Page 1

Ultrasound System
Service Manual
Page 2

P03309-01 08/2003
Copyright 2003 by SonoSite, Inc.
All rights reserved. Printed in the USA.
ii
Page 3

Manufactured by
SonoSite, Inc.
21919 30th Drive SE
Bothell, WA 98021-3904
USA
Telephone: 1-888-482-9449 or 1-425-951-1200
Fax: 1-425-951-1201
SonoSite Ltd
Alexander House
40A Wilbury Way
Hitchin, Herts
SG4 OAP UK
T: +44-1462-444800
F: +44-1462-444801
Caution: United States federal law restricts this device to sale by or on the order of a physician.
“TITAN” and “SonoSite TITAN” are trademarks of SonoSite, Inc.
Kensington is a registered trademark of Kensington Technology Group.
Non-SonoSite product names may be trademarks or registered trademarks of their respective owners.
SonoSite products may be covered by one or more of the following U.S. patents: 4454884, 4462408, 4469106, 4474184, 4475376, 4515017, 4534357,
4542653, 4543960, 4552607, 4561807, 4566035, 4567895, 4581636, 4591355, 4603702, (4607642), 4644795, 4670339, 4773140, 4817618, 4883059,
4887306, 5016641, 5050610, 5095910, 5099847, 5123415, 5158088, 5197477, 5207225, 5215094, 5226420, 5226422, 5233994, 5255682, (5275167),
5287753, 5305756, 5353354, 5365929, 5381795, 5386830, 5390674, 5402793, (5,423,220), 5438994, 5450851, 5456257, 5471989, 5471990, 5474073,
5476097, 5479930, 5482045, 5482047, 5485842, 5492134, 5517994, 5529070, 5546946, 5555887, 5603323, 5606972, 5617863, (5634465), 5634466,
5636631, 5645066, 5648942, 5669385, (5706819), 5715823, 5718229, 5720291, 5722412, 5752517, 5762067, 5782769, 5800356, 5817024, 5833613,
5846200, 5860924, 5893363, 5916168, 5951478, 6036643, 6102863, 6104126, 6113547, 6117085, 6142946, 6203498 B1, 6371918, 6135961, D0280762,
D0285484, D0286325, D0300241, D0306343, D0328095, D0369307, D0379231. Other patents pending.
iii
Page 4

iv
Page 5

Contents
Chapter 1: Introduction
1.1 Audience .......................................................................................1
1.2 Conventions Used in This Service Manual ..............................1
1.3 Product Upgrades and Updates ................................................1
1.4 Customer Comments ...................................................................1
1.5 About the System .........................................................................2
1.6 About the System Software ........................................................4
1.7 Software Licensing .......................................................................4
Chapter 2: Safety
2.1 Electrical Safety ............................................................................5
2.2 Equipment Safety .........................................................................6
2.3 Battery Safety ................................................................................7
2.4 Biological Safety ...........................................................................8
2.5 Labeling Symbols .........................................................................8
Chapter 3: System Overview
3.1 System Overview .........................................................................9
3.2 Theory of Operation ....................................................................9
3.2.1 Transducer ........................................................................10
3.2.2 Front End Subsystem ......................................................10
3.2.3 Digital Signal Processing Subsystem ............................12
3.2.4 Backend Subsystem .........................................................12
3.2.5 Control Subsystem ...........................................................14
3.2.6 Power Supply and Control Subsystem .........................15
3.3 System Specifications ................................................................16
3.3.1 System Dimensions .........................................................16
3.3.2 Display Dimensions ........................................................16
3.3.3 Transducers ......................................................................16
3.3.4 Imaging Modes ................................................................16
3.3.5 Applications ......................................................................16
3.3.6 Image Storage ...................................................................17
3.3.7 Accessories ........................................................................17
3.3.8 Peripherals ........................................................................17
3.3.9 Temperature, Pressure, and Humidity Limits ............18
3.3.10 Electrical ..........................................................................18
3.3.11 Electromechanical Safety Standards ...........................18
3.3.12 EMC Standards Classification ......................................19
3.3.13 Airborne Equipment Standards ...................................19
3.3.14 ECG Standard .................................................................19
3.3.15 DICOM Standard ...........................................................19
Chapter 4: Setup and Operation
4.1 System Controls .........................................................................21
4.2 System Components ..................................................................22
4.3 Setup ............................................................................................23
4.4 Touchpad ....................................................................................24
4.5 Accessories ..................................................................................24
v
Page 6

4.6 Preparing the System for Operation .......................................25
4.6.1 Installing and Removing the Battery ............................25
4.6.2 Using AC Power/Charging Battery .............................26
4.6.3 Connecting to AC Power ................................................27
4.6.4 Connecting and Removing Transducers ......................28
4.6.5 Turning the System On and Off ....................................28
4.7 Upgrading the System Software ..............................................29
4.7.1 Obtaining a License Key .................................................32
4.7.2 Installing a License Key ..................................................33
4.7.3 To Display the System Information Screen ..................34
4.7.4 To Display the License Update Screen .........................35
Chapter 5: Cleaning and Disinfecting
5.1 Universal Precautions ...............................................................37
5.2 Receipt of Suspected Contaminated Materials ......................37
5.3 Recommended Disinfectants ....................................................38
Chapter 6: Troubleshooting
6.1 Basic Troubleshooting ...............................................................39
6.2 Periodic Maintenance ................................................................40
6.3 System and Subsystem Diagnosis ...........................................41
6.4 System Repair .............................................................................41
6.5 Test Equipment ..........................................................................41
6.6 Failure Modes .............................................................................41
6.6.1 Display ...............................................................................41
6.6.2 Control Panel ....................................................................41
6.6.3 System/Main PCBA ........................................................42
6.6.4 Battery ................................................................................42
6.7 Troubleshooting Flow Diagrams .............................................43
6.7.1 Display ...............................................................................43
6.7.2 Control Panel ....................................................................44
6.7.3 System ................................................................................45
6.7.4 Battery ................................................................................46
6.7.5 Mini-Dock/Mobile Docking System .............................47
Chapter 7: Replacement Procedures
7.1 Display Replacement .................................................................49
7.1.1 Required Parts ..................................................................49
7.1.2 Required Tools .................................................................49
7.1.3 Display Removal ..............................................................49
7.1.4 Display Replacement .......................................................51
7.1.5 Test the Display ................................................................52
7.2 Control Panel Subassembly Replacement ..............................52
7.2.1 Required Parts ..................................................................52
7.2.2 Required Tools .................................................................52
7.2.3 Control Panel Removal ...................................................52
7.2.4 Control Panel Replacement ............................................52
7.3 Main System Disassembly for Repair and/or Replacement 53
7.3.1 Required Parts ..................................................................53
7.3.2 Required Tools .................................................................53
7.3.3 Main PCBA Removal ......................................................53
vi
Page 7

Chapter 8: Performance Testing
8.1 Overview .....................................................................................59
8.2 Test Equipment ..........................................................................59
8.3 Setting Up Performance Tests ..................................................59
8.3.1 Scan Reference Orientation ............................................60
8.4 Testing 2D Performance ............................................................60
8.4.1 2D Image Quality ............................................................. 60
8.4.2 Axial Measurement Accuracy ........................................60
8.4.3 Lateral Measurement Accuracy .....................................61
8.4.4 Penetration ........................................................................61
8.5 Additional Performance Tests .................................................. 62
8.5.1 CPD ....................................................................................62
8.5.2 Directional Color Power Doppler (DCPD) ..................62
8.5.3 M Mode Imaging .............................................................63
8.5.4 Tissue Harmonic Imaging ..............................................63
8.5.5 Pulsed Wave (PW) Doppler Imaging ...........................63
8.5.6 Image Quality Verification Test/Livescan ...................64
8.5.7 Image Review ...................................................................64
8.5.8 Printer ................................................................................64
8.5.9 Battery Charging ..............................................................64
8.5.10 Video Output ..................................................................64
8.6 Returning Products to SonoSite ...............................................65
8.6.1 Contacting SonoSite Technical Support .......................65
8.6.2 Shipping Instructions ......................................................65
Chapter 9: Accessory Service
9.1 Mobile Docking System ............................................................67
9.2 Mini-Dock ...................................................................................69
9.3 Connectivity ................................................................................69
9.4 Block Diagrams and Schematics ..............................................70
9.5 Theory of Operation ..................................................................78
9.5.1 Video ..................................................................................78
9.5.2 Power Distribution ..........................................................78
9.6 Replacement Procedures ...........................................................79
9.6.1 Required Tools .................................................................79
9.6.2 Cup Surround ...................................................................79
9.6.3 Casters ...............................................................................79
9.6.4 Power Supply ...................................................................80
9.6.5 Locking Handle ................................................................82
9.6.6 Deflector ............................................................................82
9.6.7 Mini-Dock .........................................................................83
Appendix A: Parts List
A.1 Replacement Parts List .............................................................85
A.1.1 Display ............................................................................. 85
A.1.2 Control Panel ...................................................................86
A.1.3 Replacement Parts, System ...........................................87
A.1.4 Transducer Nest Frame Assembly ...............................93
A.1.5 AC Adapter .....................................................................94
A.1.6 Mini-Dock ........................................................................95
A.1.7 Mobile Docking System .................................................96
A.2 Ordering Replacement Parts ................................................... 98
vii
Page 8

Appendix B: Service Event Report
Index
.................................................................................................101
viii
Page 9

Chapter 1: Introduction
Before servicing the TITANTM high-resolution ultrasound system, please read the information in this
manual. This text applies only to the SonoSite TITAN ultrasound system product manufactured after
June 19, 2003. Please find service information about products manufactured before June 17, 2003 in
C1.51 Ultrasound System Service Manual (P00715), C1.75 Ultrasound System Service Manual (P01118), C1.9
PLUS Ultrasound System Service Manual (P02287), and C1.99 PLUS and ELITE Ultrasound System Service
Manual (P02913).
1.1 Audience
The intended audience of this manual is properly trained field and in-house service personnel.
1.2 Conventions Used in This Service Manual
These conventions are used in this service manual:
• A Warning describes precautions necessary to prevent injury or loss of life.
• A Caution describes precautions necessary to protect the products.
• When the steps in the operating instructions must be performed in a specific order, the steps are
numbered.
• Bulleted lists present information in list format, but they do not imply a sequence.
• The system handle is on the front of the system, and the battery compartment is on the back of the
system.
1.3 Product Upgrades and Updates
SonoSite may offer software upgrades and new features that may improve system performance.
Service manual updates, explaining the effects of upgrades and new features on system performance,
will accompany the upgrades.
1.4 Customer Comments
Questions and comments are encouraged. SonoSite is interested in your feedback regarding the
service manual. Please call SonoSite at 1-877-657-8118. If you are outside the USA, call the nearest
SonoSite representative. You can also send electronic mail (e-mail) to SonoSite at the following
address:
service@sonosite.com
Chapter 1: Introduction 1
Page 10

1.5 About the System
The ultrasound system has multiple configurations and feature sets. All are described in this service
manual but not every option may apply to your system. System features are dependent on your
system configuration, transducer, and exam type.
Figure 1.1 TITAN System Front View
Table 1.1: TITAN System Front Features
4
1
2
5
3
Number Feature
1 Control panel
2 Transducer connection
3Handle
4Display
5 CompactFlash™ slots (front for image storage, back for system updates)
2 Chapter 1: Introduction
Page 11

1
3 42
Figure 1.2 TITAN System Rear View
Table 1.2: TITAN System Rear Connectors
Number Feature
1 DC input connector
2 I/O connector
3Battery
4 ECG connector (available on future releases)
The TITAN system is a portable, software-controlled, ultrasound system using all-digital architecture.
The system is used to acquire and display high-resolution, real-time ultrasound images: 2D, color
power Doppler (CPD), directional color power Doppler (DCPD), Tissue Harmonic Imaging (THI),
M Mode, and pulsed wave (PW) Doppler. The system has cine buffer, image zoom, labeling, biopsy,
measurements, calculations, a USB connection for image transfer, image storage, image review,
printing, recording, and the ability to archive Doppler with audio output to a videotape.
Currently, the system supports the following broadband transducers:
• C11/8-5 MHz 11 mm microcurved array
• C15/4-2 MHz 15 mm microcurved array
• C60/5-2 MHz 60 mm curved array
• HST/10-5 MHz 25 mm linear array
• ICT/8-5 MHz 11 mm intracavitary array
• L38/10-5 MHz 38 mm linear array
System accessories include the TITAN mobile docking system, the TITAN mini-dock, a power supply,
a battery, video and printer cables, and SiteLink Image Manager 2.0 software.
System peripherals include medical grade (conforming to the requirements of EN60601-1) and
non-medical (commercial) grade products. System medical grade peripherals include a printer and
VCR. System non-medical grade peripherals include a CompactFlash card and a Kensington Security
Cable. Use of peripherals is covered in the manufacturers’ instructions, which accompany each
peripheral.
Chapter 1: Introduction 3
Page 12

1.6 About the System Software
The ultrasound system contains software that controls its operation. A software upgrade may be
required. SonoSite will provide you with a CompactFlash card containing the software. Typically new
software provides new capabilities. A single CompactFlash card can be used to update one or more
systems. Software upgrades use the back CompactFlash slot on the right hand side of the system.
CompactFlash cards installed in the front CompactFlash slot do not upgrade the system.
1.7 Software Licensing
Use of the software that you receive from SonoSite is controlled by a license key. A license key is a
number sequence containing exactly 12 decimal digits.
License keys are obtained from SonoSite or from its authorized representatives. You must obtain one
key for each system that will use the new software. See “Obtaining a License Key” on page 32 for
information on obtaining a license key.
Software that you receive from SonoSite may be installed and will operate for a short period of time
without requiring a valid license key. We refer to this period of time as the “grace period.” The grace
period is variable.
When you first install your software, your SonoSite system prompts you for a license key. If you have
not yet obtained a valid license key, you can elect to use the software as long as the grace period time
has not been fully consumed.
When a system is running in the grace period, all system functions are available. As you use the
system, the grace period is slowly consumed. When the grace period has expired, the system will not
be usable until a valid license key has been entered. Grace period time is not consumed while the
system is powered off or when it is in “sleep” mode. Whenever a system is running in the grace period,
the grace period time remaining is available on the license update screen.
Caution: When the grace period expires, all system functions except for licensing are
unavailable until a valid license key is entered into the system.
4 Chapter 1: Introduction
Page 13

Chapter 2: Safety
Read this information before using the ultrasound system. The information in this manual applies to
the ultrasound system, transducer, accessories, and peripherals. This chapter contains safety
information.
A Warning describes precautions necessary to prevent injury or loss of life.
A Caution describes precautions necessary to protect the products.
2.1 Electrical Safety
This system meets EN60601-1, Class I/internally-powered equipment requirements and Type BF
isolated patient-applied parts safety requirements.
This system complies with the applicable medical equipment requirements published in the Canadian
Standards Association (CSA), European Norm Harmonized Standards, and Underwriters
Laboratories (UL) safety standards.
For maximum safety observe the following warnings and cautions:
Warning: To avoid the risk of electrical shock or injury, do not open the system enclosures. All
internal adjustments and replacements, except battery replacement, must be made by a
qualified technician.
To avoid the risk of injury, do not operate the system in the presence of flammable
gasses or anesthetics. Explosion can result.
To avoid the risk of electrical shock, use only properly grounded equipment. Shock
hazards exist if the power supply is not properly grounded. Grounding reliability can
only be achieved when equipment is connected to a receptacle marked “Hospital
Only” or “Hospital Grade” or the equivalent. The grounding wire must not be
removed or defeated.
To avoid the risk of electrical shock, before using the transducer, inspect the transducer
face, housing, and cable. Do not use the transducer if the transducer or cable is
damaged.
To avoid the risk of electrical shock, always disconnect the power supply from the
system before cleaning the system.
To avoid the risk of electrical shock, do not use any transducer that has been immersed
beyond the specified cleaning or disinfection level. See Chapter 5, “Cleaning and
Disinfecting.”
To avoid the risk of electrical shock and fire hazard, inspect the power supply, AC
power cord and plug on a regular basis. Ensure they are not damaged.
To avoid the risk of electrical shock, use only accessories and peripherals
recommended by SonoSite, including the power supply. Connection of accessories and
peripherals not recommended by SonoSite could result in electrical shock. Contact
SonoSite or your local representative for a list of accessories and peripherals available
from or recommended by SonoSite.
Chapter 2: Safety 5
Page 14

Warning: To avoid the risk of electrical shock, use commercial grade peripherals recommended
by SonoSite on battery power only. Do not connect these products to AC mains power
when using the system to scan or diagnose a patient/subject. Contact SonoSite or your
local representative for a list of the commercial grade peripherals available from or
recommended by SonoSite.
To avoid the risk of electrical shock, inspect the interconnect cables on a regular basis
for damage.
To avoid the risk of electrical shock to the patient/subject, do not touch the system
battery contacts while simultaneously touching a patient/subject.
To prevent injury to the operator/bystander, the transducer must be removed from
patient contact before the application of a high-voltage defibrillation pulse.
Caution: Although your system has been manufactured in compliance with existing EMC/EMI
requirements (EN60601-1-2), use of the system in the presence of an electromagnetic
field can cause degradation of the ultrasound image. If this occurs often, SonoSite
suggests a review of the system environment. Identify and remove the possible sources
of the emissions or move your system.
Electrostatic discharge (ESD), or static shock, is a naturally occurring phenomenon.
ESD is common in conditions of low humidity, which can be caused by heating or air
conditioning. Static shock is a discharge of the electrical energy from a charged body to
a lesser or non-charged body. The degree of discharge can be significant enough to
cause damage to a transducer or an ultrasound system. The following precautions can
help reduce ESD: anti-static spray on carpets, anti-static spray on linoleum, and
anti-static mats.
Do not use the system if an error message appears on the display: note the error code;
call SonoSite or your local representative; turn off the system by pressing and holding
the power key until the system powers down.
To avoid increasing the system and transducer connector temperature, do not block
the airflow to the ventilation holes on the side of the system.
2.2 Equipment Safety
To protect your ultrasound system, transducer, and accessories, follow these precautions.
Caution: Excessive bending or twisting of cables can cause a failure or intermittent operation.
To avoid damaging the power supply, verify the power supply input is within the
correct voltage range. See “Electrical” on page 18 in Chapter 3.
Improper cleaning or disinfecting of any part of the system can cause permanent
damage. For cleaning and disinfecting instructions, see Chapter 5, “Cleaning and
Disinfecting.”
Do not use solvents such as thinner or benzene, or abrasive cleaners on any part of the
system.
Remove the battery from the system if the system is not likely to be used for some time.
Do not spill liquid on the system.
6 Chapter 2: Safety
Page 15

2.3 Battery Safety
To prevent the battery from bursting, igniting, or emitting fumes and causing equipment damage,
observe the following precautions.
Warning: The battery has a safety device. Do not disassemble or alter the battery.
Charge the batteries only when the ambient temperature is between 0° and 45°C (32°
and 113°F).
Do not short-circuit the battery by directly connecting the positive and negative
terminals with metal objects.
Do not heat the battery or discard it in a fire.
Do not expose the battery to storage temperatures over 60°C (140°F). Keep it away
from fire and other heat sources.
Do not charge the battery near a heat source, such as a fire or heater.
Do not leave the battery in direct sunlight.
Do not pierce the battery with a sharp object, hit it, or step on it.
Do not use a damaged battery.
Do not solder a battery.
The polarity of the battery terminals is fixed and cannot be switched or reversed. Do
not force the battery into the system.
Do not connect the battery to an electrical power outlet.
Do not continue recharging the battery if it does not recharge after two successive six
hour charging cycles.
Caution: To prevent the battery from bursting, igniting, or emitting fumes and causing
equipment damage, observe the following precautions.
Do not immerse the battery in water or allow it to get wet.
Do not put the battery into a microwave oven or pressurized container.
If the battery leaks or emits an odor, remove it from all possible flammable sources.
If the battery emits an odor or heat, is deformed or discolored, or in any way appears
abnormal during use, recharging or storage, immediately remove it and stop using it.
If you have any questions about the battery, consult SonoSite or your local
representative.
Store the battery between -20°C (-4°F) and 60°C (140°F).
Use only SonoSite batteries.
Do not use or charge the battery with non-SonoSite equipment. Only charge the battery
with the TITAN system.
Chapter 2: Safety 7
Page 16

2.4 Biological Safety
Observe the following precautions related to biological safety.
Warning: Non-medical (commercial) grade peripheral monitors have not been verified or
validated by SonoSite as being suitable for diagnosis.
Do not use the system if it exhibits erratic or inconsistent behavior. Discontinuities in
the scanning sequence are indicative of a hardware failure that must be corrected
before use.
Do not use the system if it exhibits artifacts on the LCD screen, either within the clinical
image or in the area outside of the clinical image. Artifacts are indicative of hardware
and/or software errors that must be corrected before use.
Some transducer sheaths contain natural rubber latex and talc, which can cause allergic
reactions in some individuals. Refer to 21 CFR 801.437, User labeling for devices that
contain natural rubber.
Perform ultrasound procedures prudently. Use the ALARA (as low as reasonably
achievable) principle.
SonoSite does not currently recommend a specific brand of acoustic standoff.
2.5 Labeling Symbols
Labeling symbols for SonoSite products can be found in the user guide for each product.
8 Chapter 2: Safety
Page 17

Chapter 3: System Overview
3.1 System Overview
The system houses the system electronics, display, control panel, and the system batteries. It provides
basic connections for external power, and the transducer connector and a general purpose docking
connector for all other interfaces. The system operates with external transducers and optional
peripheral equipment. The types of external devices that may be used are:
• Transducer(s)
• AC Power Supply/Charger
• Mobile Docking System/Mini-dock
• External Peripherals
The transducer connects to the main unit through the scanhead connector. The transducer contains
data, which the system uses to drive the transducer in the scanhead, process the data received back
and format and display the data for the user. The interface is backward compatible to previous
systems and scanheads.
The AC power supply not only provides power from the AC mains for operating the system, it also
contains the charger for charging the internal system battery. This may be used if a mobile docking
system or mini-dock is not desired or available.
The mobile docking system provides power to run the system, contains the charger to charge the
internal system battery and provides fixed external power, video, RS-232, and USB connections. The
docking system may also provide additional control surfaces and monitors. The unit interfaces to the
docking system through connections on the back of the unit. It provides a convenient place for the unit
to be operated and stored under certain usage scenarios.
The mini-dock provides the breakout for all the connectors from the docking connector for remote use
where a docking system may not be available and the external connections are desired. The use of a
mini-dock allows the main unit to be more portable when the connections are not required.
External OEM peripherals are items such as monitor, printers, and VCRs. These can be connected to
the mobile system or directly to the system with the use of the mini-dock using the video and/or
printer control input/outputs.
3.2 Theory of Operation
The system has six major functional groups: the transducer, the frontend subsystem, the digital signal
processing subsystem, the backend subsystem, the control subsystem, and the power supply and
control subsystem. Figure 3.1 shows how these functions interact.
Chapter 3: System Overview 9
Page 18
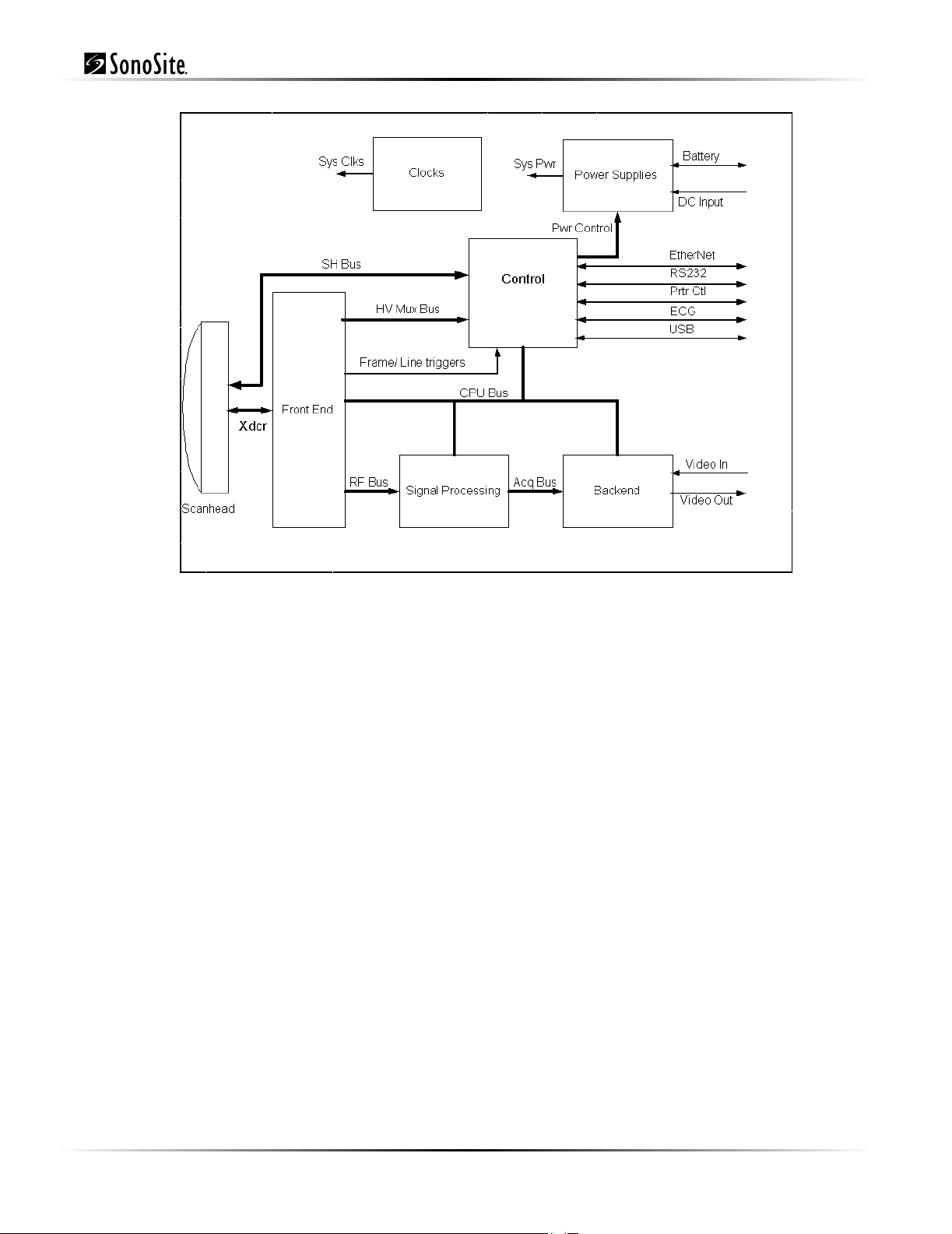
Figure 3.1 TITAN Block Diagram
3.2.1 Transducer
The transducer elements convert the pulser voltage to acoustic energy during the “transmit” portion
of the ultrasound acquisition cycle. Also, the transducer elements convert the acoustic echo to voltage
in the “receive” portion of the acquisition cycle. The system transducers have 64 to 128 elements. The
front end subsystem senses the voltage developed on the transducer elements.
3.2.2 Front End Subsystem
The Front End is designed to support various imaging modalities such as 2D, spectral Doppler and
color Doppler. From the Front End's perspective all modes can be grouped into a few basic types:
single mode, simultaneous modes and triggered modes. All these modes are built from similar, basic
transmit and receive sequences controlled within the Front End. A generic top level block diagram of
a typical Front End is in the following figure.
10 Chapter 3: System Overview
Page 19
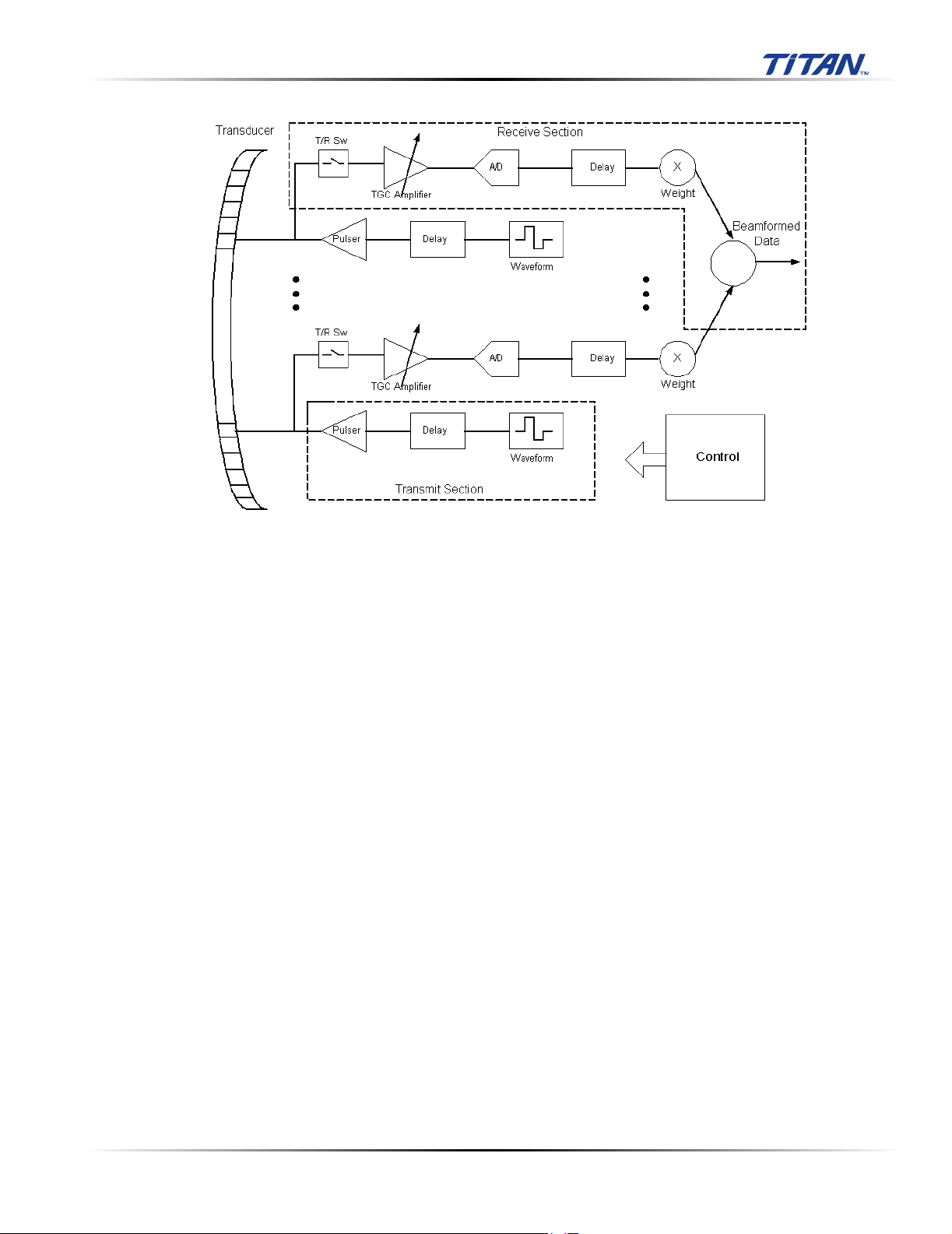
Figure 3.2 Front End Subsystem
The transmit section consists of a waveform generator, delay block, and high power high voltage
driver to excite the transducer element. Multiple elements are driven with delays determined by the
time of flight in the medium from the elements to the point in space where the beam is to be focused.
The longer the time of flight is to the focal point the smaller the delay is for a given transmit element
to allow all to arrive at the focal point at the same time. The number of elements driven is determined
by element sensitivity off axis and depth of field considerations. The waveform is selected to drive the
transducer at a certain center frequency, bandwidth, and power and is optimized for the given mode.
The receive section consists of a transmit/receive switch to protect the receiver from the transmit
voltage, a variable gain receiver to amplify and condition the return echoes, an A/D to digitize the
data, a delay block to focus the return signals and a weight block to scale the return echoes for each
channel. All the signals are then summed together to generate the beamformed receive data. The
analog gain varies with depth to compensate for signal attenuation through the medium. The delays
and weights are independent for each channel. The delay and weight for the receive channel can
typically be changed dynamically to keep the receive beam in continuous focus. The delay is simply
set by the time of flight in the medium from the point of interest to the element, which starts at skinline
and proceeds to the deepest depth of interest.
The control section drives the data to the various data path elements on a line by line basis, controls
the timing for the transmit and receive sections, and controls the tagged information and timing of the
data to the rest of the system.
Unique transmit and receive sequences, lines or PRIs, are arranged into repeated groups or frames.
The simplest frame is for a single mode where the line does not change, for example M Mode or PW
Doppler. Here the same line characteristics; aperture size, delay, weights, and waveform information,
are continually repeated. A scanned single mode, such as 2D, keeps the same transmit aperture size
but the delays and receive weights change due to the aperture translation or steering changes with
each line acquired. Simultaneous modes may also change the transmit waveform and aperture size
Chapter 3: System Overview 11
Page 20
and the delays and receive weights. Downstream processing also changes, due to the unique
processing requirements for the different types of data. Triggered modes are the same as the previous
modes except that the frames are started and stopped on user or external inputs.
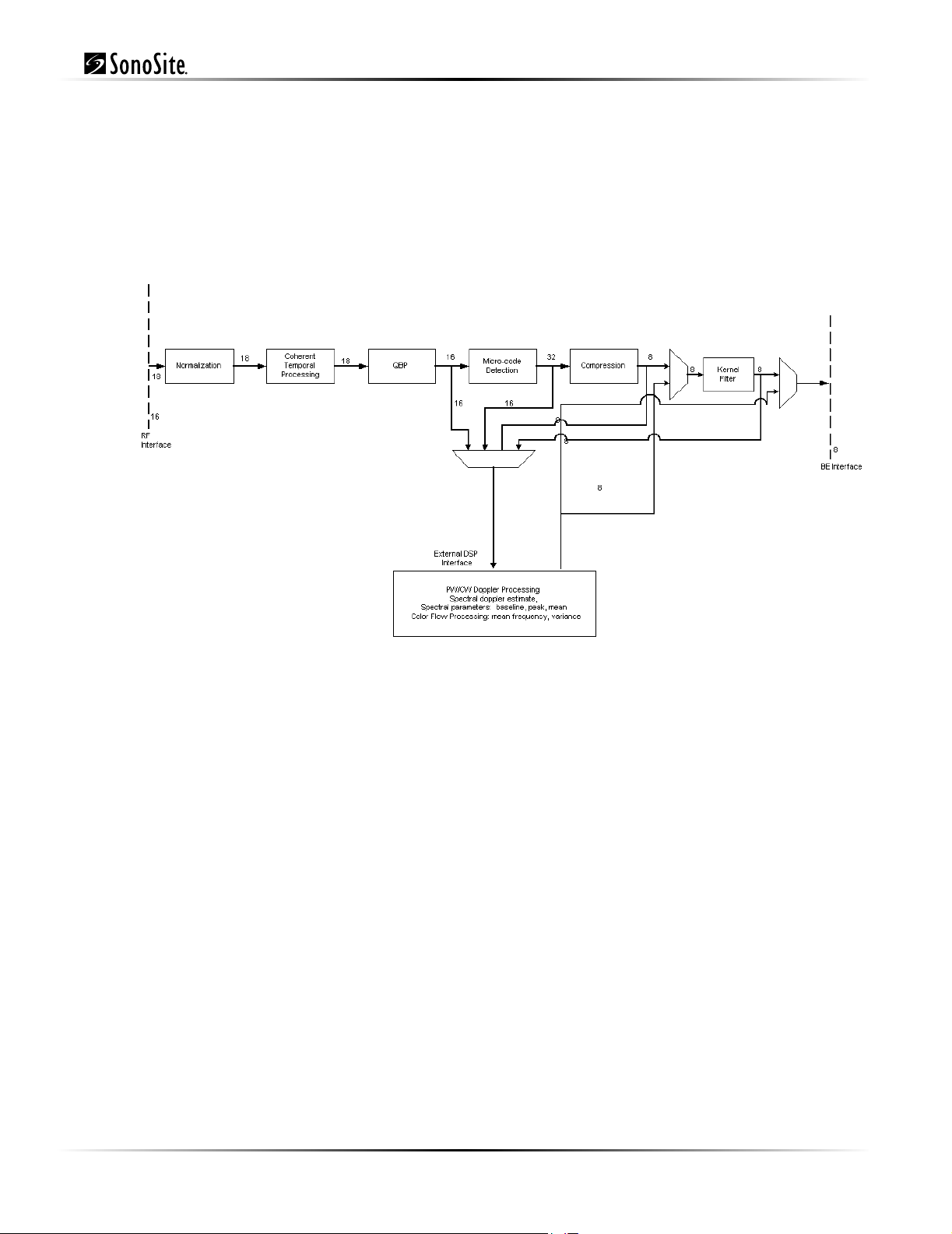
3.2.3 Digital Signal Processing Subsystem
The DSP subsystem receives data from the front end chip sets, performs processing to enhance the
signal-to-noise ratio of signal features of interest, and prepares data for raster scan conversion and
display.
Figure 3.3 Digital Signal Processing Subsystem
3.2.4 Backend Subsystem
The Backend subsystem is responsible for the conversion of raw acquisition data into a raster image
ready for display. This includes the acquisition data path with flash suppression and temporal
filtering, and the display data path with scan conversion into raster space. The Backend subsystem
also contains the video data path that supports generation of video comprising of the ultrasound
image as well as graphics annotation. Video generation of both standard composite interlaced video
and progressive scan video is supported. Most functionality is within the ASIC. However, the memory
resources for acquisition memory, and display memory are found in external memory components.
The conversion from PC type video to TV type video is also performed externally.
Control is received initially from the CPU to setup each functional block and afterward the hardware
is completely data driven. This control takes the form of programming setup registers inside the blocks
and setting up scan conversion tables. Each block provides temporary storage as required to buffer
data and keep their respective processing pipeline full and operating. Also note that the block
diagrams show only the data path, but each block is responsible for generating any necessary memory
addresses for their respective input data stream.
12 Chapter 3: System Overview
Page 21
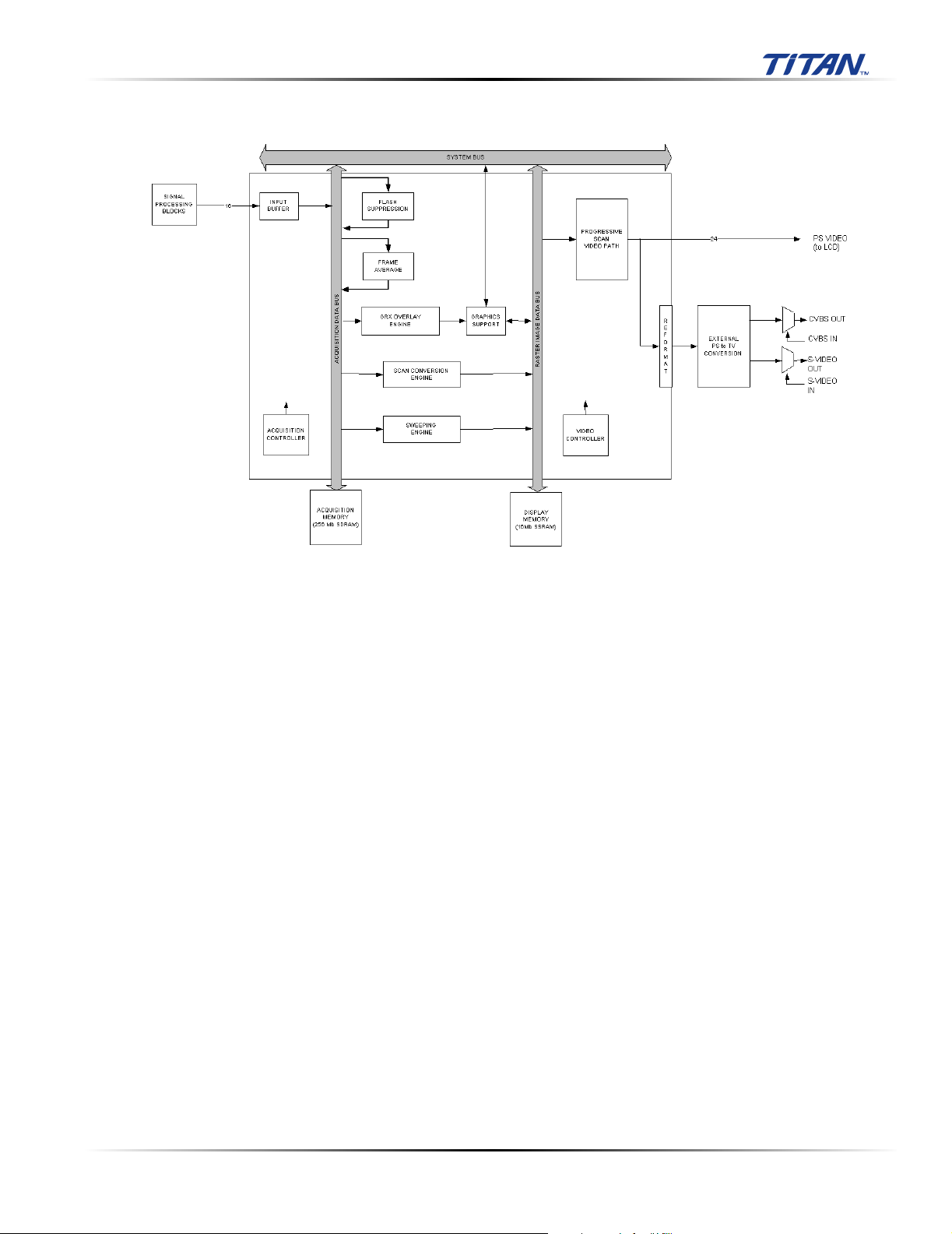
The BackEnd subsystem is shown in the figure below.
Figure 3.4 BackEnd Subsystem Block Diagram
The backend subsystem performs processing encompassing three main data domains, acquisition
data, raster data, and video data.
Support for acquisition data includes the input buffer, flash suppression, frame average, and external
ACQ memory. Cine buffer management is performed by the acquisition controller.
Conversion from acquisition data to raster data is performed by the graphics overlay, scan conversion
engine, sweeping engine, and 3D engine. Raster data is stored in an external DISPLAY memory. Also
supporting raster operations is the graphics support block that provides acceleration hardware for
pixel operations from the CPU and graphics overlay engine.
Video data is processed as progressive scan (60 Hz) and supplied externally on a digital bus. In
addition, interlaced (30 Hz) video is supplied in both composite and S-video formats. The progressive
video path includes buffers, priority logic, and LUTs. External video in signals are input and
multiplexed onto the external video out path to allow for external sources to display information on
connected displays, VCRs, or printers.
Chapter 3: System Overview 13
Page 22
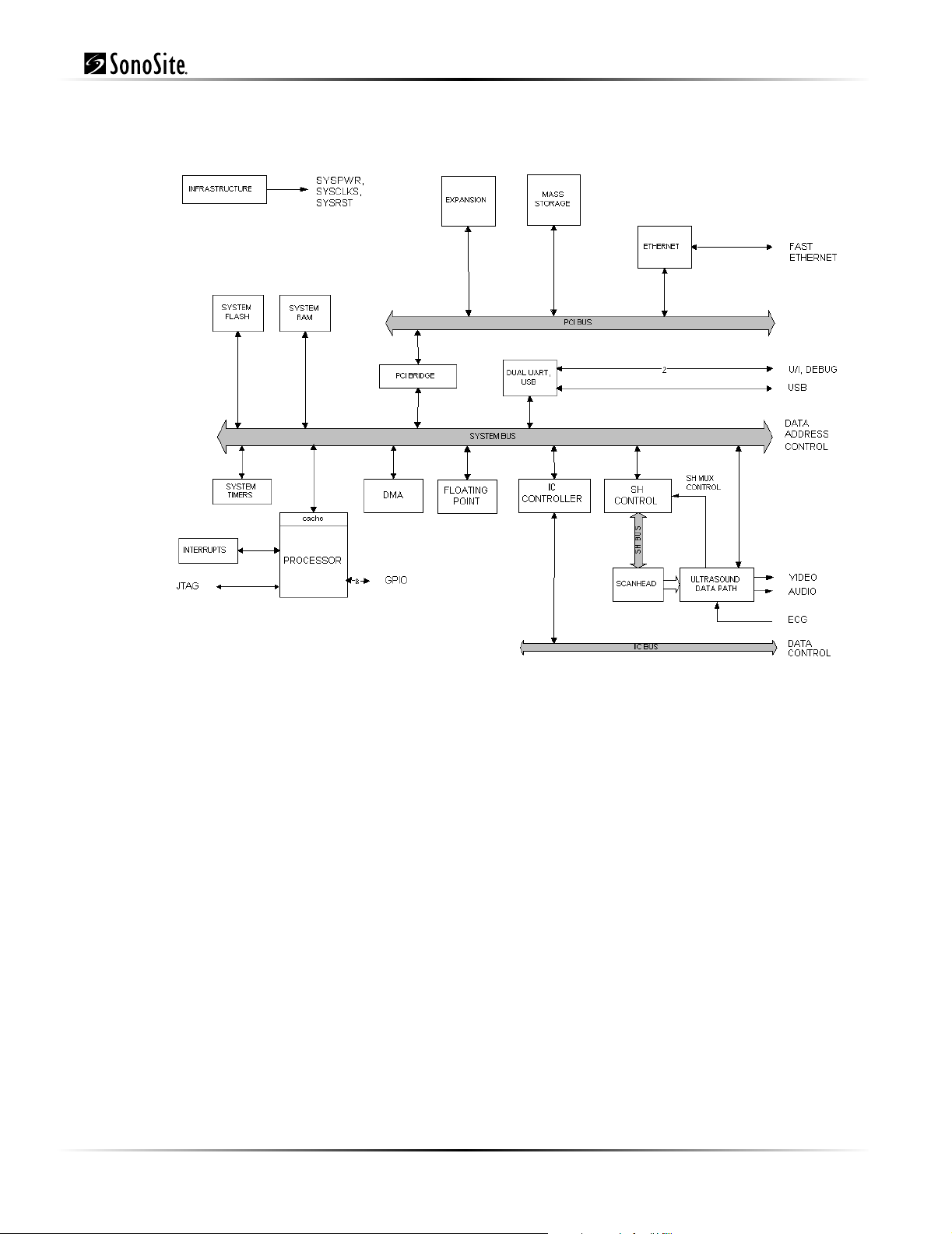
3.2.5 Control Subsystem
The control subsystem is shown in the figure below.
Figure 3.5 Control Subsystem
The core control subsystem contains the processor, the system bus, the system memory resources of
FLASH and RAM, the interrupt logic, system timers, a DMA engine, and a floating point unit.
Support for the ultrasound subsystem consists of a scanhead interface, scanhead mux control, a
portion of the system FLASH for storage of saved images, and a control path to program the
ultrasound datapath.
Communication interfaces consist of an Ethernet interface, USB port, two general purpose serial bus
interfaces, and the I2C bus.
14 Chapter 3: System Overview
Page 23
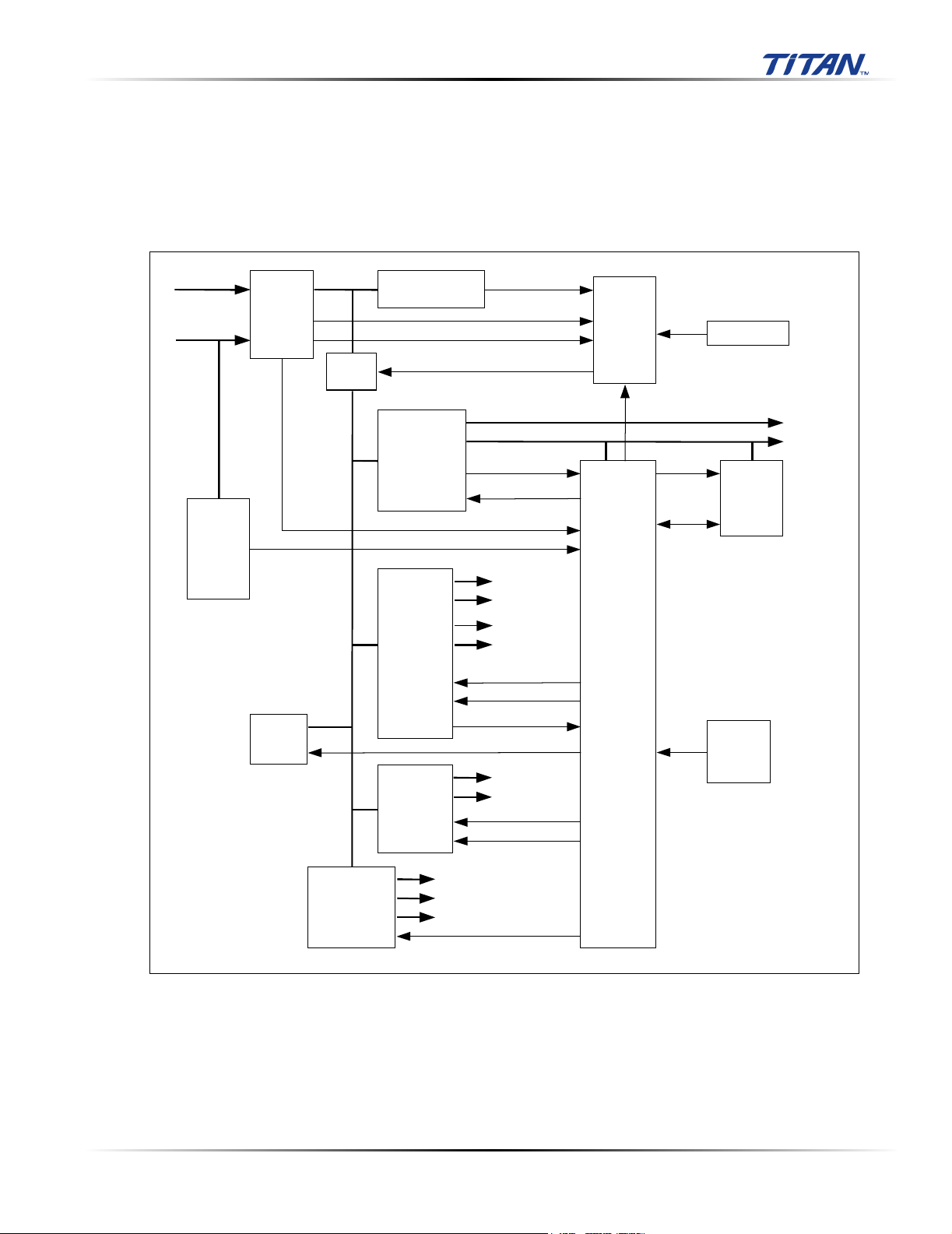
3.2.6 Power Supply and Control Subsystem
A
V
V
V
V
V
A
A
V
V
V
The system Power Supply and Control System consists of an easily replaced rechargeable battery
pack; an On/Off Key; a standby power regulator; digital, analog, display and transducer power
supplies; a power monitor and a power control system. Operating current is drawn from the battery
or an external AC/DC Adapter, which also contains circuitry for charging the battery.
The Power Supply and Control Subsystem are in the figure below.
EXT
VBAT
Battery
Pack
Power
Select
BDATA
Fan
EXTS
Power
Switch
Standby Power
Regulator
PWR
Digital
Power
Supplies
nalog
Power
Supplies
Display
Power
Supplies
STBY
EXTS
BATS
PWR_Enable
VCC1_Good
DPS_Enables
+HVB
+HV
+6V
-6V
PS_Enable
PS_Sense
Fan_Enable
BL_PWR
LCD(n)
LCD_Control
LCD_Enable
HV_ADJ
On/Off
Latch
Power
Monitor
and
Control
Off
RSTN
PS_Bus
On/Off Key
CC(n)
CC1
CPU
Temp
Sense
Transducer
Power
Supplies
SH_5V
SH_3.3V
SH_VPP
SH_Enable
Figure 3.6 Power Supply and Control System Block Diagram
Chapter 3: System Overview 15
Page 24

3.3 System Specifications
This section contains system and accessory specifications and agency approvals. The specifications for
recommended peripherals can be found in the manufacturers’ instructions.
3.3.1 System Dimensions
Length: 11.8 in. (29.97 cm)
Width: 10.9 in. (27.69 cm)
Depth: 3.0 in. (7.62 cm)
Weight: 8.3 lbs. (3.76 kg) with the C60 transducer and battery installed
3.3.2 Display Dimensions
Length: 5.1875 in. (13.18 cm)
Width: 6.75 in. (17.15 cm)
Diagonal: 8.5 in. (21.59 cm)
3.3.3 Transducers
• C11/8-5 MHz 11 mm microcurved array (5 ft./1.5 m)
• C15/4-2 MHz 15 mm microcurved array (5.5 ft./1.7 m)
• C60/5-2 MHz 60 mm curved array (5 ft./1.5 m)
• HST/10-5 MHz 25 mm linear array (8 ft./2.1 m)
• ICT/8-5 MHz 11 mm intracavitary array (5 ft./1.5 m)
• L38/10-5 MHz 38 mm linear array (5.5 ft./1.7 m)
3.3.4 Imaging Modes
2D (256 gray shades)
Color power Doppler (CPD) (256 colors)
Directional color power Doppler (DCPD) (256 colors)
MMode
Pulsed wave (PW) Doppler
Tissue Harmonic Imaging
3.3.5 Applications
Abdominal Imaging
Cardiac Imaging
Cephalic Imaging
Gynecology Imaging
Interventional and Intraoperative Imaging Applications
Obstetrical Imaging
Pediatric and Neonatal Imaging
Superficial Imaging
Vascular Imaging
16 Chapter 3: System Overview
Page 25

3.3.6 Image Storage
The number images saved to the CompactFlash card vary depending on the card storage capacity.
Cine buffer
3.3.7 Accessories
3.3.7.1 Hardware, Software, and Documentation
AIUM Ultrasound Medical Safety Guidance Document
Battery
Biopsy Guide
Mobile Docking System
Mini-Dock
Power supply
Quick Reference Guide
SiteLink Image Manager 2.0
SiteLink DICOM 2.0
SonoKnowledge education package
System User Guide
Ultrasound gel
3.3.7.2 Cables
Print control cable (10 ft./3.1 m)
Printer AC power cord (1 ft./30.5 cm)
VCR AC power cord (1.5 ft./45.7 cm)
VCR (control/audio) cable (6 ft./1.8 m)
Video cable (RCA/RCA) (10 ft./3.1 m)
Video cable (RCA/BNC) (10 ft./3.1 m)
S-video (6 ft./1.8 m)
System AC power cord (10 ft./3.1 m)
USB cable for SiteLink (10 ft./3.1 m)
3.3.8 Peripherals
See the manufacturer’s specifications for the following peripherals.
3.3.8.1 Medical Grade
Black-and-white printer
Recommended sources for printer paper: Contact Sony at 1-800-686-7669 or
www.sony.com/professional
distributor.
Color printer
Video cassette recorder
to order supplies or to obtain the name and number of the local
3.3.8.2 Non-Medical Grade
Kensington Security Cable
Chapter 3: System Overview 17
Page 26

3.3.9 Temperature, Pressure, and Humidity Limits
The temperature, pressure, and humidity limits apply only to the ultrasound system and transducers.
Operating Limits: System
• 10–40°C (50–104°F), 15–95% R.H.
• 700 to 1060hPa (0.7 to 1.05 ATM)
Shipping/Storage Limits: System without Battery
• -35–65°C (-31–149°F), 15–95% R.H.
• 500 to 1060hPa (0.5 to 1.05 ATM)
Operating Limits: Battery
• 10–40°C (50–104°F), 15–95% R.H.
Shipping/Storage Limits: Battery
• -20–60°C (-4–140°F), 0–95% R.H.*
• 500 to 1060hPa (0.5 to 1.05 ATM)
* For storage longer than 30 days, store at or below room temperature.
Operating Limits: Transducer
• 10–40°C (50–104°F), 15–95% R.H.
Shipping/Storage Limits: Transducer
• -35–65°C (-31–149°F), 15–95% R.H.
3.3.10 Electrical
Power Supply Input: 100-240 VAC, 50/60 Hz, 1.2 A Max @ 100 VAC.
Power Supply Output (system on): (1) 15 VDC, 2.7A Max (system)
(2) 12.6 VDC, 0.8A Max (battery charging)
Power Supply Output (system off): (1) 15 VDC, 2.0A Max (system)
(2) 12.6 VDC, 1.8A Max (battery charging)
Combined output not exceeding 52W.
Battery
• 6-cell, 11.25 VDC, 4.4 amp-hours, rechargeable lithium ion battery pack.
• Run time is 2 hours or more, depending on imaging mode and display brightness.
3.3.11 Electromechanical Safety Standards
EN 60601-1:1997, European Norm, Medical Electrical Equipment–Part 1. General Requirements
for Safety.
EN 60601-1-1:2001, European Norm, Medical Electrical Equipment–Part 1. General
Requirements for Safety–Section 1-1. Collateral Standard. Safety Requirements for Medical
Electrical Systems.
C22.2, No. 601.1:1990, Canadian Standards Association, Medical Electrical Equipment–Part 1.
General Requirements for Safety.
CEI/IEC 61157:1992, International Electrotechnical Commission, Requirements for the
Declaration of the Acoustic Output of Medical Diagnostic Ultrasonic Equipment.
UL 2601-1:1997, Second Edition, Underwriters Laboratories, Medical Electrical Equipment-Part
1: General Requirements for Safety.
18 Chapter 3: System Overview
Page 27

3.3.12 EMC Standards Classification
EN 60601-1-2:2001, European Norm, Medical Electrical Equipment. General Requirements for
Safety-Collateral Standard. Electromagnetic Compatibility. Requirements and Tests.
CISPR11:97, International Electrotechnical Commission, International Special Committee on
Radio Interference. Industrial, Scientific, and Medical (ISM) Radio-Frequency Equipment
Electromagnetic Disturbance Characteristics-Limits and Methods of Measurement.
The Classification for the SonoSite system, SiteStand, accessories, and peripherals when
configured together is: Group 1, Class A.
3.3.13 Airborne Equipment Standards
RTCA/DO160D:1997, Radio Technical Commission for Aeronautics, Environmental Conditions and
Test Procedures for Airborne Equipment, Section 21.0 Emission of Radio Frequency Energy,
Category B.
3.3.14 ECG Standard
ANSI/AAMI EC53-1995, Association for the Advancement of Medical Instrumentation, ECG Cables,
and Lead Wires.
The SonoSite ultrasound system meets the requirements of this standard except Section 4.4.1
(Exposure to ethylene oxide (EO) sterilization) and Section 4.5.9 (Connector retention force). The
requirement in Section 4.5.9 does not apply, because the product weighs less than 8. 4 pounds.
3.3.15 DICOM Standard
NEMA PS 3.15: 2000, Digital Imaging and Communications in Medicine (DICOM)-Part 15: Security
Profiles.
Chapter 3: System Overview 19
Page 28

20 Chapter 3: System Overview
Page 29
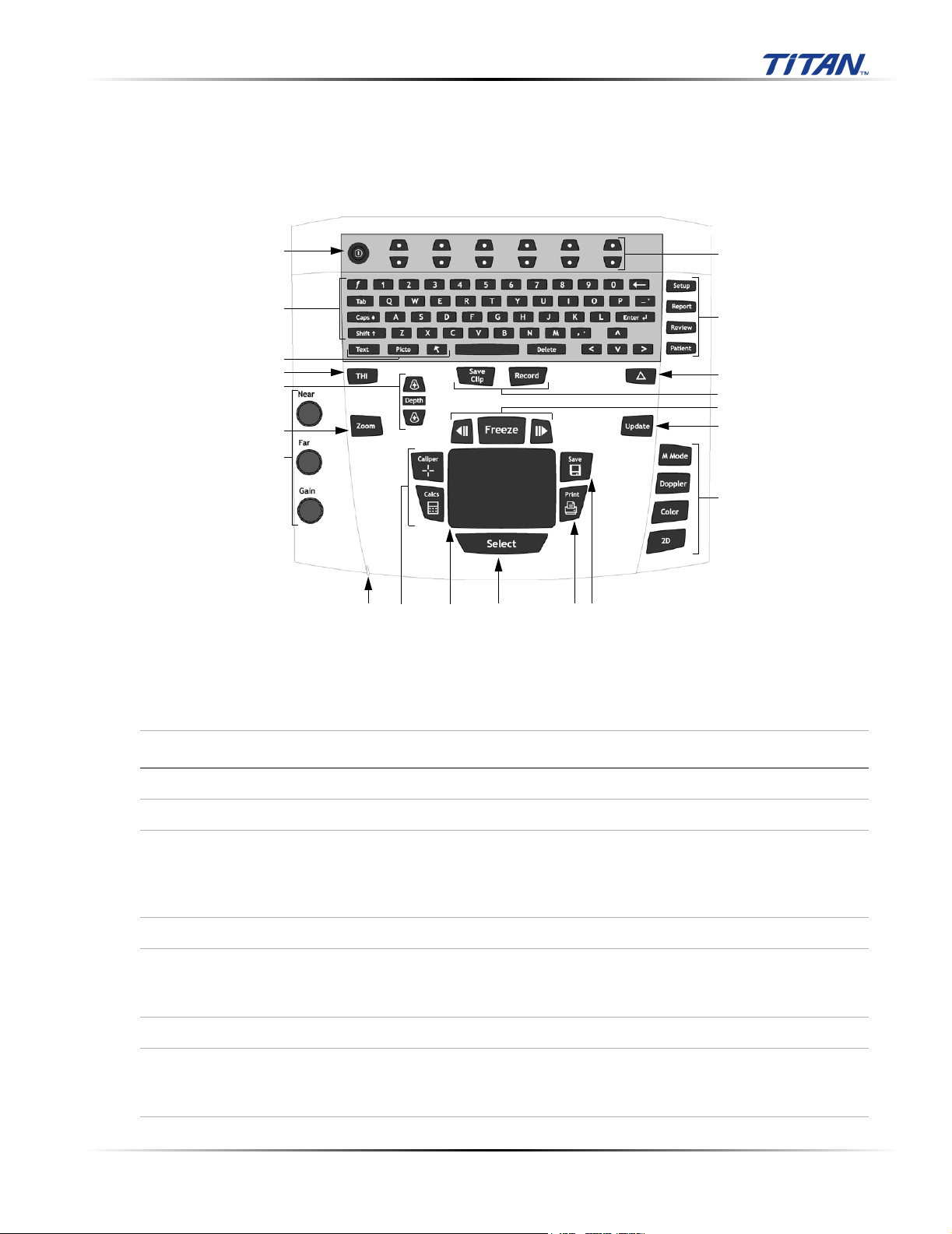
Chapter 4: Setup and Operation
4.1 System Controls
1
2
3
4
5
6
7
Figure 4.1 System Controls
8 9 11 12 13
10
14
15
16
17
18
19
20
Table 4.1: System Controls
Number System Control Description
1 Power Turns system on and off.
2 Alphanumeric Use to enter text and numbers.
3 Annotation Text Turns the keyboard on and off for text entry.
Picto Turns the pictographs/pictograph marker on and off.
Arrow Displays an arrow that can be moved and rotated within the
image area.
4 THI Turns Tissue Harmonic Imaging on and off.
5 Depth Depth Adjusts the imaging depth for 2D.
Depth Up Decreases imaging depth.
Depth Down Increases imaging depth.
6 Zoom Magnifies image 2x.
7 Gain Near Adjusts the gain applied to the near field of the image.
Far Adjusts the gain applied to the far field of the image.
Gain Adjusts the overall gain applied to the entire image.
Chapter 4: Setup and Operation 21
Page 30

Table 4.1: System Controls (Continued)
Number System Control Description
8 AC power
indicator
9 Measurements/
Calculations
10 Touchpad Use to select, adjust, and move objects on the screen.
11 Select Use to switch among touchpad control for line position (2D), text position
12 Print Prints the active image to the printer.
13 Save Saves an image to the CompactFlash card.
14 Remappable
controls
15 Forms Setup Access to the system settings.
16 (Delta key) Use as a shortcut to existing functionality in the system.
17 Video recording Record Turns VCR record on and off.
A steady green light indicates AC power is connected. A flashing green light
indicates the system is in sleep mode.
Caliper activates a measurement caliper on the screen.
Calcs turns the calculation menu on and off.
(text), calipers for measurement (calipers), pictograph marker position/angle
(picto), arrow position/orientation (arrow).
Controls features on the context menu which are adjusted based on the system
state.
Report Access to the patient report.
Review Access to the patient list and saved patient images.
Patient Access to patient information.
Save Clip (Available on future releases.)
18 Freeze Freeze Stops the live imaging and displays a frozen image.
Cine Review images stored in the cine buffer; (back/forward)
back/forward through last-in, first-out sequence.
19 Update Toggles between image modes in M Mode and Doppler, e.g., between Doppler
sample line and Doppler spectral trace.
20 Modes M Mode Turns M Mode on and off.
Doppler Turns Doppler on and off.
Color Turns CPD/DCPD on and off.
2D Turns 2D on and off.
4.2 System Components
The SonoSite system components are identified in “About the System” on page 2.
All mode images can be stored and reviewed in
the cine buffer.
22 Chapter 4: Setup and Operation
Page 31

4.3 Setup
Key click
Beep alert
Sleep delay
Power delay
OB Authors
Date
Time
1 Press the Setup key.
2 Select Audio, Battery from the on-screen menu.
3 In the Key click list, select On or Off.
1 Press the Setup key.
2 Select Audio, Battery from the on-screen menu.
3 In the Beep alert list, select On or Off.
1 Press the Setup key.
2 Select Audio, Battery from the on-screen menu.
3 In the Sleep delay list, select Off, 5, or 10 minutes.
1 Press the Setup key.
2 Select Audio, Battery, from the on-screen menu.
3 In the Power delay list, select Off, 15, or 30 minutes.
1 Press the Setup key.
2 Select Calculations from the on-screen menu.
3 In OB Authors list, select the desired OB authors.
1 Press the Setup key.
2 Select Date and Time, Presets from the on-screen menu.
3 In the Date field, enter the current date (year, month, and day).
1 Press the Setup key.
2 Select Date and Time, Presets from the on-screen menu.
3 In the Time field, enter the current time in 24 hour format (hours and
minutes).
Thermal Index
Doppler Scale
Printer
Video mode
Delta Key
1 Press the Setup key.
2 Select Date and Time, Presets from the on-screen menu.
3 In the Thermal Index list, select TIS, TIB, or TIC.
1 Press the Setup key.
2 Select Date and Time, Presets from the on-screen menu.
3 In the Doppler Scale list, select cm/s or kHz.
1 Press the Setup key.
2 Select Date and Time, Presets from the on-screen menu.
3 In the Printer list, select the desired printer from the list of
recommended printers.
1 Press the Setup key.
2 Select Date and Time, Presets from the on-screen menu.
3 In the Video mode list, select NTSC or PAL.
1 Press the Setup key.
2 Select Delta Key, F Keys from the on-screen menu.
3 Select desired functionality for the Delta key.
The Delta key will now control this function.
Chapter 4: Setup and Operation 23
Page 32

F Keys
1 Press the Setup key.
2 Select Delta Key, F Keys from the on-screen menu.
3 Type in desired text. Use the Backspace key or Delete key to correct
mistakes.
Patient Header
Mode Data
System Status
System Information
Reset
Press the Setup key to exit.
1 Press the Setup key.
2 Select Display Information from the on-screen menu.
3 Select the desired check boxes to display desired information in the
patient header.
1 Press the Setup key.
2 Select Display Information from the on-screen menu.
3 Select the desired check boxes to display imaging information on the
screen.
1 Press the Setup key.
2 Select Display Information from the on-screen menu.
3 Select the desired check boxes to display the system status on the
screen.
1 Press the Setup key.
2 Select System Information from the on-screen menu.
Note: To install a license key see “Installing a License Key” on page 33.
To return settings for this setup page to factory default, select Reset from
the on-screen menu.
4.4 Touchpad
The touchpad is used to select, adjust, and move objects on the screen. For example, it controls the
caliper position, CPD/DCPD box position, floating cursor, and more.
Note: The arrow keys control much of the same functionality as the touchpad.
4.5 Accessories
For information about accessories and other SonoSite products, refer to the user guide for each
product.
24 Chapter 4: Setup and Operation
Page 33

4.6 Preparing the System for Operation
4.6.1 Installing and Removing the Battery
Caution: Use only the specified SonoSite battery pack. For battery safety notes, see “Battery
Safety” on page 7.
The system can be powered from either a battery pack or external power.
The battery pack is a 6-cell, 11.25V (nominal), 4.4 amp-hour, Lithium-Ion, rechargeable battery pack.
The battery comprises six lithium-ion cells plus electronics, a temperature sensor, and battery contacts.
If the battery is being installed for the first time, it will need to be charged.
Warning: To avoid injury to the operator and to prevent damage to the ultrasound system,
inspect the battery for leaks prior to installing.
Locking levers
Figure 4.2 Insert Battery into System
To install the battery:
1 Turn the system upside down.
2 Place the battery into the battery compartment, at a slight angle. See Figure 4.2.
3 Slide the battery forward until it locks into place.
4 Push down on the two locking levers to secure battery.
To remove b a t ter y :
1 Push up on the two locking levers.
2 Slide the battery back.
3 Lift the battery from the compartment.
Chapter 4: Setup and Operation 25
Page 34

4.6.2 Using AC Power/Charging Battery
The battery charges when the system is connected to the AC power supply. If the system is off and
connected to AC power, a completely discharged battery will fully charge in 2.5 to 3.5 hours. If the
system is on and connected to AC power, a completely discharged battery will fully charge in 5 to
6hours.
The system can run on AC power or charged battery in three ways.
• Connected directly to the system
• Connected to the mini-dock (see “To operate the system using AC power (directly to system):” on
page 26)
• Connected to the mobile docking system (see “To connect AC power using the mini-dock:” on
page 27)
To operate the system using AC power (directly to system):
Caution: Verify the hospital supply voltage corresponds to the power supply voltage range. See
“Electrical” on page 18.
1 Connect the DC power cable from the power supply to the connector on the system. See Figure 1.2
on page 3.
2 Connect one end of the system AC power cord into the power supply. Then plug the other end
into a hospital-grade electrical outlet.
4.6.2.1 Battery Charge Indicators
The Battery Charge Indicator, a battery icon located on the upper right hand section of the display,
indicates the current battery level.
• All Battery Indicator segments lit mean the system battery is fully charged.
• Some Battery Indicator segments lit mean the system battery is partially charged.
• When the battery is charging the Battery Indicator segments light sequentially.
Table 4.2 contains the charging specifications for the system.
Table 4.2: System Charging Specification
System Charging Parameter Specification
Charge time to 80% capacity, with System power off 3 hours @ 25° C
Charge time to 80% capacity, with System power on 6 hours @ 25° C
26 Chapter 4: Setup and Operation
Page 35

4.6.3 Connecting to AC Power
Power Supply
Power
Strip
Power
Out
AC
Power
To AC Power
(wall outlet)
A
C
G
Mini-dock
B
Printer
VCR
Audio
In
S-Video Composite
AC In
To
PC
Video- InRemote Out
AC In
Video
In
EF
Audio
Out
D
Video
Out
RS 232
Dip switches
1-4 Down
5,6 Up
Figure 4.3 Connectivity Diagram
To connect AC power to the docking system:
Note: The AC power cord to the power supply and the DC power cord from the power supply are preinstalled.
1 Remove back panel.
2 Connect the system AC power cord to the power strip on the top shelf of the mobile docking
system.
A country specific AC power cord is provided.
3 When ready to use, route the AC power cord out the back, and replace the back panel.
4 Connect the system AC power cord to a hospital-grade electrical outlet.
To connect AC power using the mini-dock:
1 Insert the ultrasound system into the mini-dock.
2 Connect the DC power cable from the power supply to the connector on the mini-dock.
3 Connect one end of the system AC power cord into the power supply. Then plug the other end
into a hospital-grade electrical outlet.
Chapter 4: Setup and Operation 27
Page 36

4.6.4 Connecting and Removing Transducers
Warning: The transducer connector can become hot during operation. This is normal. Operate
the system in the docking system or on a flat, hard surface to allow air flow past the
connector.
Caution: The electrical contacts inside the system transducer connector may be damaged by
foreign material. Keep foreign material out of the connector.
Figure 4.4 Connect the Transducer
To connect the transducer:
1 Turn the system upside down (if not in docking system).
2 Pull the transducer latch up and rotate it clockwise.
3 Align the transducer connector with the connector on the bottom of the system.
4 Insert the transducer connector into the system connector.
5 Turn the latch counterclockwise.
6 Press the latch down, securing the transducer connector to the system.
To remove the transducer:
1 Pull the latch up and rotate it clockwise.
2 Pull the transducer connector away from the system.
4.6.5 Turning the System On and Off
To turn the system on/off:
Caution: Do not use the system if an error message appears on the display. Note the error code
and turn off the system. Call SonoSite or your local representative.
1 Locate the Power key on the top left side of the system. See Figure 4.1 on page 21.
2 Press the Power key once to turn on and once to turn off.
28 Chapter 4: Setup and Operation
Page 37

To wake up the system:
To conserve battery life, the system is configured to go into sleep mode. The system goes into sleep
mode when the lid is closed or if the system has not been touched for a preset amount of time. Press
any key, touch the touchpad, open the lid to wake up the system. To adjust the time for sleep delay,
see “Sleep delay” on page 23.
4.7 Upgrading the System Software
As described in “About the System Software” on page 4, software upgrades are provided on
CompactFlash cards, which are installed in the rear CompactFlash slot on the right hand side of the
system. Upgrades provided may be required or optional.
Whenever you install a CompactFlash card containing a newer version of software into the system, the
system will determine the level of software, prepare the system for the upgrade, and then install the
new software onto the system.
To upgrade the system software:
1 Insert CompactFlash card into the back slot.
The system displays the following message:
Figure 4.5 Upgrade System Software
2 Press Yes to accept and No to reject the upgrade.
Note: If you do not perform the upgrade:
• the new transducer is not available
• the new features are not enabled
• the new software benefits are not available
Chapter 4: Setup and Operation 29
Page 38

When you have accepted the upgrade, the system loads the new software and displays the
following message:
Figure 4.6 System Software Loading
Note: The system upgrade can take up to 10 minutes; however, many software upgrades will be completed
in less time.
When the software upgrade has prepared the system for upgrade, the system displays the
following message:
Figure 4.7 System Software Step 1 Restart
3 Press Restart.
If the software upgrade is unsuccessful, the system displays an error code and you must contact
SonoSite technical support at 1-877-657-8118.
30 Chapter 4: Setup and Operation
Page 39

After restart, the system goes into the upgrade process. The system displays the following
message:
Figure 4.8 System Software Installation
When the system preparation is completed, the system displays the following message:
Figure 4.9 System Software Step 2 Restart
4 Press Restart.
During the restart, the initial system screen shows a progress indicator. The progress indicator is
present while the system is replacing its operating software and disappears when the process is
completed.
When the operating software has been replaced, the system presents you with the license update
screen so that you may license the software.
Chapter 4: Setup and Operation 31
Page 40

Figure 4.10 System Software License Key
At this point, the software upgrade process is complete, but the system software is not yet licensed.
The following section explains how to license your software.
4.7.1 Obtaining a License Key
A license key is required to update your system. It may be obtained by contacting SonoSite, Inc.
Technical Support Department.
USA/Canada Customers
• Technical support: 1-877-657-8118
• Technical support fax: 1-425-951-6700
• Technical support e-mail: service@sonosite.com
• SonoSite website: www.sonosite.com
International Customers
• Contact your local representative
or call 425-951-1330
• Technical support fax: 425-951-6700
• Technical support e-mail: service@sonosite.com
• SonoSite website: www.sonosite.com
To receive your license key, you will need to provide the following information, which is displayed on
the system information screen of your system:
• Name of the person installing the upgrade
• System serial number (located on the bottom of your system)
• ARM version
• PCBA serial number
and select Products & Solutions and then
Technical Support
and select Products & Solutions and then
Technical Support
32 Chapter 4: Setup and Operation
Page 41

4.7.2 Installing a License Key
When you have obtained a license key for your software, you must enter it into the system. Once a
valid license key has been entered, the system remains licensed until the next time the system software
is upgraded.
1 Turn on the system.
If the software is not yet licensed, the license update screen displays.
The license update screen displays the following information: how to contact SonoSite, and the
required information to obtain the License Update number, and the grace period (time remaining)
on your system.
Figure 4.11 License Screen
Note: The software versions on your system may vary based on your upgrade and configuration.
2 Enter your license key in the license number field.
If the license key that you entered is recognized by the system as being valid for your system and
the software you installed, Done appears on-screen.
3 Select Done from the on-screen menu to install the license key and license your software.
If the license key that you entered is not recognized by the system, the Cancel button remains on
the screen as long as the defined grace period has not expired.
If the grace period has expired, the menu item will indicate this by showing zero hours remaining
in the grace period. At this point, you must then enter a valid license key before you can use the
system.
Note: If you have entered a valid license key and you cannot complete the licensing procedure, verify that the
license key has been entered correctly. The license key should be exactly 12 digits (for example, 123348990552)
with no other characters or punctuation.
Note: If after confirming correct entry of the license key, you are still unable to license your system, call
SonoSite technical support. USA/Canada customers call 1-877-657-8118. International customers call your
local representative or 1-425-951-1330.
If the system is on and the grace period expires, the license update screen must be displayed from the
system information screen. See “System Information” on page 24.
Chapter 4: Setup and Operation 33
Page 42

4.7.3 To Display the System Information Screen
1 Press the Setup key.
2 Select System Information from the on-screen menu.
The system information screen displays the following information: Product, Modes, Previous License
Update, Boot Version, ARM Version, DSP Version, PCBA Serial Number, PLD, CPLD Version, SH
Database Version, and SH Serial Number.
Note: The software versions on your system may vary based on your upgrade and configuration.
Figure 4.12 System Information Screen
34 Chapter 4: Setup and Operation
Page 43

4.7.4 To Display the License Update Screen
1 Press the Setup key.
2 Select System Information from the on-screen menu.
3 On the lower section of system information screen, select the button under License.
The license update screen displays.
4 Perform the steps in “Installing a License Key” on page 33.
Figure 4.13 Setup Screen: License Key
Chapter 4: Setup and Operation 35
Page 44

36 Chapter 4: Setup and Operation
Page 45

Chapter 5: Cleaning and Disinfecting
5.1 Universal Precautions
SonoSite recommends that personnel who have regular exposure to medical devices returned for
service practice “universal precautions.” Universal precautions are an approach to infection control.
Those servicing this product should follow the prescribed standards for their area.
5.2 Receipt of Suspected Contaminated Materials
SonoSite recommends that personnel who have regular exposure to medical devices returned for
service practice “universal precautions.” Universal precautions are an approach to infection control.
Those servicing this product should follow the prescribed standards for their area.
If visual inspection suggests possible contamination when opening a product returned for service,
take proper steps to contain the contamination. Wear necessary Personal Protective Equipment (PPE)
(gloves, masks, and gowns) when opening or examining a suspect package.
Before transfer to a service area, label the suspect package “contaminated” and seal it to prevent
exposure.
Discard any packing materials removed from a package suspected of contamination in a biohazard
container.
Discard any contaminated materials received with the product in an appropriate biohazard container.
Contaminated materials may include biohazardous waste and sharps.
Maintain a disinfecting agent in case any work surface is contaminated. The recommended agent is
0.5% sodium hypochlorite (bleach) solution. To prepare the agent, mix one part household bleach
(5.25% - 6% sodium hypochlorite) to nine parts water. Spray or wipe the solution onto the work surface
and allow to air dry.
Please use these recommendations when cleaning or disinfecting your ultrasound system,
transducers, and accessories. This chapter assists in effective cleaning and disinfection, but it is also
intended to protect the system and transducers against damage during cleaning or disinfection.
For more information about cleaning or disinfecting solutions or ultrasound gels for the transducer,
call SonoSite technical support or your local representative. For information about a specific product,
call the product manufacturer.
5.3 Recommended Disinfectants
For a list of disinfectants recommended for use on the system and transducers, see the TITAN
Ultrasound System User Guide.
Chapter 5: Cleaning and Disinfecting 37
Page 46

38 Chapter 5: Cleaning and Disinfecting
Page 47

Chapter 6: Troubleshooting
6.1 Basic Troubleshooting
This chapter contains information to help you correct problems with system operation and provides
instructions on the proper care of the system, transducer, and accessories.
If you encounter difficulty with the system, use the information in this chapter to help correct the
problem. If the problem is not covered here, contact SonoSite technical support at the following
numbers or addresses:
USA/Canada Customers
• Technical support: 1-877-657-8118
• Technical support fax: 1-425-951-6700
• Technical support e-mail: service@sonosite.com
• SonoSite website: www.sonosite.com
Technical Support
International Customers
• Contact your local representative
or call 425-951-1330
• Technical support fax: 425-951-6700
• Technical support e-mail: service@sonosite.com
• SonoSite website: www.sonosite.com and select Products & Solutions and then
Technical Support
and select Products & Solutions and then
Table 6.1: Troubleshooting
Symptom Solution
System will not power on. Check all power connections.
Perform the following sequence: remove DC input connector and
battery; wait 10 seconds; connect DC input or install battery; press the
power key.
Ensure the battery is charged.
System image quality is poor. Adjust the LCD screen to improve viewing angle.
Adjust the brightness, as necessary, to improve image quality.
Adjust the gain.
Zoom does not work. Press Freeze key. Zoom does not work when the image is frozen.
No CPD image. Adjust the gain.
No DCPD image. Adjust the gain.
No OB measurement selections. Select the OB or Gyn exam type.
Print does not work. Set the correct printer in system setup.
Check the printer connections.
Check the printer to ensure that it is turned on and set up properly. See
the printer manufacturer’s instructions, if necessary.
Chapter 6: Troubleshooting 39
Page 48

Table 6.1: Troubleshooting (Continued)
Symptom Solution
VCR does not record. Check the VCR connections.
Check the VCR to ensure that it is turned on and set up properly. See
the VCR manufacturers’ instructions, if necessary.
External monitor does not work. Check the monitor connections.
Check the monitor to ensure that it is turned on and set up properly.
See the monitor manufacturers’ instructions, if necessary.
Unexpected labels using the
function keys.
Inaccurate fetal age calculation. Ensure that the patient information, date, and time are set accurately.
System does not recognize the
transducer.
Text cursor does not move when
touchpad or arrows are selected.
A maintenance icon displays
on the system screen
.
6.2 Periodic Maintenance
There is no recommended periodic or preventive maintenance required for the system, transducers,
or accessories. There are no internal adjustments or alignments required. There are no functions that
require periodic testing or calibration. All maintenance and/or performance tests are described in
Chapter 8, “Performance Testing” of this manual. Performing maintenance activities not described in
this manual may void the product warranty.
Local regulations may require electrical safety testing.
Contact SonoSite Technical Support for any maintenance questions.
Ensure labels have been assigned to the function keys.
Disconnect and reconnect the transducer.
Text cursor is constrained to one line.
This icon indicates that system maintenance may be required. Record
the number in parentheses on the C: line and contact SonoSite or your
SonoSite representative.
40 Chapter 6: Troubleshooting
Page 49

6.3 System and Subsystem Diagnosis
This section covers basic diagnostic and troubleshooting procedures you may follow if the system
does not operate properly. To diagnose system failures, consult the referenced diagnostic figures that
follow or the SonoSite Technical Support department.
Table 6.2: Troubleshooting Subassemblies and Diagnostic Figures
Subassemblies Diagnostic Figures
Display Figure 6.2
Control Panel Figure 6.3
System Figure 6.4
Battery Figure 6.5
Mini-Dock/Mobile Docking System Figure 6.6
6.4 System Repair
The system is repairable through subassembly replacement or through replacement of parts as
recommended by SonoSite in Chapter 7, “Replacement Procedures” of this manual. Component level
repair of Printed Circuit Board Assemblies is performed only at the SonoSite repair facility.
Replacement of board level components by unauthorized service facilities voids the SonoSite
warranty.
6.5 Test Equipment
Test equipment is not required for this troubleshooting section. Troubleshooting test aids include an
external monitor and a spare battery.
6.6 Failure Modes
6.6.1 Display
Attach an external monitor to the external video connector to verify display failures. For example, if
the system display is blank and the external monitor works properly, the system display requires
servicing.
Follow the Display Flow Diagram (Figure 6.2) to evaluate the cause of failure.
6.6.2 Control Panel
Go to the patient information screen and press each individual key on the keyboard to identify and
verify control panel failures. Press function keys and note their response.
Follow the Control Panel Flow Diagram (Figure 6.3) to evaluate the cause of failure.
Chapter 6: Troubleshooting 41
Page 50

6.6.3 System/Main PCBA
The main PCBA may present symptoms that are difficult to assess. Main PCBA failures typically result
in “assert codes” that are output to the display. If an assert code should display, note the assert code
and contact SonoSite technical support to clarify the failure. Figure 6.1 shows an assert code and a
maintenance icon displayed on the system screen.
Follow the System Flow Diagram (Figure 6.4) to evaluate the cause of failure.
Figure 6.1 Assert Screen
Assert code
6.6.3.1 Clearing a System Assert Code
After the assert code has been recorded, power down the system.
1 Press the Power key on the system and release it.
2 Turn the power back on to check if the fault cleared or if the condition remains.
If the condition cleared, you may use the system. If the condition remains, corrective action must
be taken before the system can be used. Contact SonoSite Technical Support for assistance or repair
parts.
3 If the Power key is not functional, all sources of power must be removed to allow the system to
power down.
6.6.4 Battery
If the system does not operate or does not run for the expected duration for a given charge, battery
failure may have occurred.
Follow the Battery Flow Diagram (Figure 6.5) to evaluate the cause of failure.
42 Chapter 6: Troubleshooting
Page 51

6.7 Troubleshooting Flow Diagrams
6.7.1 Display
Figure 6.2 Display Flow Diagram
Chapter 6: Troubleshooting 43
Page 52

6.7.2 Control Panel
Figure 6.3 Control Panel Flow Diagram
44 Chapter 6: Troubleshooting
Page 53

6.7.3 System
Figure 6.4 System Flow Diagram
Chapter 6: Troubleshooting 45
Page 54

6.7.4 Battery
Figure 6.5 Battery Flow Diagram
46 Chapter 6: Troubleshooting
Page 55

6.7.5 Mini-Dock/Mobile Docking System
Figure 6.6 Mini-Dock and Mobile Docking System Flow Diagram, Part 1
Chapter 6: Troubleshooting 47
Page 56

Figure 6.7 Mini-Dock and Mobile Docking System Flow Diagram, Part 2
48 Chapter 6: Troubleshooting
Page 57

Chapter 7: Replacement Procedures
7.1 Display Replacement
Note: Consult Chapter 6, “Troubleshooting” before making any repairs.
7.1.1 Required Parts
Service Assembly, Display, TITAN (P03861)
7.1.2 Required Tools
• #1 Phillips screwdriver
• Torque screwdriver, 2.0–10.0 in./lb. (0.23–1.1 newton meter)
• 8 mm nut driver
• An anti-static mat
•A wrist grounding strap
Caution: Always use correct ESD procedures. ESD damage is cumulative and may not be
noticeable at first. Initial ESD symptoms may be slightly degraded performance or
image quality.
7.1.3 Display Removal
1 Remove the battery from the system. See Section 4.6.1, “Installing and Removing the Battery,” on
page 25 for battery removal.
2 Remove the two screws from the back of the system per Figure 7.1.
Screws (2)
Figure 7.1 System Rear
3 Lay the system on the top and remove the two screws from the bottom of the system per Figure 7.2.
Chapter 7: Replacement Procedures 49
Page 58

Screws (2)
Figure 7.2 System Bottom
4 Turn the system over, fully open the display, and lift off the Control Panel per Figure 7.3.
Figure 7.3 Control Panel Removal
50 Chapter 7: Replacement Procedures
Page 59

5 Disconnect the two connectors from the display to the Main PCBA per Figure 7.3.3.5.
Connectors (2)
Figure 7.4 Display Connectors
6 Remove the four screws from the Display Hinges per Figure 7.5.
Screws (4)
Figure 7.5 Display Screws
7.1.4 Display Replacement
1 Set the new display in place.
2 Install the four screws that hold the Display in place. Torque the screws to 5.5 inch pounds.
3 Connect the two connectors that connect the Display to the Main PCBA.
4 Place the Control Panel in place.
5 Reinstall the four screws that hold the Control Panel in place. Torque the screws to 5.5 inch
pounds.
Chapter 7: Replacement Procedures 51
Page 60

7.1.5 Test the Display
1 Replace the battery, attach an external power supply, or attach a mini-dock.
2 Press the Power key to apply power to the system.
3 Verify the display operates correctly.
7.2 Control Panel Subassembly Replacement
7.2.1 Required Parts
• P03862 Service Assembly, Control Panel TITAN, English or
• P03863 Service Assembly, Control Panel TITAN, English, International, or
• P03864 Service Assembly, Control Panel TITAN, French, or
• P03865 Service Assembly, Control Panel TITAN, German, or
• P03866 Service Assembly, Control Panel TITAN, Italian, or
• P03867 Service Assembly, Control Panel TITAN, Spanish, or
• P03868 Service Assembly, Control Panel TITAN, Portuguese
7.2.2 Required Tools
• #1 Phillips screwdriver
• Torque screwdriver, 2.0–10.0 in./lb. (0.23–1.1 newton meter)
• 8mm nut driver
• An anti-static mat
• A wrist grounding strap
Caution: Always use correct ESD procedures. ESD damage is cumulative and may not be
noticeable at first. Initial ESD symptoms may be slightly degraded performance or
image quality.
7.2.3 Control Panel Removal
1 Remove the two screws from the rear of the system per Figure 7.1.
2 Remove the two screws from the bottom of the system per Figure 7.2.
3 Turn the system over, fully open the display, and lift off the Control Panel per Figure 7.3.
7.2.4 Control Panel Replacement
1 Place the new control panel in place.
2 Install the four screws removed in Section 7.2.3. Torque the screws to 5.5 inch pounds.
52 Chapter 7: Replacement Procedures
Page 61

7.3 Main System Disassembly for Repair and/or Replacement
7.3.1 Required Parts
Parts for the Main System Repair could include any of the following
• P03871 Service Assembly Main PCBA, TITAN
• P03870 Service Assembly Power Supply, TITAN
• P03869 Service Assembly TGC, TITAN
• P03872 Service Assembly Speaker, TITAN
• P03873 Service Assembly Upper Enclosure, TITAN
• P03874 Service Assembly Lower Enclosure, TITAN
Note: Replacing the enclosure bottom requires printing a new label for the product. This must be printed
prior to shipping the enclosure bottom. You will be required to provide the information to print this label.
• Nest Frame Assembly, TITAN (order these parts individually)
• P00364 Connector, Interposer
• P00524 Screw, Shoulder, Thrust Plate
• P00353 Wear Plate
• P00646 Spring, Thrust Plate
• P02860 Nest Plate, Interposer
• P00352 Shield, Perimeter, Long
• P00527 Shield, Perimeter, Short
• P02861 Post, Mounting
7.3.2 Required Tools
• #1 Phillips screwdriver
• Torque screwdriver, 2.0–10.0 in./lb. (0.23–1.1 newton meter)
• 8 mm nut driver
• An anti-static mat
•A wrist grounding strap
Caution: Always use correct ESD procedures. ESD damage is cumulative and may not be
noticeable at first. Initial ESD symptoms may be slightly degraded performance or
image quality.
7.3.3 Main PCBA Removal
1 Remove the display and the control panel from the system following the removal procedures in
Section 7.1.3 and Section 7.2.3.
2 Remove the additional 4 screws from the bottom of the system per Figure 7.6.
Chapter 7: Replacement Procedures 53
Page 62

Screws (4)
Figure 7.6 Bottom Screws
3 Turn the system over and remove the top enclosure from the main PCBA. This exposes all of the
replaceable parts for the main system per Figure 7.7.
54 Chapter 7: Replacement Procedures
Page 63

Main PCBA
Power supply
Figure 7.7 System Components
7.3.3.1 Speaker Replacement
1 Press on the connector release and pull the connector out of the receptacle.
2 Gently pry off the retaining clip with a flat bladed pry tool. See Figure 7.8.
Nest frame
assembly
TGC assembly
SpeakerSpeaker
Connector
Retaining clip
Figure 7.8 Speaker Replacement
Chapter 7: Replacement Procedures 55
Page 64

7.3.3.2 Power Supply
1 Gently pry the shield from the power supply and set it aside. This part will be used in reassembly.
Note that the shield fits only one way. See Figure 7.9.
Power supply
shield
Figure 7.9 Power Supply Shield
2 Remove the 7 screws that hold down the power supply PCB per Figure 7.10.
3 Gently lift the power supply and shield assembly away from the Main PCBA.
Screws (7)
Figure 7.10 Power Supply Screws
56 Chapter 7: Replacement Procedures
Page 65

7.3.3.3 TGC PCBA
1 Remove the TGC knobs identified in Figure 7.11.
TGC knobs (3)
Figure 7.11 TGC Knobs
2 Remove the flex cable from the TGC PCB by lifting on the flex release tab. See Figure 7.12.
3 Remove the flex cable from the Main PCBA by lifting gently on the flex release tab.
4 Remove the two screws holding the TGC PCBA in place.
Figure 7.12 TGC Removal
Release tabs Screws (2)
Chapter 7: Replacement Procedures 57
Page 66

7.3.3.4 Main PCBA
1 Remove the 3 screws holding the Main PCBA in place per Figure 7.13.
2 Remove the 4 shoulder bolts holding the transducer nest frame assembly in place. As you remove
the nest frame assembly from the PCBA tilt the PCBA and enclosure to almost vertical to avoid
spilling the Interposer Connectors from the Assembly.
3 Disconnect the speaker wires from the Main PCBA.
4 Remove the TGC PCBA following the procedure in Section 7.3.3.3.
Screws (3)
CompactFlash
release buttons
Shoulder bolts (4)
Figure 7.13 Main PCBA Screws
5 Lift on the edge of the Main PCBA closest to the system handle.
Note: As the Main PCBA is removed press in on the CompactFlash release buttons so they clear the opening in
the bottom enclosure.
7.3.3.5 Main PCBA Replacement
Replace the Main PCBA by following the reverse of the removal procedure. Do not tighten all the
screws until everything is in place.
1 Replace the Main PCBA.
2 Replace the Nest Frame Assembly.
3 Reconnect the speaker wires.
4 Replace the power supply assembly.
5 Replace the TGC assembly.
6 Reinstall the shield to the Power Supply.
7 Tighten all screws to their specified torque of 5.5 inch pounds.
58 Chapter 7: Replacement Procedures
Page 67

Chapter 8: Performance Testing
8.1 Overview
Warning: Critical Test Function — A failure of the system functions tested in this section could
affect safety or effectiveness of the system adversely. While performing the steps in this
section, verify that the images on the system display and on the external monitor are
acceptable.
• Verify that all controls operate smoothly over their full range and that the system responds
properly.
• To obtain 2D images, SonoSite recommends using the RMI 413A Soft Tissue Phantom or the RMI
403 GS Multipurpose Phantom. Any equivalent Phantom is acceptable.
• To obtain Power Doppler images, SonoSite recommends using the RMI 425 Doppler Phantom or
the RMI 1425A Doppler Phantom. Any equivalent Phantom is acceptable.
• When making penetration measurements on a phantom, apply the phantom reference value and
tolerance to the measurement.
8.2 Test Equipment
• SonoSite ultrasound system under test
• C60/5-2 MHz transducer
• C15/4-2 MHz transducer
• RMI 413A Soft Tissue Phantom, RMI 403 GS Multipurpose Phantom, or the equivalent
• RMI 425 Doppler Phantom, RMI 1425A Doppler Phantom, or the equivalent
• Video Printer
•External Monitor
•Acoustic gel
8.3 Setting Up Performance Tests
To set up the performance tests:
1 Attach the C60/5-2 MHz transducer to the system.
2 Select general for optimization and OB for exam type.
3 Couple the transducer to the phantom, adjusting gain settings and transducer for a proper
phantom image (e.g., pins are high-level echoes positioned in straight lines; cysts are sonolucent,
edges are sharp, and graphite particles of the phantom are mid-grays).
Chapter 8: Performance Testing 59
Page 68

8.3.1 Scan Reference Orientation
To set up the scan reference orientation:
1 Verify that the correct transducer name appears in the upper right corner of the system display.
2 Verify that the scan plane orientation mark in the image located near the skinline corresponds to
element #1 on the transducer. To test, put your finger on the probe and run it across the transducer
face. Your finger touching the transducer face should appear at the orientation mark on the display
image format.
3 With the array pointing down and the orientation mark to the operator’s left, element #1
corresponds with the left side of the array.
8.4 Testing 2D Performance
To test 2D performance:
1 Use a C60/5-2 MHz transducer in 2D mode.
2 Adjust the position of the C60/5-2 MHz transducer on the phantom.
3 Use the 2D system controls to obtain a clear image that shows both the horizontal and vertical rows
of pins.
8.4.1 2D Image Quality
To test 2D image quality:
1 Verify that the ultrasound image appears uniform in both the axial and lateral direction, with no
dropouts or intensity variations.
2 Verify that the cystic structure at the focal zone is clearly differentiated from the surrounding
tissue and is echo-free, while solid tissue, with numerous echo sources, appears solid.
8.4.2 Axial Measurement Accuracy
Note: Measurements must be performed while the image is frozen.
To set up axial accu racy:
1 Acquire the image.
2 Press the Freeze key.
3 Press the Caliper key. The caliper appears on the image display. The screen menu indicates Cal 1,
Cal 2, and Ellipse. (If the caliper line setup is on, then a dotted line connects the two calipers. See
the TITAN Ultrasound System User Guide, if necessary.) The Cal 1 caliper is active by default.
4 Use the touchpad to position one of the calipers.
5 Press the Select key to fix the caliper and enable the other caliper.
6 Use the touchpad to move the other caliper. The results update as you move the caliper, and the
measurement is complete when you finish moving the calipers. (Press the Select key to alternate
the active caliper, and adjust the measurement with the touchpad.)
7 To perform another distance measurement on the image, select the other distance icon and repeat
the preceding steps.
60 Chapter 8: Performance Testing
Page 69

To test axial accuracy:
1 Measure the distance, center to center, of any two pins that are 5-12 cm apart vertically.
2 Verify that the distance measured is within the tolerance listed in Table 8.1.
8.4.3 Lateral Measurement Accuracy
To set up the lateral measurement accuracy:
Perform steps 1 through 7 in Section 8.4.2.
To test the lateral measurement accuracy:
1 Measure the distance, center to center, of any two pins that are 4-10 cm apart horizontally.
2 Verify that the distance measured is within the tolerance listed in Table 8.1.
3 Press the Freeze key to return the system to live 2D mode.
Table 8.1: System Measurement Accuracy
Measurements Tolerance
Axial Distance +/- 2%
Lateral Distance +/- 2%
8.4.4 Penetration
To test penetration:
1 Adjust the system controls to obtain a clear image that shows the limits of echo penetration as
shown in Table 8.2.
2 Measure from the center of the skinline to the deepest vertical position—where the scatter echoes
start to break up and tissue definition is lost.
Table 8.2: Imaging Performance
Imaging Performance C60 ICT C15 L38 C11
2D Penetration 11.5 cm 5.0 cm 19.0 cm 6.0 cm 5.0 cm
Chapter 8: Performance Testing 61
Page 70

8.5 Additional Performance Tests
8.5.1 CPD
To test CPD:
Note: Use the RMI 425 Doppler Phantom or the RMI 1425A Doppler Phantom.
1 Connect any transducer and set up the system for CPD mode.
2 Acquire the image.
3 Press and release the Color key for CPD/DCPD mode. Select CPD from the on-screen menu. A
Region of Interest (ROI) box is displayed on top of the grayscale image. (Press the 2D key to return
to 2D imaging.)
To move the CPD image:
• Use the touchpad to move the CPD ROI. While you are moving the CPD ROI, you will see an
outline of the new position moving on the display. When you stop moving, the new position will
display the CPD ROI. (The size of the CPD ROI is fixed. There is no control to change it.)
To adjust CPD gain:
Turn gain clockwise to increase the amount of CPD gain. (While in CPD imaging, near and far affect
only the 2D image; they do not affect the CPD image.)
Turn gain counterclockwise to decrease the amount of CPD gain.
1 Image the vessel using a Doppler phantom. Verify that as the gain controls increase and decrease,
Doppler echo intensity increases and decreases to correspond. Verify that no flow exists outside
the vessel.
2 Save a CPD image by pressing the Freeze key and then the Save key.
8.5.2 Directional Color Power Doppler (DCPD)
To test DCPD:
Note: Use the RMI 425 Doppler Phantom or the RMI 1425A Doppler Phantom.
1 Set up the system per Section 8.5.1 for CPD mode.
2 Select DCPD. Press DCPD on the on-screen menu while in CPD mode. A Region of Interest (ROI)
box is displayed on top of the 2D grayscale. (Press the 2D key to return to 2D imaging.)
To move the DCPD image:
• Use the touchpad to move the DCPD ROI. While you are moving the DCPD ROI, you will see an
outline of the new position moving on the display. When you stop moving, the new position will
display the DCPD ROI. (The size of the DCPD image is fixed. There is no control to change it.)
62 Chapter 8: Performance Testing
Page 71

To adjust DCPD gain:
• Turn gain clockwise to increase the amount of DCPD gain. (While in DCPD imaging, near and far
affect only the 2D image; they do not affect the DCPD image.)
• Turn gain counterclockwise to decrease the amount of DCPD gain.
1 Image the vessel using a Doppler phantom. Verify that as the gain controls increase and decrease,
Doppler echo intensity increases and decreases to correspond. Verify that no flow exists outside
the vessel.
2 Save a DCPD image by pressing the Freeze key and then the Save key.
8.5.3 M Mode Imaging
To test M Mo de Imaging:
1 Attach a C60 transducer and acquire an image.
2 Press the MMode key for the M Mode sample line.
3 Position the M Mode sample line over the image using the touchpad.
4 Press the MMode key again to turn on M Mode.
5 Select the desired sweep speed from the on-screen menu (slow, med, or fast). The on-screen menu
will show the selected sweep speed.
6 Press the Freeze key to freeze the image. Press it again to return to live imaging.
7 Press the 2D key to return to 2D imaging.
8.5.4 Tissue Harmonic Imaging
To test THI Imaging:
1 Attach the C60 transducer and acquire an image.
2 Set the depth to maximum and note the depth at which echo information is lost.
3 Press the THI key on the control panel so it displays THI on the display. Tissue Harmonic Imaging
in now active.
4 Observe a decrease in dot size and a significant loss in penetration due to the higher frequency.
Image resolution increases.
5 Press the THI key again to turn off Tissue Harmonic Imaging.
8.5.5 Pulsed Wave (PW) Doppler Imaging
To test PW Doppler Imaging:
1 Attach the C15 transducer.
2 Press the Doppler key for the Doppler sample gate.
3 Press the Doppler key again for the Doppler spectral trace.
4 Place a large drop of ultrasound gel on the transducer lens.
5 Gently tap the top of the gel and observe a reflection on the spectral trace and the sound from the
speakers.
6 Press the 2D key to return to 2D imaging.
Chapter 8: Performance Testing 63
Page 72

8.5.6 Image Quality Verification Test/Livescan
• Products with replaced subassemblies, or products that have been otherwise disassembled, must
undergo an Image Quality Verification Test/Livescan.
• The Image Quality Verification Test/Livescan should be performed after successfully completing
Section 8.3, “Setting Up Performance Tests,” on page 59 and Section 8.5.1, “CPD,” on page 62.
• The test is completed before returning the system to service.
• A certified sonographer must perform the test.
• The Livescan test performed is at the discretion of the Sonographer and will represent their
acceptance of a successful service event.
8.5.7 Image Review
Review all saved images and verify that the images are displayed properly.
8.5.8 Printer
To test printer operation:
1 Print two images in rapid succession and verify proper operation.
2 Verify that the print control on the system functions correctly.
8.5.9 Battery Charging
To test battery charging operation:
1 Remove the system from the Docking System and insert a battery into the system.
2 Press the Power key to turn the system on. Allow the battery to discharge. The battery indicator
icon on the display, below the Transducer Type indicator, will extinguish from left to right as the
battery discharges.
Note: The battery may take 1–2 hours to discharge.
3 Reattach the system to the Docking System and attach the AC power cord to the power connector.
4 Note that the battery indicator indicates that the battery is charging. The sections of the battery
indicator will light sequentially from left to right as the battery charges.
5 If charging is not evident, see Chapter 6, “Troubleshooting” for troubleshooting procedures.
8.5.10 Video Output
Caution: Use only the recommended video monitor, printer, or VCR when verifying the video
output at the video receptacle.
To test the video output:
1 Attach an external video monitor to the video connector using the video cable.
2 Turn on the system power and verify that the video on the external monitor matches the video on
the system display.
If the video does not appear similar, or there is no display on the external monitor, see Chapter 6,
“Troubleshooting” for troubleshooting procedures.
64 Chapter 8: Performance Testing
Page 73

8.6 Returning Products to SonoSite
8.6.1 Contacting SonoSite Technical Support
For technical support of any SonoSite product, do one of the following:
• USA/Canada customers: 1-877-657-8118
• International customers: 425-951-1330
• Technical support fax: 1-425-951-6700
• Technical support e-mail: service@sonosite.com
• SonoSite website: www.sonosite.com
Technical Support
You will be asked to provide the following information by telephone or e-mail:
• Contact name and phone number
• Product name
• Serial number
• Description of the problem
8.6.2 Shipping Instructions
Please contact SonoSite to get a return material authorization number (RMA). Contact SonoSite before
returning any product.
The shipping address for all returned products is:
SonoSite, Inc.
Attn: Technical Support RMA ___________________
21919 30th Drive SE
Bothell, Washington 98021
USA
and select Products & Solutions and then
Chapter 8: Performance Testing 65
Page 74

66 Chapter 8: Performance Testing
Page 75

Chapter 9: Accessory Service
This chapter contains information on servicing the TITAN mobile docking system (docking system)
and the TITAN mini-dock. For information about installation and operating instructions for these
peripherals consult the TITAN Ultrasound System User Guide.
9.1 Mobile Docking System
The docking system provides a mobile work platform and recharges the internal battery. The docking
system includes the TITAN mini-dock with connections to an external printer and/or monitors, power
supply, USB connection to SiteLink Image Manager, VGA connection, and S-Video connection. The
docking system provides a storage area for the transducers and other supplies.
1
8
2
3
4
5
6
7
Front view Rear view
9
10
11
12
13
14
15
Figure 9.1 Docking System, Front and Rear Views
Table 9.1: Docking System Features
Number Description
1 Ultrasound system
2 Transducer, connector, and gel storage on both sides of docking system
Chapter 9: Accessory Service 67
Page 76

Table 9.1: Docking System Features (Continued)
Number Description
3Side panels
(Use to lift, raise, or transport the docking system)
4 Handle release button
5 Transducer storage
6 Height adjustment pedal
(Press down on foot pedal, grab sides and raise/lower)
7 Locking wheels
(Press down/pull up on locks to lock/unlock)
8 CompactFlash shield
9 Wrist rest/handle
10 Cable hooks
11 Printer bay (black/white only)
12 VCR bay
13 Mini-dock cable cover
14 Mini-dock
15 Storage
To insert system:
1 Push in the handle release button and lift the handle to the upper most position.
2 Close the lid on the ultrasound system and slide into the docking system. Connection will be made
when handle is pushed down.
3 Press the handle down until an audible click is heard.
Warning: To prevent injury, do not use the handle to lift the docking system over an obstacle or
threshold. Use the side panels to lift over an obstacle or threshold.
To remove s ystem:
1 Close the lid on the ultrasound system.
2 Push in the handle release button and lift the handle to the upper most position.
3 Remove the ultrasound system.
68 Chapter 9: Accessory Service
Page 77

9.2 Mini-Dock
The TITAN mini-dock (mini-dock) provides connectivity for the TITAN ultrasound system when the
system is out of the docking system. The mini-dock provides connections to an external printer and/or
monitors, a power supply, USB connection to SiteLink Image Manager, VGA connection, and S-video
connection.
9.3 Connectivity
Figure 9.2 Mini-Dock Connections
Table 9.2: Mini-Dock Connections
Symbol Definition
DC input
Print control
USB
Ethernet (available on future releases)
RS-232 (VCR control/audio)
S-video out
S-video in (available on future releases)
RGB video out
Composite video out
Audio out
ECG (available on future releases)
Chapter 9: Accessory Service 69
Page 78

Power Supply
Power
Strip
Power
Out
AC
Power
A
C
G
Mini-dock
B
Printer
VCR
Audio
In
S-Video Composite
To
PC
Video- InRemote Out
AC In
Video
In
EF
Audio
Out
D
Video
Out
RS 232
Dip switches
1-4 Down
5,6 Up
To AC Power
(wall outlet)
AC In
Figure 9.3 Connectivity Diagram
9.4 Block Diagrams and Schematics
Figure 9.4 Mini-Dock PCB and I/O PCB Component Placement
70 Chapter 9: Accessory Service
Page 79

Figure 9.5 TITAN Dock Interface Diagram
Chapter 9: Accessory Service 71
Page 80

Figure 9.6 Mini-Dock Video Distribution
72 Chapter 9: Accessory Service
Page 81

CLK_ENCODER
page3
CLK_ENCODER
CLK_PAL_ENC
CLK_NTSC_ENC
RESETn
SDA_3V
SCL_3V
VideoDecoder
CLK_14_318182
CLK_17_734475
RESETn
SCL_3V
SDA_3V
page4
page2
CLK_ENCODER
SCL_3V
SDA_3V
RESETn
SCL_3V
SDA_3V
RESETn
CLK_NTSC_ENC
CLK_PAL_ENC
SystemIo
DvEncoder
PAL Frequency is 17.734475
NTSC Frequency is 14.318182
Signals are connected properly, with net names reversed.
Figure 9.7 Mini-Dock Connectors Schematic, Page 1
Chapter 9: Accessory Service 73
Page 82

1
2
3
4
C93
5V
LUMACHROMA
L2
L1
3VDAVDD
3.3V
CLK_PAL_ENC
80p Diffline CONN
0.1UF
16V
D
R44
1
ENA
4
VCC
INA+
5
100
R40
C41
2.7UH
100
R39
C38
2.7UH
R16
100
C37
0.1UF
16V
D
R15
47.5K
R14
47.5K
CLK_NTSC_ENC
SDA_3V
CLK_ENCODER
REV
SCHEMATIC DIAGRAM
<=+/-.XX=+/-
.XXX= +/-
WHICH IS PROPRIETA RY TO
SONOSITE, INC.
THIS DOCUMENT CONTAINS
CONFIDENTIAL IN FORMATION
C88
0.1UF
4
GND
Vin
GND
Vout
LT1790BCS6-1.25
6
C87
4.7UF
D
BOUT5
BOUT7
BOUT6
DV_ENABLE
R33
R32
49.9
136
ADGND
ADGND
DAGND36DAGND38DAGND
ADVDD
DAVDD34DAVDD44DAVDD
45
C86
0.1UF
C85
0.1UF
3VDAVDD
FB5
0805
3.3V
136
146
156
130
GND
GND
GND
VCC
151
128
50V
50V
D D
FB6
50V
D
50V
D
3.3V
C2 DOCK
NEITHER THE DOCUM ENT NOR
16V
1
2
16V
47.5K
C89
18PF
40
16V
16V
PLLGND
PLLVCC
2 4
D02457
Friday, Decem ber 20, 2 002
DATE SH. OF
1
NONE
B A
SIZE
SCALE
PART, WITHOUT THE EXPRESS
OR REPRODUCED , IN WHOL E OR
WRITTEN CONSENT OF
SONOSITE, INC.
THEREIN SHALL BE DI SCLOSED
THE INFORMATIO N CONTAINED
D
D
D
ADV7120KST30
2
26
DV_CLK_OUT
D
50V
D
D
AL128
D
D
3
D
AL300
C90
0.1UF
16V
D
0805
4
P2-32
18
31
19
30
33
32
D
37.4
R43
7
OUTA
500
500
ENB
INA-
6
3109
D
D
C43
330PF
50V
18PF
50V
C42
330PF
50V
D D
R20
100
D
D
C40
330PF
50V
18PF
50V
C39
330PF
50V
D D
D
30
D
TVCSYNC32TVHSYNC
VRT
134
VRB
135
7
AR
137
AG
132
AB
129
PAL
2
1
DEC
4
INC
3
5
6
152
153
154
158
159
SCL_3V
SDA_3V
SCL_3V
INB+
ADEN
8
OUTB
L3
31
MENU
XOUT2
XOUT1
37.4
500
INB-
D
CVID
151
TVCLK
TVVSYNC
RGBOUT
SELECT
CLKTYPE
XIN2/FIN2
XIN1/FIN1
D
R19
R18
R17
D
D
37.4
R42
14
OUTC
D
11
VEE
500
500
U15
ENC
INC+
500
INC-
2
MAX4019
D
12
13
100
R41
D
C46
330PF
50V
18PF
C44
50V
2.7UH
C45
330PF
50V
D D
R21
100
RSET chosen to give 1V peak to peak analog
video outputs.
C47
0.1UF
16V
D
R23
1.00K
D
R22
150
D
ACMP
AY
AC
RSET
VREF
MD3
MD1
MD2
MD0
41
39
43
35
42
37
MD088MD187MD286MD385MD483MD582MD681MD780MD855MD9
VREF
RSET
COMP
AC/BOUT
AY/GOUT
ACMP/ROUT
GHSDIV
GHSOUT
GHSOUT228GVSOUT
GVSYNC
GHSYNC
GVSOUT227GCLK
146
47.5K
47.5K
47.5K
RED0
145
147
141
140
143
107
106
100
RA0
90
89
88
86
85
84
83
82
81
43
42
41
39
XIN
40
38
50
49
48
47
46
D D
TVCLK1TVHREF2TVVS3TVHS4GVS6GHS7GHREF8GCLK9RIN0/YIN018RIN1/YIN117RIN2/YIN216RIN3/YIN315RIN4/YIN414RIN5/YIN513RIN6/YIN612RIN7/YIN711GIN0/UVIN027GIN1/UVIN126GIN2/UVIN225GIN3/UVIN324GIN4/UVIN423GIN5/UVIN522GIN6/UVIN621GIN7/UVIN720BIN036BIN135BIN234BIN333BIN432BIN531BIN630BIN7
D
MQ11
MQ10
MQ9
MQ8
DO044DO143DO242DO341DO439DO538DO637DO7
U10
DI01DI12DI23DI34DI46DI57DI68DI7
MD10
MD12
MD11
MD9
MD8
MQ0
MQ3
MQ2
MQ4 MQ12
MQ1
DO044DO143DO242DO341DO439DO538DO637DO7
U9
DI01DI12DI23DI34DI46DI57DI68DI7
MD4
MD2
MD0
MD1
MD3
MD[15..0]
MQ[15..0]
MD8
MD7
MD4
MD5
MD6
RED1
RED2
RED3
RED4
RED5
RED6
105
104
103
102
101
RA199RA298RA397RA495RA594RA693RA7
PDSPEN
PVS
PHS
SCLK
PCLKB
PCLKA
GOUT3
GOUT2
GOUT1
SDA
SCL
IREQ
XOUT
HOSTCLK
TEST2
TEST1
YUVIN
I2CADDR
PWRDN
MQ14
MQ15
MQ13
36
9
MD14
MD13
MD15
MQ5
MQ6
MQ7
36
9
MD6
MD5
MD7
MD11
MD12
MD10
MD9
54
MD1053MD1152MD1250MD1349MD1448MD15
RED7
GREEN0
GREEN1
100
117
116
110
109
92
GA0
GA1
30
15
IRDY
IE11RESET
30
15
IRDY
IE11RESET
3.3V
MD13
MD15
MD14
47
U2A
GREEN2
GREEN3
GREEN4
115
114
112
111
108
107
105
104
GA2
GA3
GA4
GA5
3.3V3.3V
ORDY
WCK13WE10WRST14RCK32RE35RRST31OE34TST
PLRTY
16
D
ORDY
WCK13WE10WRST14RCK32RE35RRST31OE34TST
PLRTY
16
D
MQ1
MQ2
MQ0
98
MQ197MQ296MQ0
AL128
GREEN5
GREEN6
GREEN7
110
109
103
102
120
GA6
GA7
C51
0.1UF
16V
18
5
40
VDD29VDD
VDD
AVDD
27
17
OEn
RESETn
C50
0.1UF
16V
18
5
40
VDD29VDD
VDD
AVDD
17
27
RESETn
OEn
MQ3
MQ5
MQ6
MQ4
MQ395MQ493MQ592MQ691MQ790MQ865MQ9
BLUE0
BLUE1
BLUE2
BLUE3
127
126
125
124
119
118
117
115
BA0
BA1
BA2
BA3
BA4
D
22
D
22
MQ7
BLUE4
122
114
BA5
3.3V
12
GND
AGND
3.3V
12
GND
AGND
MQ8
MQ9
64
BLUE5
BLUE6
121
120
113
112
BA6
BA7
U1A
33
26
GND
SDA25SCL24SDAEN
3.3V
33
26
GND
SDA25SCL24SDAEN
3.3V
MQ10
MQ1063MQ1162MQ1260MQ1359MQ14
BLUE7
119
AL300
C49
0.1UF
16V
D
AL440B
NC1
19
GND
NC2
20
NC3
21
NC4
28
23
C48
0.1UF
16V
D D
D
NC1
19
GND
NC2
20
NC3
21
NC4
28
23
AL440B
R34
10K
D
MWENH
MQ11
MQ12
MQ13
MQ15
MQ14
77
57
58
MQ15
SCL13SDA
I2CADDR11RESET
I2C
10
12
SDA_3V
SCL_3V
47.5K
R24
3.3V
RB060RB159RB258RB357RB455RB554RB653RB7
P2-28
P2-29
P2-26
28
29
26
DV_VSYNC_OUT
DV_HSYNC_OUT
D
MRRST
MREN
MWENL
MWRST
78
69
71
66
MREN
MWENL
MWRST
MWENH
INTYPE0
INTYPE1
157
156
148
149
D
52
GB070GB169GB268GB367GB465GB564GB663GB7
29
D
3.3V
MWCLK
73
MRRST
MWCLK
PWRDWN
140
P2-27
27
C52
0.1UF
MRCLK
68
MRCLK
MEMTYP
MEMCONFIG1
MEMCONFIG0
TEST12
TEST11
TEST10
TEST9
TEST8
TEST7
TEST6
TEST5
TEST4
TEST3
TEST2
TEST1
ROMDATA0
ROMDATA1
ROMDATA2
139
138
P2-21
P2-20
21
20
16V
D
ANALOG_R
2.7UH
L4
3.3V
R25
47.5K
72
75
D
76
9
15
16
17
18
20
21
22
23
25
26
33
RESETn
62
BB080BB179BB278BB377BB475BB574BB673BB7
ROMDATA3
ROMDATA4
ROMDATA5
ROMDATA6
137
135
134
133
132
D
C53
ROMDATA7
D
R100
122
C55
18PF
50V
C54
R26
75
RESETB
126
330PF
50V
330PF
50V
100
72
ROMADDR15
ROMADDR14
ROMADDR13
ROMADDR12
ROMADDR11
ROMADDR10
ROMADDR9
ROMADDR8
ROMADDR7
ROMADDR6
ROMADDR5
ROMADDR4
ROMADDR3
ROMADDR2
ROMADDR1
ROMADDR0
OPLLCLK
OHSFB
OHSREF
IHSREF
OCLK
VCOIN
129
D
L5
D
D
U11
160
159
158
157
155
154
153
152
150
149
148
147
145
144
143
142
127
125
124
123
VCOIN1
P2-22
P2-23
22
23
D
ANALOG_G
18PF
C56
2.7UH
R28
5V
FB4
5VRGBDAC
31
VAA12VAA30VAA
GND1GND14GND15GND27GND28GND38GND39GND
R040R141R242R343R444R545R646R7
ROUT3
ROUT1
ROUT2
ROUT0
PLLCLK
R27
47.5K
C58
0.1UF
C57
0.01UF
P2-25
P2-24
25
24
ANALOG_B
C60
330PF
50V
DD
50V
2.7UH
L6
C59
330PF
50V
75
D
0805
C63
0.1UF
16V
D
C62
0.1UF
16V
D
C61
0.1UF
16V
D
G02G13G24G35G46G57G68G7
47
ROUT4
ROUT5
GOUT1
ROUT6
ROUT7
GOUT0
GND14GND19GND29GND51GND56GND67GND74GND84GND94GND
U2B
8
3.3V
16V
D
50V
D
D
C70
330PF
50V
DD
18PF
C68
50V
C69
330PF
50V
R29
75
D
IOB
IOG
IOR
D
48
32
29
33
IOB
IOR
IOG
9
GOUT6
GOUT5
GOUT4
GOUT2
GOUT3
GOUT7
108
VDD
VDD
VDD99VDD89VDD79VDD70VDD61VDD46VDD24VDD
123
113
C78
0.01UF
C67
0.1UF
16V
C66
0.1UF
16V
C77
0.01UF
DDD
C76
0.01UF
C65
0.1UF
16V
D D
C75
0.01UF
C64
0.1UF
16V
D
3.3V
GND5GND10GND28GND45GND56GND71GND76GND87GND
U1B
19
C74
0.1UF
C73
0.1UF
C72
0.1UF
C71
0.1UF
3.3V
Video Encoder and RGB
UNLESS OTHERWISE SPECIFIED,
DIMENSIONS ARE IN INCH ES.
TOLERANCES ARE :
5VRGBDAC
C80
0.1UF
16V
C79
0.1UF
16V
U12
VREF
R31
562
35
34
36
VREF
COMP
FS_ADJUST
B016B117B218B319B420B521B622B723BLANK10SYNC11CLOCK
BOUT2
BOUT3
BOUT4
BOUT0
BOUT1
128
133
118
142
155
160
GND
GND
GND
GND
ADGND
VDD
VDD
ADVDD
ADVDD
VDD
150
139
130
131
138
144
50V
D
50V
50V
D
50V
D
101
106
131
GND
GND
VCC
VCC
VCC
VCC
VCC96VCC91VCC66VCC61VCC51VCC37VCC
141
121
116
111
16V
D
C84
0.01UF
16V
D
C83
0.01UF
16V
D
C82
0.01UF
16V
D
C81
0.01UF
3.3V
P2-18
P2-31
P2-19
P2-30
P2-33
RESETn
D
ROUT0
ROUT1
ROUT2
ROUT5
ROUT4
ROUT3
ROUT6
ROUT7
GOUT1
GOUT3
GOUT2
GOUT0
GOUT6
BOUT1
BOUT0
GOUT7
GOUT5
GOUT4
BOUT2
BOUT5
BOUT4
BOUT3
BOUT6
DV_VSYNC_OUT
DV_ENABLE
DV_CLK_OUT
DV_HSYNC_OUT
22
57
24
60
21
28
59
62
50
32
47
35
20
23
26
29
P1-20
P1-23
P1-26
P1-29
56
38
44
61
53
58
41
P1-57
P1-60
P1-62
P1-50
P1-32
P1-47
P1-35
P1-56
P1-38
P1-44
P1-61
P1-53
P1-58
P1-41
D D
30
25
27
P1-22
P1-24
P1-21
P1-28
P1-59
P1-30
P1-25
P1-27
42
40
36
39
34
33
43
31
37
P1-42
P1-40
P1-36
P1-39
P1-34
P1-33
P1-43
P1-31
P1-37
C C
BOUT7
52
45
48
54
49
46
55
51
P1-52
P1-45
P1-48
P1-54
P1-49
P1-46
P1-55
P1-51
RESETn
B B
A A
Figure 9.8 Mini-Dock Connectors Schematic, Page 2
74 Chapter 9: Accessory Service
Page 83

REV
3 4
C2 DOCK
D02457
Friday, December 20, 2002
SCHEMATIC DIAGRAM
1
Video Decoder
UNLESS OTHERWISE SPECIFIED,
DIMENSIONS ARE IN INCH ES.
TOLERANCES ARE :
200 PIN
DOCK CONN
P1-63
P1-66
63
66
P1-93
P1-78
P1-69
69
P1-96
P1-99
P1-81
P1-101
P1-87
P1-72
P1-75
78
72
75
P1-104
P1-84
P1-90
P1-100
P1-105
P1-64
P1-103
P1-102
93
96
99
81
87
84
90
101
104
64
100
105
103
102
D
P1-77
P1-68
P1-67
P1-70
P1-65
68
67
70
65
P1-83
P1-85
P1-73
P1-82
P1-71
P1-76
P1-80
P1-74
P1-79
77
83
85
73
82
71
76
80
74
79
P1-98
P1-97
P1-91
P1-94
P1-88
P1-92
P1-89
P1-95
P1-86
98
97
91
94
88
92
89
95
86
DATE SH. OF
1
NONE
B A
SIZE
SCALE
<=+/-.XX=+/-
.XXX= +/-
PART, WITHOUT THE EXPRESS
OR REPRODUCED , IN WHOL E OR
WRITTEN CONSENT OF
SONOSITE, INC.
THEREIN SHALL BE DI SCLOSED
WHICH IS PROPRIETA RY TO
SONOSITE, INC.
NEITHER THE DOCUM ENT NOR
THIS DOCUMENT CONTAINS
THE INFORMATIO N CONTAINED
CONFIDENTIAL IN FORMATION
2
D
47.5K
R7
47.5K
R6
47.5K
R5
3.3V
3
4
SCL_3V
SDA_3V
SDA_3V
SCL_3V
D D
RIN0
RIN1
RIN3
RIN2
RIN6
RIN4
RIN7
RIN5
GIN4
GIN2
GIN6
GIN1
GIN7
GIN3
GIN5
GIN0
BIN0
BIN1
DV_CLK_IN
DV_ENABLE_IN
DV_HSYNC_IN
DV_VSYNC_IN
110
RA199RA298RA397RA495RA594RA693RA7
PDSPEN
PVS
PHS
SCLK
PCLKB
PCLKA
GOUT3
GOUT2
GOUT1
SDA
SCL
IREQ
XOUT
XIN
HOSTCLK
TEST2
TEST1
YUVIN
I2CADDR
PWRDN
TV_CLK
TV_HREF
92
TV_VSYNC
TV_HSYNC
109
GA0
Y0Y1Y2Y3Y4Y5Y6
100
RA0
90
89
88
86
85
84
83
82
81
43
42
41
39
40
38
D
50
49
48
47.5K
R8
47
46
D
TVCLK1TVHREF2TVVS3TVHS4GVS6GHS7GHREF8GCLK9RIN0/YIN018RIN1/YIN117RIN2/YIN216RIN3/YIN315RIN4/YIN414RIN5/YIN513RIN6/YIN612RIN7/YIN711GIN0/UVIN027GIN1/UVIN126GIN2/UVIN225GIN3/UVIN324GIN4/UVIN423GIN5/UVIN522GIN6/UVIN621GIN7/UVIN720BIN036BIN135BIN234BIN333BIN432BIN531BIN630BIN7
Y045Y146Y247Y348Y453Y554Y655Y7
BIN2
120
119
118
108
107
105
104
103
102
BA0
BA1
GA1
BA2
GA2
GA3
GA4
GA5
GA6
GA7
Y7
UV0
UV1
56
C033C134C235C336C437C538C639C7
U5A
AY084AY186AY288AC090AC192AC294XTALI8XTALO7RST
10
C13
0.1UF
16V
CLK_26_8Mhz
C14
0.1UF
16V
PAL_NTSCn
R10
75
R9
75
D D
3.3V
D
CHROMA_IN
LUMA_IN
34
P2-34
80 PIN CONN TO
CONNECTOR PCB
RESETn
35
36
P2-35
P2-36
RESETn
C C
BIN5
BIN3
BIN7
BIN4
BIN6
117
115
113
112
114
BA3
BA4
BA6
BA7
BA5
RB060RB159RB258RB357RB455RB554RB653RB7
U4A
AL300
UV2
UV3
UV4
UV5
UV6
UV7
44
EXV016EXV127EXV228EXV361EXV462EXV563EXV668EXV7
PORTA58SCH(PORTB)24TESTEN57TEST96VRT77VRB78COMP297SCLK75AEX069SDAT72AEX1
D
C91
7
5
VDD
CLKA6CLKB
U14
XTALIN3XTALOUT4FS
8
70
C17
0.1UF
16V
D
C16
0.1UF
16V
D
C15
0.1UF
16V
D
SCL_3V
SDA_3V
0.01UF
50V
D
1
CLKC
CY22381FI
GND
2
D
CLK_ENCODER
52
GB070GB169GB268GB367GB465GB564GB663GB7
29
71
25
HAV
HS126HS2(IIC)76VS
D
TV_HSYNC
CLK_PAL_ENC
CLK_NTSC_ENC
140
5
22
EHAV
ROMDATA0
ROMDATA1
ROMDATA2
139
138
137
73
74
17
PID
ODD
CCDAT
VAV(OENC0)3EVAV(OENC1)4OEN15CK
23
TV_VSYNC
R11
10K
62
BB080BB179BB278BB377BB475BB574BB673BB7
ROMDATA3
ROMDATA4
ROMDATA5
ROMDATA6
ROMDATA7
135
134
133
132
D
21
CK2
CCEN
KS0127B
18
R12
10K
D
D
B B
72
ROMADDR15
160
ROMADDR14
159
ROMADDR13
158
ROMADDR12
157
ROMADDR11
155
ROMADDR10
154
ROMADDR9
153
ROMADDR8
152
ROMADDR7
150
ROMADDR6
149
ROMADDR5
148
ROMADDR4
147
ROMADDR3
145
ROMADDR2
144
ROMADDR1
143
ROMADDR0
142
OPLLCLK
127
OHSFB
125
OHSREF
124
IHSREF
123
OCLK
VCOIN
RESETB
126
129
122
RESETn
R13
47.5K
C24
0.1UF
16V
D
VCOIN2
C23
0.01UF
50V
D
VSS6VSS13VSS14VSS19VSS40VSS41VSS60VSS64VSS65VSS83VSS87VSS91VSS
U5B
VDD20VDD59VDD311VDD312VDD342VDD343VDD366VDD367VDDA85VDDA89VDDA93VDDA98VDDA1
C22
0.01UF
50V
D
C19
0.1UF
16V
D
C21
0.01UF
50V
C18
0.1UF
16V
D
C20
0.01UF
50V
3.3V
D D
3.3V
5V
101
GND5GND10GND28GND45GND56GND71GND76GND87GND
U4B
19
VCC
VCC
VCC96VCC91VCC66VCC61VCC51VCC37VCC
116
111
C30
0.1UF
16V
DD
C29
0.1UF
16V
D
C28
0.1UF
16V
D
C27
0.1UF
16V
3.3V
3.3V
95
NCP1NCP2NCP29NCP30NCP31NCP32NCP49NCP50NCP51NCP52NCP79NCP80NCP81NCP82NCP99NCP
9
C26
0.1UF
16V
DD
C25
0.1UF
16V
C31
0.1UF
16V
D
0805
0805
FB2
FB1
5V
A A
106
131
136
GND
GND
GND
VCC
VCC
VCC
151
141
121
C35
0.01UF
50V
C34
0.01UF
50V
C33
0.01UF
50V
C32
0.01UF
50V
2
D
130
146
156
GND
GND
PLLGND
AL300
PLLVCC
128
C36
0.1UF
16V
D
D
D
FB3
0805
D
D
3.3V
3
D
100
KS0127B
4
Figure 9.9 Mini-Dock Connectors Schematic, Page 3
Chapter 9: Accessory Service 75
Page 84

REV
4 4
C2 DOCK
D02457
Friday, December 20, 2002
SCHEMATIC DIAGRAM
1
V+3V-7VCC
FORCEON14FORCEOFF
20
C11
0.1UF
16V
C10
0.1UF
16V
GND
19
D
D
3_3VIN
RESETn
System I/O
UNLESS OTHERWISE SPECIFIED,
DIMENSIONS ARE IN INCH ES.
D
18
MAX3225E
C12
0.1UF
TOLERANCES ARE :
16V
SYSTEM JTAG INTERFACE:
Allow for System JTAG
chain to be accessed.
MII Interface:
Allow Ethernet Physical Layer to exist in
Dock if necessary.
DOCK_TRSTN
DOCK_TMS
DOCK_TDI
DOCK_TCK
DOCK_TDO
171
172
173
169
170
P1-171
P1-172
P1-173
P1-169
P1-170
200 PIN DOCK CONN
2
VPWR
Dock Power Soft Start Switch
3
182
192
175
168
P1-175
P1-168
D2
6
5
D1
D2
8
7
D1
187
183
191
185
180
189
179
181
176
177
P1-182
P1-192
P1-187
P1-183
P1-191
P1-185
P1-180
P1-189
P1-179
P1-181
P1-176
P1-177
10V
C5
330UF
5V3.3V
Q1B
SI4920DY
S
3
G
4
SI4920DY
Q1A
S
G
2
R35
33.2K
10V
C4
330UF
1
Q2
32
1
C92
4.7UF
16V
D
R37
R36
100K
D
RESETn
0.1UF
16V
C1
D
RESETn
D
3.3V
5
1
2
VDD
GND
U6
RESET
TPS3820-33DB
MR3WDI
4
178
197
188
174
199
198
193
P1-193
2N3904
184
195
194
P1-197
P1-195
P1-194
D D
D
10K
R38
100K
190
186
P1-178
P1-188
P1-174
P1-199
P1-198
P1-184
P1-190
P1-186
D
SDA_3V
R3
10K
SDA_3V
3
1
2
VL
IOL
GND
D1
MBR0540T1
IOC4SHDN5VCC
U7
6
MAX3371
D
200
MTG2
196
MTG1
P1-200
P1-202
P1-196
P1-201
P2-43
P2-42
P2-44
P2-45
43
42
44
45
80 PIN DIFF LINE CONN
3.3V
SCL_3V
R4
10K
SCL_3V
C6
D
2
3
1
VL
IOL
GND
IOC4SHDN5VCC
MAX3371
U8
6
5VIN
D
4
8
6
5
SCL
VSS
SDA
VCC
U13
WC#
NC1E12E2
M24C04-WDW6T
7
3
D
VPWR
0.1UF
16V
D
3_3VIN
0.1UF
16V
C7
D
VCR_CTS
VCR_TX
VCR_RTS
VCR_RX
1
9
11
8
RDY
R1IN16R2IN
T1OUT17T2OUT
INVALID
C1+2C1-4C2+5C2-
R1OUT15R2OUT10T1IN13T2IN
U3
6
12
0.1UF
16V
C9
0.1UF
16V
C8
DATE SH. OF
1
NONE
B A
SIZE
SCALE
<=+/-.XX=+/-
.XXX= +/-
PART, WITHOUT THE EXPRESS
WRITTEN CONSENT OF
SONOSITE, INC.
OR REPRODUCED , IN WHOL E OR
THEREIN SHALL BE DI SCLOSED
THE INFORMATIO N CONTAINED
SONOSITE, INC.
NEITHER THE DOCUM ENT NOR
WHICH IS PROPRIETA RY TO
THIS DOCUMENT CONTAINS
CONFIDENTIAL IN FORMATION
2
D
3
47.5K
R2
P2-80
P2-6363P2-7979P2-64
80
64
47.5K
R1
3.3V 3.3V
D
PRINTER_SENSE
D
RATE
DOCK_SENSEB
DOCK_SENSEA
ON_OFF
19
144
17
16
18
P1-19
P1-144
P1-17
P1-16
DOCK_SENSE1
DOCK_SENSE0
DOCK_SENSE2
145
147
146
P1-145
P1-147
P1-146
SYSTEM_SENSE_ENA
SYSTEM_STATUS0
SYSTEM_SENSE0
SYSTEM_SENSE1
148
151
150
149
P1-148
P1-151
P1-150
P1-149
3_3VIN
5VIN
0.1UF
C2
SYSTEM_STATUS1
SYSTEM_STATUS2
153
155
152
154
P1-153
P1-155
P1-152
P1-154
0.1UF
16V
C3
16V
D
D
156
157
P1-156
P1-157
P2-6
P2-1
P2-7
P2-8
P2-4
P2-2
P2-3
P2-5
6
1
7
8
4
2
3
5
D
D
EXT_TXC
EXT_RTSC
EXT_CTSC
EXT_RXC
SDA_5V
SCL_5V
D
107
160
108
161
106
158
159
110
109
P1-107
P1-160
P1-108
P1-161
P1-106
P1-158
P1-159
P1-110
P1-109
P2-9
9
132
P1-132
P2-11
P2-10
11
10
ECG_ANALOG
ECG_FILTER_CLK
134
133
P1-134
P1-133
P2-16
P2-13
P2-14
P2-12
P2-15
16
13
14
12
15
D
ECG_POWER_CLK
ECG_SENSE
AUD_L_OUT
AUD_R_OUT
VPWR
141
167
140
135
137
136
P1-141
P1-167
P1-140
P1-135
P1-137
P1-136
P2-40
P2-38
P2-39
P2-37
P2-17
38
37
17
AUD_R_IN
AUD_L_IN
HEADPHONE_SNS
D
142
138
139
P1-142
P1-138
P1-139
P2-41
40
39
41
MONO_OUT
MONO_IN
163
143
164
111
165
166
162
P1-163
P1-143
P1-164
P1-111
P1-165
P1-166
P1-162
P2-59
P2-47
P2-56
P2-58
P2-46
P2-51
P2-50
P2-48
P2-53
P2-49
P2-57
59
47
56
58
46
51
50
48
53
49
57
USB_D-
USB_D+
ENET_RX+
ENET_TX-
ENET_TX+
ENET_RX-
VCR_TXC
VCR_RTSC
VCR_CTSC
VCR_RXC
USB_POWER
118
113
115
117
116
112
114
P1-118
P1-113
P1-115
P1-117
P1-116
P1-112
P1-114
ACTLED
123
126
121
124
122
119
120
125
P1-123
P1-126
P1-121
P1-124
P1-122
P1-119
P1-120
P1-125
P2-54
54
LILED
127
P1-127
P2-55
55
SPEEDLED
128
P1-128
P2-52
P2-61
P2-60
52
61
60
ENET_LED_PWR
3.3V
STAT_FRAME
130
129
P1-130
P1-129
P2-82
P2-81
P2-62
62
D
MTG2
MTG1
CTRL_LINE
D
131
4
P1-131
4
P2-67
P2-65
P2-68
P2-69
P2-66
67
65
68
69
66
80 PIN DIFF LINE CONN
VPWR_IN
1
2
3
4
5
P1-1
P1-2
P1-3
P1-4
P1-5
P2-78
P2-73
P2-75
P2-74
P2-71
P2-70
P2-76
P2-72
P2-77
78
73
75
74
71
70
76
72
77
VCHGR
VPWR_IN_RTN
6
P1-6
VBAT_SNS
13
7
14
8
12
10
9
11
P1-13
P1-7
P1-14
P1-8
P1-12
P1-10
P1-9
P1-1515P1-18
P1-11
200 PIN DOCK CONN
System
Sense /
DC Power /
Battery Charger
Interface
D D
Status
C C
ECG / Honda
Connector
Interface
USB
SIGNALS
Ethernet
Printer
Control /
Audio I/O
VCR SERIAL
PORT CMOS
B B
API Test
A A
Figure 9.10 Mini-Dock Connectors Schematic, Page 4
76 Chapter 9: Accessory Service
Page 85

3
0805
600 OHM@100MHZ
FB1
LUMA_IN Y_IN
34
P10-34
LUMACHROMA
S-Video DIN - Front
P11
4
YIN_RTN
C3
100PF
50V
L1
32
C2
100PF
50V
C1
100PF
50V
YCIN_RTN
35
P10-35
1 4
1
2
80 Pin Diff-Line Connector
REV
C
1 1
Ethernet
with
Transformer
R25
0.00
12
7
6
5
RTN RTN
0.00
R24
CIN_RTN
C_IN
C6
100PF
50V
L2
32
1 4
600 OHM@100MHZ
R3
1.00K
C5
100PF
50V
R2
1.00K
FB2
0805
C4
100PF
50V
AUD_L_OUT
AUD_R_OUT
CHROMA_IN
36
R1
0.00
D
P10-36
OEM Serial
Port
P7-1
P7-10
P7-6
P7-11
P7-4
P7-2
P7-5
P7-3
P7-8
P7-9
1
9
C10
0.001UF
50V
C9
0.001UF
50V
P7-7
6
4
2
5
3
8
7
C20
47PF
50V
GND1
GND2
C19
47PF
50V
C12
0.001UF
50V
C18
47PF
50V
C17
47PF
50V
FB30805
D
0.00
MONO_OUT
40
41
P10-40
P10-41
VCR_RX
42
P10-42
FB40805
VCR_TX
43
P10-43
50V
FB50805
FB60805
VCR_RTS
VCR_CTS
ENET_TX-
ENET_TX+
D
48
44
45
50
46
P10-48
P10-44
P10-45
P10-50
P10-46
C11
0.001UF
R9
1.00K
R6
1.00K
R10
AUD_R_IN
AUD_L_IN
MONO_IN
38
37
39
P10-38
P10-37
P10-39
8 POS RJ45
GND1
GND2
Differential 100 ohm lines critical routing
ENET_RX+
47
P10-47
1234568
RDC
TDC
D2
D1
ENET_LED_PWR
ENET_RX-
52
53
51
49
P10-52
P10-53
P10-51
P10-49
P2
C47
0.001UF
50V
D
USB Connector
0.1UF
16V
D
50V
D
P8-1
P8-2
P8-3
P8-4
P8-5
1
2
3
4
GND1
L4
32
1 4
600 OHM@100MHZ
3.3V
D
Differential 100 ohm lines critical routing
USB_VBUS
USB_D+
USB_D-
57
58
59
60
P10-57
P10-58
P10-59
P10-60
GREEN
GREEN
54
P10-54
C46
C45
18PF
LILED
ACTLED
SPEED LED
55
56
P10-55
P10-56
R14
P8-6
P12
GND2
R16
R15
1.00K
499K
STAT_FRAME
CTRL_LINE
D
62
61
P10-62
P10-61
Printer Control / API
3 POS PHONE
123
11
C35
100PF
50V
1.00K
C34
100PF
50V
R17
10K
R18
47.5K
DD
C30
0.1UF
16V
D
VPWR_IN
67
65
68
69
P10-67
P10-6666P10-72
P10-65
P10-68
P10-69
PRINTER_SENSE
63
P10-63
64
P10-64
Power Input
Connector
P9-1
P9-2
1
2
VPWR_IN_RTN
71
72
70
P10-71
P10-70
P9-3
P10-73
D0191 1
C2 Doc k Connecto r
Friday, May 10, 2002
SCHEMATIC DIAGRAM
DATE S H. OF
1
NONE
B
SIZE
SCALE
P9-4
P9-5
P9-6
P9-7
3
4
5
GND1
GND2
Place near P9
VCHGR
VBAT_SNS
RATE
D
73
74
P10-74
75
P10-75
76
P10-76
77
P10-77
78
P10-78
79
P10-79
80
P10-80
MTG2
P10-82
MTG1
P10-81
<=+/-.XX=+/-
.XXX=+/-
SONOSITE, INC.
WRITTEN CONSE NT OF
PART, WITHOU T THE EXP RESS
THE INFORMATION CONTAINED
OR REPRODUCED , IN WHOLE OR
THEREIN SHALL BE DISCL OSED
WHICH IS PRO PRIETARY TO
THIS DOCUMENT CONTAINS
CONFIDENTIAL IN FORMATION
SONOSITE, INC.
NEITHER THE DO CUMENT NOR
UNLESS OTHER WISE SPEC IFIED,
DIMENSIONS A RE IN INC HES.
TOLERANCES A RE:
R30
0.00
D
2
3
Honda Connector: ECG /
Serial Debug / Audio
P5-16
P5-7
P5-13
P5-5
P5-6
P5-8
P5-18
P5-17
P5-10
P5-15
P5-11
6
8
10
11
MTG2
MTG4
MTG3
MTG1
R23
0.00
Place near P5
ECG_SDA
ECG_SCL
EXT_RXC
EXT_TXC
D
5
6
3
2
1
4
4
P10-5
P10-6
P10-3
P10-2
P10-1
P10-4
P5-12
P5-2
P5-4
P5-9
P5-14
P5-3
P5-1
7
5
2
4
9
3
1
13
12
14
C8
0.001UF
ECG_AUDIO_LEFT
C7
0.001UF
ECG_AUDIO_RIGHT
VPWR
EXT_RTSC
EXT_CTSC
ECG_ANALOG
ECG_SENSE
EGC_FILTER_CLK
ECG_PWR_CLK
D
8
9
13
10
12
7
11
14
P10-8
P10-9
P10-13
P10-10
P10-12
P10-7
P10-11
P10-14
R7
100K
3.3V
50V
R8
50V
R5
1.00K
R4
1.00K
Headphone Audio Output
P4
123
11
H_AUDIO_L
H_AUDIO_R
H_AUDIO_SNS
R12
1.00K
1.00K
R11
10K
AUD_R_OUT
HEADPHONE_SNS
AUD_L_OUT
15
17
16
P10-15
P10-17
P10-16
80 Pin Diff-Line Connector
D D
Figure 9.11 Dock Connectors Schematic
R32
12
C_OUT
32
600 OHM@100MHZ
FB12
CHROMA_OUT
32
P10-32
0.00
7
3
6
5
RTN RTN
R31
0.00
C_RTN
C44
100PF
50V
L9
1 4
C43
100PF
50V
C42
100PF
50V
R22
0.00
D
4
33
P10-33
3
Mounting
15 POS VGA
Output
P3
PHONO
1
3 POS PHONE
C15
C14
C13
2
C16
0.001UF
50V
0.001UF
50V
0.001UF
50V
0.1UF
16V
D
R26
C23
100PF
50V
CVID_OUT
L3
32
1 4
600 OHM@100MHZ
C22
100PF
50V
FB7
0805
C21
100PF
50V
CVID
R13
0.00
CVID_RTN
19
18
P10-18
P10-19
C C
P1-6
P1-1
P1-2
P1-7
6
1
2
7
R27
0.00
R_OUT
0.00
0805
D
G_OUTANALOG_G
C29
100PF
C26
L5
32
600 OHM@100MHZ
C25
FB8
C24
ANALOG_R
20
P10-20
50V
100PF
50V
L6
32
1 4
1 4
600 OHM@100MHZ
100PF
50V
C28
100PF
50V
FB9
0805
100PF
50V
C27
100PF
50V
RGB_RTN
21
23
22
P10-21
P10-23
P10-22
Composite Video
Connector
Not Used
P1-5
P1-4
P1-3
P1-14
P1-8
5
4
3
8
14
R28
0.00
B_OUTANALOG_B
C33
100PF
50V
R29
0.00
L7
32
R20
1 4
600 OHM@100MHZ
C32
100PF
50V
VSYNC
FB10
0805
24
P10-24
D
C31
100PF
50V
R19
0.00
25
26
P10-25
P10-26
B B
Holes
Key Pin
Not Used
P1-16
P1-15
P1-13
P1-1010P1-17
P1-9
P1-12
P1-11
9
15
13
12
11
GND1
GND2
C37
100PF
50V
C36
100PF
50V
49.9
R21
49.9
HSYNC
3.3V
D
C38
0.1UF
16V
27
29
28
P10-27
P10-29
P10-28
LUMACHROMA
S-Video DIN - Front
P6
4
Y_RTN
Y_OUT
C41
100PF
50V
L8
32
1 4
600 OHM@100MHZ
C40
100PF
50V
FB11
0805
0805
C39
100PF
50V
D
LUMA_OUT
YCOUT_RTN
30
31
P10-31
P10-30
A A
Chapter 9: Accessory Service 77
Page 86

9.5 Theory of Operation
9.5.1 Video
The video data originates on the main PCBA in 640 × 480, RGB-888 square pixel format at 60Hz refresh
rate NTSC and 50Hz for PAL video.
9.5.1.1 VGA Video Output
The digital RGB data is converted to progressive scan analog RGB on the dock PCB. The analog RGB
drives a VGA style connector and is also the input for the video encoder.
9.5.1.2 Analog Interlaced Video Outputs
The digital RGB data is converted to analog interlaced composite and S-Video outputs on the dock
PCB. A video processor converts RGB and output in the selected video format.
For NTSC video mode, the processor is converting from progressive scan to interlaced and is
responsible for the color space conversion converting from RGB to composite and S-Video.
For PAL video mode, the processor is doing the interlacing and color space conversion and outputs
768 × 576 lines at 50Hz.
9.5.1.3 Video Decoders
A video decoder digitizes the input S-Video data. A separate component performs the de-interlace and
converts to digital RGB data.
9.5.2 Power Distribution
The battery charger and VPWR signals provided by the AC power supply are intended to be passed
through the dock so that the system behaves the same whether the AC power supply is plugged into
the dock or directly to the system (with no dock).
The dock uses the 3.3V and 5V power supplies available on the Main PCB. When the dock is
connected, the dock provides necessary surge limiting and reset circuitry necessary to ensure proper
operation of all components.
The dock provides the capability to turn OFF 3.3V and 5V power supplies to as much of the dock as
possible to allow for a minimum power state.
78 Chapter 9: Accessory Service
Page 87

9.6 Replacement Procedures
Replacement procedures for all replaceable parts are not discussed in this manual. Replacement of
many mechanical parts is intuitive to properly trained service personnel and due to the simple nature
of these kinds of repairs procedures are determined to be not necessary.
9.6.1 Required Tools
• #1 Phillips screwdriver
• 1/8 inch, 5/32 inch, and 1/4 inch hex wrench, ball type
• 3/8 inch and 1/2 inch wrench or socket
• 13/16 inch and 3/4 inch open end wrench
9.6.2 Cup Surround
1 Remove the six (6) screws attaching the cup surround to the top of the docking system.
3/8" Hex nuts (2)
1/8" Allen screws (3)
Figure 9.12 Cup Surround Screws
2 Lift the cup surround from the docking system.
9.6.3 Casters
1 Tilt the docking system in any direction and lay it on its side.
2 Remove the caster by turning the 13/16 inch nut on the caster shaft counter-clockwise until the
caster is removed from the docking system base.
3 Install the new caster in the same manner.
1/8" Allen screws (3)
Chapter 9: Accessory Service 79
Page 88

9.6.4 Power Supply
1 Remove the rear cover on the docking system by pressing on the black lever at the bottom of the
cover per Figure 9.13.
Rear cover
release lever
Figure 9.13 Mobile Docking System Rear Cover
2 Pull the cover down and away from the docking system.
3 The power supply is located on the upper shelf on the left side.
Power supply
Figure 9.14 Mobile Docking System Power Supply
Power strip
Printer/video
cables
80 Chapter 9: Accessory Service
Page 89

4 Unplug the power connector from the power strip and from the power supply per Figure 9.14.
5 Remove cable cover from top rear of docking system to expose mini-dock wiring.
6 Remove the power supply from the docking system by unplugging the cable from the mini-dock
per Figure 9.15 and removing the cable tie that attaches it to the docking system.
Power supply
cable
Cable tie
Figure 9.15 Mini-Dock Connectors
7 The power supply cable is threaded through the TITAN support leg from the power supply in the
rear up to the mini-dock.
8 Lower the docking system to the lowest point.
9 Tilt the docking system toward the rear and carefully lower it so it lays on the ground.
10 In the right support leg, as you face it from the bottom, you will notice the cables attached to a cable
tie. Remove the cable tie and the tape from around the cables.
11 Separate the cables and pull the cable from the power supply down to the bottom of the docking
system support leg.
12 Tilt the docking system back to the upright position and pull the power supply from the rear of
the docking system, pulling the cable up from the support leg.
13 Install the new power supply into the rear of the docking system and thread the cable down the
support leg.
14 Lay the docking system back down, pull the excess cable free then thread the cable up the support
leg to the top of the docking system.
15 Attach the power supply connector to the mini-dock and replace the cable tie.
16 Retape the wires and reattach the wires to the support leg with a new cable tie.
17 Install the power supply power cord.
Chapter 9: Accessory Service 81
Page 90

9.6.5 Locking Handle
1 Remove the six (6) screws attaching the cup surround to the top of the docking system per
Figure 9.12.
2 Lift the cup surround from the docking system.
3 Remove the two 3/8 inch locking nuts from the handle screws per Figure 9.12.
4 Remove the two 1/8 inch hex screws holding the handle in place per Figure 9.16.
Retaining clip
1/8" hex screw
Figure 9.16 Handle Screws
5 Lift the handle from the docking system. Remove the retaining clip from the latch link assembly
and remove the handle.
6 Install the new handle and reassemble the docking system in the reverse order.
Deflector
Video/power
cables
9.6.6 Deflector
1 Remove the six (6) screws attaching the cup surround to the top of the docking system per
Figure 9.12.
2 Lift the cup surround from the docking system.
3 Peel the old deflector from the frame per Figure 9.16.
4 Clean any adhesive residue from the frame.
5 Install the new deflector in place of the old deflector noting the notches cut out for the attaching
hardware.
6 Reinstall the cup surround.
82 Chapter 9: Accessory Service
Page 91

9.6.7 Mini-Dock
1 Remove the cover from the top of the docking system just behind the mini-dock per Figure 9.17 to
expose the cables attached to the mini-dock.
2 Disconnect all of the cables.
Figure 9.17 Mobile Docking System Cable Cover
3 Raise the docking system to the highest point by pressing on the foot pedal and lifting on the outer
edges of the cup surround.
4 Looking under the top of the docking system note two access holes per Figure 9.18. These access
holes are to remove the two (2) screws holding the mini-dock in place.
Cable cover
Access holes
Figure 9.18 Mini-Dock Access Holes
Chapter 9: Accessory Service 83
Page 92

5 Looking from the front of the docking system find the two screws holding the mini-dock in place
and remove them using a Phillips screwdriver.
Phillips screw
Access hole
Figure 9.19 Mini-Dock Screws
6 Lift the mini-dock from the docking system.
7 Reinstall the mini-dock by following these steps in reverse order.
84 Chapter 9: Accessory Service
Page 93

Appendix A: Parts List
This section contains a list of field-replaceable parts.
A.1 Replacement Parts List
The following tables contain all the replaceable parts for the TITAN Ultrasound System. All quantities
are one unless otherwise noted.
A.1.1 Display
Table A.1: Display
Part Number Description
P03861 Service Assembly Display TITAN
Appendix A: Parts List 85
Page 94

A.1.2 Control Panel
Table A.2: Control Panel
Part Number Description
P03862 Service Assembly Control Panel, TITAN, English
P03863 Service Assembly Control Panel, TITAN, English, International
P03864 Service Assembly Control Panel, TITAN, French
P03865 Service Assembly Control Panel, TITAN, German
P03866 Service Assembly Control Panel, TITAN, Italian
P03867 Service Assembly Control Panel, TITAN, Spanish
P03868 Service Assembly Control Panel, TITAN, Portuguese
86 Appendix A: Parts List
Page 95

A.1.3 Replacement Parts, System
5
1
2 2
3
4
Table A.3 : System
Find Number Part Number Description
1 P03870 Service Assembly Power Supply, TITAN
2 P03872 Service Assembly Speaker, TITAN
3 P03869 Service Assembly TGC, TITAN
4 P03873 Service Assembly Upper Enclosure, TITAN
not shown P03874 Service Assembly Lower Enclosure, TITAN
Note: This part requires printing a replacement label for the product.
Contact SonoSite Technical Support when ordering this part to have the
label printed and placed on the part.
5 P03871 Service Assembly Main PCBA, TITAN
Note: This part does not include the transducer nest frame assembly. Those
parts must be ordered separately if needed to complete the replacement of
the Main PCBA.
Appendix A: Parts List 87
Page 96

Figure A.1 Power Supply, P03870
Figure A.2 Speaker Assembly, P03872
88 Appendix A: Parts List
Page 97

Figure A.3 TGC Assembly, P03869
Figure A.4 Upper Enclosure, P03873
Appendix A: Parts List 89
Page 98

Figure A.5 Lower Enclosure Assembly, P03874 (top view)
90 Appendix A: Parts List
Page 99

Figure A.6 Lower Enclosure, P03874 (bottom view)
Appendix A: Parts List 91
Page 100

Figure A.7 Main PCB Assembly, P03871
92 Appendix A: Parts List
 Loading...
Loading...