Page 1
3B SCIENTIFIC® PHYSICS
Boyle’s Law Apparatus U30046
Instruction Sheet
11/08 ALF
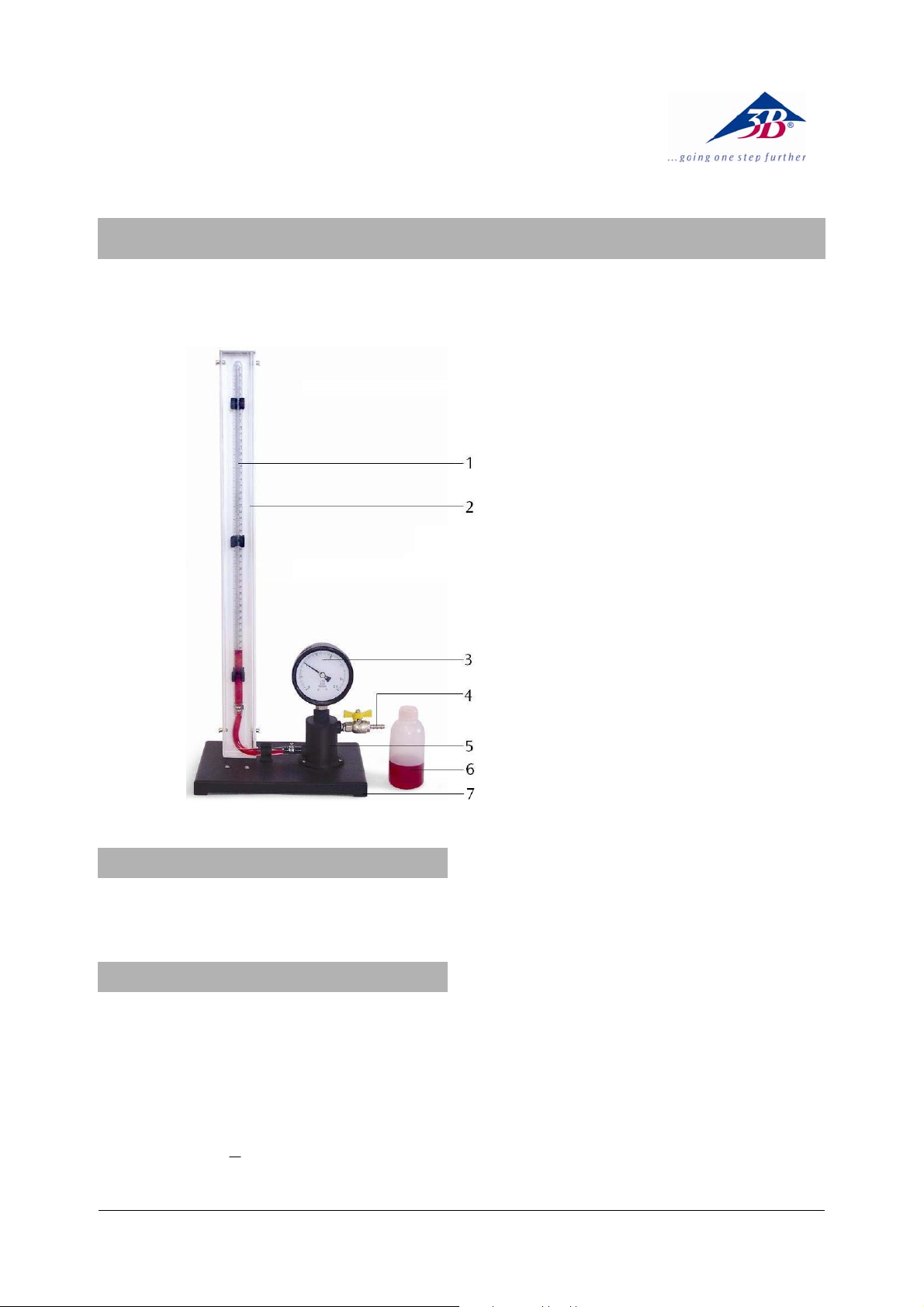
1 Calibrated glass tube
2 Metal plate
3 Bourdon gauge
4 Hose connection
5 Oil reservoir
6 Spare oil
7 Base plate
1. Safety instructions
• Avoid going beyond range on pressure meter.
2. Description
Boyle’s Law apparatus is used for the experimentbased determination of the relationship between the
volume and the pressure of a gas (air) at constant
temperature (Boyle’s Law).
Boyle’s law states that for a given mass of gas (air) at a
constant temperature the product made up of the
volume V and the pressure p is constant:
kVP =⋅ ⇒
kp1⋅=
V
The apparatus is essentially a calibrated glass tube
mounted on a white metal plate. The glass tube is
extra strong and additionally protected by a plastic
safety screen. It is connected to an oil reservoir on
which a Bourdon gauge is fitted. By means of a hand
pump coloured oil is gradually pumped from the oil
reservoir into the tube creating over pressure. Whilst
the volume of the trapped is read from a scale clearly
visible at the tube, pressure is measured by a Bourdon
gauge, which reads in Pa x 10
1.01325 x 10
transparent plastic back to allow students to see its
working parts.
1
5
Pa). The Bourdon gauge is fitted with a
5
. (Standard pressure =
Page 2
3. Technical data
Hose nipple: 10 mm dia.
Pressure max.: 3.4 x 10
5
Pa
Dimensions: approx. 350 x 200 x 760 mm³
4. Additionally required equipment
1 Vacuum hand pump U20500
5. Operation
5.1 Assembly and set up
• Insert the glass tube carefully into the clamps on
the metal plate and mount it on to the base plate.
• Open the stop cock and fill up the oil reservoir so
that at normal atmospheric pressure the oil just
reaches the bottom calibration on the tube. Be
careful not to fill in too much oil, because
otherwise it might flow out through the hose
connection into the pump.
• Screw on the Bourdon gauge carefully.
• Attach the hand pump.
5.2 Experiment procedure
• Record the reading on the tube (the volume) and
the reading on the manometer in a table (refer to
table 1).
• Use the pump to increase the pressure slightly,
and then allow a minute for the apparatus to
return to room temperature.
• Repeat the readings of pressure and volume.
• Repeat this process until you have sufficient
readings.
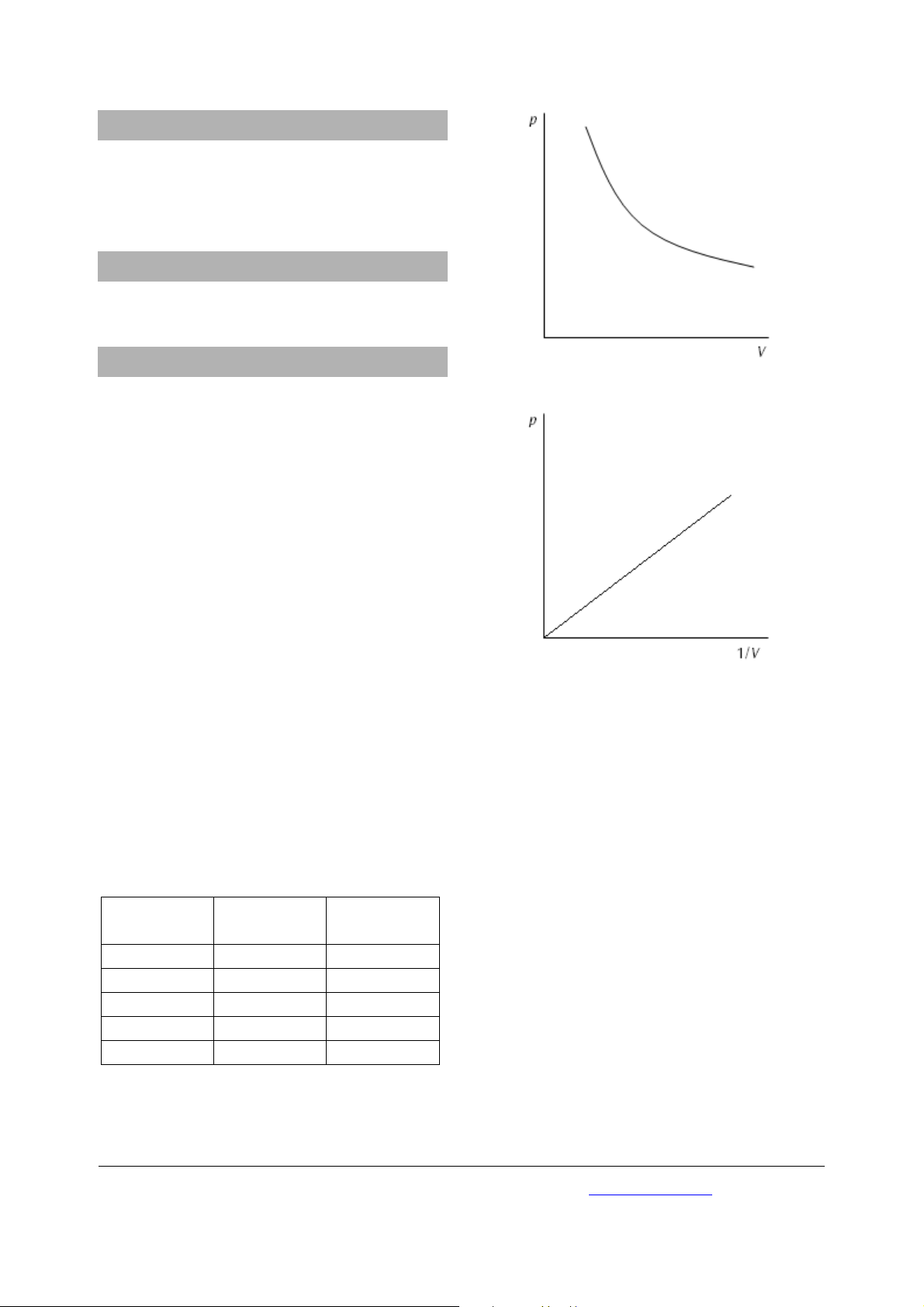
• Plot the values in a graph of p against V and p
against 1/V (refer to fig. 1 and 2).
Volume of air,
(V /ml)
Pressure p
(Pa x 105)
1/V (ml
-1
)
Fig. 1 Graph of pressure against volume
Fig. 2 Graph of pressure against 1/V
Table 1 Measuring values
3B Scientific GmbH • Rudorffweg 8 • 21031 Hamburg • Germany • www.3bscientific.com
Subject to technical amendment
© Copyright 2008 3B Scientific GmbH
 Loading...
Loading...