Page 1

IM-EN-PR23AC-VACC-A
Appendix
to PR-23 Instruction Manual for Vaisala K-PATENTS®
Products Intended for Use in Vaccine Production
Vaisala K-PATENTS® Pharma Refractometer PR-23-AC
Page 2

Appendix to the Instructions Manual PR-23
Do not underestimate or neglect the laboratory and factory safety rules:
●
Before you start, assess the workplace to determine if hazards are present, or are
likely to be present, which necessitate the use of personal protective equipment, e.g.:
●
protective clothing and shoes
●
safety goggles
●
protective gloves
●
respiratory shields and devices
●
Locate the nearest safety equipment, extinguishers, eyewash, and emergency shower
2
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 3

Contents
Section 1 Vaisala K-PATENTS® products for vaccine production and sucrose gradient
ultracentrifugation ............................................................................................................... 5
1.1 Designqualication........................................................................................................ 6
Section 2 The Pharma Refractometer PR-23-AC ................................................................................ 7
2.1 System description ........................................................................................................ 7
2.2 System components ...................................................................................................... 8
2.2.1 Checklist of components .................................................................................... 8
2.3 Pharma Refractometer Sensor PR-23-AC- 62-HSS-SC ............................................. 10
2.3.1 Sensor model code .......................................................................................... 10
2.3.2 Pharma Mini Flow Cell model code ................................................................. 10
2.4 Indicating Transmitter DTR-GP-SC ............................................................................. 12
2.4.1 Indicating Transmitter model code ................................................................... 12
2.5 Pharma vaccines accessories ..................................................................................... 13
Section 3 Installation of PR-23 Pharma Refractometer ................................................................... 15
3.1 Hardware and software requirements ......................................................................... 15
3.2 Mechanical and electrical requirements ...................................................................... 15
3.3 Sensor installation for use in pharmaceutical batch manufacturing ............................ 15
3.4 Indicating Transmitter installation for use in table top ................................................. 19
3.5 Wiring transmitter connections to sensor, power cable and computer ....................... 20
3.6 Refractometerinstrumentverication .......................................................................... 21
Section 4 Electronic data capture and storage ................................................................................ 23
4.1 Ethernet connection ..................................................................................................... 23
Section 5 Complying with documentation and validation regulations ......................................... 25
5.1 Documentation ............................................................................................................. 25
5.2 Qualication ................................................................................................................. 25
5.3 Protocol acceptance by customer and list of tests performed .................................... 25
5.4 Electronic data management and data storage ........................................................... 26
5.5 Electronic signatures/audit trail .................................................................................... 26
5.6 Record keeping ............................................................................................................ 26
5.7 Security ........................................................................................................................ 26
5.8 System validation ......................................................................................................... 26
5.9 Vaisala K-PATENTS
Section 6 Onsitequalicationprotocolsandrecords:InstallationQualication ....................... 29
6.1 Authorization and responsibilities ................................................................................ 29
6.1.1 Documents and procedures ............................................................................ 29
6.1.2 Authorizedofciator ......................................................................................... 30
6.1.3 Execution ......................................................................................................... 30
6.2 System ......................................................................................................................... 30
®
refractometer system adherence to Part 11 ............................. 27
© Copyright Vaisala 2020. All rights reserved.
3
Page 4

Appendix to the Instructions Manual PR-23
6.2.1 Qualifying the system ...................................................................................... 30
6.2.2 Manufacturers and suppliers ........................................................................... 31
6.3 IQ protocol ................................................................................................................... 31
6.3.1 Scope of delivery ............................................................................................. 31
6.3.2 Damage ............................................................................................................ 32
6.4 Documentation ............................................................................................................. 32
6.5 Operating environment ................................................................................................ 33
6.6 Installation .................................................................................................................... 33
6.7 Setting up the system components and devices ......................................................... 34
6.8 Electrical connections and wiring ................................................................................ 35
6.9 Ethernet connection ..................................................................................................... 36
6.10 Initial check and switching the device on ................................................................... 36
6.11 InstallationQualicationsummaryreport .................................................................... 37
Section 7 Onsitequalicationprotocolsandrecords:OperationalQualication ...................... 39
7.1 Individual module and system components check ...................................................... 39
7.2 InstallationQualicationhasbeenperformedsuccessfully ........................................ 39
7.3 Test procedure ............................................................................................................. 40
7.4 Authorizedofciator ..................................................................................................... 40
7.5 Systemqualication ..................................................................................................... 41
7.6 Setting up the system components and devices ......................................................... 41
7.7 Instrumentvericationwithsampleholderandrefractiveindexliquids ...................... 42
7.8 OperationalQualicationsummaryreport .................................................................. 43
Section 8 Routine operation phase ................................................................................................... 45
Section 9 Preventive maintenance .................................................................................................... 47
Section 10 Other documentation ......................................................................................................... 49
4
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 5
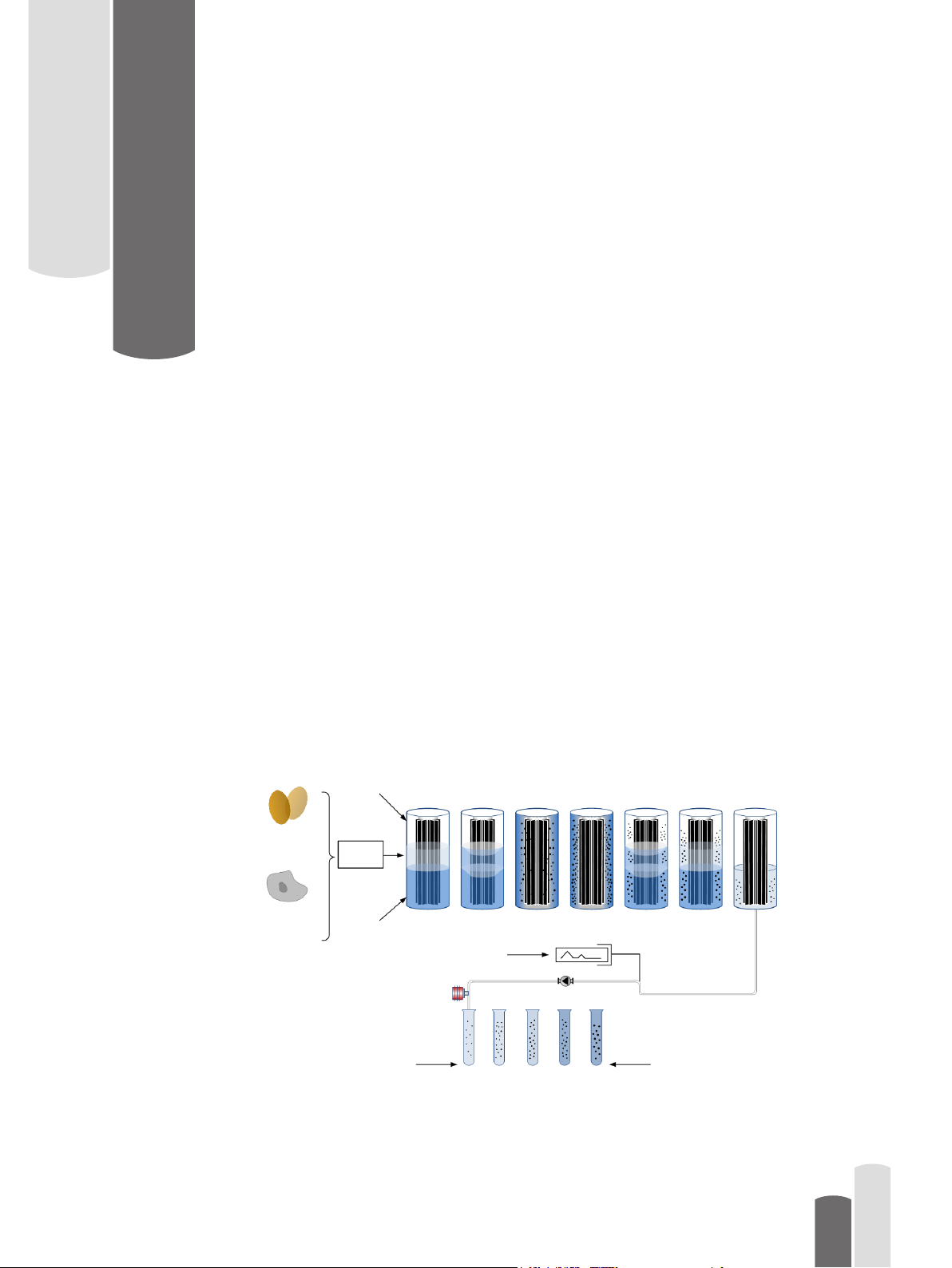
Vaisala K-PATENTS® products for
1
vaccine production and sucrose
gradient ultracentrifugation
This instruction manual appendix covers Vaisala K-PATENTS® Pharma Refractometer
PR-23-AC when used in the production of viral vaccines. The vaccines are either produced
by inoculating viruses into specic pathogen-free eggs or in animal cell culture based
process. The allantoic uid of these processes is harvested and puried by centrifugation
and stabilised with buffer containing sucrose. The centrifugation process typically uses
density gradient continuous ow ultracentrifuge for the purication of the virus particles.
The internal subviral core of the virus is separated and fractionated on the basis of its’
sedimentation rate, and the buoyant sucrose density. Pharma Refractometer PR-23-AC is
used for accurate measurement of these sucrose densities. The measurement signal is used
for reliable and timely determination of the product peak in the density gradient (0 to 60%
w/w sucrose), and the subsequent collection of the virus rich fraction (Figure 1.1.).
Pharma Refractometer PR-23-AC can be installed in the vaccines fractionation unit for
in-line process control. The output of the transmitter is a 4 to 20mA DC output signal
proportional to sucrose solution density or Brix. Process data can also be downloaded to a
computer via an Ethernet cable.
High Density
Solution
• Inoculation egg
with virus
• Incubation
• Inoculation
preparation
• Cell expansion
• Virus propagation
Figure 1.1 Ultracentrifugation density gradient purication process steps.
© Copyright Vaisala 2020. All rights reserved.
Inactivation
of virus
Low Density
Solution
Sucrose gradient purication by zonal centrifugation
Fractionation and separation
Chart Recorder
Filling
Low Density High Density
Rotor unloading
5
Page 6

Appendix to the Instructions Manual PR-23
1.1 Design qualication
Design Qualication (DQ) typically consists of manufacturer’s documentation to verify
that the proposed design of the Vaisala K-PATENTS
intended purpose.
Pharma Refractometer PR-23-AC is an in-line real-time instrument that is designed to meet
the pharmaceutical industry standards and guidelines including PAT, GMP, CIP/SIP and
validation. Pharma Refractometer PR-23-AC wetted parts materials comply with the contactcompatibility of a substance with pharmaceutical materials. Gasket materials conform to the
FDA requirements 21 CFR 177.2600 and to biocompatibility standards according to USP
Class VI. Meeting the FDA and USP criteria guarantees that the seal material is acceptable
for sanitary process applications and the material, or extracts from the material will not
be harmful to human health. No animal derived ingredients (ADI) have been used in the
machining and polishing processes. The PR-23-AC also meets the 3-A Sanitary Standard
and is tested for in-place-cleanability according to the Euopean Hygienic Engineering Design
Group (EHEDG) test.
The refractometer has an Ethernet communications solution. The transmitter uses the
IP protocol to communicate over the Ethernet to any type of computer. This eliminates
human error and allows for easy capture of the refractometer generated measurement
and diagnostic data for storage, analysis and reporting. Access to the refractometer
and the generated data can be restricted to authorized personnel using password and
padlock protection.
®
Refractometer is suitable for the
Vaisala K-PATENTS refractometers are designed, manufactured and serviced under
ISO 9001 quality system and procedures that guarantee the accuracy and repeatability
of the measurement results. Each refractometer sensor is provided with a calibration
certicate comparing a set of standard liquids to the actual sensor output. Vaisala veries
the calibration of all delivered instruments according to the procedure similar to the
one described in the PROCESS REFRACTOMETER PR-23 INSTRUCTION MANUAL,
Section 13.
The quality system is ISO 9001 certied by Det Norske Veritas. The quality performance
is improved by critical self-assessment, internal auditing and feedback system. The chain
of quality starts from the subcontractors with whom Vaisala maintains a quality contracting
and regular auditing system. The internal quality functions, from verication of incoming
products to packing and delivery, are based on dened procedures. Vaisala provides full
traceability of the wetted parts materials. Certicates of Origin, and any other required
quality documentation is available upon request at time of order.
Vaisala K-PATENTS process refractometers and support services are available to customers
anywhere in the world. Application, installation and technical assistance are provided both
locally by the representatives and by the headquarters in Finland and a branch in the U.S.
Vaisala warrants that all products made by Vaisala shall be free of defects in material and
workmanship. Vaisala agrees either to replace or repair free of charge any such product or
part thereof which shall be returned to the nearest authorized Vaisala K-PATENTS repair
facility within two (2) years from the date of delivery.
6
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 7

2
The Pharma Refractometer
PR-23-AC
2.1 System description
The recommended system for the vaccines production process comprises of a
Pharma Refractometer PR-23-AC unit and a Pharma Mini Flow Cell PMFC-HSS
that allows the sensor connection to the zonal ultracentrifuge rotor unloading and
fractionation phase.
The standard Ethernet communication solution allows for simultaneous data
logging and continuous monitoring of the measurement values and diagnostic
data from the Indicating Transmitter DTR to a computer via an Ethernet connection.
© Copyright Vaisala 2020. All rights reserved.
7
Page 8

Appendix to the Instructions Manual PR-23
2.2 System components
2.2.1 Checklist of components
Pharma Refractometer Sensor PR-23-AC-62-HSS-SC- EP calibrated with raw
1
measurement data refractive index (n
Indicating Transmitter DTR-M/U-GP-SC that calculates and displays the process liquid
2
concentration based on the refractive index and temperature, installed in a stainless steel
2a
enclosure that contains a key
Table top stand PR-7603-SS for Indicating Transmitter,
3
contains a set of two M5x10 A2 DIN 912 screws
3a
Wall mounting screws kit for mounting the Indicating Transmitter DTR on the wall
4
Interconnecting cable between transmitter and sensor PR-8230
5
PR-8820 Crossover cable for Ethernet connection between Indicating Transmitter and
6
computer, length 5 m (16 inch), contains cable gland for enclosure connection
Table top stand PR-7605 -SS with an integral support rod and
7
2.5” Sanitary Clamp for the Pharma Refractometer Sensor PR-23-AC-62-HSS
7a
contains a M5x16 A4 DIN 912 screw
7b
Pharma Mini Flow Cell PMFC-HSS
8
PR-9244-USP O-ring for the Pharma Mini Flow Cell, 22.2x3.0 EPDM
9
Two sets of PR-9235 0.5” Sanitary Clamp for the Pharma Mini Flow Cell connection
10
Two sets of PR-9236 -USP Sanitary gasket EPDM for the 0.5” Sanitary Clamps
11
Two sets of PR-9237 0.5” Sanitary ferrule (lenght 1.5 cm) for the inlet and outlet hose
12
connections and Pharma Mini Flow Cell
Universal sample holder PR-1012
13
R.I. Liquid set PR-2300, consists of Cargille Cer ticate for the liquids
14
) and temperature (T)
D
8
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 9
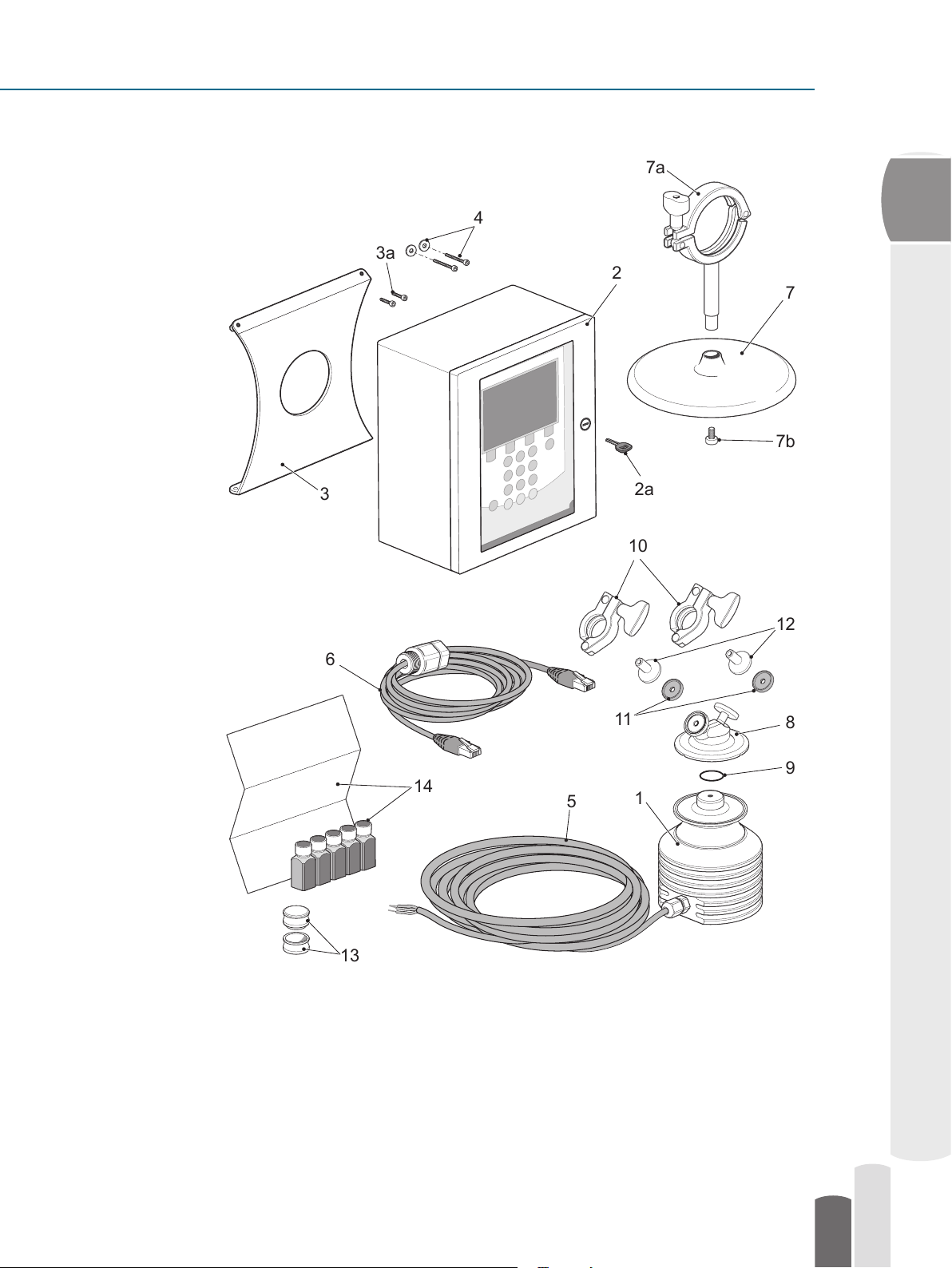
The Pharma Refractometer PR-23-AC
1
13
2
11
8
9
10
7b
7a
5
3
3a
14
2a
7
12
6
4
2
Figure 2.1 System components provided by Vaisala.
© Copyright Vaisala 2020. All rights reserved.
9
Page 10

Appendix to the Instructions Manual PR-23
2.3 Pharma Refractometer Sensor PR-23-AC-62-HSS-SC
2.3.1 Sensor model code
Model and Description Model
PR-23 = Sensor PR-23
Sensor model
-A = 3A approved -A
Sensor type
C = Compact type for pipe line installation C
Refractive Index range limits
-62 = R.I. 1.320....1.530 ( 0-100 Brix ) -62
Process connection -SC
-H = Sanitary 3A-clamp, 2 ½ inch -H
Sensor wetted parts material
SS = AISI 316 L SS
Electrical classication
-GP = General purpose -GP
-AX = ATEX certied EX II 3 G Eex nA II T4 (up to Zone 2) -AX
-IA = ATEX and IECEx certied EX II 1 G Ex ia II C T4 Ga (up to Zone 0) (A) -IA
Sensor housing
-SC = Stainless steel -SC
Sensor wetted parts surface treatment option
-EP = Electropolished process wetted parts (Ra 0.4µm, 15 µ inch ) -EP
(A) Available with STR- Indicating Transmitter and IS Isolator only
10
2.3.2 Pharma Mini Flow Cell model code
The wetted parts materials for the Pharma Mini Flow Cell are AISI 316 stainless
steel standard Ra 0.4µm, 15 µ inch and EPDM (ethylene propylene diene
monomer) for the O-ring sealing.
Model and Description Model
PMFC = Pharma Mini Flow Cell PMFC
Sensor connection
-H = Sanitary 3A-clamp, 2 ½ inch -H
Material of Construction
SS = AISI 316 SS
Process connection
-H = Sanitary mini tting -H
Pipe section diameter
04 = 4 mm 04
05 = 5 mm 05
06 = 6 mm 06
Options
-EP = Electropolished process wetted parts (Ra 0.4µm, 15 µ inch ) -EP
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 11

The Pharma Refractometer PR-23-AC
2
Figure 2.2 Pharma Refractometer PR-23-AC-62-HSS-EP sensor and Pharma Mini Flow Cell
Figure 2.3 Pharma Mini Flow Cell PMFC-HSS-EP.
PMFC-HSS-EP with PR-7605 -SS table top stand.
© Copyright Vaisala 2020. All rights reserved.
11
Page 12

Appendix to the Instructions Manual PR-23
2.4 Indicating Transmitter DTR-GP-SC
Indicating Transmitter DTR is a specialized computer designed to process data
received from the refractometer sensor. Indicating Transmitter (Figure 2.4) contains
a front panel with a backlit Liquid Crystal Display (LCD) and a keyboard. A lock and
a key are included in the enclosure’s door to prevent unauthorized access. Please
note that neither any power cables nor any external power switches are included in
the standard delivery.
Materials for the Pharma Indicating Transmitter Enclosure DTR-M/U-GP-SC are:
Stainless steel enclosure and polycarbonate window.
2.4.1 Indicating Transmitter model code
Model and Description Model
DTR = Indicating Transmitter (connectivity for two sensors)
STR = Indicating Transmitter (connectivity for one –IA/-IE sensor)
Cable connection
-U = ½ inch NPT-type conduit hubs -U
-M = M20x1,5 metric cable glands -M
Electrical classication
-GP = General purpose -GP
Enclosure
-SC = Stainless Steel enclosure with window -SC
Transmitter options (A) (leave this section blank, if AC supply is specied)
-AC = Power supply 100-240 VAC 50/60 Hz -AC
-DC = Power supply 24 V DC -DC
(A) Note standard power supply is 100-240 VAC 50/60 Hz
DTR
STR
12
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 13
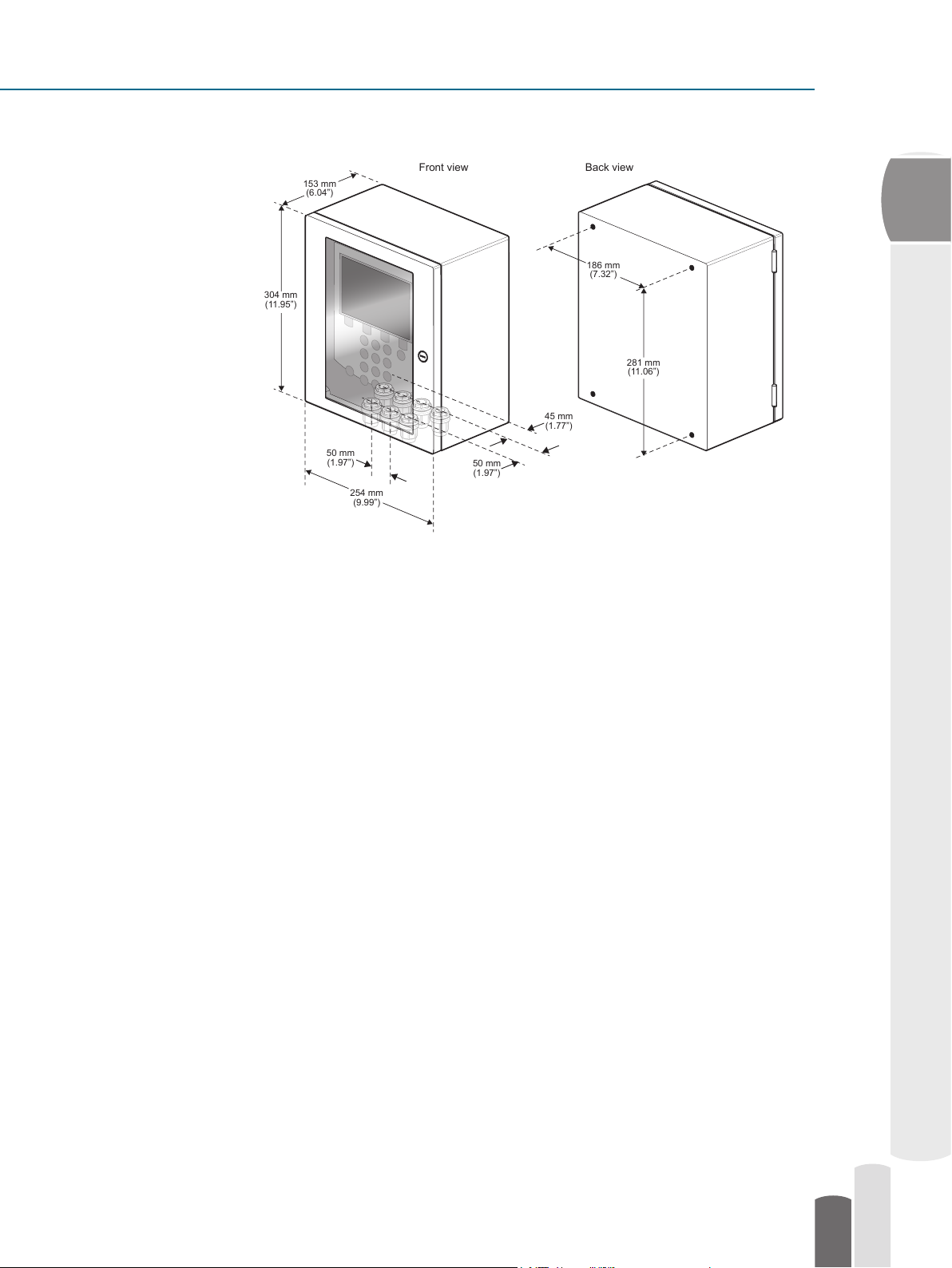
The Pharma Refractometer PR-23-AC
Front view Back view
186 mm
(7.32”)
281 mm
(11.06”)
153 mm
(6.04”)
304 mm
(11.95”)
254 mm
(9.99”)
50 mm
(1.97”)
50 mm
(1.97”)
45 mm
(1.77”)
2
Figure 2.4 Indicating Transmitter DTR-GP-SC with Stainless steel enclosure; dimensions (mm/in).
2.5 Pharma vaccines accessories
Vaisala recommended accessories for the vaccines production application contain
Ethernet crossover cable, IQ and OQ documentation (this document) and parts for
verication and usage of Pharma Refractometer sensor and Indicating Transmitter
mounted on a table top or a trolley via metal support stands. The recommended
accessories and corresponding part numbers are:
●
PR-7603-SS Table top stand for Indicating Transmitter (contains a set of two M5x10
A2 DIN screws)
●
PR-7605-SS Table top stand with the intregral support rod and 2.5” Sanitary Clamp
for the Pharma Refractometer Sensor PR-23-AC (contains a screw)
●
PR-8820 Crossover cable for Ethernet connection between Indicating Transmitter
and computer, length 5 m (16 feet)
●
Parts for off-line instrument verification:
PR-1012 Sample holder
PR-2300 R.I. liquid set 5 x ¼ .oz.; Including: 1.33; 1.37; 1.42; 1.47; 1.52
●
IM-EN-PR23AC-VACC IQ and OQ Documentation for Equipment qualification
© Copyright Vaisala 2020. All rights reserved.
13
Page 14

Appendix to the Instructions Manual PR-23
14
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 15

3
Installation of PR-23 Pharma
Refractometer
3.1 Hardware and software requirements
PR-23 software is included in the Indicating Transmitter DTR and it comprises the
following functions:
●
Automatic temperature compensation
●
Ethernet connection for data download
●
Sensor diagnostics and verication
3.2 Mechanical and electrical requirements
Power supply for the refractometer is AC input 100-240 VAC/50-60 Hz, optional 24 VDC,
30 VA.
3.3 Sensor installation for use in pharmaceutical batch
manufacturing
Laboratory table top or trolley installation and key considerations for the site
preparation
1. Physical dimensions of the instrument and accessories: make sure there is enough
space to accommodate them.
2. Suitable recommended operational environment for the instrument and for the Cargille
Refractive Index Liquids should be maintained between 20 – 30 °C (68 – 86°F).
3. Utilities: 100-240 VAC/50-60 Hz (optional 24 VDC, 30 VA) electrical power supply and
computer network connection.
© Copyright Vaisala 2020. All rights reserved.
15
Page 16

Appendix to the Instructions Manual PR-23
Attaching table top stand PR-7605-SS for Sensor and Mini Flow cell
Attach the 2.5” Sanitary clamp and the support rod to the base plate. The supplied screw
(M5x16 A4 DIN 912) for attaching the stand is inserted from the bottom of the base plate
through the bottom hole (Figure 3.1). To assemble the Pharma Mini Flow Cell rst locate the
PR-9244-USP O-ring 22.2x3.0 EPDM inside the Mini Flow Cell (Figure 3.2). Then insert the
2.5” Sanitary clamp for the sensor and Mini Flow Cell.
Finally insert the two PR-9236-USP Sanitary EPDM gaskets and the two PR-9237
0.5” Sanitary ferrules for the inlet and outlet connectios using the two PR-9235 0.5”
Sanitary Clamps.
Sensor can now be connected to exible hoses and used as a free standing tabletop unit,
see Figure 3.3.
1
Sanitary clamp
and support
Base plate
Retaining screw
Sensor stand assembly
Figure 3.1 Sensor stand assembly: Attaching the clamp and support rod to the base plate.
16
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 17

Installation of PR-23 Pharma Refractometer
2
3
Figure 3.2 Sensor stand assembly: Installing the sensor and Mini Flow Cell to the stand.
3
PR-9236-USP Sanitary gasket
EPDM for the 0.5”
PR-9237-USP
0.5” Sanitary ferrule
PR-9235 0.5” Sanitary Clamp
Figure 3.3 Sensor assembly: Attaching the ferrules for the feed pipes.
© Copyright Vaisala 2020. All rights reserved.
17
Page 18

Appendix to the Instructions Manual PR-23
Figure 3.4 Sensor stand assembled.
4
18
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 19

Installation of PR-23 Pharma Refractometer
3.4 Indicating Transmitter installation for use
in table top
Attaching table top stand PR-7603- SS for Indicating Transmitter
Unlock and open the transmitter cabinet door, then unscrew the retaining screw for the keypad
panel and open the panel. The supplied screws (M5x10 A2 DIN 912) for attaching the stand
are inserted from the inside through the top two holes located at the back of the cabinet. These
are aligned and screwed into the threaded attachment points located at the top of the stand.
The enclosure can now be used as a free standing tabletop unit, see Figure 3.2.
3
1
Figure 3.5 Attaching the laboratory table top stand to the Transmitter for use in the laboratory.
© Copyright Vaisala 2020. All rights reserved.
19
Page 20

Appendix to the Instructions Manual PR-23
3.5 Wiring transmitter connections to sensor,
power cable and computer
For Indicating Transmitter DTR wiring and Ethernet connections instructions see PROCESS
REFRACTOMETER PR-23 INSTRUCTION MANUAL, Section 3 and Section 12. When the
wiring connections have been made sensor calibration and verication can commence.
Indicating Transmitter
DTR-M-GP-SC
Pharma Mini Flow Cell
PMFC-HSS-EP
Pharma Refractometer
PR-23-AC-62-HSS-EP
Interconnecting cable
PR-8230
PC -Windows
(not supplied by Vaisala)
Figure 3.6 Connection diagram
Power Supply
Cable (not supplied
by Vaisala)
Crossover Cable
20
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 21

Installation of PR-23 Pharma Refractometer
3.6 Refractometer instrument verication
The operational procedure checking the refractometer calibration accuracy, linearity and
short-term repeatability and reproducibility consists of verication tests using Cargille
standard refractive index n
The verication of the refractometer calibration is performed whenever a new Vaisala
laboratory refractometer is qualied as a part of the validation process, and also if any of the
following occurs:
●
There is a replacement of optical parts (prism and prism gasket).
●
Refractometer readings reect an unusual shift, or are outside of the acceptable limits,
and other means of assessing and correcting unacceptable control values fail to
identify and correct the problem.
Verication is recommended to be performed once every 12 months (or more frequently if
specied in the client’s own quality system) as a routine quality control check. Verication
is carried out using the Sample Holder PR-1012 and the set of Cargille standard refractive
index n
PR-1012 consists of a sample receptacle with O-ring seal around the bottom aperture.
Before commencing the verication process make sure that your refractometer and sample
holder are at normal room temperature. Preferably take all components to the laboratory
already one day prior to the verication. Check the condition and expiry date of your
standard refractive index liquids and that you also have the required cleaning solution
(e.g. Isopropyl alcohol) and cleaning tissue to clean the sensor wetted surfaces and the
sample holder.
liquids. A set (R.I. Liquid Set PR-2300) is supplied by Vaisala. The Sample Holder
D
liquids.
D
3
For full Sensor verication instructions see PROCESS REFRACTOMETER PR-23,
INSTRUCTION MANUAL Section 13.
After verication of the PR-23 sensor, further verication of the Laboratory Test Cuvette and
Sensor combination can be carried out.
© Copyright Vaisala 2020. All rights reserved.
21
Page 22

Appendix to the Instructions Manual PR-23
22
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 23

4
Electronic data capture and storage
4.1 Ethernet connection
In addition to software operation via the hardware, the DTR can be considered as a web
server and is accessible via a web-browser (e.g. Internet Explorer, Firefox, Chrome etc.).
The Ethernet connection enables data download from an Indicating Transmitter DTR to a
computer and replaces the traditional paper-based data collection methods and streamlines
data collection. The connection works both directly between the DTR and a computer, or via
a hub or a switch, local area network (LAN), wireless network (WLAN) or ber Ethernet.
Any type of computer (PC, Mac, PDA, mainframe…) with a compatible network connection
can be congured to download data from the DTR.
For connecting and operating instructions of the Ethernet connection see PROCESS
REFRACTOMETER PR-23 INSTRUCTION MANUAL Section 12.
© Copyright Vaisala 2020. All rights reserved.
23
Page 24

Appendix to the Instructions Manual PR-23
24
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 25

5
Complying with documentation
and validation regulations
5.1 Documentation
When a pharmaceutical company purchases new measuring instruments, they must take
into account the documentation requirements covered by national and international laws
and directives, for example, the US Food and Drug Authority’s Code of Federal Regulations
(CFR). FDA’s validation requirements leave it up to the manufacturer to determine what
data is essential to prove control over their processes. Therefore, the requirements vary
from company to company, and each pharmaceutical company is responsible for dening
and maintaining its own documentation requirements list. Some areas to consider and their
Vaisala K-PATENTS solutions are presented in the sections below.
5.2 Qualication
The qualication action consists of proving and documenting that the equipment and
ancillary systems are properly installed, operating correctly, and producing veried results.
Qualication is a part of the validation process, but the individual qualication stages alone
do not constitute process validation.
Installation Qualication, Operational Qualication and Performance Qualication protocols
are normally required to document that the correct refractometer model and parts have been
ordered, delivered and installed according to Vaisala’s recommendations, and also to check
that the refractometer meets its performance specication and is able to reliably measure
typical samples using the selected measurement method. Users are able to create their
own protocols using the relevant information from this manual appendix and the product
manual, and/or using their own templates. The complete qualication process must be
fully documented.
5.3 Protocol acceptance by customer and list of
tests performed
A qualication protocol which provides details about the system, the scope and constraints
of the qualication, the qualication tests, test procedures and acceptance criteria should
be available for review and approval before the qualication begins. The protocol should
also contain an exception log to record any out of the specication results, investigation and
problem resolution. After the qualication, the test results must be reviewed and approved
before the instrument can be put into routine use.
© Copyright Vaisala 2020. All rights reserved.
25
Page 26

Appendix to the Instructions Manual PR-23
5.4 Electronic data management and data storage
The Code of Federal Regulations (CFR) FDA 21, Part 11 requires that pharmaceutical
companies use electronic (i.e. software-maintained) data recording and storage, rather
than paperwork. In case of instrument measurements, the code requires that every reading
taken with the instrument must be logged and permanently stored electronically, and the
data is password-protected ensuring alteration accountability (i.e. which operator makes
an alteration) and tracking.
Part 11 describes four basic system elements that must be addressed. They are:
●
Electronic signatures and tracking
●
Data storage and logs
●
Security
●
System validation.
5.5 Electronic signatures/audit trail
Data records must be linked to the relevant electronic signatures so that when accessed,
either electronically or through printout, the signatures will be openly displayed along with
the date and time of execution.
5.6 Record keeping
Data records must be stored in a format that the FDA can reasonably expect to be able to
read. These records must be retained for the length of time required by the predicate rule.
5.7 Security
System access can be restricted to authorized individuals using the lock on the Indicating
Transmitter door and password protected access to the transmitter and to the computer.
There are also four input switches behind the front panel of Indicating Transmitter. An input
switch can be congured to seal the calibration and to prevent access to the calibration
and to conguration, see PROCESS REFRACTOMETER PR-23 INSTRUCTION MANUAL,
Section 6.4.
The actions of these authorized individuals in relation to the data must be openly accounted
for throughout the audit trail.
5.8 System validation
The system must be validated to prove that it complies with the technical requirements of
Part 11. The Installation Qualication, Operation Qualication, and Performance Qualication
(IQ/OQ/PQ) should also be performed.
26
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 27

Complying with documentation and validation regulations
5.9 Vaisala K-PATENTS® refractometer system
adherence to Part 11
It is not possible to supply a system readily in compliance with Part 11. This is because the
requirements of Part 11 fall into two categories: those that are handled technically (through
software features), and those that are handled procedurally (such as through system
validation, SOPs, policies, etc.).
Part 11 applies to all computerized systems that create, modify, maintain, archive, or
retrieve records required by the FDA. Pharma Refractometer generates electronic records
via Ethernet connection. These records can be stored as digital les and printed out for
signature or led and maintained as hard copies. The computer les are subject to Part 11
regulation. The instrument parameter and conguration changes also fall into this category.
These computer les may be used in either of the two ways:
1. as a non-subject system by printing results, signing by hand, and maintaining
hard copies
2. as an electronic record-keeping system subject to Part 11 regulation.
Systems described by number 1 would be subject only to predicate rules, not Part 11.
Systems described by number 2 must comply with Part 11.
Please note: While Vaisala has taken account of the FDA Part 11 rules during development of
the Pharma Refractometer package and in the compilation of the instructions and guidelines
contained in this Instruction manual appendix, the system described has not been approved
or mandated by the FDA or any other government agencies. So all compliance responsibility
lies with the end user and Vaisala makes no claims that the completion of all the procedures
described here will exempt these companies or individuals from FDA sanctions.
5
© Copyright Vaisala 2020. All rights reserved.
27
Page 28

Appendix to the Instructions Manual PR-23
28
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 29

6
Onsite qualication protocols and
records: Installation Qualication
This Installation Qualication (IQ) involves documented verication of the complete
system: Pharma Refractometer PR-23-AC and Pharma Mini Flow Cell PMFC-HSS with
Ethernet connection, as installed and connected to a fractionation unit and a computer,
and in compliance with the approved design, the manufacturer’s recommendations and
user requirements.
6.1 Authorization and responsibilities
6.1.1 Documents and procedures
The following documents and procedures are inspected:
●
Scope and Procedure for Qualication
●
Report on Installation Qualication
●
Protocol for Installation Qualification
The authorized ofcial (client) hereby declares that the execution of the Installation
Qualication (IQ) for the Pharma Refractometer and Pharma Mini Flow Cell have been
approved in accordance with this document/log. The authorized ofcial is responsible for all
relevant matters in regard to the installation qualification.
Release by superior department:
Name:
Function:
Date:
Signature:
Initials:
Authorization by a higher-level authority is a prerequisite for carrying out the
qualication procedure. If no valid written authorization is available, terminate the
Installation Qualification.
© Copyright Vaisala 2020. All rights reserved.
29
Page 30

Appendix to the Instructions Manual PR-23
6.1.2 Authorized ofciator
Selection of the individual authorized to carry out the Installation Qualication of the Pharma
Refractometer system should be in accordance to their relevant ability to undertake the
procedure. The authorized ofciator’s signature is required for the next stage to validate
Date/Initials in the Installation Qualication log and reports.
Name:
Function:
Date:
Signature:
Initials:
6.1.3 Execution
As it is executed, each described step of the Installation Qualication requires initialing
and dating. If any deviations occur, the qualication must either be aborted or a detailed
explanation of the deviations must be entered in the subsequent “Deviation, evaluation,
corrective actions” logs and must be documented appropriately.
6.2 System
6.2.1 Qualifying the system
Location of the Pharma Refractometer Sensor and Pharma Mini Flow Cell:
Location of the Indicating Transmitter:
Device Serial Number Supplier Manufacturer
Pharma Refractometer:
Sensor PR-23-AC-62-HSS
Pharma Refractometer: Indicating
Transmitter DTR-M/U-GP-SC
Computer
Vaisala Oyj
Vaisala Oyj
30
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 31

Onsite qualication protocols and records: Installation Qualication
6.2.2 Manufacturers and suppliers
Full address of the manufacturers and suppliers:
Manufacturer: Supplier:
Vaisala Oyj
Postal address: P.O. Box 26,
FI-00421 Helsinki, Finland
Street address:
Vanha Nurmijärventie 21,
FI-01670 Vantaa, Finland
Tel. Int.+358 9 89491
Fax Int.+358 9 8949 2227
www.vaisala.com
6.3 IQ protocol
6.3.1 Scope of delivery
Description of requirements
Check that the delivery is complete and that all the listed instrument components and
accessories are included in the delivery.
6
Requirement acceptance values
Compliance with the component checklist “System components provided by Vaisala”,
included in the Manual Appendix (this document) Section 2.2.
Failure to meet delivery values
If any essential component is missing terminate the installation qualication and call your
support, otherwise check conditional pass and move with the IQ, inform your support.
Terminate the IQ.
*
Date Signature Pass
*Conditional pass:
Conditional Pass Fail
© Copyright Vaisala 2020. All rights reserved.
31
Page 32

Appendix to the Instructions Manual PR-23
6.3.2 Damage
Description of requirements
Inspection of all components and devices to check they are undamaged and functional.
Damage or malfunction detected
Terminate the IQ.
Report to:
*
Date Signature Pass
*Conditional pass:
6.4 Documentation
Description of requirements
Make sure that the Operating Instructions and all other required documentation are complete
and accessible.
Type of document Document/Revision No.
Instruction Manual for Inline
Refractometer
PR-23(-...-AX/FM/CS/IA/IF)
Appendix to Instruction
Manual
Operating Manual for:
Material Safety Data Sheet
for Cargille Refractive Index
Liquids
Conditional Pass Fail
Requested
Present Missing Not requested
IM-EN-PR23
IM- EN- PR23AC-VACC- A
32
Date Signature Pass
*Conditional pass:
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
*
Conditional Pass Fail
Page 33

Onsite qualication protocols and records: Installation Qualication
6.5 Operating environment
Description of requirements
Ensuring that the appropriate power supply and power switch are available.
Requirement acceptance values
An electrical power supply with a voltage and frequency of 100-230 VAC/50-60 Hz (Optional
24 VDC). A computer (PC, Mac, PDA or mainframe).
Failure to meet any of the acceptance values
A new environment must be established and the qualication performed again from Section
6.3.1 (of this document) onwards.
Date Signature Pass
*Conditional pass:
6.6 Installation
Requirement description
The authorized operator who in accordance with Section 6.2 (of this document), must read
the installation instructions in Section 3 (of this document).
6
*
Conditional Pass Fail
Requirement acceptance values
The relevant sections have been read.
Date Signature Pass
*Conditional pass:
*
Conditional Pass Fail
© Copyright Vaisala 2020. All rights reserved.
33
Page 34

Appendix to the Instructions Manual PR-23
6.7 Setting up the system components and devices
Description of requirements
The Pharma Refractometer system with Refractometer sensor, Pharma Mini Flow Cell and
Indicating Transmitter, are assembled and mounted correctly as described in the Section
3 (this document). Also the ancillary sample system is connected in accordance with the
Section 3 (this document). The ancillary equipment is switched on in accordance with the
corresponding operating manuals.
Requirement acceptance values
The system and devices are complete and have been set up in compliance with
the instructions.
Failure to meet the acceptance values
Terminate the IQ.
*
Date Signature Pass
*Conditional pass:
Conditional Pass Fail
34
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 35

Onsite qualication protocols and records: Installation Qualication
6.8 Electrical connections and wiring
Description of requirements
The frequency of the power supply must match the frequency indicated on the instrument’s
rating plate. The electrical wiring connections have been connected in accordance with
the instructions laid down in the PROCESS REFRACTOMETER PR-23 INSTRUCTION
MANUAL Section 12.
Requirement acceptance values
All electrical wiring connections have been connected in compliance with the instructions laid
down in the PROCESS REFRACTOMETER PR-23 INSTRUCTION MANUAL Section 12.
*
Date Signature Pass
*Conditional pass:
Description of requirements
Ethernet connections and wiring have been connected and set up in accordance with the
PROCESS REFRACTOMETER PR-23 INSTRUCTION MANUAL Section 12.
Conditional Pass Fail
6
Requirement acceptance values
The Ethernet connections comply with the PROCESS REFRACTOMETER PR-23
INSTRUCTION MANUAL Section 12.
*
Date Signature Pass
*Conditional pass:
Conditional Pass Fail
© Copyright Vaisala 2020. All rights reserved.
35
Page 36

Appendix to the Instructions Manual PR-23
6.9 Ethernet connection
Description of requirements
Ethernet connections and wiring have been connected and set up in accordance with the
PROCESS REFRACTOMETER PR-23 INSTRUCTION MANUAL Section 12.
Requirement acceptance values
The Ethernet connections comply with the PROCESS REFRACTOMETER PR-23
INSTRUCTION MANUAL Section 12.
*
Date Signature Pass
*Conditional pass:
Conditional Pass Fail
6.10 Initial check and switching the device on
Description of requirements
The initial check has been performed and the electrical power has been connected in
accordance with the PROCESS REFRACTOMETER PR-23 INSTRUCTION MANUAL
Section 5.1.1.
Requirement values
The corresponding screen displays occur in accordance with the PROCESS
REFRACTOMETER PR-23 INSTRUCTION MANUAL Section 5.1.1.
Failure to meet acceptance values
Terminate the IQ.
*
36
Date Signature Pass
*Conditional pass:
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Conditional Pass
Fail
Page 37

Onsite qualication protocols and records: Installation Qualication
6.11 Installation Qualication summary report
Successful completion of the preceding activities and checks indicates that this instrument
has been satisfactorily delivered and installed. This instrument has passed the Installation
Qualication and may now be submitted for Operational Qualification.
IQ completed by
Name:
Function:
Date:
Signature:
Signature:
IQ deviations approved by
Name:
Function:
Date:
Signature:
Signature:
6
IQ approved by
Name:
Function:
Date:
Signature:
Signature:
© Copyright Vaisala 2020. All rights reserved.
37
Page 38

Appendix to the Instructions Manual PR-23
Comments (including discrepancies)
38
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 39

7
Onsite qualication protocols and
records: Operational Qualication
Operational Qualication (OQ) is documented verication stating that the equipment and
systems, as installed for the rst time or after repairs and major incidents, perform as
intended throughout the required operating ranges. The OQ is to ensure that the Pharma
Refractometer meets predened specications, and all system components function
correctly and according to specications within a specic environment.
7.1 Individual module and system components check
Checking the operation of the refractometer as an individual module, and as a system that
comprises also of the Pharma Mini Flow Cell, the computer, the Ethernet connection and
Ancillary equipment such as fractionation unit.
●
Operational check on the refractometer consists of refractive index n
linearity and short-term repeatability and reproducibility verication tests with Cargille
standard refractive index n
●
In addition to the system components, testing functional challenge, testing the system
software operation, should be conducted.
●
Stage by stage operational procedure checking. A pre-determined set of instructions
can be input stage by stage into the system. The system responses are then
compared to the expected outcome of the instructions to determine any problems in
their fulfillment.
●
Sign off when successfully completed.
liquids.
D
accuracy,
D
7.2 Installation Qualication has been performed
successfully
Description of Requirement
An Installation Qualication has been performed for the system.
Requirement Acceptance values
The Installation Qualication has been carried out successfully with the required approval.
Date of Installation Qualification:
Performed by:
Do not proceed with the Operational Qualication until a valid Installation Qualication has
been successfully completed and signed off.
© Copyright Vaisala 2020. All rights reserved.
39
Page 40

Appendix to the Instructions Manual PR-23
7.3 Test procedure
The Operational Qualication of the system is performed in accordance with a set plan in
which the following points are tested and documented sequentially:
●
The required documents, measuring instruments, refractive index liquids, and required
cleaning materials are available
●
Functional checks and verication of the refractometer performance
●
Functional checks have been made for the ancillary equipment.
The authorized ofcial (client) hereby declares that the performance of the Operational
Qualication (OQ) for the Pharma Refractometer and Pharma Mini Flow Cell has been
approved in accordance with this document/protocol. The authorized ofcial is responsible
for all relevant matters in regard to the operational qualification.
Release by superior department:
Name:
Function:
Date:
Signature:
Initials:
Authorization by a higher-level authority is a prerequisite for carrying out the
qualication procedure. If no valid written authorization is available, terminate the
Operational Qualification.
7.4 Authorized ofciator
Selection of the individuals authorized to carry out the Operational Qualication of the
Pharma Refractometer system should be in accordance with their relevant ability to
undertake the procedure. The authorized ofciator’s signature is required for the next stage
to validate Date/Initials in the Operational Qualication log and reports.
Name:
Function:
40
Date:
Signature:
Initials:
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 41

7.5 System qualication
Onsite qualication protocols and records: Operational Qualication
Check that the system is the same as dened in the IQ, with no changes.
Denition of requirements
All system equipment remains the same as for the IQ and the ancillary equipment IQ is valid.
Date Signature Pass Conditional Pass Fail
Conditional pass:
7.6 Setting up the system components and devices
Description of requirements
The Pharma Refractometer system comprised of refractometer sensor, Pharma Mini Flow
Cell and Indicating Transmitter, is assembled and mounted correctly as described in the
Section 3 (this document). Initial startup checks for the Refractometer have been made
according to PROCESS REFRACTOMETER PR-23 INSTRUCTION MANUAL, Section 5.
Also the ancillary fractionation unit and the sample delivery system (if required) are
connected and the ancillary equipment is switched on and functional checks are made in
accordance with the corresponding operating manuals.
7
Requirement acceptance values
The system and devices are complete and have been set up in compliance with
the instructions.
Failure to meet any of the acceptance values
Terminate the OQ.
Date Signature Pass Conditional Pass Fail
Conditional pass:
© Copyright Vaisala 2020. All rights reserved.
41
Page 42

Appendix to the Instructions Manual PR-23
7.7 Instrument verication with sample holder and
refractive index liquids
Description of requirements
Refractometer, Sample Holder PR-1012 and a set of ve standard refractive index
liquids PR-2300 with Cargille Certication are allowed to be settled to laboratory
ambient temperature (between 20-30 °C, 77-86°F) 24 hours prior to commencement of
the qualification.
Requirement acceptance values
Refractometer, sample holder and Refractive index liquids positioned in the laboratory 24
hours prior to verication with the ambient temperature at between 20-30 °C (77-86°F).
Date Signature Pass Conditional Pass Fail
Conditional pass:
Description of requirements
The procedure is done with all ve liquids using a sample holder and verication instructions
at PROCESS REFRACTOMETER PR-23 INSTRUCTION MANUAL, Section 13.
Nominal R.I. values:
●
1.330
●
1.370
●
1.42 0
●
1.470
●
1.520
Requirement values
The verication results are OK for all samples and acceptance / deviation values (not
more than + 0.0004 of the nominal values) are received for each sample. The Instrument
Verication page in the browser for the complete verication test procedure shows
Verication result: pass.
Failure to meet acceptance values
Terminate the OQ.
Date Signature Pass Conditional Pass Fail
42
Conditional pass:
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 43

Onsite qualication protocols and records: Operational Qualication
7.8 Operational Qualication summary report
Successful completion of the preceding activities and checks indicates that this instrument
performs satisfactorily. The Operational Qualication has been accepted.
OQ completed by
Name:
Function:
Date:
Signature:
Signature:
OQ deviations approved by
Name:
Function:
Date:
Signature:
Signature:
7
OQ approved by
Name:
Function:
Date:
Signature:
Signature:
© Copyright Vaisala 2020. All rights reserved.
43
Page 44

Appendix to the Instructions Manual PR-23
Comments (including discrepancies)
44
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 45

8
Routine operation phase
After the instrument is qualied, it can be used to measure analytical data. A Standard
Operating Procedure (SOP) has to be written for the new instrument. Operational
instructions, maintenance and calibration should be included in the SOP. It is unnecessary
to copy the complete operation manual into the SOP. Writing down simple instructions
referencing the related manual sections is more effective. The particular tasks and the
frequency they should be performed during maintenance should be clearly stated in the
maintenance section. Tests required to verify the instrument, the acceptance criteria and the
frequency for each test should be covered in the calibration section of the SOP.
Denitions of major and minor repairs, which necessitate partial or full system re-qualication,
should be included as well. For example, the replacement of a Teon pad in the sample mixer
does not require a full re-qualication. Replacement of optical parts (prism) will warrant full
re-qualication.
Good system maintenance starts with the users. Proper care, which can be as simple as
a good system rinsing and clean up after use, will reduce the possibility of system failure
during runs and will extend the useful life of the instrument.
Maintain good usage and service records for the instrument for Good Manufacturing
Practice (GMP) purposes. Records of usage allow the users to be alerted to any system or
instrument calibration failure. The user may have to do an impact assessment to determine
whether the failure would have affected the reliability of the results generated by the system.
The service records will also provide useful information about the system, which may
simplify trouble shooting in some cases.
The GMP requirements dictate that the refractometer calibration verication (see Section
3.1) should be performed at suitable intervals in accordance with an established schedule.
Any instrument failing to meet established specications shall not be used. Each Pharma
Refractometer is recommended to have a calibration verication label applied with the
relevant status information on the system, date of the last calibration verication, who carried
out the verication and the scheduled date for the next verification.
© Copyright Vaisala 2020. All rights reserved.
45
Page 46

Appendix to the Instructions Manual PR-23
46
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 47

9
Preventive maintenance
The need for Pharma Refractometer regular maintenance is minimal, due to the construction
with no moving parts, no mechanical adjustments, no trimpots and with a solid-state light
source, see Section 7, PROCESS REFRACTOMETER PR-23 INSTRUCTION MANUAL.
The following checks should be performed for Mini Flow Cell at suitable intervals in
accordance with an established schedule:
●
Check the condition of the O-ring (PR-9244-USP O-ring 22.2x3.0 EPDM) of the
Pharma Mini Flow Cell
●
Check the condition of the two sanitary gaskets (PR-9236-USP EPDM) of the 0.5”
sanitary clamps
© Copyright Vaisala 2020. All rights reserved.
47
Page 48

Appendix to the Instructions Manual PR-23
48
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 49

10
Other documentation
You may want to include the following documents in your les concerning this Vaisala
K-PATENTS
●
●
●
●
®
instrument:
Delivery Data Sheet (supplied with the instrument)
Certicate of Traceability for Standard Refractive Index liquids
(supplied with the liquids PR-230 0)
Material Traceability Certicate of Compliance in accordance with EN 1024-3.1b.
Note: This document is delivered on request and it must be specied when ordering.
Vaisala ISO 9001 certificate
© Copyright Vaisala 2020. All rights reserved.
49
Page 50

Appendix to the Instructions Manual PR-23
Notes
50
Document/Revision No. IM-EN-PR23AC-VACC/A Effective: March 1, 2020
Page 51

Page 52

www.vaisala.com
 Loading...
Loading...