Page 1

Infant Flow® SiPAP™
Operator’s manual
Page 2
ii Infant Flow
®
SiPAP™
This document is protected by United States and International Copyright laws.
This document may not be copied, reproduced, translated, stored in a retrieval system,
transmitted in any form, or reduced to any electronic medium or machine-readable form,
in whole or in part, without the written permission of CareFusion. Information in this
document is subject to change without notice.
This document is for informational purposes only and should not be considered as
replacing or supplementing the terms and conditions of the License Agreement.
© 2009–2010 CareFusion Corporation or one of its subsidiaries. All rights reserved. Infant
Flow is a registered trademark and SiPAP is a trademark of CareFusion Corporation or one
of its subsidiaries. All other trademarks are the property of their respective owners.
USA
CareFusion
22745 Savi Ranch Parkway
Yorba Linda, California 92887-4668
Authorized European Representative
CareFusion Germany 234 GmbH
Leibnizstrasse 7
97204 Hoechberg, Germany
District Court Wuerzburg HRB7004
800.231.2466 tel
+1.714.283.2228 tel
+49.931.4972.0 tel
+49.931.4972.423 fax
+1.714.283.8493 fax
carefusion.com
Literature number: 675–101–101 Revision K
675–101–101 Rev. K
Page 3
Infant Flow® SiPAP™ iii
R evision History
Date Revision Changes
September 2003 675-101(3) Release
August 2004 D Release manual in VIASYS Respiratory Care template using
VIASYS Respiratory Care nomenclature. Revise part number
list in Appendix B approved accessories.
November 2004 E
March 2005 F Updated the contact information.
May 2006 G Updated the company name.
Revised contact/ordering information.
Ch 4. Sec. 5.b revised transducer LED illumination conditions.
Appendix A corrected units from "Pm" to bpm.
Appendix E added dimension ranges to bonnet sizes.
Appendix E Was 467350 Transducer Assembly Is: 677-002
Transducer Interface.
Updated the Declaration of Conformity Notice.
Updated the Contact and Ordering Information.
Update the figures.
Added a Caution regarding back pressure. Added a Note
regarding the Hudson RCI Humidification System.
Added the sentence “Ensure there is a minimum 8 LPM set
on the NCPAP/PRES Low Flow meter”
under “Two Point O
Changed step 8 regarding the nCPAP pressure.
Changed the second and third paragraphs under Changing a
Control.
Sensor Calibration.
2
to the first paragraph
Added “Setting a Manual Breath.”
Added a note regarding the enabling of manual breath or backup apnea breath.
Added a warning concerning infant flow consumables.
Added the statement “Disconnect the air and oxygen gas
sources when the Infant Flow SiPAP
Removed Appendix E.
February 2009 H Changed Ti to T-High and Inspiratory Time to Time High.
Replaced reference to VIASYS Respiratory Care accessories
with reference to Cardinal Health accessories.
Added “TM” superscript to “SiPAP”.
Added reference to AirLifeTM Infant nCPAP System Generator.
Removed “inspiratory time” or “inspiration time”.
Replaced “Inspiratory Time (Time High)” with “Time High
675–101–101 Rev. K
™ is not in use.”
Page 4
iv Infant Flow
Date Revision Changes
(Thigh)”
Changed 1 cmH2O to 1.5 cmH2O; Added “or 60 psi” to clarify 4
bar.
Added the parts list for both Infant Flow® Products and AirLifeTM
Products.
Added reference to Cardinal Health contact information on page
v.
Added reference to AirLifeTM Infant nCPAP System accessories.
Added a warning about using an external oxygen monitor.
Added reference to factory trained technician and Service
Manual P/N 675-120.
Added “®” (registered symbol) superscript to Infant Flow.
Updated CAUTION label: from “Back pressure from the
humidifier chamber to some auto-feed water bags may occur.”
To “Back pressure from some auto-feed humidifier chambers
may cause the water bags to fill with air.”
®
SiPAP™
Replaced Figure 5.
Add content concerning a depleted or damaged internal oxygen
cell.
Added a warning about using an external oxygen monitor.
Added content to explain fault code E5X.
Replaced “key” with “button”; clarified oxygen alarm by adding
“the audible”; added clarification of the internal monitoring being
disabled and that an external oxygen monitor must be used.
Added a Note regarding the 2nd Flow Meter being used for
manual breath delivery;
Added hyphen in “T-High”.
Clarified the “Mode Select Screen”
Added “Directions for using the AirLifeTM Infant nCPAP System.
Changed 1 cmH2O to 1.5 cmH2O; Corrected low battery voltage
level from 10 to 11.10.
Added “or trained biomedical engineer”.
Added a table entry for the oxygen monitor and alarms disable.
Changed 1 cmH2O to 1.5 cmH2O; corrected low battery voltage
level from 10 to 11.10.
Updated Table 10.
Updated Table 11.
Clarified the meaning of T-High.
February 2010 J Revised to comply with the revised Medical Device Directive
2007/42/EC.
675–101–101 Rev. K
Page 5

Infant Flow® SiPAP™ v
Date Revision Changes
March 2010 K Rebranded the manual to the CareFusion style.
Updated the part number table.
675–101–101 Rev. K
Page 6

vi Infant Flow
Warranty
Infant Flow® SiPAPTM is warranted to be free from defects in material and
workmanship and to meet the published specifications for One (1) year from date of
shipment.
The liability of CareFusion (referred to as the Company) under this warranty is limited
to replacing, repairing or issuing credit, at the discretion of the Company, for parts
that become defective or fail to meet published specifications during the warranty
period; the Company will not be liable under this warranty unless (A) the Company is
promptly notified in writing by Buyer upon discovery of defects or failure to meet
published specifications; (B) the defective unit or part is returned to the Company,
transportation charges prepaid by Buyer; (C) the defective unit or part is received by
the Company for adjustment no later than four weeks following the last day of the
warranty period; and (D) the Company’s examination of such unit or part shall
disclose, to its satisfaction, that such defects or failures have not been caused by
misuse, neglect, improper installation, unauthorized repair, alteration or accident.
Any authorization of the Company for repair or alteration by the Buyer must be in
writing to prevent voiding the warranty. In no event shall the Company be liable to
the Buyer for loss of profits, loss of use, consequential damage or damages of any
kind based upon a claim for breach of warranty, other than the purchase price of any
defective product covered hereunder.
®
SiPAP™
The Company warranties as herein and above set forth shall not be enlarged,
diminished or affected by, and no obligation or liability shall arise or grow out of the
rendering of technical advice or service by the Company or its agents in connection
with the Buyer's order of the products furnished hereunder.
Limitation of Liabilities
This warranty does not cover normal maintenance such as cleaning, adjustment or
lubrication and updating of equipment parts. This warranty shall be void and shall not
apply if the equipment is used with accessories or parts not manufactured by the
Company or authorized for use in writing by the Company or if the equipment is not
maintained in accordance with the prescribed schedule of maintenance.
The warranty stated above shall extend for a period of One (1) year from date of
shipment, with the following exceptions:
1. Components for monitoring of physical variables such as temperature,
pressure, or flow are warranted for ninety (90) days from date of receipt.
2. Elastomeric components and other parts or components subject to
deterioration, over which the Company has no control, are warranted for sixty
(60) days from date of receipt.
3. Internal batteries are warranted for ninety (90) days from the date of receipt.
The foregoing is in lieu of any warranty, expressed or implied, including, without
limitation, any warranty of merchantability, except as to title, and can be amended
only in writing by a duly authorized representative of the Company.
675–101–101 Rev. K
Page 7

Infant Flow® SiPAP™ vii
Contents
Revision History ......................................................................................................... iii
Warranty .................................................................................................................... vi
Contents ................................................................................................................... vii
List of Figures .......................................................................................................... viii
List of Tables ........................................................................................................... viii
Notices ....................................................................................................................... ix
Chapter 1 - Product Description ................................................................................. 1
Chapter 2 - Product Specifications............................................................................. 3
Chapter 3 - Summary of Warnings and Cautions....................................................... 7
Chapter 4 - Unpacking & Setup ............................................................................... 11
Chapter 5 - Operation .............................................................................................. 23
Chapter 6 - Operating Modes .................................................................................. 33
Chapter 7 - Alarms and Indicators ........................................................................... 35
Chapter 8 - Maintenance & Cleaning ....................................................................... 41
Chapter 9 – Explanation of Symbols ........................................................................ 43
Appendix A - Product Configurations ....................................................................... 49
Appendix B - Pneumatic Diagram ............................................................................ 51
Appendix C - Alarm Troubleshooting ....................................................................... 53
Appendix D - Fault Management ............................................................................. 57
Glossary ................................................................................................................... 63
Index ........................................................................................................................ 65
675–101–101 Rev. K
Page 8

viii Infant Flow
List of Figures
Figure 1 – Stand unpacking and assembly ........................................................ 11
Figure 2 – Stand and Driver assembly ............................................................... 12
Figure 3 – Driver assembled with patient circuit and /humidifier ........................ 13
Figure 4 – Attaching the Abdominal Respiratory Sensor ................................... 14
Figure 5 – Flow Pressure Nomogram ................................................................ 15
Figure 6 – Front Panel ....................................................................................... 23
Figure 7 – Rear Panel ........................................................................................ 24
Figure 8 – Set Up Screen .................................................................................. 27
Figure 9 – Alarm set/confirm Screen ................................................................. 28
Figure 10 – Mode Select Screen ....................................................................... 29
Figure 11 – Parameter Adjust Screens .............................................................. 29
Figure 12 – Main Screen .................................................................................... 30
®
SiPAP™
Figure 13 – Monitored Parameters Screen ........................................................ 30
Figure 14 – NCPAP ........................................................................................... 33
Figure 15 – BiPhasic .......................................................................................... 33
Figure 16 – BiPhasic tr....................................................................................... 34
Figure 17 - Flat Battery screen .......................................................................... 37
List of Tables
Table 1 – Functions and Accessories .................................................................. 2
Table 2 - Soft-key operation ............................................................................... 25
Table 3 – Parameter Default Value .................................................................... 27
Table 4 – Alarm Symbols and Indicators ........................................................... 38
Table 5 – Equipment Symbols ........................................................................... 43
Table 6 – Button Symbols .................................................................................. 45
Table 7 – Non-US Configuration Parameters .................................................... 49
Table 8 – US Configuration Parameters ............................................................ 49
Table 9 – Alarm Troubleshooting ....................................................................... 53
Table 10 – Fault Classification ........................................................................... 57
Table 11 – Fault Recovery ................................................................................. 58
675–101–101 Rev. K
Page 9

Infant Flow® SiPAP™ ix
Notices
E MC Notic e
This equipment radiates and is susceptible to radio frequency energy. If not installed
and used in accordance with the instructions in this manual, electromagnetic
interference may result. The equipment has been tested and found to comply with
the limits set forth in BS EN60601-1-2 for Medical Electrical Equipment Part 1-2:
General requirements for safety-collateral standard. Electromagnetic compatibility –
requirements and tests. These limits provide reasonable protection against
electromagnetic interference when operated in the intended use environments (e.g.
hospitals) described in this manual.
This device is also designed and manufactured to comply with the following
standards:
Safety: UL 60601-1: 2003 Medical Electrical Equipment, Part 1: General
Requirements for Safety.
CAN/CSA C22.2 No 601.1-M90, Medical Electrical Equipment - Part
1: General Requirements for Safety including C22.2 No. 601.1S1-94
(IEC601-1, Amendment 1:1991) Supplement No. 1-94 to CAN/CSA
22.2 No. 601.1-M90
Electrical Safety:
Class 1 equipment
Contains type BF patient applied parts
Continuous Operation
MR I Notice
This equipment contains electromagnetic components whose operation can be affected
by intense electromagnetic fields.
Do not operate this device in a MRI environment or in the vicinity of high-frequency
surgical diathermy equipment, defibrillators, or short-wave therapy equipment.
Electromagnetic interference could disrupt the operation of the device.
Intended Use Notice
The Infant Flow® SiPAP™, consisting of a Driver and Generator plus NCPAP Prongs
and Masks, is intended for the provision of Bi-Level CPAP (SiPAP™) to produce a sigh.
The system is for use in Hospitals, Hospital Type facilities and intra-Hospital transport
environments and is indicated for the treatment of Newborn and Infant patients. The
Infant Flow
personnel, under the direction of a physician.
®
SiPAP™ should only be operated by properly trained clinical
675–101–101 Rev. K
Page 10
x Infant Flow
R egulatory Notice
Federal law restricts the sale of this device except by or on order of a physician.
Reuse of single-patient use accessories may degrade the performance of the
product or cause cross contamination.
Clas sification
Type of Equipment: Medical Equipment, Class 1 and internally powered, IPX1
Protected, and uses type BF applied parts. Equipment is not suitable for use in
presence of flammable anesthetics.
Declaration of Conformity Notice
This medical equipment complies with the Medical Device Directive, 93/42/EEC, and
the following Technical Standards, to which Conformity is declared:
EN60601-1 and EN60601-1-2
EN 10993
EN 14971
®
SiPAP™
EU Notified Body:
BSI (Reg. No. 0086)
Trade names:
®
Infant Flow
SiPAP™
Manufactured by:
CareFusion
22745 Savi Ranch Parkway
Yorba Linda, California 92887-4668
If you have a question regarding the Declaration of Conformity for this product,
please contact CareFusion.
675–101–101 Rev. K
Page 11

Infant Flow® SiPAP™ 1
Chapter 1 - Product Description
Infant Flow® SiPAP™ provides a non-invasive form of respiratory support designed
for infants in hospital environments such as Neonatal and Pediatric Intensive Care
Units. It can also be used when transporting these patients within the hospital
environment.
Infant Flow
configuration. The Plus configuration provides NCPAP and time triggered BiPhasic
modes with and without breath rate monitoring. The Comprehensive* configuration
offers these features plus a patient triggered BiPhasic mode with apnea backup
breaths. The Infant Flow
LCD touch screen display, pressure time waveform graphics, integrated patient
monitoring, alarms for high and low pressure and FiO
battery power.
As a result of the unique, patented design, the Infant Flow
System Generator has been proven to provide the most stable CPAP at the lowest
work of breathing for patients compared to other devices
performance of the Infant Flow
expiratory flows. This system has been designed and tested to perform optimally
when used only with accessories available from CareFusion. These accessories
include circuits and generators, prong and mask patient interfaces and bonnets.
®
SiPAP™ is currently available in a Plus or Comprehensive*
®
SiPAP™ comes standard in all configurations with an
and up to 2 hours of backup
2
®
or AirLifeTM Infant nCPAP
(1)
®
Generator is irrespective of patient demand or
. The outstanding
Infant Flow® SiPAP™ Features
The expanded capabilities of the Infant Flow® SiPAP™ Plus and Comprehensive*
configurations allow for applications to broader range of patients who may otherwise
not be candidates for non-invasive respiratory support from NCPAP alone
NCPAP – continuous positive airway pressure based on clinician set pressure.
Breath rate monitoring/alarm can be activated in this mode.
BiPhasic – time triggered pressure assists are delivered based on clinician set
Time-High, rate and pressure criteria. Breath rate monitoring/alarm can be activated
in this mode.
BiPhasic tr* – patient triggered pressure assists delivered based on clinician set
Time-High and pressure criteria. Breath rate monitoring/alarm and Apnea backup
breaths are automatically active in this mode.
Patented Infant Flow
®
Flow
Generator is a fluidic device for the generation of consistent infant nasal CPAP
with a low work of breathing compared to other devices
Fully integrated alarm package – Supply gases failure, High Patient Pressure, Low
patient pressure, high and low delivered Oxygen concentration, change from AC to
DC power source, low and flat battery charge status and Low breath rate/apnea
alarm.
®
or AirLifeTM Infant nCPAP System Generator - The Infant
(2,3)
.
(1)
.
Battery Backup – Up to 2 hours of battery backup allows for intra-hospital transport.
Clear indicators are provided for power supply in use (AC or DC), and battery charge
level.
675–101–101 Rev. K
Page 12
2 Chapter 1 - Product Description
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
Screen Lock – After 120 seconds of no screen inputs, the screen changes to the
Locked Screen to prevent inadvertent changes. Upon activation of a high priority
alarm the screen changes to an unlocked state to allow access to controls.
Table 1 – Functions and Accessories
Functions & Accessories Plus Comprehensive*
NCPAP
NCPAP with breath rate monitoring
and alarm
BiPhasic
BiPhasic with breath rate monitoring
and alarm
BiPhasic tr*
Internal Battery
Manual Breath
Apnea Back up rate
Screen lock
Prioritization of alarms
*Comprehensive configuration not available for sale in the United States
(1) Decreased imposed work with a new nasal continuous positive airway pressure device.
Klausner, James F., PhD, Lee, Amy., Hutchison, Alastair A., FRACP. Pediatric Pulmonology 22:
188-194; 1996
(2) A Prospective Randomized, Controlled Trial Comparing Synchronized Nasal Intermittent Positive
Pressure Ventilation versus Nasal Continuous Positive Airway Pressure as Modes of Extubation.
Khalaf Nabeel, M., Brodsky Nancy, Hurley John, Bhandari Vineet. PEDIATRICS 108 (1): 13-17:
2001
(3) Efficacy of Nasal Intermittent Positive Pressure Ventilation in Treating Apnea of Prematurity. Lin
Chyi-Her, MD, Wang Shan-Tair, PhD, Lin Yuh-Jyh, MD, Yeh Tsu-Fuh, MD:Pediatric Pulmonolgy:
26 (5): 349-53; 1996
675–101–101 Rev. K
Page 13

Infant Flow® SiPAP™ 3
Chapter 2 - Product Specifications
Modes
• NCPAP
• NCPAP with breath rate monitoring and low rate alarm
• BiPhasic (time triggered)
• BiPhasic (time triggered) with breath rate monitoring and low rate alarm
• BiPhasic tr (patient triggered) with breath rate monitoring, low breath rate
alarm and apnea back up (Comprehensive models only)
Controls
• Time High (T-High) – 0.1 – 3.0 seconds
• Rate (R)
1-120 (Non-U.S. Configuration Parameters)
1-54 (U.S. Configuration Parameters)
• Apnea Interval
• (T
• (TLBR) – 10-30 seconds; 5 second intervals (U.S. Configuration
• NCPAP / Pres Low flow meter – 0-15 L/min, accuracy ± 15% of selected
output
• Pres High flow meter – 0-5 L/min, accuracy ± 15% of selected output
• Manual Breath – X 1
• %O
Monitors
• CPAP
• PEEP
• MAP
• PIP
) – 10-30 seconds, 5 second intervals (Non-U.S. Configuration
apnea
Parameters)
Parameters)
– 21 -100%
2
• %O
• I:E ratio
• Spontaneous rate (Rsp)
• Battery charge level
675–101–101 Rev. K
2
Page 14

4 Chapter 2 - Product Specifications
Alarms
• High airway pressure – 3 cmH2O above measured airway pressure
• Airway over-pressure limit alarm
• maximum 11 cmH
• maximum 15 cmH2O in patient triggered BiPhasic tr mode
• Low airway pressure – 2 cmH
1.5 cmH
O if otherwise would be zero
2
• High and Low delivered Oxygen concentration ±5% of setting. Minimum and
maximum delivered FiO2 is 18 and 104% respectively.
• Low breath rate alarm
• Low battery charge level
• Flat battery
• Input gases failure
• Alarm volume (electronic alarms) 70 dBa at 1 meter
Pneumatic Supply
• Patient Gas Outlet: 15 mm standard taper fitting
• Patient Pressure Input: 4.5 mm Luer taper fitting
• Gas Supply: Nominal 4 bar or 60 psi, clean, dry medical air and oxygen
O in NCPAP and time triggered BiPhasic mode
2
O below measured airway pressure or
2
• Range: 40.61 to 87 PSI; Maximum differential pressure 29 PSI
• Manometer: Range 0 to + 20 cmH
• Gas Connections: Standard DISS, NIST or Air Liquide connectors
Electrical Supply
• Input Voltage: 100-230 VAC
• Input Frequency: 50/60 Hz
• Power Consumption: 50 VA maximum
• Fuse Rating For 220 V nominal operation: “T” Type 2.5 A at 250 V
• Device Housing Protection rating level: IPX1
• Battery Working Time: 2 hours (from fully charged state)
• Battery Charging Time: max. 16 hours
O, accuracy, ± 2% of span
2
675–101–101 Rev. K
Page 15

Infant Flow® SiPAP™ 5
Atmospheric & Environmental
• Temperature Range
Operating: 5 – 40° C
Storage: - 20 - 50° C
• Relative Humidity -Operating: 0 – 95% non-condensing
• Storage: 0 – 95% non-condensing
Physical
• Dimensions (Driver only)-
• (W x H x D) 26 x38 x 23.5 cm
• (W x H x D) 10.25 x15 x 9.25 in
• Weight (Driver only)-
• 8.8 kg
• 19.5 lb
Accessories
• Silencer / Bacterial Filter - The additional resistance of the D1420/100
Silencer / Bacterial Filter and adaptor is less than 0.56 cmH
and less than 0.40 cmH
O at 5 LPM.
2
O at 15 LPM,
2
675–101–101 Rev. K
Page 16

6 Chapter 2 - Product Specifications
F&P 730
773386–102
Patient Circuit w/Generator (box of 20)
773387–101
Patient Circuit (box of 20)
773388–105
Patient Circuit RCI 16V (box of 20)
Prongs
Masks
777086–101
Nasal Mask - Small (box of 10)
006905
NCPAP Generator Kit
006915
Nasal Prongs - Medium
006920
Nasal Prongs - Large
Part No. Description
Infant Flow® Products
D1420/100 Silencer (box of 20)
11541–101 Patient Circuit Assembly (box of 20)
11541–102 Patient Circuit Assembly w/Generator (box of 20)
773386–101 Patient Circuit (box of 20)
F&P 850
12204–101 Patient Circuit (box of 20)
12204–102 Patient Circuit w/Generator (box of 20)
12233–102 Patient AirLife Circuit w/Generator (box of 20)
12233–101 Patient AirLife Circuit (box of 20)
RCI
773388–103 Patient Circuit w/Generator RCI 16V (box of 20)
773389–105 Patient Circuit RCI 21V (box of 20)
773389–104 Patient Circuit w/Generator RCI 21V (box of 20)
11513–101 Nasal Prongs - Small (box of 10)
11513–102 Nasal Prongs - Medium (box of 10)
11513–103 Nasal Prongs - Large (box of 10)
777086–102 Nasal Mask - Medium (box of 10)
777086–103 Nasal Mask - Large (box of 10)
777086–104
Nasal Mask Extra Large (box of 10)
AirLifeTM Products (U.S.A. only)
Prongs
006910 Nasal Prongs - Small
Masks
006925 Nasal Mask - Small
006930 Nasal Mask - Medium
006935 Nasal Mask - Large
675–101–101 Rev. K
Page 17

Infant Flow® SiPAP™ 7
Chapter 3 - Summary of Warnings and Cautions
Please review the following safety information prior to operating the Infant Flow
SiPAP™. Attempting to operate this equipment without fully understanding its
features and functions may result in unsafe operating conditions.
Warnings and Cautions, which are general to the use of the device under all
circumstances, are included in this section. Some Warnings and Cautions are also
inserted within the manual where they are most meaningful.
Notes are also located throughout the manual to provide additional information
related to specific features.
If you have a question regarding the installation, set up, operation, or maintenance of
the device, contact CareFusion (see page v).
Terms
WARNINGS identify conditions or practices that could result in serious adverse
CAUTIONS identify conditions or practices that could result in damage to the
NOTES identify supplemental information to help you better understand how
®
reactions or potential safety hazards.
driver or other equipment.
the driver works.
Warnings
• Infant Flow® SiPAP™ is intended for use by a trained practitioner, under the
direct supervision of a qualified physician.
• When the Infant Flow
care professional should be in attendance at all times to react to an alarm or
other indications of a problem.
• Always have an alternate means of ventilation available whenever the Infant
Flow
• Do not attach the Generator to the patient until User Verification and initial set
up into NCPAP mode is complete.
• Water in the air supply can cause malfunction of this equipment.
• The operator should not touch the electrical connectors of the Infant Flow®
SIPAP™ or its accessories, and the patient simultaneously.
• An audible alarm indicates an anomalous condition and should never go
unheeded.
®
®
SiPAP™ is in use.
SiPAP™ is connected to a patient, a trained health
675–101–101 Rev. K
Page 18

8 Chapter 3 - Summary of Warnings and Cautions
• Anti-static or electrically conductive hoses or tubing should not be used within
the patient circuit.
• If a mechanical or electrical problem is recognized while operating the Infant
®
Flow
SiPAP™, it must be removed from use and referred to qualified service
personnel for servicing. Using inoperative equipment may result in patient
injury.
• Prior to patient application, ensure that all User Verification testing and
calibration procedures are successfully completed. User Verification testing
and calibration procedures must be done off patient.
• The
indicates a connection between the Transducer Assembly and the
driver. It does not indicate attachment or correct positioning of the Abdominal
Respiratory Sensor.
• Under certain conditions (minimum supply pressure and maximum gas
demand, including auxiliary output) output flow rates and therefore pressure
delivered to the generator may be reduced.
• The Pres High
flow meter must be adjusted to zero when not required for the
patient.
• Whenever a patient is attached to respiratory care equipment, constant
attendance is required by qualified personnel. The use of an alarm or
monitoring system does not give absolute assurance of warning for every
malfunction that may occur in the system. In addition, some alarm conditions
may require immediate attention.
• Nasal CPAP treatment in general can cause nasal irritation, septal distortion,
skin irritation and pressure necrosis. Adherence to the recommended usage
instructions for the Infant Flow
®
SiPAP™ and AirLifeTM Infant nCPAP System
accessories may reduce the incidence of these complications.
• It is strongly recommended that regular monitoring for gastric distention be
carried out for patients receiving non-invasive ventilatory support. Refer to
your facility’s policy and procedure for further guidance.
• This device exhausts O
during normal operation. Oxygen vigorously
2
accelerates combustion. To avoid fire hazard, do not place flammable
materials or sources of heat close to the exhaust.
• The Abdominal Respiratory Sensor is used only to enable features
associated with certain modes from the Infant Flow
®
SiPAP™. When using
the Abdominal Respiratory Sensor, always use an additional, external device
for monitoring of the respiratory rate and detection of apneic episodes as well
as an appropriate monitor for continuous SaO
®
• If the Infant Flow
SiPAP™ driver is shelf mounted, ensure that the driver is
monitoring.
2
stable and that all circuit tubing, hoses and cables are restrained to avoid
hazard of toppling.
• Check that the water trap is empty before use and empty it frequently during
use.
• Do not block or restrict the exhaust port located on the instrument back panel.
Equipment malfunction may result.
675–101–101 Rev. K
Page 19

Infant Flow® SiPAP™ 9
• Do not use the equipment without the expiratory tubing connected to the
generator.
• Only use the supplied AC cable to connect to the power supply.
• The Transducer LED indicator on the front panel of the driver only signifies
connection to the driver. It does not indicate connection to or proper
positioning of the Abdominal Respiratory Sensor.
• Do not overload the pole and stand.
• Oxygen vigorously accelerates combustion. To avoid explosion hazard, do
not use any instrument or other equipment that may have been exposed to oil
or grease contamination.
• When a low gas supply alarm occurs, the oxygen concentration delivered to
the patient will differ from that set on the %O
control.
2
• A source gas failure will change the FiO2 and may result in patient injury.
• The functioning of this equipment may be adversely affected by the operation
of other equipment nearby, such as high frequency surgical (diathermy)
equipment, defibrillators, short-wave therapy equipment, “walkie-talkies”, or
cellular phones.
• Due to possible explosion hazard, the Infant Flow
®
SiPAP™ should not be
used in the presence of flammable anesthetics.
• Electric shock hazard – Do not remove any of the Infant Flow
®
SiPAP™
covers or panels. Refer all servicing to an authorized CareFusion service
technician or factory trained technician (see Service Manual P/N 675-120).
• A protective ground connection by way of the grounding conductor in the
power cord is essential for safe operation. Upon loss of protective earth
ground, all conductive parts including knobs and controls that may appear to
be insulated can render an electrical shock. To avoid electrical shock, plug
the power cord into a properly wired receptacle, use only the power cord
supplied with the ventilator, and make sure the power cord is in good
condition.
• The Infant Flow
®
SiPAP™ is designed to ensure that the user and patient are
not exposed to excessive leakage current per applicable standards.
However, this cannot be guaranteed when external devices are attached to
the driver. In order to prevent the risk of excessive enclosure leakage current
from external equipment attached to the driver, isolation of the protective
earth paths must be provided to ensure proper connection. This isolation
should ensure that the cable shields are isolated at the peripheral end of the
cable.
• When the Infant Flow
internal oxygen monitor is disabled, the Infant Flow
®
SiPAPTM unit is connected to a patient, and the
®
SiPAPTM unit must be
used with an external oxygen monitor.
675–101–101 Rev. K
Page 20

10 Chapter 3 - Summary of Warnings and Cautions
Cautions
• Before use, verify that this equipment has been authorized for use by
qualified technical service personnel.
• Ensure that the voltage and installed fuses are set to match the voltage of the
wall outlet, or damage may result.
• A battery that is fully drained (i.e. void of any charge) may cause damage to
the driver and should be replaced.
• All accessory equipment that is connected to the driver should comply with
CSA/IEC601/ETL.
• Although failure of any of the above tests will not prevent the ventilator from
functioning, it should be checked to make sure it is operating correctly before
use on a patient.
• The Infant Flow
CareFusion accessories. Only accessories approved for use by CareFusion
should be used. If in doubt, please contact your local sales representative.
®
SiPAP™ has been designed and tested using only
Notes
• Employ safe lifting procedures when assembling the unit.
• Do not sterilize the driver. The internal components are not compatible with
sterilization techniques.
• Do not submerge the driver or pour cleaning liquids over or into the driver.
• Following each alarm verification test, ensure that control settings and alarm
limits are reset as instructed before proceeding to the next test.
• CareFusion cannot ensure product performance as stated in this manual with
the use of Non-CareFusion accessories.
675–101–101 Rev. K
Page 21
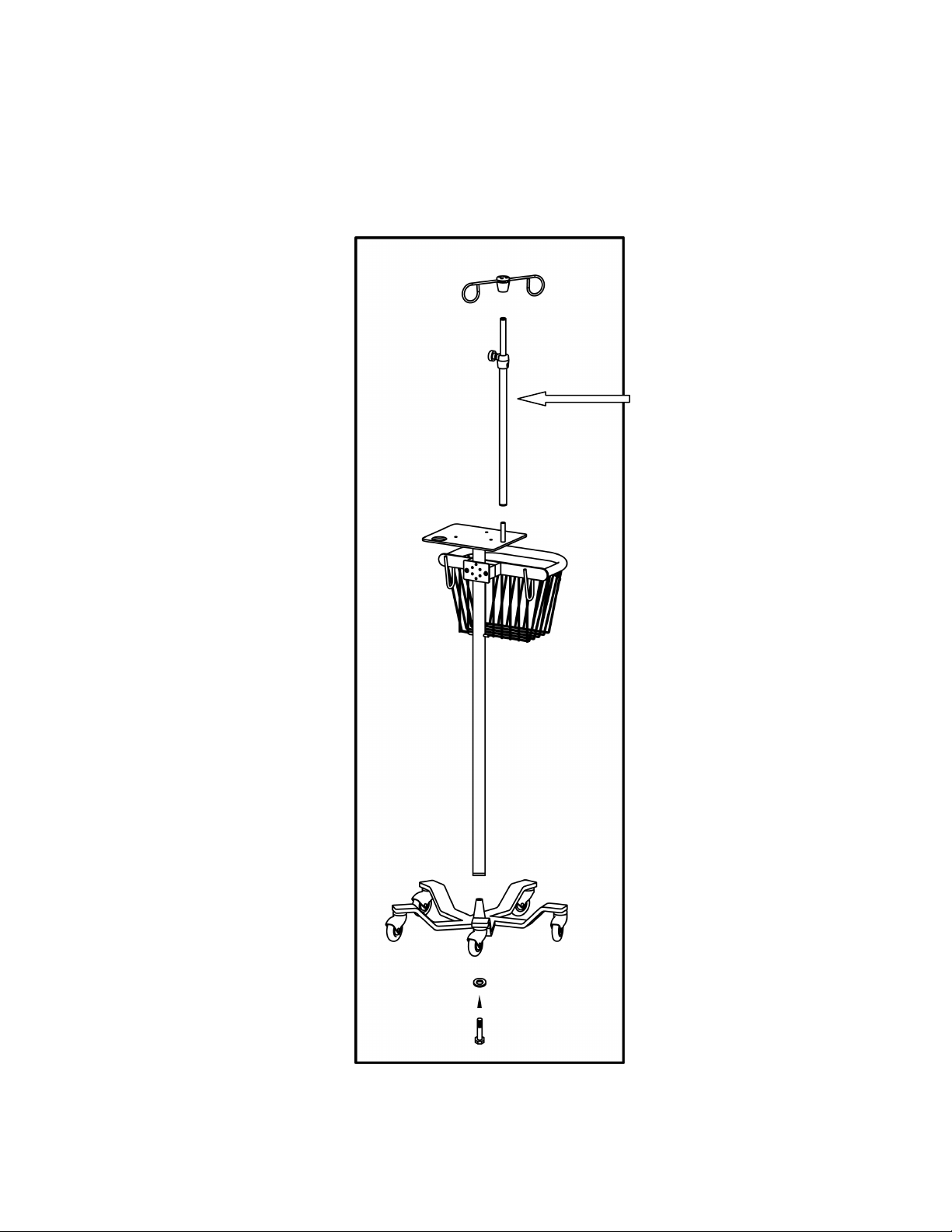
Infant Flow® SiPAP™ 11
Chapter 4 - Unpacking & Setup
Assembly and physical setup
Optional
Accessory
Figure 1 – Stand unpacking and assembly
675–101–101 Rev. K
Page 22
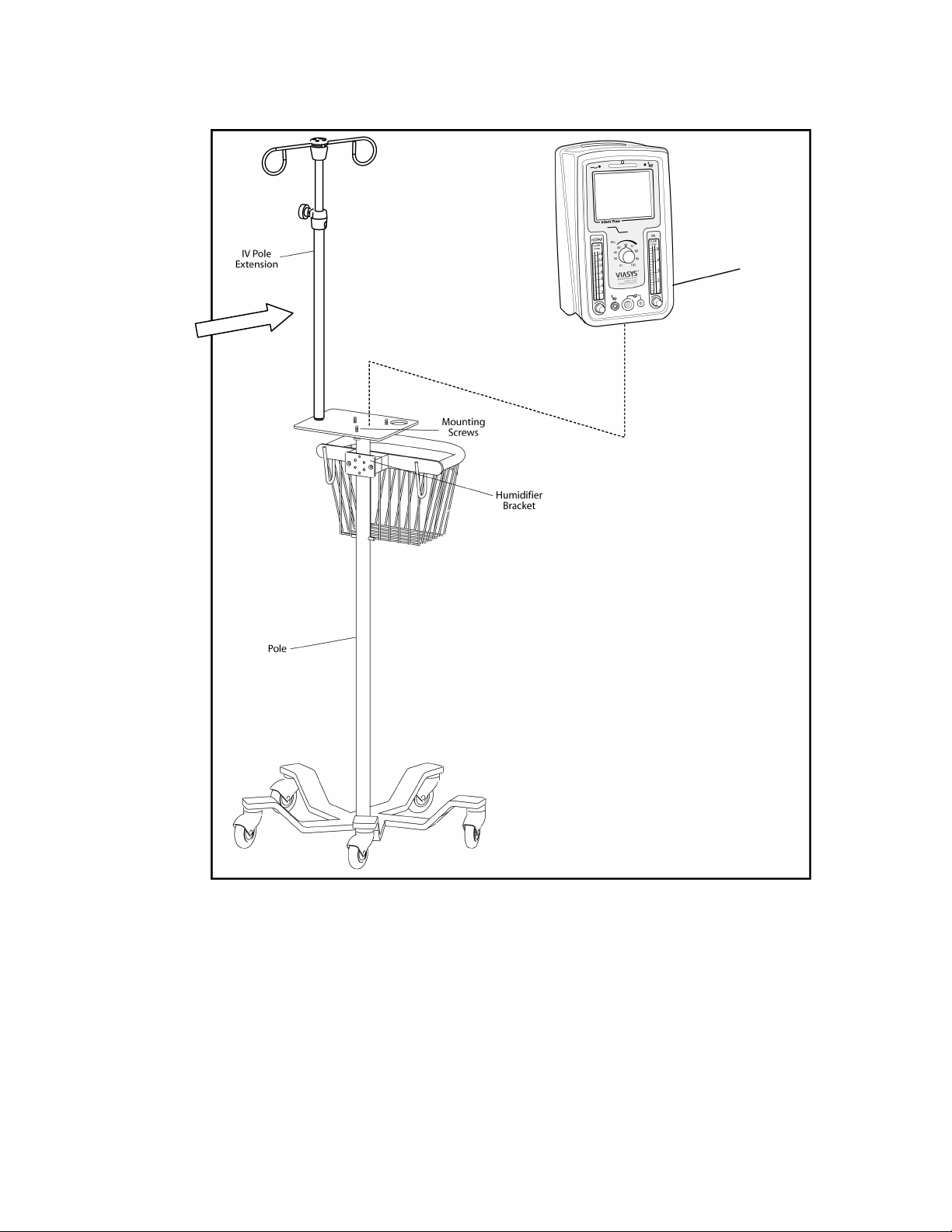
12 Chapter 4 - Unpacking & Setup
Infant Flow
SiPAP Driver
Optional
Accessory
Figure 2 – Stand and Driver assembly
675–101–101 Rev. K
Page 23
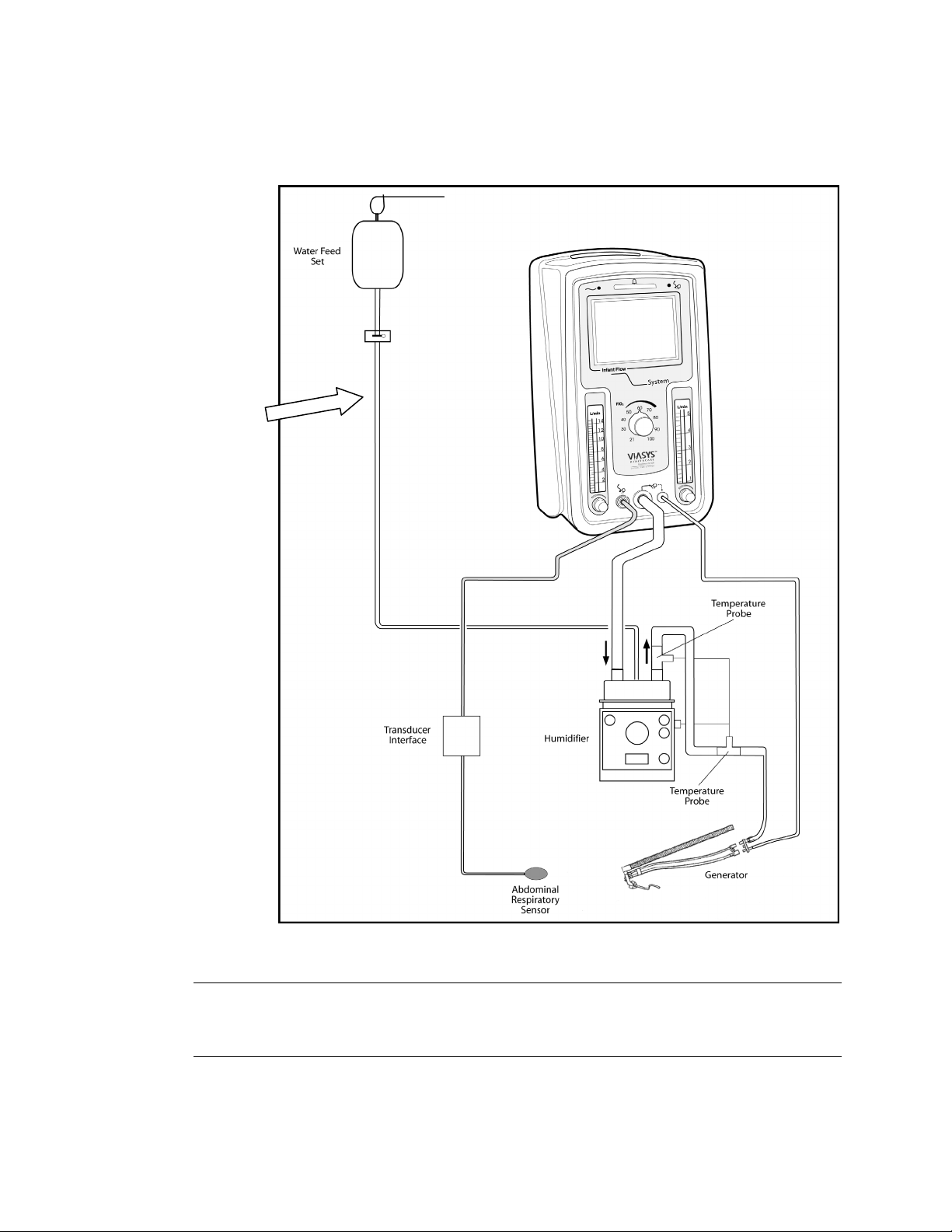
Infant Flow® SiPAP™ 13
Attaching a patient circuit
Infant Flow
SiPAP Driver
Optional
Accessory
Figure 3 – Driver assembled with patient circuit and /humidifier
Note
We recommend between 96.8 °F (36 °C) and 98.6 °F (37 °C) but never higher than
98.6 °F (37 °C) for inspired gases.
675–101–101 Rev. K
Page 24

14 Chapter 4 - Unpacking & Setup
CAUTION
Back pressure from some auto-feed humidifier chambers may cause the water bags
to fill with air. Ensure that the humidifier chambers are adequately filled according to
the manufacturer’s instructions.
Note
When the Hudson RCI Humidification System is being used with Infant Flow®
SiPAP
TM
, it is recommended that the standard compliance column be used.
Attaching the Abdominal Respiratory Sensor
Figure 4 – Attaching the Abdominal Respiratory Sensor
1. Connect the Transducer Assembly to the front panel of the driver (Fig. 3)
2. Connect Abdominal Respiratory Sensor to the Transducer interface.
3. Apply gentle compression to sensor. Verify function with illumination of LED on
transducer interface.
4. Apply sensor with suitable tape (Fig 4).
a. Pressure line perpendicular to tape
b. Sensor between umbilicus and xiphisternum
c. Placement on the side of the abdomen may be necessary
5. Verify correct placement
a. Observe spontaneous breathing
b. Transducer LED illuminates on expiration; Front panel Transducer LED
illuminates on inspiration
675–101–101 Rev. K
Page 25

Infant Flow® SiPAP™ 15
Flow / Pressure Relationship
The Infant Flow® SiPAP™ is subject to a direct relationship between the controlled
enriched gas flow and airway pressure. A nomogram illustrating the relationship
between constant airway pressure and flow settings is shown in Figure 5. For
example, 8 L/min gas flow provides approximately 5 cmH
2
O.
Note
Individual devices have a tolerance of up to ± 10% from that illustrated in the
nomogram and in particular, at pressures below 2 cmH
2
O.
Flow Pressure Nomogram (for
reference only) to show typical flow
pressure relationships. This is not
meant to establish actual product
performance.
Figure 5 – Flow Pressure Nomogram
675–101–101 Rev. K
Page 26

16 Chapter 4 - Unpacking & Setup
User Verification Test
WARNING
Do not attach Generator to the patient until User Verification and initial set up
into NCPAP mode is complete.
CAUTION
Although failure of any of the above tests will not prevent the ventilator from
functioning, it should be checked to make sure it is operating correctly before use on
a patient.
Power-on Check
This test is run automatically on power up of the driver and automatically performs
the following checks:
• Flash ROM
• Hardware Inputs/Outputs
• Audible and visual alarms indicators
• Test and calibration of pressure sensor
• Test of dump valve
The unit carries out a full functional check during this time. If unsuccessful, the
screen remains darkened and the warning bar remains on. In this case, check for the
following;
• Power Supply not connected
• Battery voltage low
If the checks are successful, the screen changes to Power Up Screen. After two
seconds, the screen changes to Power Up Check Screen.
During the Power Up check:
• Screen image shown in negative
• Warning bar comes on for one second
• Transducer Assembly LED comes on for one second
• Audible alarm sounds for one second
• Dump valve is tested
• Pressure is set to zero
675–101–101 Rev. K
Page 27

Infant Flow® SiPAP™ 17
After two seconds screen changes to Set Up Screen. Alarm limits are disabled and a
flashing question mark appears under the NCPAP / Pres Low flow meter screen
indicator.
Two Point O2 Sensor Calibration
Enter the Calibration Screen from the Set Up Screen by pressing the calibration
button on the lower right hand corner of the touch screen. Ensure there is a minimum
8 LPM set on the NCPAP/PRES Low Flow meter. In addition, ensure there is a
minimum of 3 LPM set on the NCPAP/High Flow meter. Adjust the %O
21%. Allow the %O
associated flashing button.
display to stabilize. Confirm the calibration by touching the
2
control to
2
Adjust the %O
touching the associated flashing button. Return to the Start up Screen by pressing
the Exit button.
control to 100%. Allow the %O2 display to stabilize. Confirm by
2
Note
If O2 calibration fails, a red “X” is shown. Refer to the Service Manual.
If the internal oxygen cell is depleted or damaged, it may not be possible to calibrate
the O
button. This will disable oxygen monitoring and the audible oxygen alarms until the
device is powered off. Whenever the device is operating with oxygen monitor and
alarms disabled, a fault code E5x displays, and measured FiO
sensor. The internal oxygen monitor may be disabled using the Disable O2
2
displays as dashes.
2
WARNING!
When the Infant Flow® SiPAPTM unit is connected to a patient, and the internal
oxygen monitor is disabled, the Infant Flow® SiPAPTM unit must be used with
an external oxygen monitor.
If calibration is attempted, and fails, or if the oxygen cell fails while the device is in
normal use, a Fault Code E5X displays, as tabulated in appendix D, and a high
priority alarm is indicated visually and audibly. To enable continued operation, the
internal oxygen monitoring may be disabled by pressing and holding the alarm mute /
reset button for 3 seconds. This disables the internal oxygen monitor and alarms and
clears the alarm condition. The E5X code remains to indicate that the oxygen
monitor is inoperative. An external oxygen monitor must be used.
675–101–101 Rev. K
Page 28

18 Chapter 4 - Unpacking & Setup
Leak Test
1. Have the patient circuit and generator assembled as shown in Fig 3.
2. Connect the patient interface (prong or mask) to the generator (see Chapter 5,
Step by Step Fixation) and occlude the opening to the patient.
3. If not powered up already, switch on the power to the driver.
4. Adjust the NCPAP / Pres Low flow meter to 8 L/min. Verify that the measured
pressure is 5 ± 1 cmH
5. Adjust %O2 control as prescribed for the current patient. Verify that the blender
setting, and the measured oxygen value, are within 3%. Touch the associated
flashing screen icon to confirm.
6. Adjust the Pres High flow meter as prescribed for the current patient. Touch
the associated flashing screen icon to confirm.
7. Connect the Transducer Interface to the front panel of the driver if breath
monitoring is desired in treatment. Touch the associated flashing screen icon
to confirm.
8. The display screen changes to the Alarm Set/Confirm Screen. Press the
NCPAP button or Alarm Mute/Reset button to set alarms and begin monitoring.
9. Monitored parameter for CPAP should be 4-5 cmH
leaks or blockages, (including the humidification system).
10. Remove the occlusion to the patient interface. The monitored CPAP display
should be 0-2 cmH
O. Touch the associated flashing screen icon to confirm.
2
O. If not, check circuit for
2
O. If not, check that the interface is not still occluded.
2
675–101–101 Rev. K
Page 29

Infant Flow® SiPAP™ 19
Alarm Test Initial Settings
2
nCPAP System Patient Circuit
Pres High flow meter
For Step 9 Use the settings provided below
Alarms Test
WARNING
Prior to patient application, ensure that all User Verification testing and
calibration procedures are successfully completed. User Verification testing
and calibration procedures must be done off patient.
NOTE
Following each alarm verification test, ensure that control settings and alarm limits
are reset as instructed before proceeding to the next test.
Air Supply Pressure
O
Supply Pressure
Patient Circuit
Generator
NCPAP / Pres Low flow meter
% O2
Mode
Rate
T-High
Tapnea (Non-U.S. Configuration) /
T
(U.S. Configuration)
LBR
> 30 psig (2.1 bar)
> 30 psig (2.1 bar)
Infant Flow® or AirLifeTM Infant
Infant Flow® or AirLifeTM Infant
nCPAP System Generator
8 L/min (for delivery of 5 cmH2O)
30%
3 L/min
NCPAP
30 bpm
0.3 sec
20 sec
675–101–101 Rev. K
Page 30

20 Chapter 4 - Unpacking & Setup
Perform the Alarms Test on the Infant Flow® SiPAP™ using the following steps and
the initial settings provided above.
1. Make appropriate connections for air and O
to appropriate AC outlet. Attach patient circuit, generator and patient interface
(mask or prong) as shown in Figure 3. Occlude the opening to the patient.
2. Power up the driver and allow Power On Check to complete.
3. Low airway pressure alarm: From NCPAP operating mode, with alarms set,
remove occlusion from opening to patient. Verify that the low airway pressure
alarm activates. Restore the patient interface occlusion and press the Alarm
Mute / Silence button for 3 seconds to reset the alarms.
4. High airway pressure alarm: Adjust the NCPAP / Pres Low flow meter to 11
L/min. Verify that the high airway pressure alarm activates. Return the NCPAP
/ Pres Low flow meter to 8 L/min and press the Alarm Mute / Silence button for
3 seconds to reset the alarms.
5. High %O
Alarm: Adjust the % O2 control to 35%. Verify that the High %O
2
alarm activates. Return the O2 control setting to 30%. Reset alarms by
pressing the Alarm Mute / Reset button for 3 seconds.
6. Low % O
Alarm: Adjust the % O2 control to 25%. Verify that the Low %O
2
alarm activates. Return the O2 control setting to 30%. Reset alarms by
pressing the Alarm Mute / Reset button for 3 seconds.
7. Loss AC Alarm: Disconnect the AC power cord from the wall outlet. Verify that
the Loss AC alarm activates. Reconnect the AC power cord. Clear the alarm
by pressing the Alarm Mute / Reset button.
8. High Circuit Pressure Alarm: Increase nCPAP pressure to 11.1 cmH
increasing the NCPAP/PRES Low Flow meter. Verify that the High Circuit
pressure alarm activates. Return NCPAP/PRES Low Flow meter to 8 LPM and
press the Alarm Mute/Silence button for three seconds to reset the alarms.
9. Low Breath Rate (Apnea) Alarm: Select and confirm BiPhasic+Apnea/LBR
(U.S. configuration). Using the abdominal sensor, manually tap the abdominal
sensor to simulate a spontaneous breath rate. The default mandatory breath
rate should be left alone. No alarms should be present. Change the mandatory
rate control setting to 1 bpm and stop tapping the abdominal sensor. Verify that
the Low Breath rate alarm activates after the default interval of 20 seconds.
Resume simulating spontaneous breath rate, turn the rate control to the default
setting and clear the alarm by pressing the Alarm Mute/Reset button for 3
seconds. Note: a transducer must be connected to perform the alarm check.
gas supply. Connect power cord
2
2
2
O by
2
675–101–101 Rev. K
Page 31

Infant Flow® SiPAP™ 21
Two Point O2 Sensor Calibration
Patient Circuit Leak test
2
Low O
2
Alarm
Infant Flow® SiPAP™ User Verification Test Checklist
Driver Serial Number:_____________________ Test Date:_________________
TEST PASS FAIL
Automated Tests
Power On Check
Manual Tests
Manual Alarms Checks
Low Airway Pressure Alarm
High Airway Pressure Alarm
High O
Loss AC Alarm
High Circuit Pressure Alarm
Low Breath Rate (Apnea) Alarm
Alarm
Signature of tester:_______________________________________________
Title___________________________________________________________
675–101–101 Rev. K
Page 32

22 Chapter 4 - Unpacking & Setup
Infant Flow®
SiPAP™
675–101–101 Rev. K
Page 33

Infant Flow® SiPAP™ 23
Chapter 5 - Operation
Front Panel Indicators and Controls
The front panel consists of a LCD touch screen display with key pad, separate flow
meter controls for adjustment of NCPAP /Pres Low and Pres High and a %O
blender control. Patient circuit connections are located along the bottom panel. LEDs
along the top of the front panel indicate power on, connection to wall AC, active
alarms and Transducer Interface connection to the driver. An ambient light sensor is
located under the front panel to adjust the backlight of the screen display in high and
low light environments
2
Figure 6 – Front Panel
675–101–101 Rev. K
Page 34

24 Chapter 5 - Operation
Rear Panel
Figure 7 – Rear Panel
675–101–101 Rev. K
Page 35

Infant Flow® SiPAP™ 25
Table 2 - Soft-key operation
Description Example
A button which is enabled.
A button which is inhibited due to non-availability of the designated feature
or pending acknowledgement of an active alarm condition.
A selected mode or control pending confirmation is visually highlighted
and intermittently flashes between yellow and white text.
While a button is pressed the edges are highlighted to provide a pressed
appearance.
When there is an active alarm associated with a measured value the
measured value concerned is displayed with RED FLASHING text. The
associated limit value (if any) is displayed in RED.
When an alarm that is associated with a measured value is resolved, the
device remains in a LOW priority alarm state, with the measured value
displayed in YELLOW FLASHING text and the associated limit displayed
in YELLOW, until the alarms are cleared by the operator.
When parameter adjustments cause a reduction in another parameter to
maintain requirements for minimum breath interval, the reduced
parameter is displayed in RED for 15 seconds
Changing a control
When a control such as Time-High is selected, increase and decrease buttons
appear. The control and the displayed value for the selected parameter are
highlighted. Use the decrease or increase keys to adjust the parameter. You may
accept the action by pressing the control button again. If no action is taken, the new
parameter will take effect after 15 seconds.
In normal treatment screens, parameter changes take immediate effect.
When you change the mode, such as NCPAP to BiPhasic, press the flashing mode
button to accept the change.
If no screen interactions occur for a period of 120 seconds and there are not active
alarms, then the screen goes to a ‘locked’ state to prevent inadvertent entries. To
unlock the screen, press the screen lock button. In the case of a high priority alarm,
the screen immediately unlocks to allow access to controls.
CAUTION!
When changing a control, use only your finger. Damage to the touch screen could
result if a pen or similar item is used to make changes.
675–101–101 Rev. K
Page 36

26 Chapter 5 - Operation
Increase / Decrease buttons
Pressing the ‘increase’ or ‘decrease’ buttons causes a currently-selected control to
be changed to the next valid greater or lesser value. Each press of the increase or
decrease button is accompanied by an audible click. If the control limit is reached an
audible beep sounds to alert the operator.
Displays of calculated values (such as I:E ratio) dependent on a control setting
change will change with acceptance of the parameter change.
Incompatible Control Settings
When a change to one control requires a change to a separate control to avoid an
incompatible control setting, the required change is made automatically by the driver
software.
If the adjusted control setting is restored prior to 15 seconds elapsed time or prior to
pressing any other control, then the required change action is reversed.
For example in BiPhasic mode with T-High = 2.0, as R is increased above 28 b/min
the constraint on minimum T-Low can be met only through a reduction in T-High. If R
is increased to 29, then T-High shall reduce automatically to 1.9s. If R is then
immediately reduced to 28, the previous setting for T-High shall be restored.
Parameter default value on change of mode
Some controls are active in more than one operating mode. In these instances, there
is a separate default value for operating modes as illustrated with the following table.
Settings that are changed by the operator in one specific mode will be maintained if
the mode is changed to another mode within the same mode group. All defaults shall
revert to factory default on power-cycling or software restart.
Setting a Manual Breath
The manual-breath function is available in CPAP, Biphasic, and Biphasic tr modes.
For manual breath to be active when the manual button is selected, the pre-use
pressure-high check has been completed and the pressure high-flow meter is set for
preferred manual breath. One manual breath is delivered per button-press.
Note:
The Pressure High Flow Meter must have flow above zero in order to deliver manual
breaths.
675–101–101 Rev. K
Page 37

Infant Flow® SiPAP™ 27
apnea
LBR
Table 3 – Parameter Default Value
Mode
Parameter
T-High Default for NCPAP apply Default for BiPhasic apply Default for
Rate Default for BiPhasic apply
Rb Default for
T
/ T
NCPAP NCPAP + rate
monitoring
This setting applies to all modes: system-wide default applies to all modes
BiPhasic BiPhasic + rate
monitoring
BiPhasic tr
BiPhasic tr
BiPhasic tr
User Interface Display
Screen Displays
1. Set Up Screen – The Set Up screen prompts the user to confirm settings for
base line pressure level (NCPAP / Pres Low), %O
High) controls and confirmation of connection of the Transducer Interface (XDCR) to
the driver.
, upper level pressure (Pres
2
Figure 8 – Set Up Screen
Adjust the NCPAP / Pres Low flow meter counter clockwise to increase the control to
the required flow rate and clockwise to decrease the flow. Touch the associated
flashing icon to confirm. The icon changes to a check mark and the next button
flashes.
Set the %O
as prescribed. Touch the associated flashing button to confirm. The
2
icon changes to a check mark and the next button flashes.
Adjust the Pres High flow meter as desired for delivery of BiPhasic, BiPhasic tr or
manual breaths using counterclockwise turns to increase and clockwise turns to
decrease the flow. Touch the associated flashing button to confirm. The icon
changes to a check mark and the next button flashes.
675–101–101 Rev. K
Page 38

28 Chapter 5 - Operation
If breath rate monitoring is desired, attach the Transducer Interface and the
abdominal sensor. Refer to Chapter 4 for instructions on application of the
Abdominal Respiratory Sensor. Touch the flashing button to confirm.
If an alarm is activated as a result of any of the settings, the button displays a
flashing “X”. Alarm conditions must be cleared and all settings must be confirmed
with a green check mark before other screens can be entered.
2. Alarm set/confirm Screen
Figure 9 – Alarm set/confirm Screen
Touch the NCPAP button or the Alarm Mute / Reset button for 3 seconds to set the
alarms and to move to the next screen. If either button is not touched within 2
minutes, the alarm limits will be set automatically. When the alarm limits have been
set, the screen display changes to the Mode Select Screen with the driver operating
in NCPAP mode.
Note:
Press High Flow meter must be checked through the Start-up screen and be set
during operation. This enables manual breath or back-up apnea breath, where
applicable, to be active.
675–101–101 Rev. K
Page 39

Infant Flow® SiPAP™ 29
Figure 10 – Mode Select Screen
3. Mode Select Screen - Here the operator can select the desired mode of
operation. Once selected, the operator has the ability to adjust the screen
controls for the mode selected. Only the relevant controls available for the
selected mode are visible.
To make a change to a control, touch the control. Both the control and its
associated numeric display highlight and the adjust buttons appear. Press up
or down buttons to adjust the setting as desired. Confirm the change by
pressing the control again.
4. Parameter Adjust Screen – During normal operation, active controls for the
current operating mode can be adjusted by touching the control, using the
increase or decrease arrows to make the adjustments and then pressing the
control again to confirm the change.
Figure 11 – Parameter Adjust Screens
5. Main screen– The Main Screen provides the operator with displays of current
mode of operation, alarm status, battery charge status, monitored parameters
and a pressure time graphic display. Active controls are available for
adjustment in this screen.
675–101–101 Rev. K
Page 40

30 Chapter 5 - Operation
Figure 12 – Main Screen
6. Monitored Parameter Screen – This screen is accessed by pressing the
change screen button. The monitored parameters screen displays measured
values and control settings. Adjustments to controls active for the currently
selected mode are possible from this screen. To return to the main screen
press the screens button again.
Figure 13 – Monitored Parameters Screen
675–101–101 Rev. K
Page 41

Infant Flow® SiPAP™ 31
Step by Step Fixation for Infant Flow System Generators
Following this fixation technique closely helps to ensure:
• Enhanced stability of the Generator
• Minimal disturbance to the infant
WARNING
Do not attach Generator to the patient until User Verification and initial set up
into NCPAP mode is complete.
Patient Interfaces from CareFusion accommodate a wide range of patients.
Application of an incorrectly sized prong, mask or bonnet will affect stability of the
generator. The clinician may consider alternating the use of prong and mask
interfaces at set intervals for a single patient in order to change pressure points on
the infant’s face and reduce the risk of skin breakdown.
1. Measure for prong/mask size using the nose guide. Connect the interface to
the generator.
2. Measure for bonnet size from the middle of the forehead to the nape of the
neck and then back to the middle forehead. DO NOT use a “head
circumference” measurement to determine bonnet size.
3. Loosely weave Generator straps through the buttonholes. Begin from the
inside of the colour coded buttonhole. Place the Generator on top of the
bonnet above the central Velcro strip.
4. Place the bonnet onto the infant’s head, checking that the ears are in a normal
position. Ensure the bonnet is pulled well down over the ears and down to the
nape of the neck.
Switch on the power to the driver and complete Set Up Screen steps to enter
NCPAP mode with the prescribed settings for the current patient.
5. Lift the Generator from the top of the bonnet and bring towards the nose.
Gently insert the nasal prongs/mask into position while supporting the
Generator. Secure the generator straps horizontally across the infant’s cheeks.
Do not over tighten.
6. Secure all three tubes from the Generator with the central Velcro strip. Split the
inspiratory and pressure lines and secure with secondary Velcro strips. Tie the
open end of the bonnet if desired.
7. Final check:
• Nose in neutral position; eyes visible; ears not folded
• Desired upper and lower pressure levels and FiO
• Infant settles quickly after fixation
675–101–101 Rev. K
are delivered
2
Page 42

32 Chapter 5 - Operation
Every hour
Repeat checks listed above in Final Check.
Every 3-4 hours
Loosen the generator straps and release the tubes from the central Velcro strip. The
nasal area can be cleaned with warm sterile water. Do not apply creams or
ointments.
Ensure that:
• Nasal prongs/mask is not occluded with mucus/water droplets
• Patient prongs/mask and bonnet continue to fit appropriately.
• Re-apply the generator as described above.
Directions for using the AirLife™ Infant nCPAP System
Please refer to P/N 36-5569 included in the AirLifeTM Infant nCPAP Fixation Device
for the Directions for Use.
WARNING!
CareFusion consumables are specifically designed to be used with Infant
Flow® Drivers and are the only consumables validated for use with Infant Flow®
devices.
675–101–101 Rev. K
Page 43

Infant Flow® SiPAP™ 33
Chapter 6 - Operating Modes
NCPAP
The Nasal CPAP mode can be enabled to have breath rate monitoring displayed
(NCPAP +Apnea Mode, or NCAP +LBR Mode), or the system can operate without
having the breath rate monitoring displayed (NCPAP Mode). Breath rate monitoring
requires the use of the Transducer Assembly (part number 677-002) and the
Abdominal Respiratory Sensor (part number 467349).
Figure 14 – NCPAP
BiPhasic
Allows for time triggered pressure assists, with or without breath rate monitoring and
adjustable low breath rate alarm, delivered based on clinician set Time High (T-High)
criteria, rate and pressure settings.
Figure 15 – BiPhasic
675–101–101 Rev. K
Page 44

34 Chapter 6 - Operating Modes
BiPhasic tr*
Allows for patient triggered pressure assists with breath rate monitoring enabled,
adjustable apnea time interval, apnea alarm and adjustable apnea back up rate. The
upper level pressure is delivered based on operator set Time High (T-High) and
pressure settings.
Figure 16 – BiPhasic tr
* This mode available in Comprehensive configurations only.
675–101–101 Rev. K
Page 45

Infant Flow® SiPAP™ 35
Chapter 7 - Alarms and Indicators
Audible and visual indications are given to alert the Operator to specified conditions
that affect operation. The electronic alarm limits are automatically set after two
minutes without the necessity of Operator inputs.
Alarms can be manually set at any time if required (i.e. after settings change or
patient disconnect/ reconnect) by pressing the Alarm Mute / Reset button for 3
seconds.
Alarm Priority
On activation of a MEDIUM or HIGH priority alarm, a locked screen will automatically
revert to the unlocked display. The alarm status indicator flashes intermittently,
based on the current, highest alarm priority. Distinct audible alarms represent a
HIGH, MEDIUM or LOW priority alarm.
Measured parameters and alarm limits associated with a high or medium priority
alarm condition flash RED (HIGH priority) or YELLOW (MEDIUM priority) to denote
alarm condition and priority.
Silencing audible alarms
Pressing the Alarm Mute / Reset button will silence active alarms for up to 30
seconds. If a new high priority alarm condition occurs during the alarm silence time
period, the silence will be cancelled to alert the operator of the new alarm condition.
Resetting alarms
Press the Alarm Mute / Reset button for 3 seconds to clear resolved and LOW
Priority alarms and to reset alarm limits (i.e. after a control setting change). Where
the alarm cause remains, the appropriate alarm will immediately reoccur.
Audible alarm priority
High Priority – a series of 10 tones (2 groups of 3 tones followed by a pause and 2
more tones) every 10 seconds
Medium Priority – three audible tones every 15 seconds
Low Priority – two audible tones every 30 seconds
675–101–101 Rev. K
Page 46

36 Chapter 7 - Alarms and Indicators
Alarm Types
The following alarm systems are provided with the Infant Flow® SiPAP™. Electronic
alarms are set after 2 minutes of operation without operator intervention although the
operator can manually set or reset them if required. Refer to Appendix C for
information on troubleshooting alarms.
Supply Gases Failure
If the differential pressure between the two inlet gases falls outside of the limit of
30 PSI (2.0 bar) or one gas fails completely, an alarm will sound and the gas at the
higher pressure only will be delivered to the patient.
High Airway Pressure
A HIGH priority audible and visual high pressure alarm activates when pressure
reaches 3 cmH
Airway Over-Pressure Limit Alarm
A HIGH priority audible and visual high pressure alarm activates at 11 cmH2O during
NCAP and time triggered BiPhasic modes and 15 cmH
activation of this alarm, the patient circuit pressure drops to near zero. Pressure is
restored after 3 seconds, and reduces to near zero again should the condition
causing the alarm remain.
O above the measured airway pressure.
2
O in BiPhasic tr mode. Upon
2
Low Airway Pressure
A HIGH priority audible and visual low pressure alarm activates if pressures fall to
2 cmH
otherwise be less than zero.
O below the measured airway pressure or at 1.5 cmH2O, if this would
2
High and Low % O2
HIGH priority audible and visual alarms are provided at ± 5% of the measured FiO2
with an upper maximum limit of 104% and a lower minimum limit of 18%.
A low hazard warning occurs at 18% Oxygen or below.
Low Battery Charge
If the battery charge falls below 40% the battery charge indicator changes from
green to red as a warning indicator. In this instance, plug the driver into an approved
AC power source.
Low Battery Voltage
If the battery voltage falls to < 11.10 V for 5 seconds a MEDIUM priority audible and
visual alarm is activated. In this instance, plug the driver into an approved AC power
source.
675–101–101 Rev. K
Page 47

Infant Flow® SiPAP™ 37
Flat Battery
If the battery charge is too low to reliably power the analogue and valve driver
circuits, the unit enters a safe ‘flat battery’ screen, until it is either switched off, or
plugged into a suitable external power source. The screen display will go completely
blank when the battery charge is too low to power it. While sufficient power is
available, audible and visual indication of the high-priority alarm is maintained.
Figure 17 - Flat Battery screen
Operation without Electrical Power (No AC or DC power)
The Infant Flow® SiPAP™ will continue to deliver NCPAP flow only as set from the
NCPAP / Pres Low flow meter and the set %O
DC power. In this mode, visual indications and audible alarm warnings are not given
except for the supply gases failure alarm.
in the event of a total loss of AC and
2
Error code indication
When a unit error is active, and this does not cause complete device failure, then a
non-mutable HIGH priority alarm is activated with the error code displayed in flashing
RED text in the upper right hand of the display screen, alternating with any currently
displayed mode information. Refer to Appendix D for a listing of error codes. Remove
driver from service and refer to a qualified service technician.
675–101–101 Rev. K
Page 48

38 Chapter 7 - Alarms and Indicators
Alarm Symbols and Indicators
The following displays are shown within the graphical display. As needed displays in
this table are shown separately as Domestic US configuration displays (left-hand
column) and non-US configuration displays (right-hand column).
Table 4 – Alarm Symbols and Indicators
Indicator Meaning
Battery status/charge level; Green indicates full charge; red
indicates charge < 40%)
External power source not connected
Flat battery
Battery fault (battery unable to hold charge) or supply fault
Respiratory transducer interface has become detached during
breath monitoring
Indication during pre-use checks that the NCPAP / Pres Low
flow meter should be set as desired and pressure verified
Indication during pre-use checks that the Pres High flow meter
should be set as desired and pressure verified
Indication during pre-use checks and/or calibration that % O2
should be set and verified.
675–101–101 Rev. K
Page 49

Infant Flow® SiPAP™ 39
Indicator Meaning
Indication during pre-use checks that operator should attach
the respiratory sensor (cross indicates that transducer
assembly is not connected)
Indication during pre-use checks that the operator should
attach the respiratory sensor (indicates that transducer
assembly is connected)
Does not verify attachment of sensor to patient
Refer to manual
Power has failed; re-connect external power source
Display of % O2 measured value and associated alarms
Display of NCPAP airway pressure measured value and
alarms (NCPAP modes only)
Display of mean airway pressure measured value and alarms
(BiPhasic and BiPhasic tr* modes only)
I:E ratio
Spontaneous breathing rate
Set parameter T-High
Set parameter Rate (BiPhasic modes only; mandatory rate)
Set parameter RB (BiPhasic tr* mode only; backup rate)
Set parameter low breath rate or apnea alarm timeout
675–101–101 Rev. K
Page 50

40 Chapter 7 - Alarms and Indicators
Indicator Meaning
Display of measured airway pressure graph. With breath
monitoring active, spontaneous breaths are indicated in yellow,
below delivered airway pressure graph.
Device fault (fault code will be indicated). Refer to manual.
Contact qualified service technician.
Note
Provision of labeling in this manual for any function should not be taken as evidence
that the function is available. For example parameter RB relates to BiPhasic tr*
mode, not currently approved for use in the US.
675–101–101 Rev. K
Page 51

Infant Flow® SiPAP™ 41
Chapter 8 - Maintenance & Cleaning
Cleaning
Examine the exterior of the case and the stand for damage and dirt. If necessary
clean the unit and stand. If damage to either is apparent, always seek qualified
Technical advice.
Clean the exterior surfaces of the driver, Transducer Assembly and stand with a mild
soap or liquid disinfectant solution. Do not use cleaning agents that contain
abrasives. Make sure that cleaning agents do not enter the driver through patient
connections or other ports.
CAUTION
Do not immerse any part of the Infant Flow® SiPAP™ driver in water or sterilize it
with gas or steam.
Maintenance
No special maintenance is required by the operator other than that listed below.
There are no operator serviceable parts. The unit must only be maintained and
serviced by an approved service supplier or trained biomedical engineer. Only parts
approved by CareFusion may be used in this unit. Refer to the Service Manual or
your Service Supplier for an approved service parts list
WARNING
Oxygen vigorously accelerates combustion. To avoid explosion hazard, do not
use any instrument or other equipment that may have been exposed to oil or
grease contamination.
Calibrate the oxygen analyzer regularly. Calibration of the oxygen analyzer must
be done with the unit off patient.
Regularly check and empty the water trap accessed from the rear panel of the driver
enclosure. Push the button on the bottom of the water trap to release the water into a
suitable waste receptacle.
Disconnect the air and oxygen gas sources when the Infant Flow
use.
®
SiPAP™ is not in
675–101–101 Rev. K
Page 52

42 Chapter 8 - Maintenance & Cleaning
Storage and Battery Care
Store the unit in a clean dry location. Make sure that all connections and ports are
suitably covered to prevent the ingress of dirt, moisture and foreign objects. If the
unit is not being used for a long period of time, remove the battery (refer to the
Service Manual or your Service Technician).
Dispose of scrap units in accordance with the local regulations. Refer to the Service
Manual or your Service Supplier.
675–101–101 Rev. K
Page 53

Infant Flow® SiPAP™ 43
IEC 60878
IEC 30878
IEC 60878
Chapter 9 – Explanation of Symbols
Equipment Symbols
The following symbols may be referenced on the Infant Flow® SiPAP™ driver or in
accompanying documentation.
Table 5 – Equipment Symbols
Symbol
Symbol #5031 IEC
Source /
Compliance
Symbol #03-02
IEC 60878
Symbol #5016 IEC
60417
60417
Symbol #5019 IEC
60417
Symbol #01-20
Meaning
Indicates ATTENTION, consult ACCOMPANYING
DOCUMENTS
This symbol indicates a FUSE.
This symbol indicates DIRECT CURRENT (DC)
This symbol indicates protective EARTH (ground).
Symbol #5021 IEC
60417
Symbol # 01-24
IEC 60878
Symbol # 5333
IEC 60417
Symbol #02-03
IEC 60878
Symbol #5032 IEC
60417
Symbol #01-14
Symbol #5007
IEC 60417
Symbol #01-01
IEC 60878
Symbol #5008
IEC 60417
Symbol #01-02
MDD Directive
93/42/EEC
This symbol indicates the EQUIPOTENTIAL connection used
to connect various parts of the equipment or of a system to
the same potential, not necessarily being the earth (ground)
potential (e.g., for local bonding).
This symbol indicates TYPE B equipment, which indicates
equipment that provides a particular degree of protection
against electric shock, particularly with regards to allowable
leakage current and reliability of the protective earth
connection.
This symbol is located on the rating plate. It indicates the
equipment is suitable for alternating current.
Indicates ON (Power)
Indicates OFF (Power)
CE Mark
CareFusion
Symbol
675–101–101 Rev. K
This symbol indicates an INTERNAL BATTERY FUSE
Page 54

44 Chapter 9 – Explanation of Symbols
ISO 15223:2000
Symbol
2.5A/T 250 V
101010
XDCR
Source /
Compliance
ISO 7000:2004
(2616)
ISO 15223:2000
(3.11)
EN 980:2003
(5.7.3)
N/A
CareFusion
Symbol
IEC 60878:1988
(01-41)
ISO 7000:2004
(2301)
IEC 60878:1988
(02-03)
Meaning
Electrical AC inlet
Operating temperature range of unit
Fuse holder and fuse rating
Transducer Assembly
Warning Bell
Type BF patient applied part
ETL Mark and Registration Number
Intertek Group
ISO 15223: 2000
(3.13)
EN 980:2003 (4.4)
IEC 60878 (03-02)
ISO 15223:2000
(3.1.4)
EN 980:2003 (4.4)
(3.12)
EN 980:2003 (4.3)
ISO 15223:2000
(3.2)
EN 980:2003 (4.2)
Year of Manufacture
Read Accompanying Documents
Unique Batch Number Identifier
Use Before Expiry Date shown
Year-Month
Single Use Only - Do NOT Re-use
675–101–101 Rev. K
Page 55

Infant Flow® SiPAP™ 45
Symbol
Source /
Compliance
ISO 15223:2000
(3.8)
ISO 15223:2000
(3.8)
Meaning
Keep Dry
Keep Away from Heat
Symbols used on buttons
The following symbols are used to label user input areas within the graphical display.
As needed displays in this table are shown separately as Domestic US configuration
displays (left-hand column) and non-US configuration displays (right-hand column).
Table 6 – Button Symbols
Symbol Description
High Priority Alarm Active, red flashing
Medium Priority Alarm Active, yellow flashing
Low Priority Alarm Active, yellow, does not flash.
No alarms are present, green, does not flash
Active alarm silenced
Adjust BiPhasic rate
Adjust BiPhasic tr* backup rate
Adjust apnea alarm timeout
Adjust low breath rate alarm timeout
Adjust BiPhasic, BiPhasic tr* on time, and NCPAP
manual breath function
675–101–101 Rev. K
Page 56

46 Chapter 9 – Explanation of Symbols
Symbol Description
Decrease / Increase currently selected parameter
Go to mode select screen.
Nasal CPAP mode
Nasal CPAP mode with breath rate monitoring
BiPhasic mode
BiPhasic mode with breath rate monitoring
BiPhasic tr* mode with breath rate monitoring
Manual Breath. Single BiPhasic cycle at current
. One BiPhasic
2
settings for T-High, Pres High and % O
cycle is delivered regardless of button press duration
Toggle between Main Screen and Monitored
Parameter Screen
Go to user calibration screen
Confirm
Wait
Completed
Action has failed
Press to un-lock keypad
Warning message. To clear, press any of the three
icons.
Oxygen monitor and alarms disable
675–101–101 Rev. K
Page 57

Infant Flow® SiPAP™ 47
Note
Provision of labeling in this manual for any function should not be taken as evidence
that the function is available. For example parameter R
not currently approved for use in the US.
relates to BiPhasic tr* mode,
B
675–101–101 Rev. K
Page 58

48 Chapter 9 – Explanation of Symbols
Infant Flow®
SiPAP™
675–101–101 Rev. K
Page 59

Infant Flow® SiPAP™ 49
3
High
1
15%
1
Appendix A - Product Configurations
Non-US Configuration Parameters
Table 7 – Non-US Configuration Parameters
Parameter Min Max Accuracy Units Default
Set Oxygen concentration, %O2 21 100
NCPAP / Pres Low flow rate 0 15
Pres High flow rate 0 5
BiPhasic / BiPhasic tr* on time, T-
0.1 3.0
±
±15%
± 15%
± 0.005
% N/A
L/min N/A
L/min N/A
seconds .3 sec
BiPhasic rate, R (mandatory rate) 1 120
BiPhasic tr* backup rate, Rb (apnea
backup rate)
Apnea timeout, Tapnea 10 30
1 120
± 0.5
± 0.5
±
bpm 30 bpm
bpm 10 bpm
seconds 20 sec
Note
BiPhasic tr mode not currently available in the United States. In non-US
configurations, T-High automatically reduces at higher R and Rb rate settings to
maintain a minimum off time of 100 milliseconds
US Configuration Parameters
Table 8 – US Configuration Parameters
Parameter Min Max Accuracy Units Default
Set Oxygen concentration, %O2 21 100
NCPAP / Pres Low flow rate 0 15
Pres High flow rate 0 5
BiPhasic on time, T-High 0.1 3.0 *
BiPhasic rate, R (Mandatory rate) 1 54
Low Breath Rate timeout, T
10 30
LBR
.
±3
±
± 15%
± 0.005
± 0.5
±
% N/A
L/min N/A
L/min N/A
seconds 1.0 sec
bpm 10 bpm
Seconds 20 sec
Note
In US configurations, T-High automatically reduces at higher R and Rb rate settings
to maintain a minimum off time of 1.0 seconds.
675–101–101 Rev. K
Page 60

50 Appendix A - Product Configurations
Infant Flow®
SiPAP™
675–101–101 Rev. K
Page 61

Infant Flow® SiPAP™ 51
Appendix B - Pneumatic Diagram
675–101–101 Rev. K
Page 62

52 Appendix B - Pneumatic Diagram
Infant Flow®
SiPAP™
675–101–101 Rev. K
Page 63

Infant Flow® SiPAP™ 53
Appendix C - Alarm Troubleshooting
Table 9 – Alarm Troubleshooting
Alarm
%O2 < 18% High
%O2 > 104% High
High %O2
(> 5% above
setting for 15
seconds).
Low %O2
(> 5 % below
setting for 15
seconds).
Over
pressure
(> 11 cmH
in NCPAP
and BiPhasic
modes)
Over
pressure
(> 15 cmH
in BiPhasic
tr* mode)
Low battery
charge
(< 40%).
Priority Possible Cause
• O
2
required.
• O
2
High
required.
• Blender setting
changed.
• Supply gas failure
• Water trap overflow
High
• Blender setting
changed.
• Supply gas failure
• Water trap overflow
High
O
2
• Flow rate set too
high.
• Occlusion of
exhalation limb
• Blocked
silencer/bacteria
filter
High
O
2
• Flow rate set too
high.
• Occlusion of
exhalation limb
• Blocked
silencer/bacteria
filter
Warning
indication.
• Battery status
indicator changes
from green to red.
calibration
calibration
Actions
• Restore FiO2 level to above the
minimum limit
• Press Alarm Reset for 3 seconds.
• Recalibrate O
as soon as
2
practicable.
• Restore FiO2 level to below the
maximum limit
• Press Alarm Reset for 3 seconds.
• Recalibrate O
as soon as
2
practicable.
• Press Alarm Mute to silence the
alarm
• Correct delivered oxygen
concentration
• Press Alarm Reset for 3 seconds to
set new limits
• Press Alarm Mute to silence the
alarm
• Correct delivered oxygen
concentration
• Press Alarm Reset for 3 seconds to
set new limits
• Check exhaust tube / filter
• Reduce flow rate to achieve
pressure below high pressure limit
• Press Alarm reset for 3 seconds to
set new limits
• Check exhaust tube / filter
• Reduce flow rate to achieve
pressure below high pressure limit
• Press Alarm reset for 3 seconds to
set new limits
• Connect external power
675–101–101 Rev. K
Page 64

54 Appendix C - Alarm Troubleshooting
Alarm
Priority Possible Cause
Battery fault High (Cannot be
reset)
Low battery
Medium
voltage
< 11.10 V for
5 seconds).
AC power
High
disconnected
High
High
NCPAP /
Pres Low
(CPAP > 3
O
cmH
2
above set
for 15
seconds).
Low NCPAP
High
/ Pres Low
( CPAP < 2
O
cmH
2
below set
for 15
seconds)
or
< 1.5 cmH
O
2
at any time).
High
High
BiPhasic or
BiPhasic tr*
pressure
(MAP > 3
O
cmH
2
above
set for 15
seconds).
BiPhasic or
BiPhasic tr*)
High (Cannot be
silenced)
mode fails to
operate as
set.
• Battery
disconnected
• Battery failing to
hold charge
• Battery
disconnected
• Battery failing to
hold charge
• AC power
disconnected
• NCPAP / Pres Low
setting change
• Circuit disconnect /
reconnect
• NCPAP / Pres Low
setting change
• Circuit disconnect /
reconnect
• Circuit leak
• Pres High setting
change
• Circuit disconnect /
reconnect
• See description of
error code
displayed
Actions
• Push Alarm Mute button for 3
seconds to silence alarm
• Refer to Service Engineer.
• Push Alarm Mute button for 3
seconds to silence alarm
• Connect AC power
• Push Alarm Mute button for 3
seconds to silence alarm
• Reconnect the AC power.
• Push Alarm Mute button for 3
seconds to silence and reset alarm
limits
• Reset alarm limits after setting
change and patient circuit
disconnect / reconnect
• Push Alarm Mute button for 3
seconds to silence and reset alarm
limits
• Reset alarm limits after setting
change and patient circuit
disconnect / reconnect
• Check for leaks in patient circuit
• Push Alarm Mute button for 3
seconds to silence and reset alarm
limits
• Reset alarm limits after setting
change and patient circuit
disconnect / reconnect
• Revert to nCPAP mode
• Refer to Service Technician
675–101–101 Rev. K
Page 65

Infant Flow® SiPAP™ 55
rate
fails completely
power
Alarm
Low breath
rate
Flow meter
fault.
Gas Supply
failure
Oxygen cell
calibration
error.
Electrical
fault.
Water trap
blocked
Software
fault
Software not
running with
unit
connected to
Priority Possible Cause
High
• Plus: audible /
visual alarms
only
• Comprehensive
: audible /
visual alarms
and backup
N/A
N/A
High
(Cannot be reset)
High
(Cannot be reset)
• Rr = 0 for > low
breath (apnea)
interval timeout
• No flow indications
• Flow can’t be
adjusted.
• Differential
pressure between
the two inlet gases
falls outside of the
limit of 30 PSI
(2.0 bar) or one gas
• Oxygen cell
incorrectly
calibrated,
damaged or
depleted
• AC power LED
does not match
screen icon.
• Full or leaking
• Filter blocked
• Loss of wall
pressure
• Imbalance in gas
supply
• See description of
error code
displayed
• See description of
error code
displayed
Actions
• Push Alarm Mute button once to
silence alarm
• Restore patient breathing.
• Check placement / connection of
abdominal Respiratory Sensor
• Refer to Service Technician.
• Check gas inlet supplies
• Check inlet water trap
• Refer blender to service technician
• Calibrate or replace oxygen cell.
• Refer to Service Manual.
• Refer to Service Technician
• Refer to Service Technician
• Refer to Service Technician
• Refer to Service Technician
*BiPhasic tr mode not currently available in the United States
675–101–101 Rev. K
Page 66

56 Appendix C - Alarm Troubleshooting
Infant Flow®
S iPAP™
675–101–101 Rev. K
Page 67

Infant Flow® SiPAP™ 57
and BiPhasic tr*)
worst error code
Appendix D - Fault Management
The general philosophy when handling a software detectable fault condition is to still
allow a basic level of treatment to be applied to the patient - with over pressure
protection, oxygen alarms and apnea monitoring (where possible), but inhibiting the
higher level features of the unit (such as BiPhasic modes).
Fault classification
Each fault condition is classified according to the severity ratings (where means
not available under software control,
(
) means may or may not be available depending on other severity rating 3 and 4
conditions, which may occur individually or simultaneously):
Table 10 – Fault Classification
means available under software control, and
Measurements
Control Modes and
Features
Severity
1
(major)
2
3
Classification
Un-usable
Restricted
Untriggerable
Impact On Unit
Functionality
Unit is
inoperable under
software control,
but can still be
used in an unpowered
pneumatic mode
Unit functionality
is restricted to
NCPAP modes
only
Patient trigger
functions not
available
(NCPAP and
BiPhasic with
breath
monitoruing on
Reporting
Mechanism
A list of error
codes are
presented to the
user via the
"Fault lockout"
display
Where
applicable, error
codes listed on
mode selection
screen. Status
bar mode
alternates with
worst error code
Where
applicable, error
codes listed on
mode selection
screen. Status
bar mode
alternates with
O
O2 %
2
CmH
NCPAP
+apnea
Biphasic
tr
biphasic
Unit will not
4a
(minor)
No
backup
operate on
battery when the
external power is
removed
Battery status
appears as if flat
battery
() () ()
675–101–101 Rev. K
Page 68

58 Appendix D - Fault Management
worst error code
error code.
Clas-
Software
Corrective
4b
(minor)
5
(minor)
6
(minor)
Severity
Classification
Spurious
Spurious
No
oxygen
monitor
Impact On Unit
Functionality
Spurious
software
exception
trapped
Non-fatal error
trapped
Oxygen monitor
and alarm
functions are not
available.
Reporting
Mechanism
Software restarts,
and status bar
extended mode
alternates with
High-priority
alarm; status bar
extended mode
alternates with
worst error code
High priority
alarm may be
cleared by
operator reset;
status bar
extended mode
alternates with
Measurements
O2 %
Control Modes and
Features
O
2
CmH
NCPAP
() () ()
+apnea
Biphasic
tr
biphasic
Fault recovery / action
If a detectable fault condition occurs (either before treatment begins or while being
applied) the software will respond in the following way:
Table 11 – Fault Recovery
E## Fault condition Consequence
Program memory
checksum error
Battery too flat
(<6.5V) to
operate LCD,
-
analogue and
valve driver
circuits (no
external power)
Battery too flat
(<10.25V) to
operate analogue
and valve driver
circuits but
sufficient for LCD
driver (no
external power)
Software corrupt execution
inhibited
No user interface
display
Sensor readings
invalid
sification
Unusable
Unusable
Unusable
Response
Hardware held in
permanent reset
condition with
alarm bar lit
(status LED on)
Hardware held in
reset condition
with alarm bar lit
(status LED off)
until external
power applied
User lockout:
"Plug in external
power" prompt
Action Required
Service: Reload
software
User: Plug in
external power
User: Plug in
external power
675–101–101 Rev. K
Page 69

Infant Flow® SiPAP™ 59
calibration data
and Flow)
flat)
"E##" alarm
circuits
rail)
prompt
sense)
E## Fault condition Consequence
Unable to
Non-volatile
E10
memory fault
Calibration data
E11
lost
retrieve/set unit
configuration and
Sensor readings
invalid
Configuration DIP
settings and/or
PT PRESENT
E12
different to nonvolatile
Possible
incomplete unit
set-up performed
configuration
record
Charged battery
voltage too low
E20
(<11V) when
Battery capacity
low
under test load
External supply
voltage too low
E21
(<14V) to charge
battery (battery
Battery will not
charge
Clas-
sification
Unusable
Unusable
Unusable
No backup
No backup
Software
Response
User lockout:
Error "E##"
prompt
User lockout:
Error "E##"
prompt
User lockout:
Error "E##"
prompt
Battery fault icon
flashes and "E##"
alarm
Battery low alarm
continues even
through external
power applied -
Corrective
Action Required
Service: Fix or
replace PCB
Service: Low
level calibration
, Pressure
(O
2
Service: Perform
set-up procedure
Service: Fix
Battery or
charger
User: Plug in
correct external
supply
Service: Fix PSU
Analogue supply
E22
rails out of limits
Valve driver
E23
supply rails out of
limits
Hardware 'safe-
E24
start' watchdog
disabled
Pressure sensor
E30
fault (ADC hits
Zero valve not
E31
connected (via
sense)
Zero valve
E32
activation fault
(via sense)
Unable to auto-
E33
zero pressure
sensor
Dump valve not
E41
connected (via
Dump valve
E42
activation fault
(via sense)
Unreliable sensor
readings
Valve operations
unreliable
Unusable
Unusable
Valves disabled Unusable
Pressure sensor
readings invalid
Unusable
Pressure sensor
readings
Unusable
unreliable
Pressure sensor
readings
Unusable
unreliable
Pressure sensor
readings
Unusable
unreliable
No over pressure
protection
No over pressure
protection
Restricted
Restricted
User lockout:
Error "E##"
prompt
User lockout:
Error "E##"
prompt
User lockout:
Error "E##"
prompt
User lockout:
Error "E##"
User lockout:
Error "E##"
prompt
User lockout:
Error "E##"
prompt
User lockout:
Error "E##"
prompt
Restricted mode:
Error "E##" alarm
Restricted mode:
Error "E##" alarm
Service: Fix
circuits
Service: Fix valve
supply rail
Service: Fix
reset/safe-start
circuits
Service: Fix
sensor/circuits
Service: Fix
valve/circuits
Service: Fix
valve/circuits
Service: Fix
valve/sensor/circ
uits
Service: Fix
valve/circuits
Service: Fix
valve/circuits
675–101–101 Rev. K
Page 70

60 Appendix D - Fault Management
or high gain)
blender.
timeout)
blender.
report Apnea
E## Fault condition Consequence
Oxygen sensor
E50
fault (ADC hits
rail)
Oxygen sensor
can not be
E51
recalibrated by
user (bad offset
Oxygen sensor
calibrates but the
E52
fuel cell is wornout (low gain)
Oxygen sensor
readings invalid
Possible fuel cell,
electronic,
blender or gas
supply fault
Oxygen sensor
readings
unreliable
Oxygen sensor
Oxygen sensor
readings
unreliable
E53
too noisy to
calibrate
(calibration
Oxygen
calibration may
be invalid (O
E54
reading below
17% or above
2
Oxygen sensor
readings
unreliable
104% detected)
Oxygen sensor
E55
disabled by the
operator
BiPhasic valve
E61
not connected
(via sense)
BiPhasic valve
E62
activation fault
(via sense)
Oxygen sensor
readings
unreliable
BiPhasic modes
unusable
BiPhasic modes
unusable
Apnea and/or
PT transducer
disconnected
patient trigger
unusable/interrup
ted
PT module fault
E70
(PTRDY or CAN
bus failure)
No breath signal
from PT module
E71
although CAN
data does not
Apnea and
patient trigger
unusable
Patient may be in
apnea but PT
module
dysfunctional?
Clas-
sification
No oxygen
monitor
No oxygen
monitor
No oxygen
monitor
No oxygen
monitor
No oxygen
monitor
No oxygen
monitor
Restricted
Restricted
Un-
triggerable
Un-
triggerable
Untriggera
ble
Software
Response
High priority
alarm; Error
"E##" prompt
High priority
alarm; Error
"E##" prompt
High priority
alarm; Error
"E##" prompt
High priority
alarm; Error
"E##" prompt
High priority
alarm; Error
"E##" prompt
Error “E##”
prompt
Restricted mode:
Error "E##" alarm
Restricted mode:
Error "E##" alarm
NCPAP and
BiPhasic modes
with breath
monitoring on,
inhibited (or low
breath rate alarm
given if treatment
started)
Reduced
functionality "E##" alarm
Reduced
functionality "E##" alarm
Corrective
Action Required
Service: Fix
sensor/circuits.
User: Check gas
supplies.
Service: Fix
sensor/circuits/
User: Check gas
supplies.
Service: Replace
sensor.
User: Check gas
supplies.
Service: Fix
sensor/circuits/
User: recalibrate
the oxygen cell.
User: re-power
the device to reenable oxygen
monitoring
Service: Fix
valve/circuits
Service: Fix
valve/circuits
User: Reconnect
PT transducer
Service: Fix
PT/circuits
Service: Fix
PT/circuits
No trigger signal
E72
from PT module
BiPhasic tr* mode
inoperable.
Untriggera
ble
Reduced
functionality "E##" alarm
Service: Fix
PT/circuits
675–101–101 Rev. K
Page 71

Infant Flow® SiPAP™ 61
E## Fault condition Consequence
Spurious
software
interrupt, XTAL
(E9
fails, stack
0)
overflow/underflo
w, CPU Class B
exception
Abnormal
hardware,
E90
software or
watchdog reset
Internal software
E91
error detected
Unknown error
E99
detected
Software
interrupted and
restarts (possibly
during treatment)
Software restarts
possibly during
treatment
Software
unreliable
Software
unreliable
[1] Error codes in parentheses (brackets) are generated as an indirect consequence of the problem.
* Biphasic tr mode not currently available in the United States
Clas-
sification
Spurious
Spurious
Unusable
Unusable
Software
Response
Hardware
reinitialized
(disabled) with
alarm bar lit and
beeper sounding
to identify root
cause
Software restarts
- "E##" alarm
User lockout:
Error "E##"
prompt
User lockout:
Error "E##"
prompt
Corrective
Action Required
Software: Fix
persistent
exceptions
Software: Fix
persistent
exceptions
Service: Fix
abnormal reset
Software: Fix
software error
Software: Fix
software error
Fault code display screen
The fault lockout screen shall incorporate item ref. (as appropriate to build) and shall
display a list of all active fault codes. Faults not resulting in user lockout shall result
in indication on the status bar.
675–101–101 Rev. K
Page 72

62 Appendix D - Fault Management
Infant Flow®
SiPAP™
675–101–101 Rev. K
Page 73

Infant Flow® SiPAP™ 63
Term
Meaning
Apnea
Temporary inability to breathe.
mandatory)
CPAP
Continuous Positive Airway Pressure
mask
NCPAP+Apnea
NCPAP with Low Breath Rate monitoring (non-US labeling)
Rate
Mandatory rate (per minute); active in BiPhasic mode
RSP
Patient’s spontaneous respiratory rate (per minute)
s / sec
Seconds
US labeling
Labeling using English text in place of symbols and/or icons
PEEP
Positive End-Expiratory Pressure
PIP
Peak Inspiratory Pressure
Glossary
LBR Low Breath Rate
Bpm
Breaths per minute (applies to each of spontaneous, triggered and
Generator
BiPhasic
BiPhasic+LBR BiPhasic with Low Breath Rate monitoring (US labeling)
BiPhasic tr*
BiPhasic
tr+Apnea*
NCPAP Nasally applied CPAP
NCPAP+LBR NCPAP with Low Breath Rate monitoring (US labeling)
RB
T
/ T
apnea
T-High Length of time (in seconds) for a sigh or manual breath (Time High).
Non-US labeling
LBR
Patient attachment for delivering CPAP, used with nasal prongs or
Time triggered, time cycled pressure assists at two separate pressures
levels.
Patient triggered, time cycled pressure assists at two separate pressure
levels. *This mode currently not available in the United States.
BiPhasic tr* with Low Breath Rate monitoring (non-US labeling). *This
mode currently not available in the United States.
Backup ventilator rate (in BiPhasic mode during apnea alarm, per
minute; non-US labeling)
Apnea Interval (non-US labeling) or Low Breath Rate (LBR) monitor
alarm time (US-labeling); both in seconds
This mnemonic may also be associated with an alarm icon
Labeling using non-linguistic symbols in place of English text wherever
possible
Pres Low
Pres High
675–101–101 Rev. K
Adjustable lower baseline pressure level control in BiPhasic and
BiPhasic tr modes
Adjustable upper pressure level control in BiPhasic and BiPhasic tr
modes
Page 74

64
Infant Flow®
SiPAP™
675–101–101 Rev. K
Page 75

Infant Flow® SiPAP™ 65
Index
fixation, 31
A
Absominal Respiratory Sensor, 14
Accessories, 2
airway pressure, 15
alarm priority, 35
Alarm set/confirm Screen, 28
Alarm Symbols, 38
alarms, 4
Alarms, 35
Alarms Test, 19
assembly, 11
B
Battery Backup, 1
Battery Care, 42
BiPhasic, 1, 33
BiPhasic tr, 1, 34
button symbols, 45
Flat Battery alarm, 37
front panel, 23
Fully integrated alarm package, 1
G
gas flow, 15
H
High Airway Pressure alarm, 36
High and Low % O
High Patient Circuit Pressure alarm, 36
alarm, 36
2
I
incompatible control settings, 26
increase buttons, 26
Indicators and Controls, 23
intended use, ix
C
calibration
O2 sensor, 17
cautions, 7
Circuit Occlusion Alarm, 20
classification, x
Cleaning, 41
configuration
Comprehensive, 1
Plus, 1
Configurations, 49
control buttons, 25
controls, 3
D
decrease buttons, 26
E
electrical supply, 4
electromagnetic components, ix
EMC, ix
environmental requirements, 5
Error code indication alarm, 37
F
fault lockout screen, 61
faults, 57
features, 1
L
Leak Test, 18
Low Airway Pressure alarm, 36
Low Battery Charge alarm, 36
Low Battery Voltage alarm, 36
M
Main Screen, 29
Maintenance, 41
manual breath
setting, 26
Mode Confirm Screen, 29
modes, 3
Monitored Parameter Screen, 30
monitors, 3
MRI, ix
N
NCPAP, 1, 33
notes, 7
O
O2 sensor
calibration, 17
Operating Modes, 33
Operation without Electrical Power alarm, 37
675–101–101 Rev. K
Page 76

66 Index
P
Parameter Adjust Screen, 29
parameter default values, 26
Patented Infant Flow
patient circuit, 13
physical specifications, 5
Pneumatic Diagram, 51
pneumatic supply, 4
power-on check, 16
®
Generator, 1
R
radio frequency energy, ix
Rear Panel, 24
reset alarm limits, 35
S
safety information, 7
Screen Displays, 27
Screen Lock, 2
Set Up screen, 27
silence active alarms, 35
Silencer / Bacterial Filter, 5
Soft-key operation, 25
specifications, 3
Storage, 42
Supply Gases Failure alarm, 36
symbols, 43
T
Troubleshooting, 53
U
User Interface, 27
User Verification Test, 16
User Verification Test Checklist, 21
W
warnings, 7
warranty, vi
675–101–101 Rev. K
 Loading...
Loading...