Page 1

Bio-Rad
Protein Assay
For Technical Service
Call Your Local Bio-Rad Office or
in the U.S. Call 1-800-4BIORAD
(1-800-424-6723)
Page 2

Table of Contents
Section 1 Introduction..................................................................... 1
1.1 Principle..................................................................................... 1
1.2 Product Description.................................................................... 3
1.3 Materials Required but Not Supplied......................................... 3
Section 2 Instructions...................................................................... 4
2.1 Reconstituting the Standard....................................................... 4
2.2 Standard Procedure.................................................................... 4
2.3 Microassay Procedure................................................................ 5
2.4 Microtiter Plate Protocols .......................................................... 6
Section 3 Common Questions......................................................... 8
Section 4 Troubleshooting Guide ................................................... 12
Section 5 Ordering Information..................................................... 13
Section 6 Safety Information.......................................................... 14
Section 7 References........................................................................ 24
Page 3

Section 1
Introduction
The Bio-Rad Protein Assay, based on the method of Bradford, is a
simple and accurate procedure for determining concentration of solubilized protein. It involves the addition of an acidic dye to protein solution,
and subsequent measurement at 595 nm with a spectrophotometer or
microplate reader. Comparison to a standard curve provides a relative
measurement of protein concentration.
1.1 Principle
The Bio-Rad Protein Assay is a dye-binding assay in which a differential color change of a dye occurs in response to various concentrations
of protein.1The absorbance maximum for an acidic solution of
Coomassie®Brilliant Blue G-250 dye shifts from 465 nm to 595 nm when
binding to protein occurs.
basic and aromatic amino acid residues, especially arginine.5Spector
found that the extinction coefficient of a dye-albumin complex solution
was constant over a 10-fold concentration range. Thus, Beer’s law may be
applied for accurate quantitation of protein by selecting an appropriate
ratio of dye volume to sample concentration.
Interferences may be caused by chemical-protein and/or chemical-dye
interactions. Table 1 lists those chemical reagents not directly affecting
the development of dye color. (Note: Basic buffer conditions and detergents interfere with this assay.) Since every protein-chemical reagent
combination has not been assayed, it is possible that some of the listed
reagents produce interference in combination with certain proteins.
However, with respect to proteins such as bovine serum albumin and
gamma globulin, the listed reagents show little or no interference. The
acceptable concentrations of reagents for the Standard Procedure are
shown in Table 1. Equivalent concentrations of reagents for the
Microassay Procedure (see Section 2) are 1/40 of those listed in this table,
due to the difference of sample-to-dye ratios between the Standard and
Microassay Procedures.
2,3,4
The Coomassie blue dye binds to primarily
6
1
Page 4

Table 1. Reagents Compatible with the Bio-Rad Protein Assay
When Using the Standard Procedure.*
Acetate, 0.6 M KCI, 1.0 M
Acetone Malic acid, 0.2 M
Adenosine, 1 mM MgCl
Amino Acids Mercaptoethanol, 1.0 M
Ammonium sulfate, 1.0 M MES, 0.7 M
Ampholytes, 0.5% Methanol
Acid pH MOPS, 0.2 M
ATP, 1 mM NaCl, 5 M
Barbital NAD, 1 mM
BES, 2.5 M NaSCN, 3 M
Boric acid Peptones
Cacodylate-Tris, 0.1 M Phenol, 5%
CDTA, 0.05 M Phosphate, 1.0 M
Citrate, 0.05 M PIPES, 0.5 M
Deoxycholate, 0.1% Polyadenylic acid, 1 mM
Dithiothreitol, 1 M Polypeptides (MW<3000)
DNA, 1 mg/ml Pyrophosphate, 0.2 M
EDTA, 0.1 M rRNA, 0.25 mg/ml
EGTA, 0.05 M tRNA, 0.4 mg/ml
Ethanol total RNA, 0.30 mg/ml
Eagle’s MEM SDS, 0.1%
Earle’s salt solution Sodium phosphate
Formic acid, 1.0 M Streptomycin sulfate, 20%
Fructose Triton X-100, 0.1%
Glucose Tricine
Glutathione Tyrosine, 1 mM
Glycerol, 99% Thymidine, 1 mM
Glycine, 0.1 M Tris, 2.0 M
Guanidine-HCI Urea, 6 M
Hank's salt solution Vitamins
HEPES buffer, 0.1 M
, 1.0 M
2
* Interference may be caused by chemical-protein and/or chemical-dye interactions. Table 1 lists
those chemical reagents not directly affecting the development of dye color. Since every protein-
chemical reagent combination has not been assayed, it is possible that some of the listed reagents
produce interference in combination with certain proteins. However, with respect to proteins such as
bovine albumin and globulin, the above listed reagents show little or no interference.
2
Page 5

1.2 Product Description
Protein Assay Dye Reagent Concentrate (catalog number 500-0006)
contains 450 ml of solution containing dye, phosphoric acid, and
methanol. One bottle of dye reagent concentrate is sufficient for 450
assays using the standard assay procedure, or 2,250 assays using the
microassay procedure.
The Dye Reagent Concentrate can be purchased in a kit with one of
two standards: Bovine gamma globulin (Kit I, catalog number 500-0001)
or bovine serum albumin (Kit II, catalog number 500-0002).
The Bio-Rad Protein Assay is for research use only.
1.3 Materials Required but Not Supplied
For standard assay
Spectrophotometer set to 595 nm
Cuvettes with 1 cm path length matched to laboratory spectropho-
tometer. Bio-Rad’s disposable polystyrene cuvettes (catalog number
223-9950) are recommended
13 x 100 mm test tubes
Test tube rack for 13 x 100 mm test tubes
Vortex mixer
Whatman #1 filter (or equivalent) and funnel for dye reagent prepara-
tion
Graduated cylinders, pipets, and containers for reagent preparation
and storage
Pipets accurately delivering 100 µl and 5.0 ml
For microplate assay
Microtiter plates
Pipets accurately delivering 200 µl and 800 µl
3
Page 6

Section 2
Instructions
2.1 Reconstituting the Standard
To reconstitute the lyophilized bovine gamma globulin and bovine
serum abumin standards, add 20 ml of deionized water and mix until dissolved. If the standard will not be used within 60 days, it should be
aliquoted and frozen at -20 °C.
Note: The standards contain buffer salts required for solubilizing the protein.
2.2 Standard Procedure
1. Prepare dye reagent by diluting 1 part Dye Reagent Concentrate with
4 parts distilled, deionized (DDI) water. Filter through Whatman #1
filter (or equivalent) to remove particulates. This diluted reagent may
be used for approximately 2 weeks when kept at room temperature.
2. Prepare three to five dilutions of a protein standard, which is repre-
sentative of the protein solution to be tested. The linear range of the
assay for BSA is 0.2 to 0.9 mg/ml, whereas with IgG the linear range
is 0.2 to 1.5 mg/ml. (See Common Questions, question 4, for more
information.)
3. Pipet 100 µl of each standard and sample solution into a clean, dry
test tube. Protein solutions are normally assayed in duplicate or tripli-
cate.
4. Add 5.0 ml of diluted dye reagent to each tube and vortex.
5. Incubate at room temperature for at least 5 minutes. Absorbance will
increase over time; samples should incubate at room temperature for
no more than 1 hour.
6. Measure absorbance at 595 nm.
4
Page 7
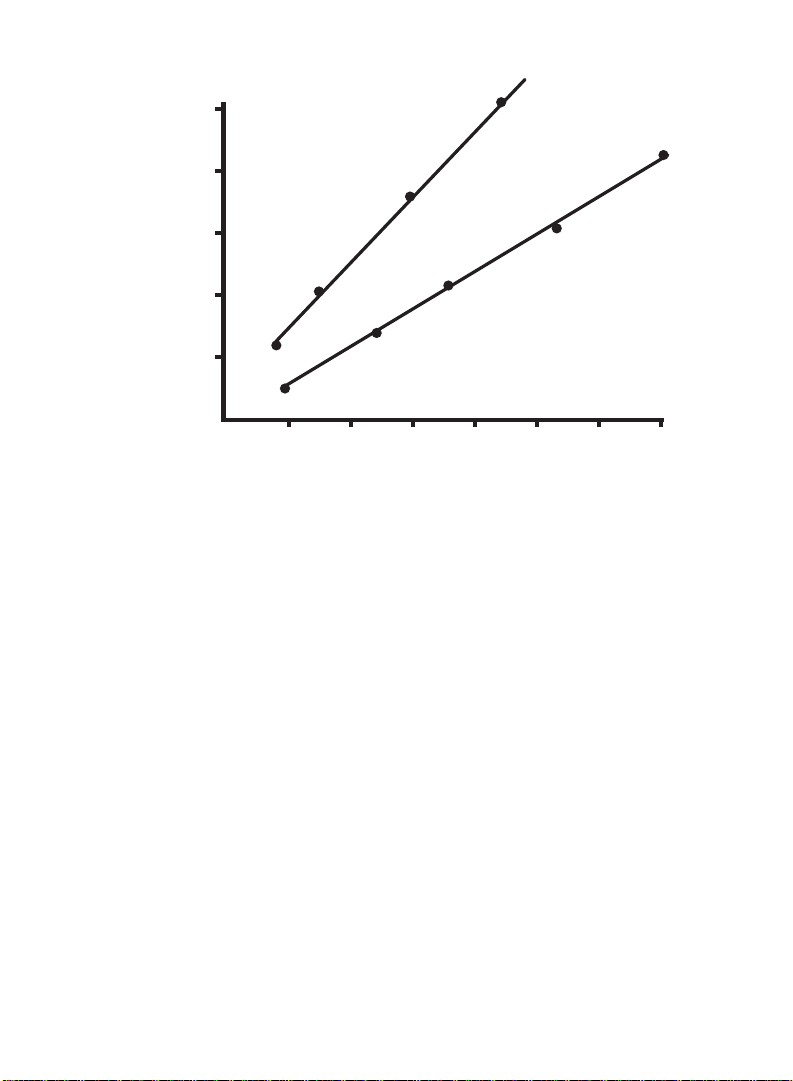
Standard procedure
(20-140 µg)
Protein (mg/ml)
BSA
IgG
O.D.
595
0.20
0.2
0.4
0.6
0.40 0.60 0.80 1.00 1.20 1.40
0.8
1.0
Fig. 1. Typical standard curve for the Bio-Rad Protein Assay, bovine gamma
globulin (standard I), bovine serum albumin (standard II). O.D.
blank - 200-1,400 µg/ml x 0.1 ml = 20-140 µg protein.
corrected for
595
2.3 Microassay Procedure
1. Prepare three to five dilutions of a protein standard which is represen-
tative of the protein solution to be tested. The linear range of the assay
for BSA is 1.2 to 10.0 µg/ml, whereas with IgG the linear range is 1.2
to 25 µg/ml. (See Common Questions, question 4, for more informa-
tion.)
2. Pipet 800 µl of each standard and sample solution into a clean, dry
test tube. Protein solutions are normally assayed in duplicate or tripli-
cate.
3. Add 200 µl of dye reagent concentrate to each tube and vortex.
4. Incubate at room temperature for at least 5 minutes. Absorbance will
increase over time; samples should incubate at room temperature for
no more than 1 hour.
5. Measure absorbance at 595 nm.
5
Page 8
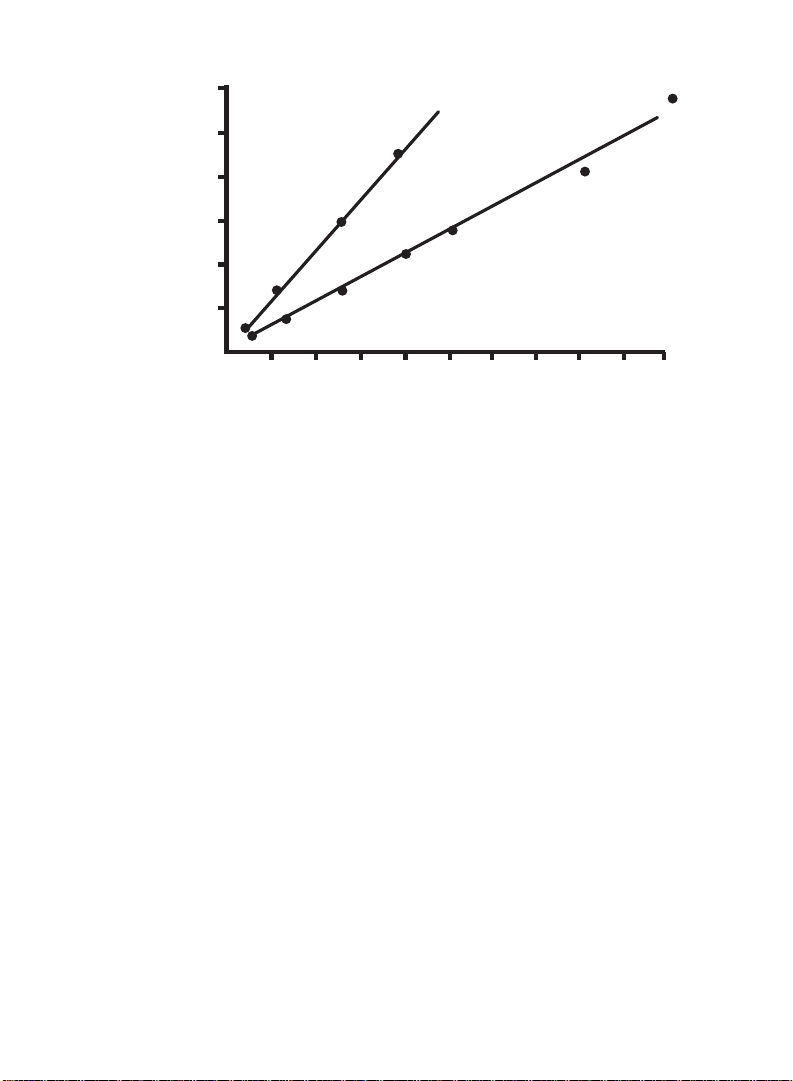
Fig. 2. Typical standard curve for the Bio-Rad Protein Microassay (1-20 µg/ml),
Microassay procedure
(1-20 µg)
Protein (µg,ml)
BSA
IgG
O.D.
595
2.5 5 7.5
10
12.5 15 17.5 20
22.5
25
0.1
0.2
0.3
0.4
0.5
0.6
bovine gamma globulin (standard I), bovine serum albumin (standard II).
O.D.
corrected for blank. 1.25-25 µg/ml x 0.8 ml = 1-20 µg protein.
595
2.4 Microtiter Plate Protocols
The Bio-Rad Protein Assay can also be used with a microplate reader,
such as Bio-Rad's Model 450 and 3550 Microplate Readers. The linear
range of the Standard and Microassay procedures when used in the
microtiter plate format is slightly changed, since the ratio of sample to dye
is modified.
Standard Procedure for Microtiter Plates
1. Prepare dye reagent by diluting 1 part Dye Reagent Concentrate with
2. Prepare three to five dilutions of a protein standard, which is repre-
4 parts DDI water. Filter through a Whatman #1 filter (or equivalent)
to remove particulates. This diluted reagent may be used for about 2
weeks when kept at room temperature.
sentative of the protein solution to be tested. The linear range of this
microtiter plate assay is 0.05 mg/ml to approximately 0.5 mg/ml.
Protein solutions are normally assayed in duplicate or triplicate.
6
Page 9

3. Pipet 10 µl of each standard and sample solution into separate
microtiter plate wells.
4. Add 200 µl of diluted dye reagent to each well. Mix the sample and
reagent thoroughly using a microplate mixer. Alternatively, use a
multi-channel pipet to dispense the reagent. Depress the plunger
repeatedly to mix the sample and reagent in the well. Replace with
clean tips and add reagent to the next set of wells.
5. Incubate at room temperature for at least 5 minutes. Absorbance will
increase over time; samples should incubate at room temperature for
no more than 1 hour.
6. Measure absorbance at 595 nm.
Microassay Procedure for Microtiter Plates
1. Prepare three to five dilutions of a protein standard, which is repre-
sentative of the protein solution to be tested. The linear range of the
assay is 8.0 µg/ml to approximately 80 µg/ml. Protein solutions are
normally assayed in duplicate or triplicate.
2. Pipet 160 µl of each standard and sample solution into separate
microtiter plate wells.
3. Add 40 µl of dye reagent concentrate to each well. Mix the sample
and reagent thoroughly using a microplate mixer. Alternatively, use a
multi-channel pipet to dispense the reagent. Depress the plunger
repeatedly to mix the sample and reagent in the well. Replace with
clean tips and add reagent to the next set of wells.
4. Incubate at room temperature for at least 5 minutes. Absorbance will
increase over time; samples should incubate at room temperature for
no more than 1 hour.
5. Measure absorbance at 595 nm.
7
Page 10

Section 3
Common Questions
1. The buffer that I normally use is not listed in the reagent compatibility
list. How will I know if it interferes with the Bio-Rad Protein Assay?
It is best to run two standard curves, one with protein in the same
buffer as your sample and one with protein in water, and then plot a
graph of protein concentration versus absorbance. If the buffer does
not interfere, the two graphs of the standard curve will have identical
slope. Partial interference can be compensated for by adding the
buffer or interfering component to the standard curve for the actual
protein assay.
2. My sample contains a detergent concentration which is incompatible
with the Bio-Rad Protein Assay. How can I assay for protein?
If the protein concentration is high enough, a sample with detergent
can be diluted so that the concentration of detergent is reduced to
0.1% or less. Excess detergent can also be removed from a protein
solution with Bio-Beads®SM-2 adsorbent.
Alternatively, the Bio-Rad DC (detergent compatible) Protein Assay
can be used. The DC Protein Assay is a modified Lowry assay which
works in the presence of 1% ionic or nonionic detergent. This two-
step method is ready to evaluate in just 15 minutes, and each kit will
assay up to 500 samples with the standard method, or 10,000 samples
with the microplate method.
3. Which protein assay method should I choose, the Bio-Rad (Bradford)
Protein Assay or the DC (detergent compatible) Protein Assay?
There are distinct advantages for each assay depending upon the
application. The Bio-Rad Protein Assay, based on the Bradford
method, can be used in the presence of sugars, DTT, and 2-mercap-
toethanol which may interfere with the Bio-Rad DC Protein Assay.
Alternatively, the Bio-Rad DC Protein Assay can be used in the pres-
ence of detergents and sodium hydroxide, two components known to
interfere with the Bradford assay. If the sample is in a buffer that is
compatible with both assays, then either may be used.
8
Page 11

4. My sample is a mixture of proteins. Which protein standard should I
use to generate the standard curve?
In any protein assay, the best protein to use as a standard is a purified
preparation of the protein being assayed. In the absence of such an
absolute reference protein, one must select another protein as a rela-
tive standard. The best relative standard to use is one which has simi-
lar properties to, and which gives a color yield similar to that of, the
protein(s) being assayed.
Any purified protein can be selected as a reference standard if only
relative protein values are desired. Bio-Rad offers two standards,
Bovine gamma globulin (Standard I, catalog number 500-0005) and
bovine serum albumin (Standard II, catalog number 500-0007).
5. How does the Bio-Rad Protein Assay compare to other assay methods?
The Bio-Rad Protein Assay compares favorably with two other pro-
tein assay methods, the Biuret and the Lowry. In the table below,
results are tabulated from dilutions made to gravimetrically prepared
10 mg/ml solutions of commercially available proteins. Bio-Rad
Protein Standard I (bovine gamma globulin) was used for the Lowry
and Bio-Rad Protein Assays, with bovine serum albumin was used for
the Biuret method. All three assays display considerable variation in
response to different proteins, but the averages were comparable.
9
Page 12

Table 2. Comparison of Bio-Rad, Lowry, and Biuret Protein
Assays for 23 Proteins
All assays were performed on appropriate dilutions of gravimetrically prepared 10 mg/ml solutions of commercially obtained proteins. Standards
were as described in the text.
Assay Results (Protein mg/ml)
Biuret Lowry Bio-Rad
1. Alcohol dehydrogenase 5.8 5.0 7.8
2. α-Amylase 6.8 6.0 8.3
3. Bovine serum albumin 9.7 8.4 21.1
4. Carbonic anhydrase 8.8 8.9 13.0
5. Catalase 7.6 6.3 9.7
6. α-Chymotrypsin 9.4 11.6 7.8
7. Cytochrome c* 25.7 11.3 25.3
8. Ovalbumin 10.2 10.1 9.4
9. Fibrinogen 6.2 7.3 7.8
10. Gamma globulin (rabbit) 9.4 11.8 8.0
11. β-Galactosidase 9.5 9.9 7.9
12. Hemoglobin (bovine)* 16.2 8.3 19.9
13. Histones 9.7 9.2 15.8
14. Hemocyanin 6.6 5.4 9.2
15. Lysozyme 10.4 12.6 9.9
16. Myoglobin* 13.7 7.9 20.7
17. Ovomucoid 7.8 8.3 5.9
18. Pepsin 9.8 12.4 4.1
19. Ribonuclease 11.8 15.9 5.3
20. Trypsin inhibitor (soy) 9.1 10.3 6.1
21. Transferrin 8.5 9.0 12.6
22. Trypsin 11.4 15.5 4.9
23. Thyroglobulin 7.7 8.2 9.3
Average 10.1 9.5 10.9
*Average 8.8 9.6 9.2
* The second average eliminates from the figures the values for the three colored proteins shown by
asterisks.
10
Page 13

6. Is any sample preparation required?
In general, no. However, the protein must be solubilized. (The sample
can not be a suspension or an unfiltered homogenate.)
7. Can protein bound to a solid phase be quantitated?
Yes, according to a recent reference in Analytical Biochemistry.
Consult Anal. Biochem. 200, 195 (1992) for additional information.
8. What is the shelf life of the dye reagent concentrate and the standards?
The Dye Reagent Concentrate is stable for 1 year. For optimum shelf
life, store at 4 ˚C.
Lyophilized preparations of Protein Standard I (bovine gamma globu-
lin) and Protein Standard II (bovine serum albumin), if included,
should be refrigerated upon arrival. These lyophilized preparations
have a shelf life of 1 year at 4 ˚C. Rehydrated and stored at 4 ˚C, the
protein solutions should be used within 60 days. Rehydrated and stored
in aliquots at -20 ˚C, the protein solutions should be used within 5
years. Avoid repeated freeze and thaw cycles of the protein standard.
9. Does the binding of the blue dye to cuvettes skew results?
The amount of dye that binds to cuvettes, especially quartz cuvettes,
is negligible.1Therefore, removal of the residual blue color between
each sample reading is unnecessary. However, since the cuvettes may
be used for subsequent procedures, there are several recommended
treatments for dye removal:
(a) Rinse cuvette with methanol, or
(b) Rinse cuvette with concentrated glassware detergent, fol-
lowed by DDI water, then acetone, or
(c) Soak cuvette in 0.1 N HCl for a few hours, then wash as in
(b).
Bio-Rad's disposable polystyrene cuvettes (catalog number 223-9950)
are recommended for the protein assay. They absorb less blue dye
than glass cuvettes, and the cuvettes can be used as the mixing vessels
for the standard assay if sample and reagent volumes are reduced to
50 µl and 2.50 ml, respectively. This technique eliminates the need for
test tubes, and yields twice as many assays per bottle of reagent.
11
Page 14

Section 4
Troubleshooting Guide
1. How can protein with a typical low dye response be assayed?
Occasionally a protein will be assayed which yields exceptionally low
color response to the Bio-Rad Protein Assay. One such protein is
gelatin. Although the standard range (20-140 µg) is not detectable
when the standard assay procedure is used, quantitation of the protein
is possible when the sample-to-dye ratio is changed. By using the
sample-to-dye ratio of the microassay procedure (800 µl sample + 200
µl dye reagent concentrate) a usable standard curve for moderate con-
centrations of gelatin is produced.
Therefore, for a protein which yields exceptionally low color response
to the Bio-Rad Protein Assay, quantitation in the standard range may
be possible when the microassay sample-to-dye ratio is used.
Other modifications of this dye binding assay to increase sensitivity
have been reported.
2. Absorbance of the protein solution is very low. What is the likely
cause?
The dye reagent concentrate may be old. If it is over 1 year old,
replace with a new bottle of reagent.
The sample may contain a substance which interferes with the reac-
tion, such as detergent or sodium hydroxide. Check the compatibility
guide (Table 1). Dilute the sample or switch to the DC Protein Assay
if necessary.
The molecular weight of the protein is low; the lower limit of detec-
tion for this method is approximately 3,000 to 5,000 daltons.
7,8
12
Page 15

Section 5
Ordering Information
Catalog Number Description
500-0001 Bio-Rad Protein Assay Kit I, includes 450 ml dye reagent
concentrate and lyophilized bovine gamma globulin standard
500-0002 Bio-Rad Protein Assay Kit II, includes 450 ml dye reagent
concentrate and lyophilized bovine serum albumin standard
500-0006 Bio-Rad Protein Assay Dye Reagent Concentrate, 450 ml
Related Materials
500-0005 Protein Standard I, bovine gamma globulin, lyophilized.
When reconstituted, it provides 20 ml of 1.4 ± 0.2 mg/ml
solution.
500-0007 Protein Standard II, bovine serum albumin, lyophilized.
When reconstituted, it provides 20 ml of 1.4 ± 0.2 mg/ml
solution.
500-0116 Bio-Rad
250 ml Reagent A, 2000 ml Reagent B, and 5 ml Reagent S.
500-0111 Bio-Rad
Reagents Package (catalog 500-0116) and lyophilized
bovine gamma globulin standard.
500-0112 Bio-Rad
Reagents Package (catalog 500-0116) and lyophilized
bovine serum albumin standard.
223-9950 Disposable Polystyrene Cuvettes, 100 - 3.5 ml cuvettes
223-9955 Disposable Polystyrene Cuvettes, 100 - 1.5 ml cuvettes
224-0096 Costar 96 Well Flat Bottom EIA Plate, polystyrene
microtiter plates, 5 per package, carton of 100
DC
Protein Assay Reagents Package, includes
DC
Protein Assay Kit I, includes contents of
DC
Protein Assay Kit II, includes contents of
13
Page 16

Section 6
Safety Information
MATERIAL SAFETY DATA SHEET
I. PRODUCT IDENTIFICATION
TRADE NAME: Methanol (as used in Protein Assay Kits I and II and
Dye Reagent Concentrate)
Catalog No.: 500-0001, 500-0002, 500-0006
Chemical identity, Common names: Methanol; Methyl alcohol;
Carbinol; Wood alcohol; Wood naphtha, Methyl hydroxide.
Formula: CH3OH M.W.: 32.04
MANUFACTURER’S NAME:
BIO-RAD LABORATORIES EMERGENCY PHONE No:
2000 ALFRED NOBEL DRIVE 510/741-1000
HERCULES, CALIFORNIA 94547
DATE PREPARED OR REVISED: March 24, 1994
NAME OF PREPARER: Roy Wood
II. HAZARDOUS INGREDIENTS
This product contains the following toxic chemical subject to the
reporting requirements of section 313 of the Emergency Planning and
Community Right-To-Know Act of 1986 and of 40 CFR 372:
Chemical CAS Exposure Limits in Air
Names Numbers Percent* ACGIH TLV OSHA PEL Other
Methanol 67-56-1 25% 200 ppm 200 ppm ACGIH TLV
(TWA) (TWA) 250 ppm
(STEL)
LD50:5628 mg/kg oral-rat (RTECS)
TWA = 8-hour Time Weighted Average
STEL = Short Term Exposure Limit (15 minute)
* The other 75% of the mix is 25% water and 50% phosphoric acid
[see MSDS #500-0001(b)].
14
Page 17

III. PHYSICAL/CHEMICAL CHARACTERISTICS
BOILING POINT: 64.5 ˚C (148 ˚F)
VAPOR PRESSURE: 97 mm Hg at 20 ˚C
EVAPORATION RATE (BUTYL ACETATE = 1): 5.9
SOLUBILITY IN WATER: Complete
APPEARANCE AND COLOR: Clear liquid, alcohol odor.
SPECIFIC GRAVITY(H2O = 1): 0.79
MELTING POINT: -98 °C (-144 ˚F)
VAPOR DENSITY(AIR = 1): 1.1
IV. FIRE AND EXPLOSION HAZARD DATA
FLASH POINT: 101 ˚F (for a 25% solution)
(METHOD USED): Closed Cup
FLAMMABLE LIMITS: LEL=6% UEL=36%
EXTINGUISHING MEDIA: Alcohol foam, carbon dioxide, dry chemi-
cal, or water fog.
SPECIAL FIRE FIGHTING PROCEDURES: Fire fighters should
wear full protective clothing and self-contained breathing apparatus with
full facepiece. Use water to keep fire-exposed containers cool.
UNUSUAL FIRE AND EXPLOSION HAZARDS: Vapor is heavier
than air and may travel along the ground. Never use a welding or cutting
torch near container (even empty). This material may burn with a flame
which is invisible in daylight.
V. HEALTH HAZARD INFORMATION
SYMPTOMS OF OVEREXPOSURE (for each potential route of exposure):
INHALED: Excessive inhalation can cause nasal and respiratory irritation, visual disturbance, blurred vision, dizziness, giddiness, weakness,
fatigue, nausea, vomiting, headache, possible unconsciousness, and
asphyxiation.
CONTACT WITH SKIN OR EYES: Can cause irritation, tearing,
blurred vision, drying of skin, and dermatitis.
15
Page 18

ABSORBED THROUGH SKIN: May be absorbed through intact skin
and produce systemic effects.
SWALLOWED: Can cause gastrointestinal irritation, headache, nausea,
vomiting, blindness, unconsciousness, and death.
HEALTH EFFECTS OR RISKS FROM EXPOSURE
ACUTE: Poison! Swallowing or breathing high concentrations of
methanol may produce headache, vomiting, nausea, irritation, dizziness,
weakness, fatigue, giddiness, blurred vision, unconsciousness, blindness,
and death. Methanol is extremely corrosive to the eyes.
Brief eye contact with the liquid or mist will severely damage the eyes
and prolonged eye contact can cause permanent eye injury which may be
followed by blindness.
Skin contact may irritate the skin, causing dermatitis. Methanol may be
absorbed through intact skin, causing systemic effects.
CHRONIC: Marked impairment of vision, central nervous system damage, and death have been reported after prolonged or repeated exposure.
Methyl alcohol may cause liver damage.
FIRST AID: EMERGENCY PROCEDURES
EYE CONTACT: Flush with large amounts of water for at least 15 minutes, lifting upper and lower lids occasionally. Get medical attention.
SKIN CONTACT: Flush skin with large amounts of water for at least 15
minutes, while removing contaminated clothing and shoes. Wash clothes
before reuse. Get medical attention.
INHALED: Remove victim to fresh air. If breathing has stopped, give
artificial respiration. Keep person warm. Get medical attention.
SWALLOWED: If conscious, immediately induce vomiting by giving
two glasses of water and sticking a finger down the throat. After vomiting, give milk or water. Never give anything by mouth to an unconscious
or convulsing person. Get medical attention immediately.
16
Page 19

SUSPECTED CANCER AGENT
X NO: THIS PRODUCT’S INGREDIENTS ARE NOT FOUND IN
THE LISTS BELOW.
YES: _____FEDERAL OSHA ______NTP ______IARC
MEDICAL CONDITIONS AGGRAVATED BY EXPOSURE
Those individuals with diseases of the eyes, liver, kidneys, and skin
may be at increased risk from exposure.
VI. REACTIVITY DATA
STABLE X UNSTABLE_________
CONDITIONS TO AVOID: Heat, sparks, and open flame.
INCOMPATIBILITY(Materials to avoid): Strong oxidizing agents,
many metals, certain plastics, chloroform.
HAZARDOUS DECOMPOSITION PRODUCTS: Carbon oxides and
formaldehyde may form when heated to decomposition.
HAZARDOUS POLYMERIZATION MAY OCCUR______ WILL
NOT OCCUR X
CONDITIONS TO AVOID: Heat, sparks, and open flame.
VII. SPILL, LEAK, AND DISPOSAL PROCEDURES
SPILL RESPONSE PROCEDURES: Dike and cover spill with noncombustible absorbent. Wear protective clothing including rubber apron,
rubber gloves, rubber boots, chemical goggles, and face shield. Wear an
appropriate NIOSH-approved respirator. Eliminate all ignition sources.
PREPARING WASTES FOR DISPOSAL: Comply with all applicable
federal, state, and local regulations on spill reporting, waste handling, and
waste disposal.
17
Page 20

VIII. SPECIAL HANDLING INFORMATION
VENTILATION AND ENGINEERING CONTROLS: Provide sufficient mechanical explosion-proof ventilation to maintain exposure below
exposure limits.
RESPIRATORY CONTROLS: Wear a NIOSH-approved respirator
appropriate for the vapor concentration at the point of use. Appropriate
respirators may be a supplied-air respirator or a self-contained breathing
apparatus.
EYE PROTECTION: Chemical splash goggles or face shield.
GLOVES: Wear resistant gloves such as neoprene.
OTHER CLOTHING AND EQUIPMENT: Impervious clothing and
boots.
WORK PRACTICES, HYGIENIC PRACTICES: Use good laboratory
practices. Wash hands after using and before eating. Do not eat, drink, or
smoke in the work area. Keep product away from heat, sparks, and
flames.
OTHER HANDLING AND STORAGE REQUIREMENTS: Store in
closed containers. All handling equipment should be electrically grounded. Store in a cool, dry, well-ventilated place away from incompatible
materials. Store in a flammable liquid storage area or cabinet. An eye
wash and safety shower should be nearby and ready for use.
PROTECTIVE MEASURES DURING MAINTENANCE OF CONTAMINATED EQUIPMENT: Protective clothing and appropriate respi-
ratory protection should be worn. Do not cut, grind, weld, or drill on or
near containers. Electrically ground all equipment and use only nonsparking tools.
We believe that the information contained herein is current as of the
date of this Material Safety Data Sheet. Since the use of this information
and conditions of use of the product are not within the control of Bio-Rad,
it is the user’s responsibility to handle the product under conditions of
safe use.
18
Page 21

MATERIAL SAFETY DATA SHEET
I. PRODUCT IDENTIFICATION
TRADE NAME: Phosphoric Acid (as used in Protein Assay Kits I & II
and the Dye Reagent Concentrate).
Catalog No.: 500-000l, 500-0002, 500-0006
Chemical identity, Common names: Phosphoric acid, ortho-phosphoric
acid, white phosphoric acid.
Formula: H3PO
4
M.W.: 98.00
MANUFACTURER’S NAME:
BIO-RAD LABORATORIES EMERGENCY PHONE No:
2000 ALFRED NOBEL DRIVE 510/741-1000
HERCULES, CALIFORNIA 94547
DATE PREPARED OR REVISED: March 31, 1993
NAME OF PREPARER: Roy Wood
II. HAZARDOUS INGREDIENTS
This product contains the following toxic chemical subject to the
reporting requirements of section 313 of the Emergency Planning and
Community Right-To-Know Act of 1986 and of 40 CFR 372:
Chemical CAS Exposure Limits in Air
Names Numbers Percent* ACGIH TLV OSHA PEL Other
Phosphoric 7664-38-2 50% (in mix) 1 mg/m
acid (TWA) (TWA) 3 mg/m
TWA=8-hour Time Weighted Average
STEL=Short Term Exposure Limit (l5 minute)
* The other 50% of the mix is 25% water and 25% methanol
[see MSDS #500-0001(a)].
3
1 mg/m3ACGIH TLV
3
(STEL)
19
Page 22

III. PHYSICAL/CHEMICAL CHARACTERISTICS
BOILING POINT: l58 ˚C (3l6 ˚F)
VAPOR PRESSURE: 0.03 mm Hg at 20 ˚C
EVAPORATION RATE (BUTYL ACETATE = 1): N/A
SOLUBILITY IN WATER: Complete
APPEARANCE AND COLOR: Clear, colorless, syrupy liquid.
Odorless.
SPECIFIC GRAVITY(H2O = 1): l.69
MELTING POINT: 2l ˚C (70 ˚F)
VAPOR DENSITY(AIR = 1): 3.4
IV. FIRE AND EXPLOSION HAZARD DATA
FLASH POINT: N/A (not flammable) FLAMMABLE LIMITS: N/A
(METHOD USED): N/A
EXTINGUISHING MEDIA: Water fog or water spray
SPECIAL FIRE FIGHTING PROCEDURES: Water may be used to
keep fire-exposed containers cool until fire is out. Fire fighters should
wear full protective clothing and self-contained breathing apparatus with
full facepiece.
UNUSUAL FIRE AND EXPLOSION HAZARDS: Phosphoric acid
reacts with most metals to release hydrogen gas which can form explosive
mixtures with air.
V. HEALTH HAZARD INFORMATION
SYMPTOMS OF OVEREXPOSURE (for each potential route of exposure):
INHALED: Irritation to nose, throat, and respiratory tract; coughing,
chest pain, and difficulty in breathing.
CONTACT WITH SKIN OR EYES: Corrosive; may cause redness,
burns, pain, blurred vision, severe irritation, and tissue damage.
ABSORBED THROUGH SKIN: Liquid can cause severe irritation and
burns to the skin.
20
Page 23

SWALLOWED: Corrosive; may cause sore throat, abdominal pain, nausea, and severe burns.
HEALTH EFFECTS OR RISKS FROM EXPOSURE
ACUTE: Phosphoric acid is extremely corrosive to the eyes, skin, nose,
mouth, throat, and mucous membranes. Bronchitis, pulmonary edema,
and chemical pneumonitis may occur with inhalation of vapors or mists.
Breathing high concentrations may result in death. Brief eye contact with
the liquid or mists will severely damage the eyes and prolonged contact
may cause permanent eye injury which may be followed by blindness.
Vapors will severely irritate skin.
Liquid and mists will severely burn the skin. Prolonged liquid contact will
burn or destroy surrounding tissue and death may occur if burns extend
over large portions of the body. Swallowing the liquid burns the tissues,
causes severe abdominal pain, nausea, vomiting, and collapse.
Swallowing large quantities can cause death.
CHRONIC: Skin contact may result in areas of destruction of skin tissue
or primary irritant dermatitis. Similarly, inhalation of vapors or mists may
cause damage to tissues and increase susceptibility to respiratory illness.
FIRST AID: EMERGENCY PROCEDURES
EYE CONTACT: Flush with large amounts of water for at least l5 minutes, lifting upper and lower lids occasionally. Get medical attention.
SKIN CONTACT: Flush skin with large amounts of water for at least l5
minutes, while removing contaminated clothing and shoes. Wash clothes
before reuse. Get medical attention.
INHALED: Remove to fresh air. If not breathing, give artificial respiration. Get medical attention.
SWALLOWED: DO NOT induce vomiting. If conscious, give large
quantities of milk or water. Never give anything by mouth to an unconscious or convulsing person. Get medical attention immediately.
21
Page 24

SUSPECTED CANCER AGENT
x NO: THIS PRODUCT’S INGREDIENTS ARE NOT FOUND IN
THE LISTS BELOW.
YES: _____FEDERAL OSHA ______NTP ______IARC
MEDICAL CONDITIONS AGGRAVATED BY EXPOSURE
History of respiratory or skin disease may increase risk from exposures.
VI. REACTIVITY DATA
STABLE x UNSTABLE_________
CONDITIONS TO AVOID: Contact with metals can form hydrogen gas
and be an explosion hazard.
INCOMPATIBILITY(Materials to avoid): Strong caustics, metals,
cyanides, sulfides, and sulfites. A strong mineral acid, contact with water
can cause heat generation and violent splattering.
HAZARDOUS DECOMPOSITION PRODUCTS: Toxic fumes of
phosphorous oxides.
HAZARDOUS POLYMERIZATION MAY OCCUR______ WILL
NOT OCCUR x
CONDITIONS TO AVOID: N/A
VII. SPILL, LEAK, AND DISPOSAL PROCEDURES
SPILL RESPONSE PROCEDURES: Dike and cover spill with
absorbent. Small spills can be carefully neutralized with sodium bicarbonate of lime. Wear protective clothing including an acid resistant suit, rubber gloves, rubber boots, chemical goggles, and face shield. Wear an
appropriate NIOSH-approved respirator.
PREPARING WASTES FOR DISPOSAL: Comply with all applicable
federal, state, and local regulations on spill reporting, waste handling, and
waste disposal.
22
Page 25

VIII. SPECIAL HANDLING INFORMATION
VENTILATION AND ENGINEERING CONTROLS: A local exhaust
system is recommended to maintain levels below exposure limits.
RESPIRATORY CONTROLS: Wear a NIOSH-approved respirator
appropriate for the vapor or mist concentration at the point of use.
Appropriate respirators may be a full facepiece air-purifying respirator
equipped with high efficiency cartridges, a supplied-air respirator, or a
self-contained breathing apparatus.
EYE PROTECTION: Wear chemical safety goggles and a full face
shield, where splashing is possible. Contact lenses should not be worn
when working with this material.
GLOVES: Wear chemical resistant gloves such as neoprene, nitrile rubber, or natural rubber.
OTHER CLOTHING AND EQUIPMENT: Impervious protective
clothing and boots.
WORK PRACTICES, HYGIENIC PRACTICES: Use good laboratory
practices. Wash hands after using and before eating. Do not eat, drink, or
smoke in the work area.
OTHER HANDLING AND STORAGE REQUIREMENTS: Keep in
tightly sealed container. Store in a cool, dry, well-ventilated place away
from incompatible materials. Corrosive to mild steel. Store in rubber lined
or 3l6 stainless steel. An eye wash and safety shower should be nearby
and ready for use.
PROTECTIVE MEASURES DURING MAINTENANCE OF CONTAMINATED EQUIPMENT: Protective clothing and appropriate respi-
ratory protection should be worn. Do not cut, grind, weld, or drill on or
near containers.
We believe that the information contained herein is current as of the
date of this Material Safety Data Sheet. Since the use of this information
and conditions of use of the product are not within the control of Bio-Rad,
it is the user’s responsibility to handle the product under conditions of
safe use.
23
Page 26

Section 7
References
1. Bradford, M., Anal. Biochem., 72, 248 (1976).
2. Reisner, A. H., Nemes, P. and Bucholtz, C., Anal. Biochem., 64, 509 (1975).
3. Fazakes de St. Groth, S. et al., Biochim. Biophys. Acta,71, 377 (1963).
4. Sedmack, J. J. and Grossberg, S. E., Anal. Biochem., 79, 544 (1977).
5. Compton, S. J. and Jones, C. G., Anal. Biochem., 151, 369 (1985).
6. Spector, T., Anal. Biochem., 86, 142 (1978).
7. Duhamel, R. C., Meezan, E. and Brendal, K., J. Biochem. Biophys. Methods,
5, 67 (1981).
8. Macart, M. and Gerbaut, L., Clin. Chim. Acta, 122, 93 (1982).
*Coomassie is a trademark of ICI.
24
Page 27

U.S. (800) 4BIORAD • California Ph. (510) 741-1000 • New York
Ph. (516) 756-2575
•
Australia Ph. 02-805-5000
•
Austria Ph. (1) 877
89 01
•
Belgium Ph. 09-385 55 11 • Canada Ph. (905) 712-2771 •
China
Ph. (01) 2046622 • Denmark Ph. 45-39 17 99 47 • France
Ph. (1) 49 60 68 34
•
Germany Ph. 089 31884-0
•
India Ph. 91-11-461-
0103
•
Italy Ph. 02-21609 1 • Japan Ph. 03-3534-7665 • Hong Kong
Ph. 7893300
•
The Netherlands Ph. 0318-540666 • New Zealand
Ph. 09-443 3099
•
Singapore Ph. (65) 4432529 • Spain Ph. (91) 661 70
85
•
Sweden Ph. 46 (0) 735 83 00 • Switzerland Ph. 01-809 55 55 •
United Kingdom Ph. 0800 181134
Life Science
Group
SIG 093094 Printed in USA
Bio-Rad
Laboratories
LIT33 Rev C
 Loading...
Loading...