Page 1

3M Separation and Purication Sciences
™
3M
Zeta Plus
Depth Filters
High Performance, Scalable,
Single-Use System
™
Page 2
3M™ Zeta Plus™ Encapsulated System
The System of Choice for Single-Use Depth Filtration
High Performance
Filter Media
The 3M™ Zeta Plus™ Encapsulated
System utilizes the high performing
Zeta Plus depth lter series
media, including the single and
dual layer.
• Positive charge is capable of
reducing negatively charged
DNA, endotoxins and other host
cell proteins
™
• The 3M
media enhances the contaminant
holding capacity of the lter media.
This allows for larger particles to
be trapped in the upstream zone
of the more open lter media and
smaller particles to be trapped in
the downstream zone, reducing
premature plugging and helping
extend service life of the media.
• Can be used for post fermentation
cell culture clarication or
downstream impurity removal
• Can be employed independently or
in conjunction with centrifugation or
tangential ow ltration (TFF)
Zeta Plus™ dual layer
3M™ Zeta Plus™ Depth Filter Quick Start Guide
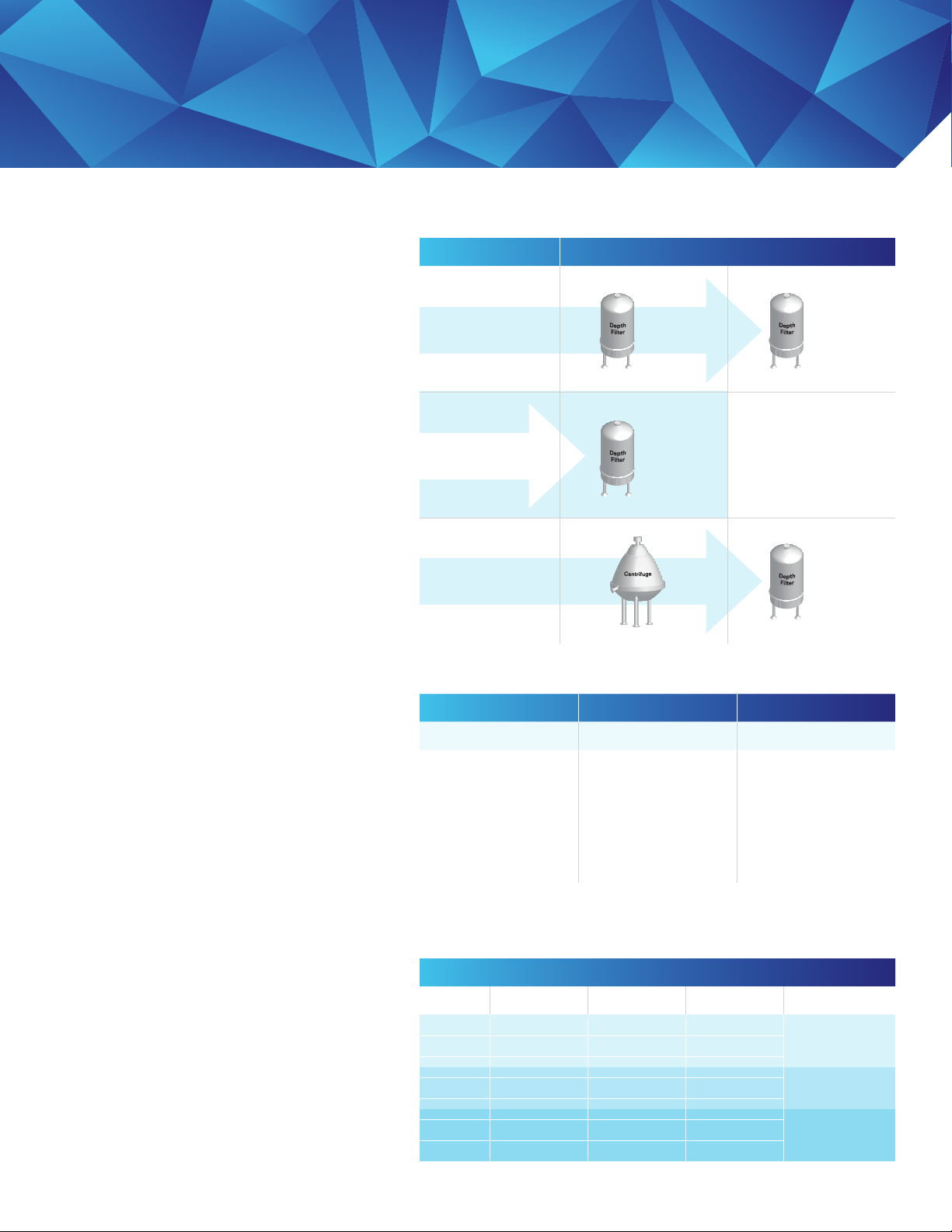
Application Stage / Product
First Stage Second Stage
Two Stage
Operations
Single Stage
Operations
Post Centrifuge
Operations
05SP01
10SP02
60SP02
90ZB08
90SP08
60ZB05
60SP05
90ZB08
90SP08
60ZB05
60SP05
Media Series
SP Media LA Media ZB Media
Widest Range Cleanest Highly Charged
SP has the widest nominal
pore size range relative to
other 3M Zeta Plus media
oerings, including a
greater number of grades
as well as grades with
larger nominal pore sizes
than LA or ZB media.
LA is the cleanest
3M Zeta Plus media family
offered. 3M
series low aluminum (LA)
filter media are designed
to provide low levels of
extractables, especially
aluminum.
™
Zeta Plus™ LA
ZB media offers a higher
charge level than SP or LA
media, and offers single
layer and dual layer grades
with a smaller nominal
pore size than either the
SP media family or the LA
media family.
• Activated carbon and lipid removal
media also available
2 | 3M™ Zeta Plus™ Single-Use Depth Filtration Product Portfolio
Sizing Guide
Pore Size Options: 3M™ Zeta Plus™ SP, LA and ZB Media
Media Family
Grades SP ZB LA Application
5 X
10 X
30 X X X
50 X
60 X X X
90 X X X
120 X
For reference only. Retention ratings may vary depending on application.
Primary
Secondary
Centrate
Page 3

Features & Benets
Capsule/Manifold Design
Translucent plastic shell
(standard capsules,
polycarbonate shells)
Fully encapsulated shell
around solid core
Self guiding locking
mechanism
Lenticular style capsule
design
3M™ Zeta Plus™ Capsules:
Encapsulated Standard Capsule
with Polycarbonate Shells
• Easy detection of the liquid
level inside, providing real time
monitoring of the filtration
process.
• Eliminates the need for a
stainless steel housing and the
cleaning step after filtration.
• Fast and reliable capsule-to-
capsule connectivity.
• Consistency between
single-use and conventional
depth filtration.
3M™ Zeta Plus™ Capsules: Encapsulated
Capsule with Alkaline Resistant*
Polyphenylene Oxide/Polystyrene
3M™ Zeta Plus™ Filter Media
Model# 16EZB
• Both single and dual layer Zeta Plus filter
media are available.
• Excellent performance in throughput and
filtration efficiency with proper media
selection and sizing.
3M™ Zeta Plus™ Capsule Family
* Based on testing with 1M NaOH and 5% NaClO (Bleach).
• The 3M
a single use depth filtration system
• The complete system is comprised of a
holder, two manifolds and the desired number
of capsules
• The polycarbonate capsules feature a
translucent shell that allows for easy fluid
level observation
• A self-guiding locking mechanism ensures fast
and reliable connections between capsules
Self Guiding Locking
Mechanism Enables
Fast and Reliable
Capsule-To-Capsule
Connection
™
Zeta Plus™ Encapsulated System is
3M™ Zeta Plus™ Single-Use Depth Filtration Product Portfolio | 3
Page 4

3M™ Zeta Plus™ Encapsulated System
The System of Choice for Single-Use Depth Filtration
Ergonomically Designed
Large Filter Holders
Traditional depth ltration systems utilize lenticular style
cartridge lters and a vertical ltration ow path to allow
easy access to process liquids and ecient utilization of
lter media. However, stacking cartridges from bottom
to top can be cumbersome, and dismantling the spent
cartridges is often labor intensive.
Features & Benets
Ergonomically Designed Holder System
3M™ Encapsulated System
Holder, Large (Model
#16EZB): holder is pivoted
between horizontal and
vertical positions
Vertical flow path
• Enables loading and unloading at
waist height.
• Central inlets and outlets minimize
fluid spills during post use handing.
• Holder and capsule design
allows the combination of multiple
3M Zeta Plus media types or even
multiple 3M filtration products
in a single holder.
• Reduced footprint during operation.
4 | 3M™ Zeta Plus™ Single-Use Depth Filtration Product Portfolio
Page 5

Recognizing the need for a depth ltration system that is fast, easy and clean, 3M designed lter holders
(Model# 16EZB) that can be pivoted between the horizontal position for loading and unloading the
capsules and manifolds, and the vertical position for ltration. Allowing loading and unloading at waist
height eliminates the need for operators to lift capsules above their heads and reduces the risk of uid
spills when handling spent capsules. The use of the vertical ow path allows for full media utilization and
a small system footprint during ltration.
3M™ Encapsulated System Holders, Small
(Model# 16EZA)
The small holder is available for laboratory and pilot scale-up
studies, in addition to low volume production ltration. The
1-high holder can accommodate from one to four 0.23 m²
capsules, or one 1.6 m² (dual layer) or 2.5 m² (single layer)
capsule. The 2-high holder can accommodate up to two 1.6 m²
(dual layer) or 2.5 m² (single layer) capsules. The 3 high holder
can accommodate up to three 1.6 m² (dual layer) or 2.5 m²
(single layer) capsules. Either single stage or two-stage depth
ltration can be performed within the same holder. The 1-high
small holder has a built-in torque limiter that will signal the
operator when the holder assembly is properly sealed. All
small holders have been designed to be fully autoclavable for
applications where that may be required.
3M™ Encapsulated System Holders, Large
(Model# 16EZB)
The large holder can accommodate up seven 1.6 m² (dual layer)
or 2.5 m² (single layer) capsules. This holder is best suited for
use in small to large production scale purication processes.
However, this holder can also accommodate a single 1.6 m²
(dual layer) or 2.5 m² (single layer) capsule should choose to use
it for scale up studies.
Two Stage Operations
For two stage purication operations a
second pair of manifolds is required between
each stage of multistage operations.
Manifold and capsule materials should
always be the same.
Figure 1. 3M™ Zeta Plus™ Encapsulated System
3M™ Zeta Plus™ Single-Use Depth Filtration Product Portfolio | 5
Page 6

Innovative Capsule/Manifold Design
Two capsule congurations are available for use
™
with the 3M
Zeta Plus™ Encapsulated System.
05SP01A
• Single cell and multicell capsules are available
2
• Single cells have 0.23 m
• Multicells have 1.6 m
2
or 2.5 m
of single layer media
of ltration media
2
of dual layer media
• Alkaline resistant capsules available
• Dual stage ltration can be performed in
the same holder by using an additional set
of manifolds
*Based on testing with 1M NaOH and 5% NaClO (Bleach).
10SP02A
30SP02A
60SP02A
60SP05A
60ZB05A
90SP05A
90ZB05A
90ZB08A
120ZB08A
0.1 0.5 1 2 4 6 10
Micron
Figure 2. Nominal Retention Ratings for
3M™ Zeta Plus™ Dual Layer Grades
(For reference only. Retention ratings may vary depending on application.)
Additional Formats Available
In addition to the 3M™ Zeta Plus™ Encapsulated system, cartridge and sheet options are also available in most media types and grades.
Zeta Plus Cartridges:
• Produced in 8 inch, 12 inch and 16 inch sizes
• Multiple lenticle and construction configurations
• Dual Layer cartridges are available in SP, ZB and LA
media families
• Stainless steel housings for each size available
• Uses the same media as encapsulated systems
6 | 3M™ Zeta Plus™ Single-Use Depth Filtration Product Portfolio
Page 7

Scalability
The 3M™ Zeta Plus™ Encapsulated System retains the lenticular lter design and vertical ow path that
™
are characteristics of traditional depth ltration systems. A full range of 3M
available from benchtop to production scale, which allows for lab scale, pilot testing and scale-up with
the same ltration media.
Figure 3. The 3M™ Zeta Plus™
Encapsulated System
Zeta Plus™ capsules is
Capacity
Single-Use Depth Filtration
Product Portfolio
BC25 (25 cm2)
E0170 (170 cm²) and
E0340 (340 cm²)
E01020 (1020 cm²)
16EZA
Double layer: 1.6 m²
Single layer: 2.5 m²
16EZB
Double layer: 11.2 m²
Single layer: 17.5 m²
Process Volume
Zeta Plus Sheets:
• Available in SP, ZB and LA media families
• Activated carbon and lipid removal media
available on request
• May be die cut to match specific requirements
• Designed for use with commercially available
filter presses
Contact your local sales rep for additional information about these formats.
3M™ Zeta Plus™ Single-Use Depth Filtration Product Portfolio | 7
Page 8

Table 2a. 3M™ Zeta Plus™ Laboratory Capsules: Filter Specifications
BC25, Luer BC25, Sanitary
Dimensions
Single Layer (height by diameter)
Dual Layer (height by diameter)
Weight
Dry - Single Layer ≈ 60 g ≈ 64 g
Dry - Dual Layer ≈ 69 g ≈ 75 g
Wet Post Blow-Down - Single Layer ≈ 70 g ≈ 75 g
Wet Post Blow-Down - Dual Layer ≈ 86 g ≈ 93 g
Materials of Construction
Shells Polypropylene
Ring Seal (dual layer media) Polypropylene
Edge Seal Overmold Glass Fiber Filled Polypropylene
Luer Cap & Luer-barb Connector Polypropylene
Volume
Capsule Fill Volume¹ - Single Layer ≈ 17 mL
Capsule Fill Volume¹ - Dual Layer ≈ 25 mL
Post Blow-Down Hold-up Volume² - Single Layer ≈ 11 mL
Post Blow-Down Hold-up Volume² - Dual Layer ≈ 17 mL
Miscellaneous
Effective Filtration Area 25 cm² 25 cm²
Connector Luer
1
Volume of liquid required to fill capsule (experimentally measured).
2
Capsule Post blow-down hold-up volume. Estimated volume of residual preconditioning flush liquid after air/gas blow-down, using water as the flush
fluid and calculated by post-blow-down weight and flush fluid density. Actual amount depends upon exact blow-down conditions, media type in
capsule, the number of capsules in the system, the process fluid, and loading level of the capsule.
6.5 cm × 7.6 cm
(2.6 inches × 3 inches)
6.9 cm × 7.6 cm
(2.7 inches × 3 inches)
Can accommodate both ½" and ¾"
7.9 cm × 7.6 cm
(3.1 inches × 3 inches)
8.3 cm × 7.6 cm
(3.3 inches × 3 inches)
Sanitary Style
Laboratory Capsule Filter Schematics
Inlet
Vent
Outlet
BC-25 Sanitary Lab Capsule
8 | 3M™ Zeta Plus™ Single-Use Depth Filtration Product Portfolio
Vent
BC-25 Luer Lab Capsule
Inlet
Outlet
Page 9

Table 2b. 3M™ Zeta Plus™ Scale-Up Capsules: Filter Specifications
170 cm² Capsule 340 cm² Capsule 1020 cm² Capsule
Dimensions
Height × Diameter
Weight
Dry - Single Layer 1.0 kg (2.2 lb) 1.0 kg (2.2 lb) 1.4 kg (3.0 lb)
Dry - Dual Layer 1.0 kg (2.2 lb) 1.0 kg (2.3 lb) 1.6 kg (3.5 lbs)
Wet Post Blow-Down - Single Layer 1.1 kg (2.4 lb) 1.1 kg (2.5 lb) 1.8 kg (4.0 lb)
Wet Post Blow-Down - Double Layer 1.2 kg (2.6 lb) 1.3 kg (2.9 lb) 2.4 kg (5.2 lb)
Materials of Construction
Capsule Shells Polysulfone
Separator, Spacer, Vent Cap Polypropylene
O-ring Fluorocarbon
Endcap & Edge Seals
Hold-up Volume
Capsule Fill Volume
Post Blow-Down Hold-up
2
Volume
1
Single Layer ≈ 0.67 L (≈ 1.5 gal) ≈ 0.69 L (≈ 1.5 gal) ≈ 1.7 L (≈ 3.7 gal)
Dual Layer ≈ 0.63 L (≈ 1.4 gal) ≈ 0.65 L (≈ 1.4 gal) ≈ 1.6 L (≈ 3.5 gal)
Single Layer ≈ 0.12 L (≈ 0.26 gal) ≈ 0.16 L (≈ 0.35 gal) ≈ 0.46 L (≈ 1.0 gal)
Dual Layer ≈ 0.15 L (≈ 0.34 gal) ≈ 0.26 L (≈ 0.58 gal) ≈ 0.80 L (≈ 1.8 gal)
Miscellaneous
Effective Filtration Area 170 cm² (0.18 ft²) 340 cm² (0.37 ft²) 1020 cm² (1.10 ft²)
Connector
1
Volume of liquid required to fill capsule (experimentally measured).
2
Capsule Post blow-down hold-up volume. Estimated volume of residual preconditioning flush liquid after air/gas blow-down, using water as the flush
fluid and calculated by post-blow-down weight and flush fluid density. Actual amount depends upon exact blow-down conditions, media type in
capsule, the number of capsules in the system, the process fluid, and loading level of the capsule.
IMPORTANT NOTICE: Always operate the filter system within the maximum differential pressure of 2.4 bar (35 psig).
4.1" × 8.5" (10.3 cm × 21.6 cm) 6.0" × 8.5" (15.2 cm × 21.6 cm)
Thermoplastic Elastomer
1/2" Sanitary Style
Scale-Up Capsule Filter Schematics
Outlet not shown
VentInlet
170 cm2 & 340 cm2 Capsules
Vent
Inlet Outlet
1020 cm2 Capsule
3M™ Zeta Plus™ Single-Use Depth Filtration Product Portfolio | 9
Page 10

Table 2c. 3M™ Zeta Plus™ Production Capsules: Filter Specifications
Configuration
Single Cell Capsule Multi-Cell Capsule
Standard Alkaline Resistant
Dimensions (Height × Diameter)
5.7 cm × 45.2 cm (2.2" × 17.8") 20.3 cm × 45.2 cm (8.0" × 17.8")
Weight
Dry 3.3 kg (7 lbs) 3.4 kg (8 lbs) 10.0 kg (22 lbs) 10.7 kg (24 lbs)
Wet (post Blow-Down) 4.4 kg (10 lbs) 4.8 kg (11 lbs) 19.3 kg (43 lbs) 19.7 kg (43 lbs)
Materials of Construction
Filter Media Filter aids, cellulose, binding resin Filter aids, cellulose, binding resin
Outer Shell Polycarbonate
Polyphenylene oxide /
Polystyrene
O-rings Silicone Silicone
Separators, Spacers and Connectors
Polypropylene Polypropylene
Edge Seals Thermoplastic Elastomer Thermoplastic Elastomer
Handles N/A Nylon
Hold-up Volume
Single Layer E16E01 & E16R01: ≈ 3.8 L (≈ 1.0 gal) E16E11 & E16R11: ≈ 18.8 L (≈ 5.0 gal)
Capsule Fill Volume
Post Blow-Down
Hold-up Volume³
2
Dual Layer E16E01 & E16R01: ≈ 3.4 L (≈ 0.9 gal) E16E07 & E16R07: ≈ 18.1 L (≈ 4.8 gal)
Single Layer E16E01 & E16R01: ≈ 0.7 L (≈ 0.2 gal) E16E11 & E16R11: ≈ 7.5 L (≈ 2.0 gal)
Dual Layer E16E01 & E16R01: ≈ 1.3 L (≈ 0.4 gal) E16E07 & E16R07: ≈ 9.0 L (≈ 2.4 gal)
Maximum Operating Line Pressure 3.4 bar (50 psig) 3.4 bar (50 psig)
Maximum Differential Pressure
Forward 2.4 bar (35 psid) 2.4 bar (35 psid)
Effective Filtration Area 0.23 m² (2.4 ft²)
1
Based on testing with 1M NaOH and 5% NaClO (Bleach).
² Volume of liquid required to fill capsule (experimentally measured).
³ Capsule Post blow-down hold-up volume. Estimated volume of residual preconditioning flush liquid after air/gas blow-down, using water as the
flush fluid and calculated by post-blow-down weight and flush fluid density. Actual amount depends upon exact blow-down conditions, media type
in capsule, the number of capsules in the system, the process fluid, and loading level of the capsule.
1
Standard Alkaline Resistant
Polycarbonate
Polyphenylene oxide /
Polystyrene
Double layer: 1.6 m² (17.2 ft²)
Single layer: 2.5 m² (27.0 ft²)
1
Single-Use Capsule Filter Schematic
Female Connector Female Connector
Plastic Shell
E16 Single-Cell Capsule
(with double layer or single layer media)
10 | 3M™ Zeta Plus™ Single-Use Depth Filtration Product Portfolio
0.23 m
2
Capsule
Straight Handle
T Handle
Capsule Stop
E16 Multi-Cell Capsule
1.6 m2 Capsule
(with double layer media)
2.5 m2 Capsule
(with single layer media)
Page 11

Table 2d. 3M™ Encapsulated System Manifold Specifications
Configuration
Standard Alkaline Resistant
Dimensions (Height × Diameter)
Connector
Material Polycarbonate Polyphenylene oxide / Polystyrene
Weight 4.4 kg (9.6 lbs) 4.7 kg (10.4 lbs)
Hold up Volume Per Set < 250 mL (<0.07 gal)
5.2 cm × 45.2 cm ( 2.0" × 17.8" )
1.5" Sanitary Style
1
Single-Use Manifold Filter Schematic
Upstream Vent
Downstream Vent
Manifold Stop
O-Rings
Male Connector
Top Manifold Bottom Manifold
Female Connector
Outlet
Inlet
Manifold Stop
Table 3. 3M™ Encapsulated System Holder Specifications
Holder Model
Small Holder
(Model# 16EZA)
Maximum Operating Pressure
Materials of Construction
Frame 304 Stainless Steel 304 Stainless Steel
End Plates 304 Stainless Steel 304 Stainless Steel
Support Rods 440 Stainless Steel 316 Stainless Steel
Stand 304 Stainless Steel 304 Stainless Steel
Hand Wheels 300 Series Stainless Steel 300 Series Stainless Steel
Gear Box N/A
Locking Bar N/A 304 Stainless Steel
Casters Stainless Steel Stainless Steel
Wheels Phenolic Polyurethane
Material
Standard Mechanical Polish Finish (4552601) Mechanical Polish Finish (6123502)
Special Electropolish Finish (4552602) N/A
5.2 cm × 45.2 cm ( 2.0" × 17.8" )
Epoxy Coated Cast Iron Cover
Shrouded in 304 Stainless Steel
Large Holder
(Model# 16EZB)
3M™ Zeta Plus™ Single-Use Depth Filtration Product Portfolio | 11
Page 12

Table 4. 3M™ Encapsulated System Holder Capacity
Model
16EZA 4 1 2 N/A
16EZB N/A 7 N/A 6
E16E01 Capsule E16E07/E16E11 Capsule E16E01 Capsule E16E07/E16E11 Capsule
Table 4a. 3M™ Single Cell Capsule Capacities
Single Stage Two Stage
Holder
Single Stage Filtration (one set of manifolds) Two Stage Filtration* (two sets of manifolds)
Small, 1-high (Part #4552601) up to 4 2 to 3
Small, 2-high (Part #4552603) up to 9 5 to 8
Small, 3-high (Part #4552604) up to 11 6 to 9
Large (Part #6123502) 4 to 26 2 to 23
Single Cell Capsules (E16E01, E16R01, BV800)
Table 4b. 3M™ Multi-Cell Capsule Capacities
Holder
Single Stage Filtration (one set of manifolds) Two Stage Filtration* (two sets of manifolds)
Small, 1-high (Part #4552601) 1 n/a
Small, 2-high (Part #4552603) 2 2
Small, 3-high (Part #4552604) 3 2
Large (Part #6123502) up to 7 2 to 6
Multi-Cell Capsules (E16E07, E16R07, E16E11, E16R11, BV5600)
* Number of 3M production capsules which will fit in a 3M holder along with two sets of 3M manifolds. For example, 2 single cell production
capsules in the first stage followed by 1 single cell production capsule in the second stage meets the maximum of 3 single cell production capsules
for Part Number 4552601.
Figure 9. Small Holder Family (Model# 16EZA) Dimensions
121 cm
(47.48”)
Max
98 cm
(38.48”)
Min
Depth (z)
64.5 cm (25.4”)
65.6 cm
(25.81”)
3M™ Encapsulated System Holder,
Small, One-High Part #4552601
148 cm
(58.38”)
Max
120 cm
(47.38”)
Min
Depth (z)
64.5 cm (25.4”)
65.6 cm
(25.81”)
3M™ Encapsulated System Holder,
Small, Two-High Part #4552603
169 cm
(65.38”)
Max
141 cm
(55.38”)
Min
Depth (z)
64.5 cm (25.4”)
65.6 cm
(25.81”)
3M™ Encapsulated System Holder,
Small, Three-High Part #4552604
12 | 3M™ Zeta Plus™ Single-Use Depth Filtration Product Portfolio
Page 13

Figure 10. Large Holder (Model# 16EZB) Dimensions
205.9 cm (81.1”)
Maximum
86.4 cm
(34.0”)
Horizontal Position
113.9 cm
(45.5”)
Z = depth = 109 cm (42.75”).
Depth includes the handle.
3M™ Encapsulated System Holder, Large, Part #6123502
109 cm
(42.75”)
Vertical Position
210.7 cm
(83”)
3M™ Zeta Plus™ Single-Use Depth Filtration Product Portfolio | 13
Page 14
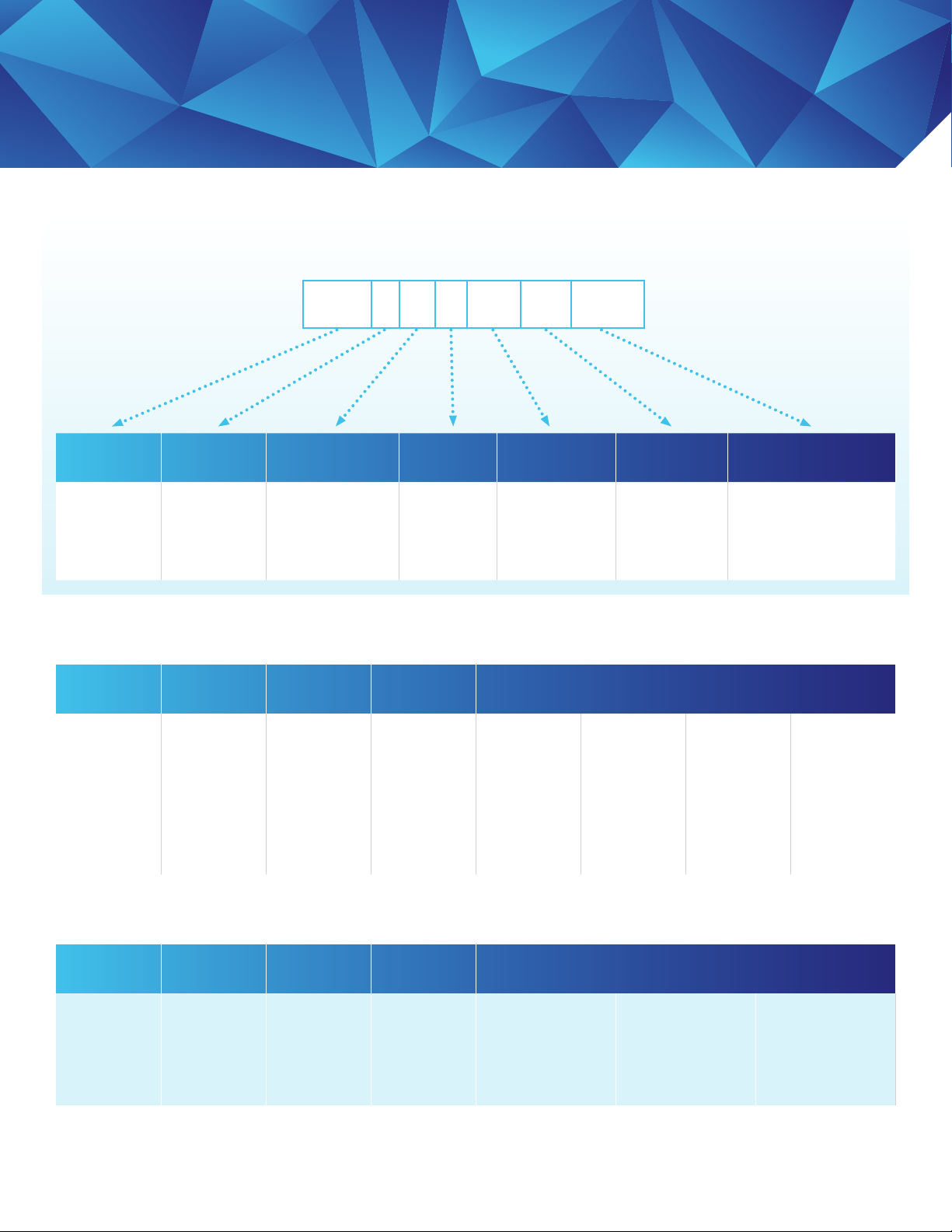
Capsule Ordering Guide
Capsule Product Naming Convention
E16 E 11 A 30 SP 01A
Diameter Configuration Number of Cells
16"
Production
E - Standard
R - Alkaline
Resistant
1, 7 or 11 Cells
1 or 11 = Single
1 or 7 = Dual
Gasket
Material
A-Silicone 05, 10, 30,
Grade Media Second Layer
50, 60, 90
are available
for SP
DELI or DELP
Capsule Filter Ordering Information - Double Layer (U.S. Customers)
Catalog
Number
E16
Configuration
E - Standard
R - Alkaline
Resistant
*
Number of
Cells
01 - 1 Cell
07 - 7 Cell
Gasket
Material
A-Silicone
05SP01A
10SP02A
30SP02A
30SP03A
60SP01A
60SP02A
60SP03A
60SP05A
90SP05A
90SP08A
60LA05A
90LA05A
90LA08A
SP, ZB, LA,
Grade
01A, 02A, 03A, 05A
indicate grade of
second layer
120ZB05A
120ZB08A
120ZB10A
60ZB05A
90ZB05A
90ZB08A
DELP08A
Capsule Filter Ordering Information - Single Layer (U.S. Customers)
*
Number of
Cells
01 - 1 Cell
11 - 11 Cell
Catalog
Number
E16
14 | 3M™ Zeta Plus™ Single-Use Depth Filtration Product Portfolio
Configuration
E - Standard
R - Alkaline
Resistant
Gasket
Material
A-Silicone
30LA
60LA
90LA
Grade
10SP
30SP
50SP
60SP
90SP
30ZB
60ZB
90ZB
120ZB
DELP
Page 15

Manifold Ordering Information
Manifold Part 3M PI Part Number 3M ID
Manifold Set (Standard) 6128901 70020256221
Manifold Set (Alkaline Resistant*) 6129001 70020262369
Filter Holder Ordering Information
Model Name 3M Catalog ID (U.S. Customers) Description 3M ID
™
Encapsulated System Holder,
3M
Small, One-High
™
Encapsulated System Holder,
3M
Small, Two-High
™
Encapsulated System Holder,
3M
Small, Three-High
™
Encapsulated System Holder,
3M
Large
16EZA
16EZB
4552601
4552603
4552604
6123502
Scale-Up Capsules - Dual Layer
70020310846
70020310861
70020310879
70020252899
3M Catalog ID
(U.S. Customers)
E
EFA cm
2
0170
0340
1020
Scale-Up Capsules - Single Layer
3M Catalog ID
(U.S. Customers)
E
EFA cm
2
0170
0340
1020
Material
Code
FSA
Material
Code
FSA
05SP01A
10SP02A
30SP02A
30SP03A
60SP01A
60SP02A
60SP03A
60SP05A
90SP05A
90SP08A
05SP
10SP
30SP
50SP
60SP
90SP
Grade
60LA05A
90LA05A
90LA08A
60ZB05A
90ZB05A
90ZB08A
120ZB05A
120ZB08A
120ZB10A
Grade
30LA
50LA
60LA
90LA
DELI08A
DELP08A
30ZB
60ZB
90ZB
120ZB
DELI
DELP
* Based on testing with 1M NaOH and 5% NaClO (Bleach). See Chemical Compatibility Guide (70-0202-2023-5/LITPHG03) for more information.
3M™ Zeta Plus™ Single-Use Depth Filtration Product Portfolio | 15
Page 16

Intended Use: 3M™ Zeta Plus™ single-use lter products are intended for use in biopharmaceutical processing applications of aqueous and chemical
based pharmaceuticals (drugs) and vaccines in accordance with the product instructions and specications, and cGMP requirements, where applicable.
Since there are many factors that can aect a product’s use, the customer and user remain responsible for determining whether the 3M product is
suitable and appropriate for the user’s specic application, including user conducting an appropriate risk assessment and evaluating the 3M product in
user’s application.
Restricted Use: 3M advises against the use of these 3M products in any application other than the stated intended use(s), since other applications have
not been evaluated by 3M and may result in an unsafe or unintended condition. Do not use in any manner whereby the 3M product, or any leachable
from the 3M product, may become part of or remains in a medical device that is regulated by any agency, and/or globally exemplary agencies, including
but not limited to: a) FDA, b) European Medical Device Directive (MDD), c) Japan Pharmaceuticals and Medical Devices Agency (PMDA) or in
applications involving permanent implantation into the body; Life-sustaining medical applications; Applications requiring food contact compliance.
Product Selection and Use: Many factors beyond 3M’s control and uniquely within user’s knowledge and control can aect the use and performance of
a 3M product in a particular application. As a result, end-user is solely responsible for evaluating the product and determining whether it is appropriate
and suitable for end-user’s application, including completing a risk assessment that considers the product leachable characteristics and its impact on
drug safety conducting a workplace hazard assessment and reviewing all applicable regulations and standards (e.g., OSHA, ANSI, etc.). Failure to
properly evaluate, select, and use a 3M product and appropriate safety products, or to meet all applicable safety regulations, may result in injury,
sickness, death, and/or harm to property.
Warranty, Limited Remedy, and Disclaimer: Unless a dierent warranty is specically stated on the applicable 3M product packaging or product
literature (in which case such warranty governs), 3M warrants that each 3M product meets the applicable 3M product specication at the time 3M ships
the product. 3M MAKES NO OTHER WARRANTIES OR CONDITIONS, EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED TO, ANY IMPLIED
WARRANTY OR CONDITION OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, OR ARISING OUT OF A COURSE OF DEALING,
CUSTOM, OR USAGE OF TRADE. If a 3M product does not conform to this warranty, then the sole and exclusive remedy is, at 3M’s option, replacement
of the 3M product or refund of the purchase price.
Limitation of Liability: Except for the limited remedy stated above, and except to the extent prohibited by law, 3M will not be liable for any loss or damage
arising from or related to the 3M product, whether direct, indirect, special, incidental, or consequential (including, but not limited to, lost prots or
business opportunity), regardless of the legal or equitable theory asserted, including, but not limited to, warranty, contract, negligence, or strict liability.
3M Separation and Purification Sciences Division
400 Research Parkway
Meriden, CT 06450 USA
Phone: 1-800-243-6894
1-203-237-5541
Web: www.3M.com/bioprocessing
Please recycle. Printed in U.S.A. © 3M 2019.
3M and Zeta Plus are trademarks of 3M Company.
All rights reserved. 70-0202-3967-2 Rev 12/2019
 Loading...
Loading...