 0 50 X X O O —
Sodium citrate 0 60 0 38 — O X O —
0 38 0 10 O — O O —
Sodium cyanide
0 100 0 100 — — O O —
Sodium formaldehyde O — O — —
Sodium formaldehyde bisulfate O — O — —
Sodium formaldehyde sulfoxylate — — O — —
Sodium formate 0 100 0 50 O — O X —
Sodium gluconate O — O — —
Sodium hydrosulfate 0 82 0 20 X X O O —
0 60 0 12 O — O O —
Sodium hydrosulfide
0 93 12 60 X X C — —
Sodium hydrosulfite 0 50 0 40 O O — —
0 104 0 70 X X O O O
86 Micro Motion Corrosion Guide
Page 87

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
316L 304L C22 Ti Zr
Low High Low High
Temp °C Conc. % wt
(1)
X O O O
(1)
X O O O
(1)
X O C O
(1)
X O X O
(1)
X C X X
Sodium hydroxide
0 53 0 15 O
0 53 15 20 O
0 53 20 50 O
53 86 0 50 X
86 120 0 100 X
0 60 0 16 X X O O O
Sodium hypochlorite
60 80 16 20 X X X O X
Sodium hypophosphite O — O — —
Sodium laureth
sulfate
0 38 0 70 O — — — —
Sodium lauryl sulfate 0 38 0 70 O — O — —
Sodium metabisulfite — — O — —
Sodium metal — — O — —
Sodium methoxide 0 90 0 30 X X O X —
Sodium methylate 0 90 0 30 X X O X —
0 112 0 60 O O O O —
Sodium nitrate
0 120 60 100 O — — O —
Sodium nitrite — — O O —
Sodium omandine — — O O —
Sodium perchlorlate 0 65 0 100 X X O O —
Sodium persulfate — — O — —
Sodium phenolate 0 120 0 100 — — O — —
Sodium phosphate 0 100 X X O O —
Sodium polyphosphate — — O — —
Sodium silicate O — C O O
Sodium sulfate 0 100 0 20 O O O O O
Sodium sulfide 0 100 0 50 X X O O O
0 60 0 10 O O O O —
Sodium sulfite
60 120 0 10 X X O O —
Sodium thiosulfate 0 38 0 30 O — O O —
Sodium xylene sulphonate O — O — —
Soy oil O — O O —
Soy protein 0 18 — — O O —
Soy sauce — — O O —
User Guide 87
Page 88

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Stannic chloride X X X O —
Stannous chloride
Starch syrup O — O — —
Stearic acid O X O O —
Styrene O — O — —
Sucrose 0 93 0 62 O — O — —
Sulfamic acid 0 30 X X C X —
Sulfanilic acid O — O C —
Sulfite liquor X X O X —
Sulfolane O — O — —
Sulfonic acid X X O X —
Sulfonylchloride X X O X —
Sulfur (molten) 0 120 0 100 O — O — —
Sulfur dichloride X X O X —
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
0 75 0 10 X X O O O
0 120 10 100 X X O O —
Sulfur monochloride X X O X —
24 0 20 O X O X O
Sulfuric acid
–18
–18 24 20 50 X X O X O
–18 24 50 75 X X O X X
–18 24 75 85 X X O X X
–18 24 85 93 X X O X X
–18 24 93 98 O X O X X
–18 24 98 100 O O C X X
24 38 0 10 O X O X O
24 38 10 50 X X O X O
24 38 50 75 X X X X X
24 38 75 85 X X X X X
24 38 85 93 X X O X X
24 38 93 98 O X O X X
24 38 98 100 O O C X X
38 52 0 5 O X O X O
38 52 5 25 X X O X O
38 52 25 50 X X C X O
88 Micro Motion Corrosion Guide
Page 89

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
38 52 50 75 X X X X X
38 52 75 90 X X C X X
38 52 90 98 X X O X X
38 52 98 100 X O X X X
52 54 0 5 O X O X O
52 54 5 10 X X O X O
52 54 10 50 X X X X O
52 54 50 85 X X X X X
52 54 85 98 X X O X X
52 54 98 100 X O X X X
54 66 0 10 X X O X O
54 66 10 50 X X X X O
54 66 50 99 X X X X X
54 66 99 100 X O X X X
66 93 0 50 X X X X O
66 93 50 100 X X X X X
93 204 0 100 X X X X C
Sulfuric fluoride X X O X X
Sulfuryl chloride X X C X C
Sulphurous acid X X C O X
(1) Observe the chloride limits shown in Halogens.
7.20 T chemical tables
Table 7-19: Synonyms for T chemicals
Synonym Listed under
Table salt Sodium chloride
Tallow Animal fat
Tear gas Chloropicrin
Tectilon blue Anthraquinone
Tetrabromoethane, tetrabromoethane,
tetrabromoacetylene
Tetrachloroethylene Perchloroethylene
Acetylene tetrabromide
Tetrachloromethane Carbon tetrachloride
User Guide 89
Page 90

Material compatibility tables User Guide
October 2020 GI-00415
Table 7-19: Synonyms for T chemicals (continued)
Synonym Listed under
Tetramethylene oxide Tetrahydrofuran
Tin dichloride Stannous chloride
Tin tetrachloride Stannic chloride
Toluol Toluene
Transformer oil Oil, transformer
Trichloroethylene, trichlorethylene, trichloroethene Acetylene trichloride
Trichloromethane Chloroform
Turpentine oil Oil, turpentine
7.20.1 T chemicals with Coriolis meters
Chemical
Tall oil fatty acid — O — — —
Tall oil rosin — O X O —
Tall oil soap X O O X —
Tar 150 200 O O X O X
Tar acid 0 200 0 100 X O X O —
Tea O O O O O
Terephthalic
acid
Tetrachloroethane
Tetrachloroethylene
Tetrachloroethylene
(anhydrous)
Tetrachloroethylene sulfide X O — — —
Tetrachlorosilane X O — O —
Temp °C Conc. % wt
Low High Low High
100 160 0 100 O
0 70 0 100 X O O O O
0 120 0 100 X O O — —
0 120 100 100 O O O — —
SS C22 Tz Ta Ti
(1)
O X O —
Tetrafluoroethane O O O — —
Tetrahydrafluorine (anhydrous) — — O — —
Tetrahydrofuran O O X — X
Tetramethylammonium
hydroxide
Tetrasodium EDTA O O — — —
90 Micro Motion Corrosion Guide
0 60 0 35 X O O X —
Page 91

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
SS C22 Tz Ta Ti
Low High Low High
Thinner O O — O O
Thiodichloric acid X O — O —
Thionyl chloride X X O O X
Tin liquor X O X O —
Temp °C Conc. % wt
Titanium
chloride
0 100 X O O O O
Titanium dioxide O O O O O
Titanium iron sulfate solution — — O — —
Titanium
tetrachloride
0 100 X O O O O
Toluene O O O O O
Toluene diisocyanate O O — O —
Toluenesulfonic acid
0 125 0 94 C O
(2)
X O X
Tomato paste O O O — —
Triacetin O O — — —
Tribromomethane X — O O —
Trichloroacetic
acid
0 120 0 50 X O X O X
Trichloroacetyl chloride X X — O —
Trichlorobenzene X O O O —
Trichlorobromomethane X — O O —
Trichloroethane X X X O O
Trichloroethylene (anhydrous) O O — O O
Trichloromethylpyridine X O — O —
Trichloromonofluoroethane O O — — —
Trichlorosilane (anhydrous) O O — O —
Trichlorotrifluoroethane O O O — X
Triethanolamine
0 95 0 100 O O O — O
Triethyl aluminum O O — O X
Triethylamine O O — O —
Triethylene glycol O O X O O
Trifluoroacetic acid X O X — —
Trimethylchlorocyanate X O O O —
User Guide 91
Page 92

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Trimethyl sulfonium bromide X — — O —
Triphenylmethylchloride X O — O —
Triphenyl phosphite O O X O O
Trisodiumphosphate
Trityl chloride X O — O —
Tungsten
hexafluoride
Turpentine O O O O X
(1) Avoid bromides and chlorides
(2) Maintain a velocity of < 10 ft/sec (3 m/sec)
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 200 0 90 X O X O —
0 60 0 50 O O O O —
0 50 100 100 X O — — —
7.20.2 T chemicals with fork density and viscosity meters
Chemical
Tall oil fatty acid — — O — —
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
Tall oil rosin — — O — —
Tall oil soap — — O — —
Tar 150 200 O — O — —
Tar acid 0 200 0 100 — — O — —
Tea O — O O —
Terephthalic acid 100 160 0 100 C — O O —
Tetrachloroethane 0 70 0 100 — — O — —
Tetrachloroethylene (anhydrous) O X O X —
Tetrachloroethylene (wet) X X O X —
Tetrachloroethylene sulfide — — O — —
Tetrachlorosilane — — O — —
Tetrafluoroethane (dry) O — O — —
Tetrahydrofuran O — O X —
Tetramethylammonium
hydroxide
Tetrasodium EDTA O — O — —
Thinner O — O — —
0 60 0 35 — — O — —
Thionyl chloride X X O X —
92 Micro Motion Corrosion Guide
Page 93

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
Thiodichloric acid — — O — —
Tin liquor — — O — —
Titanium chloride 0 100 X X O O —
Titanium dioxide O — O O —
Titanium iron sulfate solution — — O O O
Titanium
tetrachloride
Toluene O — O — —
Toluene diisocyanate O — O — —
Toluenesulfonic acid 0 125 0 94 X X O X —
Tomato paste O — O — —
Triacetin O — O — —
Tribromomethane X X O — —
Trichloroacetic acid 0 120 0 50 X X O X X
Trichloroacetyl chloride — — O — —
Trichlorobenzene X X O — —
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
0 100 X X O O —
Trichlorobromomethane — — O — —
Trichloroethane O — O O O
Trichloroethylene (dry) O — O X —
Trichloromethylpyridine — — O — —
Trichloromonofluoroethane O — O — —
Trichlorosilane (anhydrous) O — O — —
Trichlorotrifluoroethane O — O — —
Triethanolamine 0 95 0 100 O — O O —
Triethyl aluminum O — O X —
Triethylamine O — O — —
Triethylene glycol O — O O —
Trifluoroacetic acid X X O X —
Trimethyl sulfonium bromide X X — — —
Trimethylchlorocyanate — X O — —
Triphenylmethylchloride X X O — —
Triphenyl phosphite O — O — —
Trisodiumphosphate
0 200 0 90 — — O — —
0 60 0 50 O O O O O
User Guide 93
Page 94

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Tritylchloride X X O — —
Turpentine O — O X —
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
7.21 U chemical tables
7.21.1 U chemicals with Coriolis meters
Chemical
Urea 0 90 0 100 O O O O O
7.21.2 U chemicals with fork density and viscosity meters
Chemical
Urea 0 90 0 100 O — O O O
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
7.22 V chemical tables
Table 7-20: Synonyms for V chemicals
Synonym Listed under
Vinegar acid Acetic acid
Vinyl benzene Styrene
Vinyl cyanide Acrylonitrile
Vinylformic acid Acrylic acid
Vinyltrichlorosilane Methyldichlorosilane
Vitriol brown oil Sulfuric acid
7.22.1 V chemicals with Coriolis meters
Chemical
Temp °C Conc. % wt
Low High Low High
Vanadium benzene O O — — —
Vanadium chloride X O O O —
Vanadium oxychloride X O — O —
Vanadium oxytrichloride X O O O —
SS C22 Tz Ta Ti
94 Micro Motion Corrosion Guide
Page 95

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
Vanadium tetrachloride X O O O —
Vanadium triacetylacetonate X O X O —
Varnish O O O O —
Vazo X O — — —
Vegetable
tanning liquor
Vinegar O O X O O
Vinyl acetate O O O — O
Vinyl acetate polymer residues O O — — —
Vinyl chloride
(latex)
Vinyl chloride
(monomer)
Vinyl fluoride X — O X —
Vinylidene chloride X O O O X
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 79 0 100 — O O O —
0 60 0 100 — O O O O
0 65 0 100 O O O O O
7.22.2 V chemicals with fork density and viscosity meters
Chemical
Vanadium benzene O — O — —
Vanadium chloride X X O — —
Vanadium oxychloride X X O — —
Vanadium oxytrichloride X X O — —
Vanadium tetrachloride X X O — —
Vanadium triacetylacetonate — — O — —
Varnish O — O — —
Vazo — X O — —
Vegetable tanning
liquor
Vinegar O — O O —
Vinyl acetate O — O — —
Vinyl acetate polymer residues O — O — —
Vinyl chloride (latex) 0 60 0 100 — — O — —
Temp °C Conc. % wt
Low High Low High
0 79 0 100 — — O — —
316L 304L C22 Ti Zr
Vinyl chloride
(monomer)
User Guide 95
0 65 0 100 O — O — —
Page 96

Material compatibility tables User Guide
October 2020 GI-00415
Chemical
Vinyl fluoride — — O — —
Vinylidene chloride X X O — O
Vitamin E O — O — —
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
7.23 W chemical tables
Table 7-21: Synonyms for W chemicals
Synonym Listed under
Waste liquor Black liquor
Waste oil Oil, waste
Water glass Sodium silicate
7.23.1 W chemicals with Coriolis meters
Chemical
(1)
Water
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
0 200 0 100 O O O O O
Water, flour, starch, and corn syrups — O O O O
Wax emulsion O O O — O
Whey and milk O O O O O
Whiskey O O O O O
White liquor 20 50 0 100 X O O X —
(1) Observe chloride limits shown in Halogens
7.23.2 W chemicals with fork density and viscosity meters
Chemical
Temp °C Conc. % wt
Low High Low High
Water 0 200 0 100 O
Water/Flour/Starch/Corn syrup — — O O —
Wax emulsion O — O O —
Whey/Milk O — O O —
Whisky O O O — —
White liquor 20 50 0 100 — — O — —
316L 304L C22 Ti Zr
(1)
(1)
O
O O O
96 Micro Motion Corrosion Guide
Page 97

User Guide Material compatibility tables
GI-00415 October 2020
Chemical
Wine O — O O —
(1) Observe the chloride limits shown in Halogens.
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
7.24 X chemical tables
7.24.1 X chemicals with Coriolis meters
Chemical
Xylene 20 120 0 100 O O O O O
7.24.2 X chemicals with fork density and viscosity meters
Chemical
Xylene 20 120 0 100 O — O O O
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
Temp °C Conc. % wt
316L 304L C22 Ti Zr
Low High Low High
7.25 Y chemical tables
7.25.1 Y chemicals with Coriolis meters
Chemical
Yeast O O — — —
Yogurt O O O O —
7.25.2 Y chemicals with fork density and viscosity meters
Chemical
Yeast O — O — O
Yogurt O — O — —
SS C22 Tz Ta Ti
316L 304L C22 Ti Zr
User Guide 97
Page 98

Material compatibility tables User Guide
October 2020 GI-00415
7.26 Z chemical tables
7.26.1 Z chemicals with Coriolis meters
Chemical
Zeolite
Maximum slurry velocity is 6 ft/sec (1.8 m/sec)
Zinc carbonate
slurry
Zinc chloride 0 107 0 71 X O O O O
Zinc dialkyl dithiophosphate X O — O —
Zinc
hydrosulfite
Zinc sulfate 0 111 0 34 X O O O O
Zirconium
chloride
Temp °C Conc. % wt
SS C22 Tz Ta Ti
Low High Low High
— O O — —
0 21 0 100 — O O O —
21 82 0 100 — O O O —
0 120 0 10 X O O O —
0 85 0 25 X O O O —
7.26.2 Z chemicals with fork density and viscosity meters
Chemical
Temp °C Conc. % wt
Low High Low High
Zeolite — — O — —
316L 304L C22 Ti Zr
Zinc carbonate slurry
Zinc chloride 0 107 0 71 X X O O —
Zinc dialkyl dithiophosphate — — O — —
Zinc hydrosulfite 0 120 0 10 — — O — —
Zinc sulfate 0 111 0 34 — — O O —
Zirconium chloride 0 85 0 25 X X O — —
98 Micro Motion Corrosion Guide
0 21 0 100 — — O — —
21 82 0 100 — — O — —
Page 99

User Guide
GI-00415 October 2020
User Guide 99
Page 100

*GI-00415*
GI-00415
Rev. I
2020
Micro Motion Inc. USA
7070 Winchester Circle
Boulder, Colorado USA 80301
T +1 303-527-5200
T +1 800-522-6277
F +1 303-530-8459
www.emerson.com
Micro Motion Asia
Emerson Automation Solutions
1 Pandan Crescent
Singapore 128461
Republic of Singapore
T +65 6363-7766
F +65 6770-8003
©
2020 Micro Motion, Inc. All rights reserved.
The Emerson logo is a trademark and service mark of Emerson Electric Co. Micro Motion, ELITE,
ProLink, MVD and MVD Direct Connect marks are marks of one of the Emerson Automation
Solutions family of companies. All other marks are property of their respective owners.
Micro Motion Europe
Emerson Automation Solutions
Neonstraat 1
6718 WX Ede
The Netherlands
T +31 (0) 318 495 555
T +31 (0) 70 413 6666
F +31 (0) 318 495 556
www.emerson.com/nl-nl
Micro Motion United Kingdom
Emerson Automation Solutions
Emerson Process Management Limited
Horsfield Way
Bredbury Industrial Estate
Stockport SK6 2SU U.K.
T +44 0870 240 1978
F +44 0800 966 181
 Loading...
Loading...