CareFusion Infant Flow SiPAP User manual

Infant Flow® SiPAP™
Operator’s manual

ii |
Infant Flow® SiPAP™ |
This document is protected by United States and International Copyright laws.
This document may not be copied, reproduced, translated, stored in a retrieval system, transmitted in any form, or reduced to any electronic medium or machine-readable form, in whole or in part, without the written permission of CareFusion. Information in this document is subject to change without notice.
This document is for informational purposes only and should not be considered as replacing or supplementing the terms and conditions of the License Agreement.
© 2009–2010 CareFusion Corporation or one of its subsidiaries. All rights reserved. Infant Flow is a registered trademark and SiPAP is a trademark of CareFusion Corporation or one of its subsidiaries. All other trademarks are the property of their respective owners.
USA |
Authorized European Representative |
CareFusion |
CareFusion Germany 234 GmbH |
22745 Savi Ranch Parkway |
Leibnizstrasse 7 |
Yorba Linda, California 92887-4668 |
97204 Hoechberg, Germany |
|
District Court Wuerzburg HRB7004 |
800.231.2466 tel |
+49.931.4972.0 tel |
+1.714.283.2228 tel |
+49.931.4972.423 fax |
+1.714.283.8493 fax |
|
carefusion.com |
|
Literature number: 675–101–101 Revision K
675–101–101 Rev. K
Infant Flow® SiPAP™ iii
R evis ion His tory
Date |
Revision |
Changes |
September 2003 |
675-101(3) |
Release |
|
|
|
August 2004 |
D |
Release manual in VIASYS Respiratory Care template using |
|
|
VIASYS Respiratory Care nomenclature. Revise part number |
|
|
list in Appendix B approved accessories. |
|
|
|
November 2004 |
E |
Revised contact/ordering information. |
|
|
|
|
|
Ch 4. Sec. 5.b revised transducer LED illumination conditions. |
|
|
Appendix A corrected units from "Pm" to bpm. |
|
|
Appendix E added dimension ranges to bonnet sizes. |
|
|
Appendix E Was 467350 Transducer Assembly Is: 677-002 |
|
|
Transducer Interface. |
|
|
|
March 2005 |
F |
Updated the contact information. |
|
|
Updated the Declaration of Conformity Notice. |
|
|
|
May 2006 |
G |
Updated the company name. |
|
|
|
|
|
Updated the Contact and Ordering Information. |
|
|
|
|
|
Update the figures. |
|
|
|
|
|
Added a Caution regarding back pressure. Added a Note |
|
|
regarding the Hudson RCI Humidification System. |
|
|
|
|
|
Added the sentence “Ensure there is a minimum 8 LPM set |
|
|
on the NCPAP/PRES Low Flow meter” to the first paragraph |
|
|
under “Two Point O2 Sensor Calibration. |
|
|
Changed step 8 regarding the nCPAP pressure. |
|
|
|
|
|
Changed the second and third paragraphs under Changing a |
|
|
Control. |
|
|
|
|
|
Added “Setting a Manual Breath.” |
|
|
|
|
|
Added a note regarding the enabling of manual breath or back- |
|
|
up apnea breath. |
|
|
|
|
|
Added a warning concerning infant flow consumables. |
|
|
|
|
|
Added the statement “Disconnect the air and oxygen gas |
|
|
sources when the Infant Flow SiPAP™ is not in use.” |
|
|
|
|
|
Removed Appendix E. |
|
|
|
February 2009 |
H |
Changed Ti to T-High and Inspiratory Time to Time High. |
|
|
|
|
|
Replaced reference to VIASYS Respiratory Care accessories |
|
|
with reference to Cardinal Health accessories. |
|
|
|
|
|
Added “TM” superscript to “SiPAP”. |
|
|
|
|
|
Added reference to AirLifeTM Infant nCPAP System Generator. |
|
|
Removed “inspiratory time” or “inspiration time”. |
|
|
|
|
|
Replaced “Inspiratory Time (Time High)” with “Time High |
|
|
|
675–101–101 Rev. K
iv |
|
|
|
Infant Flow® SiPAP™ |
|
|
Date |
|
Revision |
|
Changes |
|
|
|
|||
|
|
|
|
|
(Thigh)” |
|
|
|
|
|
|
|
|
|
|
|
Changed 1 cmH2O to 1.5 cmH2O; Added “or 60 psi” to clarify 4 |
|
|
|
|
|
bar. |
|
|
|
|
|
|
|
|
|
|
|
Added the parts list for both Infant Flow® Products and AirLifeTM |
|
|
|
|
|
Products. |
|
|
|
|
|
|
|
|
|
|
|
Added reference to Cardinal Health contact information on page |
|
|
|
|
|
v. |
|
|
|
|
|
|
|
|
|
|
|
Added reference to AirLifeTM Infant nCPAP System accessories. |
|
|
|
|
|
Added a warning about using an external oxygen monitor. |
|
|
|
|
|
Added reference to factory trained technician and Service |
|
|
|
|
|
Manual P/N 675-120. |
|
|
|
|
|
|
|
|
|
|
|
Added “®” (registered symbol) superscript to Infant Flow. |
|
|
|
|
|
Updated CAUTION label: from “Back pressure from the |
|
|
|
|
|
humidifier chamber to some auto-feed water bags may occur.” |
|
|
|
|
|
To “Back pressure from some auto-feed humidifier chambers |
|
|
|
|
|
may cause the water bags to fill with air.” |
|
|
|
|
|
|
|
|
|
|
|
Replaced Figure 5. |
|
|
|
|
|
|
|
|
|
|
|
Add content concerning a depleted or damaged internal oxygen |
|
|
|
|
|
cell. |
|
|
|
|
|
Added a warning about using an external oxygen monitor. |
|
|
|
|
|
Added content to explain fault code E5X. |
|
|
|
|
|
Replaced “key” with “button”; clarified oxygen alarm by adding |
|
|
|
|
|
“the audible”; added clarification of the internal monitoring being |
|
|
|
|
|
disabled and that an external oxygen monitor must be used. |
|
|
|
|
|
|
|
|
|
|
|
Added a Note regarding the 2nd Flow Meter being used for |
|
|
|
|
|
manual breath delivery; |
|
|
|
|
|
Added hyphen in “T-High”. |
|
|
|
|
|
|
|
|
|
|
|
Clarified the “Mode Select Screen” |
|
|
|
|
|
|
|
|
|
|
|
Added “Directions for using the AirLifeTM Infant nCPAP System. |
|
|
|
|
|
Changed 1 cmH2O to 1.5 cmH2O; Corrected low battery voltage |
|
|
|
|
|
level from 10 to 11.10. |
|
|
|
|
|
|
|
|
|
|
|
Added “or trained biomedical engineer”. |
|
|
|
|
|
|
|
|
|
|
|
Added a table entry for the oxygen monitor and alarms disable. |
|
|
|
|
|
|
|
|
|
|
|
Changed 1 cmH2O to 1.5 cmH2O; corrected low battery voltage |
|
|
|
|
|
level from 10 to 11.10. |
|
|
|
|
|
|
|
|
|
|
|
Updated Table 10. |
|
|
|
|
|
|
|
|
|
|
|
Updated Table 11. |
|
|
|
|
|
|
|
|
|
|
|
Clarified the meaning of T-High. |
|
|
|
|
|
|
|
February 2010 |
|
J |
|
Revised to comply with the revised Medical Device Directive |
|
|
|
|
|
2007/42/EC. |
|
|
|
|
|
|
675–101–101 Rev. K
Infant Flow® SiPAP™ |
v |
Date |
Revision |
Changes |
March 2010 |
K |
Rebranded the manual to the CareFusion style. |
|
|
Updated the part number table. |
|
|
|
675–101–101 Rev. K
vi |
Infant Flow® SiPAP™ |
Warranty
Infant Flow® SiPAPTM is warranted to be free from defects in material and workmanship and to meet the published specifications for One (1) year from date of shipment.
The liability of CareFusion (referred to as the Company) under this warranty is limited to replacing, repairing or issuing credit, at the discretion of the Company, for parts that become defective or fail to meet published specifications during the warranty period; the Company will not be liable under this warranty unless (A) the Company is promptly notified in writing by Buyer upon discovery of defects or failure to meet published specifications; (B) the defective unit or part is returned to the Company, transportation charges prepaid by Buyer; (C) the defective unit or part is received by the Company for adjustment no later than four weeks following the last day of the warranty period; and (D) the Company’s examination of such unit or part shall disclose, to its satisfaction, that such defects or failures have not been caused by misuse, neglect, improper installation, unauthorized repair, alteration or accident.
Any authorization of the Company for repair or alteration by the Buyer must be in writing to prevent voiding the warranty. In no event shall the Company be liable to the Buyer for loss of profits, loss of use, consequential damage or damages of any kind based upon a claim for breach of warranty, other than the purchase price of any defective product covered hereunder.
The Company warranties as herein and above set forth shall not be enlarged, diminished or affected by, and no obligation or liability shall arise or grow out of the rendering of technical advice or service by the Company or its agents in connection with the Buyer's order of the products furnished hereunder.
Limitation of Liabilities
This warranty does not cover normal maintenance such as cleaning, adjustment or lubrication and updating of equipment parts. This warranty shall be void and shall not apply if the equipment is used with accessories or parts not manufactured by the Company or authorized for use in writing by the Company or if the equipment is not maintained in accordance with the prescribed schedule of maintenance.
The warranty stated above shall extend for a period of One (1) year from date of shipment, with the following exceptions:
1.Components for monitoring of physical variables such as temperature, pressure, or flow are warranted for ninety (90) days from date of receipt.
2.Elastomeric components and other parts or components subject to deterioration, over which the Company has no control, are warranted for sixty (60) days from date of receipt.
3.Internal batteries are warranted for ninety (90) days from the date of receipt.
The foregoing is in lieu of any warranty, expressed or implied, including, without limitation, any warranty of merchantability, except as to title, and can be amended only in writing by a duly authorized representative of the Company.
675–101–101 Rev. K
Infant Flow® SiPAP™ |
vii |
Contents |
|
Revision History ......................................................................................................... |
iii |
Warranty .................................................................................................................... |
vi |
Contents ................................................................................................................... |
vii |
List of Figures .......................................................................................................... |
viii |
List of Tables ........................................................................................................... |
viii |
Notices....................................................................................................................... |
ix |
Chapter 1 - Product Description................................................................................. |
1 |
Chapter 2 - Product Specifications............................................................................. |
3 |
Chapter 3 - Summary of Warnings and Cautions....................................................... |
7 |
Chapter 4 - Unpacking & Setup ............................................................................... |
11 |
Chapter 5 - Operation .............................................................................................. |
23 |
Chapter 6 - Operating Modes .................................................................................. |
33 |
Chapter 7 - Alarms and Indicators ........................................................................... |
35 |
Chapter 8 - Maintenance & Cleaning....................................................................... |
41 |
Chapter 9 – Explanation of Symbols........................................................................ |
43 |
Appendix A - Product Configurations....................................................................... |
49 |
Appendix B - Pneumatic Diagram............................................................................ |
51 |
Appendix C - Alarm Troubleshooting ....................................................................... |
53 |
Appendix D - Fault Management ............................................................................. |
57 |
Glossary................................................................................................................... |
63 |
Index ........................................................................................................................ |
65 |
675–101–101 Rev. K
viii |
Infant Flow® SiPAP™ |
List of Figures |
|
Figure 1 – Stand unpacking and assembly........................................................ |
11 |
Figure 2 – Stand and Driver assembly............................................................... |
12 |
Figure 3 – Driver assembled with patient circuit and /humidifier |
........................13 |
Figure 4 – Attaching the Abdominal Respiratory Sensor ................................... |
14 |
Figure 5 – Flow Pressure Nomogram ................................................................ |
15 |
Figure 6 – Front Panel ....................................................................................... |
23 |
Figure 7 – Rear Panel........................................................................................ |
24 |
Figure 8 – Set Up Screen .................................................................................. |
27 |
Figure 9 – Alarm set/confirm Screen ................................................................. |
28 |
Figure 10 – Mode Select Screen ....................................................................... |
29 |
Figure 11 – Parameter Adjust Screens.............................................................. |
29 |
Figure 12 – Main Screen.................................................................................... |
30 |
Figure 13 – Monitored Parameters Screen........................................................ |
30 |
Figure 14 – NCPAP ........................................................................................... |
33 |
Figure 15 – BiPhasic.......................................................................................... |
33 |
Figure 16 – BiPhasic tr....................................................................................... |
34 |
Figure 17 - Flat Battery screen .......................................................................... |
37 |
List of Tables |
|
Table 1 – Functions and Accessories .................................................................. |
2 |
Table 2 - Soft-key operation............................................................................... |
25 |
Table 3 – Parameter Default Value.................................................................... |
27 |
Table 4 – Alarm Symbols and Indicators ........................................................... |
38 |
Table 5 – Equipment Symbols ........................................................................... |
43 |
Table 6 – Button Symbols.................................................................................. |
45 |
Table 7 – Non-US Configuration Parameters .................................................... |
49 |
Table 8 – US Configuration Parameters ............................................................ |
49 |
Table 9 – Alarm Troubleshooting....................................................................... |
53 |
Table 10 – Fault Classification........................................................................... |
57 |
Table 11 – Fault Recovery................................................................................. |
58 |
675–101–101 Rev. K
Infant Flow® SiPAP™ |
ix |
Notices
E MC Notice
This equipment radiates and is susceptible to radio frequency energy. If not installed and used in accordance with the instructions in this manual, electromagnetic interference may result. The equipment has been tested and found to comply with the limits set forth in BS EN60601-1-2 for Medical Electrical Equipment Part 1-2: General requirements for safety-collateral standard. Electromagnetic compatibility – requirements and tests. These limits provide reasonable protection against electromagnetic interference when operated in the intended use environments (e.g. hospitals) described in this manual.
This device is also designed and manufactured to comply with the following standards:
Safety: UL 60601-1: 2003 Medical Electrical Equipment, Part 1: General Requirements for Safety.
CAN/CSA C22.2 No 601.1-M90, Medical Electrical Equipment - Part 1: General Requirements for Safety including C22.2 No. 601.1S1-94 (IEC601-1, Amendment 1:1991) Supplement No. 1-94 to CAN/CSA 22.2 No. 601.1-M90
Electrical Safety:
Class 1 equipment
Contains type BF patient applied parts
Continuous Operation
MR I Notice
This equipment contains electromagnetic components whose operation can be affected by intense electromagnetic fields.
Do not operate this device in a MRI environment or in the vicinity of high-frequency surgical diathermy equipment, defibrillators, or short-wave therapy equipment. Electromagnetic interference could disrupt the operation of the device.
Intended Us e Notice
The Infant Flow® SiPAP™, consisting of a Driver and Generator plus NCPAP Prongs and Masks, is intended for the provision of Bi-Level CPAP (SiPAP™) to produce a sigh. The system is for use in Hospitals, Hospital Type facilities and intra-Hospital transport environments and is indicated for the treatment of Newborn and Infant patients. The
Infant Flow® SiPAP™ should only be operated by properly trained clinical personnel, under the direction of a physician.
675–101–101 Rev. K

x |
Infant Flow® SiPAP™ |
R egulatory Notice
Federal law restricts the sale of this device except by or on order of a physician.
Reuse of single-patient use accessories may degrade the performance of the product or cause cross contamination.
C las s ification
Type of Equipment: Medical Equipment, Class 1 and internally powered, IPX1 Protected, and uses type BF applied parts. Equipment is not suitable for use in presence of flammable anesthetics.
Declaration of C onformity Notice
This medical equipment complies with the Medical Device Directive, 93/42/EEC, and the following Technical Standards, to which Conformity is declared:
EN60601-1 and EN60601-1-2 EN 10993
EN 14971
EU Notified Body:
BSI (Reg. No. 0086)
Trade names:
Infant Flow®
SiPAP™
Manufactured by:
CareFusion
22745 Savi Ranch Parkway
Yorba Linda, California 92887-4668
If you have a question regarding the Declaration of Conformity for this product, please contact CareFusion.
675–101–101 Rev. K
Infant Flow® SiPAP™ |
1 |
Chapter 1 - Product Description
Infant Flow® SiPAP™ provides a non-invasive form of respiratory support designed for infants in hospital environments such as Neonatal and Pediatric Intensive Care Units. It can also be used when transporting these patients within the hospital environment.
Infant Flow® SiPAP™ is currently available in a Plus or Comprehensive* configuration. The Plus configuration provides NCPAP and time triggered BiPhasic modes with and without breath rate monitoring. The Comprehensive* configuration offers these features plus a patient triggered BiPhasic mode with apnea backup breaths. The Infant Flow® SiPAP™ comes standard in all configurations with an LCD touch screen display, pressure time waveform graphics, integrated patient monitoring, alarms for high and low pressure and FiO2 and up to 2 hours of backup battery power.
As a result of the unique, patented design, the Infant Flow® or AirLifeTM Infant nCPAP System Generator has been proven to provide the most stable CPAP at the lowest work of breathing for patients compared to other devices (1). The outstanding performance of the Infant Flow® Generator is irrespective of patient demand or expiratory flows. This system has been designed and tested to perform optimally when used only with accessories available from CareFusion. These accessories include circuits and generators, prong and mask patient interfaces and bonnets.
Infant Flow® SiPAP™ Features
The expanded capabilities of the Infant Flow® SiPAP™ Plus and Comprehensive* configurations allow for applications to broader range of patients who may otherwise not be candidates for non-invasive respiratory support from NCPAP alone (2,3).
NCPAP – continuous positive airway pressure based on clinician set pressure. Breath rate monitoring/alarm can be activated in this mode.
BiPhasic – time triggered pressure assists are delivered based on clinician set Time-High, rate and pressure criteria. Breath rate monitoring/alarm can be activated in this mode.
BiPhasic tr* – patient triggered pressure assists delivered based on clinician set Time-High and pressure criteria. Breath rate monitoring/alarm and Apnea backup breaths are automatically active in this mode.
Patented Infant Flow® or AirLifeTM Infant nCPAP System Generator - The Infant Flow® Generator is a fluidic device for the generation of consistent infant nasal CPAP with a low work of breathing compared to other devices(1).
Fully integrated alarm package – Supply gases failure, High Patient Pressure, Low patient pressure, high and low delivered Oxygen concentration, change from AC to DC power source, low and flat battery charge status and Low breath rate/apnea alarm.
Battery Backup – Up to 2 hours of battery backup allows for intra-hospital transport. Clear indicators are provided for power supply in use (AC or DC), and battery charge level.
675–101–101 Rev. K
2 |
Chapter 1 - Product Description |
Screen Lock – After 120 seconds of no screen inputs, the screen changes to the Locked Screen to prevent inadvertent changes. Upon activation of a high priority alarm the screen changes to an unlocked state to allow access to controls.
Table 1 – Functions and Accessories
Functions & Accessories |
Plus |
Comprehensive* |
|
|
|
NCPAP |
• |
• |
|
|
|
NCPAP with breath rate monitoring |
• |
• |
and alarm |
|
|
|
|
|
BiPhasic |
• |
• |
|
|
|
BiPhasic with breath rate monitoring |
• |
• |
and alarm |
|
|
|
|
|
BiPhasic tr* |
|
• |
|
|
|
Internal Battery |
• |
• |
|
|
|
Manual Breath |
• |
• |
|
|
|
Apnea Back up rate |
|
• |
|
|
|
Screen lock |
• |
• |
|
|
|
Prioritization of alarms |
• |
• |
|
|
|
*Comprehensive configuration not available for sale in the United States
(1)Decreased imposed work with a new nasal continuous positive airway pressure device. Klausner, James F., PhD, Lee, Amy., Hutchison, Alastair A., FRACP. Pediatric Pulmonology 22: 188-194; 1996
(2)A Prospective Randomized, Controlled Trial Comparing Synchronized Nasal Intermittent Positive Pressure Ventilation versus Nasal Continuous Positive Airway Pressure as Modes of Extubation. Khalaf Nabeel, M., Brodsky Nancy, Hurley John, Bhandari Vineet. PEDIATRICS 108 (1): 13-17:
2001
(3)Efficacy of Nasal Intermittent Positive Pressure Ventilation in Treating Apnea of Prematurity. Lin Chyi-Her, MD, Wang Shan-Tair, PhD, Lin Yuh-Jyh, MD, Yeh Tsu-Fuh, MD:Pediatric Pulmonolgy: 26 (5): 349-53; 1996
675–101–101 Rev. K
Infant Flow® SiPAP™ |
3 |
Chapter 2 - Product Specifications
Modes
•NCPAP
•NCPAP with breath rate monitoring and low rate alarm
•BiPhasic (time triggered)
•BiPhasic (time triggered) with breath rate monitoring and low rate alarm
•BiPhasic tr (patient triggered) with breath rate monitoring, low breath rate alarm and apnea back up (Comprehensive models only)
Controls
•Time High (T-High) – 0.1 – 3.0 seconds
•Rate (R)
1-120 (Non-U.S. Configuration Parameters)
1-54 (U.S. Configuration Parameters)
•Apnea Interval
•(Tapnea) – 10-30 seconds, 5 second intervals (Non-U.S. Configuration Parameters)
•(TLBR) – 10-30 seconds; 5 second intervals (U.S. Configuration Parameters)
•NCPAP / Pres Low flow meter – 0-15 L/min, accuracy ± 15% of selected output
•Pres High flow meter – 0-5 L/min, accuracy ± 15% of selected output
•Manual Breath – X 1
•%O2 – 21 -100%
Monitors
•CPAP
•PEEP
•MAP
•PIP
•%O2
•I:E ratio
•Spontaneous rate (Rsp)
•Battery charge level
675–101–101 Rev. K
4 |
Chapter 2 - Product Specifications |
Alarms
•High airway pressure – 3 cmH2O above measured airway pressure
•Airway over-pressure limit alarm
•maximum 11 cmH2O in NCPAP and time triggered BiPhasic mode
•maximum 15 cmH2O in patient triggered BiPhasic tr mode
•Low airway pressure – 2 cmH2O below measured airway pressure or 1.5 cmH2O if otherwise would be zero
•High and Low delivered Oxygen concentration ±5% of setting. Minimum and maximum delivered FiO2 is 18 and 104% respectively.
•Low breath rate alarm
•Low battery charge level
•Flat battery
•Input gases failure
•Alarm volume (electronic alarms) 70 dBa at 1 meter
Pneumatic Supply
•Patient Gas Outlet: 15 mm standard taper fitting
•Patient Pressure Input: 4.5 mm Luer taper fitting
•Gas Supply: Nominal 4 bar or 60 psi, clean, dry medical air and oxygen
•Range: 40.61 to 87 PSI; Maximum differential pressure 29 PSI
•Manometer: Range 0 to + 20 cmH2O, accuracy, ± 2% of span
•Gas Connections: Standard DISS, NIST or Air Liquide connectors
Electrical Supply
•Input Voltage: 100-230 VAC
•Input Frequency: 50/60 Hz
•Power Consumption: 50 VA maximum
•Fuse Rating For 220 V nominal operation: “T” Type 2.5 A at 250 V
•Device Housing Protection rating level: IPX1
•Battery Working Time: 2 hours (from fully charged state)
•Battery Charging Time: max. 16 hours
675–101–101 Rev. K
Infant Flow® SiPAP™ |
5 |
Atmospheric & Environmental
•Temperature Range Operating: 5 – 40° C
Storage: - 20 - 50° C
•Relative Humidity -Operating: 0 – 95% non-condensing
•Storage: 0 – 95% non-condensing
Physical
•Dimensions (Driver only)-
•(W x H x D) 26 x38 x 23.5 cm
•(W x H x D) 10.25 x15 x 9.25 in
•Weight (Driver only)-
•8.8 kg
•19.5 lb
Accessories
•Silencer / Bacterial Filter - The additional resistance of the D1420/100
Silencer / Bacterial Filter and adaptor is less than 0.56 cmH2O at 15 LPM, and less than 0.40 cmH2O at 5 LPM.
675–101–101 Rev. K
6 |
Chapter 2 - Product Specifications |
Part No. |
|
Description |
Infant Flow® Products |
|
|
D1420/100 |
|
Silencer (box of 20) |
F&P 730 |
|
|
11541–101 |
|
Patient Circuit Assembly (box of 20) |
11541–102 |
|
Patient Circuit Assembly w/Generator (box of 20) |
773386–101 |
|
Patient Circuit (box of 20) |
773386–102 |
|
Patient Circuit w/Generator (box of 20) |
F&P 850 |
|
|
12204–101 |
|
Patient Circuit (box of 20) |
12204–102 |
|
Patient Circuit w/Generator (box of 20) |
773387–101 |
|
Patient Circuit (box of 20) |
12233–102 |
|
Patient AirLife Circuit w/Generator (box of 20) |
12233–101 |
|
Patient AirLife Circuit (box of 20) |
RCI |
|
|
773388–105 |
|
Patient Circuit RCI 16V (box of 20) |
773388–103 |
|
Patient Circuit w/Generator RCI 16V (box of 20) |
773389–105 |
|
Patient Circuit RCI 21V (box of 20) |
773389–104 |
|
Patient Circuit w/Generator RCI 21V (box of 20) |
Prongs |
|
|
11513–101 |
|
Nasal Prongs - Small (box of 10) |
11513–102 |
|
Nasal Prongs - Medium (box of 10) |
11513–103 |
|
Nasal Prongs - Large (box of 10) |
Masks |
|
|
777086–101 |
|
Nasal Mask - Small (box of 10) |
777086–102 |
|
Nasal Mask - Medium (box of 10) |
777086–103 |
|
Nasal Mask - Large (box of 10) |
777086–104 |
|
Nasal Mask Extra Large (box of 10) |
AirLifeTM Products (U.S.A. only) |
||
006905 |
|
NCPAP Generator Kit |
Prongs |
|
|
006910 |
|
Nasal Prongs - Small |
006915 |
|
Nasal Prongs - Medium |
006920 |
|
Nasal Prongs - Large |
Masks |
|
|
006925 |
|
Nasal Mask - Small |
006930 |
|
Nasal Mask - Medium |
006935 |
|
Nasal Mask - Large |
675–101–101 Rev. K
Infant Flow® SiPAP™ |
7 |
Chapter 3 - Summary of Warnings and
Cautions
Please review the following safety information prior to operating the Infant Flow® SiPAP™. Attempting to operate this equipment without fully understanding its features and functions may result in unsafe operating conditions.
Warnings and Cautions, which are general to the use of the device under all circumstances, are included in this section. Some Warnings and Cautions are also inserted within the manual where they are most meaningful.
Notes are also located throughout the manual to provide additional information related to specific features.
If you have a question regarding the installation, set up, operation, or maintenance of the device, contact CareFusion (see page v).
Terms
WARNINGS identify conditions or practices that could result in serious adverse reactions or potential safety hazards.
CAUTIONS identify conditions or practices that could result in damage to the driver or other equipment.
NOTES identify supplemental information to help you better understand how the driver works.
Warnings
•Infant Flow® SiPAP™ is intended for use by a trained practitioner, under the direct supervision of a qualified physician.
•When the Infant Flow® SiPAP™ is connected to a patient, a trained health care professional should be in attendance at all times to react to an alarm or other indications of a problem.
•Always have an alternate means of ventilation available whenever the Infant Flow® SiPAP™ is in use.
•Do not attach the Generator to the patient until User Verification and initial set up into NCPAP mode is complete.
•Water in the air supply can cause malfunction of this equipment.
•The operator should not touch the electrical connectors of the Infant Flow® SIPAP™ or its accessories, and the patient simultaneously.
•An audible alarm indicates an anomalous condition and should never go unheeded.
675–101–101 Rev. K
8 |
Chapter 3 - Summary of Warnings and Cautions |
•Anti-static or electrically conductive hoses or tubing should not be used within the patient circuit.
•If a mechanical or electrical problem is recognized while operating the Infant Flow® SiPAP™, it must be removed from use and referred to qualified service personnel for servicing. Using inoperative equipment may result in patient injury.
•Prior to patient application, ensure that all User Verification testing and calibration procedures are successfully completed. User Verification testing and calibration procedures must be done off patient.
•The  indicates a connection between the Transducer Assembly and the driver. It does not indicate attachment or correct positioning of the Abdominal Respiratory Sensor.
indicates a connection between the Transducer Assembly and the driver. It does not indicate attachment or correct positioning of the Abdominal Respiratory Sensor.
•Under certain conditions (minimum supply pressure and maximum gas demand, including auxiliary output) output flow rates and therefore pressure delivered to the generator may be reduced.
•The Pres High flow meter must be adjusted to zero when not required for the patient.
•Whenever a patient is attached to respiratory care equipment, constant attendance is required by qualified personnel. The use of an alarm or monitoring system does not give absolute assurance of warning for every malfunction that may occur in the system. In addition, some alarm conditions may require immediate attention.
•Nasal CPAP treatment in general can cause nasal irritation, septal distortion,
skin irritation and pressure necrosis. Adherence to the recommended usage instructions for the Infant Flow® SiPAP™ and AirLifeTM Infant nCPAP System accessories may reduce the incidence of these complications.
•It is strongly recommended that regular monitoring for gastric distention be carried out for patients receiving non-invasive ventilatory support. Refer to your facility’s policy and procedure for further guidance.
•This device exhausts O2 during normal operation. Oxygen vigorously accelerates combustion. To avoid fire hazard, do not place flammable materials or sources of heat close to the exhaust.
•The Abdominal Respiratory Sensor is used only to enable features associated with certain modes from the Infant Flow® SiPAP™. When using the Abdominal Respiratory Sensor, always use an additional, external device for monitoring of the respiratory rate and detection of apneic episodes as well as an appropriate monitor for continuous SaO2 monitoring.
•If the Infant Flow® SiPAP™ driver is shelf mounted, ensure that the driver is stable and that all circuit tubing, hoses and cables are restrained to avoid hazard of toppling.
•Check that the water trap is empty before use and empty it frequently during use.
•Do not block or restrict the exhaust port located on the instrument back panel. Equipment malfunction may result.
675–101–101 Rev. K
Infant Flow® SiPAP™ |
9 |
•Do not use the equipment without the expiratory tubing connected to the generator.
•Only use the supplied AC cable to connect to the power supply.
•The Transducer LED indicator on the front panel of the driver only signifies connection to the driver. It does not indicate connection to or proper positioning of the Abdominal Respiratory Sensor.
•Do not overload the pole and stand.
•Oxygen vigorously accelerates combustion. To avoid explosion hazard, do not use any instrument or other equipment that may have been exposed to oil or grease contamination.
•When a low gas supply alarm occurs, the oxygen concentration delivered to the patient will differ from that set on the %O2 control.
•A source gas failure will change the FiO2 and may result in patient injury.
•The functioning of this equipment may be adversely affected by the operation of other equipment nearby, such as high frequency surgical (diathermy) equipment, defibrillators, short-wave therapy equipment, “walkie-talkies”, or cellular phones.
•Due to possible explosion hazard, the Infant Flow® SiPAP™ should not be used in the presence of flammable anesthetics.
•Electric shock hazard – Do not remove any of the Infant Flow® SiPAP™ covers or panels. Refer all servicing to an authorized CareFusion service technician or factory trained technician (see Service Manual P/N 675-120).
•A protective ground connection by way of the grounding conductor in the power cord is essential for safe operation. Upon loss of protective earth ground, all conductive parts including knobs and controls that may appear to be insulated can render an electrical shock. To avoid electrical shock, plug the power cord into a properly wired receptacle, use only the power cord supplied with the ventilator, and make sure the power cord is in good condition.
•The Infant Flow® SiPAP™ is designed to ensure that the user and patient are not exposed to excessive leakage current per applicable standards. However, this cannot be guaranteed when external devices are attached to the driver. In order to prevent the risk of excessive enclosure leakage current from external equipment attached to the driver, isolation of the protective earth paths must be provided to ensure proper connection. This isolation should ensure that the cable shields are isolated at the peripheral end of the cable.
•When the Infant Flow® SiPAPTM unit is connected to a patient, and the internal oxygen monitor is disabled, the Infant Flow® SiPAPTM unit must be used with an external oxygen monitor.
675–101–101 Rev. K
10 |
Chapter 3 - Summary of Warnings and Cautions |
Cautions
•Before use, verify that this equipment has been authorized for use by qualified technical service personnel.
•Ensure that the voltage and installed fuses are set to match the voltage of the wall outlet, or damage may result.
•A battery that is fully drained (i.e. void of any charge) may cause damage to the driver and should be replaced.
•All accessory equipment that is connected to the driver should comply with CSA/IEC601/ETL.
•Although failure of any of the above tests will not prevent the ventilator from functioning, it should be checked to make sure it is operating correctly before use on a patient.
•The Infant Flow® SiPAP™ has been designed and tested using only CareFusion accessories. Only accessories approved for use by CareFusion should be used. If in doubt, please contact your local sales representative.
•Employ safe lifting procedures when assembling the unit.
•Do not sterilize the driver. The internal components are not compatible with sterilization techniques.
•Do not submerge the driver or pour cleaning liquids over or into the driver.
•Following each alarm verification test, ensure that control settings and alarm limits are reset as instructed before proceeding to the next test.
Notes
•CareFusion cannot ensure product performance as stated in this manual with the use of Non-CareFusion accessories.
675–101–101 Rev. K
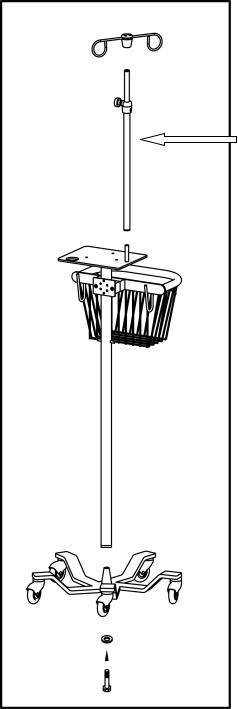
Infant Flow® SiPAP™ |
11 |
Chapter 4 - Unpacking & Setup
Assembly and physical setup
Optional
Accessory
Figure 1 – Stand unpacking and assembly
675–101–101 Rev. K
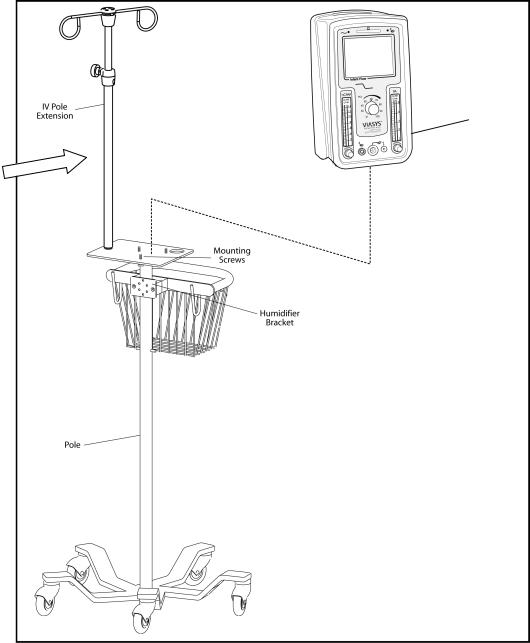
12 |
Chapter 4 - Unpacking & Setup |
Infant Flow
SiPAP Driver
Optional
Accessory
Figure 2 – Stand and Driver assembly
675–101–101 Rev. K
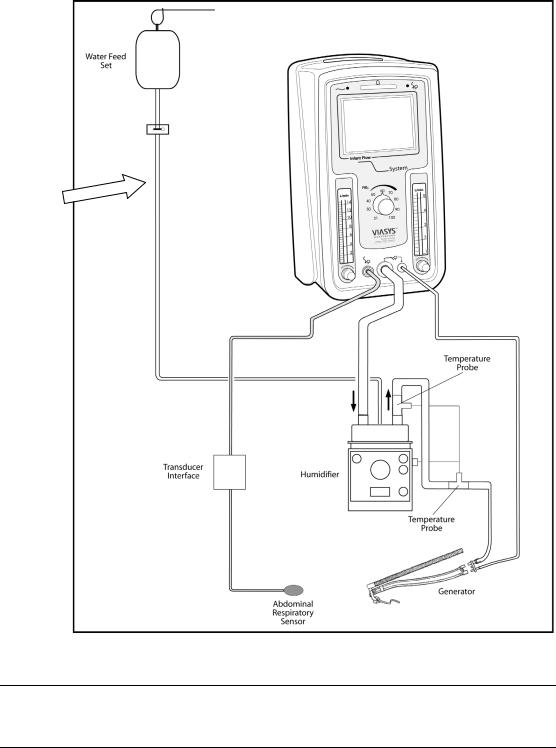
Infant Flow® SiPAP™ |
13 |
Attaching a patient circuit
Infant Flow
SiPAP Driver
Optional
Accessory
Figure 3 – Driver assembled with patient circuit and /humidifier
Note
We recommend between 96.8 °F (36 °C) and 98.6 °F (37 °C) but never higher than 98.6 °F (37 °C) for inspired gases.
675–101–101 Rev. K
 Loading...
Loading...