CSZ Electri-Cool II 767 User manual

OPERATION
MANUAL
ELECTRI-COOL® II
MODEL 767

Table of Contents
1.0
2.0
3.0
4.0
5.0 Set Up / Operation / Cleaning
6.0 Specifications
7.0 General Maintenance and Checkout Procedures
7.10
7.11
8.0 Troubleshooting Guide
Warnings / Cautions
Symbols
Receiving and Inspection
Approvals
Introduction
4.1
Purpose 6
4.2
Clinical Indications 6
4.3
Contraindications 7
4.4
Description 7
4.5
Accessories 8
4.6
Transporting and Storing 9
5.1
Equipment Set Up 9
5.2
Filling Instructions 11
5.3
Startup Instructions 11
5.4
Setting Temperature 11
5.5
Pad Instructions 14
5.6
Disposal 14
5.7
Cleaning 14
5.8
Storage 14
7.1
Exterior Physical Inspection 16
7.2
Air Filter Inspection
7.3
Flow Test
7.4
Temperature Test 16
7.5
Low Water Switch/Alarm Test 16
7.6
Leakage Current Test 17
7.7
Ground Continuity Test 17
7.8
Internal Heatsink High-Limit Thermostat Test/ Redundant Internal Heatsink
High-Limit Thermal Fuse Test
Water Temperature Low-Limit Thermistor Test 17
Internal Physical Inspection 17
3
5
9
15
16
16
17
19
6
6
6
16
8.1
General 19
8.2
Alarm Indications 19
9.0 Parts List
10.0
11.0
Fluid Circuit Disinfection/Dry Storage Procedure
Customer Service & Support
20
23
27
56269-D ECN M508-3187
Page 2 of 27
• Read Operation Manual before operating.
• At least every 20 minutes, check patient’s temperature and skin condition of areas in contact with
blanket; also, check blanket water temperature. Pediatric, temperature-sensitive patients with
vascular disease, and operating room patients should be checked more frequently. Notify physician
promptly of any change, or if the patient’s temperature is not responding properly, or does not
reach the prescribed temperature in the prescribed time, or there is a change in the prescribed
temperature range. Failure to inform physician of the deviation may result in injury to the
patient.
• Observe patient’s skin condition frequently due to individual differences in sensitivity and
susceptibility to injury from cold and/or externally applied chemicals or pressure. Patients
at greatest risk are those unconscious, on prolonged therapy, diabetics, children, and
persons incapacitated or with insensitive skin areas or poor circulation.
• Prevent excessive and/or prolonged tissue pressure and shearing forces, especially over bony
prominences to prevent skin damage that may result.
• Do not place additional cold sources between the patient and blanket. Skin damage may result.
• The area between the patient and the blanket should be kept dry to avoid injury to patient.
• Do not wrap pad so tightly as to constrict blood flow. Do not use pins to secure pad or
hoses. Do not allow pad or hoses to come in contact with any sharp object.
• Do not use the Electri-Cool® II system in the presence of flammable anesthetics. Risk of explosion
can result.
• The Electri-Cool® II system has been designed and tested according to UL-60601-1 and has passed
testing regarding the reception and emission of electromagnetic interference. However, operation of
the Electri-Cool® II system near sources of electromagnetic interference or near sources that may
receive electromagnetic interference may cause abnormal operation of this unit or other devices. If
interference of this type is observed, re-locate the unit away from such devices.
• Power interruption will cause the Electri-Cool® II to revert to the default range of 40°F-45°F (5°C-7 °C).
Follow instructions for First Time Set-Up/System Test Routine to resume operation. Failure to
resume the desired therapy could result in injury.
• This device is still energized when the inlet switch is in the off position. To completely
disconnect the device from the power source, remove the appliance plug from the back of the
unit.
• Electric shock hazard. Do not remove cover. Service to be performed by qualified
personnel only.
• For continued protection against risk of fire, replace only with same type and rating of fuse.
WARNING
Page 3 of 27

CAUTION
• Use distilled water only. Failure to use distilled water may result in poor performance and/or
damage to the Electri-Cool® II voiding the warranty.
• Do not use De-ionized Water. The majority of de-ionizers do not maintain a neutral pH of 7. If the
de-ionized water is acidic, it will cause a battery effect, the metal will begin to deteriorate and may
cause a leak in the system.
• Do not use alcohol. Alcohol may cause blanket deterioration.
• Do not overfill. Overfilling may result in overflow when the water in the blanket drains back into the
system when the system is turned off.
• Power cord has “HOSPITAL GRADE” plug (domestic units only). Grounding reliability can
only be achieved when connected to an equivalent receptacle marked “HOSPITAL
GRADE.”
• Do not operate pump without a pad connected or with pad clamps in the “CLOSED” position.
Always check clamps to make sure they are fully open.
• Check hose couplings to be certain they are properly locked together.
• Make sure hose and pads are free of kinks that might restrict flow.
• Complete folding of pad may restrict flow and reduce therapy to patient.
• The pad surface should be checked for damage prior to each application.
• To ensure maximum therapy is delivered, it may be necessary to retain the pad on the patient with
straps for adequate contact with the patient.
• Follow Pad instructions and Hospital/Physician instructions for applying, storing, and disposal of
product.
Page 4 of 27
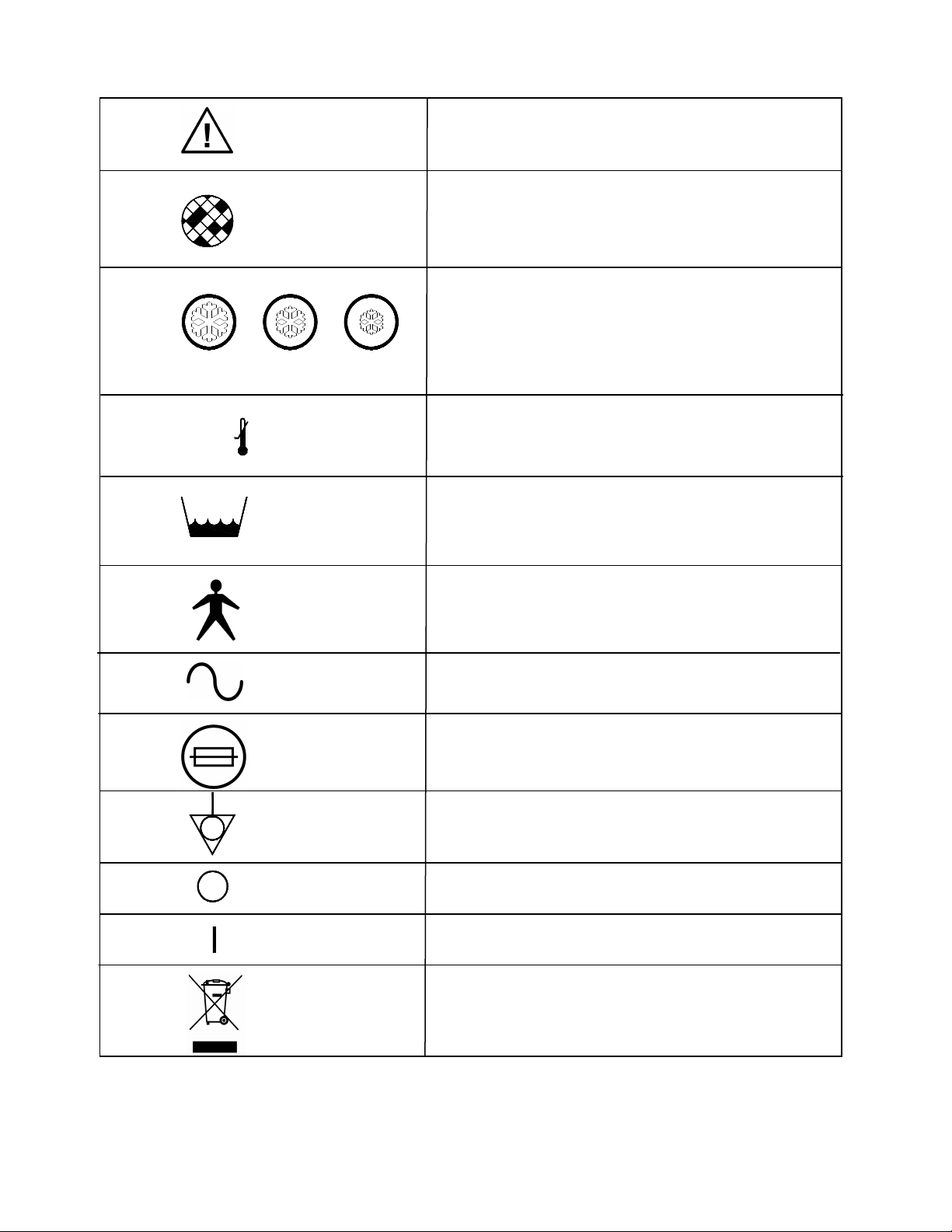
1.0 Symbols
See Accompanying Documentation
Inspect and Change Filter Periodically
5 - 7°C 8 - 10°C 11 -13°C
40 - 45°F 46 - 50°F 51 - 55°F
Temperature Settings
2°C/36°F
Low Water Temperature
Low Water Level
Type B Applied Part
Voltage, Alternating Current
Externally Accessible Fuse Rating
Potential Equalization Connection (Grounding)
Power OFF ( Located on Switch)
Power ON (Located on Switch)
Separate collection for electrical and Electronic
equipment
Page 5 of 27
2.0 Receiving Inspection
2.1 After unpacking the Electri-Cool ® II Cold Therapy Unit, inspect the system for concealed damage.
Retain all packing material and carefully record or photograph any damage. Notify the carrier at once
and ask for an inspection (in writing). Failure to do this within 15 days may result in loss of claim. Do
not return the equipment to Cincinnati Sub-Zero. Call our Medical Technical Service department for
further instruction.
3.0 Approvals
3.1 General Safety and Electrical Safety
Classified by Underwriters Laboratory Inc. ® with
respect to Electric Shock, Fire and Mechanical
Hazards only in accordance with UL 60601-1,
CAN/CSZ-C22.2 No. 601-1, 5R37
5R37
3.2 Electromagnetic Compatibility (EMC)
Tested and passed in accordance with IEC 60601-1-2 EMC requirements
3.3 European
4.0 Introduction
4.1 Purpose
The purpose of this manual is to provide operation and maintenance instructions for the Electri-Cool ®
II Cold Therapy Unit.
4.2 Clinical Indications
Cold therapy has been found to be clinically effective in accelerating the healing of damaged tissue,
and in retarding metabolism within the tissue cells.
Localized cold therapy is used in the operating room, recovery room, intensive care unit, physical
therapy, and in individual patient rooms. It is used effectively to treat surgical incisions, as well as
wounds and inflammation caused by traumatic injury. It is prescribed with many types of surgery, for
example:
• Orthopedic (Arthroscopy, Anterior Cruciate Ligament Repair, Arthroplasty for Hip, Knee, and other
Total Joint Replacement)
• Neurologic/Orthopedic (Laminectomy)
• Abdominal (Hernia, Cholecystectomy)
• Oral/Maxillofacial/Plastic (Dental Extractions, Rhinoplasty, Augmentations, Face Lifts)
• Urological (Vasectomy, Prostatectomy, Urological Implants)
• Obstetrical/Gynecological (“C” Section, Episiotomy, Hysterectomy)
In compliance with the Medical Device Directive 93/42/EEC
Page 6 of 27
In most cases, cold therapy is applied immediately following the surgical procedure directly to the
wound site. This acts to help reduce swelling and pain, while also helping to reduce or eliminate the
need for pain-killing medications. For the specialty of hand surgery, cold therapy is also applied to the
upper extremity before and during the procedure in conjunction with a tourniquet.
Other conditions indicating the application of cold therapy are:
• Acute injuries • Chronic pain
• Alopecia • Low back pain
• Arthritis • Muscle spasms
• Bruises/contusions • Sprains
• Cellulitis • Strains
4.3 Contraindications
Observe patient's skin condition frequently due to individual differences in sensitivity and susceptibility
to injury from cold and/or externally applied chemicals or pressure. Patients at greatest risk are those
unconscious, on prolonged therapy, diabetics, children, and persons incapacitated or with insensitive
skin or compromised circulation.
4.4 Description
The Electri-Cool® II Cold Therapy Unit has been designed to provide maximum thermal transfer
efficiency at optimum safety. The Electri-Cool® II consists of a plastic reservoir for holding distilled
water; a float switch to sense water level; a pump for circulating water through an external pad; a
thermoelectric sub-assembly to cool the water; a microprocessor-based electronic control to regulate
water temperature; and a fan to transfer absorbed heat to the ambient air. The user can select
among three pre-set temperature ranges: 51ºF-55ºF (11ºC-13ºC); 46ºF-50ºF (8ºC-10ºC); and 40ºF45ºF (5ºC-7ºC). In addition to the aforementioned features, this model includes audible alarms for low
temperature and low water with visual indicators. The unit also shuts down entirely in the event of an
internal component over temperature condition.
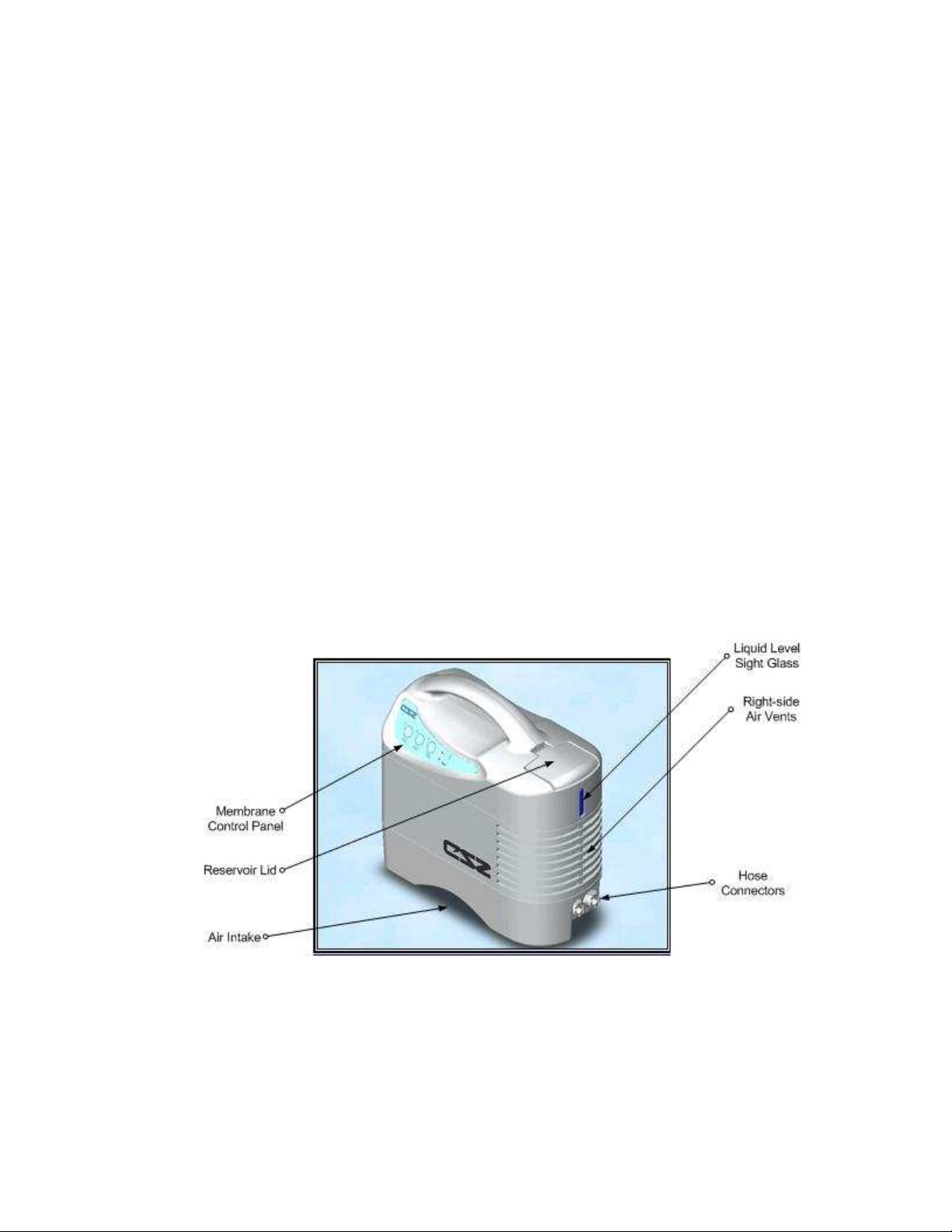
Figure 1.1: Electri-Cool® II Front View
Page 7 of 27
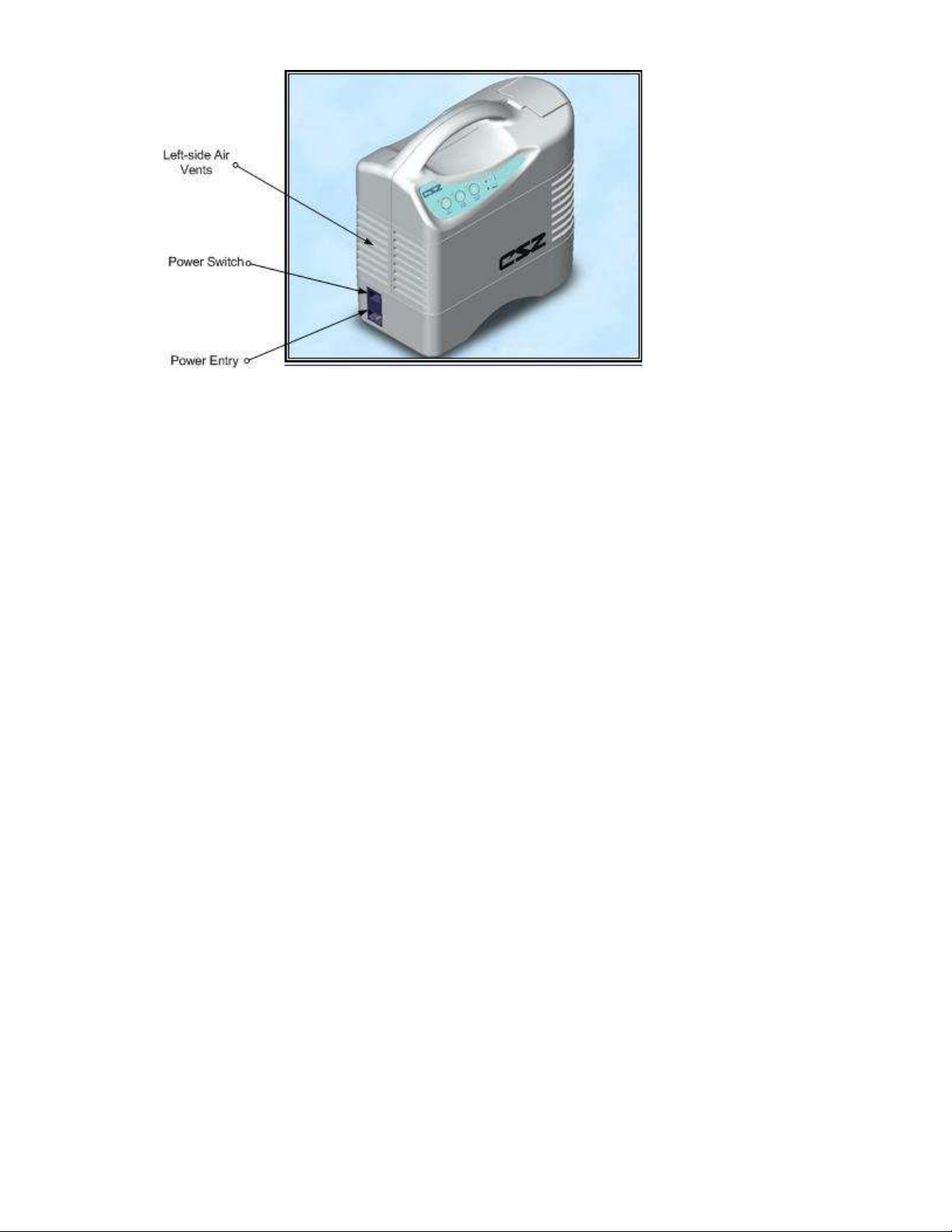
Figure 1.2: Electri-Cool® II Left-side View
4.5 Accessories
The essential accessories for the Electri-Cool® II Unit are listed below:
The Thermal Pads consist of two layers of plastic sealed together to provide multiple passageways for
water flow in a random-flow pattern designed to prevent occlusion.
Temp-Pad® Localized Cold Therapy Pads
Urethane with Foam Backing Sterile w/o Straps Sterile w/Straps
3” x 5” (7.6cm x 12.7cm) 305-USS
3" x 18" (7.6cm x 45.7cm) 318-US
5" x 10" (12.7cm x 25.4cm) 510-US 510-USS
8" x 14" (20.3cm x 35.6cm) 814-US 814-USS
11" x 10" (27.9cm x 25.4cm) CT-99
11" x 12" (27.9cm x 30.5cm) 1112-US 1112-USS
12" x 15" (30.5cm x 38.1 cm) 1215-US
ImmobilICE® Supports consist of a Temp-Pad® Pad contained within a polypropylene felt body,
brushed nylon outer laminate and Tietex 100% polyester with hook and loop closures.
ImmobilICE® Supports, Sterile
Universal Back Support 814-IMB
Universal Hip Support 814-IMH
Temp-Pad Wraps
Shoulder Wrap - small/medium 707-WSS
Shoulder Wrap - large/x-large 707-WSL
Knee Wrap 707-WKN
The pads are connected to the unit via a hose with self-sealing “quick-connect” couplings containing
automatic shut-off valves at each end.
Insulated Hose
Insulated Connecting Hose, 6' (1.83m) 757-HIN
Other Accessories
Bed Bracket 767-BBK
Disposable Filter 767-FIL
Mobile Stand (Requires 767-BBK) 767-MST
Page 8 of 27
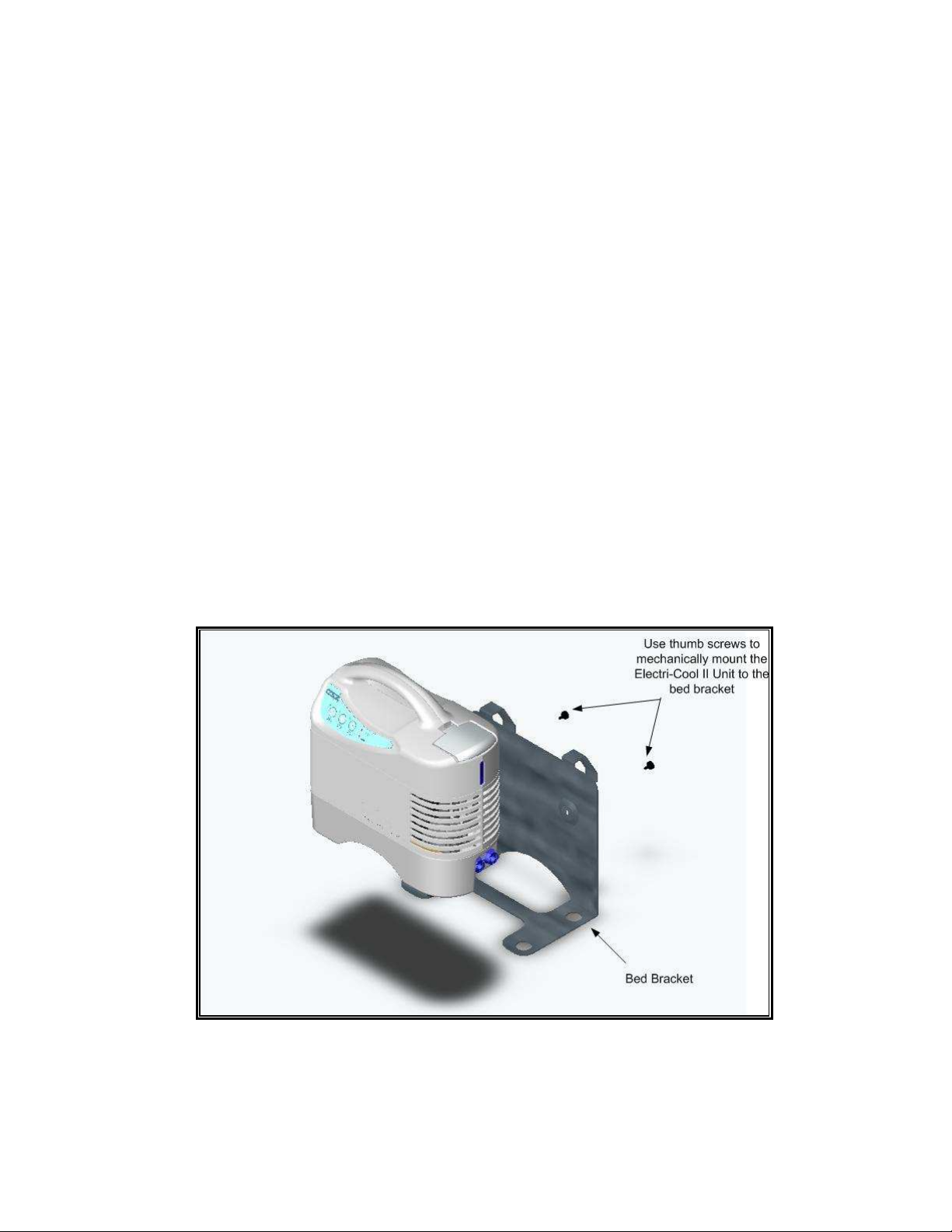
4.6 Transporting and Storing
Drain all water from the reservoir before shipment or storage. The Electri-Cool® II unit can be transported via
normal shipping methods via ground, air, or water when packaged in its approved packaging material.
During transportation and storage, packaging should not be exposed to conditions that fall out of the ranges
below:
4.6.1 Temperature: -40°C to 70°C (–40°F to 158°F)
4.6.2 Humidity: 10% to 100%
4.6.3 Atmospheric Pressure: 500 to 1060hPa (7.25 to 15.37 PSIA)
5.0 Set Up / Operation / Cleaning & Storage
5.1 Equipment Set Up
5.1.1 Before use, check the air filter for accumulated dust. If filter is dirty, replace the filter with a new
one. Refer to, “General Maintenance and Checkout Procedures”, for filter replacement
instructions.
5.1.2 Check to ensure that the water level in the reservoir is at its maximum level. The maximum level
is at the lowest level of the fill spout (or threaded neck). Refer to “Filling Instructions”.
Use distilled water only. Failure to use distilled water may result in poor performance and/or
damage to the Electri-Cool® II voiding the warranty.
5.1.3 The Electri-Cool® II unit should be placed on a secure, flat surface at least 61cm (2 feet) from
any wall or object that may restrict airflow to the unit. A bed bracket or mounting stand as listed
in “Accessories” section can also be used within proximity of the patient such that the attached
hoses will reach the desired therapy site.
Figure 5.1: Mounting the Electri-Cool II to the Bed Bracket
Page 9 of 27
 Loading...
Loading...