Smith & Nephew Renasys EZ User Manual
User guide
For use with Soft Port
RENASYS™ EZ RENASYS EZ PLUS
Negative Pressure Wound
Therapy
User guide
RENASYS™ EZ
RENASYS EZ PLUS
Negative Pressure Wound Therapy
Table of contents |
page |
Introduction |
4 |
Device description |
4 |
Indications for use |
6 |
Contraindications |
6 |
Warnings |
6 |
Precautions |
6 |
Glossary of symbols |
8 |
Physician orders |
9 |
Canister selection |
9 |
Canister installation |
9 |
Dressing changes |
10 |
Removing or changing the canister |
10 |
Operating the device |
11 |
Safety alarms |
12 |
Troubleshooting guide |
14 |
Maintenance |
17 |
Cleaning |
17 |
Battery operation |
17 |
Fuse replacement |
17 |
Returning the device |
18 |
Storage |
18 |
Electromagnetic compatibility |
19 |
Specifications |
21 |
Customer assistance/warranty |
22 |
Introduction
This user manual contains important information regarding the safe and effective operation of RENASYS™ EZ (p/n 66800059) and RENASYS EZ Plus (p/n 66800697) Negative Pressure Wound Therapy [NPWT] devices. This manual is intended to aid in training of personnel and to provide a reference for experienced users.
Also included are instructions for commissioning the device, maintenance, cleaning and disposal.
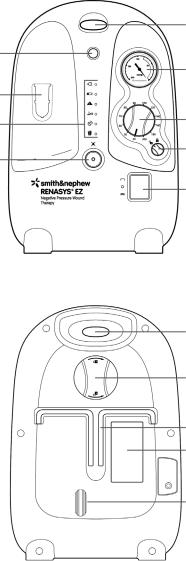
Device description |
|
|
Canister holder bracket |
|
|
Handle |
|
|
Mode of operation switch |
Vacuum port |
|
|
||
Vacuum port |
|
|
Pressure selector |
Canister |
|
Pressure selector lock |
holder bracket |
|
Status lights |
||
|
||
Vacuum gauge |
|
|
Status lights |
Audio pause |
|
Audio pause button and status light |
button and |
|
status light |
||
Air exhaust outlet |
|
|
AC power inlet and fuse |
|
|
IV pole lock knob |
|
|
IV pole pad |
|
|
Bed hooks |
|
|
Specification badge |
|
|
Rubber feet |
|
The following Smith & Nephew components are required for the proper and effective use of the device:
RENASYS-G Gauze Dressing Kit with Soft Port
RENASYS-F Foam Dressing Kit with Soft Port
RENASYS AB Abdominal Dressing Kit with Soft Port
250ml S-Canister Kit (p/n 66800913)
800ml S-Canister Kit (p/n 66800912)
Bacterial Overflow Guard (p/n 66800194)
Front view of device
(RENASYS EZ shown)
Handle
Vacuum gauge
Pressure selector
Pressure selector lock
Mode of operation switch
Handle
IV pole lock knob
Bed hooks
Specification badge
IV pole pad
4
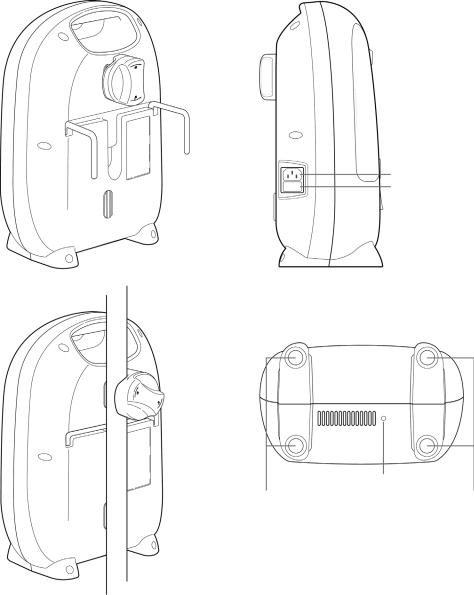
Rear view of device showing bed hooks and |
Left view of device |
IV pole mount in use |
|
AC power inlet
Fuse cover
Underside of device
|
Air exhaust outlet |
Rubber feet |
Rubber feet |
5
Indications for use
RENASYS™ EZ and RENASYS EZ Plus devices are indicated for patients who would benefit from a suction device (negative pressure wound therapy), as it
may promote wound healing via removal of fluids, including irrigation and body fluids, wound exudates and infectious materials.
Examples of appropriate wound types include:
•Chronic
•Acute
•Traumatic
•Sub-acute and dehisced wounds
•Ulcers (such as pressure or diabetic)
•Partial-thickness burns
•Flaps and grafts
Contraindications
The use of the device is contraindicated in the presence of:
•Necrotic tissue with eschar
•Untreated osteomyelitis
•Malignancy in wound (with exception of palliative care to enhance quality of life)
•Exposed arteries, veins, organs or nerves
•Non-enteric and unexplored fistulas
•Anastomotic sites
Warnings
1.Carefully monitor patients for signs of sudden or increased bleeding. If such symptoms are observed, immediately discontinue therapy, take
appropriate measures to control the bleeding, and contact the treating clinician.
2.Patients suffering from difficult hemostasis or who are receiving anticoagulant therapy have an increased risk of bleeding. During negative
pressure therapy, avoid using hemostatic products that may increase the risk of bleeding.
3.Do not use on exposed blood vessels or organs. Sharp edges such as bone fragments must be covered or removed prior to initiating therapy, due to risk of puncturing organs or blood vessels drawn closer under the action of negative pressure.
4.Foam or gauze must not be tightly packed or forced into any wound area.
5.In the event defibrillation is required, disconnect the device from the wound dressing prior to defibrillation. Remove the wound dressing only if its location will interfere with defibrillation.
6.RENASYS devices are not MRI compatible. Do not bring the RENASYS device into the MRI suite.
7.When operating, transporting, repairing or disposing of RENASYS devices and accessories, the risk of infectious liquids being aspirated, or contamination of the device assembly through incorrect use, cannot be eliminated. Universal precautions should be observed whenever working with potentially contaminated parts or equipment.
8.NPWT has not been studied on pediatric patients. Patient size and weight should be considered when prescribing RENASYS devices.
9.RENASYS devices are unsuitable for use in areas where there is danger of explosion (e.g., hyperbric oxygen unit, or in the presence of a flammable anesthetic mixture with air or nitrous oxide). Disconnect the device from the dressing prior to entering an area where this equipment will be used.
10.Canister kits are provided non-sterile and should not be placed within a sterile field.
Precautions
1.Precautions should be taken for patients who are or may be:
-Receiving anticoagulant therapy or platelet aggregation inhibitors, actively bleeding or have friable blood vessels or organs
-Suffering from abnormal wound hemostasis
-Untreated for malnutrition
-Noncompliant or combative
-Suffering from wounds in close proximity to blood vessels or delicate fascia.
2.CT scans and x-ray have the potential to interfere with some electronic medical devices. Where possible, move the device out of the x-ray or scanner range. If the device has been taken
into the CT scan or x-ray range, check that it is functioning correctly following the procedure.
6
3.As a condition of use, the RENASYS™ device should only be used by qualified and authorized personnel. The user must have the necessary knowledge of the specific medical application for which NPWT is being used.
4.If the RENASYS device has been at temperatures below freezing the device must be brought to room temperature prior to use or the pump unit may be damaged.
5.Inspect the Bacterial Overflow Guard on the canister and replace the canister as necessary. At minimum, the canister should be changed weekly. Always use the smallest canister volume possible
– do not use a large canister on patients with a high risk of bleeding.
6.If any liquids penetrate the RENASYS device, discontinue use and return to your authorized provider for service.
7.Do not use a dressing kit with breached or damaged packaging.
8.The use of NPWT presents a risk of tissue ingrowth. Tissue in-growth may be reduced by reducing therapy pressure, using a wound contact layer or by increasing the frequency of dressing changes.
9.Underlying structures, such as tendons, ligaments and nerves should be covered with natural tissue or a non-adherent dressing layer prior to applying the NPWT dressing kit.
10.If multiple pieces of foam or gauze are needed to fill the wound profile, count and record how many foam pieces are present to ensure all the foam pieces are removed at a dressing change.
11.Infected wounds may require more frequent dressing changes. Regular monitoring of the wound should be maintained to check for signs of infection.
12.NPWT should remain on for the duration of the treatment. If the patient must be disconnected, the ends of the tubing should be protected using the tethered cap. The length of time a patient may be disconnected from the RENASYS device is a clinical decision based on individual characteristics of the patient and the wound.
Factors to consider include the location of the wound, the volume of drainage, the integrity of the dressing seal, the assessment of bacterial burden and the patient's risk of infection.
13.Ensure that tubing and Soft Port are installed completely and without any kinks to avoid leaks or blockages in the vacuum circuit. Position the RENASYS device and tubing appropriately to avoid the risks of causing a trip hazard. Whenever possible, the device and system tubing should be positioned level with or below the wound.
14.When bathing or showering, the patient must disconnect from the RENASYS device and should protect both ends of the tubing using the tethered caps. Ensure that the aeration disc, located near the Quick Click Connector, is free of excess moisture before reactivation of therapy.
15.NPWT should not be painful. If the patient reports discomfort, consider reducing the pressure.
16.Maintain regular monitoring of the RENASYS device and wound site during therapy to ensure therapeutic treatment and patient comfort.
17.As with all adhesive products apply and remove the dressing carefully from sensitive or fragile skin to avoid skin stripping, especially after frequent dressing changes.
Precaution specific to foam:
1.Foam should be cut to fit loosely into the wound bed. Never force or tightly pack foam into any areas of the wound, to avoid damaging underlying tissue.
2.Never place foam into blind or unexplored tunnels. If a tunnel of known depth presents, cut the foam longer than the tunnel, to ensure direct contact is made with the foam in the primary wound cavity.
3.Do not cut the foam directly over the wound cavity to avoid foam fragments from falling into the wound. Rub the edges of the foam, away from the open wound, to remove loose fragments after cutting.
7
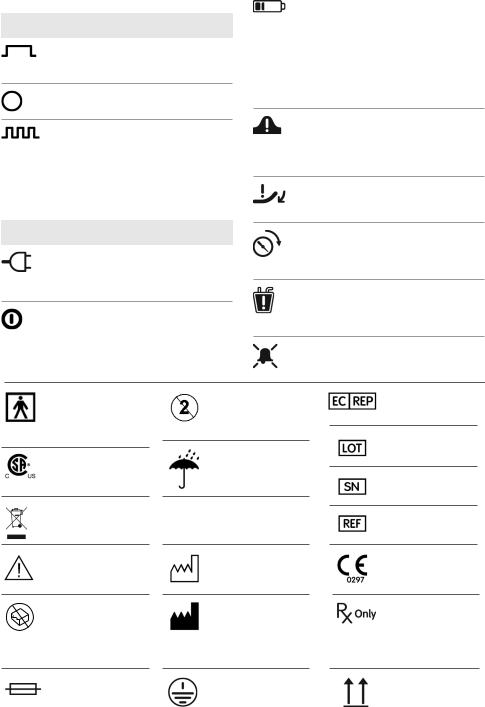
Glossary of symbols
Operation switch
Continuous therapy Device will maintain the preset vacuum level without stopping until switched off
OFF position Device stops delivering NPWT
Intermittent therapy
•RENASYS™ EZ device produces vacuum for approximately 32 seconds and turns off for approximately 16 seconds
•RENASYS EZ Plus device produces vacuum for approximately 5 minutes and turns off for approximately 2 minutes
Status lights
Mains power (Feature available only on RENASYS EZ) When the system is connected to an AC outlet, the status light will illuminate green; does not indicate device is turned on
On/off (Feature available only on RENASYS EZ Plus) When the operation switch is in the Continuous or Intermittent mode, the status light will illuminate green
Battery indicator
•Battery full: solid green status light
•Battery charging: blinking green status light
•Battery low: blinking yellow status light and audible alarm
•Battery fault (feature available only on RENASYS EZ Plus): solid yellow status light
Over vacuum When the system encounters excessively high vacuum (of >235mmHg) the device will stop delivering NPWT. The audible alarm will sound and the status light will flash yellow
Leak When the system detects a significant leak the audible alarm will sound and the status light will flash yellow
Low vacuum If the vacuum level is lower than set point of therapy by >15mmHg, the audible alarm will sound and the status light will flash yellow
Blockage/Canister full When the system detects that the canister is full or that there is a blockage in the system the audible alarm will sound and the status light will flash yellow
Alarm suppress Pressing the alarm suppress button will silence the alarm for approximately 2-4 minutes
Equipment classification
Isolation type BF applied part
CSA international classification
EU:
not for general waste
Caution:
see instructions for use
Do not use if package is damaged
Single use
do not reuse
Keep dry
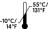 Storage temperature
Storage temperature
Date of manufacture
Place of manufacture
European representative
Lot number
Serial number
Product catalog number
CE mark
Caution: U.S. Federal law restricts this device to sale by or on the order
of a physician
Fuse |
Earthing |
Keep upright |
|
location of protective |
|
|
earthing terminals |
|
8
 Loading...
Loading...