Samsung MySono U6 Service manual

Service Manual

Samsung Medison provides the following warranty to the purchaser of this unit. This warranty is valid for
a period of one year from the date of installation and covers all problems caused by faulty workmanship
or faulty material. Samsung Medison will, as sole and exclusive remedy and at no charge, replace any such
defective unit returned to Samsung Medison within the designated warranty period.
The warranty does not cover damages and loss caused by outside factors including, but not limited to, re,
ood, storm, tidal wave, lightning, earthquake, theft, abnormal conditions of operation, and intentional
destruction of the equipment. Damage caused by equipment relocation is not covered.
The warranty is void in cases where the equipment has been damaged as a result of an accident, misuse,
abuse, dropping, or when attempts to modify or alter any part or assembly of the equipment have taken
place.
Parts with cosmetic defects or deterioration will not be replaced. Replacement of batteries, training
materials, and supplies are not covered.
Samsung Medison will not be responsible for incidental or consequential damages of any kind arising
from or connected with the use of the equipment.
Samsung Medison will not be responsible for any loss, damage, or injury resulting from a delay in services
rendered under the warranty
This limited warranty is in lieu of all other warranties expressed or implied, including warranties of
merchant ability or tness for any particular use. No representative or other person is authorized to
represent or assume for Samsung Medison any warranty liability beyond that set forth herein.
Defective equipment shipped from you to Samsung Medison must be packed in the replacement
cartons. Shipping and insurance costs are the responsibility of the customer. To return defective material
to Samsung Medison contact the Samsung Medison Customer Service Department.
Samsung Medison or a local distributor will make available, upon request, circuit diagrams, a component
parts list, descriptions, calibration instructions and other information which will assist your appropriately
quali ed technical personnel to repair those parts of the equipment which are designed by Samsung
Medison as repairable.
CAUTION: United State federal law restricts this device to sale by or on the order of physicians.
WARRANTY
MANUFACTURER : SAMSUNG MEDISON CO., LTD.
Samsung Medison Bldg., 42, Teheran-ro 108-gil, Gangnam-Gu, Seoul,135-280 Korea
SAMSUNG MEDISON CO., LTD. Customer Service Department
TEL : 82-2-2194-1234 FAX : 82-2-2194-1071
Website: www.samsungmedison.com
EC REPRESENTATIVE
SONOACE Deutschland GmbH Elbestrasse 10, 45768 Marl, Germany
TEL : 49-2365-924-3810
FAX : 49-2365-924-3830

WARRANTY
Samsung Medison provides the following warranty to the purchaser of this unit. This warranty is valid for
a period of one year from the date of installation and covers all problems caused by faulty workmanship
or faulty material. Samsung Medison will, as sole and exclusive remedy and at no charge, replace any such
defective unit returned to Samsung Medison within the designated warranty period.
The warranty does not cover damages and loss caused by outside factors including, but not limited to, re,
ood, storm, tidal wave, lightning, earthquake, theft, abnormal conditions of operation, and intentional
destruction of the equipment. Damage caused by equipment relocation is not covered.
The warranty is void in cases where the equipment has been damaged as a result of an accident, misuse,
abuse, dropping, or when attempts to modify or alter any part or assembly of the equipment have taken
place.
Parts with cosmetic defects or deterioration will not be replaced. Replacement of batteries, training
materials, and supplies are not covered.
Samsung Medison will not be responsible for incidental or consequential damages of any kind arising
from or connected with the use of the equipment.
Samsung Medison will not be responsible for any loss, damage, or injury resulting from a delay in services
rendered under the warranty
This limited warranty is in lieu of all other warranties expressed or implied, including warranties of
merchant ability or tness for any particular use. No representative or other person is authorized to
represent or assume for Samsung Medison any warranty liability beyond that set forth herein.
Defective equipment shipped from you to Samsung Medison must be packed in the replacement
cartons. Shipping and insurance costs are the responsibility of the customer. To return defective material
to Samsung Medison contact the Samsung Medison Customer Service Department.
Samsung Medison or a local distributor will make available, upon request, circuit diagrams, a component
parts list, descriptions, calibration instructions and other information which will assist your appropriately
quali ed technical personnel to repair those parts of the equipment which are designed by Samsung
Medison as repairable.
CAUTION: United State federal law restricts this device to sale by or on the order of physicians.
MANUFACTURER : SAMSUNG MEDISON CO., LTD.
Samsung Medison Bldg., 42, Teheran-ro 108-gil, Gangnam-Gu, Seoul,135-280 Korea
SAMSUNG MEDISON CO., LTD. Customer Service Department
SONOACE Deutschland GmbH Elbestrasse 10, 45768 Marl, Germany
TEL : 82-2-2194-1234 FAX : 82-2-2194-1071
Website: www.samsungmedison.com
EC REPRESENTATIVE
TEL : 49-2365-924-3810
FAX : 49-2365-924-3830


Service Manual
Version 1.00.00
English
CSD-SMDU6


Safety Classications
Classications:
X
Type of protection against electrical shock: Class I
X
Degree of protection against electrical shock (Patient connection):Type BF equipment
X
Degree of protection against harmful ingress of water: Ordinary equipment
X
Degree of safety of application in the presence of a ammable anesthetic material with air or
with oxygen or nitrous oxide: Equipment not suitable for use in the presence of a ammable
anesthetic mixture with air or with oxygen or nitrous oxide.
X
Mode of operation: Continuous operation
Electromechanical safety standards met:
X IEC/EN 60601-1 Medical Electrical Equipment, Part 1General Requirements for Safety.
X IEC/EN 60601-1-1 Safety requirements for medical electrical systems.
X IEC/EN 60601-1-2 Electromagnetic compatibility -Requirements and tests.
X IEC/EN 60601-2-37 Particular requirements for the safety of ultrasonic medical diagnostic and
monitoring equipment.
X IEC 61157 Declaration of acoustic output parameters.
X ISO 10993-1 Biological evaluation of medical devices.
X UL 60601-1 Medical Electrical Equipment, Part 1 General Requirements for Safety.
X CSA 22.2, 601.1 Medical Electrical Equipment, Part 1 General Requirements for Safety.

Declarations
This is CSA symbol for Canada and United States of America
This is manufacturer’s declaration of product compliance with applicable EEC
directive(s) and the European notied body.
This is manufacturer’s declaration of product compliance with applicable EEC
directive(s).
This is GMP symbol for Good Manufacturing Practice of Korea quality system
regulation.
Certicate of Excellent Service Quality is to certify that the above company
has served customers with excellent services by the Ministry of Knowledge
Economy Republic of Korea.
Before Using This Product
Read this service manual to familiarize yourself thoroughly with repair procedures and important safety
information before attempting to service the product.
Failure to follow this information may cause an accident such as electric shock, as well as mechanical or
other hazards to the service engineer, product operator, and/or patient.
1. Refer to the service manual when you
2. You are strongly urged to familiarize yourself with the operational safety information contained in
Chapter 2 Safety’.
‘
3. This product is an ultrasound diagnosis device and cannot be used from the user’s PC. We are not
responsible for errors that occur when the system is run on the user’s PC.
4. This product may only be serviced by the Global Service Group of Samsung Medison
authorized engineer.
5. Samsung Medison is not responsible for any problems caused by an unauthorized person servicing
the product.
6. The manufacturer is not responsible for any damage to this product caused by user carelessness
and/or neglect.
7. The content of this manual may be changed without prior notice.
8. The following terms are used to highlight safety precautions that the user must
DANGER: Disregarding this instruction may result in death, serious injury, or other dangerous
situations.
WARNING: Follow this information to prevent a serious accident or damage to property.
service the product.
or an
be aware of:
CAUTION: Hazards or unsafe practices that may result in minor personal injury or property
damage.
NOTE: The accompanying information covers an installation, operation, or maintenance
procedure that requires careful attention from the user, but has little chance of leading directly to
a dangerous situation.
If You Need Help
If you need help regarding the product, please contact the Samsung Medison Global Service Group in
charge of servicing this product.
Revision History
The revision history of this manual is as the follows.
Document No. Date REASON FOR CHANGE
CSD-SMDU6 2011-12-28 Initial Release

Table of Contents
Table of Contents
Chapter 1 Introduction
1.1 Overview ...............................................................................................................................................1-3
1.2 Main Features of MySono U6 ........................................................................................................1-4
1.3 Product Conguration .....................................................................................................................1-5
1.3.1 Console ............................................................................................................................................................1-5
1.3.2 Probes ............................................................................................................................................................... 1-6
1.3.3 Dedicated Cart (Optional) ........................................................................................................................1-6
1.4 Product Specications .....................................................................................................................1-7
Chapter 2 Safety
2.1 Overview ...............................................................................................................................................2-3
2.2 Safety Information ............................................................................................................................2-4
2.2.1 Safety Symbols .............................................................................................................................................. 2-4
2.2.2 Location of Labels ........................................................................................................................................2-5
2.3 Electrical Safety ..................................................................................................................................2-6
2.3.1 Prevention of Electric Shock ....................................................................................................................2-6
2.3.2 ESD ....................................................................................................................................................................2-7
2.3.3 EMI ......................................................................................................
2.3.4 EMC ...................................................................................................................................................................2-8
2.4 Mechanical Safety .......................................................................................................................... 2-14
2.4.1 Safety Notes .................................................................................................................................................2-14
2.4.2 Moving the Equipment ...........................................................................................................................2-14
2.5 Biological Safety .............................................................................................................................. 2-15
2.5.1 ALARA Principle ..........................................................................................................................................2-15
2.6 Protecting the Environment ....................................................................................................... 2-30
2.7 Battery ................................................................................................................................................. 2-31
...............................................................2-7
11

Service Manual
Chapter 3 Installing the Product
3.1 Overview ...............................................................................................................................................3-3
3.2 Transporting ........................................................................................................................................3-4
3.7.1 Caution on Transporting ...........................................................................................................................3-4
3.7.2 Humidity and Temperature ...................................................................................................................... 3-4
3.3 Unpacking ............................................................................................................................................3-5
3.7.3 Dismantling the Product Box ..................................................................................................................3-5
3.7.4 Product Components .................................................................................................................................3-5
3.7.5 MySono U6 Cart (sold separately) ...........................
3.7.6 MySono U6 Cart (Optional), 2 Probe Connector ..............................................................................3-7
3.4 Installation Environment ................................................................................................................3-8
3.7.7 Caution.............................................................................................................................................................3-8
3.5 Installing the Product .......................................................................................................................3-9
3.7.8 Installation Safety ........................................................................................................................................3-9
3.7.9 AC Adapter Connection ............................................................................................................................3-9
3.7.10 Probe Connection ......................................................................................................................................3-10
..............................................................................3-6
3.6 Turning the Product On .............................................................................................................. 3-11
3.7 Shutting Down the Product ....................................................................................................... 3-12
3.8 Connecting Peripherals ................................................................................................................ 3-13
3.9 Connecting the Battery ................................................................................................................ 3-14
3.7.11 Battery Icons ................................................................................................................................................3-15
3.10 System Settings ............................................................................................................................... 3-16
3.7.12 General System Settings (Setup-General) ........................................................................................3-16
3.7.13 Display ...........................................................................................................................................................3-19
3.7.14 Annotate .......................
3.7.15 Peripherals ....................................................................................................................................................3-25
3.7.16 User Dened Key ..........................................................................
3.7.17 Miscellaneous ..............................................................................................................................................3-28
3.7.18 Option .........................................................................................................................................................
3.7.19 DICOM ............................................................................................................................................................3-30
3.7.20 Auto Calc .......................................................................................................................................................3-43
3.7.21 About ...................................
................................................................................................................................3-23
..............................................................3-27
...3-29
...........................................................................................................................3-44
12

Table of Contents
Chapter 4 Inspecting the Product
4.1 Overview ...............................................................................................................................................4-3
4.2 Turning On the Product ..................................................................................................................4-4
4.3 Monitor ..................................................................................................................................................4-5
4.3.1 Screen Layout ................................................................................................................................................4-5
4.3.2 Screen Brightness Adjustment ...............................................................................................................4-6
4.4 Control Panel .......................................................................................................................................4-7
4.4.1 Functions of the Control Panel ...............................................................................................................4-7
4.4.2 Alphanumeric Keyboard .........................................................................................................................4-10
4.5 Inspecting Functions ..................................................................................................................... 4-11
4.5.1 Basic Inspections ........................................................................................................................................4-11
4.5.2 Detailed Inspections .................................................................................................................................4-12
Chapter 5 Product Structure
5.1 Overview ...............................................................................................................................................5-3
5.2 System Block Diagram .....................................................................................................................5-4
5.2.1 System Overview ........................................................................................................................................5-4
5.3 Basic Structure of MySono U6 ......................................................................................................5-5
5.3.1 Overview .........................................................................................................................................................5-5
5.3.2 Ultrasound System Part ............................................................................................................................. 5-6
5.3.3 User Interface Part ........................................................
5.3.4 Miscellaneous Part.......................................................................................................................................5-7
5.4 PSA ..........................................................................................................................................................5-8
5.4.1 Main Functions .............................................................................................................................................5-8
5.4.2 Specication ..................................................................................................................................................5-8
5.4.3 Operational Principles of the High Voltage Switching
5.5 Main Board ........................................................................................................................................ 5-10
5.5.1 Main Functions ...........................................................................................................................................5-10
5.5.2 Beamformer Part ........................................................................................................................................5-11
5.5.3 CW Part ..........................................................................................................................................................5-14
5.5.4 Back End Part .................................................................
...............................................................................5-7
Process ..................................................5-9
..............................................................................5-17
13

Service Manual
5.6 PCI Part ............................................................................................................................................... 5-21
5.6.1 Main Functions ...........................................................................................................................................5-21
5.7 Motor Control Part ......................................................................................................................... 5-22
5.7.1 Main Functions ...........................................................................................................................................5-22
5.7.2 Block Diagram .............................................................................................................................................5-22
5.7.3 Specication ................................................................................................................................................5-22
5.7.4 Operational Principles ..............................................................................................................................5-23
5.8 PC Module ......................................................................................................................................... 5-24
5.8.1 Main Functions ...........................................................................................................................................5-24
5.9 Software DSC ................................................................................................................................... 5-25
5.9.1 Main Functions ...........................................................................................................................................5-25
5.9.2 Operational Principles ..............................................................................................................................5-25
5.10 Control Panel .................................................................................................................................... 5-26
5.10.1 Main Functions ...........................................................................................................................................5-26
5.11 Power Supply ................................................................................................................................... 5-27
5.11.1 Power adapter .............................................................................................................................................5-27
5.11.2 DC to DC Power Module .........................................................................................................................5-27
Chapter 6 Basic Maintenance
6.1 Overview ...............................................................................................................................................6-3
6.2 System Information ..........................................................................................................................6-4
6.3 Windows Mode ..................................................................................................................................6-5
6.4 Version Updates .................................................................................................................................6-6
6.4.1 Software Version Updates ........................................................................................................................6-6
6.4.2 Hardware Version Updates ....................................................................................................................... 6-6
6.5 Admin Mode ........................................................................................................................................6-7
6.5.1 Entering Admin Mode ................................................................................................................................6-7
6.5.2 Admin Mode Functions .............................................................................................................................6-8
6.6 Adding and Deleting Options ................................................................................................... 6-13
6.6.1 Types of Option ..........................................................................................................................................6-13
6.6.2 Adding an Option ......................................................................................................................................6-14
6.6.3 Removing an Option ................................................................................................................................6-17
14

Table of Contents
Chapter 7 Troubleshooting
7.1 Overview ...............................................................................................................................................7-3
7.2 Power .....................................................................................................................................................7-4
7.2.1 Power Fails to Turn On................................................................................................................................7-4
7.2.2 Power Fails to Turn O ...............................................................................................................................7-4
7.2.3 Power Turns O Spontaneously ....................................................................................
7.3 Monitor ..................................................................................................................................................7-6
7.3.1 Nothing is Displayed on Screen .............................................................................................................7-6
7.3.2 Screen is Discolored ....................................................................................................................................7-6
7.4 Error Messages ....................................................................................................................................7-7
7.4.1 Error Occurs and Product Stops while Booting ................................................................................7-7
7.4.2 Error Occurs but Product Works ............................................................................................................. 7-7
7.5 Image .....................................................................................................................................................7-8
7.5.1 No BW Mode Image Echo; No BW Mode Image Format ...............................................................7-8
7.5.2 Rain-like Streaking in BW Mode Image (Noise) ................................................................................ 7-8
7.5.3 PW Doppler Mode, CW Doppler Mode, Color Doppler Mode, M Mode Trouble.................7-8
.........................7-5
Chapter 8 Disassembly and Reassembly
8.1 Overview ...............................................................................................................................................8-3
8.2 Basic Disassembly and Reassembly ...........................................................................................8-4
8.2.1 Preparation ..................................................................................................................................................... 8-4
8.2.2 HDD & Battery Pack .....................................................................................................................................8-4
8.2.3 Middle of System Disassembly and Reassembly .............................................................................8-5
8.3 Ultrasound System Part Disassembly and Reassembly ......................................................8-7
8.3.1 Preparation ..................................................................................................................................................... 8-7
8.3.2 MAIN ASSY .....................................................................................................................................................8-7
8.3.3 Sub Board Disassembly and Reassembly .........................................................................
8.4 Control Panel Disassembly and Reassembly ..........................................................................8-9
8.4.1 Preparation ..................................................................................................................................................... 8-9
8.4.2 Trackball .......................................................................................................................................................... 8-9
8.4.3 Control Panel Board ..................................................................................................................................8-10
8.4.4 Alpha numeric Keyboard & Speaker ASSY .......................................................................................8-11
8.5 LCD Part Disassembly and Reassembly ................................................................................. 8-12
8.5.1 Preparation ...................................................................................................................................................8-12
8.5.2 LCD Module ..................................................................................................................................................8-12
..................8-8
15

Service Manual
Chapter 9 Probes
9.1 Overview ...............................................................................................................................................9-3
9.2 Probe List ..............................................................................................................................................9-4
9.2.1 Probe Application and Preset..................................................................................................................9-4
9.2.2 Function list ....................................................................................................................................................9-5
9.3 Thermal Index (TI) Table ..................................................................................................................9-9
9.4 Ultrasound transmission Gel ...................................................................................................... 9-10
9.5 Sheaths ............................................................................................................................................... 9-11
9.5.1 Applying Sheath .........................................................................................................................................9-11
9.6 Probe Safety Precautions ............................................................................................................. 9-12
9.6.1 Use and Infection Control of the Probe .............................................................................................9-12
9.6.2 Electric Shocks ............................................................................................................................................9-13
9.7 Cleaning and Disinfecting the Probe ...................................................................................... 9-14
9.7.1 Information on Detergent, Disinfectant, and Ultrasound Gel ..................................................9-14
9.7.2 Cleaning ........................................................................................................................................................9-21
9.7.3 Disinfection ..................................................................................................................................................9-22
Chapter 10 Maintenance
10.1 Overview ............................................................................................................................................ 10-3
10.2 Operational Environment ............................................................................................................ 10-4
10.2.1 Installing and Storing the Product ......................................................................................................10-4
10.3 Product Maintenance ................................................................................................................... 10-5
10.3.1 Cleaning ........................................................................................................................................................10-5
10.3.2 Disinfection ..................................................................................................................................................10-6
10.3.3 Accuracy Check...........................................................................................................................................10-6
10.4 Battery Pack Management .......................................................................................................... 10-7
10.4.1 Battery Pack Removal ...............................................................................................................................10-7
10.4.2 Battery Pack Installation ..........................................................................................................................10-7
10.4.3 Recharging the Battery Pack .........................................................................
10.4.4 Storing the Battery Pack ..........................................................................................................................10-9
10.4.5 Disposing of the Battery Pack ...............................................................................................................10-9
........................................10-8
16

Table of Contents
10.5 Information Maintenance .........................................................................................................10-10
10.5.1 User Setting Back-up .............................................................................................................................10-10
10.5.2 Patient Information Restore ................................................................................................................10-10
10.5.3 Software ..................................................................................................................................................... 10-11
Chapter 11 Service Part List
11.1 Overview ............................................................................................................................................ 11-3
11.2 Cover ................................................................................................................................................... 11-4
11.3 System................................................................................................................................................. 11-5
11.4 Control Panel .................................................................................................................................... 11-7
11.5 LCD ....................................................................................................................................................... 11-8
11.6 Mechanism & Chassis .................................................................................................................... 11-9
11.7 Option ...............................................................................................................................................11-10
11.8 Probe .................................................................................................................................................11-11
17

Chapter 1
Introduction
1.1 Overview ....................................................... 1-3
1.2 Main Features of MySono U6 .................... 1-4
1.3 Product Conguration ............................... 1-5
1.3.1 Console ...................................................................... 1-5
1.3.2 Probes ......................................................................... 1-6
1.3.3 Dedicated Cart (Optional) ................................... 1-6
1.4 Product Specications ............................... 1-7


1.1 Overview
Chapter 1 describes important information about MySono U6 that you must know before
servicing the product. The product’s main features, conguration, and specication are
explained.
MySono U6 is a high-resolution, deep-penetration color diagnostic ultrasound system that
oers a wide variety of convenient exam options.
Chapter 1 Introduction
1-3

Service Manual
1.2 Main Features of MySono U6
Cutting-edge Digital Beamforming technology: Utilizes proprietary technology developed
by Samsung Medison.
Diverse applications: Can be used for such diverse applications as general, obstetrics,
gynecology, abdomen, vascular, extremities, cardiac, urology, and chest
Diverse diagnosis modes: Features an array of diagnosis modes including 2D mode, M
mode, Color Doppler mode (C mode), Power Doppler mode (PD mode), and PW Spectral
Doppler mode (D mode).
3D image feature: Provides detailed three-dimensional images in 3D and 4D Image modes.
Measurement and report features: In addition to measurements of distance, area,
circumference, and volume, various measurement features for each application are
provided. A report feature for utilizing the measurements is also provided.
Scan image review feature: Up to 2621-frame Cine images and 4086-line Loop images are
provided.
SONOVIEW feature: An integrated image management system facilitates storage and
accessing of images and ensures compatibility of data.
Digital Imaging and Communication in Medicine (DICOM) feature: Save, transfer, or print
images over the network.
Ease of connecting peripherals: Various peripherals can be connected and used with ease.
1-4

1.3 Product Conguration
MySono U6 consists of the main console, probes, and an optional cart.
1.3.1 Console
The inside of the console contains ultrasound imaging components, and the outside features
various connectors and a handle.
Chapter 1 Introduction
Probe Connector
USB Port
[Figure 1.1 MySono U6 Console]
DVI-I Port
LAN Port
DC Power Port
[Figure 1.2 MySono U6 Side View]
Security
MIC Port
Audio Port
USB Port
1-5

Service Manual
1.3.2 Probes
Probes are devices that generate ultrasound waves and then process the reected wave data
to form images.
NOTE: For detailed information, refer to ‘Chapter 9. Probes’.
1.3.3 Dedicated Cart (Optional)
The MySono U6 cart can be used as a base station for your MySono U6, or to move it around.
For more information on using and setting up the MySono U6 Cart, refer to the accompanying
manual.
1-6
[Figure 1.3 MySono U6 Cart]

1.4 Product Specications
Height: 75.4mm
Physical Dimensions
Battery Pack
Monitor
Probe connections
Probes
(Type BF / IPX7)
Electrical Parameters
Pressure Limits
Humidity Limits
Temperature Limits
Input / Output
Connections
Width: 360mm
Depth: 291mm
Weight: more than 4.8kg (without battery)
Hight: 23.5mm
Width: 224.5mm
Depth: 78.5mm
Weight: less than 700g
15 inch LCD monitor
One probe port
Two probe ports for option
Curved Linear Array: C2-5, C2-8, C4-9
Linear Array: LN5-12
Phased Array: P2-4
Endocavity Curved Linear Array: EVN4-9
Volume Probe: 3DC2-6, 3D4-9
CW Probe: CW2.0
Input: 100-240VAC, 0.7-1.63A, 47-63Hz
Output: 19VDC, 7.9A, 150W Max
Operating: 700hPa - 1060hPa
Storage: 700hPa - 1060hPa
Operating: 30% - 75%
Storage & Shipping: 20% - 90%
Operating: 10°C - 35°C
Storage & Shipping: -25°C - 60°C
Video (DVI-I) port
Network port
USB port
Microphone port
Audio port
Chapter 1 Introduction
Auxiliary
Application
USB ECG
USB Foot Switch(IPX1)
External DVD Multi
USB Video Printer
USB Laser Printer
USB Hard Disk Drive
USB Flash Memory Media
Abdomen, Obstetrics, Gynecology, Musculoskeletal, Small Parts, Vascular, Cardiac,
Pediatric Cardiology, TCD, Urology
1-7
Service Manual
Imaging modes
Focusing
Gray Scale
Measurement Packages
Measurement
Image Storage
Signal processing
(Pre-processing)
Signal processing
(Post-processing)
2D imaging mode
M imaging mode
Color Doppler Imaging (CDI) mode
Power Doppler Imaging (PDI) mode
Directional Power Doppler Imaging (DPDI) mode
Power Pulse Inversion Imaging (PPII) mode
Pulse Wave (PW) Spectral Doppler imaging mode
Tissue Doppler Imaging (TDI) mode
Tissue Doppler Wave mode
3D imaging mode
4D imaging mode
Dual modes
Combined modes
Simultaneous mode
Zoom mode
Transmit focusing, maximum of eight points (four points simultaneously
selectable)
Digital dynamic receive focusing (continuous)
256 (8 bits)
Obstetrics, Gynecology, Cardiac, Carotid, Urology, Fetal Echo, LE Artery, UE Artery,
LE Vein, UE Vein, Radiology, TCD, Thyroid, Breast, Testicle, Supercial, Pediatric
Hips, MSK
* Refer to the Chapter 5 for additional information.
Trackball operation of multiple cursors
2D mode: Linear measurements and area measurements using elliptical
approximation or trace
M mode: Continuous readout of distance, time and slope rate
Doppler mode: Velocity and trace
Maximum 2,621 frames for CINE memory
Maximum 8,192 Lines for LOOP memory
Image ling system
TGC control
Mode-independent gain control
Acoustic power control (adjustable)
Dynamic aperture
Dynamic apodization
Dynamic range control (adjustable)
Image view area control
M-mode sweep speed control
Frame average
Edge Enhancement / Blurring
Gamma-scale windowing
Image orientation (left/right and up/down, rotation)
White on black/black on white
Zoom
1-8
Chapter 2
Safety
2.1 Overview ....................................................... 2-3
2.2 Safety Information ...................................... 2-4
2.2.1 Safety Symbols ........................................................ 2-4
2.2.2 Location of Labels .................................................. 2-5
2.3 Electrical Safety ........................................... 2-6
2.3.1 Prevention of Electric Shock .............................. 2-6
2.3.2 ESD ...............................................................................2-7
2.3.3 EMI ............................................................................... 2-7
2.3.4 EMC .............................................................................2-8
2.4 Mechanical Safety ..................................... 2-14
2.4.1 Safety Notes ...........................................................2-14
2.4.2 Moving the Equipment ......................................2-14
2.5 Biological Safety ........................................ 2-15
2.5.1 ALARA Principle ....................................................2-15
2.6 Protecting the Environment ...................2-30
2.7 Battery ......................................................... 2-31


2.1 Overview
Chapter 2 contains important information for servicing MySono U6 safely.
It is relevant to the ultrasound system, the probes, the recording devices, and any of the
optional equipment.
MySono U6 is intended for use by, or by the order of, and under the supervision of, a licensed
physician who is qualied for direct use of the medical device.
This equipment should not be used by any healthcare professional or individual who is not
properly qualied to operate it. Prolonged use of three-dimensional ultrasound (3D, 4D) by
an unqualied individual, such as to produce a commemorative photograph or video of the
fetus, may have an adverse eect on the fetus.
Be sure to use the three-dimensional ultrasound diagnostic imaging system only for its
intended purposes, since using it for purposes other than diagnosing the fetus may have an
adverse eect on the fetus.
Chapter 2 Safety
2-3
Service Manual
2.2 Safety Information
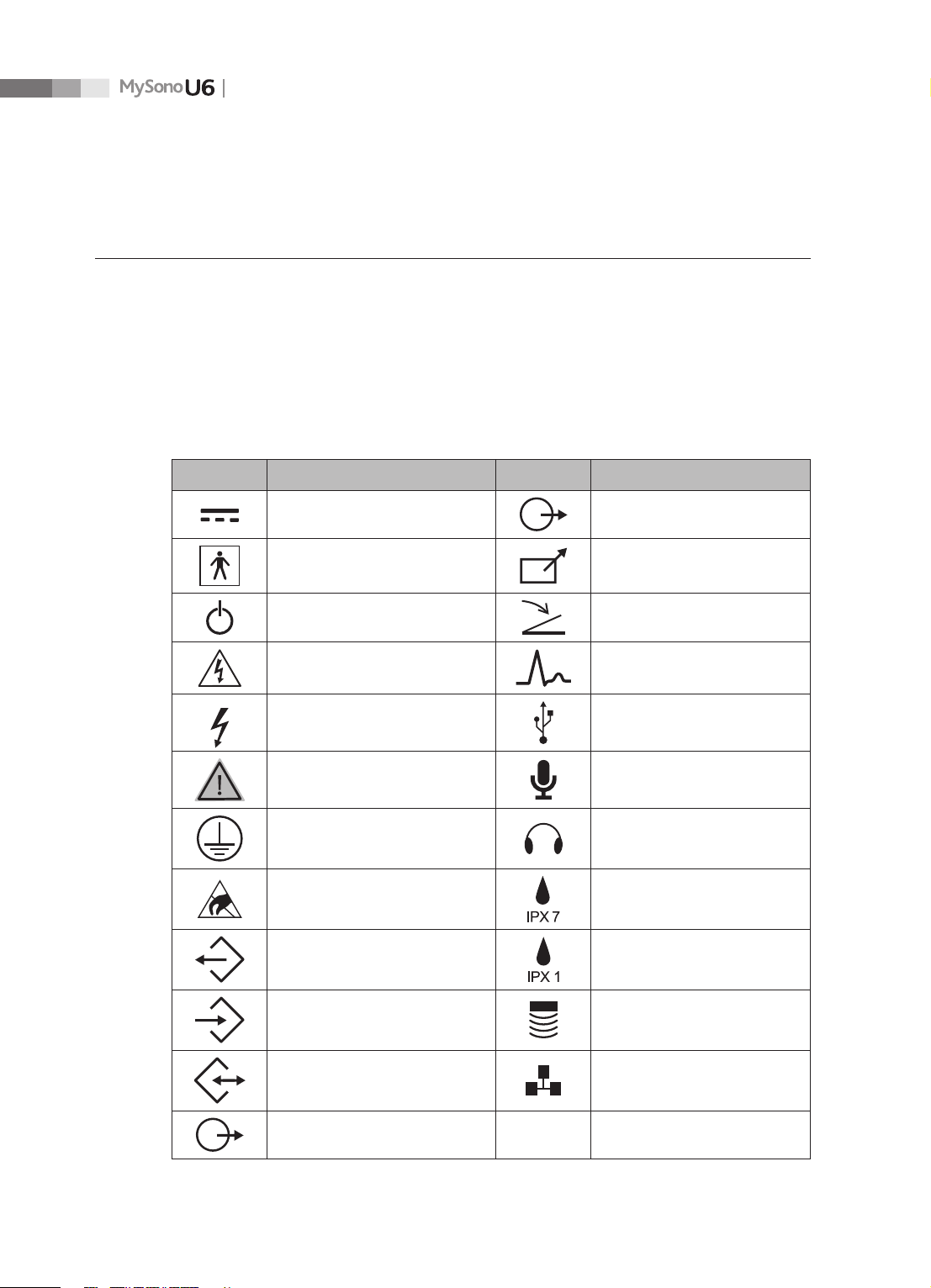
2.2.1 Safety Symbols
The International Electro Technical Commission (IEC) has established a set of symbols for
medical electronic equipment, which classify a connection or warn of potential hazards. The
classications and symbols are shown below.
Symbol Description Symbol Description
DC (direct current) voltage source
Isolated patient connection (Type
BF applied part)
Power switch (Supplies/cuts the
power for product)
Caution: Electric shock risk,
electricity
Indicates dangerous voltages
over 1,000V AC or over 1,500V DC.
Danger, warning, caution MIC input port
Protective grounding terminal Audio Port
ESD (electrostatic discharge)
warning
DATA output port
Left and right Audio / Video
output
Remote print output
Foot switch connector
ECG connector
USB connector
Protection against the eects of
immersion
Protection against dripping
water
2-4
DATA input port Probe connector
DATA input/output port Network port
Left and right Audio / Video input
 Loading...
Loading...