Page 1

USER’S GUIDE
o
™
o
MODELS CA-3000 & CA-3200
MODELS CM-3000 & CM-3200
M
EDICAL PRODUCT INNOVATIONS SINCE
192
8
Page 2
TABLE OF CONTENTS
S ECTION
1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . .
2 Warnings and Cautions . . . . . . . . . . . . . . . . . .
3 Specifications . . . . . . . . . . . . . . . . . . . . . . . . .
4 Controls, Connectors and Visual Indicators . . . .
5 Operating Procedure . . . . . . . . . . . . . . . . . . . .
2
3
6
7
10
6 Cleaning and Disinfection . . . . . . . . . . . . . . . .
7 Accessories . . . . . . . . . . . . . . . . . . . . . . . . . .
8 Preventive Maintenance and Troubleshooting Guide
9 Warranty and Service . . . . . . . . . . . . . . . . . . .
14
15
16
18
1
Page 3

SECTION 1
I NTRODUCTION
The Emerson CoughAssist™ Mechanical In-Exsufflator (MI-E) assists patients in
clearing retained bronchopulmonary secretions by gradually applying a positive
pressure to the airway, then rapidly shifting to a negative pressure. This rapid
shift in pressure, via a facemask, mouthpiece or an endotracheal or tracheostomy
tube, produces a high expiratory flow rate from the lungs, simulating a cough, a
technique referred to as “mechanical insufflation-exsufflation.” The automatic
CoughAssist MI-E (CA-3000, CA-3200) has timing mechanisms to automate the
inspiratory and expiratory cycles as well as a manual control. The manual
CoughAssist MI-E (CM-3000, CM-3200) uses a manually operated valve to shift
from positive to negative pressure and back.
Those who might benefit from the use of the CoughAssist MI-E include any patient
with an ineffective cough due to muscular dystrophy, myasthenia gravis, poliomyelitis,
or other neurologic disorder with some paralysis of the respiratory muscles, such
as spinal cord injury. It may also be used to treat ineffective cough due to other
bronchopulmonary diseases, such as emphysema, cystic fibrosis and bronchiectasis.
It is effective for both trached and non-invasively ventilated patients.
V
Indications for Use: Any patient unable to cough or clear secretions effectively
due to reduced peak cough expiratory flow (less than 2 to 3 liters per second),
resulting from high spinal cord injuries, neuro-muscular deficits or severe
fatigue associated with intrinsic lung disease, is a candidate for this device.
V
Contraindications: Any patient with a history of bullous emphysema,
known susceptibility to pneumothorax or pnuemo-mediastinum, or known to
have had any recent barotrauma, should be carefully considered before use.
2
Page 4
SECTION 2
WARNINGS AND
C AUTIONS
EQUIPMENT CLASSIFICATION
Per IEC 60601-1, Medical Electrical Equipment, General Requirements for Safety, the
CoughAssist MI-E is classified as follows:
Class 1 Equipment: Equipment in which protection against electric shock does not
rely on basic insulation only, but includes a grounding pin on the power cord. For
ground reliability always plug the power cord into an AC grounded outlet.
Type BF Equipment: Type B piece of equipment with an F-Type applied part.
A Type B piece of equipment is one that provides a particular degree of protection
against electric shock, particularly regarding allowable leakage current and reliability
of the protective earth connection (grounding).
F-Type applied part is one that extends from the patient into the equipment and is
isolated from all other parts of the equipment.
Water Ingress: This device does not have protection against ingress of water.
Disinfection: With the exception of the patient circuit, this device can be disinfec-
ted using 70% isopropyl alcohol or equivalent. (See Section 6: Cleaning and
Disinfection.)
Flammable Anesthetics: This device is not suitable for use in the presence of a
flammable anesthetic mixture with air, or in the presence of a flammable anesthetic
mixture with oxygen or nitrous oxide.
Intermittent Operation: This device is designed for Intermittent Operation Only
and not for continuous use. The device should not be cycled continuously for more
than 5 minutes. After such time, the unit should either be turned off or left idling with
the blower on for at least 5 minutes.
3
Page 5

IMPORTANT SAFEGUARDS
D EFINITIONS
Throughout this guide the following definitions apply:
• Warning/DANGER: A condition that could cause electrocution or injury to a user
or operator if instructions are not followed.
• CAUTIONS: A condition that could cause damage to equipment or cause inaccurate
function.
WARNINGS
V
Patients known to have cardiac instability should be monitored for pulse and oxygen
saturation very closely.
V
Soreness and/or pain in the chest from a pulled muscle may occur in patients
using the CoughAssist MI-E for the first time if the positive pressure used exceeds
pressures, which the patient normally receives during Positive Pressure Therapy.
Such patients should start at a lower positive pressure during treatment, and gradually
(over several days, or as tolerated) increase the positive pressure used.
1
V
Do not use in the presence of flammable anesthetics.
V
Connection should be made to a grounded outlet only.
V
Do not place or store the device where it can fall or be pulled into a tub or sink.
V
If the device comes into contact with water, unplug the unit.
V
Never operate the CoughAssist MI-E if it has a damaged cord or plug, is not working properly, or has been dropped, damaged or immersed in water.
1 Positive Pressure Therapy includes the use of a volume ventilator, nasal or mask ventilation or CPAP (Continuous
Positive Airway Pressure), or IPPB (Intermittent Positive Pressure Breathing).
4
Page 6
WARNINGS AND
C AUTIONS
V
Replace fuses only with ones having the same ratings for blow characteristics,
current and voltage.
V
Do not remove the cover; there are no serviceable parts inside the unit. Refer
all service to authorized personnel.
V
Always check time and pressure settings before each treatment.
AUTIONS
C
• Federal Law (USA) restricts this device to use by, or at the direction of a physician.
• Position the CoughAssist MI-E so that the air intake ports on the side and
rear of the unit are not blocked.
• Never operate the device unless a bacterial/viral filter is attached to the
patient circuit.
• Always use a new filter when using the device on a new patient.
• This device is designed for Intermittent Operation Only and not for continuous
use. The device should not be cycled continuously for more than 5 minutes.
After such time, the unit should either be turned off or left idling with the
blower on for at least 5 minutes.
• Turn the unit off when not in use.
• Keep the cord away from heated surfaces.
• Do not sterilize with ethylene oxide gas or steam sterilize the pump or
pump housing.
• This device should only be used by trained personnel.
5
Page 7

SECTION 3
S PECIFICATIONS
Maximum Positive Pressure:
Maximum Negative Pressure:
Standards:
Maximum Inhalation Flow:
Maximum Exhalation Flow Capacity:
Pressure Gauge:
Mode of Operation:
Inhalation, Exhalation, Pause Times:
“Off” Position:
Blower Type:
Input Voltage:
60 cm H2O (44 mm Hg)
60 cm H2O (44 mm Hg)
Conforms to UL STD 2601-1, certified to CAN/CSA - STD C22.2 No. 601.1-M90, 0413
3.3 liters/second with inhale flow set to minimum; if set to maximum inhalation, the flow is
the same as the exhalation flow
10 liters/second; actual flow depends on maximum pressure and on airway resistance
-70 to 0 to +70 cm H
Models CA-3000, CA-3200: Automatic and Manual Timing
Models CM-3000, CM-3200: Manual Timing
CA-3000, CA-3200: Automatic mode, 0 to 5 seconds
CM-3000, CM-3200: User variable
Yes - connects to ambient
Two-stage centrifugal blower with AC/DC universal motor
CA-3000, CM-3000: 100-120 VAC, 60Hz
CA-3200, CM-3200: 220-240 VAC, 50Hz
O; accuracy +/-2% of full scale
2
Input Power:
Dimensions:
Weight:
Temperature Specifications:
Humidity Specifications:
CA-3000, CM-3000: 300 VA
CA-3200, CM-3200: 600 VA
292mm x 279mm x 419mm (HxWxD)
(11.5” x 11” x 16.5”)
CA-3000, CA-3200: 11 kg (24 lbs)
CM-3000, CM-3200: 9.3kg (20.6 lbs)
Operating temperature range: +10 to +40°C (+50 to +104°F)
Storage temperature range: -20 to +50°C (-4 to +122°F)
Operating humidity range: 30% to 75%
Storage humidity range: 10% to 90%
6
Page 8

SECTION 4
CONTROLS
o
, CONNECTORS & VISUAL INDICATORS
CA-3000, CA-3200
RONT PANEL
F
1. Manual/Auto: Changes the cycling mechanism to either manual or automatic mode.
2. Inhale: Sets time interval (sec) for Inhale phase of automatic cycling.
Not operative in the manual mode.
3. Exhale: Sets time interval (sec) for Exhale phase of automatic cycling.
Not operative in the manual mode.
4. Pause: Sets time interval (sec) for Pause phase of automatic cycling.
Not operative in the manual mode.
CONTROLS
5. Power: The power switch turns on or off the CoughAssist MI-E. The “I” symbol on the
switch designates the on position. When activated a green light within the switch will
illuminate. The “O” symbol designates the off position.
6. Manual Control Lever: Use to manually cycle the unit to inhale or exhale. Not operative
in the automatic mode.
7. Pressure: Varies the inhalation and exhalation pressures together (also see Inhale Pressure).
8. Handle: Recessed carrying handle.
9. Patient Port: Connection for patient circuit.
10. : Symbol for Type B Equipment with F-Type applied part.
11. Inhale Flow: There are two inhalation flow settings: Full ( ) and Reduced ( ).
NOTE: When using reduced inhalation flow there will be a small reduction in inhale pressure.
12. Inhale Pressure: Varies the inhalation pressure between 50% and 100% of the
exhale pressure.
7
13. Pressure Gauge Zero Adjust: Access to “zero” adjustment on the pressure gauge.
Use only if the gauge does not return to “O” when the unit is turned off (see Section 8).
14. Pressure Gauge: Indicates pressure in the patient circuit calibrated in cm H
O.
2
Page 9
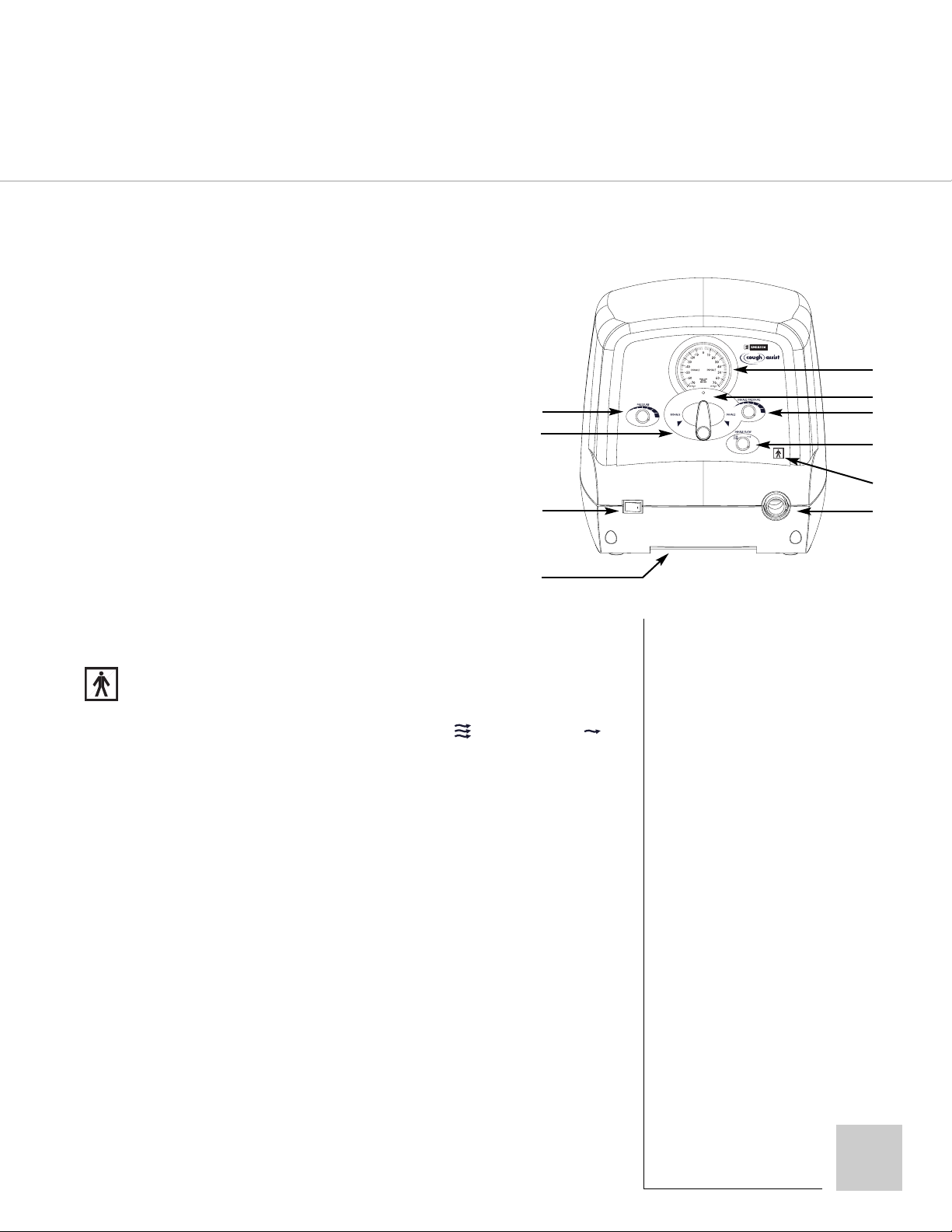
CM-3000, CM-3200
RONT PANEL C ONTROLS
F
1. Pressure: Varies the inhalation and exhalation pressures together
(also see Inhale Pressure).
1
2. Manual Control Lever: Use to manually cycle the unit to inhale
or exhale.
3. Power: The power switch turns on or off the unit. The “I” symbol
on the switch designates the on position. When activated a green
light within the switch will illuminate. The “O” symbol designates
the off position.
4. Handle: Recessed carrying handle.
5. Patient Port: Connection for patient circuit.
6. : Symbol for Type B Equipment with F-Type applied part.
7. Inhale Flow: There are two inhalation flow settings: Full ( ) and Reduced ( ).
NOTE: When using reduced inspiratory flow there will be a small reduction in inspiratory pressure.
8. Inhale Pressure: Varies the inhalation pressure between 50% and 100% of the
exhale pressure.
2
3
4
10
9
8
7
6
o
5
9. Pressure Gauge Zero Adjust: Access to “zero” adjustment on the pressure gauge.
Use only if the gauge does not return to “0” when the unit is turned off (see Section 8).
10. Pressure Gauge: Indicates pressure in the patient circuit calibrated in cm H2O.
8
Page 10

BACK PANEL (ALL MODELS)
1. Cord Wrap/Breathing Hose Holder
2. Power Cord Receptacle: Securely connects the power cord to the
receptacle.
3. Replacement fuse location
4. This symbol, located next to the protective earth terminal inside
the unit, signifies that the device is earthed (grounded).
5. This symbol signifies the year of manufacture of the
device. The name and address of our authorized representative for
Europe appears below it.*
6. Marking of conformity to European Medical Device Directive,
plus the 4-digit number signifying the applicable notified body.*
7. Listing of product safety standards
8. Product Label Description:
9
Page 11

SECTION 5
O PERATING
P ROCEDURE
INITIAL S ET- UP
1. Install the power cord right angle connector to the receptacle on the rear of the
device. Run the cord inside of the lower cord wrap to act as a strain relief.
2. Position the unit on a suitable surface within easy reach of the patient, or the operator
of the unit. CAUTION: Position the device so that the air intake ports on the side
and rear of the unit are not blocked.
3. Assemble the patient circuit (filter, breathing hose and patient interface) as follows:
a. Attach the bacterial/viral filter to the patient port on the front panel.
b. Attach the 3-foot 22mm ID smooth bore breathing hose to the bacterial/viral filter.
c. Attach the appropriate patient interface to the breathing hose. Patient interface
options include a facemask and adapter, mouthpiece, lip seal or tracheostomy
tube adapter. (A facemask and adapter are included with each unit.)
4. Plug the power cord into
an AC grounded outlet of
appropriate voltage.
NOTE: There are no requirements regarding avoidance with other devices
CoughAssist MI-E meets IEC 60601-1-2 for Electromagnetic Interference and Immunity, Medical Electrical Equipment,
2
Part 1, General Requirements for Safety, 2. Collateral Standard: Electromagnetic Compatibility Requirements and Tests,
which defines standards for both electromagnetic emissions and immunity.
2
.
Page 12

PRESSURE
Each individual patient may require special settings for the maximum positive
(inhalation) and negative (exhalation) pressures. For a patient using this
device for the first time, it is advisable to begin with lower pressures, such
as 10-15 cm H2O positive and negative, to familiarize the patient with the
feel of mechanical insufflation-exsufflation. During subsequent treatments,
pressures can be increased as necessary to achieve adequate secretion
clearance. See WARNING (page 4).
Note that at these lower pressures the CoughAssist MI-E may have limited effectiveness in clearing secretions. Increasing pressures should improve the effectiveness.
1. Turn on the power switch.
2. Set the inhale flow to full or reduced.
3. Attach the patient circuit to the unit and block the end of the breathing hose.
ADJUSTMENT
4. Set the manual/auto switch to manual (automatic models only).
5. Push the manual control lever to the exhalation phase (to the left). Observe the
pressure gauge on the device and adjust the maximum pressure (negative) using the
pressure knob to achieve the correct reading on the gauge.
6. Shift the manual control lever to the inhalation phase (push to the right). Adjust the
pressure reading by turning the inhale pressure knob to achieve the correct reading
on the pressure gauge (clockwise to increase pressure and counterclockwise to
decrease pressure).
7. Cycle the manual control lever from inhale (positive) to exhale (negative) and back
a few times to ensure that the pressure and suction readings are correct.
8. Release the manual control lever to ensure that the pressure immediately returns to
0cm H2O. If it does not, refer to the Maintenance Section of this guide.
11
Page 13

O PERATING
P ROCEDURE
TIMING A DJUSTMENT
(CA-3000, CA-3200 MODELS ONLY)
If the automatic feature of the CoughAssist MI-E is to be used, adjust the times as follows:
1. Each cough cycle consists of an inhalation phase, an exhalation phase and a pause
phase, after which inhalation begins again. The time for each phase is set with the
three knobs on the left side of the front panel. Normally, inhale time and exhale time
are set to 1 to 3 seconds and the pause time can be set up to 5 seconds, or eliminated
by setting the pause time knob to 0 seconds, depending on the patient’s preference.
2. Set the manual/auto switch to the auto position and observe that the unit cycles from
positive to negative pressure, then to zero pressure, and repeats until the switch is set
back to manual. When set to the manual position, the unit should return to 0 cm H
REATMENT
T
O.
2
Treatment usually consists of 4 or 5 coughing cycles in succession. The patient is then
allowed to rest for 20 to 30 seconds, which helps avoid hyperventilation. The cycles can
then be repeated 4 to 6 times for a full treatment.
WARNING: Always check time and pressure settings before each treatment.
Manual Operation (All models):
1. Attach the appropriate patient interface to the patient.
2. Shift the manual/auto switch to the manual position (automatic models only). Shift
the manual control lever to the inhale position (to the right) and observe the pressure gauge to see the pressure build slowly over 2 to 3 seconds.
3. Rapidly shift the manual control lever to the exhale position (to the left) to induce
the cough, holding it there for 1 to 2 seconds. The lever can then either be left in
the neutral position for a few seconds, or can be shifted immediately to the positive
pressure phase for another cough cycle, depending on the patient’s preference.
4. After 4 to 5 cycles, remove the patient interface from the patient and allow time for a
normal breathing pattern to return (20 to 30 seconds), or place the patient back on
the ventilator if currently in use. Avoid prolonged periods connected to the device.
During this resting period, clear secretions that may have become visible in the
mouth, throat or tracheostomy tube.
12
Page 14

PERATING P ROCEDURE
O
Automatic Operation (CA-3000, CA-3200 models only):
1. Attach the appropriate interface to the patient.
2. To operate the unit automatically, set the manual/auto switch to the auto position.
The unit will cycle from inhale (positive) to exhale (negative) to zero pressure, and
back to inhale.
3. After 4 to 5 cycles, set the manual/auto switch back to the manual position. Remove
the patient interface from the patient and allow time for a normal breathing pattern to
return (20 to 30 seconds), or place the patient back on the ventilator if currently in
use. Avoid prolonged periods connected to the device. During this resting period, clear
secretions that may have become visible in the mouth, throat or tracheostomy tube.
CAUTION: This unit is designed for Intermittent Operation Only and not for continuous
use. The device should not be cycled continuously for more than 5 minutes. After such
time, the unit should either be turned off or left idling with the blower on for at least
5 minutes.
OPERATION VERIFICATION (ALL MODELS)
It is recommended that the CoughAssist MI-E be periodically tested to ensure that the
cycling valve returns to the neutral, or pause, position after either the inhale or exhale
phase. To determine this, follow these steps:
1. Attach a patient circuit to the unit and block the end of the hose.
2. Turn the power switch ON.
3. Set the manual/auto switch to the manual position (automatic models only).
4. Set the pressure knob fully clockwise (maximum pressure).
5. Cycle the manual control lever from inhale to exhale and observe the pressure gauge
to ensure that positive and negative pressure is being applied to the patient circuit.
6. Release the manual control lever from the inhale position and observe that the pressure
immediately drops to 0 cm H
pressure does not drop to zero, the unit should be returned for repair.
O. Repeat for the exhale position. In either case, if the
2
13
Page 15

SECTION 6
CLEANING
IRCUIT
PATIENT
Institutional (hospital) Use:
1. Breathing Hose, Patient Interface and Adapters: If the device is to be used by more
than one patient, the circuit must be replaced.
2. Bacterial/V
must be replaced to prevent cross contamination. Do not try to wash the filter.
NOTE: The patient circuit should not be sterilized for reuse.
Home (individual) Use:
1. Br
eathing Hose, Patient Interface and Adapters: After use, the breathing hose and
patient interface should be washed thoroughly in soap and water. These parts must
be completely dry before reuse.
C
iral Filter: If the device is to be used by more than one patient the filter
& D
ISINFECTION
2. Bacterial/V
material from the patient, can be left in place as long as it is not blocked by sputum or trapped moisture. Do not try to wash the filter.
NOTE: The patient circuit should not be sterilized.
iral Filter: The filter, which protects the device from entraining foreign
EXTERNAL H OUSING
The exterior of the pump and housing may be washed with a mild detergent and water,
or with a bactericidal cleaning solution such as 70% isopropyl alcohol.
CAUTION: Do not sterilize with ethylene oxide gas or steam sterilize the pump
or pump housing.
14
Page 16
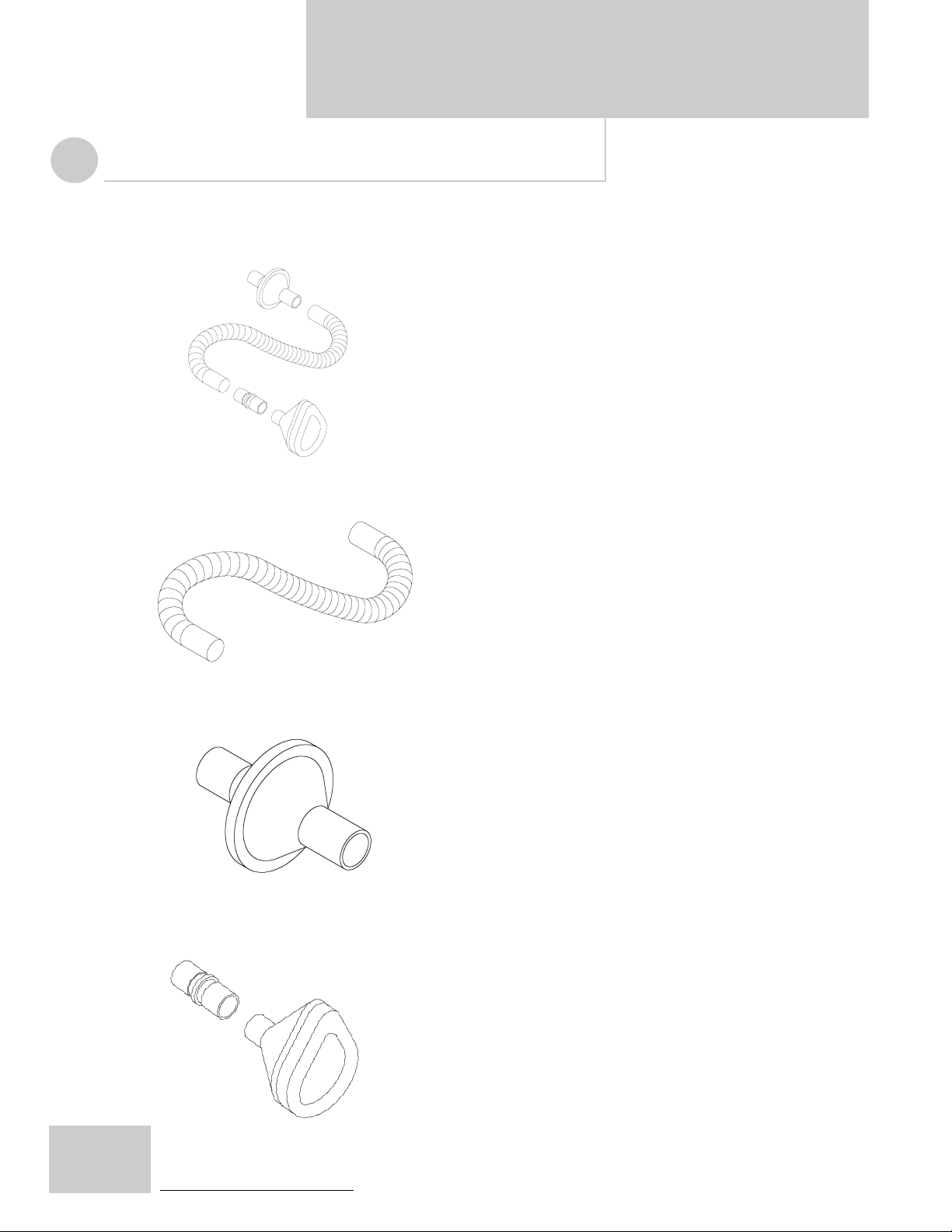
SECTION 7
ACCESSORIES
The following replacement accessories may be obtained from J.H. Emerson Co.
CoughAssist MI-E Patient Circuit
The CoughAssist MI-E Patient Circuit consists of one 3-foot long
flexible smooth bore tube, a bacterial/viral filter, an adult facemask and an adapter.
Part No. 325-9217
Breathing Hose
Part No. 732-1136
Part No. 740-1008
3-foot long flexible smooth bore tubing with 22mm interior diameter.
NOTE: Corrugated tubing may cause a small reduction in flow
rates as well as cause a whistling sound. The use of tubing
greater than 3-feet in length may cause a small reduction in
flow rates as well.
Bacterial/Viral Filter
Bacterial/Viral filter.
Part No. 740-1006
Facemask and Adapter
Facemask and Adapter, 22mm outside diameter x 22mm
outside diameter.
15
Part No. 740-1007
Page 17

SECTION 8
AINTENANCE &
PREVENTIVE
T
ROUBLESHOOTING G UIDE
PREVENTIVE M AINTENANCE
This device has been designed to provide virtually maintenance-free operation for
extended periods of time. Sharp blows to the unit or dropping the unit is to be avoided.
No routine maintenance is required.
GENERAL S UGGESTIONS
1. Keep the unit’s exterior clean.
2. Check that the air intake ports are not blocked.
3. Keep the CoughAssist MI-E away from curtains, blankets or any heat generating device.
M
WARNING: Do not remove the cover; there are no serviceable parts inside the unit.
Refer all service to authorized personnel.
Technical Information: J.H. Emerson Co. will make available on request a list
of all repairable exterior parts with descriptions. Interior schematics and circuit
diagrams will be made available to qualified technical personnel only.
16
Page 18
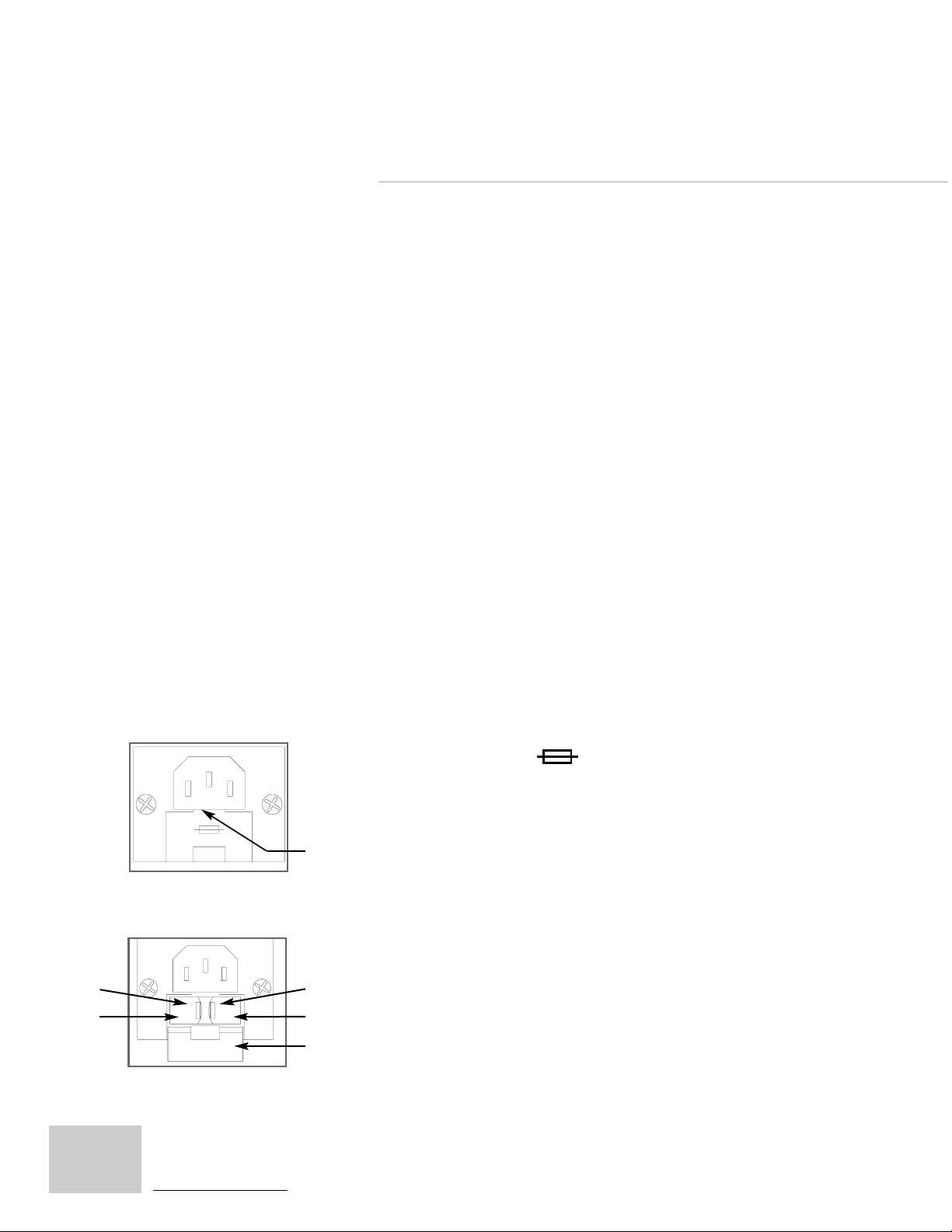
TROUBLESHOOTING G UIDE
1. Pressure Gauge Adjustment: If the pressure
gauge does not go to “0” when the device is turned
off it must be readjusted. Remove the adhesive cover
over the pressure gauge zero adjust and turn the adjust
screw to “0” using a screwdriver. If unable to make this
adjustment, the unit should be returned for servicing.
2. Fuse Replacement: If the unit is connected to the
proper power source and the green light within the
power switch does not illuminate when the switch is
actuated, one or both of the two safety fuses may have
blown. The procedure for replacement of a blown fuse
is as follows:
a. Disconnect the unit from any power outlet and
disconnect the power cord from the receptacle on
the rear of the unit (see Back Panel diagram on
page 9).
Spring Clip
Fuse Holder
17
Door Latch
Spring Clip
Fuse Holder
Access Door
b. Locate the access door on the receptacle labeled
with the symbol: . Open the access door
by prying the latch at the top with a small screw
driver or fingernail. Pivot the door down to reveal
the two fuse holders.
c. Press each of the two spring clips to the side
(the left one to the left, the right one to the right)
and slide both fuse holders out of the receptacle.
d. Inspect both fuses and replace, if necessary, with
fuses with equivalent ratings, as shown on the
rear Product Label Description (page 9). To
replace a fuse, slide the damaged one out of the
holder and slide the new fuse in its place.
e. Reinsert each fuse and holder into the receptacle,
close the access door and reconnect the power cord.
Page 19

SECTION 9
W ARRANTY
WARRANTY
The CoughAssist MI-E is
warranted to be free of
defects in material or
workmanship for one year.
USTOMER
C
For questions about this product please contact Customer Service at:
SERVICE
:
& S
ERVICE
J.H. Emerson Co.
22 Cottage Park Avenue
Cambridge, MA 02140-1691
Phone: 800-252-1414 or 617-864-1414
Fax: 617-868-0841
Email: info@jhemerson.com
Web: www.coughassist.com
AUTHORIZED
REPRESENTATIVE
FOR THE EUROPEAN
COMMUNITY
Emergo Europe
P.O. Box 149
:
4300 AC Zierikzee
The Netherlands
18
Page 20

O.
J.H. EMERSON
22 Cottage Park Avenue
Cambridge, MA 02140-1691
Phone: 800-252-1414 or 617-864-1414
Fax: 617-868-0841
Email: info@jhemerson.com
Web: www.coughassist.com
C
910-1111-2
 Loading...
Loading...