Page 1

Service Manual
TM
Valleylab
FT Series Energy Platform
FT10
Page 2

Page 3

Service Manual
ValleylabTM FT10
FT Series Energy Platform
For use with software version 2.0x
Part Number: PT00047922
Page 4

Preface
Preface
This manual and the equipment it describes are for use only by qualified medical
professionals trained in the particular technique and surgical procedure to be performed.
It is intended as a guide for servicing the Covidien Valleylab FT10 FT Series Energy Platform
only. Additional user information is available in the Valleylab FT10 FT Energy Platform
User’s Guide.
Equipment covered in this manual
Valleylab FT10 FT Series Energy Platform (VLFT10GEN) with software version 2.0x
The latest version of the VLFT10GEN user’s guide and service manual is available at
http://www.medtronic.com/covidien/support/biomed-connect/electrosurgery.
Call these numbers to request a hardcopy of the service manual
• USA and Canada: 1 800 255 8522 Option 2
• International: 1 303 476 7996
Additional technical information may be available from Covidien Technical Service (see
page 10-6).
For a complete list of service centers world wide, please refer to the Covidien web site: http://www.medtronic.com/covidien/support/service-centers.
ii Valleylab FT10 Energy Platform Service Manual
Page 5

Limited Warranty
Covidien warrants the covered product listed below to be free from defects in material and
workmanship for normal use and service for the period(s) set forth below. Covidien’s
obligation under this warranty is limited to the repair or replacement, at its sole option, of
any product, or part thereof, which has been returned to it (or its authorized distributor)
within the applicable time period shown below after delivery of the product to the original
purchaser, and which examination discloses, to Covidien’s satisfaction, that the product is
defective. This limited warranty does not apply to any product, or part thereof, which has
been repaired or altered in a way so as, in Covidien’s judgment, to affect its stability or
reliability, or which has been subjected to misuse, neglect, or accident.
The warranty period for this Covidien product is as follows:
Valleylab™ FT10 FT Series Energy Platform One year from date of shipping
Limited Warranty
Notwithstanding any other provision herein or in any other document or communication,
Covidien’s liability with respect to this limited warranty and the products sold hereunder
shall be limited to the aggregate purchase price for the products sold to the customer. This
limited warranty is non-transferable and runs only to the original purchaser of the covered
product(s). There are no warranties which extend beyond the terms hereof. Covidien
disclaims any liability hereunder or elsewhere in connection with the sale of products and
for any form of indirect, tort, or consequential damages.
This limited warranty and the rights and obligations hereunder shall be construed under
and governed by the laws of the State of Colorado, USA. The sole forum for resolving
disputes arising under or relating in any way to this limited warranty is the District Court
of the County of Boulder, State of Colorado, USA.
Covidien reserves the right to make changes in covered products built or sold by it at any time without incurring any obligation to make the same or similar changes to equipment previously built or sold by it.
THE OBLIGATION TO REPAIR OR REPLACE A DEFECTIVE OR NONPERFORMING PRODUCT IS THE SOLE REMEDY OF THE CUSTOMER UNDER THIS LIMITED WARRANTY. EXCEPT AS
EXPRESSLY PROVIDED HEREIN, COVIDIEN DISCLAIMS ALL OTHER WARRANTIES,
WHETHER EXPRESS OR IMPLIED, ORAL OR WRITTEN, WITH RESPECT TO
PRODUCTS, INCLUDING WITHOUT LIMITATION ALL IMPLIED WARRANTIES,
WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE.
Valleylab FT10 Energy Platform Service Manual iii
Page 6

Software License
Software License
Customer hereby acknowledges that Covidien LP and or its affiliates (collectively called
“COVIDIEN” herein) owns the entire right, title, and interest in and to the Software, as
may be installed in the Products or Equipment addressed herein or provided separately
(“Software”) (including all of the computer code, source and object, comprising the
Software and all components and elements thereof), and all associated manuals,
drawings, technical information and Documentation (collectively, the “Documentation”),
including, without limitation, all patent, copyright, trademark, trade secret and other
intellectual property or proprietary rights (“Intellectual Property Rights”) in and to the
Software and all components and elements thereof all of which shall remain the sole and
exclusive property of Covidien. The amount paid by the Customer for the Products and/or
Equipment incorporating the Software includes as a portion of that amount, a license fee
granting Customer only the rights set forth in this Software License. This Software
License will be superseded by any express Software agreement between Covidien and
Customer The use of term “Product” herein includes Products and/or Equipment as
applicable.
1. Single User License Grant: COVIDIEN grants to Customer a limited, nonexclusive,
non-sublicensable, nontransferable and revocable license to use the Software, exclusively
at location identified by Customer on the order form as the ship-to location of the
Products, solely in machine-readable object code form, and only on a single central
processing unit embedded in the Products as provided by COVIDIEN under this
agreement, and solely for the Customer’s internal business purpose in the operation of
the Products provided by COVIDIEN under this agreement. Notwithstanding anything to
the contrary contained in this Agreement, the Software is licensed to be used on only
one computing device or Product, and a valid license must be obtained under this
Agreement for each computing device or Product with which the Software is used or in
which the Software is embedded.
2. Restrictions on Use: Except to the extent expressly authorized in these Software
License Terms or by law, Customer shall not and shall not cause any third part to; (i)
decompile, disassemble, or reverse engineer the Software; (ii) modify or create any
derivative works (including, without limitation, translations, transformations, adaptations
or other recast or altered versions) of or based on the Software, or alter the Software in
any way; (iii) merge the Software with any other software or product not supplied by
Covidien; (iv) use, copy, sell, sublicense, lease, rent, loan, assign, convey or otherwise
transfer the Software except as expressly authorized by this Agreement; (v) distribute,
disclose or allow use of the Software, in any format, through any timesharing service,
service bureau, network or by any other means, to or by third parties; (vi) remove or
modify any copyright, confidential or proprietary markings, legends or restrictions that
are in the Software originally supplied to Customer; or (vii) violate any obligations with
regard to Covidien’s Confidential Information (as defined below). To the extent that
Customer is expressly permitted by applicable mandatory law to undertake any of the
activities listed in the preceding sentence, Customer will not exercise those rights unless
and until Customer has given Covidien not less than 30 days’ prior written notice of
Customer’s intent to exercise any such rights unless an order of a government agency of
competent jurisdiction will not so allow. This License will terminate immediately upon
notice from Covidien if Customer fails to comply with any provision of this License or any
agreement.
iv Valleylab FT10 Energy Platform Service Manual
Page 7

3. Reservation of Rights: Notwithstanding anything to the contrary contained in this
Agreement, or any order form, purchase order or agreement between the parties, all
rights not expressly granted by Covidien to Customer are reserved to and retained by
Covidien and Covidien expressly is not selling, assigning or otherwise transferring to
Customer, and Customer is not purchasing or otherwise acquiring or obtaining, any of
Covidien’s Intellectual Property Rights or other rights in or to the Software or
Documentation.
4. Confidentiality: Customer agrees that the Software and the Documentation, and all
components and elements of the Software and Documentation, including, without
limitation, the specific design and structure of individual programs, constitutes
confidential information and trade secrets of Covidien (the “Confidential Information”).
Customer agrees not to disclose, provide, or otherwise make available such Confidential
Information, including, without limitation, any trade secrets or copyrighted material, in
any form to any third party. Customer agrees that it will make the Software available only
to those employees, contractors, or consultants of Customer with a specific need to
know, who are obligated to comply with the restrictions contained in these Software
License Terms and to maintain secrecy of the Software and all other Confidential
Information and are properly trained in its use. Customer is responsible for the
compliance of all users of the Software and Products with these obligations and shall
cause all users of the Software and Products to comply with these obligations. Customer
acknowledges that the Software embodies proprietary trade secrets of Covidien
including, without limitation, technical and non-technical information regarding the
Software and the development and manufacture of the same. Customer hereby agrees
to maintain the confidentiality of such trade secrets using at least as great a degree of
care as Customer uses to maintain the confidentiality of its own most confidential
information. Customer shall communicate these obligations to those employees and
agents of Customer who come into contact with the Software, and shall use its best
efforts to ensure their compliance with all confidentiality obligations applicable to
Customer.
5. Change Orders: Covidien shall have the right, at any time during the Term, by written
request to Customer (an “Update Notice”), to require that Customer return the Products
and Software to Covidien for such periods of time as are required by Covidien (“Update
Periods”) or to allow Covidien to access the Software at the Customer’s location for the
purpose of enabling Covidien to incorporate Software revisions, updates or modifications
from time to time. Upon receipt of an Update Notice, Customer shall return the
requested Products and Software to Covidien at Covidien’s cost and expense, or work
with Covidien to find a suitable time for Covidien to access the Software at the
Customer’s location. Customer acknowledges and agrees that during Update Periods, if
the Software must be returned to Covidien, the Products and Software will be
unavailable to Customer and in Covidien’s possession. Covidien will use reasonable
commercial efforts to perform the revisions, updates or modifications and to return the
revised, updated or modified Products and Software to Customer as soon as is reasonably
practicable.
6. Software License Term: The term of the Software license granted under this Software License shall be for the commercial life of the associated Product or Equipment.
Software License
7. Limited Warranty: Covidien represents and warrants to Customer that the Software
will perform substantially as described in Covidien's then current Documentation for such
Valleylab FT10 Energy Platform Service Manual v
Page 8

Software License
Software and the remaining warranty, or extended warranty, if any, applicable to the
Product or Equipment with which such Software was delivered. If Customer notifies
Covidien of defects during the applicable warranty period, and those defects are verified
by Covidien, as Customer’s sole and exclusive remedy, Covidien will replace the defective
Software or, at its option, terminate this Software License and refund to Customer the
amount paid by Customer to Covidien for the Software (if provided separately from a
Product) or for the Product in which the defective Software is installed (if embedded within
a Product). Customer’s remedy for breach of this limited warranty shall be limited to the
foregoing replacement or refund and shall not encompass any other damages. No dealer,
distributor, agent or employee of Covidien is authorized to make any modification or
addition to the warranty and remedies stated herein.
Notwithstanding these limited warranty provisions, all of Covidien’s obligations with
respect to such warranties shall be contingent on Customer’s use of the Software in
accordance with this Agreement and in accordance with Covidien’s instructions as
provided by Covidien in the Documentation provided by Covidien, as such instructions
may be amended, supplemented, or modified by Covidien, in its sole discretion, from time
to time. Covidien shall have no warranty obligations with respect to any failures of the
Software that are the result of accident, abuse, misapplication, extreme power surge or
extreme electromagnetic field, or any other cause outside of Covidien’s control.
This limited warranty does not apply to any damages, malfunctions, or non-conformities
caused to or by; (i) Customer’s use of Software in violation of these Software License Terms
or in a manner inconsistent with any Documentation or instructions provided by Covidien;
(ii) use of non-Covidien furnished equipment, software, or facilities with its equipment or
Products; (iii) Customer’s failure to follow Covidien’s installation, operation, repair or
maintenance instructions; (iv) Customer’s failure to permit Covidien timely access, remote
or otherwise, to Products; (v) failure to implement all features, revisions, modifications,
updates, patches, “bug fixes”, or new versions of or to the Software provided by Covidien
under this Agreement or otherwise; (vi) Products with there original manufacturer’s serial
numbers altered, defaced or deleted; (vii) Products that been altered, serviced or modified
by a party other than Covidien; or (viii) Software that has been subjected to abnormal
physical or electrical stress, misuse, negligence or accident by Customer or a third party.
8. Export Laws: THESE SOFTWARE TERMS ARE EXPRESSLY MADE SUBJECT TO ANY AND
ALL LAWS, REGULATIONS, ORDERS, OR OTHER RESTRICTIONS WITH RESPECT TO THE
EXPORT FROM THE UNITED STATES OF AMERICA OF THE SOFTWARE. BUYER SHALL NOT
EXPORT OR RE-EXPORT THE SOFTWARE (I) WITHOUT FULL COMPLIANCE WITH SUCH
LAWS, REGULATIONS, ORDERS AND OTHER RESTRICTIONS, INCLUDING, WITHOUT
LIMITATION, OBTAINING ALL NECESSARY APPROVAL FROM ALL REQUIRED
GOVERNMENTAL AGENCIES AND (II) WITHOUT THE PRIOR WRITTEN CONSENT OF
COVIDIEN.
9. U.S. Government Rights. The Software is a “commercial item” developed exclusively
at private expense, consisting of “commercial computer software” and “commercial
computer software Documentation” as such terms are defined or used in the applicable
U.S. acquisition regulations. The Software is licensed hereunder (i) only as a commercial
item and (ii) with only those rights as are granted to all other customers pursuant to the
terms and conditions of this License. Customer shall not use, duplicate, or disclose the
Software in any way not specifically permitted by this License. Nothing in this License
requires Covidien to produce or furnish technical data for or to Customer.
vi Valleylab FT10 Energy Platform Service Manual
Page 9

10. Survival. Sections 2, 3, 4, 8, 9 and this Section 10 shall survive the termination or expiration of these Software License Terms.
Software License
Valleylab FT10 Energy Platform Service Manual vii
Page 10

Page 11

Table of Contents
Preface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ii
Limited Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .iii
Software License . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .iv
Chapter 1. Overview and General Features
The Valleylab FT10 Energy Platform. . . . . . . . . . . . . . . . . . . . . . 1-2
Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
Front Panel. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Rear Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-4
Modes & Settings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-5
Bipolar Resection. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-10
LigaSure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-11
System Conventions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-14
The Touchscreen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-14
System Buttons . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-16
Interface Conventions . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-17
Chapter 2. Warnings and Precautions for
Patient and Operating Room Safety
Conventions Used in this Guide . . . . . . . . . . . . . . . . . . . . . . . . . 2-1
General Warnings and Precautions. . . . . . . . . . . . . . . . . . . . . . . 2-2
Fire/Explosion Hazards . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-2
System Setup Warnings and Precautions. . . . . . . . . . . . . . 2-3
Warnings and Precautions for the Energy Platform . . . . . 2-6
Warnings and Precautions for Active Instruments . . . . . . 2-7
Warnings for Implanted Electronic Devices (IEDs). . . . . . . 2-8
Post Surgery Safety Issues . . . . . . . . . . . . . . . . . . . . . . . . . 2-9
Warnings and Precautions for Monopolar Procedures . . . . . . . 2-9
Warnings and Precautions for
Patient Return Electrodes . . . . . . . . . . . . . . . . . . . . . . . . . 2-10
Inadvertent Radio Frequency (RF) Burns . . . . . . . . . . . . . 2-11
Warnings and Cautions for Laparoscopic Procedures . . . . . . . 2-13
Warnings and Precautions for Bipolar Procedures . . . . . . . . . 2-14
Warnings and Precautions for LigaSure Procedures . . . . . . . . 2-14
Warnings and Precautions for Bipolar Resection . . . . . . 2-15
Servicing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-15
Shunt Cords . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-16
Conductive Fluid In the Surgical Site . . . . . . . . . . . . . . . . . . . . 2-16
Valleylab FT10 Energy Platform Service Manual ix
Page 12

Chapter 3. Principles of Operation
Block Diagram . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-2
Electronic Assemblies Principles of Operation . . . . . . . . . . . . . . 3-4
High-Voltage/Low-Voltage Power Supply Principles of
Operation. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
Controller PCBA. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
RF PCBA . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
Steering Relay PCBA . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
Display PCBA . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
Mechanical Assemblies Principles of Operation. . . . . . . . . . . . . 3-5
IT Network Connectivity Principles of Operation. . . . . . . . . . . . 3-5
Chapter 4. Technical Specifications
VLFT10GEN Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
General. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
Dimensions and Weight . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
Environmental Parameters . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Input Power . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Power Cord Specifications. . . . . . . . . . . . . . . . . . . . . . . . . . 4-4
Backup Power . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-4
Equipotential Ground Connection . . . . . . . . . . . . . . . . . . . 4-4
EKG Blanking and Smoke Evacuation . . . . . . . . . . . . . . . . 4-4
Internal Memory . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Duty Cycle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Leakage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Radio Frequency Identification (RFID) . . . . . . . . . . . . . . . . 4-6
Wireless Fidelity (WiFi) . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-6
Ethernet . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-7
Symbols Used . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-8
Standards and IEC Classifications . . . . . . . . . . . . . . . . . . . . . . . 4-10
Class I Equipment (IEC 60601-1) . . . . . . . . . . . . . . . . . . . . 4-10
Type CF Equipment/Defibrillator Proof
(IEC 60601-1, IEC 60601-2-2, and ANSI/AAMI HF18) . . . . 4-10
IP21 Liquid Ingress/Spillage
(IEC 60601-1 and IEC 60601-2-2) . . . . . . . . . . . . . . . . . . . . 4-11
Voltage Transients – Energy Platform Mains Transfer
(IEC 60601-1, IEC 60601-2-2, and ANSI/AAMI HF18) . . . . 4-11
CISPR 11 Class A . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-11
Electromagnetic Compatibility
(IEC 60601-1-2 and IEC 60601-2-2) . . . . . . . . . . . . . . . . . . 4-11
Cables Used for EMC Compliance Testing . . . . . . . . . . . . 4-16
Return Electrode Monitor (REM) . . . . . . . . . . . . . . . . . . . 4-18
x Valleylab FT10 Energy Platform Service Manual
Page 13

Auto Bipolar . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-18
Audio Tones. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-19
Energy Output Characteristics. . . . . . . . . . . . . . . . . . . . . . . . . . 4-21
Output Waveforms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-23
Output Power vs. Resistance Graphs. . . . . . . . . . . . . . . . . . . . . 4-25
Monopolar Graphs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-25
Bipolar Graphs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-40
Discontinuous Power Curves. . . . . . . . . . . . . . . . . . . . . . . 4-49
Chapter 5. System Setup
Setup. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-2
Unpacking the System. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-2
Before Starting the System . . . . . . . . . . . . . . . . . . . . . . . . . 5-2
Turning On the VLFT10GEN . . . . . . . . . . . . . . . . . . . . . . . . 5-3
Turning Off the VLFT10GEN . . . . . . . . . . . . . . . . . . . . . . . . 5-3
System Functions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-4
On/Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-4
Restore Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-4
Audio Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-5
Service and Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-6
Logs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-7
DEMO Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-7
System Menu . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-8
Service Menu . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-10
Language Selection Menu . . . . . . . . . . . . . . . . . . . . . . . . 5-11
Feature Enabling Menu. . . . . . . . . . . . . . . . . . . . . . . . . . . 5-11
Chapter 6. Testing Setup and Functional Tests
Periodic Safety Check (Routine Maintenance) . . . . . . . . . . . . . . 6-2
Inspecting the System and Accessories. . . . . . . . . . . . . . . . 6-3
Inspecting Internal Components (when required) . . . . . . 6-5
Power Up Check. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-6
Verifying Power Output . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-7
RF Output Test Screen. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-7
Testing Monopolar 1 COAG Output - 500 Ω Load. . . . . . . 6-8
Testing Monopolar 2 COAG Output - 500 Ω Load . . . . . . 6-9
Testing Monopolar 2 COAG Output - 100 Ω Load . . . . . 6-10
Testing Monopolar 2 CUT Output - 300 Ω Load . . . . . . . 6-11
Testing Monopolar 2 VALLEYLAB Mode
Output - 300 Ω Load . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-12
Testing Bipolar Energy Channel Output - 100 Ω Load . . 6-13
Testing LigaSure/Bipolar Output . . . . . . . . . . . . . . . . . . . 6-14
Valleylab FT10 Energy Platform Service Manual xi
Page 14

Verifying REM Function . . . . . . . . . . . . . . . . . . . . . . . . . . 6-15
Verifying Cross Coupling. . . . . . . . . . . . . . . . . . . . . . . . . . 6-16
Checking High-Frequency Leakage Current . . . . . . . . . . . . . . . 6-19
Checking Monopolar High-Frequency
Leakage Current . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-19
Checking Patient Return High-Frequency
Leakage Current . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-20
Checking Bipolar High-Frequency Leakage Current . . . . 6-21
Checking LigaSure/Bipolar High-Frequency
Leakage Current . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-22
Safety Testing in Accordance with IEC 60601-1 . . . . . . . . . . . . 6-23
Checking Low-Frequency Leakage Current . . . . . . . . . . . 6-23
Chassis or Earth Leakage. . . . . . . . . . . . . . . . . . . . . . . . . . 6-24
Total Patient Leakage Source Current . . . . . . . . . . . . . . . 6-24
Total Patient Leakage Sink Current . . . . . . . . . . . . . . . . . 6-24
Ground Continuity Testing . . . . . . . . . . . . . . . . . . . . . . . . 6-25
Preventive Maintenance Check Sheet. . . . . . . . . . . . . . . . . . . . 6-25
Chapter 7. Calibrations
Touchscreen Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-2
Energy Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-4
Periodic Safety Check (Routine Maintenance) . . . . . . . . . . . . . . 7-7
Inspecting the System and Accessories. . . . . . . . . . . . . . . . 7-8
Inspecting Internal Components (when required) . . . . . 7-10
Power Up Check. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-11
Chapter 8. Troubleshooting
General Troubleshooting Guidelines . . . . . . . . . . . . . . . . . . . . . 8-2
REM Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-2
Correcting a REM-Alarm Condition . . . . . . . . . . . . . . . . . . 8-2
Correcting Malfunctions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-3
System Errors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-8
Chapter 9. Replacement Procedures
Tools Required for Component Replacement . . . . . . . . . . . . . . 9-2
Touch-up Paint Instructions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-2
Top-Level Assemblies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-4
Base Chassis Replacement. . . . . . . . . . . . . . . . . . . . . . . . . . 9-4
Controller Fan Replacement . . . . . . . . . . . . . . . . . . . . . . . . 9-8
Controller PCBA Replacement . . . . . . . . . . . . . . . . . . . . . 9-10
PCBA Battery Replacement. . . . . . . . . . . . . . . . . . . . . . . . 9-14
Display PCBA Replacement . . . . . . . . . . . . . . . . . . . . . . . . 9-17
xii Valleylab FT10 Energy Platform Service Manual
Page 15

E-PAC Foam Replacement. . . . . . . . . . . . . . . . . . . . . . . . . 9-20
Power-Supply Fan Replacement . . . . . . . . . . . . . . . . . . . . 9-23
Power-Supply Module Replacement . . . . . . . . . . . . . . . . 9-26
Speaker Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-28
RF Fan Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-30
RF PCBA Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-32
Steering Relay PCBA Replacement . . . . . . . . . . . . . . . . . . 9-34
Top Cover Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . 9-37
Front-Panel Assemblies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-39
Front Bezel Replacement . . . . . . . . . . . . . . . . . . . . . . . . . 9-39
Bipolar Receptacle Replacement . . . . . . . . . . . . . . . . . . . 9-43
LigaSure/Bipolar Receptacle Replacement . . . . . . . . . . . 9-45
Monopolar 1 Receptacle Replacement . . . . . . . . . . . . . . 9-47
Monopolar 2 Receptacle Replacement . . . . . . . . . . . . . . 9-49
VIBE Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-51
REM Assembly Replacement. . . . . . . . . . . . . . . . . . . . . . . 9-53
LCD Module Replacement. . . . . . . . . . . . . . . . . . . . . . . . . 9-56
Touchscreen Assembly Replacement . . . . . . . . . . . . . . . . 9-59
Rear-Panel Assemblies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-63
EMI Filter Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-63
Equipotential Ground Connection Replacement . . . . . . 9-66
FTSW PCBA Replacement . . . . . . . . . . . . . . . . . . . . . . . . . 9-68
Fuse Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-70
WiFi Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-71
Back Panel Disassembly and Re-assembly . . . . . . . . . . . . 9-72
Chapter 10. Maintenance and Repair
Responsibility of the Manufacturer . . . . . . . . . . . . . . . . . . . . . 10-2
Routine Maintenance and Periodic Safety Checks. . . . . . . . . . 10-2
Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-3
Product Service. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-3
Returning the Energy Platform for Service . . . . . . . . . . . 10-4
Software Updates. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-5
Updating Energy Platform Software . . . . . . . . . . . . . . . . 10-5
Service Manual Copies and Updates . . . . . . . . . . . . . . . . . . . . . 10-6
Covidien Technical Service. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-6
Training/Education. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-7
Valleylab FT10 Energy Platform Service Manual xiii
Page 16

Page 17
Chapter 1
Overview and General Features
This chapter provides an overview of the features and functions of the Valleylab FT10 FT Series Energy Platform.
Precaution
Read the instructions, warnings, and precautions provided with this energy platform and associated accessories before using. Specific instructions for electrosurgical instruments are not included in this manual.
Valleylab FT10 Energy Platform Service Manual 1-1
Page 18

The Valleylab FT10 Energy Platform
The Valleylab FT10 Energy Platform
Introduction
The Valleylab FT10 FT Series Energy Platform (VLFT10GEN) provides RF energy for
monopolar and bipolar surgical applications, and tissue-fusion and vessel-sealing
applications. It features a touchscreen divided into four quadrants for viewing and user
input of settings and options available for any application. The energy platform
automatically detects coded handsets and configures the energy platform accordingly.
Safety and diagnostic functionality include automatic fail-safe functions.
The VLFT10GEN, applied parts (patient return electrodes and active instruments) are designed to work as a system. Covidien offers a selection of patient return electrodes and active instruments that are fully compatible with this energy platform.
• Refer to each instrument’s instructions for use (IFU) for indications, warnings, and specific contraindications.
• When considering other manufacturers’ patient return electrodes and/or active instruments, customers should seek detailed user instructions and warning information from the manufacturer.
The generator is intended for use in general surgery and such surgical specialties as urologic, vascular, thoracic, plastic, gynecologic, reconstructive, and colorectal surgery.
Indications for Use
The VLFT10GEN is a high frequency electrosurgical generator intended for use with
monopolar and bipolar accessories for cutting and coagulating tissue. When used with
compatible sealing devices, it is indicated for sealing vessels up to and including 7mm,
tissue bundles, and lymphatics.
The generator can also be used with compatible resectoscopes for endoscopically controlled removal or coagulation of tissue using 0.9% NaCl solution as the irrigation medium.
The tissue fusion function has not been shown to be effective for tubal sterilization or tubal coagulation for sterilization procedures. Do not use this function for these procedures.
Contraindications
None known.
1-2 Valleylab FT10 Energy Platform Service Manual
Page 19
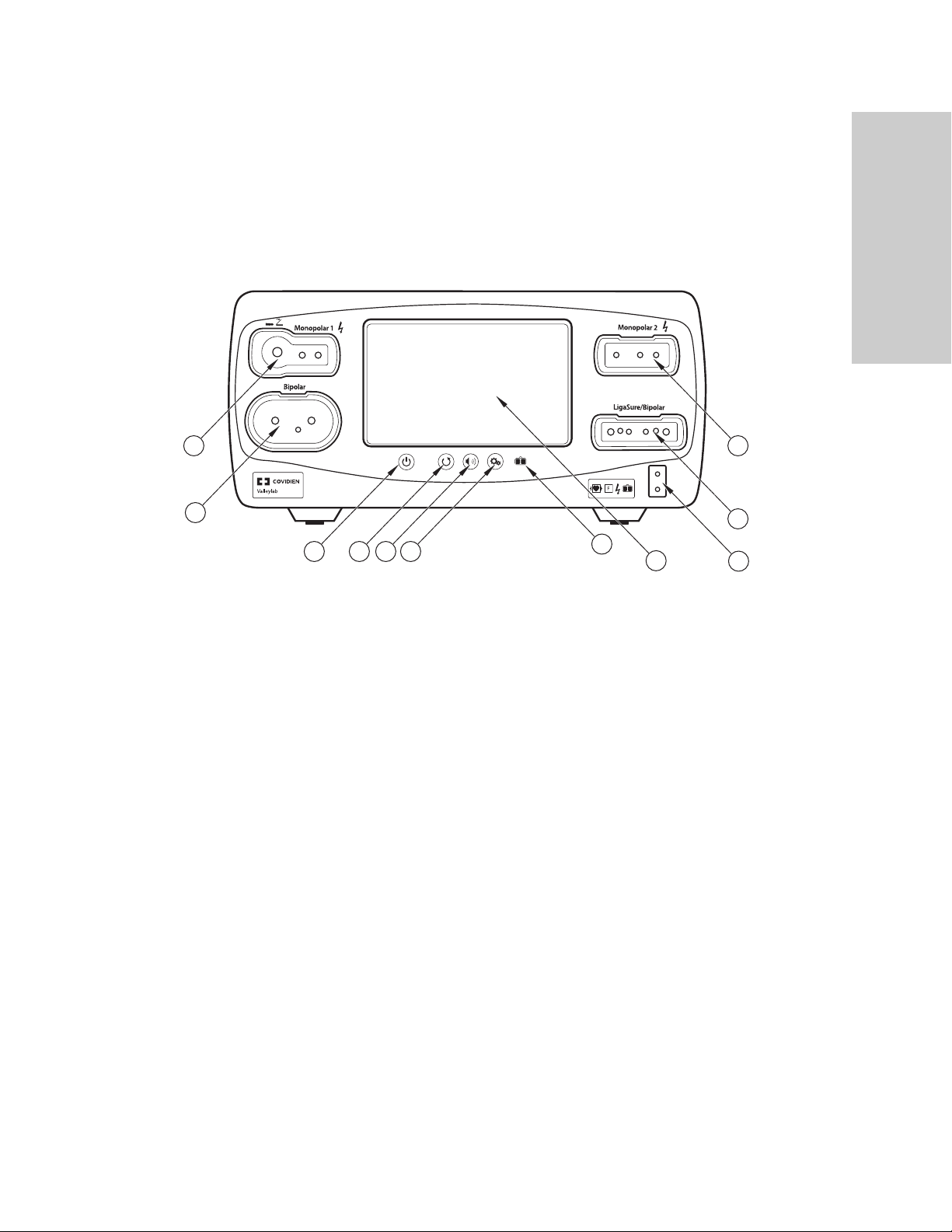
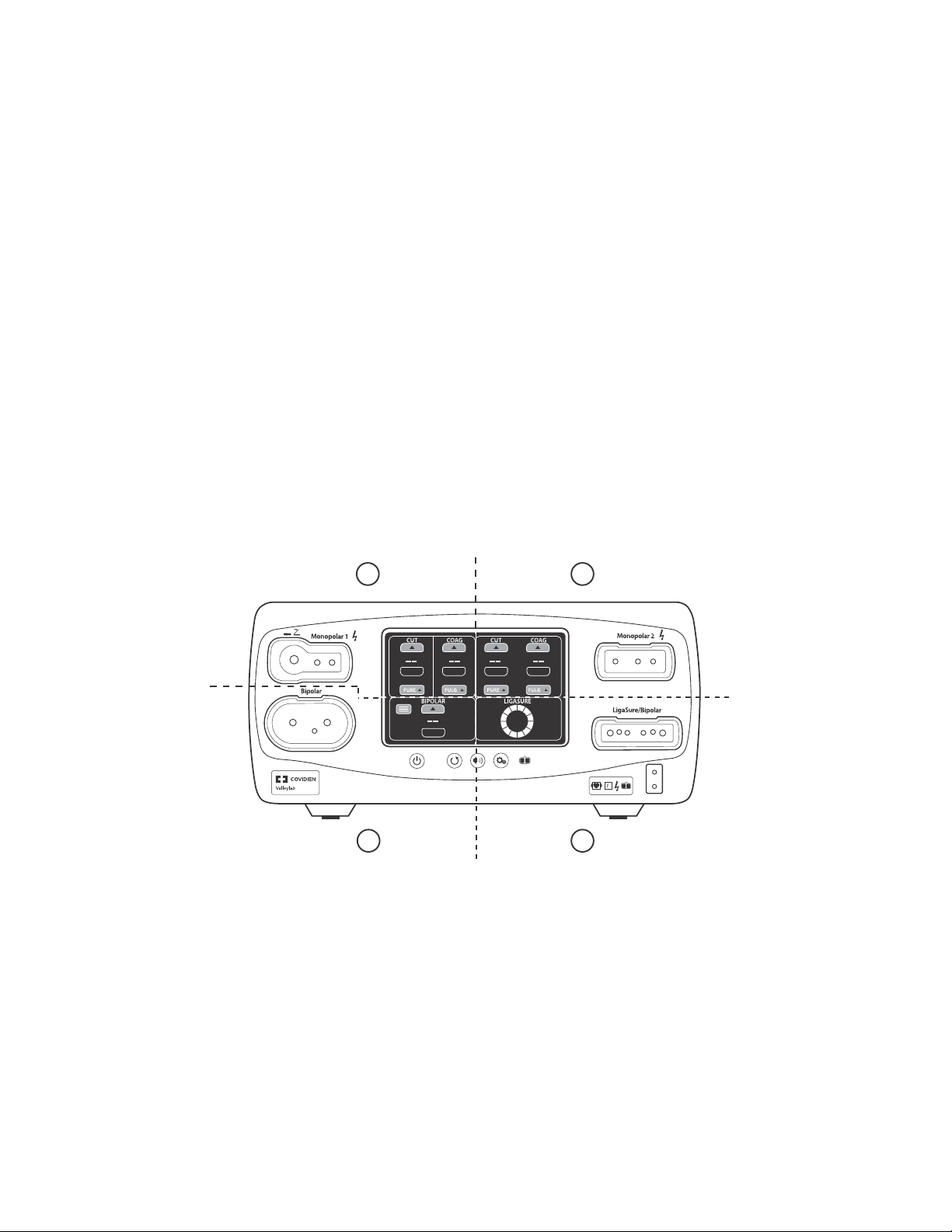
Front Panel
The Valleylab FT10 Energy Platform
Overview and General Features
10
FT10
11
1
2
4
3
On/Off button
ཱ Restore Settings button
ི Audio Volume control button
ཱི Service/Settings button
ུ REM™ (Return Electrode Monitoring) indicator
ཱུ Interface touchscreen
ྲྀ REM patient return electrode receptacle
ཷ LigaSure™/Bipolar receptacle
ླྀ Monopolar 2 instrument receptacle
ཹ Monopolar 1 Universal Foot-Pedal Port (UFP) receptacle
ེ Bipolar instrument receptacle
9
8
5
6
7
Valleylab FT10 Energy Platform Service Manual 1-3
Page 20
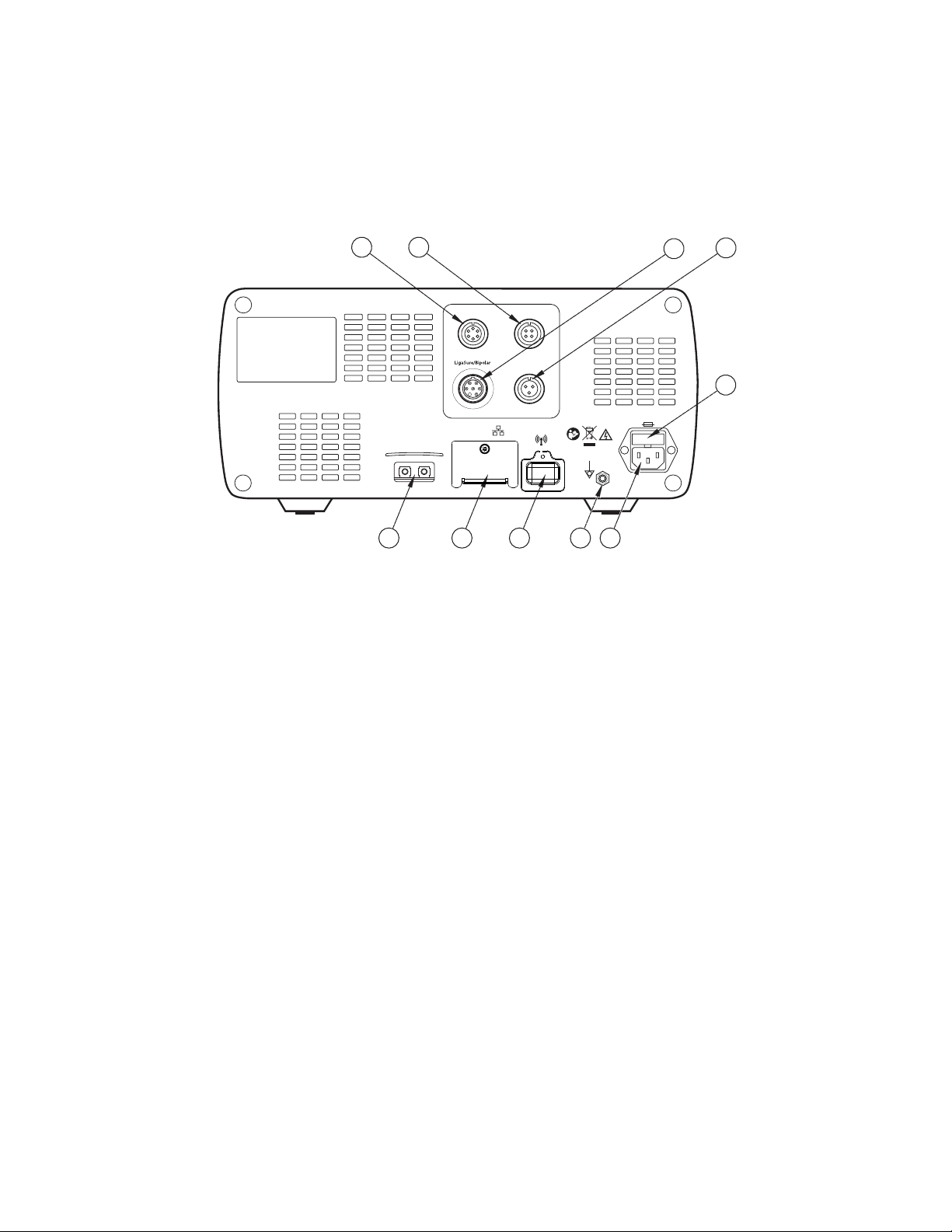
The Valleylab FT10 Energy Platform
Rear Panel
1
2
Monopolar 2 Monopolar 1
Bipolar
3
4
5
Warning: Risk of Fire.
Replace Fuse as Marked
250V, F10.0A (100-127Vac)
250V, F6.3A (220-240Vac)
Monopolar1Monopolar
2
Avertissement: Risque du feu.
Remplacez les fusibles
comme marqués.
250V, F10.0A (100-127Vac)
250V, F6.3A (220-240Vac)
10
89
6
7
Monopolar 2 foot-pedal receptacle (requires included adapter to connect standard
four-pin monopolar foot pedal)
ཱ Monopolar 1 foot-pedal receptacle
ི LigaSure/Bipolar foot-pedal receptacle (requires included adapter to connect bipolar
resection foot pedal)
ཱི Bipolar foot-pedal receptacle
ུ Fuse drawer
ཱུ Power cord receptacle
ྲྀ Equipotential ground connection
ཷ WiFi antenna (Covered. For service only.)
ླྀ Ethernet receptacle (Covered. For service only.)
ཹ Interlink cable receptacles for EKG blanking and smoke-evacuation control
(independently linked to Monopolar 1 and Monopolar 2 activation)
1-4 Valleylab FT10 Energy Platform Service Manual
Page 21

The Valleylab FT10 Energy Platform
Modes & Settings
The VLFT10GEN provides the following modes and settings for a variety of surgical procedures:
Monopolar modes Power-Setting Ranges Peak Voltage
· CUT
- PURE Off, 1–300 W 1287 V
- BLEND Off, 1–200 W 2178 V
· VALLEYLAB 5–60 W 2783 V
· COAG
Overview and General Features
- SOFT Off, 1–120 W 264 V
- FULGURATE Off, 1–120 W 3448 V
-SHARED FULGURATE
- SPRAY Off, 1–120 W 3932 V
- SHARED SPRAY
Bipolar effects
· LOW Off, 1–15 W 133 V
· MEDIUM 16–40 W 214 V
· HIGH 45–95 W 462 V
LigaSure (tissue fusion) No power settings 244 V
Bipolar Resection effect
· CUT 1–6 742 V
· COAG 1–6 318 V
Off, 1–120 W 3448 V
Off, 1–120 W 3932 V
Valleylab FT10 Energy Platform Service Manual 1-5
Page 22

The Valleylab FT10 Energy Platform
Monopolar Modes
The system produces six modes of monopolar power output.
Precaution
To provide expected functionality from a hand piece, proper insertion is required. Refer to the alignments dots below the receptacles for proper insertion orientation.
CUT Modes
PURE CUT provides a clean, precise cut in any tissue with little or no hemostasis.
BLEND CUT is a conventional blended waveform that provides slower cutting with
simultaneous hemostasis.
VALLEYLAB Mode
VALLEYLAB mode is a unique combination of hemostasis and dissection that allows the
user to slow down for more hemostasis and speed up for faster dissection.
COAG Modes
SOFT desiccates tissue at a relatively slower rate with deeper thermal penetration. It is
typically performed with a ball electrode.
FULGURATE coagulates tissue by sparking from the active electrode, through air, to the patient tissue.
SPRAY delivers wider fulguration; penetration is shallower and the affected tissue area is larger than with the FULGURATE mode.
SHARED allows two monopolar instruments to activate simultaneously in either FULGURATE or SPRAY modes. A single power setting is provided and power is shared between the two instruments.
Note: SOFT and SHARED COAG modes are features that are not available on the clinical screen until they have been enabled. These features can be enabled through the Feature Enabling menu of the service screen. See Feature Enabling Menu on page 3-10.
Compatible Monopolar Instruments & Devices
The following Covidien catalog numbers for monopolar surgical instruments, return electrodes, foot pedals, and adapters are fully compatible with the VLFT10GEN.
Monopolar UFP Instruments (connect only to Monopolar 1)
E05021 Monopolar Adapter
E050212 Monopolar Adapter
The Monopolar 1 UFP-receptacle, identified by a blue ring, accepts UFP connectors with
diameters of 4 mm to 8 mm, and lengths of 15.2 mm to 41.7 mm. UFP connectors with
a diameter of less than 4 mm require an adapter to connect to the Monopolar 1 UFP
receptacle.
1-6 Valleylab FT10 Energy Platform Service Manual
Page 23
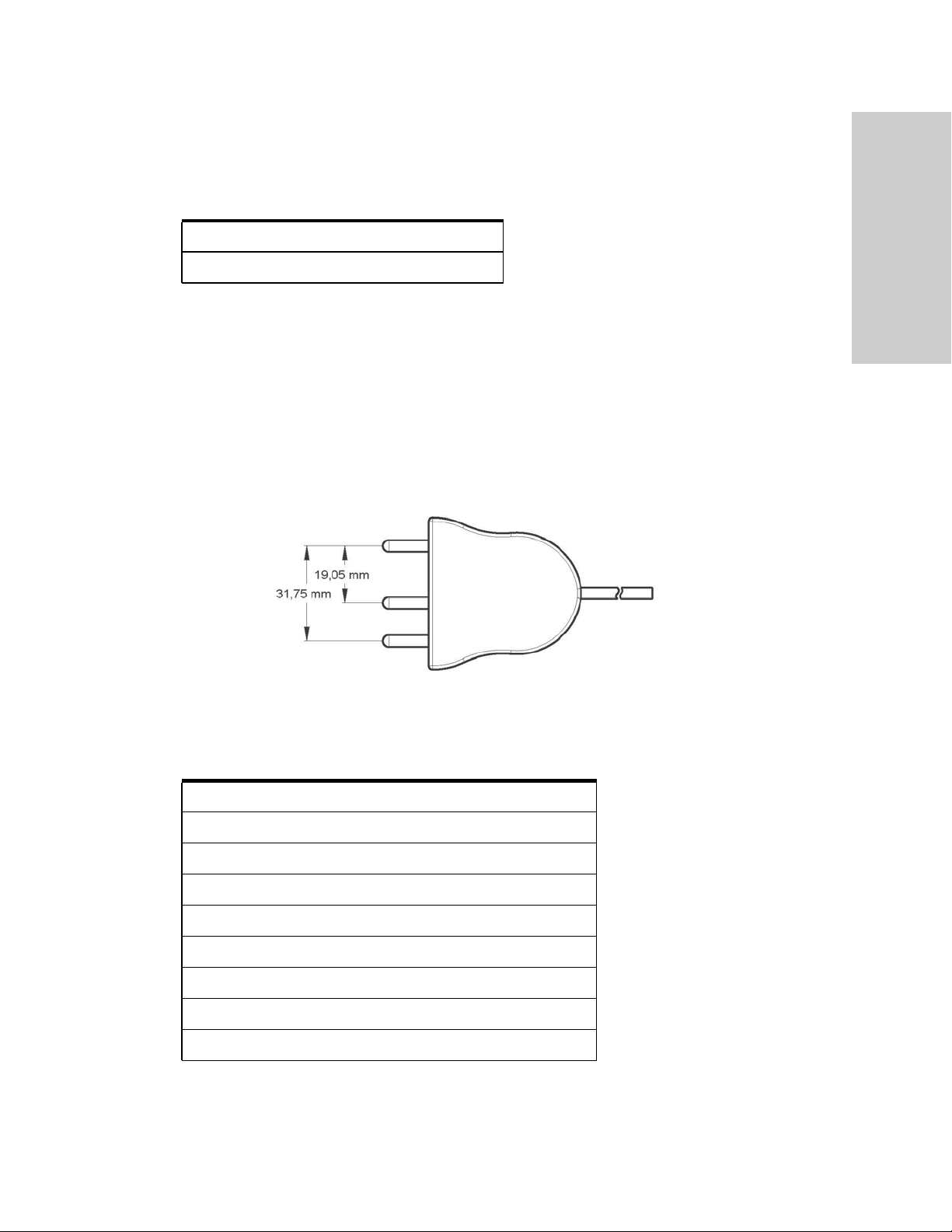
The Valleylab FT10 Energy Platform
Monopolar Instruments (connect only to Monopolar 2 receptacle)
FT3000DB Force TriVerse™ Electrosurgical Device
FT3000 Force TriVerse Electrosurgical Device
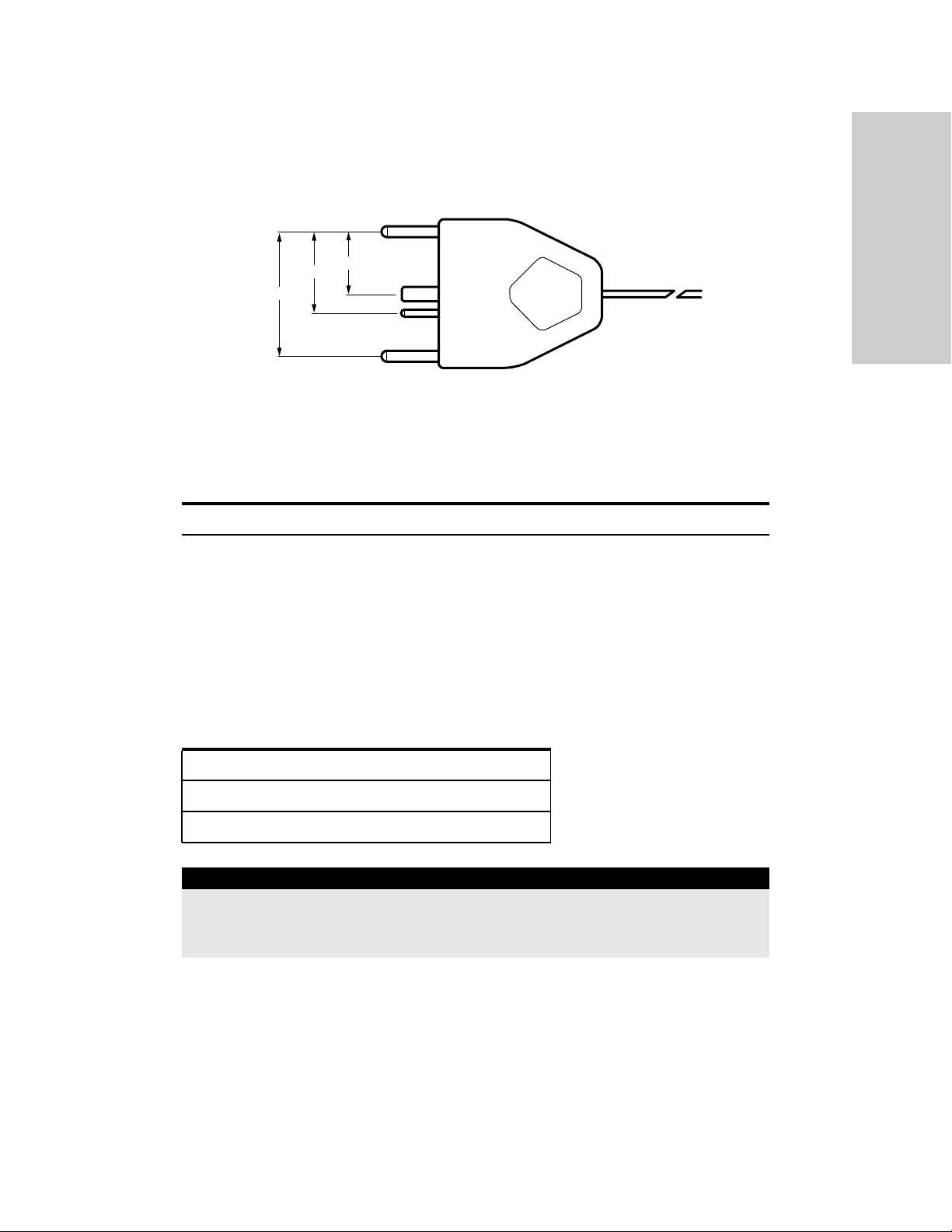
Monopolar Instruments (connect to either Monopolar 1 or Monopolar 2)
This generator is designed for use with Covidien monopolar instruments. However,
other monopolar instruments are compatible with the VLFT10GEN if they have a
connector that matches the following figure and are rated for peak voltages of at least
3932 V.
Monopolar
Overview and General Features
Utilizes 4 mm banana pins
Return Electrodes (Monitoring)
E0560 Valleylab REM Patient Return Electrode Cord
E7507 REM Polyhesive™ Adult Patient Return Electrode
E7507DB REM Polyhesive Adult Patient Return Electrode
E7508 REM Polyhesive Adult Cordless Patient Return Electrode
E7509 REM Polyhesive Adult Cordless Patient Return Electrode
E7509B REM Polyhesive Adult Cordless Patient Return Electrode
E7510-25 REM Polyhesive Infant Patient Return Electrode
E7510-25DB REM Polyhesive Infant Patient Return Electrode
E7512 REM Polyhesive Neonatal Patient Return Electrode
Valleylab FT10 Energy Platform Service Manual 1-7
Page 24
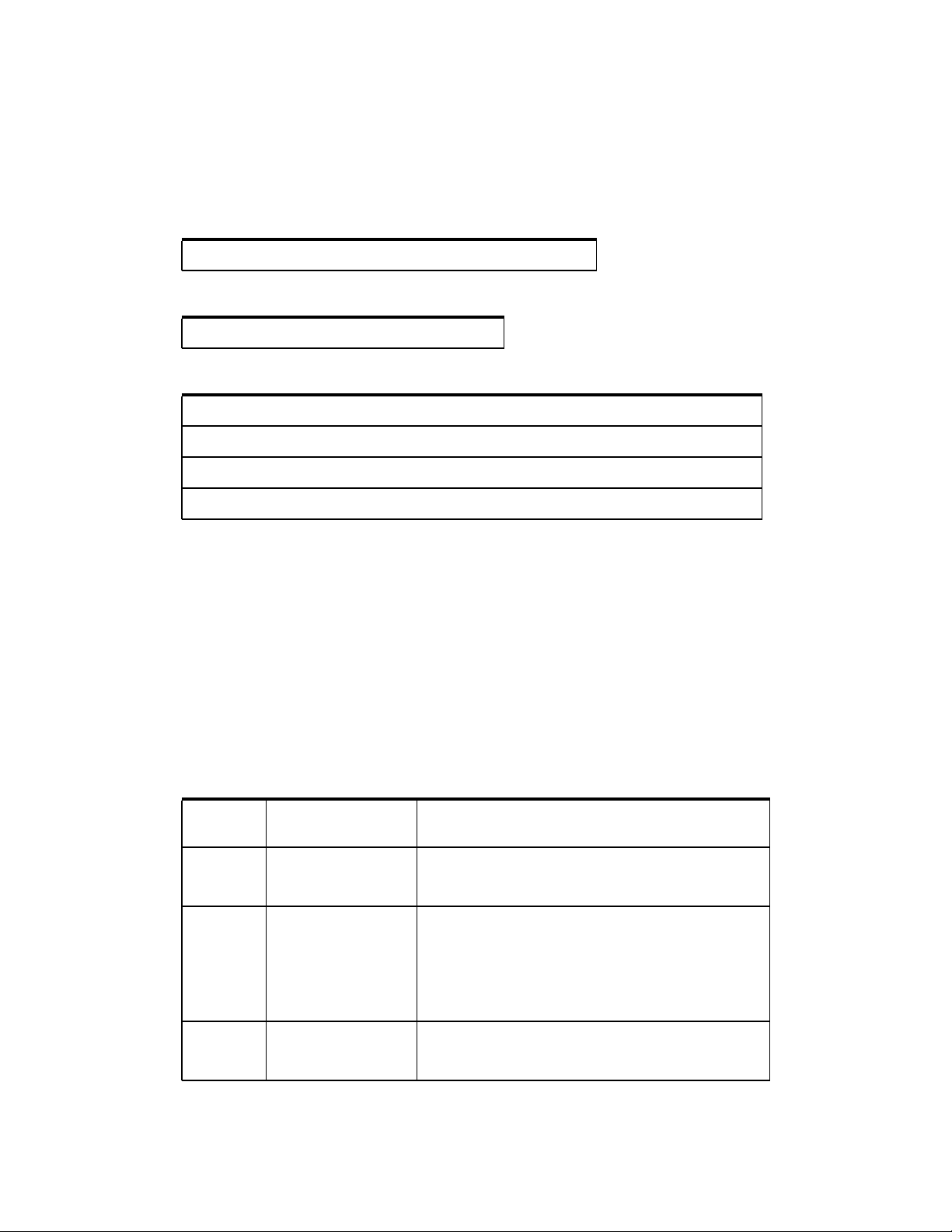
The Valleylab FT10 Energy Platform
Return Electrodes (Non-Monitoring, for use in DEMO mode only. Not for clinical use.)
E7506 Non-REM Polyhesive Patient Return Electrode
REM Connector
E0507B Valleylab Multiple Return/S Cord Adapter
Foot Pedals
FT6003 ForceTriad Three-Pedal Footswitch (Monopolar 2 only)
E6008 Valleylab Monopolar Footswitch (Monopolar 1, Monopolar 2 with adapter)
E6008B Valleylab Monopolar Footswitch (Monopolar 1, Monopolar 2 with adapter)
1017577 6-Pin to 4-Pin Monopolar Footswitch Adapter (Monopolar 2 only)
Bipolar Effects
Selection of bipolar effects and power settings is dependent on surgeon preferences, tissue characteristics, accessories selection, and the intended clinical application.
LOW effect is for power selections of 1–15 watts. It delivers low-voltage output for precision and fine control of the amount of desiccation typically used with small-surface area instruments.
MEDIUM effect is for power selections of 16–40 watts. It is a conventional bipolar output typically used with medium-surface area electrodes.
HIGH effect is for power selections of 45–95 watts. Power remains constant over a wide range of tissue types and may be used for large electrodes.
Effect Setting
LOW 1—15 watts • Small-surface area instruments
MEDIUM 16—40 watts • Medium-surface area instruments
Power Setting Range
Optimized Instrumentation
• Micro-tip forceps (0.4—2.2 mm)
• Micro-tip forceps (1.0—2.2 mm)
• Small paddle lap forceps
• Bipolar scissors
HIGH 45—95 watts
(5 watt increments)
• Large-surface area instruments
• Large paddle lap forceps
1-8 Valleylab FT10 Energy Platform Service Manual
Page 25
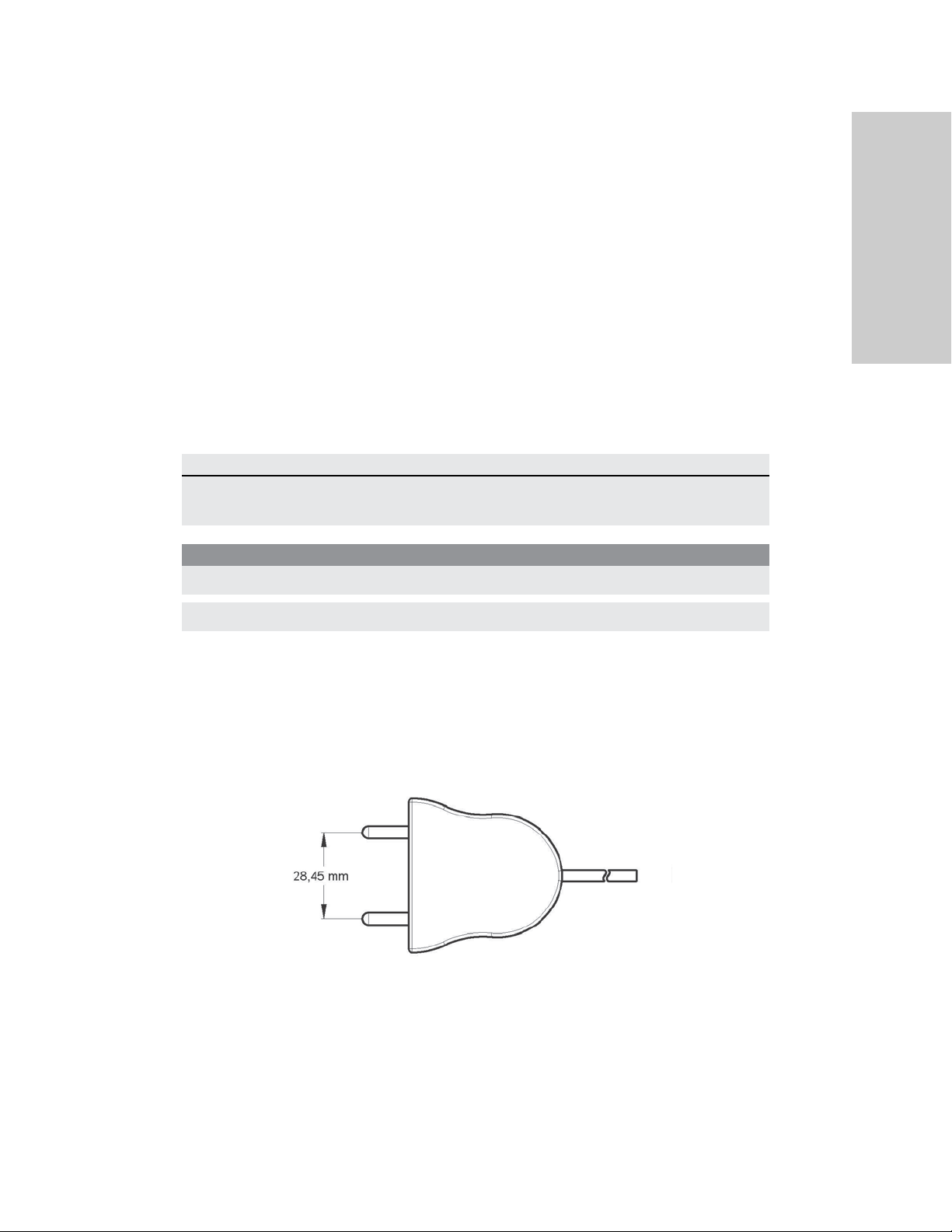
The Valleylab FT10 Energy Platform
Auto Bipolar
The Auto Bipolar feature senses tissue impedance between the two bipolar electrodes,
then uses the impedance information to automatically start or stop bipolar RF energy
delivery. Optionally, the user may select a timed activation delay for auto start of RF
activation.
Note: When using Auto Bipolar, the tissue in the grasp of the bipolar device must have an
impedance less than 2200 . The activation impedance safety feature will not deliver RF
power to the tissue if it is not within the specified range. This is a factory-set value that
cannot be reset by the user.
Note: Auto Bipolar is a feature that is not available on the clinical screen until it is enabled. This feature can be enabled through the Feature Enabling Menu of the service screen. See Feature Enabling Menu on page 3-10.
Important
If the VLFT10ADP1 Bipolar Adapter is used, only one bipolar instrument can set the Auto Bipolar feature to ON.
Overview and General Features
Precaution
Do not use instruments with flying leads with the VLFT10GEN.
Do not use the FT0501 ForceTriad™ Bipolar Adapter with the VLFT10GEN.
Compatible Bipolar Instruments & Devices
The VLFT10GEN is designed for use with Covidien bipolar instruments. However, other bipolar instruments are compatible with the VLFT10GEN if their connectors match the following illustration and are rated for peak voltages of at least 531 V.
Bipolar
Utilizes 4 mm banana pins
Valleylab FT10 Energy Platform Service Manual 1-9
Page 26
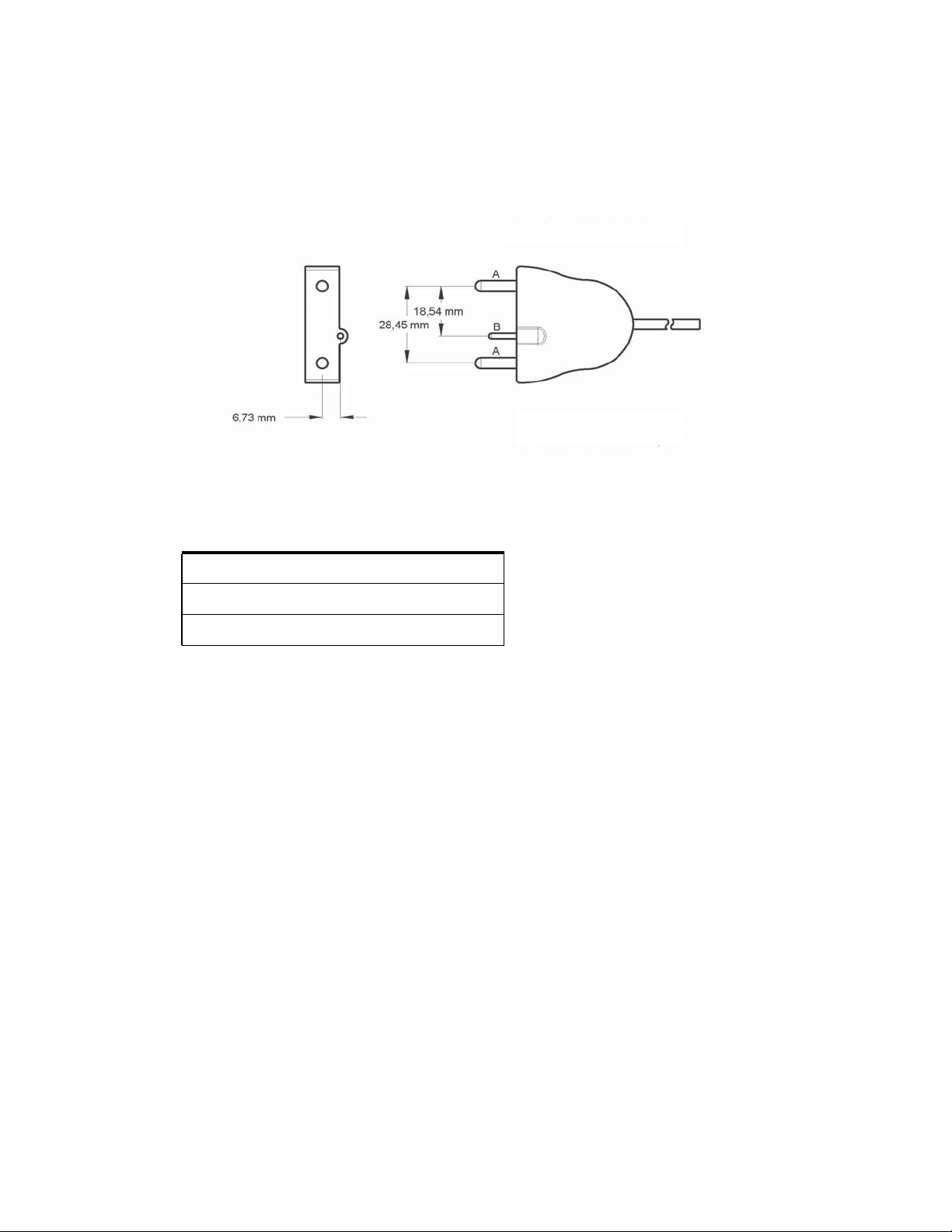
The Valleylab FT10 Energy Platform
The following Covidien catalog numbers for bipolar foot pedals are fully compatible with the VLFT10GEN.
Bipolar with Handswitching
A. Utilizes 4 mm banana pins
B. Utilizes 2 mm banana pins
Foot Pedals
E6009 Valleylab Bipolar Standard Footswitch
E6009B Valleylab Bipolar Standard Footswitch
E6019 Valleylab Bipolar Dome Footswitch
Bipolar Adapter
The VLFT10ADP1 Bipolar Adapter allows bipolar instruments to connect to the Ligasure/ Bipolar receptacle, thereby enabling the use of a second bipolar instrument.
Bipolar Resection
Bipolar Resection configures the LigaSure/Bipolar receptacle to use bipolar-resection resectoscopes.
1-10 Valleylab FT10 Energy Platform Service Manual
Page 27

Accessories
FT0021S ForceTriad Bipolar Resection Cord
FT0022W ForceTriad Bipolar Resection Cord
FT6009 ForceTriad FT Series Bipolar Resection Footswitch (with adapter)
1060355 Valleylab FT10 Bipolar Resection Footswitch Adapter
Effect-Settings Reference Chart
The Valleylab FT10 Energy Platform
Overview and General Features
Effect Setting CUT Initiation
Current (amps)
1 1.8 80 25
2 1.8 120 50
3 2.1 120 75
4 2.4 120 100
5 2.4 160 125
6 2.4 200 150
CUT (watts) COAG (max RMS volts)
LigaSure
LigaSure tissue fusion can be used on arteries, veins, pulmonary vasculature, and
lymphatics—up to and including 7 mm in diameter—and tissue bundles. When used with
compatible instruments, the system provides precise energy delivery and electrode
pressure to vessels for a controlled time period to achieve a complete and permanent
fusion of the vessel lumen. The system has been designed to produce minimal sticking,
charring, and thermal spread to adjacent tissue.
Warning
The tissue fusion function has not been shown to be effective for tubal sterilization or tubal coagulation for sterilization procedures. Do not use this function for these procedures.
LigaSure Instruments
The LigaSure instruments that complete the VLFT10GEN tissue-fusion system include
reusable and single-use instruments for open and laparoscopic procedures. Each reusable
instrument requires a corresponding single-use electrode. The LigaSure function is only
available when using compatible instruments.
Valleylab FT10 Energy Platform Service Manual 1-11
Page 28
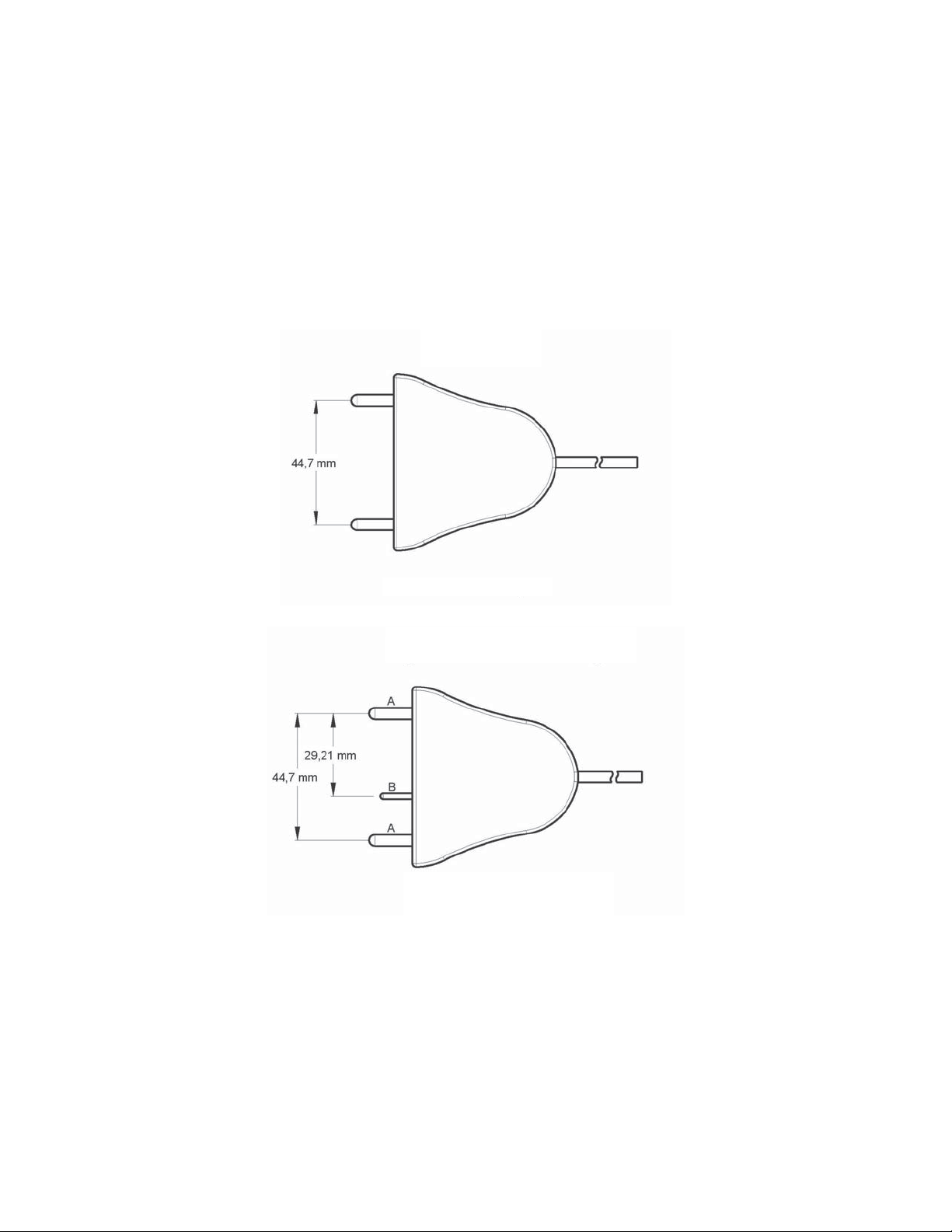
The Valleylab FT10 Energy Platform
Compatible LigaSure Instruments & Devices
This generator is designed for use with Covidien LigaSure instruments that have a
connector that matches the following figures and are rated for peak voltages of at least
244 V. However, it does not recognize all LigaSure instruments. Please refer to the cover
of the instructions for use to confirm if a specific LigaSure catalog number is compatible
with the VLFT10GEN.
LigaSure
Utilizes 4 mm banana pins
LigaSure with Switching
A. Utilizes 4 mm banana pins
B. Utilizes 2 mm banana pins
1-12 Valleylab FT10 Energy Platform Service Manual
Page 29
The Valleylab FT10 Energy Platform
Overview and General Features
A
29,21 mm
44,7 mm
22,4 mm
B
A
A. Utilizes 4 mm banana pins
B. Utilizes 2 mm banana pins
The following LigaSure foot pedal is fully compatible with the VLFT10GEN.
Foot Pedal
LS0300 Tissue Fusion Footswitch, Purple
Connection to External Systems
The VLFT10GEN can be connected to an external system. For example, connections can
be made to enable smoke evacuation or EKG blanking during monopolar activation, or
provide argon-enhanced coagulation. There are two external system receptacles on the
back of the VLFT10GEN that can signal RF activation. The receptacles are labeled
Monopolar 1 and Monopolar 2. They both signal RF activation whenever RF energy from
any of the RF receptacles is initiated. Refer to the external system user’s guide for detailed
instructions regarding how to connect it to the generator.
External Systems Compatible with the VLFT10GEN
SEA3730 RapidVac™ Smoke Evacuator Interlink Cable
SE3690 RapidVac Smoke Evacuator
Force™ Argon II-20 Argon Gas Delivery Unit II
Warning
Only medical devices compliant with IEC 60601-1 may be connected to the external system receptacles. The use of any external system connected to the VLFT10GEN should be evaluated by qualified personnel.
Valleylab FT10 Energy Platform Service Manual 1-13
Page 30
System Conventions
System Conventions
The Touchscreen
The VLFT10GEN features a user-friendly touchscreen interface to control system functions. The touchscreen is divided into quadrants; each of the four sections is associated with an adjacent instrument receptacle.
• Quadrant 1—Settings entered in the touchscreen control an instrument attached to
the Monopolar 1 receptacle.
• Quadrant 2—Settings entered in the touchscreen control an instrument attached to
the Monopolar 2 receptacle.
• Quadrant 3—Settings entered in the touchscreen control an instrument attached to
the Bipolar receptacle.
• Quadrant 4—The touchscreen displays instrument-specific options and activation of
LigaSure and Bipolar Resection devices.
1 2
FT10
34
1-14 Valleylab FT10 Energy Platform Service Manual
Page 31

Generator States
The appearance of touchscreen components indicates one of the three states of the system.
Edit
When the system is powered on and no instrument is attached, the instrument controls in the monopolar and bipolar sections can be preset. The following illustration shows monopolar controls edited prior to inserting an instrument.
System Conventions
Overview and General Features
Inserted
The controls change from the edit stage’s flat gray to a brightly illuminated color when an instrument is inserted into the associated receptacle. The following illustration shows the monopolar controls when a two-button pencil is inserted.
Valleylab FT10 Energy Platform Service Manual 1-15
Page 32

System Conventions
Activation
The black background illuminates brightly when the instrument is activated. The following
illustration shows the two-button pencil is currently delivering energy for PURE CUT. The
mode controls are locked during activation preventing any change in the mode. Power
settings can be changed during activation.
System Buttons
There are four buttons on the energy platform’s front panel:
Turns system power on and off. To turn power on, hold
On/Off
Restore Settings
Audio Volume Displays the volume pop-up menu to adjust sound levels.
Service/Settings Displays the service/settings menu.
the button for 0.25 seconds To turn off, hold the button
for 1 second. To reset a non-responsive system, hold for 10
seconds.
Restores settings from the last time the system was powered down using the On/Off button.
1-16 Valleylab FT10 Energy Platform Service Manual
Page 33

Interface Conventions
Interface Element Name Description
System Conventions
Overview and General Features
Pop-up window/ menu
Up/Down arrows Up and down arrows indicate additional
Software Buttons Options and confirmations are represented by
Pop-up windows and menus appear on screen
when user input is needed or requested. Popups close if the user touches anywhere
outside of the pop-up window.
values or selections are available for the
current setting.
For numeric values, press the up or down arrow to increase or decrease the displayed value. When the value is at its maximum or minimum available setting, the appropriate arrow button becomes inactive.
virtual buttons on the touchscreen. Touch onscreen buttons to select preference.
Toggle Switches Touch the virtual toggle switches to enable
(ON) or disable (OFF) options or functions.
ON
Valleylab FT10 Energy Platform Service Manual 1-17
Page 34

Page 35

Chapter 2
Warnings and Precautions for Patient and Operating Room Safety
The safe and effective use of electrosurgery depends to a large degree upon
factors solely under the control of the operator. There is no substitute for a
properly trained and vigilant surgical team. It is important that the operating
instructions supplied with this or any electrosurgical equipment be read,
understood, and followed.
Electrosurgery has been used safely in millions of procedures. Before starting any
surgical procedure, the surgeon should be trained in the particular technique and
surgical procedure to be performed, should be familiar with the medical literature
related to the procedure and potential complications, and should be familiar with
the risks versus the benefits of utilizing electrosurgery in the procedure.
Conventions Used in this Guide
Warning
Indicates a hazardous situation which, if not avoided, could result in death or serious injury.
Precaution
Indicates a hazardous situation which, if not avoided, may result in minor or moderate injury.
Notice
Indicates a hazard which may result in product damage.
Important
Indicates an operating tip or maintenance suggestion.
Valleylab FT10 Energy Platform Service Manual 2-1
Page 36

General Warnings and Precautions
General Warnings and Precautions
Fire/Explosion Hazards
Warning
Danger - Explosion Hazard Do not use electrosurgery in the presence of flammable anesthetics
or oxidizing gases (such as nitrous oxide (N
solvents (such as ether or alcohol).
Fire Hazard Do not place active instruments near or in contact with flammable materials (such as gauze or surgical drapes). Electrosurgical instruments that are activated or hot from use can cause a fire. When not in use, place electrosurgical instruments in a safety holster or safely away from patients, the surgical team, and flammable materials.
Sparking and heating associated with electrosurgery can be an ignition source. Keep gauze and
sponges wet. Keep electrosurgical electrodes away from flammable materials and oxygen (O
enriched environments.
O and oxygen) or in close proximity of to volatile
2
)
2
Use of electrosurgery in O
to reduce the O
If possible, stop supplemental oxygen at least one minute before and during use of electrosurgery.
The use of non-flammable agents is recommended for cleaning and disinfecting wherever possible. If flammable agents are used, do not activate the energy platform until flammable vapors from skin-preparation solutions and tinctures have dissipated.
There is a risk of pooling of flammable solutions under the patient or in body depressions, such as the umbilicus, and in body cavities, such as the vagina. Any fluid pooled in these areas should removed before activating the energy platform.
Avoid the accumulation of naturally occurring flammable gases that may accumulate in body cavities such as the bowel.
Prevent the accumulation of flammable or oxidizing gases or vapors under surgical drapes or near the surgical site.
Tissue buildup (eschar) on the tip of an active electrode may create embers that pose a fire hazard, especially in oxygen-enriched environments. Keep the electrode clean and free of all debris.
Facial and other body hair is flammable. Water soluble surgical lubricating jelly may be used to cover hair close to the surgical site to decrease flammability.
Verify that all anesthesia circuit connections are leak free before and during use of electrosurgery.
Fire Hazard During Oropharyngeal Surgery
Verify endotracheal tubes are leak free and that the cuff seals properly to prevent oxygen leaks.
concentration at the surgical site.
2
rich environments increases the risk of fire. Therefore, take measures
2
If an uncuffed tube is in use, pack the throat with wet sponges around the uncuffed tube, and be sure to keep sponges wet throughout the procedure.
Question the need for 100% O
If necessary, scavenge excess O
Do not attempt to recharge the generator’s lithium battery. This can cause the battery to explode.
during oropharyngeal or head and neck surgery.
2
with separate suction.
2
2-2 Valleylab FT10 Energy Platform Service Manual
Page 37

General Warnings and Precautions
System Setup Warnings and Precautions
Warning
Electric Shock Hazard Connect the system power cord to a properly grounded power receptacle.
Do not use power plug adapters.
Electric Shock Hazard When taking measurements or troubleshooting the system, take appropriate precautions, such as using isolated tools and equipment, using the “one hand rule,” etc.
Electric Shock Hazard Do not touch any exposed wiring or conductive surfaces while the system is disassembled and energized. Never wear a grounding strap when working on an energized system.
Warnings and Precautions for
Patient and Operating Room
Electric Shock Hazard To allow stored energy to dissipate after power is disconnected, wait at least 5 minutes before replacing parts.
Position the generator where it can be easily unplugged in an emergency.
Fire Hazard Do not plug into a power strip or extension cord.
Patient Safety Use the energy platform only if the power-on self-test has been completed as
described in this manual, otherwise inaccurate power outputs may result.
Hazardous Electrical Output This equipment is for use only by trained, licensed physicians. Do not use electrosurgical equipment unless properly trained to use it in the specific procedure being undertaken. Use of this equipment without such training can result in serious, unintended patient injury, including bowel perforation and unintended, irreversible tissue necrosis.
Do not touch the patient while touching a connector or fuse contact at the same time. Simultaneous contact can cause electric shock or burns.
Do not wrap the instrument cords or patient-return-electrode cords around metal objects. This may induce currents (capacitive coupling) that could lead to shocks, fires, or injury to the patient or surgical team.
Electric Shock Hazard Do not connect wet instruments to the energy platform. Ensure that all instruments and adapters are correctly connected and that no metal is exposed at any connection points.
Confirm proper power settings before proceeding with surgery. If the proper power settings are
not known, set the power to a low setting and cautiously increase the power until the desired
effect is achieved. If increased power settings are requested, check the patient return electrode and
all instrument connections before major power-setting adjustments.
Safety
Contact between the active electrode and any metal will greatly increase current flow and can result in unintended surgical effect.
Valleylab FT10 Energy Platform Service Manual 2-3
Page 38

General Warnings and Precautions
Warning
While using electrosurgery, the user and patient should not be allowed to come into direct contact
with grounded metal objects (e.g., surgical-table frame, instrument table, etc.). If this is not
possible during certain procedures (e.g., those in which noninsulated head frames are used), use
extreme caution to maximize patient safety:
• Use the lowest power setting that achieves the desired effect.
• Place the patient return electrode as close to the surgical site as possible.
• Place dry gauze between the patient and the grounded object if possible.
• Continually monitor the contact point(s).
• Do not use metal needle monitoring electrodes.
Warning
Ensure the UFP connector is fully inserted in the generator prior to use. A partially inserted connector may result in injury to the surgical team if the exposed connector is touched during use.
Precaution
Read the instructions, warnings, and precautions provided with this energy platform and associated accessories before using. Specific instructions for electrosurgical instruments are not included in this manual.
Read the instructions, warnings, and precautions provided with electrosurgical instruments before using. Specific instructions for electrosurgical instruments are not included in this manual.
Always use the lowest power setting that achieves the desired surgical effect. The active electrode
should be utilized only for the minimum time necessary in order to lessen the possibility of
unintended burn injury. Accidental and unintended burn injury has occurred during procedures in
small surgical fields and on small appendages. Pediatric applications and/or procedures performed
on small anatomic structures may require reduced power settings. The higher the current flow and
the longer the current is applied, the greater the possibility of unintended thermal damage to
tissue, especially during use on small structures.
Certain devices or accessories may present an unacceptable risk at low power settings. For
example, with argon beam coagulation, the risk of gas embolism increases if there is insufficient
high frequency (HF) power to produce a rapid, impermeable eschar on the target tissue.
For surgical procedures where the current could flow through delicate parts of the body, the use of bipolar techniques may be desirable in order to avoid unwanted coagulation.
Connect only Covidien-approved devices. Using devices from other manufacturers may cause equipment malfunction or patient injury.
Examine all instruments and connections to the system before using. Improper connection may result in arcs, sparks, instrument malfunction, or unintended surgical effects.
Do not operate the generator for clinical use while cables are connected to the utility WiFi
receptacle or Ethernet receptacles on the back of the generator. This may cause a system error that
would halt the procedure and require restarting the generator.
2-4 Valleylab FT10 Energy Platform Service Manual
Page 39

General Warnings and Precautions
Precaution
Do not turn the activation tone down to an inaudible level. The activation tone alerts the surgical team when the energy platform is delivering RF energy.
When using a smoke evacuator in conjunction with the VLFT10GEN, set the system volume control at a level that ensures the activation tones can be heard.
A non-functioning VLFT10GEN may cause an interruption of surgery. A backup system should be available for use.
Inadvertent activation may occur while installing, removing, or bending electrodes. Ensure that the instrument cord is not connected to the VLFT10GEN or that the system is off.
Leads connected to the patient should be positioned in such a way that contact with the patient
or other leads is avoided because the capacitance between the electrode cable and the patient may
result in some local high current densities. When not in use, place electrosurgical instruments in a
safety holster or safely away from patients, the surgical team, and flammable materials.
Warnings and Precautions for
Patient and Operating Room
Safety
Studies have shown that smoke generated during electrosurgical procedures can be potentially harmful to patients and the surgical team. These studies recommend adequately ventilating the smoke by using a surgical-smoke evacuator or other means.
1. U.S. Department of Health and Human Services. National Institute for Occupational Safety and
Health (NIOSH). Control of Smoke from Laser/Electric Surgical Procedures. HAZARD CONTROLS,
Publication No. 96-128, September, 1996
Notice
Connect the power cord to a properly grounded power receptacle having the correct voltage. Otherwise, product damage may result.
The VLFT10GEN requires special precautions regarding EMC and needs to be installed and put into service according to the EMC information provided in Chapter 4, Technical Specifications.
Portable and mobile RF communications equipment can affect the VLFT10GEN. Refer to the EMC information provided in Chapter 4, Technical Specifications.
The system should not be used adjacent to or stacked with equipment other than specified in the Valleylab FT10 Energy Platform User Guide and Service Manual. If adjacent or stacked use is necessary, the system should be observed to verify normal operation in the configuration in which it will be used.
The system intentionally applies RF energy for diagnosis or treatment during activation. Observe
other electronic medical equipment in the vicinity during the system activation for any possible
adverse electromagnetic effects. Ensure adequate separation of electronic medical equipment
based on observed reactions.
1
The use of accessories, other than specified in the Valleylab FT10 Energy Platform User Guide and Service Manual, may result in increased emissions or decreased immunity of the system.
Before plugging the generator into a power receptacle, verify that the installed fuses are appropriate for the local input line voltage. See Input Power on page 4-3.
Valleylab FT10 Energy Platform Service Manual 2-5
Page 40

General Warnings and Precautions
Notice
Calibration must be performed on a non-conductive surface. Do not use antistatic bench-top mats. When performed on a conductive surface, calibration values may not be accurate.
After completing calibration, the system will reboot to the clinical screen to save the values or abort the calibration.
Important
The VLFT10GEN is intended for use in a hospital or medical center environment.
If required by local codes, connect the energy platform to the hospital potential equalization system with an equipotential cable.
The operator of the generator may be as far away from the generator as 2 feet (direct product interaction), 5 feet (inside the sterile field), and 13 feet (across the room working with other equipment).
RFID and WiFi function may be interfered with by other equipment even if that other equipment complies with CISPR emission requirements.
Log files are maintained when the system is powered down. The time when the system was powered down or experiences a total loss of power is also logged.
When log files reach capacity, the earliest log is deleted to make room for the newest log.
Generator log files can be uploaded for viewing on a computer using Valleylab Exchange. Refer to the Valleylab Exchange Remote Software System User’s Guide available at http://www.medtronic.com/covidien/support/valleylab-exchange.
The VLFT10GEN contains substances of very high concern as defined in Article 57 and Annex XIV
of Regulation (EC) No 1907/2006 (Registration, Evaluation, Authorization, and Restriction of
Chemicals [REACH]). Specifically, it contains di-(2-ethylhexyl)phthalate (DEHP)
(CAS number 117-81-7; EC number EN 204-211-0) and 1,2 dimethoxyethane, ethylene glycol
dimethyl ether (EGDME) (CAS number 110-71-4; EC number EN 203-794-9) in concentrations
above 0.1% by weight.
Warnings and Precautions for the Energy Platform
Warning
Each instrument receptacle on this energy platform is designed to accept only one instrument at a time. Follow the instructions provided with electrosurgical instruments for proper connection and use.
Failure of the generator could result in an unintended increase of output power or activation.
Only medical devices compliant with IEC 60601-1 may be connected to the external system receptacles. The use of any external system connected to the VLFT10GEN should be evaluated by qualified personnel.
2-6 Valleylab FT10 Energy Platform Service Manual
Page 41

General Warnings and Precautions
Precaution
Do not stack equipment on top of the energy platform or place the energy platform on top of electrical equipment. This is an unstable configuration and does not allow for adequate cooling.
Provide at least 4” to 6“ (10 to 15 cm) of unobstructed space round the top and sides of the generator to ensure proper cooling.
Provide as much distance as possible between the energy platform and other electronic equipment (such as monitors). Do not cross or bundle cords from electronic devices. This energy platform may cause interference with other electronic equipment.
The use of monitoring systems that incorporate high-frequency current-limiting devices is recommended to reduce interference with the monitoring device.
Warnings and Precautions for
Patient and Operating Room
The system contains electrostatic-sensitive components. When repairing the system, work at a
static-control workstation. Wear a grounding strap when handling electrostatic-sensitive
components, except when working on an energized system. Handle Printed Circuit Board
Assemblies (PCBA) by their non-conductive edges. Use an antistatic container for transport of
electrostatic-sensitive components and PCBAs.
Notice
Make no modifications to the electrosurgical generator. Any modifications to the system will void the warranty.
When testing RF equipment, follow these test procedures. Keep test leads to the minimum length
usable; lead inductance and stray capacitance can adversely affect readings. Carefully select
suitable ground points to avoid ground loop error in measurements.
The accuracy of most RF instruments is approximately 1%–5% of full scale. Using uncompensated scope probes causes large errors when measuring high-voltage RF waveforms.
Warnings and Precautions for Active Instruments
Warning
Energy applied to an electrosurgical instrument can convert liquids to steam. The thermal energy
of steam may cause unintended injury in close proximity to the tip of the instrument. Care should
be taken in surgical procedures occurring in confined spaces in anticipation of this possibility.
Safety
Do not activate the energy platform in an open-circuit condition. To reduce the chances of unintended burns, activate the energy platform only when the active electrode is near or touching the target tissue.
Use the lowest power setting that achieves the desired surgical effect and use a low-voltage waveform (PURE CUT, BLEND, or VALLEYLAB mode) to lessen the potential for the creation of capacitive currents.
If energy delivery from the generator cannot be stopped, remove the handpiece from the patient and disconnect the handpiece or power cord.
Valleylab FT10 Energy Platform Service Manual 2-7
Page 42

General Warnings and Precautions
Warning
Do not activate the instrument when not in contact with target tissue as this may cause injuries due to capacitive coupling.
The surface of the active electrode may remain hot enough to cause burns after the RF current is deactivated.
Keep the active electrodes clear. Build-up of eschar may reduce the instrument's effectiveness. Do not activate the instrument while cleaning. Injury to operating room personnel may result.
Precaution
Read the instructions, warnings, and precautions provided with electrosurgical instruments before using. Specific instructions for electrosurgical instruments are not included in this manual.
Inspect instruments and cords—especially for laparoscopic/endoscopic instruments—for breaks,
cracks, nicks, and other damage before every use. If damaged, do not use. Damaged instruments
or cords may result in injury or electrical shock to the patient or surgical team.
Use only instruments that can withstand the maximum output (peak) voltage for each output
mode as listed in Chapter 4, Technical Specifications. Using an instrument with a voltage rating
that is lower than the maximum output voltage may result in injury to the patient or the operator,
or damage to the instrument.
Information on voltage ratings for non-Covidien instruments should be obtained from the instrument’s manufacturer.
Notice
All Covidien instruments have voltage ratings that are greater than the maximum output voltages in the VLFT10GEN.
Inspect instrument plugs for wear before every use. Worn plugs may result in a loose or stuck connection to the generator.
Warnings for Implanted Electronic Devices (IEDs)
IEDs include, but are not limited to, pacemakers, neurostimulators, implantable cardioverter defibrillators (ICDs), ventricular assist devices (VAD), spinal cord stimulators, cochlear implants, infusion pumps, and bone growth stimulators.
Warning
Use the system with caution in the presence of internal or external pacemakers or other implanted
devices. Interference produced by electrosurgical equipment can cause a pacemaker or other
device to enter an unsafe mode or permanently damage the device. Consult the device
manufacturer or responsible hospital department for further information when use is planned in
patients with implanted medical devices.
2-8 Valleylab FT10 Energy Platform Service Manual
Page 43

Warnings and Precautions for Monopolar Procedures
Post Surgery Safety Issues
Warning
Shock Hazard Before cleaning or servicing the unit, disconnect the power plug from the power
outlet in order to completely isolate the generator from mains power.
Notice
Do not clean the energy platform with abrasive cleaning or disinfectant compounds, solvents, or other materials that could scratch the panels or damage the energy platform.
Warnings and Precautions for
Patient and Operating Room
Warnings and Precautions for Monopolar Procedures
Warning
Simultaneously activating suction/irrigation and electrosurgical current may result in increased arcing at the electrode tip, burns to unintended tissues, or shocks and burns to the surgical team.
Power output of a two- or three-button pencil (COAG selection) can change during use when another monopolar instrument is activated.
Some surgeons may elect to “buzz the hemostat” during surgical procedures. It is not
recommended, and the hazards of such a practice probably cannot be eliminated. Burns to the
surgeon’s hands are possible. To minimize the risk take these precautions:
• Do not buzz the hemostat with a needle electrode.
• Do not lean on the patient, the table, or the retractors while buzzing the hemostat.
• Activate CUT rather than COAG. CUT has a lower voltage than COAG.
• Firmly grasp as much of the hemostat as possible before activating the energy platform. This disperses the current over a larger area and minimizes the current concentration at the finger tips.
• Buzz the hemostat below hand level (as close as possible to the patient) to reduce the opportunity for current to follow alternate paths through the surgeon’s hands.
Safety
• Use the lowest power setting possible for the minimum time necessary to achieve hemostasis.
• Activate the energy platform after the instrument makes contact with the hemostat. Do not arc to the hemostat.
• When using a coated- or nonstick-blade electrode, place the edge of the electrode against the hemostat or other metal instrument.
DEMO mode delivers monopolar energy without the use of a patient return electrode, and is intended for demonstration purposes only. Chance of burns to the patient significantly increase when DEMO mode is used for clinical procedures.
Valleylab FT10 Energy Platform Service Manual 2-9
Page 44

Warnings and Precautions for Monopolar Procedures
Precaution
To provide expected functionality from a hand piece, proper insertion is required. Refer to the alignment dots below the receptacles for proper insertion orientation.
The use of modes that produce electrical arcs between the active electrode and tissue may result in neuromuscular stimulation.
Warnings and Precautions for Patient Return Electrodes
Warning
It is not possible to foresee what combination of current and duty cycle may be safely used in every
situation—for example, when higher currents and/or longer duty cycles are used on procedures
such as tissue lesioning, tissue ablation, tissue vaporization; and procedures where conductive fluid
is introduced into the surgical site. Under these conditions a greater risk may exist that the heating
under a fully applied return electrode may be high enough to injure the patient.
When using a Covidien energy platform or a patient return electrode during these types of surgical
procedures, the user should seek written guidance in the form of detailed user instructions from
the manufacturer of the active accessory regarding the currents and duty cycles that can be
expected. In some instances, the application of additional patient return electrodes may help
mitigate the increased risk.
Do not attempt to use patient return electrodes that disable the Return Electrode Monitoring (REM)
system. The VLFT10GEN REM system will function correctly only with contact quality monitoring
(CQM) split-style patient return electrodes. Other patient-return-electrode products may not
identify loss of safe contact between the return electrode and the patient, thereby failing to
provide an auditory alarm and causing patient injury or product damage.
The safe use of monopolar electrosurgery requires proper placement of the patient return electrode. To avoid electrosurgical burns beneath the patient return electrode, follow all directions provided with the product.
Do not cut a patient return electrode to reduce its size. Patient burns due to high current density may result.
To avoid patient burns, ensure that the patient return electrode makes firm and complete contact
with the skin. Always check the patient return electrode periodically, after the patient is
repositioned, and during procedures involving long periods of activation.
Use of duty cycles greater than 25% (10 seconds active followed by 30 seconds inactive) will
increase the risk that heat build-up under a return electrode may be high enough to injure the
patient. Do not continuously activate for longer than one minute.
Apparent low-power output at the normal operating settings may indicate faulty application of the
return electrode. Verify the return electrode is correctly placed and attached to the patient as
described in the electrode’s instructions for use. Verify the connection between the electrode and
the generator before selecting a higher output power.
2-10 Valleylab FT10 Energy Platform Service Manual
Page 45

Warnings and Precautions for Monopolar Procedures
Warning
DEMO mode does not monitor the quality of pad contact with the patient. No warning will be issued from the generator when a non-REM return electrode’s pad-to-patient contact degrades when in DEMO mode.
Precaution
Covidien REM Polyhesive patient return electrodes are recommended for use with the VLFT10GEN. Return electrodes from other manufacturers may not provide proper impedance to work correctly with the energy platform.
Important
A statement of compatibility from the CQM patient return electrode manufacturer should be obtained prior to the use of a non-Covidien CQM patient return electrode.
A patient return electrode is not necessary in bipolar or LigaSure procedures.
Warnings and Precautions for
Patient and Operating Room
Safety
Inadvertent Radio Frequency (RF) Burns
Warning
Electrodes and probes used with monitoring, stimulation, and imaging devices (or similar equipment) can provide a path for high frequency current even if the electrodes or probes are isolated at 50 –60 Hz, insulated, and/or battery operated.
Do not use needles as monitoring electrodes during electrosurgical procedures. Inadvertent electrosurgical burns may result.
To reduce the risk of an inadvertent electrosurgical burn at the monitoring electrode or probe site,
place the electrode and/or probe as far away as possible from the electrosurgical site and/or patient
return electrode. Protective impedances (resistors or RF inductors) installed in the monitoring leads
may reduce the risk of such burns. Consult the hospital biomedical engineer for further
information.
Valleylab FT10 Energy Platform Service Manual 2-11
Page 46

Warnings and Precautions for Monopolar Procedures
Warning
In some circumstances, the potential exists for alternate site burns at points of skin contact (e.g.,
between the arm and the side of the body). This occurs when electrosurgical current seeks a path
to the patient return electrode that includes the skin-to-skin contact point. Current passing
through small skin-to-skin contact points is concentrated and may cause a burn. This is true for
ground referenced and isolated output electrosurgical energy systems.
To reduce the potential for alternate site burns, do one or more of the following:
• Avoid skin-to-skin contact points, such as fingers touching leg or knee touching knee when positioning the patient.
• Place insulation, such as dry gauze or towel, between contact points to ensure that contact does not occur.
• Position the patient return electrode to provide a direct current route between the surgical site and the return electrode which avoids skin-to-skin contact areas.
• In addition, place patient return electrodes according to the manufacturer’s instructions.
2-12 Valleylab FT10 Energy Platform Service Manual
Page 47

Warnings and Cautions for Laparoscopic Procedures
Warnings and Cautions for Laparoscopic Procedures
Warning
For laparoscopic procedures, be alert to these potential hazards:
• Laparoscopic surgery may result in gas embolism due to insufflation of gas in the abdomen.
• The electrode tip or LigaSure jaws may remain hot enough to cause burns after the electrosurgical current is deactivated.
• Inadvertent activation or movement of the activated instrument electrode or jaws outside of the field of vision may result in injury to the patient.
• Localized burns to the patient or physician may result from electrical currents carried through
conductive objects (such as other instrument, cannulas, or scopes). Electrical current may be
generated in conductive objects through direct contact with the active electrode or jaws,
capacitative coupling, or by the active instrument (electrode or cable) being in close proximity
to the conductive object.
• Do not use hybrid trocars that have a non-conductive locking anchor placed over a conductive
sleeve. For the operative channel, use all-metal or all-plastic systems. At no time should
electrical energy pass through hybrid systems. Capacitive coupling of RF current may cause
unintended burns.
• When using laparoscopic instrumentation with metal cannulas, the potential exists for burns
to the abdominal wall due to direct electrode contact or capacitive coupling of RF current. This
is most likely to occur in instances where the energy platform is activated for extended periods
at high power levels inducing high current levels in the cannula.
• Carefully insert and withdraw LigaSure instruments from cannulas to avoid possible damage to the devices and/or injury to the patient.
• Ensure that the insulation of single-use and reusable laparoscopic instrumentation is intact and
uncompromised. Compromised insulation may lead to inadvertent metal-to-metal sparking
and neuromuscular stimulation and/or inadvertent sparking to adjacent tissue.
Warnings and Precautions for
Patient and Operating Room
Safety
• Do not activate the LigaSure function in an open-circuit condition. Activate the energy platform only when the instrument is near or in direct contact with the target tissue to reduce the possibility of unintended burns.
Covidien recommends against the use of laparoscopic surgery on pregnant patients.
Valleylab FT10 Energy Platform Service Manual 2-13
Page 48

Warnings and Precautions for Bipolar Procedures
Warnings and Precautions for Bipolar Procedures
Warning
Use of different Covidien cord models or cords from other manufacturers may not achieve proper
electrical output for this device, thereby failing to produce the desired clinical effect. For example,
Auto Bipolar activation/deactivation settings may not work properly using cords other than those
specified by Covidien.
In the Auto Bipolar setting, activation may occur with contact of any material. When not in use, place electrosurgical instruments in a safety holster or safely away from patients, the surgical team, and flammable materials.
Precaution
Bipolar instruments must be connected to the Bipolar instrument receptacle only. Improper connection may result in inadvertent system activation.
Do not use instruments with flying leads with the VLFT10GEN.
Do not use the FT0501 ForceTriad Bipolar Adapter with the VLFT10GEN.
Bipolar forceps should not be set down while Auto Bipolar is active. Contact with any material may cause activation. Turn off Auto Bipolar before setting down an instrument.
Warnings and Precautions for LigaSure Procedures
Warning
LigaSure instruments are intended for use only with compatible Covidien generators and energy
platforms. See the front cover of the instrument’s instructions for a list of compatible generators.
Use of these instruments with other generators may not result in electrical output that they were
designed for, and may not result in the desired clinical effect.
The tissue fusion function has not been shown to be effective for tubal sterilization or tubal coagulation for sterilization procedures. Do not use this function for these procedures.
Tissue fusion requires the application of RF energy and pressure from the instrument. Ensure
grasping pressure is maintained until the seal cycle is complete.Tissue to be sealed must be firmly
grasped between the instrument jaw electrodes. Tissue in the jaw hinge or outside the instrument
jaw may not be sealed even if thermal blanching occurs.
If the seal-cycle-complete tone has not sounded, an optimal seal may not have been achieved. Reactivate the RF energy until a seal-cycle-complete tone is heard.
Do not activate the energy platform until the tissue-fusion or LigaSure instrument has been applied
with the proper pressure. Do not release the pressure on the tissue until the end tone has sounded.
Activating the energy platform with improper grasping pressure applied may result in an improper
seal and may increase thermal spread to tissue outside the surgical site.
2-14 Valleylab FT10 Energy Platform Service Manual
Page 49

Warning
Fluid in the body cavity should be kept to a minimum during treatment. Conductive fluids (e.g.,
blood or saline) in direct contact with, or in close proximity to, the instrument may carry electrical
current or heat, which may cause unintended burns to the patient. Aspirate fluid from around the
instrument jaws before activating the instrument.
Do not attempt to seal or cut over clips or staples as incomplete seals may be formed.
Servicing
Precaution
Energy-based devices, such as electrosurgical pencils or ultrasonic scalpels, that are associated with thermal spread should not be used to transect seals.
Warnings and Precautions for Bipolar Resection
Warning
Bipolar Resection mode is intended to be used only with compatible resectoscopes for endoscopically controlled removal (resection) or coagulation of tissue using 0.9% NaCI solution (saline) as the irrigation medium
Precaution
Bipolar Resection mode is supported only if a Covidien-specified bipolar resection instrument is
connected to the LigaSure/Bipolar receptacle with a Covidien bipolar resection cord. In addition,
Bipolar Resection mode can be activated only with the Covidien Bipolar Resection foot pedal
connected to the LigaSure/Bipolar foot-pedal receptacle. See the cords IFU listed in Accessories on
page 1-11 for the list of supported bipolar resection instruments and cords.
Servicing
Warnings and Precautions for
Patient and Operating Room
Safety
Warning
Electric Shock Hazard Do not remove the energy platform cover. Contact qualified personnel for
service.
Do not dispose of electrical appliances as unsorted municipal waste. Use separate collection facilities.
Electrical appliances that are incorrectly disposed in dumps or landfills can leach dangerous substances causing contamination of soil and groundwater, and damaging the environment.
Contact your local government, or point of sale for information regarding the collection of waste electrical appliances
Valleylab FT10 Energy Platform Service Manual 2-15
Page 50

Shunt Cords
Notice
Refer to this service manual for maintenance recommendations, and function and output-power verification procedures.
Do not spray cleaning fluids directly on the generator as damage to the generator may occur.
The latest version of the Valleylab FT10 Energy Platform Service Manual is available at http://www.medtronic.com/covidien/support/biomed-connect/electrosurgery. Call these numbers to request a hardcopy of the service manual
• USA and Canada: 1 800 255 8522 Option 2
• International: 1 303 476 7996
Shunt Cords
Warning
Some surgical instruments (e.g., colonoscopes) may allow substantial leakage current that could
burn the surgeon. If the instrument manufacturer recommends the use of a shunt cord (s-cord) to
direct the current back to the energy platform, an E0507-B adaptor must also be used. To avoid a
REM alarm, a REM Polyhesive patient return electrode with the E0507-B adaptor must be used.
Conductive Fluid In the Surgical Site
Warning
When this energy platform is used in monopolar procedures where conductive fluid (including, but
not limited to saline or lactated Ringer’s) is introduced into the surgical site for distention or to
conduct RF current, higher than normal currents (greater than one amp) may be produced. In this
situation, use one or more adult-size return electrode. Do not use return electrodes labeled for
children, infants, babies, neonatal use, or pediatric use.
Use of duty cycles greater than 25% (10 seconds active followed by 30 seconds inactive) will
increase the risk that heat build-up under a return electrode may be high enough to injure the
patient. Do not continuously activate for longer than one minute.
2-16 Valleylab FT10 Energy Platform Service Manual
Page 51

Chapter 3
Principles of Operation
This chapter provides information about how the VLFT10GEN functions and how the internal components interact.
Valleylab FT10 Energy Platform Service Manual 3-1
Page 52

Block Diagram
Block Diagram
Bipolar
BIP
Receptacle
#13
Ligasure/
Ligasure Flex
Bipolar
Receptacle
REM
MONO 2
Receptacle
MONO 1
Receptacle
RF
#12
REM Assembly
#11
Mono 2 Flex
#10
Mono 1 Flex
#9
Steering Relay
VIBE
#2
LCD + BL
#6
#4
LED Backlight
LCD-Mezzanine Display
Lig / BIP RF Flex
#14
Mono RF Flex
#14
VIBE RFID Barcode Scanner Assembly
TOUCH
SCREEN
#8
Touch Screen Assembly
FP MEZZANINE
#15
Ladder Flex
#21
HVDC Power Supply
Controller
#1
FP Mezzanine
USB A
RJ45
EKG1
USB B
EKG2
#20
#19
Control
LVDC Power
Power Supply
#24
POWER SUPPLY
# 22
Power Entry
Line
Neut
Line Filter
Power Entry
#16
GND Betelgeuse
Controller Fan
#23
GND Entry
GND
#18
#17
Fan Power Supply
Fan Controller Board
PS Fan
RF Fan
WIFI
Dongle
#27
Fan RF Board
Speaker Assembly
Speaker
#25
Footswitch - Controller
P2
FOOTSWITCH BOARD
Mono1 Mono2 BIP LIG
Ligasure FS
#26
LS
Purple
J4 P1
J2
J1
Discrete cable
Flex cable Assembly
Off the Shelf Cable
Assembly
Off the Shelf Flex
Aseembly
3-2 Valleylab FT10 Energy Platform Service Manual
Page 53

Cable Part Numbers
Cable Number Cable Name
Block Diagram
#1 FP Mezzanine
#2 VIBE RFID Scanner Assy
#4 LCD-Mezzanine Display
#6 LED Backlight
#8 Touch Screen Assembly
#9 Mono 1 Flex
#10 Mono 2 Flex
#11 REM Assembly
#12 LigaSure Flex
#13 Bipolar
#14 Mono RF Flex
#14 Lig/BIP RF Flex
#15 Ladder Flex
#16 Fan Controller Board
#17 Fan Power Supply
#18 Fan RF Board
#19 Power Supply Control
#20 LVDC Power
#21 HVDC Power Supply
#22 Power Entry
#23 GND Entry
#24 GND Controller
#25 Foot-switch - Controller
Principles of Operation
#26 LigaSure FS
#27 Speaker Assembly
Valleylab FT10 Energy Platform Service Manual 3-3
Page 54

Electronic Assemblies Principles of Operation
Electronic Assemblies Principles of Operation
High-Voltage/Low-Voltage Power Supply Principles of Operation
The High-Voltage/Low-Voltage power-supply module provides a complete main power supply solution. It is contained within its own chassis and is cooled by a dedicated system fan.
Controller PCBA
The Controller Printed Circuit Board Assembly (PCBA) serves as the main digital control of the system. It comprises the following features:
• Control of the user interface (touchscreen, push-button, foot pedal, and handpiece activation inputs, LCD, and LEDs)
• Closed-loop control of RF power delivery
• External communications (WiFi and Ethernet)
• Control of smoke-evacuation devices or blanking functionality of an EKG device
• Audio control
• Power-supply control
RF PCBA
The RF PCBA serves primarily to convert a DC voltage sourced from the High Voltage
Power supply output to a 434 kHz AC source with an output impedance that is tailored
to match the energy modality being sourced. The RF PCBA also houses the RF energy
current and voltage sensors which are used to provide closed loop control of the energy
delivered to the patient.
Steering Relay PCBA
The Steering Relay PCBA accepts energy from the RF PCBA and directs it toward the
intended energy-output receptacle based on energy modality. Non-activated energy
receptacles are disconnected from the energy source by way of high-voltage relays. The
Steering Relay PCBA houses the REM module, the ISOFirm modules, and the isolated
power supply for the REM and ISOFirm modules.
Display PCBA
The Display PCBA houses the touchscreen controller, the LCD backlight driver, and provides an interface for the LCD, VIBE module, and User Interface buttons.
3-4 Valleylab FT10 Energy Platform Service Manual
Page 55

Mechanical Assemblies Principles of Operation
Mechanical Assemblies Principles of Operation
The Chassis assembly consists of 4 major metal mechanical subassemblies (front bezel,
chassis base, chassis top, and rear bezel), 3 cooling fans, and a molded foam PCBA
mounting structure (Electronic Packaging Assembly Concept—E-PAC™*).
IT Network Connectivity Principles of Operation
The generator connects to an IT network, usually through Valleylab Exchange, for the
purpose of registering the generator and its components, updating software, setting the
country code, and transmitting error and event logs. The generator cannot connect to an
IT network while in clinical mode. Because the generator cannot connect to an IT network
while in clinical mode, there are no OR-related hazardous situations. The generator has to
be in the service screens for IT network connectivity. The generator can be 1) connected
to another computer (point to point connection), 2) connected remotely to a router or to
a DHCP server via a WiFi access point broadcasting a non-hidden SSID, or 3) connected via
Ethernet cable to a router or directly to the DHCP server. The generator allows wired
connections to be either static (assigned by the user) or assigned by the DHCP server. The
MAC address is unique to each generator.
Connection of the generator to an IT network that includes other equipment could result
in previously unidentified risks. The user should identify, analyze, evaluate, and control
these risks. Subsequent changes to the IT network could introduce new risks and require
additional analysis. Changes to the IT network include:
• changes to the IT network configuration
• connection of additional items to the IT network
• disconnecting items from the IT network
• update of equipment connected to the IT network
• upgrade of equipment connected to the IT network
Principles of Operation
Valleylab FT10 Energy Platform Service Manual 3-5
Page 56

Page 57

Chapter 4
Technical Specifications
Precaution
Read the instructions, warnings, and precautions provided with this energy platform and associated accessories before using. Specific instructions for electrosurgical instruments are not included in this manual.
Valleylab FT10 Energy Platform Service Manual 4-1
Page 58

VLFT10GEN Specifications
VLFT10GEN Specifications
General
Output configuration Isolated output
Cooling Natural convection and fan
Display 7.0 in. (17.8 cm.) LCD touchscreen
Connector receptacles LED illuminated connector readers on the
Enclosure Magnesium
LigaSure/Bipolar receptacle
Mounting • Valleylab Universal Generator Cart (VLFTCRT)
• Operating-room boom systems
• Any stable, flat surface such as a table or cart top
Operating System Linux™*
Important
The VLFT10GEN contains substances of very high concern as defined in Article 57 and Annex XIV
of Regulation (EC) No 1907/2006 (Registration, Evaluation, Authorization, and Restriction of
Chemicals [REACH]). Specifically, it contains di-(2-ethylhexyl)phthalate (DEHP) (CAS number 11781-7; EC number EN 204-211-0) and 1,2 dimethoxyethane, ethylene glycol dimethyl ether
(EGDME) (CAS number 110-71-4; EC number EN 203-794-9) in concentrations above 0.1% by
weight.
Dimensions and Weight
Height 7.0 in. (17.8 cm)
Width 14.5 in. (36.8 cm)
Length 18.2 in. (46.2 cm)
Weight 22.3 lb. (10.1 kg)
4-2 Valleylab FT10 Energy Platform Service Manual
Page 59

Environmental Parameters
VLFT10GEN Specifications
Operation Transport and Storage
Ambient temperature range
Relative humidity 15% to 85% non-condensing 15% to 90% non-condensing
Atmospheric pressure
1. The system can be stored for up to one year without performance degradation upon use.
2. If the generator is stored at a temperature outside the normal operating range of 10 to 40 °C,
the system is ready for use after at least one hour at ambient temperature 20 °C ± 5 °C.
50 to 104 °F (10 to 40 °C) 14 to 140 °F (-10 to +60 °C)
700 to 1060 millibars 500 to 1060 millibars
1,2
Input Power
Nominal Line Voltage
100–127 VAC 220–240 VAC Units
Line Ranges
Line Voltage Full Regulation Range
90–140 198–264 VAC
Line Frequency 47–63 47–63 Hz
Max VA nominal line Voltage
Max Mains Current
Max Heat Dissipation 180 180 W
Fuses 10
Power Cord NEMA 3-prong hospital-grade connector
1. Max VA and current are based on nominal line voltages.
1
1
950 950 VA
9.5 4.8 A
Fuses (2) – 5 mm x 20 mm 10 A, 250 V fast blow
6.3
Fuses (2) – 5 mm x 20 mm 6.3 A, 250 V fast blow
A
RMS
Valleylab FT10 Energy Platform Service Manual 4-3
Technical Specifications
Page 60

VLFT10GEN Specifications
Power Cord Specifications
This system is factory equipped with a 110 VAC hospital grade NEMA 5-15 power cord.
Should the AC power cord need to be replaced to match another plug configuration, the
replacement plug/cable/receptacle configuration must meet or exceed the following
specifications:
• 100-127 VAC
Cable - SJT16/3, IEC color code, maximum length 15 ft. (5 m) Plug - minimum 10 A - 125 VAC Unit receptacle - IEC female, minimum 10 A - 125 VAC
• 220-240 VAC
Cable - H05VVF3G1.0 VDE, maximum length 15 ft. (5 m) Plug - minimum 6 A - 250 VAC Unit receptacle - IEC female, minimum 6 A - 250 VAC
Important
Contact your local Covidien representative for alternative internationally approved power-cord options.
Backup Power
The VLFT10GEN retains all user programmed features, calibration, and statistical data when switched off and unplugged. The VLFT10GEN operates within specification when switched over to a supplied-line power by hospital backup systems.
Real time clock battery Battery type – Lithium CR1620 or CR1632
Battery life – 75 mAh minimum
Equipotential Ground Connection
An equipotential ground connection is provided to allow connection of the VLFT10GEN to a common ground by way of a potential equalization connector. This connection meets IEC 60601-1 requirements.
EKG Blanking and Smoke Evacuation
Interlink cable receptacles are provided to signal other devices, such as an EKG or smoke
evacuator, that the VLFT10GEN is active. The receptacle is a 2.5 mm mono jack. It is
electrically isolated from the internal ground referenced electronics. The shell is electrically
isolated from chassis ground.
4-4 Valleylab FT10 Energy Platform Service Manual
Page 61

VLFT10GEN Specifications
Internal Memory
Storage capacity 8 GB
Duty Cycle
The VLFT10GEN is capable of operating a duty cycle of 25%, defined as 10 seconds active and 30 seconds inactive, in any mode for a period of four hours.
Precaution
Use of duty cycles greater than 25% (10 seconds active followed by 30 seconds inactive) will
increase the risk that heat build-up under a return electrode may be high enough to injure the
patient. Do not continuously activate for longer than one minute.
Leakage
Leakage Currents and Patient Auxiliary Currents (IEC 60601-1:2012)
Touch Current < 100 µA NC, < 500 µA SFC
Earth Leakage Current < 500 µA NC, < 1000 µA SFC
Patient Auxiliary Current (< 1kHz) < 10 µA NC, < 50 µA SFC
Patient Auxiliary Current (> 1kHz) Scaled with frequency per IEC 60601-1:2012, but
does not exceed 10mA NC/SFC
Patient Leakage Current < 10 µA NC, < 50 µA SFC
Total Patient Leakage Current < 50 µA NC, < 100 µA SFC
NC—Normal Condition
SFC—Single Fault Condition (as defined in IEC 60601-1:2012)
Total Patient Leakage Current—Measurement of patient leakage current with all patient outputs connected together
High Frequency Leakage (IEC 60601-2-2)
Bipolar (short leads) < 68.9 mARMS
Technical Specifications
Monopolar measured directly at the ESU terminals < 100 mA
LigaSure/BPR measured directly at the ESU terminals < 100 mA
RMS
RMS
Valleylab FT10 Energy Platform Service Manual 4-5
Page 62

VLFT10GEN Specifications
Radio Frequency Identification (RFID)
The RFID module is located above the LigaSure/Bipolar receptacle. The intended use of the RFID module is to identify the inserted LigaSure instrument and configure the generator with the data included in the RFID tag.
Transmit/Receive Frequency Range 13.56MHz
RF Output Power 68.17 dBu V/m @3 meter
Type of Antenna Integral Loop Antenna
Modulation Amplitude-shift keying (ASK)
Mode of Operation (Simplex / Duplex) Duplex
Contains Module FCC ID 2AAVI-JDK1901
Contains IC ID 11355A-JDK1901
Wireless Fidelity (WiFi)
The WiFi module is located on the back of the generator. The intended use of the WiFi module is to perform service operations on the generator.
Transmit/Receive Frequency Range 2.4000 ~ 2.4835 GHz
(Industrial Scientific Medical Band)
Standards IEEE 802.11b, 802.11g, 802.11n
RF Output Power 11b: 17 ± 1.5 dBm
11g: 15 ± 1.5 dBm 11n: 14 ± 1.5 dBm
Data Rate 11b: 1/2/5.5/11 Mbps
11g: 6/9/12/24/36/48/54 Mbps 11n: (20 MHz): MCSO-7 (Up to 72 Mbps) 11n: (40 MHz): MCSO-7 (Up to 150 Mbps)
Securities WPA2
Type of Antenna Internal Antenna (1T1R)
Contains Module FCC ID NDD9578111008
Contains IC ID 4701A-78111306
4-6 Valleylab FT10 Energy Platform Service Manual
Page 63

VLFT10GEN Specifications
IFETEL Radio Standards Warning Statement
The operation of radios within this equipment is subject to the following two conditions:
1) They shall not cause harmful interference, and
2) They must accept any interference received, including interference that may cause undesired operation.
Industry Canada Statement
This device complies with Industry Canada’s licence-exempt RSSs. Operation is subject to the following conditions:
1) This device may not cause interference; and
2) This device must accept any interference, including interference that may cause undesired operation of the device.
RF Exposure Statement
This device meets the requirements for RF exposure when operated at a minimum distance of 20 cm from the user or nearby persons.
EU Short Form Declaration
Hereby, Covidien llc, declares that this wireless LAN and RFID is in compliance with the essential requirements and other relevant provisions of Directive 1999/5/EC.
WiFi Device Installation Guide
The translated Quick Installation Guide (QIG) for the WiFi device (EW-7811Un) is available
on the Edimax™* website in many languages. It is at the following location:
http://www.edimax.com/edimax/download/download/data/edimax/global/download/
for_home/wireless_adapters/wireless_adapters_n150/ew-7811un.
Ethernet
The Ethernet connection is located on the back of the generator. The intended use of the Ethernet connection is to perform service operations on the generator.
Connected Speed 10/100/1000Base-T
Technical Specifications
Standards IEEE 802.3, IPv4
Protocols SFTP (for file transfer through port 22), UDP (non-file transfer), TCP/IP
Valleylab FT10 Energy Platform Service Manual 4-7
Page 64

Symbols Used
Symbols Used
For prescription use only
Consult instructions for use
Follow instructions for use (blue)
Caution: Consult accompanying documents
The energy platform output is floating (isolated) with respect to ground.
Defibrillation proof
DANGER
Explosion risk if used with flammable anesthetics
To reduce the risk of electric shock, do not remove the cover. Refer servicing to qualified service personnel.
1) Unit produces non-ionizing radiation
2) Unit includes RFID
Unit includes WiFi
4-8 Valleylab FT10 Energy Platform Service Manual
Page 65

3164410
3164410
Conformsto ANSI/AAMI std. ES60601-1:2005
Conformsto ANSI/AAMI std. ES60601-1:2005
Conformsto IEC std. 60601-2-2 Ed. 5 2009
Conformsto IEC std. 60601-2-2 Ed. 5 2009
Symbols Used
Classified with respect to electrical shock, fire, and mechanical hazards only in accordance with AAMI ES standard 60601-1; certified to CSA standard C22.2 60601-1:2008.
IP21
Liquid Ingress/Spillage Classification
On/Off
Restore Settings
Audio Volume
Service/Settings
High voltage
REM patient return electrode
Monopolar 1 Universal Foot-Pedal Port (UFP)
Ethernet
Fuse
Valleylab FT10 Energy Platform Service Manual 4-9
Technical Specifications
Page 66

Standards and IEC Classifications
Foot pedal
Volume adjustment for activation tones
Bipolar Menu button
Equipotential grounding point
Equipment should not be disposed in trash
Do not reuse (Single use only)
Standards and IEC Classifications
The VLFT10GEN meets all pertinent clauses of the IEC 60601-1 editions 2.0 and 3.1, IEC 60601-2-2 editions 4.0 and 5.0, IEC 60601-1-2 editions 2.1 and 3.0 and 4.0, and IEC 60601-1-8 edition 2.1.
Class I Equipment (IEC 60601-1)
Accessible conductive parts cannot become live in the event of a basic insulation failure because of the way in which they are connected to the protective earth conductor.
Type CF Equipment/Defibrillator Proof (IEC 60601-1, IEC 60601-2-2, and ANSI/AAMI HF18)
This VLFT10GEN provides a high degree of protection against electric shock, particularly regarding allowable leakage currents. It is type CF isolated (floating) output and may be used for procedures involving the heart.
4-10 Valleylab FT10 Energy Platform Service Manual
Page 67

Standards and IEC Classifications
IP21 Liquid Ingress/Spillage (IEC 60601-1 and IEC 60601-2-2)
The VLFT10GEN is constructed so that liquid spillage in normal use does not wet electrical insulation or other components which when wetted are likely to adversely affect the safety of the equipment.
Voltage Transients – Energy Platform Mains Transfer (IEC 60601-1, IEC 60601-2-2, and ANSI/AAMI HF18)
The VLFT10GEN continues to operate normally with no errors or system failures when transfer is made between line AC and an emergency system-voltage source. The system may momentarily shut down in a safe mode depending on the switchover time.
CISPR 11 Class A
The emissions characteristics of this equipment make it suitable for use in industrial areas
and hospitals (CISPR 11 class A). If it is used in a residential environment (for which CISPR
11 class B is normally required), this equipment may not offer adequate protection to
radio-frequency communication services. The user may need to take mitigation measures,
such as relocating or re-orienting the equipment.
Important
RFID and WiFi function may be interfered with by other equipment even if that other equipment complies with CISPR emission requirements.
Electromagnetic Compatibility (IEC 60601-1-2 and IEC 60601-2-2)
The VLFT10GEN complies with the appropriate IEC 60601-1-2 and 60601-2-2 specifications regarding electromagnetic compatibility.
Notice
The VLFT10GEN requires special precautions regarding EMC and needs to be installed and put into service according to the EMC information provided in Chapter 4, Technical Specifications.
Portable and mobile RF communications equipment can affect the VLFT10GEN. Refer to the EMC information provided in Chapter 4, Technical Specifications.
The system should not be used adjacent to or stacked with equipment other than specified in the Valleylab FT10 Energy Platform User Guide and Service Manual. If adjacent or stacked use is necessary, the system should be observed to verify normal operation in the configuration in which it will be used.
Technical Specifications
Valleylab FT10 Energy Platform Service Manual 4-11
Page 68

Standards and IEC Classifications
Notice
The system intentionally applies RF energy for diagnosis or treatment during activation. Observe
other electronic medical equipment in the vicinity during the system activation for any possible
adverse electromagnetic effects. Ensure adequate separation of electronic medical equipment
based on observed reactions.
The use of accessories, other than specified in the Valleylab FT10 Energy Platform User Guide and Service Manual, may result in increased emissions or decreased immunity of the system.
Guidance and manufacturer’s declaration - electromagnetic emissions
The Valleylab FT10 FT Series Energy Platform is intended for use in the electromagnetic environment specified below. The customer or the user of the Valleylab FT10 FT Series Energy Platform should assure that it is used in such an environment.
Emissions test Compliance Electromagnetic
environment - guidance
RF emissions
CISPR 11
RF emissions
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/flicker
emissions
IEC 61000-3-3
Group 2 The Valleylab FT10 FT Series
Energy Platform must emit
electromagnetic energy in
order to perform its intended
function. Nearby electronic
equipment may be affected.
Class A The Valleylab FT10 FT Series
Energy Platform is suitable for
use in all establishments other
Class A
Complies
than domestic and those
directly connected to the
public low-voltage power
supply network that supplies
buildings used for domestic
purposes.
The essential performance requirement per IEC60601-1 does not apply to VLFT10GEN. Basic safety is the performance requirement used during immunity testing.
4-12 Valleylab FT10 Energy Platform Service Manual
Page 69

Standards and IEC Classifications
Guidance and manufacturer’s declaration - electromagnetic immunity
The Valleylab FT10 FT Series Energy Platform is intended for use in the electromagnetic environment specified below. The customer or the user of the Valleylab FT10 FT Series Energy Platform should assure that it is used in such an environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic
environment -
guidance
Electrostatic discharge
(ESD)
IEC 61000-4-2
Electrical fast
transient/burst
IEC 61000-4-4
Surge IEC
IEC 61000-4-5
Voltage dips, short
interruptions and
voltage variations on
power supply input
lines
IEC 61000-4-11
Power frequency (50/ 60 Hz) magnetic field
IEC 61000-4-8
± 8 kV contact
± 15 kV air
± 8 kV contact
± 15 kV air
Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30%.
± 2 kV for power
supply lines
± 1 kV for input/
output lines
± 1 kV differential
mode
± 2 kV common mode
< 5% U
in U
(> 95% dip
T
) for 0.5 cycle
T
< 40% UT (> 60% dip
in U
) for 5 cycles
T
< 70% U
in U
< 5% U
T
) for 25 cycles
T
(> 95% dip
T
in U
) for 5 sec
T
for 1 cycle
0% U
T
(> 30% dip
± 2 kV for power
supply lines
± 1 kV for input/
output lines
± 1 kV differential
mode
± 2 kV common mode
a
< 5% U
< 40% U
< 70% U
< 5% U
in U
) for 0.5 cycle
T
in U
) for 5 cycles
T
in U
) for 25 cycles
T
in U
T
0% U
T
T
T
T
) for 5 sec
for 1 cycle
T
(> 95% dip
(> 60% dip
(> 30% dip
(> 95% dip
Mains power quality should be that of a typical commercial or hospital environment.
Mains power quality should be that of a typical commercial or hospital environment.
Mains power quality should be that of a typical commercial or hospital environment.
30 A/m 30 A/m Power frequency
magnetic fields should
be at levels
characteristic of a
typical location in a
typical commercial or
hospital environment.
Technical Specifications
a. 1 cycle is at 50 Hz or 20 ms
NOTE: U
is the a.c. mains voltage prior to the application of the test level.
T
Valleylab FT10 Energy Platform Service Manual 4-13
Page 70

Standards and IEC Classifications
Guidance and manufacturer’s declaration - electromagnetic immunity
The Valleylab FT10 FT Series Energy Platform is intended for use in the electromagnetic environment specified below. The customer or the user of the Valleylab FT10 FT Series Energy Platform should assure that it is used in such an environment.
Immunity
test
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
IEC 60601 test
level
3 V
rms
150 kHz to 80 MHz
3 V/m
80 MHz to 2.7 GHz
Compliance
level
3 V
rms
Electromagnetic environment -
guidance
Portable and mobile RF communications
equipment should be used no closer to
any part of the Valleylab FT10 FT Series
Energy Platform, including cables, than
the recommended separation distance
calculated from the equation applicable
to the frequency of the transmitter.
Recommended separation distance:
d=1.2ÖP
3 V/m d=1.2ÖP 80 MHz to 800 MHz
d=2.3ÖP 800 MHz to 2.7 GHz
Where P is the maximum output power
rating of the transmitter in watts (W)
according to the transmitter
manufacturer and d is the recommended
separation distance in meters (m).
Field strengths from fixed RF transmitters,
as determined by an electromagnetic site
compliance level in each frequency
a
survey
, should be less than the
range.
b
Interference may occur in the vicinity of
equipment marked with the following
symbol.
Continued
4-14 Valleylab FT10 Energy Platform Service Manual
Page 71

Standards and IEC Classifications
Guidance and manufacturer’s declaration - electromagnetic immunity
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects, and people.
a. Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones
and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be
predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF
transmitters, an electromagnetic site survey should be considered. If the measured field strength in
the location in which the Valleylab FT10 FT Series Energy Platform is used exceeds the applicable RF
compliance level above, the Valleylab FT10 FT Series Energy Platform should be observed to verify
normal operation. If abnormal performance is observed, additional measures may be necessary, such
as reorienting or relocating the Valleylab FT10 FT Series Energy Platform.
b. Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
Recommended separation distances between portable and mobile RF communication
equipment and the Valleylab FT10 FT Series Energy Platform
The VLFT10GEN is intended for use in an electromagnetic environment in which radiated RF
disturbances are controlled. The customer or the user of the system can help prevent
electromagnetic interferences by maintaining a minimum distance between portable and mobile RF
communications equipment (transmitters) and the system as recommended below, according to the
maximum output power of the communications equipment.
Rated maximum
output power of
transmitter (W)
0.01 0.12 0.12
0.1 0.37 0.37 0.74
1 1.2 1.2 2.3
10 3.7 3.7 7.4
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation
distance d in meters (m) can be estimated using the equation applicable to the frequency of the
transmitter, where P is the maximum output power rating of the transmitter in watts (W) according
to the transmitter manufacturer.
Separation distance according to frequency of transmitter (m)
150 kHz to 80 MHz
d=1.2 x ÖP
80 MHz to 800 MHz
d=1.2 x ÖP
a
800 MHz to 2.5 GHz
d=2.7 x ÖP
a
0.23
Technical Specifications
a. For frequencies listed in the following table, use a separation distance of 0.3 m.
Continued
Valleylab FT10 Energy Platform Service Manual 4-15
Page 72

Standards and IEC Classifications
Recommended separation distances between portable and mobile RF communication
equipment and the Valleylab FT10 FT Series Energy Platform
Frequency ranges and text condition on transmitter bands
Bands (MHz) Test Frequencies
(MHz)
380 – 390 385 Pulse
430 – 470 450 FM ± 5 kHz Deviation
704 – 787 710, 745, 780 Pulse
800 – 960 810, 870, 930 Pulse
1700 – 1990 1720, 1845, 1970 Pulse
2400 – 2570 2450 Pulse
5100 – 5800 5240, 5500, 5785 Pulse
Modulation Claimed Compliance
Level V/m
a
– 18 Hz 27
or Pulse
a
– 18 Hz
a
– 217 Hz 9
a
– 18 Hz 28
a
– 217 Hz 28
a
– 217 Hz 28
a
– 217 Hz 9
28
a. Pulse Modulation is defined as a square wave input with a 50% duty cycle at the listed frequency.
NOTE 1 The minimum dwell time during test is 7 s.
NOTE 2 A minimum separation distance of 0.3 meters should be maintained between any device
transmitting in this band and the VLFT10GEN. This includes devices such as mobile phones, PDAs,
Wireless LANs, RFID and Bluetooth™*.
Cables Used for EMC Compliance Testing
The following cables, lengths, and instruments were used to determine compliance levels.
Model No. Description Shielded Length Location Classification Tests
Included
207002060 A/C cable No 15 ft.
(4.6 m)
1017178 Equipotential
cable
E2450HDB 2-button
pencil
No 15 ft.
(4.6 m)
No 15 ft.
(4.6 m)
Rear
panel
Rear
panel
Front
panel
AC Emissions/
Immunity
EQ Emissions/
Immunity
Patient Emissions/
Immunity
Mono 1
FT3000DB Force
TriVerse
pencil
No 15 ft.
(4.6 m)
Front
panel
Mono 2
Patient Emissions/
Immunity
Continued
4-16 Valleylab FT10 Energy Platform Service Manual
Page 73

Standards and IEC Classifications
Model No. Description Shielded Length Location Classification Tests
Included
E7507DB Adult REM
pad
E0020V/
E4073CT
LF1644 LigaSure No 10 ft.
FT0021S/
FT27050SL
E6008B Monopolar
E6019 Dome Bipolar
LS0300 LigaSure
Foot
switching
bipolar cord/
bayonet
forceps
Cord Storz/
Storz
Resectoscope
footswitch
footswitch
footswitch
No 15 ft.
(4.6 m)
No 15 ft.
(4.6 m)
No 15 ft.
(4.6 m)/
No 15 ft.
(4.6 m)
No 15 ft.
(4.6 m)
No 15 ft.
(4.6 m)
(3 m)
NA
Front
panel
REM
Front
panel
bipolar
Front
panel
LigaSure
Front
panel
Adv.
Energy
Rear
panel
Mono 1
Rear
panel
bipolar
Rear
panel
LigaSure
Patient Emissions/
Immunity
Patient Emissions/
Immunity
Patient Immunity
Patient Emissions
SIP/SOP Emissions/
Immunity
SIP/SOP Emissions/
Immunity
SIP/SOP Emissions/
Immunity
Any Ethernet No 15 ft.
(4.6 m)
min.
SEA3730 Coax No 10 ft.
(3 m)
SEA3730 Coax No 10 ft.
(3 m)
Rear
panel
Rear
panel
smoke
evac.
Rear
panel
ECG
blank
SIP/SOP Emissions/
Immunity
SIP/SOP Emissions/
Immunity
SIP/SOP Emissions/
Immunity
Valleylab FT10 Energy Platform Service Manual 4-17
Technical Specifications
Page 74

Standards and IEC Classifications
Return Electrode Monitor (REM)
REM Specification
Interrogation Frequency 64–76 kHz
Interrogation Current < 100 µA
Contact Resistance Sense Range 5 to 135
Contact Resistance Accuracy (RF not activated) ± 7
Contact Resistance Accuracy (RF Activated) Greater of ± 14 or 20%
REM Alarm Activation
REM patient return electrode: When the measured resistance exceeds the standard range
of safe resistance (below 5 ohms or above 135 ohms) or when the measured contact
resistance increases by 40% over the baseline, the REM alarm is activated and RF output
is disabled.
Auto Bipolar
The VLFT10GEN is equipped with an Auto Bipolar feature that allows for automatic activation of bipolar energy.
Warning
Use of different Covidien cord models or cords from other manufacturers may not achieve proper
electrical output for this device, thereby failing to produce the desired clinical effect. For example,
Auto Bipolar activation/deactivation settings may not work properly using cords other than those
specified by Covidien.
In the Auto Bipolar setting, activation may occur with contact of any material. When not in use, place electrosurgical instruments in a safety holster or safely away from patients, the surgical team, and flammable materials
Auto Bipolar Specifications
Interrogation Frequency 434 kHz ± 10%
Interrogation Current < 10 µA
Activation Impedance ≤ 2200 ± 20%
Deactivation Impedance > 4000 ± 20%
averaged over 1 second
RMS
Keying Delay User selectable in 0.5 second increments from 0 sec. to 2.5 sec.
Minimum Power 1 W
4-18 Valleylab FT10 Energy Platform Service Manual
Page 75

Audio Tones
Activation Tones
Standards and IEC Classifications
Activation Tones
CUT 660 Hz ± 5% Entire Activation Duration
COAG 940 Hz ± 5% Entire Activation Duration
SHARED COAG 988 Hz ± 5% Entire Activation Duration
VALLEYLAB 800 Hz ± 5% Entire Activation Duration
BIPOLAR 940 Hz ± 5% Entire Activation Duration
LIGASURE 440 Hz ± 5% Entire Activation Duration
Tone Duration Volume
User adjustable from 45 dBA to 65 dBA (-0/+6 dBA @ 1 m)
Valleylab FT10 Energy Platform Service Manual 4-19
Technical Specifications
Page 76

Standards and IEC Classifications
Alarm Tones
Alarm Tones Tone(s) Duration Volume
REM 660 Hz ± 5% Two 500 msec. tones
separated by 500 msec. of
silence
Incomplete Seal Cycle (Regrasp/ Reactivate)
Seal Cycle Complete
High System Alarm
Medium System Alarm
High 784 Hz ± 5%
Low 587 Hz ± 5%
988 Hz ± 5% Two 175 msec. tones
Simultaneously play the following tones:
784 Hz ± 5%
1568Hz ± 5%
2352 Hz ± 5%
3136 Hz ± 5%
Simultaneously play the following tones:
784 Hz ± 5%
1568Hz ± 5%
2352 Hz ± 5%
Four 175 msec. tones (high, low, high, low)
separated by 175 msec. of
silence
Ten 138 msec. tones separated by 88, 88, 312, 88, 825, 88, 88, 312, and 88 msec. of silence
Three 188 msec. tones separated 188 msec. of silence
65 dBA (-0/+6 dBA @ 1 m)
3136 Hz ± 5%
Low System Alarm
Simultaneously play the following tones:
784 Hz ± 5%
1568Hz ± 5%
2352 Hz ± 5%
3136 Hz ± 5%
One 188 msec. tone
4-20 Valleylab FT10 Energy Platform Service Manual
Page 77

Alarm Tones Tone(s) Duration Volume
Energy Output Characteristics
Informational Alarm
Notice
Alarm characteristics cannot be changed.
Alarm response time is less than one second.
Simultaneously play the following tones:
784 Hz ± 5%
1568Hz ± 5%
2352 Hz ± 5%
3136 Hz ± 5%
One 188 msec. tone
User adjustable from 45 dBA to 65 dBA (-0/+6 dBA @ 1 m)
Energy Output Characteristics
The VLFT10GEN automatically senses the tissue resistance and adjusts the output voltage
to maintain a consistent effect across different tissue density. The adjustment is based on
the selected mode or effect, power setting, and the level of tissue resistance. The
maximum output voltage is controlled to reduce capacitive coupling and video
interference and to minimize undesired sparking. The following table outlines the rated
power, peak voltages, and maximum output current for a given modality. The peak voltage
includes tolerance and identifies the maximum value across all loading conditions. Output
power can vary ± 15%. Current nominal maximum does not include tolerance.
Valleylab FT10 Energy Platform Service Manual 4-21
Technical Specifications
Page 78

Energy Output Characteristics
Rated
Load
(Ω)
Rated
Output
Power
Peak
Voltage
(V)
(W)
Monopolar CUT
PURE
BLEND
300
300
300
200
1287
2178
Valleylab
VALLEYLAB 300 200 2783
Monopolar COAG
SOFT
FULGURATE
SHARED FULGURATE
SPRAY
SHARED SPRAY
100
500
500
500
500
120
120
4
120
120
4
120
264
3448
3448
3932
3932
Bipolar
LOW (1–15 W)
MEDIUM (16–40 W)
HIGH (45–95 W)
100
100
100
15
40
95
133
214
462
1
Current
Nominal
Max (A)
1.25
1.0
1.0
1.55
1.0
1.0
1.0
1.0
1.0
2.0
2.0
Typical
Crest
Factor
1.6
2.2
Duty Cycle
2
100%
50%
3.2 25%
1.5
5.4
5.4
6.2
6.2
1.8
1.8
1.7
100%
6.25%
6.25%
4.76%
4.76%
100%
100%
100%
LigaSure
LIGASURE
20 350 244 5.5 1.9 100%
Bipolar Resection
• CUT
• COAG
500
100
200
175
3
742
318
2.4
3.2
1.4
1.6
100%
100%
1. Per IEC 60601-2-2: 2009 clause 201.7.9.2.2.101(c)(2), whenever the peak voltage is greater than 1600 V, the calculated variable y is less than the actual crest factor. The peak voltage at rated load can be calculated using the equation: V
peak(Umax
) = TypicalCrestFactor √Power·RatedLoad
2. At rated load.
3. Bipolar Resection CUT maximum power is 432 W, including tolerance.
4. Rated load in SHARED COAG modes is the total energy channel load. For SHARED dual
activation using two activating instruments, it is the parallel combination of the two loads. For
SHARED single activation using one activating instrument, it is the load connected to the
activating electrode only.
4-22 Valleylab FT10 Energy Platform Service Manual
Page 79

Energy Output Characteristics
Output Waveforms
TissueFect Tissue Sensing Technology, an automatic adjustment, controls all modes and
effects. As tissue resistance increases from zero, the energy platform outputs constant
current, followed by constant power, followed by constant voltage. The maximum output
voltage is controlled to reduce capacitive coupling and video interference and to minimize
sparking.
Bipolar
LOW 434 kHz ±10% continuous sinusoid
MEDIUM 434 kHz ±10% continuous sinusoid
HIGH 434 kHz ±10% continuous sinusoid
Monopolar CUT
CUT 434 kHz ±10% continuous sinusoid
BLEND 434 kHz ±10% bursts of sinusoid, recurring at 27.13
kHz ±10% intervals
50% duty cycle
VALLEYLAB
VALLEYLAB 434 kHz ±10% bursts of sinusoid, recurring at 27.13
kHz ±10% intervals
25% duty cycle
Technical Specifications
Valleylab FT10 Energy Platform Service Manual 4-23
Page 80

Energy Output Characteristics
Monopolar COAG
SOFT 434 kHz ±10% continuous sinusoid
FULGURATE 434 kHz ±10% damped sinusoidal bursts with a
SHARED FULGURATE 434 kHz ±10% damped sinusoidal bursts with a
SPRAY 434 kHz ±10% damped sinusoidal bursts with a
repetition frequency of 27.13 kHz ±10%
6.25% duty cycle
repetition frequency of 27.13 kHz ±10%
6.25% duty cycle
repetition frequency of 20.67 kHz ±10%
4.76% duty cycle
SHARED SPRAY 434 kHz ±10% damped sinusoidal bursts with a
repetition frequency of 20.67 kHz ±10%
4.76% duty cycle
LigaSure
LIGASURE 434 kHz ±10% continuous sinusoid
Bipolar Resection
BIPOLAR RESECTION 434 kHz ±10% continuous sinusoid
4-24 Valleylab FT10 Energy Platform Service Manual
Page 81

Output Power vs. Resistance Graphs
Output Power vs. Resistance Graphs
For the values stated in the graphs provided in this section:
• Output power at rated load is within the greater of 15% or 8 W.
• Peak voltage includes tolerances.
• The generator measures resistance within the greater of 15% or 5 .
• Output power is measured using compatible instruments, not test leads.
Monopolar Graphs
PURE CUT
Output power versus resistance for Pure CUT power
1
Output power (watts)
ཱ Load resistance (ohms)
100%
50%
2
Technical Specifications
Valleylab FT10 Energy Platform Service Manual 4-25
Page 82

Output Power vs. Resistance Graphs
Output power versus power setting for Pure CUT power
1
2
Output power (watts)
ཱ Power setting
Note: Rated load
Peak voltage versus power setting for Pure CUT power
1
2
Peak voltage (volts)
ཱ Power setting
Note: Open circuit
4-26 Valleylab FT10 Energy Platform Service Manual
Page 83

BLEND
Output power versus resistance for BLEND power
Output Power vs. Resistance Graphs
1
2
Output power (watts)
ཱ Load resistance (ohms)
Output power versus power setting for BLEND power
1
100%
50%
2
Output power (watts)
ཱ Power setting
Note: Rated load
Valleylab FT10 Energy Platform Service Manual 4-27
Technical Specifications
Page 84

Output Power vs. Resistance Graphs
Peak voltage versus power setting for BLEND power
1
Peak voltage (volts)
2
ཱ Power setting
Note: Open circuit
VALLEYLAB
Output power versus resistance for VALLEYLAB power
1
2
Output power (watts)
ཱ Load resistance (ohms)
100%
50%
4-28 Valleylab FT10 Energy Platform Service Manual
Page 85

Output power versus power setting for VALLEYLAB power
1
2
Output power (watts)
Output Power vs. Resistance Graphs
ཱ Power setting
Note: Rated load
Peak voltage versus power setting for VALLEYLAB power
1
2
Peak voltage (volts)
ཱ Power setting
Note: Open circuit
Technical Specifications
Valleylab FT10 Energy Platform Service Manual 4-29
Page 86

Output Power vs. Resistance Graphs
SOFT COAG
Output power versus resistance for SOFT COAG power
125
100
75
1
50
25
0
0
100%
50%
400
800 1200
1600
2000
2
Output power (watts)
ཱ Load resistance (ohms)
Output power versus power setting for SOFT COAG power
120
96
72
1
48
24
0
0 30 60 90 120
2
Output power (watts)
ཱ Power setting
Note: Rated load
4-30 Valleylab FT10 Energy Platform Service Manual
Page 87

Peak voltage versus power setting for SOFT COAG power
280
224
168
1
112
56
Output Power vs. Resistance Graphs
0
0
24 48 72 96 120
2
Peak voltage (volts)
ཱ Power setting
Note: Open circuit
FULGURATE
Output power versus resistance for FULGURATE power
1
100%
50%
Technical Specifications
2
Output power (watts)
ཱ Load resistance (ohms)
Valleylab FT10 Energy Platform Service Manual 4-31
Page 88

Output Power vs. Resistance Graphs
Output power versus power setting for FULGURATE power
1
2
Output power (watts)
ཱ Power setting
Note: Rated load
Peak voltage versus power setting for FULGURATE power
1
2
Peak voltage (volts)
ཱ Power setting
Note: Open circuit
4-32 Valleylab FT10 Energy Platform Service Manual
Page 89

Output Power vs. Resistance Graphs
SHARED FULGURATE - Single Activation (One Instrument Activated)
Output power versus resistance for SHARED FULFURATE (single activation) power
1
2
100%
50%
Output power (watts)
ཱ Load resistance (ohms)
Output power versus power setting for SHARED FULGURATE (single activation) power
1
2
Output power (watts)
ཱ Power setting
Note: Rated load
Valleylab FT10 Energy Platform Service Manual 4-33
Technical Specifications
Page 90

Output Power vs. Resistance Graphs
Peak voltage versus power setting for SHARED FULGRUATE (single activation) power
1
Peak voltage (volts)
2
ཱ Power setting
Note: Open circuit
SHARED FULGURATE - Dual Activation (Two Instruments Activated)
Output power versus resistance for SHARED FULFURATE (dual activation) power
1
2
Output power (watts)
ཱ Load resistance (ohms)
100%
50%
4-34 Valleylab FT10 Energy Platform Service Manual
Page 91

Output Power vs. Resistance Graphs
Output power versus power setting for SHARED FULGURATE (dual activation) power
1
2
Output power (watts)
ཱ Power setting
Note: Rated load
Peak voltage versus power setting for SHARED FULGRUATE (dual activation) power
1
2
Peak voltage (volts)
ཱ Power setting
Note: Open circuit
Technical Specifications
Valleylab FT10 Energy Platform Service Manual 4-35
Page 92

Output Power vs. Resistance Graphs
SPRAY
Output power versus resistance for SPRAY power
1
100%
50%
2
Output power (watts)
ཱ Load resistance (ohms)
Output power versus power setting for SPRAY power
1
2
Output power (watts)
ཱ Power setting
Note: Rated load
4-36 Valleylab FT10 Energy Platform Service Manual
Page 93

Peak voltage versus power setting for SPRAY power
1
2
Peak voltage (volts)
Output Power vs. Resistance Graphs
ཱ Power setting
Note: Open circuit
SHARED SPRAY - Single Activation (One Instrument Activated)
Output power versus resistance for SHARED SPRAY (single activation) power
100%
1
2
Output power (watts)
50%
Technical Specifications
ཱ Load resistance (ohms)
Valleylab FT10 Energy Platform Service Manual 4-37
Page 94

Output Power vs. Resistance Graphs
Output power versus power setting for SHARED SPRAY (single activation) power
1
2
Output power (watts)
ཱ Power setting
Note: Rated load
Peak voltage versus power setting for SHARED SPRAY (single activation) power
1
2
Peak voltage (volts)
ཱ Power setting
Note: Open circuit
4-38 Valleylab FT10 Energy Platform Service Manual
Page 95

Output Power vs. Resistance Graphs
SHARED SPRAY - Dual Activation (Two Instruments Activated)
Output power versus resistance for SHARED SPRAY (dual activation) power
1
2
100%
50%
Output power (watts)
ཱ Load resistance (ohms)
Output power versus power setting for SHARED SPRAY (dual activation) power
1
2
Output power (watts)
ཱ Power setting
Note: Rated load
Valleylab FT10 Energy Platform Service Manual 4-39
Technical Specifications
Page 96

Output Power vs. Resistance Graphs
Peak voltage versus power setting for SHARED SPRAY (dual activation) power
1
Peak voltage (volts)
2
ཱ Power setting
Note: Open circuit
Bipolar Graphs
Bipolar LOW Effect
Note: Bipolar LOW effect is utilized for power settings of OFF, 1-15 W inclusive within
Bipolar mode (1-95 W).
Output power versus resistance for Bipolar LOW power
1
100%
50%
2
Output power (watts)
ཱ Load resistance (ohms)
4-40 Valleylab FT10 Energy Platform Service Manual
Page 97

Output power versus power setting for Bipolar LOW power
2
1
2
2
Output Power vs. Resistance Graphs
Output power (watts)
ཱ Power setting
Note: Rated load
Peak voltage versus power setting for Bipolar LOW power
1
2
Peak voltage (volts)
Technical Specifications
ཱ Power setting
Note: Open circuit
Valleylab FT10 Energy Platform Service Manual 4-41
Page 98

Output Power vs. Resistance Graphs
Bipolar MEDIUM Effect
Note: Bipolar MEDIUM effect is utilized for power settings of 16–40 W inclusive within
Bipolar mode (1-95 W).
Output power versus resistance for Bipolar MEDIUM power
1
100%
50%
2
Output power (watts)
ཱ Load resistance (ohms)
Output power versus power setting for Bipolar MEDIUM power
1
2
Output power (watts)
ཱ Power setting
Note: Rated load
4-42 Valleylab FT10 Energy Platform Service Manual
Page 99

Peak voltage versus power setting for Bipolar MEDIUM power
1
2
Output Power vs. Resistance Graphs
Peak voltage (volts)
ཱ Power setting
Note: Open circuit
Bipolar HIGH Effect
Note: Bipolar HIGH Effect is utilized for power settings of 45–95 W inclusive within Bipolar
mode (1-95 W).
Output power versus resistance for Bipolar HIGH power
3
100%
50%
1
Technical Specifications
2
Output power (watts)
ཱ Load resistance (ohms)
ི See Discontinuous Power Curves on page 4-49 for additional details of this region of
the power curve.
Valleylab FT10 Energy Platform Service Manual 4-43
Page 100

Output Power vs. Resistance Graphs
Output power versus power setting for Bipolar HIGH power
1
2
Output power (watts)
ཱ Power setting
Note: Rated load
Peak voltage versus power setting for Bipolar HIGH power
400
320
240
1
160
80
0
45 55 65 75 85 95
2
Peak voltage (volts)
ཱ Power setting
Note: Open circuit
4-44 Valleylab FT10 Energy Platform Service Manual
 Loading...
Loading...