Page 1
Infant Flow® LP SiPAP and SiPAP Model M675
Service manual
Page 2

ii Infant Flow
®
LP SiPAP Model M675
This document is protected by United States and International Copyright laws.
This document may not be copied, reproduced, translated, stored in a retrieval system, transmitted in any
form, or reduced to any electronic medium or machine-readable form, in whole or in part, without the
written permission of CareFusion. Information in this document is subject to change without notice.
This document is for informational purposes only and should not be considered as replacing or
supplementing the terms and conditions of the License Agreement.
© 2008 – 2011 CareFusion Corporation or one of its subsidiaries. All rights reserved Infant Flow is a
registered trademark of CareFusion Corporation or one of its subsidiaries. All other trademarks are
property of their respective owners.
USA
CareFusion
22745 Savi Ranch Parkway
Yorba Linda, California 92887-4668
Authorized European Representative
CareFusion Germany 234 GmbH
Leibnizstrasse 7
97204 Hoechberg, Germany
800.231.2466 tel
+1.714.283.2228 tel
+1.714.283.8493 fax
carefusion.com
Literature number: 675–120 Revision G
+49.931.4972.0 tel
+49.931.4972.423 fax
675–120 Rev. G
Page 3
Service Manual iii
September 2003
675-120(1)
Release
page 88.
Update list of service parts
Updated Table 6 starting on page 43
Revision History
Date Revision Changes
November 2004 B
December 2004 C
May 2005 D
Release manual in VIASYS Healthcare template using
VIASYS Healthcare Respir ator y Care nomenclature.
Revised per EO 27980. Removed the picture
from the title page.
Deleted the ESD warning from page 19.
Removed “O2 Sensor” and added the word “measured”
on page 30.
Changed “O2 sensor” to “fuel cell” on pages 39, 45, 62,
68, 69, 73, 86, and 87.
Replaced the O2 senor row on page 50.
Updated the error codes on pages 56 and 57.
Replaced the warning on page 61.
Changed the Fitting procedure on pages
64, 68, and 75.
Changed step 6 on page 65.
Added Addendum A – Oxygen Leak Test
Changed Transducer Assy. To Transducer Interface on
Revised per ECO 60329
Update address/contact info
Update battery remove/install procedure
Added note for fuel cell disposal
Update check valve assembly remove/install procedure
Update water trap & restrictor remove/install procedure
Delete redundant leak test
August 2008 E
675-120 Rev. G
Updated company information
Updated company information
Corrected Table 2 and updated Table 3
Updated Figure 6
Updated Figure 10
Updated the section “Diagnostics.”
Page 4
iv Infant Flow
FRONT”
Model M675
Date Revision Changes
April 2010 F Changed Ti to T-High throughout
Figure 5 - Diagnostic screen updated
Spelling correction from “GRD” to “GND”
Added “Note: transducer must be attached.”
Changed “10 V” to “11.10 V”
Updated Table 6 - Error Codes (E50 - E54) and added
E55
Removed “Blender (Check Valves and Filter) Annually”
from table 7.
Added “, and two screws on the case-bottom”.
Added “and document L2879”
Replaced Ti symbol with T-High symbol
Changed “PL5” to “PL2”
Replaced Figure 3
Replaced Figure 4 and 5. Changed “value doesn’t” to
“value on the external oxygen analyzer doesn’t”.
Changed “467352” to “68289”; changed “777245” to
“52700A”; removed items 677-005A, 675-311, S117635,
467461, 467460.
Removed item S117641; changed “675-310” to “M675A-
®
LP SiPAP Model M675
May 2011 G
Added instructions and specifications for LP SiPAP
675-120 Rev. G
Page 5

Service Manual v
Warranty
The Infant Flow® LP SiPAP and SiPAP systems are warranted to be free from
defects in material and workmanship and to meet the published specifications for
One (1) year from date of shipment.
The liability of CareFusion, (referred to as the Company) under this warranty is
limited to replacing, repairing or issuing credit, at the discretion of the Company, for
parts that become defective or fail to meet published specifications during the
warranty period; the Company will not be liable under this warranty unless (A) the
Company is promptly notified in writing by Buyer upon discovery of defects or failure
to meet published specifications; (B) the defective unit or part is returned to the
Company, transportation charges prepaid by Buyer; (C) the defective unit or part is
received by the Company for adjustment no later than four weeks followin g t he last
day of the warranty period; and (D) the Company’s examination of such unit or part
shall disclose, to its satisfaction, that such defects or failures have not been caused
by misuse, neglect, improper installation, unauthorized repair, alteration or accident.
Any authorization of the Company for repair or alteration by the Buyer must be in
writing to prevent voiding the warranty. In no event shall the Company be liable to
the Buyer for loss of profits, loss of use, consequential damage or damages of any
kind based upon a claim for breach of warranty, other than the purchase price of any
defective product covered hereunder.
The Company warranties as herein and above set forth shall not be enlarged,
diminished or affected by, and no obligation or liability shall arise or grow out of the
rendering of technical advice or service by the Company or its agents in connect ion
with the Buyer's order of the products furnished hereunder.
Limitation of Liabilities
This warranty does not cover normal maintenance such as cleaning, adjustment or
lubrication and updating of equipment parts. This warranty shall be void and shall not
apply if the equipment is used with accessories or parts not manufactured by the
Company or authorized for use in writing by the Company or if the equipment is not
maintained in accordance with the prescribed schedule of maintenance.
The warranty stated above shall extend for a period of One (1) year fr om date of
shipment, with the following exceptions:
1. Components for monitoring of physical variables such as temperature, pressure,
or flow are warranted for ninety (90) days from date of receipt.
2. Elastomeric components and other parts or components subject to deterioration,
over which the Company has no control, are warranted for sixty (60) days from
date of receipt.
3. Internal batteries are warranted for ninety (90) days from the date of receipt.
The foregoing is in lieu of any warranty, expressed or implied, including, without
limitation, any warranty of merchantability, except as to title, and can be amended
only in writing by a duly authorized representative of the Company.
675-120 Rev. G
Page 6

vi Infant Flow
®
LP SiPAP Model M675
Contents
Revision History ................................................................................ iii
Warranty ............................................................................................ v
Limitation of Liabilities ........................................................................ v
Notices ............................................................................................ viii
EMC Notice ............................................................................................................... viii
MRI Notice ................................................................................................................. viii
Intended Use Notice ................................................................................................... ix
Regulatory Notice ....................................................................................................... ix
Classification .............................................................................................................. ix
Declaration of Conformity Notice ................................................................................. x
Chapter 1 – Product Description ................................................................................ 1
Chapter 2 – Product Specifications ............................................................................ 3
Modes .......................................................................................................................... 3
Controls ....................................................................................................................... 3
Monitors ....................................................................................................................... 3
Alarms ......................................................................................................................... 4
Pneumatic Supply ........................................................................................................ 4
Electrical Supply .......................................................................................................... 4
Atmospheric & Environmental ...................................................................................... 5
Physical ....................................................................................................................... 5
Chapter 3 – Warnings and Cautions .......................................................................... 7
Terms .......................................................................................................................... 7
Warnings ..................................................................................................................... 7
Cautions ...................................................................................................................... 9
Chapter 4 – System Construction ............................................................................ 11
Touch Screen ............................................................................................................ 15
Alarm Conditions ....................................................................................................... 17
Diagnostic Screen ...................................................................................................... 17
Chapter 5 – Theory of Operation .............................................................................. 19
Gas Flow ................................................................................................................... 19
Electronic Functions .................................................................................................. 22
E
lectrical Layout ........................................................................................................ 23
Fault Management ..................................................................................................... 24
675-120 Rev. G
Page 7

Service Manual vii
Chapter 6 – Operational Setup ................................................................................ 25
Preparing and Connecting the Equipment ....................................... 25
Switching On the IFSD .................................................................... 29
User Verification Test ...................................................................... 30
Power-on Check ........................................................................................................ 30
Two Point O
Leak Test ................................................................................................................... 32
Alarms Test ................................................................................................................ 33
Infant Flow
Setting up the Equipment ........................................................................................... 36
Setting the NCPAP Parameters ................................................................................. 37
Setting the BiPhasic Parameters ................................................................................ 38
Setting the Triggered BiPhasic Parameters ................................................................ 39
Calibration .................................................................................................................. 41
Giving a Manual Timed Sigh ...................................................................................... 41
Operation Without Electrical Power ............................................................................ 41
Fault Indications ......................................................................................................... 42
Diagnostics ................................................................................................................ 48
Sensor Calibration ................................................................................ 31
2
®
LP SiPAP/SiPAP User Verification Test Checklist .................................. 35
Chapter 7 – Maintenance ......................................................................................... 55
Maintenance Frequencies ................................................................ 55
PM Procedure ............................................................................................................ 55
Tools Required........................................................................................................... 55
Cleaning .......................................................................................... 56
General ...................................................................................................................... 56
Removal and Fitting of Case ...................................................................................... 59
Removal and Fitting of Battery ................................................................................... 60
Removal and Fitting of Oxygen Filter ......................................................................... 61
Removal and Fitting of Fuel Cell Filter/Restrictor ....................................................... 62
Removal and Fitting of the Oxygen Sensor ................................................................ 63
Removal and Fitting of Blender and Components ...................................................... 64
Removal and Fitting of Water Trap Filter .................................................................... 68
Removal of the Pilot Drive Check Valves ................................................................... 68
R
emoval and Fitting of Case Bleed Filtered Restrictor ............................................... 69
Removal and Fitting of Valve/Sensor PCB ................................................................. 70
Removal and Fitting of the Muffler/Filter ..................................................................... 70
Chapter 8 – Explanation of Symbols ........................................................................ 71
Appendix A – Oxy g en Leak Test.............................................................................. 75
Appendix B – Produc t C on fig ur ati ons ...................................................................... 79
Non-US Configuration Parameters ............................................................................. 79
US Configuration Parameters .................................................................................... 79
Appendix C – Spare Parts ....................................................................................... 81
Appendix D – Pneumatics Assembly ....................................................................... 83
Glossary ................................................................................................................... 85
675-120 Rev. G
Page 8

viii Infant Flow
Notices
EMC Notice
This equipment radiates and is susceptible to radio frequency energy. If not installed
and used in accordance with the instructions in this manual, electromagnetic
interference may result. The equipment has been tested and found to comply with
the limits set forth in BS EN60601-1-2 for Medical Electrical Equipment Part 1-2:
General requirements for safety-collateral standard. Electromagnetic compatibility –
requirements and tests. These limits provide reasonable protection against
electromagnetic interference when operated in the intended use environments (e.g.
hospitals) described in this manual.
This device is also designed and manufactured to comply with the following
standards;
Safety: UL 60601-1: 2003 Medical Electrical Equipment, Part 1: General
Requirements for Safety.
CAN/CSA C22.2 No 601.1-M90, Medical Electrical Equipment - Part
1: General Requirements for Safety including C22.2 No. 601.1S1-94
(IEC601-1, Amendment 1:1991) Supplement No. 1-94 to CAN/CSA
22.2 No. 601.1-M90
®
LP SiPAP Model M675
With regards to Electrical Safety:
Class 1 equipment
Contains type BF patient applied parts
Continuous Operation
MRI Notice
This equipment contains electromagnetic components whose operation can be
affected by intense electromagnetic fields.
Do not operate this device in a MRI environment or in the vicinity of high-frequency
surgical diathermy equipment, defibrillators, or short-wave therapy equipment.
Electromagnetic interference could disrupt the operation of the device.
675-120 Rev. G
Page 9

Service Manual ix
Intended Use Not ice
The Infant Flow® LP SiPAP system, consisting of a Driver and Generator plus
NCPAP Prongs and Masks, is a medical device intended for the provision of Bi-Level
CPAP to produce a sigh. This system is for use in Hospital, Hospital Type facilities
and intra-Hospital transport environments and is indicated for the treatment of
Newborn and Infant patients.
Operators of this equipment and Se rvice En gineers are required to read and thoroughly
understand the contents of this manual before using or maintaining the equipment.
This manual is intended for use by a competent, fully qualified Service
Engineer. It includes a description of the unit and how it w or ks. It also contains
operating and diagnostic procedures and maintenance instructions. For usage
of associated equipment, refer to the Manufacturer’s literature.
Regulatory No t ice
Federal law restricts the sale of this device except by or on order of a physician.
Classification
Type of Equipment: Medical Equipment, Class 1 and internally powered, IPX1
Protected, and uses type BF applied parts. Equipment is not suitable for use in
presence of flammable anesthetics.
675-120 Rev. G
Page 10
x Infant Flow
Declaration of Conformity Notice
This medical equipment complies with the Medical Device Directive, 93/42/EEC, and
the following Technical Standards, to which Conformity is declared:
Council Directive(s): MDD 93/42/EEC Annex II (excluding section 4)
Safety: EN 60601-1, EN 794-1
EMC: EN 60601-1-2:2001
Conformity Assessment: MDD Annex II
Quality System: ISO 13485
EU Notified Body: BSI (Reg. No. 0086)
Device Classification: IIb
®
LP SiPAP Model M675
EU Notified Body:
BSI (Reg. No. 0086)
Trade names:
Infant Flow LP SiPAP
Manufactured by:
CareFusion
22745 Savi Ranch Parkway
Yorba Linda, CA 92887, USA
If you have a question regarding the Declaration of Conformity for this product,
please contact CareFusion.
675-120 Rev. G
Page 11

Service Manual 1
Chapter 1 – Product Description
The Infant Flow® LP SiPAP and SiPAP systems are non-invasive forms of respiratory
support designed for use in hospital environments such as Neonatal and Pediatric
Intensive Care Units. They can also be used when transporting patients within the
hospital environment.
The Infant Flow
Comprehensive configuration. The Plus configuration provides NCPAP and time
triggered, BiPhasic modes with and without breath-rate monitoring. The
Comprehensive configuration offers these features plus patient BiPhasic mode with
apnea backup breaths. The Infant Flow
standard in all configurations with an LCD touch screen display, pressure time
waveform graphics, integrated patient monitoring, alarms for high and low pressure
and FiO
and up to 2 hours of backup battery power.
2
As a result of the unique, patented design, the Infant Flow
systems have been proven to provide the most stable CPAP at the lowest work-ofbreathing for patients compared to other devices. The outstanding performance of
the Infant Flow
expiratory flows. This system has been designed and tested to perform optimally
when used only with accessories available from CareFusion. These accessories
include circuits and generators, prong and mask patient interfaces and bonnets.
®
LP SiPAP and SiPAP systems are currently available in the Plus or
®
LP SiPAP and SiPAP systems come
®
LP SiPAP and SiPAP
®
LP SiPAP and SiPAP systems is irrespective of patient demand or
Infant Flow® LP SiPAP and SiPAP Features
The expanded capabilities of the Infant Flow® LP SiPAP and SiPAP, Plus and
Comprehensive configurations, allow for applications to a broader range of patients
who may otherwise not be candidates for non-invasive respiratory support.
NCPAP. Allows for continuous positive airway pressure based on clinician set
pressure. Breath rate monitoring/alarm can be activated in this mode.
BiPhasic. Allows for time triggered pressure assists to be delivered based on
clinician set inspiratory time, rate, and pressure criteria. Breath rate monitoring/alarm
can be activated in this mode.
BiPhasic tr*. Allows for patient triggered pressure assists to be delivered based on
clinician set inspiratory time and pressure criteria. Breath rate monitoring/alarm, and
Apnea backup breaths are automatically active in the mode.
Patented Infant Flow
device for the generation of consistent infant nasal CPAP with a low work of
breathing. The Infant Flow LP system has a lower driving pressure than other
variable-flow devices.
Fully integrated alarms packages. Supply gases failure, High Patient Pressure,
Low patient pressure, high and low delivered Oxygen concentration, change from AC
to DC power source, low and flat battery charge status and Low breath rate/apnea
alarm.
®
LP Generator. The Infant Flow® LP Generator is a fluidic
Battery Backup. Up to 2 hours of battery backup allows for intra-hospital transport.
Clear indicators are provided for power supply in use (AC or DC), and battery charge
level.
675-120 Rev. G
Page 12
2 Infant Flow
NCPAP
•
•
NCPAP with breath rate monitoring
and alarm
• •
BiPhasic
•
•
and alarm
• •
BiPhasic tr*
•
Internal Battery
•
•
Manual Breath
•
•
Apnea Back up rate
•
Screen lock
•
•
Prioritization of alarms
•
•
LP. Refers to the low driving pressure of the Infant Flow LP Generator. The Infant
Flow LP SiPAP system is compatible only with the Infant Flow LP Generator. T he
prescribed nCPAP level will not be obtained if other variable flow generators are
used.
Screen Lock. After 120 seconds of no screen inputs, the screen changes to the
Locked Screen to prevent inadvertent changes. Upon activation of a high priority
alarm the screen changes to an unlocked state to allow for immediate interventions
as required.
Table 1 - Functions and Accessories
Functions & Accessories Plus Comprehensive*
®
LP SiPAP Model M675
BiPhasic with breath rate monitoring
*Comprehensive configuration not available for sale in the United States
CAUTION!
The Infant Flow® LP SiPAP and SiPAP systems have been designed and tested
as complete systems using Infant Flow® accessories. Only accessories approved
for use should be used. If in doubt, please contact your local CareFusion
representative.
WARNING!
The Infant Flow® LP SiPAP system is compatible only with the Infant Flow
LP Generator. The prescribed nCPAP level will not be obtained if other
variable flow generators are used.
675-120 Rev. G
Page 13

Service Manual 3
Chapter 2 – Product Specifications
Modes
• NCPAP
• NCPAP with breath rate monitoring and low rate alarm
• BiPhasic (t im e triggered)
• BiPhasic (t im e triggered) with breath rate monitoring and low rate alarm
• BiPhasic tr (patient triggered ) with breath rate monitoring, low breath rate
alarm, and apnea back up
Controls
• Inspiratory Time (T-High): 0.1-3.0 seconds
• Rate (R): 1-120 (Comprehensive only. The comprehensive configuration is
not available for sale in the United States.)
• Rate (R): 1-54 (Plus only)
• Apnea Inter val (T
• Apnea Inter val (T
• NCPAP/Pres Low flowmeter: 0-15LPM, accuracy +/- 15% of selected output
• NCPAP/Pres High flowmeter: 0-5LPM, accuracy +/- 15% of selected output
• Manual Breath: X 1
• Rate monitoring on/off: NCPAP
• %O
Monitors
• CPAP
• PEEP
• MAP
• PIP
• %O
• I:E r a ti o
apnea): 10-30 seconds, 5 second intervals (Comp* only)
LBR): 10-30 seconds, 5 second intervals (Plus only)
: 21 - 100% - accuracy +/-3%
2
2
• Spontaneous rate (Rs)
• Battery charg e level
675-120 Rev. G
Page 14

4 Infant Flow
Alarms
• High airway pressure – 3 cmH20 above measured airway pressure
• Hig h cir cuit pressure – maximum 11 cmH20 in time triggered Biphasic mode
• Hig h cir cuit pressure – maximum 15 cmH20 in patient triggered Biphasic tr
mode (Comprehensive only. The comprehensive configuration is not
available for sale in the United States.)
• Low airway pressure – 2 cmH20 below measured airway pressure or 1
cmH20 if otherwise would be zero
• Hig h and Low delivered Oxygen concentration +5% of setting
• Low breath r ate alarm
• Low or Flat battery charge level
• Alarm volume (electronic alarms) 70 dBa at 1 meter
Pneumatic Supply
• Patient Gas Outlet: 15 mm standard taper fitting
®
LP SiPAP Model M675
• Patient Pressure Input: 4.5 mm Luer taper fitting
• Gas Supply: Nominal 4 bar or 60 psi, clean, dry medical air and oxygen
• Range: 2.8 to 6 bar (40 to 90 psi); maximum differential pressure 2 bar
(30 psi)
• Manometer: Range 0 to + 20 cmH
• Gas Connecti ons: Standard DISS or NIST connectors
Electrical Supply
• Input Voltage:100-230 VAC
• Input Frequency: 50/60 Hz
• Power Consumption: 50 VA maximum
• Fuse Rating For 220 V nominal operation: “T” Type 2.5 A at 250 V
• Device Housing Prot ection rating level: IPX1
• Battery Worki ng Time: 2 hours (from fully charged state)
• Battery Charging Time: max. 16 hours
O, accuracy, ± 2% of span
2
675-120 Rev. G
Page 15

Service Manual 5
Atmospheric & Environmental
• Temperatur e Range-Operating: 5 – 40° C
• Storage: 0 - 50° C
• Relative Humidit y -Operating: 0 – 90% non-condensing
• Storage: 0 – 90% non-condensing
Physical
• Dimensions (driver only) -(W x H x D) 26 x38 x 23.5 cm /
10.25 x15 x 9.25 in
• Weight (driver only)-8.8 kg / 19.5 lb
675-120 Rev. G
Page 16

6 Infant Flow
®
Infant Flow
LP SiPAP an d SiPAP
Model M675
®
LP SiPAP Model M675
675-120 Rev. G
Page 17

Service Manual 7
Chapter 3 – Warnings and Cautions
Please review the following safety information before operating the Infant Flow® LP
SiPAP or SiPAP system. Attempting to operate this equipment without fully
understanding its features and functions may result in unsafe operating conditions.
Warnings and Cautions, which are general to the use of the device under all
circumstances, are included in this section. Some Warnings and Cautions are also
inserted within the manual where they are most meaningful.
Notes are also located throughout the manual to provide additional information
related to specific features.
If you have a question regarding the installation, set up, operation, or maintenance of
the device, contact technical support at CareFusion.
Terms
WARNINGS identify conditions or practices that could result in serious adverse
reactions or potential safety hazards.
CAUTIONS identify conditions or practices that could result in damage to the
ventilator or other equipment.
NOTES identify supplemental information to help you better understand how
Warnings
• Whenever a patient is attached to respiratory care equipment, constant
attendance is required by qualified personnel. The use of an alarm or
monitoring system does not give absolute assurance of warning for every
malfunction that may occur in the system. In addition, some problems may
require immediate attention.
• The gas blender incorporated in this product is designed to mix medical
grade air and oxygen only. Do not modify the inlets to accommodate other
source gases such as anesthetic gases.
• Check that the water trap is empty before use and empty it frequently during
use.
• Liquid water or other contaminants in either gas supply, particularly the air
supply, may cause malfunction of this equipment and equipment connected
to it.
• When f illing a humidifier, do not move the stand. Moving or transporting the
stand while refilling may cause the stand and equipment to over balance.
the ventilator works.
• The Infant Flow LP SiPAP system is compatible only with the Infant Flow LP
675-120 Rev. G
Generator. The prescribed nCPAP level will not be obtained if other variable
flow g ener ators are used.
Page 18

8 Infant Flow
• Do not use conductive patient circuits with the Infant Flow® LP SiPAP Driver.
• Nasal CPAP can cause nasal irritation, septal distortion, skin irritation and
pressure necrosis. Observe the usage guidelines to minimize these
complications.
®
LP SiPAP Model M675
• This device exhausts O
during normal operation. Oxygen vigorously
2
accelerates combustion. To avoid fire hazard, do not place flammable
materials or sources of heat close to the exhaust.
• Do not use the equipment without the exhaust tube fitted (refer to Figure 2).
• To reduce trip hazard, always ensure cable and tubes are restrained away
from walking areas.
• The Abdominal Respiratory Sensor will not detect all forms of apnea.
Independent monitoring should always be used with this device.
• If the unit is shelf mounted, ensure that the unit is stable and that hoses and
cables are restrained to avoid hazard of toppling.
• This equipment is not suitable for use in the presence of a flammable
anesthetic mixture.
• The NCPAP Pres High flowmeter must be adjusted to zero when not required
for the patient.
• Under extr eme conditions (minimum supply pressure and maximum gas
demand, including auxiliary output) output flow rates and delivered pressure
may be reduced.
• Only use the supplied AC cable to connect to the power supply.
• Do not attach the Generator to the patient until the initial set up is complete.
• The
indicates a connection between the transducer interface and the
unit. It does not indicate correct positioning of the Abdominal Respiratory
Sensor.
• Calibration m ust only be done when the unit is not connected to the patient.
• Verif y that the displayed value for delivered FiO
corresponds to the value set
2
on the blender. Refer to Faults and Indications.
• Oxygen vigor ously accelerates combustion. To avoid explosion hazard, do
not use any instrument or other equipment that may have been exposed to oil
or grease contamination.
675-120 Rev. G
Page 19

Service Manual 9
Cautions
• Federal Law (USA) restricts this device to sale by or on the order of a
physician.
• The precision gas blender in this product may become non-functional or
damaged if used without the protective water trap and filters provided.
• The power switch on this unit does not isolate the external power supply.
Disconnect the power supply cable to ensure complete isolation.
• Before use, verif y that this equipment has been authorized for use by a
qualified person.
• The Infant Flow
tested as complete systems using Infant Flow
accessories approved for use should be used. If in doubt, please contact your
local CareFusion representative.
• Where the integrit y of the external protective earth conductor is in doubt, the
equipment shall be powered by its internal power source (battery).
• Do not immerse any part of the IFSD in water or sterilize it with gas or steam.
®
LP SiPAP and SiPAP systems have been designed and
®
SiPAP accessories. Only
• Ensure patient breathing circuit is replaced at regular intervals.
675-120 Rev. G
Page 20

10 Infant Flow
®
Infant Flow
LP SiPAP and SiPAP
Model M675
®
LP SiPAP Model M675
675-120 Rev. G
Page 21
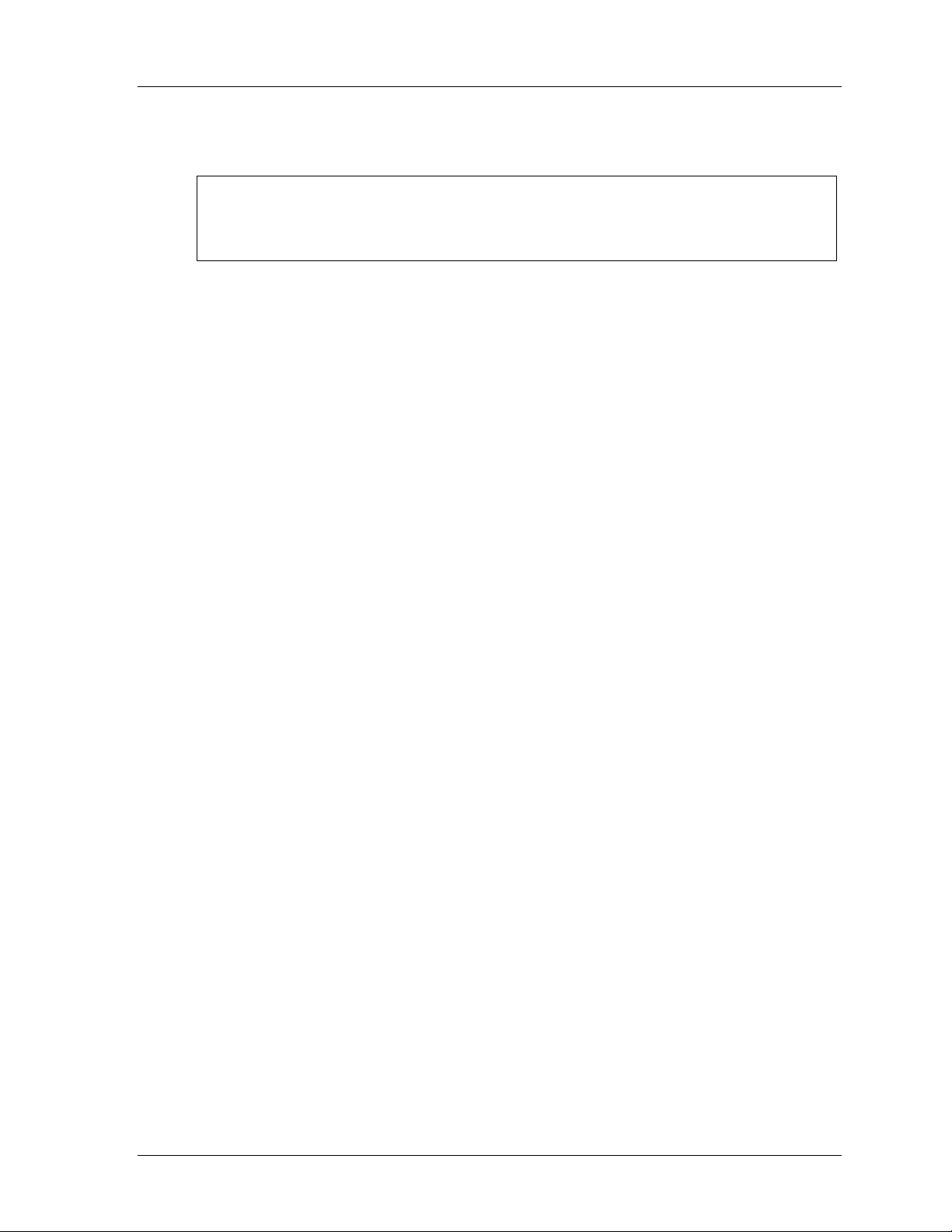
Service Manual 11
Chapter 4 – System Construction
CAUTION!
Where the integrity of the external protective earth conductor is in doubt the
equipment shall be powered by its internal power source (battery).
The IFSD is AC powered with an integral rechargeable DC battery that provides
power for up to two hours without any interruption of performance or function. If the
AC power supply fails or is disconnected, the IFSD automatically switches to battery
power and gives an audio and visual alarm.
The IFSD is enclosed in a case with Operator controls and input connectors on the
front and rear panel. The front panel is shown in Figure 1. The back panel is shown
in Figure 2. The case incorporates non slip feet for table top use or must be fitted to
a dedicated stand. The major components within the casing are:
• a gas module
• an electronics module
• a front panel module
• a patient trigger module
• a firmware module
675-120 Rev. G
Page 22

12 Infant Flow
®
LP SiPAP Model M675
Figure 1: IFSD Front Panel
Gas Module
The function of the gas module is to take air and oxygen, blend them into the
required mixture and deliver this mixture to the patient at the prescribed flow rat e.
The gas module also measures the oxygen concentration, measures the patient
pressure, provides an auxiliary gas outlet (optional) and provides switched Biphasic flow.
The main components are an air/oxygen blender, a flow manifold, a vent valve, an
exhaust manifold with alarm whistle, NCPAP Pres low and NCPAP Pres high flowmeters,
and a valve/sensor PCB. The inlet gas connections are on an interc han geable i nlet bl ock
to allow for different gas fittings. The exhaust manifold discharges gas to the outside of
the case and is positioned away from the electrical connectors and switch to reduce any
potential explosive hazard.
675-120 Rev. G
Page 23
Service Manual 13
Leak Test
Electronics Module
The function of the electronics module is to power the unit either by AC mains supply
or DC emergency battery supply, to control the gas module and read the gas module
sensors. The main components are a power supply unit, a rechargeable batt ery, a
main processor PCB, LED PCB, Valve/Sensor PCB and a LCD screen (touch
screen). The LCD screen includes a back-light which is always on when the IFSD is
powered. The touch screen displays information and receives inputs from the Operator
via the touch screen keyboard.
Figure 2: IFSD Back Panel
Port
675-120 Rev. G
Page 24
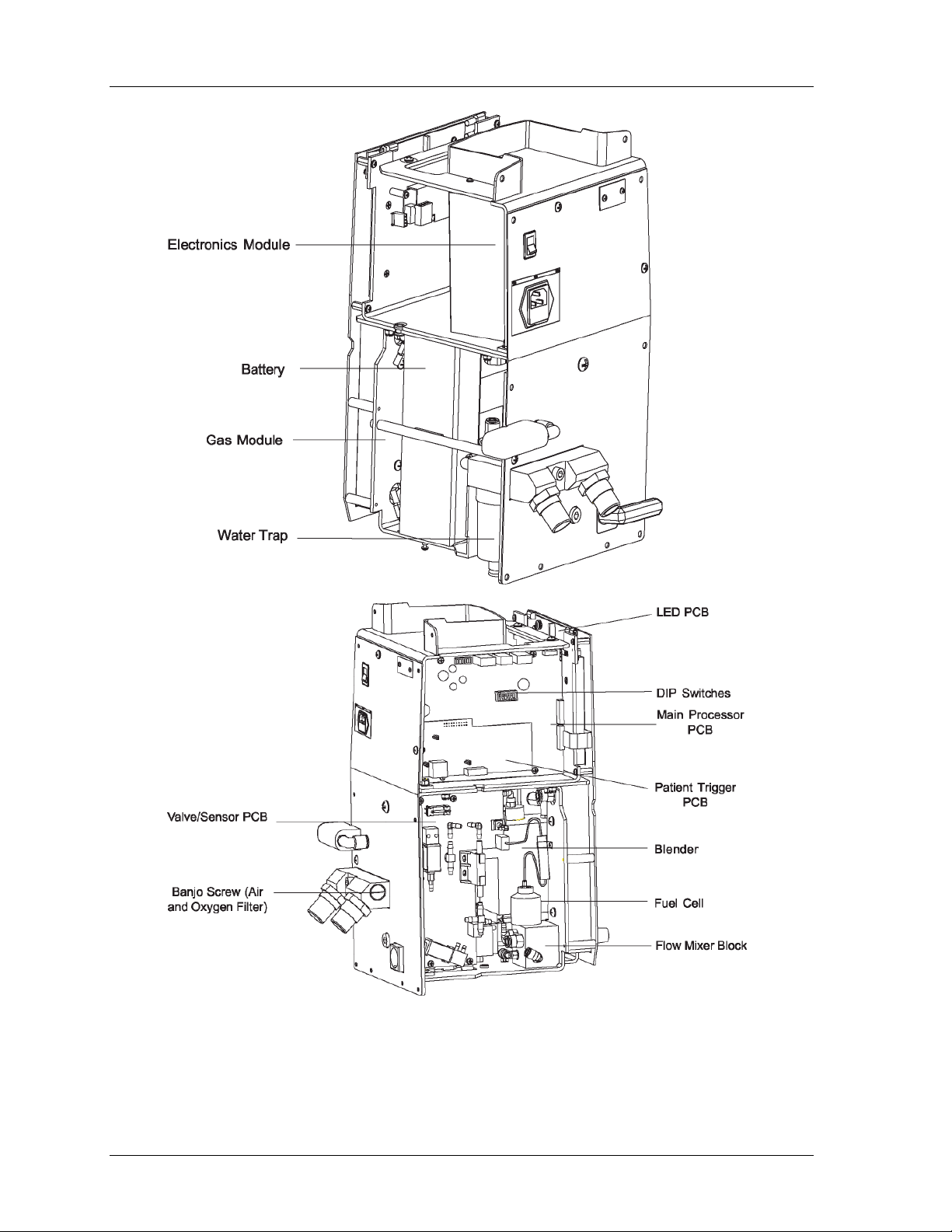
14 Infant Flow
®
LP SiPAP Model M675
Figure 3: IFSD Internal Components
675-120 Rev. G
Page 25
Service Manual 15
Front Panel Module
The function of the front panel module is to house the gas and electrical connections
to the patient, Operator controls and indicators. The module consists of a front panel
plate, the touch screen with key pad, flowmeters and FiO
and indicators and an ambient light sensor. The backlight on the touch screen is
decreased if the ambient light sensor detects a low ambient light level and increased
if it detects a high ambient light level.
The green Power light is always on when AC power is connected to the unit.
control, patient connectors
2
The Alarm Warning Bar
Patient Trigger Module (P lus and Comprehensive Models)
The patient trigger module consists of a PCB which plugs into the main processor
PCB. Its function is to detect patient breaths and apnea and give this information to the
main processor in the electronics module. The main processor uses the signals from the
patient trigger module to instruct the biphasic pressure control to provide a timed sigh
to the patient.
Firmware Module
The firmware module is the unit’s embedded software. Its function is to instruct the
microprocessor how to control the unit and to interact with the Operator.
Touch Screen
The touch screen provides the Operator with a series of screens with icons to enable
settings, calibration and fault diagnosis. The Start Up Screen is shown in Figure 4.
The display includes a status bar which incorporates a battery status, mode
indicator, alarm button and patient trigger indicator. The center part of the display
shows icons which relate to the function being selected or performed. The display also
includes a key pad with six keys. The icon in each key changes depending on the
function being performed.
flashes red to indicate an alarm.
Battery Status and Chargi ng Indicator
If the battery status shows three bars or less, the display flashes alternately between
red bars on a white background and a pink background.
Alarm Button
The alarm button alerts the Operator to fault conditions. An audible alarm is activated
at the same time. In the unalarmed condition the button is green
high-priority alarm condition, the button flashes
heard. If the alarm button is pressed (to silence the audible alarm), the button
changes to
flashes
condition, the button is solid
675-120 Rev. G
. In the
red and an audible alarm is
and flashes red. In a medium-priority alarm condition, the button
yellow and an audible alarm is heard. In a low-priority alarm
yellow with an audible alarm. If any alarm
Page 26

16 Infant Flow
condition resets itself, the yellow alarm bar remains to alert the clinician of a previous
problem.
Caution/Information
The Caution/Information icon alerts the Operator to read this manual. It flashes
during an alarm condition.
Mode Indicator
The Mode Indicator shows four question marks when in Start up or Adjust and
changes to show the applicable mode in use (e.g. NCPAP).
®
LP SiPAP Model M675
Figure 4: Touch Screen Display – Start Up
Patient Trigger Indicator (Plus and Comprehensive Models)
The Patient Trigger indicator changes to when the transducer interface is
connected and ready to be connected to the patient and indicates a
transducer interface is not connected.
when the
675-120 Rev. G
Page 27

Service Manual 17
Alarm Conditions
Audible and visible alarm indications are given to alert the Operator to specified
conditions that affect the operation of the unit. The electronic alarm limits are
automatically set after two minutes without Operator inputs but the Operator can
manually set the alarm parameters for certain conditions if required. The IFSD has
the following alarms:
• an audible W histle which sounds a constant tone when the gas imbalance
limits are exceeded.
• an audible Alarm which sounds a constant tone or two different intermittent
tones; medium or high level (medium level sounds beep.beep.beep every 15
seconds and high level sounds beep .beep .beep beep beep every 10 seconds).
• a visual Warning Bar which flashes red continuously, flashes intermittently or
comes on continuously.
• an Al arm Button on the touch screen status bar which flashes continuously.
Other indications are given to indicate the status of the condition. Refer to Table 2 for
alarms and indications for specified conditions.
Diagnostic Screen
A diagnostic screen is provided for low level calibration, test procedures for valves
and an error log. Figure 5 shows the screen display. Labeling of Diagnostic screen
will be the same regardless of configuration.
Figure 5: Diagnostic Screen
675-120 Rev. G
Page 28
18 Infant Flow
Box
Description
PCB. Position 1 is on and 0 is off.
INDICATOR
contrast.
successfully completed and a if the calibration fails.
Box
Description
S/W
The S/W box shows the version and serial number of the loaded firmware.
(E2P).
PT
The PT box shows the status of the Patient Trigger module.
inspiratory time, and breath rate (controlled by DIP 1).
(BLL) and if the backlight is operative (BLF - 1 for fail, O for on).
(BAT), the battery charge status (CHG) and the temperature (TMP).
8.2 and 10 V).
RS232
The RS232 box shows factory set data.
values.
(OSEN) and the software offset (OFFS) and gain (GAI N) values.
for the zero valve and the Z sensor (ZSEN) voltage.
for the dump valve and the D sensor (DSEN) voltage.
for the pressure assist valve and the P sensor (PSEN) voltage.
Table 2 - Diagnostics Screen Boxes
DIPS The DIPS box shows the position of the DIP switches on the main processor
®
LP SiPAP Model M675
CONTRAST
CALIBRATION
INDICATOR
The contrast indicator box is for use in the factory for setting the screen
The calibration indicator box shows
during calibration, a when
The following display boxes show if the values are within t he set parameters, a
if the values are outside the set parameters, a if the function is not
calibrated and a
if the function is disabled on the DIP switch.
Table 3 - Additional Diagnostics Screen Boxes
H/W The H/W box shows the status of the watchdog t i mer (WDG) and the EEPROM
CONFIG The CONFIG box shows the softw are con figurati on based on the DIP swit ch
settings and requirements. LAB = INTL or US (controlled by DIP 3), which is the
Comprehensive or Plus model; tBiP = 1 or 0 (controlled by DIP 2 and 5), which
indicates Triggered BiPhasic is enabled (1) or disabled (2); APB = 1 or 0
(controlled by D IP 2 ), w hi ch in dicates that Apnea BiPhasic is enabled (1) or
disabled (0 ); The re maining sy mbols provi de the de fault and r ange s for
LCD The LCD box shows the ambient light sensor voltage (AMB), the back light level
PSU The PSU box shows the external power supply voltage (EXT), the battery voltage
RAILS The RAILS box shows the ground voltage (GND), and the control voltages (6.5,
PRESSURE The PRESSURE box shows cm H2O pressure value, the voltage from the
pressure sensor (PSEN) and the software offset (OFFS) and gain (GAIN)
OXYGEN The OXYGEN box shows the FiO2 %, the voltage from the oxygen sensor
ZVALVE The ZVALVE box shows if the Z DRIVE is operative (ZDRV - 1 for fail, O for on)
DVALVE The DVALVE box shows if the D DRIVE is operative (DDRV - 1 for fail, O for on)
PVALVE The PVALVE box shows if the P DRIVE is operative (PDRV - 1 for fail, O for on)
675-120 Rev. G
Page 29

Service Manual 19
Chapter 5 – Theory of Operation
Gas Flow
With the oxygen and air connections connected and the power switch ed on, oxygen
and air at 386 kPa (56 psig) flows to the blender. The air passes thr ough a water trap
with an integral filter where any moisture in the air is removed. Oxygen and air are
filtered before entering a non-return valve in the blender. The blender mixes the
oxygen and air in the proportion set by the position of the FiO
blender supplies blended gas between 2.5 and 30 LPM.
The blender incorporates an alarm/bypass module. If a single gas supply fails or
there is an imbalance between the inlet gas pressures exceeding 200 kPa (30 psig),
the module directs the flow through a reed whistle to warn the Operator of the
condition and at the same time connects the inlet gas at the hig her pressure to the
blender outlet.
The blended gas is filtered before passing through a flow control to an oxygen
analyzer. The oxygen analyzer utilizes a galvanic fuel cell to measure and display
the measured delivered oxygen concentration.
control valve. The
2
A vent valve on the exhaust manifold incorporates a solenoid operated pilot valve
and a large orifice spool valve. The vent valve is normally held in the open position
by a spring to vent high flow rates with low pressure drops so that the patient
pressure is minimal. The electrically actuated pilot valve is operated by blended gas
so that the vent valve continues to operate if one gas supply is lost.
Blended gas flows through an electronically operated dump valve which is normally
in the closed position. If over pressurization occurs, the sensors detect the condition
and the controller signals the dump valve to open, sounds an alarm buzzer and at
the same time closes the vent valve to rapidly reduce the patient pressure to near
zero. When the over pressure condition stops, the controller signals the vent valve to
open and the dump valve to close.
The NCPAP flowmeter is set by the Operator to the required flow. The flow rate is
shown on the gauge on the flowmeter. When the flowmeter control knob is turned
fully clockwise, the flow of gas is turned off.
675-120 Rev. G
Page 30

20 Infant Flow
®
LP SiPAP Model M675
Figure 6: IFSD Gas Flow Schematic
675-120 Rev. G
Page 31

Service Manual 21
The patient pressure is shown on the touch screen and is monitored by a pressure
sensor which sends the signal to the main processor. A zeroing valve automatically
checks the pressure readings against atmospheric pressure to ensure accuracy of
the patient pressure readings.
A mechanical pop-off valve is factory set to limit the delivered pressure to a safe
level sufficient to achieve the maximum required patient pressure at the generator.
Adjustment of the pop-off valve is not required by Operators.
Auxiliary Output (Opti ona l)
Blended gas flows through a fixed flow control and non-return valve to a standard
DISS fitting on the back panel to supply a maximum 15 LPM for delivery to the
patient via other sources.
Inspiratory Pressure
Blended gas passes through a variable outlet flow control and through the NCPAP
Pres High Flowmeter. The NCPAP Pres High Flowmeter is set by the Operator to the
required flow. The outlet from the NCPAP Pr es High Flowmeter when not delivering to
the patient flows through a restrictor to exhaust, allowing the flowmeter to be
adjusted without delivering gas to the patient. The flow rate is shown on the gauge
on the flowmeter. When the flowmeter control knob is turned fully clockwise, the flow
of gas is turned off. The flow is directed to the patient via a high speed selector valve
which operates when requested by the NCPAP Pres high timing.
WARNING!
The NCPAP Pres high must be adjusted to zero w hen not required for the
patient.
Triggered BiPhasic
For BiPhasic Tr, a micro controller provides reliable indications of breaths derived
from the patient abdominal respiratory sensor. It sends signals to operate the
NCPAP Pres high valve to provide a timed sigh.
WARNING!
Under extreme conditions (minimum supply pressure and maximum gas
demand, including auxiliary output) output flow rates may reduce.
Operation without E lectrical Power
The IF SD can be used without electrical power. In this mode, NCPAP Pres low flow
only is delivered, set by the NCPAP Pres Low Flowmeter and the required FiO
In this mode, visual indications and warnings are not given except for the gas failure
alarm.
level.
2
675-120 Rev. G
Page 32

22 Infant Flow
Electronic Functions
Sensors mounted on the valve/sensor PCB monitor pressure, flow rate and oxygen.
Sense signals for each of the valves allow the micro-controller to monitor the valve’s
state and determine whether the valve is connected or short circuited. Current to the
valve sensor PCB is limited via the fuses below a 10 VA limit to ensure safety in a
possible oxygen enriched environment.
The patient trigger PCB interfaces with the main processor PCB via a 20 way head.
Communication between the two PCB’s is via a CAN bus. The main processor PCB
also supplies +5 V power to this PCB and monitors other control signals.
®
LP SiPAP Model M675
Figure 7: IFSD Electronic Enclosure
675-120 Rev. G
Page 33

Service Manual 23
Electrical Layo ut
Figure 8 shows the electrical wiring and PCB connector layout.
Figure 8: IFSD Electrical Wiring Diagram
675-120 Rev. G
Page 34

24 Infant Flow
BiPhasic
BiPhasic
Fault lockout
Unit inoperable by
Error code(s)
alternates
Unit restricted to
Error code(s)
Patient trigger
Battery status
Unit will not
Fault Management
When a software detectable fault condition occurs, the unit still allows a basic level of
treatment to the patient. Table 4 shows the fault conditions and the level of control
available.
Table 4 - Faults, Available Modes and Control Functions
Measurements Software Control Modes
®
LP SiPAP Model M675
Alarm Functionality FiO2 CmH2O NCPAP
screen shows
error
code(s).
shown
on mode
selection
screen.
Status bar
mode
shown
on mode
selection
screen.
Status bar
mode
software, operable
in
unpowered
NCPAP and
NCPAP+Apnea
modes.
(NCPAP+Apnea,
BiPhasic+Apnea
and
BiPhasic Tr)
modes not
available.
X X X X X X
NCPAP+
Apnea
X
+ Apnea
X X
Tr
X
675-120 Rev. G
shows
discharged
battery.
operate
on battery power
when external
power
supply is
removed.
Page 35

Service Manual 25
Chapter 6 – Operational Setup
The operating procedures below show the procedures for all models. Reference
should only be made to the procedures for the model in use. Read the Warnings and
Cautions at the beginning of this manual before you start the procedures.
Preparing and Connecting the Equipment
1 Connect the Medical Air and Oxygen hoses to the IFSD connections on the
back panel.
WARNING!
Only use the supplied AC cable to connect to the power supply.
2 Connect the power supply cable to a suitable power supply outlet. The green
power light will come on regardless of the position of the power switch.
3 Connect the patient circuit to the IFSD as required. Figure 9 shows a typical
configuration, the actual configuration may vary dependent on the type of ancillary
equipment used and the clinical needs prescribed for the patient. For functional
test purposes, the Generator nasal prongs can be occluded to simulate patient
responses.
4 If desired, connect the transducer module to the sensor and to the IFSD.
WARNING!
The Infant Flow LP SiPAP system is compatible only with the Infant
Flow LP Generator. The prescribed nCPAP level will not be obtained if
other variable generators are used.
675-120 Rev. G
Page 36

26 Infant Flow
Infant Flow® LP Generator
Infant Flow® LP
®
LP SiPAP Model M675
SiPAP Driver or
SiPAP Driver
Figure 9: Patient Connections
675-120 Rev. G
or Generator
WARNING!
Check that the water trap is empty before use and empty it fr equently
during use.
Page 37

Service Manual 27
5 Make sure that the water trap is empty. If necessary, empty any water from the
trap (refer to Chapter 3, Maintenance).
6 Gas Flow Pressure Setting
The IFSD provides a virtually constant airway pressure irrespective of patient
demand or expiratory flows via the specially designed generator and nasal prongs. This
is the reason for the IFSD’s ability to provide superior NCPAP. The IFSD is subject to
a direct relationship between controlled enriched gas flow and NCPAP pressure.
Nomograms illustrating the relationship between constant airway pressure and flow
settings are shown in Figure 10 and Figure 11. Example: 8 LPM gas flow provides 5
cm H
O NCPAP.
2
Verify the type of IFSD you are using, LP SiPAP or 675–CFG–XXX, to determine
which nomogram to use.
Note:
Individual devices have a tolerance of up to ± 10% from that illustrated in the
nomogram and in particular, at pressures below 2 cm H
2
O.
Figure 10: Flow Pressure Nomogram for 675-CFG-XXX
675-120 Rev. G
Page 38

28 Infant Flow
performance.
Flow Pressure Nomogram (for
reference only) to show typical flow
pressure relationships. This is not
meant to establish actual product
®
LP SiPAP Model M675
Figure 11 – Flow Pressure Nomogram for LP SiPAP
Individual devices have a tolerance of up to ±15 percent from that illustr ated in the
nomogram and, in particular, at pressures below 2cmH
2
O.
675-120 Rev. G
Page 39

Service Manual 29
Switching On the IFSD
WARNING!
Do not attach the Generator to the patient until the initial set up is
complete.
Put the power switch on the back panel to the position.
• the warning bar comes on
• the green power light remains on
• the audible alarm sounds
The unit carries out a full functional check. If the checks are not successful (prog r am
memory fault, power supply not connected or emergency battery voltage low), the
screen remains black and the warning bar stays on.
If the checks are successful, the warning bar goes off and the screen changes to the
Power Up Screen.
After two seconds the screen changes to the Power Up Check Screen.
During the power up checks:
• the screen image is shown in negative
• the warning bar comes on for one second
• the Apnea light comes on for one second
• the audible alarm sounds for one second
• the dump valve is tested
• the pressu r e is set to zero
After two seconds the screen changes to the Start Up Screen.
• the alarm limits are disabled
A flashing question mark alternating with a red cross appears under the first
adjustment to be made.
To calibrate
the O2 fuel cell, refer to the section “Calibration” on page 41.
Note:
Where a mode is not applicable (e.g. Patient Trigger), the button is blank and the
icons are not shown.
675-120 Rev. G
Page 40

30 Infant Flow
User Verification Test
WARNING!
Do not attach the Infa nt Flow® LP Generator or the I nfant Flow® Generator
to the patient until User Verification and initial setup into NCPAP mode is
complete.
CAUTION!
Although failure of any of the above tests will not prevent the ventilator from
functioning, it should be checked to make sure it is operating correctly before use
on a patient.
Power-on Check
This test is run automatically on power up of the driver and automatically performs
the following checks:
• Flash ROM
®
LP SiPAP Model M675
• Hardware Input s/Out puts
• Audible and visual alarms indicators
• Test and calibrat ion of pressure sensor
• Test of dump val ve
The unit carries out a full functional check during this time. If unsuccessful, the
screen remains darkened and the warning bar remains on. In this case, check for the
following;
• Power Supply not connected
• Battery voltage low
If the checks are successful, the screen changes to Power Up Screen. After two
seconds, the screen changes to Power Up Check Screen.
During the Power Up check:
• Screen image shown in negative
• Warning bar comes on for one second
• Transducer Assembly LED comes on for one second
• Audible alarm sounds for one second
• Dump valve is tested
• Pressure is set to zero
After two seconds screen changes to Set Up Screen. Alarm limits are disabled and a
flashing question mark appears under the NCPAP / Pres Low flowmeter screen
indicator.
675-120 Rev. G
Page 41

Service Manual 31
Two Point O2 Sensor Calibratio n
1. Enter the Calibration Screen from the Setup Screen by pressing the calibration
button on the lower right hand corner of the touch screen.
2. Ensure there is a min i mum 8 LPM set on the NCPAP/PRES Low Flowmeter. In
addition, ensure there is a minimum of 3 LPM set on the NCPAP/High
Flowmeter.
3. Adjust the %O
calibration by touching the associated flashing button.
4. Adjust the %O
touching the associated flashing button.
5. Return to the Start up Screen by pressing the Exit button.
control to 21%. Allow the %O2 display to stabilize. Confirm the
2
control to 100%. Allow the %O2 display to stabilize. Confirm by
2
Note:
If O2 calibration fails, a red “X” is shown.
If the internal oxygen cell is depleted or damaged, it may not be possible to calibrate
the O
button. This will disable oxygen monitoring and the audible oxyg en alarms unt il the
device is powered off. Whenever the device is operating with oxygen monitor and
alarms disabled, a fault code E55 displays, and measured FiO
sensor. The internal oxygen monitor may be disabled using the Disable O2
2
displays as dashes.
2
WARNING!
When the Infant Flow® LP SiPAP system unit or SiPAP system unit (part
number 675-CFG-xxx) is connected to a patient and the internal oxygen
monitor is disabled, the Infant Flow® LP SiPAP system unit or SiPAP
system unit must be used with an external oxygen monitor.
If calibration is attempted, and fails, or if the oxygen cell fails while the device is in
normal use, a Fault Code E55 displays, as tabulated in appendix D, and a high
priority alarm is indicated visually and audibly. To enable continued operation, the
internal oxygen monitoring may be disabled by pressing and holding the alarm mute /
reset button for three seconds. This disables the internal oxygen monitor and alarms
and clears the alarm condition. The E55 code remains to indicate that the oxygen
monitor is inoperative. An external oxygen monitor must be used.
675-120 Rev. G
Page 42

32 Infant Flow
Leak Test
1. Have the patient circuit and generator assembled as shown in Figure 3.
2. Connect the patient interface (prong or mask) to the generator (see Chapter 5,
Step by Step Fixation) and occlude the opening to the patient.
3. If not powered up already, switch on the power to the driver.
4. For LP SiPAP, adjust the NCPAP/Pres Low Flowmeter to 9 LPM. Verify that the
measured pressure is 5 ±1 cmH
confirm.
For SiPAP, part number 675-CFG-XXX, adjust the NCPAP/Pres Low Flowmeter
to 8 LPM. Verify that the measured pressure is 5 ±1 cmH
associated flashing screen icon to confirm.
O. Touch the associated flashing screen icon to
2
®
LP SiPAP Model M675
O. Touch the
2
5. Adjust %O
control as prescribed for the current patient. Verify that the blender
2
setting and the measured oxygen value are within 3%. Touch the associated
flashing screen icon to confirm.
6. Adjust the Pres High Flowmeter as prescribed for the current patient. Touch the
associated flashing screen icon to confirm.
7. If desired, connect the Transducer Interface to the front panel of the driver if
breath monitoring is required during treatment. Touch the associated flashing
screen icon to confirm.
8. The display screen changes to the Alarm Set/Confirm Screen. Press the NCPAP
button or Alarm Mute/Reset button to set alarms and begin monitoring.
9. Monitored parameter for CPAP should be 4-5 cmH
O. If not, check circuit for
2
leaks or blockages, (including the humidification system).
10. Remove the occlusion to the patient interface. The monitored CPAP display
should be 0-2 cmH
O. If not, check that the interface is not still occluded.
2
675-120 Rev. G
Page 43

Service Manual 33
Alarm Test Initial Settings
Air Supply Pressure
O
2
Supply Pressure
Patient Circuit
Generator
Infant Flow® LP Generator
8 LPM for SiPAP
% O2
Pres High Flowmeter
Mode
Alarms Test
WARNING!
Prior to patient application, ensure that all User Verification testing and
calibration procedures are successfully completed. User Verification
testing and calibration procedures must be done off patient.
Note:
Following each alarm verification test, ensure that control settings and alarm limits
are reset as instructed before proceeding to the next test.
> 30 psig (2.1 bar)
> 30 psig (2.1 bar)
Infant Flow® LP Patient Circuit
NCPAP / Pres Low Flowmeter
9 LPM for LP SiPAP or
30%
3 LPM
NCPAP
Perform the Alarms Test on the Infant Flow® LP SiPAP system or Infant Flow SiPAP
system using the following steps and the initial settings provided above.
1. Make appropriate connections for air and O
gas supply. Connect power cord to
2
appropriate AC outlet. Attach patient circuit, generator and patient interface
(mask or prong) as shown in Figure 3. Occlude the opening to the patient .
2. Power up the driver and allow Power On Check to complete.
3. Low airway pressure alarm: From NCPAP operating mode, with alarms set,
remove occlusion from opening to patient. Verify that the low airway pressure
alarm activates. Restore the patient interface occlusion and press the Alarm
Mute / Silence button for 3 seconds to reset the alarms.
4. High airway pressure alarm: Adjust the NCPAP / Pres Low Flowmeter to 11
LPM. Verify that the high airway pressure alarm activates. Return the NCPAP /
Pres Low Flowmeter to 9 LPM for LP SiPAP or 8 LPM for SiPAP and press the
Alarm Mute / Silence button for 3 seconds to reset the alarms.
5. High %O
activates. Return the O
Alarm: Adjust the % O2 control to 35%. Verify that the High %O2 alarm
2
control setting to 30%. Reset alarms by pressing the
2
Alarm Mute / Reset button for 3 seconds.
675-120 Rev. G
Page 44

34 Infant Flow
6. Low % O2 Alarm: Adjust the % O2 control to 25%. Verify that the Low %O2 alarm
activates. Return the O
control setting to 30%. Reset alarms by pressing the
2
Alarm Mute / Reset button for 3 seconds.
7. Loss AC Alarm: Disconnect the AC power cor d from the wall outlet. Verify that
the Loss AC alarm activates. Reconnect the AC power cord. Clear the alarm by
pressing the Alarm Mute / Reset button.
8. High Circuit Pressure Alarm: Increase nCPAP pressure to 11.1 cmH
increasing the NCPAP/PRES Low Flowmeter. Verify that the High Circuit
pressure alarm activates. Return NCPAP/PRES Low Flowmeter to 8 LPM and
press the Alarm Mute/Silence button for three seconds to reset the alarms.
Note:
If staff is using a transducer (P/N 677-002) for Apnea Monitoring, proceed to
step 9. If staff is not using a transducer, mark N/A on checkout sheet and testing
is finished.
9. Low Breath Rate (Apnea) Alarm: Attach the transducer and abdominal sensor to
the unit. Set Mode to BiPhasic+Apnea/LBR (U.S. Configuration) or Biphasic +
LBR, rate 30 Bpm, T-High 0.3 seconds and Tapnea or TLBR to 20 sec.
Manually squeeze the abdominal sensor to simulate a spontaneous breath. No
alarms should be active. Change the mandatory rate control to setting for rate to
1 Bpm and stop squeezing the abdominal sensor. Verify that the Low Breath
rate alarm activates after the default interval of 20 seconds. Return the rate
control to 30 Bpm and clear the alarm by pressing the Alarm Mute / Reset
button for 3 seconds.
®
LP SiPAP Model M675
O by
2
675-120 Rev. G
Page 45

Service Manual 35
TEST
PASS
FAIL
N/A
Automated Tests
Power On Check
Manual Tests
Two Point O2 Sensor Calibration
Patient Circuit Leak test
Manual Alarms Checks
Low Airway Pressure Alarm
High Airway Pressure Alarm
High O
2
Alarm
Low O
2
Alarm
Loss AC Alarm
High Circuit Pressure A larm
Low Breath Rate (Apnea) Alarm
Infant Flow® LP SiPAP/SiPAP User Verification Test Checklist
Driver Serial Number:_____________________ Test Date:_________________
Signature of tester:_______________________________________________
Title___________________________________________________________
675-120 Rev. G
Page 46

36 Infant Flow
Setting up the Equipment
The procedures show the screen set up for all modes. If a mode is not applicable to
the model in use, go to the next applicable step.
Adjust the NCPAP Pres Low Flowmeter to indicate the required flow rate. When
®
LP SiPAP Model M675
done, touch the flashing
The button icon changes to a
Set the FiO
confirm each time.
and NCPAP Pres high flow as appropriate, touching the button to
2
button to confirm the initial setting
and the next button starts flashing.
WARNING!
Ensure that the pressure is consistent with the flow rate.
WARNING!
Verify that the displayed value for delivered FiO2 corresponds to the value
set on the blender. Refer to Faults and Indications.
If an alarm is activated, the button displays a flashing cross. The alarm condition
must be cleared before the setting is confirmed (refer to Table 1).
WARNING!
The indicates a connection between the transducer interface and the
unit. It does not indicate correct positioning of the abdominal respiratory
sensor.
Note:
All triggered modes (NCPAP+Apnea, BiPhasic+Apnea, BiPhasic Tr) are
automatically confirmed with a
When all of the initial settings have been confirmed (NCPAP Pres low, FiO2, NCPAP
Pres high and/or Respiratory sensor) the screen changes to the Adjust Screen.
• The alarm limits remain disabled.
To set the alarm limits, touch the NCPAP button or the alarm button f or three
seconds. If a button is not touched within two minutes, the alarm limits will be
automatically set.
if the patient trigger is connected.
Note:
NCPAP+Apnea, BiPhasic+Apnea, BiPhasic Tr selection buttons are not lit if the
transducer interface box is not connected to the IFSD.
675-120 Rev. G
Page 47

Service Manual 37
When the alarm limits have been set, the screen changes to the Mode Selection
Screen and defaults to the nCPAP mode.
• The alarm limits are set.
Touch NCPAP, NCPAP+Apnea, BiPhasic, BiPhasic+Apnea, or BiPhasic Tr to enter
the Parameter Set up Screen for each mode.
Note:
When the Mode Selection screen is showing, NCPAP treatment will always be
delivered.
After two minutes if the Operator has not made any inputs, the screen changes to the
Locked Screen.
• The key pad is locked.
• The display remains as shown for patient monitoring.
Touch the
• The display returns to the Mode Selection Screen.
button for three seconds to unlock the key pad.
Note:
If a high priority alarm occurs, the keypad automatically unlocks.
Setting the NCPAP Parameters
For NCPAP+Apnea function the transducer interface and the abdominal respiratory
sensor must be connected.
From the Mode Select Screen touch the NCPAP + Apnea
The screen changes to the Parameter Set Up Screen and shows:
• The patient respiration rate (Rsp).
• The delay time for the alarm to come on Tapnea.
• The Inspiratory time for a manual sigh.
Use the
buttons to set the alarm delay time.
button.
Confirm the settings by touching the flashing
been accepted, the screen changes to the Locked Screen.
Touch the
• The display changes to the Parameter Adjust Screen.
675-120 Rev. G
button. When the settings have
button to unlock the key pad.
Page 48

38 Infant Flow
Note:
If a high priority alarm occurs, the key pad automatically unlocks.
To return to the Mode Selection Screen, touch the button.
Setting the BiPh asic Parameters
For BiPhasic+Apnea function the transducer interface and the abdominal respiratory
sensor must be connected.
®
LP SiPAP Model M675
From the Mode Select Screen touch the Biphasic
The screen changes to the Parameter Set Up Screen and shows:
• The NCPAP Pres high inspiration time (T
• The NCPAP Pres high respiration rate (R)
• The Inspiration/Expiration ratio (I/E)
-High)
button.
Note:
If a transducer interface is connected to the unit, is displayed and BiPhasic +
Apnea monitoring mode is enabled. In addition to the above parameters, the screen
shows:
• The detected breath bar graph.
• The detected breath rate (Rsp)
• The delay time for the alarm to come on (Tapnea)
Push the individual buttons to select between the parameters T
Tapnea. Use the buttons to set the parameter for T-High, Rate, and
Tapnea.
-High, Rate, and
Note:
The I/E rate changes accordingly.
Confirm the BiPhasic settings by touching the flashing BiPhasic or BiPhasic + Apnea
button.
When the settings have been accepted, the screen changes to the locked screen
and treatment starts.
To adjust the parameters, touch the
The screen changes to the BiPhasic or BiPhasic + Apnea Adjust Screen.
Parameters T
675-120 Rev. G
-High and Rate can now be adjusted.
button.
Page 49

Service Manual 39
Note:
The I/E rate changes accordingly.
To return to the Mode Selection Screen, touch the button.
Note:
The button is shown with a pink background if an alarm condition occurs. If this
occurs, the button cannot be operated until the alarm condition has been cleared or
silenced using the alarm button.
After two minutes if the Operator has not made any inputs, the screen changes to the
Locked Screen.
• The key pad is locked.
• The display remains as shown for patient monitoring.
Touch the
• The display returns to the BiPhasic or BiPhasic + Apnea Adjust Screen.
button to unlock the keypad.
Note:
If a high priority alarm occurs, the keypad automatically unlocks.
Setting the Trigg er ed BiPhasic Parameters
From the Mode Select Screen touch the BiPhasic Tr button.
Note:
The transducer interface must be connected to enter this mode.
The screen changes to the Parameter Set Up Screen and shows:
• The patient’s respiration rate (Rsp)
• The NCPAP Pres high inspiration time (T
• The NCPAP Pres high backup respiration rate (Rb)
• The delay time for the apnea alarm to come on (Tapnea)
-High)
Press each individual button to select between the parameters T
Tapnea. Use the
Confirm the BiPhasic Tr settings by touching the flashing BiPhasic Tr button.
When the settings have been accepted, the screen changes to the locked screen
and treatment starts.
675-120 Rev. G
-High, Rb and
buttons to set the parameter for T-High, Rb and Tapnea.
Page 50

40 Infant Flow
To adjust the parameters, touch the button.
The screen changes to the BIPHASIC TR Adjust Screen.
®
LP SiPAP Model M675
Parameters T
To return to the Mode Selection Screen, touch the button.
-High, Rb and Tapnea can now be adjusted.
Note:
The button is blank if an alarm condition occurs. If this occurs, the button
cannot be operated until the alarm condition has been cleared or silenced using the
alarm button.
After two minutes if the Operator has not made any inputs, the screen changes to the
Locked Screen.
• The key pad is locked.
• The display remains as shown for patient monitoring.
Touch the
• The display returns to the BiPhasic Tr Adjust Screen.
button to unlock the key pad.
Note:
If a high priority alarm occurs, the key pad automatically unlocks.
675-120 Rev. G
Page 51

Service Manual 41
Calibration
WARNING!
Calibration must only be done when the unit is not connected to the
patient.
From the Start Up Screen touch the button.
The screen changes to the Calibration Screen.
• The alarm limits are disabled.
Turn the FiO
Turn the FiO
Touch the
The screen returns to the Start Up Screen.
control to 21 and confirm by touching the flashing button.
2
control to 100 and confirm by touching the flashing button.
2
button.
Note:
If the calibration procedure fails, a red is shown in the applicable button. Recalibrate and if necessary, replace the O
fuel cell.
2
Giving a Manual Timed Sigh
In the Mode Select or Adjust screens of NCPAP+Apnea, BiPhasic+Apnea or BiPhasic
Tr - touch the
button to give the patient a manual timed sigh.
Operation Without Electrical Power
The IFSD can be used without mains or battery power. To use the IFSD in this
mode, set the required NCPAP flow on the NCPAP Pres Low Flowmeter and the
required FiO
except for the gas failure alarm /bypass whi ch wi ll operate un til pressures are b alanced.
level. All audible and visual indications and warnings are not given
2
675-120 Rev. G
Page 52

42 Infant Flow
Fault Indications
Refer to Table 1 (all models) and Table 2 (BiPhasic and BiPhasic T r models only) for
the fault indications for specific faults and the procedures for resetting or canceling.
Discharged Battery
When the battery voltage is too low to power the circuits, the screen changes to the
Power Down Screen.
• All functions and controllable inputs are disabled.
• The controller waits for the power source to be connected.
• When external power is restored, the screen changes to the Power Up
Screen.
• The screen goes blank when the battery power is too low to power the Power
Down Screen.
Fault Lockout
®
LP SiPAP Model M675
If a fault occurs which is detectable by the software and prevents the unit from
operating correctly, the screen changes to the Fault Lockout Screen.
• All functions and controllable inputs are disabled, but the unit can still be used
without electrical power.
• The related fault code numbers are shown on the screen.
• The alarm bar comes on and the predominant fault code number is shown in
the status bar.
If an error code is shown, refer to the Service Manual or contact your Service
Engineer to rectify the faults.
Over Pressure Indications
If an over pressure occurs, the software opens the dump valve to r elease the
pressure.
• The upper pressure limit is shown in red.
• The pressure display flashes.
• When the pressure drops below the lower limit, the lower limit is shown in red
and the pressure flashes alternately between the limits.
Rectify the fault by adjustment of the high pressure and touch the warning button for
three seconds to reset.
675-120 Rev. G
Page 53

Service Manual 43
returns to normal.
Table 5 - Faults and Indications
Alarm Method of Setting
Minimum oxygen
concentration
18 FiO2).
(<
Over pressure
(Patient pressure
> 11 cmH
O when in
2
Always active when
power is on.
Always active when
power is on.
NCPAP mode).
Indications and
Actions
Intermittent high level
Audible Alarm.
Warning Bar flashes.
display flashes.
FiO
2
Digital O
alarm low
2
limit is highlighted.
Alarm Button flashes.
Intermittent high level
Audible Alarm.
Warning Bar flashes.
Pressure display
flashes.
Digital high-pressure
alarm limit is
highlighted.
Alarm Button flashes.
Dump valve actuated
for three seconds to
stop flow to patient and
repeated until flow
Method of Resetting
or Canceling
Restore FiO
level to
2
above the low lim it
then push Alarm
Button for three
seconds.
Reduce pressure to
below the high
pressure limit then
push the Alarm
Button for three
seconds.
High oxygen concentration
5 FiO2 above set point
(>
at time alarm is set for 15
seconds,
or
104 FiO2 for > 15
>
seconds).
Set automatically on
entering NCPAP
mode for the first time
after start up or by
pushing the Alarm
Button for three
seconds at any time.
Intermittent high level
Audible Alarm.
Warning Bar flashes.
digital display
O
2
flashes.
Digital O
alarm high
2
limit is highlighted.
Alarm Button flashes.
Push the Alarm
Button once to stop
the Audible Alarm for
30 seconds (Alarm
Button flashes, digital
alarm high limit
O
2
stays highlighted,
Warning Bar still
flashes).
Push the Alarm
Button for three
seconds to reset the
limit (alarms clear).
675-120 Rev. G
Page 54

44 Infant Flow
®
LP SiPAP Model M675
Alarm Method of Setting
Low oxygen concentration
5 FiO2 below set point
(<
at time alarm is set for 15
seconds,
or
20 FiO2 for > 15
<
seconds).
High NCPAP pressure (>
3 cmH
O above set point
2
at time alarm is set for 15
seconds).
Set automatically on
entering NCPAP
mode for the first time
after start up or by
pushing the alarm
button for three
seconds at any time.
Set automatically on
entering NCPAP
mode for the first time
after start up or by
pushing the alarm
button for three
seconds at any time.
Indications and
Actions
Intermittent high level
Audible Alarm.
Warning Bar flashes.
digital display
O
2
flashes.
Digital O
alarm low
2
limit is highlighted.
Alarm Button flashes.
Intermittent high level
Audible Alarm.
Warning Bar flashes.
Pressure display
flashes.
Digital pressure high
limit is highlighted.
Alarm Button flashes.
Method of Resetting
or Canceling
Push the Alarm
Button once to stop
the Audible Alarm for
30 seconds (Alarm
Button flashes, digital
alarm low limit
O
2
stays highlighted,
Warning Bar still
flashes).
Push the Alarm
Button for three
seconds to reset the
limit (alarms clear).
Push the Alarm
Button once to stop
the audible alarm for
30 seconds (Alarm
Button flashes, digital
pressure high limit
stays highlighted,
Warning Bar still
flashes). Push the
Alarm Button for
three seconds to
reset the limit (alarms
clear).
Low pressure
2 cmH2O below set
(<
point for 15 seconds)
or
1 cmH2O at any time).
<
Set automatically on
entering NCPAP
mode for the first time
after start up or by
pushing the alarm
button for three
seconds at any time.
Intermittent high level
Audible Alarm.
Warning Bar flashes.
Pressure display
flashes.
Digital pressure low
limit is highlighted.
Alarm Button flashes.
Push the Alarm
Button once to stop
the Audible Alarm for
30 seconds (Alarm
Button flashes, digital
pressure low limit
stays highlighted,
Warning Bar still
flashes ).
Push the Alarm
Button for three
seconds to reset the
limit (alarms clear).
675-120 Rev. G
Page 55

Service Manual 45
Alarm Method of Setting
Over pressure (Patient
pressure
> 11 cmH
O when in
2
Always active when
power is on.
BiPhasic or BiPhas ic Tr
mode).
High BiPhasic/ BiPhasic
Tr pressure
(MAP >
3 cmH2O above
set point at time alarm is
set for 15 seconds).
Set automatically on
entering NCPAP
mode for the first time
after start up or by
pushing the alarm
button for three
seconds at any time.
Indications and
Actions
Intermittent high level
Audible Alarm.
Warning Bar flashes.
digital display
O
2
flashes.
Digital O
alarm low
2
limit is highlighted.
Alarm Button flashes.
Intermittent high level
Audible Alarm.
Warning Bar flashes.
High MAP pressure
display flashes.
Digital high MAP
pressure limit is
highlighted.
Alarm Button flashes.
Method of Resetting
or Canceling
Reduce pressure to
below the high
pressure limit then
push the Alarm
Button for three
seconds.
Push the Alarm
Button once to stop
the Audible Alarm for
30 seconds (Alarm
Button flashes, digital
high MAP pressure
limit stays
highlighted, Warning
Bar still flashes).
Push the Alarm
Button for three
seconds to reset the
limit (alarms clear).
Low battery charge
40%).
(<
Low battery voltage
11.10 V for 5 seconds).
(<
Automatic Battery status indicator
changes from gray to
red.
Automatically
constantly monitored
with the power switch
in the on position with
no external power
connected.
Intermittent medium
level Audible Alarm.
Warning Bar flashes
intermittently.
Battery status indicator
flashes.
Unit starts a controlled
shut down at a set limit.
Connect external
power.
Push the Alarm
Button once to stop
the Audible Alarm for
three minutes (Alarm
Button flashes,
battery status
indicator stays
flashing).
Connect external
power (alarms clear).
Visual alarms remain
until battery charge
state is above low
675-120 Rev. G
Page 56

46 Infant Flow
number).
®
LP SiPAP Model M675
Alarm Method of Setting
Battery fault
(Battery disconnected or
failing to take or hold
charge).
External power
disconnected.
Automatic Intermittent high level
Automatic Intermittent high level
Indications and
Actions
Audible Alarm .
Warning Bar flashes
intermittently.
Battery status indicator
flashes.
Screen displays
flashing fault code (E
number)
Audible Alarm.
Warning Bar flashes
intermittently.
Battery status indicator
and power indicator
alternately flash.
Method of Resetting
or Canceling
Cannot be reset.
Push the Alarm
Button once to stop
the Audible Alarm for
60 seconds.
Refer to Service
Engineer.
Push the Alarm
Button once to stop
the audible alarm.
(The Warning Bar
stops flashing and
the battery status
indicator is
displayed.)
Reconnect the
external power.
Software fault Automatic Intermittent high level
Audible Alarm.
Warning Bar flashes
intermittently.
Screen displays
flashing fault code (E
number).
Software not running with
unit connected to power
Blender whist le Water trap blocked,
Oxygen cell calibration
error.
Automatic Constant Audible
Alarm.
Warning Bar on.
Screen displays
flashing fault code (E
Audible Alarm. Refer to Service
full or leaking; filters
blocked; loss of wall
pressure; imbalance
of wall gas supply.
Automatic monitoring
(oxygen cell
incorrectly calibrated,
damaged or depleted).
Difference between
displayed and set
value.
Cannot be reset.
Refer to Service
Engineer.
Cannot be reset.
Refer to Service
Engineer.
Engineer.
Calibrate or replace
oxygen cell. Refer to
Service Manual.
675-120 Rev. G
Page 57

Service Manual 47
Alarm Method of Setting
Flowmeter fault. Flowmeter fault No flow indications or
Electrical fault. Electrical fault External power light
Low breath rate
(Rr = 0 for >
timeout as determined by
patient trigger module).
breath rate
Set automatically on
entering BiPhasic Tr,
NCPAP + Apnea or
BiPhasic +Apnea
modes if patient
trigger is installed and
selected.
Indications and
Actions
flow cannot be
adjusted.
does not match screen
icon.
Intermittent high level
Audible Alarm.
Warning Bar flashes.
Breath rate digital
display flashes.
Breath rate icon
flashes.
In BiPhasic models
with monitored NCPAP
+ Apnea, a single sigh
is given.
In BiPhasic Tr Mode
backup sighs at the set
rate are given.
Alarm Button flashes.
The timer is reset and
the applicable sigh
function is repeated
until breathing
restoration is detected
or the Operator
intervenes.
Method of Resetting
or Canceling
Refer to Service
Engineer.
Refer to Service
Engineer.
Push the Alarm
Button once to stop
the Audible Alarm for
30 seconds (Alarm
Button flashes,
breath rate icon and
digital display still
flashes, Warning Bar
still flashes, in
BiPhasic Tr Mode
backup sighs at the
set rate continue).
Restore patient
breathing.
If breath detection is
restored before the
next breath rate
timeout period has
elapsed, the alarm
condition is
automatically
cleared.
BiPhasic/BiPhasic Tr
mode fails to operate as
set.
675-120 Rev. G
Automatic monitoring Intermittent high level
Audible Alarm.
Warning Bar flashes
intermittently.
NCPAP Pres high on
time display flashes.
Screen displays
flashing fault code (E
number).
Cannot be silenced
Revert to NCPAP
mode or refer to
Service Engineer.
Page 58

48 Infant Flow
Diagnostics
Diagnostic mode is accessed by enabling DIP switch 6 and exited by disabling it.
Enable DIP switch 6 during Start Up, Adjust or Mode Selection.
The screen changes to the Diagnostics Menu Screen (for a description of the
displayed information, refer to pages 17-18):
• The alarm limits are disabled.
• Low level calibration procedure for pressure and oxygen sensors are
available via the key pad.
• Test procedures for valves and user interface are available via the key pad.
• The error log is accessible via the key pad.
Press to carry out a pressure calibration
The diagnostic main screen stays the same and the key pad at the bottom of the
screen changes. With n othing attached to the pressure line inlet port, touch the first
key to calibr ate at 0 cm H
and connect the circuit to the unit and the occlude prongs. Adjust the nCPAP
Flowmeter until pressure reads 10 cm H
gauge, and touch the second key to calibrate at 10 cm H
O. Connect the pressure gauge inline on a patient circuit
2
O ±0.2 cm H2Oon the external pressure
2
O.
2
®
LP SiPAP Model M675
To return to the Diagnostics Menu Screen, touch the
button.
Press
to carry out an oxygen calibration
The diagnostic main screen stays the same and the key pad at the bottom of the
screen changes. Set the blender knob at 21% and touch the first key to calibrate at
21 %. Set the blender knob at 100% O
100 % O
.
2
To return to the Diagnostics Menu Screen, touch the
and touch the second key to calibrate at
2
button.
is currently inactive..
Press to carry out a valve test
The diagnostic main screen stays the same and the key pad at the bottom of the
screen changes. Touch the first key to test the zero valve and listen for click of valve,
the second key to test the dump valve and listen for click of the valve, and the third
key to test the PA valve and listen for the click of the valve.
To return to the Diagnostics Menu Screen, touch the
675-120 Rev. G
button.
Page 59

Service Manual 49
Code
Warning Bar on.
power applied.
power).
assistance.
Press to carry out a user interface test.
The diagnostic main screen stays the same and the key pad layout changes. Touch
the first key to test the battery, the second key to test the audible alarm and the third
to test the respiratory indicator.
To return to the Diagnostics Menu Screen, touch the
Press the
The screen changes to the Error Log Screen and shows a list of error code numbers.
The error code is:
• Shown normal if it has occurred since the last reset
• Shown grayed if it is inactive since the last reset
• Flashing if the error is active since power up or last cleared
To mute flashing errors, the
Refer to Table 6 for the details of error codes and corrective action.
When the corrective action has been taken, to clear all faults, press the
Error
- Program memory
Fault Condition Consequence
checksum error.
button to show the Error Log Screen
button.
Table 6 - Error Codes
Unusable – Software
corrupt, execution
inhibited.
button.
Software
Response
Hardware held in
permanent reset
condition with
button.
Corrective
Action
Reload
software.
- Battery too discharged
(<6.5 V) to operate LCD,
analogue and valve driver
circuits (no external
power).
- Battery too discharged
(<10 V) to operate
analogue and valve driver
circuits but sufficient for
LCD driver (no external
E10 Non-volatile memory fault. Unusable - unable to
675-120 Rev. G
Unusable - no user
interface display.
Unusable – sensor
readings invalid.
retrieve/set unit
configuration and
calibration data.
Hardware held in
reset condition
with Warning Bar
(status LED off)
on until external
User lockout
‘Plug in external
power’ prompt
User lockout
‘Error number’
prompt.
Plug in
external
power.
Plug in
external
power.
Call
CareFusion
technical
support for
Page 60

50 Infant Flow
Code
sensors.
normal mode.
prompt.
alarm prompt.
support.
support
®
LP SiPAP Model M675
Error
Fault Condition Consequence
E11 Calibration data lost. Unusable – sensor
readings invalid.
E12 Configuration DIP settings
and/or PT PRESENT
different to non-volatile
Unusable – possible
incomplete unit set up
performed.
configuration record.
Software
Response
User lockout
‘Error number’
prompt.
User lockout
‘Error number’
prompt.
Corrective
Action
Put the unit
into
diagnostics
mode (DIP6
ON) and recalibrate O
2
and pressure
Ensure that all
the DIP switch
settings are
correct and
the patient
trigger
board/cable
are correctly
attached (if
required). Put
the unit into
diagnostics
mode, clear
the E12 error,
then return to
E20 Charged battery voltage
too low (<11 V) when
under test load.
E21 External supply voltage too
low (<14 V) to charge
battery (battery flat).
E22 Analog supply rails out of
limits.
E23 Valve driver supply rails
out of limits.
No backup – battery
capacity low.
No backup – battery will
not charge.
Unusable - unreliable
sensor readings.
Unusable - unreliable
valve operations.
Battery fault icon
flashes and ‘Error
number’ alarm
Battery low alarm
continues even
when external
power applied.
‘Error number’
User lockout
‘Error number’
prompt.
User lockout
‘Error number’
prompt.
Replace the
battery.
Contact
CareFusion
technical
support.
Contact
CareFusion
technical
Contact
CareFusion
technical
675-120 Rev. G
Page 61

Service Manual 51
Code
support.
sensors.
sensors.
sensors.
sensors.
sensors.
Error
Fault Condition Consequence
E24 Hardware safe-start
watchdog disabled.
E30 Pressure sensor fault
(ADC hits rail).
E31 Zero valve not connected
(via sense).
E32 Zero valve activation fault
(via sense).
Unusable – valve
disabled.
Unusable – pressure
sensor readings invalid.
Unusable – pressure
sensor readings
unreliable.
Unusable – pressure
sensor readings
unreliable.
Software
Response
User lockout
‘Error number’
prompt.
User lockout
‘Error number’
prompt.
User lockout
‘Error number’
prompt.
User lockout
‘Error number’
prompt.
Corrective
Action
Power cycle
unit. If fault
still occurs,
Contact
CareFusion
technical
Replace the
Valve/Sensor
PCB and recalibrate O
2
and pressure
Replace the
Valve/Sensor
PCB and recalibrate O
2
and pressure
Replace the
Valve/Sensor
PCB and recalibrate O
2
and pressure
E33 Unable to auto zero
pressure sensor.
E41 Dump valve not connected
(via sense).
E42 Dump valve activation fault
(via sense).
Unusable – pressure
sensor readings
unreliable.
Restricted - no over
pressure protection.
Restricted - no over
pressure protection.
User lockout
‘Error number’
prompt.
Restricted mode
‘Error number’
alarm prompt.
Restricted mode
‘Error number’
alarm prompt.
Replace the
Valve/Sensor
PCB and recalibrate O
2
and pressure
Replace the
Valve/Sensor
PCB and recalibrate O
2
and pressure
sensors.
Replace the
Valve/Sensor
PCB and recalibrate O
2
and pressure
675-120 Rev. G
Page 62

52 Infant Flow
Code
sensors
circuits.
O2 sensor.
circuits.
calibrate.
®
LP SiPAP Model M675
Error
Fault Condition Consequence
E50 Oxygen sensor fault (ADC
hits rail).
E51 Oxygen sensor cannot be
calibrated by user (bad
offset or high gain).
No oxygen monitor –
oxygen sensor
readings invalid.
No oxygen monitor –
possible fuel cell,
electronic, blender or
gas supply fault.
Software
Response
”E50” displayed;
high priority
alarm.
”E51 displayed;
high priority
alarm.
Corrective
Action
Replace the
O2 Sensor
and recalibrate O
2
Check the
Error Log
screen in
diagnostics
mode. If an
E52 error also
occurs,
replace the
fuel cell and
re-calibrate the
sensor-
O
2
otherwise
check the
blender ,gas
supplies, or
E52 Oxygen sensor calibrates
but the fuel cell is worn out
(low gain).
E53 Oxygen sensor too noisy
to calibrate (calibration
timeout).
Oxygen calibration suspect
(oxygen value has been
E54
measured as outside range
18 – 104%)
Oxygen sensor disabled by
the operator
E55
No oxygen monitor –
oxygen sensor
readings unreliable.
No oxygen monitor –
oxygen sensor
readings unreliable.
Oxygen sensor
readings unreliable
No oxygen or alarm
monitoring
”E52” displayed;
high priority
alarm.
”E53 displayed;
high priority
alarm.
”E54 displayed;
high priority
alarm.
“E55” alarm
displayed.
Replace the
fuel cell
O
2
and recalibrate the
Replace the
fuel cell
O
2
and recalibrate the
sensor or
O
2
Recalibrate
sensor. If
O
2
this fails
replace the O
cell and re-
Re-power the
device to reenable
oxygen
monitoring.
2
675-120 Rev. G
Page 63

Service Manual 53
Code
sensor.
sensor.
connected.
Apnea.
dysfunctional.
alarm prompt.
support
alarm prompt.
support
support
support
Error
Fault Condition Consequence
E61 NCPAP Pres high valve
not connected (via sense).
E62 NCPAP Pres high valve
activation fault (via sense).
E70 PT module fault (PTRDY
or CAN bus failure).
Restricted -
BiPhasic/BiPhasic
unusable.
Restricted BiPhasic/BiPhasic Tr
unusable.
Untriggerable - Apnea
and PT unusable.
Software
Response
Restricted mode.
‘Error number’
alarm prompt.
Restricted mode.
‘Error number’
alarm prompt.
Reduced
functionality
‘Error number’
alarm prompt.
Corrective
Action
Replace the
Valve/Sensor
PCB and recalibrate the
and
O
2
pressure
Replace the
Valve/Sensor
PCB and recalibrate the
and
O
2
pressure
Check the
Patient
Trigger
interface
module/cables
are correctly
E71 No breath signal from PT
module although CAN
data does not report
E72 No trigger signal from PT
module in BiPhasic Tr
mode.
E90* Spurious software
interrupt, XTAL fails, stack
overflow/ underflow, CPU
Class B exception.
E90 Abnormal hardware,
software or watchdog
reset.
E91 Internal software error
detected.
Untriggerable - Patient
may be in Apnea but
PT module may be
Untriggerable – No
BiPhasic Tr treatment
given to patient.
Spurious – software
interrupted and restarts
(possibly during
treatment).
Spurious – software
restarts possibly during
treatment.
Unusable – software
unreliable.
Reduced
functionality
‘Error number’
Reduced
functionality
‘Error number’
Hardware
reinitialized
(disabled) with
alarm bar on and
audible alarm
sounding to
identify root
cause.
Software restarts
‘Error number’
alarm prompt.
User lockout
‘Error number’
prompt.
Contact
CareFusion
technical
Contact
CareFusion
technical
Contact
CareFusion
technical
support.
Contact
CareFusion
technical
Contact
CareFusion
technical
675-120 Rev. G
Page 64

54 Infant Flow
Code
support
®
LP SiPAP Model M675
Error
E99 Unknown error detected. Unusable – software
* - Generated as a c onsequenc e of the fault.
Fault Condition Consequence
unreliable.
Software
Response
User lockout
‘Error number’
prompt.
Corrective
Action
Contact
CareFusion
technical
675-120 Rev. G
Page 65

Service Manual 55
Chapter 7 – Maintenance
Maintenance Frequencies
There are three different types of Preventative Maintenances required for the LP
SiPAP/SiPAP system.
1. Annual PM- Placement of annual PM Kit, P/N 777242-101 in unit. Contents
include: battery, O2 sensor, various filters and O-rings.
2. Two Year PM- Placement of annual PM Kit and blender overhaul. Annual PM Kit
and Low Flow MicroBlender Overhaul Kit P/N 10003 required.
3. Five Year PM- Placement Five Year PM Kit, P/N 777242-103. Contents include:
annual PM kit and Valve/Sensor PCB.
PM Procedure
1. Replacement of designated parts
2. User Verification Test (UVT)
3. Oxygen Leak Test
Tools Required
• #1 Phillips Screwdriver
• #2 Phillips Screwdriver
• Flathead Screwdriver
• Pliers
• Circle Plie r s
• If unit has blender knob P/N 22562, blender knob removal tool P/N 93244
• If unit has blender knob P/N467472, 5/16 nut driver
675-120 Rev. G
Page 66

56 Infant Flow
Cleaning
CAUTION!
Do not immerse any part of the IFSD in water or sterilize it with gas or steam.
1 Clean the exterior surfaces of the IFSD and the transducer interface with a mild
soap or liquid disinfectant solution. Do not use cleaning agents that contain
abrasives.
2 Spray cleaning solution onto a cloth, and then wipe the exterior of the unit. DO
NOT spray the solution directly onto the driver, because liquid may get inside
the unit and cause damage .Make sure that cleaning agents do not enter the
unit through patient connection ports.
WARNING!
Oxygen vigorously accelerates combustion. To avoid explosion
hazard, do not use any instrument or other equipment that may have
been exposed to oil or grease contamination.
®
LP SiPAP Model M675
General
1 Examine the exterior of the case for damage and dirt. If necessary clean the
unit.
2 Check the water trap on the back panel. If water is visible in the water tr ap, push
the button on the bottom of the water trap to release the water .
3 Technical Assistance can be obtained from the offices listed at the beg inning of
this manual.
4 Store the unit in a clean dry location. Make sure that the connections and ports
are suitably blanked to prevent the ingress of dirt, moisture and foreign objects. If
the unit is not being used for a long period of time, remove the battery.
5 Dispose of scrap units in accordance with the local regulations
Removal and Fitting of Parts – General
1 Only parts approved by CareFusion may be used in this unit.
2 Remove all attached patient connections before removal of parts. Disconnect
the unit from the power supply.
3 Place the unit on a stable, clean work surface before removal of parts.
4 Numbers in parenthesis in the Removal/Fitting procedures are the item numbers
on the associated figure.
675-120 Rev. G
Page 67

Service Manual 57
Number
777244
Battery
60
06804
Oxygen Filter
61
465474
Banjo Screw O-Ring
61
465457
68289
O2 Sensor
63
465476
O-Ring on Front Panel
64 – 65, Steps 1 – 12
467269
Water Trap Filter
68
675-230
Pilot Drive Check Valves
68
Restrictor
11507
Muffler Filter
70
Table 7 shows the part numbers, components and page reference for removal
directions for the annual pm kit.
Table 7- Annual PM Kit Components
Part Number Components
672-024-SA
465474
Fuel Cell Filter/Restr ictor
and O-rings
467542 Case Bleed Filtered
Removal Directions Page
62
69
675-120 Rev. G
Page 68

58 Infant Flow
Number
11329 or 11798
Blender
64 – 67
Overhaul Kit
Service Manual
777242-101
Annual PM Kit
See Table 7
Number
777242-101
Annual PM Kit
See Table 7
52700A
Valve/Sensor PCB
70
Table 8 shows the two year pm components and page number references.
Table 8- Two Year PM Components
Part Number Component
Removal Direction Page
®
LP SiPAP Model M675
10003 Low Flow MicroBlender
Table 9 shows the fiver year PM components and page number references.
Table 9- Five Year PM Components
Part Number Component
L1130 Low Flow MicroBlender
Removal Direction Page
675-120 Rev. G
Page 69

Service Manual 59
Removal and Fitting of Case
Refer to Figure 12.
Removal
1 Remove eight screws (1) on the rear panel and two screws (1) on the case-
bottom.
2 Pull off the case (2).
Fitting
1 Install the case (2) over the main frame, making sure that all wires and tubing
are secure and cannot be trapped by the case.
2 Install the ten screws (1) on the rear panel and tighten.
3. Carry out an Oxygen Leak check (see Appendix A on page 75) and functional
check of the IFSD.
Note:
An Oxygen Leak test must be done after every service that requires removal of the
case to ensure no buildup of oxygen is present within the case.
2
Figure 12: Case Removal/Fitting
675-120 Rev. G
1
1
1
Page 70

60 Infant Flow
Removal and Fitting of Battery
Refer to Figure 13.
Removal
1 Remove the case.
2 Disconnect the battery lead connector SK3 (1) (cable 5) from the plug PL3 on
the Main Processor PCB. Remove the screw (2) on the rear panel and remove the
spacer (3). Note position of cable tie securing battery, then cut and remove
cable tie.
3 Pull the battery (4) complete with lead from the compartment.
Note:
The battery is held in position in the compartment by a tab on the frame and
secured by cable tie P/N 461964.
4 Feed the battery lead through the frame, pull the grommet (6) from the recessed
hole in the frame and remove the battery.
5. Disconnect the positive and negative leads from the battery. Make note of where
each lead goes.
®
LP SiPAP Model M675
Note:
Exhausted batteries and oxygen fuel cells both contain lead and must be
disposed of according to local regulations.
Fitting
1 Feed the battery lead through the frame to plug PL3 on the Main Processor
PCB.
2. Reconnect positive and negative leads to battery.
CAUTION!
Verify that the leads are connected correctly. If leads are connected
incorrectl y , damag e to the system will result.
3 Slide the battery into the compartment against the tab (5) and position the
grommet (6) in the recessed hole in the frame.
4 Install the spacer (3) and secure in position with the screw (2). Tighten the
screw (2).
5 Connect the battery lead connector SK3 (1) to the plug PL3 on the Main
Processor PCB.
6 Install new cable tie securing the battery against the metal tab.
675-120 Rev. G
Page 71

Service Manual 61
Figure 13: Battery Removal/Fitting
Removal and Fitting of Oxygen Filter
Note:
This procedure can be done without removing the case.
Refer to Figure 14.
Removal
1 Remove the banjo screw (1) on the end of the air/oxygen mounting block (2).
2 Remove the filter (3).
3 Remove and discard the O-ring (4).
Fitting
1 Install a new O-ring (P/N 465476) (4) on the banjo screw (1).
2 Install a new filter (P/N 467464) (3) in the air/oxygen mounting block and install
the banjo screw (1).
3 Tighten the banjo screw (1).
Figure 14: Oxygen Filter Removal/Fitting
675-120 Rev. G
Page 72

62 Infant Flow
®
LP SiPAP Model M675
Removal and Fitting of Fuel Cell Filter/Restrictor
Refer to Figure 15.
Removal
1 Remove the case.
2 Remove the oxygen filter screw (1) from the flow mixer block (2). Remove the
filter/restrictor (3). Remove and discard the O-ring (4) and the O-ring (5) from
the oxygen filter screw (1).
Fitting
Install a new O-ring (P/N 465474) (5) on the oxygen filter screw (1). Install a new Oring (P/N 465457) (4) and the filter/ restrictor (P/N 672-024-SA) (3) into the flow
mixer block (2). Install the oxygen filter screw (1) and tighten.
Figure 15: Oxygen Filter Restrictor Removal/Fitting
675-120 Rev. G
Page 73

Service Manual 63
Removal and Fitting of the Oxygen Sensor
Refer to Figure 16.
Removal
1 Remove the case.
2 Disconnect the connector SK12 (1) from the top of the oxygen sensor.
3 If necessary, remove the screw (2) securing the C clip (3) and release the C clip
securing the cable (cable 7) and the ferrite.
4 Unscrew the oxygen sensor (4) from the flow mixer block (5).
Note:
The oxygen sensor is installed finger tight, but it may be necessary to use a
suitable wrench on the flats at the top of the oxygen sensor to remove it. Th e
leak compensation tubing (6) can also be disconnected from the elbow to gain
access.
5 Discard the oxygen sensor (4) and attached O-ring.
Note:
Exhausted batteries and oxygen sensor both contain lead and must be disposed
of according to local regulations.
Fitting
1 Check the expiration date of the new sensor. Remove the O2 Sensor (P/N
68289) from the sealed package and check that the fuel cell has an O-ring
installed above the threads.
2 Screw the sensor (4) into the flow mixer block (5) and tighten finger tight.
3 If removed, secure the cable (cable 7) and the ferrite with C clip (3) and the
screw (2).
4 Connect the connector SK12 (1) to the sensor.
675-120 Rev. G
Page 74

64 Infant Flow
Figure 16: Fuel Cell Removal/Fitting
®
LP SiPAP Model M675
Removal and Fitting of Blender and Components
Refer to Figure 17.
Note:
Two different blenders are used in the LP SiPAP, P/N 11329 and 11798. These
blenders are NOT interchangeable. Contact CareFusion technical support for further
assistance.
Removal
1 Remove the case.
2 Remove the battery.
3 Disconnect the connector SK1 (1) on the LED PCB.
4 Disconnect the connector PL5 (2) on the Main Processor PCB.
5 Disconnect the connector at PL2 (3) on the Patient Trigger PCB.
1
6 Remove the input connection tube (4) by pulling the elbow off the
tube, taking care not to break the barbs on the elbow.
/8 “ silicone
Note:
There are trwo types of blender knobs used on the LP SiPAP. P/N 467472 has a
removable front cap and the knob can be removed with a 5/16 Nut driver. PN
22562 requires the blender knob removal tool, P/N 93244.
675-120 Rev. G
Page 75

Service Manual 65
7 For blender knob P/N 467472, remove the cover (6) from the front of the FiO2
control knob (7). Using the 5/16 nut driver, unscrew the collet on the front of the
FiO
control knob (7), remove the collet and pull off the control knob (7).
2
For blender knob P/N 22562, use the blender knob removal tool, P/N 93244, to
remove the blender knob.
8 Remove the two covers (8) from the front of the NCPAP Pres low and NCPAP
Pres high flow control knobs (9) by gently prying out of the recess in the knob
with a small screwdriver.
9 Using a screwdriver or Circle pliers, unscrew the central securing nuts for the
flowmeter knobs and pull off the control knobs (9).
10 Remove the eight screws (10) from the front panel (11).
11 Pull the front panel assembly (11) off the frame, taking care not to damage any
tubes.
12 Remove and discard the O-ring (12) from the Pop Off valve on the Front Panel.
Replace the O-Ring (P/N 46546)
13 Remove the Valve/Sensor PCB (13) as follows:
a Disconnect the fuel cell connector SK1 (14) at PL1 on the Valve/Sensor
PCB.
b Disconnect the dump/vent valve connector SK3 (15) at PL3 on the
Valve/Sensor PCB.
c Remove the f ive screws (16).
d Pull the Valve/Sensor PCB (13) downwards and out forward to
disconnect the PCB from the D plug PL2.
e Disconnect the exhaust tubes from the back of the PCB and the PA flow
tube and remove the Valve/Sensor PCB.
14 At the blender, disconnect the air supply (17), the oxygen supply (18), the
whistle outlet tube (19) and the main outlet (20).
15 Remove the four screws (21) securing the blender (22) and remove the blender.
Do not remove the collet knob on the front of the blender.
16 Overhaul blender per instructions in Low Flow MicroBlender Service Manual P/N
L1130.
17 To replace the alarm reed components, locate the reed alarm cap.
This will be located either on the back of the unit to the right of t he inlet block or
on the inlet gas block itself. Refer to directions in the blender service manual to
replace the reed alarm.
Note:
If the alarm cap is not in either location, contact CareFusion technical support.
675-120 Rev. G
Page 76

66 Infant Flow
®
LP SiPAP Model M675
Figure 17: Blender and Components Removal/Fitting
675-120 Rev. G
Page 77

Service Manual 67
Fitting
1 Fit the blender or refurbished blender (22) with the four screws (21) into the front
frame panel.
2 Connect the air supply (17), the oxygen supply (18), the whistle outlet tube (19)
and the main outlet (20).
Note:
See Appendix D for the tubing diagram.
3 Fit the Valve/Sensor PCB (13) as follows:
a Connect the exhaust tubes and the PA flow tube.
b Fit the Valve/Sensor PCB (13) to the D plug PL2.
c Fit the five screws (16).
d Connect the fuel cell connector SK1 (14) at PL1 on the Valve/Sensor
PCB.
e Connect the dump/vent valve connector SK3 (15) at PL3 on the
Valve/Sensor PCB.
4 Fit the front panel assembly (11) and install the eight screws (10).
5 Set the FiO
so that the pointer is in the 21 % position and install the control knob. For P/N
467472, install the cover (6).
6 Install the NCPAP Pres low and NCPAP Pres high flow control knobs (9) on the
spindles. Using a screwdriver or Circle pliers tighten the central securing nuts.
Install the covers (8).
7 Install the input connection tube (4) on the elbow of the
8 Connect the connector at PL2 (3) on the Patient Trigger PCB.
9 Connect the connector PL5 (2) on the Main Processor PCB.
10 Connect the connector SK1 (1) on the LED PCB.
11 Install the battery.
collet on the blender to the 21 % position. Align the contr ol knob (7)
2
1
/8 “ silicone tube.
675-120 Rev. G
Page 78

68 Infant Flow
®
Removal and Fitting of Water Trap Filter
Refer to Figure 18.
Removal
1 Remove the filter bowl (1) using the water trap tool (P/N 673-051-A) included
with the IFSD.
2 Pull out and discard the filter (2) from the water trap.
Fitting
1 Install the new filter (P/N 467269) (2) in the water trap.
2 Screw on the water trap bowl (1) and tighten using the water trap tool.
Removal of the Pilot Drive Check Valves
LP SiPAP Model M675
Removal
1 Locate the pilot valve check valve behind the NCPAP flowmeter.
2. Cut the cable tie securing the valve to the flowmeter.
3. Remove the pilot drive check valve from tubing and discard.
4. Locate the pilot drive check valve behind the Pressure High Flowmeter.
5 Cut the cable tie securing the valve to the flowmeter.
6. Remove the pilot drive check valve from tubing and discard.
Fitting
1 Install the pilot drive check valve in tubing behind NCPAP Flowmeter .
2. Zip tie the check valve to the flowmeter.
3. Install the pilot drive check valve in tubing behind the Pressure Hig h
Flowmeter.
4 Zip-tie the check valve to the flowmeter.
675-120 Rev. G
Page 79

Service Manual 69
Removal and Fitt ing of Case Bleed Filt ered Restrictor
Refer to Figure 18.
Removal
1 Remove the case and loosen the clamp securing the restrictor to sheet metal.
2 Note restrictor flow direction, then remove the case bleed filtered restrictor (3)
from the 4mm tube.
3 Discard the case bleed filtered restrictor (3).
Fitting
1 Install the new case bleed filtered restrictor (P/N 467542) (3) to the tube in the
proper orientation.
2 Re-secure the restrictor to the sheet metal..
Figure 18: Water Trap Filter Removal/Fitting
675-120 Rev. G
Page 80

70 Infant Flow
®
LP SiPAP Model M675
Removal and Fitting of Val ve/Sensor PCB
See Figure 17.
Removal
1 Remove the case.
2 Disconnect the fuel cell connector SK1 (14) at PL1 on the Valve/sensor PCB.
3 Disconnect the dump/vent/valve connector SK3 (15) at PL3 on the Valve/Sensor
PCB.
4. Remove the five screws (16).
5. Pull the Valve/Sensor PCB (13) downwards and out forward to disconnect the
PCB from the D Plug PL-2
6 Disconnect the exhaust tubes on the back of the PCB and the flow tube and
remove the Valve/Sensor PCB.
Fitting
1 Connect the exhaust tubes to back of PCB and PA Flow tube.
2 Fit the Valve/Sensor PCB (13) into the D plug PL2.
3. Fit the five screws (16).
4. Connect the fuel cell connector SK! (14) at PL1 on the Valve/Sensor PCB.
5 Connect the dump/vent valve connector to SK3 (15) at PL3 on the Valve/Sensor
PCB.
Removal and Fitting of the Muffler/Filter
Removal
1. Remove the screw that secures the Muffler/Filter to the rear panel.
2. Remove the Muffler/Filter from the tubing and discard.
Fitting
1 Insert the Muffler/Filter (P/N 11507) into the tubing.
2 Install screw that secures Muffler/Filter to the rear panel.
675-120 Rev. G
Page 81

Service Manual 71
Symbol
Source / Compliance
Meaning
DOCUMENTS
Symbol #01-14 IEC 30878
Symbol #5031 IEC 60417
This symbol indicates DIRECT CURRENT (DC)
Symbol #01-01 IEC 60878
Symbol #01-02 IEC 60878
Chapter 8 – Explanation of Symbols
The following symbols may be referenced on the Infant Flow® LP SiPAP or SiPAP
driver or in accompanying documentation
2.5A/T 250 V
101010
Symbol #03-02 IEC 60878
Symbol #5016 IEC 60417 This symbol indicates a FUSE.
CareFusion Symbol
Symbol #5032 IEC 60417
Symbol #5019 IEC 60417
Symbol #01-20 IEC 60878
Symbol #5021 IEC 60417
Symbol # 01-24 IEC 60878
Symbol #5007 IEC 60417
Indicates ATTENTION, consult ACCOMPANYING
This symbol indicates an INTERNAL BATTERY
FUSE
Fuse holder and fuse rating
Electrical AC in le t
The equipment is suitable for alternating current.
This symbol indicates protective EARTH (ground).
This symbol indicates the EQUIPOTENTIAL
connection used to connect various parts of the
equipment or of a system to the same potential, not
necessarily being the earth (ground) potential (e.g.,
for local bonding).
Power ON
675-120 Rev. G
Symbol #5008 IEC 60417
Power ON (for part of the equipment)
MDD Directive 93/42/EEC CE Mark
Power OFF
Power OFF (for part of the equipment)
ETL Mark and Registration Number
Page 82

72 Infant Flow
Symbol
Source / Compliance
Meaning
connection.
®
LP SiPAP Model M675
CareFusion Symbol
CareFusion Symbol Respiratory Connection and Indicator
Symbol # 5333 IEC 60417
Symbol #02-03 IEC 60878
Operating temperature range of unit
Warning Bell
This symbol indicates TYPE B equipment, which
indicates equipment that provides a particular
degree of protection against electric shock,
particularly with regards to allowable leakage
current and reliability of the protective earth
Type BF patient applied part
Year of Manufacture
Unique Batch Number Identifier
Use Before Expiry Date shown
Year-Month
Single Use Only - Do NOT Re-use
Keep Dry
Keep Away from Heat
RS232 Connection
675-120 Rev. G
Page 83

Service Manual 73
Symbol
Description
Symbols used on buttons:
The following symbols are used to label user input areas within the g r aphical display.
As needed displays in this table are shown separately as Domestic US config urat ion
displays (left-hand column) and International configuration displays (right-hand
column).
High Priority Alarm Active
Medium Priority Alarm Active
Low Priority Alarm Active; medium and high priority
alarms are resolved. Alarm does not flash.
No alarms (active or resolved) are present
Active alarm silenced
Adjust BiPhasic rate
Adjust BiPhasic Tr backup rate
Adjust apnea alarm timeout
Adjust low breath rate alarm timeout
Adjust BiPhasic, BiPhasic Tr on time, and NCPAP
manual breath function
Decrease / Increase currently selected parameter
Go to mode select screen.
Nasal CPAP mode
675-120 Rev. G
Page 84

74 Infant Flow
cycle is delivered regardless of button press duration
Nasal CPAP mode with breath rate monitoring
BiPhasic mode
BiPhasic mode with breath rate monitoring
BiPhasic Tr mode with breath rate monitoring
Manual Breath. Single breath cycle at current settings
-High, NCPAP Pres high and O
for T
Toggle between Main Screen and Monitored Parameter
Screen
Go to user calibration mode screen
2
®
LP SiPAP Model M675
%. One breath
Confirm
Wait
Completed
Action has failed
Press to un-lock keypad
Warning message. To clear, press any of the three
icons.
Note:
Provision of labeling in this manual for any function should not be taken as evidence
that the function is available. For example parameter R
not currently approved for use in the US.
relates to BiPhasic Tr mode,
B
675-120 Rev. G
Page 85

Service Manual 75
Appendix A – Oxygen Leak Test
Oxygen Leak Test
This test should be performed after and Preventative Maintenance and/or repair has been
done on the LP SiPAP. This test will check for leaks within the enclosure.
Test equipment needed:
Calibrated oxygen analyzer
1. Connect the driver to the air and oxygen supplies. Plug unit into Main AC.
2. Connect the oxygen analyzer sampling tube to the driver by replacing the M6 pan
screw with an M6 fitting. Before operating the driver, take an initial O2
concentration reading. Plug the fitting.
3. Attach circuit and generator with prongs to unit. Switch the power on.
4. Press the Cal Icon on the touch screen and do a 2 point calibration of the O2
sensor (Figure A-1).
5. Set the NCPAP (Low Pressure) Flowmeter to about 8 LPM
SiPAP,
and then push the soft key button below the left Flowmeter icon (Figure A–
1).
, or 9 LPM on the LP
Figure A–1
6. Set the %O2 to 100% then push the soft key button below the blender icon.
675-120 Rev. G
Page 86

76 Infant Flow
7. Set the pre ssure Hi gh Flowmeter to about 2 LPM then push its soft key button
under the right Flowmeter icon.
The Respiratory Sensor option is not required, but to finish, select this soft key
button.
8. Accept the NCPAP settings by selecting the NCPAP soft key. (%O2 should
display 100) (Figure A–2).
®
LP SiPAP Model M675
Figure A–2
9. The next screen varies but select BiPhasic soft key (Figure A–3).
Figure A–3
675-120 Rev. G
Page 87

Service Manual 77
10. Accept the default BiPhasic settings by selecting the BiPhasic soft key again
(Figure A–4).
Figure A–4
11. Verify that the measured value on the external oxygen analyzer doesn’t exceed
23% FiO
. If so, then troubleshoot for an oxygen leak. Check all the fittings and
2
connections and repeat the Oxygen Leak Test (Figure A–5).
Figure A–5
675-120 Rev. G
Page 88

78 Infant Flow
®
Infant Flow
LP SiPAP an d SiPAP
Model M675
®
LP SiPAP Model M675
675-120 Rev. G
Page 89

Service Manual 79
Parameter
Min
Max
Accuracy
Units
Set Oxygen concentr ati on, %O2
21
100
3
%
15%
15%
BiPhasic on time, T
(inspiration time)
0.1
3.0 *
± 0.005
seconds
BiPhasic rate, R (respiration rate)
1
120
0.5
pm
respiration rate)
1
Parameter
Min
Max
Accuracy
Units
3
15%
Bi-level additional flow rate
0
5
15%
LPM
time)
0.5
Breath rate timeout
10
30
1
Seconds
DEFAULT SETTINGS
Inspiration Time
T
: 0.3 seconds (1.0 sec U.S) +/- 1%.
Rate during BiPhasic modes
R: 30 / minute (10 / minute U.S.)
Back Up Rate During BiPhasic Tr
Rb: 10 / minute
Apnea Interval
T
apnea
: 20 seconds (+/- 1%)
Appendix B – Product Configurations
Non-US Configuration Parameters
±
CPAP flow rate 0 15
Bi-level additional flow rate 0 5
-High
BiPhasic Tr backup rate, Rb (backup
1 120
±
±
±
± 0.5
LPM
LPM
pm
Breath rate timeout 10 30
* T-High automatically reduces at higher R and Rb rate settings to maintain a minimum off time of 100
milliseconds.
US Configuration Parameters
Inspired oxygen fraction, FiO2 21 100
CPAP flow rate 0 15
BiPhasic on time, T-High (inspiration
BiPhasic rate, R (respiration rate) 1 54
* T-High automatically reduces at higher R and Rb rate settings to maintain a minimum off time of 1000
milliseconds.
0.1 3.0 *
-High
±
±
±
±
± 0.005
±
±
seconds
%
LPM
seconds
Pm
675-120 Rev. G
Page 90

80 Infant Flow
®
Infant Flow
LP SiPAP an d SiPAP
Model M675
®
LP SiPAP Model M675
675-120 Rev. G
Page 91

Service Manual 81
Part Number
Description
467472
BLENDER CONTROL KNOB (for blender part number 11329)
467468
CAP, BLENDER CONTROL KNOB
467471
POINTER, BLENDER CONTROL KNOB
467469
FLOWMETER KNOB
467470
CAP, FLOWMETER KNOB
675-308
CASE
467467
FOOT, POLYURETHANE .5OD
467466
WASHER M3 CRINKLE BECU (FOR CASE)
675-200
FLOWMETER MK3 HIGH
675-201
FLOWMETER MK 3 LOW
777244
PACKAGED BATTERY, MK3
47072
SPACER ROUND M4X155MM NYLON
467595
COMPRESSION PA D
673-051-A
WATERTRAP TOOL
465476
O-RING, PATIENT CONNECTOR
672-024-SA
ASSEMBLY, FILTER
465457
O-RING, O2 SCREW/FLOWMETER/GAS BLOCK
465474
O-RING, O2 FILTER
68289
FUEL CELL
675-230
PILOT DRIVE DUCKBILL ASSY
467542
CASE BLEED FILTER RESTRICTOR
52700A
VALVE/SENSO R PCB
467459
FUSE TIME LAG 2.5A 250V
467458
POWER INPUT TWIN FUSE
675-307
AUX FLOW MOUNTING BLOCK
11798
ASSY BLENDER (Manufactured in 2008 or later)
10003
MAINT KIT MCRBLNDR+LOFLO
675-300
EXHAUST TUBE 90 DEGREE
675-101-xxx
OPS MAN INF FLOW LP SiPAP SYSTEM
677-002
TRANSDUCER INTERFACE
467349
ABDOMINAL RESP SENSOR 25PK
03895
VALVE CHECK DCKBL BLACK
06804
FILTER NYLON CONE INL
467269
FILTER ELEMENT (WATERTRAP)
Appendix C – Spare Parts
09220 FLOWMETER, AUXILIARY
465464 FILTER, WATERTRAP
675-120 Rev. G
Page 92

82 Infant Flow
Part Number
Description
467455
SWITCH COVER
22562
BLENDER KNOB (for blender part number 11798)
675-256
SWITCH CABLE ASSY
675-310-EN
FRONT PANEL (English)
675-310-FR
FRONT PANEL (French)
675-310-RO
FRONT PANEL (Icon)
675-316
MK3 TRIGGER LEUR
675-209
O2 SENSOR CABLE
777242-101
SERVICE KIT, Mk3 DRIVER ANNUAL
777242-103
SERVICE KIT, Mk3 DRIVER 5 YEAR
777072-101
ASSY O2 /AIR FITTINGS MARK III DISS
777072-102
ASSY O2 AIR FITTINGS MARK III NIST
777072-103
ASSY O2 /AIR FIT MARKIII FEMALE DISS
777072-103
ASSY O2 AIR FIT MARKIII AIR LIQUIDE
461964
CABLE TIE, BATTERY
52000-00239
CABLE TIE, CHECK VALVE ASSEMBLY
11329
ASSEMBLY BLENDER (MANUFACTURED BEFORE 2008)
93244
BLENDER KNOB REMOVAL TOOL FOR KNOB P/N 22562
L1130
LOW FLOW MICROBLENDER SERVIC E MANUAL
675-312 CONNECTOR PATIENT INLET
®
LP SiPAP Model M675
11431 ASSEMBLY, AUXILLIARY FLOWMETER
675-316 PROXIMAL PRESSURE LINE PORT
675-120 Rev. G
Page 93

Service Manual 83
Appendix D – Pneumatics Assembly
675-120 Rev. G
Page 94

84 Infant Flow
®
LP SiPAP Model M675
675-120 Rev. G
Page 95

Service Manual 85
Glossary
Term Meaning
Apnea
Bpm
cmH2O Centimeters of Water (Pressure)
CPAP Continuous Positive Airway Pressure
FiO2 Fraction of Inspired Oxygen
Generator
I/E Inspiration/Expiration ratio
IFSD Infant Flow® LP SiPAP Driver
LBR Low Breath Rate monitoring (US labeling)
LPM Liters per minute
MAP Mean Airway Pressure
BiPhasic
BiPhasic+Apnea BiPhasic Ventilation with Apnea monitoring (non-US labe ling)
Temporary inability to breathe; monitoring of breathing (non-US
labeling)
Breaths per minute (applies to each of spontaneous, triggered
and mandatory)
Patient attachment for delivering CPAP, used with nasal prongs or
mask
BiPhasic Ventilation - CPAP with additional pressure pulses,
time-triggered and time-cycled
BiPhasic +LBR BiPhasic with low breath rate monitoring (US labeling)
BiPhasic Tr
NCPAP Nasally applied CPAP
NCPAP+LBR NCPAP with Low Breath Rate monitoring (US labeling)
NCPAP+Apnea NCPAP with Low Breath Rate monitoring (non-US labeling)
Non-US labeling Labeling using non-linguistic symbols in place of English text
PEEP Positive End-Expiratory Pressur e
PIP Peak Inspired Pressure
BiPhasic Flow Inspiratory Pressure
Rate Mandatory BiPhasic rate ( per minute)
RB
RSP Patient ’s spontaneous respiratory rate (per minute)
s / sec Seconds
Sigh A single pulse of additional gas flow
Triggered BiPhasic Ventilation - CPAP with additional pressure
pulses, patient-triggered and time-cycled
Backup ventilator rate (in BiPhasic Tr mode during apnea
alarm, per minute; non-US labeling)
675-120 Rev. G
Page 96

86 Infant Flow
Term Meaning
Apnea Interval (non-US labeling) or Low Breath Rate (LBR)
T
apnea
/ T
LBR
T-High Machine breath inspiration time (seconds)
monitor alarm time (US-labeling); both in seconds
This mnemonic may also be associated with an alarm icon
®
LP SiPAP Model M675
Treatment
Application of NCPAP, BiPhasic, BiPhasic Tr, with or without
breath monitoring, to the patient
US labeling Labeling using English text in place of symbols and/or icons
675-120 Rev. G
 Loading...
Loading...