Page 1

Sonicator® Plus 930 Specifications
General Specifications:
Input: 90–240 VAC, 50–60 Hz, 2.3 Amp Nom.
Certification: The Sonicator Plus 930 complies with the ultrasound
performance standards set forth in the Code of Federal
Regulations, Title 21 (Food and Drugs), Part 1050.10
ETL and C-ETL Listed: Model ME 930 (9801427)
Classification: Protective Class I Equipment
Type BF Equipment
Enclosed equipment without protection against ingress of
water.
Equipment not suitable for use in the presence of a
flammable anesthetic mixture with air or with nitrogen
oxide
Year 2000 Compliant Yes
U.S. Patent Numbers: 4,966,131 and 5,095,890
Weight: 8.7 pounds
4.0 kg
Dimensions: 6 in (H) x 12 in (W) x 12 in (D)
15.2 cm (H) x 30.5 cm (W) x 30.5 cm (D)
Operating Temperature: +50°F to +104°F
+10°C to +40°C
Humidity:
Storage Temperature: -40°F to 167°F
Timer Accuracy: ±0.5 minutes for times less than 5 minutes
Maximum Treatment Time: 60 minutes–electrical stimulation
Treatment Timer: Treatment time counts down to zero when a time is set, or
Operating, 30% to 75% Relative Humidity at 104°F
Nonoperating, 5 to 95% Relative Humidity, noncondensing
-40°C to 75°C
±10% for times from 5 to 10 minutes
±1.0 minute for times greater that 10 minutes
30 minutes–ultrasound or combination therapy
up to 60 or 30 minutes when no time is set. The digital
timer indicates time in minutes and seconds. The timer
also indicates the remaining or elapsed treatment time
during the “Hold” period.
Page 2
Ultrasonic Generator Specifications:
Frequency: 1.0 MHz ±5%
3.2 MHz ±5%
Modes: Continuous
Pulsed—20% duty cycle
Pulse Repetition Rate: 100 Hz ±20%
Pulse Duration: 2 msec ±20%, 20% duty cycle
Temporal Peak/ average
5:1 ±20%, 20% duty cycle
intensity ratio:
Maximum output power: 11 W with a 5 cm² applicator, (ME 7513)
Maximum intensity: 2.2 W/cm²
Indication accuracy: ±20% (for any level above 10% of maximum)
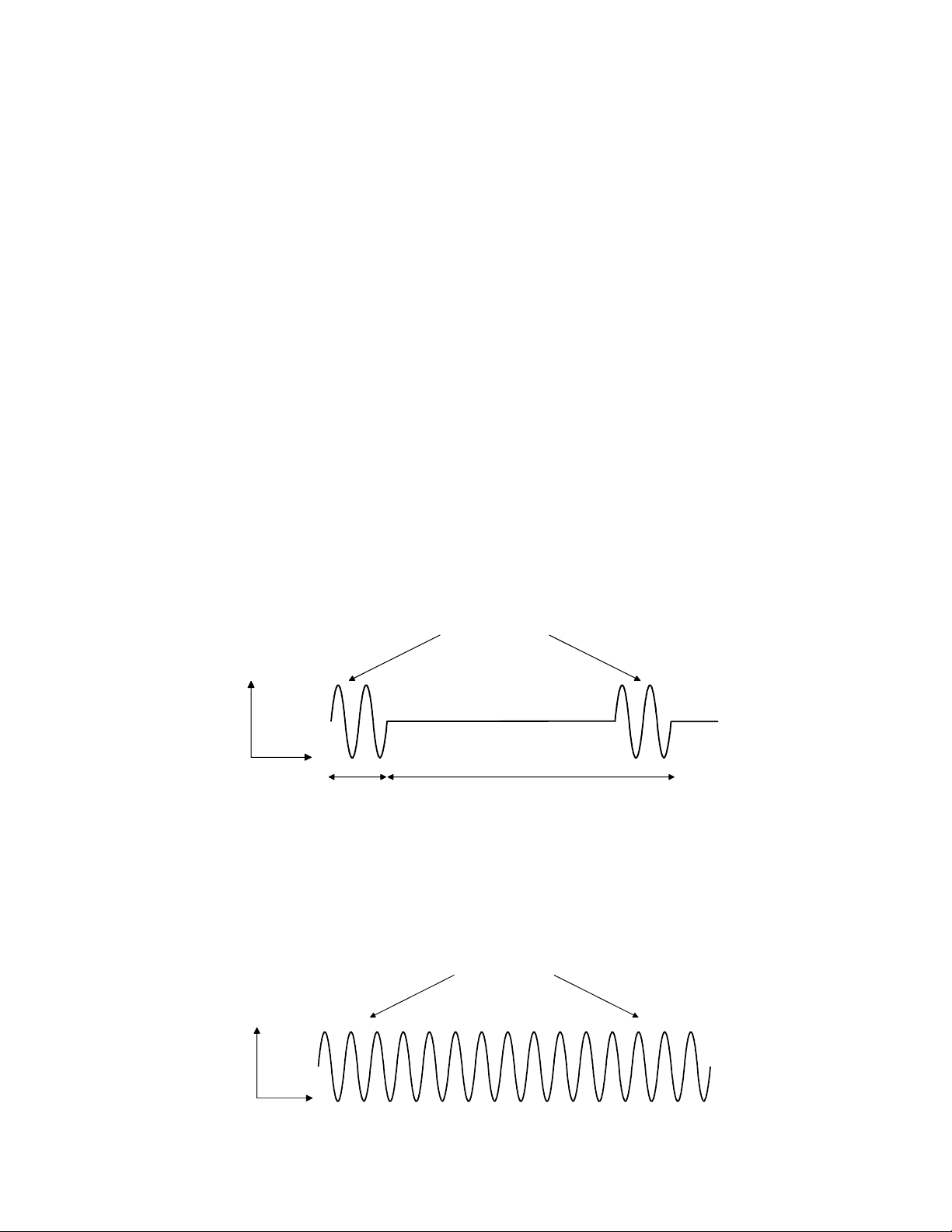
Output description: The output waveform is continuous or pulsed as
programmed by the membrane panel control. In the pulse
mode the 1 or 3.2 MHz sine wave pulses
are modulated. The power level is adjusted by varying the
pulse amplitude. The pulse waveforms are shown
below:
1 or 3.2 MHz
Ultrasound
Output
Time
2 ms
Pulse
Width
10 ms
Pulse Space
Pulse Waveform—20% Duty Cycle
In the continuous mode, the power is on at least 95% of the
time the timer is running. The continuous mode waveform
is shown below:
1 or 3.2 MHz
Ultrasound
Output
Time
Continuous Waveform
Page 3

Ultrasonic Applicator Specifications:
Piezoelectric discs: The output transducer utilizes a barium titanate disc with
a specially coated face.
Individual Applicator Specifications:
Applicator Part Number Frequency Effective Radiating Area
ME 7513 1 or 3.2 MHz ±5% 5 cm² ±20%
Maximum Beam
Non–Uniformity Ratio: 6:1
Spatial Pattern: The applicator produces a collimated (cylindrical) beam
with an area of 5 cm², measured 5 mm from the ceramic
disc surface when the radiation is emitted into the
equivalent of an infinite medium of distilled water at 30° C.
The beam of the applicator is circular in all planes parallel
to the applicator face. A few inches from the face, it is a
single smooth bell-shaped curve. Nearer the face the
pattern varies more due to phase cancellations. Sample
curves measured in the far field from the surface are shown
in Figures 9.3 and 9.4.
5 cm² Applicator (1 MHz), ME 7513,—Three Dimensional Beam Pattern
5 cm² Applicator (3.2 MHz), ME 7513,—Three Dimensional Beam Pattern
Page 4

Waveform Specifications:
Interferential Mode
Interferential Waveform
Waveform Type: Sinewave
Polarity: None
Volts: 0–65 volts RMS, 1 Kohm load
Current: 0–65 mA RMS, 1 Kohm load
Average current at
maximum intensity
and frequency: 65 mA RMS
Maximum current
density under 2"
diameter electrode. 3.2 mA/cm²
Frequency: Channel 1 = 4000 Hz
Frequency Modulation: 1–15 Hz
80–150 Hz
1–150 Hz
xx–xx Hz,
xx=any value from
1 to 250 Hz
Channel 2 = 4000 to 4250
Hz variable frequency sine
wave
Premodulated Mode
Premodulated Waveform
Phase Duration: 125 µs
Available Amplitude
Modulation Options: Vector rotation
Available Channels: Channels 1 & 2
Waveform Type: Amplitude modulated
sine wave
Polarity: None
Volts: 0–50 volts RMS, 1 Kohm load
Current: 0–50 mA RMS,
1 Kohm load
Average current at
maximum intensity
and frequency: 50 mA RMS
Maximum current
density under 2"
diameter electrode. 2.5 mA/cm²
Frequency: 4,000 Hz
Frequency Modulation: 1–15 Hz
80–150 Hz
1–150 Hz
xx–xx Hx,
Page 5

Medium Frequency Mode
Medium Frequency (Russian)
Waveform
xx=any value from
1 to 250 Hz
Phase Duration: 125 µs internal sine wave
4–1,000 ms beat envelope
Available Amplitude
Modulation Options: Continuous
Surge
Reciprocation
Available Channels: All
Waveform Type: Burst modulated sine wave
Polarity: None
Volts: 0–50 volts RMS, 1 Kohm load
Current: 0–50 mA RMS, 1 Kohm load
Average current at
maximum intensity
and frequency: 50 mA RMS
Maximum current
density under 2"
diameter electrode. 2.5 mA/cm²
Frequency: 2500 Hz, Burst at
10 ms on and 10 ms off
Frequency Modulation: No
Phase Duration: 200 µs
Available Amplitude
Modulation Options: Continuous
Surge
Reciprocation
Available Channels: All
Page 6

Amplitude Modulation Specifications:
Vector rotation:
-50% amplitude modulation in
Surge Mode:
Up ramp: 3 seconds
Down ramp: 2 seconds
Preset on/off times: 5 seconds on, 5 seconds off
Reciprocation mode:
Up and down ramps: 1 second, reciprocation only
Reciprocation time: 2–240 seconds, (On time = off time)
Interferential Mode Only,
anti phase with an eight second modulation period.
Premodulated and Medium Frequency
10 seconds on, 10 seconds off
10 seconds on, 30 seconds off
10 seconds on, 50 seconds off
Premodulated and Medium Frequency
 Loading...
Loading...