Page 1

3B SCIENTIFIC
Boyle’s Law Apparatus E 1017366
Instruction manual
12/13 SD/ALF
®
PHYSICS
1 Manometer
2 Inlet and outlet valve
3 Safety valve
Boyle’s law apparatus E is for investigating the
relationship between volume and pressure in a
body of air inside an enclosed space at constant temperature. It also serves to confirm
Boyle’s law.
The apparatus consists of an enclosed perspex
cylinder with a piston which can be moved in
order to modify the volume enclosed. There is
also a scale for determining the volume and a
manometer to measure the pressure. The piston is moved by turning a threaded shaft with a
crank handle. The force needed to achieve this
is not very large since the ring gaskets on the
4 Piston with ring-shaped
gaskets
5 Main cylinder
1. Description
6 Crank with threaded shaft
7 Scale
piston are lubricated with a small amount of
silicone oil.
An air inlet/outlet valve can equalise pressure
with the surrounding atmosphere with the piston
in any position. Any movement of the piston
thereafter results in the pressure changing to
above or below atmospheric pressure depending on the initial conditions.
A safety valve opens if the excess pressure
should rise to more than 3.5 bars.
1
Page 2
2. Technical data
Main cylinder:
Length: 230 mm
Internal diameter: 50 mm
Maximum pressure: 3.5 bars
Piston: 22 mm x 50 mm diam.
Volume: 410 cm³
Dead space volume V
: 20 cm³ approx.
0
Scale:
Length: 200 mm
Divisions: 1 mm
Manometer:
Pressure range: 0 – 4 bars
Diameter: 100 mm
Tolerance class 1
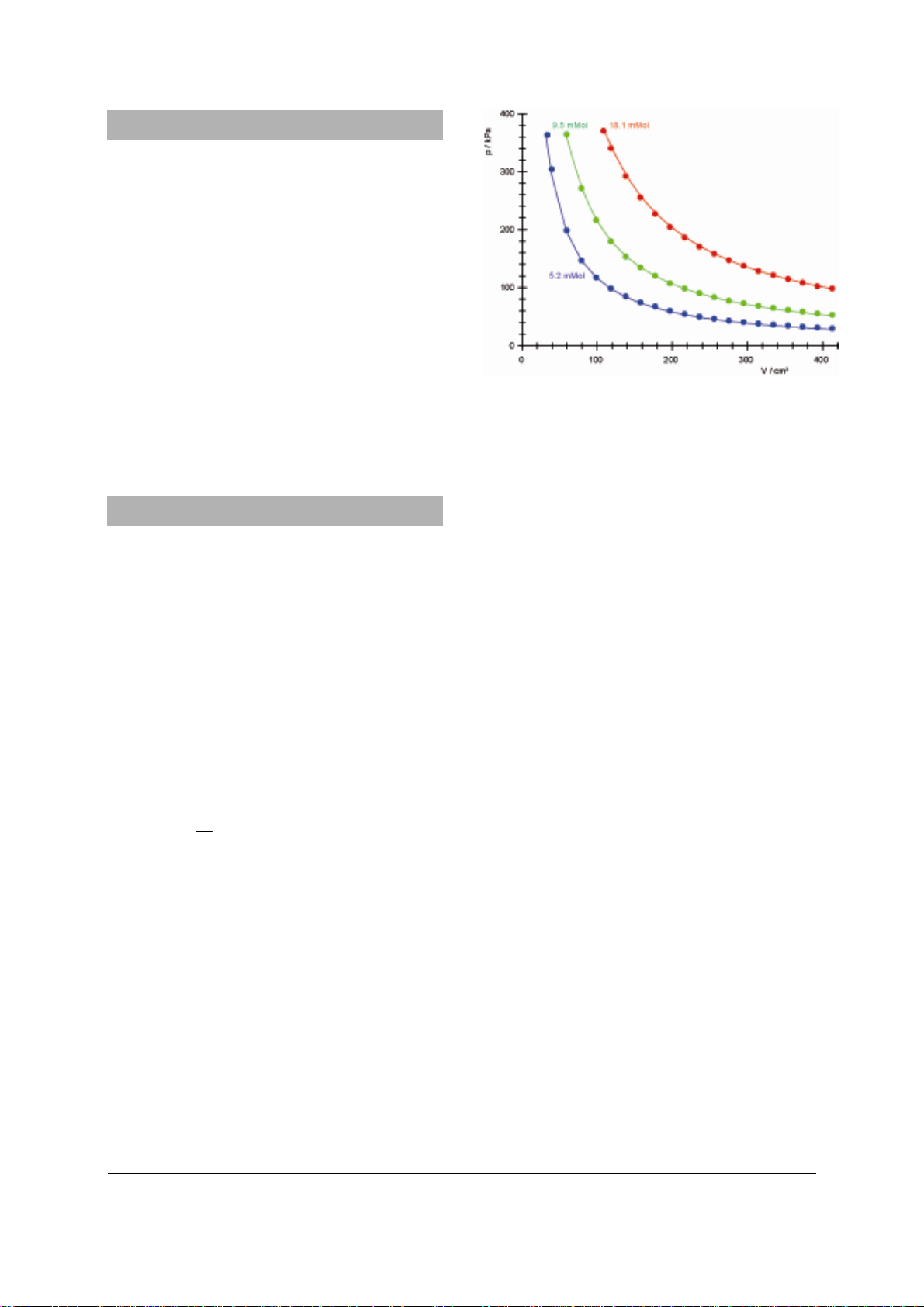
3. Operation
• Turn the piston back and forth a little so that
the gaskets come into contact with the silicone oil.
• Set the piston to the desired point, e.g. 20
cm and let air into the cylinder to equalise
the air inside with the surrounding atmos-
phere (p = 1 bar).
• Close the air inlet/outlet valve.
• Move the piston to a new position by turn-
ing the threaded shaft.
• Read off the piston position s and the pres-
sure p.
• Calculate the volume V using the following
expression
Vs V=⋅π⋅ +
4
where d = 50 mm, V
0
= 20
0
2
d
cm³
• Plot the measurements on a graph.
Note: the amount of air with which the Boyle’s
law apparatus is filled depends on the position
of the piston when the pressure was equalised
with the surrounding atmosphere. The maximum quantity of air inside is reached when the
piston is at 20 cm.
Fig. 1 Pressure-volume diagram for air at room temperature with three different amounts of substance
3B Scientific GmbH ▪ Rudorffweg 8 ▪ 21031 Hamburg ▪ Germany ▪ www.3bscientific.com
Subject to technical amendments
© Copyright 2013 3B Scientific GmbH
 Loading...
Loading...