Page 1

M-Turbo
TM
Ultrasound System
Service Manual
Page 2

SonoSite, Inc.
21919 30th Drive SE
Bothell, WA 98021-3904
USA
Telephone: 1-888-482-9449 or 1-425-951-1200
Fax: 1-425-951-1201
SonoSite Ltd
Alexander House
40A Wilbury Way
Hitchin, Herts
SG4 OAP UK
T: +44-1462-444800
F: +44-1462-444801
Caution:
M-Turbo, SiteLink, SonoCalc, SonoHD, SonoMB, and SonoSite are registered trademarks or trademarks of SonoSite, Inc.
DICOM is the registered trademark of the National Electrical Manufacturers Association for its standards publications relating to digital communications
of medical information.
Non-SonoSite product names may be trademarks or registered trademarks of their respective owners.
Protected by U.S. patents: 5722412, 5817024, 5893363, 6135961, 6364839, 6371918, 6383139, 6416475, 6471651, 6569101, 6648826, 6962566, 7169108,
D456509, D538432. Patents pending.
Federal (United States) law restricts this device to sale by or on the order of a physician.
P08144-01 12/2007
Copyright 2007 by SonoSite, Inc.
All rights reserved.
ii
Page 3

Contents
Chapter 1: Introduction
Audience ........................................................................................................................... 1
Conventions ....................................................................................................................1
Contact Information ..................................................................................................... 1
Chapter 2: System Overview
About the System .......................................................................................................... 3
Theory of Operation ..................................................................................................... 4
Description of Operating Modes .................................................................... 5
Additional System Feature Performances ................................................... 7
ECG Module ............................................................................................................ 8
DICOM ...................................................................................................................... 8
IMT ............................................................................................................................. 8
System Specifications .................................................................................................. 8
System Dimensions ............................................................................................. 9
Display Dimensions ............................................................................................. 9
Transducers ............................................................................................................ 9
Imaging Modes ..................................................................................................... 9
Image and Clips Storage .................................................................................... 9
Accessories ............................................................................................................. 9
Peripherals ............................................................................................................10
Temperature, Pressure, and Humidity Limits ...........................................11
Electrical ................................................................................................................11
Battery ....................................................................................................................11
Electromechanical Safety Standards ...........................................................12
EMC Standards Classification .........................................................................12
Airborne Equipment Standards ....................................................................12
DICOM Standard .................................................................................................12
HIPAA Standard ...................................................................................................12
Chapter 3: Troubleshooting
Periodic Maintenance ................................................................................................13
System and Subsystem Diagnosis .........................................................................13
System Repair ...............................................................................................................13
Test Equipment ............................................................................................................13
Failure (Assert) Codes .................................................................................................14
Verifying a System Assert Code .....................................................................14
DICOM ....................................................................................................................15
Chapter 4: Replacement Procedures
Display Replacement .................................................................................................17
Required Parts .....................................................................................................17
Required Tools .....................................................................................................17
Display Removal .................................................................................................17
Display Replacement ........................................................................................20
Test the Display ...................................................................................................20
Control Panel Subassembly Replacement ..........................................................21
Required Parts .....................................................................................................21
Required Tools .....................................................................................................21
Control Panel Removal .....................................................................................21
Control Panel Replacement ............................................................................21
Main System Disassembly for Repair and/or Replacement .........................22
Required Parts .....................................................................................................22
iii
Page 4

Required Tools .....................................................................................................22
System Disassembly ..........................................................................................22
Chapter 5: Performance Testing
Overview ........................................................................................................................33
Test Equipment ............................................................................................................33
Setting Up Performance Tests ................................................................................33
Basic Operational Tests ..............................................................................................34
2D Performance Tests ................................................................................................34
2D Performance / Image Quality ..................................................................34
Axial Measurement Accuracy ........................................................................35
Lateral Measurement Accuracy .....................................................................35
Penetration ...........................................................................................................35
Additional Performance Tests .................................................................................36
Color Doppler (Color) ........................................................................................36
Color Power Doppler (CPD) ............................................................................36
M Mode Imaging ................................................................................................37
Tissue Harmonic Imaging ................................................................................37
Pulsed Wave (PW) Doppler Imaging ...........................................................37
Continuous Wave (CW) Doppler Imaging .................................................38
Image Quality Verification Test/Livescan ...................................................38
Printer .....................................................................................................................38
Battery Charging ................................................................................................39
Video Output .......................................................................................................39
Appendix A: Replacement Parts List
Display .............................................................................................................................41
Control Panel .................................................................................................................42
System .............................................................................................................................43
Transducer Nest Frame Assembly .........................................................................46
Ordering Replacement Parts ...................................................................................46
Appendix B: Service Event Report
Service Event Report Form .......................................................................................48
Service Event Report Instructions .........................................................................49
Returning Products to SonoSite .............................................................................50
Shipping Instructions .......................................................................................50
Index ........................................................................................................................ 51
iv
Page 5

Chapter 1: Introduction
Before servicing the M-Turbo ultrasound system, please read this manual. The information applies only to the
SonoSite M-Turbo ultrasound system product manufactured after December 5, 2007.
The ultrasound system has multiple configurations and feature sets. All are described in this service manual but
not every option may apply to your system. System features depend on your system configuration, transducer, and
exam type.
Refer to the M-Turbo Ultrasound System User Guide for additional information regarding safety, system controls,
operation, capabilities, and specifications.
Audience
The intended audience of this manual is properly trained field and in-house service personnel.
Conventions
These conventions are used in this service manual:
•A WAR NIN G describes precautions necessary to prevent injury or loss of life.
•A Caution describes precautions necessary to protect the products.
• Numbered steps must be performed in a specific order.
• Bulleted lists present information in list format but do not imply a sequence.
Labeling symbols are in the user guide.
Contact Information
Questions and comments are encouraged. SonoSite is interested in your feedback regarding the service manual.
If you encounter difficulty with the system, use the information in this manual to help correct the problem. If the
problem is not covered here, contact SonoSite Technical Support as follows:
Technical Support (USA, Canada) 1-877-657-8118
Technical Support fax: 1-425-951-6700
Technical Support e-mail: service@sonosite.com
SonoSite website: www.sonosite.com (Select Resources > Support & Service)
International Technical Support: Contact your local representative or call (USA) +425-951-1330
European Service Center +44-(0)1462-444-800
Japan Service Center +81-3-5304-5337
e-mail: uk.service@sonosite.com
Chapter 1: Introduction 1
Page 6

2 Chapter 1: Introduction
Page 7

Chapter 2: System Overview
About the System
The SonoSite M-Turbo high-resolution ultrasound system is a portable, full featured, general purpose, software
controlled, diagnostic ultrasound system using all digital architecture. The system is used to acquire and display
high-resolution, real-time ultrasound data in 2D, M Mode, Pulsed Wave (PW) Doppler, Continuous Wave (CW)
Doppler, Color Power Doppler (CPD), and color Doppler (Color) or in a combination of these modes.
The system has an electrocardiography (ECG) display feature and supports a 3-lead ECG cable assembly to collect
data for M Mode and Doppler measurements. The system provides measurement capabilities for anatomical
structures and fetal biometry that provide information used for clinical diagnostic purposes. The system has a PW
and CW Doppler audio output feature and cine review, image zoom, labeling, biopsy, measurements and
calculations, image storage and review, printing, and recording capabilities.
The system includes the ability to measure the intima-media thickness (IMT) of the carotid artery using digital
ultrasound images. The IMT measurement of the carotid artery may be used adjunctively with other medical data
obtained by a physician to help assess the cardiovascular health of a patient.
The system includes Digital Imaging and Communications (DICOM) capabilities as well as general computer
communication capabilities to provide the acceptance, transfer, display, storage, and digital processing of
ultrasound images and loops. Security support is also provided to facilitate HIPAA compliance.
The system/transducer is capable of exceeding a TI or an MI of 1.0 in certain operating modes or mode
combinations. The system displays the current output level in terms of one of two bioeffects indices (“Mechanical
Index [MI]” and “Thermal Index [TI]”) in accordance with the AIUM/NEMA Standard for Real Time Display of
Thermal and Mechanical Acoustic Output Indices on Diagnostic Ultrasound Equipment.
Chapter 2: System Overview 3
Page 8
Theory of Operation
AQ BusRF Bus
Acquisition
subsystem
Processing
subsystem
Transduce r
Display
subsystem
Control Bus
Control
subsystem
User
interface
Battery
pack
assembly
Pulser voltage
Video
External video to monitor,,
printer
Power
subsystem
Power
adapter
External power
IrDA
Serial Bus
Logic power
Display power
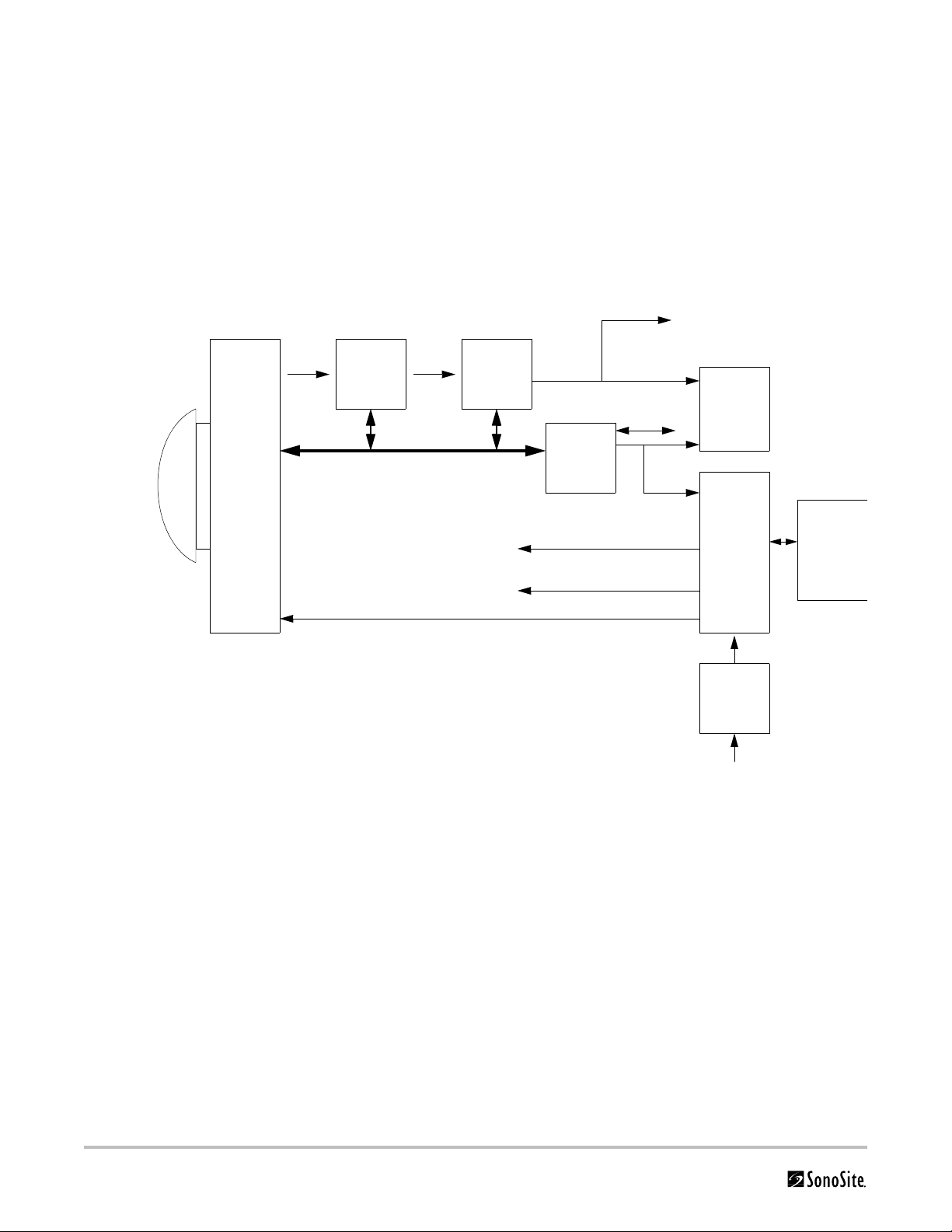
The M-Turbo ultrasound system has seven (7) major functional groups:
•Transducer
•Acquisition Subsystem
• Processing Subsystem
•Display Subsystem
• Control Subsystem
• User Interface Subsystem
•Power Subsystem
Figure 2.1 is a system block diagram that shows the relationship of the functional groups.
Figure 2.1 SonoSite High-Resolution Ultrasound System (M-Turbo) Block Diagram
The Transducer elements convert the pulser voltage to acoustic energy during the transmit portion of the
ultrasound acquisition cycle. The elements convert the acoustic echo to voltage in the receive portion of the
acquisition. The voltage developed on the transducer elements is sensed by the acquisition subsystem. The system
transducers have 64 to 192 elements.
The Acquisition Subsystem consists of the beamformer and interface to the transducer. The beamformer controls
the timing of the transmit pulses to focus the acoustic beam. The beamformer amplifies the low-level received
echos and controls the receive focusing. The system beamformer transmits on up to 128 elements and receives on
64 elements.
The Processing Subsystem includes capabilities for interfacing with the beamformer and performing high speed
processing. The processing subsystem demodulates, filters, detects, and compresses the signal supplied by the
beamformer into display information.
4 Chapter 2: System Overview
Page 9

The Display Subsystem converts the detected ultrasound data into picture elements (pixels). The software user
interface graphics are combined with the ultrasound information and converted to a video stream. The external
video port supports NTSC and PAL format.
The Control Subsystem consists of the central processing unit, program and video memory, permanent image
storage and retrieval memory, external communication interface ports, and connection to the user interface keys.
The control software includes the acoustic power and intensity software subsystem, power group monitors, and a
beamformer monitor. This software guarantees a level of patient safety by ensuring the system is operating within
acoustic power and intensity limits.
The User Interface Subsystem represents the software interface and form factor. The software interface is the
interaction between the user and the screen layout components. The form factor is the type of physical buttons,
location, and grouping of the buttons and the device size, shape, and weight. Dedicated controls are for high usage
activities and grouped according to the user workflow.
The Power Subsystem provides the system power and protects the hardware from destructive and/or unsafe
conditions by detecting failures in the system through hardware and software monitors. Detection of a fault results
in disabling of the pulser supply, and signaling of an error to the Control Group. The power subsystem includes the
battery pack and battery charging electronics.
Description of Operating Modes
2D Mode 2D mode is a two dimensional image of the amplitude of the echo signal. It is used for
location and measurement of anatomical structures and for spatial orientation during
operation of other modes. In 2D, a two-dimensional cross-section of a 3-dimensional soft
tissue structure such as the heart is displayed in real time. Ultrasound echoes of different
intensities are mapped to different gray scale or color values in the display. The outline of the
2D cross-section may be a rectangle, parallelogram, trapezoid, sector, or a full circle,
depending on the particular transducer used. 2D mode can be used in combination with any
other modes.
MMode M Mode is also known as “T-M mode” or “time-motion” mode. It is used primarily for cardiac
measurements such as valve timing and septal wall thickness when accurate timing
information is required.
Ultrasound echoes of different intensities are mapped to different gray scale values in a
scrolling display. M Mode displays time motion information of the ultrasound data derived
from a stationary beam. Depth is arranged along the vertical axis with time along the
horizontal axis. M Mode can be used alone but is normally used in conjunction with a 2D
image for spatial reference. The 2D image has a graphical line (M-line) superimposed on the
2D image indicating where the M Mode beam is located.
Chapter 2: System Overview 5
Page 10

Color
Doppler
(Color)
In color Doppler, a real-time, two-dimensional cross-section of blood flow is displayed. The
2D cross-section may be presented as a rectangle, parallelogram, trapezoid, sector, or a full
circle, depending on the particular transducer used.
The 2D cross-section is presented as a full color display, with various colors being used to
represent the velocity, both positive and negative, of the blood flow echoes. Often, to
provide spatial orientation, the full color blood flow cross-section is overlaid on top of the
gray scale cross-section of soft tissue structure (2D echo). For each pixel in the overlay, the
decision of whether to display VCD, gray scale (echo) information or a blended combination
is based on the relative strength of echoes from the soft-tissue structures and from the red
blood cells.
A high pass filter (wall filter) is used to remove the signals from stationary or slowly moving
structures. Tissue motion is discriminated from blood flow by assuming that blood is moving
faster than the surrounding tissue, although additional parameters may also be used to
enhance the discrimination. The remaining signal after wall filtering may be averaged over
time (persistence) to present a steady state image of blood flow distribution. Variance
information may also be displayed to provide information when large variance is observed in
the velocity information.
Color Power
Doppler
(CPD)
Continuous
Wave (CW)
Doppler
In CPD, a real-time two-dimensional cross-section of blood flow is displayed. The 2D
cross-section may be presented as a rectangle, parallelogram, trapezoid, sector, or a full
circle, depending on the particular transducer used.
The 2D cross-section is presented as a full color display, with various colors being used to
represent the power in blood flow echoes. Often, to provide spatial orientation, the full color
blood flow cross-section is overlaid on top of the gray scale cross-section of soft tissue
structure (2D echo). For each pixel in the overlay, the decision of whether to display CPD, gray
scale (echo) information or a blended combination is based on the relative strength of
echoes from the soft-tissue structures and from the red blood cells.
A high pass filter (wall filter) is used to remove the signals from stationary or slowly moving
structures. Tissue motion is discriminated from blood flow by assuming that blood is moving
faster than the surrounding tissue, although additional parameters may also be used to
enhance the discrimination. The power in the remaining signal after wall filtering may be
averaged over time (persistence) to present a steady state image of blood flow distribution.
CW provides a real-time representation of blood flow and is displayed as a
velocity-versus-time sweeping output. Velocity (or frequency) is presented as the vertical
axis with time along the horizontal axis. The magnitude of the detected signal is represented
as different gray scale values.
CW Doppler mode provides the clinician with the ability to obtain blood flow velocities
focused about a user specified focal region. A continuous transmit waveform of ultrasound
energy with a known frequency is transmitted and focused by the system; on the receive
side, the transducer receive echoes are continuously amplified, focused about the focal
region and converted to a base band quadrature signal. The signal is analyzed by a
quadrature phase detector that establishes two receive channels to allow detection of flow
direction. These two channels are then analyzed by a fast complex Fourier transform (FFT)
circuit to establish the spectrum of frequencies present in the echoes. The data are displayed
as spectrum frequencies with respect to time.
CW can be used alone but is normally used in conjunction with a 2D image for spatial
reference. The 2D image has a graphical line (D-line) superimposed on the 2D image
indicating where the M-mode beam is located.
6 Chapter 2: System Overview
Page 11

Pulsed Wave
(PW) Doppler
PW provides a real-time representation of blood flow and is displayed as a
velocity-versus-time sweeping output. Velocity (or frequency) is presented as the vertical
axis with time along the horizontal axis. The magnitude of the detected signal is represented
as different gray scale values. The ultrasound data is derived from a single area, the sample
volume, on a stationary beam.
PW Doppler mode provides the clinician with the ability to obtain blood flow velocities
about a spatial sample volume. A burst of ultrasound with a known spectrum is transmitted
by the system; on the receive side, the transducer receive echoes are amplified and range
gated at the appropriate depth. The signal is analyzed by a quadrature phase detector that
establishes two receive channels to allow detection of flow direction. These two channels are
then analyzed by a fast complex Fourier transform (FFT) circuit to establish the spectrum of
frequencies present in the echoes. The data are displayed as spectrum frequencies with
respect to time.
PW can be used alone but is normally used in conjunction with a 2D image for spatial
reference. The 2D image has a graphical line (D-line) superimposed on the 2D image
indicating where the M-mode beam is located. The sample volume position (depth) and size
are also indicated on the D-Line.
Additional System Feature Performances
Broadband Imaging This ultrasound acquisition system uses high resolution broadband technology in
the transmit pulsers, transducer, and receivers. The receive path can capture and
process signals over a wide spectrum, from below 2.0 MHz to beyond 10 MHz. For
each application, the transmit pulse is designed to produce an appropriate
bandwidth. For example, in 2D grayscale imaging, a wide band pulse is used to
support good axial resolution. For Doppler modes, a narrower band pulse is used,
which improves the spectral resolution of the detected Doppler signal.
In addition to transmit pulse control, programmable digital signal processing is used
in the receive path to further refine the bandwidth used to produce the final image.
Digital filters are applied to the digitized received signal to limit and shape the
spectral bandwidth used to generate the displayed output.
Tissue Specific
Imaging
Biopsy Guidance The system can display a pair of biopsy guidelines that represent the anticipated
Measurement and
Calculation
Capabilities
In this feature, parameters for signal and image processing are optimized to
maximize the image quality or to obtain the best compromise of resolution and
penetration for different specific clinical applications. These parameters include: the
order of received filters, the bandwidth, the dynamic range, the compression curve,
the gain setting and parameters for compounding frequency band, etc. For
example, different system parameter setups are used for abdominal or peritoneal
scanning. This feature is for ease of use for the operator by automatically setting up
system control parameters rather than manually adjusting settings for best
performance.
path of the biopsy needle. The image of an anatomical target, biopsy guidelines, a
scan plane marker, and a biopsy needle are displayed to assist in guiding the biopsy
needle to the target. The system also provides needle guidance for vascular access
procedures. For additional information, see the biopsy user guides.
The system offers a variety of measurements and calculations, specific to exam type
and transducer. A list of them , and author references, are in the system user guide.
Measurement accuracy is also discussed.
Chapter 2: System Overview 7
Page 12

Continuous Wave
Doppler Audio
Output
The system provides for audio output of the CW velocity information. This can be
presented as stereo information, with flow moving towards the transducer on one
channel and flow away on the other, or as a mono output with the single audio
output representing the summation of the flow directions.
Pulsed Wave Doppler
Audio Output
Electrocardiograph
(ECG) Display
ECG Module
The ECG module allows a representation of the heart electrical activity to be displayed in real time with ultrasound
images acquired and displayed on the system video display.
The ECG module interfaces to the patient through three (3) ECG leads: Right Arm ECG lead (RA), Left Arm ECG lead
(LA), and Left Leg ECG lead (LL). The ECG received signal from the ECG electrodes are isolated, amplified, and
filtered by the ECG module before it is sent to the system for further processing and display.
The ECG module and cable are an integrated assembly. The module receives power from the system. Patient
isolation is provided by the ECG module, allowing the connection and signals to the system to be system-ground
referenced. The isolation between the patient and the system meets the requirements of IEC 601-1 for Type BF
equipment.
The system provides for audio output of the PW velocity information. This can be
presented as stereo information, with flow moving towards the transducer on one
channel and flow away on the other, or as a mono output with the single audio
output representing the summation of the flow directions.
ECG is provided to measure the electrical signal generated by the heart. A three lead
interface: Right Arm (RA), Left Arm (LA) and Left Leg (LL), is provided on the system.
The ECG signal is displayed as an amplitude-versus-time sweeping output.
Amplitude is presented on the vertical axis with time along the horizontal axis.
DICOM
The system features Digital Imaging and Communications (DICOM) capability to provide the acceptance, transfer,
display, storage, and digital processing of single ultrasound images as well as loops of ultrasound images.
IMT
The system includes the ability to measure the intima-media thickness (IMT) of the carotid artery using digital
ultrasound images. The intima is that region of the arterial wall from and including the endothelial surface at the
lumen to the luminal margin of the media. The media layer extends from the intima to the adventitia of the vessel
wall. The adventitia is normally quite echogenic on ultrasound images when compared to the media. The IMT
measurement of the carotid artery may be used adjunctively with other medical data obtained by a physician to
help assess the cardiovascular health of a patient.
System Specifications
This section contains system and accessory specifications and agency approvals. The specifications for
recommended peripherals can be found in the manufacturers’ instructions. See the applicable SonoSite accessory
user guide for information on the accessories.
8 Chapter 2: System Overview
Page 13

System Dimensions
Length: 11.8 in. (29.97 cm)
Width: 10.8 in. (27.43 cm)
Height: 3.1 in. (7.87 cm)
Weight: 8.5 lbs. (3.9 kg) with the C60x transducer and battery installed
Display Dimensions
Length: 8.4 in. (21.34 cm)
Height: 6.3 in. (16 cm)
Diagonal: 10.4 in. (26.4 cm)
Transducers
C11x/5-2 MHz 11 mm curved array (6 ft./1.8 m)
C60x/5-2 MHz 60 mm curved array (5.5 ft./1.7 m)
HFL38x/13-6 MHz 25 mm linear array (5.6 ft./1.7 m)
ICTx/8-5 MHz 11 mm intracavitary array (5.5 ft./1.7 m)
L25x/13-6 MHz 25 mm linear array (7.5 ft./2.3 m)
L38x/10-5 MHz 38 mm linear array (5.5 ft./1.7 m)
P21x/5-1 MHz 21 mm phased array (6 ft./1.8 m)
Imaging Modes
2D (256 gray shades)
Color power Doppler (CPD) (256 colors)
Color Doppler (Color) (256 colors)
Continuous Wave (CW) Doppler
MMode
Pulsed wave (PW) Doppler
Tissue Doppler Imaging (TDI)
Tissue Harmonic Imaging (THI)
Image and Clips Storage
The number of images and clips you can save varies with imaging mode and file format.
Accessories
Hardware, Software, and Documentation
Barcode Scanner
Battery
Biopsy Guide
Carry case
Chapter 2: System Overview 9
Page 14

ECG Cable (6 ft/1.8m)
External display
Footswitch
Kensington Security Cable
Mini-Dock
Mobile Docking System Lite II (MDS Lite II)
Mobile Docking System M Series (MDSm)
Needle Guide
Power supply
Quick Reference Guide
SiteLink Image Manager 4.0
SonoCalc IMT
System User Guide
System AC PowerCcord (10 ft / 3.1 m)
Triple Transducer Connect
Video and printer cables
Cables
See the M-Turbo Ultrasound System User Guide, MDSm User Guide, and the MDS Lite II User Guide for information on
cables.
Peripherals
Peripherals include the following medical grade (conforming to the requirements of EN60601-1) and non-medical
grade (commercial) products. Manufacturer’s instructions accompany each peripheral. System setup instructions
are in the M-Turbo Ultrasound System User Guide. Instructions for using peripherals with the system are in the
applicable SonoSite accessory user guide.
Medical Grade
Black-and-white printer
Recommended sources for printer paper: Contact CIVCO at 1-800-445-6741 or www.civco.com to order
supplies or to find the local distributor.
Color printer
DVD recorder
15” External monitor
Non-Medical Grade
USB Memory Stick
10 Chapter 2: System Overview
Page 15

Temperature, Pressure, and Humidity Limits
Note: The temperature, pressure, and humidity limits apply only to the ultrasound system and transducers.
Operating Limits: System
• 10–40°C (50–104°F), 15–95% R.H.
• 700 to 1060hPa (0.7 to 1.05 ATM)
Operating Limits: Battery
• 10–40°C (50–104°F), 15–95% R.H.
• 700 to 1060hPa (0.7 to 1.05 ATM)
Operating Limits: Transducer
10–40°C (50–104°F), 15–95% R.H.
Shipping/Storage Limits: System without Battery
• -35–65°C (-31–149°F), 15–95% R.H.
• 500 to 1060hPa (0.5 to 1.05 ATM)
Shipping/Storage Limits: Battery
• -20–60°C (-4–140°F), 0–95% R.H.*
• 500 to 1060hPa (0.5 to 1.05 ATM)
* For storage longer than 30 days, store at or below room temperature.
• 10–40°C (50–104°F), 15–95% R.H.
Shipping/Storage Limits: Transducer
• -35–65°C (-31–149°F), 15–95% R.H.
Electrical
Power Supply Input: 100-240 VAC, 50/60 Hz, 2.0 A Max @ 100 VAC.
Power Supply Output 1 15 VDC, 5.0A Max (system)
Power Supply Output 2 12 VDC, 2.3A Max (battery)
Combined output not exceeding 75W.
Battery
6-cell, 11.2 VDC, 5.2 amp-hours, rechargeable lithium ion battery pack.
Run time is up to 2 hours, depending on imaging mode and display brightness.
Chapter 2: System Overview 11
Page 16

Electromechanical Safety Standards
EN 60601-1:1997, European Norm, Medical Electrical Equipment–Part 1. General Requirements for Safety.
EN 60601-1-1:2001, European Norm, Medical Electrical Equipment–Part 1. General Requirements for
Safety–Section 1-1. Collateral Standard. Safety Requirements for Medical Electrical Systems.
EN 60601]2]37:2001 + Amendment A1:2005, European Norm, Particular requirements for the safety of ultrasonic
medical diagnostic and monitoring equipment.
CAN/CSA C22.2, No. 601.1]M90, Canadian Standards Association, Medical ElectricalEquipment.Part 1. General
Requirements for Safety (including CSA 601.1 Supplement 1:1994 and CSA 601.1 Amendment 2:1998)
.CEI/IEC 61157:1992, International Electrotechnical Commission, Requirements for the Declaration of the Acoustic
Output of Medical Diagnostic Ultrasonic Equipment.
UL 60601]1 (1st Edition), Underwriters Laboratories, Medical Electrical Equipment] Part 1: General Requirements
for Safety.
EMC Standards Classification
EN 60601-1-2:2001, European Norm, Medical Electrical Equipment. General Requirements for Safety-Collateral
Standard. Electromagnetic Compatibility. Requirements and Tests.
CISPR11:2004, International Electrotechnical Commission, International Special Committee on Radio Interference.
Industrial, Scientific, and Medical (ISM) Radio-Frequency Equipment Electromagnetic Disturbance
Characteristics-Limits and Methods of Measurement.
The Classification for the SonoSite system, SiteStand, accessories, and peripherals when configured together is:
Group 1, Class A.
Airborne Equipment Standards
RTCA/DO]160E:2004, Radio Technical Commission for Aeronautics, Environmental Conditions and Test Procedures
for Airborne Equipment, Section 21.0 Emission of Radio Frequency Energy, Category B.
DICOM Standard
NEMA PS 3.15: 2000, Digital Imaging and Communications in Medicine (DICOM)-Part 15: Security Profiles.
HIPAA Standard
The Health Insurance and Portability and Accountability Act, Pub.L. No. 104-191 (1996).
45 CFR 160, General Administrative Requirements.
45 CFR 164, Security and Privacy.
12 Chapter 2: System Overview
Page 17

Chapter 3: Troubleshooting
This chapter contains information to help you correct problems with system operation and provides instructions
on the proper care of the system, transducer, and accessories.
Periodic Maintenance
There is no recommended periodic or preventive maintenance required for the system, transducers, or accessories.
There are no internal adjustments or alignments required. There are no functions that require periodic testing or
calibration. Performance tests are described in Chapter 5, “Performance Testing” of this manual. Performing
maintenance activities not described in this manual may void the product warranty.
Local regulations may require electrical safety testing.
Contact SonoSite Technical Support for any maintenance questions.
System and Subsystem Diagnosis
This section covers basic diagnostic and troubleshooting procedures you may follow if the system does not
operate properly. To diagnose system failures, consult the referenced diagnostic figures that follow or SonoSite
Technical Suppor t.
Table 3.1: Troubleshooting Subassemblies and Diagnostic Figures
Subassemblies Diagnostic Figures or Table
DICOM Tab le 3. 2
Dipslay TBA
Battery TBA
Control Panel TBA
System Repair
The system is repairable through subassembly replacement or through replacement of parts as recommended by
SonoSite in Chapter 4, “Replacement Procedures.” Component level repair of Printed Circuit Board Assemblies is
performed only at the SonoSite repair facility. Replacement of board level components by unauthorized service
facilities voids the SonoSite warranty.
Test Equipment
Test equipment is not required for this troubleshooting section. Troubleshooting test aids include an external
monitor and a spare battery.
Chapter 3: Troubleshooting 13
Page 18
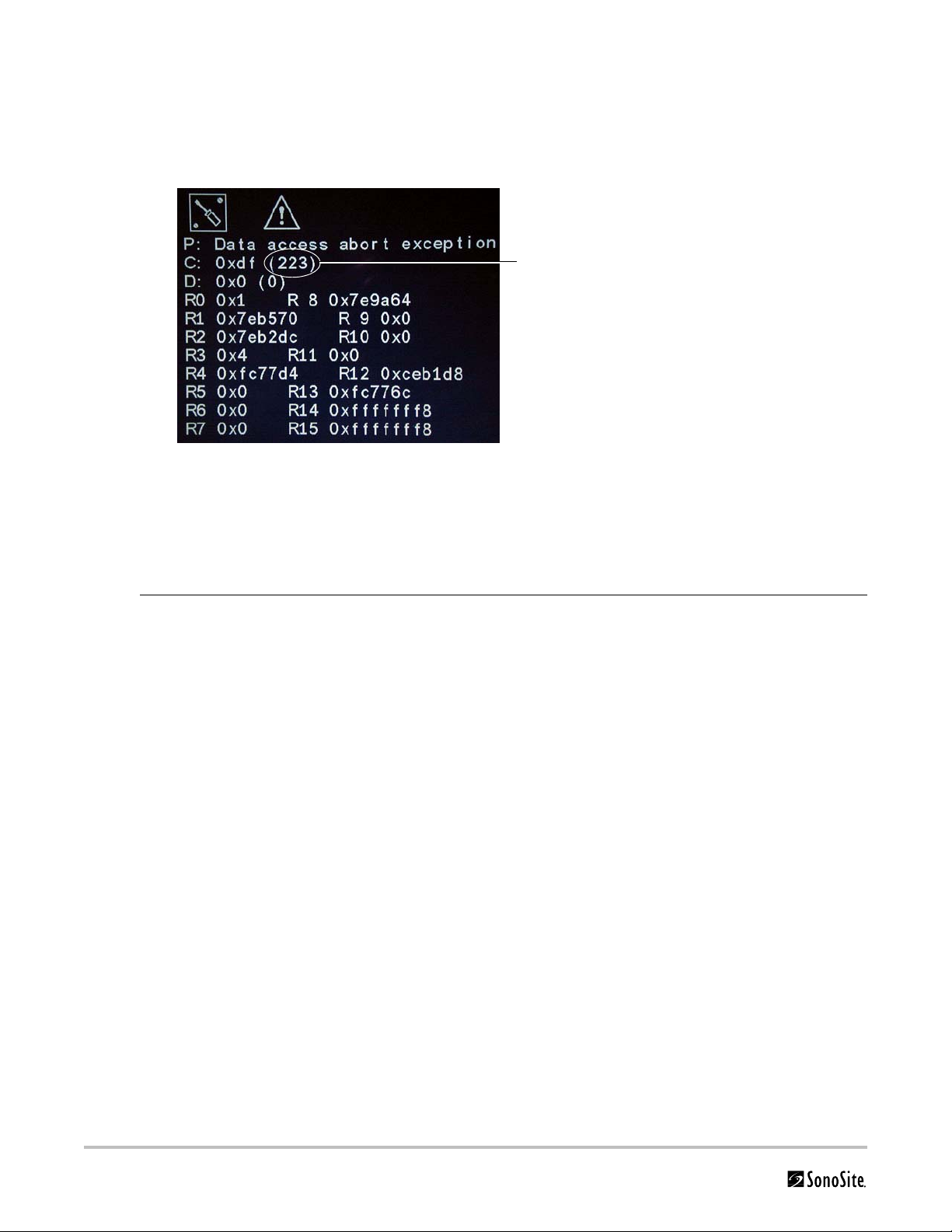
Failure (Assert) Codes
Assert code
The system displays an “assert screen” for hardware and software issues related to main PCBA failures. Main PCBA
failures typically result in “assert codes” that are output to the display. If an assert screen appears, note the assert
code and contact SonoSite Technical Support to clarify the failure. Figure 3.1 shows an assert screen. The assert
code is the bracketed number on the line labeled “C:”.
Figure 3.1 Assert Screen
Verifying a System Assert Code
System asserts are caused by hardware and/or software faults. Hardware asserts typically require main PCBA
replacement. Software asserts can be reset and the system may recover. A simple method to identify the cause of
the assert is identified here:
Assert Cause 1 Record the assert code.
2 Press and release the Power button to power the system down.
3 Press the Power button again to power on the system.
• If the system powers on normally, it has recovered from the fault (software assert) and
you may use the system.
• If the assert condition remains, corrective action must be taken; usually replacement of
the main PCBA is required. Contact SonoSite Technical Support for assistance and to
obtain repair parts.
If the Power button is not functional, all sources of power must be removed to allow the
system to power down. I.e., disconnect AC power and remove the battery.
14 Chapter 3: Troubleshooting
Page 19

DICOM
Table 3.2: DICOM Troubleshooting
Error Message Tiller Error Code Cause Troubleshooting
Socket
communication
failed
Archiver
transaction
failed
Printer
transaction
failed
TSOCKET_CONNECT_FAILURE Invalid network
configuration.
Wrong port
number.
Application is not
running.
Printer is offline.
TDICARCH_OPEN_FAILURE Wrong Capture
Type selected
TDICPRNT_OPEN_FAILURE Wrong Image
settings
Using Ping, verify that the
Printer/Archiver is connected.
• If Ping fails, check the devices IP
address, M-Turbo IP address,
Subnet mask, and Gateway IP
address.
• If Ping is OK, use Verify to check
if device is available.
If Verify fails:
a) Check the Printer/Archiver’s
Port configuration on the
M-Turbo.
b) Ensure that the Printer is
online and the Archiver’s
application is running.
Verify that the Archiver supports
the selected Capture Type setting,
e.g., US Image, SC Image or
US-Ret Image.
Verify that the printer supports
the selected Image settings. E.g,.
Color (RGB) or Grayscale
(Monochrome)
DICOM network
communication
failed
Internal failure
detected
TDNETWORK_OPEN_FAILURE Device does not
recognize
M-Turbo, rejects
association
TDNETWORK_READ_FAILURE Invalid DICOM
Attribute
Verify that M-Turbo AE Title or IP
address is correctly configured on
the Printer/Archiver.
Note: Some devices require that the
Imaging modality (M-Turbo) be
recognized in order to accept
images. This requires configuration
on the device.
Check M-Turbo Printer DICOM
settings for correctness (e.g., film
size, format)
Chapter 3: Troubleshooting 15
Page 20

16 Chapter 3: Troubleshooting
Page 21

Chapter 4: Replacement Procedures
Screws (2)
Caution:
Caution:
Always use correct ESD procedures. ESD damage is cumulative and may not be noticeable at first.
Initial ESD symptoms may be slightly degraded performance or image quality.
All fasteners should be torqued to 5.5 inch pounds except where noted.
Display Replacement
Required Parts
Service Assembly, LCD Display, M-Turbo (P08659)
Required Tools
• #1 Phillips screwdriver
• Torque screwdriver, 2.0–10.0 inch pounds (0.23–1.1 newton meter)
• An anti-static mat
• A wrist grounding strap
Display Removal
Display
Removal
Figure 4.1 System Rear
1 Remove the battery from the system.
2 Remove the two screws from the back of the system per Figure 4.1.
Chapter 4: Replacement Procedures 17
Page 22

3 Lay the system on the top, and remove the two screws from the bottom of the system per
Screws (2)
Figure 4.2.
Figure 4.2 System Bottom
4 Turn the system over, fully open the display, and lift off the Control Panel per Figure 4.3.
Figure 4.3 Control Panel Removal
18 Chapter 4: Replacement Procedures
Page 23

5 Disconnect the two connectors from the display to the Main PCBA per Figure 4.4.
Connectors (2)
Remove Screw Caps and
Screws (2)
Display rear enclosure slides
up
Figure 4.4 Display Connectors
6 The replacement Display Assembly does not include the Display Rear Enclosure. Remove
the Display Rear Enclosure by removing the two screw caps, two screws, and then sliding
the rear enclosure up and away from the display as show in Figure 4.5.
Figure 4.5 Remove Display Back Enclosure
Chapter 4: Replacement Procedures 19
Page 24

7 Remove the four screws from the Display Hinges per Figure 4.6.
Screws (4)
Figure 4.6 Display Screws
Display Replacement
Display
Replacement
Test the Display
Test Display 1 Replace the battery or attach an external power supply.
1 Set the new display in place.
2 Install the four hinge screws that hold the Display in place. Torque the screws to 5.5 inch
pounds.
3 Reinstall the Display Rear Enclosure, screws (2) and screw caps.
4 Connect the two connectors that connect the Display to the Main PCBA.
5 Place the Control Panel in place.
6 Reinstall the four screws that hold the Control Panel in place. Torque the screws to 5.5 inch
pounds.
2 Press the Power key to apply power to the system.
3 Verify the display operates correctly.
20 Chapter 4: Replacement Procedures
Page 25

Control Panel Subassembly Replacement
Required Parts
One of the following:
• P08856 Service Assembly, Control Panel M-Turbo, English
• P08878 Service Assembly, Control Panel M-Turbo, French
• P08879 Service Assembly, Control Panel M-Turbo, German
• P08880 Service Assembly, Control Panel M-Turbo, Italian
• P08881 Service Assembly, Control Panel M-Turbo, Spanish
• P08882 Service Assembly, Control Panel M-Turbo, Portuguese
Required Tools
• #1 Phillips screwdriver
• Torque screwdriver, 2.0–10.0 inch pounds (0.23–1.1 newton meter)
• An anti-static mat
• A wrist grounding strap
Caution:
Always use correct ESD procedures. ESD damage is cumulative and may not be noticeable at first.
Initial ESD symptoms may be slightly degraded performance or image quality.
Control Panel Removal
Control Panel
Removal
1 Remove the two screws from the rear of the system per Figure 4.1.
2 Remove the two screws from the bottom of the system per Figure 4.2.
3 Turn the system over, fully open the display, and lift off the Control Panel per Figure 4.3.
Control Panel Replacement
Control Panel
Replacement
1 Place the new control panel in place.
2 Install the four screws removed in “Control Panel Removal” on page 21. Torque the screws
to 5.5 inch pounds.
Chapter 4: Replacement Procedures 21
Page 26

Main System Disassembly for Repair and/or Replacement
Required Parts
Parts for the Main System Repair could include any of the following:
• P08939 Service Assembly Main PCBA, M-Turbo
• P08850 Service Assembly Power Supply, M-Turbo
• P05470 Service Assembly TGC, MicroMaxx (compatible with MicroMaxx and M-Turbo)
• P05473 Service Assembly Speaker, M-Turbo
• Nest Frame Assembly, M-Turbo (order these parts individually as necessary)
• P00364 Connector, Interposer (Qty 8)
• P00924 Screw, Shoulder, Thrust Plate (Qty 4)
• P00353 Wear Plate
• P00646 Spring, Thrust Plate (Qty 4)
• P07750 Nest Frame
• P03834 Shield, Perimeter, Long (Qty 2)
• P03833 Shield, Perimeter, Short (Qty 2)
• P08200 M2.5-.45x10 Socket Head Cap Screw (Qty 4)
Required Tools
• #1 Phillips screwdriver
• Torque screwdriver, 2.0–10.0 inch pounds (0.23–1.1 newton meter)
•2 mm allen key
•Scissors
•Q-Tips
• An anti-static mat
• A wrist grounding strap
Caution:
Always use correct ESD procedures. ESD damage is cumulative and may not be noticeable at first.
Initial ESD symptoms may be slightly degraded performance or image quality.
System Disassembly
System
Disassembly
1 Remove the battery.
2 Remove the control panel from the system following the removal procedures in “Control
3 Remove the 4 remaining screws from the bottom of the system.
4 Remove the bottom enclosure. This exposes all of the replaceable parts for the main system
Panel Removal” on page 21.
per Figure 4.7.
22 Chapter 4: Replacement Procedures
Page 27

Major System Components
Nest frame
assembly
TGC assembly
SpeakerSpeaker
Power supply
Main PCBA
SD Card
Daughter-card
Connectors
Retaining clips
Figure 4.7 System Components
Speaker Replacement
Caution:
Use caution when removing the left speaker connector to prevent damage to the Main PCBA
components around the connector.
Speaker
Replacement
1 Press on the connector release and pull the connector out of the receptacle.
2 Gently pry off the retaining clip with a flat bladed pry tool. See Figure 4.8.
3 Replace the speakers by reversing steps 1-2.
Figure 4.8 Speaker Replacement
Chapter 4: Replacement Procedures 23
Page 28

Power Supply PCBA Replacement
Power supply
shield
Power Supply
Removal
1 Gently pry the shield from the power supply and set it aside. This part will be used in
reassembly. Note that the shield fits only one way. See Figure 4.9.
Figure 4.9 Power Supply Shield
2 Remove the 7 screws that hold down the power supply PCB per Figure 4.10.
3 Gently lift the power supply away from the Main PCBA.
4 Install the new Power Suppply PCBA by reversing steps 1-3.
24 Chapter 4: Replacement Procedures
Power supply
screws (7x)
Figure 4.10 Power Supply Screws
Page 29

SD Card Daughter-card
SD Card Daughter-card
under copper tape
SD Card
Daughter-card
screws (4x)
Long screw
SD Card
1 Carefully remove the copper tape from the SD Card Daughter-card. See Figure 4.11.
Daughter-card
Removal
Figure 4.11 SD Card Daughter-card copper tape
2 Remove the 4 screws that hold down the SD Card Daughter-card per Figure 4.12. Note the
location of the one longer screw for reassembly.
3 Gently lift the SD Card Daughter-card straight up away from the Main PCBA.
Figure 4.12 SD Card Daughter-card screws
Chapter 4: Replacement Procedures 25
Page 30

SD Card
Copper tape applied to
side of Power Supply
Frame
Ventilation cut-outs
SD Card
Daughter-card
alignment pins
Note: Kapton tape is used to
retain the SD Cards in place
Daughter-card
Replacement
1 Remove Power Supply frame assembly from the Main PCBA.
2 Apply one strip of 1” x 5” self adhesive copper tape to the edge of the Power Supply frame
as shown in Figure 4.13.
3 The copper tape must be cut away from the ventilation holes in the frame or failure of the
Main PCBA will occur.
Figure 4.13 SD Card Daughter-card copper tape
4 Place the Power Supply frame back onto the Main PCBA.
5 Place the Power Supply PCBA in the frame and secure with the 7 screws
6 Install the SD Card Daughter-card onto the Main PCBA frame using the alignment
holes/pins on the card and frame.SeeFigure 4.14.
Caution: Improper installation of the SD Card Daughter-card will cause all or part of the
internal image storage memory to not be recognized by the system.
7 Install the screws ensuring proper location of longer screw.
26 Chapter 4: Replacement Procedures
Page 31

Figure 4.14 SD Card Daughter-card alignment
8 Fold the copper strip installed in Step 1 over the top of the SD Card Daughter-card.
9 Install a second strip of 1” x 5” self-adhesive copper tape over the SD Card Daughter-card on
the edge closest to the Power Supply frame as show in Figure 4.15.
Figure 4.15 Copper Tape Installation
10 Install a third strip of 1” x 5” self-adhesive copper tape over the SD Card Daughter-card as
shown in Figure 4.16.
Figure 4.16 Copper Tape Installation
11 The adhesive on the copper strips must be activated by rubbing the entire surface of the
copper tape using a Q-tip as shown in Figure 4.17.
Chapter 4: Replacement Procedures 27
Page 32

Figure 4.17 Activating Copper Tape Adhesive
Note: Rub the entire surface
of the copper tape to ensure
proper adhesion.
TGC knobs (3)
TGC PCBA Replacement
TGC PCBA
Removal
1 Remove the Control Panel if not already removed.
2 Remove the TGC knobs identified in Figure 4.18.
Figure 4.18 TGC Knobs
28 Chapter 4: Replacement Procedures
Page 33

3 Remove the flex cable from the TGC PCB by lifting on the flex release tab. See Figure 4.19.
Release tabs Screws (2)
Screws (3)
4 Remove the flex cable from the Main PCBA by lifting gently on the flex release tab.
5 Remove the two screws holding the TGC PCBA in place.
6 Reverse steps 1-5 to reinstall the TGC PCB.
Figure 4.19 TGC PCBA Removal
Main PCBA Replacement
Main PCBA
Removal
1 Remove the Power Supply PCBA, SD Card Daughter-card, and TGC PCBA as described in the
2 Remove the 3 screws holding the Main PCBA in place per Figure 4.20. Dissconnect the
previous steps.
speaker wires from the Main PCBA.
Figure 4.20 Main PCBA Screws
Chapter 4: Replacement Procedures 29
Page 34

Figure 4.21 Nest Frame Top Screws
2.5mm Socket Head Cap
Screws (4x)
3 Turn the system over.
4 Remove the 4 Socket Head Cap Screw as shown in Figure 4.21. This releases the Nest Frame
and will allow the Main PCBA to be removed.
5 As you remove the nest frame assembly from the PCBA, tilt the PCBA and enclosure to
almost vertical to avoid spilling the Interposer Connectors from the assembly.
6 Lift on the edge of the Main PCBA closest to the system handle.
30 Chapter 4: Replacement Procedures
Page 35

Main PCBA
Replacement
Replace the Main PCBA by following the reverse of the removal procedure. Do not tighten all the
screws until everything is in place.
1 Replace the Main PCBA.
2 Reinstall the Nest Frame Assembly. The Nest Frame Socket Head Cap Screws should be
torqued to 4.5 inch pounds
3 Reconnect the speaker wires.
4 Reinstall the Power Supply PCBA.
5 Reinstall the SD Card Daughter-card and copper tape.
6 Reinstall the TGC assembly.
7 Reinstall the shield to the Power Supply.
8 Tighten all screws to their specified torque of 5.5 inch pounds.
9 Reinstall the Control Panel.
10 Reinstall the bottom enclosure.
Chapter 4: Replacement Procedures 31
Page 36

32 Chapter 4: Replacement Procedures
Page 37

Chapter 5: Performance Testing
Overview
WAR NIN G:
To obtain 2D images, SonoSite recommends using the RMI 413A Soft Tissue Phantom or the RMI 403 GS
Multipurpose Phantom. A .7db/cm phantom is required for performing penetration measurements. Any
equivalent .7db/cm Phantom is acceptable.
When making penetration measurements on a phantom, apply the phantom reference value and tolerance to the
measurement.
Some features and capabilities are optional and therefore may be uavailable to test.
Critical Test Function — A failure of the system functions tested in this section could affect
safety or effectiveness of the system adversely. While performing the steps in this section, verify
that the images on the system display and on the external monitor are acceptable.
Test Equipment
• SonoSite ultrasound system under test
• C60x/5-2 MHz transducer
• P21x/5-1 MHz transducer
• RMI 413A Soft Tissue Phantom, RMI 403 GS Multipurpose Phantom, or equivalent. A referenced .7db/cm
phantom is required for performing penetration measurements.
• Video Printer
• External Monitor
•Acoustic gel
Setting Up Performance Tests
Set up
Performance
Te st s
1 Attach the C60x/5-2 MHz transducer to the system.
2 Select Gen for optimization and OB for exam type.
3 Couple the transducer to the phantom, adjusting gain settings and transducer for a proper
phantom image (e.g., pins are high-level echoes positioned in straight lines; cysts are
sonolucent, edges are sharp, and graphite particles of the phantom are mid-grays).
Chapter 5: Performance Testing 33
Page 38

Basic Operational Tests
Basic System
Operation
Te st s
1 Verify that the correct transducer name appears in the upper right corner of the system
display.
2 Verify proper date and time.
3 Verify that the scan plane orientation mark in the image located near the skinline
corresponds to element #1 on the transducer. To test, put your finger on the probe and run
it across the transducer face. Your finger touching the transducer face should appear at the
orientation mark on the display image format.
4 Verify that all of the keyboard keys are functional. Verify that all controls operate smoothly
over their full range and that the system responds properly.
5 Verify that all of the softkeys are functional.
6 Verify that as the Gain controls are increased and decreased, there is a corresponding
increase and decrease in echo intensity.
7 Capture a Cineloop buffer. Exercise the Cineloop controls and verify proper operation.
8 Close the lid and verify the unit goes into sleep mode. Open the lid and verify the unit
returns to normal operation.
9 Verify the airflow from the vent on the left side of the system is blowing out.
2D Performance Tests
2D Performance / Image Quality
Test 2D
Performance
and Image
Quality
1 Use a C60x/5-2 MHz transducer in 2D mode.
2 Adjust the position of the C60x/5-2 MHz transducer on the phantom.
3 With the array pointing down and the orientation mark to the operator’s left, element #1
corresponds with the left side of the array.
4 Use the 2D system controls to obtain a clear image that shows both the horizontal and
vertical rows of pins.
5 Verify that the ultrasound image appears uniform in both the axial and lateral direction,
with no dropouts or intensity variations.
6 Verify that the cystic structure at the focal zone is clearly differentiated from the
surrounding tissue and is echo-free, while solid tissue with numerous echo sources, appears
solid.
7 Press the Freeze key and then save the image. Press the Freeze key again to return to live
imaging.
34 Chapter 5: Performance Testing
Page 39

Axial Measurement Accuracy
Note: Measurements must be performed while the image is frozen.
Set Up Axial
Measurement
Accuracy
Test Axial
Measurement
Accuracy
1Acquire the image.
2 Press the Freeze key.
3 Press the Caliper key. The caliper appears on the image display. (See the M-Turbo Ultrasound
System User Guide, if necessary, for caliper operation.)
4 Use the touchpad to position one of the calipers.
5 Press the Select key to fix the caliper and enable the other caliper.
6 Use the touchpad to move the other caliper. The results update as you move the caliper, and
the measurement is complete when you finish moving the calipers. (Press the Select key to
alternate the active caliper, and adjust the measurement with the touchpad.)
1 Measure the distance, center to center, of any two pins that are 5-12 cm apart vertically.
2 Verify that the distance measured is within the tolerance listed in Tab le 5 .1.
Lateral Measurement Accuracy
Set Up Lateral
Measurement
Accuracy
Test Lateral
Measurement
Accuracy
Perform “Set Up Axial Measurement Accuracy” on page 35.
1 Measure the distance, center to center, of any two pins that are 4-10 cm apart horizontally.
2 Verify that the distance measured is within the tolerance listed in Tab le 5 .1.
3 Press the Freeze key to return the system to live 2D mode.
Table 5.1: System Measurement Accuracy
Measurements Tolerance
Axial Distance +/- 2%
Lateral Distance +/- 2%
Penetration
Caution:
Te st
Penetration
A referenced .7db/cm phantom is required for performing penetration measurements
1 Adjust the system controls to obtain a clear image that shows the limits of echo penetration
as shown in Table 5. 2.
2 Set the system exam type and optimization mode settings to the values shown in Table 5. 2.
3 Measure from the center of the skinline to the deepest vertical position—where the scatter
echoes start to break up and tissue definition is lost.
4 When making penetration measurements on a phantom, apply the phantom reference
value and tolerance to the measurement.
5 Press the Freeze key and then save the image. Press the Freeze key again to return to live
imaging.
Chapter 5: Performance Testing 35
Page 40

Table 5.2: Imaging Performance
Imaging
Performance
Exam type Nerve OB OB Small
Optimization Gen Gen Gen Res Res Res Pen
2D Penetration 6.8cm 14.0 cm 6.5 cm 4.5 cm 4.3 cm 5.7 cm 21.0 cm
C11x C60x ICTx HFL38 L25x L38x P21x
Additional Performance Tests
Color Doppler (Color)
Test Colo r 1 Connect any transducer.
2 Press the Color key. “Color” should be annotated in the top left corner of the display.
3 A Region of Interest (ROI) box is displayed on top of the grayscale image. Use the touchpad
to move the CPD ROI. Verify that the ROI moves to the new position on the display.
4Adjust the Depth control for minimum depth in the image.
5Adjust the Gain control so that color speckles just appear inside the ROI box.
6 Gently tap the face of the transducer and observe that the ROI box fills with color
information.
7 Press the Freeze key and then save the image. Press the Freeze key again to return to live
imaging.
Sup Breast ABD
Parts
Color Power Doppler (CPD)
Test CP D 1 Connect any transducer.
2 Press the Color key. A Region of Interest (ROI) box is displayed on top of the grayscale image.
3 Press the Color softkey to switch to CPD. “CPD” should be annotated in the top left corner
of the display.
4Adjust the Depth control for minimum depth in the image.
5Adjust the Gain control so that color speckles just appear inside the ROI box.
6 Gently tap the face of the transducer and observe that the ROI box fills with color
information.
36 Chapter 5: Performance Testing
Page 41

MMode Imaging
Test M Mod e
Imaging
1 Attach a C60x transducer and acquire an image.
2 Press the MMode key for the M Mode sample line.
3 Position the M Mode sample line over the image using the touchpad.
4 Press the MMode key again to turn on M Mode.
5 Select the desired sweep speed from the on-screen menu (slow, med, or fast). The on-screen
menu will show the selected sweep speed.
6 Press the Freeze key to freeze the image. Save the image. Press the Freeze key again to
return to live imaging.
7 Press the 2D key to return to 2D imaging.
Tissue Harmonic Imaging
Test THI
Imaging
1 Attach the C60x transducer and acquire an image.
2 Set the depth to maximum and note the depth at which echo information is lost.
3 Press the THI key on the control panel so it displays THI on the display. Tissue Harmonic
Imaging in now active.
4 Observe a decrease in dot size and a significant loss in penetration due to the higher
frequency. Image resolution increases.
5 Press the Freeze key and then save the image. Press the Freeze key again to return to live
imaging.
6 Press the THI key again to turn off Tissue Harmonic Imaging.
Pulsed Wave (PW) Doppler Imaging
Test PW
Doppler
Imaging
1 Attach the P21x transducer.
2 Press the Doppler key for the Doppler sample gate.
3 Press the Doppler key again for the Doppler spectral trace.
4 Place a large drop of ultrasound gel on the transducer lens.
5Adjust the Gain control as necessary and then gently tap the top of the gel and observe a
reflection on the spectral trace and the sound from the speakers.
6 Press the Freeze key and then save the image. Press the Freeze key again to return to live
imaging.
7 Press the 2D key to return to 2D imaging.
Chapter 5: Performance Testing 37
Page 42

Continuous Wave (CW) Doppler Imaging
Test CW
Doppler
Imaging
1 Attach the P21x transducer.
2 Press the Patient key.
3 Select the Cardiac exam type.
4 Press the Done softkey.
5 Press the Doppler key for the Doppler sample gate.
6 Press the PW softkey to switch to CW Mode.
7 Press the Doppler key again for the Doppler spectral trace.
8 Place a large drop of ultrasound gel on the transducer lens.
9Adjust the Gain control as necessary and then gently tap the top of the gel and observe a
reflection on the spectral trace and the sound from the speakers.
10 Press the Freeze key and then save the image. Press the Freeze key again to return to live
imaging.
11 Press the 2D key to return to 2D imaging.
Image Quality Verification Test/Livescan
• Products with replaced subassemblies, or products that have been otherwise disassembled, must undergo an
Image Quality Verification Test/Livescan.
• The Image Quality Verification Test/Livescan should be performed after successfully completing all applicable
performance tests listed prior in this chapter.
• The test is completed before returning the system to service.
• A certified sonographer must perform the test.
• The Livescan test performed is at the discretion of the Sonographer and will represent their acceptance of a
successful service event.
• Review all saved images and verify that the images are displayed properly.
Printer
Test Print e r
Operation
1 Verify proper printer type is configured in the system Setups page.
1 Press the print button and verify that the printer begins to print an image. After the image
begins to emerge from the printer, press the print button again. The printer should ignore
the second print command.
2 Verify the proper content of the printed image.
38 Chapter 5: Performance Testing
Page 43

Battery Charging
Test Batt e r y
Charging
Operation
Video Output
Caution:
Test Video
Output
1 Remove the system from the docking system and insert a battery into the system.
2 Press the Power key to turn the system on. Allow the battery to discharge. The battery
indicator icon on the display, below the Transducer Type indicator, will extinguish from left
to right as the battery discharges.
Note: The Power and Sleep delays in the Setup page should be selected to “Off” to properly
perform this test. The battery may take 1–2 hours to discharge.
3 Reattach the system to the Docking System and attach the AC power cord to the power
connector.
4 Note that the battery indicator indicates that the battery is charging. The sections of the
battery indicator will light sequentially from left to right as the battery charges.
Use only the recommended video monitor or printer when verifying the video output at the
video receptacle.
1 Attach an external video monitor to the video connector using the video cable.
2 Turn on the system power and verify that the video on the external monitor matches the
video on the system display.
If the video does not appear similar, or there is no display on the external monitor, see
Chapter 3, “Troubleshooting” for troubleshooting procedures.
Chapter 5: Performance Testing 39
Page 44

40 Chapter 5: Performance Testing
Page 45

Appendix A: Replacement Parts List
1
2
3
The following tables contain all the field-replaceable parts for the M-Turbo ultrasound system. Quantities are one
unless otherwise noted.
Display
Table A.1: Display
Find Number Part Number Description
1 P08659 Service Assembly Display M-Turbo
Note: The Display Assembly does not include the rear Display Enclosure (item
3). This should be retained from the unit being replaced.
2P08060Hinge
P08855 Service Assy, Display Enclosure, Gray (Olympic Mist), M-Turbo
P08874 Service Assy, Display Enclosure, Blue (Glacier Sky), M-Turbo
3
P08875 Service Assy, Display Enclosure, Green (Pacific Pine), M-Turbo
P08876 Service Assy, Display Enclosure, Brown (Copper River), M-Turbo
P08877 Service Assy, Display Enclosure, Pink (Alpine Berry), M-Turbo
Appendix A: Replacement Parts List 41
Page 46

Control Panel
Table A.2: Control Panel
Part Number Description
P08856 Service Assembly Control Panel, M-Turbo, English
P08878 Service Assembly Control Panel, M-Turbo, French
P08879 Service Assembly Control Panel, M-Turbo, German
P08880 Service Assembly Control Panel, M-Turbo, Italian
P08881 Service Assembly Control Panel, M-Turbo, Spanish
P08882 Service Assembly Control Panel, M-Turbo, Portuguese
42 Appendix A: Replacement Parts List
Page 47

System
3
4 4
6
5
2
1
Table A.3: System
Find Number Part Number Description
1 P07442 SD Card Daughter-card
2 P09202 2GB SD Card
not shown P09216-01 Copper Tape for SD Card Daughter-card (Note: Part number referenced is
per inch of copper tape. Approximately 15 inches of 1” wide tape is required
per system.)
3 P08850 Service Assembly, Power Supply, M-Turbo
4 P03872 Service Assembly, Speaker
5 P08939 Service Assembly Main PCBA, M-Turbo
Note: This part does not include the transducer nest frame assembly. Those
parts must be ordered separately if needed to complete the replacement of the
Main PCBA.
6 P05470 Service Assembly, TGC PCB
Not shown P00361 Foot
Appendix A: Replacement Parts List 43
Page 48

Figure A.1 Power Supply, P08850
Figure A.2 Speaker Assembly, P03872
44 Appendix A: Replacement Parts List
Page 49

Figure A.3 TGC Assembly, P05470
1
2
3
Table A.4: TGC Assembly
Find Number Part Number Description
1 P02317 Assembly, PCB, TGC
2 P06287 Knob, TGC
3 P02308 FFC, 12 Position Jumper
Figure A.4 Main PCB Assembly, P08939
Appendix A: Replacement Parts List 45
Page 50

Transducer Nest Frame Assembly
2
6
1
7
5
4
3
Figure A.5 Nest Frame Parts
Table A.5: Nest Frame Assembly
Find Number Part Number Description
1 P07750 Nest Frame Assembly
2 P00364 Connector, Interposer (8x)
3 P03833 Shield, Perimeter, Short (2x)
4 P03834 Shield, Perimeter, Long (2x)
5 P00924 Screw, Shoulder, Thrust Plate (4x)
6 P00353 Wear Plate
not shown P00646 Spring, Thrust Plate, .047 wire (4x)
7 P08200 Socket Head Cap Screw, M2.5-.45x10mm (4x)
Ordering Replacement Parts
To order parts, contact SonoSite Technical Support as indicated in “Contact Information” on page 1.
46 Appendix A: Replacement Parts List
Page 51

Appendix B: Service Event Report
The Service Event Report provides information about product failures to the manufacturer and to authorized
service facilities, which provide approved warranty services for SonoSite products. For all repairs completed,
complete the form and return a copy of it to the following address:
SonoSite, Inc.
Technical Suppor t
21919 30th Drive SE
Bothell, Washington 98021
USA
To co nt a c t SonoSite Technical Suppor t , s e e“Contact Information” on page 1.
Appendix B: Service Event Report 47
Page 52

Service Event Report Form
48 Appendix B: Service Event Report
Page 53

Service Event Report Instructions
Instructions for completing the Service Event Report
Sections highlighted in yellow must be completed for SonoSite to accept the Service Event Report. If additional information is
required for certain circumstances you will be advised.
Forward the completed form to:
Email: service@sonosite.com
Fax: +1-425-951-6700
Service Type
x Out of Box Failure: the item has arrived from SonoSite with failures.
x Warranty Service: the item has failed after arrival and is covered by either the included warranty or a valid extended
warranty.
x Out of Warranty Service: the item has failed and it is no longer covered by a warranty.
Parts Status
x Check One.
Service Provider
x Name: the name of the technician performing the work.
x Provider Reference: a unique number used by the Provider to track Service Event Reports. Any format is acceptable.
x Company: the name of the Distributor or authorized repair facility.
x Address: the address replacement parts will be shipped to.
x Date Reported: the date the failure was reported to SonoSite.
x Phone Number: the phone number to contact the service technician.
x Fax Number: the fax number to contact the service technician.
x Email Address: the email address to contact the service technician.
Device Description:
x Name: the description of the failed product.
x Ref Number: the reference number from the part number label of the failed product.
x Serial Number: the serial number from the part number label of the failed product.
x Lot Number: if applicable, the Lot Number from the device identification label.
x ARM/SHDB Version: the software level of the failed device. Typically found on the system information screen.
x Configuration: for configurable devices, the optional features enabled.
Event Description
x A description of the problem in the words of the user. Typically what the user reports to the repair facility.
Diagnosis
x A description of what the repair technician found. Include a list of the suspect parts.
Service Performed
x A description of the work performed to repair the system. Typically only completed if it is repaired from stock repair parts.
Parts Removed
x Part Name: the name of the failed/suspect part to be replaced.
x Part Number: the part number of the failed/suspect part.
x Serial Number: the serial number from the failed/suspect part.
x Lot Number: the lot number if applicable.
x Rev: the revision of the failed/suspect part if available.
x Replaced By: the person replacing the part.
Parts Installed
x The same information as the Parts Removed except from the parts installed if work has already been performed. If you are
waiting for parts to be ordered, leave this section blank.
Tests Performed
x The results of any testing performed, if testing has already been performed.
Appendix B: Service Event Report 49
Page 54

Returning Products to SonoSite
You will be asked to provide the following information:
• Contact name and phone number
•Product name
•Serial number
• Description of the problem
Shipping Instructions
Please contact SonoSite to get a return material authorization number (RMA). Contact SonoSite before returning
any product.
The shipping address for all returned products is:
SonoSite, Inc.
Attn: Technical Support RMA ___________________
21919 30th Drive SE
Bothell, Washington 98021
USA
50 Appendix B: Service Event Report
Page 55

Index
Numerics
2D performance tests
axial measurement accuracy 35
image quality 35
lateral measurement accuracy 35
penetration 35
A
accessories 8
assert code 14
assistance, customer 1
B
battery
specifications 11
storage and shipping 11
battery charging test 39
C
cable specifications 10
control panel assembly
replacement procedure 21
conventions used 1
D
display assembly
replacement procedure 17
I
image
quality verification test 38
review 38
M
main PCBA
failures 14
replacement procedure 22
monitor 8
P
performance tests
2D 34
battery 39
CPD 36
CW 38
M-Mode 37
overview 33
printer 38
PW 37
THI 37
Velocity Color 36
video output 39
periodic maintenance 13
peripherals 10
power supply replacement procedure 24
printer
test 38
product failures, reporting 47
products, returning 50
R
replacement parts
list 41
ordering 46
replacement procedures 17
Display replacement 17
Main PCBA replacement 29
Power Supply PCBA replacement 24
SD Card Daughter-card replacement 25
Speaker replacement 23
TGC PCBA replacement 28
return material authorization number (RMA) 50
S
service event report 47
shipping instructions 50
speaker replacement 23
subassembly replacement 13
system
dimensions 8
measurement accuracy 35
overview 3
specifications 8, 11
T
TGC PCBA replacement procedure 28
theory of operation 4
transducer
specifications 9, 11
storage and shipping 11
V
video output tests 39
Index 51
Page 56

52 Index
Page 57

Page 58

P08144-01
*P08144-01*
 Loading...
Loading...