Page 1

C1.9 PLUS Ultrasound System
Service Manual
Page 2

P02287-03 01/2003
Copyright 2003 by SonoSite, Inc.
All rights reserved. Printed in the USA.
ii C1.9 PLUS Ultrasound System Service Manual
Page 3

Manufactured by
SonoSite, Inc.
21919 30th Drive SE
Bothell, WA 98021-3904
USA
Telephone: 1-888-482-9449 or +1-425-951-1200
Fax: +1-425-951-1201
SonoSite European Headquarters
Baystrait House, Station Road
Biggleswade SG18 8AL
UK
Telephone: +44-1767-313-117
Fax: +44-1767-312-400
CAUTION:
` United States federal law restricts this device to sale by or on the order of a
physician.
“SiteCharge,” “SitePack,” “SiteStand,” and “SonoHeart,” are trademarks of SonoSite, Inc.
Non-SonoSite product names may be trademarks or registered trademarks of their respective
owners.
SonoSite products may be covered by one or more of the following U.S. patents: 4454884, 4462408,
4469106, 4474184, 4475376, 4515017, 4534357, 4542653, 4543960, 4552607, 4561807, 4566035,
4567895, 4581636, 4591355, 4603702, (4607642), 4644795, 4670339, 4773140, 4817618,
4883059, 4887306, 5016641, 5050610, 5095910, 5099847, 5123415, 5158088, 5197477, 5207225,
5215094, 5226420, 5226422, 5233994, 5255682, (5275167), 5287753, 5305756, 5353354,
5365929, 5381795, 5386830, 5390674, 5402793, (5,423,220), 5438994, 5450851, 5456257,
5471989, 5471990, 5474073, 5476097, 5479930, 5482045, 5482047, 5485842, 5492134, 5517994,
5529070, 5546946, 5555887, 5603323, 5606972, 5617863, (5634465), 5634466, 5636631,
5645066, 5648942, 5669385, (5706819), 5715823, 5718229, 5720291, 5722412, 5752517,
5762067, 5782769, 5800356, 5817024, 5833613, 5846200, 5860924, 5893363, 5916168, 5951478,
6036643, 6102863, 6104126, 6113547, 6117085, 6142946, 6203498 B1, D0280762, D0285484,
D0286325, D0300241, D0306343, D0328095, D0369307, D0379231. Other patents pending.
iii
Page 4

iv C1.9 PLUS Ultrasound System Service Manual
Page 5
Table of Contents
CHAPTER 1 Introduction 1
1.1 About the System......................................................................1
1.2 Audience ...................................................................................2
1.3 Conventions Used in This Manual............................................2
1.4 About the System Software ......................................................2
1.5 Software Licensing ...................................................................3
CHAPTER 2 Safety 5
2.1 Electrical Safety........................................................................5
2.1.1 Equipment Protection.........................................................7
2.2 Battery Safety............................................................................8
2.3 Biological Safety.......................................................................9
2.4 Labeling Symbols .....................................................................9
CHAPTER 3 System Overview 11
3.1 Theory of Operation................................................................11
3.1.1 Transducer........................................................................12
3.1.2 Acquisition Subsystem.....................................................12
3.1.3 Processing Subsystem ......................................................12
3.1.4 Display Subsystem ...........................................................12
3.1.5 Control Subsystem ...........................................................12
3.1.6 User Interface Subsystem.................................................12
3.1.7 Power Subsystem .............................................................13
3.2 Components ............................................................................13
3.3 Controls...................................................................................14
3.4 Accessories .............................................................................15
3.4.1 Battery Pack .....................................................................15
3.4.1.1 Battery Charge Indicators ............................................15
3.4.2 External Power .................................................................16
3.4.2.1 External System Connections ......................................16
3.4.3 Power Adapter..................................................................17
3.5 System Specifications .............................................................17
3.5.1 Physical Dimensions ........................................................17
3.5.2 Monitor.............................................................................17
3.5.3 Transducers ......................................................................18
3.5.4 Imaging Modes.................................................................18
v
Page 6

3.5.5 Image Storage...................................................................18
3.5.6 Temperature, Pressure, and Humidity Limits ..................18
3.5.6.1 System Operating.........................................................18
3.5.6.2 System Shipping/Storage ............................................18
3.5.6.3 Battery Operating.........................................................18
3.5.6.4 Battery Shipping/Storage .............................................18
3.5.6.5 Transducers Operating .................................................18
3.5.6.6 Transducers Shipping/Storage .....................................19
3.5.7 Electrical...........................................................................19
3.6 Battery.....................................................................................19
3.7 Safety Requirements...............................................................19
3.7.1 Meets Electromechanical Safety Standards .....................19
3.7.2 Meets EMC/EMI Standards .............................................20
3.7.3 Meets Airborne Equipment Standards (without ECG Cable
Attached) ..........................................................................20
3.7.4 Meets ECG Standard........................................................20
CHAPTER 4 Setup and Operation 21
4.1 Connecting and Removing Transducers .................................21
4.2 Turning the System On and Off..............................................22
4.3 Installing and Removing the Battery ......................................23
4.4 Using AC Power .....................................................................24
4.5 Upgrading the System Software .............................................25
4.6 Obtaining A License Key .......................................................26
4.6.1 Installing A License Key..................................................26
4.6.1.1 Displaying the System Information Screen .................28
4.7 Checking and Charging the Battery........................................28
CHAPTER 5 Cleaning and Disinfecting 31
5.1 Universal Precautions .............................................................31
5.2 Receipt of Suspected Contaminated Materials .......................31
5.3 Recommended Disinfectants ..................................................32
CHAPTER 6 Troubleshooting 33
6.1 System and Subsystem Diagnosis...........................................33
6.2 System Repair .........................................................................33
6.3 Test Equipment.......................................................................33
vi C1.9 PLUS Ultrasound System Service Manual
Page 7

6.4 Failures....................................................................................34
6.4.1 Display..............................................................................34
6.4.2 Control Panel....................................................................34
6.4.3 Trackball...........................................................................34
6.4.4 Main PCBA ......................................................................34
6.4.5 Clearing the Main PCBA Failure.....................................35
6.4.6 Battery ..............................................................................35
CHAPTER 7 Replacement Procedures 43
7.1 Display Subassembly Replacement........................................43
7.1.1 Required Parts ..................................................................43
7.1.2 Required Tools and Materials ..........................................43
7.1.3 Removing the Display Subassembly................................44
7.1.4 Replacing the Display Subassembly ................................46
7.2 Control Panel Subassembly Replacement ..............................48
7.2.1 Required Parts ..................................................................48
7.2.2 Required Tools .................................................................48
7.2.3 Removing the Control Panel Subassembly ......................49
7.2.4 Replacing the Control Panel Subassembly.......................51
7.3 Trackball .................................................................................52
7.3.1 Required Parts ..................................................................52
7.3.2 Required Tools .................................................................52
7.3.3 Removing the Trackball ...................................................52
7.3.4 Replacing the Trackball....................................................55
7.4 Main PCBA Subassembly Replacement.................................57
7.4.1 Required Parts ..................................................................57
7.4.2 Required Tools .................................................................58
7.4.3 Removing the Main PCBA Subassembly ........................58
7.4.4 Replacing the Main PCBA Subassembly.........................59
7.5 Transducers.............................................................................62
7.6 AC Power Adapter..................................................................62
7.7 OEM Peripherals.....................................................................63
7.7.1 External Monitor (Sony) ..................................................63
7.7.2 VCR (Sony)......................................................................63
7.7.3 Printer (Sony) ...................................................................63
vii
Page 8

CHAPTER 8 Performance Tests 65
8.1 Overview.................................................................................65
8.2 Test Equipment:......................................................................65
8.3 Setting Up Performance Tests ................................................66
8.3.1 Scan Reference Orientation..............................................66
8.4 Testing 2D Performance .........................................................66
8.4.1 2D Image Quality.............................................................66
8.4.2 Axial Measurement Accuracy..........................................67
8.4.3 Lateral Measurement Accuracy .......................................67
8.4.4 Penetration........................................................................68
8.5 Additional Performance Tests ................................................68
8.5.1 CPD ..................................................................................68
8.5.2 PowerMap DCPD.............................................................69
8.5.3 M-mode Imaging..............................................................69
8.5.4 Tissue Harmonic Imaging ................................................70
8.5.5 Pulsed Wave (PW) Doppler Imaging...............................70
8.5.6 ECG Monitoring...............................................................70
8.5.7 Image Quality Verification Test.......................................71
8.5.8 Image Review...................................................................71
8.5.9 Printer ...............................................................................71
8.5.10 Battery Charging ..............................................................71
8.5.11 Video Output ...................................................................71
8.6 Returning Products to SonoSite..............................................72
8.6.1 Contacting SonoSite Technical Support ..........................72
8.6.2 Shipping Instructions........................................................72
APPENDIX A Parts List 73
APPENDIX B Service Event Report 85
Index 87
viii C1.9 PLUS Ultrasound System Service Manual
Page 9
CHAPTER 1 Introduction
Before servicing the SonoSite ultrasound system, please read the information in this
manual. This text applies to SonoSite ultrasound system products manufactured after
April 27, 2001. Please find service information about products manufactured before
April 27, 2001 in C1.51 Ultrasound System Service Manual (P00715) and C1.75
Ultrasound System Service Manual (P01118).
1.1 About the System
The SonoSite system has various configurations and features. All are described in
this manual but not every option may apply to your system. System features are
dependent on your system configuration, transducer and exam type.
The SonoSite system is a portable, software-controlled, ultrasound system. It has an
all-digital architecture. It is used to acquire and display high-resolution, real-time,
2D, Color Power Doppler (CPD), PowerMap
Doppler (DCPD), Tissue Harmonic Imaging (THI), M-mode and Pulsed Wave (PW)
Doppler ultrasound images. The system has electrocardiography (ECG), cine
review, image zoom, labeling, biopsy, measurements and calculations, serial port
connection for image transfer, image storage and review, printing and recording
capabilities. The system setup also has a selection to support optical character
recognition (OCR) of the English character set for time, date, patient name, and
patient identification. The OCR screen characters are optimized for use with the ALI
NewPORT DICOM image capture station peripheral available from ALI. For more
information about the ALI NewPORT 2.1, refer to the ALI NewPORT 2.1 Image
Capture Station User’s Guide.
™
(PM) Directional Color Power
Currently, the system supports the following broadband transducers:
• C60/5-2 MHz 60-mm curved array
• C15/4-2 MHz 15-mm micro-curved array
• C11/7-4 MHz 11-mm micro-curved array
• ICT/7-4 MHz 11-mm intracavitary
• L38/10-5 MHz 38-mm linear array
Chapter 1: Introduction 1
Page 10

System accessories include a SiteStand® mobile docking station and accessories,
SiteCharge
printer cables, SiteLink image manager software, SiteStand
CRT Stand, ScanPack quick access carrier, and SitePack
System peripherals include medical grade (conforming to the requirements of
EN60601-1) and non-medical (commercial) grade products. System medical grade
peripherals include an external color monitor, video printers, and VCRs. System
non-medical grade peripherals include a digital video recorder, a battery charger, a
lithium-ion battery, printer, and a handheld monitor. Use of peripherals is covered in
the manufacturers’ instructions, which accompany each peripheral. System setup
instructions for the use of peripherals are covered in Chapter 3 in the SonoSite
Ultasound System User Guide.
™
dual battery charger, a power adapter, a battery, ECG cable, video and
®
basket, Basic Stand,
™
protective carry pack.
1.2 Audience
The intended audience of this manual is properly trained field and in-house service
personnel.
1.3 Conventions Used in This Manual
These conventions are used in this user guide:
• Warnings and cautions are identified with the arrow symbol.
• Control names and references to display elements are presented in bold-face
type.
• Operating instructions are introduced with a statement in bold-face type that
ends with a colon. For example: To read this user guide:
• When the steps in the operating instructions must be performed in a specific
order, the steps are numbered.
• Bulleted lists present information in list format, but they do not imply a
sequence.
• Screen display text is shown in Arial 10 pt. For example:
• The left side of the system is to your left as you face the system. The system
handle is at the top of the system, the battery compartment is at the bottom of the
system.
• Note: A note cites information that is a general rule for a procedure, gives an
exception to a rule, or provides noncritical information of general interest.
Successful upgrade.
1.4 About the System Software
Your SonoSite system contains software that controls its operation. From time to
time, SonoSite may provide new software for use with your system. This software is
provided using a software update module or a transducer. This software may be
either required or optional. A single module or transducer can be used to update one
or more systems.
When the new software is required, you must install it if you wish to use the new
software features (e.g., new transducer). If you choose not to install it, you must
2 C1.9 PLUS Ultrasound System Service Manual
Page 11

remove the transducer and replace it with one that is compatible with the software
that is currently installed in your system.
When the software is optional, you can either install it or choose to use your existing
software. If you choose not to install the software, the system will prompt you again
whenever the system is started, and whenever the transducer is disconnected and
then reconnected to the system. For more information on software upgrades, refer to
Chapter 4.5, Upgrading the System Software, on page 25
1.5 Software Licensing
Use of the software that you receive from SonoSite is controlled by a license key. A
license key is a number sequence containing exactly 12 decimal digits.
License keys are obtained from SonoSite or from its authorized representatives. You
must obtain one key for each system that will use the new software. See
Chapter 4.6, Obtaining A License Key, on page 26
license key.
Software that you receive from SonoSite may be installed and will operate for a
short period of time without requiring a valid license key. We refer to this period of
time as the “grace period.” The grace period is variable.
When you first install your software, your SonoSite system will prompt you for a
license key. If you have not yet obtained a valid license key, you can elect to use the
software as long as the grace period time has not been fully consumed. We refer to
this mode of operation as “running in the grace period.”
.
for information on obtaining a
When a system is running in the grace period, all system functions are available. As
you use the system, the grace period is slowly consumed. When the grace period has
expired, the system will not be usable until a valid license key has been entered.
Grace period time is not consumed while the system is powered off or when it is in
“sleep” mode. Whenever a system is running in the grace period, the grace period
time remaining is available on the license update screen. Refer to Chapter 4.6.1.1,
Displaying the System Information Screen, on page 28
CAUTION:
.
` When the grace period expires, all system functions except for licensing will be
unavailable until you enter a valid license key into the system.
Chapter 1: Introduction 3
Page 12

This page intentionally left blank.
4 C1.9 PLUS Ultrasound System Service Manual
Page 13

CHAPTER 2 Safety
Please read this information before using the SonoSite ultrasound system. It applies
to the ultrasound system, transducers, accessories, and peripherals.
A WARNING describes precautions necessary to prevent injury or loss of life.
A CAUTION describes precautions necessary to protect the products.
2.1 Electrical Safety
This system meets EN60601-1, Class I/internally-powered equipment requirements
and Type BF isolated patient-applied parts safety requirements. The ECG cable
meets safety requirements of EN 60601-2-25 for Type CF patient-applied part.
This system complies with the applicable medical equipment requirements
published in the Canadian Standards Association (CSA), European Norm
Harmonized Standard, and Underwriters Laboratories (UL) safety standards. See
Chapter 3.5, System Specifications, on page 17
For maximum safety observe the following warnings and cautions:
.
WARNINGS:
` To avoid discomfort or minor risk of patient injury, keep hot surfaces away from
the patient.
` Under certain circumstances, the transducer connector and back of the display
enclosure can reach temperatures that exceed EN60601-1 limits for patient
contact, therefore only the operator shall handle the system. This does not include
the transducer face.
` To avoid discomfort or minor risk of operator injury when handling the transducer
connector, the system should not be operated for more than 60 minutes
continuously in a live-scan mode (as opposed to freeze or sleep modes).
Chapter 2: Safety 5
Page 14

` To avoid the risk of electrical shock or injury, do not open the system enclosures.
All internal adjustments and replacements, except battery replacement, must be
made by a qualified technician.
` To avoid the risk of injury, do not operate the system in the presence of flammable
gasses or anesthetics. Explosion can result.
` To avoid the risk of electrical shock, use only properly grounded equipment.
Shock hazards exist if the AC power adapter is not properly grounded. Grounding
reliability can only be achieved when equipment is connected to a receptacle
marked “Hospital Only” or “Hospital Grade” or the equivalent. The grounding
wire must not be removed or defeated.
` To avoid the risk of electrical shock, before using the transducer, inspect the
transducer face, housing, and cable. Do not use the transducer, if the transducer or
cable is damaged.
` To avoid the risk of electrical shock, always disconnect the AC power adapter
from the system before cleaning the system.
` To avoid the risk of electrical shock, do not use any transducer that has been
immersed beyond the specified cleaning or disinfection level. Refer to the
SonoSite Ultrasound System User Guide for cleaning and disinfection levels.
` To avoid the risk of electrical shock and fire hazard, inspect the AC power adapter
cord and plug on a regular basis. Ensure they are not damaged.
` To avoid the risk of electrical shock, use only accessories and peripherals
recommended by SonoSite. Connection of accessories and peripherals not
recommended by SonoSite could result in electrical shock. Contact SonoSite or
your local representative for a list of accessories and peripherals available from or
recommend by SonoSite.
` To avoid the risk of electrical shock, use commercial grade peripherals
recommended by SonoSite on battery power only. Do not connect these products
to AC mains power when using the system to scan or diagnose a patient/subject.
Contact SonoSite or your local representative for a list of the commercial grade
peripherals available from or recommended by SonoSite.
` To prevent injury, only use market cleared ECG electrodes and cables.
` To avoid the risk of electrical shock to the patient/subject, ensure proper assembly
of the ECG electrodes and cables.
` To avoid the risk of electrical shock to the patient/subject, do not touch the system
battery contacts while simultaneously touching a patient/subject.
` To prevent injury to the operator/bystander, the transducer must be removed from
patient contact before the application of a high-voltage defibrillation pulse.
CAUTIONS:
` Although your system has been manufactured in compliance with existing EMC/
EMI requirements (EN60601-1-2), use of the system in the presence of
an electromagnetic field can cause degradation of the ultrasound image. If this
occurs often, SonoSite suggests a review of the system environment. Identify and
remove the possible sources of the emissions or move your system.
6 C1.9 PLUS Ultrasound System Service Manual
Page 15

` Electrostatic discharge (ESD), or static shock, is a naturally occurring
phenomenon. ESD is common in conditions of low humidity, which can be caused
by heating or air conditioning. Static shock is a discharge of the electrical energy
from a charged body to a lesser or non-charged body. The degree of discharge can
be significant enough to cause damage to a transducer or an ultrasound system.
The following precautions can help reduce ESD: anti-static spray on carpets, antistatic spray on linoleum, and anti-static mats.
` Do not use the system if an error message appears on the image display: note the
error code; call SonoSite or your local representative; turn off the system by
pressing and holding the power switch until the system powers down.
` To avoid increasing the system and transducer connector temperature, do not
block the airflow to the ventilation holes on the back of the system.
2.1.1 Equipment Protection
To protect your ultrasound system, transducer, and accessories, follow these
precautions.
To protect your ultrasound system, transducer, and accessories, follow these
precautions.
CAUTIONS:
` The ECG cable emits electromagnetic interference when connected to the
SonoSite system. It is not approved for use in-flight on aircraft.
` Excessive bending or twisting of cables can cause a failure or intermittent
operation.
` Improper cleaning or disinfecting of any part of the system can cause permanent
damage. For cleaning and disinfecting instructions, refer to the SonoSite
Ultrasound System User Guide.
` Do not submerge the transducer connector in solution. The cable is not liquid-tight
beyond the transducer connector/cable interface.
` Do not use solvents such as thinner or benzene, or abrasive cleaners on any part
of the system.
` Remove the battery from the system if the system is not likely to be used for some
time.
` Do not spill liquid on the system.
` The top membrane of the phantom is delicate and can be damaged if handled
improperly. Use minimum force when coupling the transducer to the phantom.
` Do not handle PCBs without proper static protection. Improper handling may
damage components.
` Incorrect assembly or configuration or using an improper power source may
damage the system.
` Do not touch the scanhead connector pins.
Chapter 2: Safety 7
Page 16

2.2 Battery Safety
To avoid the risk of injury, follow the warnings and cautions to make sure that the
battery does not burst, ignite, or generate heat or fumes.
WARNINGS:
` The battery has a safety device. Do not disassemble or alter the battery.
` Charge the batteries only when the ambient temperature is between 0° and 40°C
(32° and 104°F).
` Do not short-circuit the battery by directly connecting the positive and negative
terminals with metal objects.
` Do not heat the battery or discard it in a fire.
` Do not expose the battery to temperatures over 60°C (140°F). Keep it away from
fire and other heat sources.
` Do not charge the battery near a heat source, such as a fire or heater.
` Do not leave the battery in direct sunlight.
` Recharge the battery only with the SiteCharge™ dual battery charger or the
system.
` Do not pierce the battery with a sharp object, hit it, or step on it.
` Do not use a damaged battery.
` Do not solder a battery.
` When connecting the battery to the SiteCharge™ dual battery charger or to the
system, never reverse the polarity of the battery terminals.
` The polarity of the battery terminals are fixed and cannot be switched or reversed.
Do not force the battery into the system or the SiteCharge™ dual battery charger.
` Do not connect the battery to an electrical power outlet.
` Do not continue recharging the battery if it does not recharge after two successive
six hour charging cycles.
CAUTIONS:
` To avoid the battery bursting, igniting, or fumes from the battery and causing
equipment damage, observe the following precautions:
` Do not immerse the battery in water or allow it to get wet.
` Do not put the battery into a microwave oven or pressurized container.
` If the battery leaks or emits an odor, remove it from all possible flammable
sources.
` If the battery emits an odor or heat, is deformed or discolored, or in any way
appears abnormal during use, recharging or storage, immediately remove it and
stop using it. If you have any questions about the battery, consult SonoSite or your
local representative.
` Store the battery between -20°C (-4°F) and 60°C (140°F).
` Use only SonoSite batteries.
8 C1.9 PLUS Ultrasound System Service Manual
Page 17

2.3 Biological Safety
Observe the following precautions related to biological safety.
WARNINGS:
` To prevent misdiagnosis, do not use the ECG trace to diagnosis cardiac rhythms.
The SonoSite ECG option is a non-diagnostic feature.
` To prevent injury, only use market cleared ECG electrodes and cables.
` Non-medical (commercial) grade peripheral monitors have not been verified or
validated by SonoSite as being suitable for diagnosis.
` Do not use the system if it exhibits erratic or inconsistent behavior. Discontinuities
in the scanning sequence are indicative of a hardware failure that must be
corrected before use.
` Do not use the system if it exhibits artifacts on the LCD screen, either within the
clinical image or in the area outside of the clinical image. Artifacts are indicative
of hardware and/or software errors that must be corrected before use.
` Some transducer covers contain natural rubber latex and talc, which can cause
allergic reactions in some individuals. Refer to the FDA Medical Alert, March 29,
1991.
` Perform ultrasound procedures prudently. Use the ALARA (as low as reasonably
achievable) principle.
` SonoSite does not currently recommend a specific brand of acoustic standoff.
2.4 Labeling Symbols
Labeling symbols for SonoSite products can be found in the user guide for each
product.
Chapter 2: Safety 9
Page 18

This page intentionally left blank.
10 C1.9 PLUS Ultrasound System Service Manual
Page 19
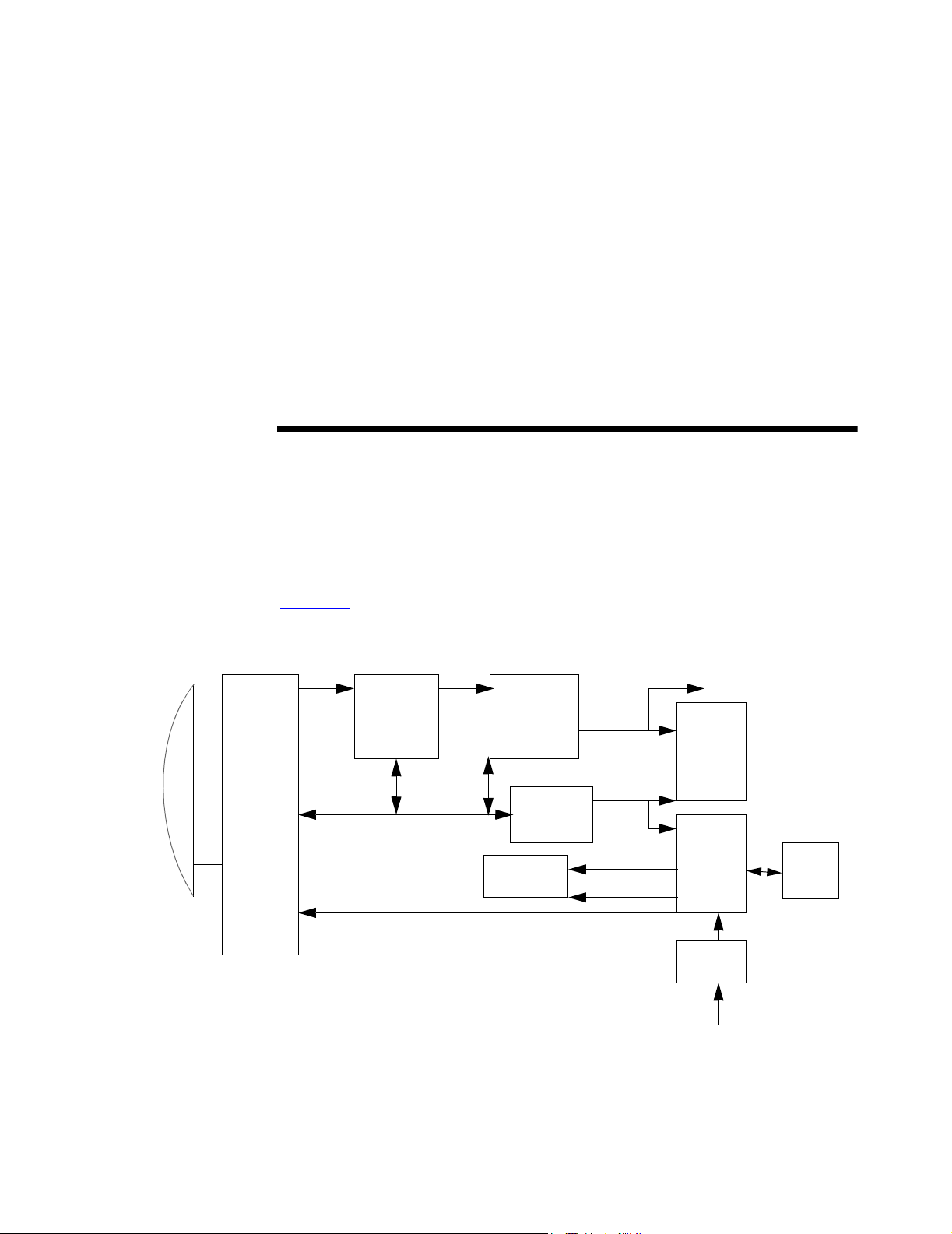
CHAPTER 3 System Overview
3.1 Theory of Operation
The SonoSite ultrasound system has seven major functional groups: the transducer,
the acquisition subsystem, the processing subsystem, the display subsystem, the
control subsystem, the user interface subsystem, and the power subsystem.
Figure 3.1
shows how these functions interact.
Transducer
Acquisition
subsystem
RF bus
Processing
subsystem
Pulser voltage
AQ bus
Control bus
Display
subsystem
Control
subsystem
To all
subsystems
Figure 3.1 System Block Diagram
Video bus
Serial bus
Display power
Logic power
External video to monitor,
VCR, printer ports
User
interface
subsystem
Power
subsystem
Power
adapter
External power
Battery
pack
Chapter 3: System Overview 11
Page 20

3.1.1 Transducer
The transducer elements convert the pulser voltage to acoustic energy during the
“transmit” portion of the ultrasound acquisition cycle. Also, the transducer elements
convert the acoustic echo to voltage in the “receive” portion of the acquisition cycle.
The system transducers have more than 64 elements. The acquisition subsystem
senses the voltage developed on the transducer elements.
3.1.2 Acquisition Subsystem
The acquisition subsystem consists of a beamformer and an interface to the
transducer. The beamformer times the “transmit” pulses to focus the acoustic beam.
Also, the beamformer amplifies the low-level echo signal and times and focuses the
“receive” information.
3.1.3 Processing Subsystem
The high-speed processing subsystem interfaces with the beamformer. The
processing subsystem demodulates, filters, detects, and compresses the signal
supplied by the beamformer. Next, it sends this data to the display subsystem.
3.1.4 Display Subsystem
The display subsystem converts the detected ultrasound data into picture elements
(pixels). The software user interface graphics are combined with the ultrasound
information and converted to a video stream. The external video ports support NTSC
and PAL format.
3.1.5 Control Subsystem
The control subsystem consists of the central processing unit, program and video
memory, permanent image storage and retrieval memory, and a connection to the
user interface keys. The control software includes the acoustic power and intensity
software power group monitors, and a beamformer monitor. This software makes the
system operate within acoustic power and intensity limits, which guarantees a level
of patient safety.
3.1.6 User Interface Subsystem
The user interface subsystem comprises the software user interface and the form
factor. The software user interface is the interaction between the user and the screen
layout components. The form factor is the device’s physical attributes: buttons,
location and grouping of buttons and the device size, shape, and weight. Dedicated
controls, or often-used features, are grouped according to user workflow.
12 C1.9 PLUS Ultrasound System Service Manual
Page 21

3.1.7 Power Subsystem
The power subsystem provides power and protects the hardware from destructive or
unsafe conditions. This subsystem’s hardware and software monitors detect failures
in the device. Upon detecting a fault, the system disables the pulser supply, and
signals an error to the control subsystem. The power subsystem includes the battery
pack and the battery charging electronics.
3.2 Components
The SonoSite system components are identified in Chapter 1, section 1.2.
Chapter 3: System Overview 13
Page 22
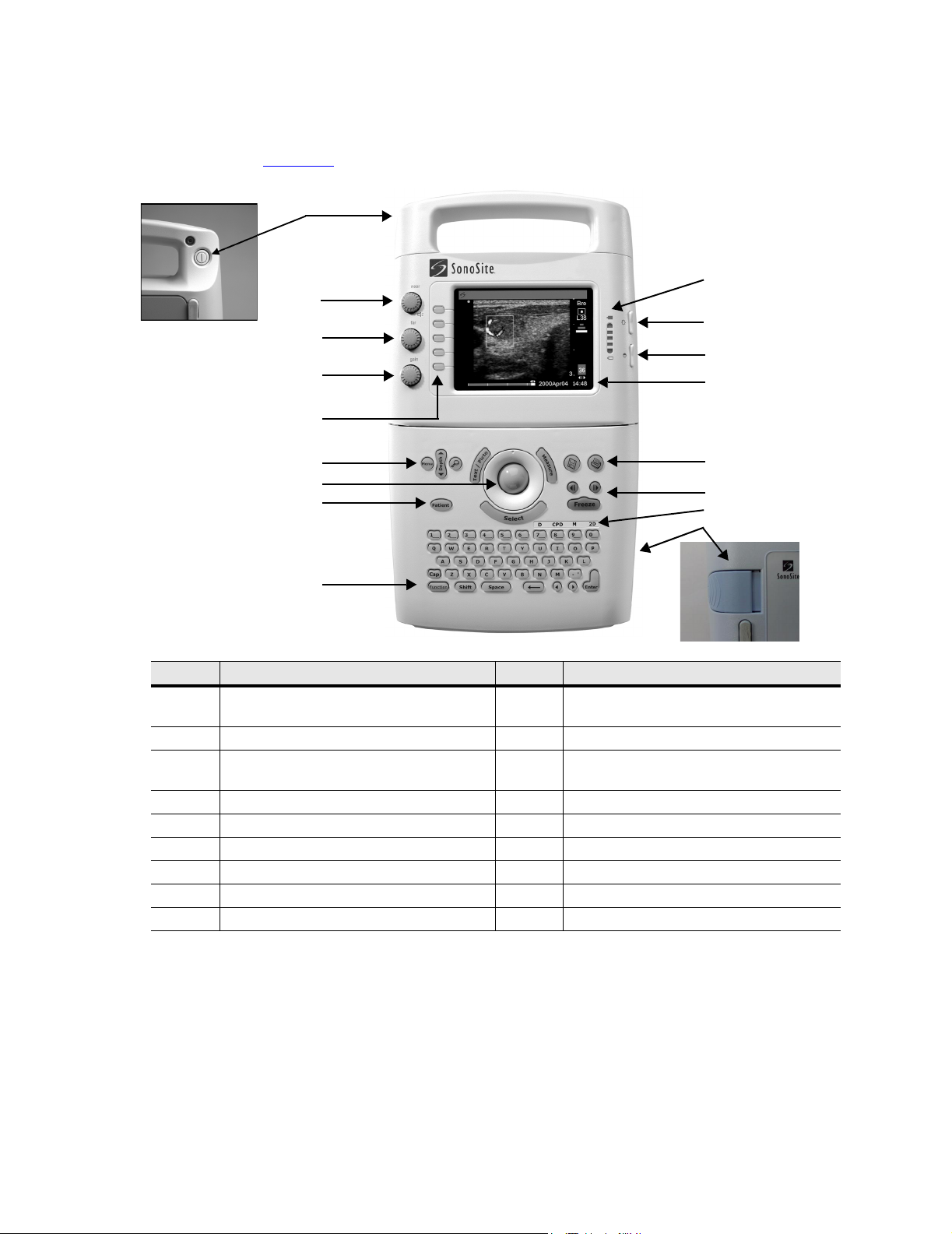
3.3 Controls
Figure 3.2 shows the SonoSite system controls.
1
2
rear view
3
4
5
10
11
12
13
6
7
8
14
15
16
17
9
Number Feature Number Feature
1 power switch, located on the rear of the
system handle
2 near, affects gain of shallow echoes 10 battery charge indicators
3 far, affects gain of deeper echoes 11 LCD (liquid crystal display) brightness
4 gain, affects overall gain 12 LCD contrast control
5 menu select controls 13 LCD
6 Menu, Depth, and Zoom 14 print key
7 trackball 15 cine arrow keys and Freeze key
8 Patient 16 mode controls
9 Function key
control
17 battery release
Figure 3.2 SonoSite Ultrasound System Controls
14 C1.9 PLUS Ultrasound System Service Manual
Page 23
3.4 Accessories
For information about accessories and other SonoSite products, refer to the user
guide for each product.
3.4.1 Battery Pack
CAUTIONS:
` Use only the specified SonoSite battery pack. For battery safety notes, see
Chapter 2, Safety, on page 5
The system can be powered from either a battery pack or external power.
The system is powered by a rechargeable, six-cell, 11.1 V dc, 3.0 amp-hours,
lithium-ion battery. A fully-charged battery has a run time of 1.5 to 4 hours,
depending upon operating conditions. The battery pack case is made of injection
molded plastic. When in use, it is inserted into the system. The battery pack has no
user-serviceable parts. The operating life of the battery is 1-2 years, depending on
how you use the system. Table 3.2 contains battery operating specifications.
Table 3.1 Battery Pack Operation Specifications
.
BATTERY PACK OPERATION
PARAMETER
Operation time during use model 1.5 hours @ 77°F (25°C)
Operation time during power off
(leakage and self discharge)
Number of charge discharge cycles
(100% depth of discharge)
3.4.1.1 Battery Charge Indicators
The battery charge indicators, which consist of light-emitting diodes (LEDs) on the
system, indicate the current battery level.
• All LEDs lit mean the system battery is fully charged.
• Some LEDs lit mean the system battery is partially charged.
Table 3.3 contains the charging requirements for the system.
Table 3.2 System Charging Requirements
SYSTEM CHARGING PARAMETER SPECIFICATION
Charge time to 80% capacity (internal
charger) with the system off
Charge time to 80% capacity (internal
charger) with the system on
SPECIFICATION
14 days @ 77°F (25°C)
500 @ 70°F (21°C)
3 hours @ 77°F (25°C)
12 hours @ 77°F (25°C)
Chapter 3: System Overview 15
Page 24
3.4.2 External Power
The external power connection provides the system electricity via the power adapter.
External power charges the battery pack and powers the system in low battery
conditions.
3.4.2.1 External System Connections
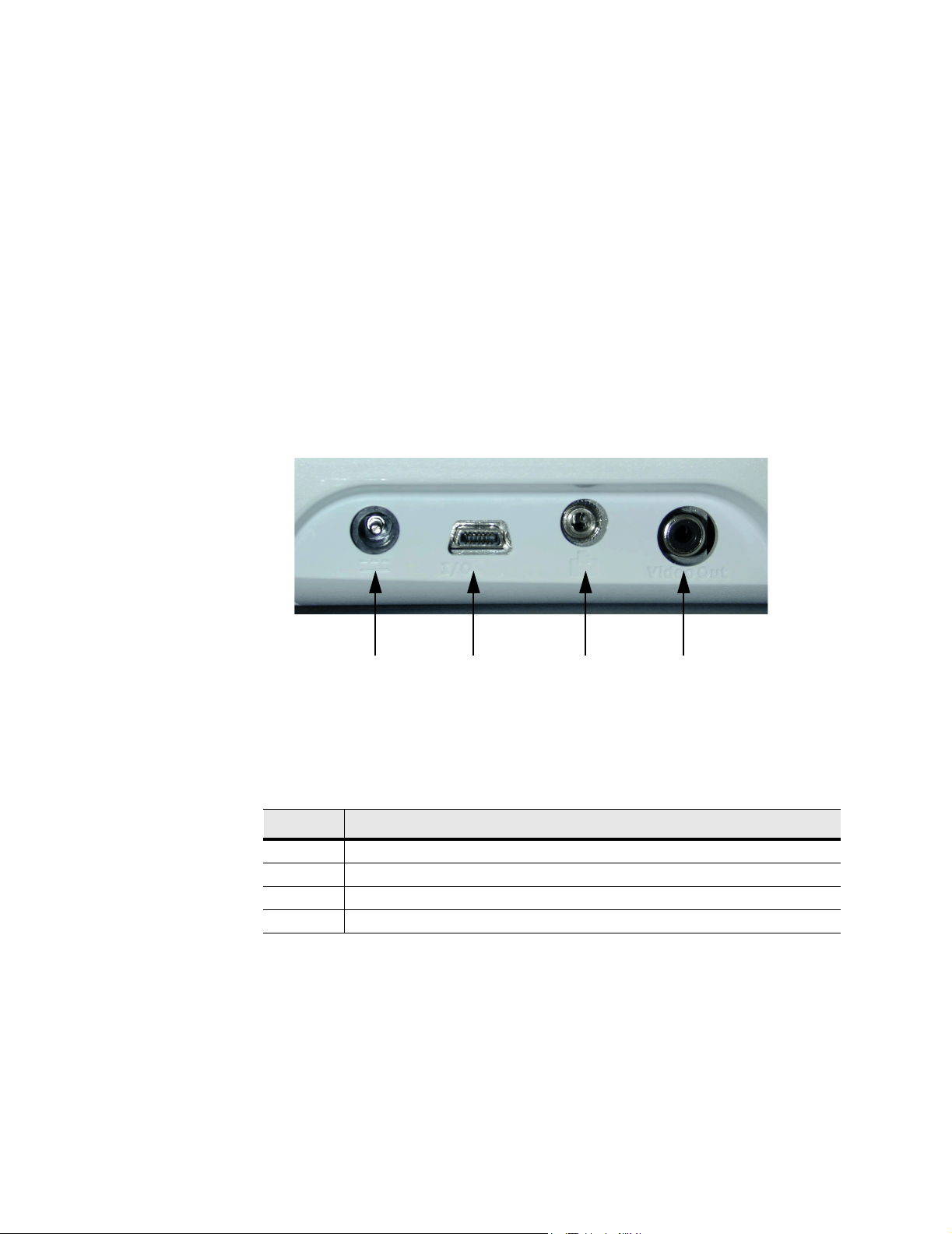
Figure 3.3 shows the following external system connections:
• A DC line voltage connector (1) connects the system to a power adapter.
• An I/O connector (2) provides for connection to a PC or input of an ECG signal
for ECG display on the monitor.
• A print control connector (3) connects the system to a printer.
• A video out connector (4) provides a composite video signal for a VCR, printer,
or external video monitor.
12 34
Figure 3.3 External System Connections
Table 3.3 External System Connections
NUMBER FEATURE
1 DC line voltage connector
2 I/O connector
3 Print control connector for a recommended printer
4 Video out connector for a recommended VCR, printer, or external video monitor
16 C1.9 PLUS Ultrasound System Service Manual
Page 25
3.4.3 Power Adapter
CAUTION:
` Use only the specified SonoSite power adapter.
A universal power adapter (50–60 Hz, 100–240 V ac) can power the SonoSite
system. When the system is plugged into a wall outlet, the battery pack recharges.
Recharging a battery which is not fully discharged will not decrease battery life.
Keep the ambient temperature between 32
battery successfully. To maintain battery charge, attach the power adapter to the
system when the device is not in use.
Power Cord
Table 3.4 Power Cord Specifications
° and 104°F (0° and 40°C) to charge a
CONFIGU-
RATION
100-120 V ac
ACC/ 60 Hz
230 V ac /50 Hz /
PAL
230 V ac / 60 Hz
LENGTH RATING MALE PLUG
9 ft. 10 in. 250 V ac MA 5-15P
Hospital grade,
grounding type
molded on
3 m 250 V ac CEE-7/VII
grounding type
with 4.8 mm pins
molded on
3.5 System Specifications
This section provides specifications for the SonoSite ultrasound system.
3.5.1 Physical Dimensions
Height: 13.3 in. (33.8 cm)
Width: 7.6 in. (19.3 cm)
Depth: 2.5 in. (6.35 cm)
FEMALE
CONNEC-
TOR
CEE-22,
molded on
CEE-22,
molded on
APPRO
-VALS
UL,
CSA
EU Manufac-
MARKING
Manufacturer,
Agency
Approvals
turer,
Agency
Approvals
Weight: 5.4 lbs (2.46 kg) with the C60 transducer connected
3.5.2 Monitor
Height: 3.1 in. (7.9 cm)
Width: 4.3 in. (10.9 cm)
Diagonal: 5 in. (12.7 cm)
Brightness control
Contrast control
Chapter 3: System Overview 17
Page 26

3.5.3 Transducers
C60/5-2 MHz 60-mm
C15/4-2 MHz 15-mm
C11/7-4 MHz 11-mm
ICT/7-4 MHz 11-mm
L38/10-5 MHz 38-mm
3.5.4 Imaging Modes
2D Imaging (256 gray shades)
CPD Imaging (64 colors)
M-mode
PowerMap DCPD Imaging (64 colors)
Pulsed Wave (PW) Doppler
Tissue Harmonic Imaging
3.5.5 Image Storage
Up to 119 images (depending on the configuration of the system)
Cine review
3.5.6 Temperature, Pressure, and Humidity Limits
3.5.6.1 System Operating
• 50–104°F (10–40°C), 15–95% R.H.
• 700-1060hPa (0.7 ATM to 1.05 ATM)
3.5.6.2 System Shipping/Storage
• -31–149°F (-35–65° C), 15–95% R.H.
• 500-1060hPa (0.5ATM to 1.05 ATM)
3.5.6.3 Battery Operating
• 50–104°F (10–40°C), 15–95% R.H.
3.5.6.4 Battery Shipping/Storage
• -4–140°F (-20–60°C), 0–95% R.H.
3.5.6.5 Transducers Operating
• 50–104°F (10–40°C), 15–95% R.H.
18 C1.9 PLUS Ultrasound System Service Manual
Page 27

3.5.6.6 Transducers Shipping/Storage
• -31–149°F (-35–65°C), 15–95% R.H
3.5.7 Electrical
• System optional: 100-120/220-240 V ac, 50/60 Hz input, 16.0 V dc output
power adapter
• SiteCharge dual battery charger input voltage: 16.0 V dc, 2.8 A
• SiteCharge dual battery charger output voltage: 12.6 V dc, 3.0 A (2x)
• AC power adapter input: 100-120/220-240 V ac, 50/60 Hz, 1.0-0.50 A
• AC power adapter output: + 16.0 V dc, 2.8 A
3.6 Battery
• 6-cell, 11.1 V dc, 3.0 amp-hours, rechargeable, lithium-ion battery pack
• Run time: 1.5 to 4 hours, depending upon operating conditions
3.7 Safety Requirements
3.7.1 Meets Electromechanical Safety Standards
EN 60601-1:1997, European Norm, Medical Electrical Equipment–Part 1. General
Requirements for Safety.
EN 60601-1-1:1993, European Norm, Medical Electrical Equipment–Part 1.
General Requirements for Safety–Section 1-1. Collateral Standard. Safety
Requirements for Medical Electrical Systems.
EN 60601-1-2:1998, European Norm, Medical Electrical Equipment. General
Requirements for Safety-Collateral Standard. Electromagnetic Compatibility.
Requirements and Tests.
EN 60601-2-25: 1996, European Norm, Medical Electrical Equipment–Part 2.
Particular Requirements for Safety–Section 25. Specification for
Electrocardiographs.
C22.2, No. 601.1:1998, Canadian Standards Association, Medical Electrical
Equipment–Part 1. General Requirements for Safety.
CEI/IEC 61157:1992, International Electrotechnical Commission, Requirements for
the Declaration of the Acoustic Output of Medical Diagnostic Ultrasonic
Equipment.
UL 2601-1:1999, Underwriters Laboratories, Medical Electrical Equipment-Part 1:
General Requirements for Safety.
Chapter 3: System Overview 19
Page 28

3.7.2 Meets EMC/EMI Standards
IEC 61000-4-2:1999, International Electrotechnical Committee, Electromagnetic
Compatibility (EMC)-Part 4. Testing and Measurement Techniques-Section 4.2:
Electrostatic Discharge/Immunity Test-Basic EMC Publication
IEC 61000-4-3:1997, International Electrotechnical Committee, Electromagnetic
Compatibility (EMC)-Part 4. Testing and Measurement Techniques-Section 3:
Radiated Radio-Frequency, Electromagnetic Field Immunity Test.
IEC 61000-4-4:1995, International Electrotechnical Committee, Electromagnetic
Compatibility (EMC)-Part 4. Testing and Measurement Techniques-Section 4,
Electrical Fast Transient/Burst Immunity Test-Basic EMC Publication.
IEC 61000-4-5:1999, International Electrotechnical Committee, Electromagnetic
Compatibility (EMC)-Part 4. Testing and Measurement Techniques-Section 5,
Surge Immunity Test.
CISPR11:97, International Electrotechnical Commission, International Special
Committee on Radio Interference. Industrial, Scientific, and Medical (ISM) RadioFrequency Equipment Electromagnetic Disturbance Characteristics-Limits and
Methods of Measurement.
3.7.3 Meets Airborne Equipment Standards (without ECG Cable Attached)
RTCA/DO160D:1997, Radio Technical Commission for Aeronautics,
Environmental Conditions and Test Procedures for Airborne Equipment, Section
21.0 Emission of Radio Frequency Energy, Category B.
3.7.4 Meets ECG Standard
AAMI EC53:1995, Association for the Advancement of Medical Instrumentation,
ECG Cables and Lead Wires.
20 C1.9 PLUS Ultrasound System Service Manual
Page 29
CHAPTER 4 Setup and Operation
WARNING:
` CRITICAL TEST FUNCTION - Skipping the functional tests in this procedure
could adversely affect safety or effectiveness of the system.
Operation of the SonoSite ultrasound system can be found in the user guide provided
with each system.
4.1 Connecting and Removing Transducers
The system comes with one or more transducers. Only one transducer can be
connected to the system at a time.
WARNING:
` The transducer connector can become hot during operation. This is normal.
Operate the system in the SiteStand mobile docking station or on a flat, hard
surface to allow air flow past the connector.
CAUTION:
` The electrical contacts inside the system transducer connector may be damaged by
foreign material or by rough handling. Do not touch the electrical contacts. Keep
foreign material out of the connector. Keep a transducer connected to the system
whenever possible.
To connect the transducer:
1. On the transducer connector, pull the latch up and rotate it clockwise until it
snaps to a stop (Figure 4.1). The latch should be easy to move. Do not force the
latch.
2. Align the transducer connector with the connector on the rear of the system and
insert it by pushing the transducer connector into the system connector. The
transducer connector should be easy to insert. Do not force the transducer
connector.
3. Turn the latch counterclockwise until it snaps to a stop.
Chapter 4: Setup and Operation 21
Page 30

4. Press the latch down until it snaps into place, securing the transducer connector
to the system.
Transducer connector Transducer connector latch
Figure 4.1 Connecting the Transducer
To remove the transducer:
1. On the transducer connector, pull the latch up and rotate it clockwise until it
snaps to a stop (Figure 4.1).
2. Carefully pull the transducer connector away from the system.
4.2 Turning the System On and Off
When turning power on or off, you must push and hold the Power switch for
approximately one second before the system responds. This feature prevents battery
discharge, resulting from accidentally turning the system on. It also prevents
accidentally turning the system off during an exam.
The first time you turn on the system, set the date and time. See the SonoSite
Ultrasound System User Guide.
CAUTION:
` Do not use the system if an error message appears on the image display. Note the
error code. Call SonoSite or your local representative. When an error code occurs,
turn off the system by pressing and holding the power switch until the system
powers down.
To turn on power:
1. Locate the Power switch on the back of the left side of the system handle. See
Figure 3.2, SonoSite Ultrasound System Controls, on page 14.
2. Press and hold the Power switch until the system beeps or until you see the
image display.
3. Release the Power switch.
22 C1.9 PLUS Ultrasound System Service Manual
Page 31

To turn off power:
1. Press and hold the Power switch.
You will hear the system emit two sets of “high-low” beeps. The system powers
off after the second set of beeps.
2. Release the Power switch.
To wake up the system:
• The system has a sleep delay, which is activated through the sleep delay system
setup. When the battery charge indicators are blinking, but the other system
lighting is off, press any system control to wake up the system.
4.3 Installing and Removing the Battery
The battery comprises six lithium-ion cells (two sets of three connected in series)
plus electronics, a temperature sensor, and the battery contacts. When in use, it is
inserted into the system.
WARNING:
` If you are holding the system, when you remove the battery, place your hand
beneath the battery. If it falls to the floor, it could be damaged, or cause personal
injury.
If the battery is being installed for the first time, it will need to be charged. Make
sure to remove the protective tape from the battery contacts before charging the
battery.
To install the battery:
1. Locate the battery compartment at the bottom of the system (Figure 4.2).
2. Install a new battery (label side up) into the battery compartment by pushing it
into the compartment until it clicks in place. (Do not force the battery into the
compartment, check the battery orientation if it is difficult to install.) Ensure that
both sides of the battery are fully connected and that the battery release button is
not pressed.
Figure 4.2 Inserting the Battery
Chapter 4: Setup and Operation 23
Page 32

To remove the battery:
1. Turn off the system.
2. Locate the battery compartment at the bottom of the system.
WARNING:
` If you are holding the system, when you remove the battery, place your hand
beneath the battery. If it falls to the floor, it could be damaged, or cause personal
injury.
3. To release the battery, press the battery release button (lower, right side) on the
system.
4.4 Using AC Power
The battery charges when the system is using AC power. If the system is off and
connected to AC power, a fully discharged battery will charge in about three hours.
To operate the system using AC power:
1. Connect the line cord to the AC power adapter.
2. Connect the line cord to a hospital-grade electrical outlet.
3. Connect the AC power adapter to the system using the upper, left connector on
the left side of the system. (Figure 4.3).
Note: You must wait approximately 10 seconds after connecting to AC power before
turning on the power switch.
12 34
Figure 4.3 System Connections
Table 4.1 System Connections
NUMBER FEATURE
1 DC line voltage connector
2 I/O connector
3 Print control connector for a recommended printer
4 Video out connector for a recommended VCR, printer, or external video
monitor
24 C1.9 PLUS Ultrasound System Service Manual
Page 33

4.5 Upgrading the System Software
Transducers that you receive from SonoSite may contain either required or optional
upgrades to the system software that resides on your SonoSite system.
Whenever you connect a transducer to a SonoSite system, the system communicates
with the transducer to determine if it contains software that would upgrade the
system.
CAUTION:
` Initiating any upgrade of the system software erases any images stored on your
system. Do not upgrade the system software until you have determined that any
stored images are no longer needed.
To upgrade the system software:
1. When you first connect a transducer with new software and turn the system on,
the following message displays:
Do you want to upgrade the system software?
For required upgrades:
You must perform the software upgrade or replace the transducer with one that is
compatible with the software currently installed on your system. Do one of the
following:
• Select no (disconnect transducer) to reject the system software upgrade.
• Select yes (up to 20 minutes) to accept the system software upgrade and go to
step 2.
For optional upgrades:
You may either install the new software or continue to use the existing software.
SonoSite recommends that you install these optional upgrades soon after receiving
them. Do one of the following: Select no (continue) to use the system without
upgrading.
• Select yes (up to 20 minutes) to accept the upgrade and go to step 2.
2. When you have accepted the upgrade, the system loads the new software and
displays the following message:
Upgrade in progress (20 minutes total).
Note: The system upgrade can take up to 20 minutes; however, many software
upgrades will be completed in less time.
To cancel the upgrade in progress, select cancel.
If this is a required upgrade:
• The existing software remains installed.
• The system displays the following message:
Incompatible transducer, upgrade the system software.
If this is an optional upgrade:
• The existing software remains installed.
• The system will go to live scan.
3. When the system has loaded the new software, the following message displays:
Successful upgrade.
If the software upgrade is unsuccessful, the system displays an error code and
you must contact SonoSite technical support. U.S. customers, call 1-877-657-
8118. International customers, call 1-425-951-1330 or contact your local
representative.
Chapter 4: Setup and Operation 25
Page 34

4. Select reboot to restart your system.
During the restart, the initial system screen will show two progress indicator
bars. These progress indicator bars are present while the system is replacing its
operating software and will disappear when the process is complete.
When the operating software has been replaced, the system presents you with
the license update screen so that you may license the software. At this point, the
software upgrade process is complete, but the system software is not yet
licensed. The following section explains how to license your software.
4.6 Obtaining A License Key
A license key is required to update your SonoSite system. To obtain a license key,
do one of the following:
If you encounter difficulty with the system, use the information in this chapter to
help correct the problem. If the problem is not covered here, call SonoSite technical
support at the following numbers or addresses:
USA/Canada Customers
• Technical support: 1-877-657-8118
• Technical support fax and email: 1-425-951-6700; service@sonosite.com
• SonoSite website: www.sonosite.com
International Customers
• Call your local representative or 1-425-951-1330.
To receive your license key, you will need to provide the following information,
which is displayed on the system information screen of your system (except for the
person installing the upgrade and the system serial number):
• Name of the person installing the upgrade
• System serial number (SN) (located on the back of your system)
• ARM Ver: (version)
• PCBA Serial No: (number)
See Section 4.6.1.1, Displaying the System Information Screen, on page 28. If the
system is on and the grace period expires, view the license information screen on the
system information screen.
4.6.1 Installing A License Key
When you have obtained a license key for your software, you must enter it into the
system. Once a valid license key has been entered, the system remains licensed until
the next time the system software is upgraded.
1. Turn on the system. If the software is not yet licensed, the license update screen
displays (Figure 4.4).
The license update screen displays the following information: the License Update
number, the ARM Ver (version), the PCBA Serial No. (number), the SonoSite web
site address and telephone number, the license number, the register later or done
, and the grace period (time remaining) on your system.
26 C1.9 PLUS Ultrasound System Service Manual
Page 35

Figure 4.4 License Update Screen
Note: The software versions on your system may vary based on your upgrade
and configuration.
2. Enter your license key in the license number field.
If the license key that you entered is recognized by the system as being valid for
your system and the software you installed, done appears on-screen. Select
done from the on-screen menu to install the license key and license your
software.
If the license key that you entered is not recognized by the system, the register
later button remains on the screen as long as the defined grace period has not
expired.
If the grace period has expired, the menu item will indicate this by showing zero
hours remaining in the grace period. At this point, you must then enter a valid
license key before you can use the system with this or any other transducer.
Note: If you have entered a valid license key and you cannot complete the
licensing procedure, verify that the license key has been entered correctly. The
license key should be exactly 12 digits (for example, 123348990552) with no
other characters or punctuation.
Note: If after confirming correct entry of the license key, you are still unable to
license your system, call SonoSite technical support. USA/Canada customers call
1-877-657-8118. International customers call your local representative or
1-425-951-1330.
If the system is on and the grace period expires, the license update screen must
be displayed from the system information screen.
Chapter 4: Setup and Operation 27
Page 36

4.6.1.1 Displaying the System Information Screen
To display the system information screen:
1. Press and release the Function
2. Press and release I. The system information screen displays (Figure 4.5).
The system information screen displays the following information: the Boot/PIC
Vers (version), the ARM/DSP Vers, the PCBA Serial No (number), the Product
Name, the Status, the PLD 1, 2, Vers, CPLD SH Ver, SHDB Ver (scanhead
database version), and the Sh Serial No (scanhead serial number).
Note: The software versions on your system may vary based on your upgrade
and configuration.
key.
Figure 4.5 System Information Screen
To display the license update screen:
1. On the system information screen, select the unlock icon (upper left corner of
the screen).
The license update screen displays.
2. Perform the steps in Section 4.6.1, Installing A License Key, on page 26.
4.7 Checking and Charging the Battery
To check the battery:
Note: Disconnect the system from AC power before checking the battery charge.
Five LEDs (light-emitting diodes) on the right side of the system monitor allow you
to check the battery condition. If all LEDs are lit, the battery is fully charged. A solid
dark gray battery icon in the lower right portion of the system display indicates a low
battery. A solid white battery icon indicates approximately 10 minutes of battery life
remaining. A flashing white battery icon indicates approximately 2 minutes of
battery life remaining.
28 C1.9 PLUS Ultrasound System Service Manual
Page 37

The system will operate on a fully-charged battery for 1.5 to 4 hours, depending
upon use. Ensure the battery is charged at all times to provide the longest possible
battery operation. You can set the sleep delay and power delay to prolong battery
life.
When the system is not likely to be used for some time, to prevent total battery
discharge, remove the battery from the system.
To charge the battery:
CAUTION:
` Charge batteries only when the ambient temperature is between 32° and 104°F (0°
and 40°C).
1. Connect the AC line cord of the AC power adapter to a hospital-grade electrical
outlet.
2. Connect the DC line cord of the AC power adapter to the power connector on
the system. (1) (Figure 4.6 and Table 4.2
3. Charge the battery until it is fully charged.
Note: It takes about three hours to charge a battery when the system is off.
4. Disconnect the system from AC power.
5. Turn the system on to check the battery charge.
).
12 34
Figure 4.6 System Connections
Table 4.2 System Connections
NUMBER FEATURE
1 DC line voltage connector
2 I/O connector
3 Print control connector for a recommended printer
4 Video out connector for a recommended VCR, printer, or external video monitor
Chapter 4: Setup and Operation 29
Page 38

This page intentionally left blank.
30 C1.9 PLUS Ultrasound System Service Manual
Page 39

CHAPTER 5 Cleaning and Disinfecting
5.1 Universal Precautions
SonoSite recommends that personnel who have regular exposure to medical devices
returned for service practice “universal precautions.” Universal precautions are an
approach to infection control. Those servicing this product should follow the
prescribed standards for their area.
5.2 Receipt of Suspected Contaminated Materials
• If visual inspection suggests possible contamination when opening a product
returned for service, take proper steps to contain the contamination. Wear
necessary Personal Protective Equipment (PPE) (gloves, masks, and gowns)
when opening or examining a suspect package.
• Before transfer to a service area, label the suspect package “contaminated” and
seal it to prevent exposure.
• Discard any packing materials removed from a package suspected of
contamination in a biohazard container.
• Discard any contaminated materials received with the product in an appropriate
biohazard container. Contaminated materials may include biohazardous waste
and sharps.
• Maintain a disinfecting agent in case any work surface is contaminated. The
recommended agent is 0.5% sodium hypochlorite (bleach) solution. To prepare
the agent, mix one part household bleach (5.25% - 6% sodium hypochlorite) to
nine parts water. Spray or wipe the solution onto the work surface and allow to
air dry.
Please use these recommendations when cleaning or disinfecting your ultrasound
system, transducers, and accessories. This chapter assists in effective cleaning and
Chapter 5: Cleaning and Disinfecting 31
Page 40

disinfection, but it is also intended to protect the system and transducers against
damage during cleaning or disinfection.
For more information about cleaning or disinfecting solutions or ultrasound gels for
the transducer, call SonoSite technical support or your local representative. For
information about a specific product, call the product manufacturer.
5.3 Recommended Disinfectants
For a list of disinfectants recommended for use on the SonoSite ultrasound system
and transducers, see the SonoSite Ultrasound System User Guide.
32 C1.9 PLUS Ultrasound System Service Manual
Page 41

CHAPTER 6 Troubleshooting
6.1 System and Subsystem Diagnosis
This section covers basic diagnostic and troubleshooting procedures you may need if
the system does not operate properly. To diagnose system failures, consult Table 6.1
and the referenced diagnostic figures that follow.
Table 6.1 Troubleshooting Subassemblies and Diagnostic Figures
SUBASSEMBLIES DIAGNOSTIC FIGURES
Display Figure 6.2
External Display Figure 6.3
Control Panel Figure 6.4
Trackball Figure 6.5
System Figure 6.6
Battery Figure 6.7
6.2 System Repair
The system is repairable through subassembly replacement.
6.3 Test Equipment
There is no test equipment required for this troubleshooting section. Test aids
include an external monitor, a spare battery, and a SiteCharge™ dual battery
charger.
Chapter 6: Troubleshooting 33
Page 42

6.4 Failures
Maint
6.4.1 Display
Attach an external monitor to the external video connector to verify display failures.
For example, if the system display is blank and the external monitor works properly,
the system display requires servicing.
6.4.2 Control Panel
Go to the patient information screen and press each individual key on the keyboard
to identify and verify control panel failures. Press function keys and note their
response.
6.4.3 Trackball
Intermittent function or loss of control indicates trackball failures. Clean the
trackball by removing the retainer ring and then removing the ball.
6.4.4 Main PCBA
The main PCBA can present symptoms that may be difficult to assess. Main PCBA
failures result in “assert codes” that are output to the display. Note these assert codes
and contact SonoSite technical support, per Appendix B, Service Event Report, on
page 85, to clarify the failure. Figure 6.1
icon displayed on the system screen.
shows an assert code and a maintenance
enance icon
Assert code
Figure 6.1 Assert Code and Maintenance Icon
34 C1.9 PLUS Ultrasound System Service Manual
Page 43

6.4.5 Clearing the Main PCBA Failure
After the assert code has been recorded, power down the system.
1. Press the Power switch on the system until the power turns off (approximately
5–10 seconds).
2. Turn the power back on to check if the fault cleared or if the condition remains.
If the condition cleared, you may use the system. If the condition remains,
corrective action must be taken before the system can be used.
6.4.6 Battery
If the system does not operate or does not run for the expected duration for a given
charge, battery failure is likely.
Chapter 6: Troubleshooting 35
Page 44

If System
Display Is
Blank, Attach
An External
Monitor
Ext.
Monitor
OK?
Yes
Replace
Display
Corrected?
Replace
No
Main PCBA
No
Replace
No Corrected?
Control Panel
Corrected?
No
Yes
Return
System to
SonoSite
Perform
Image Quality
Tests
Yes
Perform
Image Quality
Tests
Yes
Perform
Image Quality
Tests
Figure 6.2 Display Diagnosis
36 C1.9 PLUS Ultrasound System Service Manual
Page 45

If External
Monitor
Display
Becomes
Blank, Check
The System
Display
Sys.
Display
OK?
No
Replace
Main PCBA
Corrected?
Yes
Ext.
Monitor
Cable
OK?
No
Connect
Cable
Stop
Yes
Check And
Adju st
Video
Format
No
Corrected?
Yes
Stop
Corrected? StopYes Yes
No
Correct
Monitor?
Yes
Replace
Cable
Corrected?
No
Yes
Replace
External
Monitor
Stop
No
Return
System to
SonoSite
No
Replace
Main PCBA
Return
Monitor to
SonoSite
Corrected?No Yes Stop
Figure 6.3 External Monitor Diagnosis
Chapter 6: Troubleshooting 37
Page 46

LED Burned Out?
Key Not
Working?
Replace
Control Panel
Corrected?
No
Replace
Main PCBA
Yes
Stop
Corrected?
Yes
Perform
Image Quality
Tests
Figure 6.4 Control Panel Diagnosis
No
Return
System to
SonoSite
38 C1.9 PLUS Ultrasound System Service Manual
Page 47

Trackball
Movement
Erratic?
Trackball
Doesn't Move
Cursor?
Keybd.
OK?
No
Clean
Trackball
Corrected? StopYes Yes
No
Replace
Trackball
Replace
Control
Panel
Corrected? StopYes
No
Corrected? StopYes
No
Replace
Main PCB
Figure 6.5 Trackball Diagnosis
Corrected? StopYes
No
Return
System to
SonoSite
Chapter 6: Troubleshooting 39
Page 48

No Indication Of
Power When You
Press The ON
Button?
Try Battery
Power Only
Make Sure
Battery Is
Fully Inserted
Listen For Two Beeps
And Look For A
Video Display.
If System Works On
Battery, Verify
Operation On AC
Power
First Remove
Battery And
Then Connect
AC Power
Working?
No
Battery
Charged?
Yes
Working?
No
Replace
Main PCB
Corrected? Yes
Yes
No
Yes
Try AC
Power
Charge or
Replace
Battery
Try AC
Power
Perform
Image Quality
Tests And
Verify AC
Power
Operation
YesWorking?
No
Replace
AC
Adapter
Working? System OKYes
No
Replace
Main PCB
Corrected? Yes
No
No Problem
Found
Perform
Image Quality
Tests And
Verify AC
Power
Operation
No
Return
System To
SonoSite
Figure 6.6 System Diagnosis
40 C1.9 PLUS Ultrasound System Service Manual
Page 49

No Indication of
Power When You
Press the ON Button?
Did You Hear Two
Beeps When You
Pressed the Power
Button? Is There A
Video Display?
No
Yes
No Problem
Found
Is Battery
Fully Inserted?
No
Insert
Battery and
Try Power
Again
Working?
No
Charge or
Replace
Battery
Yes
Yes
First Remove
Battery And
Then Connect
AC Power
No Problem
Found
Working? Yes
No
Go To System
Diagram
Replace
Battery and
Try Again
Working?
No
Go To System
Diagram
Yes
Problem
Solved
Figure 6.7 Battery Diagnosis
Chapter 6: Troubleshooting 41
Page 50

This page intentionally left blank.
42 C1.9 PLUS Ultrasound System Service Manual
Page 51

CHAPTER 7 Replacement Procedures
7.1 Display Subassembly Replacement
Note: Consult Chapter 6, Troubleshooting, on page 33 before making any repairs.
7.1.1 Required Parts
Service Assembly, Display, C1.9 (P02081)
Service Assembly, Display, C1.9 Japanese (P02545)
The Display Assembly does not include a label. One of the following labels must be
ordered and installed on the display. It must match the label on the display that is
being removed.
Label “180PLUS” (P01991)
Label “SonoHeart PLUS” (P01992)
Label “180 Vet” (P01993)
Label “180 II” (P01994)
7.1.2 Required Tools and Materials
• A #1 Phillips screwdriver, 7.0 in. (17.8 cm / 177.8 mm)
• A torque driver, 2.0–10.0 in./lb. (0.23–1.1 newton meter)
• An anti-static mat
• A wrist grounding strap
CAUTION:
` Always use correct ESD procedures. ESD damage is cumulative and may not be
noticeable at first. Initial ESD symptoms may be slightly degraded performance
or image quality.
Chapter 7: Replacement Procedures 43
Page 52

7.1.3 Removing the Display Subassembly
1. Press the battery release on the lower right side of the system to remove the
system battery.
2. Remove the two screws in the battery compartment with a #1 Phillips
screwdriver; this releases the Control Panel Subassembly from the top housing
(Figure 7.1).
Screws (2)
Battery
compartment
Figure 7.1 Removing the Control Panel Subassembly
3. Insert an anti-static mat between the Control Panel Subassembly and the Display
Subassembly to prevent damaging the display LCD (Figure 7.2).
4. Lay the Control Panel Subassembly over onto the Display Subassembly to
expose the wire harness and flex circuits.
5. Carefully disconnect the 100-pin flex circuit from the bottom module.
6. Disconnect the Display Subassembly Wire Harness and unlatch two flex circuits
connectors from the Control Panel Subassembly (Figure 7.2).
7. Set the Control Panel Subassembly aside.
44 C1.9 PLUS Ultrasound System Service Manual
Page 53

100-pin flex
circuit
Anti-static mat
40-pin flex circuit
40-pin flex circuit
Figure 7.2 Disconnecting the Display Wire Harness and Flex Circuits
Chapter 7: Replacement Procedures 45
Page 54

8. Remove the four screws that secure the Display Subassembly hinges to the top
housing with a #1 Phillips screwdriver (Figure 7.3).
9. Carefully guide the ends of the display wire harness and two 40-pin flex circuits
out through the slot in the top housing and remove the old Display Subassembly.
Top housing
Figure 7.3 Removing the Display Subassembly
7.1.4 Replacing the Display Subassembly
1. Carefully guide the ends of the replacement display wire harness and two 40-pin
flex circuits through the slot in the top housing (Figure 7.4).
Screws (4)
Display hinges
Figure 7.4 Replacing the Top Housing
Display Wire
Harness
40-pin flex
circuits (2)
46 C1.9 PLUS Ultrasound System Service Manual
Page 55

2. Secure the four screws on the replacement Display Subassembly hinges to the
top housing with a #1 Phillips screwdriver (Figure 7.3). Torque screws to
6.1 in./lb. (0.7 newton meter).
3. Place the Control Panel Subassembly onto the Display Subassembly.
4. Install the display wire harness and flex circuits.
Note: Check that the reference designators on the flex circuits match the reference
designators on the PCBA connectors.
5. Replace the 100-pin flex circuit on the bottom module (Figure 7.5).
Display
100-pin flex
circuit
Display Wire
Harness
Control Panel
Trackball
Anti-static mat
Figure 7.5 Placing the Control Panel Subassembly onto the Display
6. Carefully replace the Control Panel Subassembly by inserting the tabs on the top
of the Control Panel Subassembly into the slots.
7. Set the Control Panel into place and replace the two screws inside the battery
compartment (Figure 7.6). Torque screws to 7.1 in./lb. (0.8 newton meter).
8. Place the battery in the battery compartment.
9. Turn on the system.
10. Perform the Display tests in Chapter 8, Performance Tests, on page 65
to
verify that the Display Subassembly is functioning properly.
Chapter 7: Replacement Procedures 47
Page 56

7.2 Control Panel Subassembly Replacement
7.2.1 Required Parts
• Service Assembly, Control Panel C1.9, English (P02082), or
• Service Assembly, Control Panel C1.9, French (P02083), or
• Service Assembly, Control Panel C1.9, German (P02084), or
• Service Assembly, Control Panel C1.9, Italian (P02085), or
• Service Assembly, Control Panel C1.9, Portuguese (P02086), or
• Service Assembly, Control Panel C1.9, Spanish (P02087), or
• Service Assembly, Control Panel C1.9, Japanese (P02544), or
• Service Assembly, Trackball C1.9 (P02088)
Note: The Service Assembly, Control Panel C1.9 does not include a trackball. To
install a trackball, see Section 7.3, Trackball, on page 52.
7.2.2 Required Tools
• A #1 Phillips screwdriver, 7.0 in. (17.8 cm / 177.8 mm)
• A torque driver, 2.0–10.0 in./lb (0.2–1.1 newton meter)
• An anti-static mat
• A wrist grounding strap
CAUTION:
` Always use correct ESD procedures. ESD damage is cumulative and may not be
noticeable at first. Initial ESD symptoms may be slightly degraded performance
or image quality.
48 C1.9 PLUS Ultrasound System Service Manual
Page 57

7.2.3 Removing the Control Panel Subassembly
1. Press the battery release on the lower right side of the system to remove the
system battery.
2. Remove the two screws in the battery compartment with a #1 Phillips
screwdriver; this releases the Control Panel Subassembly from the top housing
(Figure 7.6).
Screws (2)
Battery
compartment
Figure 7.6 Removing the Control Panel Subassembly
3. Insert an anti-static mat between the Control Panel Subassembly and the Display
Subassembly to prevent damaging the Display LCD.
4. Carefully lay the Control Panel Subassembly over onto the display to expose the
display wire harness and the two 40-pin flex circuits.
5. Carefully disconnect the 100-pin flex circuit from the bottom module.
6. Disconnect the display wire harness and the two 40-pin flex circuits from the
Control Panel Subassembly (Figure 7.7).
7. Remove the Control Panel Subassembly.
Chapter 7: Replacement Procedures 49
Page 58

100-pin flex
circuit
Anti-static mat
Display Wire
Harness
40-pin flex circuits (2)
Figure 7.7 Disconnecting the Control Panel Subassembly
50 C1.9 PLUS Ultrasound System Service Manual
Page 59

7.2.4 Replacing the Control Panel Subassembly
1. Place the Control Panel Subassembly onto the Display Subassembly.
2. Install the wire harness and the two 40-pin flex circuits.
3. Replace the 100-pin flex circuit on the bottom module (Figure 7.7).
4. Carefully replace the Control Panel Subassembly by inserting the tabs on the top
of the assembly into the slots.
5. Set the Control Panel Subassembly in place; replace the two screws inside the
battery compartment using a #1 Phillips screwdriver. Torque the screws to
7.1 in./lb. (0.8 newton meter) (Figure 7.8).
Screws (2)
Battery
compartment
Figure 7.8 Replacing the Control Panel Subassembly
6. Place the battery in the battery compartment.
7. Turn on the system.
8. To verify proper operation of the new Control Panel Subassembly, perform the
Control Panel tests in Chapter 8, Performance Tests, on page 65
Chapter 7: Replacement Procedures 51
.
Page 60

7.3 Trackball
7.3.1 Required Parts
Service Assembly, Trackball, C1.9 (P02088)
7.3.2 Required Tools
• A #1 Phillips screwdriver, 7.0 in. (17.8 cm / 177.8 mm)
• A torque driver, 2.0–10.0 in./lb (0.2–1.1 newton meter)
• A small, blunt punch
• An anti-static mat
• A wrist grounding strap
• CAUTION: Always use correct ESD procedures. ESD damage is cumulative
and may not be noticeable at first. Initial ESD symptoms may be slightly
degraded performance or image quality.
7.3.3 Removing the Trackball
1. Press the battery release on the lower right side of the system to remove the
system battery.
2. Remove the two screws in the battery compartment using a #1 Phillips
screwdriver; this releases the Control Panel Subassembly from the top housing
(Figure 7.8).
3. Insert an anti-static mat between the Control Panel Subassembly and the display
LCD to prevent damaging the display LCD.
4. Lay the Control Panel Subassembly over onto the display LCD to expose the
wire harness and flex circuits.
5. Carefully disconnect the 100-pin flex circuit from the bottom module.
6. Disconnect the display wire harness and the two 40-pin flex circuits from the
Control Panel Subassembly.
7. Remove the Control Panel Subassembly.
8. Remove the trackball retaining ring. Carefully insert the tip of a blunt punch into
the indent and turn the trackball retaining ring slightly counterclockwise. This
releases the ring and allows you to remove it (Figure 7.9).
52 C1.9 PLUS Ultrasound System Service Manual
Page 61

Trackball retaining ring
Figure 7.9 Removing the Trackball Retaining Ring
9. Remove the ball.
10. Disconnect the trackball wire harness from the Control Panel Subassembly.
11. Remove the foam backing from the Trackball to expose the four screws
connecting the Trackball to the Control Panel Subassembly (Figure 7.10).
Figure 7.10 Removing the Foam Backing
Foam backing
Chapter 7: Replacement Procedures 53
Page 62

12. Remove the four screws that secure the Trackball to the Control Panel
Subassembly using a #1 Phillips screwdriver (Figure 7.11).
Screws (4)
Figure 7.11 Removing the Trackball
13. Holding the Trackball in place, carefully turn the Control Panel Subassembly
over and allow the Trackball to drop off of the Control Panel Subassembly, to
prevent losing the Trackball parts (Figure 7.12).
14. Remove the Trackball and note the location of its components.
Figure 7.12 Removing the Trackball
Trackball
Trackball Wire
Harness
54 C1.9 PLUS Ultrasound System Service Manual
Page 63

7.3.4 Replacing the Trackball
1. Remove the four screws that hold the top cover on the new Trackball using a #1
Phillips screwdriver. Discard the cover and screws. Be careful not to tip the
Trackball and spill the Trackball parts (Figure 7.12).
2. Holding the Trackball, carefully turn the Control Panel Subassembly over onto
the new Trackball and set it in place.
3. Carefully turn the Control Panel Subassembly over, holding the Trackball in
place.
4. Tighten the four screws securing the Trackball using a #1 Phillips screwdriver
(Figure 7.13). Torque the screws to 3.6 in./lb. (0.4 newton meter).
Screws (4)
Figure 7.13 Securing the Trackball
5. Place the foam backing on the Trackball (Figure 7.14).
Figure 7.14 Placing the Foam Backing
Chapter 7: Replacement Procedures 55
Foam backing
Page 64

6. Turn the Control Panel Subassembly over and install the new trackball retaining
ring. With your fingers, press and turn the ring slightly clockwise until it locks in
place (Figure 7.15).
Trackball retaining ring
Figure 7.15 Installing the Trackball Retaining Ring
7. Turn the Control Panel Subassembly over and secure the trackball wire harness
to the Control Panel Subassembly.
8. Place the Control Panel Subassembly onto the display LCD.
9. Install the display wire harness and the two 40-pin flex circuits (Figure 7.16).
10. Replace the 100-pin flex circuit on the bottom module.
100-pin flex
circuit
30-pin flex
circuits (2)
Figure 7.16 Installing the Display Wire Harness and Flex Circuits
11. Carefully replace the Control Panel Subassembly by inserting the tabs on the top
of the Control Panel Subassembly into the slots.
56 C1.9 PLUS Ultrasound System Service Manual
Page 65

12. Set the Control Panel Subassembly in place and replace the two screws inside
the battery compartment (Figure 7.17). Torque the screws to 7.1 in./lb. (0.8
newton meter).
Screws (2)
Battery
compartment
Figure 7.17 Replacing the Control Panel
13. Place the battery in the battery compartment.
14. Turn on the system.
15. Perform the trackball tests in Chapter 8, Performance Tests, on page 65
verify proper operation of the new trackball.
7.4 Main PCBA Subassembly Replacement
7.4.1 Required Parts
• Service Assembly, PCB C1.9 Main, English (P02075), or
• Service Assembly, PCB C1.9 Main, French (P02076), or
• Service Assembly, PCB C1.9 Main, German (P02077), or
• Service Assembly, PCB C1.9 Main, Italian (P02078), or
• Service Assembly, PCB C1.9 Main, Portuguese (P02079), or
• Service Assembly, PCB C1.9 Main, Spanish (P02080)
• Service Assembly, PCB C1.9 Main, Japanese (P02546)
to
Chapter 7: Replacement Procedures 57
Page 66

7.4.2 Required Tools
• A #1 Phillips screwdriver, 7.0 in. (17.8 cm / 177.8 mm)
• A torque driver, 3.6 in./lb (0.4 newton meter)
• An anti-static mat
• A wrist grounding strap
CAUTION:
` Always use correct ESD procedures. ESD damage is cumulative and may not be
noticeable at first. Initial ESD symptoms may be slightly degraded performance
or image quality.
7.4.3 Removing the Main PCBA Subassembly
1. Press the battery release on the lower right side of the system to remove the
system battery.
2. Place the system face down.
3. Remove the eight screws securing the bottom housing to the top housing using a
#1 Phillips screwdriver (Figure 7.18).
Screws (8)
Figure 7.18 Removing the Bottom Housing from the Top Housing
4. Carefully separate the bottom housing from the top housing; do not damage the
100-pin flex circuit that connects the two.
5. Disconnect the 100-pin flex circuit from the Main PCBA Subassembly.
6. Remove the bottom housing that holds the Main PCBA Subassembly.
58 C1.9 PLUS Ultrasound System Service Manual
Page 67

7. Remove the four screws that connect the Main PCBA Subassembly to the
bottom housing using a #1 Phillips screwdriver (Figure 7.19).
Screws (4)
40-pin flex
Figure 7.19 Removing the Main PCBA Subassembly from the Bottom Housing
8. Carefully disconnect the 40-pin flex circuit that connects the Main PCBA
Subassembly to the Dock Interface PCBA (located under the Main PCBA).
9. Remove the Main PCBA Subassembly.
7.4.4 Replacing the Main PCBA Subassembly
1. Set the replacement Main PCBA Subassembly onto the bottom housing
40 Pin flex
Figure 7.20 Replacing the Main PCBA Subassembly
2. Secure the four screws that connect the Main PCBA Subassembly to the bottom
housing (Figure 7.21). Torque the screws to 3.6 in./lb. (0.4 newton meter).
Chapter 7: Replacement Procedures 59
Page 68

3. Connect the 40-pin flex circuit to the Main PCBA Subassembly.
4. Carefully set the top housing onto the bottom housing, connecting the 100-pin
flex circuit as it moves into position (Figure 7.22).
Screws (4)
Figure 7.21 Securing the Main PCBA to the Bottom Housing
60 C1.9 PLUS Ultrasound System Service Manual
Page 69

Main PCBA
100-pin flex
circuit
Figure 7.22 Setting the Top Housing Onto the Bottom Housing
Chapter 7: Replacement Procedures 61
Top housing
Page 70

5. Carefully mate the bottom housing to the top housing and secure the eight
screws using a #1 Phillips screwdriver (Figure 7.23). Torque the screws to
7.1 in./lb. (0.8 newton meter).
Screws (8)
Figure 7.23 Securing the Bottom Housing to the Top Housing
6. Place the battery into the battery compartment.
7. Turn on the system.
8. Verify that the Main PCBA Subassembly functions properly by performing the
tests in Chapter 8, Performance Tests, on page 65
7.5 Transducers
The transducers have no user-serviceable parts; return transducers
to SonoSite for exchange.
7.6 AC Power Adapter
The AC power adapter has no user-serviceable parts. Return power adapters to
SonoSite for exchange.
.
62 C1.9 PLUS Ultrasound System Service Manual
Page 71

7.7 OEM Peripherals
If you need assistance to locate a nearby service center, contact SonoSite technical
support.
7.7.1 External Monitor (Sony)
To return a failed display, contact the Sony Service Center at 714-220-9100. For
service, ship the display to the original OEM or send Sony displays to the following
address:
Sony Service Center
10833 Valley View
Cypress, CA 90630
United States
7.7.2 VCR (Sony)
To return a failed VCR, contact the Sony Service Center at 800-282-2848 or 972931-2497. For service, ship the VCR to the original OEM or send Sony VCRs to the
following address:
Sony Electronics, Inc.
7517 Campbell Road
Dallas, TX 75248
United States
7.7.3 Printer (Sony)
To return a failed printer, contact the Sony Service Center at 770-263-9888. For
service, ship the printer to the original OEM or send Sony printers to the
following address:
Sony Service
3175 A Northwood Parkway
Norcross, GA 90071
United States
Chapter 7: Replacement Procedures 63
Page 72

This page intentionally left blank.
64 C1.9 PLUS Ultrasound System Service Manual
Page 73

CHAPTER 8 Performance Tests
8.1 Overview
WARNING:
` Critical Test Function - A failure of the system functions tested in this section
could affect safety or effectiveness of the system adversely. While performing the
steps in this section, verify that the images on the system display and on the
external monitor are acceptable.
• Verify that all controls operate smoothly over their full range and that the system
responds properly.
• To obtain 2D images, SonoSite recommends using the RMI 413A Soft Tissue
Phantom or, the RMI 403 GS Multipurpose Phantom. Any equivalent Phantom
is acceptable.
• To obtain Power Doppler images, SonoSite recommends using the RMI 425
Doppler Phantom or the RMI 1425A Doppler Phantom. Any equivalent
Phantom is acceptable.
• When making penetration measurements on a phantom, apply the phantom
reference value and tolerance to the measurement.
8.2 Test Equipment:
• SonoSite ultrasound system with a C60/5-2 MHz transducer and an L38/10-5
MHz transducer
• RMI 413A Soft Tissue Phantom, RMI 403 GS Multipurpose Phantom, or the
equivalent
• RMI 425 Doppler Phantom, RMI 1425A Doppler Phantom, or the equivalent
• ECG Simulator
• Video Printer
• External Monitor
• Acoustic gel
Chapter 8: Performance Tests 65
Page 74

8.3 Setting Up Performance Tests
To set up the performance tests:
1. Attach the C-60/5-2 MHz transducer to the system.
2. Select general for optimization and OB for exam type.
3. Couple the transducer to the phantom, adjusting gain settings and transducer for
a proper phantom image (e.g., pins are high-level echoes positioned in straight
lines; cysts are sonolucent, edges are sharp, and graphite particles of the
phantom are mid-grays).
8.3.1 Scan Reference Orientation
1. Verify that the correct transducer name appears in the upper right corner of the
system display.
2. Verify that the scan plane orientation mark in the image located near the skinline
corresponds to element #1 on the transducer. To test, put your finger on the
probe and run it across the transducer face. Your finger touching the transducer
face should appear at the orientation mark on the display image format.
3. With the array pointing down and the orientation mark to the operator’s left,
element #1 corresponds with the left side of the array.
8.4 Testing 2D Performance
To test 2D performance:
1. Use a C60/5-2 MHz transducer in 2D mode.
2. Adjust the position of the C60/5-2 MHz transducer on the phantom.
3. Use the 2D system controls to obtain a clear image that shows both the
horizontal and vertical rows of pins.
8.4.1 2D Image Quality
To test 2D image quality:
1. Verify that the ultrasound image appears uniform in both the axial and lateral
direction, with no dropouts or intensity variations.
2. Verify that the cystic structure at the focal zone is clearly differentiated from the
surrounding tissue and is echo-free, while solid tissue, with numerous echo
sources, appears solid.
66 C1.9 PLUS Ultrasound System Service Manual
Page 75

8.4.2 Axial Measurement Accuracy
Note: Measurements must be performed while the image is frozen.
To test axial accuracy:
1. Acquire the image.
2. Press Freeze.
3. Press Measure. Two calipers appear on the image display. A menu appears, on
which are listed two distance icons, an ellipse icon, a delete icon, and a calcs
icon, if applicable. (If the caliper line setup is on, then a dotted line connects the
two calipers. See the SonoSite Ultrasound System User Guide, if necessary.) The
first caliper in the menu is active by default.
4. Use the trackball to position one of the calipers.
5. Press Select to fix the caliper and enable the other caliper.
6. Use the trackball to move the other caliper. The results update as you move the
caliper, and the measurement is complete when you finish moving the calipers.
(Press Select to alternate the active caliper, and adjust the measurement with the
trackball.)
7. To perform another distance measurement on the image, select the other
distance icon and repeat the preceding steps.
8. Measure the distance, center to center, of two pins that are 5-12 cm apart
vertically.
9. Verify that the distance measured is within the tolerance listed in Table 8.1.
8.4.3 Lateral Measurement Accuracy
To test the lateral measurement accuracy:
1. Perform steps 1 through 7 in Section 8.4.2.
2. Measure the distance, center to center, of two pins that are 4-10 cm. apart
horizontally.
3. Verify that the distance measured is within the tolerance listed in Table 8.1.
4. Press Freeze to return the system to live 2D mode.
Table 8.1 System Measurement Accuracy
MEASUREMENTS TOLERANCE
Axial Distance +/- 2%
Lateral Distance +/- 2%
Chapter 8: Performance Tests 67
Page 76

8.4.4 Penetration
To test penetration:
1. Adjust the system controls to obtain a clear image that shows the limits of echo
penetration as shown in Table 8.2.
2. Measure from the center of the skinline to the deepest vertical position—where
the scatter echoes start to break up and tissue definition is lost.
Table 8.2 Imaging Performance
IMAGING
PERFORMANCE
2D Penetration 11.5 cm 6.0 cm 5.0 cm 19.0 cm 6.0 cm 5.0 cm
C60 ICT ICT HF C15 L38 C11
8.5 Additional Performance Tests
8.5.1 CPD
To test CPD:
Note: Use the RMI 425 Doppler Phantom or the RMI 1425A Doppler Phantom.
1. Connect any transducer and set up the system for CPD mode.
2. Acquire the image.
3. Press and release the 8 key for CPD mode. A Region of Interest (ROI) box is
displayed on top of the grayscale image. (Press the 0 key to return to 2D
imaging.)
To move the CPD image:
•Use the trackball to move the CPD ROI. While you are moving the CPD
ROI, you will see an outline of the new position moving on the display. When
you stop moving, the new position will display the CPD ROI. (The size of the
CPD ROI is fixed. There is no control to change it.)
To adjust CPD gain:
•Turn gain clockwise to increase the amount of CPD gain. (While in CPD
imaging, near and far affect only the 2D image; they do not affect the CPD
image.)
•Turn gain counterclockwise to decrease the amount of CPD gain.
4. Image the vessel using a Doppler phantom. Verify that as the gain controls
increase and decrease, Doppler echo intensity increases and decreases to
correspond. Verify that no flow exists outside the vessel.
5. Save a CPD image by pressing Freeze and then Save.
68 C1.9 PLUS Ultrasound System Service Manual
Page 77

8.5.2 PowerMap DCPD
To test PowerMap DCPD:
Note: Use the RMI 425 Doppler Phantom or the RMI 1425A Doppler Phantom.
1. Set up the system per section 8.5.1 for CPD mode.
2. Press the 8 key while in CPD mode for PowerMap DCPD. A Region of Interest
(ROI) box is displayed on top of the 2D grayscale. (Press the 0 key to return to
2D imaging.)
To move the PowerMap DCPD image:
•Use the trackball to move the PowerMap DCPD ROI. While you are moving
the PowerMap DCPD ROI, you will see an outline of the new position
moving on the display. When you stop moving, the new position will display
the PowerMap DCPD ROI. (The size of the PowerMap DCPD image is fixed.
There is no control to change it.)
To adjust PowerMap DCPD gain:
•Turn gain clockwise to increase the amount of PowerMap DCPD gain.
(While in PowerMap DCPD imaging, near and far affect only the 2D image;
they do not affect the PowerMap DCPD image.)
•Turn gain counterclockwise to decrease the amount of PowerMap DCPD
gain.
3. Image the vessel using a Doppler phantom. Verify that as the gain controls
increase and decrease, Doppler echo intensity increases and decreases to
correspond. Verify that no flow exists outside the vessel.
4. Save a PowerMap DCPD image by pressing Freeze and then Save.
8.5.3 M-mode Imaging
1. Attach a C60 transducer and acquire an image.
2. Press the 9 key for the M-mode sample line.
3. Position the M-mode sample line over the image by moving the trackball left
or right.
4. Press the 9 key again to turn on M-mode.
5. Adjust the sweep speed by pressing the Menu key and pressing the sweep speed
key from the on-screen menu.
6. Press the Freeze key to freeze the image. Press it again to return to live imaging.
7. Press the 0 key again to return to 2D imaging.
Chapter 8: Performance Tests 69
Page 78

8.5.4 Tissue Harmonic Imaging
1. Attach the C60 transducer and acquire an image.
2. Set the depth to maximum and note the depth at which echo information is lost.
3. Press the Menu key.
4. Press the T
Imaging in now active.
5. Observe a decrease in dot size and a significant loss in penetration due to the
higher frequency. Image resolution increases.
6. Press the T
H key on the on-screen menu so it displays TH (on). Tissue Harmonic
H key again to turn off Tissue Harmonic Imaging.
8.5.5 Pulsed Wave (PW) Doppler Imaging
1. Attach the C60 transducer.
2. Press the 7 key for the Doppler sample gate.
3. Press the 7 key again for the Doppler spectral trace.
4. Place a large drop of ultrasound gel on the transducer lens.
5. Gently tap the top of the gel and observe a reflection on the spectral trace and the
sound from the speakers.
6. Press the 0 key to return to 2D imaging.
8.5.6 ECG Monitoring
1. Connect an ECG Cable to the I/O connector located on the left side of the
system.
2. Connect an ECG simulator set for 60 BPM, Normal Sinus Rhythm.
3. Set the ECG amplitude to 0.5 mV and verify the trace stabilizes and shows the
appropriate waveform.
4. Set the ECG amplitude to 2.0 mV and verify the trace stabilizes and shows the
appropriate waveform.
5. Save an ECG image.
70 C1.9 PLUS Ultrasound System Service Manual
Page 79

8.5.7 Image Quality Verification Test
To test the image quality:
• Products with replaced subassemblies, or products that have been disassembled
otherwise, must undergo an Image Quality Verification Test.
• The Image Quality Verification Test should be performed after completing
(successfully) Section 8.3, Setting Up Performance Tests, on page 66 and
Section 8.5.1, CPD, on page 68.
• The test is completed before returning the system to service.
• A certified sonographer must perform the test.
8.5.8 Image Review
Review all saved images and verify that the images are displayed properly.
8.5.9 Printer
To test printer operation:
1. Print two images in rapid succession and verify proper operation.
2. Verify that the print control receptacles on the system and on the stand function
correctly.
8.5.10 Battery Charging
To test battery charging operation:
1. Insert a battery into the system.
2. Remove AC power from the system AC power connector.
3. Press and hold the Power switch to turn the system on. Allow the battery to
discharge. The battery indicator LEDs (to the right of the display) extinguish
from top to bottom as the battery discharges.
Note: The battery may take 1–2 hours to discharge.
4. Re-attach the AC power cord to the AC power connector.
5. Note that the battery indicator LEDs light from bottom to top as the battery
charges.
6. If charging is not evident within 30–60 minutes, refer to Chapter 6,
Troubleshooting, on page 33
8.5.11 Video Output
CAUTION:
` Use only the recommended video monitor, printer, or VCR when verifying the
video output at the video receptacle.
for troubleshooting procedures.
Chapter 8: Performance Tests 71
Page 80

To test the video output:
1. Attach an external video monitor to the video connector using the video cable.
2. Turn on the system power and verify that the video on the external monitor
matches the video on the system display.
If the video does not appear similar, or there is no display on the external monitor,
refer to Chapter 6, Troubleshooting, on page 33
for troubleshooting procedures.
8.6 Returning Products to SonoSite
8.6.1 Contacting SonoSite Technical Support
For technical support of any SonoSite product, do one of the following:
• U.S. and Canadian customers, call 1-877-657-8118.
• International customers, call +425-951-1330.
• Connect to SonoSite on the World Wide Web at www.sonosite.com
Products, then choose Technical Support
•E-mail service@sonosite.com
.
. Select
You will be asked to provide the following information by telephone or e-mail:
• Contact name and phone number
• Product name
• Serial number
• Description of the problem
8.6.2 Shipping Instructions
Please contact SonoSite to get a return material authorization number (RMA).
Contact SonoSite before returning any product.
The shipping address for all returned products is:
• SonoSite, Inc.
• Attn: Technical Support RMA ___________________
• 21919 30th Drive SE
• Bothell, Washington 98021-3904
•USA
72 C1.9 PLUS Ultrasound System Service Manual
Page 81

APPENDIX A Parts List
This section contains a list of field-replaceable parts.
A.1 Replacement Parts List
The following tables contain all the replaceable parts for the SonoSite ultrasound
system. All quantities are one unless otherwise noted.
A.2 Ordering Replacement Parts
To order parts, contact SonoSite Technical Support as indicated in Chapter 8.6,
Returning Products to SonoSite, on page 72
.
Appendix A: Parts List 73
Page 82

A.3 Display
234
18
5
976
Table A.1 Display
FIND
NUMBER
Note: One of the following labels must be ordered for the display.
6 P01991 Label “180PLUS”
6 P01992 Label “SonoHeart PLUS”
6 P01993 Label “180 Vet”
6 P01994 Label “180 II”
Note: The following parts are available for purchase separately.
1 P01213 Flex circuit, Display
2 P00806 Knob, TGC Assembly
3 P00457 Label, SonoSite logo
PART
NUMBER
P02081 Service Assembly, Display, C1.9
P02545 Service Assembly, Display, C1.9, Japanese
DESCRIPTION
4 P00343 Hinge, Display
5 P00518 Screw, k30x14, cross recess panhead, tf
7 P00340 Lens, display
8 P00611 Assembly, flex circuit, frame
9 P02362 LCD 5”
74 C1.9 PLUS Ultrasound System Service Manual
Page 83

A.4 Control Panel
Table A.2 Control Panel
1
FIND
NUMBER
1 P01792 Label function keys, English
1 P02429 Label function keys, Japanese 180 PLUS
PART
NUMBER
P02082 Service Assembly, Control Panel C1.9, English
P02083 Service Assembly, Control Panel C1.9, French
P02084 Service Assembly, Control Panel C1.9, German
P02085 Service Assembly, Control Panel C1.9, Italian
P02086 Service Assembly, Control Panel C1.9, Portuguese
P02087 Service Assembly, Control Panel C1.9, Spanish
P02544 Service Assembly, Control Panel C1.9, Japanese
DESCRIPTION
Appendix A: Parts List 75
Page 84

A.5 Trackball
1
Table A.3 Trackball
FIND
NUMBER
Note: The following parts are available for purchase separately.
1 P00093 Retaining ring
PART
NUMBER
P02088 Service Assembly, trackball
P00725 Screw, k25x16, cross recess panhead
DESCRIPTION
76 C1.9 PLUS Ultrasound System Service Manual
Page 85

A.6 Main PCBA
For replacement procedures, see Chapter 7.4, Main PCBA Subassembly
Replacement, on page 57
.
1
2
3
4
9
5
6
7
8
Appendix A: Parts List 77
Page 86

Table A.4 Main PCBA
FIND
NUMBER
1 P00441 Screw, flathead, 6-32, ss, 3/8
2 P00354 Backer plate
3 P00353 Wear plate
4 P00524 Screw, shoulder, thrust plate
5 P00709 Gasket, nest frame
6 P00364 Connector, interposer
7 P00646 Spring, thrust plate,.047 wire
8 P00351 Nest frame assembly, cast
9 P01904 Speaker assembly
PART
NUMBER
P00029 Assembly flex circuit, 100-pin (not shown)
P03482
Service assembly, PCB C1.9 Main
Please provide system serial number, language,
and system model (SonoHeart PLUS or 180PLUS)
when ordering
DESCRIPTION
78 C1.9 PLUS Ultrasound System Service Manual
Page 87

A.7 Power Supply and Power Cable
1
Table A.5 Power Supply and Power Cable
2
FIND
NUMBER
1 P00538 Power supply, 85-264 V ac, 50-60 HZ, 16 V, 45 W
2 P00848 Power cable, North America
PART
NUMBER
P00849 Power cable, Australian
P00850 Power cable, UK/Ireland
P00851 Power cable, India/South Africa
P00852 Power cable, Israel
P00853 Power cable, Russian
P00854 Power cable, Argentina
P00855 Power cable, Japanese
P00856 Power cable, Italian
P00857 Power cable, Chinese
P00858 Power cable, Switzerland
P00859 Power cable, Danish
P00864 Power cable, International, no ground pin
DESCRIPTION
P00875 Power cable, Japanese, two prong with jumper wire
P02905 Power cable, Olympus
Appendix A: Parts List 79
Page 88

A.8 Accessory Cables
1
4
6
8
23
5
9
10
11
7
80 C1.9 PLUS Ultrasound System Service Manual
Page 89

Table A.6 Accessory Cables
INDEX
NUMBER
1 P01314 Cable, BNC/BNC video, 3.5 ft.
2 P01111 Cable, RCA/RCA video
3 P02046 Cable, serial, PC Direct
4 P01167 Cable, RCA/stereo
5 P00536 Cable, RCA/BNC video
6 P01592 Cable, ECG
7 P02223 ECG patient lead assembly
8 P00762 Cable, printer control, 1 ft.
9 P00537 Cable, printer, control
10 P00763 Cable, BNC/BNC video, 1 ft.
11 P01568 Cable, serial, null modem
PART
NUMBER
DESCRIPTION
Appendix A: Parts List 81
Page 90

A.9 Additional Spare Parts
1
2
3
4
6
Table A.7 Additional Spare Parts
INDEX
NUMBER
1 P00104 Key, on/off
2 P00361 Foot
3 P01795 Assembly, PCB, dock interface
PART
NUMBER
7
8
9
5
1012 11
DESCRIPTION
4 P00362 Contact, battery clip, x3
5 P00360 Spring, clip
6 P00110 Enclosure, bottom, ui
7 P00301 Assembly, flex circuit 40-pin
8 P00640 Spring, battery ejection, x2
9 P00356 Button, release
10 P00357 Slide, release
82 C1.9 PLUS Ultrasound System Service Manual
Page 91

Table A.7 Additional Spare Parts, Continued
INDEX
NUMBER
11 P00358 Retainer, slide
12 P00359 Pivot, slide
PART
NUMBER
P00107 Contact, dock interface clip (not shown)
P00113 Enclosure, top, UI (not shown)
DESCRIPTION
Appendix A: Parts List 83
Page 92

84 C1.9 PLUS Ultrasound System Service Manual
Page 93

APPENDIX B Service Event Report
The Service Event Report provides information about product failures to the
manufacturer and to authorized service facilities, which provide approved warranty
services for SonoSite products. For all repairs completed, complete the form and
return a copy of it to the following address:
SonoSite, Inc.
Technical Support
21919 30th Drive SE
Bothell, Washington 98021-3904
telephone: 1-877-657-8118 (U.S. customers)
+425-951-1330 (international customers)
facsimile: +425-951-6700
e-mail: service@sonosite.com
website: www.sonosite.com
Select Products, then Technical Support.
Appendix B: Service Event Report 85
Page 94

Service Event Report
Service Provider
Name: Date:
Company:
Address:
Phone Number: Fax Number:
E-mail address:
Device Description
Name: Serial Number:
Part Number: Lot Number: Revision:
Software Version: Other Identifiers:
Event Description
Diagnosis
Service Performed
Performed By: Date:
Actions:
Parts Removed
Part Name Part Number Serial Number Lot Number Rev Replaced By
Parts Installed
Part Name Part Number Serial Number Lot Number Rev Replaced By
Tests Performed (attach test data)
Test: Test:
Performed By: Performed By:
Result: Pass Fail Result: Pass Fail
Page ____ of ____ F00019 Rev B
Attach additional sheets as required
86 C1.9 PLUS Ultrasound System Service Manual
Page 95

Index
Numerics
2D imaging 1
2D performance tests
axial measurement accuracy 67
image quality 66
lateral measurement accuracy 67
penetration 68
A
AC power
see also power
using 24
AC power adapter 62
accessories 17
acquisition subsystem 12
B
battery 2
charge indicators 15
charging 29
checking 28
diagnosis figure 41
installing 23
safety 8
specifications 19
storage and shipping 18
battery charging
test 71
battery pack
operating specifications 15
biological safety 9
control subsystem 12
conventions 2
CPD imaging 1
D
diagnosis figures 33
disinfectants
recommended 32
display
diagnosis figures 36
failures 34
display assembly 43
removing 44
replacing 46
required parts 43
required tools and materials 43
display subsystem 12
E
electrical
safety 5
specifications 19
environmental specifications 18
equipment protection 7
error message 7
external power 15
power supply 17
F
fetal tables 17
C
C11/7-4 transducer 1
C15/4-2 transducer 1
C60/5-2 transducer 1
cable 2
cleaning and disinfecting 31–32
precautions 31
Color Power Doppler
adjusting gain 68, 69
contaminated materials
receipt of 31
control panel assembly 48
removing 49
replacing 51
required parts 48
required tools 48
G
grace period 3
H
humidity limits 18
I
ICT/7-4 transducer 1
image
quality verification test 71
review 71
storage 18
87
Page 96

imaging
modes specified 18
introduction 1–26
K
keyboard
diagnosis figure 38
L
L38/10-5 MHz 38-mm 18
L38/10-5 transducer 1
labeling
symbols 9
license key 3
installing 26
obtaining 26
license update screen
displaying 28
M
main PCBA 57–62
failures 34
removing 58
replacing 59
required parts 57
required tools 58
monitor 17
external diagnosis 37
specifications 17
moving image 68, 69
P
parts list 73–82
additional spare parts 82
control panel assembly 75
display assembly 74
main PCBA 77
trackball assembly 76
performance tests 65–72
overview 65
peripheral output 71
printer 71
required test equipment 65
video output 71
peripherals 17, 63
medical grade 2
non-medical grade 2
printer 63
VCR 63
phantoms
PFS-A-3-1-G2 UHDC Flow System
(Doppler) 65
RMI 1425A Doppler 65
RMI 403 GS Multipurpose 65
RMI 413A Soft Tissue 65
RMI 425 Doppler 65
physical dimensions 17
power
battery charge indicators 15
battery pack 15
connections 16
external 16
line cord specifications 17, 29
subsystem 13
supply 17
turning off 23
turning on 22
power adapter 2
PowerMap DCPD
imaging 1
printers
test 71
processing subsystem 12
product failures
reporting 85
products
returning 72
R
replacement parts
list 73
ordering 73
replacement procedures 43–63
return material authorization number (RMA) 72
S
safety 5–9
battery 8
biological 9
electrical 5
equipment protection 7
labeling symbols 9
service event report 85
form 86
setup and operation 5, 21
shipping
instructions 72
shipping specifications 18
SiteCharge dual battery charger 2, 62
SiteLink Image Management software 2
SitePack carrying case 2
SiteStand mobile docking station 2
88 C1.9 PLUS Ultrasound System Service Manual
Page 97

software
licensing 3
upgrading 25
SonoSite technical support
contacting 72
standards
airborne equipment 20
electromechanical safety 19
EMC/EMI 20
storage
image 18
specifications 18
Subassembly 44, 58, 59
subassembly
replacement 33
symbols, labeling 9
system
charging requirements 15
components 1
controls 14
depth 17
diagnosis figure 40
dimensions 17
height 17
measurement accuracy 67
overview 11–20
software 2
specifications 17
temperature limit 18
turning off 22
turning on 22
waking up 23
weight 17
width 17
system and subsystem
diagnosis 33
system information screen
displaying 28
T
theory of operation 11–13
trackball
diagnosis figure 39
trackball assembly 52
removing 52
replacing 52, 55
required parts 52
required tools 52
transducer 17
acquisition subsystem 11
C15/4-2 MHz 15mm 18
C60/5-2 MHz 60mm 18
connecting 21
ICT/7-4 MHz 11mm 18
removing 22
servicing 62
specifications 18
troubleshooting 33–41
failures 34
test equipment 33
U
universal precautions 31
user interface subsystem 12
V
video
output tests 71
W
warnings 7
89
Page 98

90 C1.9 PLUS Ultrasound System Service Manual
 Loading...
Loading...