Page 1

original instruction for use
treadmill h/p/cosmos
®
150/50 LC
IMPORTANT! READ CAREFULLY BEFORE USE!
KEEP FOR FUTURE REFERENCE!
firmware version: v3.03.x
article number: cos105000-2016-00 en
run ahead of time
®
Carefusion LE200 LC
Page 2

page 2
cos105000-2016-00 en
These instructions for use are only valid for the original conguration of the rst delivery of the devices pictured below.
If the device has been changed, please refer to the latest version of this document, available at:
www.h-p-cosmos.com
Product family: treadmill h/p/cosmos 150/50 LC
Models:
pluto
®
| Carefusion LE200 LC
(cos30027va08)
Page 3

page 3
cos105000-2016-00 en
Dear customer,
Thank you for choosing this premium device.
Since its establishment in 1988, h/p/cosmos
®
has strongly inuenced sports, athletics,
ergometry, rehabilitation, and science through the development and distribution of new
products, software, system solutions, and application methodologies.
During this time the company, based in Traunstein, Germany, has developed into THE
German specialist for manufacturing treadmill ergometers and systems for tness, sports,
sports science, sports medicine, athletics, biomechanics, medicine, rehabilitation, therapy,
ergometry, performance diagnostics, and scientic research.
Many developments and pioneering work from h/p/cosmos
®
have inuenced not only product
design and functionality but also their usage and methodologies.
Your success with our devices is the primary goal of h/p/cosmos.
This is why we offer individual devices as well as comprehensive system solutions.
You will nd a wide range of options and accessories in these instructions for use and at
www.h-p-cosmos.com.
At h/p/cosmos, the quality and safety of our products is our highest priority.
These instructions for use include all of the information needed to operate the device correctly
and safely.
Please read them carefully before use and keep them available at all times.
We hope you will have a lot of fun and success as you work with your h/p/cosmos device.
Franz Harrer
President & CEO
h/p/cosmos sports & medical gmbh
Franz Harrer
President & CEO
h/p/cosmos sports & medical gmbh
Page 4

page 4
cos105000-2016-00 en
Content
1 Symbols and Labels ........................................................................................................................................7
1.1 Symbols used (general) ............................................................................................................................7
1.2 Symbols used (transport) ..........................................................................................................................8
1.3 Labels on device .......................................................................................................................................9
2 Description .....................................................................................................................................................10
2.1 Illustration ................................................................................................................................................10
2.2 Function ...................................................................................................................................................10
3 Intended Use .................................................................................................................................................. 11
3.1 Intended use ............................................................................................................................................11
3.2 Intended operator ....................................................................................................................................11
3.3 Intended location .....................................................................................................................................11
3.4 Intended duration ....................................................................................................................................11
3.5 Contraindications .....................................................................................................................................12
4 Safety ..............................................................................................................................................................13
4.1 Safety information – Forbidden use ........................................................................................................13
4.2 Fall prevention devices ............................................................................................................................15
4.3 Emergency dismount ...............................................................................................................................17
4.4 Emergency off .........................................................................................................................................18
4.5 Emergency stop ......................................................................................................................................18
4.6 Unauthorized access ...............................................................................................................................19
4.7 Residual risk / Side effects ......................................................................................................................19
4.8 Fireghting ...............................................................................................................................................19
4.9 All-pole disconnection ..............................................................................................................................19
5 Preparation .....................................................................................................................................................20
6 UserTerminal ..................................................................................................................................................22
6.1 Keys and displays ...................................................................................................................................22
6.2 Standard vs. “lt” devices ..........................................................................................................................23
6.3 Connection of external devices / Interfaces ............................................................................................24
7 Position of Patient and Operator .................................................................................................................25
8 Operation .......................................................................................................................................................26
8.1 General application procedure ...............................................................................................................26
8.2 Overview of operation modes .................................................................................................................27
8.3 Manual mode ..........................................................................................................................................28
8.4 Prole mode ...........................................................................................................................................29
8.5 Cardio mode (optional) ...........................................................................................................................31
8.6 Test mode ...............................................................................................................................................33
Page 5

page 5
cos105000-2016-00 en
8.7 Interfere with automatic program ............................................................................................................35
8.8 Pause function .........................................................................................................................................36
8.9 Acceleration levels ...................................................................................................................................37
8.10 User Options .........................................................................................................................................38
9 Accessories / compatible devices ...............................................................................................................40
9.1 Creating Systems ....................................................................................................................................40
9.2 Overview of accessories .........................................................................................................................40
9.3 Compatible devices .................................................................................................................................41
10 Disinfection / Cleaning ................................................................................................................................42
11 Maintenance .................................................................................................................................................43
11.1 Intervals and competences ....................................................................................................................43
11.2 Daily inspection .....................................................................................................................................43
11.3 Lubrication .............................................................................................................................................44
11.4 Adjustment of running belt .....................................................................................................................45
11.5 Issues for qualied service personnel ...................................................................................................46
11.6 Safety inspection ...................................................................................................................................46
11.7 Spare parts and consumables ...............................................................................................................46
12 Troubleshooting ..........................................................................................................................................47
12.1 General troubleshooting ........................................................................................................................47
12.2 RS232 troubleshooting ..........................................................................................................................48
12.3 Error messages .....................................................................................................................................48
13 Technical data ..............................................................................................................................................49
13.1 UserTerminal .........................................................................................................................................49
13.2 Dimensions ............................................................................................................................................49
13.3 Loads .....................................................................................................................................................49
13.4 Emissions ..............................................................................................................................................49
13.5 Essential performance characteristics ...................................................................................................49
13.6 Environmental conditions ......................................................................................................................50
13.7 Technical and legal requirements ..........................................................................................................50
13.8 EMC tests ..............................................................................................................................................50
13.9 Classication .........................................................................................................................................51
13.10 Certicates ..........................................................................................................................................51
13.11 Interfaces (RS232, D-SUB, 9-pole) .....................................................................................................51
13.12 Voltage, Current, Performance ............................................................................................................52
13.13 All-pole disconnection ..........................................................................................................................52
14 Liability and Warranty .................................................................................................................................53
15 Expected Lifetime ........................................................................................................................................53
Page 6

page 6
cos105000-2016-00 en
16 Disposal ........................................................................................................................................................53
17 Annex I ..........................................................................................................................................................54
17.1 Installation .............................................................................................................................................54
17.2 Instruction protocol ................................................................................................................................54
17.3 Instruction protocol, signatures .............................................................................................................55
17.4 User Options (details) ............................................................................................................................56
18 Annex II (Pre- & self-dened tests) ............................................................................................................61
18.1 UKK walk test ........................................................................................................................................61
18.2 Graded test ............................................................................................................................................62
18.3 Conconi test ..........................................................................................................................................62
18.4 Bruce protocol .......................................................................................................................................63
18.5 Naughton protocol .................................................................................................................................63
18.6 Balke protocol ........................................................................................................................................63
18.7 Cooper protocol .....................................................................................................................................64
18.8 Ellestad A protocol .................................................................................................................................64
18.9 Ellestad B protocol ................................................................................................................................64
18.10 Ramp prole ........................................................................................................................................65
18.11 Gardner test protocol ...........................................................................................................................65
18.12 Self-dened tests .................................................................................................................................66
19 Annex III (Accessories) ...............................................................................................................................68
19.1 Arm support, adjustable [cos12013] ......................................................................................................68
19.2 Arm support, optional stop button [cos10107, cos10108] .....................................................................69
19.3 Crossbar front rail [cos102426] .............................................................................................................70
19.4 Elevation 0% to +25% [cos102927] ......................................................................................................71
19.5 Emergency stop retrotting [cos15933, cos100548, cos15294] ............................................................72
19.6 Handrail, long 1358mm [cos102918] .....................................................................................................73
19.7 Handrail, pediatric [cos102400] .............................................................................................................74
19.8 Heart rate measurement POLAR non-coded [cos102818] ....................................................................75
19.9 Heart rate measurement POLAR W.I.N.D. coded [cos100106] ............................................................76
19.10 PC-software para control [cos10071-v4.1.0] .......................................................................................77
19.11 robowalk expander [cos30022, cos30023] ..........................................................................................78
19.12 Safety arch for treadmill families 150/50 LC and 150/50 [cos10079] ..................................................79
19.13 Wheelchair ramp [cos102931] .............................................................................................................80
20 Contact .........................................................................................................................................................81
Page 7
page 7
cos105000-2016-00 en
1 Symbols and Labels
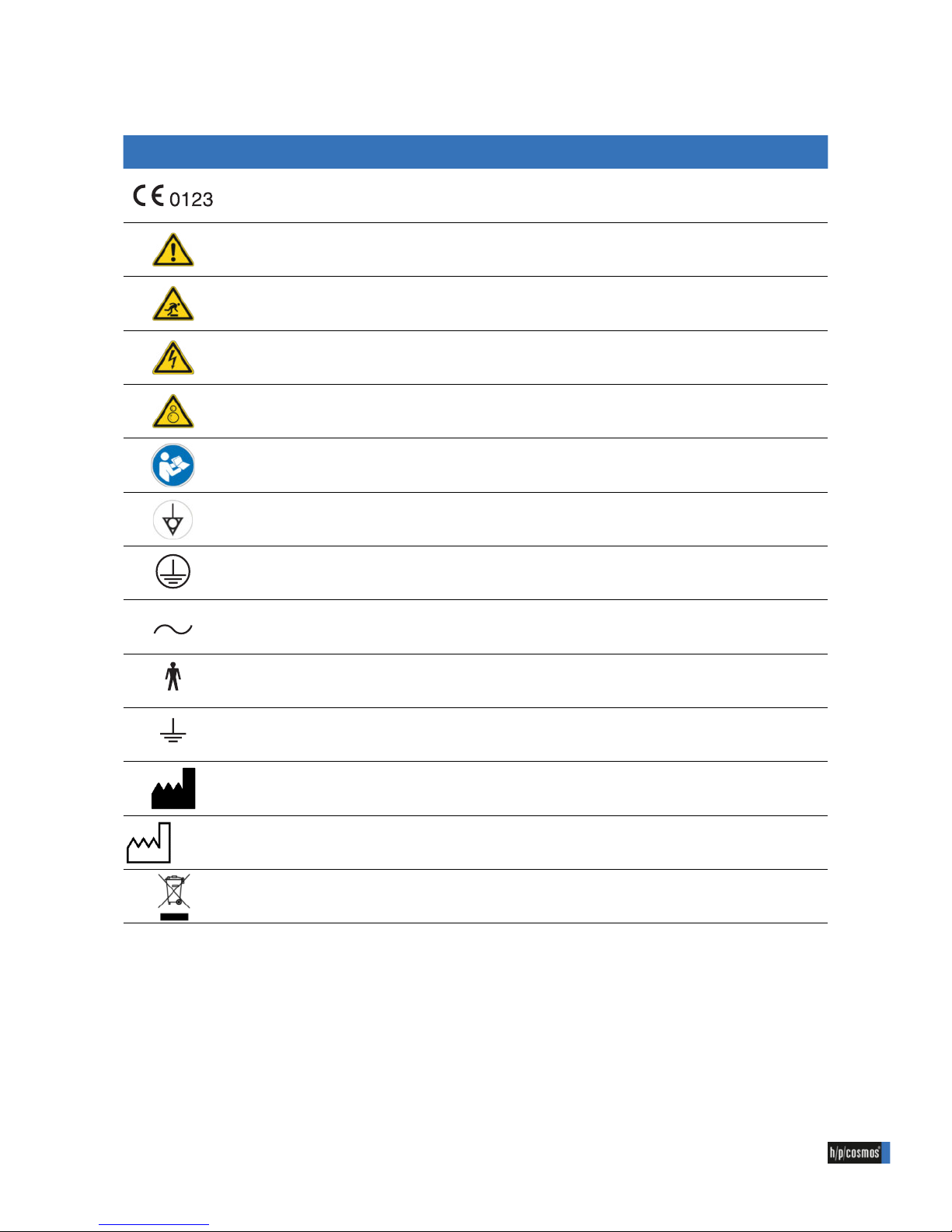
1.1 Symbols used (general)
Illustraion Description Reference
CE sign, proof that the essential requirements
(here with number of notied body) were met
(according to medical device directive
93/42/EEC)
General warning (danger, warning or caution statements) (acc. to DIN EN ISO 7010 W001)
Warning of obstacles (stumbling) (acc. to DIN EN ISO 7010 W007)
Warning of electrical voltage (acc. to DIN EN ISO 7010 W012)
Warning of counter rotating rollers (trapping zones) (acc. to DIN EN ISO 7010 W025)
Follow instructions for use (acc. to DIN EN ISO 7010 M002)
Potential equalization (acc. to IEC 60445)
Protection ground (acc.to IEC 60417-5019)
Alternating current (AC) (acc. to IEC 60417-5032)
Applied part of type B (acc. to IEC 60417-5840)
Connection point for Neutral line (acc. to IEC 60445)
Manufacturer (acc. to ISO 15223-1)
2015-10-01
Manufacturing date (acc. to ISO 15223-1)
Separate collection for electrical and electronic equipment (acc. to 2012/19/EU)
Page 8
page 8
cos105000-2016-00 en
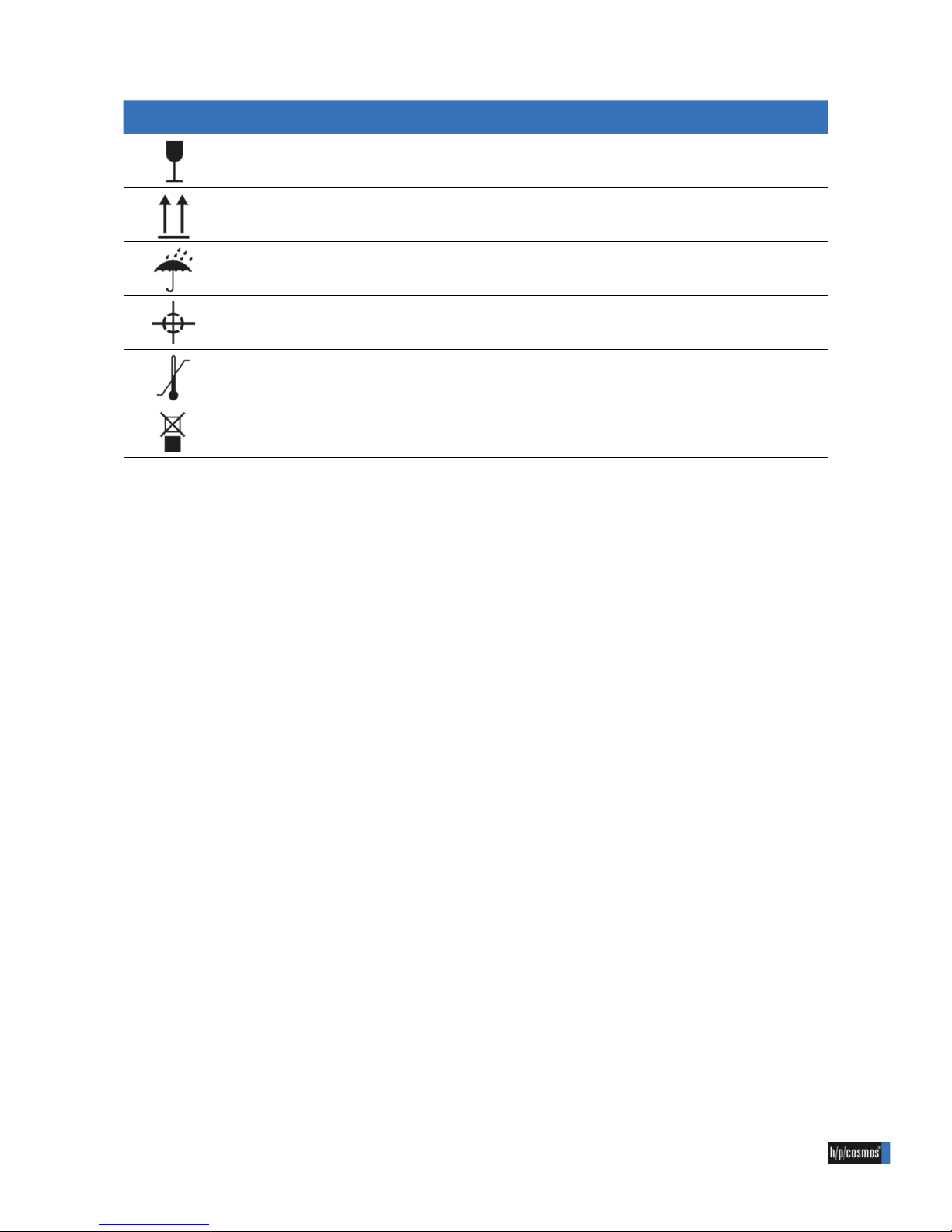
Illustraion Description Reference
Fragile, Handle with care (acc. to ISO7000-0621)
This way up (acc. to ISO7000-0623)
Keep dry (acc. to ISO7000-0626)
Centre of gravity (acc. to ISO7000-0627)
Temperature limitations (acc. to ISO7000-0632)
Do not stack (acc. to ISO7000-2402)
1.2 Symbols used (transport)
Page 9
page 9
cos105000-2016-00 en
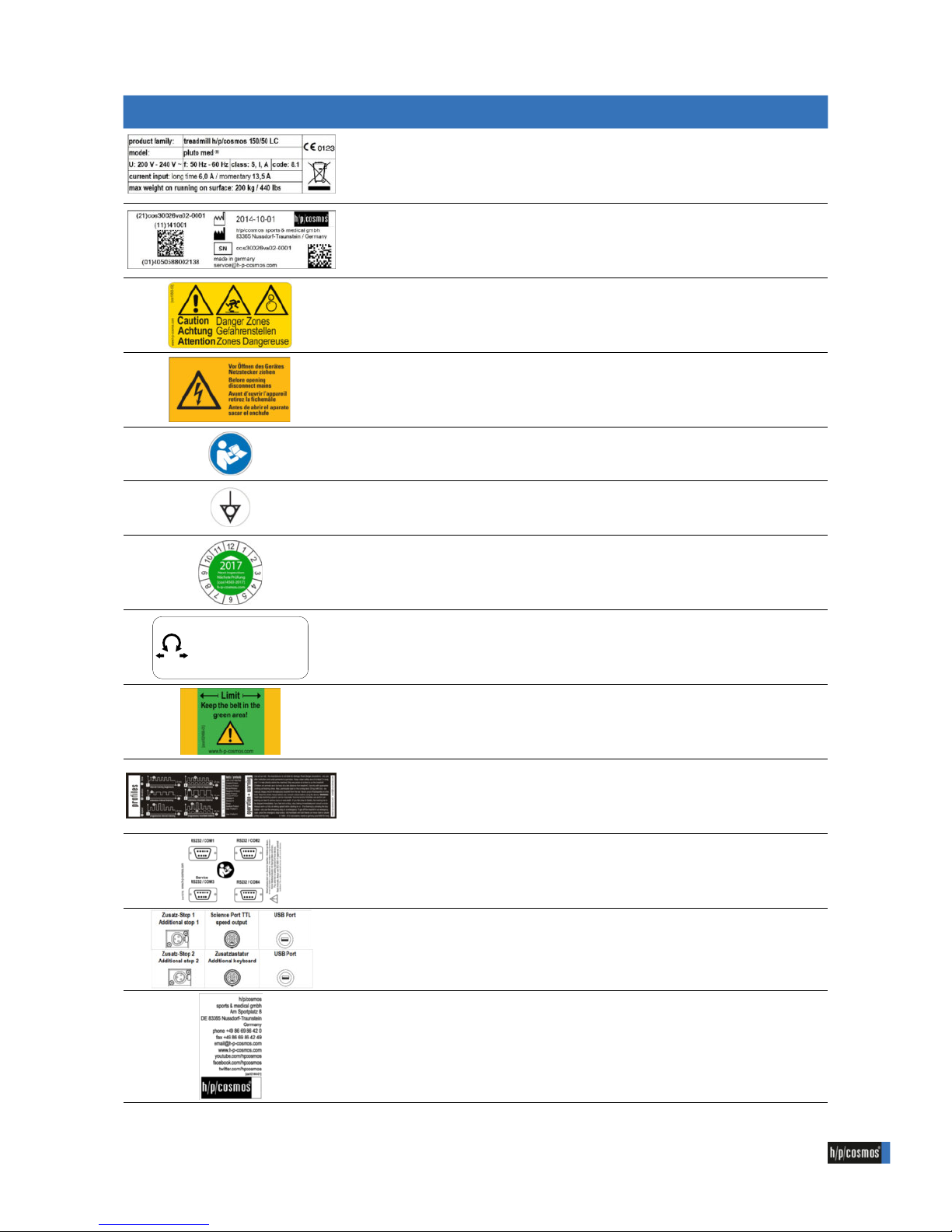
Illustraion Description Reference
name plate -
UDI name plate -
label "caution danger zones" cos10508-03
label "before opening disconnect mains" cos11880
label "follow instructions" cos101380
label "potential equalization" cos101594
label “inspection 20xx” + base label cos14543-20xx + cos11787
label "adjust running belt" cos10512
label "limit running belt shaft cover" cos102466-01
operation & safety instructions cos100578-01-xx
label "interface backplate 150/50 LC" cos102788
label "rear panel UserTerminal 5B outs." cos11933-01
label "h/p/cosmos address" cos10144-01
Laufgurteinstellung
Adjust running belt
Anleitung lesen
Read manual
[COS10512]
LR
www.h-p-cosmos.com
Farbprofil: Generisches CMYK-Druckerprofil
Komposit Standardbildschirm
1.3 Labels on device
Page 10

page 10
cos105000-2016-00 en
No. Description Illustration
1. UserTerminal
2.
Pull cord safety stop
(ripcord / safety lanyard)
3. Emergency off
4. Crossbar-frontrail
5. Side handrail
6. Motor cover
7. Foot rail
8. Non slip surface
9. Running deck
10. Running belt
11. Rear roller
12. Rear roller protective cover
13. Marking of running surface
14. Safety arch
15. Safety harness
2.1 Illustration
2 Description
2.2 Function
The treadmill has two essential performance characteristics: Speed and elevation.
The rotation of the running belt represents the speed.
The raising of the whole treadmill frame incl. running deck enables the elevation.
Both parameters are manually adjustable on the UserTerminal.
Furthermore, operation is possible via pre- and self-dened modes.
Operation is also possible via external devices (PC, ECG, etc.).
The chapter “operation” gives a detailed description of all functions.
The chapter “technical data” shows technical details.
The treadmill is driven by powerful motors.
For that reason it is very important to follow the safety information, in order to avoid injury or death.
As previously described, the treadmill contains a number of standardized protocols.
Nevertheless, the treadmill does not provide recommendations for treatment.
The decision regarding the correct load is the responsibility of the medical doctor.
Depending on the application, the load includes speed, elevation, distance, heart rate, body weight or motion support, etc..
Page 11

page 11
cos105000-2016-00 en
3 Intended Use
3.1 Intended use
h/p/cosmos medical treadmills are intended for walking or running in place for
❚
Recreational tness training
❚
Gait training (with or without body weight support)
h/p/cosmos medical treadmills can be used in combination with external devices for walking or running in place as
❚
Loading devices for neuromuscular and biomechanical measurements (e.g. EEG, EMG, motion analysis)
❚
Loading devices for cardiovascular measurements (e.g. ECG)
❚
Loading devices for cardiopulmonary measurements (e.g. ergospirometry)
For any medical treadmill application, a fall prevention system is prescribed and absolutely obligatory.
h/p/cosmos medical treadmills may be operated with healthy subjects for non-medical uses in non-medical environments as well.
For use with healthy subjects, please apply the instructions for use for sports devices, available at
www.h-p-cosmos.com
3.2 Intended operator
❚
Medical staff only
❚
that has been carefully trained according to these instructions for use
❚
that is working according to the prescription of the medical doctor, where applicable and necessary
❚
The patient is not the intended operator.
But the intended operator is authorized to allow the patient to control the device according to the instructions of the intended
operator and under the permanent observation of the intended operator.
This means the operation of the device remains the responsibility of the intended operator at all times, taking the physical and
mental condition of the patient into account.
The intended operator has to be within reach of at least one emergency stop / off at all times.
3.3 Intended location
❚
Medical facilities only
❚
No use at home or in home healthcare environments (acc. to IEC 60601-1-11)
❚
No outdoor use
❚
No direct sunlight
❚
Sufciently lighted for proper readability of warning, labels, displays and operation elements
❚
Proper environmental conditions (see “Technical Data”)
❚
Stationary training equipment: Not moved during use
3.4 Intended duration
❚
Depending on the prescription of the medical doctor
Page 12

page 12
cos105000-2016-00 en
3.5 Contraindications
Absolute contraindications
(have to be excluded before the treadmill is used)
❚
Acute myocardial infarction (within 2 days)
❚
Instable angina pectoris
❚
Cardiac arrhythmia pathology and/or limited hemodynamics
❚
Symptomatic massive aortic stenosis
❚
Uncompensated / uncontrolled heart insufciency
❚
Acute pulmonary embolism or pulmonary infarction
❚
Acute endocarditis, myocarditis, pericarditis
❚
Acute aortic dissection
❚
Acute coronary syndrome
❚
Acute phlebothrombosis of the lower extremities
❚
Febrile infections
❚
Pregnancy
❚
Acute thrombosis
❚
Fresh wounds e.g. after surgery
❚
Acute fracture
❚
Damaged disc or traumatic disease of the spine
❚
Epilepsy
❚
Inammations
❚
Acute migraine
Relative contraindications
(The application may be started if the possible benets exceed the risks.
The decision has to be made by the medical doctor before the treadmill is used)
❚
Left main coronary stenosis
❚
Main artery disease
❚
Cardiac valve disease of moderate severity
❚
Known electrolyte imbalance
❚
Arterial hypertonia (RR > 200 mm Hg syst. > 110 mm Hg diast.)
❚
Tachyarrhythmia or bradyarrhythmia
❚
Hypertrophic cardiomyopathy and other forms of outow tract obstruction
❚
Higher degree atrioventricular AV-blocking
❚
Anemia
❚
Physical and/or mental disabilities leading to inability to exercise adequately
❚
Partially invasive medical devices (probes, infusions, catheters, external xators, etc.)
❚
Cardiac pacemaker
❚
Visual impairment (vision < 30% acc. to WHO)
Further contraindications may occur. This has to be evaluated by the medical doctor.
A second person has to be present during maintenance.
Sources:
http://leitlinien.dgk.org (German Cardiac Society)
www.acc.org (American College of Cardiology Foundation)
www.americanheart.org (American Heart Association)
http://my.americanheart.org/idc/groups/ahaecc-internal/@wcm/@sop/documents/downloadable/ucm_423807.pdf
Page 13

page 13
cos105000-2016-00 en
4 Safety
h/p/cosmos medical treadmills may be operated with healthy subjects as well.
For applications with healthy subjects, please apply the instructions for use for sports devices, available at
www.h-p-cosmos.com
4.1 Safety information – Forbidden use
Obey the following danger, warning and caution statements stricktly in order to prevent serious injury or death!
❚
For any medical treadmill application, a fall prevention device is prescribed and absolutely obligatory.
❚
Do not use the safety harness on bare skin.
❚
WARNING! Heart rate monitoring systems may be inaccurate.
❚
Incorrect or over exercising may result in serious injury or death.
❚
Advice the patient: If you feel faint or dizzy stop exercising immediately and consult a medical doctor.
❚
Exclude overloading or overstressing of the patient.
❚
Only carefully trained medical staff is allowed to use the device.
❚
The patient has to be checked by a medical doctor before using the device.
❚
A debrillator must be present at any time.
❚
The intended operator has to be in reach of at least one emergency stop/off at any time.
❚
Obey all information given in these instructions for use.
❚
Do not use the device against the intended use.
❚
Do not use the device in case one or more of the listed contraindications prevail.
❚
In case of relative contraindications permanent observation of the patient by medical staff is obligatory.
❚
Neither patient nor operator must be under the inuence of alcohol, drugs or anesthetics.
❚
Start the use of the treadmill with slow walking, especially for beginners.
❚
Make sure the space under the treadmill is free from persons, body parts or objects, especially when switching on (treadmill will
lower during initialization) and when changing the elevation.
❚
Do not enter the device when running belt is rotating.
❚
Do not step on rear roller.
❚
Do not stand on or enter the running deck when device is in elevation (running belt might slip through due to gravity).
❚
Make sure no towels, jewellery, cell phones, containers with liquid etc. can fall into the device or onto the running surface.
❚
Do not enter the device without appropriate shoes without high heels, spikes or studs.
❚
Do not use the device with wheels (bikes, wheelchairs, inline skates, etc.).
❚
Do not turn around, walk sideways or backwards; do not jump on or off the running belt while it is in motion.
❚
Do not touch the running belt while it is in motion (besides contact with feet).
❚
Do not lean on the UserTerminal - do not apply pressure to the displays - press keys softly.
❚
Ensure assist mean, accessories, cables etc. do not extend into the running area.
Page 14

page 14
cos105000-2016-00 en
❚
Do not insert metal objects such as a pin or a wire into any gap or any outlet on the device.
❚
Do not touch the patient and external electrical devices at the same time.
❚
Always the latest command will be executed, regardless of whether it came via interface or from the UserTerminal during one of
the four modes. Only stop command has higher priority and cannot be overwritten.
❚
WARNING: To avoid the risk of electric shock, this equipment must only be connected to a supply mains with protective earth.
❚
WARNING: Do not use portable high frequency communication devices in the patient environment (see “position of patient and
user”). Disregard can cause loss of performance.
❚
Free standing equipment has to be installed on a stable and levelled base.
❚
Choose proper oor, clothing and humidity, in order to prevent electrostatic discharge (also see technical data).
❚
Do not use the device without instruction by authorized personnel acc. to the instruction protocol.
❚
Regard safety area behind device of 2.0 m x width of treadmill.
❚
Do not use the device with children <12 months.
❚
Exclude access of unsupervised children (< 14 years) onto or near any parts of the device (incl. accessories, packaging, lubrication
and service material).
❚
In case of application with children (> 1, < 14 years) permanent observation of the patient by medical staff is obligatory.
❚
Animals must not be in the same room with the device.
❚
The automatic modes must only be performed on the prescription of the medical doctor.
❚
Operator and patient have to be aware of automatic load changes during prole, cardio and test mode.
❚
During stress tests a medical doctor has to be available at any time.
❚
Unmeant trapping hazards: Take off ties, scarfs or other clothes that may be trapped. Secure long hair and ribbons during
maintenance and training in order to prevent being captured in trapping zones.
❚
Perform a daily visual inspection (see chapter “maintenance”).
❚
Obey the maintenance intervals claimed in chapter “maintenance”.
❚
Obey the competences claimed in chapter “maintenance”.
❚
A second person has to be present during maintenance.
❚
In case of any visible or assumed defects or malfunctions (of the device, accessories, software, etc.), unplug device, exclude
reconnection, mark clearly and inform h/p/cosmos service personnel via telephone and writing.
❚
In case of any visible or assumed wear and tear (of the device, accessories, labels, etc.), unplug device, exclude reconnection,
mark clearly and inform h/p/cosmos service personnel via telephone and writing.
❚
In case of any uid entering into the device, unplug device, exclude reconnection, mark clearly and inform h/p/cosmos service
personnel via telephone and writing.
❚
Do not modify the device, congurations, accessories or software in any way.
❚
Do not connect any devices, accessories or software, not listed in “accessories / compatible devices”.
❚
Disinfect the device before and after every treatment.
❚
Disconnect the device and all accessories from mains power supply before cleaning or disinfection.
Page 15

page 15
cos105000-2016-00 en
4.2 Fall prevention devices
A fall prevention device is the only effective way to protect the patient from falling.
For any medical treadmill application, a fall prevention device is prescribed and absolutely obligatory.
h/p/cosmos provides fall prevention devices in the form of a safety arch or a body weight support device (airwalk).
It is up to the operator to use any other device that prevents the patient from falling and complies with IEC60601-1.
The pull-cord safety stop is not a fall prevention.
Treadmill with safety arch Body weight support device airwalk
®
Further information see “annex II (accessories)”
Description Ilustration
Put on the safety harness so that the h/p/cosmos logo is at the
front.
The h/p/cosmos logo has to be visible in the back as well.
Close buckles.
Page 16

page 16
cos105000-2016-00 en
Description Ilustration
Tighten shoulder and chest straps.
Use carabiner to connect safety harness with rope.
Explain function to subject.
Adjust the length of the rope in a way the patient has to
maintain the correct position (see “position of patient and
operator”).
Secure rope with rope arrest.
In order to re-open the buckle, press the fastener with thumb
and index nger.
Page 17

page 17
cos105000-2016-00 en
4.3 Emergency dismount
Patient is conscious and aware of danger.
❚
Patient grabs the handrails
❚
Patient jumps off the running belt onto the foot rails
❚
Operator / patient hits the emergency off
Patient is conscious but not aware of danger.
❚
Patient stumbles and falls into fall prevention device.
❚
Treadmill stops
❚
Operator / patient hits the emergency off
❚
Operator helps patient stand up again.
❚
Operator helps patient exit the device.
Patient lost consciousness and is hanging in the fall prevention device.
❚
Hit the emergency off.
❚
Call a medical doctor.
❚
Call one or more persons, strong enough to carry the patient.
❚
Inform the third person that you will open the buckle of the safety harness
❚
Open the buckle of the safety harness.
❚
Patient will slide into the other person’s arms.
❚
Render rst aid.
Page 18

page 18
cos105000-2016-00 en
4.4 Emergency off
Do only use in case of emergency.
Do not use as normal stop button.
Do not stand on or enter the running deck when device is in elevation (running belt might slip through due to gravity).
The operator has to be in reach of the emergency off at any time.
If the operator is not able to reach the emergency off button at the UserTerminal (body height, obstacles, etc.), the operator must
install an additional emergency stop within reach (see accessories).
4.5 Emergency stop
Do only use in case of emergency.
Do not use as normal stop button.
Do not stand on or enter the running deck when device is in elevation (running belt might slip through due to gravity).
Operation Result Release Restart
UserTerminal
Power supply to load
generating components
interrupted
Running belt spins out (until
0 km/h) with
undened deceleration
Movement of elevation
system stops
UserTerminal off
Interface communication
interrupted
Push button
Pull button Switch the device on.
Pull cord safety stop
Pull rope
Reconnect rope And restart application.
Operation Result Release Restart
Safety arch
Running belt stops with
predened deceleration
Movement of elevation
system stops
UserTerminal displays “pull
stop”
Mains connection and
Interface communication
not interrupted
Pull rope
Release rope Restart application
Page 19

page 19
cos105000-2016-00 en
4.6 Unauthorized access
See OP 40 … 44 in the User Options to lock the whole device or individual modes.
4.7 Residual risk / Side effects
After risk reduction only 13 of more than 230 causes are in the "widely acceptable" region.
In case fall prevention is not applied or not applied correctly, there are residual risks, such as falling of a person resulting in skin
abrasions, bruises, fractures or in worst case even death.
Furthermore there is residual risk such as unintended overload of the patient caused by wrong operation, wrong assessment, or
wrong application of the operator and also incorrect data transfer (e.g. electromagnetic interferences, software failure, etc.). Even
the best software and hardware safety concepts can never completely rule out a failure of software or hardware and thereby a
theoretically possible overloading of the patient.
Since the treadmill is an electrically operated device, an electric shock, which might result in death can never be ruled out, although
the design and verication is according to the relevant standards for electrical safety of medical devices, an electric shock, which can
result in death, can never be ruled out completely.
The residual risk of strangulation can not be excluded as well. The risk is reduced by safety information within the IFU.
It cannot be excluded that unintended or forbidden use might cause further not yet regarded risks and that already regarded risks
might have been estimated incorrectly. It can also not be excluded that the daily use of the medical product might show further risks.
In ergometry, diagnostics and therapy there are alternatives to treadmill application such as bicycle ergometry (without natural
gait movement) or overground gait therapy (secured only by the therapist), etc. the benet of treadmill training in contrast to these
alternatives is clearly outweighing the residual risk of falling or overload with the known consequences.
In this risk analysis the "present state" of the device has been evaluated.
Having carried out the evaluation and validation of the product, the risk of appearance of a not acceptable risk is very low.
The device (it´s construction, it´s function as well as the intended application) does - under normal conditions - not represent any
unjustiable risk for the patient, the user, the operator or third persons.
4.8 Fireghting
Do not use liquid reghting resources.
Use CO2.
4.9 All-pole disconnection
The following options are available for all-pole disconnection:
❚
Unplug device from power socket.
❚
Unplug cable from device.
❚
Switch off device protection switch
Maintain enough free space to ensure access to cables and the circuit breaker (see “position of patient and operator”).
Page 20

page 20
cos105000-2016-00 en
5 Preparation
Description Ilustration
Perform daily inspection as described in “daily inspection”.
Explain device and application to patient.
Explain and apply fall prevention as described in “fall
prevention”.
Guide patient onto treadmill.
❚
Do not enter the device when running belt is rotating.
❚
Do not step on rear roller.
❚
Do not stand on or enter the running deck when device is in
elevation (running belt might slip through due to gravity).
If possible, the patient should hold both handrails for stability
when entering the treadmill.
Holding handrails during use affects exercise results.
Page 21

page 21
cos105000-2016-00 en
Description Ilustration
Apply pull-cord safety stop.
(Attach clip to patients’ clothing.)
Adjust the length of the rope so that the patient has to maintain
the correct position (see “position of patient and operator”).
Page 22

page 22
cos105000-2016-00 en
6 UserTerminal
6.1 Keys and displays
Element
Primary function
Secondary functions
Displays currently activated mode -
Displays current speed in m/min, km/h, m/s or mph (see LED)* Displays max. speed when selecting modes
Displays covered distance in m, km or miles (see LED)* -
Displays elapsed time in minmin:ss or hh:minmin Displays duration when selecting proles
Displays current elevation in % or degrees (see LED)*
Displays current prole step / number (see
LED)
Displays MET, energy and power in MET, kJ or Watt (see LED) -
Displays measured heart rate
Displays parameter when setting cardio
mode or UKK walk test
The displays may show service information and error messages as well (see “trouble shooting”).
*adjust units and decimals with OP 12-14
Element
Primary function
Secondary functions
Decrease / increase current speed Navigate through settings, adjust parameters
Decrease / increase current elevation See “interfere with automatic program”
Start the operation Conrm setting (“enter”)
Stop the operation
Not an emergency stop / off!
Abort setting (“cancel”)
Exit user options (“cancel”)
See “emergency off”
In case of emergency, press the emergency off button!
-
Depending on the operation mode, the keys have additional functions (see “operation”).
Press the keys softly. As conrmation, you will hear a beep.
Always the latest command will be executed, regardless of whether it came via interface or from the UserTerminal during one of the
four modes. Only stop command has higher priority and cannot be overwritten.
Page 23

page 23
cos105000-2016-00 en
6.2 Standard vs. “lt” devices
Most h/p/cosmos treadmills are available as standard or “lt” (light) devices.
“lt” devices have no UserTerminal (no display, no keyboard).
Standard device “lt” device
Control via
- UserTerminal
- Software (see “accessories”)
- additional keyboard (see “accessories”)
- ECG, spirometry, etc. (see “compatible devices”)
Control via
- Software (see “accessories”)
- additional keyboard (see “accessories”)
- ECG, spirometry, etc. (see “compatible devices”)
Page 24

page 24
cos105000-2016-00 en
6.3 Connection of external devices / Interfaces
The back of the UserTerminal has RS232 interfaces.
For USB-RS232 converter, see “accessories”.
The person combining a medical device with any other device for the rst time is creating a Medical Electrical System.
Requirements for ME-Systems, see “creating systems”.
Unused RS232 interfaces must be covered with dust caps [cos102973] for isolation purposes.
Description Ilustration
RS232 / COM1
Possible connection of external
devices.
(standard)
RS232 / COM2
Possible connection of external
devices.
(optional)
RS232 / COM3
Possible connection for service
only
(optional)
RS232 / COM4
Possible connection of external
devices.
(optional)
Additional interfaces are located at the front of the device below the motor cover.
Description Ilustration
Additional Stop 1
Possible connection for
emergency stop devices.
Additional keyboard
Possible connection for remote
control
Additional Stop 2
Possible connection for
emergency stop devices.
N/A
Page 25

page 25
cos105000-2016-00 en
7 Position of Patient and Operator
1) Position of patient (initial contact)
1a) Optimal position 40%, front
1b) Tolerated position 30%, middle running area
1c) Not tolerated position / buffer zone 30%, rear
2) Intended position of operator
The operator must be within reach of the emergency off at all times.
If the operator is not able to reach the emergency off button at the UserTerminal (body height, obstacles, etc.), the operator
must install an additional emergency stop within reach (see accessories).
3) Training area acc. to ISO 20957-1 patient + device
4) Free area acc. to ISO 20957-1 training area + 0.6 m must be free at all times
(except operator)
5) Safety area acc. to DIN EN 957-6 2.0 m behind device must be free at all times
(except operator)
6) Patient environment acc. to IEC 60601-1 device + 1.5 m
There must be no electrical devices within this area, which are not part of an ME-System with the device.
Do not touch the patient and external electrical devices at the same time.
Page 26
page 26
cos105000-2016-00 en
8 Operation
8.1 General application procedure
Description Illustration
Disinfect the device (see “cleaning”).
Disconnect the device and all accessories from mains
power supply before cleaning or disinfection.
Make sure…
…the PE-cable is connected to electrical installation
and device,
…the device is directly plugged into the dedicated wall
socket,
…the device protection switch on the front of the device
is switched on (light on),
…all emergency offs are released.
Switch the running machine on with the on/off switch
on the UserTerminal (light goes on).
Make sure the space under the treadmill is free from
persons, body parts or objects, especially when
switching on (treadmill will lower during initialization)
and when changing the elevation.
Normal condition: When starting, all displays show
“0”-values.
Select an operation mode.
For a detailed description, see following chapters.
Perform application. -
Switch the running machine off with the on/off switch
on the UserTerminal (light goes off)
Disinfect the device (see “cleaning”).
Disconnect the device and all accessories from mains
power supply before cleaning or disinfection.
Page 27

page 27
cos105000-2016-00 en
8.2 Overview of operation modes
For control, remote control and supervising purposes the free PC software para control is available.
Manual mode
Select mode
Conrm mode
Select speed
Select elevation
Terminate application
Cardio mode
Select mode
Conrm mode
Adjust parameters, indicated by LEDs
Conrm prole
Terminate application
Prole mode
Select mode
Conrm mode
Select prole
Conrm prole
Terminate application
Test mode
Select mode
Conrm mode
Select test
Conrm test
Terminate application
Page 28

page 28
cos105000-2016-00 en
8.3 Manual mode
Basic functions Buttons / displays Further information
Select “manual mode” with “+” or
“-“ button
Selected mode ashes.
Device must be in “mode selection”
(one of the mode LEDs ashes)
To get there, cancel all other
activities by pressing the “stop”button.
Conrm with “enter”
Running belt starts with predened
starting speed (default = 0.5 km/h).
To predene starting speed, see
OP09 in “User Options”.
To request body weight for
calculation of energy and power,
see OP16 in “User Options”.
Conrm with “enter”
Running belt starts with predened
starting speed (default = 0.5 km/h).
Pressing and holding the
key accelerates with dened
acceleration level (see “acc.
levels”).
Decelerate to 0 km/h to pause the
operation (see “pause function”).
Adjust elevation with “up” or “down“
Current elevation is displayed in
“elevation” display
Terminate the operation with “stop”
To predene stop time, see OP08 in
“User Options”.
Page 29

page 29
cos105000-2016-00 en
8.4 Prole mode
❚
The automatic modes must only be performed on the prescription of the medical doctor.
❚
Operator and patient have to be aware of automatic load changes during prole, cardio and test mode.
Start and load changes within the automatic modes are indicated by acoustic signals (beep).
Furthermore, the displays show the next load parameters (ashing).
The prole mode covers six load proles, representing interval training sessions.
These basic proles are scalable (see OP11 in “User Options”, default = off).
Scaled proles cannot be stored. For self-dened tests see “test mode”.
Proles 2 and 5 (prole 2 without elevation)
step v in km/h t in min elev. in %
1 7.2 05:00 5
2 9.0 03:00 0
3 7.2 02:00 10
4 9.0 03:00 0
5 7.2 02:00 10
6 9.0 03:00 0
7 7.2 02:00 10
8 9.0 03:00 0
9 7.2 02:00 10
0
5
10
15
20
25
0
5
10
15
0 5 10 15 20
elev. in %
v in km/h
t in min
profile 1 / 4
0
5
10
15
20
25
0
5
10
15
0 5 10 15 20
elev. in %
v in km/h
t in min
profile 2 / 5
Proles 1 and 4 (prole 1 without elevation)
step v in km/h t in min elev. in %
1 6.5 04:00 0
2 9.0 00:30 5
3 6.5 03:00 0
4 9.0 00:30 10
5 6.5 03:00 0
6 9.0 00:30 10
7 6.5 03:00 0
8 9.0 00:30 10
9 6.5 03:00 0
10 9.0 00:30 10
11 6.5 03:00 0
12 9.0 00:30 10
13 6.5 03:00 0
0
5
10
15
20
25
0
5
10
15
0 5 10 15 20
elev. in %
v in km/h
t in min
profile 1 / 4
Proles 3 and 6 (prole 3 without elevation)
step v in km/h t in min elev. in %
1 10.1 04:00 0
2 11.5 02:00 10
3 10.1 02:00 0
4 13.0 01:00 7.5
5 10.1 02:00 0
6 14.4 01:00 5
7 10.1 02:00 0
8 13.0 01:00 7.5
9 10.1 02:00 0
10 11.5 01:00 5
11 10.1 02:00 0
12 11.5 01:00 5
13 10.1 04:00 0
0
5
10
15
20
25
0
5
10
15
0 5 10 15 20
elev. in %
v in km/h
t in min
profile 1 / 4
0
5
10
15
20
25
0
5
10
15
0 5 10 15 20
elev. in %
v in km/h
t in min
profile 2 / 5
0
5
10
15
20
25
0
5
10
15
0 5 10 15 20
elev. in %
v in km/h
t in min
profile 3 / 6
Page 30

page 30
cos105000-2016-00 en
Basic functions Buttons / displays Further information
Select “prole mode” with “+” or “-“
button.
Selected mode ashes.
Device must be in “mode selection”
(one of the mode LEDs ashes)
To get there, cancel all other
activities by pressing the “stop”button.
Conrm with “enter”.
Prole 1 is displayed.
Select prole with “+” or “-“.
Current prole is displayed in
“elevation” display.
Max. speed is displayed in “speed”
display.
Duration is displayed in “time”
display.
Conrm with “enter”.
The selected prole starts with the
rst prole step after countdown.
The operation stops automatically
after the last step.
To scale the proles, activate OP11
(User Options).
If scaling is performed, the max.
parameters are displayed (see
above).
Terminate the operation with “stop”.
For possibilities to interfere with an automatic program, see “interfere with an automatic program”.
Page 31

page 31
cos105000-2016-00 en
8.5 Cardio mode (optional)
❚
WARNING! Heart rate monitoring systems may be inaccurate.
❚
Incorrect or over exercising may result in serious injury or death.
❚
If you feel faint or dizzy stop exercising immediately and consult a medical doctor.
❚
Exclude overloading or overstressing of the patient.
❚
The automatic modes must only be performed on the prescription of the medical doctor.
❚
Operator and patient have to be aware of automatic load changes during prole, cardio and test mode.
❚
In case of any visible or assumed defects or malfunctions (of the device, accessories, software, etc.), unplug device, exclude
reconnection, mark clearly and inform h/p/cosmos service personnel via telephone and writing.
Start and load changes within the automatic modes are indicated by acoustic signals (beeps).
Furthermore, the displays show the next load parameters (ashing).
The cardio mode allows training within pre-dened heart rate limits.
In order to stay within the limits, the treadmill adjusts speed and elevation automatically, rst speed, then elevation.
A POLAR heart rate sensor detects the heart rate.
Moisten the contact areas of the POLAR heart rate sensor.
Place the transmitter directly under the pectoral muscle (see picture).
Basic functions Buttons / displays Further information
Select “cardio mode” with “+” or “-“
button
Selected mode ashes.
Conrm with “enter”.
Device must be in “mode selection”
(one of the mode LEDs ashes)
To get there, cancel all other
activities by pressing the “stop”button.
Set
− max. speed,
− age,
− heart rate upper limit and
− heart rate lower limit
with “+” or “-“
Conrm each parameter with
“enter”.
The running belt starts
automatically.
To avoid high speed, set a low max.
speed. The treadmill will adjust the
load via elevation.
In order to avoid elevation, set a
high max. speed. The treadmill will
adjust the load via speed.
Terminate the operation with “stop”.
Page 32

page 32
cos105000-2016-00 en
If the heart rate signal totally fails, an acoustic warning signal occurs and the heart rate display shows no value any more.
Furthermore, the device reduces speed and elevation to 0 within one minute.
For possibilities to interfere with an automatic program, see “interfere with an automatic program”.
The treadmill adjusts speed and elevation according to following matrices.
Deviation from lower limit Speed (km/h) Elevation (%) Reaction time (s)
< 5 heart beats 0.2 0.1 25
6 … 15 0.4 0.2 25
16 … 30 0.6 0.4 25
31 … 50 0.8 0.8 20
> 50 heart beats 1.0 1.0 20
Deviation from lower limit Speed (km/h) Elevation (%) Reaction time (s)
< 5 heart beats 0.3 0.3 12
6 … 15 0.8 0.8 12
16 … 30 1.0 1.0 10
31 … 50 1.5 1.2 8
> 50 heart beats 2.0 1.6 7
Page 33

page 33
cos105000-2016-00 en
8.6 Test mode
❚
WARNING! Heart rate monitoring systems may be inaccurate.
❚
Incorrect or over exercising may result in serious injury or death.
❚
If you feel faint or dizzy stop exercising immediately and consult a medical doctor.
❚
Exclude overloading or overstressing of the patient.
❚
During stress tests a medical doctor has to be available at any time.
❚
The automatic modes must only be performed on the prescription of the medical doctor.
❚
Operator and patient have to be aware of automatic load changes during prole, cardio and test mode.
❚
In case of any visible or assumed defects or malfunctions (of the device, accessories, software, etc.), unplug device, exclude
reconnection, mark clearly and inform h/p/cosmos service personnel via telephone and writing.
Start and load changes within the automatic modes are indicated by acoustic signals (beeps).
Furthermore, the displays show the next load parameters (ashing).
The h/p/cosmos treadmills are equipped with pre-dened tests.
❚
As described before, the treadmill contains a number of standardized protocols.
Nevertheless, the treadmill does not give recommendations for treatment.
The decision about the correct load is the duty of the medical doctor.
Depending on the application the load includes speed, elevation, distance, heart rate, body weight or motion support etc..
The annex covers a detailed explanation of all pre-dened tests (see “Annex II”).
The annex also covers a detailed explanation how to create a self-dened test.
No. Description No. Description
01 UKK walk test 07 Cooper protocol
02 Graded test 08 Ellestad A protocol
03 Conconi test 09 Ellestad B protocol
04 Bruce protocol 10 Ramp prole
05 Naughton protocol 11 Gardner test protocol
06 Balke protocol 21 – 28 Freely denable
Basic functions Buttons / displays Further information
Select “test mode” with “+” or “-“
button.
Selected mode ashes.
Conrm with “enter”.
Device must be in “mode selection”
(one of the mode LEDs ashes)
To get there, cancel all other
activities by pressing the “stop”button.
Page 34

page 34
cos105000-2016-00 en
Basic functions Buttons / displays Further information
Select test with “+” or “-“
Conrm with “enter”.
The running belt starts
automatically after countdown.
Following tests require further
settings:
01 UKK walk test
02 Graded test
03 Conconi test
10 Ramp prole
Terminate the operation with “stop”.
For possibilities to interfere with an automatic program, see “interfere with an automatic program”.
Page 35

page 35
cos105000-2016-00 en
8.7 Interfere with automatic program
Basic functions Buttons / displays Further information
Alter speed
Press “+” or “-“.
Prole / test mode:
Only valid for current step.
Cardio mode:
Reduce the speed with “-“ or
exceed the max. speed with “+”;
This sets a new max. speed
Decelerate to 0 km/h to pause the
operation (see “pause function”).
Alter elevation
Press “up” or “down”.
Prole / test mode:
Only valid for current step.
Switch between modes
Press “enter” together with “+” or
“-“.
Switch to automatic mode:
Further settings are necessary to
continue.
Prole or test mode:
Switch between steps
Press “enter” together with “up” or
“down“.
Cardio mode only:
Change heart rate upper limit
Press “enter” together with “up” or
“down“.
Heart rate lower limit follows
according to initial range.
Page 36

page 36
cos105000-2016-00 en
8.8 Pause function
Reducing the speed with the “-“ key to 0.00 km/h, triggers the “pause” function.
The running belt stops.
The speed display shows “PAUS”.
Pressing the “start” key, starts the running belt at the preset starting speed (default: 0.5 km/h). All values are maintained.
Pressing the “+” key, starts the running belt at 0.1 km/h. All values are maintained.
Pressing the “stop” key one time, terminates the application. All values remain on the display for 2 minutes.
Pressing the “stop” key a second time, resets all values.
Page 37

page 37
cos105000-2016-00 en
8.9 Acceleration levels
❚
Start the use of the treadmill with slow walking, especially for beginners.
There are seven acceleration / deceleration levels for any kind of operation.
The acceleration levels are dened by the time it takes to accelerate from 0 km/h to maximum speed.
Example: With acceleration level 3, it takes 33 seconds from 0 km/h to maximum speed (see table below).
In order to access a certain acceleration level press the “+” or “-“ button several times, then hold it.
The number of times the button is pressed before holding denes the acceleration level.
Example: Pressing “+” 3 times, then holding “+” results in an acceleration with acceleration level 3.
Acceleration levels 1 - 4 are freely accessible.
Acceleration levels 5 - 7 are locked by the administrator options. For access, please contact our service department.
The high acceleration of the levels 5 - 7 is dangerous for patients and must only be applied during sports medicine and athletic use.
In order to limit the accessibility of the acceleration levels, see OP 27 - 29 of the “User Options”.
Acc. level 0 to max in s Acc. in m/s²
1 131 0.038
2 66 0.076
3 33 0.152
4 16 0.313
5 8 0.625
6 5 1.000
7 3 1.667
0,000
0,200
0,400
0,600
0,800
1,000
1,200
1,400
1,600
1,800
1 2 3 4 5 6 7
acceleration in m/s²
acceleration level
acc. levels treadmill family 150/50 LC
Page 38

page 38
cos105000-2016-00 en
Basic functions Buttons / displays Further information
Press “+”, “-“ and “stop” together for
at least 3 s.
“speed “ display shows “OP 01”.
Select option with “+” or “-“
Conrm with “enter”.
Adjust option with “+”or “-”.
Conrm with “enter”.
Leave options by pressing “cancel”.
8.10 User Options
Page 39

page 39
cos105000-2016-00 en
No. function Adjustable range (default setting)
OP 01 Reset error messages.
OP 02 Total distance (km) Report only
OP 03 Total time (h), operation + stand-by Report only
OP 04 Total time (h), operation only Report only
OP 05 Firmware version Report only
OP 06 Real time clock Setting of current date/time
OP 07 Acoustic heart rate signal
OFF or ON
OP 08 Deceleration time
2 ... 30 s (5s)
OP 09 Starting speed (manual and cardio mode)
0.5 km/h ... 5.0 km/h (0.5 km/h)
OP 11 Scaling of prole mode
0 (off)
1 all parameters together
2 each parameter separately
OP 12 Unit for displaying speed
0 = x.x km/h, 1 = x.x m/s, 2 = x.x mph, 3 = x m/min
20 = x.xx km/h, 21 = x.xx m/s, 22 = x.xx mph, 23 = x.x m/min
OP 13 Unit for displaying distance
0: km;1: miles; 2: m
OP 14 Unit for angle of elevation
0 = % / 1 = ° (degrees)
OP 15 Default body weight
10 ... 250 kg (65 kg)
OP 16 Bodyweight request OFF / ON
OP 17 Unit of energy consumption
JOUL = kJoule, CALO = kcal
OP 18 Max. speed (default) in cardio mode 0.0 ... max. speed (6.0 km/h)
OP19 Setting of polar W:I:N:D system
0000 0000 all senders accepted
xxxx xxxx only specic sender accepted
9999 9999 next sender will be accepted
OP 20 RS 232 interface protocol: COM 1
OFF, 1 … 20 (1 = h/p/cosmos coscom)
OP 21 RS 232 interface protocol: COM 2
OFF, 1 ... 18 (1 = h/p/cosmos coscom)
OP 23 RS 232 interface protocol: COM 4
OFF, 18 … 23 (20 = h/p/cosmos coscom v3)
OP 27 Min. acceleration and deceleration level
1 ... 5
OP 28 Max. acceleration and deceleration level
1 ... 7 (4)
OP 29 Acceleration and deceleration level via RS232
1 ... 5 (4)
OP 40 Locking and unlocking the treadmill
OFF = locked, ON = unlocked
OP 41 Locking and unlocking the manual mode
OFF = locked, ON = unlocked
OP 42 Locking and unlocking the prole mode
0 (all locked) ... 6 (all unlocked)
OP 43 Locking and unlocking the cardio mode
OFF = locked, ON = unlocked
OP 44 Locking and unlocking the test mode
0 ... 94 (28, unlocked up to test 28)
OP 45 Report mode display „index“
0 (display alternates)
1 (MET), 2 (kJ), 3 (Watt)
cannot be stored beyond restart
OP 46 Report mode display „elevation“
0 (display alternates)
1 (elevation), 2 (step)
Cannot be stored beyond restart
OP 47 Sustain values in display resp. automatic „Reset“
OFF = RESET with 1 x STOP
ON= RESET with 2 x STOP
OP 48 Countdown of program-step
OFF = count-up, ON = countdown
OP 52 Output interval for printer protocol
0 = no individual values, 1 ... 100 s (60 s)
OP 53 Language settings for printer protocol
English, German, French, Spanish, Portuguese, Hungarian
Table of User Options (details see chapter 17.4 “User Options (details)”)
Page 40

page 40
cos105000-2016-00 en
9 Accessories / compatible devices
❚
Do not modify the device, congurations, accessories or software in any way.
❚
Do not connect any devices, accessories or software, not listed in “accessories / compatible devices”.
Read and obey all instructions for use of all accessories and compatible devices.
The list of accessories / compatible devices may vary.
Therefore always refer to the most recent version of these instructions for use, available at www.h-p-cosmos.com.
9.1 Creating Systems
The person combining a medical device with any other device for the rst time is creating a Medical Electrical System (ME-System
acc. to IEC 60601-1, 16).
Depending on the combination, this system might even be a Programmable Electrical Medical System (PEMS acc. to IEC 60601-1,
14).
It is obligatory to perform a risk management when creating an ME-System / PEMS.
Risk management, safety, compliance, and maintenance are the responsibility of the manufacturer of the ME-system / PEMS, not
the responsibility of h/p/cosmos.
For all ME-Systems / PEMS a potential equalization must be provided.
Furthermore, the person who puts devices bearing the CE marking together, must meet the corresponding requirements, stated in
the European Medical Device Directive (MDD 93/42/EEC, Article 12).
9.2 Overview of accessories
Following accessories are available for this device:
(For illustrations and detailed descriptions, see annex or www.h-p-cosmos.com.)
Article number Accessory Purpose Information
cos10079 h/p/cosmos safety arch Fall prevention and safety stop Mandatory (*)
cos10223 Potential equalization cable Potential equalization Included
cos10071-v4.1.0 Para control PC software Remote control software Included
cos00097010034 Interface connection cable RS 232 5m Connection Included
cos00097010035 Interface connection cable RS 232 10m Connection Optional
cos12769-01 USB-RS232 converter Connection Optional
cos00098010025 COM 2 interface RS232 Connection Optional
cos16487 COM 3 interface RS232 Connection Optional
cos16488 COM 4 interface RS232 Connection Optional
cos102400 Pediatric handrail Pediatric application Optional
cos102918 Handrail long 1358 mm Body weight support Optional
cos102426 Cross-bar front rail Body weight support Optional
cos102931 Wheelchair ramp Wheelchair access Optional
cos100106 POLAR heart rate receiver board WIND Heart rate measurement Optional
cos102818 Heart rate measurment Polar & control Heart rate measurement Optional
cos12013 Arm support adjustable in height and width Body weight support Optional
cos100680 Additional keyboard for arm support Remote control Optional
cos12922 Extension cord 2m additional keyboard Remote control Optional
cos10111-01 Keyboard holder for arm support Remote control Optional
cos14135 Holder f. optional keyboard o. handrail 60 Remote control Optional
cos10107 Optional stop button in right arm support Emergency stop Optional
Page 41

page 41
cos105000-2016-00 en
Article number Accessory Purpose Information
cos10108 Optional stop button in left arm support Emergency stop Optional
cos100548 Emergency stop button magnet holder 10m Emergency stop Optional
cos15294 Emergency stop ext. without xation 5m Emergency stop Optional
cos15294L10m Emergency stop ext. without xation 10m Emergency stop Optional
cos15294 L15m Emergency stop ext. without xation 15m Emergency stop Optional
cos15933 Emergency stop-button magnet holder 5m Emergency stop Optional
cos12410 Drink-bottle holder Drinking bottle Optional
cos15485 Drink-bottle Drinking bottle Optional
cos102927 Elevation 0% to +25% Elevation Optional
cos30022 Robowalk expander F Motion support / resistance Optional
cos30023 Robowalk expander B Motion support / resistance Optional
cos101277 Science port speed output TTL Measurement Optional
cos14376 Sensor f speed & distance measurem. 150-50 Measurement Optional
cos14005 Floor protection mat treadmill 150/50 Floor protection, stability Optional
cos12607-00 Base plate 150/50 for h/p/cosmos airwalk Floor protection, stability Optional
cos100755c Fixing disc leveling socket 150/50 set Floor protection, stability Optional
(*) At least one kind of fall prevention is mandatory, see “safety”.
9.3 Compatible devices
A number of ECG and CPET (cardiopulmonary exercise test) devices as well as software products are compatible with h/p/cosmos
treadmills via coscom v3 interface protocol.
Please contact service@h-p-cosmos.com for the list of compatible devices.
The risk management of this device covers the inuence of the compatible devices on this device.
The risk management of this device does not cover the inuence of this device on the compatible devices.
Make sure, this device is listed as compatible device in the instructions for use of the compatible device.
Page 42

page 42
cos105000-2016-00 en
10 Disinfection / Cleaning
❚
Disinfect the device before and after every treatment.
❚
Disconnect the device and all accessories from mains power supply before cleaning or disinfection.
Description Illustration
Disinfection
− Unplug the device.
− Apply some disinfectant to a cloth.
− Wipe all surfaces the patient might have touched.
− Wipe all surfaces that may have come into contact with body
uids.
− Wipe the safety harness.
h/p/cosmos recommends Bacillol plus, order number [cos12179].
Clean the device regularly
− Unplug the device.
− Use a damp cloth (not wet).
− Wipe all surfaces.
− Wash safety harness acc. to label.
The h/p/cosmos devices are neither sterile nor can they be sterilized.
Page 43

page 43
cos105000-2016-00 en
11 Maintenance
❚
Obey the maintenance intervals claimed in chapter “maintenance”.
❚
Obey the competences claimed in chapter “maintenance”.
❚
A second person has to be present during maintenance.
❚
In case of any visible or assumed defects or malfunctions (of the device, accessories, software, etc.), unplug device, exclude
reconnection, mark clearly and inform h/p/cosmos service personnel via telephone and writing.
❚
In case of any visible or assumed wear and tear (of the device, accessories, labels, etc.), unplug device, exclude reconnection,
mark clearly and inform h/p/cosmos service personnel via telephone and writing.
❚
In case of any uid entering into the device, unplug device, exclude reconnection, mark clearly and inform h/p/cosmos service
personnel via telephone and writing.
Do not perform maintenance during use.
Proper maintenance is an important pre-condition for safety, reliability, function and accuracy of the device.
Support h/p/cosmos service personnel with the documents needed.
11.1 Intervals and competences
Maintenance Interval Competence
Daily inspection Daily Operator
Lubrication When OIL message occurs Operator
Adjustment of running belt If due Operator
Safety inspection 12 months h/p/cosmos service personnel only
Change of safety arch rope 24 months h/p/cosmos service personnel only
Tightening of the running belt If due h/p/cosmos service personnel only
Adjustment of levelling sockets If due h/p/cosmos service personnel only
Installation and repair work If due h/p/cosmos service personnel only
h/p/cosmos recommends entering into a service contract with an authorized h/p/cosmos service technician.
A service contract provides the best preventive maintenance and care for the device.
The service contract is available at service@h-p-cosmos.com.
To receive information on becoming h/p/cosmos service personnel, please contact service@h-p-cosmos.com.
11.2 Daily inspection
❚
Perform a daily visual inspection (see chapter “maintenance”).
Before daily use, check the whole device for wear and tear.
Description Illustration
Pay special attention to components with high probability of wear
and tear:
1. Running belt and non-slip surfaces
2. External cabling
3. All textile parts
4. Fall prevention incl. safety harness, buckle, carabiner, rope
and rope arrest
If there is any visible or assumed wear and tear, unplug device,
exclude reconnection and call h/p/cosmos service personnel.
Page 44

page 44
cos105000-2016-00 en
Description Illustration
Perform a functional check of all safety equipment:
5. Fall prevention
6. Pull cord safety stop
7. Emergency off button on UserTerminal
8. Further safety equipment
If there is any visible or assumed defects or malfunctions, unplug
device, exclude reconnection and call h/p/cosmos service
personnel.
Description Illustration
When OIL message occurs, check the oil lm on the running
belt. Do the same if there are dry grinding noises during
operation.
The OIL messages occurs every 1000 km by default.
The oil interval may be varied, depending on environmental
conditions and use.
Unplug the treadmill.
Move one hand / tissue for 1 m through the center between
running belt and running deck.
A slight lm of oil must remain on the hand.
If the running belt is too dry, it must be lubricated.
For further and precise analysis a detailed instructions regarding the
tissue lubrication test are available at service@h-p-cosmos.com.
Fill the syringe with 10 ml of the lubricant (h/p/cosmos special
oil, only) and attach the tube to the syringe.
Pump the lubricant under the running belt very slowly and
remove the syringe.
11.3 Lubrication
Page 45
page 45
cos105000-2016-00 en
Description Illustration
Start the running belt with 2 km/h and walk on the belt for 2 min.
Vary the position in order to distribute the lubricant.
Reset the OIL message (see OP01 in “User options”).
Description Illustration
The maximum allowed lateral position of the running belt is
marked with this label.
Operate the device at 10 km/h.
Turn the LEFT trimming screw very slowly
(¼ rotation – observe – ¼ rotation – observe…).
Turn clockwise to adjust belt to the right.
Turn counter-clockwise to adjust belt to the left.
After adjustment, observe the running belt at 10 km/h for at
least 2 min.
Belt must maintain the position.
Remove Allen key from screw.
11.4 Adjustment of running belt
❚
Unmeant trapping hazards: Take off ties, scarfs or other clothes that may be trapped. Secure long hair and ribbons during
maintenance and training in order to prevent being captured in trapping zones.
❚
Do not touch the running belt while it is in motion.
❚
A second person has to be present during maintenance.
Page 46

page 46
cos105000-2016-00 en
11.6 Safety inspection
In order to maintain the safety of the device, h/p/cosmos prescribes performance of an annual safety inspection.
Refer to the date on the inspection sticker on your device for the next inspection date.
h/p/cosmos bases the annual safety inspection on German laws and regulations.
It is the operator’s responsibility to comply with national laws and regulations.
The inspection label on the device also certies the optional equipment and the accessories.
However, inspection intervals for optional equipment and accessories may deviate.
After 12 months or 5000 km there will be a safety inspection reminder (see below).
The error message will be reset by the h/p/cosmos service personnel performing the safety inspection.
11.7 Spare parts and consumables
Spare parts must only be replaced by qualied h/p/cosmos service personnel.
Information about spare parts is available at service@h-p-cosmos.com.
A list of consumables is included in the accompanying documents.
11.5 Issues for qualied service personnel
All maintenance work that is not explained in detail, must not be performed by the operator.
Safety inspections, installation and repair work, must also not be performed by the operator.
This kind of work must be performed by h/p/cosmos service personnel according to the “h/p/cosmos service instructions”.
The “h/p/cosmos service instructions” are available at service@h-p-cosmos.com.
Page 47

page 47
cos105000-2016-00 en
12 Troubleshooting
12.1 General troubleshooting
❚
In case of any visible or assumed defects or malfunctions (of the device, accessories, software, etc.), unplug device, exclude
reconnection, mark clearly and inform h/p/cosmos service personnel via telephone and writing.
❚
In case of any visible or assumed wear and tear (of the device, accessories, labels, etc.), unplug device, exclude reconnection,
mark clearly and inform h/p/cosmos service personnel via telephone and writing.
Problem Solution
Device cannot be switched on Release emergency off (see “emergency off”)
Check power supply connection
Check device protection switch
Check power socket (test with another device)
Elevation does not work (E21) Switch off
Wait 10 min (in order to cool down)
Switch on again
In case E21 still appears, unplug device, exclude reconnection, mark clearly
and inform h/p/cosmos service personnel via telephone and writing.
Device does not start but displays speed value Switch off and on again
Check error messages (see below)
Oil leakage Unplug device
Remove excess oil besides running belt
Remove excess oil under running belt
Check the next days and repeat if necessary.
Device indicates “pull stop” Release emergency stops (see “safety”)
Electrostatic discharge Choose proper oor, clothing and humidity
Bouncing noise Device may not stand rmly
Contact service@h-p-cosmos.com
Grinding noises Check lubrication (see “lubrication”)
Check adjustment of running belt (see “adjustment of running belt”)
Running belt outside of lateral limits See “adjustment of running belt”
Problems with optional heart rate measurement See “accessory: wireless heart rate measurement …”
Any other problem unplug device, exclude reconnection, mark clearly and inform h/p/cosmos
service personnel via telephone and writing.
Page 48

page 48
cos105000-2016-00 en
Problem Solution
No connection via RS232
(wrong cable)
For connection to PC with h/p/cosmos software and most external devices
use the included RS 232 interface connection cable [cos00097010034].
No connection via RS232
(cable defect)
Check cable and plugs for defects.
Replace defective cable.
No connection via RS232
(wrong COM port)
Do not use COM 3 on the device
(see “connection of external devices”).
No connection via RS232
(wrong settings)
Choose correct interface protocol on device (“User Options” OP 20 or 21).
Choose correct interface protocol on periphery.
Check installation of peripheral software.
No connection via RS232
(blocked COM port)
Restart peripheral software.
Restart peripheral device.
Acceleration via external device too slow. Check max. acceleration and deceleration level via RS232
(“User Options” OP 29).
Any other problem unplug device, exclude reconnection, mark clearly and inform h/p/cosmos
service personnel via telephone and writing.
Error code Acoustic code
(x = short beep,
o = long beep)
Error message Action
E01 ooooo xoooo Oil Help See “lubrication”
E02 ooooo xxooo Service Help See “safety inspection”
E20 xxooo ooooo Elev Help
Unplug device,
exclude reconnection and
contact service@h-p-cosmos.com
E21 xxooo xoooo Incr Help
E30 xxxoo ooooo Setup Help
E31 xxxoo xoooo Setup Help
E32 xxxoo xxooo Setup Help
E41 xxxxo xoooo Setup Help
E50 xxxxx ooooo FU Help
E51 xxxxx xoooo FU Help
E52 xxxxx xxooo FU Help
12.2 RS232 troubleshooting
12.3 Error messages
Following error messages may be displayed on the UserTerminal:
Page 49

page 49
cos105000-2016-00 en
13 Technical data
13.1 UserTerminal
Description Data
Displays 6 seven-segment LCD displays,
LED displays of mode and unit
Keyboard 6-key keyboard lm
“lt” devices have no UserTerminal (no display, no keyboard).
13.2 Dimensions
Description Data
Device dimensions 2095 x 850 x 1310 mm (L x W x H)
Running surface dimensions 1500 x 500 mm (L x W)
Track access height 220 mm
Handrail dimensions D = 60 mm, L = 620 mm
Data may be inuenced by accessories.
13.3 Loads
Description Data
Max. patient weight * 200 kg
Device weight 230 kg
Substitutional load to oor (EN 1991) 3.0 kN / m²
Load on each support (wheels + feet) 4 x 1.3 kN
Data may be inuenced by accessories.
13.4 Emissions
Description Data
Heat emission approx. 53°C (on/off button, contact < 1 min)
A-weighted emission sound pressure level at the
trainer’s ear (EN 957-6)
LpA <70 dB A (63 dB)
(Noise emission under load is higher than without load.)
13.5 Essential performance characteristics
Description Data
Speed 0.5 … 18.0 km/h
Min. speed increment 0.1 km/h
Speed accuracy * ± 5 % (above 2 km/h), ± 0.1 km/h (up to 2 km/h)
Elevation 0.0 ... 20.0 %
Min. elevation increment 0.1 %
Elevation accuracy * ± 5 % (above 2 % elevation)
Page 50

page 50
cos105000-2016-00 en
13.6 Environmental conditions
Operation Data
Temperature 0 … 40° C
Humidity 20 … 90%, without condensation
Pressure 700 … 1060 hPa
Altitude max. 3000 m, without pressurization
(altitudes >1000m can cause minor loss of performance)
Oxygen saturation <= 25%
Exclude danger of explosions or inammability. Exclude high voltage lines / devices in near vicinity.
Transport & Storage Data
Temperature -25°C … 40°C
Humidity 20 … 90%, without condensation
Pressure 700 … 1060 hPa
Altitude max. 3000 m, without pressurization
When storing for more than 6 months without power connection, the batteries of the MCU may discharge.
Please contact service@h-p-cosmos.com in case of re-installation after storage.
13.7 Technical and legal requirements
Description Data
Stationary training equipment ISO 20957-1, EN 957-6
Medical electrical equipment IEC 60601-1
Electromagnetic compatibility IEC 60601-1-2
Usability IEC 60601-1-6, IEC 62366-1
Software IEC 62304
Medical device directive MDD 93/42/EEC
Machinery directive MD 2006/42/EC
Legal requirements German medical device act (MPG)
13.8 EMC tests
Description Data
Measurement of conducted emission acc. to EN 55011, Group 1, Class B
Measurement of radiated emission acc. to EN 55011, Group 1, Class B
Electrostatic discharge immunity test acc. to EN 61000-4-2
Radiated, radio-frequency, electromagnetic eld
immunity test
acc. to EN 61000-4-3
Electrical fast transient immunity test acc. to EN 61000-4-4
Surge immunity test acc. to EN 61000-4-5
Immunity to conducted disturbances, induced by
radio-frequency elds
acc. to EN 61000-4-6
Power frequency magnetic eld immunity test acc. to EN 61000-4-8
Voltage dips, short interruptions and voltage
variations immunity tests
acc. to EN 61000-4-11
Testing of voltage changes, voltage uctuations
and icker in public low-voltage supply systems
acc. to EN 61000-3-3
Variation of mains frequency acc. to DIN EN 60601-1
Page 51

page 51
cos105000-2016-00 en
13.9 Classication
Description Data
MDD 93/42/EEC Notied body
MDD 93/42/EEC Risk class IIb
active therapeutic device and active diagnostic device
60601-1 Protection against electric
shock
Class I,
IEC 60601-1 Protection against harmful
ingress of water or particulate
matter
IP00
IEC 60601-1 Mode of operation Continuous operation
IEC 60601-1 Overvoltage category II (2500 Vpeak mains transient voltage)
IEC 60601-1 Applied part
Type B
(whole device)
IEC 60601-1 Pollution degree Degree 2
ISO 20957-1 Usage class S (Studio): professional and / commercial use
I (inclusive): professional and/or commercial use provided for inclusive use for
people with special needs
EN 957-6 Accuracy class A
IEC 62304 Risk class B
13.10 Certicates
Description Data
MDD 93/42/EEC CE declaration of conformity
MDD 93/42/EEC EC certicate, quality assurance
MDD 93/42/EEC Free sales certicate
EN ISO 13485 Certicate, quality management medical devices
IEC 60601-1 CB certicate
UL 60601-1 NRTL certicate
Certicates see accompanying documents and
https://www.h-p-cosmos.com/en/contact-support/media-downloads/security-certicates
13.11 Interfaces (RS232, D-SUB, 9-pole)
Description Data
COM 1 (standard) Baud rate 9600 bps / 115200 bps
COM 2 (optional) Baud rate 9600 bps
COM 3 (service) Baud rate 115200 bps
COM 4 (optional) Baud rate 115200 bps
Page 52

page 52
cos105000-2016-00 en
13.12 Voltage, Current, Performance
Description Data
Input voltage * 200 V - 240 V ~ (f: 50 – 60 Hz)
Current input (long time) * 6.0 A
Current input (momentary) * 13.5 A
Energy consumption (long time) ≤ 1440 VA
Energy consumption (momentary) ≤ 3240 VA
Energy efciency N/A
Device protection switch (circuit breaker) 16 A
Drive motor capacity 2200 W
Elevation motor capacity 470 W
Earth leakage current ≤ 0.2 mA
Isolation transformer 1840 VA
Power supply cord detachable, 3 m
13.13 All-pole disconnection
There are the following options for all-pole disconnection:
❚
Unplug device from power socket.
❚
Unplug cable from device.
❚
Switch off device protection switch
Regard free area in order to maintain access to cables and circuit breaker (see “position of patient and operator”).
* Overload or weak power supply may lead to reduced speed accuracy or tripping the fuse.
Subject to technical alterations without prior notice. E & OE.
Page 53

page 53
cos105000-2016-00 en
14 Liability and Warranty
Following will cause loss of liability and warranty and may result in serious injury or death or damage to the device:
❚
Use other than explicitly mentioned as intended use
❚
Unauthorized maintenance or lack of maintenance, safety checks or repairs
❚
Unauthorized modications or extensions
❚
Unauthorized installation, commissioning or instruction
❚
Use of any unauthorized or non-original h/p/cosmos parts, spare parts, consumables, sensors or detectors
❚
Disregard of safety information (danger, warning and caution statements)
❚
Any modications to the device, software, congurations and accessories
❚
Connection of accessories, software or devices, not listed in “accessories / compatible devices”
The “safety information – forbidden use” list does not claim to be exhaustive and may be extended during market phase (post market
surveillance). The latest version of these instructions for use is always available at: www.h-p-cosmos.com
Limited liabilities apply:
If h/p/cosmos or h/p/cosmos organizational bodies, senior management or agents can be held accountable for the payment of
damages pertaining to slight negligence (breach of material contractual obligations), the damages shall be limited to damages
that could typically have been foreseen. Liability pertaining to slight negligence excludes liability as a result of loss of production,
interruption of business and loss of prots.
Further details see website: www.h-p-cosmos.com/en/gtcb.
15 Expected Lifetime
❚
Obey the maintenance intervals claimed in chapter “maintenance”.
❚
Obey the competences claimed in chapter “maintenance”.
The expected lifetime of the entire device is 10 years, provided, that
❚
all maintenance intervals are maintained.
❚
wear and tear parts are replaced by h/p/cosmos service personnel during annual maintenance.
16 Disposal
Dispose the device according to European directive 2012/19/EU and the corresponding local disposal law.
Dispose the lubrication material according to the corresponding local disposal law.
Contact service@h-p-cosmos.com to receive further information or an offer regarding correct disposal by the manufacturer.
Page 54

page 54
cos105000-2016-00 en
17 Annex I
17.1 Installation
This device must only be unpacked, transported and installed by h/p/cosmos service personnel (see maintenance).
If the packaging has been damaged, please contact service@h-p-cosmos.com immediately.
It is the customer’s responsibility to ensure the following conditions before the installation:
❚
There must be a separate power circuit for the device (dedicated line).
❚
There must be a separate wall socket for the device (electrically interlocked with circuit breaker 16A, type C).
❚
The wall socket has to be marked with the serial number of the device.
❚
The wall socket has to be accessible at all times.
❚
The intended location must meet the environmental conditions (see “technical data”).
❚
The intended location must be capable of bearing the load of the device (see “technical data”).
❚
The intended location must provide the safety area and free area as stated in “position of patient and operator”.
❚
The intended location must provide a ceiling height, high enough for device + accessories (fall prevention).
❚
The intended location must provide a stable and levelled base in order to prevent noise or bouncing.
❚
The intended location must maintain the local requirements for electrical installation.
❚
The intended location must provide a suitable protective earth condition (e.g. PE-bolt).
❚
The intended location must provide the requirements for electrical installation acc. to “technical data”.
The manufacturer does not assume liability for any damage, complaints or missing parts that are not reported immediately upon
delivery on the packing list/delivery note.
17.2 Instruction protocol
When installing the device, the h/p/cosmos service personnel instructs the intended operator according to the instructions for use,
following this instruction protocol.
With the name and signature on the instruction protocol, the instructed persons conrm that they fully know how to operate the
device safely. The instructed persons conrm they are able to instruct further operators according to this protocol.
No. Information Chapter Check
1. These instructions for use are available as print version at service@h-p-cosmos.com. -
2. The instructions for use are to be read in full before starting with the operation. -
3. The safety information is explained in detail. Safety
4. The safety information must be displayed within sight of the device. Safety
5. The function of all safety equipment is explained in detail. Safety
6. The use of the prescribed fall prevention is explained in detail. Safety
7. The position of patient and operator is explained. Position of P+O
8. The safety area (2 m behind device) is specically pointed out especially. Position of P+O
9. The function of the UserTerminal is explained in detail. UserTerminal
10. The general use is explained (incl. manual, prole, cardio and test mode). Operation
11. The competences and intervals for maintenance are explained. Maintenance
12. The adjustment of the running belt is explained in detail. Maintenance
13. The lubrication of the device is explained in detail. Maintenance
14. The accompanying documents are explained and handed over. -
Page 55

page 55
cos105000-2016-00 en
17.3 Instruction protocol, signatures
By signing this protocol, the authorized h/p/cosmos service personnel and the customer conrm the receipt and understanding of all
safety information, the performed instruction and commissioning according to the instruction protocol [cos15228-03]. The customer
conrms the receipt of the listed devices including all accessories and options according to the h/p/cosmos delivery note. Disregard
of safety information, intended or forbidden use, as well as unauthorized maintenance or lack of maintenance and regular safety
checks may lead to injury or even death and can damage the device. Furthermore, this will result in loss of liability and warranty.
Please ll out the instruction protocol and send it back to h/p/cosmos by fax (+49 8669 8642 49), email (sales@h-p-cosmos.com) or
mail).
h/p/cosmos device, model name device serial number
instructor
name in clear block letters h/p/cosmos dealer / technician date and signature
instructed persons (customer, operator, etc.)
name in clear block letters h/p/cosmos dealer / technician date and signature
h/p/cosmos sports & medical gmbh
Am Sportplatz 8
DE-83365 Nussdorf-Traunstein
Germany
customer´s stamp / address:
Page 56

page 56
cos105000-2016-00 en
17.4 User Options (details)
Option Description Comment / Display
OP01 Reset (delete) error messages
Note: This option exclusively resets the causing
variable. If, for example, the E.02 service-timeinterval is being reset with this operation, the
distance-interval is not reset and vice versa.
Only administrator option OP 47 resets all three
values simultaneously.
The required maintenance work must be performed before deleting
error messages. The h/p/cosmos service department must be
contacted before one of the service displays or error codes is deleted!
Conrmation on display
indicating "donE"
Info: This option only resets the error message. If the error still exists,
you are not able to reset the error message. In this case consult an
authorized service engineer
The following intervals are reset by this operation:
E.01: Oil-interval A-OP 35
E.02: Service-time-interval A-OP 37
E.02: Service-distance-interval A-OP 38
OP02 Total distance covered (km)
and indicates: Total distance covered in km
indicates km
OP03 Indication of total hours of operation
= stand-by-time including runtime of motor/
running belt (h)
and show: Operation hours
reports: h
OP04 Indication of total hours of
runtime of motor/running belt (h)
and report: Operation hours
shows: h
OP05 Indication of rmware version and date
reports “OP05” reports “typE”
indicates device type, e.g. “1.4”
indicates: „MCU 5“
reports version, e.g. “1.01.1”
reports default type, e.g. 1.3
OP06 Adjustment of actual date and the real time clock
shows: rtc for Real Time Clock
reports ashing: Date / time, year, month, date, hours, minutes,
seconds
OP07 Acoustic heart rate signal This function is normally used to control the regularity of the heart rate
or to nd reasons for transmitting problems
indicates: OFF or ON
OFF: No acoustic heart-rate-signal
ON: Acoustic heart rate signal for every beat
OP08 Stop time / deceleration time after STOP key in
relation to the max. speed
indicates: Stop time in sec.
indicates: sec for seconds
Adjustable from 2.... 30 seconds
OP09 Start speed (manual mode or cardio mode) for
feedback after START key has been pressed.
This value can be reduced to 0.0 km/h for
advanced users.
indicates: start speed in km/h
max ashing
set unit is ashing
Adjustable from 0.0 km/h ... 5.0 km/h
Page 57

page 57
cos105000-2016-00 en
Option Description Comment / Display
OP11 Scaling of the proles in prole mode (not for
test mode)
shows: Scaling possibilities
0: No scaling (standard)
1: Scaling 1...6, which is shown in the prole mode on the INDEX
display, refers to all parameters together (speed, elevation, time)
2: Scaling 1...6 refers to each parameter individually (speed, elevation,
time)
OP12 Unit for display of speed
indicates: Unit of speed
... without decimal place:
3 = m/min
... with one decimal place:
0 = km/h 1 = m/s 2 = mph 23 = m/min
... with two decimal places:
20 = km/h 21 = m/s 22 = mph
km/h, m/s, mph or m/min ashes
OP13
Unit for display of distance
indicates: Unit of distance
0 = km 1 = miles 2 = m
m, km, or miles ashes
OP14 Unit for angle of elevation
indicates: Unit of elevation
0 = % (per cent) 1 = ° (degree)
% or ° ashes
OP15 Subject's body weight
(default value)
indicates: 10 ... 250 (estimated weight)
weight ashes
The personal body weight is necessary for a more correct calculation
(estimation) of power and energy consumption.
OP16 Request for body weight
before manual or automatic start
0 = OFF. Request for body weight before starting a program is not
required. Calculation of energy consumption and power is based on
the body weight entered in option no. 15.
1 = ON. Input of body weight before starting a program is required.
Calculation of energy consumption and power is based on the entered
body weight.
OP17 Unit for energy consumption JOUL = kJoule is the unit of energy consumption
CALO = kcal is the unit of energy consumption
OP18 Maximum speed in cardio mode (default value)
(this option is only available for treadmillergometers, not for ladder-ergometers)
reports: 0.0 ... max
for the default value for maximum speed in the cardio mode.
adjusted unit ashes, max. ashes
The value of max. speed in cardio mode can be changed online by
pressing
OP19 Setting of sender for POLAR W.I.N.D. system 0000 0000 = all senders are accepted (can also be set with UP and
DOWN)
xxxx xxxx = only specic sender with special ID is accepted, must
be set with
and
9999 9999 = next sender will be accepted, saved and ltered
(can also be set with UP and DOWN)
(further settings in user OP23 and administrator OP16 are necessary)
Page 58

page 58
cos105000-2016-00 en
Option Description Comment / Display
OP20 RS232 interface protocol: COM 1
Do not connect any devices, accessories or
software, not listed in “accessories / compatible
devices”.
indicates ashing: number of the RS232 interface protocol
and reports:
OFF = RS232 not active / no protocol / interface deactivated
1 = h/p/cosmos coscom v1, v2, v3 with baud rate 9600 bps
(standard setting COM 1 and COM 2)
3 = printer protocol (serial printer or converter required)
7 = TM treadmill emulation in km/h
if available use: h/p/cosmos coscom (= 1)
8 = TM treadmill emulation in miles per hour
if available use: h/p/cosmos coscom (= 1)
10 = Loop Back Test (special test plug required, available
at h/p/cosmos)
11 = SunTech Tango blood pressure monitor
(signal tunnel/loop only)
12 = Remote Control Hardware Terminal MCU 4
(special hardware required)
20 = h/p/cosmos coscom v3 with baud rate 115200 bps
For advanced h/p/cosmos coscom v3 connections with baud rate
115200, please select OP20=20. Please note that the connected
devices/software must be approved for h/p/cosmos coscom v3 with
baud rate 115200 (for example h/p/cosmos para control
®
4.1).
OP21 RS232 interface protocol: COM 2 See descriptions above
OP23 RS232 interface protocol COM 4
indicates ashing: Number of the RS232 interface protocol
and reports:
OFF = RS232 not active / no protocol / interface deactivated
18 = Chip-card reader PROXOMED (special hardware required)
20 = h/p/cosmos coscom v3 / baud rate 115200 bps
22 = POLAR W:I:N:D: - System (more adjustments in user option
OP19 und administrator option OP16)
23 = Chip-card reader ProMedPlus (special hardware required)#
OP27 Minimum acceleration and deceleration level
The selected minimum level is valid for all
acceleration and deceleration processes in all
modes and proles.
ashes, reports the min. acceleration / deceleration for all
modes and proles (standard: level 1)
Level of settings: 1 ... 5 but not higher than the value in option 28. For
safety reasons, the acceleration / deceleration levels 5, 6 and 7 cannot
be selected. Note: The selected acceleration and deceleration level is
NOT valid for control and operation via RS232-interface. In this case
the acceleration and deceleration level is set in option 29 or in the
corresponding command of the h/p/cosmos coscom protocol.
OP28 Maximum acceleration and deceleration level
The selected maximum level is valid for all
acceleration and deceleration processes in all
modes and proles
ashes, reports the max. acceleration / deceleration for all
modes and proles (standard: level 4)
The maximum acceleration and deceleration level is NOT valid
for control and operation via V24/RS232 interface. In this case
the acceleration and deceleration level is set in option 29 or in the
corresponding command of the h/p/cosmos coscom protocol.
Page 59

page 59
cos105000-2016-00 en
Option Description Comment / Display
OP29 Standard acceleration and deceleration level for
RS 232 interface
The selected acceleration and deceleration level is valid for control
and operation via RS232 interface. This option is very helpful if the
peripheral equipment (e.g. ECG, ergospirometry, PC) does not offer a
menu for acceleration and deceleration levels.
ashing, reports: 1 ... 5, (standard: 1) for the acceleration and
deceleration level for all speed commands via RS 232. The maximum
adjustable value depends on the setting of option 28.
Note: If the peripheral equipment sends an acceleration and
deceleration command via the h/p/cosmos coscom protocol, the
selected level in option 29 or in the corresponding command of the
h/p/cosmos coscom protocol.
OP40 Locking and unlocking of the treadmill OFF = After switching on, the treadmill is completely locked / not
accessible. To unlock the treadmill, press the buttons +, - and START
simultaneously.
While locked the display shows "no ACCESS"
ON = treadmill is unlocked / accessible (standard)
OP41 Locking and unlocking the manual mode OFF = manual mode is locked / not accessible
ON = manual mode is unlocked / accessible (standard)
OP42 Locking and unlocking the prole mode OFF = prole mode is locked / not accessible
1 ... 6 = prole mode is unlocked / accessible up to the selected prole
number standard: 6
Example: Selected prole number = 3: The proles 1-3 can be
selected, the proles 4 – 6 cannot be selected
OP43 Locking and unlocking the cardio mode OFF = cardio mode is locked / not accessible
ON = cardio mode is unlocked (standard)
OP44 Locking and unlocking the test mode OFF = test mode is locked / not accessible
1 ... 94 = test mode is unlocked / accessible up to the selected test
prole number standard: 24
selected test number = 5: The test proles 1-5 can be selected, the
test proles 6 – 94 cannot be selected
OP45 Report mode display “Index“
0 = Display alternates (default)
1 = Display permanently in MET
2 = Display permanently in kJ
3 = Display permanently in Watt
After total switch off the default value 0 will be valid again.
OP46
Report mode display “Elevation“
in prole
mode and test mode
0 = Display alternates (default)
1 = Display permanently in % or degree (°), depending on OP14
2 = Display permanently in “Step“
After total switch off the default value 0 will be valid again.
OP47 Sustain values in display resp. automatic “Reset“ OFF = Display values are deleted after pressing START again or
automatically 2 minutes after having pressed STOP (default)
ON = Display values will be continued (added) after pressing START
again and will not be automatically deleted by pressing STOP.
Display values can only be deleted by pressing the STOP key twice
(time, distance, energy).
Page 60

page 60
cos105000-2016-00 en
Option Description Comment / Display
OP48 Program step countdown OFF = The time display counts up each program step
ON = The time display counts down each program step
OP52 Output interval for printer protocol By entering a value between 0 and 100, the output interval is set in
seconds for a printer directly connected to the treadmill. Standard: 60
(= printout of all values once per minute). The value 0 disables printing
of individual values, but not printing of headers and end results (UKK).
OP53 Language settings for printer protocol Select the language for printouts on a printer directly connected to
the treadmill. One of six languages can be chosen. Both, the protocol
printout and the test result and training recommendation of the UKK 2
km walking test are printed in the selected language.
EnGL = English (standard) SPAn = Spanish
GErM = German POrt = Portuguese
FrEn = French HUnG = Hungarian
For correct printout, the connected printer must be compatible with
PCL printer language. For special characters the ISO 8859-1 (Latin-1)
font is used.
Page 61

page 61
cos105000-2016-00 en
18 Annex II (Pre- & self-dened tests)
18.1 UKK walk test
UKK stands for Urho Kaleka Kekkonen, founder of the UKK Institute in Tampere, Finland.
The UKK walk test is a tness test, calculating the UKK Fitness Index based on the measured heart rate within a 2 km walk at max.
walking speed. The test requires POLAR heart rate measurement.
A UKK Fitness Index of 100 represents an average tness.
A UKK Fitness Index <100 represents below average tness, a UKK Fitness Index >100 represents above agerage tness.
The UKK Fitness Index is calculated as follows (according to gender):
Men: Fitness Index = 420 + A x 0.2 – (T x 0.19338 + HR x 0.56 + [W : (H x H) x 2.6])
Women: Fitness Index = 304 + A x 0.4 – (T x 0.1417 + HR x 0.32 + [W : (H x H) x 1.1])
A (age) = Age in years, HR (heart rate) = average heart rate during test in bpm, T (time) = walking time for 2 km in s,
W (weight) = subject weight in kg, H (height) = subject height in m
Before performing the UKK walk test, the subject must warm up and determine the max. walking speed.
During the test, the subject must walk as fast as possible for 2 km (heart rate approx. 80% of max). The subject must not run.
The treadmill measures the heart rate (via polar heart rate measurement) every 500 m.
Affter performing the test, the UKK Fitness Index is displayed.
The UKK walk test is suitable for subjects between 20 and 65 years old.
For subjects older than 65 years or overweight subject the results will be less accurate.
Athletes usually do not reach the required heart rate.
Refer to “test mode” for safety information and adjustment.
Basic functions Buttons / displays Further information
Select “test mode” with “+” or “-“
button
Selected mode ashes.
Conrm with “enter”.
“PR 01” ashes.
Conrm with “enter”.
Device must be in “mode selection”
(one of the mode LEDs ashes)
To get there, cancel all other
activities by pressing the “stop”button.
Set
− gender,
− age,
− height
− weight
with “+” or “-“
(corresponding LED ashes)
Conrm each parameter with “enter”.
Select walking speed with “+” or “-“.
After 2,0 km, the speed is reduced
to 50%.
After 5 more minuntes the test is
terminated.
At the end the “index” display
shows the UKK tness index.
In case the device receives no or incorrect heart rate signals, an acoustic warning signal occurs.
Page 62

page 62
cos105000-2016-00 en
18.2 Graded test
18.3 Conconi test
Description Illustration
(e.g. for performance diagnostics based on lactate measurement)
Refer to “test mode” for safety information and adjustment.
Parameter Default value
Starting speed 8.0 km/h
Increment 2.0 km/h
Acceleration level 4
Step length 3:00 min
Break time 0:30 min
Each parameter is adjustable.
STOP must be activated manually by the medical doctor.
Skip remaining break time:
Press “start” once restart after countdown
Press “start” twice restart immediately
Prolong break:
Press “-“ within break “pause” is indicated
Press “start” to continue test continues with remaining break time
Description Illustration
(e.g. for performance diagnostics based on heart rate measurement)
Refer to “test mode” for safety information and adjustment.
Endurance test (max. heart rate test)
Standard load prole:
❚
Starting speed: 8.0 km/h, must be changed according to the condition
of the subject
❚
Circuit (lap length): 200 m (can be changed)
❚
Increment: 0.5 km/h (can be changed)
STOP must be activated manually by the medical doctor
Page 63
page 63
cos105000-2016-00 en
18.4 Bruce protocol
18.5 Naughton protocol
18.6 Balke protocol
Description Illustration
e.g. for ECG stress test
Refer to “test mode” for safety information and adjustment.
Step Duration (min) Speed (km/h) Elevation (%)
1
3:00
2.7 10
2 4.0 12
3 5.4 14
4 6.7 16
5 8.0 18
6 8.8 20
7 9.6 22
Description Illustration
e.g. for ECG stress test
Regard chapter “test mode” for safety information and adjustment.
Step Duration (min) Speed (km/h) Elevation (%)
1
3:00 3.0
0.0
2 3.5
3 7.0
4 10.5
5 14.0
6 17.5
Description Illustration
e.g. for ECG stress test
Refer to “test mode” for safety information and adjustment.
Step Duration (min) Speed (km/h) Elevation (%)
1
2:00 5.0
2.5
2 5.0
3 7.5
4 10.0
5 12.5
6 15.0
7 17.5
8 20.0
9 22.5
10 25.0
Page 64

page 64
cos105000-2016-00 en
18.8 Ellestad A protocol
Description Illustration
e.g. for ECG stress test
Regard chapter “test mode” for safety information and adjustment.
Step Duration (min) Speed (km/h) Elevation (%)
1
3:00
2.7
10.0
2 4.8
3 6.4
4 8.0
18.9 Ellestad B protocol
Description Illustration
e.g. for ECG stress test
Refer to “test mode” for safety information and adjustment.
Step Duration (min) Speed (km/h) Elevation (%)
1
3:00
2.7 10.0
2 4.8 10.0
3 6.4 10.0
4 8.0 10.0
5 8.0 15.0
6 9.6 15.0
18.7 Cooper protocol
Description Illustration
e.g. for ECG stress test
Refer to “test mode” for safety information and adjustment.
❚
Start at 5.3 km/h and 0% elevation
❚
After 1 minute elevation increases to 2 %
❚
After another minute, the elevation is increased by 1% every minute
❚
When elevation is 25 % elevation stays constant and the speed is
increased by 0.32 km/h every minute
STOP must be activated manually by the medical doctor.
Page 65

page 65
cos105000-2016-00 en
18.11 Gardner test protocol
Description Illustration
For application in angiology
Refer to “test mode” for safety information and adjustment.
Pre-test phase: Patient stands on the footboards, not on the belt.
The Gardner test protocol serves to evaluate the
maximum walking distance of peripheral arterial
disease patients with intermittent claudication.
The test is to be performed under constant supervision
of a medical doctor.
The patient rst stands on the side footboards of the
running machine and not on the belt. Start test prole
11 and the belt speeds up to 3.2 km/h. As the patient
steps onto the running belt, the doctor presses the
START key again. By pressing the START key the
second time, the displays will be reset to zero.
After completing the test, the results can be printed on
a host printer if connected.
Step
Duration
(min:sec)
Speed
(km/h)
Elevation
(%)
Total time
(min:sec)
0
until START
is pressed
3.2 0
until START
is pressed
Test phase: Patient steps onto the running belt.
1 02:00 3.2 0 2:00
2 02:00 3.2 2 4:00
3 02:00 3.2 4 6:00
4 02:00 3.2 6 8:00
5 02:00 3.2 8 10:00
6 02:00 3.2 10 12:00
7 02:00 3.2 12 14:00
8 02:00 3.2 14 16:00
9 02:00 3.2 16 18:00
10 02:00 3.2 18 20:00
11 30:00 3.2 18 50:00
18.10 Ramp prole
Description Illustration
(not available for every model)
Refer to “test mode” for safety information and adjustment.
Ramp prole with 2 parameters:
❚
Target speed standard: 10.0 km/h; adjustable from 0 to maximum
speed of the treadmill.
❚
Time for reaching target speed in seconds: standard: 10 seconds;
adjustable from 0 to 99 seconds
Page 66
page 66
cos105000-2016-00 en
Basic functions Buttons / displays Further information
Select “test mode”
with “+” or “-“ button
Selected mode ashes.
Conrm with “enter”.
Device must be in “mode selection”
(one of the mode LEDs ashes)
To get there, cancel all other
activities by pressing the “stop”button.
Select self-dened test (21 – 28)
with “+” or “-“
Conrm with “enter” for at least 5 s.
Select speed for step 1
with “+” or “-“.
Conrm with “enter”.
Select distance for step 1
with “+” or “-“.
Conrm with “enter”.
Select distance “0” in order to
program this step by time.
Select time for step 1
with “+” or “-“.
Conrm with “enter”.
Select time “0:00” in order to
program this step by distance.
Select elevation for step 1
with “+” or “-“.
Conrm with “enter”.
18.12 Self-dened tests
Tests 21 – 28 are freely denable with up to 40 program steps.
Follow the instructions below to program an individual test.
The “heart rate” display will show the current program step.
Use “up” and “down” to scroll through the program steps.
Page 67

page 67
cos105000-2016-00 en
Basic functions Buttons / displays Further information
Select acceleration for step 1
with “+” or “-“.
Conrm with “enter”.
The acceleration level is displayed
in the “index” display.
Proceed the same way for further
steps.
Make sure all steps after the last one
have a speed of “0 km/h”.
Press “start” for at least 5 s to save
the program and exit programming.
Page 68

page 68
cos105000-2016-00 en
19 Annex III (Accessories)
19.1 Arm support, adjustable [cos12013]
Title Description
Short description The h/p/cosmos arm supports are a simple solution for unweighting of the patient.
Height and width adjustability offers a wide eld of application.
Illustration
Application Adjust the arm support by pulling
the locking element and turning
the segments.
Hold free segments with other
hand.
Scales on each joint allow
reproducibility.
Additional safety information
❚
Do not adjust under load.
❚
Use caution at squeeze and shear points.
❚
Make sure the hand grips are in upright position during use.
❚
Do not use for running.
❚
Position arm supports outside of training area when running.
❚
Do not use on bare skin.
❚
Do not leave the arm support in a position that projects into running area
❚
Before loading, make sure the adjustment elements are correctly locked.
❚
Do not use the arm supports with reverse belt rotation.
Technical data Adjustability: Height and width via 3 joints
Measurements: 480 x 425 x 260 mm each (packed)
Weight: 2.2 kg each
Max. patient weight: 140 kg
Max. patient weight of treadmill is reduced when combined with arm support.
Additional accessories cos100680 additional keyboard for arm support
cos14135 keyboard holder for arm support
cos10107 additional stop button in right arm support
cos10108 additional stop button in left arm support
Installation By h/p/cosmos service personnel, only
Further information https://www.h-p-cosmos.com/en/products/individual-products/adjustable-arm-supports-
scale-0deg-handrail-shape
Page 69

page 69
cos105000-2016-00 en
19.2 Arm support, optional stop button [cos10107, cos10108]
Title Description
Short description Additional emergency stop, integrated into arm support
Illustration
Application Operation Result Release Restart
Running belt stops
with predened
deceleration
Movement of
elevation system
stops
UserTerminal
displays “pull stop”
Mains connection
and interface
communication not
interrupted
Restart application
Push button Release button
Additional safety information N/A
Technical data N/A
Additional accessories N/A
Installation By h/p/cosmos service personnel, only
Further information https://www.h-p-cosmos.com/en/products/individual-products/additional-stop-button-right
Page 70

page 70
cos105000-2016-00 en
19.3 Crossbar front rail [cos102426]
Title Description
Short description Crossbar for additional balance control
Illustration
Application Patient may hold crossbar front rail for balance control
Holding handrails during use affects exercise results.
Additional safety information N/A
Technical data Length: 700 mm
Diameter: 40 mm
Weight: 1.3 kg
Technical data N/A
Installation By h/p/cosmos service personnel, only
Further information https://www.h-p-cosmos.com/en/products/individual-products/handrail-crossbar-5036-
arched-grip-cover
Page 71

page 71
cos105000-2016-00 en
19.4 Elevation 0% to +25% [cos102927]
Title Description
Short description Extends elevation to 25% (22.5°)
Illustration N/A
Application N/A
Additional safety information N/A
Technical data Max. elevation: 25%
22.5°
Additional accessories N/A
Installation No retro-tting
Further information N/A
Page 72

page 72
cos105000-2016-00 en
19.5 Emergency stop retrotting [cos15933, cos100548, cos15294]
Title Description
Short description Additional emergency stop buttons
cos15933 Emergency stop button with magnet holder 5m
cos100548 Emergency stop button with magnet holder 10m
cos15294 Emergency stop ext. without attachement 5m
cos15294 L10m Emergency stop ext. without attachement 10m
cos15294 L15m Emergency stop ext. without attachement 15m
Illustration
with magnet holder without attachement
Application Operation Result Release Restart
Running belt stops
with predened
deceleration
Movement of
elevation system
stops
UserTerminal
displays “pull stop”
Mains connection
and interface
communication not
interrupted
Restart application
Push button Release button
Additional safety information N/A
Technical data N/A
Additional accessories N/A
Installation By operator
Further information https://www.h-p-cosmos.com/en/products/individual-products/emergency-stop-button-
magnet-holder-5-m-spiral-cable
Page 73

page 73
cos105000-2016-00 en
19.6 Handrail, long 1358mm [cos102918]
Title Description
Short description Long handrail for additional safety
Illustration
Application Patient has to hold both handrails for stability when entering the treadmill.
Patient may hold handrails for balance control.
Holding handrails during use affects exercise results.
Additional safety information N/A
Technical data Length: 1358 mm
Diameter: 40 mm
Weight: 9.5 kg (4.0 kg additional weight)
Additional accessories N/A
Installation By h/p/cosmos service personnel, only
Further information N/A
Page 74
page 74
cos105000-2016-00 en
19.7 Handrail, pediatric [cos102400]
Title Description
Short description Additional handrail for small patients
Illustration
Application Patient may hold handrails for balance control.
Holding handrails during use affects exercise results.
Additional safety information N/A
Technical data Length: 910 mm
Width: 855 mm
Height: 543 mm
Max. patient weight: 50 kg
Max patient weight of treadmill is reduced when combined with arm support.
Additional accessories N/A
Installation By h/p/cosmos service personnel, only
Further information N/A
Page 75
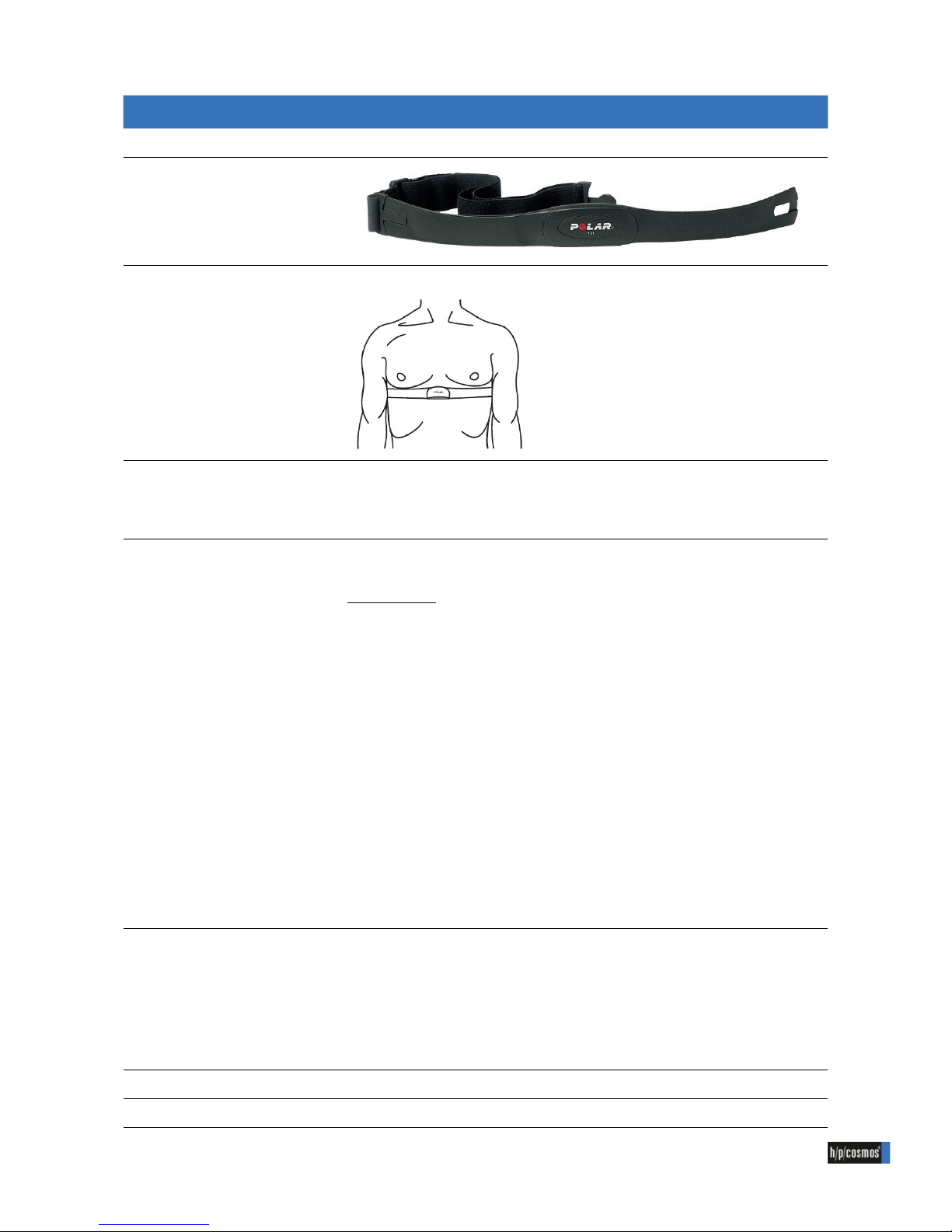
page 75
cos105000-2016-00 en
19.8 Heart rate measurement POLAR non-coded [cos102818]
Title Description
Short description Heart rate measurement, non-coded
Illustration
Application Apply chest belt as shown:
Additional safety information
❚
WARNING! Heart rate monitoring systems may be inaccurate.
❚
Incorrect or over exercising may result in serious injury or death.
❚
If you feel faint or dizzy stop exercising immediately and consult a medical doctor.
Technical data
Transmission radius: approx. 1m
Further data see accompanying POLAR documents or www.polar.com.
Troubleshooting:
In case the heart rate is not displayed:
- Chest belt might be applied incorrectly (see application above)
- Other chest belt than POLAR T31 or T34 is used (see print)
If the heart rate is not displayed or displayed incorrectly:
There might be interferences with
- Screens, computers, printers, mobile phones and any radio engineering systems
- Electric devices, electric motors, transformers
- High-voltage transmission lines, also from trains
- Strong uorescent tubes nearby
- Central heating radiators
- Other electric devices
In order to prevent interference of the running machine, place the device at some
distance away from such sources of interference. Do not rely on the indicated values if
you suspect interference.
Please refer to the instructions provided by the manufacturer, POLAR.
Additional accessories cos10905 POLAR chest belt XS
cos10906 POLAR chest belt S
cos10165 POLAR chest belt M
cos10907 POLAR chest belt L
cos10902 POLAR transmitter set T31
cos15178 POLAR transmitter set T34 (extended range)
Installation By h/p/cosmos service personnel, only
Further information N/A
Page 76

page 76
cos105000-2016-00 en
19.9 Heart rate measurement POLAR W.I.N.D. coded [cos100106]
Title Description
Short description Heart rate measurement coded
Illustration
Application Apply chest belt as shown:
Additional safety information
❚
WARNING! Heart rate monitoring systems may be inaccurate.
❚
Incorrect or over exercising may result in serious injury or death.
❚
If you feel faint or dizzy stop exercising immediately and consult a medical doctor.
Technical data
Transmission radius: approx. 10m
Further data see accompanying POLAR documents or www.polar.com.
Troubleshooting:
In case the heart rate is not displayed:
- Chest belt might be applied incorrectly (see application above)
- Other chest belt than POLAR W.I.N.D. is used (see print)
- Device and chest belt are not connected (see User Option OP 19)
Additional accessories cos100420b POLAR WIND transmitter TRX24
cos100420c POLAR WIND WearLink chest belt
Installation By h/p/cosmos service personnel, only
Further information
https://www.h-p-cosmos.com/en/products/individual-products/polar-heart-rate-receiver-wind
Page 77
page 77
cos105000-2016-00 en
19.10 PC-software para control [cos10071-v4.1.0]
Title Description
Short description The h/p/cosmos para control is designed for remote control of medical devices.
But it does not perform medical diagnosis or evaluation.
The read out data shall not be the basis for diagnosis or evaluation.
For remote control of all h/p/cosmos running machine and ladder-ergometers with
MCU2, MCU3, MCU4 and MCU5. The parameters and the keyboard of the treadmill
are displayed on the PC and keys can be simulated for remote control of the running
machine or the ladder ergometer.
Illustration
Application See separate instructions for use.
Additional safety information See separate instructions for use.
Technical data Min. processor: Pentium IV
OS Windows XP / Vista / 7
RAM 1 GB (2 GB recommended)
Free HD 200 MB
Resolution 1280 x 1024
Microsoft .NET Framework 3.5 Service Pack 1
Microsoft® DirectX 9.c
Additional accessories cos12769-01 USB-RS232 converter
cos00097010034 Interface connection cable RS 232 5 m
cos00097010035 Interface connection cable RS 232 10 m
Installation By operator
Install from h/p/cosmos demo & info DVD or download from website (see below).
Further information https://www.h-p-cosmos.com/en/products/software/hpcosmos-para-control-410
Page 78

page 78
cos105000-2016-00 en
19.11 robowalk expander [cos30022, cos30023]
Title Description
Short description The h/p/cosmos robowalk expander supports gait training.
Expander ropes, attached to the limbs, support or load the patient.
Illustration
Application See separate instructions for use.
Additional safety information See separate instructions for use.
Technical data
robowalk front [cos30022]
Height: approx. 110 cm (depending on treadmill)
Weight: approx. 15 kg (depending on treadmill)
Max. pulling force: 50 N per rope
Technical data
robowalk back [cos30023]
Height: approx. 80 cm (depending on treadmill)
Weight: approx. 25 kg (depending on treadmill)
Max. pulling force: 50 N per rope
Additional accessories cos101051-XS leg cuff XS shank for robowalk expander
cos101050-S leg cuff S thigh for robowalk expander
cos101050-M leg cuff M thigh for robowalk expander
cos101050-L leg cuff L thigh for robowalk expander
Installation By h/p/cosmos service personnel, only
Further information https://www.h-p-cosmos.com/en/products/individual-products/robowalk-expander-f-15050
Page 79

page 79
cos105000-2016-00 en
19.12 Safety arch for treadmill families 150/50 LC and 150/50 [cos10079]
Title Description
Short description The h/p/cosmos safety arch one option to secure the patient from falling.
Furthermore, the safety arch stops the treadmill in case of falling.
Illustration
Application See “fall prevention”
Additional safety information See safety information for the treadmill.
Technical data Max. patient weight: 200 kg
Max. patient height: 200 cm
Min. triggering force: approx. 100N (~10kg)
Height: 250 cm (treadmill at 0% elevation)
260 cm (treadmill at max. elevation)
Additional accessories cos14903-03-XXS chest belt system XXS safety arch harness
cos14903-03-XS chest belt system XS safety arch harness
cos14903-03-S chest belt system S safety arch harness
cos14903-03-M chest belt system M safety arch harness
cos14903-03-L chest belt system L safety arch harness
cos14903-03-XL chest belt system XL safety arch harness
Installation By h/p/cosmos service personnel, only
Further information https://www.h-p-cosmos.com/en/products/individual-products/safety-arch-50-harness-
chest-belt-fall-stop-prevention
Page 80

page 80
cos105000-2016-00 en
19.13 Wheelchair ramp [cos102931]
Title Description
Short description Wheelchair ramp supports entering the device with wheelchair patients.
Illustration
Application Push patient with wheelchair onto treadmill.
Connect patient to fall prevention device.
Support patient so they can stand upright.
Remove wheelchair.
Start application.
Additional safety information
❚
Do not use the device with wheels (bikes, wheelchairs, inline skates, etc.).
❚
Unmeant trapping hazards: Take off ties, scarfs or other clothes that may be trapped.
Secure long hair and ribbons during maintenance and training in order to prevent being
captured in trapping zones.
The ramp must not touch the running belt.
Make sure the ramp cannot slip.
Always enter from the back, not from the side.
Do not install the ramp when running belt is in motion.
Technical data Length
Width
Height
Weight
Additional accessories N/A
Installation By operator
Further information N/A
Page 81
page 81
cos105000-2016-00 en
20 Contact
For any service or sales enquiries, please have the model type and serial number of your device ready.
For service support, we recommend using Skype with webcam.
Sales & Service
carefusion GmbH
Leibnizstrasse 7
DE 97204 Hoechberg-Wuerzburg
Germany
Sales department
phone +49 9 31 49 72 280
fax +49 9 31 49 72 423
email info@carefusion.com
Service department
phone +49 9 31 49 72 146
fax +49 9 31 49 72 864
email info@carefusion.com
Manufacturer
h/p/cosmos sports & medical gmbh
Am Sportplatz 8
DE 83365 Nussdorf-Traunstein, Germany
email email@h-p-cosmos.com
web www.h-p-cosmos.com
 Loading...
Loading...