Page 1

Ultra Comfort SE™ support surface
1703
1704
Operations Manual
2017/05 D.0 1704-009-001 REV D www.stryker.com
Page 2

sample text
Page 3
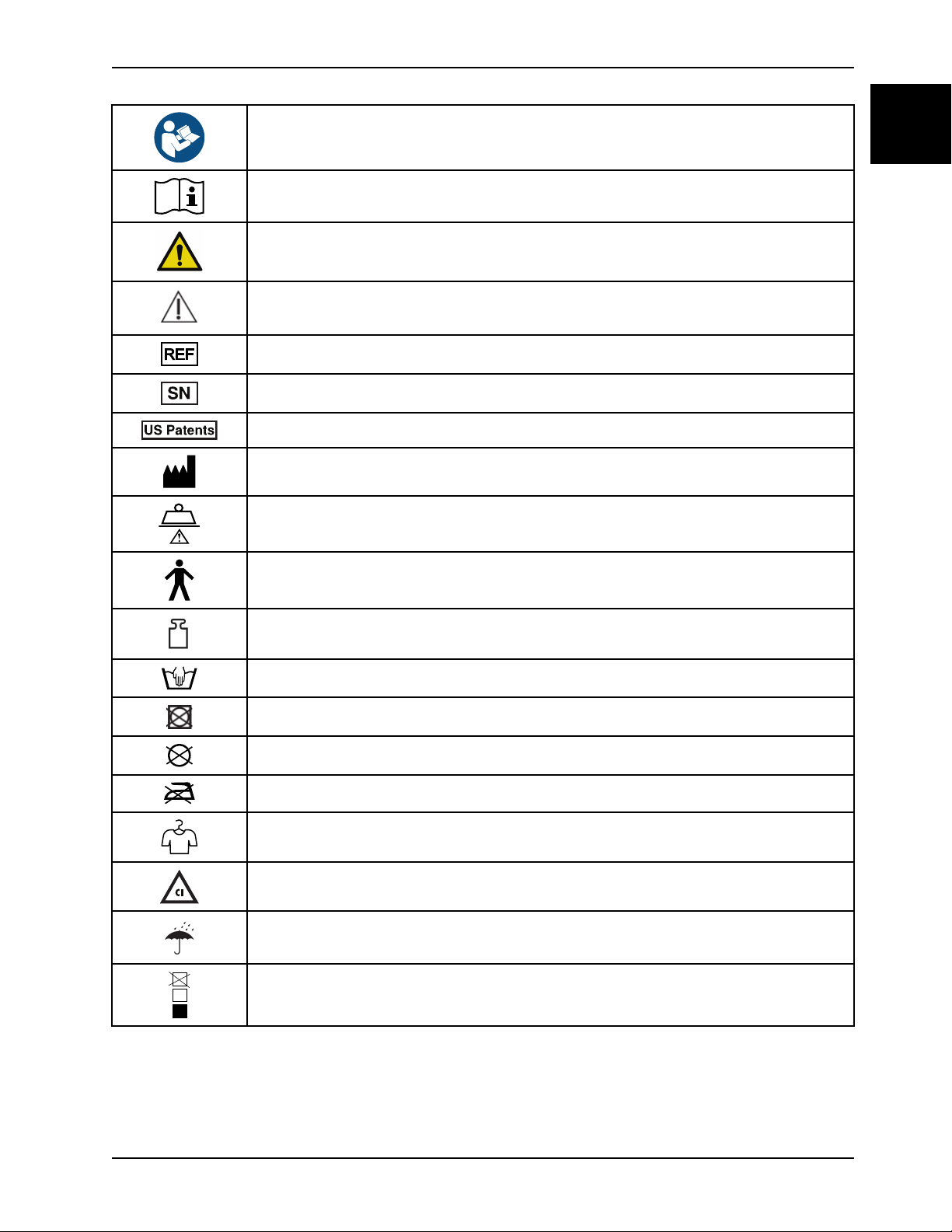
Symbols
15
Refer to instruction manual/booklet
Operating instructions / Consult instructions for use
General warning
Caution
Catalogue number
Serial number
For US Patents see www.stryker.com/patents
Manufacturer
English
EN
Safe working load
Type B applied part
Mass of product
Wash by hand
Do not tumble dry
Do not dry clean
Do not iron
Allow to completely air dry
Chlorinated bleach
Keep dry
Do not stack more than 15 high (applies to model 1703)
www.stryker.com 1704-009-001 REV D
Page 4
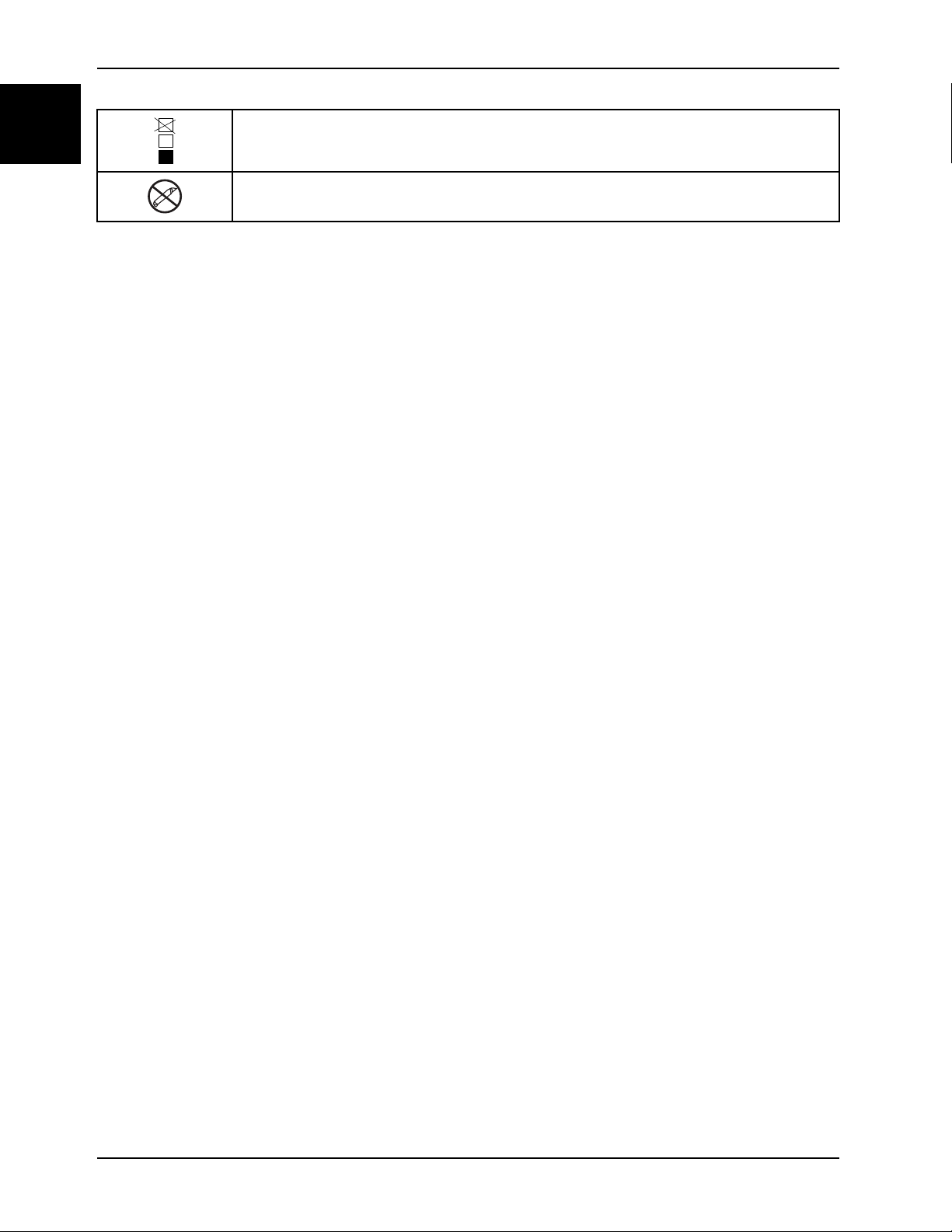
English
11
EN
Symbols
Do not stack more than 11 high (applies to model 1704)
Do not use sharp objects to open the package.
1704-009-001 REV D www.stryker.com
Page 5

Ultra Comfort SE™ non-powered support surface
1703
1704
Operations Manual
2017/05 D.0 1704-009-001 REV D www.stryker.com
Page 6

sample text
Page 7
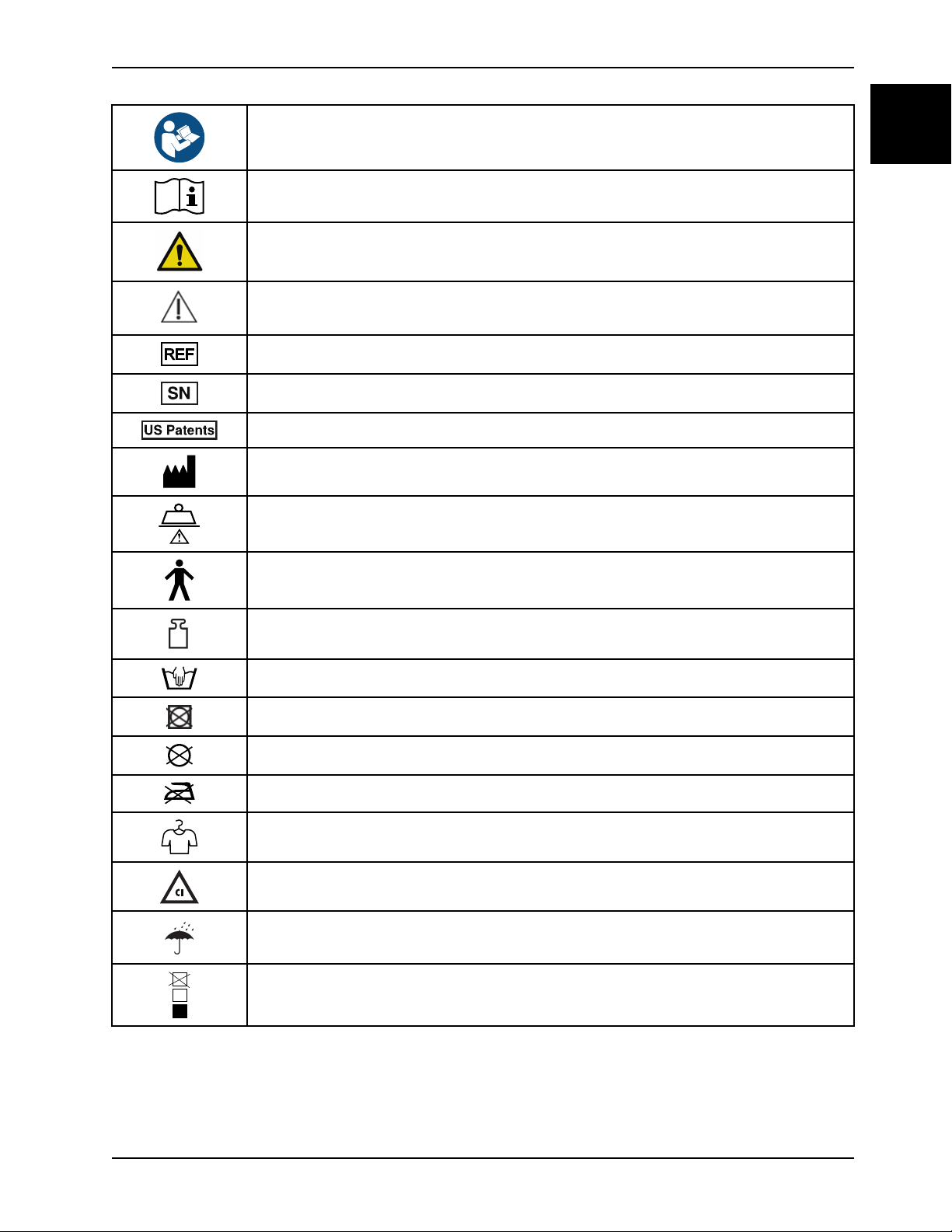
Symbols
15
Refer to instruction manual/booklet
Operating instructions / Consult instructions for use
General warning
Caution
Catalogue number
Serial number
For US Patents see www.stryker.com/patents
Manufacturer
English
EN
Safe working load
Type B applied part
Mass of product
Wash by hand
Do not tumble dry
Do not dry clean
Do not iron
Allow to completely air dry
Chlorinated bleach
Keep dry
Do not stack more than 15 high (applies to model 1703)
www.stryker.com 1704-009-001 REV D
Page 8
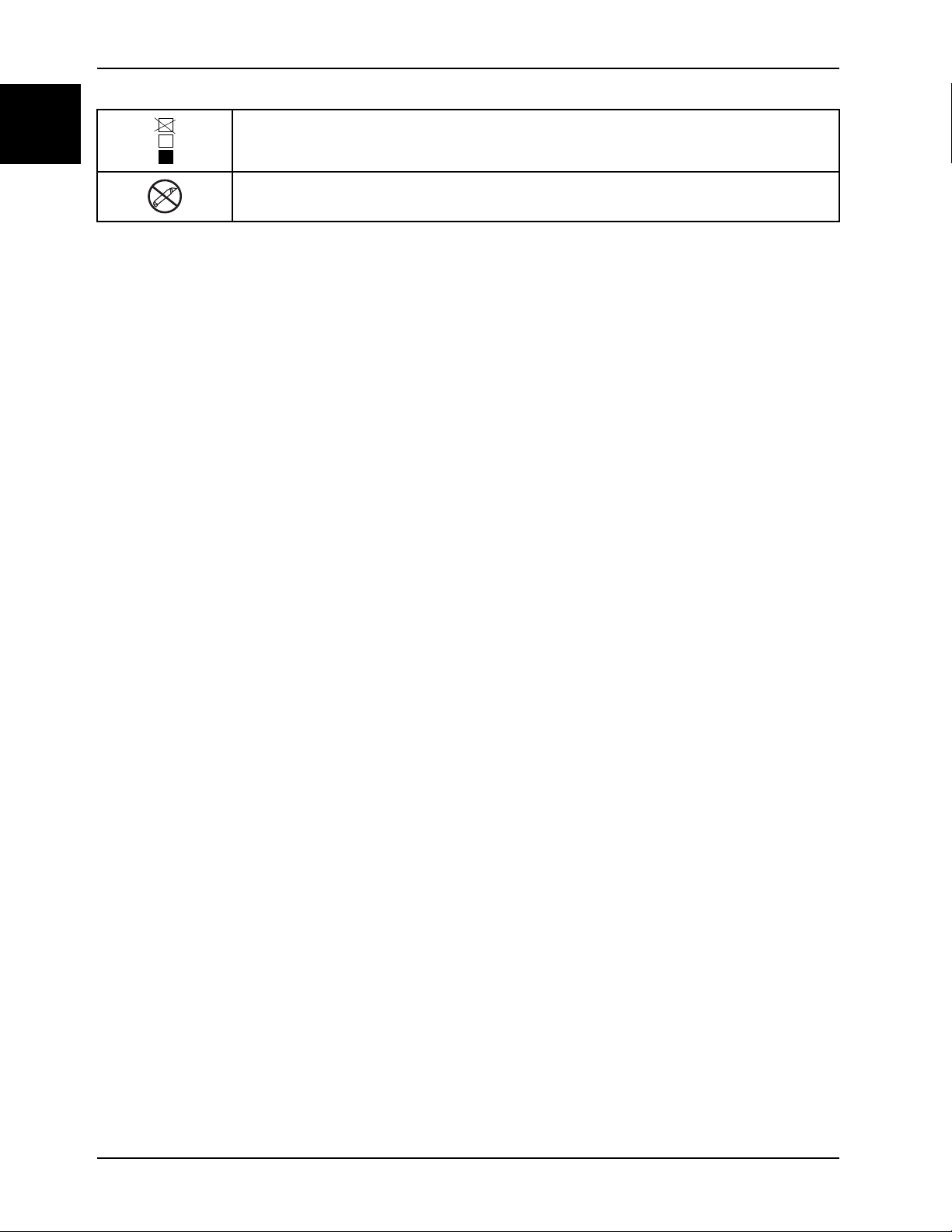
English
11
EN
Symbols
Do not stack more than 11 high (applies to model 1704)
Do not use sharp objects to open the package.
1704-009-001 REV D www.stryker.com
Page 9

Table of Contents
Warning/Caution/Note Definition ............................................................................................................... 1-2
Summary of safety precautions........................................................................................................... 1-2
Introduction........................................................................................................................................... 1-4
Product description........................................................................................................................... 1-4
Indications for use ............................................................................................................................ 1-4
Expected life................................................................................................................................... 1-4
Contraindications ............................................................................................................................. 1-5
Specifications.................................................................................................................................. 1-5
Contact information .......................................................................................................................... 1-5
Serial number location ...................................................................................................................... 1-6
Date of manufacture......................................................................................................................... 1-6
Operation ............................................................................................................................................. 1-7
Installing the support surface.............................................................................................................. 1-7
Transferring a patient from one patient support platform to another............................................................ 1-7
Managing incontinence and drainage ................................................................................................... 1-8
Selecting the appropriate CPR protocol ................................................................................................ 1-8
Support surface care ........................................................................................................................ 1-8
Preventive maintenance .................................................................................................................... 1-9
Applying Velcro® to the 0747 stretcher............................................................................................... 1-10
Warranty ............................................................................................................................................ 1-12
Warranty exclusion and damage limitations ......................................................................................... 1-12
To obtain parts and service .............................................................................................................. 1-12
Return authorization........................................................................................................................ 1-12
Damaged product........................................................................................................................... 1-12
International warranty clause ............................................................................................................ 1-12
English
EN
www.stryker.com 1704-009-001 REV D 1-1
Page 10

Warning/Caution/Note Definition
English
EN
The words WARNING, CAUTION, and NOTE carry special meanings and should be carefully reviewed.
WARNING
Alerts the reader about a situation which, if not avoided, could result in death or serious injury. It may also describe
potential serious adverse reactions and safety hazards.
CAUTION
Alerts the reader of a potentially hazardous situation which, if not avoided, may result in minor or moderate injury to the
user or patient or damage to the product or other property. This includes special care necessary for the safe and
effective use of the device and the care necessary to avoid damage to a device that may occur as a result of use or
misuse.
Note: Provides special information to make maintenance easier or important instructions clearer.
Summary of safety precautions
Always read and strictly follow the warnings and cautions listed on this page. Service only by qualified personnel.
WARNING
• Always check patient’s skin regularly. Consult a physician if erythema or skin breakdown occurs. Serious injury
could result if the patient’s skin condition is left untreated.
• Always use extra caution and supervision to help reduce the risk of a patient fall. Patient stability and siderail
coverage may be compromised with the use of an overlay.
• Always consider the use of siderails. The safe use of the support surface is maximized when used in conjunction
with siderails and there may be an increased risk of falls when siderails are not present. Serious injury or death can
result from the use (potential entrapment) or non-use (potential patient falls) of siderails or other restraints.
Consider local policies regarding the use of siderails. The physician operator, or responsible parties should
determine whether and how to use siderails based on each patient’s individual needs.
• Always use extra caution with a patient at risk of a fall (such as agitated or confused) to help reduce the likelihood
of a fall.
• Do not use the support surface on a larger or smaller frame width or length. This product is intended to match the
stretcher litter deck. This is to avoid the risk of the support surface sliding and patient injury.
• Always inspect for foreign objects between support surface and support platform. Foreign objects may cause the
support surface to slide on the support platform.
• Do not use the support surface when gaps are present. The risk of entrapment can develop when the support
surface is placed on frames that leave gaps of even a few inches between the support surface and the headboard,
footboard, and siderails.
• Do not stick needles into a support surface through the support surface cover. Holes may allow body fluids to enter
the inside (inner core) of the support surface and could cause cross-contamination, product damage, or product
malfunction.
• Do not use the support surface as a transfer device.
• Do not exceed the safe working load of the support surface. Excess weight could cause unpredictable safety and
performance of this product.
• Always make sure that the patient support platforms and their respective transfer gaps are adequate to support the
patient. If the space between the two patient support platforms is greater than 3 in. (7.6 cm), use a transfer bridge
to fill the gap. A transfer bridge is meant to ease transfer of a patient from one patient support platform to another.
• Always make sure that the opposite siderail is raised when placing a patient on the support surface to reduce the
risk of patient fall.
• Always monitor the patient condition at regular intervals for patient safety.
1-2 1704-009-001 REV D www.stryker.com
Page 11
Warning/Caution/Note Definition
Summary of safety precautions (Continued)
WARNING (CONTINUED)
• Do not wash the internal components of this support surface. Discard the support surface if a contamination is
found inside.
• Do not immerse the support surface.
• Do not allow liquid to pool on the support surface.
• Always inspect support surface covers (top and bottom) for tears, punctures, excessive wear, and misaligned
zippers before each use. If compromised immediately remove the support surface from service.
• Always make sure that you wipe each product with clean water and thoroughly dry each product after any
application of chemical solutions. Some chemical solutions are corrosive in nature and may cause damage to the
product if you use them improperly. If you do not properly rinse and dry the product, a corrosive residue may be left
on the surface of the product that may cause premature degradation of critical components. Failure to follow these
instructions may void your warranty.
• Always apply the supplied Velcro to the 0747 stretcher to make sure that the mattress is secure. Non-use may
result in patient harm due to mattress movement.
CAUTION
• Improper usage of the product can cause injury to the patient or operator. Operate the product only as described in
this manual.
• Do not modify the product or any components of the product. Modifying the product can cause unpredictable
operation resulting in injury to patient or operator. Modifying the product also voids its warranty.
• Always be aware of devices or equipment that are placed on the top of the support surface. Damage to the surface
may occur due to the weight of the equipment, heat generated by the equipment, or sharp edges on the equipment.
• Do not put overlays or accessories inside the cover to avoid the risk of reducing pressure redistribution
performance.
• Always evaluate the appropriate CPR protocol to be used with this product before operating.
• Do not allow liquid to seep into the zipper area or watershed cover barrier when washing the underside of the
support surface. Fluids allowed to come in contact with the zipper may leak into the support surface.
• Do not iron, dry-clean, or tumble dry the support surface covers.
• Do not power wash the support surface as this may damage the product.
• Always completely dry the support surface covers before storing, adding linens, or placing a patient on the surface.
Drying the product helps to prevent the performance of the product from being impaired.
• Do not over expose the covers to higher concentration chemical solutions as these may degrade the covers.
• Do not use accelerated hydrogen peroxides or quaternaries that contain glycol ethers as they may damage the
cover and reduce the legibility of the graphics.
• Failure to follow manufacturing instructions may also affect useful life of the support surface cover.
English
EN
www.stryker.com 1704-009-001 REV D 1-3
Page 12

Introduction
English
EN
This manual assists you with the operation or maintenance of your Stryker product. Read this manual before operating
or maintaining this product. Set methods and procedures to educate and train your staff on the safe operation or
maintenance of this product.
CAUTION
• Improper usage of the product can cause injury to the patient or operator. Operate the product only as described in
this manual.
• Do not modify the product or any components of the product. Modifying the product can cause unpredictable
operation resulting in injury to patient or operator. Modifying the product also voids its warranty.
Notes
• This manual is a permanent part of the product and should remain with the product even if the product is sold.
• Stryker continually seeks advancements in product design and quality. This manual contains the most current
product information available at the time of printing. There may be minor discrepancies between your product and
this manual. If you have any questions, contact Stryker Customer Service or Technical Support at 1-800-327-0770.
Product description
Ultra Comfort SE™ is a non-powered support surface that assists in improving human patient outcomes by
redistributing pressure. This product is available in two different widths, 26” or 30”. The dimensions are intended to
match the stretcher litter deck.
Ultra Comfort SE utilizes foam to redistribute pressure and to help with immersion.
Indications for use
This support surface is for use with human patients with existing or at risk of incurring pressure injuries. The safe
working load for Ultra Comfort SE is 700 lb (317 kg).
Ultra Comfort SE assists in the prevention and treatment of all pressure injury stages (including stages 1, 2, 3, 4,
Unstageable and Deep Tissue Pressure Injury or all pressure injuries) and is recommended to be implemented in
combination with clinical evaluation of risk factors and skin assessments made by a healthcare professional.
Ultra Comfort SE is intended to match the stretcher litter deck. This support surface is intended to be used for shortterm stays (treatment and recovery). Additionally, this product is not intended to be used within a home healthcare
setting.
Ultra Comfort SE shall be used with a support surface cover at all times. The support surface cover can interact with all
external skin.
Operators of this support surface include healthcare professionals (such as nurses, nurse aids, or doctors).
This support surface is for use by patients in an acute care setting. This may include emergency department, Preoperative, Transport, Endoscopy, GI, critical care, step down, progressive care, med/surg, sub-acute care, and post
anesthesia care unit (PACU), operating room, or other locations as prescribed. This product is not intended to be sterile,
include a measuring function, or be used in a home healthcare environment.
Expected life
The Ultra Comfort SE has a 1 year expected life under normal use, conditions, and with appropriate periodic
maintenance.
1-4 1704-009-001 REV D www.stryker.com
Page 13

Introduction
104 °F
(40 °C)
50 °F
(10 °C)
158 °F
(70 °C)
-40 °F
(-40 °C)
75%
30%
95%
0%
1060 hPa
700 hPa
1060 hPa
500 hPa
Contraindications
None known.
Specifications
700 lb (317 kg)
Safe working load
Model 1703-034-300 1704-034-300 1704-034-600
Length 76 in 193 cm 76 in 193 cm 76 in 193 cm
Width 30 in. 76 cm 30 in. 76 cm 26 in. 66 cm
Thickness 3 in. 7.6 cm 4 in. 10 cm 4 in. 10 cm
Product weight 6.5 lb 2.9 kg 8.5 lb 3.9 kg 7.3 lb 3.3 kg
Top cover material Thermoplastic polyurethane (TPU)
support surface material polyurethane foam
Product compliance without
fire barrier
Note: The patient must not exceed safe working load specified by the support surface.
USA 16 CFR 1632
CALTB117
English
EN
Environmental conditions
Operation Storage and transportation
Ambient temperature
Relative humidity (non-condensing)
Atmospheric pressure
Stryker reserves the right to change specifications without notice.
Contact information
Contact Stryker Customer Service or Technical Support at: 1-800-327-0770.
Stryker Medical
3800 E. Centre Avenue
Portage, MI 49002
USA
To view your operations or maintenance manual online, see https://techweb.stryker.com/.
Have the serial number (A) of your Stryker product available when calling Stryker Customer Service or Technical
Support. Include the serial number in all written communication.
www.stryker.com 1704-009-001 REV D 1-5
Page 14

Introduction
A
English
EN
Serial number location
Date of manufacture
The year of manufacture is the first four digits of the serial number.
1-6 1704-009-001 REV D www.stryker.com
Page 15

Operation
Installing the support surface
WARNING
• Always check patient’s skin regularly. Consult a physician if erythema or skin breakdown occurs. Serious injury
could result if the patient’s skin condition is left untreated.
• Always use extra caution and supervision to help reduce the risk of a patient fall. Patient stability and siderail
coverage may be compromised with the use of an overlay.
• Always consider the use of siderails. The safe use of the support surface is maximized when used in conjunction
with siderails and there may be an increased risk of falls when siderails are not present. Serious injury or death can
result from the use (potential entrapment) or non-use (potential patient falls) of siderails or other restraints.
Consider local policies regarding the use of siderails. The physician operator, or responsible parties should
determine whether and how to use siderails based on each patient’s individual needs.
• Always use extra caution with a patient at risk of a fall (such as agitated or confused) to help reduce the likelihood
of a fall.
• Do not use the support surface on a larger or smaller frame width or length. This product is intended to match the
stretcher litter deck. This is to avoid the risk of the support surface sliding and patient injury.
• Always inspect for foreign objects between support surface and support platform. Foreign objects may cause the
support surface to slide on the support platform.
• Do not use the support surface when gaps are present. The risk of entrapment can develop when the support
surface is placed on frames that leave gaps of even a few inches between the support surface and the headboard,
footboard, and siderails.
• Do not stick needles into a support surface through the support surface cover. Holes may allow body fluids to enter
the inside (inner core) of the support surface and could cause cross-contamination, product damage, or product
malfunction.
English
EN
CAUTION
• Always be aware of devices or equipment that are placed on the top of the support surface. Damage to the surface
may occur due to the weight of the equipment, heat generated by the equipment, or sharp edges on the equipment.
• Do not put overlays or accessories inside the cover to avoid the risk of reducing pressure redistribution
performance.
To install the support surface:
1. Make sure that the support surface properly fits the frame on that the product is being placed.
2. Align the low profile Velcro® on the bottom cover to the frame.
3. Place linens on the support surface per hospital protocols.
Transferring a patient from one patient support platform to another
WARNING
• Do not use the support surface as a transfer device.
• Do not stick needles into a support surface through the support surface cover. Holes may allow body fluids to enter
the inside (inner core) of the support surface and could cause cross-contamination, product damage, or product
malfunction.
• Do not exceed the safe working load of the support surface. Excess weight could cause unpredictable safety and
performance of this product.
www.stryker.com 1704-009-001 REV D 1-7
Page 16

Operation
English
EN
Transferring a patient from one patient support platform to another (Continued)
WARNING (CONTINUED)
• Always make sure that the patient support platforms and their respective transfer gaps are adequate to support the
patient. If the space between the two patient support platforms is greater than 3 in. (7.6 cm), use a transfer bridge
to fill the gap. A transfer bridge is meant to ease transfer of a patient from one patient support platform to another.
• Always make sure that the opposite siderail is raised when placing a patient on the support surface to reduce the
risk of patient fall.
To transfer the patient from one patient support surface to another:
Prerequisite: Follow hospital protocols required to transfer a patient from one support surface to another.
1. Position one patient support platform alongside the other patient support platform while minimizing the gap between
the two platforms.
2. Set the brakes to on for both patient support platforms.
3. Adjust the patient support platform heights so that they are level with one another.
4. Transfer the patient following all applicable safety rules and institution protocols for patient and operator safety.
Managing incontinence and drainage
WARNING
Always monitor the patient condition at regular intervals for patient safety.
You can use disposable diapers or incontinence pads to manage incontinence. Always provide appropriate skin care
after each incontinence episode.
Selecting the appropriate CPR protocol
CAUTION
Always evaluate the appropriate CPR protocol to be used with this product before operating.
Support surface care
WARNING
• Do not wash the internal components of this support surface. Discard the support surface if a contamination is
found inside.
• Do not immerse the support surface.
• Do not allow liquid to pool on the support surface.
• Always inspect support surface covers (top and bottom) for tears, punctures, excessive wear, and misaligned
zippers before each use. If compromised immediately remove the support surface from service.
1-8 1704-009-001 REV D www.stryker.com
Page 17

Operation
Support surface care (Continued)
WARNING (CONTINUED)
• Always make sure that you wipe each product with clean water and thoroughly dry each product after any
application of chemical solutions. Some chemical solutions are corrosive in nature and may cause damage to the
product if you use them improperly. If you do not properly rinse and dry the product, a corrosive residue may be left
on the surface of the product that may cause premature degradation of critical components. Failure to follow these
instructions may void your warranty.
CAUTION
• Do not allow liquid to seep into the zipper area or watershed cover barrier when washing the underside of the
support surface. Fluids allowed to come in contact with the zipper may leak into the support surface.
• Do not iron, dry-clean, or tumble dry the support surface covers.
• Do not power wash the support surface as this may damage the product.
• Always completely dry the support surface covers before storing, adding linens, or placing a patient on the surface.
Drying the product helps to prevent the performance of the product from being impaired.
• Do not over expose the covers to higher concentration chemical solutions as these may degrade the covers.
• Do not use accelerated hydrogen peroxides or quaternaries that contain glycol ethers as they may damage the
cover and reduce the legibility of the graphics.
• Failure to follow manufacturing instructions may also affect useful life of the support surface cover.
English
EN
The support surface cover is resistant to the following chemical solutions:
• Quaternaries (active ingredient - ammonium chloride)
• Phenols (active ingredient - o-phenylphenol)
• Chlorinated bleach solution (use 1 part bleach (5.25% sodium hypochlorite) to 10 parts of water which equals 4773
ppm of available chlorine (400 mL of a 5.25% bleach solution per 4000 mL water ))
• 70% isopropyl alcohol
Follow hospital protocol for support surface care in between patients to avoid the risk of cross-contamination and
infection.
Preventive maintenance
At a minimum, check all items listed during annual preventive maintenance for all Stryker Medical products. You may
need to perform preventive maintenance checks more frequently based on your level of product usage.
Remove product from service before performing preventive maintenance.
Note: Perform support surface care before inspection, if applicable.
Inspect the following items:
Zipper and covers (top and bottom) are free of tears, cracks, cuts, holes, or other openings
Internal components for signs of staining from fluid ingress or contamination by fully unzipping the covers
Labels for legibility, proper adherence, and integrity
Foam has not degraded or come apart
Product serial number:
Completed by:
www.stryker.com 1704-009-001 REV D 1-9
Date:
Page 18

Operation
A
English
EN
Applying Velcro® to the 0747 stretcher
WARNING
Always apply the supplied Velcro to the 0747 stretcher to make sure that the mattress is secure. Non-use may result in
patient harm due to mattress movement.
• (1) Fowler (A) Velcro pile (0785-034-007) • (2) Velcro pile (adhesive back) (B) (0785-034-005)
Note: This kit is to attach the mattress to the Model 0747 Transport Stretcher frame. If the Velcro pattern on the
mattress does not match the pattern on the frame, please follow these instructions.
Tools required
• Tape measure
Procedure
Note: Remove the product from service before you perform this upgrade.
1. Raise the product to the highest height position.
2. Remove and save the mattress.
3. Push down on the brake pedal to apply the brake.
4. Wipe the surface with isopropyl alcohol (70% alcohol).
5. Allow the surface to dry for at least two minutes.
6. Remove the backing on the fowler Velcro (A) and apply the Velcro to the surface of the fowler (Figure 1-1 on page
1-10).
Figure 1-1: Apply Velcro to the fowler
7. Press on the center of the Velcro and all edges to secure to the surface.
8. Allow the adhesive to cure for at least one hour before you return the product to service.
Note: For best results, allow the adhesive to cure for 24 hours before you return the product to service.
1-10 1704-009-001 REV D www.stryker.com
Page 19

Operation
B
Applying Velcro® to the 0747 stretcher (Continued)
9. Repeat steps 1-8 to replace the Velcro (B) at the foot end (Figure 1-2 on page 1-11).
Figure 1-2: Apply Velcro to the foot end
10. Verify proper operation of the product before you return it to service.
English
EN
www.stryker.com 1704-009-001 REV D 1-11
Page 20

Warranty
English
EN
Stryker Medical, a division of Stryker Corporation (“Stryker”), warrants that its Model 1703 and 1704 Ultra Comfort SE
products will be free from defects in material and workmanship for a period of 1 year for after date of delivery. Stryker’s
obligation under this warranty is expressly limited to supplying replacement parts and labor for, or replacing, at its option,
any product which is, in the sole discretion of Stryker, found to be defective. If requested by Stryker, products or parts
for which a warranty claim is made shall be returned prepaid to the factory. Any improper use or any alteration or repair
by others in such manner as in Stryker’s judgment affects the product materially and adversely shall void this warranty.
Any repair of Stryker products using parts not provided or authorized by Stryker shall void this warranty. No employee or
representative of Stryker is authorized to change this warranty in any way.
The foam assembly of mattress will naturally compress over time.
The above noted Warranty period applies only to the original purchaser of the Ultra Comfort SE products and begins on
the date of delivery to such original purchaser.
Warranty exclusion and damage limitations
The express warranty set forth herein is the only warranty applicable to the product. Any and all other warranties,
whether express or implied, including any implied warranty of merchantability or fitness for a particular purpose
are expressly excluded by Stryker. In no event shall Stryker be liable for incidental or consequential damages.
To obtain parts and service
Stryker products are supported by a nationwide network of dedicated Stryker Field Service Representatives. These
representatives are factory trained, available locally, and carry a substantial spare parts inventory to minimize repair
time. Simply call your local representative or call Stryker Customer Service at 1-800-327-0770.
Return authorization
Product cannot be returned without prior approval from the Stryker Customer Service Department. An authorization
number will be provided which must be printed on the returned product. Stryker reserves the right to charge shipping
and restocking fees on returned product. Special, modified, or discontinued products are not subject to return.
Damaged product
ICC Regulations require that claims for damaged product must be made within fifteen (15) days of receipt of the product.
Do not accept damaged shipments unless such damage is noted on the delivery receipt at the time of receipt. Upon
prompt notification, Stryker will file a freight claim with the appropriate carrier for damages incurred. Claims will be
limited in amount to the actual replacement cost. In the event that this information is not received by Stryker within the
fifteen (15) day period following the delivery of the product, or the damage was not noted on the delivery receipt at the
time of receipt, the customer will be responsible for payment of the original invoice in full within thirty (30) days of
receipt. Claims for any incomplete shipments must be made within thirty (30) days of invoice.
International warranty clause
This warranty reflects U.S. domestic policy. Warranty outside the U.S. may vary by country. Contact your local Stryker
Medical representative for additional information.
1-12 1704-009-001 REV D www.stryker.com
Page 21

Ultra Comfort SE™ surface de support non motorisée
1703
1704
Manuel d’utilisation
2017/05 D.0 1704-009-001 REV D www.stryker.com
Page 22

sample text
Page 23

Symboles
15
11
Consulter le manuel d'utilisation/notice
Mode d’emploi/Consulter le mode d’emploi
Avertissement général
Mise en garde
Numéro de référence
Numéro de série
Pour les brevets américains, consulter www.stryker.com/patents
Fabricant
Français
FR
Charge maximum admissible
Pièce appliquée de type B
Masse du produit
Nettoyer à la main
Ne pas sécher au sèche-linge
Ne pas nettoyer à sec
Ne pas repasser
Laisser sécher complètement à l’air
Eau de Javel
Maintenir au sec
Ne pas empiler plus de 15 dispositifs (s’applique au modèle 1703)
Ne pas empiler plus de 11 dispositifs (s’applique au modèle 1704)
www.stryker.com 1704-009-001 REV D
Page 24

Français
FR
Symboles
Ne pas utiliser un objet acéré pour ouvrir l’emballage.
Marquage CE
1704-009-001 REV D www.stryker.com
Page 25

Table des matières
Définition de « Avertissement », « Mise en garde » et « Remarque » ..................................................................2-2
Résumé des mesures de sécurité ........................................................................................................ 2-2
Introduction........................................................................................................................................... 2-5
Description du produit ....................................................................................................................... 2-5
Indications d'utilisation....................................................................................................................... 2-5
Durée de vie utile prévue ................................................................................................................... 2-6
Contre-indications ............................................................................................................................ 2-6
Caractéristiques techniques ............................................................................................................... 2-6
Informations de contact ..................................................................................................................... 2-6
Emplacement du numéro de série........................................................................................................ 2-7
Date de fabrication ........................................................................................................................... 2-7
Fonctionnement ..................................................................................................................................... 2-8
Installation de la surface de support..................................................................................................... 2-8
Transfert d’un patient d’une plate-forme de support à une autre................................................................ 2-9
Prise en charge de l’incontinence et du drainage.................................................................................... 2-9
Sélection du protocole de RCP approprié.............................................................................................. 2-9
Entretien de la surface de support ..................................................................................................... 2-10
Maintenance préventive................................................................................................................... 2-10
Mise en place du Velcro® sur le brancard 0747 ................................................................................... 2-11
Garantie ............................................................................................................................................. 2-13
Exclusion de garantie et limitations des dommages ............................................................................... 2-13
Pièces de rechange et service technique ............................................................................................ 2-13
Autorisation de retour...................................................................................................................... 2-13
Produit endommagé........................................................................................................................ 2-13
Clause de garantie internationale ...................................................................................................... 2-14
Français
FR
www.stryker.com 1704-009-001 REV D 2-1
Page 26

Définition de « Avertissement », « Mise en garde » et
« Remarque »
Les termes AVERTISSEMENT, MISE EN GARDE et REMARQUE ont une signification particulière et doivent faire l’objet
d’une lecture attentive.
Français
FR
AVERTISSEMENT
Avertit le lecteur d’une situation qui, si elle n’est pas évitée, pourrait entraîner la mort ou des blessures graves. Peut
également attirer l’attention sur l’existence potentielle d’effets indésirables graves ou de risques d’accident.
MISE EN GARDE
Avertit le lecteur d’une situation potentiellement dangereuse qui, si elle n’est pas évitée, peut causer des blessures
mineures ou modérées à l’utilisateur ou au patient ou endommager le matériel en question ou d’autres biens. Couvre
notamment les précautions à prendre afin d’assurer l’utilisation sûre et efficace du dispositif et d’éviter les dommages
qui pourraient découler de l’usage ou du mésusage du matériel.
Remarque : Fournit des informations spécifiques destinées à faciliter l’entretien ou à clarifier des instructions
importantes.
Résumé des mesures de sécurité
Toujours lire et respecter strictement les avertissements et les mises en garde indiqués sur cette page. Toute réparation
doit être effectuée exclusivement par du personnel qualifié.
AVERTISSEMENT
• Toujours vérifier la peau du patient régulièrement. Consulter un médecin si un érythème ou une plaie cutanée
apparaît. Une lésion grave peut se produire si l’affection cutanée du patient n’est pas traitée.
• Toujours faire preuve de prudence et de supervision particulières pour limiter le risque de chute du patient. La
stabilité du patient et la protection assurée par les barrières peuvent être compromises lors de l’utilisation d’un
surmatelas.
• Toujours penser à utiliser les barrières. La sécurité d’utilisation de la surface de support est optimale lorsque la
surface est employée avec les barrières ; il peut y avoir un risque accru de chutes lorsque les barrières ne sont pas
en place. Une blessure grave ou le décès peut résulter de l’utilisation (possibilité de coincement) ou de la nonutilisation (chute potentielle du patient) des barrières ou d’autres dispositifs de maintien. Tenir compte des
politiques locales en ce qui concerne l’utilisation des barrières. Le médecin opérateur ou les parties responsables
doivent déterminer si et comment les barrières doivent être utilisées en fonction des besoins spécifiques du patient.
• Toujours faire preuve d’une prudence particulière avec un patient présentant un risque de chute (patient agité ou
confus, par exemple) pour réduire le risque de chute.
• Ne pas utiliser la surface de support sur un châssis dont la largeur ou la longueur est plus grande ou plus petite. Ce
produit est destiné à correspondre à la plate-forme du plan de couchage du brancard. Cela permet d'éviter le
risque de glissement de la surface de support et de blessure du patient.
• Toujours rechercher la présence de corps étrangers entre la surface de support et la plate-forme de support. Les
corps étrangers peuvent provoquer le glissement de la surface de support sur la plate-forme de support.
• Ne pas utiliser la surface de support lorsque des espaces sont présents. Le risque de coincement peut apparaître
lorsque la surface de support est placée sur des châssis qui laissent des espaces, même de quelques centimètres,
entre la surface de support et la tête de lit, le pied du lit et les barrières.
• Ne pas planter d’aiguille dans une surface de support à travers la housse. La formation de trous risque de
provoquer l’infiltration de liquides corporels à l’intérieur de la surface de support (dans la partie interne), ce qui
pourrait entraîner une contamination croisée ou un dysfonctionnement du produit, ou endommager ce dernier.
• Ne pas utiliser la surface de support comme dispositif de transfert.
2-2 1704-009-001 REV D www.stryker.com
Page 27

Définition de « Avertissement », « Mise en garde » et «
Remarque »
Résumé des mesures de sécurité (Suite)
AVERTISSEMENT (SUITE)
• Ne pas dépasser la charge maximum admissible de la surface de support. Une charge excessive pourrait rendre
imprévisible la sécurité et les performances de ce produit.
• Toujours veiller à ce que les plates-formes de support du patient et les espaces de transfert respectifs entre ces
plates-formes soient adéquats pour supporter le patient. Si l’espace entre les deux plates-formes de support du
patient est supérieur à 7,6 cm, utiliser la planche de transfert pour combler l'espace. La planche de transfert est
destinée à faciliter le transfert d’un patient d’une plate-forme de support à une autre.
• Toujours s’assurer que la barrière opposée est levée lorsque l’on place un patient sur la surface de support afin de
réduire le risque de chute du patient.
• Toujours contrôler l’état du patient à des intervalles réguliers pour la sécurité du patient.
• Ne pas laver les composants internes de cette surface de support. Jeter la surface de support si une contamination
est observée à l’intérieur.
• Ne pas immerger la surface de support.
• Ne pas laisser de liquide s’accumuler sur la surface de support.
• Toujours inspecter les housses de la surface de support (supérieure et inférieure) pour déceler toute déchirure,
perforation, usure excessive, ainsi que des bords de fermeture éclair non alignés, avant chaque utilisation. Si
l'intégrité de la surface de support est compromise, la mettre immédiatement hors service.
• Toujours veiller à rincer chaque produit à l’eau propre et à sécher soigneusement chaque produit après toute
application de solutions chimiques. Certaines solutions chimiques sont de nature corrosive et peuvent endommager
le produit si elles ne sont pas utilisées correctement. Si le produit n’est pas rincé et séché correctement, un résidu
corrosif peut rester sur la surface du produit et peut entraîner une dégradation prématurée des composants
essentiels. Le non-respect de ces instructions peut annuler la garantie.
• Toujours mettre le Velcro fourni en place sur le brancard 0747 afin d'assurer que le matelas est bien fixé. Si le
Velcro n’est pas utilisé, cela peut entraîner des blessures chez le patient en cas de mouvement du matelas.
Français
FR
MISE EN GARDE
• L’utilisation incorrecte du produit est susceptible de causer des blessures au patient ou à l’utilisateur. Utiliser le
produit uniquement de la manière décrite dans ce manuel.
• Ne pas modifier le produit ni aucun de ses composants. Toute modification du produit peut entraîner un
fonctionnement imprévisible, susceptible de causer des blessures au patient ou à l’utilisateur. La garantie du
produit serait en outre invalidée par toute modification du produit.
• Toujours faire attention aux dispositifs ou équipements qui sont déposés sur la surface de support. Un
endommagement de la surface peut se produire à cause du poids de l’équipement, de la chaleur générée par
l’équipement ou des bords tranchants de l’équipement.
• Ne pas placer de surmatelas ou d’accessoires à l’intérieur de la housse pour éviter le risque de réduire la
performance de redistribution de la pression.
• Toujours déterminer le protocole de RCP approprié à appliquer avec ce produit avant son utilisation.
• Ne pas laisser de liquide s’infiltrer dans la zone de la fermeture éclair ou dans le rabat de la fermeture éclair lors
du lavage du côté inférieur de la surface de support. Les liquides qui parviennent à entrer en contact avec la
fermeture éclair peuvent pénétrer dans la surface de support.
• Ne pas repasser, ni nettoyer à sec, ni sécher au sèche-linge les housses de la surface de support.
• Ne pas laver la surface de support sous pression sous risque d'endommager le produit.
www.stryker.com 1704-009-001 REV D 2-3
Page 28

Français
FR
Définition de « Avertissement », « Mise en garde » et «
Remarque »
Résumé des mesures de sécurité (Suite)
MISE EN GARDE (SUITE)
• Toujours sécher complètement les housses de la surface de support avant de les stocker, de poser des draps ou
d’installer un patient sur la surface de support. Le séchage du produit aide à éviter une altération de la
performance du produit.
• Ne pas surexposer les housses à des solutions chimiques de concentration plus élevée car ces solutions peuvent
dégrader les housses.
• Ne pas utiliser de peroxydes d’hydrogène accélérés ou de mélanges quaternaires contenant des éthers glycoliques,
car ils peuvent endommager la housse et réduire la lisibilité des éléments graphiques.
• Le non-respect des instructions de fabrication peut également avoir un impact sur la durée de vie utile de la housse
de la surface de support.
2-4 1704-009-001 REV D www.stryker.com
Page 29

Introduction
Ce manuel vous aide à utiliser ou entretenir votre produit Stryker. Lire ce manuel avant d’utiliser ce produit ou d’en
effectuer la maintenance. Il convient d'établir des procédures et techniques visant à éduquer et à former le personnel
quant au fonctionnement et à l’entretien sécuritaires de ce produit.
MISE EN GARDE
• L’utilisation incorrecte du produit est susceptible de causer des blessures au patient ou à l’utilisateur. Utiliser le
produit uniquement de la manière décrite dans ce manuel.
• Ne pas modifier le produit ni aucun de ses composants. Toute modification du produit peut entraîner un
fonctionnement imprévisible, susceptible de causer des blessures au patient ou à l’utilisateur. La garantie du
produit serait en outre invalidée par toute modification du produit.
Remarques :
• Ce manuel doit être considéré comme faisant partie du produit et doit l’accompagner à tout moment, même en cas
de vente ultérieure du produit.
• Stryker cherche continuellement à améliorer le design et la qualité de ses produits. Ce manuel contient les
informations produit les plus récentes disponibles au moment de l’impression. Il peut y avoir de légères divergences
entre le produit et ce manuel. Pour toute question, contacter le service clientèle ou le support technique de Stryker
au +1-800-327-0770.
Description du produit
Ultra Comfort SE™ est une surface de support non motorisée qui permet d’améliorer les résultats pour les patients
humains en redistribuant la pression. Le produit est disponible en deux largeurs : 66 cm ou 76 cm. Les dimensions sont
destinées à correspondre à celles de la plate-forme du plan de couchage du brancard.
Ultra Comfort SE emploie de la mousse pour redistribuer la pression et faciliter l’immersion.
Indications d'utilisation
Français
FR
Cette surface de support est prévue pour être utilisée chez les patients humains présentant des plaies de pression
existantes ou susceptibles d’en développer. La charge maximale admissible pour Ultra Comfort SE est de 317 kg.
Ultra Comfort SE aide à prévenir et à traiter toutes les plaies de pression quel que soit leur stade (y compris stades 1, 2,
3, 4, inclassable et plaies de pression des tissus profonds ou toutes les plaies de pression) et son utilisation est
recommandée en association avec une évaluation clinique des facteurs de risque et des évaluations de la peau
réalisées par un professionnel de santé.
Les dimensions de Ultra Comfort SE sont destinées à correspondre à celles de la plate-forme du plan de couchage du
brancard. La surface de support est destinée à être utilisée pour les séjours à court terme (traitement et récupération).
De plus, ce produit n'est pas destiné à être utilisé dans un environnement de soins de santé à domicile.
Ultra Comfort SE doit être utilisé avec une housse de surface de support à tout moment. La housse de la surface de
support peut interagir avec toute la peau externe.
Les opérateurs de cette surface de support sont, entre autres, des professionnels de santé (tels que des infirmiers, des
aides-soignants ou des médecins).
Cette surface de support est destinée aux patients dans les environnements de soins actifs. Il peut s'agir du service des
urgences, de soins préopératoires, de soins pendant le transport, du service d'endoscopie, de soins de santé gastrointestinale, de l'unité de soins intensifs, de soins intermédiaires, de soins gradués, de soins médicaux/chirurgicaux, de
soins pour affections subaiguës et de l'unité de soins post-anesthésie (USPA), de soins en salle d'opération ou d'autres
lieux, selon les indications. Ce produit n'est pas destiné à être stérile, à inclure une fonctionnalité de mesure ou à être
utilisé dans un environnement de soins de santé à domicile.
www.stryker.com 1704-009-001 REV D 2-5
Page 30

Français
104 °F
(40 °C)
50 °F
(10 °C)
140 °F
(60 °C)
-40 °F
(-40 °C)
75%
30%
95%
10%
1060 hPa
700 hPa
1060 hPa
500 hPa
FR
Introduction
Durée de vie utile prévue
Le Ultra Comfort SE a une durée de vie utile prévue de 1 an dans des conditions d’utilisation normales et en respectant
l'entretien périodique approprié.
Contre-indications
Aucune connue.
Caractéristiques techniques
Charge maximum admissible
317 kg (700 livres)
Remarque : Le patient ne doit pas dépasser la charge maximale admissible spécifiée
pour la surface de support.
Modèle
1703-034-300 1704-034-300 1704-034-600
Longueur 76 po. 193 cm 76 po. 193 cm 76 po. 193 cm
Largeur 30 po. 76 cm 30 po. 76 cm 26 po. 66 cm
Épaisseur
3 po. 7,6 cm
4 po.
10 cm
4 po.
10 cm
Poids du produit 6,5 livres 2,9 kg 8,5 livres 3,9 kg 7,3 livres 3,3 kg
Matériau de la housse
Polyuréthane thermoplastique (TPU)
supérieure
Matériau du matelas Mousse de polyuréthane
Conformité de protection antifeu du produit
Conditions ambiantes Fonctionnement
USA 16 CFR 1632
CALTB117
Stockage et transport
Température ambiante
Humidité relative (sans
condensation)
Pression atmosphérique
Stryker se réserve le droit de modifier ces caractéristiques sans préavis.
Informations de contact
Contacter le service clientèle ou le support technique de Stryker au +1-800-327-0770.
2-6 1704-009-001 REV D www.stryker.com
Page 31

Introduction
A
Informations de contact (Suite)
Stryker Medical
3800 E. Centre Avenue
Portage, MI 49002
États-Unis
Pour consulter votre mode d'emploi ou votre manuel d'entretien en ligne, rendez-vous sur https://techweb.stryker.com/.
Avoir le numéro de série (A) du produit Stryker à disposition avant d’appeler le service clientèle ou le support technique
de Stryker. Inclure le numéro de série dans toutes les communications écrites.
Emplacement du numéro de série
Français
FR
Date de fabrication
Les quatre premiers chiffres du numéro de série correspondent à l'année de fabrication.
www.stryker.com 1704-009-001 REV D 2-7
Page 32

Français
FR
Fonctionnement
Installation de la surface de support
AVERTISSEMENT
• Toujours vérifier la peau du patient régulièrement. Consulter un médecin si un érythème ou une plaie cutanée
apparaît. Une lésion grave peut se produire si l’affection cutanée du patient n’est pas traitée.
• Toujours faire preuve de prudence et de supervision particulières pour limiter le risque de chute du patient. La
stabilité du patient et la protection assurée par les barrières peuvent être compromises lors de l’utilisation d’un
surmatelas.
• Toujours penser à utiliser les barrières. La sécurité d’utilisation de la surface de support est optimale lorsque la
surface est employée avec les barrières ; il peut y avoir un risque accru de chutes lorsque les barrières ne sont pas
en place. Une blessure grave ou le décès peut résulter de l’utilisation (possibilité de coincement) ou de la nonutilisation (chute potentielle du patient) des barrières ou d’autres dispositifs de maintien. Tenir compte des
politiques locales en ce qui concerne l’utilisation des barrières. Le médecin opérateur ou les parties responsables
doivent déterminer si et comment les barrières doivent être utilisées en fonction des besoins spécifiques du patient.
• Toujours faire preuve d’une prudence particulière avec un patient présentant un risque de chute (patient agité ou
confus, par exemple) pour réduire le risque de chute.
• Ne pas utiliser la surface de support sur un châssis dont la largeur ou la longueur est plus grande ou plus petite. Ce
produit est destiné à correspondre à la plate-forme du plan de couchage du brancard. Cela permet d'éviter le
risque de glissement de la surface de support et de blessure du patient.
• Toujours rechercher la présence de corps étrangers entre la surface de support et la plate-forme de support. Les
corps étrangers peuvent provoquer le glissement de la surface de support sur la plate-forme de support.
• Ne pas utiliser la surface de support lorsque des espaces sont présents. Le risque de coincement peut apparaître
lorsque la surface de support est placée sur des châssis qui laissent des espaces, même de quelques centimètres,
entre la surface de support et la tête de lit, le pied du lit et les barrières.
• Ne pas planter d’aiguille dans une surface de support à travers la housse. La formation de trous risque de
provoquer l’infiltration de liquides corporels à l’intérieur de la surface de support (dans la partie interne), ce qui
pourrait entraîner une contamination croisée ou un dysfonctionnement du produit, ou endommager ce dernier.
MISE EN GARDE
• Toujours faire attention aux dispositifs ou équipements qui sont déposés sur la surface de support. Un
endommagement de la surface peut se produire à cause du poids de l’équipement, de la chaleur générée par
l’équipement ou des bords tranchants de l’équipement.
• Ne pas placer de surmatelas ou d’accessoires à l’intérieur de la housse pour éviter le risque de réduire la
performance de redistribution de la pression.
Pour installer la surface de support :
1. Veiller à ce que la surface de support soit parfaitement adaptée au châssis sur lequel le produit doit être placé.
2. Aligner le Velcro® bas profil de la housse inférieure sur le châssis.
3. Placer les draps sur la surface de support selon les protocoles hospitaliers.
2-8 1704-009-001 REV D www.stryker.com
Page 33

Fonctionnement
Transfert d’un patient d’une plate-forme de support à une autre
AVERTISSEMENT
• Ne pas utiliser la surface de support comme dispositif de transfert.
• Ne pas planter d’aiguille dans une surface de support à travers la housse. La formation de trous risque de
provoquer l’infiltration de liquides corporels à l’intérieur de la surface de support (dans la partie interne), ce qui
pourrait entraîner une contamination croisée ou un dysfonctionnement du produit, ou endommager ce dernier.
• Ne pas dépasser la charge maximum admissible de la surface de support. Une charge excessive pourrait rendre
imprévisible la sécurité et les performances de ce produit.
• Toujours veiller à ce que les plates-formes de support du patient et les espaces de transfert respectifs entre ces
plates-formes soient adéquats pour supporter le patient. Si l’espace entre les deux plates-formes de support du
patient est supérieur à 7,6 cm, utiliser la planche de transfert pour combler l'espace. La planche de transfert est
destinée à faciliter le transfert d’un patient d’une plate-forme de support à une autre.
• Toujours s’assurer que la barrière opposée est levée lorsque l’on place un patient sur la surface de support afin de
réduire le risque de chute du patient.
Pour transférer un patient entre deux surfaces de support :
Condition préalable : Respecter les protocoles hospitaliers relatifs au transfert de patient entre deux surfaces de
support.
Français
FR
1. Placer une plate-forme de support de patient le long d’une autre plate-forme de support, en veillant à minimiser
l'espace entre elles.
2. Serrer les freins sur les deux plate-formes de support de patient.
3. Régler les deux plate-formes de support de patient à la même hauteur.
4. Pour assurer la sécurité du patient et de l’opérateur, observer toutes les règles de sécurité et protocoles hospitaliers
en vigueur lors du transfert du patient.
Prise en charge de l’incontinence et du drainage
AVERTISSEMENT
Toujours contrôler l’état du patient à des intervalles réguliers pour la sécurité du patient.
Il est possible d’utiliser des couches jetables ou des serviettes pour incontinent pour prendre en charge l’incontinence.
Toujours dispenser les soins cutanés appropriés après chaque épisode d’incontinence.
Sélection du protocole de RCP approprié
MISE EN GARDE
Toujours déterminer le protocole de RCP approprié à appliquer avec ce produit avant son utilisation.
www.stryker.com 1704-009-001 REV D 2-9
Page 34

Français
FR
Fonctionnement
Entretien de la surface de support
AVERTISSEMENT
• Ne pas laver les composants internes de cette surface de support. Jeter la surface de support si une contamination
est observée à l’intérieur.
• Ne pas immerger la surface de support.
• Ne pas laisser de liquide s’accumuler sur la surface de support.
• Toujours inspecter les housses de la surface de support (supérieure et inférieure) pour déceler toute déchirure,
perforation, usure excessive, ainsi que des bords de fermeture éclair non alignés, avant chaque utilisation. Si
l'intégrité de la surface de support est compromise, la mettre immédiatement hors service.
• Toujours veiller à rincer chaque produit à l’eau propre et à sécher soigneusement chaque produit après toute
application de solutions chimiques. Certaines solutions chimiques sont de nature corrosive et peuvent endommager
le produit si elles ne sont pas utilisées correctement. Si le produit n’est pas rincé et séché correctement, un résidu
corrosif peut rester sur la surface du produit et peut entraîner une dégradation prématurée des composants
essentiels. Le non-respect de ces instructions peut annuler la garantie.
MISE EN GARDE
• Ne pas laisser de liquide s’infiltrer dans la zone de la fermeture éclair ou dans le rabat de la fermeture éclair lors
du lavage du côté inférieur de la surface de support. Les liquides qui parviennent à entrer en contact avec la
fermeture éclair peuvent pénétrer dans la surface de support.
• Ne pas repasser, ni nettoyer à sec, ni sécher au sèche-linge les housses de la surface de support.
• Ne pas laver la surface de support sous pression sous risque d'endommager le produit.
• Toujours sécher complètement les housses de la surface de support avant de les stocker, de poser des draps ou
d’installer un patient sur la surface de support. Le séchage du produit aide à éviter une altération de la
performance du produit.
• Ne pas surexposer les housses à des solutions chimiques de concentration plus élevée car ces solutions peuvent
dégrader les housses.
• Ne pas utiliser de peroxydes d’hydrogène accélérés ou de mélanges quaternaires contenant des éthers glycoliques,
car ils peuvent endommager la housse et réduire la lisibilité des éléments graphiques.
• Le non-respect des instructions de fabrication peut également avoir un impact sur la durée de vie utile de la housse
de la surface de support.
La housse de la surface de support résiste aux solutions chimiques suivantes :
• Quaternaires (substance active : chlorure d’ammonium)
• Phénoliques (substance active : o-phénylphénol)
• Solution d'eau de Javel (utiliser 1 partie d'eau de Javel [hypochlorite de sodium à 5,25 %] pour 10 parties d'eau, ce
qui équivaut à 4 773 ppm de chlore actif [400 ml d'une solution d'eau de Javel à 5,25 % pour 4 000 ml d'eau])
• Alcool isopropylique à 70 %
Toujours respecter le protocole hospitalier pour l'entretien de la surface de support entre deux patients pour éviter le
risque de contamination croisée et d’infection.
Maintenance préventive
Au minimum, vérifier tous les éléments mentionnés pendant l’entretien préventif annuel pour tous les produits Stryker
Medical. Il peut être nécessaire d’effectuer les vérifications d’entretien préventif plus fréquemment en fonction du degré
d’utilisation du produit.
Mettre le produit hors service avant de procéder à l’entretien préventif.
2-10 1704-009-001 REV D www.stryker.com
Page 35

Fonctionnement
Maintenance préventive (Suite)
Remarque : Procéder à l’entretien de la surface de support avant l’inspection, le cas échéant.
Inspecter les éléments suivants :
La fermeture éclair et les housses (supérieure et inférieure) sont exemptes de déchirures, fissures, entailles,
trous ou autres ouvertures.
Les composants internes ne présentent pas de signes de taches liées à la pénétration de liquide ou la
contamination (vérification avec les housses entièrement ouvertes).
Bonne lisibilité, adhésion et intégrité des étiquettes
La mousse ne n’est pas dégradée ni détachée
Numéro de série du produit :
Effectué par :
Date :
Mise en place du Velcro® sur le brancard 0747
AVERTISSEMENT
Toujours mettre le Velcro fourni en place sur le brancard 0747 afin d'assurer que le matelas est bien fixé. Si le Velcro
n’est pas utilisé, cela peut entraîner des blessures chez le patient en cas de mouvement du matelas.
• (1) Partie velours du Velcro pour relève-buste (A)
(0785-034-007)
Remarque : Ce kit sert à fixer le matelas au châssis du brancard de transport modèle 0747. Si le schéma du Velcro sur
le matelas ne correspond pas à celui sur le châssis, suivre ces instructions.
• (2) Partie velours du Velcro (dos adhésif) (B) (0785-034-
005)
Français
FR
Outils requis
• Mètre à ruban
Procédure
Remarque : Retirer le produit du service avant de réaliser cette mise à niveau.
1. Élever le produit à la hauteur maximum.
2. Enlever et mettre de côté le matelas.
3. Appuyer sur la pédale d’enclenchement du frein pour appliquer le frein.
4. Essuyer la surface avec de l'alcool isopropylique (alcool à 70 %).
5. Laisser sécher la surface pendant au moins deux minutes.
www.stryker.com 1704-009-001 REV D 2-11
Page 36

Français
A
B
FR
Fonctionnement
Mise en place du Velcro® sur le brancard 0747 (Suite)
6. Retirer le dos adhésif du Velcro pour relève-buste (A) et appliquer le Velcro sur la surface du relève-buste (Figure
2-1 à la page 2-12).
Figure 2-1 : Mettre le Velcro en place sur le relève-buste
7. Appuyer sur la partie centrale du Velcro et sur tous les bords afin de le fixer à la surface.
8. Laisser durcir l'adhésif pendant au moins une heure avant de remettre le produit en service.
Remarque : Pour obtenir un résultat optimal, laisser durcir l'adhésif pendant 24 heures avant de remettre le produit
en service.
9. Répéter les étapes 1 à 8 pour remplacer le Velcro (B) du côté pieds (Figure 2-2 à la page 2-12).
Figure 2-2 : Mettre le Velcro en place sur le côté pieds
10. Vérifier le fonctionnement correct du produit avant de le remettre en service.
2-12 1704-009-001 REV D www.stryker.com
Page 37

Garantie
Stryker Medical, division de Stryker Corporation (« Stryker »), garantit que son produit Ultra Comfort SE modèles 1703
et 1704 sera exempt de défaut matériel et de fabrication pendant une période de 1 an à compter de la date de livraison.
L’obligation de Stryker en vertu de la présente garantie se limite expressément à la fourniture de pièces de rechange et
de main-d’œuvre ou au remplacement, au gré de la société, de tout produit que Stryker, à sa seule discrétion, aura jugé
défectueux. Le cas échéant, à la demande de Stryker, tout produit ou pièce faisant l’objet d’une réclamation de
garantie doit être renvoyé en port payé à l’usine. Tout usage incorrect ou toute modification ou réparation réalisée par
un tiers ayant, selon l’avis de Stryker, un effet matériel et indésirable sur le produit, annulera la présente garantie. Toute
réparation de produits Stryker effectuée avec des pièces non fournies ou non agréées par Stryker annulera cette
garantie. Aucun employé ou représentant de Stryker n’est autorisé à modifier la présente garantie de quelque manière
que ce soit.
L’ensemble mousse du matelas se déformera naturellement au fil du temps.
La période de garantie indiquée ci-dessus s’applique uniquement pour l’acheteur original des produits Ultra Comfort
SE et commence à la date de la livraison à cet acheteur original.
Exclusion de garantie et limitations des dommages
La garantie expresse décrite ici est la seule garantie appliquée au produit. Toute autre garantie, qu’elle soit expresse
ou implicite, y compris toute garantie de qualité marchande ou d’adéquation à un usage particulier, est
expressément exclue par Stryker. En aucun cas, Stryker ne pourra être tenue responsable de tout dommage
accessoire ou indirect.
Français
FR
Pièces de rechange et service technique
Les produits Stryker bénéficient du soutien d’un réseau national de réparateurs-représentants locaux spécialisés. Ces
représentants locaux formés dans nos usines ont des stocks importants de pièces détachées qui permettent de réduire
au minimum les délais de réparation. Appeler le représentant local ou contacter le service clientèle de Stryker au +1800-327-0770.
Autorisation de retour
Le retour de produits ne peut pas être effectué sans l’accord préalable du service clientèle de Stryker. Le numéro
d’autorisation qui sera fourni doit être inscrit sur le produit retourné. Stryker se réserve le droit de facturer des frais
d’expédition et de restockage pour les articles retournés. Les articles spéciaux, modifiés ou sans suite ne peuvent pas
faire l’objet d’un retour.
Produit endommagé
La réglementation ICC (Interstate Commerce Commission) exige que les réclamations relatives à un produit
endommagé soient effectuées dans les quinze (15) jours suivant la réception du produit. Ne pas accepter de livraisons
endommagées à moins que lesdits dommages ne soient signalés sur le bordereau de livraison au moment de la
réception. Dès la réception de la notification prompte, Stryker soumettra une réclamation au transporteur approprié pour
les dommages encourus. Le montant des réclamations sera limité au coût de remplacement réel. Si cette information
n'est pas reçue par Stryker dans les quinze (15) jours suivant la livraison du produit, ou si les dommages ne sont pas
signalés sur le bordereau de livraison au moment de la réception, le client restera redevable du paiement intégral de la
facture d'origine dans un délai de trente (30) jours à compter de la réception. Les réclamations pour livraison
incomplète doivent être déposées dans les trente (30) jours suivant la date de la facture.
www.stryker.com 1704-009-001 REV D 2-13
Page 38

Français
FR
Garantie
Clause de garantie internationale
La présente garantie reflète les dispositions en vigueur aux États-Unis. Hors des États-Unis, la garantie peut différer
selon le pays. Contacter le représentant Stryker Medical local pour obtenir plus d’informations.
2-14 1704-009-001 REV D www.stryker.com
Page 39

Page 40

Stryker Medical
3800 E. Centre Avenue
Portage, MI 49002
USA
2017/05 1704-009-001 REV D www.stryker.com
 Loading...
Loading...