
Preparative separation of a binary
compound mixture – recovery of pure
compounds and solvent consumption
Abstract
This Application Note describes the separation of two compounds from
a binary compound mixture using the Agilent 1100 Series purification
system. The parameters purity, recovery, analysis run time, solvent con-
sumption and liquid phase composition are monitored and their interre-
lation is explained. Further, we discuss how the analysis can be opti-
mized with regard to these parameters.
Application
Udo Huber
Introduction
Separation of discrete compounds
from a mixture is a typical task in
preparative liquid chromatography. The simplest task is isolating
two compounds from a binary
mixture, however, it is also possible to isolate pure compounds
from more complex compound
mixtures, such as natural product
extracts 1, for example. The compounds to isolate are typically isomers or enantiomers
2,3
.
In this Application Note we
demonstrate the separation of two
compounds from a binary compound mixture using the Agilent
1100 Series purification system.
The method developed on an analytical column was scaled up to
preparative scale and the compounds were separated in milligram quantities. The separation
was done repeatedly, varying the
composition of the liquid phase to
achieve a separation with good
recovery and purity with minimum
solvent consumption.
Equipment
The system included two Agilent
1100 Series preparative pumps, an
Agilent 1100 Series diode array
detector, an Agilent 1100 Series
column organizer and an Agilent
220 micro plate sampler modified
for higher flow rates. The system
was controlled using the Agilent
ChemStation (revision A.08.04)
and the micro plate sampling software (revision A.03.02).
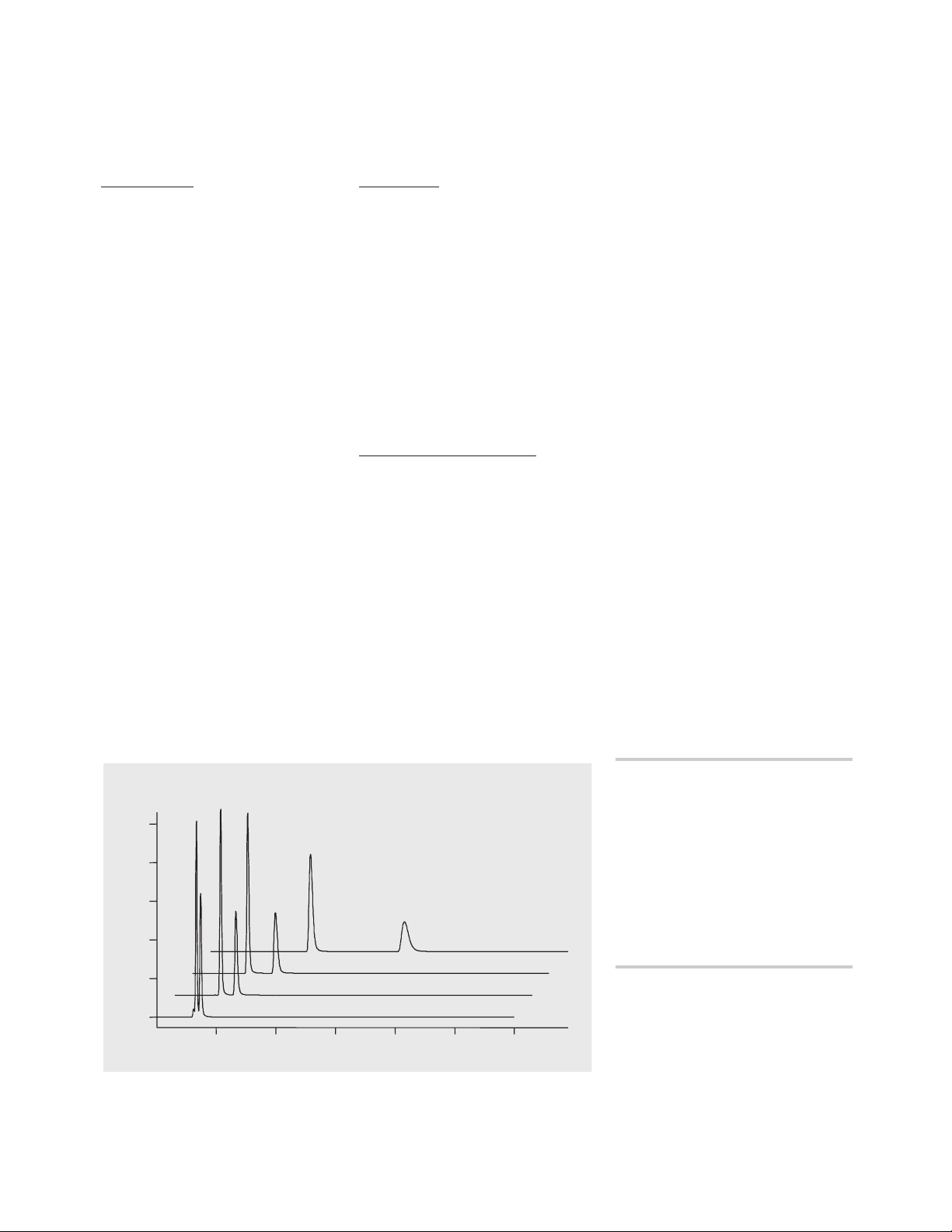
Results and Discussion
Overloading of the analytical column
The separation of the binary compound mixture was first done on
an analytical column. Separation
was achieved isocratically with a
water/ acetonitrile mixture
(figure 1).
Figure 1
Analytical separation of binary compound mixtures
Time [min]
24681012
Absorbance
[mAU]
0
500
1000
1500
2000
2500
30 % B
20 % B
15 % B
10 % B
Mobile phase: water = A
acetonitrile = B
Isocratic: between 10 and 30 % B
Stop time: 12 min
Column: Zorbax SB-C18
3 x 150 mm, 5 µm
Flow: 0.6 ml/min
Injection: 5 µl
Column temperature: ambient
UV detector: DAD 270 nm/16
(reference 360 nm/100)
Standard cell
(10 mm pathlength)

Since the crude product was very
soluble concentration overloading
was possible. Figure 2 shows that
the analytical column could be
loaded with up to 500–1000 µg of
each compound
Scale up to preparative scale
The scale-up from the analytical to
the preparative column was calculated using the formulae shown in
figure 3. After the first preparative
run the flow rate was changed to
25 ml/min to achieve comparable
retention times.
0.025 µg
0.25 µg
2.5 µg
25 µg
250 µg
Isocratic: 10 % B
Time [min]
2 4 6 8 10 12 14
Absorbance
[mAU]
0
1000
2000
3000
4000
5000
500 µg
1000 µg
Analytical column
Zorbax SB-C18
3 x 150 mm, 5 µm
Preparative column
Zorbax SB-C18
21.2 x 150 mm, 5 µm
Flow: 0.6 ml/min
Flow ~ 30 ml/min
Volume: 15 µl/injection
Volume: 750 µl/injection
x
1
=
r
2
1
×π
x1 = max. volume column 1
r1 = radius column 1
x2 = max. volume column 2
r2 = radius column 2
CL= ratio lengths of columns
= 15 µl
= 1.5 mm
= ?
= 10.6 mm
= 1
x
2
r
2
2
×π
×
1
C
L
V
1
V
2
r
1
r
2
2
2
=
.
.
Figure 2
Overloading of the analytical column
Figure 3
Scale up from analytical preparative column

taining the compound mixture.
The overall recovery of the compounds was 24.5 mg of compound
1 (98 %) and 23.9 mg of compound
2 (94 %). The overall analysis run
time could be minimized to about
2.6 minutes, that is, solvent consumption was about 65 ml per
analysis.
Fractionation under isocratic conditions with 20 % B in the mobile
phase
Figure 5a shows the preparative
run and the fractionation based on
retention time windows, figure 5b
shows the fractionation result.
About 21 mg of compound 1 with
a purity higher than 95 % could be
isolated, which is a recovery of
85 %. The isolated 23.5 mg of
Fractionation under isocratic conditions with 30 % B in the mobile
phase
Figure 4a shows the preparative
run and the fractionation based on
retention time windows, figure 4b
shows the fractionation result. It
can be seen that only about 12 mg
of compound 1 with a purity higher than 90 % could be isolated
together with many fractions con-
Figure 4
Fractionation with 30 % mobile phase B, b) isolation results, isocratic conditions
Mixed fractions
Analysis with 20 % B
0
5
10
15
20
25
30
0.5 1.5 2.5 3.5 4.5
Time [min]
Amount
[mg]
Pure
compound 1
024
Absorbance
[mAU]
0
500
1000
1500
2000
2500
P 2- _1
P 2- _3
P 2- _5
P 2- _7
P 2- _9
P 2- _11
P 2- _13
P 2- _15
P 2- _17
P 2- _19
Time [min]
a)
Fractions: 1 - 5 min
Width: 0.2 min
b)
Compound 1
Compound 1
Analysis with 30 % B
0
5
10
15
20
25
0.7 1.1 1.5 1.9 2.3 2.7 3.1
Time [min]
Compound 1
Compound 2
Mixed
fractions
Pure
compound 1
Amount
[mg]
4
Time [min]
02
Absorbance
[mAU]
0
500
1000
1500
2000
2500
P 2- _1
P 2- _2
P 2- _3
P 2- _4
P 2- _5
P 2- _6
P 2- _7
P 2- _8
P 2- _9
P 2- _10
P 2- _11
P 2- _12
P 2- _13
Fractions: 0.6 - 3 min
Width: 0.2 min
a) b)
Figure 5
a) Isolation results, isocratic conditions, 20 % mobile phase B, b) isolation results, isocratic conditions

compound 2 (93 % recovery) had a
purity of slightly less than 90 %.
The overall recovery was 24.3 mg
of compound 1 (97 %) and 23.5 mg
of compound 2 (93 %). The overall
analysis run time could be minimized to about 3.4 minutes, that
is, solvent consumption was about
85 ml per analysis.
Fractionation under isocratic conditions with 15 % B in the mobile phase
Figure 6a shows the preparative
run and the fractionation based on
retention time windows, figure 6b
shows the fractionation results. All
isolated 24 mg of compound 1 had
a purity higher than 98 %. The
recovery was 97 %. The isolated
23 mg of compound 2 (90 % recovery) had a purity of 96 %. The
overall analysis run time could be
minimized to about 4 minutes, that
is, solvent consumption was about
100 ml per analysis.
Fractionation under isocratic conditions with 10 % B in the mobile
phase
Figure 7a shows the preparative
run and the fractionation based on
retention time windows, figure 7b
shows the fractionation results.
24.4 mg of compound 1 were
Analysis with 15 % B
Compound 1
Compound 2
Pure
compound 1
Pure compound 2
Time [min]
0246
Absorbance
[mAU]
0
500
1000
1500
2000
2500
P2-_1
P2-_3
P2-_5
P2-_7
P2-_9
P2-_11
P2-_13
P2-_15
P2-_17
P2-_19
Amount [mg]
Fractions: 1 - 12 min
Width: 0.2 min
a)
b)
Analysis with 10 % B
Compound 1
Compound 2
Pure
compound 1
Pure
compound 2
a)
b)
Amount
[mg]
Time [min]
0246810
Absorbance
[mAU]
0
500
1000
1500
2000
P 2- _1
P 2- _5
P 2- _10
P 2- _15
P 2- _20
P 2- _25
P 2- _30
P 2- _35
P 2- _40
P 2- _45
P 2- _50
P 2- _54
Fractions:
1-12 min
Width:
0.2 min
Figure 6
a) Fractionation with 15 % mobile phase B, b)isolation results, isocratic conditions
Figure 7
a) Fractionation with 10 % mobile phase B, b) isolation results, isocratic conditions
30
25
20
15
10
5
0
0.5 1.5 2.5 3.5 4.5
Time [min]
30
25
20
15
10
5
0
0.5 2.5 4.5 6.5 8.5 10.5

isolated with a purity of 98 %
which is a recovery of 96 %. Compound 2 could be isolated with
97 % recovery (24.6 mg) and a
purity of over 98 %. Despite these
results the performance of the
separation is not optimum. There
are several fractions with no
product between the fractions
containing the pure compounds.
The overall analysis run time of
the separation is about 10.2 minutes, which means a solvent consumption of 255 ml per analysis.
Recovery against purity
Figures 8a and 8b show the recovered amount of the two compounds against purity. It can be
clearly seen that the recovered
amount with high purity increases
with less acetonitrile in the mobile
phase. Therefore, the analysis has
to be adjusted depending on
which parameter has higher priority. If high purity is required, for
example for activity testing, and
the recovery is not important, an
analysis with more acetonitrile
can be done. Both compounds
can be isolated with good purity
with relative short run times. On
the other hand, if good recovery
is required but purity is less
important, for example for isolation of intermediates in a synthesis sequence, less acetonitrile
should be used in the mobile
phase.
a)
b)
60
70
80
90
100
0 5 10 15 20 25
30 % B
Compound 2Compound 1
Recovered Amount [mg]
10 % B
Purity [%]
Purity [%]
Recovered Amount [mg]
Figure 8
a) Recovery against purity for compound 1, b) recovery against purity for compound 2
100
90
80
0 5 10 15 20 25
30 % B
20 % B
15 % B
20 % B
15 % B
10 % B

Recovery and solvent consumption
Another aspect of the separation
to keep in mind is the solvent consumption. Solvents for preparative
LC have to be of high purity and
are therefore rather expensive.
Since waste disposal and environmental protection are nowadays
important issues the chemist
should try to avoid unnecessary
solvent consumption. Figure 9
shows the recovery (purity > 90 %)
against solvent usage.
It can be seen that the solvent
consumption for down to 15 %
acetonitrile in the liquid phase is
less than 100 ml per analysis.
Using only 10 % acetonitrile in the
liquid phase more than doubles
the analysis run time and therefore the solvent consumption
(> 250 ml per analysis). Since the
results for recovery and purity are
already excellent for 15 % acetonitrile in the mobile phase it is not
necessary to run the analysis with
less acetonitrile.
Conclusion
This Application Note describes
the separation of two compounds
from a binary mixture using the
Agilent 1100 Series purification
system. The purification was performed isocratically using different liquid phase compositions. The
collected fractions were re-analyzed and the recovery and purity
of the compounds determined.
Further, the influence of the liquid
0
20
40
60
80
100
302015
10
% B
0
50
100
150
200
250
300
Recovery [%] (>90 % pure)
Solvent consumption [ml]
Compound 1
Compound 2
Solvent
Figure 9
Recovery against solvent consumption
phase composition on the parameters recovery, purity and analysis run time were investigated.
Also, the correlation of purity and
solvent consumption, which is an
important parameter regarding
costs, waste disposal and environment protection, was shown.

Copyright © 2000 Agilent Technologies
All Rights Reserved. Reproduction, adaptation
or translation without prior written permission
is prohibited, except as allowed under the
copyright laws.
Printed 10/2000
Publication Number 5988-0638EN
References
1.
“Isolation and purification of
hydroxyanthraquinones using the
Agilent 1100 Series purification
system”, Agilent Application Note,
2000, publication number 59880637EN
2.
B.R. Sadler, K. Chae, K.S. Ishaq,
K.S. Korach “Separation of indenestrol A and B isomeres and enantiomeres by high-performance liquid chromatography”, J. Chro-
matogr. A, 1998, 799(1-2), 117-124
3.
A.D. Cooper, T.M. Jefferies “Semipreparative high-performance liquid-chromatographic resolution of
brompheniramine enantiomeres
using beta-cyclodextrin in the
mobile phase” J. Chromatogr.,
1993, 637(2), 137-143
Udo Huber is an application
chemist based at Agilent
Technologies, Waldbronn,
Germany.
www.agilent.com/chem
 Loading...
Loading...