Page 1

L52 Series Transducer
User Guide
Page 2

Page 3

L52 Series Transducer User Guide
Benutzerhandbuch zum Schallkopf der L52-Series
Manual para el usuario del transductor
L52 Series
Guide d’utilisation de la sonde série L52
English Deutsch Español Français Italiano PortuguêsFrançais
Manuale dell’utente del trasduttore serie L52
Manual do Usuário do Transdutor Série L52
Page 4

Page 5

Brugervejledning til transducere af typen L52
Brukerhåndbok for transduser i L52-serien
Användarhandbok för transduktor L52-serien
Οδηγός χρήσης του μορφοτροπέα σειράς L52
Dansk Norsk Svenska Ελληνικά Русский Türkçe
Руководство пользователя датчика серии L52
L52 Series Dönüştürücü Kullanıcı Kılavuzu
L52 Series
换能器用户指南
L52 Series 轉換器使用者手冊
简体中文 繁體中文
Page 6

Manufactured by
SonoSite, Inc.
21919 30th Drive SE
Bothell, WA 98021
USA
T: 1-888-482-9449 or 1-425-951-1200
F: 1-425-951-1201
SonoSite Ltd
Alexander House
40A Wilbury Way
Hitchin
Herts SG4 OAP
UK
T: +44-1462-444800
F: +44-1462-444801
Caution:
180PLUS, M-Turbo, MicroMaxx, NanoMaxx, S Series, SonoSite, SonoSite TITAN, TITAN, and the SonoSite logo are registered (in some
jurisdictions) or unregistered trademarks owned by SonoSite, Inc.
Non-SonoSite product names may be trademarks or registered trademarks of their respective owners.
The SonoSite ultrasound system(s) referenced in this document may be covered by one or more of the following U.S. patents: 5722412,
5817024, 5893363, 6135961, 6203498, 6364839, 6371918, 6383139, 6416475, 6447451, 6471651, 6569101, 6648826, 6575908, 6604630,
6817982, 6835177, 6962566, 7169108, 7449640, 7534211, 7549961, 7588541, 7591786, 7604596, 7643040, 7686766, 7694814, 7727153,
7740586, 7804970, 7809400, 7 819807, 7841575, 7849250, 7867168, 7883276, D456509, D461895, D509900, D538432, D544962, D558351,
D559390, D591423, D592750, D592760, D625014, D625015, and by the following counterpart foreign patents: AU727381, AU730822,
CA2372152, CA2372158, CA2373065, CN ZL 97113678.5, CN ZL 98106133.8, CN ZL 98108973.9, CN ZL 200830007734.8, DE60021552.0,
DE60029777.2, DE60034670.6, DE69730563.5, DE6980539.6, DE69831698.3, DE60 2004 23 816.3-08, FR0815793, FR0875203, FR0881492,
FR1175713, FR1180970, FR1589878, GB0875203, GB0881492, GB1175713, GB1180970, GB1180971, GB1589878, IT0815793, IT0881492,
IT1175713, IT1589878, KR528102, KR532359, NO326202, NO326814, NZ542968, RCD000897368-0001, SP0815793, SP0881492,
SP1589878. Patents pending.
United States federal law restricts this device to sale by or on the order of a veterinarian.
P07895-06 03/2011
Copyright 2011 by SonoSite, Inc.
All rights reserved. Printed in the USA.
ii
Page 7

L52 Series Transducer User Guide
Introduction .................................................................................................................................................1
Imaging .........................................................................................................................................................1
Measurements and Calculations ..........................................................................................................4
Safety ..............................................................................................................................................................5
Troubleshooting and Maintenance ..................................................................................................21
Introduction
This user guide supplements the following ultrasound system user guides:
• NanoMaxx Ultrasound System User Guide
• M-Turbo Ultrasound System User Guide
• S Series Ultrasound System User Guide
• MicroMaxx Ultrasound System User Guide
• TITAN Ultrasound System User Guide
• SonoSite Ultrasound System User Guide
It describes the following transducers, which are for veterinary use only:
• L52n/10-5 MHz (L52n) transducer on the NanoMaxx® ultrasound system
• L52x/10-5 MHz (L52x) transducer on the M-Turbo® ultrasound system, S Series™ ultrasound
system, or MicroMaxx® ultrasound system
• L52e/10-5 MHz (L52e) transducer on the MicroMaxx® ultrasound system
• L52/10-5 MHz (L52) transducer on the TITAN® high-resolution ultrasound system or
SonoSite®180PLUS™ ultrasound system
See the ultrasound system user guide for additional safety information; for instructions on
preparation, use, and maintenance of the ultrasound system; and for intended uses for each exam
type and imaging mode.
English Deutsch Español Français Italiano Português
Imaging
Transducer, Exam Type, and Imaging Mode
The following table describes the transducer, exam type, imaging mode, and optimization that may
be available on your system.
1
Page 8

Transducer, Exam Type, and Imaging Mode (NanoMaxx)
Imaging Mode
Transducer Exam Type
L52n OB X X X
Vascular (Vas) X X X
Musculoskeletal (Msk) X X X
2D
MMode
CPD Color
Transducer, Exam Type, and Imaging Mode (M-Turbo or MicroMaxx)
Imaging Mode
Transducer Exam Type
L52x OB X X X X —
Vascular (Vas) X X X X —
Muscle (Msk) X X X X —
2D
MMode
CPD Color PW CW
Transducer, Exam Type, and Imaging Mode (S Series)
Imaging Mode
Transducer Exam Type
L52x OB X X X X
Vascular (Vas) X X X X
Muscle (Msk) X X X X
2
2D
M Mode
CPD Color PW
Page 9

Transducer, Exam Type, and Imaging Mode (MicroMaxx)
Imaging Mode
English Deutsch Español Français Italiano Português
Transducer
Exam
Typ e
2D
MMode
THI
2DMB2D
S
CPD Color PW
TDI
PW
L52e OB X — X X X X X — —
Vascular
X—XXXXX——
(Vas)
Muscle
X—XXXXX——
(Msk)
Transducer, Exam Type, and Imaging Mode (TITAN)
Imaging Mode
Transducer
Exam
Typ e
2D THI CPD DCPD Color M Mode PW CW
L52 OB X — X — — X — —
Vascular
X—X — — X ——
(Vas)
Muscle
X—X — — X ——
(Msk)
CW
Transducer, Exam Type, and Imaging Mode (180PLUS)
Imaging Mode
Transducer Exam Type 2D CPD
L52 OB res, gen, pen low, med, high
Vascular (Vas) res, gen, pen low, med, high
3
Page 10

Measurements and Calculations
Calculations
This table shows the calculations available by exam type for the L52 Series transducer.
Calculations for L52n (NanoMaxx)
Exam Type Calculations
OB OB
Calculations for L52x (M-Turbo and MicroMaxx)
Exam Type Calculations
Musculoskeletal (Msk) Percent Reduction
Volume
OB OB
Vascular (Vas) Percent Reduction
Vascular
Volume
Volume Flow
Calculations for L52x (S Series)
Exam Type S Series System Calculations
OB S-VetMed OB
Calculations for L52e/L52 (MicroMaxx and TITAN)
Exam Type Calculations
Muscle (Msk) Percent Reduction
Volume
OB OB
Vascular (Vas) Percent Reduction
Vascular
Volume
Volume Flow
4
Page 11

Calculations for L52 (180PLUS)
Exam Type Calculations
OB OB
Vascular (Vas) Volume
Safety
Guidelines for reducing MI and TI
The following are general guidelines for reducing MI or TI. If multiple parameters are given, the best
results may be achieved by minimizing these parameters simultaneously. In some modes, changing
these parameters does not affect MI or TI. Changes to other parameters may also result in MI and TI
reductions. Please note the MI and TI values on the right side of the screen.
Table 1: MI
Transducer Depth
English Deutsch Español Français Italiano Português
Volume Flow
L52n (NanoMaxx) ↑
L52x (M-Turbo, S Series, or MicroMaxx) ↑
L52e (MicroMaxx) ↑
L52 (TITAN) ↑
L52 (180PLUS) ↑
↓ Decrease or lower setting of parameter to reduce MI.
↑ Raise or increase setting of parameter to reduce MI.
5
Page 12

Table 2: TI (TIS, TIC, TIB)
Color Power Doppler
Transducer
PRF Depth
L52n (NanoMaxx) — ↑ —
L52x (M-Turbo or MicroMaxx) ↓↑Sample Vol ↓
L52x (S Series) ↓↑ —
L52e (MicroMaxx) ↓↑Sample Vol ↑
L52 (TITAN) ↓↑ —
L52 (180PLUS) — — —
↓ Decrease or lower setting of parameter to reduce TI.
↑ Raise or increase setting of parameter to reduce TI.
— Data are not applicable for this transducer/mode.
Transducer surface temperature rise
Tab le 3 lists the measured surface temperature rise (in °C) from ambient (23°C ± 3°C) of transducers
used on the ultrasound system. The temperatures were measured in accordance with EN 60601-2-37
section 42 where controls and settings were positioned to give maximum temperatures.s
Table 3: Transducer Surface Temperature Rise IEC 60601-2-37 (Internal Use)
Settings
PW Settings
Te st
TITAN
L52n on
NanoMaxx
Still air
Simulated use
6
7.5 8.8 8.2 13.0 9.3 10.8
5.6 5.9 5.6 5.5 2.4 2.4
L52x on
M-Turbo
or S Series
L52x on
MicroMaxx
L52e on
MicroMaxx
L52 on
L52 on
180PLUS
Page 13

Output display
Table 4: TI or MI is ≥ 1.0
English Deutsch Español Français Italiano Português
Transducer Model Index
2D/
MMode
Color CPD
PW
Doppler
L52n (NanoMaxx) MI Yes Yes Yes n/a
TI No No No n/a
L52x (M-Turbo) MI Yes Yes Yes Yes
TI No No No Yes
L52x (S Series) MI Yes Yes Yes —
TI No No No —
L52x (MicroMaxx) MI Yes Yes Yes Yes
TI Yes No No Yes
L52e (MicroMaxx) MI No Yes Yes Yes
TI No No No Yes
L52 (TITAN, 180PLUS)*MINoNoNoNo
TI No No No No
* The L52 transducer never exceeds or equals a MI or TI of 1.0 on the TITAN or 180PLUS system.
7
Page 14
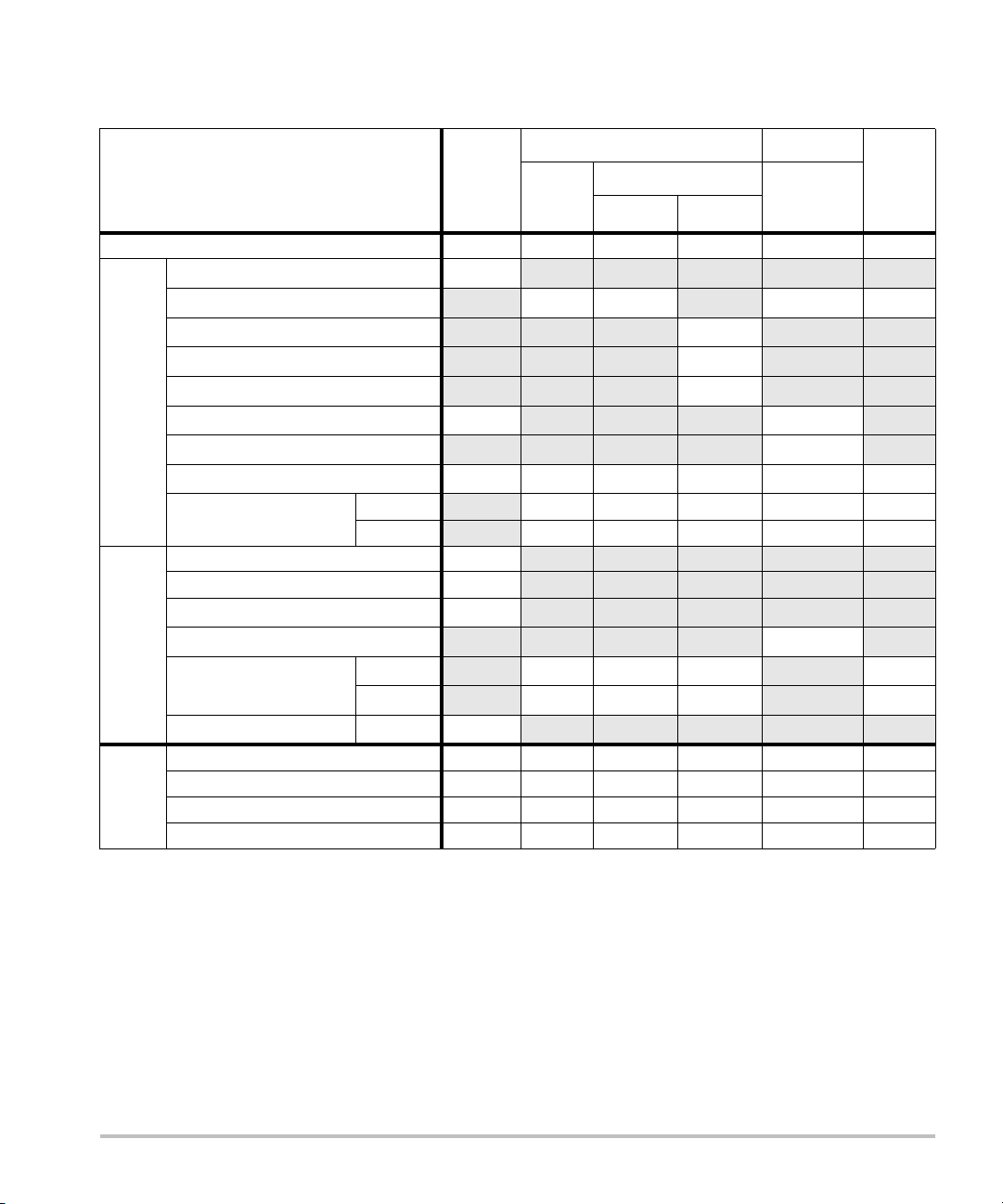
Acoustic output tables (M-Turbo and S Series)
Table 5: Transducer Model: L52x/10-5 Operating Mode: 2D
TIS TIB
A
aprt
Non-scan
≤1A
aprt
Non-scan
TIC
>1
Index Label M.I.
Scan
Global Maximum Index Value 1.01 (a) — — — (a)
p
r.3
W
0
min of [W
z
1
z
bp
z
sp
Parameter
deq(zsp)(cm)
Associated Acoustic
f
c
Dim of A
.3(z1
aprt
),I
TA. 3(z1
)] (mW)
(MPa)
(mW)
2.336
#— —#
—
(cm)
(cm)
(cm)
1.8
—
—
—
—
(MHz)
5.33 # — — — #
X(cm) #—— — #
Y(cm)
#—— — #
PD (µsec) 0.15
PRF (Hz) 7222
pr@PII
max
deq@Pll
Other
Focal Length FLx (cm)
max
Information
I
@MI
PA. 3
max
(MPa)
(cm)
FLy (cm)
(W/cm2)
3.25
—
#—— #
#—— #
329.1
Control 1: Exam Type Vas — — — — —
Control 2: Optimization Gen — — — — —
Control 3: Depth 4.2 cm — — — — —
Control
Operating
Control 4: MB (Multi Beam) Off — — — — —
Conditions
(a) This index is not required for this operating mode; value is <1.
(b) This transducer is not intended for transcranial or neonatal cephalic uses.
# No data are reported for this operating condition since the global maximum index value is not reported for the reason
listed. (Reference Global Maximum Index Value line.)
— Data are not applicable for this transducer/mode.
8
Page 15
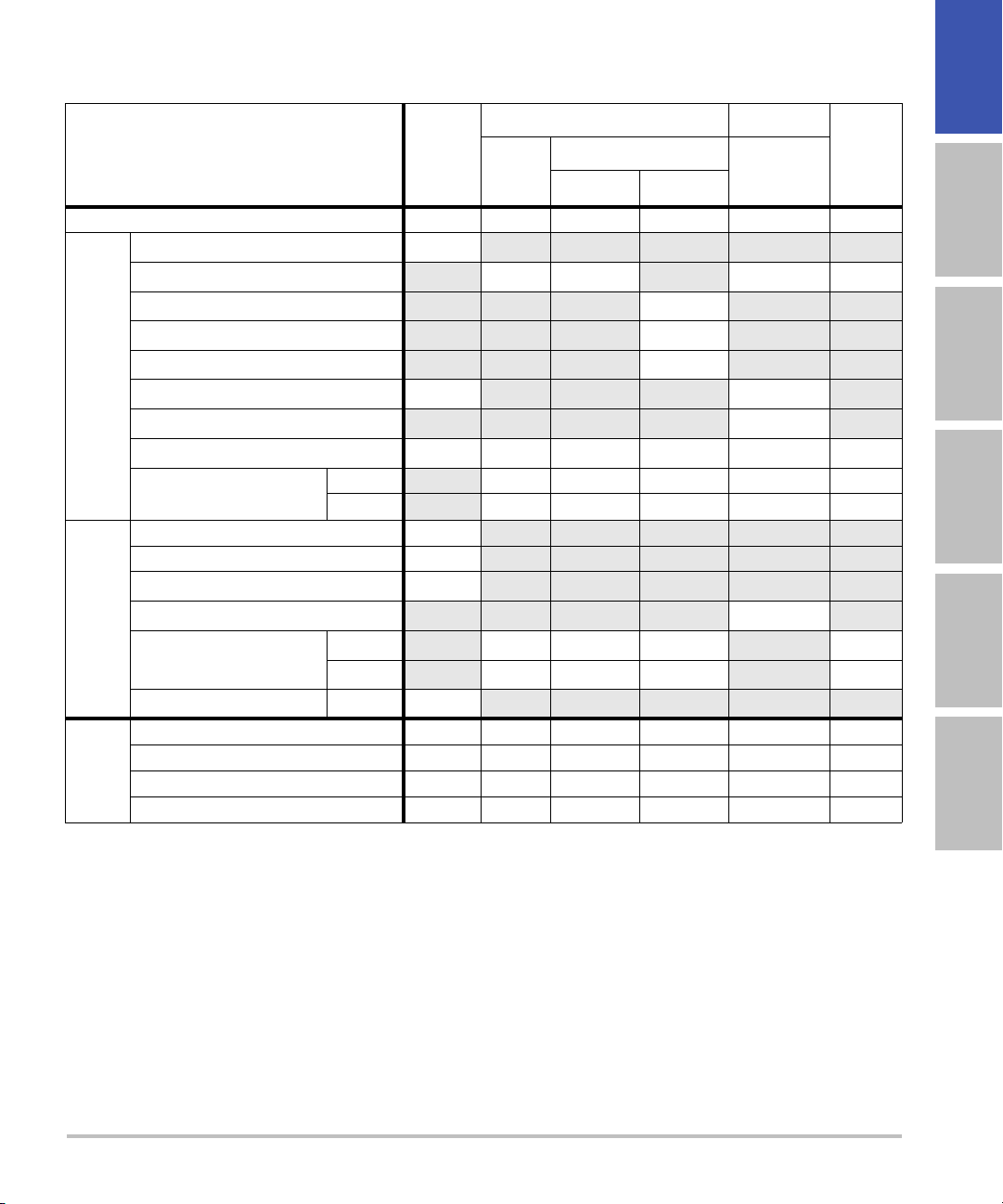
Table 6: Transducer Model: L52x/10-5 (M-Turbo only) Operating Mode: M Mode
TIS TIB
English Deutsch Español Français Italiano Português
A
aprt
Non-scan
≤1A
aprt
Non-scan
TIC
>1
Index Label M.I.
Scan
Global Maximum Index Value 1.01 — (a) — (a) (b)
p
r.3
W
0
min of [W.3(z1),I
z
1
z
bp
z
sp
Parameter
deq(zsp)(cm)
Associated Acoustic
f
c
Dim of A
TA. 3(z1
aprt
(MPa)
(mW)
)] (mW)
(cm)
(cm)
(cm)
2.336
1.8
—# ##
—
—
—
#
#
(MHz)
5.33 — # — # #
X(cm) —# — # #
Y(cm)
—# — # #
PD (µsec) 0.15
PRF (Hz) 1600
pr@PII
max
deq@Pll
Other
Focal Length FLx (cm)
max
Information
@MI
I
PA .3
max
(MPa)
(cm)
(cm)
FL
y
(W/cm2)
3.25
#
—# — #
—# — #
329.1
Control 1: Exam Type Vas — — — — —
Control 2: Optimization Gen — — — — —
Control 3: Depth 4.2 cm — — — — —
Control
Operating
Control 4: MB (Multi Beam) Off — — — — —
Conditions
(a) This index is not required for this operating mode; value is <1.
(b) This transducer is not intended for transcranial or neonatal cephalic uses.
# No data are reported for this operating condition since the global maximum index value is not reported for the reason
listed. (Reference Global Maximum Index Value line.)
— Data are not applicable for this transducer/mode.
9
Page 16
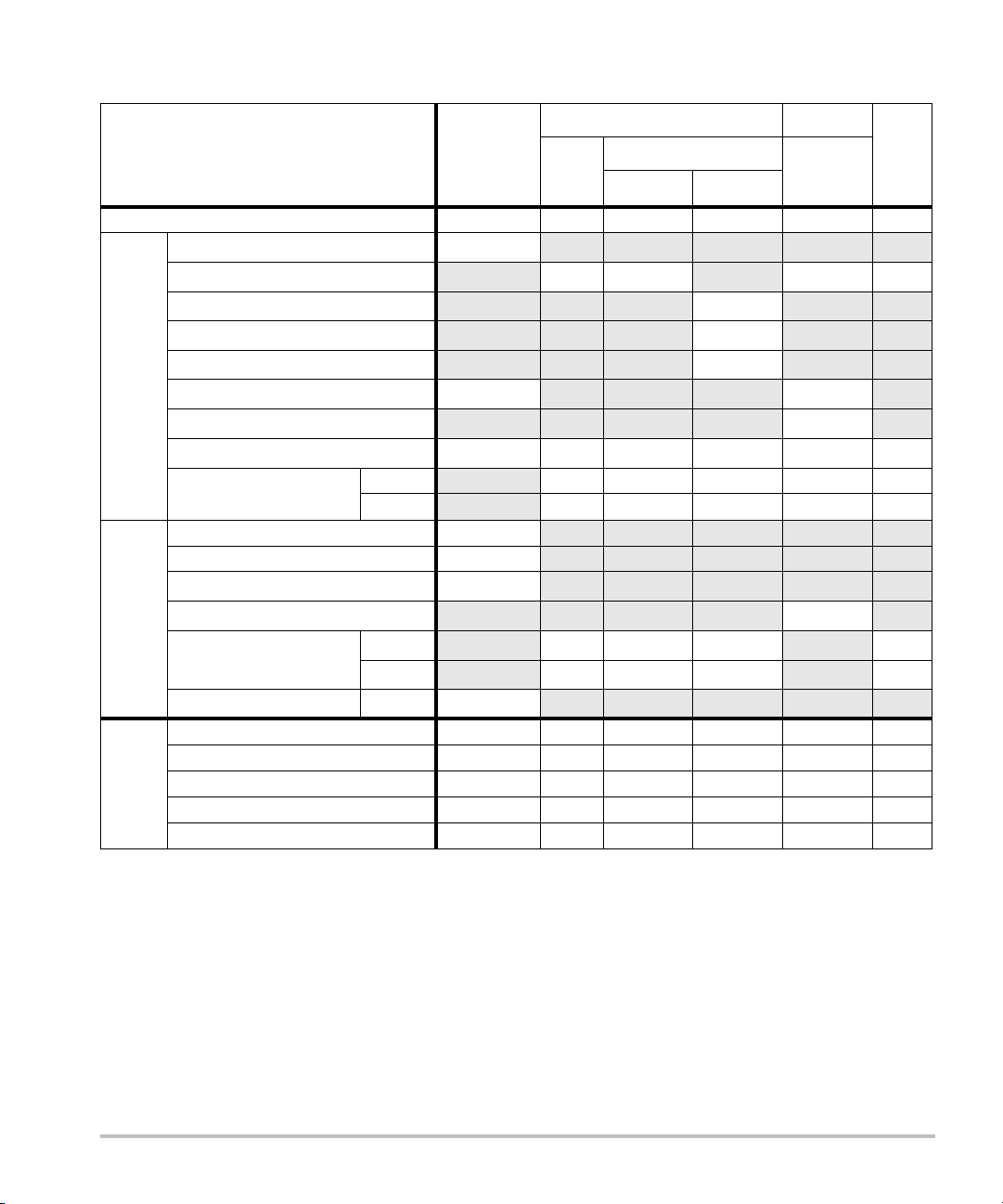
Table 7: Transducer Model: L52x/10-5 Operating Mode: Color/CPD
TIS TIB
A
aprt
Non-scan
≤1A
aprt
Non-scan
TIC
>1
Index Label M.I.
Scan
Global Maximum Index Value 1.33 (a) — — — (b)
p
r.3
W
0
min of [W.
z
1
Z
bp
Z
sp
Parameter
deq(Zsp)(cm) —
Associated Acoustic
F
c
Dim of A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2.807
(mW) #— —#
] (mW) —
(cm) —
(cm) —
(cm) 1.8 —
(MHz) 4.37 # — — — #
X(cm) #— — —#
Y(cm)
#— — —#
PD (µsec) 0.61
PRF (Hz) 5427
pr@PII
max
deq@Pll
Other
Focal Length FLx (cm) #— — #
max
Information
@MI
I
PA. 3
max
(MPa) 3.628
(cm) —
FL
(cm) #— — #
y
(W/cm2)
411.1
Control 1: Mode Any — — — — —
Control 2: Exam type Vas — — — — —
Control 3: Optimization/Depth Any/5.4 — — — — —
Control
Control 4: PRF Any — — — — —
Operating
Conditions
Control 5: Color Box Position/Size Any/Def — — — — —
(a) This index is not required for this operating mode; value is <1.
(b) This transducer is not intended for transcranial or neonatal cephalic uses.
# No data are reported for this operating condition since the global maximum index value is not reported for the reason
listed. (Reference Global Maximum Index Value line.)
— Data are not applicable for this transducer/mode.
10
Page 17
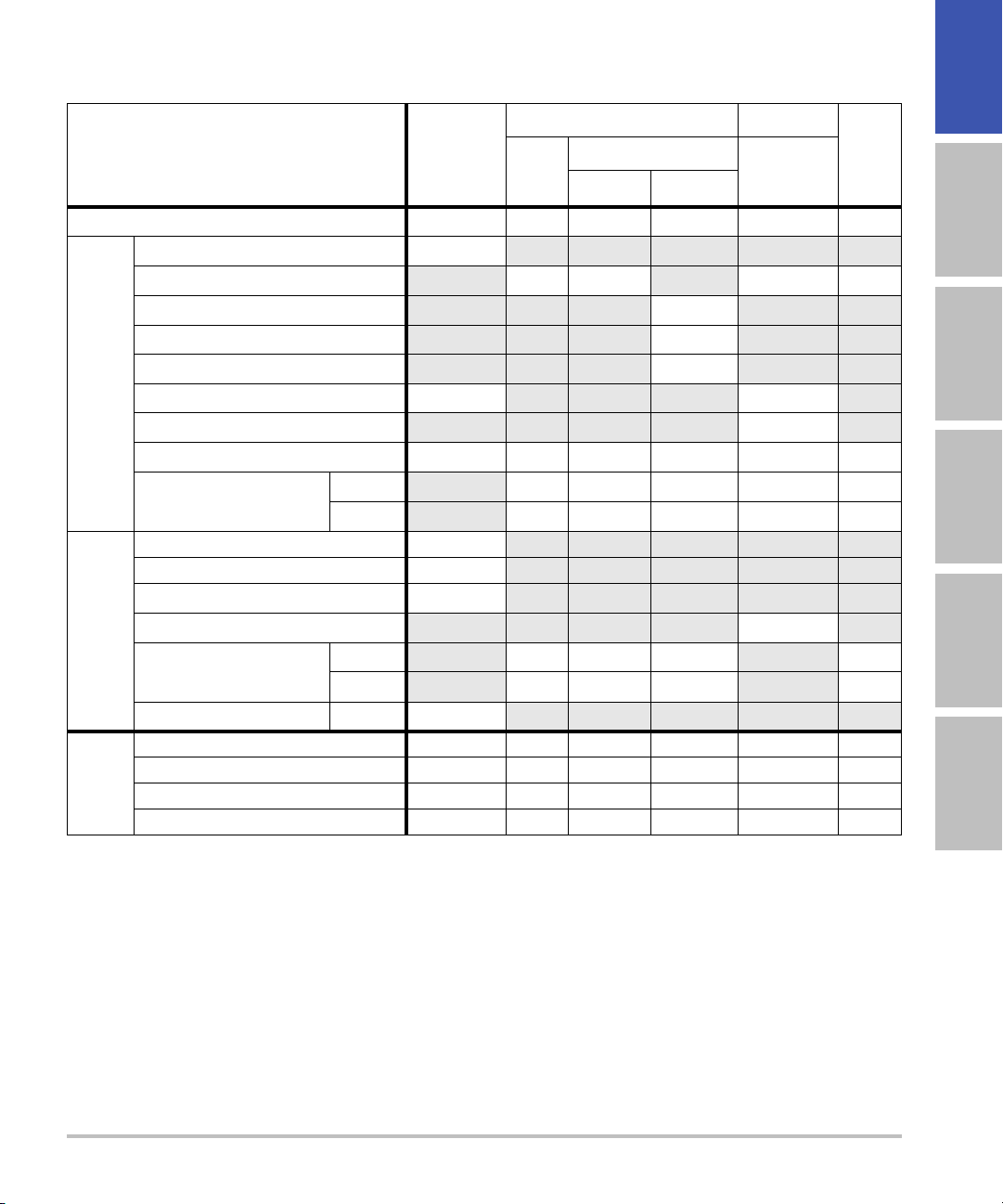
Table 8: Transducer Model: L52x/10-5 (M-Turbo only) Operating Mode: PW Doppler
TIS TIB
English Deutsch Español Français Italiano Português
Index Label M.I.
Global Maximum Index Value 1.17
p
r.3
W
0
min of [W.
z
1
Z
bp
Z
sp
Parameter
deq(Zsp)(cm) 0.45
F
Associated Acoustic
c
Dim of A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2.443
(mW)
] (mW) —
(cm) —
(cm) —
(cm) 2.1 1.5
(MHz) 4.36
X(cm)
Y(cm)
PD (µsec) 1.38
PRF (Hz) 1008
pr@PII
max
deq@Pll
Other
Focal Length FLx (cm)
max
Information
I
@MI
PA. 3
max
(MPa) 3.30
(cm) 0.34
FL
(cm)
y
(W/cm2)
288.97
Control 1: Exam Type Any — Any — Any —
Control 2: PRF 1008 Hz — 3125 Hz — 3125 Hz —
Control 3: SV Size 1 mm — 1 mm — 1 mm —
Control
Operating
Control 4: SV Position Zone 4 — Zone 6 — Zone 6 —
Conditions
Scan
—
—
—
—
—
—
—
Non-scan
Non-scan
A
aprt
≤1A
aprt
>1
1.44 — 2.22 (b)
69.42 69.42 #
4.35 — 4.35 #
1.476 — 1.476 #
0.55 — 0.55 #
5.99 — #
3.4 — #
TIC
(a) This index is not required for this operating mode; value is <1.
(b) This transducer is not intended for transcranial or neonatal cephalic uses.
# No data are reported for this operating condition since the global maximum index value is not reported for the reason
listed. (Reference Global Maximum Index Value line.)
— Data are not applicable for this transducer/mode.
11
Page 18
Acoustic output tables (NanoMaxx)
Table 9: Transducer Model: L52n/10-5 Operating Mode: 2D
TIS TIB
A
aprt
Non-scan
≤1A
aprt
Non-scan
TIC
>1
Index Label M.I.
Scan
Global Maximum Index Value 1.0 (a) — — — (b)
p
r.3
W
0
min of [W.3(z1),I
z
1
z
bp
z
sp
Parameter
deq(zsp)(cm)
Associated Acoustic
f
c
Dim of A
aprt
)] (mW)
TA. 3(z1
(MPa)
(mW)
2.34
#— —#
—
(cm)
(cm)
(cm)
1.8
—
—
—
—
(MHz)
5.33 # — — — #
X(cm) #—— — #
Y(cm)
#—— — #
PD (µsec) 0.15
PRF (Hz) 7707
pr@PII
max
deq@Pll
max
Focal Length FLx (cm)
Other Information
@MI
I
PA. 3
max
(MPa)
(cm)
(cm)
FL
y
(W/cm2)
3.25
—
#—— #
#—— #
329.1
Control 1: Exam Type Any
Control 2: Optimization Gen
Control 3: Depth 4.2 cm
Control
Control 4: MB (Multi Beam) Off or
Operating
Conditions
On
(a) This index is not required for this operating mode; value is <1.
(b) This transducer is not intended for transcranial or neonatal cephalic uses.
# No data are reported for this operating condition since the global maximum index value is not reported for the reason
listed. (Reference Global Maximum Index Value line.)
— Data are not applicable for this transducer/mode.
12
Page 19
Table 10: Transducer Model: L52n/10-5 Operating Mode: M Mode
TIS TIB
English Deutsch Español Français Italiano Português
A
aprt
Non-scan
≤1A
aprt
Non-scan
TIC
>1
Index Label M.I.
Scan
Global Maximum Index Value 1.0 — (a) — (a) (b)
p
r.3
W
0
min of [W.3(z1),I
z
1
z
bp
z
sp
Parameter
deq(zsp)(cm)
Associated Acoustic
f
c
Dim of A
aprt
)] (mW)
TA. 3(z1
(MPa)
(mW)
2.34
—# ##
—
(cm)
(cm)
(cm)
1.8
—
—
#
#
(MHz)
5.33 — # — # #
X(cm) —# — # #
Y(cm)
—# — # #
PD (µsec) 0.15
PRF (Hz) 1600
pr@PII
max
deq@Pll
max
Focal Length FLx (cm)
Other Information
I
@MI
PA. 3
max
(MPa)
(cm)
(cm)
FL
y
(W/cm2)
3.25
#
—# — #
—# — #
329.1
Control 1: Exam Type Any — — — — —
Control 2: Optimization Gen — — — — —
Control 3: Depth 4.2 cm — — — — —
Control
Control 4: MB (Multi Beam) Off or On—— — — —
Operating
Conditions
(a) This index is not required for this operating mode; value is <1.
(b) This transducer is not intended for transcranial or neonatal cephalic uses.
# No data are reported for this operating condition since the global maximum index value is not reported for the reason
listed. (Reference Global Maximum Index Value line.)
— Data are not applicable for this transducer/mode.
13
Page 20
Table 11: Transducer Model: L52n/10-5 Operating Mode: Color/CPD
TIs TIb
A
aprt
Non-scan
≤1A
aprt
Non-scan
TIc
>1
Index Label M.I.
Scan
Global Maximum Index Value 1.2 (a) — — — (b)
p
r.3
W
0
min of [W.
z
1
Z
bp
Z
sp
Parameter
deq(Zsp)(cm) —
F
Associated Acoustic
c
Dim of A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2.35
(mW) #— —#
] (mW) —
(cm) —
(cm) —
(cm) 1.8 —
(MHz) 4.37 # — — — #
X (cm) #— — —#
Y (cm)
#— — —#
PD (µsec) 0.60
PRF (Hz) 7097
pr@PII
max
deq@Pll
Other
Focal Length FLx (cm) #— — #
max
Information
@MI
I
PA. 3
max
Control 1: Mode CPD or
(MPa) 3.08
(cm) —
(cm) #— — #
FL
y
(W/cm2)
308.5
—— — — —
Color
Control 2: Exam type Msk — — — — —
Control 3: Optimization Res — — — — —
Control
Operating
Control 4: depth 5.4 — — — — —
Conditions
Control 5: Color Box Default — — — — —
(a) This index is not required for this operating mode; value is <1.
(b) This transducer is not intended for transcranial or neonatal cephalic uses.
# No data are reported for this operating condition since the global maximum index value is not reported for the reason
listed. (Reference Global Maximum Index Value line.)
— Data are not applicable for this transducer/mode.
14
Page 21
Acoustic output tables (MicroMaxx)
Table 12: Transducer Model: L52x/10-5 Operating Mode: 2D
TIS TIB
English Deutsch Español Français Italiano Português
Index Label M.I.
Scan
Global Maximum Index Value 1.0 (a)
p
r.3
W
0
min of [W.
z
1
Z
bp
Z
sp
Parameter
deq(Zsp)(cm)
F
Associated Acoustic
c
Dim of A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2.23
(mW)
] (mW)
(cm)
(cm)
(cm) 1.9
(MHz) 5.42 #
X(cm) #
Y(cm)
#— —
#
PD (µsec) 0.146
PRF (Hz) 8394
pr@PII
max
deq@Pll
Other
Focal Length FLx (cm) #
max
Information
I
@MI
PA. 3
max
(MPa) 3.19
(cm)
FL
(cm) #
y
(W/cm2)
325.3
Control 1: Exam Type OB — — — — —
Control 2: Optimization Gen — — — — —
Control 3: Depth 2.5 - 3.9 — — — — —
Control
Operating
Control 4: MB On or Off — — — — —
Conditions
Non-scan
Non-scan
A
aprt
≤1A
aprt
>1
—— —
—
—
—
—
—
—— —
—— —
—— —
—
——
——
TIC
(b)
#
#
#
#
#
#
(a) This index is not required for this operating mode; value is <1.
(b) This transducer is not intended for transcranial or neonatal cephalic uses.
# No data are reported for this operating condition since the global maximum index value is not reported for the reason
listed. (Reference Global Maximum Index Value line.)
— Data are not applicable for this transducer/mode.
15
Page 22
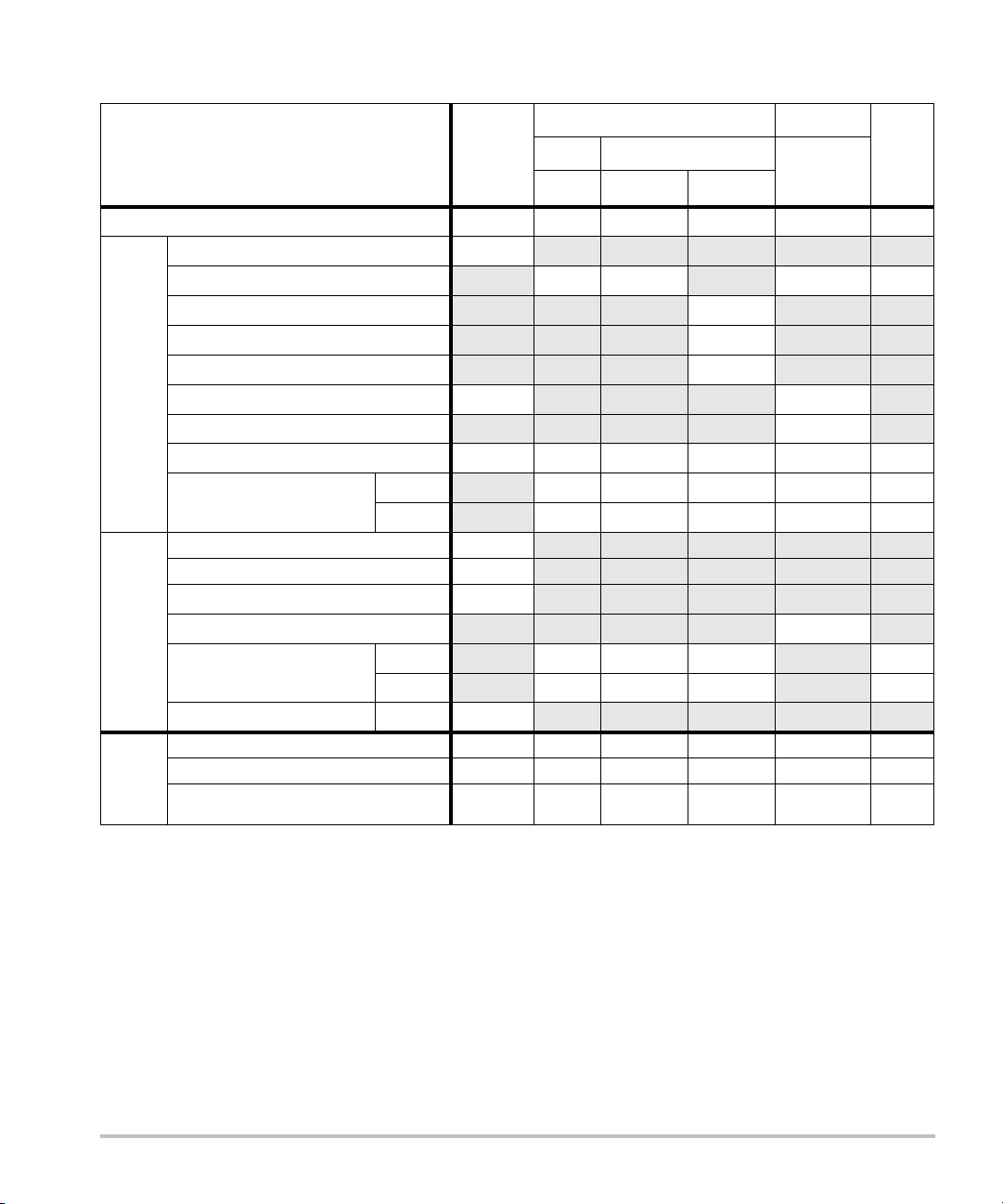
Table 13: Transducer Model: L52x/10-5 Operating Mode: M-Mode
Index Label
M.I.
Global Maximum Index Value 1.0
p
r.3
W
0
min of [W.
z
1
Z
bp
Z
sp
Parameter
deq(Zsp)(cm) 0.659
F
Associated Acoustic
c
Dim of A
3(z1),ITA. 3 (z1)
aprt
] (mW)
(MPa) 2.23
(mW)
(cm)
(cm)
(cm) 1.9 1.7
(MHz) 5.42
X(cm)
Y(cm)
PD (µsec) 0.146
PRF (Hz) 1600
pr@PII
max
deq@Pll
Other
Focal Length FLx (cm)
max
Information
I
@MI
PA. 3
max
(MPa) 3.19
(cm) 0.641
FL
(cm)
y
(W/cm2)
325.3
Control 1: Exam Type OB — — — Any —
Control 2: Optimization Gen — — — Pen —
Control 3: Depth 2.5 - 3.9 — — — 15 —
Control
Operating
Conditions
TIS TIB
Scan Non-scan Non-scan
A
—
—
≤1A
aprt
(a)
# 58.3 #
aprt
—
>1
1.2 (b)
—
—
—
—
—
—
—
—
#
#
#
#
#
—
—
—
—
—
4.35 #
2.71 #
0.55 #
TIC
#
#
(a) This index is not required for this operating mode; value is <1.
(b) This transducer is not intended for transcranial or neonatal cephalic uses.
# No data are reported for this operating condition since the global maximum index value is not reported for the reason
listed. (Reference Global Maximum Index Value line.)
— Data are not applicable for this transducer/mode.
16
Page 23

Table 14: Transducer Model: L52x/10-5 Operating Mode: Color/CPD
TIS TIB
English Deutsch Español Français Italiano Português
Index Label M.I.
Scan
Global Maximum Index Value 1.3 (a)
p
r.3
W
0
min of [W.
z
1
Z
bp
Z
sp
Parameter
deq(Zsp)(cm)
F
Associated Acoustic
c
Dim of A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2.70
(mW) #
] (mW)
(cm)
(cm)
(cm) 1.4
(MHz) 4.35 #
X(cm) #
Y(cm)
PD (µsec) 0.607
PRF (Hz) 4169
pr@PII
max
deq@Pll
Other
Focal Length FLx (cm) #
max
Information
I
@MI
PA. 3
max
(MPa) 3.33
(cm)
FL
(cm) #
y
(W/cm2)
377.1
Control 1: Mode Color — — — — —
Control 2: Exam Type Any — — — — —
Control 3: Optimization/Depth Low/
Control
Operating
Control 4: PRF <
Conditions
2.5 - 3.9
718 — — — — —
—— — — —
Control 5: Color Box Position/Size Any — — — — —
Non-scan
TIC
Non-scan
≤1A
A
aprt
—— —
— —
aprt
>1
(b)
#
—
—
—
—
—
—— —
—— —
#
—— —
#
#
#
—
——
——
#
#
(a) This index is not required for this operating mode; value is <1.
(b) This transducer is not intended for transcranial or neonatal cephalic uses.
# No data are reported for this operating condition since the global maximum index value is not reported for the reason
listed. (Reference Global Maximum Index Value line.)
— Data are not applicable for this transducer/mode.
17
Page 24
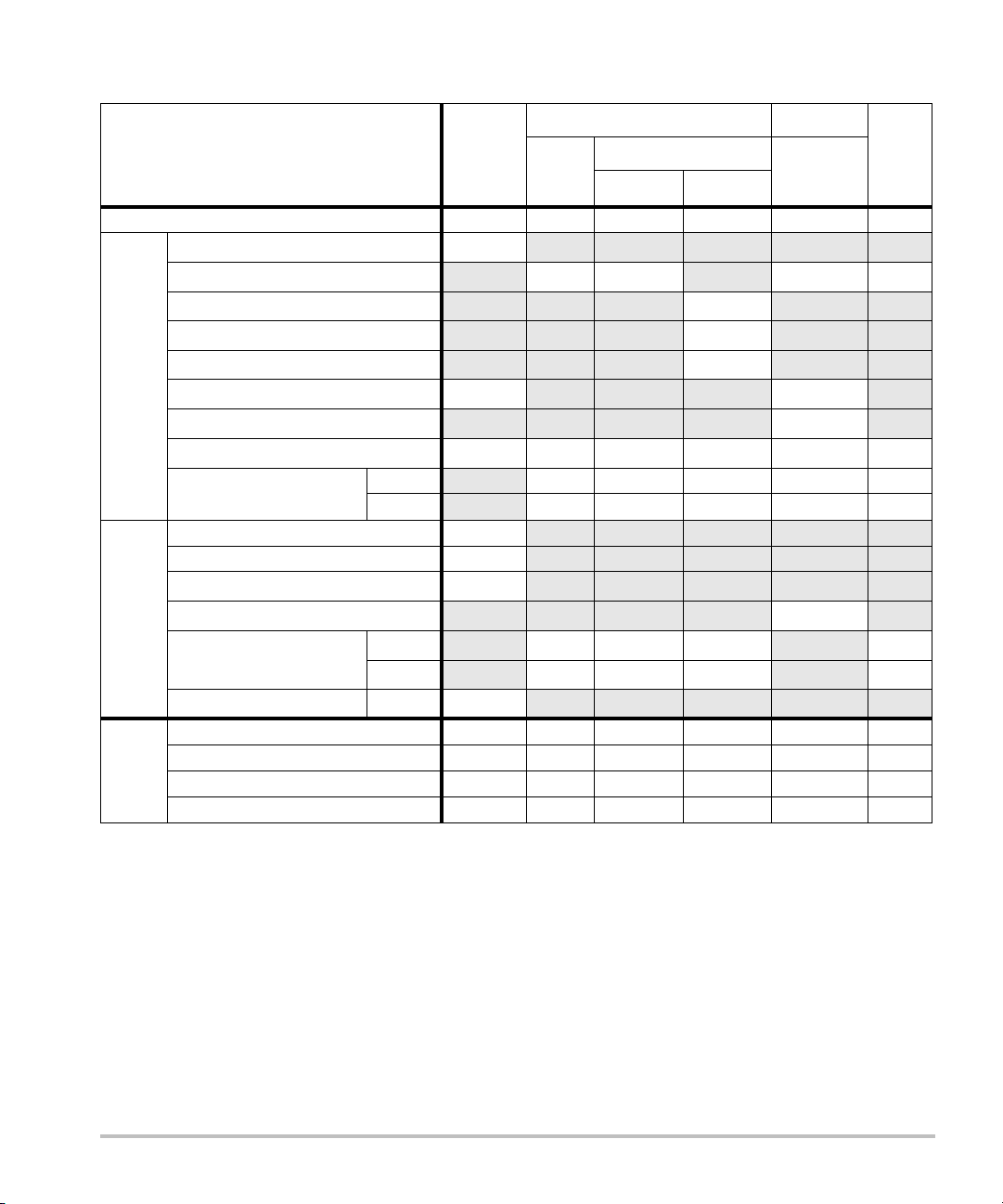
Table 15: Transducer Model: L52x/10-5 Operating Mode: PW Doppler
TIS TIB
A
aprt
Non-scan
≤1A
aprt
Non-scan
TIC
>1
Index Label M.I.
Scan
Global Maximum Index Value 1.2 — 1.4 — 2.2 (b)
p
r.3
W
0
min of [W.
z
1
Z
bp
Z
sp
Parameter
deq(Zsp)(cm) 0.19
Associated Acoustic
F
c
Dim of A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2.48
(mW) —68.5 37.5 #
] (mW) —
(cm) —
(cm) —
(cm) 2.3 2.4
(MHz) 4.36 — 4.35 — 4.36 #
X(cm) —2.05 — 0.90 #
Y(cm)
—0.55 — 0.55 #
PD (µsec) 1.39
PRF (Hz) 1008
pr@PII
max
deq@Pll
Other
Focal Length FLx (cm) —8.32 — #
max
Information
@MI
I
PA. 3
max
(MPa) 3.505
(cm) 0.18
FL
(cm) —3.5 — #
y
(W/cm2)
284.3
Control 1: Exam Type Any — Any — Any —
Control 2: Sample Volume 1 mm — 2 mm — 12 mm —
Control 3: PRF 1008 Hz — Any — 10417 Hz —
Control
Operating
Control 4: Sample Volume Position Zone 3 — Zone 7 — Zone 3 —
Conditions
(a) This index is not required for this operating mode; value is <1.
(b) This transducer is not intended for transcranial or neonatal cephalic uses.
# No data are reported for this operating condition since the global maximum index value is not reported for the reason
listed. (Reference Global Maximum Index Value line.)
— Data are not applicable for this transducer/mode.
18
Page 25

Table 16: Transducer Model: L52e/10-5 Operating Mode: Color/CPD
TIS TIB
English Deutsch Español Français Italiano Português
A
aprt
Non-scan
≤1A
aprt
Non-scan
TIC
>1
Index Label M.I.
Scan
Global Maximum Index Value 1.2 (a) — — — (b)
p
r.3
W
0
min of [W.
z
1
Z
bp
Z
sp
Parameter
deq(Zsp)(cm) —
Associated Acoustic
F
c
Dim of A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2.30
(mW) #— —#
] (mW) —
(cm) —
(cm) —
(cm) 1.6 —
(MHz) 3.92 # — — — #
X(cm) #— — —#
Y(cm)
#— — —#
PD (µsec) 0.797
PRF (Hz) 5332
pr@PII
max
deq@Pll
Other
Focal Length FLx (cm) #— — #
max
Information
@MI
I
PA. 3
max
(MPa) 2.85
(cm) —
FL
(cm) #— — #
y
(W/cm2)
257.0
Control 1: Exam Type Any — — — — —
Control 2: Color Opt Any — — — — —
Control 3: Depth 4.9 — — — — —
Control
Control 4: PRF Any — — — — —
Operating
Conditions
Control 5: Color Box Position/Size Any — — — — —
(a) This index is not required for this operating mode; value is <1.
(b) This transducer is not intended for transcranial or neonatal cephalic uses.
# No data are reported for this operating condition since the global maximum index value is not reported for the reason
listed. (Reference Global Maximum Index Value line.)
— Data are not applicable for this transducer/mode.
19
Page 26
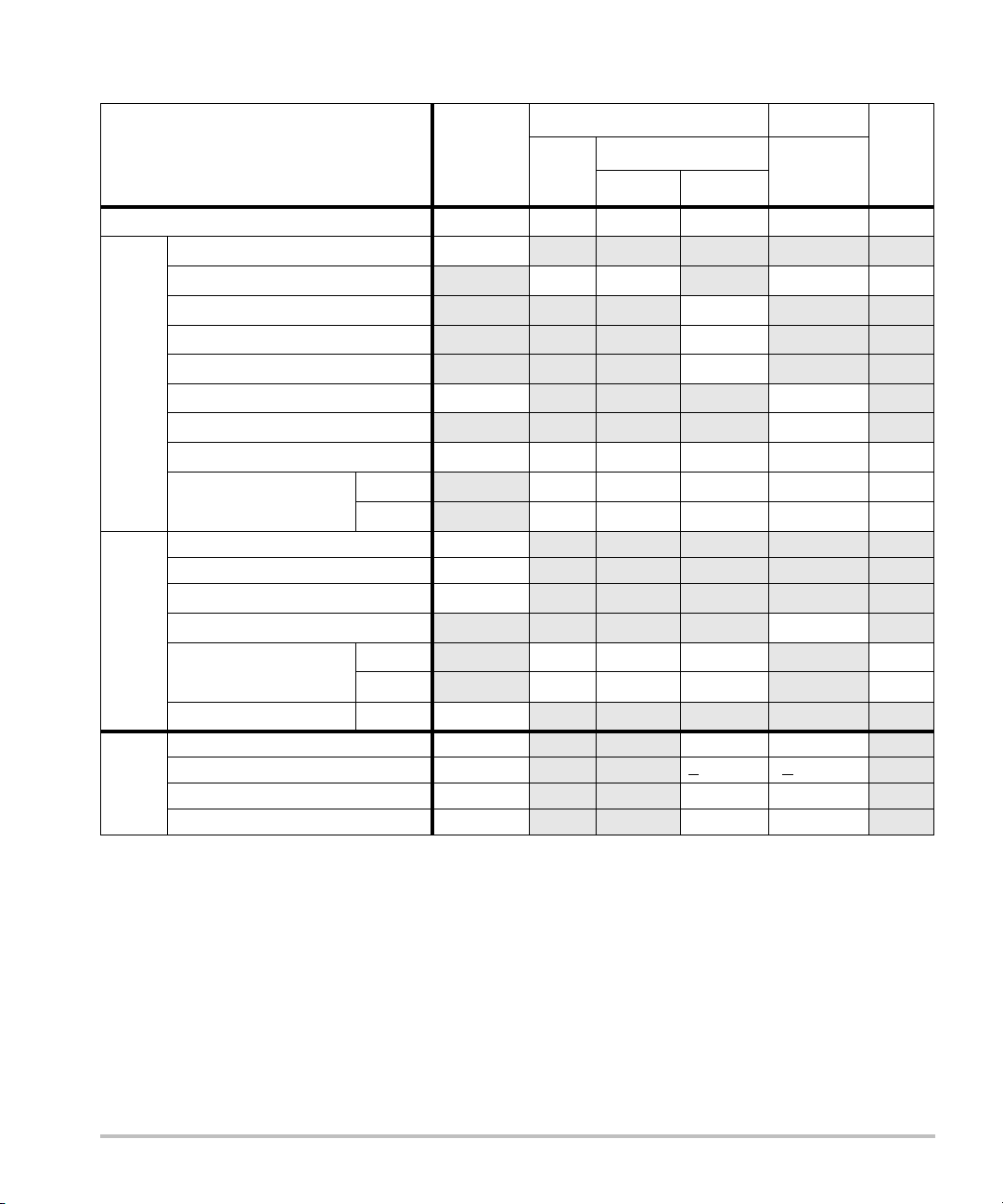
Table 17: Transducer Model: L52e/10-5 Operating Mode: PW Doppler
TIS TIB
Index Label M.I.
Global Maximum Index Value 1.2
p
r.3
W
0
min of [W.
z
1
Z
bp
Z
sp
Parameter
deq(Zsp)(cm) 0.358
F
Associated Acoustic
c
Dim of A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2.31
(mW)
] (mW) 70.59
(cm) 1.7
(cm) 1.7
(cm) 1.6 2.0
(MHz) 3.87
X(cm)
Y(cm)
PD (µsec) 1.14
PRF (Hz) 1008
pr@PII
max
deq@Pll
Other
Focal Length FLx (cm)
max
Information
I
@MI
PA. 3
max
(MPa) 2.861
(cm) 0.302
FL
(cm)
y
(W/cm2)
319.6
Control 1: Exam Type Any Any OB
Control 2: PRF 1008 Hz >1563 Hz <3125 Hz
Control 3: SV Size 1 mm 1 mm 2 mm
Control
Operating
Control 4: SV Position Zone 3 Zone 7 Zone 6
Conditions
Scan
Non-scan
≤1A
A
aprt
——
——
——
——
——
——
——
Non-scan
TIC
>1
aprt
1.3 2.2 (b)
61.29 #
3.84 3.85 #
1.886 1.23 #
0.55 0.55 #
7.38 #
3.0 #
(a) This index is not required for this operating mode; value is <1.
(b) This transducer is not intended for transcranial or neonatal cephalic uses.
# No data are reported for this operating condition since the global maximum index value is not reported for the reason
listed. (Reference Global Maximum Index Value line.)
— Data are not applicable for this transducer/mode.
20
Page 27

Troubleshooting and Maintenance
Cleaning and disinfecting transducers
To disinfect the transducer, use the immersion method or the wipe method. You can use the
immersion method only if your disinfectant is compatible with it. Check the product labeling.
For more information about cleaning and disinfecting the L52 transducer, refer to the ultrasound
system user guide.
The following table lists disinfectants that have been tested by SonoSite. See the SonoSite Web site
for updated cleaning and disinfectant information: www.sonosite.com.
Tab le 18 does not have the following regulatory information for disinfectants:
•EPA Registration
• FDA 510(k) clearance (liquid sterilant, high level disinfectant)
• CE approval
Prior to use, confirm that the regulatory status of the disinfectant is appropriate for
your jurisdiction and use.
Table 18: Disinfectant Compatibility with L52 Series Transducer
English Deutsch Español Français Italiano Português
Disinfection and
Cleaning Solution
Incidin Plus 1%
AbcoCide 14 USA Liquid Gluteraldehyde
Accel Wipes CAN Wipe Hydrogen Peroxide
Aidal Plus AUS Liquid Gluteraldehyde
Airkem A-33 USA Liquid Quaternary Ammonia
Alcohol, Ethyl USA Liquid Ethyl Alcohol
Alkacide FRA Liquid Gluteraldehyde
Alkazyme FRA Liquid Quaternary Ammonia
Anios Wipes FRA Wipes Quaternary Ammonia,
Aquatabs (1000) IRL Tablet Sodium
Country
of Origin
DEU Liquid Glucoprotamin, Isopropyl
Type Active Ingredient
Alcohol
Isopropyl Alcohol
Dichloroisocyanurate
L52x
L52n
L52
L52e
21
Page 28

Table 18: Disinfectant Compatibility with L52 Series Transducer (Continued)
Disinfection and
Cleaning Solution
Aquatabs (2000) IRL Tablet Sodium
Country
of Origin
Type Active Ingredient
L52x
L52n
L52
L52e
Dichloroisocyanurate
Ascend USA Liquid Quaternary Ammonia
Asepti-HB USA Liquid Quaternary Ammonia
Asepti-Steryl USA Spray Ethyl Alcohol
Asepti-Wipes USA Wipe Isopropyl Alcohol
Bacillocid rasant DEU Liquid Gluteraldehyde/Quaternary
Ammonia
Bacoban DEU Liquid Ethanol Isopropanol
Bacoban WB DEU Liquid Benzalkonium chloride
Diethylenglycol
Banicide USA Liquid Gluteraldehyde
Bleach USA Liquid Sodium Hypochlorite
Cavicide USA Liquid Isopropyl Alcohol
CaviWipes USA Wipes Isopropyl Alcohol
Chlor-Clean GBR Liquid Sodium
Dichloroisocyanurate
Cidalkan Lingettes FRA Wipes Ethyl Alcohol
Cidex 14 USA Liquid Gluteraldehyde
Cidex OPA USA Liquid Ortho-phthaldehyde
Cidex Plus USA Liquid Gluteraldehyde
Cleanisept Wipes DEU Wipes Alkyl Ammonium Chloride
Clorox Disinfecting
USA Wipe Isopropyl Alcohol
Wipes
Control III USA Liquid Quaternary Ammonia
Coverage Spray USA Spray Quaternary Ammonia
Coverage™ Plus Wipes USA Wipes Quaternary Ammonia
22
Page 29

Table 18: Disinfectant Compatibility with L52 Series Transducer (Continued)
English Deutsch Español Français Italiano Português
Disinfection and
Cleaning Solution
Country
of Origin
Type Active Ingredient
L52x
L52n
L52
L52e
Coverage™ Wipes USA Wipes Quaternary Ammonia
DentaSept FRA Liquid Quaternary Ammonia
DisCide Wipes USA Wipe Isopropyl Alcohol
DisOPA JPN Liquid Ortho-phthaldehyde
Dispatch USA Spray Sodium Hypochlorite
Dynacide PA FRA Liquid Peracetic Acid
End-Bac II USA Liquid Quaternary Ammonia
Endosporine FRA Liquid Gluteraldehyde
Endozime AW Plus FRA Liquid Isopropyl Alcohol
Envirocide USA Liquid Isopropyl Alcohol
Enzol USA Cleaner Ethylene Glycol
Expose USA Liquid Isopropyl Alcohol
Gigasept AF DEU Liquid Quaternary Ammonia
Gigasept FF DEU Liquid Succinic Acid
Gluteraldehyde SDS USA Liquid Gluteraldehyde
Hexanios FRA Liquid Polyhexanide/Quaternary
Ammonia
Hi Tor Plus USA Liquid Chloride
Hibiclens USA Cleaner Chlorhexidine
Hydrogen Peroxide n/a Liquid Hydrogen Peroxide (3%)
Incidin Plus 3% DEU Liquid Glucoprotamin, Isopropyl
Alcohol
Kodan Tücher DEU Spray Propanol/Alcohol
Kohrsolin ff DEU Liquid Gluteraldehyde
Korsolex basic DEU Liquid Gluteraldehyde
Korsolex extra DEU Liquid Ethanol/Propanol
23
Page 30

Table 18: Disinfectant Compatibility with L52 Series Transducer (Continued)
Disinfection and
Cleaning Solution
Country
of Origin
Type Active Ingredient
L52x
L52n
L52
L52e
LpHse USA Liquid O-phenylphenol
Lysol IC USA Liquid O-phenylphenol
Madacide 1 USA Liquid Alkyl Ammonium Chloride
Matar USA Liquid O-phenylphenol
MetriCide 14 USA Liquid Gluteraldehyde
MetriCide 28 USA Liquid Gluteraldehyde
MetriCide OPA Plus USA Liquid Ortho-phthaldehyde
MetriZyme USA Cleaner Propylene Glycol
Mikrobak forte DEU Liquid Ammonium Chloride
Mikrozid DEU Wipe Ethanol/Propanol
Nuclean FRA Spray Alcohol/Biguanide
PerCept RTU Wipes™ CAN Wipe Hydrogen Peroxide
Rely+On™ PeraSafe™ GBR Liquid Paracetic Acid
Ruthless USA Spray Quaternary Ammonia
Sagrosept DEU Wipe Isopropyl Alcohol
Salvanios pH 7 FRA Liquid Quaternary Ammonia
Sani-Cloth HB USA Wipe Quaternary Ammonia
Sani-Cloth Plus USA Wipe Quaternary Ammonia
Sekusept GER Liquid Gluteraldehyde
Sklar (4) USA Liquid Isopropyl Alcohol
Sporicidin USA Wipe Phenol
Sporicidin USA Liquid Phenol
Staphene USA Spray Ethyl Alcohol
Steranios 2% FRA Liquid Gluteraldehyde
Steranios 20% FRA Liquid Gluteraldehyde
24
Page 31

Table 18: Disinfectant Compatibility with L52 Series Transducer (Continued)
English Deutsch Español Français Italiano Português
Disinfection and
Cleaning Solution
Country
of Origin
Type Active Ingredient
L52x
L52n
L52
L52e
Super Sani-Cloth USA Wipe Isopropyl Alcohol
T-Spray USA Spray Quaternary Ammonia
T-Spray II USA Spray Alkyl/Chloride
Task 105 USA Spray Quaternary Ammonia
TBQ USA Liquid Alkyl Ammonium Chloride
Theracide Plus USA Liquid Quaternary Ammonia
Tor USA Liquid Quaternary Ammonia
Trigene Advance
Wipes
Wipe Quaternary Ammonia,
Polymeric Biguanide
Hydrochloride
Tristel GBR Liquid Chlorine Dioxide
Tristel Solo GBR Foam Hexamethylenebiguanide
Tristel Wipes GBR Wipe Chlorine Dioxide
Vesphene IIse USA Liquid Sodium/o-Phenylphenate
Virex 256 USA Liquid Ammonium Chloride
Virex TB USA Liquid Quaternary Ammonia
Virox 5 CAN Wipe Hydrogen Peroxide
Virufen FRA Liquid Alkyl Ammonium Chloride
Wavicide-06 USA Liquid Gluteraldehyde
Wet Wipe Disinfection DNK Wipe Guanidinium-chloride
Wex-Cide USA Liquid O-phenylphenol
= Acceptable
25
Page 32

26
Page 33

Schallkopf der L52-Serie
Benutzerhandbuch
Page 34

Hergestellt von
SonoSite, Inc.
21919 30th Drive SE
Bothell, WA 98021
USA
Tel.: +1-888-482-9449 oder +1-425-951-1200
Fax: +1-425-951-1201
SonoSite Ltd
Alexander House
40A Wilbury Way
Hitchin
Herts SG4 OAP
Großbritannien
Tel.: +44-1462-444800
Fax: +44-1462-444801
Vorsichtshinweis:
Laut US-Bundesgesetzen darf dieses Gerät nur an Tierärzte oder auf deren Anordnung
verkauft werden.
180PLUS, M-Turbo, MicroMaxx, NanoMaxx, S Series, SonoSite, SonoSite TITAN, TITAN und das SonoSite-Logo sind eingetragene Marken
(in einigen Rechtsprechungen) oder nicht eingetragene Marken im Besitz von SonoSite, Inc.
Nicht zu SonoSite gehörende Produktnamen sind u.U. Marken oder eingetragene Marken der jeweiligen Eigentümer.
Die in diesem Dokument genannten Ultraschallsysteme von SonoSite sind u. U. durch eines oder mehrere der folgenden
US-amerikanischen Patente geschützt: 5722412, 5817024, 5893363, 6135961, 6203498, 6364839, 6371918, 6383139, 6416475, 6447451,
6471651, 6569101, 6648826, 6575908, 6604630, 6817982, 6835177, 6962566, 7169108, 7449640, 7534211, 7549961, 7588541, 7591786,
7604596, 7643040, 7686766, 7694814, 7727153, 7740586, 7804970, 7809400, 7819807, 7841575, 7849250, 7867168, 7883276, D456509,
D461895, D509900, D538432, D544962, D558351, D559390, D591423, D592750, D592760, D625014, D625015 sowie die folgenden
entsprechenden Patente im Ausland: AU727381, AU730822, CA2372152, CA2372158, CA2373065, CN ZL 97113678.5, CN ZL 98106133.8,
CN ZL 98108973.9, CN ZL 200830007734.8, DE60021552.0, DE60029777.2, DE60034670.6, DE69730563.5, DE6980539.6, DE69831698.3,
DE60 2004 23 816.3-08, FR0815793, FR0875203, FR0881492, FR1175713, FR1180970, FR1589878, GB0875203, GB0881492, GB1175713,
GB1180970, GB1180971, GB1589878, IT0815793, IT0881492, IT1175713, IT1589878, KR528102, KR532359, NO326202, NO326814,
NZ542968, RCD000897368-0001, SP0815793, SP0881492, SP1589878. Weitere Patente sind angemeldet.
P07895-06 03/2011
Copyright 2011 von SonoSite, Inc.
Alle Rechte vorbehalten. Gedruckt in den USA.
ii
Page 35

Benutzerhandbuch zum Schallkopf der L52-Series
Einführung ...................................................................................................................................................1
Bildgebung ..................................................................................................................................................1
Messungen und Berechnungen ...........................................................................................................4
Sicherheit ......................................................................................................................................................5
Fehlersuche und Wartung ...................................................................................................................21
Einführung
Dieses Benutzerhandbuch ist eine Ergänzung der folgenden Ultraschallsystem-Benutzerhandbücher:
• NanoMaxx-Ultraschallsystem-Benutzerhandbuch
• M-Turbo-Ultraschallsystem-Benutzerhandbuch
• S Series-Ultraschallsystem-Benutzerhandbuch
• MicroMaxx-Ultraschallsystem-Benutzerhandbuch
• TITAN-Ultraschallsystem-Benutzerhandbuch
• SonoSite-Ultraschallsystem-Benutzerhandbuch
Es beschreibt die folgenden Schallköpfe, die ausschließlich für den tierärztlichen Gebrauch
bestimmt sind:
• Schallkopf L52n/10-5 MHz (L52n) auf dem NanoMaxx®-Ultraschallsystem
• L52x/10-5 MHz (L52x) Schallkopf für die Ultraschallsysteme M-Turbo®, MicroMaxx® oder S Series™
• L52e/10-5 MHz (L52e) Schallkopf für das MicroMaxx®-Ultraschallsystem
• L52/10-5 MHz (L52) Schallkopf für das TITAN®-Ultraschallsystem mit hoher Auflösung oder das
SonoSite®180PLUS™-Ultraschallsystem
Zusätzliche Sicherheitsinformationen, Anleitungen zur Vorbereitung, Benutzung und Wartung des
Ultraschallsystems sowie Angaben zu den Verwendungszwecken der einzelnen Untersuchungstypen und Bildgebungsmodi sind dem Benutzerhandbuch des Ultraschallsystems zu entnehmen.
English Deutsch Español Français Italiano Português
Bildgebung
Schallkopf, Untersuchungstyp und Bildgebungsmodus
Die folgende Tabelle enthält eine Übersicht über die möglichen Untersuchungstypen,
Bildgebungsmodi sowie eventuell verfügbaren Optimierungen der einzelnen Signalköpfe.
1
Page 36

Schallkopf, Untersuchungstyp und Bildgebungsmodus (NanoMaxx)
Bildgebungsmodus
Schallkopf Untersuchungstyp
2D
M-Mode
CPD Farbe
L52n GBH X X X
Vaskulär (Vas) X X X
Muskeln (Mus) X X X
.
Schallkopf, Untersuchungstyp und Bildgebungsmodus (M-Turbo oder MicroMaxx)
Bildgebungsmodus
Schallkopf Untersuchungstyp
2D
M-Mode
CPD Farbe PW CW
L52x GBH X X X X —
Vaskulär (Vas) X X X X —
Muskeln (Mus) X X X X —
Schallkopf, Untersuchungstyp und Bildgebungsmodus (S Series)
Bildgebungsmodus
Schallkopf Untersuchungstyp
2D
M-Mode
CPD Farbe PW
L52x GBH X X X X
Vaskulär (Vas) X X X X
Muskeln (Mus) X X X X
2
Page 37

Schallkopf, Untersuchungstyp und Bildgebungsmodus (MicroMaxx)
Bildgebungsmodus
English Deutsch Español Français Italiano Português
Schallkopf
Untersuchungstyp
2D
M-Mode
THI
2DMB2D
S
CPD Farbe PW
TDI
PW
L52e GBH X — X X X X X — —
Vaskulär
X—XXXXX——
(Vas)
Muskeln
X—XXXXX——
(Mus)
Schallkopf, Untersuchungstyp und Bildgebungsmodus (TITAN)
Bildgebungsmodus
Unter-
Schallkopf
suchungs-
2D THI CPD DCPD Farbe M-Mode PW CW
typ
L52 GBH X — X — — X — —
Vaskulär
X—X — — X ——
(Vas)
CW
Muskeln
X—X — — X ——
(Mus)
Schallkopf, Untersuchungstyp und Bildgebungsmodus (180PLUS)
Bildgebungsmodus
Schallkopf Untersuchungstyp 2D CPD
L52 GBH Aufl., Allg., Tiefe niedrig, mäßig, hoch
Vaskulär (Vas) Aufl., Allg., Tiefe niedrig, mäßig, hoch
3
Page 38

Messungen und Berechnungen
Berechnungen
Diese Tabelle enthält die möglichen Berechnungen für die einzelnen Untersuchungstypen des
Schallkopfes der L52-Serie.
Berechnungen für L52n (NanoMaxx)
Untersuchungstyp Berechnungen
GBH GBH
Berechnungen für L52x (M-Turbo und MicroMaxx)
Untersuchungstyp Berechnungen
Muskeln (Mus) Prozent-Reduktion
Volumen
GBH GBH
Vaskulär (Vas) Prozent-Reduktion
Vaskulär
Volumen
Volumenfluss
Berechnungen für L52x (S Series)
Untersuchungstyp S Series-System Berechnungen
GBH S-VetMed GBH
Berechnungen für L52e/L52 (MicroMaxx and TITAN)
Untersuchungstyp Berechnungen
Muskeln (Mus) Prozent-Reduktion
Volumen
GBH GBH
4
Page 39

Berechnungen für L52e/L52 (Forts.) (MicroMaxx and TITAN)
Untersuchungstyp Berechnungen
Vaskulär (Vas) Prozent-Reduktion
Berechnungen für L52 (180PLUS)
Untersuchungstyp Berechnungen
GBH GBH
Vaskulär (Vas) Volumen
Sicherheit
English Deutsch Español Français Italiano Português
Vaskulär
Volumen
Volumenfluss
Volumenfluss
Richtlinien zur Verringerung des MI und TI
Die folgenden Tabellen enthalten allgemeine Richtlinien zur Reduzierung der MI- und TI-Werte.
Wenn mehrere Parameter gegeben sind, lassen sich unter Umständen die besten Ergebnisse erzielen,
indem die Werte aller dieser Parameter gleichzeitig verringert werden. In manchen Modi wirkt sich
eine Änderung der Parameter nicht auf den MI- oder TI-Wert aus. Andererseits können aber auch
Änderungen an anderen Parametern eine Reduzierung von MI und TI bewirken. Bitte beachten Sie
die MI- und TI-Werte auf der rechten Seite des Bildschirms.
Tabelle 1: MI
Schallkopf Tiefe
L52n (NanoMaxx) ↑
L52x (M-Turbo, S Series oder MicroMaxx) ↑
L52e (MicroMaxx) ↑
L52 (TITAN) ↑
L52 (180PLUS) ↑
↓ Verringerung des Parameterwerts zur Reduzierung von MI.
↑ Erhöhung des Parameterwerts zur Reduzierung von MI.
5
Page 40

Tabelle 2: TI (TIW, TIC, TIK)
Farb- /Amplitu den-
Schallkopf
Doppler-Einstellungen
PRF Tiefe
L52n (NanoMaxx) — ↑ —
L52x (M-Turbo oder
↓↑Probengröße ↓
MicroMaxx)
L52x (S Series) ↓↑ —
L52e (MicroMaxx) ↓↑Probengröße ↑
L52 (TITAN) ↓↑ —
L52 (180PLUS) — — —
↓ Verringerung des Parameterwerts zur Reduzierung von TI.
↑ Erhöhung des Parameterwerts zur Reduzierung von TI.
— Für diesen Schallkopf/Betriebsmodus nicht zutreffend.
Anstieg der Schallkopfoberflächentemperatur
Tab el le 3 zeigt den gemessenen Anstieg der Oberflächentemperatur (in °C) der mit dem
Ultraschallsystem verwendeten Schallköpfe im Vergleich zur Umgebungstemperatur (23 °C ± 3 °C).
Die Temperaturen wurden gemäß EN 60601-2-37, Abschnitt 42, gemessen, wobei Bedienelemente
und Einstellungen auf die Erzielung maximaler Temperaturen reguliert wurden.
PW-Einstellungen
Tabelle 3: Anstieg der Schallkopfoberflächentemperatur gemäß IEC 60601-2-37
(innere Anwendung)
Te st
TITAN
Unbewegte
L52n mit
NanoMaxx
M-Turbo
L52x mit
oder S Series
L52x mit
MicroMaxx
L52e mit
MicroMaxx
L52 mit
7,5 8,8 8,2 13,0 9,3 10,8
L52 mit
180PLUS
Luft
Simulierte
5,6 5,9 5,6 5,5 2,4 2,4
Anwendung
6
Page 41

Ausgangsleistungsanzeige
Tabelle 4: TI oder MI ist ≥ 1,0
English Deutsch Español Français Italiano Português
Schallkopfmodell Index
2D/
M-Mode
Farbe CPD PW-Doppler
L52n (NanoMaxx) MI Ja Ja Ja n.z.
TI Nein Nein Nein n.z.
L52x (M-Turbo) MIJaJaJa Ja
TI Nein Nein Nein Ja
L52x (SSeries) MIJaJaJa —
TI Nein Nein Nein —
L52x (MicroMaxx) MI Ja Ja Ja Ja
TI Ja Nein Nein Ja
L52e (MicroMaxx) MI Nein Ja Ja Ja
TI Nein Nein Nein Ja
L52 (TITAN, 180PLUS)* MI Nein Nein Nein Nein
TI Nein Nein Nein Nein
* Auf dem TITAN- oder 180PLUS-System erreicht der L52-Schallkopf niemals einen MI- oder TI-Wert von 1,0.
7
Page 42

Schallausgangsleistungs-Tabellen (M-Turbo und S Series)
Tabelle 5: Schallkopfmodell: L52x/10-5 Betriebsmodus: 2D
TIW TIK
A
aprt
Non-scan
≤1A
aprt
Non-scan
TIC
>1
Index-Bezeichnung MI
Scan
Globaler maximaler Indexwert 1,01 (a) — — — (a)
p
r.3
W
0
Min von [W
z
1
z
bp
z
sp
Assoziierter
deq(zsp)(cm)
Akustikparameter
f
c
Dim von A
.3(z1
aprt
),I
TA. 3(z1
)] (mW)
(MPa)
(mW)
2,336
#— —#
—
(cm)
(cm)
(cm)
1,8
—
—
—
—
(MHz)
5,33 # — — — #
X(cm) #—— — #
Y(cm)
#—— — #
PD (µs) 0,15
PRF (Hz) 7222
pr@PII
max
deq@Pll
max
Fokuslänge FLx (cm)
Zusätzliche
Informationen
I
@MI
PA .3
max
(MPa)
(cm)
FLy (cm)
(W/cm2)
3,25
—
#—— #
#—— #
329,1
Regelung 1: Untersuchungstyp Vas — — — — —
Regelung 2: Optimierung Allg — — — — —
Regelung 3: Tiefe 4,2 cm — — — — —
Betriebs-
Regelung 4: MS (Mehrstrahl)
regelungs-
bedingungen
Aus — — — — —
(a) Dieser Index ist für diesen Betriebsmodus nicht erforderlich. Der Wert ist <1.
(b) Dieser Schallkopf ist nicht für den transkraniellen Einsatz und für Schädeluntersuchungen bei Neugeborenen
vorgesehen.
# Für diese Betriebsbedingung liegen keine Daten vor, da aus dem angegebenen Grund kein globaler Maximalindexwert
vorliegt. (Siehe Zeile „Globaler maximaler Indexwert“.)
— Daten für diesen Schallkopf/Betriebsmodus nicht zutreffend.
8
Page 43

Tabelle 6: Schallkopfmodell: L52x/10-5 (nur M-Turbo) Betriebsmodus: M-Mode
TIW TIK
English Deutsch Español Français Italiano Português
Index-Bezeichnung MI
Scan
Non-scan
A
aprt
≤1A
aprt
Non-scan
TIC
>1
Globaler maximaler Indexwert 1,01 — (a) — (a) (b)
p
r.3
W
0
Min von [W.3(z1),I
z
1
z
bp
z
sp
Assoziierter
deq(zsp)(cm)
Akustikparameter
f
c
Dim von A
TA. 3(z1
aprt
(MPa)
(mW)
)] (mW)
(cm)
(cm)
(cm)
2,336
1,8
—# ##
—
—
—
#
#
(MHz)
5,33 — # — # #
X(cm) —# — # #
Y(cm)
—# — # #
PD (µs) 0,15
PRF (Hz) 1600
pr@PII
max
deq@Pll
max
Fokuslänge FLx (cm)
Zusätzliche
Informationen
@MI
I
PA .3
max
(MPa)
(cm)
(cm)
FL
y
(W/cm2)
3,25
#
—# — #
—# — #
329,1
Regelung 1: Untersuchungstyp Vas — — — — —
Regelung 2: Optimierung Allg — — — — —
Regelung 3: Tiefe 4,2 cm — — — — —
Betriebs-
Regelung 4: MS (Mehrstrahl)
regelungs-
bedingungen
Aus — — — — —
(a) Dieser Index ist für diesen Betriebsmodus nicht erforderlich. Der Wert ist <1.
(b) Dieser Schallkopf ist nicht für den transkraniellen Einsatz und für Schädeluntersuchungen bei Neugeborenen
vorgesehen.
# Für diese Betriebsbedingung liegen keine Daten vor, da aus dem angegebenen Grund kein globaler Maximalindexwert
vorliegt. (Siehe Zeile „Globaler maximaler Indexwert“.)
— Daten für diesen Schallkopf/Betriebsmodus nicht zutreffend.
9
Page 44

Tabelle 7: Schallkopfmodell: L52x/10-5 Betriebsmodus: Farbe/CPD
TIW TIK
A
aprt
Non-scan
≤1A
aprt
Non-scan
TIC
>1
Index-Bezeichnung MI
Scan
Globaler maximaler Indexwert 1,33 (a) — — — (b)
p
r.3
W
0
Min. von [W.
z
1
Z
bp
Z
sp
Assoziierter
deq(Zsp)(cm) —
Akustikparameter
F
c
Dim von A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2,807
(mW) #— —#
] (mW) —
(cm) —
(cm) —
(cm) 1,8 —
(MHz) 4,37 # — — — #
X(cm) #— — — #
Y(cm)
#— — —#
PD (µs) 0,61
PRF (Hz) 5427
pr@PII
deq@Pll
max
max
(MPa) 3,628
(cm) —
Fokuslänge FLx (cm) #— — #
Zusätzliche
Informationen
@MI
I
PA. 3
max
FL
(cm) #— — #
y
(W/cm2)
411,1
Regelung 1: Modus Beliebig — — — — —
Regelung 2: Untersuchungstyp Vas — — — — —
Regelung 3: Optimierung/Tiefe Beliebig/5,4 — — — — —
Regelung 4: PRF Beliebig — — — — —
Betriebs-
regelungs-
Regelung 5: Position/Größe des
bedingungen
Farbbereichs
Beliebig/
Standard
—— — — —
(a) Dieser Index ist für diesen Betriebsmodus nicht erforderlich. Der Wert ist <1.
(b) Dieser Schallkopf ist nicht für den transkraniellen Einsatz und für Schädeluntersuchungen bei Neugeborenen
vorgesehen.
# Für diese Betriebsbedingung liegen keine Daten vor, da aus dem angegebenen Grund kein globaler Maximalindexwert
vorliegt. (Siehe Zeile „Globaler maximaler Indexwert“.)
— Daten für diesen Schallkopf/Betriebsmodus nicht zutreffend.
10
Page 45

Tabelle 8: Schallkopfmodell: L52x/10-5 (nur M-Turbo) Betriebsmodus: PW-Doppler
TIW TIK
English Deutsch Español Français Italiano Português
Index-Bezeichnung MI
Globaler maximaler Indexwert 1,17
p
r.3
W
0
Min. von [W.
z
1
Z
bp
Z
sp
Assoziierter
deq(Zsp)(cm) 0,45
Akustikparameter
F
c
Dim von A
3(z1),ITA. 3(z1)
aprt
(MPa) 2,443
(mW)
] (mW) —
(cm) —
(cm) —
(cm) 2,1 1,5
(MHz) 4,36
X(cm)
Y(cm)
PD (µs) 1,38
PRF (Hz) 1008
pr@PII
deq@Pll
max
max
(MPa) 3,30
(cm) 0,34
Fokuslänge FLx (cm)
Zusätzliche
Informationen
I
@MI
PA. 3
max
FL
(cm)
y
(W/cm2)
288,97
Regelung 1: Untersuchungstyp Beliebig — Beliebig — Beliebig —
Regelung 2: PRF 1008 Hz — 3125 Hz — 3125 Hz —
Regelung 3: SV-Größe 1 mm — 1 mm — 1 mm —
Betriebs-
Regelung 4: SV-Position Zone 4 — Zone 6 — Zone 6 —
regelungs-
bedingungen
Scan
—
—
—
—
—
—
—
Non-scan
Non-scan
A
aprt
≤1A
aprt
>1
1,44 — 2,22 (b)
69,42 69,42 #
4,35 — 4,35 #
1,476 — 1,476 #
0,55 — 0,55 #
5,99 — #
3,4 — #
TIC
(a) Dieser Index ist für diesen Betriebsmodus nicht erforderlich. Der Wert ist <1.
(b) Dieser Schallkopf ist nicht für den transkraniellen Einsatz und für Schädeluntersuchungen bei Neugeborenen
vorgesehen.
# Für diese Betriebsbedingung liegen keine Daten vor, da aus dem angegebenen Grund kein globaler Maximalindexwert
vorliegt. (Siehe Zeile „Globaler maximaler Indexwert“.)
— Daten für diesen Schallkopf/Betriebsmodus nicht zutreffend.
11
Page 46

Schallausgangsleistung-Tabellen (NanoMaxx)
Tabelle 9: Schallkopfmodell: L52n/10-5 Betriebsmodus: 2D
TIW TIK
A
aprt
Non-scan
≤1A
aprt
Non-scan
TIC
>1
Index-Bezeichnung MI
Scan
Globaler maximaler Indexwert 1,0 (a) — — — (b)
p
r.3
W
0
Min von [W.3(z1),I
z
1
z
bp
z
sp
Assoziierter
deq(zsp)(cm)
Akustikparameter
f
c
Dim von A
aprt
TA. 3(z1
)] (mW)
(MPa)
(mW)
2,34
#— —#
—
(cm)
(cm)
(cm)
1,8
—
—
—
—
(MHz)
5,33 # — — — #
X(cm) #—— — #
Y(cm)
#—— —#
PD (µs) 0,15
PRF (Hz) 7707
pr@PII
max
deq@Pll
max
Fokuslänge FLx (cm)
Zusätzliche
Informationen
@MI
I
PA. 3
max
(MPa)
(cm)
(cm)
FL
y
(W/cm2)
3,25
—
#—— #
#—— #
329,1
Regelung 1: Untersuchungstyp Beliebig
Regelung 2: Optimierung Allg
Regelung 3: Tiefe 4,2 cm
Regelung 4: MS (Mehrstrahl) Aus
Betriebs-
regelungs-
bedingungen
oder
Ein
(a) Dieser Index ist für diesen Betriebsmodus nicht erforderlich. Der Wert ist <1.
(b) Dieser Schallkopf ist nicht für den transkraniellen Einsatz und für Schädeluntersuchungen bei Neugeborenen
vorgesehen.
# Für diese Betriebsbedingung liegen keine Daten vor, da aus dem angegebenen Grund kein globaler Maximalindexwert
vorliegt. (Siehe Zeile „Globaler maximaler Indexwert“.)
— Daten für diesen Schallkopf/Betriebsmodus nicht zutreffend.
12
Page 47

Tabelle 10: Schallkopfmodell: L52n/10-5 Betriebsmodus: M-Mode
TIW TIK
English Deutsch Español Français Italiano Português
A
aprt
Non-scan
≤1A
aprt
Non-scan
TIC
>1
Index-Bezeichnung MI
Scan
Globaler maximaler Indexwert 1,0 — (a) — (a) (b)
p
r.3
W
0
Min von [W.3(z1),I
z
1
z
bp
z
sp
Assoziierter
deq(zsp)(cm)
Akustikparameter
f
c
Dim von A
aprt
TA. 3(z1
)] (mW)
(MPa)
(mW)
2,34
—# ##
—
(cm)
(cm)
(cm)
1,8
—
—
#
#
(MHz)
5,33 — # — # #
X(cm) —# — # #
Y(cm)
—# — # #
PD (µs) 0,15
PRF (Hz) 1600
pr@PII
max
deq@Pll
max
Fokuslänge FLx (cm)
Zusätzliche
Informationen
I
@MI
PA. 3
max
(MPa)
(cm)
(cm)
FL
y
(W/cm2)
3,25
#
—# — #
—# — #
329,1
Regelung 1: Untersuchungstyp Beliebig — — — — —
Regelung 2: Optimierung Allg — — — — —
Regelung 3: Tiefe 4,2 cm — — — — —
Regelung 4: MS (Mehrstrahl) Aus
Betriebs-
regelungs-
bedingungen
oder Ein
—— — — —
(a) Dieser Index ist für diesen Betriebsmodus nicht erforderlich. Der Wert ist <1.
(b) Dieser Schallkopf ist nicht für den transkraniellen Einsatz und für Schädeluntersuchungen bei Neugeborenen
vorgesehen.
# Für diese Betriebsbedingung liegen keine Daten vor, da aus dem angegebenen Grund kein globaler Maximalindexwert
vorliegt. (Siehe Zeile „Globaler maximaler Indexwert“.)
— Daten für diesen Schallkopf/Betriebsmodus nicht zutreffend.
13
Page 48

Tabelle 11: Schallkopfmodell: L52n/10-5 Betriebsmodus: Farbe/CPD
TIW TIK
A
aprt
Non-scan
≤1A
aprt
Non-scan
TIC
>1
Index-Bezeichnung MI
Scan
Globaler maximaler Indexwert 1,2 (a) — — — (b)
p
r.3
W
0
Min. von [W.
z
1
Z
bp
Z
sp
Assoziierter
deq(Zsp)(cm) —
Akustikparameter
F
c
Dim von A
3(z1),ITA. 3(z1)
aprt
(MPa) 2,35
(mW) #— —#
] (mW) —
(cm) —
(cm) —
(cm) 1,8 —
(MHz) 4,37 # — — — #
X (cm) #— — — #
Y (cm)
#— — — #
PD (µs) 0,60
PRF (Hz) 7097
pr@PII
deq@Pll
max
max
(MPa) 3,08
(cm) —
Fokuslänge FLx (cm) #— — #
Zusätzliche
Informationen
@MI
I
PA. 3
max
Regelung 1: Modus CPD oder
(cm) #— — #
FL
y
(W/cm2)
308,5
—— — — —
Farbe
Regelung 2: Untersuchungstyp Mus — — — — —
Regelung 3: Optimierung Aufl — — — — —
Betriebs-
Regelung 4: Tiefe 5,4 — — — — —
regelungs-
bedingungen
Regelung 5: Farbbereich Standard — — — — —
(a) Dieser Index ist für diesen Betriebsmodus nicht erforderlich. Der Wert ist <1.
(b) Dieser Schallkopf ist nicht für den transkraniellen Einsatz und für Schädeluntersuchungen bei Neugeborenen
vorgesehen.
# Für diese Betriebsbedingung liegen keine Daten vor, da aus dem angegebenen Grund kein globaler Maximalindexwert
vorliegt. (Siehe Zeile „Globaler maximaler Indexwert“.)
— Daten für diesen Schallkopf/Betriebsmodus nicht zutreffend.
14
Page 49

Schallausgangsleistungs-Tabellen (MicroMaxx)
Tabelle 12: Schallkopfmodell: L52x/10-5 Betriebsmodus: 2D
TIW TIK
English Deutsch Español Français Italiano Português
Index-Bezeichnung MI
Scan
Globaler maximaler Indexwert 1,0 (a)
p
r.3
W
0
Min. von [W.
z
1
Z
bp
Z
sp
Assoziierter
deq(Zsp)(cm)
Akustikparameter
F
c
Dim von A
3(z1),ITA. 3(z1)
aprt
(MPa) 2,23
(mW)
] (mW)
(cm)
(cm)
(cm) 1,9
(MHz) 5,42 #
X(cm) #
#— —
Y(cm)
PD (µs) 0,146
PRF (Hz) 8394
pr@PII
deq@Pll
max
max
(MPa) 3,19
(cm)
Fokuslänge FLx (cm) #
Zusätzliche
Informationen
I
@MI
PA. 3
max
FL
(cm) #
y
(W/cm2)
325,3
Regelung 1: Untersuchungstyp GBH — — — — —
Regelung 2: Optimierung Allg — — — — —
Regelung 3: Tiefe 2,5 - 3,9 — — — — —
Betriebs-
Regelung 4: MB Ein oder Aus — — — — —
regelungs-
bedingungen
Non-scan
TIC
Non-scan
≤1A
A
aprt
—— —
aprt
>1
(b)
#
—
—
—
—
—
—— —
—— —
#
—— —
#
#
#
—
——
——
#
#
(a) Dieser Index ist für diesen Betriebsmodus nicht erforderlich. Der Wert ist <1.
(b) Dieser Schallkopf ist nicht für den transkraniellen Einsatz und für Schädeluntersuchungen bei Neugeborenen
vorgesehen.
# Für diese Betriebsbedingung liegen keine Daten vor, da aus dem angegebenen Grund kein globaler Maximalindexwert
vorliegt. (Siehe Zeile „Globaler maximaler Indexwert“.)
— Daten für diesen Schallkopf/Betriebsmodus nicht zutreffend.
15
Page 50

Tabelle 13: Schallkopfmodell: L52x/10-5 Betriebsmodus: M-Mode
Index-Bezeichnung
MI
Globaler maximaler Indexwert 1,0
p
r.3
W
0
Min. von [W.
z
1
Z
bp
Z
sp
Assoziierter
deq(Zsp)(cm) 0,659
Akustikparameter
F
c
Dim von A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2,23
(mW)
] (mW)
(cm)
(cm)
(cm) 1,9 1,7
(MHz) 5,42
X(cm)
Y(cm)
PD (µs) 0,146
PRF (Hz) 1600
pr@PII
deq@Pll
max
max
(MPa) 3,19
(cm) 0,641
Fokuslänge FLx (cm)
Zusätzliche
Informationen
I
@MI
PA. 3
max
FL
(cm)
y
(W/cm2)
325,3
Regelung 1: Untersuchungstyp GBH — — — Beliebig —
Regelung 2: Optimierung Allg — — — Tief —
Regelung 3: Tiefe 2,5 - 3,9 — — — 15 —
Betriebs-
regelungs-
bedingungen
Scan
—
—
—
—
—
—
—
TIW TIK
Non-scan
Non-scan
A
aprt
(a)
≤1A
aprt
—
>1
1,2 (b)
# 58,3 #
—
—
—
#
#
#
#
#
—
—
—
—
—
4,35 #
2,71 #
0,55 #
TIC
#
#
(a) Dieser Index ist für diesen Betriebsmodus nicht erforderlich. Der Wert ist <1.
(b) Dieser Schallkopf ist nicht für den transkraniellen Einsatz und für Schädeluntersuchungen bei Neugeborenen
vorgesehen.
# Für diese Betriebsbedingung liegen keine Daten vor, da aus dem angegebenen Grund kein globaler Maximalindexwert
vorliegt. (Siehe Zeile „Globaler maximaler Indexwert“.)
— Daten für diesen Schallkopf/Betriebsmodus nicht zutreffend.
16
Page 51

Tabelle 14: Schallkopfmodell: L52x/10-5 Betriebsmodus: Farbe/CPD
TIW TIK
English Deutsch Español Français Italiano Português
Index-Bezeichnung MI
Scan
Globaler maximaler Indexwert 1,3 (a)
p
r.3
W
0
Min. von [W.
z
1
Z
bp
Z
sp
Assoziierter
deq(Zsp)(cm)
Akustikparameter
F
c
Dim von A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2,70
(mW) #
] (mW)
(cm)
(cm)
(cm) 1,4
(MHz) 4,35 #
X(cm) #
Y(cm)
PD (µs) 0,607
PRF (Hz) 4169
pr@PII
deq@Pll
max
max
(MPa) 3,33
(cm)
Fokuslänge FLx (cm) #
Zusätzliche
Informationen
I
@MI
PA. 3
max
FL
(cm) #
y
(W/cm2)
377,1
Regelung 1: Modus Farbe — — — — —
Regelung 2: Untersuchungstyp Beliebig — — — —
Regelung 3: Optimierung/Tiefe Niedrig/
—— — ——
2,5 - 3,9
Regelung 4: PRF <
Betriebs-
regelungs-
bedingungen
Regelung 5: Position/Größe des
718 — — — — —
Beliebig — — — — —
Farbbereichs
Non-scan
TIC
Non-scan
≤1A
A
aprt
———
— —
aprt
>1
(b)
#
—
—
—
—
—
———
———
#
———
#
#
#
—
——
——
#
#
(a) Dieser Index ist für diesen Betriebsmodus nicht erforderlich. Der Wert ist <1.
(b) Dieser Schallkopf ist nicht für den transkraniellen Einsatz und für Schädeluntersuchungen bei Neugeborenen
vorgesehen.
# Für diese Betriebsbedingung liegen keine Daten vor, da aus dem angegebenen Grund kein globaler Maximalindexwert
vorliegt. (Siehe Zeile „Globaler maximaler Indexwert“.)
— Daten für diesen Schallkopf/Betriebsmodus nicht zutreffend.
17
Page 52

Tabelle 15: Schallkopfmodell: L52x/10-5 Betriebsmodus: PW-Doppler
TIW TIK
A
aprt
Non-scan
≤1A
aprt
Non-scan
TIC
>1
Index-Bezeichnung MI
Scan
Globaler maximaler Indexwert 1,2 — 1,4 — 2,2 (b)
p
r.3
W
0
Min. von [W.
z
1
Z
bp
Z
sp
Assoziierter
deq(Zsp)(cm) 0,19
Akustikparameter
F
c
Dim von A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2,48
(mW) —68,5 37,5 #
] (mW) —
(cm) —
(cm) —
(cm) 2,3 2,4
(MHz) 4,36 — 4,35 — 4,36 #
X(cm) —2,05 — 0,90 #
Y(cm)
—0,55 — 0,55 #
PD (µs) 1,39
PRF (Hz) 1008
pr@PII
deq@Pll
max
max
(MPa) 3,505
(cm) 0,18
Fokuslänge FLx (cm) —8,32 — #
Zusätzliche
Informationen
@MI
I
PA. 3
max
FL
(cm) —3,5 — #
y
(W/cm2)
284,3
Regelung 1: Untersuchungstyp Beliebig — Beliebig — Beliebig —
Regelung 2: Probengröße 1 mm — 2 mm — 12 mm —
Regelung 3: PRF 1008 Hz — Beliebig — 10417 Hz —
Regelung 4: Position der
Betriebs-
regelungs-
bedingungen
Probengröße
Zone 3 — Zone 7 — Zone 3 —
(a) Dieser Index ist für diesen Betriebsmodus nicht erforderlich. Der Wert ist <1.
(b) Dieser Schallkopf ist nicht für den transkraniellen Einsatz und für Schädeluntersuchungen bei Neugeborenen
vorgesehen.
# Für diese Betriebsbedingung liegen keine Daten vor, da aus dem angegebenen Grund kein globaler Maximalindexwert
vorliegt. (Siehe Zeile „Globaler maximaler Indexwert“.)
— Daten für diesen Schallkopf/Betriebsmodus nicht zutreffend.
18
Page 53

Tabelle 16: Schallkopfmodell: L52e/10-5 Betriebsmodus: Farbe/CPD
TIW TIK
English Deutsch Español Français Italiano Português
A
aprt
Non-scan
≤1A
aprt
Non-scan
TIC
>1
Index-Bezeichnung MI
Scan
Globaler maximaler Indexwert 1,2 (a) — — — (b)
p
r.3
W
0
Min. von [W.
z
1
Z
bp
Z
sp
Assoziierter
deq(Zsp)(cm) —
Akustikparameter
F
c
Dim von A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2,30
(mW) #— —#
] (mW) —
(cm) —
(cm) —
(cm) 1,6 —
(MHz) 3,92 # — — — #
X(cm) #— — — #
Y(cm)
#— — — #
PD (µs) 0,797
PRF (Hz) 5332
pr@PII
deq@Pll
max
max
(MPa) 2,85
(cm) —
Fokuslänge FLx (cm) #— — #
Zusätzliche
Informationen
@MI
I
PA. 3
max
FL
(cm) #— — #
y
(W/cm2)
257,0
Regelung 1: Untersuchungstyp Beliebig — — — — —
Regelung 2: Farb-Optionen Beliebig — — — — —
Regelung 3: Tiefe 4,9 — — — — —
Regelung 4: PRF Beliebig — — — — —
Betriebs-
regelungs-
Regelung 5: Position/Größe des
bedingungen
Farbbereichs
Beliebig — — — — —
(a) Dieser Index ist für diesen Betriebsmodus nicht erforderlich. Der Wert ist <1.
(b) Dieser Schallkopf ist nicht für den transkraniellen Einsatz und für Schädeluntersuchungen bei Neugeborenen
vorgesehen.
# Für diese Betriebsbedingung liegen keine Daten vor, da aus dem angegebenen Grund kein globaler Maximalindexwert
vorliegt. (Siehe Zeile „Globaler maximaler Indexwert“.)
— Daten für diesen Schallkopf/Betriebsmodus nicht zutreffend.
19
Page 54

Tabelle 17: Schallkopfmodell: L52e/10-5 Betriebsmodus: PW-Doppler
TIW TIK
Index-Bezeichnung MI
Globaler maximaler Indexwert 1,2
p
r.3
W
0
Min. von [W.
z
1
Z
bp
Z
sp
Assoziierter
deq(Zsp)(cm) 0,358
Akustikparameter
F
c
Dim von A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2,31
(mW)
] (mW) 70,59
(cm) 1,7
(cm) 1,7
(cm) 1,6 2,0
(MHz) 3,87
X(cm)
Y(cm)
PD (µs) 1,14
PRF (Hz) 1008
pr@PII
deq@Pll
max
max
(MPa) 2,861
(cm) 0,302
Fokuslänge FLx (cm)
Zusätzliche
Informationen
I
@MI
PA. 3
max
FL
(cm)
y
(W/cm2)
319,6
Regelung 1: Untersuchungstyp Beliebig Beliebig GBH
Regelung 2: PRF 1008 Hz >1563 Hz <3125 Hz
Regelung 3: SV-Größe 1 mm 1 mm 2 mm
Betriebs-
Regelung 4: SV-Position Zone 3 Zone 7 Zone 6
regelungs-
bedingungen
Scan
Non-scan
≤1A
A
aprt
——
——
——
——
——
——
——
Non-scan
TIC
>1
aprt
1,3 2,2 (b)
61,29 #
3,84 3,85 #
1,886 1,23 #
0,55 0,55 #
7,38 #
3,0 #
(a) Dieser Index ist für diesen Betriebsmodus nicht erforderlich. Der Wert ist <1.
(b) Dieser Schallkopf ist nicht für den transkraniellen Einsatz und für Schädeluntersuchungen bei Neugeborenen
vorgesehen.
# Für diese Betriebsbedingung liegen keine Daten vor, da aus dem angegebenen Grund kein globaler Maximalindexwert
vorliegt. (Siehe Zeile „Globaler maximaler Indexwert“.)
— Daten für diesen Schallkopf/Betriebsmodus nicht zutreffend.
20
Page 55

Fehlersuche und Wartung
Reinigung und Desinfektion der Schallköpfe
Um den Schallkopf zu desinfizieren, wird er in eine Reinigungslösung eingetaucht oder damit
abgewischt. Die jeweils verwendete Reinigungsmethode muss für das eingesetzte Desinfektionsmittel geeignet sein. Beachten Sie bitte die Produktkennzeichnung.
Weitere Informationen zur Reinigung und Desinfektion des L52-Schallkopfs sind dem
Ultraschallsystem-Benutzerhandbuch zu entnehmen.
Die folgende Tabelle enthält eine Übersicht über die von SonoSite getesteten Desinfektionsmittel.
Die aktuellsten Informationen zur Reinigung und Desinfektion finden Sie auf der SonoSite-Website:
www.sonosite.com.
In Tab el le 1 8 sind die folgenden rechtlichen Informationen über die Zulassung von
Desinfektionsmitteln nicht enthalten:
• EPA-Registrierung
• Zulassung nach FDA 510(k) (flüssiges Sterilisationsmittel oder starkes
Desinfektionsmittel)
•CE-Zulassung
Vor der Verwendung ist sicherzustellen, dass der Zulassungsstatus des
Desinfektionsmittels den Gesetzen Ihres Landes entspricht und für den
Verwendungszweck geeignet ist.
English Deutsch Español Français Italiano Português
Tabelle 18: Kompatibilität der Desinfektionsmittel mit dem Schallkopf der L52-Serie
Desinfektions- und
Reinigungslösung
AbcoCide 14 USA Flüssigkeit Glutaraldehyd
Accel Wipes Kanada Wischtuch Wasserstoffperoxid
Aidal Plus AUS Flüssigkeit Glutaraldehyd
Airkem A-33 USA Flüssigkeit Quartäres Ammoniak
Alkacide FRA Flüssigkeit Glutaraldehyd
Alkazyme FRA Flüssigkeit Quartäres Ammoniak
Anios Wipes FRA Wischtuch Quartäres Ammoniak,
Aquatabs (1000) IRL Tablette Natriumdichlorisocyanurat
Aquatabs (2000) IRL Tablette Natriumdichlorisocyanurat
Herkunftsland
Typ Wi rks toff
Isopropylalkohol
L52x
L52n
L52
L52e
21
Page 56

Tabelle 18: Kompatibilität der Desinfektionsmittel mit dem Schallkopf der L52-Serie (Forts.)
Desinfektions- und
Reinigungslösung
Herkunftsland
Typ Wir kst off
L52x
L52n
L52
L52e
Ascend USA Flüssigkeit Quartäres Ammoniak
Asepti-HB USA Flüssigkeit Quartäres Ammoniak
Asepti-Steryl USA Spray Ethylalkohol
Asepti-Wipes USA Wischtuch Isopropylalkohol
Bacillocid rasant DEU Flüssigkeit Gluteraldehyd/Quartäres
Ammoniak
Bacoban DEU Flüssigkeit Ethanol, Isopropanol
Bacoban WB DEU Flüssigkeit Benzalkoniumchlorid-
Diethylenglycol
Banicide USA Flüssigkeit Glutaraldehyd
Bleiche USA Flüssigkeit Natriumhypochlorit
Cavicide USA Flüssigkeit Isopropylalkohol
CaviWipes USA Wischtuch Isopropylalkohol
Chlor-Clean GBR Flüssigkeit Natriumdichlorisocyanurat
Cidalkan Lingettes FRA Wischtuch Ethylalkohol
Cidex 14 USA Flüssigkeit Glutaraldehyd
Cidex OPA USA Flüssigkeit Ortho-Phthalaldehyd
Cidex Plus USA Flüssigkeit Glutaraldehyd
Cleanisept Wipes DEU Wischtuch Alkylammoniumchlorid
Clorox Disinfecting
USA Wischtuch Isopropylalkohol
Wipes
Control III USA Flüssigkeit Quartäres Ammoniak
Coverage Spray USA Spray Quartäres Ammoniak
Coverage™ Plus Wipes USA Wischtuch Quartäres Ammoniak
Coverage™ Wipes USA Wischtuch Quartäres Ammoniak
DentaSept FRA Flüssigkeit Quartäres Ammoniak
22
Page 57

Tabelle 18: Kompatibilität der Desinfektionsmittel mit dem Schallkopf der L52-Serie (Forts.)
English Deutsch Español Français Italiano Português
Desinfektions- und
Reinigungslösung
Herkunftsland
Typ Wi rks toff
L52x
L52n
L52
L52e
DisCide Wipes USA Wischtuch Isopropylalkohol
DisOPA JPN Flüssigkeit Ortho-Phthalaldehyd
Dispatch USA Spray Natriumhypochlorit
Dynacide PA FRA Flüssigkeit Peressigsäure
End-Bac II USA Flüssigkeit Quartäres Ammoniak
Endosporine FRA Flüssigkeit Glutaraldehyd
Endozime AW Plus FRA Flüssigkeit Isopropylalkohol
Envirocide USA Flüssigkeit Isopropylalkohol
Enzol USA Reinigungs-
Ethylenglykol
mittel
Ethylalkohol USA Flüssigkeit Ethylalkohol
Expose USA Flüssigkeit Isopropylalkohol
Gigasept AF DEU Flüssigkeit Quartäres Ammoniak
Gigasept FF DEU Flüssigkeit Succinylsäure
Gluteraldehyd SDS USA Flüssigkeit Glutaraldehyd
Hexanios FRA Flüssigkeit Polyhexanid/Quartäres
Ammoniak
Hi Tor Plus USA Flüssigkeit Chlorid
Hibiclens USA Reinigungs-
Chlorhexidin
mittel
Incidin Plus 1%
DEU Flüssigkeit Glucoprotamin,
Isopropylalkohol
Incidin Plus 3% DEU Flüssigkeit Glucoprotamin,
Isopropylalkohol
Kodan Tücher DEU Spray Propanol/Alkohol
Kohrsolin ff DEU Flüssigkeit Glutaraldehyd
Korsolex basic DEU Flüssigkeit Glutaraldehyd
23
Page 58

Tabelle 18: Kompatibilität der Desinfektionsmittel mit dem Schallkopf der L52-Serie (Forts.)
Desinfektions- und
Reinigungslösung
Herkunftsland
Typ Wir kst off
L52x
L52n
L52
L52e
Korsolex extra DEU Flüssigkeit Ethanol/Propanol
LpHse USA Flüssigkeit O-Phenylphenol
Lysol IC USA Flüssigkeit O-Phenylphenol
Madacide 1 USA Flüssigkeit Alkylammoniumchlorid
Matar USA Flüssigkeit O-Phenylphenol
MetriCide 14 USA Flüssigkeit Glutaraldehyd
MetriCide 28 USA Flüssigkeit Glutaraldehyd
MetriCide OPA Plus USA Flüssigkeit Ortho-Phthalaldehyd
MetriZyme USA Reinigungs-
Propylenglykol
mittel
Mikrobak forte DEU Flüssigkeit Ammoniumchlorid
Mikrozid DEU Wischtuch Ethanol/Propanol
Nuclean FRA Spray Alkohol/Biguanid
PerCept RTU Wipes™ Kanada Wischtuch Wasserstoffperoxid
Rely+On™ PeraSafe™ GBR Flüssigkeit Peressigsäure
Ruthless USA Spray Quartäres Ammoniak
Sagrosept DEU Wischtuch Isopropylalkohol
Salvanios pH 7 FRA Flüssigkeit Quartäres Ammoniak
Sani-Cloth HB USA Wischtuch Quartäres Ammoniak
Sani-Cloth Plus USA Wischtuch Quartäres Ammoniak
Sekusept DEU Flüssigkeit Glutaraldehyd
Sklar (4) USA Flüssigkeit Isopropylalkohol
Sporicidin USA Wischtuch Phenol
Sporicidin USA Flüssigkeit Phenol
Staphene USA Spray Ethylalkohol
24
Page 59

Tabelle 18: Kompatibilität der Desinfektionsmittel mit dem Schallkopf der L52-Serie (Forts.)
English Deutsch Español Français Italiano Português
Desinfektions- und
Reinigungslösung
Herkunftsland
Typ Wi rks toff
L52x
L52n
L52
L52e
Steranios 2% FRA Flüssigkeit Glutaraldehyd
Steranios 20% FRA Flüssigkeit Glutaraldehyd
Super Sani-Cloth USA Wischtuch Isopropylalkohol
T-Spray USA Spray Quartäres Ammoniak
T-Spray II USA Spray Alkyl/Chlorid
Task 105 USA Spray Quartäres Ammoniak
TBQ USA Flüssigkeit Alkylammoniumchlorid
Theracide Plus USA Flüssigkeit Quartäres Ammoniak
Tor USA Flüssigkeit Quartäres Ammoniak
Trigene Advance
Wipes
Wischtuch Quartäres Ammoniak,
Polymerisches
Biguanid-Hydrochlorid
Tristel GBR Flüssigkeit Chlordioxid
Tristel Solo GBR Schaum Hexamethylenbiguanid
Tristel Wipes GBR Wischtuch Chlordioxid
Vesphene IIse USA Flüssigkeit Natrium/o-Phenylphenol
Virex 256 USA Flüssigkeit Ammoniumchlorid
Virex TB USA Flüssigkeit Quartäres Ammoniak
Virox 5 Kanada Wischtuch Wasserstoffperoxid
Virufen FRA Flüssigkeit Alkylammoniumchlorid
Wasserstoffperoxid n.z. Flüssigkeit Wasserstoffperoxid (3%)
Wavicide -06 USA Flüssigkeit Glutaraldehyd
Wet-Wipe Disinfection DNK Wischtuch Guanidiniumchlorid
Wex-Cide USA Flüssigkeit O-Phenylphenol
= Zugelassen
25
Page 60

26
Page 61

Transductor L52 Series
Manual para el usuario
Page 62

Fabricado por
SonoSite, Inc.
21919 30th Drive SE
Bothell, WA 98021
EE. UU.
Tel.: +1-888-482-9449 o +1-425-951-1200
Fax: +1-425-951-1201
SonoSite Ltd
Alexander House
40A Wilbury Way
Hitchin
Herts SG4 OAP
Reino Unido
Tel.: +44-1462-444800
Fax: +44-1462-444801
Atención:
La ley federal de Estados Unidos permite la venta de este dispositivo únicamente a
veterinarios o bajo prescripción facultativa.
180PLUS, M-Turbo, MicroMaxx, NanoMaxx, S Series, SonoSite, SonoSite TITAN, TITAN y el logotipo de SonoSite son marcas comerciales
registradas (en algunas jurisdicciones) o marcas comerciales no registradas propiedad de SonoSite, Inc.
Los nombres de productos ajenos a SonoSite pueden ser marcas comerciales o registradas de sus respectivos propietarios.
Los sistemas de ecografía SonoSite a los que se hace referencia en este documento pueden estar protegidos por una o varias de las
siguientes patentes de EE. UU.: 5722412, 5817024, 5893363, 6135961, 6203498, 6364839, 6371918, 6383139, 6416475, 6447451, 6471651,
6569101, 6648826, 6575908, 6604630, 6817982, 6835177, 6962566, 7169108, 7449640, 7534211, 7549961, 7588541, 7591786, 7604596,
7643040, 7686766, 7694814, 7727153, 7740586, 7804970, 7809400, 7819807, 7841575, 7849250, 7867168, 7883276, D456509, D461895,
D509900, D538432, D544962, D558351, D559390, D591423, D592750, D592760, D625014, D625015 y por las siguientes patentes
correspondientes en otros países: AU727381, AU730822, CA2372152, CA2372158, CA2373065, CN ZL 97113678.5, CN ZL 98106133.8,
CN ZL 98108973.9, CN ZL 200830007734.8, DE60021552.0, DE60029777.2, DE60034670.6, DE69730563.5, DE6980539.6, DE69831698.3,
DE60 2004 23 816.3-08, FR0815793, FR0875203, FR0881492, FR1175713, FR1180970, FR1589878, GB0875203, GB0881492, GB1175713,
GB1180970, GB1180971, GB1589878, IT0815793, IT0881492, IT1175713, IT1589878, KR528102, KR532359, NO326202, NO326814,
NZ542968, RCD000897368-0001, SP0815793, SP0881492, SP1589878. Existen patentes pendientes de confirmación.
P07895-06 03/2011
Copyright 2011 de SonoSite, Inc.
Reservados todos los derechos. Impreso en Estados Unidos.
ii
Page 63

Manual para el usuario del transductor L52 Series
Introducción ................................................................................................................................................1
Modo de imagen ........................................................................................................................................1
Mediciones y cálculos ..............................................................................................................................4
Seguridad .....................................................................................................................................................5
Solución de problemas y mantenimiento ..................................................................................... 21
Introducción
Este manual para el usuario suplementa a los siguientes manuales para el usuario de sistema de
ecografía:
• Manual para el usuario del sistema de ecografía NanoMaxx
• Manual para el usuario del sistema de ecografía M-Turbo
• Manual para el usuario del sistema de ecografía S Series
• Manual para el usuario del sistema de ecografía MicroMaxx
• Manual para el usuario del sistema de ecografía TITAN
• Manual para el usuario del sistema de ecografía SonoSite
Describe los siguientes transductores, que están destinados exclusivamente para uso veterinario:
• Transductor L52n/10-5 MHz (L52n) del sistema de ecografía NanoMaxx®
• Transductor L52x/10-5 MHz (L52x) de los sistemas de ecografía M-Turbo® y S Series™ o sistema
de ecografía MicroMaxx®.
• Transductor L52e/10-5 MHz (L52e) del sistema de ecografía MicroMaxx®
• Transductor L52/10-5 MHz (L52) del sistema de ecografía de alta resolución TITAN® o el sistema
de ecografía SonoSite®180PLUS™
En el manual para el usuario del sistema de ecografía encontrará información adicional sobre
seguridad, instrucciones relativas a la preparación, el uso y el mantenimiento del sistema de
ecografía, y sobre los usos previstos de cada tipo de examen y modo de imagen.
English Deutsch Español Français Italiano Português
Modo de imagen
Transductor, tipo de examen y modo de imagen
La tabla siguiente describe el transductor, el tipo de examen, el modo de imagen y la optimización
disponibles en el sistema.
1
Page 64

Transductor, tipo de examen y modo de images (NanoMaxx)
Modo de imagen
Transductor Tipo de examen
2D
Modo M
CPD Color
L52n OB X X X
Vascular (Vas) X X X
Musculoesquelético (Mus) X X X
.
Transductor, tipo de examen y modo de imagen (M-Turbo o MicroMaxx)
Modo de imagen
Tra nsd uc tor
Tipo de
examen
2D
Modo M
CPD Color DP OC
L52x OB X X X X —
Vascular (Vas) X X X X —
Músculo (Mus) X X X X —
Transductor, tipo de examen y modo de imagen (S Series)
Modo de imagen
Tra nsd uc tor
Tipo de
examen
2D
Modo M
CPD Color DP
L52x OB X X X X
Vascular (Vas) X X X X
Músculo (Mus) X X X X
2
Page 65

Transductor, tipo de examen y modo de imagen (MicroMaxx)
Modo de imagen
English Deutsch Español Français Italiano Português
Tra nsd uc tor
Tipo de
examen2DModo M
THI
2DMB2D
L
CPD Color DP
DTI
DP
OC
L52e OB X —XX X X X——
Vascular
X —XX X X X——
(Vas)
Músculo
X —XX X X X——
(Mus)
Transductor, tipo de examen y modo de imagen (TITAN)
Modo de imagen
Trans duc tor
Tipo de
examen
2D THI CPD DCPD Color Modo M DP OC
L52 OB X — X — — X — —
Vascular
X—X — — X ——
(Vas)
Músculo
X—X — — X ——
(Mus)
Transductor, tipo de examen y modo de imagen (180PLUS)
Modo de imagen
Transductor Tipo de examen 2D CPD
L52 OB res, gen, pen bajo, medio, alto
Vascular (Vas) res, gen, pen bajo, medio, alto
3
Page 66

Mediciones y cálculos
Cálculos
Esta tabla muestra los cálculos disponibles por tipo de examen para el transductor L52.
Cálculos para L52n (NanoMaxx)
Tipo de examen Cálculos
OB OB
Cálculos para L52x (M-Turbo y MicroMaxx)
Tipo de examen Cálculos
Musculoesquelético (Mus) Reducción porcentual
OB OB
Vascular (Vas) Reducción porcentual
Volumen
Vascular
Volumen
Flujo de volumen
Cálculos para L52x (S Series)
Tipo de examen Sistema S Series Cálculos
OB S-VetMed OB
Cálculos para L52e/L52 (MicroMaxx y TITAN)
Tipo de examen Cálculos
Músculo (Mus) Reducción porcentual
Volumen
OB OB
4
Page 67

Cálculos para L52e/L52(Continuación) (MicroMaxx y TITAN)
Tipo de examen Cálculos
Vascular (Vas) Reducción porcentual
Cálculos para L52 (180PLUS)
Tipo de examen Cálculos
OB OB
Vascular (Vas) Volumen
Seguridad
English Deutsch Español Français Italiano Português
Vascular
Volumen
Flujo de volumen
Flujo de volumen
Directrices para reducir el índice mecánico y el índice térmico
A continuación se detallan recomendaciones generales para reducir el índice mecánico o el índice
térmico. Si se proporcionan múltiples parámetros, es posible obtener resultados óptimos al llevar al
mínimo dichos parámetros simultáneamente. En determinados modos, la modificación de dichos
parámetros no afecta al índice mecánico ni al índice térmico. Los cambios en otros parámetros
también pueden causar reducciones en el índice mecánico y en el índice térmico. Observe los valores
de MI e TI en el lado derecho de la pantalla.
Tabla 1: MI
Transductor Profundidad
L52n (NanoMaxx) ↑
L52x (M-Turbo, S Series o MicroMaxx) ↑
L52e (MicroMaxx) ↑
L52 (TITAN) ↑
L52 (180PLUS) ↑
↓ Disminuir o bajar el ajuste del parámetro para reducir el MI.
↑ Aumentar o subir el ajuste del parámetro para reducir el MI.
5
Page 68

Tabla 2: TI (TIS, TIC, TIB)
Tra nsd uc tor
Ajustes del modo Doppler de
potencia en color
Ajustes del
modo DP
FRI Profundidad
L52n (NanoMaxx) — ↑ —
L52x (M-Turbo o MicroMaxx) ↓↑Vol. de
muestra ↓
L52x (S Series) ↓↑—
L52e (MicroMaxx) ↓↑Vol. de
muestra ↑
L52 (TITAN) ↓↑—
L52 (180PLUS) — — —
↓ Disminuir o bajar el ajuste del parámetro para reducir el TI.
↑ Aumentar o subir el ajuste del parámetro para reducir el TI.
— Los datos no son aplicables a este transductor/modo.
Aumento de temperatura en la superficie de los transductores
En la Tab la 3 aparece el aumento de la temperatura medido en la superficie con respecto a la
temperatura ambiente (23 °C ± 3 °C) de los transductores utilizados en el sistema de ecografía.
Las temperaturas fueron medidas según el apartado 42 de la norma EN 60601-2-37, para lo cual
se han ajustado los controles y los parámetros para producir las temperaturas máximas.
Tabla 3: Aumento de temperatura en la superficie de los transductores según la norma
IEC 60601-2-37 (uso interno)
Método de
TITAN
L52n en
NanoMaxx
Aire en reposo
Uso simulado
6
7,5 8,8 8,2 13,0 9,3 10,8
5,6 5,9 5,6 5,5 2,4 2,4
L52x en
M-Turbo
o S Series
L52x en
MicroMaxx
L52e en
MicroMaxx
L52 en
L52 en
180PLUS
Page 69

Lectura de salida
Tabla 4: TI o MI es ≥ 1,0
English Deutsch Español Français Italiano Português
Modelo de transductor Índice
2D/
Modo M
Color CPD
Doppler
DP
L52n (NanoMaxx) MI Sí Sí Sí n/a
TI No No No n/a
L52x (M-Turbo) MISíSíSíSí
TI No No No Sí
L52x (SSeries) MISíSíSí—
TI No No No —
L52x (MicroMaxx) MI Sí Sí Sí Sí
TI Sí No No Sí
L52e (MicroMaxx)MINoSíSíSí
TI No No No Sí
L52 (TITAN, 180PLUS)*MINoNoNoNo
TI No No No No
* El transductor L52 nunca excede ni iguala un MI o TI de 1,0 en el sistema TITAN o 180PLUS.
7
Page 70

Tablas de emisión acústica (M-Turbo y S Series)
Tabla 5: Modelo de transductor: L52x/10-5 Modo de funcionamiento: 2D
TIS TIB
Etiqueta de índice M.I.
Explora-
ción
Sin exploración Sin
explora-
A
≤1A
aprt
aprt
>1
ción
TIC
Valor global de índice máximo 1,01 (a) — — — (a)
p
r.3
W
0
min av [W.3(z1),I
z
1
z
bp
z
sp
asociado
deq(zsp)(cm)
Parámetro acústico
f
c
Dim de A
aprt
TA. 3(z1
)] (mW)
(MPa)
(mW)
2,336
#— —#
—
(cm)
(cm)
(cm)
1,8
—
—
—
—
(MHz)
5,33 # — — — #
X(cm) #—— — #
Y(cm)
#—— — #
PD (µs) 0,15
FRI (Hz) 7222
pr@PII
máx
deq@Pll
Otra
Distancia focal FLx (cm)
máx
información
@MI
I
PA .3
máx
(MPa)
(cm)
(cm)
FL
y
(W/cm2)
3,25
—
#—— #
#—— #
329,1
Control 1: Tipo de examen Vas — — — — —
Control 2: Optimización Gen — — — — —
Control 3: Profundidad 4,2 cm — — — — —
Control 4: MB (multihaz)
Condiciones
de los controles
de funcionamiento
Desacti-
vado
—— — — —
(a) Este índice no es necesario para este modo de funcionamiento; el valor es <1.
(b) Este transductor no está previsto para usos cefálicos neonatales o transcraneales.
# No se han descrito datos para estas condiciones de funcionamiento, dado que no se ha indicado el valor global de índice
máximo por el motivo mostrado. (Línea del valor global de índice máximo de referencia.)
— Los datos no son aplicables a este transductor/modo.
8
Page 71

Tabla 6: Modelo de transductor: L52x/10-5 (solo M-Turbo) Modo de funcionamiento: Modo M
TIS TIB
English Deutsch Español Français Italiano Português
Etiqueta de índice M.I.
Explora-
ción
Sin exploración Sin
explora-
A
aprt
≤1A
aprt
>1
ción
TIC
Valor global de índice máximo 1,01 — (a) — (a) (b)
p
r.3
W
0
min av [W
z
1
z
bp
z
sp
asociado
deq(zsp)(cm)
Parámetro acústico
f
c
Dim de A
.3(z1
aprt
),I
)] (mW)
TA. 3(z1
(MPa)
(mW)
2,336
—# ##
—
(cm)
(cm)
(cm)
1,8
—
—
#
#
(MHz)
5,33 — # — # #
X(cm) —#— # #
Y(cm)
—#— # #
PD (µs) 0,15
FRI (Hz) 1600
pr@PII
máx
deq@Pll
Otra
Distancia focal FLx (cm)
máx
información
I
@MI
PA .3
máx
(MPa)
(cm)
FLy (cm)
(W/cm2)
3,25
#
—#— #
—#— #
329,1
Control 1: Tipo de examen Vas — — — — —
Control 2: Optimización Gen — — — — —
Control 3: Profundidad 4,2 cm — — — — —
Control 4: MB (multihaz)
Condiciones
de los controles
de funcionamiento
Desacti-
vado
——— — —
(a) Este índice no es necesario para este modo de funcionamiento; el valor es <1.
(b) Este transductor no está previsto para usos cefálicos neonatales o transcraneales.
# No se han descrito datos para estas condiciones de funcionamiento, dado que no se ha indicado el valor global de índice
máximo por el motivo mostrado. (Línea del valor global de índice máximo de referencia.)
— Los datos no son aplicables a este transductor/modo.
9
Page 72

Tabla 7: Modelo de transductor: L52x/10-5 Modo de funcionamiento: Color/CPD
TIS TIB
Etiqueta de índice M.I.
Explora-
ción
Sin exploración Sin
explora-
A
≤1A
aprt
aprt
>1
ción
TIC
Valor global de índice máximo 1,33 (a) — — — (b)
p
r.3
W
0
mín. de [W.
z
1
Z
bp
Z
sp
asociado
deq(Zsp)(cm) —
Parámetro acústico
F
c
Dim de A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2,807
(mW) #— —#
] (mW) —
(cm) —
(cm) —
(cm) 1,8 —
(MHz) 4,37 # — — — #
X(cm) #———#
Y(cm)
#———#
PD (µs) 0,61
FRI (Hz) 5427
pr@PII
máx
deq@Pll
Otra
Distancia focal FLx (cm) #—— #
máx
información
@MI
I
PA. 3
máx
(MPa) 3,628
(cm) —
(cm) #—— #
FL
y
(W/cm2)
411,1
Control 1: Modo Cualquiera — — — — —
Control 2: Tipo de examen Vas — — — — —
Control 3: Optimización/
Profundidad
Cualquiera/
5,4
—————
Control 4: FRI Cualquiera — — — — —
Condiciones
Control 5: Posición/tamaño del
de los controles
de funcionamiento
cuadro Color
Cualquiera/
Def
—————
(a) Este índice no es necesario para este modo de funcionamiento; el valor es <1.
(b) Este transductor no está previsto para usos cefálicos neonatales o transcraneales.
# No se han descrito datos para estas condiciones de funcionamiento, dado que no se ha indicado el valor global de índice
máximo por el motivo mostrado. (Línea del valor global de índice máximo de referencia.)
— Los datos no son aplicables a este transductor/modo.
10
Page 73

Tabla 8: Modelo de transductor: L52x/10-5 (solo M-Turbo) Modo de funcionamiento: Doppler DP
TIS TIB
English Deutsch Español Français Italiano Português
Etiqueta de índice M.I.
Valor global de índice máximo 1,17
p
r.3
W
0
mín. de [W.
z
1
Z
bp
Z
sp
asociado
deq(Zsp)(cm) 0,45
Parámetro acústico
F
c
Dim de A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2,443
(mW)
] (mW) —
(cm) —
(cm) —
(cm) 2,1 1,5
(MHz) 4,36
X(cm)
Y(cm)
PD (µs) 1,38
FRI (Hz) 1008
pr@PII
máx
deq@Pll
Otra
Distancia focal FLx (cm)
máx
información
I
@MI
PA. 3
máx
(MPa) 3,30
(cm) 0,34
FL
(cm)
y
(W/cm2)
288,97
Control 1: Tipo de examen Cual-
quiera
Control 2: FRI 1008 Hz — 3125 Hz — 3125 Hz —
Control 3: Tamaño del VS 1 mm — 1 mm — 1 mm —
Control 4: Posición del VS Zona 4 — Zona 6 — Zona 6 —
Condiciones
de los controles
de funcionamiento
Explora-
ción
—
—
—
—
—
—
—
Sin exploración Sin
A
aprt
1,44 — 2,22 (b)
69,42 69,42 #
4,35 — 4,35 #
1,476 — 1,476 #
0,55 — 0,55 #
5,99 — #
3,4 — #
—Cual-
quiera
≤1A
aprt
—Cual-
>1
explora-
TIC
ción
—
quiera
(a) Este índice no es necesario para este modo de funcionamiento; el valor es <1.
(b) Este transductor no está previsto para usos cefálicos neonatales o transcraneales.
# No se han descrito datos para estas condiciones de funcionamiento, dado que no se ha indicado el valor global de índice
máximo por el motivo mostrado. (Línea del valor global de índice máximo de referencia.)
— Los datos no son aplicables a este transductor/modo.
11
Page 74

Tablas de emisión acústica (NanoMaxx)
Tabla 9: Modelo de transductor: L52n/10-5 Modo de funcionamiento: 2D
TIS TIB
Etiqueta de índice M.I.
Explora-
ción
Sin exploración Sin
explora-
A
≤1A
aprt
aprt
>1
ción
TIC
Valor global de índice máximo 1,0 (a) — — — (b)
p
r.3
W
0
min av [W
z
1
z
bp
z
sp
asociado
deq(zsp)(cm)
Parámetro acústico
f
c
Dim de A
.3(z1
aprt
),I
)] (mW)
TA. 3(z1
(MPa)
(mW)
2,34
#— —#
—
(cm)
(cm)
(cm)
1,8
—
—
—
—
(MHz)
5,33 # — — — #
X(cm) #—— —#
Y(cm)
#———#
PD (µs) 0,15
FRI (Hz) 7707
pr@PII
máx
deq@Pll
Otra
Distancia focal FLx (cm)
máx
información
I
@MI
PA. 3
máx
(MPa)
(cm)
FLy (cm)
(W/cm2)
3,25
—
#—— #
#—— #
329,1
Control 1: Tipo de examen Cual-
quiera
Control 2: Optimización Gen
Control 3: Profundidad 4,2 cm
Control 4: MB (multihaz)
Condiciones
de los controles
de funcionamiento
Off u
On
(a) Este índice no es necesario para este modo de funcionamiento; el valor es <1.
(b) Este transductor no está previsto para usos cefálicos neonatales o transcraneales.
# No se han descrito datos para estas condiciones de funcionamiento, dado que no se ha indicado el valor global de índice
máximo por el motivo mostrado. (Línea del valor global de índice máximo de referencia.)
— Los datos no son aplicables a este transductor/modo.
12
Page 75

Tabla 10: Modelo de transductor: L52n/10-5 Modo de funcionamiento: Modo M
TIS TIB
English Deutsch Español Français Italiano Português
Etiqueta de índice M.I.
Explora-
ción
Sin exploración Sin
explora-
A
≤1A
aprt
aprt
>1
ción
TIC
Valor global de índice máximo 1,0 — (a) — (a) (b)
p
r.3
W
0
min av [W.3(z1),I
z
1
z
bp
z
sp
asociado
deq(zsp)(cm)
Parámetro acústico
f
c
Dim de A
aprt
)] (mW)
TA. 3(z1
(MPa)
(mW)
2,34
—# ##
—
(cm)
(cm)
(cm)
1,8
—
—
#
#
(MHz)
5,33 — # — # #
X(cm) —#— # #
Y(cm)
—# — # #
PD (µs) 0,15
FRI (Hz) 1600
pr@PII
máx
deq@Pll
Otra
Distancia focal FLx (cm)
máx
información
@MI
I
PA. 3
máx
Control 1: Tipo de examen Cual-
(MPa)
(cm)
FLy (cm)
(W/cm2)
3,25
#
—# — #
—# — #
329,1
—— — ——
quiera
Control 2: Optimización Gen — — — — —
Control 3: Profundidad 4,2 cm — — — — —
Control 4: MB (multihaz)
Condiciones
de los controles
de funcionamiento
Off u
On
—— — ——
(a) Este índice no es necesario para este modo de funcionamiento; el valor es <1.
(b) Este transductor no está previsto para usos cefálicos neonatales o transcraneales.
# No se han descrito datos para estas condiciones de funcionamiento, dado que no se ha indicado el valor global de índice
máximo por el motivo mostrado. (Línea del valor global de índice máximo de referencia.)
— Los datos no son aplicables a este transductor/modo.
13
Page 76

Tabla 11: Modelo de transductor: L52n/10-5 Modo de funcionamiento: Color/CPD
TIs TIb
Etiqueta de índice M.I.
Explora-
ción
Sin exploración Sin
explora-
A
≤1A
aprt
aprt
>1
TIC
ción
Valor global de índice máximo 1,2 (a) — — — (b)
p
W
mín. de
[W.
z
Z
Z
asociado
r.3
0
3(z1),ITA. 3 (z1)
1
bp
sp
]
(MPa) 2,35
(mW) #— —#
(mW)
—
(cm) —
(cm) —
(cm) 1,8 —
deq(Zsp)(cm) —
Parámetro acústico
F
c
Dim de A
aprt
(MHz) 4,37 # — — — #
X (cm) #———#
Y (cm)
#———#
PD (µs) 0,60
FRI (Hz) 7097
pr@PII
máx
deq@Pll
Otra
Distancia focal FLx (cm) #—— #
máx
información
@MI
I
PA. 3
máx
Control 1: Modo CPD o en
(MPa) 3,08
(cm) —
FL
(cm) #—— #
y
(W/cm2)
308,5
—— — ——
Color
Control 2: Tipo de examen Mus — — — — —
Control 3: Optimización Res — — — — —
Control 4: Profundidad 5,4 — — — — —
Condiciones
Control 5: Cuadro Color Predeter-
de los controles
de funcionamiento
minado
—— — ——
(a) Este índice no es necesario para este modo de funcionamiento; el valor es <1.
(b) Este transductor no está previsto para usos cefálicos neonatales o transcraneales.
# No se han descrito datos para estas condiciones de funcionamiento, dado que no se ha indicado el valor global de índice
máximo por el motivo mostrado. (Línea del valor global de índice máximo de referencia.)
— Los datos no son aplicables a este transductor/modo.
14
Page 77

Tablas de potencia acústica (MicroMaxx)
Tabla 12: Modelo de transductor: L52x/10-5 Modo de funcionamiento: 2D
TIS TIB
English Deutsch Español Français Italiano Português
Etiqueta de índice M.I.
Explora-
ción
Valor global de índice máximo 1,0 (a)
p
r.3
W
0
mín. de [W.
z
1
Z
bp
Z
sp
asociado
deq(Zsp)(cm)
Parámetro acústico
F
c
Dim de A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2,23
(mW)
] (mW)
(cm)
(cm)
(cm) 1,9
(MHz) 5,42 #
X(cm) #
Y(cm)
#— —
#
PD (µs) 0,146
FRI (Hz) 8394
pr@PII
máx
deq@Pll
Otra
Distancia focal FLx (cm) #
máx
información
I
@MI
PA. 3
máx
(MPa) 3,19
(cm)
FL
(cm) #
y
(W/cm2)
325,3
Control 1: Tipo de examen OB — — — — —
Control 2: Optimización Gen — — — — —
Control 3: Profundidad 2,5 - 3,9 — — — — —
Control 4: MB On u Off — — — — —
Sin exploración Sin
explora-
A
≤1A
aprt
aprt
>1
ción
—— —
—
—
—
—
—
—— —
—— —
—— —
—
——
——
TIC
(b)
#
#
#
#
#
#
Condiciones
de los controles
de funcionamiento
(a) Este índice no es necesario para este modo de funcionamiento; el valor es <1.
(b) Este transductor no está previsto para usos cefálicos neonatales o transcraneales.
# No se han descrito datos para estas condiciones de funcionamiento, dado que no se ha indicado el valor global de índice
máximo por el motivo mostrado. (Línea del valor global de índice máximo de referencia.)
— Los datos no son aplicables a este transductor/modo.
15
Page 78

Tabla 13: Modelo de transductor: L52x/10-5 Modo de funcionamiento: Modo M
M.I.
Etiqueta de índice
Valor global de índice máximo 1,0
p
r.3
W
0
mín. de [W.
z
1
Z
bp
Z
sp
asociado
deq(Zsp)(cm) 0,659
Parámetro acústico
F
c
Dim de A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2,23
(mW)
] (mW)
(cm)
(cm)
(cm) 1,9 1,7
(MHz) 5,42
X(cm)
Y(cm)
PD (µs) 0,146
FRI (Hz) 1600
pr@PII
máx
deq@Pll
Otra
Distancia focal FLx (cm)
máx
información
I
@MI
PA. 3
máx
(MPa) 3,19
(cm) 0,641
FL
(cm)
y
(W/cm2)
325,3
Control 1: Tipo de examen OB — — — Cual-
Control 2: Optimización Gen — — — Pen —
Control 3: Profundidad 2,5 - 3,9 — — — 15 —
Explora-
ción
—
—
—
—
—
—
—
TIS TIB
Sin exploración Sin
explora-
A
≤1A
aprt
(a)
aprt
—
>1
ción
1,2 (b)
# 58,3 #
—
—
—
#
#
#
#
#
—
—
—
—
—
4,35 #
2,71 #
0,55 #
quiera
TIC
#
#
—
Condiciones
de los controles
de funcionamiento
(a) Este índice no es necesario para este modo de funcionamiento; el valor es <1.
(b) Este transductor no está previsto para usos cefálicos neonatales o transcraneales.
# No se han descrito datos para estas condiciones de funcionamiento, dado que no se ha indicado el valor global de índice
máximo por el motivo mostrado. (Línea del valor global de índice máximo de referencia.)
— Los datos no son aplicables a este transductor/modo.
16
Page 79

Tabla 14: Modelo de transductor: L52x/10-5 Modo de funcionamiento: Color/CPD
TIS TIB
English Deutsch Español Français Italiano Português
Etiqueta de índice M.I.
Explora-
ción
Valor global de índice máximo 1,3 (a)
p
r.3
W
0
mín. de [W.
z
1
Z
bp
Z
sp
asociado
deq(Zsp)(cm)
Parámetro acústico
F
c
Dim de A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2,70
(mW) #
] (mW)
(cm)
(cm)
(cm) 1,4
(MHz) 4,35 #
X(cm) #
Y(cm)
PD (µs) 0,607
FRI (Hz) 4169
pr@PII
máx
deq@Pll
Otra
Distancia focal FLx (cm) #
máx
información
I
@MI
PA. 3
máx
(MPa) 3,33
(cm)
FL
(cm) #
y
(W/cm2)
377,1
Control 1: Modo Color — — — — —
Control 2: Tipo de examen Cualquiera — — — — —
Control 3:
Optimización/Profundidad
Control 4: FRI <
Condiciones
Control 5: Posición/tamaño del
de los controles
de funcionamiento
cuadro Color
Bajo/
—— — ——
2,5 - 3,9
718 — — — — —
Cualquiera — — — — —
Sin exploración Sin
TIC
explora-
A
≤1A
aprt
———
— —
aprt
>1
ción
(b)
#
—
—
—
—
—
———
———
#
———
#
#
#
—
——
——
#
#
(a) Este índice no es necesario para este modo de funcionamiento; el valor es <1.
(b) Este transductor no está previsto para usos cefálicos neonatales o transcraneales.
# No se han descrito datos para estas condiciones de funcionamiento, dado que no se ha indicado el valor global de índice
máximo por el motivo mostrado. (Línea del valor global de índice máximo de referencia.)
— Los datos no son aplicables a este transductor/modo.
17
Page 80

Tabla 15: Modelo de transductor: L52x/10-5 Modo de funcionamiento: Doppler DP
TIS TIB
Etiqueta de índice M.I.
Explora-
ción
Sin exploración Sin
explora-
A
≤1A
aprt
aprt
>1
ción
TIC
Valor global de índice máximo 1,2 — 1,4 — 2,2 (b)
p
r.3
W
0
mín. de [W.
z
1
Z
bp
Z
sp
asociado
deq(Zsp)(cm) 0,19
Parámetro acústico
F
c
Dim de A
3(z1),ITA. 3 (z1)
aprt
] (mW) —
(MPa) 2,48
(mW) —68,5 37,5 #
(cm) —
(cm) —
(cm) 2,3 2,4
(MHz) 4,36 — 4,35 — 4,36 #
X(cm) — 2,05 — 0,90 #
Y(cm)
— 0,55 — 0,55 #
PD (µs) 1,39
FRI (Hz) 1008
pr@PII
máx
deq@Pll
Otra
Distancia focal FLx (cm) —8,32 — #
máx
información
@MI
I
PA. 3
máx
Control 1: Tipo de examen Cual-
(MPa) 3,505
(cm) 0,18
(cm) —3,5— #
FL
y
(W/cm2)
284,3
quiera
—Cual-
quiera
—Cual-
quiera
—
Control 2: Volumen de muestra 1 mm — 2 mm — 12 mm —
Control 3: FRI 1.008 Hz — Cual-
— 10.417 Hz —
quiera
Condiciones
Control 4: Posición de volumen de
de los controles
de funcionamiento
muestra
Zona 3 — Zona 7 — Zona 3 —
(a) Este índice no es necesario para este modo de funcionamiento; el valor es <1.
(b) Este transductor no está previsto para usos cefálicos neonatales o transcraneales.
# No se han descrito datos para estas condiciones de funcionamiento, dado que no se ha indicado el valor global de índice
máximo por el motivo mostrado. (Línea del valor global de índice máximo de referencia.)
— Los datos no son aplicables a este transductor/modo.
18
Page 81

Tabla 16: Modelo de transductor: L52e/10-5 Modo de funcionamiento: Color/CPD
TIS TIB
English Deutsch Español Français Italiano Português
Etiqueta de índice M.I.
Explora-
ción
Sin exploración Sin
explora-
A
aprt
≤1A
aprt
>1
ción
TIC
Valor global de índice máximo 1,2 (a) — — — (b)
p
r.3
W
0
mín. de [W.
z
1
Z
bp
Z
sp
asociado
deq(Zsp)(cm) —
Parámetro acústico
F
c
Dim de A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2,30
(mW) #— —#
] (mW) —
(cm) —
(cm) —
(cm) 1,6 —
(MHz) 3,92 # — — — #
X(cm) #———#
Y(cm)
#———#
PD (µs) 0,797
FRI (Hz) 5.332
pr@PII
máx
deq@Pll
Otra
Distancia focal FLx (cm) #—— #
máx
información
@MI
I
PA. 3
máx
(MPa) 2,85
(cm) —
(cm) #—— #
FL
y
(W/cm2)
257,0
Control 1: Tipo de examen Cualquiera — — — — —
Control 2: Opción de color Cualquiera — — — — —
Control 3: Profundidad 4,9 — — — — —
Control 4: FRI Cualquiera — — — — —
Control 5: Posición/tamaño del
Condiciones
de los controles
cuadro Color
de funcionamiento
Cualquiera — — — — —
(a) Este índice no es necesario para este modo de funcionamiento; el valor es <1.
(b) Este transductor no está previsto para usos cefálicos neonatales o transcraneales.
# No se han descrito datos para estas condiciones de funcionamiento, dado que no se ha indicado el valor global de índice
máximo por el motivo mostrado. (Línea del valor global de índice máximo de referencia.)
— Los datos no son aplicables a este transductor/modo.
19
Page 82

Tabla 17: Modelo de transductor: L52e/10-5 Modo de funcionamiento: Doppler DP
TIS TIB
Etiqueta de índice M.I.
Valor global de índice máximo 1,2
p
r.3
W
0
mín. de [W.
z
1
Z
bp
Z
sp
asociado
deq(Zsp)(cm) 0,358
Parámetro acústico
F
c
Dim de A
3(z1),ITA. 3 (z1)
aprt
(MPa) 2,31
(mW)
] (mW) 70,59
(cm) 1,7
(cm) 1,7
(cm) 1,6 2,0
(MHz) 3,87
X(cm)
Y(cm)
PD (µs) 1,14
FRI (Hz) 1008
pr@PII
máx
deq@Pll
Otra
Distancia focal FLx (cm)
máx
información
I
@MI
PA. 3
máx
(MPa) 2,861
(cm) 0,302
FL
(cm)
y
(W/cm2)
319,6
Control 1: Tipo de examen Cual-
quiera
Control 2: FRI 1008 Hz >1563 Hz <3125 Hz
Control 3: Tamaño del VS 1 mm 1 mm 2 mm
Control 4: Posición del VS Zona 3 Zona 7 Zona 6
Condiciones
de los controles
de funcionamiento
Explora-
ción
Sin exploración Sin
A
aprt
——
——
——
——
——
——
——
≤1A
aprt
>1
1,3 2,2 (b)
3,84 3,85 #
1,886 1,23 #
0,55 0,55 #
7,38 #
3,0 #
Cual-
quiera
explora-
TIC
ción
61,29 #
OB
(a) Este índice no es necesario para este modo de funcionamiento; el valor es <1.
(b) Este transductor no está previsto para usos cefálicos neonatales o transcraneales.
# No se han descrito datos para estas condiciones de funcionamiento, dado que no se ha indicado el valor global de índice
máximo por el motivo mostrado. (Línea del valor global de índice máximo de referencia.)
— Los datos no son aplicables a este transductor/modo.
20
Page 83

Solución de problemas y mantenimiento
Limpieza y desinfección de los transductores
Para desinfectar el transductor puede utilizar un método de inmersión o un método de limpieza con
un paño. Sólo puede utilizar el método de inmersión si el desinfectante es compatible con él.
Compruebe la etiqueta del producto.
Para obtener más información acerca de la limpieza y desinfección del transductor L52, consulte el
manual para el usuario del sistema de ecografía.
La tabla siguiente ofrece una lista de los desinfectantes probados por SonoSite. Visite el sitio web de
SonoSite para obtener información actualizada sobre limpieza y desinfección: www.sonosite.com.
En la Tab la 18 no se incluye la siguiente información normativa para desinfectantes:
•Registro EPA
• Autorización de la FDA 510(k) (esterilizante líquido, desinfectante de calidad alta)
•Aprobación de la CE
Antes de utilizar el producto, confirme que su estado normativo sea adecuado para
su jurisdicción y uso.
Tabla 18: Compatibilidad de los desinfectantes con el transductor L52 Series
English Deutsch Español Français Italiano Português
Soluciones de
desinfección y
limpieza
AbcoCide 14 EE. UU. Líquido Glutaraldehído
Accel Wipes CAN Toallita Peróxido de hidrógeno
Aidal Plus AUS Líquido Glutaraldehído
Airkem A-33 EE. UU. Líquido Amonio cuaternario
Alcohol, etílico EE. UU. Líquido Alcohol etílico
Alkacide FRA Líquido Glutaraldehído
Alkazyme FRA Líquido Amonio cuaternario
Anios Wipes FRA Toallitas Amonio cuaternario, alcohol
Aquatabs (1000) IRL Tableta Dicloroisocianurato de sodio
Aquatabs (2000) IRL Tableta Dicloroisocianurato de sodio
Ascend EE. UU. Líquido Amonio cuaternario
País de
origen
Tipo Ingrediente activo
isopropílico
L52x
L52n
L52
L52e
21
Page 84

Tabla 18: Compatibilidad de los desinfectantes con el transductor L52 Series (Continuación)
Soluciones de
desinfección y
limpieza
País de
origen
Tipo Ingrediente activo
L52x
L52n
L52
L52e
Asepti-HB EE. UU. Líquido Amonio cuaternario
Asepti-Steryl EE. UU. Rociador Alcohol etílico
Asepti-Wipes EE. UU. Toallita Alcohol isopropílico
Bacillocid rasant DEU Líquido Glutaraldehído/Amonio
cuaternario
Bacoban DEU Líquido Etanol isopropanol
Bacoban WB DEU Líquido Cloruro de benzalconio
Dietilenglicol
Banicide EE. UU. Líquido Glutaraldehído
Cavicide EE. UU. Líquido Alcohol isopropílico
CaviWipes EE. UU. Toallitas Alcohol isopropílico
Chlor-Clean GBR Líquido Dicloroisocianurato de sodio
Cidalkan Lingettes FRA Toallitas Alcohol etílico
Cidex 14 EE. UU. Líquido Glutaraldehído
Cidex OPA EE. UU. Líquido Ortoftalaldehído
Cidex Plus EE. UU. Líquido Glutaraldehído
Cleanisept Wipes DEU Toallitas Cloruro de amonio-alquilo
Clorox Disinfecting
EE. UU. Toallita Alcohol isopropílico
Wipes
Control III EE. UU. Líquido Amonio cuaternario
Coverage Spray EE. UU. Rociador Amonio cuaternario
Coverage™ Plus Wipes EE. UU. Toallitas Amonio cuaternario
Coverage™ Wipes EE. UU. Toallitas Amonio cuaternario
DentaSept FRA Líquido Amonio cuaternario
Desinfección con paño
DNK Toallita Cloruro de guanidinio
húmedo
22
Page 85

Tabla 18: Compatibilidad de los desinfectantes con el transductor L52 Series (Continuación)
English Deutsch Español Français Italiano Português
Soluciones de
desinfección y
limpieza
País de
origen
Tipo Ingrediente activo
L52x
L52n
L52
L52e
DisCide Wipes EE. UU. Toallita Alcohol isopropílico
DisOPA JPN Líquido Ortoftalaldehído
Dispatch EE. UU. Rociador Hipoclorito de sodio
Dynacide PA FRA Líquido Ácido peracético
End-Bac II EE. UU. Líquido Amonio cuaternario
Endosporine FRA Líquido Glutaraldehído
Endozime AW Plus FRA Líquido Alcohol isopropílico
Envirocide EE. UU. Líquido Alcohol isopropílico
Enzol EE. UU. Limpiador Etilenglicol
Expose EE. UU. Líquido Alcohol isopropílico
Gigasept AF DEU Líquido Amonio cuaternario
Gigasept FF DEU Líquido Ácido succínico
Gluteraldehyde SDS EE. UU. Líquido Glutaraldehído
Hexanios FRA Líquido Polihexanida/Amonio
cuaternario
Hi Tor Plus EE. UU. Líquido Cloruro
Hibiclens EE. UU. Limpiador Clorhexidina
Incidin Plus 1%
DEU Líquido Glucoprotamin, alcohol
isopropílico
Incidin Plus 3% DEU Líquido Glucoprotamin, alcohol
isopropílico
Kodan Tücher DEU Rociador Propanol/alcohol
Kohrsolin ff DEU Líquido Glutaraldehído
Korsolex basic DEU Líquido Glutaraldehído
Korsolex extra DEU Líquido Etanol/propanol
23
Page 86

Tabla 18: Compatibilidad de los desinfectantes con el transductor L52 Series (Continuación)
Soluciones de
desinfección y
limpieza
País de
origen
Tipo Ingrediente activo
L52x
L52n
L52
L52e
Lejía EE. UU. Líquido Hipoclorito de sodio
LpHse EE. UU. Líquido O-fenilfenol
Lysol IC EE. UU. Líquido O-fenilfenol
Madacide 1 EE. UU. Líquido Cloruro de amonio-alquilo
Matar EE. UU. Líquido O-fenilfenol
MetriCide 14 EE. UU. Líquido Glutaraldehído
MetriCide 28 EE. UU. Líquido Glutaraldehído
MetriCide OPA Plus EE. UU. Líquido Ortoftalaldehído
MetriZyme EE. UU. Limpiador Propilenglicol
Mikrobak forte DEU Líquido Cloruro de amonio
Mikrozid DEU Toallita Etanol/propanol
Nuclean FRA Rociador Alcohol/biguanida
PerCept RTU Wipes™ CAN Toallita Peróxido de hidrógeno
Peróxido de hidrógeno n/a Líquido Peróxido de hidrógeno (3%)
Rely+On™ PeraSafe™ GBR Líquido Ácido peracético
Ruthless EE. UU. Rociador Amonio cuaternario
Sagrosept DEU Toallita Alcohol isopropílico
Salvanios pH 7 FRA Líquido Amonio cuaternario
Sani-Cloth HB EE. UU. Toallita Amonio cuaternario
Sani-Cloth Plus EE. UU. Toallita Amonio cuaternario
Sekusept ALE Líquido Glutaraldehído
Sklar (4) EE. UU. Líquido Alcohol isopropílico
Sporicidin EE. UU. Toallita Fenol
Sporicidin EE. UU. Líquido Fenol
24
Page 87

Tabla 18: Compatibilidad de los desinfectantes con el transductor L52 Series (Continuación)
English Deutsch Español Français Italiano Português
Soluciones de
desinfección y
limpieza
País de
origen
Tipo Ingrediente activo
L52x
L52n
L52
L52e
Staphene EE. UU. Rociador Alcohol etílico
Steranios 2% FRA Líquido Glutaraldehído
Steranios 20% FRA Líquido Glutaraldehído
Super Sani-Cloth EE. UU. Toallita Alcohol isopropílico
T-Spray EE. UU. Rociador Amonio cuaternario
T-Spray II EE. UU. Rociador Alquilo/cloruro
Task 105 EE. UU. Rociador Amonio cuaternario
TBQ EE. UU. Líquido Cloruro de amonio-alquilo
Theracide Plus EE. UU. Líquido Amonio cuaternario
Tor EE. UU. Líquido Amonio cuaternario
Trigene Advance Wipes Toallita Amonio cuaternario, clorhidrato
de biguanida polimérica
Tristel GBR Líquido Dióxido de cloro
Tristel Solo GBR Espuma Hexametilenbiguanida
Tristel Wipes GBR Toallita Dióxido de cloro
Vesphene IIse EE. UU. Líquido Sodio/o-fenilfenato
Virex 256 EE. UU. Líquido Cloruro de amonio
Virex TB EE. UU. Líquido Amonio cuaternario
Virox 5 CAN Toallita Peróxido de hidrógeno
Virufen FRA Líquido Cloruro de amonio-alquilo
Wavicide -06 EE. UU. Líquido Glutaraldehído
Wex-Cide EE. UU. Líquido O-fenilfenol
= Aceptable
25
Page 88

26
Page 89

Sonde série L52
Guide d’utilisation
Page 90

Fabriqué par
SonoSite, Inc.
21919 30th Drive SE
Bothell, WA 98021
États-Unis
Tél. : +1-888-482-9449 ou +1-425-951-1200
Fax : +1-425-951-1201
SonoSite Ltd
Alexander House
40A Wilbury Way
Hitchin
Herts SG4 OAP
Royaume-Uni
Tél. : +44-1462-444800
Fax : +44-1462-444801
Mise en garde:
En vertu de la loi fédérale américaine, cet appareil ne peut être vendu que par ou sur
ordre d’un vétérinaire.
180PLUS, M-Turbo, MicroMaxx, NanoMaxx, S Series, SonoSite, SonoSite TITAN, TITAN et le logo SonoSite sont des marques déposées (dans
certaines juridictions) ou des marques non déposées de SonoSite, Inc.
Les noms de produits non fabriqués par SonoSite peuvent être des marques ou des marques déposées de leurs propriétaires respectifs.
Le ou les échographes SonoSite référencés dans ce document peuvent être protégés par un ou plusieurs des brevets américains suivants:
5722412, 5817024, 5893363, 6135961, 6203498, 6364839, 6371918, 6383139, 6416475, 6447451, 6471651, 6569101, 6648826, 6575908,
6604630, 6817982, 6835177, 6962566, 7169108, 7449640, 7534211, 7549961, 7588541, 7591786, 7604596, 7643040, 7686766, 7694814,
7727153, 7740586, 7804970, 78094 00, 7819807, 7841575, 7849250, 7867168, 7883276, D456509, D4 61895, D509900, D538432, D544962,
D558351, D559390, D591423, D592750, D592760, D625014, D625015 et par les brevets étrangers équivalents suivants: AU727381,
AU730822, CA2372152, CA2372158, CA2373065, CN ZL 97113678.5, CN ZL 98106133.8, CN ZL 98108973.9, CN ZL 200830007734.8,
DE60021552.0, DE60029777.2, DE60034670.6, DE69730563.5, DE6980539.6, DE69831698.3, DE60 2004 23 816.3-08, FR0815793,
FR0875203, FR0881492, FR1175713, FR1180970, FR1589878, GB0875203, GB0881492, GB1175713, GB1180970, GB1180971, GB1589878,
IT0815793, IT0881492, IT1175713, IT1589878, KR528102, KR532359, NO326202, NO326814, NZ542968, RCD000897368-0001, SP0815793,
SP0881492, SP1589878. Brevets en instance.
P07895-06 03/2011
Copyright 2011 by SonoSite, Inc.
Tous droits réservés. Imprimé aux États-Unis.
ii
Page 91

Guide d’utilisation de la sonde série L52
Introduction .................................................................................................................................................1
Imagerie ........................................................................................................................................................1
Mesures et calculs ......................................................................................................................................4
Sécurité ..........................................................................................................................................................5
Dépannage et entretien .......................................................................................................................21
Introduction
Ce document est un supplément aux guides d’utilisation suivants :
• Guide d’utilisation de l’échographe NanoMaxx
• Guide d’utilisation de l’échographe M-Turbo
• Guide d’utilisation de l’échographe S Series
• Guide d’utilisation de l’échographe MicroMaxx
• Guide d’utilisation de l’échographe TITAN
• Guide d’utilisation de l’échographe SonoSite
Décrit les sondes suivantes, destinées à un usage vétérinaire exclusivement :
• Sonde L52n/10-5 MHz (L52n) sur l’échographe NanoMaxx®
• Sonde L52x/10-5 MHz (L52x) sur les échographes M-Turbo®, S Series™ ou MicroMaxx®
• Sonde L52e/10-5 MHz (L52e) sur l’échographe MicroMaxx®
• Sonde L52/10-5 MHz (L52) sur l’échographe haute résolution TITAN® ou sur l’échographe
SonoSite®180PLUS™
Consultez le guide d’utilisation de l’échographe pour plus d’informations sur la sécurité, pour obtenir
les instructions relatives à la préparation, l’utilisation et l’entretien de l’échographe, et pour connaître
les utilisations prévues pour chaque type d’examen et le mode d’imagerie.
English Deutsch Español Français Italiano Português
Imagerie
Sonde, type d’examen et mode d’imagerie
Le tableau suivant décrit la sonde, le type d’examen, le mode d’imagerie et l’optimisation disponibles
sur votre échographe.
1
Page 92

Sonde, type d’examen et mode d’imagerie (NanoMaxx)
Mode d’imagerie
Sonde Type d’examen
Mode
2D/M
DPC Couleur
L52n OB X X X
Vaisseaux (Vas) X X X
Musculo-squelettique (Msk) X X X
.
Sonde, type d’examen et mode d’imagerie (M-Turbo ou MicroMaxx)
Mode d’imagerie
Sonde
Typ e
d’examen
Mode
2D/M
DPC Couleur DP OC
L52x OB X X X X —
Vaisseaux (Vas) X X X X —
Muscle (Mus) X X X X —
Sonde, type d’examen et mode d’imagerie (S Series)
Mode d’imagerie
Sonde
Typ e
d’examen
Mode
2D/M
DPC Couleur DP
L52x OB X X X X
Vaisseaux (Vas) X X X X
Muscle (Mus) X X X X
2
Page 93

Sonde, type d’examen et mode d’imagerie (MicroMaxx)
Mode d’imagerie
English Deutsch Español Français Italiano Português
Sonde
Typ e
d’examen
Mode
2D/M
THI
2DMB2D
S
DPC Couleur DP
TDI
DP
L52e OB X — X X X X X — —
Vaisseaux
X—XXX X X——
(Vas)
Muscle
X—XXX X X——
(Mus)
Sonde, type d’examen et mode d’imagerie (TITAN)
Mode d’imagerie
Sonde
Typ e
d’examen
2D THI DPC DPCD Couleur Mode M DP OC
L52 OB X — X — — X — —
Vaisseaux
X—X — — X ——
(Vas)
Muscle
X—X — — X ——
(Mus)
OC
Sonde, type d’examen et mode d’imagerie (180PLUS)
Mode d’imagerie
Sonde Type d’examen 2D DPC
L52 OB rés, gén, pén bas, moyen, haut
Vaisseaux (Vas) rés, gén, pén bas, moyen, haut
3
Page 94

Mesures et calculs
Calculs
Ce tableau indique les calculs disponibles par type d’examen pour la sonde L52.
Calculs pour la sonde L52n (NanoMaxx)
Type d’examen Calculs
OB OB
Calculs pour la sonde L52x (M-Turbo et MicroMaxx)
Type d’examen Calculs
Musculo-squelettique (Msk) Pourcentages de réduction
OB OB
Vaisseaux (Vas) Pourcentages de réduction
Volume
Vasculaire
Volume
Débit-volume
Calculs pour la sonde L52x (S Series)
Type d’examen Échographe S Series Calculs
OB S-VetMed OB
Calculs pour L52e/L52 (MicroMaxx et TITAN)
Type d’examen Calculs
Muscle (Mus) Pourcentages de réduction
Volume
OB OB
4
Page 95

Calculs pour L52e/L52 (suite) (MicroMaxx et TITAN)
Type d’examen Calculs
Vaisseaux (Vas) Pourcentages de réduction
Calculs pour L52 (180PLUS)
Type d’examen Calculs
OB OB
Vaisseaux (Vas) Volume
Sécurité
English Deutsch Español Français Italiano Português
Vasculaire
Volume
Débit-volume
Débit-volume
Recommandations pour réduire l’IM et l’IT
Les recommandations suivantes permettent de réduire l’IM ou l’IT. Si plusieurs paramètres sont
donnés, réduisez-les simultanément pour optimiser les résultats. Dans certains modes, la
modification de ces paramètres n’affecte ni l’IM ni l’IT. La modification d’autres paramètres peut
également réduire l’IM et l’IT. Notez les valeurs IM et IT affichées dans la partie droite de l’écran.
Ta bleau 1 : I M
Sonde Profondeur
L52n (NanoMaxx) ↑
L52x (M-Turbo, S Series ou MicroMaxx) ↑
L52e (MicroMaxx) ↑
L52 (TITAN) ↑
L52 (180PLUS) ↑
↓ Baisse le réglage du paramètre afin de réduire l’IM.
↑ Augmente le réglage du paramètre afin de réduire l’IM.
5
Page 96

Tableau 2 : IT (ITM, ITC, ITO)
Réglages du Doppler
Sonde
puissance couleur
PRF Profondeur
L52n (NanoMaxx) — ↑ —
L52x (M-Turbo ou MicroMaxx) ↓↑Vol.
L52x (S Series) ↓↑ —
L52e (MicroMaxx) ↓↑Vol.
L52 (TITAN) ↓↑ —
L52 (180PLUS) — — —
↓ Baisse le réglage du paramètre afin de réduire l’IT.
↑ Augmente le réglage du paramètre afin de réduire l’IT.
— Données non applicables pour cette sonde/ce mode.
Augmentation de la température de surface des sondes
Le tableau 3 indique l’augmentation de la température de surface (en °C) mesurée par rapport à la
température ambiante (23 ± 3 °C) pour les sondes utilisées sur l’échographe. Les températures ont
été mesurées conformément à la norme EN 60601-2-37 Section 42 où les réglages et paramètres ont
été définis pour obtenir des températures maximales.
Paramètres
DP
échantillon ↓
échantillon ↑
Tableau 3 : Augmentation de la température de surface des sondes CEI 60601-2-37
(usage interne)
Te st
TITAN
Air immobile
Simulation
L52n sur
NanoMaxx
L52x sur
M-Turbo
ou S Series
L52x sur
MicroMaxx
L52e sur
MicroMaxx
L52 sur
7,5 8,8 8,2 13,0 9,3 10,8
5,6 5,9 5,6 5,5 2,4 2,4
L52 sur
180PLUS
d’utilisation
6
Page 97

Affichage de la puissance acoustique
Tableau 4 : IT ou IM ≥ 1,0
English Deutsch Español Français Italiano Português
Modèle de sonde Index
Mode
2D/M
Couleur DPC
Doppler
pulsé
L52n (NanoMaxx) IM Oui Oui Oui s/o
IT Non Non Non s/o
L52x (M-Turbo) IM Oui Oui Oui Oui
IT Non Non Non Oui
L52x (S Series) IM Oui Oui Oui —
IT Non Non Non —
L52x (MicroMaxx) IM Oui Oui Oui Oui
IT Oui Non Non Oui
L52e (MicroMaxx) IM Non Oui Oui Oui
IT Non Non Non Oui
L52 (TITAN, 180PLUS)* IM Non Non Non Non
IT Non Non Non Non
* Lorsqu’elle est utilisée sur l’échographe TITAN ou 180PLUS, la sonde L52 ne dépasse jamais un IM ou un IT de 1,0.
7
Page 98

Tableaux de puissance acoustique (M-Turbo et S Series)
Tableau 5 : Modèle de sonde : L52x/10-5 Mode de fonctionnement: 2D
ITM ITO
≤1A
aprt
Fixe
aprt
Fixe
ITC
>1
Référence de l’indice I.M.
Ba-
layage
A
Valeur de l’indice maximum global 1,01 (a) — — — (a)
p
r.3
W
0
min de [W
z
1
z
bp
z
sp
associé
.3(z1
),I
TA. 3(z1
deq(zsp)(cm)
f
c
Paramètre acoustique
Dim de A
aprt
(MPa)
(mW)
)] (mW)
(cm)
(cm)
(cm)
2,336
1,8
#— —#
—
—
—
—
—
(MHz)
5,33 # — — — #
X(cm) #—— — #
Y(cm)
#—— — #
PD (µs) 0,15
PRF (Hz) 7222
pr@PII
max
deq@Pll
Autres
Distance focale DFx (cm)
max
informations
I
@MI
PA .3
max
(MPa)
(cm)
DFy (cm)
(W/cm2)
3,25
—
#—— #
#—— #
329,1
Commande 1 : Type d’examen Vas — — — — —
Commande 2 : Optimisation Gén — — — — —
Commande 3 : Profondeur 4,2 cm — — — — —
Commande 4 : MB
(Multi-faisceaux)
Conditions
des commandes
de fonctionnement
Inactif
—— — — —
(a) Cet indice n’est pas requis pour ce mode de fonctionnement ; la valeur est <1.
(b) Cette sonde n’est pas destinée aux examens transcrâniens ou céphaliques des nouveau-nés.
# Aucune donnée n’est fournie pour ce mode de fonctionnement, dans la mesure où la valeur de l’indice maximum global
n’est pas rapportée pour la raison indiquée. (Ligne de la valeur de l’indice maximum global de référence.)
— Données non applicables pour cette sonde/ce mode.
8
Page 99

Tableau 6 : Modèle de sonde : L52x/10-5 Mode de fonctionnement: Mode M
(M-Turbo uniquement)
ITM ITO
English Deutsch Español Français Italiano Português
A
aprt
Fixe
≤1A
aprt
Fixe
ITC
>1
Référence de l’indice I.M.
Ba-
layage
Valeur de l’indice maximum global 1,01 — (a) — (a) (b)
p
r.3
W
0
min de [W
z
1
z
bp
z
sp
associé
.3(z1
),I
TA. 3(z1
)] (mW)
deq(zsp)(cm)
f
c
Paramètre acoustique
Dim de A
aprt
(MPa)
(mW)
2,336
—# ##
—
(cm)
(cm)
(cm)
1,8
—
—
#
#
(MHz)
5,33 — # — # #
X(cm) —# — # #
Y(cm)
—# — # #
PD (µs) 0,15
PRF (Hz) 1600
pr@PII
max
deq@Pll
Autres
Distance focale DFx (cm)
max
informations
I
@MI
PA .3
max
(MPa)
(cm)
DFy (cm)
(W/cm2)
3,25
#
—# — #
—# — #
329,1
Commande 1 : Type d’examen Vas — — — — —
Commande 2 : Optimisation Gén — — — — —
Commande 3 : Profondeur 4,2 cm — — — — —
Commande 4 : MB (Multi-faisceaux)
Conditions
des commandes
de fonctionnement
Inactif
—— — — —
(a) Cet indice n’est pas requis pour ce mode de fonctionnement ; la valeur est <1.
(b) Cette sonde n’est pas destinée aux examens transcrâniens ou céphaliques des nouveau-nés.
# Aucune donnée n’est fournie pour ce mode de fonctionnement, dans la mesure où la valeur de l’indice maximum global
n’est pas rapportée pour la raison indiquée. (Ligne de la valeur de l’indice maximum global de référence.)
— Données non applicables pour cette sonde/ce mode.
9
Page 100

Tableau 7 : Modèle de sonde : L52x/10-5 Mode de fonctionnement: Couleur/DPC
ITM ITO
A
aprt
Fixe
≤1A
aprt
Fixe
ITC
>1
Référence de l’indice I.M.
Ba-
layage
Valeur de l’indice maximum global 1,33 (a) — — — (b)
p
r.3
W
0
min de [W.
z
1
Z
bp
Z
sp
associé
3(z1),ITA. 3 (z1)
(MPa) 2,807
(mW) #— —#
] (mW) —
(cm) —
(cm) —
(cm) 1,8 —
deq(Zsp)(cm) —
Paramètre acoustique
F
c
Dim de A
aprt
(MHz) 4,37 # — — — #
X(cm) #— — — #
Y(cm)
#— — — #
PD (µs) 0,61
PRF (Hz) 5427
pr@PII
max
deq@Pll
Autres
Distance focale DFx (cm) #— — #
max
informations
@MI
I
PA. 3
max
(MPa) 3,628
(cm) —
DF
(cm) #— — #
y
(W/cm2)
411,1
Commande 1 : Mode Tous — — — — —
Commande 2 : Type d’examen Vas — — — — —
Commande 3 : Optimisation/
Tous /5, 4 — — — — —
Profondeur
Commande 4 : PRF Tous — — — — —
Conditions
Commande 5 : Position/Taille de la
des commandes
de fonctionnement
zone Couleur
Tous /D éf . — — — — —
(a) Cet indice n’est pas requis pour ce mode de fonctionnement ; la valeur est <1.
(b) Cette sonde n’est pas destinée aux examens transcrâniens ou céphaliques des nouveau-nés.
# Aucune donnée n’est fournie pour ce mode de fonctionnement, dans la mesure où la valeur de l’indice maximum global
n’est pas rapportée pour la raison indiquée. (Ligne de la valeur de l’indice maximum global de référence.)
— Données non applicables pour cette sonde/ce mode.
10
 Loading...
Loading...