Page 1
Separation of Oxindole Alkaloids
Application
Agrichemical
Robert Ricker
Oxindole Alkaloids are a major component (in addition to triterpenes) of root-bark extracts
from Uncaria tomentosa. These extracts have been used as folk medicine, and more
recently, to treat cancer, viral infections, intestinal and epidermal disorders, and arthritis.
Previous HPLC methods have given unsatisfactory results. Due to the potential
therapeutic importance of these compounds, a new, rugged HPLC method was developed.
Highlights
4
3
5
2
7
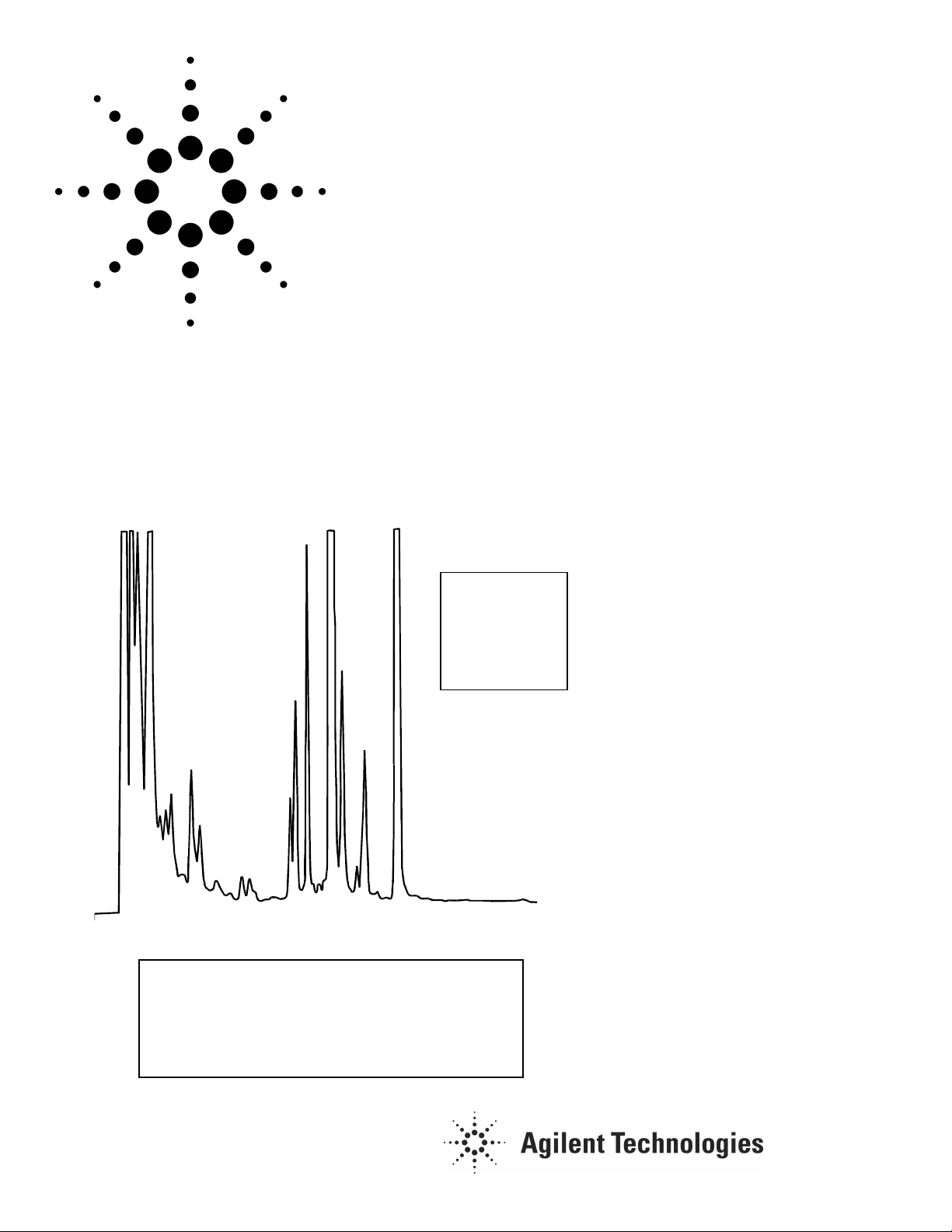
1. Spectiophyllin
2. Uncarin F
3. Mitraphyllin
4. Pteropodin
5. Rhynchophyllin
6. Isorrhynchophyllin
7. Isopteropodin
6
1
Courtesy of Dr. H. Stuppner, Inst. Pharmacognosy, Univ. of Innsbruck Austria
• High resolution and good peak shape
for a number of oxindole alkaloids on a
Rx-C18 at neutral pH.
• The good retention of the alkaloids
under these conditions separates
them from other UV absorbing
compounds in the sample.
• Use additional caution when running
silica-based columns at pH 6 and
above since silica degrades much
more quickly at higher pH.
Conditions:
Column: ZORBAX RX-C18, 4.6 x 250 mm (Agilent Part No. 880967-902)
Mobile Phase:
Gradient: 45-70% B in 35 min.
Injection: 10µL; UV: 245 nm; Flow: 1.0 mL / min.; 15°C
A 10 mM Phosphate Buffer pH 6.6
B (1:1) MeOH : ACN
Page 2

Robert Ricker is an application chemist
based at Agilent Technologies, Wilmington,
Delaware.
For more information on our products and
services, visit our website at:
www.agilent.com/chem
Copyright© 2002 Agilent Technologies, Inc.
All Rights Reserved. Reproduction,
adaptation or translation without prior
written permission is prohibited, except as
allowed under the copyright laws.
Agilent shall not be liable for errors
contained herein or for incidental or
consequential damages in connection with
the furnishing, performance, or use of this
material.
Information, descriptions, and specifications
in this publication are subject to change
without notice.
Printed in the USA
April 25, 2002
5988-6290EN
 Loading...
Loading...