3B SCIENTIFIC® PHYSICS
U17210 Boyle’s law apparatus
Instruction sheet
5/03 ALF
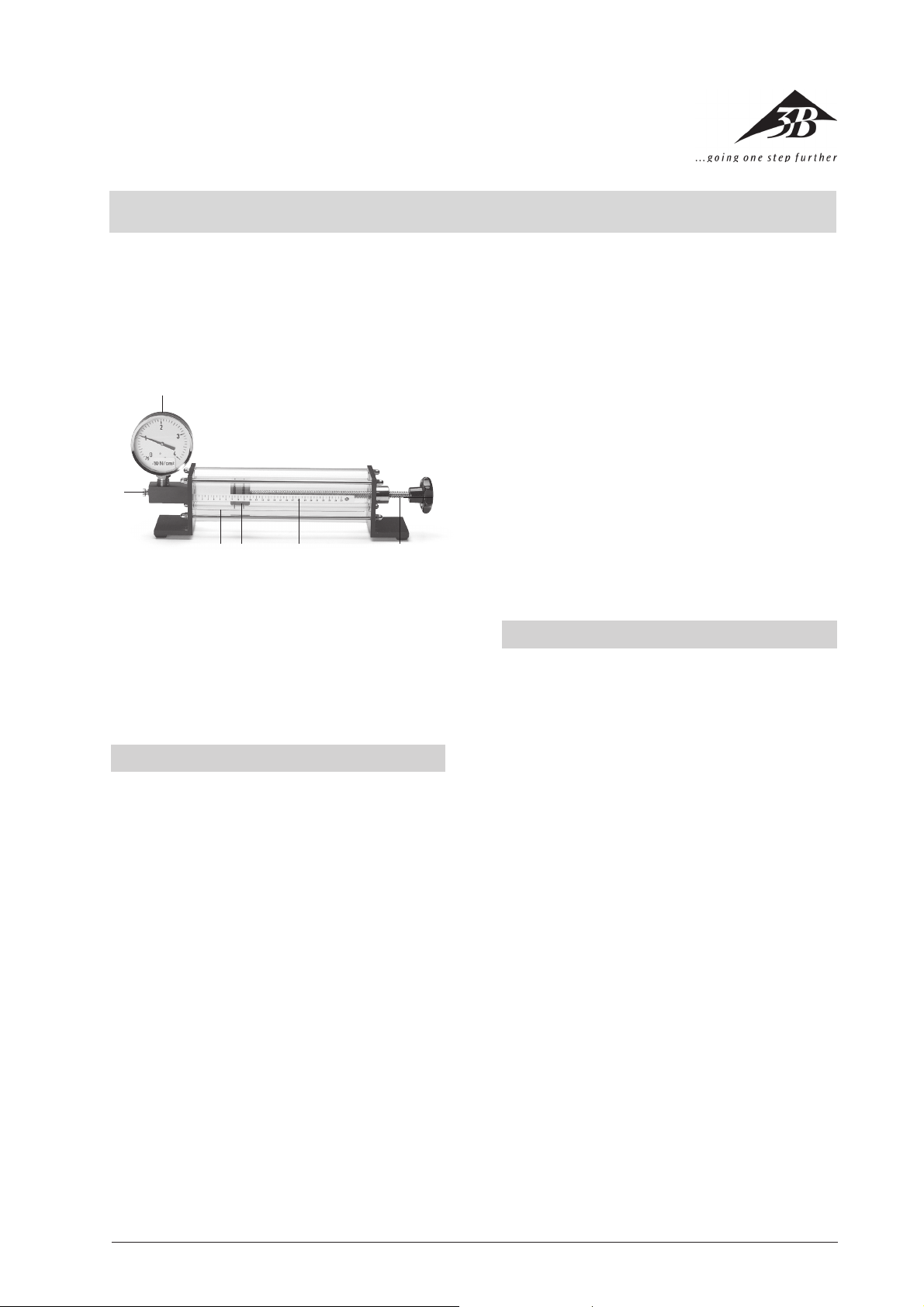
1
2
43 5
6
®
1 Manometer
2 Handscrew for the metering valve
3 Working cylinder with protective cylinder
4 Piston with O-rings
5 Scale
6 Rotary knob with threaded rod
This apparatus is used for the experiment-based determination of the relationship between the volume and
the pressure of a gas (air) at constant temperature (Boyle’s
law).
1. Description, technical data
The apparatus consists of an enclosed plexi-glass cylinder with graduated scale to determine volume and a
flange-mounted manometer for pressure readings and
includes an aeration and de-aeration valve. By turning
the knob the threaded rod moves the piston up an down
inside the cylinder thus varying the volume. This permits
the generation of over- and underpressure. Two O-rings
attached to the piston seal off the air. These are lubricated with a small amount of silicon oil. For safety reasons the power cylinder is encased in an additional plexiglass cylinder.
Power cylinder:
Length: 300 mm
Diameter: 40 mm (interior)
Piston: 30 mm x 40 mm Ø
Scale:
Length: 250 mm
Scale div.: 5 mm
Manometer:
Pressure range: –10 N/cm² - 30 N/cm²
Diameter: 100 mm
2. Operation
Perform an experiment to verify Boyle’s law which states
that for a given mass of gas (air) at a constant temperature the product made up of the volume and the pressure is constant.
The volume of the air column is computed out of the
product of the cylinder’s cross-section and the length of
the air column. As the cross-section is a fixed variable,
the change in volume can only be expressed by varying
the length of the air column.
• Ventilate the cylinder by turning the hand valve screw
to the left.
• Set the piston to the 25 cm mark. If the piston is stuck
the best remedy is to turn it slightly right to left, so
that the O-rings come into contact with the silicone
oil.
• Close the valve. The manometer gage pointed indicates an initial pressure of 1.
• Before each pressure reading tap your finger softly
against the manometer to make sure that the pointer
is on the right setting.
• Turn the rotary knob to slide the piston to the 24 cm
mark and read off and note down the next pressure
level.
• Repeat the procedure in 1 cm steps.
• Enter all the values into a graph (see Figure).
• Proceed accordingly for the case that Boyle’s law is to
be verified for decreasing pressure. Start here with an
air column length of 7 cm.
3
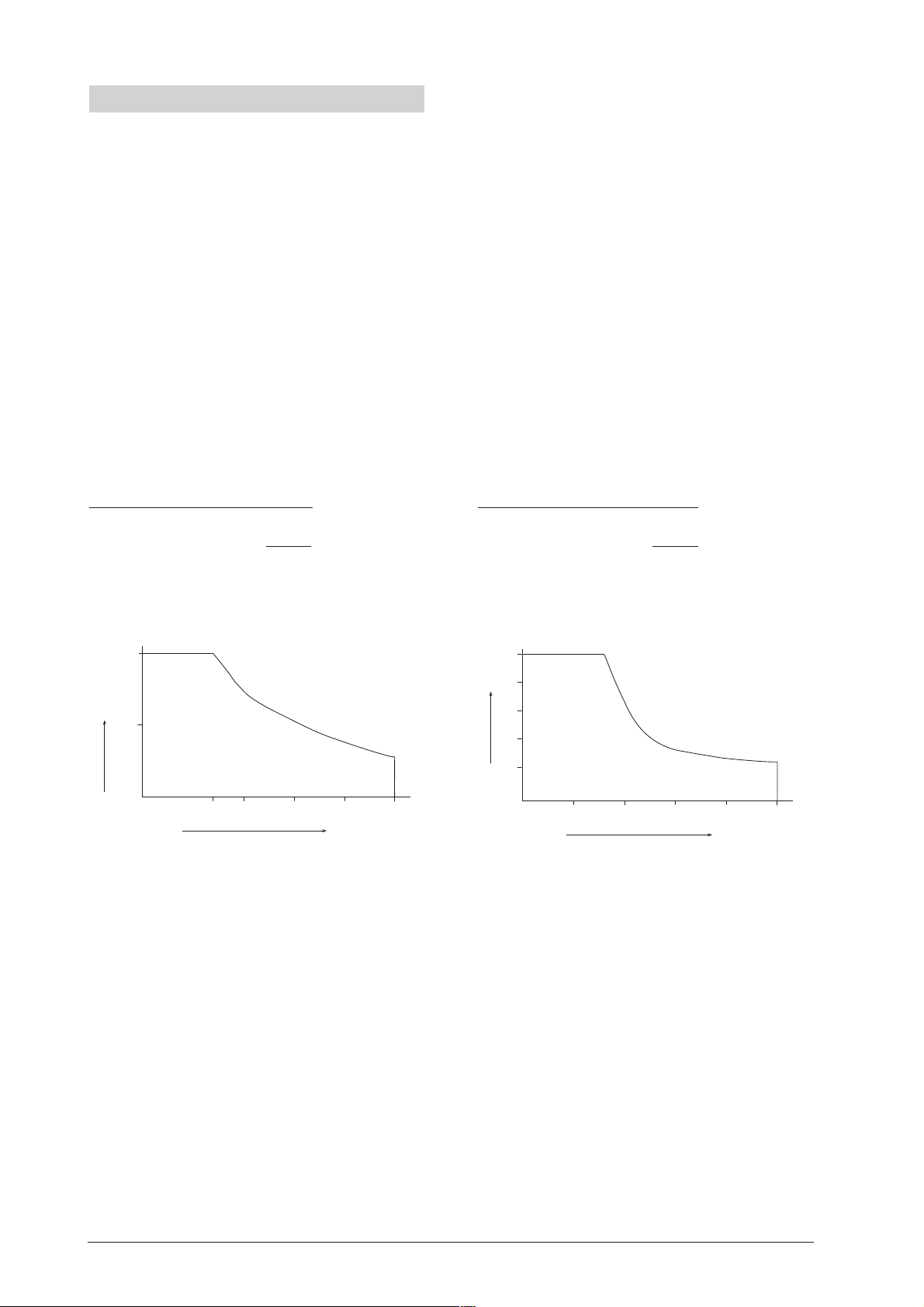
3. Measurement example
Over 10 N/cm
2
Vpp x V
25 10.0 250.0
24 10.3 247.2
23 10.8 248.4
22 11.2 246.4
21 12.0 252.0
20 12.5 250.0
19 13.2 250.8
18 14.0 252.0
17 15.0 255.0
16 16.0 256.0
15 17.0 255.0
14 18.3 256.2
13 19.8 257.4
12 21.2 254.4
11 23.2 255.2
10 25.5 255.0
9,0 28.5 256.5
8.5 30.0 255.0
Below 10 N/cm
2
V p p x V
7 10 70.0
8 8.9 71.2
9 7.9 71.1
10 6.9 69.0
11 6.1 67.1
12 5.5 66.0
13 5.1 66.3
14 4.6 64.4
15 4.5 67.5
16 4.1 65.6
17 3.5 59.5
18 3.3 59.4
19 3.1 58.9
20 3.0 60.0
21 2.9 60.9
22 2.7 59.4
23 2.5 57.5
24 2.3 55.5
25 2.1 52.5
Total 4552.5
10
2
p(N/cm )
5
0
4552.5
/18 = 252.9
10 15 20
7
p > 10 N/cm²
Average
1% = 2.53
Total 1201.8
Average
1% = 0.63
25
V
1201.8
/19 = 63.25
30
25
p
20
15
10
5
10 15 20 25
V
p < 10 N/cm²
3B Scientific GmbH • Rudorffweg 8 • 21031 Hamburg • Germany • www.3bscientific.com • Technical amendments are possible
4
 Loading...
Loading...