Page 1

Page 2

To obtain information about warranty for this product contact
Puritan Bennett Technical Support at:
1-800-255-6774
WARNING
The user should read and understand all product literature, labeling and
warnings prior to operating the Renaissance II Spirometry System
Page 3

P-495220-00 Rev. D i
Table of Contents
Listing of Warnings, Cautions, and Notes . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Indicators, Symbols, and Icons. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Introduction to the Renaissance II Spirometry System. . . . . . . . . . . . . . . 8
Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Basic Spirometry System and Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Connecting the AC Adapter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Battery Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Connecting the Pressure Tube . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Keypad Functions and Controls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Main Screen Icon Features. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Initial Configuration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Spirometry Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Introduction to Spirometry Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Obtaining Good Test Results . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
Pre-Test Procedures. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Calibration Verification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
SSD Calibration Verification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Cal Check. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Patient Preparation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Entering New Patient Data. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Pre-Med Testing Procedures. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
FVC (Forced Vital Capacity) Test Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
SVC (Slow Vital Capacity) Test Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
FVL (Flow Volume Loop) Test Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
MVV (Maximal Voluntary Ventilation) Test Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . 32
FEV6 (Forced Expiratory Volume in 6 sec.) Test Procedure . . . . . . . . . . . . . . . . . . . . . . . . 33
Post-Med Testing Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
Post-Test Procedures. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
Saving Results . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
Viewing Results . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
Page 4

ii P-495220-00 Rev. D
Table of Contents
Printing Results . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .38
Printing Reports for Multiple Patients . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .40
Deleting Patient Data. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .41
Interpretation of the Results . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Acceptability and Reproducibility. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .42
Grading Criteria . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .44
Interpretation Criteria . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .44
Lung Age Interpretation. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .47
Risk of COPD . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .47
Graphic Displays . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .48
Service and Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49
Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .49
Battery Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .49
Troubleshooting Guide . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
Electromagnetic Interference . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 54
Warranty Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .55
Technical References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 57
Product Specifications Renaissance II Spirometer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .57
Product Specifications Renaissance II Base Station . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .58
The FSII Single-Patient Use Flow Sensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .59
Predicted Normal Equations and References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .60
RS-232 Interface Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .76
Pin Function Descriptions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .76
Using the Renaissance II with a PC and Dataflow™ Software . . . . . . . . . . . . . . . . . . . . .77
System Configuration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 78
Spirometry Options (1). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .78
Device Options (2) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .80
Print Options (3) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .80
Settings (4) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .83
Display (5) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .83
Storage (8). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .83
Setup and System Configurations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .84
Printing the System Configuration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .86
Page 5

P-495220-00 Rev. D iii
Table of Contents
Barometric Pressure vs. Altitude . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 87
Glossary of Medical Terminology. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 88
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 91
Page 6
Listing of Warnings, Cautions, and Notes
1 P-495220-00 Rev. D
Listing of Warnings, Cautions, and Notes
Throughout this manual there are three indicators to convey information of a
specific nature. These indicators are warnings, cautions and notes. Carefully
read and understand these notices as they relate to adjacent text.
WARNING
Warnings alert the user to potential serious outcomes (death, injury, or
adverse events) to the patient or user.
CAUTION:
Cautions alert the user to exercise care necessary for the safe and
effective use of the Renaissance II Spirometry System.
NOTE: Indicates points of particular emphasis that make operation of the
spirometer more efficient or convenient.
Page 7

Listing of Warnings, Cautions, and Notes
P-495220-00 Rev. D 2
WARNING
The user should read and understand all product literature,
labeling and warnings prior to operating the Renaissance II
Spirometry System.
Patient Safety Warnings
• This device should be used by trained he al th care professional s an d is
not intended for patient operation.
• Physicians should assess patient’s ability to perform spirometry testing
prior to administering the test.
• Patient fainting or falling due to dizziness may occur as a result of this
test. Advise the patient to sit or stand comforta bly near a chair during
test.
Patient Data Warni ngs
• Predicted values wi ll be extrapolated for patients with age or height
outside the age and/or height limits supported by the selected author’s
normal equations.
• Results from spirometry testing should not be the sole source for
determining a patient's diagnosis and treatment. Other clinic al data,
such as patient symptoms and respiratory history, should always be
considered.
Use Environment Warnings
• The Renaissance II Spirometry System is not intended for use in an
oxygen-enriched atmosphere or in the presence of flammable
anesthetics.
• To avoid risk of electrical shock, this unit should only be used in dry
locations.
Equipment Setup Warnings
• As with all medical equipment, carefully route patient cabling to r educe
the possibility of patient entanglement or stran gulation.
• When connecting the Renaissance II spirometer to any instrument,
verify proper operation. Accessory equipment connected to the data
interface must be certified according to IEC Standard 950 for data
processing equipme nt or IEC Standard 601-1 for electromedical
equipment. All combinations of equipment must be in compliance with
IEC Standard 601-1-1 systems requirements. Anyone who connects
additional equipment to the signal input port or signal output port,
configures a medical system and is therefore responsible that the
system complies with the requirements of IEC Standard 601-1-1 and the
electromagnetic requirements of IEC Standard 601-1-2.
Page 8

Listing of Warnings, Cautions, and Notes
3 P-495220-00 Rev. D
WARNING
User Warnings
• Chemicals from a broken LCD display panel are toxic when ingested.
Use caution when handling a Renaissance II spirometer with a broken
display panel.
Flow Sensor Warnings
• Carefully read the flow sensor directions before use, including all
warnings, cautions, and instructions.
• User should visually inspect the FSII sensor for loose particles/foreign
materials prior to patient use.
CAUTION
Federal law restricts this device to sale by, or on the order of, a
physician.
Use Environment Cautions
• Do not use the Renaissance II Spirometry System in areas of high
humidity, dust, or in extreme environments.
• Place the Renaissance II Spirometry System in a secure location, where
it is unlikely to drop or fall. Do not attempt to lift or carry the
spirometer by the pressure tube or power cord.
• The Renaissance II system may be susceptible to radio frequency
interference. Refer to the electromagnetic interference section of this
manual for more information.
Equipment Setup Cautions
• The Renaissance II Spirometry System and base station are designed
for use only with the Puritan Bennett AC adapter (P-4 95208-00). Do not
connect AC Adapter (P-495208-00) to an original Renaissance system
(PB-100/110) or damage will result. Conversely, do not connect a PB100/
PB110 AC adapter (P-062521-00) to the Renaissance II Spirometry
System.
• Prior to verifying calibration, visually verify that there is no foreign
material in the pressure tube and that the tube is not damaged or
kinked.
Page 9
Listing of Warnings, Cautions, and Notes
P-495220-00 Rev. D 4
CAUTION
Battery Cautions
• The NiCad battery pack or other batteries may discharge over time.
Check batteries at least once pe r month for corrosion and verify
batteries are fully charged. Store spirometer in base station to keep
unit ready for use.
• Remove batteries if sp ir omete r wil l not be used for at least two week s.
• Dispose of batteries properly. Do not incinerate. Puritan Bennett
recommends that customers or technical service personne l follow local
governing ordinances and recycling instructions regarding disposal or
recycling of batteries.
Service Caution
• Do not remove the cover of the Renaissance II Spirometry System or
base. Removal of the cover is permitted only by qualified service
personnel. There are no user-serviceable parts inside.
• Do not spray liquids on the Renaissance II System. Follow the cleaning
instructions outlined in the Service and Maintenance section starting
on page 49 of this manual.
Flow Sensor Cautions
• Use only the FSII flow sensor specifically designed for the Renaissance
II Spirometry System.
• The FSII sensor is for single-patient use only. In the interest of
environmental protection, dispose of all sensors and nose clips
properly.
Notes
Accuracy Notes
• For test accuracy, elevation must be entered.
• V erify that the disp layed barometric pr essure is cor rect. If not corr ect,
there will be an error in the inspired volume (FIVC) during an FVL
maneuver of approximately -1.3% for every 1,000 feet above sea
level. Refer to the System Configuration section starting on page 78
for more information. The barometric pressure displayed is based on
the initial elevation setting of the spirometer. However, the
barometric pressure may be changed and the spirometer will, from
that point on, use the new value en tered.
• If you choose to obtain barometric pressure from an agency, such as
the National Weather Service, verify that the value is NOT corrected
to sea level.
Page 10
Listing of Warnings, Cautions, and Notes
5 P-495220-00 Rev. D
Calibration Notes
• The date of the last valid calibra tion check will display as part of the
spirometer's initialization sequence if a calibration check has not
been performed in the current calendar day.
• The American Thoracic Society (ATS) recommends performing a threespeed calibration check on a daily basis.
• Puritan Bennett recommends that the 3 liter calibration syringe be
recertified on an annual basis.
• Verify that the temperature of the room is the same as the
temperature noted for the calibration test. For every degree
discrepancy, there will be a corresponding 0.15% error in the test
results.
• Overestimation of the room temperature will cause lung volume to
be underestimated by 5%; conversely, if temperature is
underestimated, lung volume will be overestimated.
Test Method Notes
• The “Val” (best value) method is recommended by the American
Thoracic Society and mandated by NIOSH/ OSHA standards and should
be used for all industrial and disability testing.
• If the patient test will be submitted for Social Security Disability
(SSD) determinations, enter patient information prior to performing
the SSD calibration verification.
• Clinicians performing PFT studies should consider attendi ng NIOSH
training seminars and refresher courses to further their skills in
spirometry testing and to stay current with industry standards.
Spirometer Use Notes
• Demonstrating the test using your FSII sensor is strongly
recommended for patients that have never performed a spirometry
test before.
• Obstructing sensor opening with teeth, lips, or tongue while
performing the test will cause low readings.
Notes
Page 11
Listing of Warnings, Cautions, and Notes
P-495220-00 Rev. D 6
Battery Notes
• The Renaissance II base station allows interfacing to parallel printers
and computers and provides an alternate means for charging the
custom NiCad battery pack.
• The Renaissance II Spirometry System is designed to recharge only
the custom battery pack supplied with the system, and will not
recharge batteries from other manufacturers.
• When there is a low battery condition, the Renaissance II spirometer
beeps every 30 seconds and a low battery ic on is displayed.
• Do not mix brands or types of batteries.
• Puritan Bennett recommends replacing the NiCad battery pack at
least once per year.
• If the battery is removed, the unit will operate solely on AC power if
connected to an electrical outlet via the AC adapter.
Spirometer System Notes
• The serial numbers are located on a label affixed to the underside of
the spirometer and base station. The first letter "G" represents the
manufacturer. The next two numbers represent the year of
manufacture. The two digits following the yea r represent either a
base station (08) or a spirometer (07). The last five digits are
sequential numbers assigned during manufacture.
• Materials used to make this Renaissance II Spirometry System and
accessories contain no Latex.
• Replace the pressure tube every year.
• The LCD panel will turn off after 5 minutes (and the unit will power
off after 30 minutes) with no user input. To bring back the display
before the 30-minute limit, press any key.
Notes
Page 12
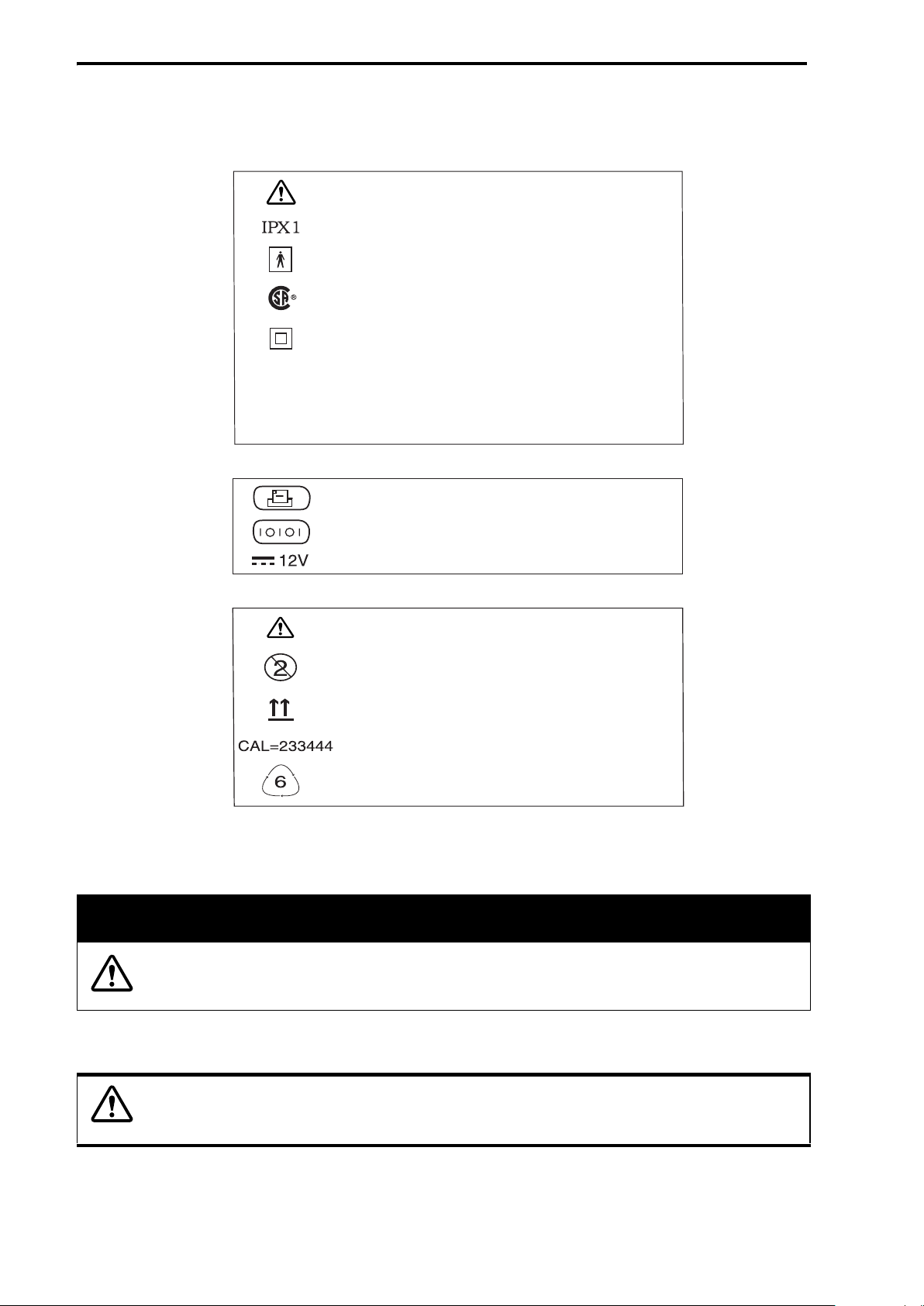
Indicators, Symbols, and Icons
7 P-495220-00 Rev. D
Indicators, Symbols, and Icons
WARNING
The Renaissance II Spirometry System is not intended for use in an oxygenenriched atmosphere or in the presence of flammable anesthetics.
CAUTION:
Federal law restricts this device to sale by or on the order of a physician.
Base Station
FSII Sensor
USC
Rx ONLY
Renaissance II Spirometer
SN
Attention, consult accompanying documents
Refers to degree of protection - Drip Proof
Type BF equipment
Agency Certification
Class II equipment
CAUTION: Federal Law (US) restricts
this device to sale by, or on the order of,
a physician
Serial Number
Connection for a printer port
I/O communications port
12 volt DC adapter connection
Attention, consult accompanying documents
Do not reuse - single patient use only
Direction of flow through the flow sensor
Recyclable plastic. The number 6 represents
polystyrene.
Bar coded calibration number
Figure 1: Renaissance II Spirometry System Indicators, Symbols, and Icons
Page 13
Introduction to the Renaissance II Spirometry System
P-495220-00 Rev. D 8
Introduction to the Renaissance II Spirometry System
The Renaissance II Spirometry System consists of a spirometer, docking base
and optional accessories, as shown in Figure 2. The Renaissance II system
provides long-term data storage capacity, and when connected to a printer
generates printouts of the data. Patient data can also be downloaded to a
computer. The spirometer test results can be compared to any of several adult
or pediatric predicted normal values. The spirometer also performs pre/post
medication comparisons.
Features
• Intuitive graphical user interface.
• Graphic display for real-time viewing of Volume-Time, Flow-Volume and
incentive displays.
• Automatically compares results to predicted values.
• Allows pre/post-medication comparisons.
• Provides clinical interpretations with COPD Risk and Lung Age
calculations.
• Optional software allows data to be downloaded to a computer.
• Memory stores demographic information, graphical data and patient
results for up to 1,000 patients.
• Operates with rechargeable NiCad batteries, alkaline batteries or an AC
adapter.
• Provides printed reports when connected to a parallel printer.
Intended Use
The intended use of the Renaissance II Spirometry System is as a diagnostic
tool to measure the maximal volume and flow of air that can be moved in and
out of a patient’s lungs. The Renaissance II spirometer obtains the spirometric
data by direct measurement of flow via the FSII sensor and pressure tube. The
flow is then electronically integrated to obtain volume. This testing can be used
for the detection, assessment and monitoring of certain lung diseases. The
system is intended for use with pediatric (4 to 17 years) and adult patients (18
to 99) in hospitals, physicians’ offices, laboratories, and occupational health
testing environments.
CAUTION:
• Place the Renaissance I I Spiro metry System in a secure location,
where it is unlikely to drop or fall. Do not attempt to lift or
carry the Renaissance II spi rometer by the pressure tube or
power cord.
• The Renaissance II system may be susceptible to radio
frequency interference. Refer to the Electromagnetic
Interference section on page 54 for more information.
Page 14
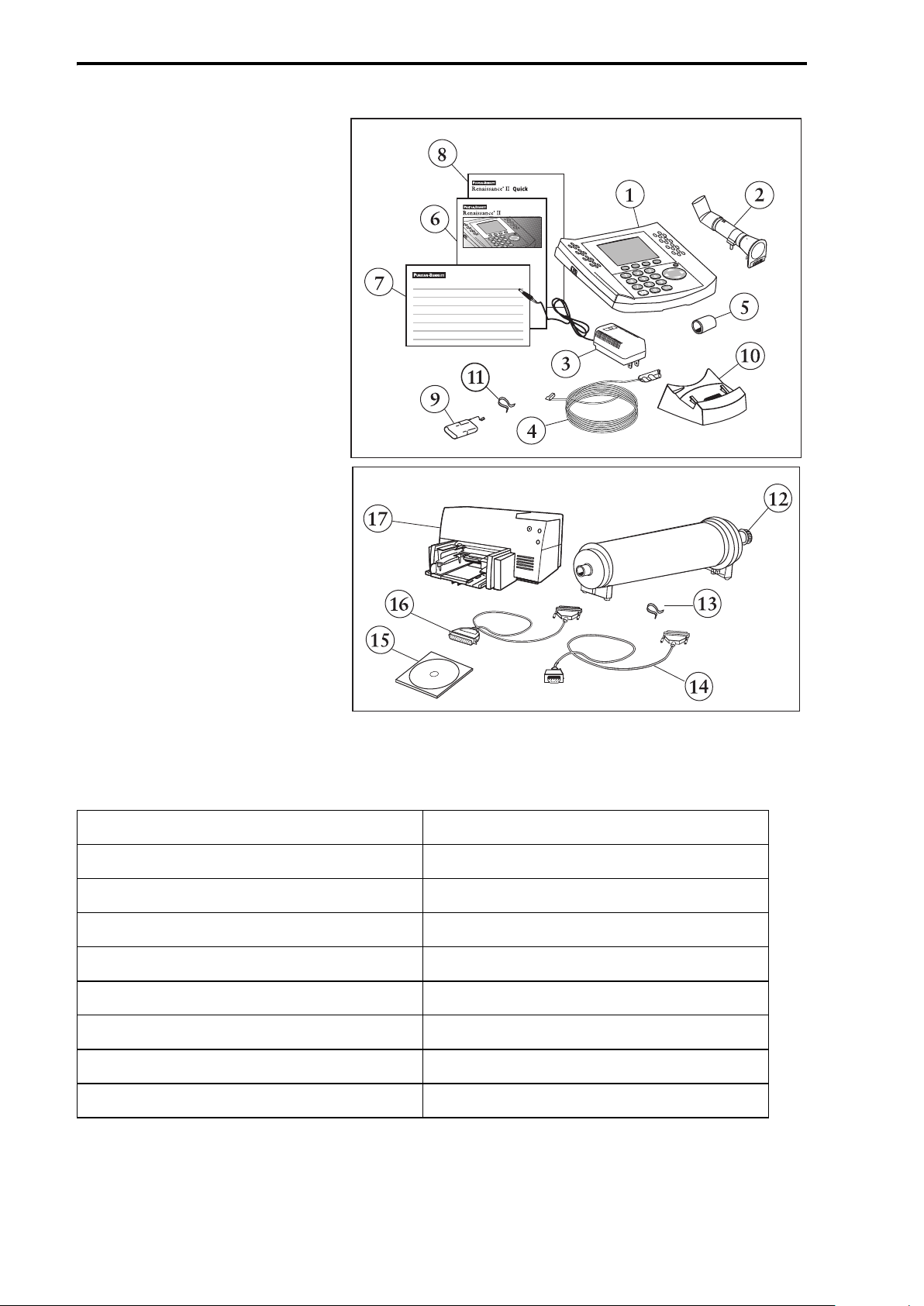
Introduction to the Renaissance II Spirometry System
9 P-495220-00 Rev. D
Basic Spirometry
System and Accessories
The Renaissance II
Spirometry System is
available in a variety of
configurations. The basic
spirometry system consists
of the spirometer, base
station, pressure tube, AC
adapter, FSII flow sensors,
syringe adapter, battery
pack, nose clips and
associated documentation
(See Table 1).
Upon receipt of your
system, verify that all
required parts are present
and undamaged. If any
parts are missing or
damaged, please contact
Puritan Bennett Technical
Support Department at 1800-255-6774.
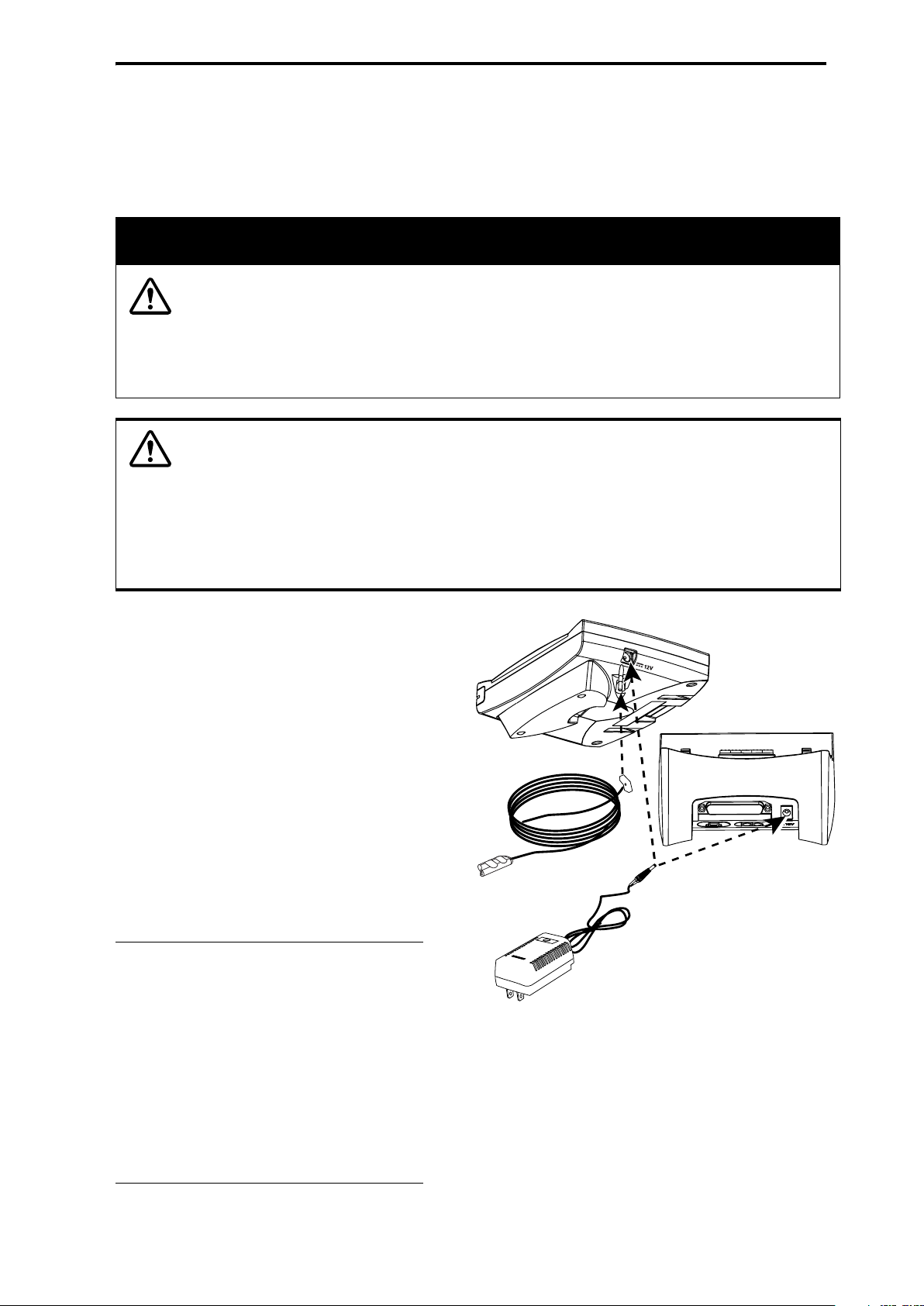
Connecting the AC Adapter
Connect the AC adapter to the 12-volt DC input jack on the side of the
Renaissance II spirometer or on the rear of the base station as shown in
Table 1: Basic Spirometry System and Accessories
1) Renaissance II Spirometer, PB-700 10) Base Station, PB-710
2) FSII Flow Sensor 11) Nose Clip, Plastic
3) AC Adapter, PB-700 Optional Accessories
4) Assy., Pressure Tube FSII 12) 3L Calibration Syringe
5) Syringe Adapter 13) Nose Clip, Plastic (25/pk)
6) User's Manual, PB-700/PB-710 14) Cable, Null Modem, NPB-510/PB-710
7) Warranty Card, PB-700/PB-710 15) DataFlow™ Data Management Software
8) Quick Guide, PB-700/PB-710 16) Cable, Printer
9) NiCad Battery Pack, PB-700 17) Printer, Spirometer Compatible
Spirometry System
User's Manual
Renaissance® II Warranty Registration Form
A
C
/
D
C
A
D
A
P
T
E
R
P
/
N
P
4
9
5
2
0
8
0
0
C
A
U
T
I
O
N
:
F
O
R
U
S
E
W
I
T
H
S
P
I
R
O
M
E
T
E
R
M
O
D
E
L
O
R
B
A
S
E
S
T
A
T
I
O
N
P
B
7
1
0
A
T
T
E
N
T
I
O
N
:
U
T
I
L
I
S
E
R
L
E
S
P
I
R
O
M
È
T
R
E
P
B
7
0
0
,
S
T
A
T
I
O
N
D
E
B
A
S
E
P
B
7
1
0
Basic System Components
Optional Accessories
Guide
Figure 2: Renaissance II Spirometry System
Page 15
Introduction to the Renaissance II Spirometry System
P-495220-00 Rev. D 10
Figure 3. A green LED indicator will light on the front panel of the
Renaissance II spirometer and on the AC adapter when properly connected to
an electrical outlet.
Battery Operation
The Renaissance II spirometer
includes a pre-installed
rechargeable custom NiCad
battery pack. As an option, the
user can install 4 AA alkaline
batteries or 4 standard AA NiCad
cells. (Refer to Battery Installation
on page 49 for installation
instructions.) If NiCad cells are
used, an external charger is
required.
WARNING
• T o avoid risk of electrical shock, this unit should only be used in
dry locations.
• As with all medical equipment, carefully route patient cabling
to reduce the possibility of patient entanglement or
strangulation.
CAUTION:
The Renaissance II Spirometry System and Base Station are
designed for use only with the Puritan Bennett AC adapter
(P-495208-00). Do not connec t AC Adapter (P-495208-00) to an
original Renaissance system (PB100/PB110) or damage will result.
Conversely, do not connect a PB100/PB110 AC adapter
(P-062521-00) to the Renaissance II Spirometry System.
NOTES:
• The custom battery pack must
be charged at least 24 hours
before portable use.
• The Renaissance II Spirometry
System is designed to recharge
only the custom battery pack
supplied with the system, and
will not recharge batteries
from other manufacturers.
Base Station
A
C
/
D
C
A
D
A
P
T
E
R
AC/DC ADAPTER
P
/
N
P
4
9
5
2
0
8
0
0
P/N P-495208-00
C
A
U
T
I
O
N
:
F
O
R
U
S
E
W
I
T
H
CAUTION: FOR USE WITH
S
P
I
R
O
M
E
T
E
R
M
O
D
E
L
SPIROMETER MODEL
P
B
7
0
0
,
PB700,
O
R
B
A
S
E
S
T
OR BASE ST
AT
I
O
N
P
B
7
1
0
TION PB710
AT
T
E
N
T
I
O
N
:
U
T
I
L
I
S
E
R
A
TTENTION: UTILISER A
V
E
C
VEC
L
E
S
P
I
R
O
M
LE SPIROM
¨T
R
E
M
O
D
TRE MOD
¨L
E
LE
P
B
7
0
0
,
S
T
PB700, ST
AT
IO
N
D
E
B
A
S
E
TION DE BASE
Spirometer
Pressure
Tube
AC Adapter
Figure 3: Setting up the System
Page 16
Introduction to the Renaissance II Spirometry System
11 P-495220-00 Rev. D
The custom NiCad battery pack has a battery life of 10-12 hours in the ON
position and a battery life of approximately 8 days in the OFF position.
The pre-installed custom NiCad battery pack will continuously charge as long
as power is connected to the spirometer through the AC adapter.
During operation, the Renaissance II spirometer continually checks battery
status. A low battery indicator will appear in the right hand corner of the screen
when fewer than 20 patient tests can be performed. If the battery voltage drops
below a reliable operating level, the unit will shut-off and not power-up until
the batteries are recharged, changed, or the AC adapter is connected.
Connecting the Pressure Tube
The Renaissance II spirometer is shipped
with a pressure tube that connects the FSII
flow sensor to the spirometer. Upon receipt,
inspect the pressure tube for damage. If the
tube is damaged, contact Puritan Bennett
Technical Support at 1-800-255-6774.
Connect the pressure tube to the underside
of the spirometer, as shown in Figure 3.
Connect the other end of the pressure tube
to the FSII flow sensor, as shown in
Figure 4.
NOTE: If the battery is removed, the unit will operate solely on AC power if
connected to an electrical outlet via the AC adapter.
CAUTION:
• The NiCad battery pack or other batteries may discharge over
time. At least once per mo nth, check batteries for corr osion and
verify batteries are fully charged. Store spirometer in base
station to keep unit ready for use.
• Remove batteries if spirometer will not be used for at least two
weeks.
NOTE: Puritan Bennett recommends replacing the NiCad battery pack at least once
per year.
FSII Flow Sensor
Pressure
Tube
Figure 4: Connecting the Pressure tube
to the FS II flow sensor
Page 17

Introduction to the Renaissance II Spirometry System
P-495220-00 Rev. D 12
After the batteries have been installed and charged and the tube is connected,
the spirometer is ready for use. The pressure tube does not need to be
disconnected from the spirometer between patients.
NOTE: Replace the pressure tube every year.
Warning
• The Renaissance II Spirometry System is not intended for use in
an oxygen-enriched atmosphere or in the pr esence of
flammable anesthetics.
• Carefully read the flow sensor directions before use, including
all warnings, caution s, an d instructio n s.
CAUTION:
Do not spray liquids on the Renaissance II System. Follow the cleaning
instructions outlined in the Service and Maintenance section starting on
page 49 of this manual.
Page 18
Introduction to the Renaissance II Spirometry System
13 P-495220-00 Rev. D
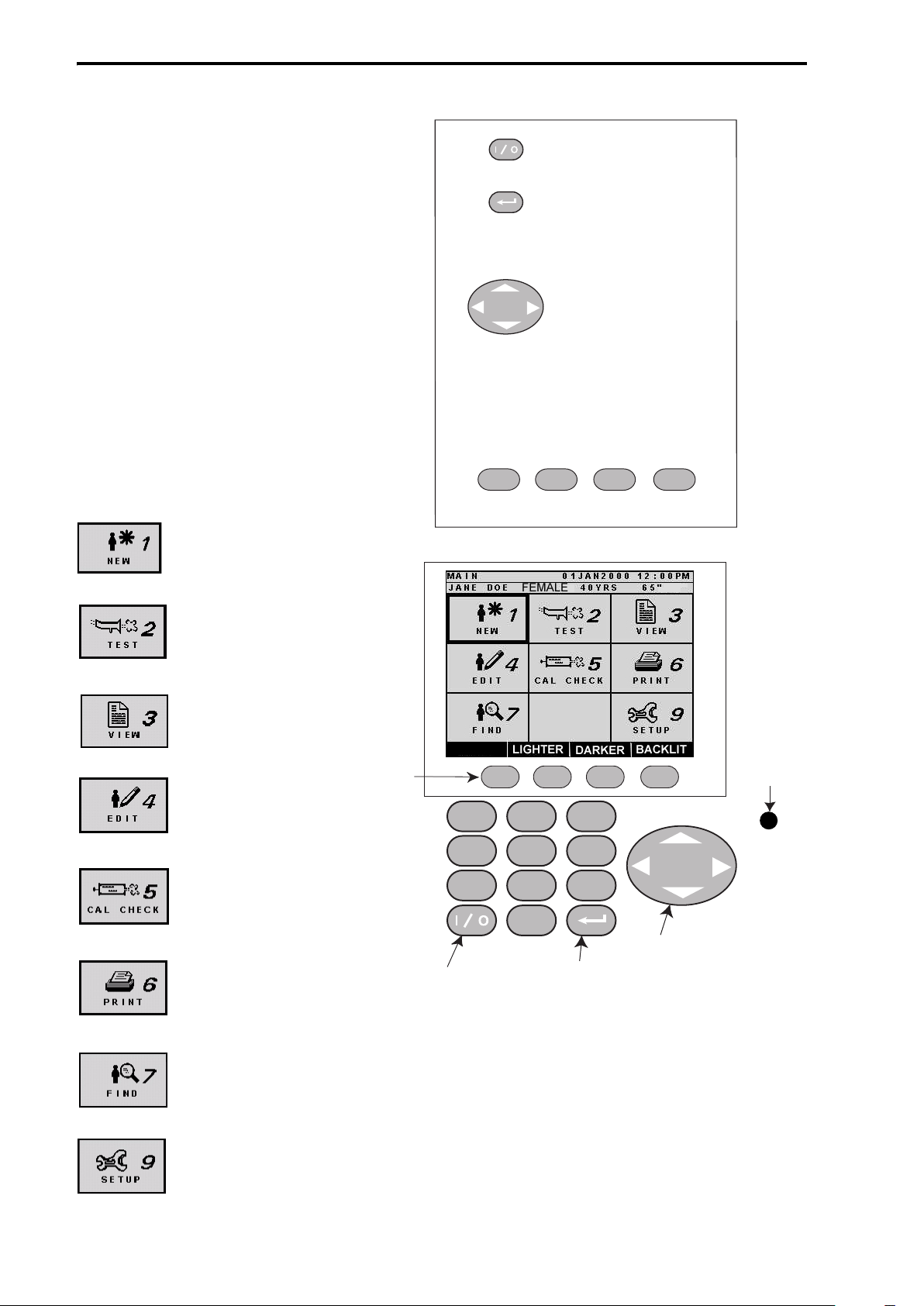
Keypad Functions and
Controls
The keypad functions and
controls are user friendly and
intuitive. The keypad and Main
screen icons, shown in Figure 5,
represent some of the most
frequently seen icons that will
be displayed. The keypad and
controls are used to access the
various functions of the
Renaissance II spirometer.
Main Screen Icon Features
Allows the user to
enter new patient data
Begins or continues a
spirometry test or
allows SSD calibration
Allows the user to view
the test results
Allows the user to edit
previously entered
patient data
Initiates one-speed
spirometer calibration
Provides a variety of
printed test reports
Locates a patient’s
previously saved test
data
Allows the user to
configure the
spirometer
Figure 5: Keypa d and Main Screen
Soft Keys
Enter
On/Off
Cursor Keys
On/Off is controlled by
the key marked "I/O".
The enter key is used to
select an option or
action in the graphic
display.
Press the up, down, left
or right arrow key to
move the cursor.
1 2 3
4 5 6
7 8
9
0
Green
Power
Indicator
Light
Cursor
Arrow
Keys
ON/OFF
ENTER
Soft
Keys
The four soft keys are used to select the
functions displayed on the screen
immediately above each key.
Page 19
Initial Configuration
P-495220-00 Rev. D 14
Initial Configuration
The Renaissance II spirometer has a number of user-selectable configuration
options which have been preset at the factory. The first time the spirometer is
powered on after leaving the factory, the user is prompted to select the
configuration options. Refer to System Configuration on page 78 for a
complete listing and description of the system configuration settings.
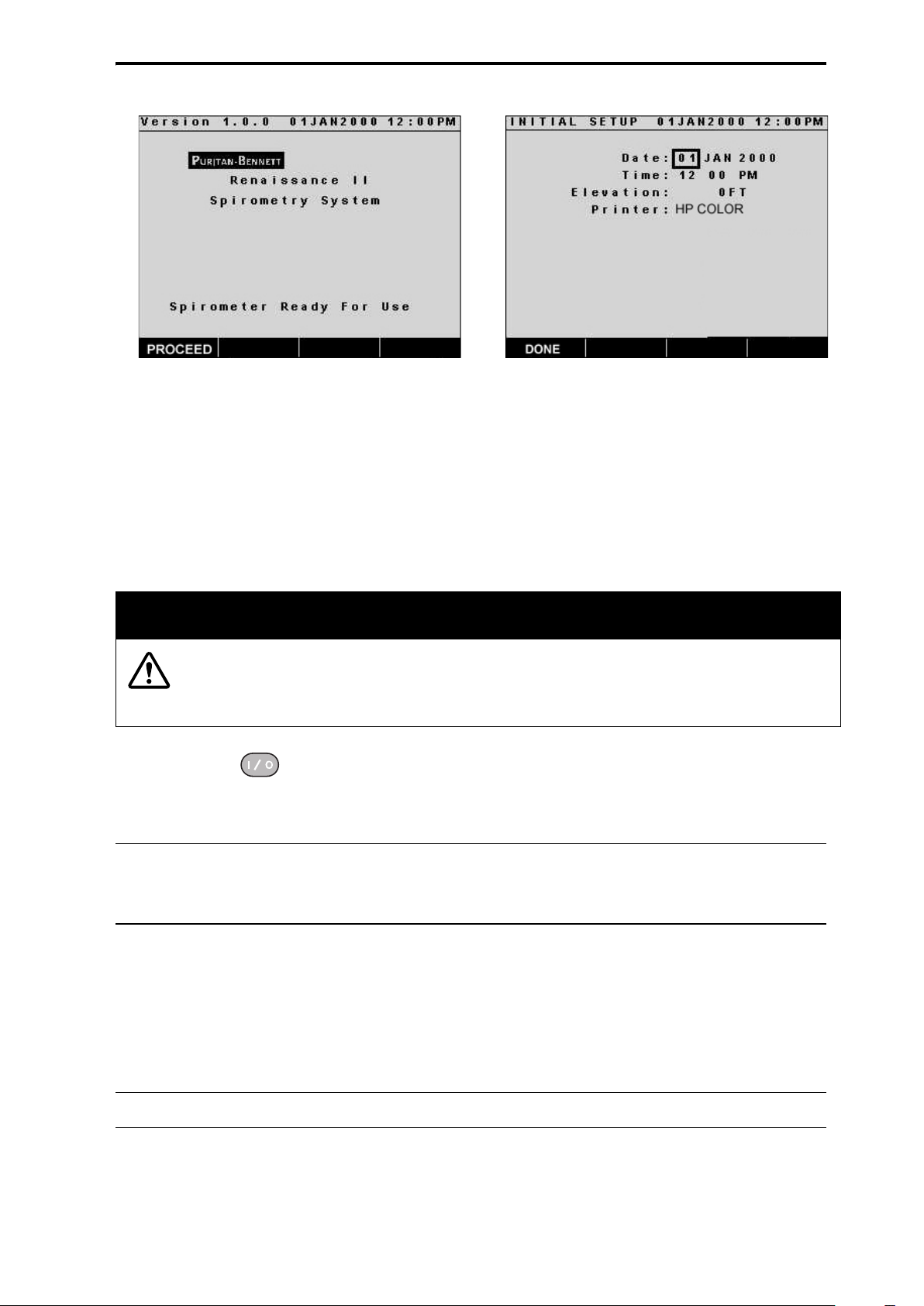
1. Press the key to power-up the spirometer. The spirometer will display
an introductory screen while a self test is performed. Press the PROCEED
soft key to go to the next screen (Figure 6).
2. In the “INITIAL SETUP” screen the user is prompted to select certain con-
figurable options. This screen will be displayed each time the spirometer is
powered-up until the user sets the displayed options (Figure 7).
3. Using the cursor key, highlight each option and enter the desired settings
using the keypad. Press the DONE soft key to go to the “MAIN” screen
(Figure 5).
4. From the “MAIN” screen, adjust the display appearance by pressing the
LIGHTER, DARKER, and BACKLIT soft keys to the desired settings.
Warning
The Renaissance II Spirometry System is not intended for use in an
oxygen-enriched atmosphere or in the presence of flammable
anesthetics.
NOTE: The date of the last valid calibration check will display as part of the
spirometer's power-up sequence if a calibration check has not been performed in
the current calendar day.
NOTE: For test accuracy, elevation must be entered.
Figure 6: Ready for Use
Figure 7: Initial Setup
Page 20

Spirometry Testing
15 P-495220-00 Rev. D
Spirometry Testing
Introduction to Spirometry Testing
The purpose of a spirometry test is to assess and monitor a patient’s lung
condition. The most common spirometry test is the Forced Vital Capacity
(FVC) test. This test requires the subject to take a deep breath and then exhale
into the spirometer as forcefully, rapidly and completely as possible. The FVC
test results report how fast the air was exhaled (flow rate) and how much air
was exhaled (volume). These parameters are compared to values derived
from ‘Predicted Normal Equations” based on the patient's age, height, gender
and race. These equations are listed starting on page 60. Depending on the
results, the healthcare professional will be able to determine whether the patient
is normal, or has an obstructive or a restrictive lung pattern.
Obstructive diseases are characterized by an increased resistance to air flow.
This resistance makes it more difficult to move air into and out of the lungs
rapidly. An obstructive pattern is characterized by a reduction in the volume
that can be exhaled in the first second of the FVC test (FEV1) and by a low
FEV1/FVC ratio. The most common obstructive diseases are asthma, chronic
bronchitis and emphysema. Asthma constricts the bronchial tubes but can be
controlled by drug therapy. Bronchitis also constricts the bronchial tubes but
may not respond to drug therapy. Emphysema is the slow, irreversible
destruction of the alveoli, leading to collapsed airways.
Restrictive diseases impair the movement of the lungs or the volume of air that
can be expelled by the lungs. They are characterized by a reduction in the total
volume of air that can be exhaled. The FEV1/FVC ratio remains normal or
increases. Gross obesity, lung fibrosis, neuromuscular diseases or paralysis can
cause restrictive diseases. Several occupational related diseases such as “black
lung” and “cotton dust lung” also result in a restrictive pattern.
In addition to the FVC test, the Renaissance II spirometer can perform FlowVolume Loop (FVL), Slow Vital Capacity (SVC), Maximal Voluntary
Ventilation (MVV), and FEV6 tests. These additional tests will sometimes
provide more information that is helpful in the diagnosis of a patient's lung
disorder.
Page 21

Spirometry Testing
P-495220-00 Rev. D 16
Obtaining Good Test Results
Unlike many other medical tests in which the patient is passive, spirometry
requires active cooperation and strenuous effort by the patient. Obtaining the
subject's full understanding and cooperation is essential.
The 10 steps to good spirometry results are listed below:
• Patient should refrain from taking bronchodilators 6-8 hours prior to
testing, unless instructed by a physician.
• Loosen any restrictive clothing. Remove loose dentures, candy, gum, etc.
• Ensure accurate input of ID#, height, weight, gender, birth date, and race.
• Patient may sit or stand, but be consistent and record position.
• The use of nose clips is optional but recommended.
• Explain procedure carefully and demonstrate how it is done.
• Coaching is critical. Remind patient to "BLAST" out the air - don't just
blow! Keep going as long, as hard, and as completely as possible (at least 6
seconds).
• Watch the patient inhale maximally and exhale forcefully and completely
with mouth and teeth firmly sealed around the mouthpiece. Watch and
listen for the incentive display.
• If the test is unacceptable, identify the reason(s) and explain how to correct
the technique.
• Obtain at least three acceptable and two reproducible tests. See pp. 1122 1123 of Reference 11 (page 91) for ATS acceptability and reproducibility
criteria. If tests are below normal, consider administering a bronchodilator
according to office protocol, then retest in 10 to 15 minutes, or as
suggested by the physician.
As the test is performed, coaching messages or incentive messages, e.g.,“Start
Test, Keep Going” appear on the display to encourage the patient. Depending
on the user's preferences, a graph of the data or an animated incentive will be
displayed during the test. These messages and graphics should be used to coach
the subject to perform the test maximally.
Warning
Patient fainting or falling due to dizziness may occur as a result of this
test. Advise the patient to sit or stand comfortably near a chair during
test.
Warning
This device should be used by trained healthcare professionals and is not
intended for patient operation.
Page 22

Pre-Test Procedures
17 P-495220-00 Rev. D
Pre-Test Procedures
Calibration Verification
The American Thoracic Society (ATS) recommends that a three-speed
calibration verification, using a calibrated syringe with a minimum volume of 3
liters, be performed on a daily basis to verify the accuracy of the system prior to
testing patients. Puritan Bennett recommends using the 3-liter calibrated syringe
specified in Table 1: Basic Spirometry System and Accessories, optional
accessory item 12 (see page 9) for verifying the calibration of the Renaissance
II. The syringe should be recertified for volume accuracy and leaks per
manufacturer recommended intervals.
The Renaissance II can perform two types of calibration verifications: SSD and
Cal Check.
The SSD calibration verification satisfies both ATS and Social Security
Disability requirements for verification at three flow rates.
The Cal Check is performed at one flow rate and can be accessed immediately
after the power-on self test, or from the Cal Check menu item (5) on the Main
screen. When running a Cal Check, calibration syringes ranging in size from 1liter to 8-liters may be used, and the Renaissance II will automatically
determine the size of the syringe. A Cal Check may be desirable in addition to
the daily three-speed (SSD) calibration to verify volume accuracy at multiple
points during studies involving a large number of maneuvers.
SSD Calibration Verification
The Renaissance II’s SSD calibration verification feature can be used to perform
either the ATS recommended daily three-speed verification, or a verification
suitable for Social Security Disability claims submissions. In both cases, the
verifications are performed using a 3-L syringe at three flow rates: 3 L/sec, 1 L/
sec, and 0.5L/sec.
To perform an ATS calibration verification, obtain a flow sensor and 3-L
calibrated syringe, and follow the instructions starting on page 19. There is no
need to enter any patient information prior to performing this verification. The
date and time of the calibration verification will be retained in memory until the
next time a calibration verification is performed.
Social Security Disability Testing requires that the calibration error at the tested
flow rates is within ± 1% of the calibrating volume. In order for the spirometer
to meet the ±1% requirement, a correction factor must be obtained to correct
the measured volume. This correction factor is then applied to the
measurements obtained during the patient tests. For this reason the sensor used
to verify the spirometer’s calibration for an SSD claims submission must also be
used for the actual patient test.
Page 23

Pre-Test Procedures
P-495220-00 Rev. D 18
NOTE: If the patient test will be submitted for Social Security Disability (SSD)
determinations, enter patient information prior to performing the SSD calibration
verification.
CAUTION:
Prior to verifying calibration, visually verify that there is no foreign
material in the pressure tube and the tube is not damaged or kinked.
Page 24
Pre-Test Procedures
19 P-495220-00 Rev. D
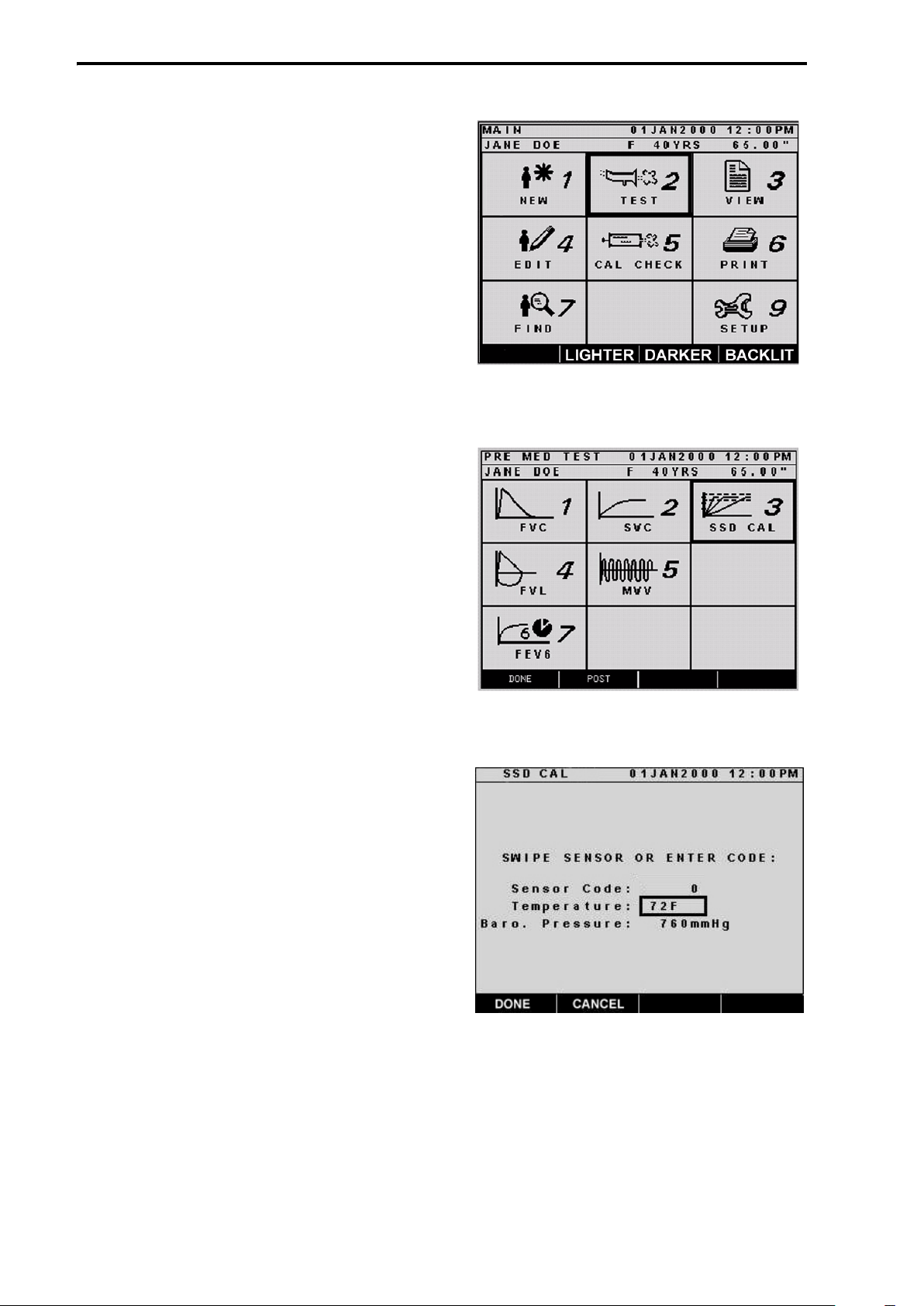
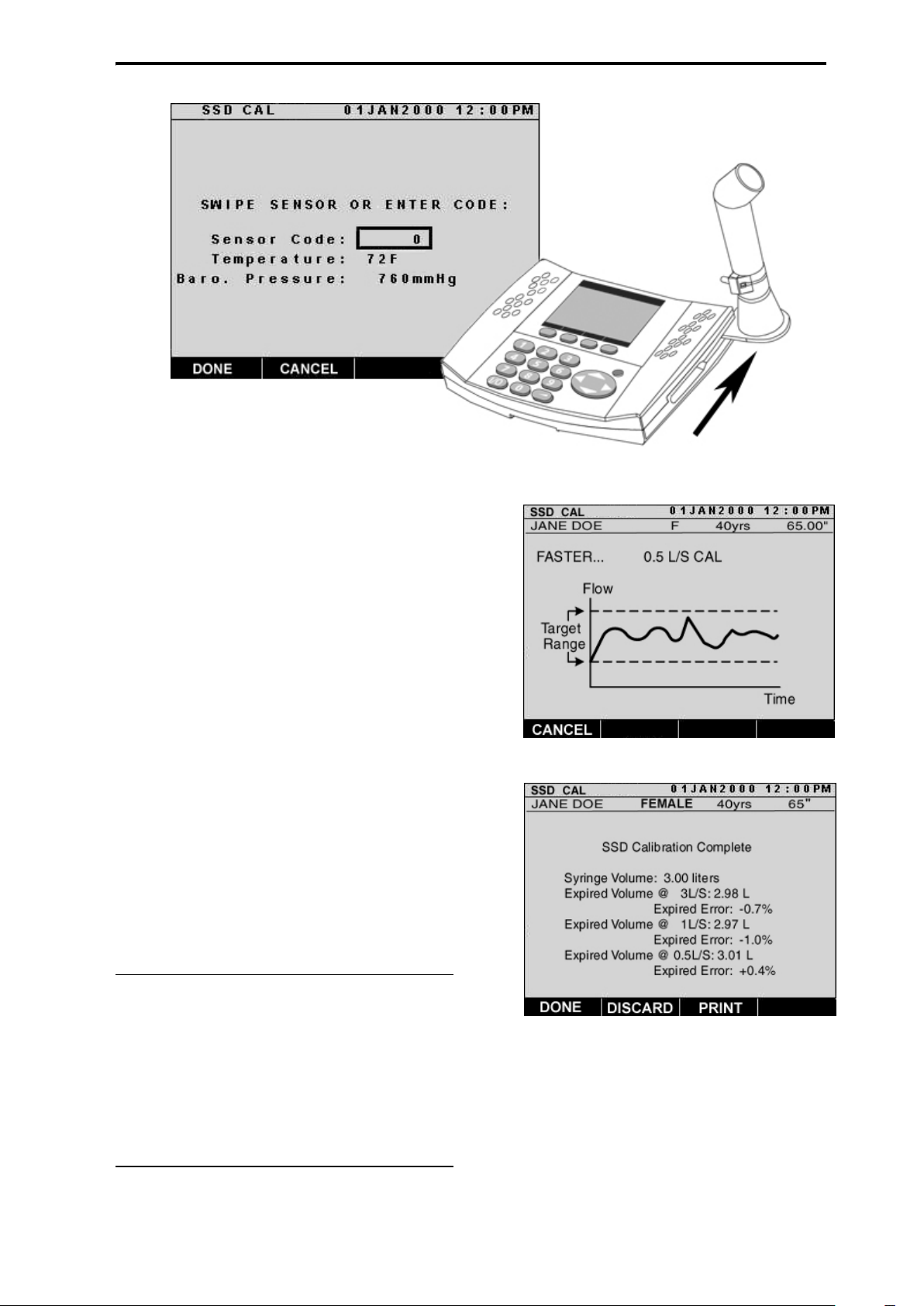
1. From the “MAIN” screen, press 2 on
the keypad or scroll to TEST using
the cursor key and press ENTER
(Figure 8).
2. From the “PRE MED TEST” screen,
press 3 on the keypad, or use the cursor key to scroll to SSD CAL and
press ENTER (Figure 9).
3. On the “SSD CAL” screen, verify
that the temperature and barometric
pressure are correct. If not, scroll to
the corresponding field and type the
correct information using the
numeric keypad before swiping the
sensor. (Figure 10.)
4. Use the cursor key to scroll to the
sensor code field and swipe the sensor (Figure 11) or enter the numeric
code and press the DONE soft key.
5. Continue to follow the screen’s
directions when prompted.
6. Push the 3-Liter syringe plunger in
smoothly over a period of approximately one-second for the 3 L/sec
verification.
7. The dotted lines appearing on the
display represent the upper and
lower limits for the flow rate. While
pushing the plunger in, the graph
will develop on the display. Try to
keep the graph within the dotted
lines. The “SSD CAL” screen will
prompt faster/slower if necessary,
(Figure 12). Repeat until you are
prompted to proceed.
8. Press the YES soft key to proceed
with the 1 L/sec verification, and follow the same procedures as before
(steps 6 through 7) when prompted on the screen for the next calibration
maneuver. Push the 3-Liter syringe plunger in over a period of approximately 3 seconds for the 1 L/sec verification.
9. Press the YES soft key to proceed with the 0.5L/sec verification.
Figure 8: Select TEST from MAIN screen
Figure 10: Enter room temperature and
barometric pressure
Figure 9: Select SSD CAL from PRE-MED
TEST screen
Page 25
Pre-Test Procedures
P-495220-00 Rev. D 20
10. Follow the directions on the screen
and push the 3-Liter syringe plunger
in over a period of approximately
6 seconds for the last SSD verification.
11. The final “SSD CAL” screen (Figure
13) will display the volume of the
calibration syringe, the corrected
measured volume and associated
percentage error for each of the three
flow rates indicated on the illustration.
12. Press DONE to save the calibration
results, DISCARD to delete, or
PRINT to print out a record of the
results. See Printing Results on page
38 for more information on printing.
NOTE: When performing either the
CAL check or SSD CAL maneuver, if
the measured flow or volume is not
within allowable range, the screen
will indicate "Unsuccessful CAL Try
Again?" Refer to the Calibration Error
section of the Troubleshooting Guide
on page 52 to resolve the problem.
Figure 11: Swipe the sensor
Figure 12: SSD CAL
Figure 13: SSD CAL Complete
Page 26

Pre-Test Procedures
21 P-495220-00 Rev. D
Cal Check
1. Connect the pressure tube to the
spirometer and to the FSII sensor.
(Shown previously in Figures 3 and
4.)
2. Following the power-up and initial
setup screens, the “CAL NOW?”
screen will appear on the display.
3. Press the YES soft key to perform the
calibration check or NO to proceed
with a test. When prompted, swipe
the sensor or enter the numeric code
printed on the sensor (Figure 14).
NOTE: Verify that the temperature of the room is the same as the temperature
noted for the calibration test. For every degree discrepancy, there will be a
corresponding 0.15% error in the test results.
NOTE: Verify that the displayed barometric pressure is correct. If not correct, there
will be an error in the inspired volume (FIVC) during an FVL maneuver of
approximately -1.3% for every 1,000 feet above sea level. Refer to the System
Configuration section starting on page 78 for more information. The barometric
pressure displayed is based on the initial elevation setting of the spirometer.
However, the barometric pressure may be changed and the spirometer will, from
that point on, use the new value entered.
CAUTION:
Prior to verifying calibration, visually verify that there is no foreign
material in the pressure tube and the tube is not damaged or kinked.
NOTE: You may also perform a Cal Check by pressing 5 on the keypad at the
“MAIN” screen or scrolling to CAL CHECK with the cursor key and pressing ENTER.
Figure 14: Enter code or swipe sensor
Page 27

Pre-Test Procedures
P-495220-00 Rev. D 22
4. Continue to follow the screen's
directions when prompted (Figure
15).
5. When the display prompts, push the
plunger in smoothly and completely over approximately one
to two seconds.
6. Press the DONE soft key if com-
plete, or pull the plunger out to
complete an INSPIRATORY CAL
check. (Figure 16.)
7. If the calibration check was suc-
cessful, the syringe volume,
measured volume and error percentage will be displayed on the
final screen. (Figure 17.)
8. To assure accurate patient testing,
the calibration check volume
error is required to be ±3% or
less. If the error is greater than
±3%, refer to the Calibration Error
section of the Troubleshooting
Guide on page 52 of this User’s
Manual.
9. Press DONE to save the Cal Check,
DISCARD to delete, or PRINT to
print out a record of the Cal Check
results. See Printing Results on page
38 for more information on printing.
NOTES:
• The ATS does not require an Inspiratory
Calibration.
• The Renaissance II’s Inspiratory
Calibration function has been validated
only for elevations below 4000 feet.
Figure 15: Attaching syringe
Figure 16: Calibratio n chec k
Figure 17: Cal Check complete
Page 28

Pre-Test Procedures
23 P-495220-00 Rev. D
Patient Preparation
Coaching the patient will result in more accurate results. There are several
possible reasons why accurate results are not obtained the first time.
• Not taking a maximal inhalation at the beginning of the maneuver.
• Not blasting the air out quickly or starting slow.
• Not blowing out completely.
Verify that the opening of the sensor is not blocked.
Instruct the patient to:
•Relax
• Loosen tight clothing, such as neckties or tight collars
• Remove dentures, candy, gum, etc.
• Elevate chin and extend the neck slightly
• Avoid leaning forward
• Use a nose clip if available (strongly recommended)
• Blast out air forcefully, completely, and as long as possible
WARNING
• Physicians should assess patient’s ability to perform spirometry
testing prior to administering the test.
• Patient fainting or falling due to dizziness may occur as a result
of this test. Advise the patient to sit or stand comfortably near a
chair during test.
• User should visually inspect the FSII sensor for loose particles/
foreign material prior to patient use.
NOTES:
• Demonstrating the test using your own FSII sensor is strongly recommended for
patients who have never performed a spirometry test before.
• The American Thoracic Society (ATS) recommends performing a three-speed
calibration check on a daily basis.
• Materials used to make this Renaissance II Spirometry System and accessories
contain no Latex.
Page 29

Pre-Test Procedures
P-495220-00 Rev. D 24
WARNING
• Results from spirometry testing should not be the sole source
for determining a patient's diagnosis and treatment. Other
clinical data, such as pa tient symptoms an d re spiratory hi story,
should always be considered.
• Predicted values will be extrapolated for patients with age or
height outside the age and/or height lim its supporte d by the
selected author’s normal equations.
NOTES:
• Verify that the temperature of the room is the same as the temperature noted
for the calibration test. For every degree discrepancy, there will be a
corresponding 0.15% error in the test results.
• Verify that the displayed barometric pressure is correct. If not correct, there will
be an error in the inspired volume (FIVC) during an FVL maneuver of
approximately -1.3% for every 1,000 feet above sea level. Refer to the System
Configuration section starting on page 78 for more information. The barometric
pressure displayed is based on the initial elevation setting of the spirometer.
However, the barometric pressure may be changed and the spirometer will, from
that point on, use the new value entered.
Page 30

Entering New Patient Data
25 P-495220-00 Rev. D
Entering New Patient Data
1. From the “MAIN” screen press 1 on the keypad or use the cursor key to
scroll to the NEW option and press ENTER.
2. When prompted to start a new patient, press the YES soft key. Pressing NO
will return you to the “MAIN” screen.
3. Enter data on the “NEW PATIENT” screen by using alpha or numeric
characters when appropriate. Press ENTER after each field is completed.
Numbers may be entered directly using the numeric keys with the spirometer in numeric mode. Alpha characters can only be entered with the spirometer in alpha mode.
Alpha character entry is modeled after
cell phones. For example, the first key
press displays the first letter, the second
press displays the second letter, and so
on until the last key press displays the
numeral. A pause in pressing the key
causes the entry point to move to the
next character.
4. When the desired character is highlighted, pause or press the cursor
key to move to the next character
space.
5. When the current field is complete,
press the ENTER key to move to
the next field and repeat the process. You must enter the patient’s
height, birth date, and gender or no
interpretation or predicted values
will be displayed.
6. Enter the patient’s race, by pressing
the corresponding number on the
keypad and then pressing ENTER.
NOTE: When in numeric mode (Figure 18), only numbers may be typed on the key
pad. Pressing the ABC... soft key puts the key pad into alpha character mode (Figure
19) allowing both letters and numbers to be entered from the key pad.
Figure 18: New Patient (Numeric)
Figure 19: New Patient (Alpha)
Page 31

Entering New Patient Data
P-495220-00 Rev. D 26
7. If desired, apply an adjustment factor to which the predicted value and
LLN calculations will be multiplied. Table 2 lists the factory default settings and the range of adjustment. See the article Spirometric Reference
Values from a Sample of the General U. S. Population
(16)
for more infor-
mation on race adjustment.
8. Continue entering the patient’s weight, and smoking history (years
smoked, cigarettes per day, and year quit), if applicable. The allowable
weight range is 30 - 440 lb. (15 - 200 kg.). See the section, Lung Age Interpretation, on page 47 for information regarding the applicability of smoking history.
9. Press the NEXT soft key to enter comments and physician, technician,
medication, and dosage information. If you need to change any informa-
tion on the previous screen press the BACK soft key.
10. When all desired information is entered, press the DONE soft key to save
the data and return to the “MAIN” screen.
Table 2: Race Adjustment Settings
Factory Default Setting* Adjustment Range
African American 88%
Asian 100%
Caucasian 100%
Hispanic 100%
Other 100%
10% - 110%
* If a race adjustment setting other than the factory default has been
entered, the spirometer retains the new setting in memory. Ensure the
race adjustment setting is correct for each new patient.
NOTE:
When Caucasian race is selected, adjustment settings other than 100% are
ignored in predicted value and LLN calculations.
Page 32

Pre-Med Testing Procedures
27 P-495220-00 Rev. D
Pre-Med Testing Procedures
FVC (Forced Vital Capacity) Test Procedure
1. From the “MAIN” screen, press 2 on the keypad or scroll to TEST using
the cursor key and press ENTER.
2. From the “PRE MED TEST” screen, press 1 on the keypad or use the cur-
sor key to scroll to FVC and press ENTER.
3. When prompted, swipe the sensor or type the six-digit numeric code and
press ENTER.
4. Enter the room temperature and barometric pressure, if necessary, and
press the DONE soft key. The sensor will zero and the spirometer will display the “FVC TEST” screen and START TEST prompt (Figure 20).
Instruct the patient to:
• Place the sensor in his/her mouth.
• Close lips and teeth around the
sensor in such a way that a tight
seal is formed.
Coach the patient enthusiastically.
"Take a good,
deep breath.
Pull, pull it all
in. Now BLAST
out Keep
blowing,
harder.....
That's good!
Squeeze it out,
squeeze it all
out.....Good
job!"
WARNING
Patient fainting or falling due to dizziness may occur as a result of this
test. Advise the patient to sit or stand comfortably near a chair during
test.
Figure 20: FVC Test
Figure 21: FVC Test Complete
Page 33

Pre-Med Testing Procedures
P-495220-00 Rev. D 28
5. The spirometer will display an incentive message, such as “Keep Going” or
“All the Way” and the elapsed time of the test, while the real-time curve is
being drawn. The patient should keep blowing until the TEST COMPLETE message appears. To display an incentive bar graph instead of the
real-time curve, press the INCENT soft key when the START TEST
prompt appears.
6. Upon completion of the test, the spirometer will display the flow-volume
curve, measured values, acceptability messages, quality grades, number of
maneuvers performed, and FVC and FEV1 variability depending on the
options enabled during system configuration (Figure 21). To view the vol-
ume-time curve, press the VT soft key. To view the results as a percentage
of the predicted values, press the %PRED soft key.
7. Press the SAVE soft key if the maneuver is acceptable. Press the DISCARD
soft key if the maneuver is unacceptable. The display returns to the “PRE-
MED TEST” screen.
8. Select FVC again and repeat the above process up to eight times, until at
least three acceptable and two reproducible maneuvers have been
obtained.
NOTE: The value for FEV6 can only be obtained by administering an FEV6 test.
Page 34

Pre-Med Testing Procedures
29 P-495220-00 Rev. D
SVC (Slow Vital Capacity) Test Procedure
1. From the “MAIN” screen, press 2 on the keypad or scroll to TEST using
the cursor key and press ENTER.
2. From the “PRE MED TEST” screen press 2 on the keypad or use the cursor key to scroll to SVC and press ENTER.
3. When prompted, swipe the sensor or type the numeric code and press
ENTER.
4. Enter the room temperature and barometric pressure, if necessary, and
press the DONE soft key. The sensor will zero and the spirometer will display the “SVC TEST” screen and START TEST prompt.
Instruct the patient to:
• Place the sensor in his/her mouth.
• Close lips and teeth around the sensor in such a
way that a tight seal is formed.
Coach the patient enthusiastically.
"Take a good, deep breath. Pull, pull it all in. Now
exhale normally. That's good! Squeeze it out, squeeze
it all out. Good job!"
5. The spirometer will display an
incentive message and the elapsed
time of the test while the real-time
curve is drawn. To display an incentive bar graph instead of the real-
time curve, press the INCENT soft
key when the START TEST prompt
appears.
6. Upon completion of the test maneuver the spirometer will display the
volume-time curve, measured, predicted, and % of predicted values,
and number of maneuvers performed (Figure 22).
7. Press the SAVE soft key if the maneuver is acceptable. Press the DISCARD
soft key if the maneuver is unacceptable. The display returns to the “PRE-
MED TEST” screen. Repeat the test up to 8 times, if necessary.
WARNING
Patient fainting or falling due to dizziness may occur as a result of this
test. Advise the patient to sit or stand comfortably near a chair during
test.
Figure 22: SVC Test Complete
Page 35

Pre-Med Testing Procedures
P-495220-00 Rev. D 30
FVL (Flow Volume Loop) Test Procedure
1. From the “MAIN” screen, press 2 on the keypad or scroll to TEST using
the cursor key and press ENTER.
2. From the “PRE MED TEST” screen, press 4 on the keypad or use the cur-
sor key to scroll to FVL and press ENTER.
3. When prompted, swipe the sensor or type the numeric code and press
ENTER.
4. Enter the room temperature and barometric pressure, if necessary, and
press the DONE soft key. The sensor will zero and the spirometer will display the “FVL TEST” screen and START TEST prompt.
Instruct the patient to:
• Place the sensor in his/her mouth
• Close lips and teeth around the sensor in such a
way that a tight seal is formed.
Coach the patient enthusiastically.
"Take a good, deep breath. Pull, pull it all in. Now BLAST out.
Keep blowing, harder. That's good! Squeeze it out, and suck it back in,
deep, deep. Good job!"
WARNING
Patient fainting or falling due to dizziness may occur as a result of this
test. Advise the patient to sit or stand comfortably near a chair during
test.
NOTES:
• Verify that the temperature of the room is the same as the temperature noted
for the calibration test. For every degree discrepancy, there will be a
corresponding 0.15% error in the test results.
• Verify that the displayed barometric pressure is correct. If not correct, there will
be an error in the inspired volume (FIVC) of approximately (-1.3%) for every
1,000 feet above sea level. Refer to the System Configuration section starting on
page 78 for more information. The barometric pressure displayed is based on
the initial elevation setting of the spirometer. However, the barometric pressure
may be changed and the spirometer will continue to use the value entered.
• If you choose to obtain barometric pressure from an agency, such as the
weather service, verify that the value is NOT corrected to sea level.
Page 36

Pre-Med Testing Procedures
31 P-495220-00 Rev. D
5. When the spirometer detects that the expiratory portion of the maneuver is
complete, the incentive message will change to "Deep Breath In!"
6. Instruct the patient to inhale as quickly, and fully as possible.
7. The spirometer will continue to display an incentive message and the
elapsed time of the test while the real-time curve is drawn. To display an
incentive bar graph instead of the real-time curve, press the INCENT soft
key when the START TEST prompt appears.
8. Upon completion of the test maneuver the spirometer will display the
flow-volume curve, measured values, quality message, quality grades,
number of maneuvers performed,
and FVC and FEV1 variability
(Figure 23). To view the volume-
time curve press the VT soft key. To
view the results as a percentage of
the predicted values, press the
%PRED soft key.
9. Press the SAVE soft key if the
maneuver is acceptable. Press the
DISCARD soft key if the maneuver is unacceptable. The display returns to
the “PRE-MED TEST” Screen.
10. Select FVL again and repeat the above process up to eight times until at
least three acceptable and two reproducible maneuvers have been obtained.
Figure 23: FVL Test Complete
Page 37

Pre-Med Testing Procedures
P-495220-00 Rev. D 32
MVV (Maximal Voluntary Ventilation) Test Procedure
1. From the “MAIN” screen, press 2 on the keypad or scroll to TEST using
the cursor key and press ENTER.
2. From the “PRE MED TEST” screen press 5 on the keypad or use the cur-
sor key to scroll to MVV and press ENTER.
3. When prompted, swipe the sensor or type the numeric code and press
ENTER.
4. Enter the room temperature and barometric pressure if necessary and press
the DONE soft key. The sensor will zero and the spirometer will display
the “MVV TEST” screen and START TEST prompt.
Instruct the patient to:
• Place the sensor in his/her mouth.
• Close lips and teeth around the sensor in such a
way that a tight seal is formed.
Coach the patient enthusiastically.
"T ake a good, deep breath. Pull, pull it all in.” “Now
breathe out and in deeply and quickly.” “Keep
going... that’s good!” “Keep going! Good job!"
5. The spirometer will display an incentive message and the elapsed time of
the test while the real-time curve is drawn. To display an incentive bar
graph instead of the real-time curve, press the INCENT soft key when the
START TEST prompt appears.
6. Upon completion of the test maneu-
ver, the spirometer will display the
volume-time curve, measured, predicted, and % of predicted values,
and number of maneuvers performed (Figure 24).
7. Press the SAVE soft key if the
maneuver is acceptable. Press the
DISCARD soft key if the maneuver
is unacceptable. The display returns
to the “PRE-MED TEST” screen.
Repeat the test if necessary.
WARNING
Patient fainting or falling due to dizziness may occur as a result of this
test. Advise the patient to sit or stand comfortably near a chair during
test.
Figure 24: MVV Test Complete
Page 38

Pre-Med Testing Procedures
33 P-495220-00 Rev. D
FEV6 (Forced Expiratory Volume in 6 sec.) Test Procedure
1. From the “MAIN” screen, press 2 on the keypad or scroll to TEST using
the cursor key and press ENTER.
2. From the “PRE MED TEST” screen press 7 on the keypad or use the cursor key to scroll to FEV6 and press ENTER.
3. When prompted, swipe the sensor or type the numeric code and press
ENTER.
4. Enter the room temperature and barometric pressure, if necessary, and
press the DONE soft key. The sensor will zero and the spirometer will display the “FEV6 TEST” screen and START TEST prompt.
Instruct the patient to:
• Place the sensor in his/her mouth.
• Close lips and teeth around the sensor in such a
way that a tight seal is formed.
Coach the patient enthusiastically.
"Take a good, deep breath. Pull, pull it all in. Now
BLAST out. Keep blowing, harder. That's good!
Squeeze it out, squeeze it all out. Good job!"
5. The spirometer will display an incentive message and the elapsed time of
the test while the real-time curve is drawn. To display an incentive bar
graph instead of the real-time curve, press the INCENT soft key when the
Start Test prompt appears.
6. The FEV6 test will automatically
terminate after six seconds. At this
time, the spirometer displays the
flow-volume curve, measured values, acceptability messages, quality
grades, number of maneuvers performed, and FEV6 and FEV1 variability (Figure 25). To view the
volume-time curve, press the VT
WARNING
Patient fainting or falling due to dizziness may occur as a result of this
test. Advise the patient to sit or stand comfortably near a chair during
test.
Figure 25: FEV6 Test Complete
Page 39

Pre-Med Testing Procedures
P-495220-00 Rev. D 34
soft key. To view the results as a percentage of the predicted values press
the %PRED soft key.
7. Press the SAVE soft key if the maneuver is acceptable. Press the DISCARD
soft key if the maneuver is unacceptable. The display returns to the “PRE-
MED TEST” screen.
8. Select FEV6 again and repeat the above process up to eight times until at
least three acceptable and two reproducible maneuvers have been
obtained.
NOTE: The value for FEV6 can only be obtained by administering an FEV6 test.
Page 40

Post-Med Testing Procedures
35 P-495220-00 Rev. D
Post-Med Testing
Procedures
After completing the pre-medication
(baseline) testing, administering the
medication (usually an inhaled
bronchodilator), and switching the
spirometer to Post-Med test mode, the
post-medication testing may begin.
The patient’s session must be retrieved
from memory if Post-Med testing is
performed on a patient whose Pre-Med
test was performed more than 30 minutes
prior (if spirometer has powered off), or if
Pre-Med testing is performed on any
patient other than the current one.
To retrieve a patient’s session from memory:
1. From the “MAIN” screen, press 7 or scroll to FIND using the cursor key.
2. Type the patient’s name or ID number (depending upon the spirometer’s
Display configuration) in the FIND PATIENT field or press the SCROLL
soft key and use the cursor arrow to select the patient’s name or ID number.
(See System Configuration on page 78 and Display (5) on page 83 for
information on how to configure the unit to show patients by name or ID.)
3. Press the following soft keys in order: MARK > CURRENT > BACK >
RECALL. The spirometer is now ready to switch to Post-Med test mode.
To switch to Post-Med test mode and perform Post-Med testing:
1. From the “PRE MED TEST” screen press the POST soft key. The
“CHANGE TO POST-MED TEST MODE?” message (Figure 26) appears.
2. Press the YES soft key. The display heading changes to “P O ST ME D
TEST” (Figure 27), and the test options appear just as they did for the
“PRE MED TEST” screen. The only test options appearing on the “POST
MED TEST” screen are those previously performed in Pre-Med testing.
3. Proceed as described in the Pre-Med testing section of this User's Manual
for each test.
NOTES:
• The LCD panel will turn off after 5
minutes (and the unit will power off
after 30 minutes) with no user input. To
bring back the display before the 30minute limit, press any key.
• After 4 hours, a Post-Med test is not
allowed and the soft key is not available.
Figure 26: Change to Post-Med Test Mode
Figure 27: Post Med Test Screen
Page 41

Post-Test Procedures
P-495220-00 Rev. D 36
Post-Test Procedures
Saving Results
The Renaissance II spirometer saves all patient data and test data, provided the
SAVE soft key is pressed after each maneuver.
Viewing Results
The patient whose test results are currently in memory may be viewed in
several formats.
1. From the “MAIN” screen press 3
on the keypad, or scroll to the
VIEW option and press ENTER.
2. The “SELECT VIEW” screen
appears, indicating the view options
as shown in Figure 28. See Table 3
for the definitions of each option.
NOTES:
• If a maneuver is saved, it is retained in memory until the entire patient session is
deleted. This includes abnormally large maneuvers that occur as a result of an
occluded flow sensor and that subsequently may be labeled as the best
maneuver.
• The spirometer will store multiple tests for each maneuver, but will only display
and print up to the best three Pre-Med and Post-Med FVC/FVL and FEV6
maneuvers, the single best Pre-Med and Post-Med SVC and MVV maneuvers,
and the last maneuver performed.
• If the user does not press the POST soft key prior to performing Post-Med tests,
the maneuvers will be stored as Pre-Med tests and cannot be transferred to
Post-Med status.
Figure 28: Select View screen
Page 42

Post-Test Procedures
37 P-495220-00 Rev. D
After selecting the desired view option, several soft key options are available:
•Pressing the NEXT soft key scrolls the display of numerical test data.
•Pressing the CURVE soft key will display the flow-volume curve(s) for
displayed FVC/FVL or FEV6 test data.
•Pressing the VT soft key while the flow-volume curves are displayed,
shows the associated volume-time curve.
•Pressing the FV soft key while the volume-time curves are displayed shows
the flow-volume graph.
•Pressing the DATA soft key returns to the numerical test data display.
•Pressing the POST soft key will change the display to the Post-medication
test results for the view option chosen.
Table 3: View Options
Displays results for the single best Pre-Med or Post-Med effort as
determined by the “Best Criteria” configured in Spirometry Options.*
Summarizes each Pre-Med or Post-Med maneuver with number of
attempts, number of acceptable tests, number of reproducible tests,
and interpretation if these options are enabled in Spirometry Options.*
Displays test results for the best three Pre-Med or Post-Med efforts as
determined by the “Best Criteria” configured in Spirometry Options.*
Displays last calibration check results.
LAST
Displays test results for the most recent maneuver only.
*See System Configuration and Spirometry Options (1) on page 78 for information on
configuring the spirometer.
Page 43

Post-Test Procedures
P-495220-00 Rev. D 38
Printing Results
The Renaissance II Spirometry System operates with selected Hewlett-Packard,
Epson, and Canon printers (see Print Options (3) on page 80 for supported
printers). The spirometer's configuration settings may be changed to match the
printer. Refer to System Configuration on page 78, for information on
changing the spirometer’s configuration.
To print a report:
1. Switch to the “SELECT REPORT”
screen (Figure 29) by pressing 6
from the “MAIN” screen or using
the cursor key to scroll to PRINT
and pressing ENTER. Table 4 lists
the available reports and their
descriptions.
2. Connect a compatible printer to the
parallel port on the base station
using the printer cable (see Table 1,
item 16). The printer should be
turned off when connecting the
cable.
3. Turn the printer on and verify that it is online and ready to print.
4. Dock the Renaissance II spirometer onto the base station. The spirometer
must stay docked in the base station for the duration of printing. If you
wish to alter the format of the printed report, press the OPTIONS soft key
and change the information. Press the DONE soft key to return to the
“SELECT REPORT” screen.
5. Press the appropriate number on the keypad or scroll to the desired report
and press ENTER to send the report to the printer.
Figure 29: Select Report screen
Page 44

Post-Test Procedures
39 P-495220-00 Rev. D
Table 4: Report Selections
Prints the results for the single best Pre-Med and Post-
Med effort for each type of maneuver as defined by the
“Best Criteria” configured in Spirometry Options.*
Prints a report suitable for Social Security Disability
claims submissions.
Prints the spirometer configuration settings.
Prints test results for the best three Pre-Med and Post-
Med FVC/FVL and FEV6 maneuvers and the single best
Pre-Med and Post-Med SVC and MVV maneuvers as
defined by the “Best Criteria” configured in Spirometry
Options.*
Prints the results of the last calibration check.
Prints the test results for the most recent maneuver.
Displays or prints a Return On Investment report.
*See System Configuration and Spirometry Options (1) on page 78 for
information on configuring the spirometer.
Page 45

Post-Test Procedures
P-495220-00 Rev. D 40
The Return on Investment (ROI) Report can be used as a cost management
tool for calculating and displaying Total Income, Total Cost, Net Income, ROI,
and Payback (in months) based upon the following values supplied by the user:
• Purchase price
• Covered tests per week
• Cost per covered test
• Reimbursement (per test)
The Renaissance II calculates ROI and Payback in the following manner:
ROI = (Reimbursement / Cost per covered test) x 100%
Payback (months) = Purchase price / Net income (monthly)
To print an ROI report:
1. From the SELECT REPORT screen, press 9 or scroll to ROI and press
ENTER.
2. Type the purchase price and press ENTER.
3. Type the number of tests performed per week and press ENTER.
4. Type the cost per test and press ENTER.
5. Type the amount reimbursed per test. The ROI is displayed on the screen.
6. Press the PRINT soft key to print the ROI report or the DONE soft key to
return to the “SELECT REPORT” screen without printing.
Printing Reports for Multiple Patients
The Renaissance II spirometer allows printing reports for multiple patients at
the same time.
To print multiple reports:
1. Prepare the printer and spirometer system as described above. The spirometer must stay docked in the base station for the duration of printing.
2. From the “MAIN” screen, press 7 on the keypad or scroll to FIND with
the cursor arrow key and press ENTER.
3. Press the SCROLL soft key.
4. Use the cursor arrow key to highlight the patient whose record you want
to print. To print all patient records, highlight any patient.
5. Press the MARK soft key.
6. Press the CURRENT soft key to select the record. Continue with the cursor arrow key and CURRENT soft key to highlight and select the patient
records you want to print. To select all patient records, press the ALL soft
key. To select all records that have been added or changed since last
NOTE: The existence of coding does not guarantee coverage or payment for any
procedure by any payer. In any case, reimbursement is only available for medically
necessary procedures (in accordance with specific payer guidelines.)
Page 46

Post-Test Procedures
41 P-495220-00 Rev. D
printed or downloaded to a PC, press the NEW soft key. When you have
finished selecting records, press the BACK soft key.
7. Press the NEXT, then PRINT soft keys. The best Pre-Med and Post-Med
result for each maneuver performed in a patient’s session is printed.
Deleting Patient Data
The Renaissance II spirometer can store data for up to 1000 patients. When the
memory becomes full or if the unit is sent in for repair or exchange, it may
become necessary to erase patient data.
To delete patient data:
1. From the “MAIN” screen, press 7 on the keypad or use the cursor arrow
key to scroll to FIND and press ENTER.
2. Press the SCROLL soft key.
3. Use the cursor arrow key to highlight the patient to be deleted. If you want
to delete all patient data, highlight any patient.
4. Press the MARK soft key.
5. Use the CURRENT or ALL soft keys to delete either the highlighted patient
or all patient data.
6. Press the BACK soft key.
7. Press the NEXT soft key.
8. Press the DELETE soft key. A confirmation screen appears if this option
has been enabled (see System Configuration on page 78 and Storage (8) on
page 83 for information on changing the spirometer’s configuration and
enabling the Confirm Before Delete option).
9. Press the YES soft key to delete.
The selected patient data is deleted. If a failure has occurred that does not allow
you to access the main screen, the memory cannot be deleted.
The optional DataFlow™ Data Management Software (Table 1, optional
accessory item 15, on page 9) allows the user to upload patient data to a PC for
archival in a database. Performing this operation prior to deleting patient data
from the Renaissance II Spirometer provides a solution for long-term patient
data storage. See the section, Using the Renaissance II with a PC and
Dataflow™ Software, on page 77 for more information.
NOTE: In order to comply with HIPAA, the user should consider deleting all patient
data prior to sending the unit to Puritan Bennett.
Page 47

Interpretation of the Results
P-495220-00 Rev. D 42
Interpretation of the Results
Acceptability and Reproducibility
Achieving high quality test results depends upon patient effort and technician
coaching. The Renaissance II spirometer determines the quality of each patient
effort by measuring the acceptability and reproducibility of the maneuver and
displaying an “Acceptability Message” that will help both you and your
patient achieve accurate results.
The Renaissance II determines the acceptability and reproducibility of
maneuvers based upon the ATS acceptability and reproducibility criteria
(11)
.
These criteria are used to determine which message from Table 5 is displayed at
the end of a maneuver.
A test is considered acceptable if the “Good Test” message is displayed at the
end of the maneuver. If a different message is displayed, use the associated
coaching instruction in Table 5 to try to improve the patient’s test outcome.
The Renaissance II measures reproducibility (also called variability) after at
least two repetitions of a particular maneuver have been performed, and
displays the measurements on the screen and in printed reports as FVC VAR
and FEV1 VAR. Results are considered reproducible if the two largest FVC
and FEV1 measurements are within 200 ml of each other.
According to the ATS, testing may be concluded when at least 3 acceptable and
2 reproducible spirograms have been obtained, or when a total of eight tests
have been performed, or the patient cannot or should not continue.
To view the number of acceptable and reproducible tests:
1. Make sure the spirometer is configured so that acceptability and reproduc-
ibility options are enabled (see the System Configuration section starting
on page 78, to configure Spirometry Options).
2. From the “MAIN” screen, press 3 on the keypad or scroll to VIEW with
the cursor arrow key and press ENTER. The “SELECT VIEW” screen
appears.
3. From the “SELECT VIEW” screen, press 2 or scroll to SUMMARY with
the cursor arrow key and press ENTER. The number of acceptable and
reproducible tests is shown in the display.
If “Accept Msgs” is enabled in the Print Options screen, the Acceptability
Message will print on the test page if the LAST test is selected for printing (see
the System Configuration section starting on page 78, to configure Spirometry
and Print Options, and Printing Results on page 38 for information regarding
printing reports).
Page 48

Interpretation of the Results
43 P-495220-00 Rev. D
Table 5: Test Acceptability Messages
Message Definition Coaching Instruction
START
FASTER
Extrapolated volume is greater than 5%
of FVC or greater than
150 ml (5% if FVC >
3 liters; 150 ml if
FVC < 3 liters).
Patient must not hesitate or leak out
any air at the beginning of the test.
AVOID
COUGHING
Substantial (> 50%) drop and recovery
in flow within the first second.
Ask the patient to clear his/her throat
and/or offer a drink of water.
BLOW OUT
LONGER
FET < 6 seconds for FVC maneuvers or
FET < 6 seconds and exhaled volume in
last 0.5 second of test > 100 ml for FEV6
maneuvers.
Coach the patient to blow out longer.
BLOW OUT
MORE
Flow > 200 ml/s during last 100 ms
before first occurrence of peak volume
or < 2 s plateau (time from end of test to
first occurrence of peak volume − 20 ml).
The patient quit before his/her lungs
were completely empty. Coach the
patient to keep blowing as long as
possible.
BLOW OUT
HARDER
Time to PEF > 120 ms (for FEV6
maneuvers only).
Instruct the patient to blast out air
forcefully.
FVC
VARIABLE
There is a difference of at least 200 ml
between the two best FVC values. The
difference must be less than 200 ml.
Observe patient performance for
differences between good tests and
those with high variability and instruct
accordingly.
FEV1
VARIABLE
There is a difference of at least 200 ml
between the two best FEV1 values. The
difference must be less than 200 ml.
Observe patient performance for
differences between good tests and
those with high variability and instruct
accordingly.
PEF
VARIABLE
There is a difference of at least 10% and
1 L/sec between the last and best PEF
values.
Observe patient performance for
differences between good tests and
those with high variability and instruct
accordingly.
FVC
GREATER
THAN FIVC
FIVC < 90% FVC. Exhalation
significantly greater than inhalation.
Instruct the patient to inhale
completely at the end of the test.
FIVC
GREATER
THAN FVC
FVC < 90% FIVC. Inhalation significantly
greater than exhalation.
Instruct the patient to blow out more
completely.
GOOD TEST! No problems detected. N/A
Page 49

Interpretation of the Results
P-495220-00 Rev. D 44
Grading Criteria
Unlike many other medical tests in which the patient is passive, spirometry
requires active cooperation and strenuous effort by the patient. The ability of
clinicians to elicit this effort varies widely, but can be improved with
experience and feedback.
The Quality Grades, which range from A to F, are displayed on screen and
printed on the Best and Best 3 reports for the FVC/FEV1 and FEV6/FEV1 tests
if the QC Grades option is enabled. The Quality Grades are an indication of
the reliability of each of these measurements, and physicians may use them to
judge their degree of confidence in the results. The grades are also an indicator
of the short-term reproducibility of the FVC/FEV1, and FEV6/FEV1
measurements for that patient.
The Renaissance II assigns a Quality Grade according to the criteria shown in
Tables 6 and 7. Use the grade in conjunction with the Acceptability Messages
and coaching instructions in Table 5 to improve patient test performance.
NOTE: It is possible to have a “Good Test” Acceptability Message accompanied by a
low QC grade. If the first FVC or FEV6 maneuver is acceptable, the Quality
Grade will be D by definition (see Tables 6 and 7). It is not until there are at
least 2 acceptable maneuvers that there is the possibility for a higher QC grade.
T able 6: Quality Grades for FVC/FEV1 Tests
A At least 3 acceptable maneuvers with reproducibility of < 120 ml.
Exceeds ATS acceptability and reproducibility criteria.
B At least 3 acceptable maneuvers with reproducibility of ≤ 200 ml.
Meets ATS acceptability and reproducibility criteria.
C At least 2 acceptable maneuvers with reproducibility of ≤ 280 ml.
Slightly below ATS acceptability and reproducibility criteria.
D At least 1 acceptable maneuver with reproducibility of ≤ 360 ml.
Substantially below ATS acceptability and reproducibility criteria.
F No acceptable maneuvers or reproducibility of > 360 ml.
All other cases.
Table 7: Quality Grades for FEV6/FEV1 Tests
A At least 2 acceptable maneuvers with reproducibility of ≤ 100 ml.
B At least 2 acceptable maneuvers with reproducibility of ≤ 150 ml.
C At least 2 acceptable maneuvers with reproducibility of ≤ 200 ml.
D At least 2 acceptable maneuvers with reproducibility of ≤ 200 ml OR at
least 1 acceptable maneuver.
F No acceptable maneuvers.
Page 50

Interpretation of the Results
45 P-495220-00 Rev. D
To ensure that the Quality Grades are displayed and printed in reports, enable
the QC Grades option in the Spirometry Options screen of the Setup menu (see
System Configuration and Spirometry Options (1) starting on page 78 for
information on configuring the spirometer).
Interpretation Criteria
Upon completion of an FVC or FEV6 test session, the Renaissance II spirometer
will generate an interpretation of the test data. The interpretation criteria are
those suggested by the American Thoracic Society statement, Lung Function
testing.
13
This computer-suggested interpretation is an option that may be
turned off in the configuration settings.
Because data from poor patient efforts may result in false positive
interpretations, careful analysis must be used in conjunction with the
interpretations, especially when maneuver quality grades are low (“D” or “F”
in Table 6 or Table 7). A flow chart of the interpretation algorithm results is
shown in Table 8.
NOTE: If Interpretation is turned OFF in the Print Options configuration, the
spirometer will not print the Comments heading.
NOTE: The patient’s height, birth date, and gender must be entered or no
interpretation will be displayed.
Page 51

Interpretation of the Results
P-495220-00 Rev. D 46
*Physiological variant is interpreted as “Undetermined” in the Renaissance II if FVC and FEV1 are
greater than 100% and FEV1% is less than 100%.
If the FEV1/FVC ratio (FEV1%) is below the lower limit of the normal range,
the patient is identified as having airway obstruction. The degree of
obstruction is then determined by the amount of reduction in the FEV1 value.
If the FVC is also reduced in a patient with an obstruction, a superimposed
restriction cannot be ruled out.
Table 8: Interpretation Results
Page 52

Interpretation of the Results
47 P-495220-00 Rev. D
When the FVC is below the lower limit of normal, and the FEV1% is normal,
the patient may have a restrictive disorder. The degree of restriction can be
determined by the amount of reduction in the FVC. Note that a low FVC is
often due to poor patient effort. If both the FVC and FEV1% are within the
normal range, the spirometry is considered to be normal.
Lung Age Interpretation
Lung age is a smoking cessation tool that, if the option is enabled, appears on
the Summary screen (by pressing the
RISK
soft key) and on the printed report
(see System Configuration and Spirometry Options (1) starting on page 78 for
information on how to enable the Lung Age option)
. The patient's lung age will
be calculated if you have entered the patient's smoking history, and the patient’s
actual age is 20 to 84 years. The lung age value is calculated by substituting the
predicted value of FEV1 with the smoker's actual FEV1. Then the FEV1
predicted equation is solved for age. The reported lung age value will never be
less than 25 or less than the patient's actual age. It should be emphasized that
the lung age parameter is intended to be used solely as a smoking cessation tool
and not as a diagnostic measurement.
(14)
Risk of COPD
The risk of COPD expresses the probability, expressed as a percentage, that the
patient will develop Chronic Obstructive Pulmonary Disease (COPD) within
the next 10 years and also provides an indication of the risk if the patient were
to quit smoking. If the option is enabled, the COPD risk appears on the
Summary screen (by pressing the RISK soft key) and on the printed report if the
patient's age and smoking history were entered during new patient setup (see
System Configuration and Spirometry Options (1) starting on page 78 for
information on how to enable the Risk of COPD option). The risk of COPD is
calculated using the patient’s age, cigarettes smoked per day, measured FEV1,
and predicted FEV1 as variables substituted into the equations documented in
the article, Risk of Chronic Obstructive Pulmonary Disease.
(15)
NOTE: Refer to “Predicted Normal Equations and References” on page 60 for
further explanation.
NOTE: Entering a smoking cessation date will eliminate the lung age value.
NOTE: The risk of COPD is calculated only for patients 64 years and younger.
Page 53

Interpretation of the Results
P-495220-00 Rev. D 48
Graphic Displays
Spirometry maneuvers are usually
illustrated by means of graphs
showing flow rates and volumes
during expiration and inspiration.
Two types of graphs are used, FlowVolume (FV) and Volume-Time (VT).
A typical Flow-Volume graph is
shown in Figure 30. The FlowVolume graph allows the user to
easily evaluate whether the maneuver
was poor due to a cough, slow start,
poor effort or early termination. In
addition, obstructive or restrictive
disorders can often be detected by
examining the waveform. Additional
guidance can be obtained on this
subject in the Introduction to
Spirometry Testing section of this
manual starting on page 15.
The Volume-Time Graph shown in
Figure 31, is the traditional
representation of the data. The
advantage of this representation is
that the FEV1 value, as well as most
other timed parameters, can be read
directly from the plot.
Either graph type can be viewed on
the graphical display. In addition, the
spirometer can be configured to print
either, both, or none of the graphs.
Figure 30: Flow-V olume Graph
Figure 31: Volume-Time Graph
Page 54

Service and Maintenance
49 P-495220-00 Rev. D
Service and Maintenance
Cleaning
Because the Renaissance II Spirometry System uses disposable single-patient use
FSII flow sensors, there is no need to clean or sterilize any part of the
spirometer or pressure tube.* Remove dust or fingerprints from the exterior by
wiping with a damp cloth.
If the need for a more thorough cleaning arises, the spirometer, base station,
and tubing can be wiped down with a solution of 70% Isopropyl Alcohol or
10% bleach. Use the established procedures at your facility for the use and
disposal of these disinfecting agents.
The pressure tube should be replaced at least once every year or if it becomes
discolored or cracked. To remove the tube, grasp the thumb grip where it
connects to the spirometer and pull gently until disconnected.
The spirometer’s bar code reader may be cleaned by wrapping a flow sensor’s
bar coded tab with an alcohol wipe and sliding it through the bar code reader
slot.
Battery Installation
The Renaissance II battery should be replaced at least once per year. Refer to
the battery label to determine battery age. When there is a low battery
condition (fewer than 20 patient tests can be performed), the Renaissance II
spirometer beeps every 30 seconds and displays a low battery icon.
*Contact Puritan Bennett Technical Support at 1.800.255.6774 for information regarding cross-
contamination studies performed on the flow sensor and pressure tube.
CAUTION:
Do not spray liquids on the Renaissance II System. Follow the cleaning
instructions outlined in this section.
CAUTION:
Do not use the Renaissance II Spirometry System in areas of high humidity
and dust, or in extreme environments.
Warning
Chemicals from a broken LCD display panel are toxic when ingested.
Use caution when handling a Rena issan c e II sp irometer with a
broken display panel.
NOTE: Before replacing the battery, print the current configuration as indicated on
page 86 to ensure no settings are lost.
Page 55

Service and Maintenance
P-495220-00 Rev. D 50
Use the following procedure to install or replace the battery:
1. If the AC adapter is connected
to the spirometer, remove the
connector from the spirometer.
2. Remove the battery door
(Figure 32) by pressing the tab
on the front of the battery door
and pulling the door off.
3. Remove the used NiCad battery
pack by disconnecting the connector and lifting the batteries
out gently.
4. Insert a new battery pack
according to the illustration on
inside of the case and plug in
the connector.
5. After the batteries have been replaced, reinstall the battery door.
6. Charge the battery for at least 24 hours before portable use. Compare the
System Configuration with your print-out to ensure settings are appropriate (see page 78 for System Configuration information).
The custom NiCad battery pack has a battery life of 10-12 hours in the ON
position and a battery life of approximately 8 days in the OFF position.
Standard AA Alkaline or NiCad batteries may be used instead of the custom
battery pack supplied with system. However, the Renaissance II will only
charge the custom NiCad battery pack supplied with the unit.
NOTE: If the battery is removed, the unit will operate solely on AC power if
connected to an electrical outlet via the AC adapter.
CAUTION:
• Dispose of batteries properly. Do not incinerate. Puritan
Bennett recommends that customers or technical service
personnel follow local gove rning ordinances and recycling
instructions regarding disposal or recycling of batteries.
• The NiCad battery pack or other batteries may discharge over
time. Check batteries at least once per month for corrosion and
verify batteries are fully charged. Store spirometer in charging
base station to keep unit ready for use.
• Remove batteries if spirometer will not be used for at least two
weeks.
Figure 32: Battery Installation
Page 56

Troubleshooting Guide
51 P-495220-00 Rev. D
Troubleshooting Guide
Unit powers up while on AC power, but not battery power
1. Verify that batteries are installed properly and properly charged.
2. Verify batteries are less than one year old. Replace batteries at least once
per year.
3. Batteries may be low. Replace or recharge the custom battery pack.
4. Contact Puritan Bennett Technical Support if results are unsuccessful.
Unit does not power up on AC or battery power
1. Verify wall outlet or power strip is on and functioning properly. If not, try
another outlet.
2. Verify power LEDs on spirometer and AC adapter illuminate.
3. Contact Puritan Bennett Technical Support if results are unsuccessful.
Unit powers up, but will not perform a test
1. If the spirometer failed the self test, record the error message, cycle power
and try again.
2. If display does not illuminate, press several keys and note if unit beeps in
response to key presses.
3. If the unit responds to key presses, the display contrast may have been
adjusted incorrectly. From the “MAIN” screen, adjust contrast darker by
pressing and holding the “DARKER” soft key (3rd key from left) until the
display appears.
4. Contact Puritan Bennett Technical Support if results are unsuccessful.
"Error Reading Sensor" is displayed in the message window
1. Barcode strip is damaged or sensor swiped incorrectly. Swipe the sensor
again or manually enter the six digit sensor calibration code.
2. Repeat the procedure with a new sensor.
3. Clean the spirometer’s bar code reader by wrapping a flow sensor’s bar
coded tab with an alcohol wipe and sliding it through the bar code reader
slot.
4. Contact Puritan Bennett Technical Support if results are unsuccessful.
NOTES:
• The Renaissance II Spirometry System is designed to recharge only the
custom battery pack supplied with the system, and will not recharge batteries
from other manufacturers.
• When there is a low battery condition, the Renaissance II spirometer beeps every
30 seconds and displays a low battery icon.
Page 57

Troubleshooting Guide
P-495220-00 Rev. D 52
Calibration error is more than ± 3%
1. Verify that the correct temperature is entered.
2. Verify that the correct barometric pressure or elevation for your location is
entered.
3. If the percent error reading is -3% or less, check the system for leaks.
Examine the pressure tube for any small punctures. Check syringe, sensor,
and pressure tube for loose connections.
4. If the percent error is +3% or more, examine the flow sensor for foreign
material contamination of the resistance medium.
5. Contact Puritan Bennett Technical Support if results are unsuccessful.
"Error Zeroing Sensor" is displayed in the message window
1. Movement sensed during zeroing. Place the sensor and pressure tube on
the table top and repeat the procedure.
2. Disconnect the pressure tube from the spirometer and rezero. If the
spirometer zeroes without the pressure tube connected, the pressure tube
may be defective. Examine pressure tube for moisture or other obstruction
in the clear portion of the tube.
3. Contact Puritan Bennett Technical Support if results are unsuccessful.
“Error Sensing Blast Out" is displayed in the message window
1. No exhalation was sensed within 20 seconds. Repeat the test.
2. Verify that the pressure tube is connected.
3. Examine pressure tube for damage.
4. Contact Puritan Bennett Technical Support if results are unsuccessful.
Spirometer auto-senses - (spirometer registers flow when no test is being
performed)
1. Disconnect pressure tube and re-initiate a test procedure. If the unit does
not auto-sense, replace the pressure tube.
2. If the unit auto-senses with nothing connected to it, contact Puritan Ben-
nett Technical Support.
NOTE: Replace the pressure tube every year. Recertify 3-liter syringes once per year.
NOTE: Replace the pressure tube every year.
NOTE: Replace the pressure tube every year.
NOTE: Replace the pressure tube every year.
Page 58

Troubleshooting Guide
53 P-495220-00 Rev. D
Patient test values displayed by the Renaissance II do not meet values
expected by the physician.
If the values are unusually high:
1. Check for damage to the flow sensor.
2. Verify that the flow sensor is not contaminated with sputum or secretions.
3. Verify that the patient data (height, birth date, gender, ethnic origin) being
used for the test is accurate for the patient.
4. If test is FVL, verify that proper room temperature and elevation or barometric pressure have been entered. (See Table 14 on page 87 in this User’s
Manual.)
5. Perform a calibration check using the sensor that the patient tested with.
Make a note of the results.
6. Contact Puritan Bennett Technical Support if results are unsuccessful.
If the values are unusually low:
1. Check flow sensor and pressure tube for leaks.
2. Verify patient is not leaking air from mouth or nose. Use nose clips.
3. Verify patient is tightly closing lips and teeth around the outside of the
sensor.
4. Perform a calibration check using the flow sensor that the patient tested
with. Make a note of the results.
5. Contact Puritan Bennett Technical Support if results are unsuccessful.
NOTES:
• Overestimation of the room temperature will cause lung volume to be
underestimated by 5%; conversely, if temperature is underestimated, lung
volume will be overestimated.
• Verify that the displayed barometric pressure is correct. If not correct, there will
be an error in the inspired volume (FIVC) during an FVL maneuver of
approximately -1.3% for every 1,000 feet above sea level. Refer to the System
Configuration section starting on page 78 for more information. The barometric
pressure displayed is based on the initial elevation setting of the spirometer.
However, the barometric pressure may be changed and the spirometer will, from
that point on, use the new value entered.
• If you choose to obtain barometric pressure from an agency, such as the National
Weather Service, verify that the value is NOT corrected to sea level.
NOTE: Obstructing sensor opening with teeth, lips, or tongue while performing the
test will cause low readings.
NOTE: Replace the pressure tube every year.
Page 59

Troubleshooting Guide
P-495220-00 Rev. D 54
Unable To Print Test Results
1. Verify that the printer is turned on and is online and ready.
2. Print a test page to ensure the printer is working properly. If the problem
seems to be isolated to the printer, contact the printer manufacturer for
technical support.
3. Verify that the proper printer has been selected in the spirometer's
configuration. See System Configuration on page 78 and Print Options (3)
on page 80 for information on configuring the printer.
4. Check the cable connections. When connecting the cable to the base
station, verify that the thumbscrews have been tightened equally to provide a flush connection with the base station connector.
5. Verify that the spirometer was docked in the base station for the duration
of printing.
6. Turn off the printer and remove the spirometer from the base station. Turn
on the spirometer and place it in the base station. Turn the printer on.
Attempt to print the test results.
7. Contact Puritan Bennett Technical Support if results are unsuccessful.
Electromagnetic Interference
The Renaissance II spirometer has been designed to provide reasonable
protection against harmful interference in a typical medical environment.
Because of the proliferation of radio frequency transmitting equipment and
other sources of electrical noise in healthcare environments, however, it is
possible that high levels of interference in close proximity may occur which
could disrupt the performance of this device. The following is a list of possible
types of radio frequency transmitting equipment and other sources of electrical
noise sometimes present in healthcare environments:
• Electrosurgical units
• Cellular phones
• Mobile two-way radios
WARNING
When connecting the Renaissance II spirometer to any instrument, verify
proper operation. Accessory equipment connected to the data interface
must be certified according to IEC Standard 950 for data processing
equipment or IEC Standard 601-1 for electromedical equipment. All
combinations of equipment must be in compliance with IEC Standard 6011-1 systems requirements . Anyone who connects additional equipment to
the signal input port or signal output port, configures a medical system
and is therefore responsible that the system complies with the
requirements of IEC Standard 601-1-1 and the electromagnetic
compatibility requirements of IEC Standard 601-1-2.
Page 60

Troubleshooting Guide
55 P-495220-00 Rev. D
During interference, the spirometer may not seem to operate correctly.
Measurements may seem inappropriate, or there may be erratic readings,
cessation of operation, or other incorrect functioning.
If erratic performance occurs, survey the location of the Renaissance II system
to determine the source of the disruption, and take appropriate action as listed
below:
• Turn other equipment in the vicinity off and on to isolate the problem.
• Reorient or relocate the offending device.
• Increase the separation between the interfering equipment and the
spirometer.
If assistance is required, contact Puritan Bennett's Technical Support
Department or your local Puritan Bennett representative.
Warranty Information
Refer to the warranty card(s) that came with your Renaissance II Spirometry
System and accessories for specific warranty periods. If Puritan Bennett
establishes a need of repair, another unit of comparable value will be dispatched
as soon as possible. The foregoing warranty shall not apply and Puritan Bennett
shall be relieved of any obligation or liability if the component has been:
• Repaired or altered, including the use of parts other than those manufactured
or approved by Puritan Bennett
• Serviced by anyone other than Puritan Bennett
• Subjected to abuse, negligence, or accident
• Reused when sold for single-patient use only
• Connected to the wrong AC adapter
Puritan Bennett does not warrant accessories for the Renaissance II Spirometry
System, such as the printer, that are not manufactured by or for Puritan
Bennett. The end user is required to seek warranty assistance directly from the
manufacturer of these accessories.
CAUTION:
The Renaissance II system may be susceptible to radio frequency
interference.
CAUTION:
Do not remove the cover of the Renaissance II spirometer or base station.
Removal of the cover is permitted only by qualified service personnel.
There are no user-serviceable parts inside.
Page 61

Troubleshooting Guide
P-495220-00 Rev. D 56
Also, the warranty may be voided if the system is used with any sensor other
than those manufactured or licensed by Puritan Bennett for use with the
Renaissance II Spirometry System. Please reference the warranty enclosed with
your system for full clarification of the warranty.
Each Renaissance II spirometer and base station is manufactured and recorded
with an individual serial number. The serial number is located on a label
attached to the underside of the unit. Please reference this information when
contacting Puritan Bennett.
NOTE: The serial numbers are located on a label affixed to the underside of the
spirometer or base station. The first letter "G" represents the manufacturer. The
next two numbers represent the year of manufacture. The two digits following the
year represent either a base station (08) or a spirometer (07). The last five digits are
sequential numbers assigned during manufacture.
Page 62

Technical References
57 P-495220-00 Rev. D
Technical References
Table 9: Product Specifications Renaissance II Spirometer
Dimensions: 5.75"(H) x 7.5"(W) x 2.25" (D)
Weight: 18 ounces
Accuracy:
*
Validated to comply with American Thoracic
Society Standards for Spirometry (1994)
(11)
Volume: ±3% of reading or 50 ml, whichever is greater;
FEV1, FEV3 and FEV6 measured by back
extrapolation
PEF: ±10% of reading or 0.40 L/sec, whichever is
greater
FEF25-75% or MMEF: ±5% of reading or 0.20 L/sec, whichever is
greater
Volume Range: 0-12 Liters BTPS
Flow Range: ±16 Liters/sec
Resistance: Less than 1.5 cm H
2
O /Liters/sec from 0-12
Liters/sec
Test Time: SVC/FVC/FVL: 30 seconds; MVV: 15 seconds
Display: 3.1" x 2.4" viewable area (78mm x 61mm), 320
x 240 dots
Parameters Measured: FVC, FEV1, FEV3, FEV6, FEV1/FVC (FEV1%),
FEF25-75, FEF25, FEF50, FEF75, PEF, FET,
VC, FVC Variability, FEV1 Variability, PEF
Variability, FIVC, PIF, FEF50/FIF50%, MVV
Time, MVV Rate, FVL, SVC
Memory Capacity: Stores up to 1,000 patient tests
Adult Predicted Normal Values: Knudson 1983, Knudson 1976, Crapo, Morris,
NHANES III
Pediatric Predicted Normal Values: Hsu, Polgar, Dockery, NHANES III
Interpretation Criteria: American Thoracic Society, 1991. Lung
Function Testing: Selection of Reference Values
and Interpretative Strategies. Am. Rev. Respir.
Dis. 144.1202-1218, NHLEP
*Contact Puritan Bennett Technical Support at 1.800.255.6774 for information regarding
validation testing.
Page 63

Technical References
P-495220-00 Rev. D 58
* Accuracy specifications are specified for 0-10,000 feet operation.
Battery: 6V rechargeable (600mAh min. capacity) NiCad
battery pack,
also supports 4 AA Alkaline batteries or NiCad
batteries
Charge life: 10-12 hrs. with unit turned ON;
approx. 8 days with unit turned OFF
NOTE: Do not mix brands or types of batteries.
Only the custom battery pack can be recharged
using the AC adapter.
Adapter/charger: Output: 12VDC, 400mA
Adapter Input: 120VAC/60 Hz/82mA/9.85VA
IEC 601-1 Medical Grade compliant
Operating Temperature: +17° to +40°C
Operating Humidity: 15% to 95% non-condensing
Operating Altitude: * Up to 15,000 feet
Storage Temperature: -20° to +60° C
Storage Humidity: 15% to 95% non-condensing
Storage Pressure: 500hPa to 1060hPa
Equipment Classification Enclosure Degree of Protection from liquid
ingress: IPX1
Applied Parts: Type BF
Mode of Operation: Short-time operation
Equipment not suitable for use in the presence of
a flammable anesthetic mixture with air or
oxygen or nitrous oxide.
Table 10: Product Specificatio ns Renaissance II Base Station
Dimensions: 6.5"(H) x 4.75" (W) x 2.5"(D)
Weight: 8oz.
Interface: Centronics-compatible IEEE 1284 parallel port for printer,
custom RS-232 compatible connection for computer interface.
Printout: 8-1/2" X 11" or A4
Adapter/charger: Output: 12VDC, 400mA
Adapter Input: 120VAC/60Hz/52mA/9.55VA
IEC 601-1 Medical Grade compliant
Table 9: Product Specifications Renaissance II Spirometer (cont.)
Page 64

Technical References
59 P-495220-00 Rev. D
The FSII Single-Patient Use Flow Sensor
The Renaissance II Spirometry System uses Puritan Bennett's unique,
individually calibrated, disposable FSII sensor. The single-patient use sensor
eliminates the need to clean or sterilize any part of the spirometry system. The
FSII sensor is designed for single-patient use only. This minimizes the effects of
cross-contamination.
The FSII disposable sensor is used for all testing procedures. A 6-digit code is
printed on the sensor in two forms:
• Numeric code
• Bar code
The code can be entered manually or by swiping the sensor through the bar
code reader located on the spirometer. The sensor code contains information
about the linearity characteristics of the sensor. The spirometer needs this
information to accurately calculate the spirometric parameters. The numeric
code is entered with each new patient, each time a calibration check is
performed or anytime a new sensor is used. Your supply of sensors should be
stored in a cool location. Each sensor should remain sealed in a plastic bag until
ready for use.
Operating Temperature: +17 to +40° C
Operating Humidity: 15% to 95% non-condensing
Operating Altitude: Up to 15,000 feet
Storage Temperature: -20° to +60° C
Storage Humidity: 15% to 95% non-condensing
Storage Pressure: 500hPa to 1060hPa
Equipment
Classification
Applied Parts: Type BF
Mode of Operation: Short-time operation
Equipment not suitable for use in the presence of a flammable
anesthetic mixture with air or oxygen or nitrous oxide.
CAUTION:
• Use only the FSII flow sensor specifically designed for the
Renaissance II Spirometry System.
• The FSII sensor is for single-patient use only. In the interest of
environmental pr ote ction , dispose of all sen sors and nos e clips
properly.
Table 10: Product Specifications Renaissance II Base Station (cont.)
Page 65

Technical References
P-495220-00 Rev. D 60
Predicted Normal Equations and References
Patient’s measured values are compared to their predicted values as one way of
judging the degree of abnormality of their lung function. (See references 1-10,
16 on page 91 of this User’s Manual for the journal articles that describe the
studies on which these equations are based.)
Equation Variables
Hi = Height in inches
Hc = Height in centimeters
A = Age in years
LLN = lower limit of normal
WARNING
• Predicted values will be extrapolated for patients with age or
height outside the age and/or height lim its supporte d by the
selected author’s normal equations.
• Results from spirometry testing should not be the sole source
for determining a patient's diagnosis and treatment. Other
clinical data, such as pa tient symptoms an d re spiratory hi story,
should always be considered.
NOTES:
• The patient’s height, birth date, and gender must be entered or no
interpretation will be displayed.
• Physiological variant is interpreted as “Undetermined” in the Renaissance II if
FVC and FEV1 are greater than 100% and FEV1% is less than 100%.
Page 66

Technical References
61 P-495220-00 Rev. D
Morris MALE
Limits age (18-90 years) height (58-80 inches)
FVC -4.241 - 0.025A + 0.148Hi (Morris, et al.,1971)
FVC
(LLN) FVC - (1.645 * 0.74) (Morris, et al.,1971)
FEV1 -1.260 - 0.032A + 0.092Hi (Morris, et al.,1971)
FEV1
(LLN) FEV1 - (1.645 * 0.55) (Morris, et al.,1971)
FEV1% 107.12 - 0.2422A - 0.3118Hi (Morris, et al.,1975)
FEV1%
(LLN) FEV1% - (1.645 * 7.79) (Morris, et al.,1971)
FEV3 FVC * .95 (Morris, et al.,1971)
FEF25-75 2.513 - 0.045A + 0.047Hi (Morris, et al.,1971)
FIVC -4.241 - 0.025A + 0.148Hi (Morris, et al.,1971)
MVV -37.94893 - 0.81621A + 3.02915Hi (Cherniack, et al.,1972)
Age < 25
PEF -8.060 + 0.166A + 0.078Hc (Knudson, et al.,1976)
FEV0.5 -3.054 + 0.043A + 0.030Hc (Knudson, et al.,1976)
FEF50 -6.3851 + 0.1150A + 0.0543Hc (Knudson, et al.,1983)
FEF75 -4.2421 - 0.0057A + 0.0397Hc (Knudson, et al.,1983)
Age ≥ 25
PEF -5.993 - 0.035A + 0.094Hc (Knudson, et al.,1976)
FEV0.5 -2.746 - 0.017A + 0.037Hc (Knudson, et al.,1976)
FEF50 -5.5409 - 0.0366A + 0.0684Hc (Knudson, et al.,1983)
FEF75 -2.4827 - 0.0230A + 0.0310Hc (Knudson, et al.,1983)
Morris FEMALE
Limits age (18-90 years) height (56-72 inches)
FVC -2.852 - 0.024A + 0.115Hi (Morris, et al.,1971)
FVC
(LLN) FVC - (1.645 * 0.52) (Morris, et al.,1971)
FEV1 -1.932 - 0.025A + 0.089Hi (Morris, et al.,1971)
FEV1
(LLN) FEV1 - (1.645 * 0.47) (Morris, et al.,1971)
FEV1% 88.70 - 0.1815A - 0.0679Hi (Morris, et al.,1975)
FEV1%
(LLN) FEV1% - (1.645 * 6.84) (Morris, et al.,1971)
FEV3 FVC * 0.95 (Morris, et al.,1971)
FEF25-75 0.551 - 0.030A + 0.060Hi (Morris, et al.,1971)
FIVC -2.852 - 0.024A + 0.115Hi (Morris, et al.,1971)
MVV -4.86957 - 0.685A + 2.1384Hi (Cherniack, et al.,1972)
Age < 20
PEF -3.916 + 0.157A + 0.049Hc (Knudson, et al.,1976)
FEV0.5 -1.738 + 0.061A + 0.019Hc (Knudson, et al.,1976)
FEF50 -2.3040 + 0.1111A + 0.0288Hc (Knudson, et al.,1983)
FEF75 -4.4009 + 0.2923A + 0.0243Hc (Knudson, et al.,1983)
-0.0075 * A^2
Page 67

Technical References
P-495220-00 Rev. D 62
Age ≥ 20
PEF -0.735 - 0.025A + 0.049Hc (Knudson, et al.,1976)
FEV0.5 -0.406 - 0.014A + 0.019Hc (Knudson, et al.,1976)
FEF50 -0.4371 - 0.0240A + 0.0321Hc (Knudson, et al.,1983)
FEF75 -0.1822 - 0.0254A + 0.0174Hc (Knudson, et al.,1983)
Age ≥ 70
PEF -0.735 - 0.025A + 0.049Hc (Knudson, et al.,1976)
FEV0.5 -0.406 - 0.014A + 0.019Hc (Knudson, et al.,1976)
FEF50 6.2402 - 0.0755A + 0.0118Hc (Knudson, et al.,1983)
FEF75 1.8894 - 0.0172A (Knudson, et al.,1983)
Knudson 1983 MALE
Limits age (18-85 years) height (58-80 inches)
Age < 25
FVC -6.8865 + 0.0739A + 0.0590Hc (Knudson, et al.,1983)
FVC
(LLN) 0.798 * FVC (Knudson, et al.,1983)
FEV1 -6.1181 + 0.0636A + 0.0519Hc (Knudson, et al.,1983)
FEV1
(LLN) 0.812 * FEV1 (Knudson, et al.,1983)
FEV3 -5.531 + 0.066A + 0.052Hc (Knudson, et al.,1976)
FEV1% 92.8965 - 1.4612FVC (Knudson, et al.,1983)
FEV1%
(LLN) 0.848 * FEV1% (Knudson, et al.,1983)
FEF25-75 -6.1990 + 0.0749A + 0.0539Hc (Knudson, et al.,1983)
PEF -8.060 + 0.166A + 0.078Hc (Knudson, et al.,1976)
FIVC -6.8865 + 0.0739A + 0.0590Hc (Knudson, et al.,1983)
FEV0.5 -3.054 + 0.043A + 0.030Hc (Knudson, et al.,1976)
FEF50 -6.3851 + 0.1150A + 0.0543Hc (Knudson, et al.,1983)
FEF75 -4.2421 - 0.0057A + 0.0397Hc (Knudson, et al.,1983)
MVV -37.94893 - 0.81621A + 3.02915Hi (Cherniack, et al.,1972)
Age ≥ 25
FVC -8.7818 - 0.0298A + 0.0844Hc (Knudson, et al.,1983)
FEV1 -6.5147 - 0.0292A + 0.0665Hc (Knudson, et al.,1983)
FEV1% 96.3074 - 0.1677A - 1.4232FVC (Knudson, et al.,1983)
FEV1%
(LLN) 0.870 * FEV1% (Knudson, et al.,1983)
FEV3 -5.245 - 0.031A + 0.063Hc (Knudson, et al.,1976)
FEF25-75 -4.5175 - 0.0363A + 0.0579Hc (Knudson, et al.,1983)
PEF -5.993 - 0.035A + 0.094Hc (Knudson, et al.,1976)
FIVC -8.7818 - 0.0298A + 0.0844Hc (Knudson, et al.,1983)
FEV0.5 -2.746 - 0.017A + 0.037Hc (Knudson, et al.,1976)
FEF50 -5.5409 - 0.0366A + 0.0684Hc (Knudson, et al.,1983)
FEF75 -2.4827 - 0.0230A + 0.0310Hc (Knudson, et al.,1983)
MVV -37.94893 - 0.81621A + 3.02915Hi (Cherniack, et al.,1972)
Age > 25 and < 39
FVC
(LLN) 0.811 * FVC (Knudson, et al.,1983)
FEV1
(LLN) 0.791 * FEV1 (Knudson, et al.,1983)
Page 68

Technical References
63 P-495220-00 Rev. D
Age > 40
FVC
(LLN) 0.734 * FVC (Knudson, et al.,1983)
FEV1
(LLN) 0.772 * FEV1 (Knudson, et al.,1983)
Knudson 1983 FEMALE
Limits age (18-88 years) height (56-72 inches)
Age < 20
FVC -4.4470 + 0.0699A + 0.0416Hc (Knudson, et al.,1983)
FVC
(LLN) 0.749 * FVC (Knudson, et al.,1983)
FEV1 -3.7622 + 0.0694A + 0.0351Hc (Knudson, et al.,1983)
FEV1
(LLN) 0.818 * FEV1 (Knudson, et al.,1983)
FEV3 -3.417 + 0.086A + 0.033Hc (Knudson, et al.,1976)
FEV1% 91.9381 + 1.5226A - 7.7593FVC (Knudson, et al.,1983)
FEV1%
(LLN) 0.833 * FEV1% (Knudson, et al.,1983)
FEF25-75 -2.8007 + 0.1275A + 0.0279Hc (Knudson, et al.,1983)
PEF -3.916 + 0.157A + 0.049Hc (Knudson, et al.,1976)
FIVC -4.4470 + 0.0699A + 0.0416Hc (Knudson, et al.,1983)
FEV0.5 -1.738 + 0.061A + 0.019Hc (Knudson, et al.,1976)
FEF50 -2.3040 + 0.1111A + 0.0288Hc (Knudson, et al.,1983)
FEF75 -4.4009 + 0.2923A + 0.0243Hc (Knudson, et al.,1983)
-0.0075 * A^2
MVV 4.86957 - 0.685A + 2.1384Hi (Cherniack, et al.,1972)
Age > 20 and < 70
FVC -3.1947 - 0.0169A + 0.0444Hc (Knudson, et al.,1983)
FEV1 -1.8210 - 0.0190A + 0.0332Hc (Knudson, et al.,1983)
FEV3 -1.633 - 0.023A + 0.035Hc (Knudson, et al.,1976)
FEV1% 113.694 - 0.2904A - 5.4024FVC (Knudson, et al.,1983)
FEV1%
(LLN) 0.854 * FEV1% (Knudson, et al.,1983)
FEF25-75 -0.4057 - 0.0309A + 0.0300Hc (Knudson, et al.,1983)
PEF -0.735 - 0.025A + 0.049Hc (Knudson, et al.,1976)
FIVC -3.1947 - 0.0169A + 0.0444Hc (Knudson, et al.,1983)
FEV0.5 -0.406 - 0.014A + 0.019Hc (Knudson, et al.,1976)
FEF50 -0.4371 - 0.0240A + 0.0321Hc (Knudson, et al.,1983)
FEF75 -0.1822 - 0.0254A + 0.0174Hc (Knudson, et al.,1983)
MVV -4.86957 - 0.685A + 2.1384Hi (Cherniack, et al.,1972)
Age > 20 and < 39
FVC
(LLN) 0.769 * FVC (Knudson, et al.,1983)
FEV1
(LLN) 0.703 * FEV1 (Knudson, et al.,1983)
Age > 40 and < 70
FVC
(LLN) 0.752 (Knudson, et al.,1983)
FEV1
(LLN) 0.779 * FEV1 (Knudson, et al.,1983)
Page 69

Technical References
P-495220-00 Rev. D 64
Age ≥ 70
FVC -0.1889 - 0.0296A + 0.0313Hc (Knudson, et al.,1983)
FVC
(LLN) 0.718 * FVC (Knudson, et al.,1983)
FEV1 2.6539 - 0.0397A + 0.0143Hc (Knudson, et al.,1983)
FEV1
(LLN) 0.726 * FEV1 (Knudson, et al.,1983)
FEV3 -1.633 - 0.023A + 0.035Hc (Knudson, et al.,1976)
FEV1% 113.694 - 0.2904A - 5.4024FVC (Knudson, et al.,1983)
FEV1%
(LLN) 0.854 * FEV1% (Knudson, et al.,1983)
FEF25-75 6.3706 - 0.0615A (Knudson, et al.,1983)
PEF -0.735 - 0.025A + 0.049Hc (Knudson, et al.,1976)
FIVC -0.1889 - 0.0296A + 0.0313Hc (Knudson, et al.,1983)
FEV0.5 -0.406 - 0.014A + 0.019Hc (Knudson, et al.,1976)
FEF50 6.2402 -0.0755A + 0.0118Hc (Knudson, et al.,1983)
FEF75 1.8894 -0.0172A (Knudson, et al.,1983)
MVV -4.86957 - 0.685A + 2.1384Hi (Cherniack, et al.,1972)
Knudson 1976 Male
Limits age (18-85 years) height (58-80 inches)
Age < 25
FVC -5.508 + 0.078A + 0.050Hc (Knudson, et al.,1976)
FVC
(LLN) 0.8150 * FVC (Knudson, et al.,1976)
FEV1 -4.808 + 0.045A + 0.046Hc (Knudson, et al.,1976)
FEV1
(LLN) 0.8175 * FEV1 (Knudson, et al.,1976)
FEV3 -5.531 + 0.066A + 0.052Hc (Knudson, et al.,1976)
FEV1% 103.64 - 0.140A - 0.087Hc (Knudson, et al.,1976)
FEV1%
(LLN) 0.8762 * FEV1% (Knudson, et al.,1976)
FEF25-75 -5.334 + 0.059Hc (Knudson, et al.,1976)
PEF -8.060 + 0.166A + 0.078Hc (Knudson, et al.,1976)
FIVC -5.508 + 0.078A + 0.050Hc (Knudson, et al.,1976)
FEV0.5 -3.054 + 0.043A + 0.030Hc (Knudson, et al.,1976)
FEF50 -6.3851 + 0.1150A + 0.0543Hc (Knudson, et al.,1983)
FEF75 -4.2421 - 0.0057A + 0.0397Hc (Knudson, et al.,1983)
MVV -37.94893 - 0.81621A + 3.02915Hi (Cherniack, et al.,1972)
Age ≥ 25
FVC -5.459 - 0.029A + 0.065Hc (Knudson, et al.,1976)
FEV1 -4.203 - 0.027A + 0.052Hc (Knudson, et al.,1976)
FEV3 -5.245 - 0.031A + 0.063Hc (Knudson, et al.,1976)
FEV1% 103.64 - 0.140A - 0.087Hc (Knudson, et al.,1976)
FEF25-75 -1.864 - 0.031A + 0.045Hc (Knudson, et al.,1976)
PEF -5.993 - 0.035A + 0.094Hc (Knudson, et al.,1976)
FIVC -5.459 - 0.029A + 0.065Hc (Knudson, et al.,1976)
FEV0.5 -2.746 - 0.017A + 0.037Hc (Knudson, et al.,1976)
FEF50 -5.5409 - 0.0366A + 0.0684Hc (Knudson, et al.,1983)
FEF75 -2.4827 - 0.0230A + 0.0310Hc (Knudson, et al.,1983)
MVV -37.94893 - 0.81621A + 3.02915Hi (Cherniack, et al.,1972)
Page 70

Technical References
65 P-495220-00 Rev. D
Age > 25 and < 35
FVC
(LLN) 0.8150 * FVC (Knudson, et al.,1976)
FEV1
(LLN) 0.8175 * FEV1 (Knudson, et al.,1976)
FEV1%
(LLN) 0.8762 * FEV1% (Knudson, et al.,1976)
Age > 35
FVC
(LLN) 0.7446 * FVC (Knudson, et al.,1976)
FEV1
(LLN) 0.7292 * FEV1 (Knudson, et al.,1976)
FEV1%
(LLN) 0.9188 * FEV1% (Knudson, et al.,1976)
Knudson 1976 FEMALE
Limits age (18-88 years) height (56-72 inches)
Age < 20
FVC -3.469 + 0.092A + 0.033Hc (Knudson, et al.,1976)
FEV1 -2.703 + 0.085A + 0.027Hc (Knudson, et al.,1976)
FEV3 -3.417 + 0.086A + 0.033Hc (Knudson, et al.,1976)
FEV1% 107.38 - 0.109A - 0.111Hc (Knudson, et al.,1976)
FEF25-75 -1.893 + 0.121A + 0.025Hc (Knudson, et al.,1976)
PEF -3.916 + 0.157A + 0.049Hc (Knudson, et al.,1976)
FIVC -3.469 + 0.092A + 0.033Hc (Knudson, et al.,1976)
FEV0.5 -1.738 + 0.061A + 0.019Hc (Knudson, et al.,1976)
FEF50 -2.3040 + 0.1111A + 0.0288Hc (Knudson, et al.,1983)
FEF75 -4.4009 + 0.2923A + 0.0243Hc (Knudson, et al.,1983)
-0.0075 * A^2
MVV -4.86957 - 0.685A + 2.1384Hi (Cherniack, et al.,1972)
Age < 25
FVC
(LLN) 0.7575 * FVC (Knudson, et al.,1976)
FEV1
(LLN) 0.7138 * FEV1 (Knudson, et al.,1976)
FEV1%
(LLN) 0.8313 * FEV1% (Knudson, et al.,1976)
Age > 25 and < 35
FVC
(LLN) 0.7575 * FVC (Knudson, et al.,1976)
FEV1
(LLN) 0.7138 * FEV1 (Knudson, et al.,1976)
FEV1%
(LLN) 0.8313 * FEV1% (Knudson, et al.,1976)
Age > 35
FVC
(LLN) 0.6646 * FVC (Knudson, et al.,1983)
FEV1
(LLN) 0.6940 * FEV1 (Knudson, et al.,1976)
FEV1%
(LLN) 0.8806 * FEV1% (Knudson, et al.,1976)
Page 71

Technical References
P-495220-00 Rev. D 66
Age ≥ 20 and < 70
FVC -1.774 - 0.022A + 0.037Hc (Knudson, et al.,1976)
FEV1 -0.794 - 0.021A + 0.027Hc (Knudson, et al.,1976)
FEV3 -1.633 - 0.023A + 0.035Hc (Knudson, et al.,1976)
FEV1% 107.38 - 0.109A - 0.111Hc (Knudson, et al.,1976)
FEF25-75 1.171 - 0.024A + 0.021Hc (Knudson, et al.,1976)
PEF -0.735 - 0.025A + 0.049Hc (Knudson, et al.,1976)
FIVC -1.774 - 0.022A + 0.037Hc (Knudson, et al.,1976)
FEV0.5 -0.406 - 0.014A + 0.019Hc (Knudson, et al.,1976)
FEF50 -0.4371 - 0.0240A + 0.0321Hc (Knudson, et al.,1983)
FEF75 -0.1822 - 0.0254A + 0.0174Hc (Knudson, et al.,1983)
MVV -4.86957 - 0.685A + 2.1384Hi (Cherniack, et al.,1972)
Age ≥ 70
FVC -1.774 - 0.022A + 0.037Hc (Knudson, et al.,1976)
FEV1 -0.794 - 0.021A + 0.027Hc (Knudson, et al.,1976)
FEV3 -1.633 - 0.023A + 0.035Hc (Knudson, et al.,1976)
FEV1% 107.38 - 0.109A - 0.111Hc (Knudson, et al.,1976)
FEF25-75 1.171 - 0.024A + 0.021Hc (Knudson, et al.,1976)
PEF -0.735 - 0.025A + 0.049Hc (Knudson, et al.,1976)
FIVC -1.774 - 0.022A + 0.037Hc (Knudson, et al.,1976)
FEV0.5 -0.406 - 0.014A + 0.019Hc (Knudson, et al.,1976)
FEF50 6.2402 - 0.0755A + 0.0118Hc (Knudson, et al.,1983)
FEF75 1.8894 - 0.0172A (Knudson, et al.,1983)
MVV -4.86957 - 0.685A + 2.1384Hi (Cherniack, et al.,1972)
Crapo MALE
Limits age (18-89 years) height (61-77 inches)
FVC -4.650 - 0.0214A + 0.0600Hc (Crapo, et al.,1981)
FVC
(LLN) FVC - 1.115 (Crapo, et al.,1981)
FEV1 -2.190 - 0.0244A + 0.0414Hc (Crapo, et al.,1981)
FEV1
(LLN) FEV1 - 0.842 (Crapo, et al.,1981)
FEV3 -3.512 - 0.0271A + 0.0535Hc (Crapo, et al.,1981)
FEF25-75 2.133 - 0.0380A + 0.0204Hc (Crapo, et al.,1981)
FEV1% 110.49 - 0.1520A - 0.1300Hc (Crapo, et al.,1981)
FEV1%
(LLN) FEV1% - 8.28 (Crapo, et al.,1981)
FEV0.5 -1.914 - 0.0152A + 0.0327Hc (Crapo, et al.,1981)
FIVC -4.650 - 0.0214A + 0.0600Hc (Crapo, et al.,1981)
MVV -37.94893 - 0.81621A + 3.02915Hi (Cherniack, et al.,1972)
Age < 25
PEF -8.060 + 0.166A + 0.078Hc (Knudson, et al.,1976)
FEF50 -6.3851 + 0.1150A + 0.0543Hc (Knudson, et al.,1983)
FEF75 -4.2421 - 0.0057A + 0.0397Hc (Knudson, et al.,1983)
Page 72

Technical References
67 P-495220-00 Rev. D
Age ≥ 25
PEF -5.993 - 0.035A + 0.094Hc (Knudson, et al.,1976)
FEF50 -5.5409 - 0.0366A + 0.0684Hc (Knudson, et al.,1983)
FEF75 -2.4827 - 0.0230A + 0.0310Hc (Knudson, et al.,1983)
Crapo FEMALE
Limits age (18-89 years) height (57-70 inches)
FVC -3.590 - 0.0216A + 0.0491Hc (Crapo, et al.,1981)
FVC
(LLN) FVC - 0.676 (Crapo, et al.,1981)
FEV1 -1.578 - 0.0255A + 0.0342Hc (Crapo, et al.,1981)
FEV1
(LLN) FEV1 - 0.561 (Crapo, et al.,1981)
FEV3 -2.745 - 0.0257A + 0.0442Hc (Crapo, et al.,1981)
FEF25-75 2.683 - 0.0460A + 0.0154Hc (Crapo, et al.,1981)
FEV1% 126.58 - 0.2520A - 0.2020Hc (Crapo, et al.,1981)
FEV1%
(LLN) FEV1% - 9.06 (Crapo, et al.,1981)
FEV0.5 -0.809 - 0.0185A + 0.0238Hc (Crapo, et al.,1981)
FIVC -3.590 - 0.0216A + 0.0491Hc (Crapo, et al.,1981)
MVV -4.86957- 0.685A + 2.1384Hi (Cherniack, et al.,1972)
Age < 20
PEF -3.916 + 0.157A + 0.049Hc (Knudson, et al.,1976)
FEF50 -2.3040 + 0.1111A + 0.0288Hc (Knudson, et al.,1983)
FEF75 -4.4009 + 0.2923A + 0.0243Hc (Knudson, et al.,1983)
-0.0075 * A^2
Age ≥ 20 and < 70
PEF -0.735 - 0.025A + 0.049Hc (Knudson, et al.,1976)
FEF50 -0.4371 - 0.0240A + 0.0321Hc (Knudson, et al.,1983)
FEF75 -0.1822 - 0.0254A + 0.0174Hc (Knudson, et al.,1983)
Age ≥ 70
PEF -0.735 - 0.025A + 0.049Hc (Knudson, et al.,1976)
FEF50 6.2402 -0.0755A + 0.0118Hc (Knudson, et al.,1983)
FEF75 1.8894 -0.0172A (Knudson, et al.,1983)
Hsu MALE
Limits age (7-17 years) height (43-75 inches)
FVC 3.58 * 10^-7 * Hc^3.18 (Hsu, et al.,1979)
FVC
(LLN) FVC * ((1.0 - 0.13)^2) (Hsu, et al.,1979)
FEV1 7.74 * 10^-7 * Hc^3.00 (Hsu, et al.,1979)
FEV1
(LLN) FEV1 * ((1.0 - 0.13) ^2) (Hsu, et al.,1979)
FEF25-75 1.33 * 10^-5 * Hc^2.46 (Hsu, et al.,1979)
PEF 5.58 * 10^-6 * Hc^2.79 (Hsu, et al.,1979)
FEV1% (PRED FEV1/PRED FVC) 100 (Hsu, et al.,1979)
FEV1%
(LLN) 0.90 * FEV1% (Hsu, et al.,1979)
FIVC 3.58 * 10^-7 * Hc^3.18 (Hsu, et al.,1979)
Page 73

Technical References
P-495220-00 Rev. D 68
FEV0.5 .7778 * FEV1 (Hsu, et al.,1979)
FEV3 .98 * FVC (Hsu, et al.,1979)
MVV -99.507 +1.267Hc (Polgar, et al.,1971)
Age < 12
FEF50 -2.5454 + 0.0378Hc (Knudson, et al.,1983)
FEF75 -1.0149 + 0.0171Hc (Knudson, et al.,1983)
Age ≥ 12
FEF50 -6.3851 + 0.1150A + 0.0543Hc (Knudson, et al.,1983)
FEF75 -4.2421 - 0.0057A + 0.0397Hc (Knudson, et al.,1983)
Hsu FEMALE
Limits age (7-17 years) height (43-71 inches)
FVC 2.57 * 10^-6 * Hc^2.78 (Hsu, et al.,1979)
FVC
(LLN) FVC * ((1.0 - 0.14)^2) (Hsu, et al.,1979)
FEV1 3.79 * 10^-6 * Hc^2.68 (Hsu, et al.,1979)
FEV1
(LLN) FEV1 * ((1.0 - 0.14) ^2) (Hsu, et al.,1979)
FEF25-75 6.32 * 10^-5 * Hc^2.16 (Hsu, et al.,1979)
PEF 4.30 * 10^-5 * Hc^2.37 (Hsu, et al.,1979)
FEV1% (PRED FEV1/PRED FVC) 100 (Hsu, et al.,1979)
FEV1%
(LLN) 0.90 * FEV1% (Hsu, et al.,1979)
FIVC 2.57 * 10^-6 * Hc^2.78 (Hsu, et al.,1979)
FEV0.5 .7778 * FEV1 (Hsu, et al.,1979)
FEV3 .98 * FVC (Hsu, et al.,1979)
MVV -99.507 +1.267Hc (Polgar, et al.,1971)
Age < 11
FEF50 0.7362 + 0.1846A (Knudson, et al.,1983)
FEF75 -0.1657 + 0.0109Hc (Knudson, et.al.,1983)
Age ≥ 11
FEF50 -2.3040 + 0.1111A + 0.0288Hc (Knudson, et al.,1983)
FEF75 -4.4009 + 0.2923A + 0.0243Hc (Knudson, et al.,1983)
-0.0075 * A^2
Page 74

Technical References
69 P-495220-00 Rev. D
Polgar MALE
Limits age (4-17 years) height (43-67 inches)
FVC 4.4 * 10^-6 * Hc^2.67 (Polgar, et al.,1971)
FVC
(LLN) FVC * (1 - (1.645 * 0.13)) (Polgar, et al.,1971)
FEV1 2.1 * 10^-6 * Hc^2.80 (Polgar, et al.,1971)
FEV1
(LLN) FEV1 * (1 - (1.645 * 0.088)) (Polgar, et al.,1971)
FEF25-75 -3.4616 + 0.0437 Hc (Polgar, et al.,1971)
PEF -7.0929 + .08738 Hc (Polgar, et al.,1971)
FEV1% 47.73 * Hc^.13 (Polgar, et al.,1971)
FEV1%
(LLN) 0.90 * FEV1% (Polgar, et al.,1971)
FIVC 4.4 * 10^-6 * Hc^2.67 (Polgar, et al.,1971)
FEV0.5 .7778 * FEV1 (Polgar, et al.,1971)
FEV3 .98 * FVC (Polgar, et al.,1971)
MVV -99.507 +1.267Hc (Polgar, et al.,1971)
Age < 12
FEF50 -2.5454 + 0.0378Hc (Knudson, et al.,1983)
FEF75 -1.0149 + 0.0171Hc (Knudson, et al.,1983)
Age ≥ 12
FEF50 -6.3851 + 0.1150A + 0.0543Hc (Knudson, et al.,1983)
FEF75 -4.2421 - 0.0057A + 0.0397Hc (Knudson, et al.,1983)
Polgar FEMALE
Limits age (4-17 years) height (43-67 inches)
FVC 3.3 * 10^-6 * Hc^2.72 (Polgar, et al.,1971)
FVC
(LLN) FVC * (1 - (1.645 * 0.13)) (Polgar, et al.,1971)
FEV1 2.1 * 10^-6 * Hc^2.80 (Polgar, et al.,1971)
FEV1
(LLN) FEV1 * (1 - (1.645 * 0.088)) (Polgar, et al.,1971)
FEF25-75 -3.4616 + 0.0437 Hc (Polgar, et al.,1971)
PEF -7.0929 + .08738 Hc (Polgar, et al.,1971)
FEV1% 63.63 * Hc^.08 (Polgar, et al.,1971)
FEV1%
(LLN) 0.90 * FEV1% (Polgar, et al.,1971)
FIVC 3.3 * 10^-6 * Hc^2.72 (Polgar, et al.,1971)
FEV0.5 .7778 * FEV1 (Polgar, et al.,1971)
FEV3 .98 * FVC (Polgar, et al.,1971)
MVV -99.507 +1.267Hc (Polgar, et al.,1971)
Age < 11
FEF50 0.7362 + 0.1846A (Knudson, et al.,1983)
FEF75 -0.1657 + 0.0109Hc (Knudson, et al.,1983)
Age ≥ 11
FEF50 -2.3040 + 0.1111A + 0.0288Hc (Knudson, et al.,1983)
FEF75 -4.4009 + 0.2923A + 0.0243Hc (Knudson, et al.,1983)
-0.0075 * A^2
Page 75

Technical References
P-495220-00 Rev. D 70
NHANES III MALE
(Hankinson, et al.,1999)
Caucasian
Age < 20
FEV1 -0.7453 - 0.04106A + 0.004477 * A^2 + 0.00014098 * Hc^2
FEV1
(LLN) -0.7453 - 0.04106A + 0.004477 * A^2+ 0.00011607 * Hc^2
FEV6 -0.3119 - 0.18612A + 0.009717 * A^2 + 0.00018188 * Hc^2
FEV6
(LLN) -0.3119 - 0.18612A + 0.009717 * A^2 + 0.00015323 * Hc^2
FVC -0.2584 - 0.20415A + 0.010133 * A^2 + 0.00018642 * Hc^2
FVC
(LLN) -0.2584 - 0.20415A + 0.010133 * A^2 + 0.00015695 * Hc^2
PEF -0.5962 - 0.12357A + 0.013135 * A^2 + 0.00024962 * Hc^2
PEF
(LLN) -0.5962 - 0.12357A + 0.013135 * A^2 + 0.00017635 * Hc^2
FEF25-75 -1.0863 + 0.13939A + 0.00010345 * Hc^2
FEF25-75
(LLN) -1.0863 + 0.13939A + 0.00005294 * Hc^2
Caucasian
Age > 20
FEV1 0.5536 - 0.01303A + 0.000172 * A^2 + 0.00014098 * Hc^2
FEV1
(LLN) 0.5536 - 0.01303A + 0.000172 * A^2 + 0.00011607 * Hc^2
FEV6 0.1102 - 0.00842A - 0.000223 * A^2 + 0.00018188 * Hc^2
FEV6
(LLN) 0.1102 - 0.00842A - 0.000223 * A^2 + 0.00015323 * Hc^2
FVC -0.1933 + 0.00064A - 0.000269 * A^2 + 0.00018642 * Hc^2
FVC
(LLN) -0.1933 + 0.00064A - 0.000269 * A^2 + 0.00015695 * Hc^2
PEF 1.0523+ 0.08272A - 0.001301 * A^2 + 0.00024962 * Hc^2
PEF
(LLN) 1.0523 + 0.08272A - 0.001301 * A^2 + 0.00017635 * Hc^2
FEF25-75 2.7006 - 0.04995A + 0.00010345 * Hc^2
FEF25-75 (LLN) 2.7006 - 0.04995A + 0.00005294 * Hc^2
African-American
Age < 20
FEV1 -0.7048 - 0.05711A + 0.004316 * A^2 + 0.00013194 * Hc^2
FEV1
(LLN) -0.7048 - 0.05711A + 0.004316 * A^2 + 0.00010561 * Hc^2
FEV6 -0.5525 - 0.14107A + 0.007241 * A^2 + 0.00016429 * Hc^2
FEV6
(LLN) -0.5525 - 0.14107A + 0.007241 * A^2 + 0.00013499 * Hc^2
FVC -0.4971- 0.15497A + 0.007701 * A^2 + 0.00016643 * Hc^2
FVC
(LLN) -0.4971- 0.15497A + 0.007701 * A^2 + 0.00013670 * Hc^2
PEF -0.2684 - 0.28016A + 0.018202 * A^2 + 0.00027333 * Hc^2
PEF
(LLN) -0.2684 - 0.28016A + 0.018202 * A^2 + 0.00018938 * Hc^2
Page 76

Technical References
71 P-495220-00 Rev. D
FEF25-75 -1.1627 + 0.12314A + 0.00010461 * Hc^2
FEF25-75
(LLN) -1.1627 + 0.12314A + 0.00004819 * Hc^2
African-American
Age > 20
FEV1 0.3411- 0.02309A + 0.00013194 * Hc^2
FEV1
(LLN) 0.3411- 0.02309A + 0.00010561 * Hc^2
FEV6 -0.0547- 0.02114A + 0.00016429 * Hc^2
FEV6
(LLN) -0.0547- 0.02114A + 0.00013499 * Hc^2
FVC -0.1517 - 0.01821A + 0.00016643 * Hc^2
FVC
(LLN) -0.1517 - 0.01821A + 0.00013670 * Hc^2
PEF 2.2257 - 0.04082A + 0.00027333 * Hc^2
PEF
(LLN) 2.2257 - 0.04082A + 0.00018938 * Hc^2
FEF25-75 2.1477- 0.04238A + 0.00010461 * Hc^2
FEF25-75
(LLN) 2.1477- 0.04238A + 0.00004819 * Hc^2
Mexican-American
Age < 20
FEV1 -0.8218 - 0.04248A + 0.004291 * A^2 + 0.00015104 * Hc^2
FEV1
(LLN) -0.8218 - 0.04248A + 0.004291 * A^2 + 0.00012670 * Hc^2
FEV6 -0.6646 - 0.11270A + 0.007306 * A^2 + 0.00017840 * Hc^2
FEV6
(LLN) -0.6646 - 0.11270A + 0.007306 *A^2 + 0.00015029 * Hc^2
FVC -0.7571- 0.09520 A + 0.006619 * A^2+ 0.00017823 * Hc^2
FVC
(LLN) -0.7571- 0.09520 A + 0.006619 * A^2 + 0.00014947 * Hc^2
PEF -0.9537- 0.19602 A + 0.014497 * A^2+ 0.00030243 * Hc^2
PEF
(LLN) -0.9537- 0.19602 A + 0.014497 * A^2 + 0.00021833 * Hc^2
FEF25-75 -1.3592 + 0.10529A + 0.00014470 * Hc^2
FEF25-75
(LLN) -1.3592 + 0.10529A + 0.00009020 * Hc^2
Mexican-American
Age > 20
FEV1 0.6306 - 0.02928A + 0.00015104 * Hc^2
FEV1
(LLN) 0.6306 - 0.02928A + 0.00012670 * Hc^2
FEV6 0.5757 - 0.02860A + 0.00017840 * Hc^2
FEV6
(LLN) 0.5757 - 0.02860A + 0.00015029 * Hc^2
FVC 0.2376 - 0.00891A - 0.000182 * A^2 + 0.00017823 * Hc^2
FVC
(LLN) 0.2376 - 0.00891A - 0.000182 * A^2 + 0.00014947 * Hc^2
Page 77

Technical References
P-495220-00 Rev. D 72
PEF 0.0870 + 0.06580A - 0.001195 * A^2 + 0.00030243 * Hc^2
PEF
(LLN) 0.0870 + 0.06580A - 0.001195 * A^2 + 0.00021833 * Hc^2
FEF25-75 1.7503 - 0.05018A + 0.00014473 * Hc^2
FEF25-75
(LLN) 1.7503 - 0.05018A + 0.00009020 * Hc^2
NHANES III FEMALE
(Hankinson, et al.,1999)
Caucasian
Age < 18
FEV1 -0.8710 + 0.06537A + 0.00011496 * Hc^2
FEV1
(LLN) -0.8710 + 0.06537A + 0.00009283 * Hc^2
FEV6 -1.1925 +0.06544A + 0.00014395 * Hc^2
FEV6
(LLN) -1.1925 +0.06544A + 0.00011827 * Hc^2
FVC -1.2082 + 0.0591A + 0.00014815 * Hc^2
FVC
(LLN) -1.2082 + 0.0591A + 0.00012198 * Hc^2
PEF -3.6181 + 0.60644A - 0.016846 * A^2 + 0.00018623 * Hc^2
PEF
(LLN) -3.6181 + 0.60644A - 0.016846 * A^2 + 0.00012148 * Hc^2
FEF25-75 -2.5284 + 0.52490A - 0.015309 * A^2 + 0.00006982 * Hc^2
FEF25-75
(LLN) -2.5284 + 0.52490A - 0.015309 * A^2 + 0.00002302 * Hc^2
Caucasian
Age >18
FEV1 0.4333 - 0.00361A - 0.000194 * A^2 + 0.00011496 * Hc^2
FEV1
(LLN) 0.4333 - 0.00361A - 0.000194 * A^2 + 0.00009283 * Hc^2
FEV6 -0.1373 + 0.01317A - 0.000352 * A^2 + 0.00014395 * Hc^2
FEV6
(LLN) -0.1373 + 0.01317A - 0.000352 * A^2 + 0.00011827 * Hc^2
FVC -0.3560 + 0.01870A - 0.000382 * A^2 + 0.00014815 * Hc^2
FVC
(LLN) -0.3560 + 0.01870A - 0.000382 * A^2 + 0.00012198 * Hc^2
PEF 0.9267 + 0.06929A - 0.001031 * A^2 + 0.00018623 * Hc^2
PEF
(LLN) 0.9267 + 0.06929A - 0.001031 * A^2 + 0.00012148 * Hc^2
FEF25-75 2.3670 - 0.01904A - 0.000200 * A^2 + 0.00006982 * Hc^2
FEF25-75
(LLN) 2.3670 - 0.01904A - 0.000200 * A^2 + 0.00002302 * Hc^2
Page 78

Technical References
73 P-495220-00 Rev. D
African-American
Age < 18
FEV1 - 0.9630 + 0.05799A + 0.00010846 * Hc^2
FEV1
(LLN) - 0.9630 + 0.05799A + 0.00008546 * Hc^2
FEV6 - 0.6370 - 0.04243A + 0.003508 * A^2 + 0.00013497 * Hc^2
FEV6
(LLN) - 0.6370 - 0.04243A + 0.003508 * A^2 + 0.00010848 * Hc^2
FVC -0.6166 - 0.04687 A + 0.003602 * A^2 + 0.00013606 * Hc^2
FVC
(LLN) -0.6166 - 0.04687 A + 0.003602 * A^2 + 0.00010916 * Hc^2
PEF -1.2398 + 0.163750A + .000109746 * Hc^2
PEF
(LLN) -1.2398 + 0.163750A + 0.00012160 * Hc^2
FEF25-75 -2.5379 + 0.43755A - 0.012154 * A^2 + 0.00008572 * Hc^2
FEF25-75
(LLN) -2.5379 + 0.43755A - 0.012154 * A^2 + 0.00003380 * Hc^2
African-America
Age > 18
FEV1 0.3433 - 0.01283A - 0.000097 * A^2 + 0.00010846 * Hc^2
FEV1
(LLN) 0.3433 - 0.01283A - 0.000097 * A^2 + 0.00008546 * Hc^2
FEV6 -0.1981 + 0.00047A - 0.000230 * A^2 + 0.00013497 * Hc^2
FEV6
(LLN) -0.1981 + 0.00047A - 0.000230 * A^2 + 0.00010848 * Hc^2
FVC -0.3039 + 0.00536A - 0.000265 * A^2 + 0.00013606 * Hc^2
FVC
(LLN) -0.3039 + 0.00536A - 0.000265 * A^2 + 0.00010916 * Hc^2
PEF 1.3597 + 0.03458A - 0.000847 * A^2 + 0.00019746 * Hc^2
PEF
(LLN) 1.3597 + 0.03458A - 0.000847 * A^2 + 0.00012160 * Hc^2
FEF25-75 2.0828 - 0.03793A + 0.00008572 * Hc^2
FEF25-75
(LLN) 2.0828 - 0.03793A + 0.00003380 * Hc^2
Mexican-American
Age < 18
FEV1 -0.9641 + 0.06490 A + 0.00012154 * Hc^2
FEV1
(LLN) -0.9641 + 0.06490 A + 0.00009890 * Hc^2
FEV6 -1.2410 + 0.07625A + 0.00014106 * Hc^2
FEV6
(LLN) -1.2410 + 0.07625A + 0.00011480 * Hc^2
FVC -1.2507 + 0.07501A + 0.00014246 * Hc^2
FVC
(LLN) -1.2507 + 0.07501A + 0.00011570 * Hc^2
PEF -3.2549 + 0.47495A - 0.013193 * A^2 + 0.00022203 * Hc^2
PEF
(LLN) -3.2549 + 0.47495A - 0.013193 * A^2 + 0.00014611 * Hc^2
Page 79

Technical References
P-495220-00 Rev. D 74
FEF25-75 -2.1825 + 0.42451A - 0.012415 * A^2 + 0.00009610 * Hc^2
FEF25-75
(LLN) -2.1825 + 0.42451A - 0.012415 * A^2 + 0.00004594 * Hc^2
Mexican-American
Age > 18
FEV1 0.4529 - 0.01178A - 0.000113 * A^2 + 0.00012154 * Hc^2
FEV1
(LLN) 0.4529 - 0.01178A - 0.000113 * A^2 + 0.00009890 * Hc^2
FEV6 0.2033 + 0.00020A - 0.000232 * A^2 + 0.00014106 * Hc^2
FEV6
(LLN) 0.2033 + 0.00020A - 0.000232 * A^2 + 0.00011480 * Hc^2
FVC 0.1210 + 0.00307A - 0.000237 * A^2 + 0.00014246 * Hc^2
FVC
(LLN) 0.1210 + 0.00307A - 0.000237 * A^2 + 0.00011570 * Hc^2
PEF 0.2401 + 0.06174A - 0.001023 * A^2 + 0.00022203 * Hc^2
PEF
(LLN) 0.2401 + 0.06174A - 0.001023 * A^2 + 0.00014611 * Hc^2
FEF25-75 1.7456 - 0.0119A + 0.000291 * A^2 + 0.00009610 * Hc^2
FEF25-75
(LLN) 1.7456 - 0.0119A + 0.000291 * A^2 + 0.00004594 * Hc^2
Dockery
(Wang, Dockery, et al., 1993)
The regression model is: x = exp (a + b ln (H)), where x is FVC(L), FEV1(L), FEV1% or
FEF25-75 (L/s), H is height in meters, and a and b are constants listed in the tables.
MALE Caucasian
Age 6-17
AGE FVC FEV1 FEV1% FEF25-75
a b ab ab a b
6 -0.024 2.470 -0.109 2.252 -0.078 -0.248 _ _
7 -0.018 2.489 -0.104 2.270 -0.086 -0.220 _ _
8 0.005 2.443 -0.089 2.257 -0.091 0.199 0.264 1.505
9 0.017 2.426 -0.063 2.197 -0.086 -0.206 0.308 1.443
10 0.030 2.407 -0.057 2.212 -0.081 -0.209 0.290 1.557
11 0.009 2.468 -0.093 2.324 -0.101 -0.147 0.242 1.738
12 -0.061 2.649 -0.161 2.512 -0.101 -0.133 0.165 1.982
13 -0.175 2.924 -0.292 2.843 -0.116 -0.085 0.007 2.396
14 -0.219 3.060 -0.329 2.983 -0.106 -0.087 0.014 2.483
15 -0.079 2.859 -0.141 2.709 -0.060 -0.155 0.241 2.163
16 0.104 2.591 0.062 2.409 -0.045 -0.178 0.503 1.764
17 0.253 2.374 0.262 2.099 0.008 -0.272 0.762 1.368
Page 80

Technical References
75 P-495220-00 Rev. D
Dockery
(Wang, Dockery, et al., 1993)
FEMALE Caucasian
Age 6-17
AGE FVC FEV1 FEV1% FEF25-75
ab ab ab ab
6 -0.013 2.007 0.109 1.949 -0.097 -0.055 _ _
7 -0.062 2.385 -0.144 2.243 -0.08 -0.132 _ _
8 -0.055 2.381 -0.137 2.239 -0.079 -0.152 0.247 1.668
9 -0.039 2.351 -0.123 2.222 -0.084 -0.128 0.254 1.710
10 -0.068 2.458 -0.161 2.364 -0.092 -0.097 0.195 1.933
11 -0.120 2.617 -0.223 2.558 -0.102 -0.061 0.161 2.091
12 -0.174 2.776 -0.264 2.709 -0.090 -0.067 0.185 2.120
13 -0.061 2.576 -0.153 2.535 -0.093 -0.040 0.294 1.976
14 0.139 2.208 0.046 2.178 -0.096 -0.026 0.450 1.711
15 0.210 2.099 0.148 2.008 -0.062 -0.093 0.581 1.486
16 0.226 2.097 0.181 1.972 -0.048 -0.120 0.654 1.366
17 0.214 2.146 0.176 1.992 -0.038 -0.154 0.688 1.290
Dockery MALE
(Wang, Dockery, et al., 1993)
FVC(LLN) 0.830 * FVC
FEV1(LLN) 0.825 * FEV1
FEV1%(LLN) 0.890 * FEV1%
Dockery FEMALE
(Wang, Dockery, et al., 1993)
FVC(LLN) 0.822 * FVC
FEV1(LLN) 0.823 * FEV1
FEV1%(LLN) 0.895 * FEV1%
NOTE: The studies upon which these equations are based were not conducted by
Puritan Bennett. The appropriate regulatory agencies should be consulted for
independent validation.
Page 81

Technical References
P-495220-00 Rev. D 76
RS-232 Interface Specifications
The Renaissance II Spirometry System
supports asynchronous communications to a computer system via a
RS-232C compatible serial port. Data
can be transmitted from the spirometer
only when it is docked in the base
station. The interface connection on
the base station is a standard 25-pin
IBM PC-AT style connector. The pin
locations for this connector are shown
in Figure 33. Puritan Bennett
recommends using a null modem cable
to connect the spirometer base station
to a PC.
Pin Function Descriptions
The function of the pins is described in Pin Function Descriptions (Table 11).
Pins 12, 13, 14, 16 and 18 are used for RS-232 communications for remote
control of the spirometer. All other pins are used for printer interfacing via
standard IEEE 1284 printer cable.
Table 11: Pin Function Descriptions
1 To Printer Data Strobe
2 To Printer Printer Data Bit 0
3 To Printer Printer Data Bit 1
4 To Printer Printer Data Bit 2
5 To Printer Printer Data Bit 3
6 To Printer Printer Data Bit 4
7 To Printer Printer Data Bit 5
8 To Printer Printer Data Bit 6
9 To Printer Printer Data Bit 7
10 To Base Printer ACK
11 To Base Printer Busy
12 To Base RS232 Clear To Send
13 To Base RS232 Receive Data
Figure 33: Pin locations (base station)
Spirometer
Base Station
(PB-710)
13 12 11 10 9 8 7 6 5 4 3 2 1
25 24 23 22 21 20 19 18 17 16 15 14
Page 82

Technical References
77 P-495220-00 Rev. D
In order for the computer system to communicate successfully with the
Renaissance II Spirometry System, an application program must first be written
on the computer system. This program must do more than act as a "terminal"
because the Renaissance II system requires responses from the computer for
setting up the proper handshaking.
Using the Renaissance II with a PC and Dataflow™ Software
Patient data can be stored and managed on a PC that has optional DataFlow™
Data Management Software installed. DataFlow software enables data transfer
to and from the Renaissance II spirometer via a null modem cable connected
between the PC and the spirometer base. DataFlow software allows you to:
•Examine trends in patient pulmonary function
•Update the spirometer with changes to patient data
•Print reports from your computer
•Query the database
•Archive data in a database
Contact Puritan-Bennett Customer Service at 1.800.635.5267 to purchase
DataFlow™ Data Management Software.
14 To PC RS232 Request To
Send
15 To Base Printer Error
16 To PC RS 232 Transmit Data
17 To Printer Printer Select
18, 19 Ground
20 Ground
21 Ground
22 Ground
23, 24, 25 Ground
Table 11: Pin Function Descriptions (cont.)
Page 83

System Configuration
P-495220-00 Rev. D 78
System Configuration
The Renaissance II Spirometry System offers a wide range of configuration
options that allow you to customize the operation and printed reports. The
Renaissance II is preset at the factory with a default configuration. These
settings can be easily changed. This section describes each configuration
option.
To view or change configuration settings:
1. From the “MAIN” screen, press 9 on the key pad or scroll to SETUP using
the cursor key and press ENTER.
2. Press the appropriate number on the key pad or scroll to the item whose
options you want to change, and press ENTER. The current configuration
and options are displayed.
3. Use the cursor arrow to tab to the selection. Enter the desired setting and
press ENTER. You may change other settings or return to the “SETU P”
menu by selecting DONE. When you have completed all of your changes,
select DONE to return to the “MAIN” screen.
The following sections describe each configuration option and the choices for
each option:
Spirometry Options (1)
Interpretation:
The interpretation option determines whether the displayed and
printed reports will include a computer generated interpretation. If you choose
to include interpretations, you can select either the ATS or Enright algorithms
for spirometry tests.
Adult Normals: This configuration determines which set of equations will be
used to generate the predicted values for patients 18 to 99 years of age. These
predicted values appear on the display and printed reports and are used to
derive the percent of predicted and the interpretation. The options are
Knudson (1983), Morris, Knudson (1976), Crapo/Morris, and NHANES III.
The option chosen will appear on each printed report. For the specified
equations and references, see the Predicted Normal Equations and References
section starting on page 60.
Pediatric Normals: This configuration determines which set of equations will
be used to generate the predicted values for patients 4 to 17 years of age. These
predicted values appear on the display and printed reports and are used to
derive the percent of predicted and the interpretation. The options are Hsu,
Polgar, Dockery, and NHANES III. The option chosen will appear on each
printed report. For the specific equations and references, see the Predicted
Normal Equations and References section starting on page 60.
NOTE: The patient’s height, birth date, and gender must be entered or no
interpretation will be displayed.
Page 84

System Configuration
79 P-495220-00 Rev. D
Reproducibility: Turning this option on displays the number of reproducible
tests performed by the patient when the SUMMARY option is selected from the
VIEW menu, or when a report is printed.
Acceptability: Turning this option on displays the number of acceptable tests
performed by the patient when the SUMMARY option is selected from the
VIEW menu, or when a report is printed.
Best Criteria: This setting determines how the values for the best test are
chosen. The three options are: “Val”, “Sum”, and “Enright.” The definition of
each option is as follows:
Val — includes the best values of FVC and FEV1 from any acceptable test.
The remainder of the values will be taken from the maneuver with the
highest sum of the FVC and the FEV1.
Sum — uses the values of FVC and FEV1 for the single maneuver with the
highest sum of the FVC and the FEV1.
Enright — uses the values of the single test with the highest sum of the
FVC, the FEV1, and 1/2 PEF.
QC Grades: This setting determines whether the display and printed reports
(Best and Best 3 only) will include the test quality grades. The grades for FVC/
FEV1 and FEV6/FEV1 tests are useful in judging the reliability of a particular
test and for evaluating and improving the coaching abilities of test technicians.
The test grades are A, B, C, D, and F. For specific grading criteria used by the
Renaissance II, refer to the Grading Criteria section on page 44.
Lung Age: If the lung age calculation option is enabled and the patient’s
smoking history has been entered, the patient’s lung age will be calculated. (See
Lung Age Interpretation on page 47, for more information on lung age.)
COPD Risk: If the COPD risk option is enabled and the patient’s smoking
history has been entered, the risk of COPD will be calculated. (See Risk of
COPD on page 47, for more information.)
Values Soft Key: By pressing the VALUES soft key, you can choose which
spirometry parameters you want to include on printed reports where you have
specified the User Defined format in the Print options (see Print Options (3) on
page 80). If you have previously specified the Clinical or Industrial print option,
and you select any or all of the following parameters, the print option
automatically changes to User Defined: FEV3, FEF25-75, FET, FEF25, PEF,
FEF50, PIF, FEF75, FEF50/FIF50.
NOTE: The “Val” (best value) method is recommended by the American Tho-
racic Society and mandated by NIOSH/OSHA standards and should be used for
all industrial and disability testing.
Page 85

System Configuration
P-495220-00 Rev. D 80
Device Options (2)
Custom Header: This configuration allows you to generate a custom header on
each page of the printed report. The custom header will appear centered at the
top of each page. The header can consist of up to two lines and each line can
have up to 20 characters.
Units: When non-metric units are selected, the patient's height is reported in
inches (in.), the patient's weight is reported in pounds (lbs.), the elevation is
reported in feet (ft.), and the ambient temperature is reported in degrees
Fahrenheit (F). When metric is selected, the units used are centimeters (cm.),
kilograms (kg.), meters (m) and degrees Celsius (C).
PEF Units: This configuration determines which units will be used when
displaying peak expiratory flow. The options are liters/second (L/S) and liters/
minute (L/M).
Clock: This setting determines how the time is entered and reported. The time
format options are twelve hour (a.m./p.m.) or twenty-four hour.
Audio: If this option is enabled, an audible beep will sound when any key is
pressed.
Audio Incentive: This setting allows you to activate or suppress the audio
incentive that is heard during the patient's effort. The audio incentive feature
can help the patient and technician achieve better test results.
Offset Curves: This setting allows you to choose how curves will be positioned
on the grid. If you disable the offset curves option, all of the curves will be
superimposed on one another and will begin at the zero point of the grid. If
you enable the offset option, all of the curves will be offset from one another.
The flow-volume curves will be offset by one liter and the volume-time curves
will be offset by one second. This selection will affect the graph sizes. Pre/postmedication comparison graphs will not be offset.
Print Options (3)
Printer: The Renaissance II Spirometry System operates with a variety of
parallel printers that use the Hewlett Packard graphic languages, as well as
several other printer protocols. The following list indicates some of the printer
types that are supported. Contact Puritan Bennett Technical Support for the
printers and model numbers currently supported.
• HP DJ Black & White, Color, and Laser
• Epson Black & White, Color
• Canon Black & White
Paper: This setting allows you to choose the printer paper size. The options are
8.5"x 11" or A4 (International Standard paper size).
Page 86

System Configuration
81 P-495220-00 Rev. D
Format: You can choose between Clinical, Industrial, or User Defined formats
for printing test results. Table 12 shows the information printed for each test
performed. If you select either the Clinical or Industrial print option, and later
change the marked selections using the VALUES soft key in Spirometry Options
(see Spirometry Options (1) on page 78), the print format will automatically
change to User Defined.
Accept Msgs: Turning this option on will print the acceptability messages
displayed during an FVC or FEV6 test, provided that the last test is selected for
printing.
Interpretation: Enabling this option will print the computer generated
interpretation in the report according to the configuration in Spirometry
options (see Spirometry Options (1) on page 78).
Predicteds: This setting determines how predicted graphical data will appear on
the printed graphs. The intent of the predicted graphical data is to produce a
graphic reference of the patient's predicted values. The options are curve,
points, or none. If you choose the curve option, a simulated Flow-Volume or
Volume-Time curve will be plotted using predicted data. If you choose the
points option, the individual data points appear on the grid as small blocks.
Table 12: Printed Values for Specified Print Format Options
Test Performed Print Format Option
Clinical Industrial User Defined
FVC FVC, FEV1,
FEV1%, FEF25-75,
FET, PEF
FVC, FEV1,
FEV1%
FVC, FEV1,
FEV1%, FEV3*,
FET*, PEF*,
FEF25-75*,
FEF25*, FEF50*,
FEF75*
FVL FIVC, PIF, FEF50/
FIF50%
FIVC FIVC, PIF*,
FEF50/FIF50%*
MVV MVV, MVV Rate MVV, MVV Rate MVV, MVV Rate
SVC SVC SVC SVC
FEV6 FEV6 FEV6 FEV6
* Any or all of these items may be specified in a User Defined
report.
Page 87

System Configuration
P-495220-00 Rev. D 82
The following predicted points are plotted on each graph:
Flow-Volume Graph Volume-Time Graph
PEF FEV0.5
FEF25 FEV1
FEF50 FEV3
FEF75 FVC or FEV6
FVC or FEV6
Graph: The graph format setting allows you to choose which graphs will
appear on the printed reports. The options are: both (prints both volume-time
(VT) and flow-volume (FV) graphs), volume-time (VT), flow-volume (FV), or
none.
Grid: This setting determines whether there will be grid lines on the graphs.
The grid lines may make it easier to read test values from the curves. The grid
lines are required for disability testing.
Size: The graph size setting allows you to choose the size of graphs that appear
on the printed report. The options are validation size and diagnostic size.
Validation size graphs are larger in scale and are designed to allow hand
validation of test values. Due to its large size, a printed report with validation
size graphs may require more than one page. All occupational and disability
testing should use the validation size.
Diagnostic will usually allow the entire report to be printed on a single page,
unless an SVC or MVV test is performed. Scaling information is printed with
all graphs.
Scale: This setting determines whether to automatically scale the graph size up
during printing, based on the size of the FVC/FVL curves stored (Auto option),
or whether to leave the graph size fixed (1x option).
All Curves: Printing a Best or Best 3 report with this option turned on will
show the best three flow-volume and volume-time graphs for each Pre-Med
and Post-Med FVC/FVL and FEV6 maneuver performed in a patient’s session.
If this option is turned off, only the single best Pre-Med and Post-Med flowvolume and volume-time graph for FVC/FVL and FEV6 tests will print. If SVC
and MVV maneuvers have been performed in the same session, only the single
best Pre-Med and Post-Med curves are shown, regardless of whether the All
Curves option is turned on or off. If there are no Post-Med tests, the three best
FVC/VFL and FEV6 curves will print regardless of the All Curves option
setting.
Page 88

System Configuration
83 P-495220-00 Rev. D
Settings (4)
Language: Allows you to select the language in which the information will be
displayed and printed.
Date: Allows you to enter the day, month, and year using the numeric keypad.
Time: Allows you to enter the time using the numeric keypad. The time will
display in 12-hr (am/pm) or 24-hr time depending upon your selection in the
device setup (see Device Options (2)).
Elevation: This setting allows the user to enter altitude above sea level. The
elevation is from 0-15,000 feet or 4572 meters. This setting allows the
spirometer to determine the BTPS correction factor.
Display (5)
Autoscale Graphs: When this option is off, volume/time and flow/volume
graphs are displayed at a fixed size. Turning this option on allows the display to
automatically scale the volume/time and flow/volume graphs based upon the
amount of stored data, up to twice the size of the fixed size graph.
Predicted: This setting allows you to choose how you want the unit to display
predicted values on the screen. The options are curve, points, and none. See
Print Options (3) for more information.
Show Patients By: This setting allows you to display patient data using the ID
number or name entered during new patient setup.
Storage (8)
Confirm Before Delete: Turning this option on requires the user to press the
YES or NO soft key before patient records are deleted. See Deleting Patient
Data on page 41 for more information.
Compress Curve Data: With this option enabled, graphical data is compressed
so that memory capacity is approximately doubled.
NOTE: Setup locations 6, 7, and 9 are not used.
Page 89

System Configuration
P-495220-00 Rev. D 84
The following table lists the factory defaults and possible settings for all
configuration options:
Table 13: Setup and System Configurations
Configuration Option Factory
Defaults
Alternate Settings
Spirometry Options
Interpretation ATS Enright, None
Adult Normals Knudson 83 Morris, Knudson 76,
Crapo/Morris,
NHANES III
Pediatric Normals Hsu Polgar, Dockery,
NHANES III
Reproducibility ON OFF
Acceptability ON OFF
Best Criteria VAL SUM, ENRIGHT
QC Grades ON OFF
Lung age ON OFF
COPD Risk ON OFF
Device Options
Header None User-specified
Units NON-METRIC
(in, lbs, ft)
METRIC (cm, kg, m)
PEF Units L/Sc L/M
Clock 12 HR 24 HR
Elevation 0 0-15,000 ft.
Audio ON OFF
Audio incent ON OFF
Offset Curves ON OFF
Page 90

System Configuration
85 P-495220-00 Rev. D
Configuration Option Factory
Defaults
Alternate Settings
Print Options
Printer HP DJ B&W HP DJ Col, HP Laser
Epson B&W, Epson Col,
Canon B&W
Paper 8.5” X 11” A4
Format Clinical Industrial, User Defined
Accept Msgs ON OFF
Interpretation ON OFF
Predicteds Curve Points, None
Graph Both VT, FV, None
Grid ON OFF
Size VAL DIAG
Scale 1X AUTO
All Curves ON OFF
Settings Options
Language
Date
Time
Elevation
Display Options
Autoscale Graphs ON OFF
Predicted Curve Points, None
Show Patients By ID Name
Storage Options
Confirm Before Delete ON OFF
Compress Curve Data ON OFF
Table 13: Setup and System Configurations (cont.)
Page 91

System Configuration
P-495220-00 Rev. D 86
Printing the System Configuration
Perform the following steps to print the spirometer’s setup and system
configuration:
1. From the “MAIN” screen press 6 on the keypad or use the cursor arrow
key to scroll to PRINT and press ENTER.
2. From the “SELECT REPORT” screen, press 3 on the keypad or use the
cursor arrow key to scroll to SETUP and press ENTER. The configuration
report is sent to the printer.
NOTE: Keep the System Configuration printout available for future reference. You
may use it to return the Spirometer to its previous setup configuration if settings
are changed.
Page 92

System Configuration
87 P-495220-00 Rev. D
Barometric Pressure vs. Altitude
Reading the Barometric Pressure vs. Altitude Chart
The barometric pressure at your elevation can be read to the nearest 100 feet
using the chart above. The following example shows how to find the
barometric pressure at an altitude of 4700 feet:
1. Find the row corresponding to 4000 feet in the vertical altitude column at
the left side of the graph.
2. Find the column corresponding to 700 feet in the horizontal altitude row at
the top of the graph.
3. Read the barometric pressure (640 mm Hg) at the point where the row and
column intersect.
Table 14: Barometric Pressure vs. Altitude
Second Edition; pg. 183 (Smithsonian 1963, Iribarne 1973)
Reference: 1984: Intermountain Thoracic Pulmonary Function Testing
Page 93

Glossary of Medical Terminology
P-495220-00 Rev. D 88
Glossary of Medical Terminology
ATPD - Ambient temperature, pressure, dry.
ATPS - Ambient temperature, pressure, saturated with water vapor.
A TPS to BTPS Conversion Factor - A factor used to convert flow and volume data from
values measured at ambient temperature (ATPS) to body temperature (BTPS).
ATS - American Thoracic Society. (http://www.thoracic.org)
Back Extrapolated Start Time - In order to measure the timed parameters more accu-
rately and consistently, the ATS recommends using a technique called back extrapolation to determine the start of maneuver. The Renaissance II spirometer uses this
technique. For more details, please reference "Standardization of Spirometry-1994
Update, ATS".
BTPS - Body temperature (37°C), pressure, saturated with water vapor.
CSA - Canadian Standards Agency.
Extrapolated Volume - The amount of air exhaled prior to the back-extrapolated start
time. If this volume is more than 5% of the FVC, the maneuver started too slowly.
This is also known as BEV (Back Extrapolated Volume).
FEF 25-75 - The mean flow rate between 25% and 75% of the Forced Vital Capacity.
This is also known as MMEF (Mean Mid Expiratory Flow).
FEF50/FIF50% - Ratio of Forced Expiratory Flow at 50% of FVC to Forced Inspira-
tory Flow at 50% of FIVC.
FET - Forced Expiratory Time measured in seconds - The time from the beginning of
the maneuver until the time at which highest volume was achieved during an FVC
maneuver.
FEV0.5 - Forced Expiratory Volume measured in one half second (liters) - The volume
of air exhaled in first half-second of an FVC maneuver.
FEV1 - Forced Expiratory Volume measured in one second (liters) - The volume of air
exhaled in the first second of an FVC maneuver.
FEV1% - The ratio of FEV1 to FVC, expressed as a percentage. Also called FEV1/
FVC%.
FEV3 - Forced Expiratory Volume measured in three seconds (liters) - The volume of air
exhaled in the first three seconds of an FVC maneuver.
FEV6 - Forced Expiratory Volume measured in 6 seconds (liters) - The volume of air
exhaled in the first six seconds of an FVC maneuver. Sometimes used as a surrogate for
FVC.
Page 94

Glossary of Medical Terminology
89 P-495220-00 Rev. D
FIVC - Forced Inspiratory Vital Capacity measured in liters -The maximum volume of
air inspired with maximum effort after a complete exhalation.
Flow-Volume Curve - A graphic printout of an FVC/FVL maneuver plotting flow vs. vol-
ume.
FVC - Forced Vital Capacity measured in liters - The maximum volume of air exhaled as
rapidly, forcefully and completely as possible from the point of maximum inhalation.
FVL - Flow Volume Loop- an FVC maneuver that is immediately followed by a maximal
inspiration.
HIPAA - Health Insurance Portability and Accountability Act of 1996.
LLN - Lower Limit of Normal - The point which is considered to be the lower limit of
the normal patient population for a given parameter. This point is defined in the various
referenced studies.
MVV - Maximal Voluntary Ventilation measured in liters/min - The volume of air that
can be exhaled during twelve seconds of rapid, deep breathing. The actual volume is
extrapolated to one minute.
NIOSH - The National Institute for Occupational Safety and Health - A government
agency established by the Occupational Safety and Health Act of 1970. NIOSH is part
of the Centers for Disease Control and Prevention (CDC) and is the only federal institute
responsible for conducting research and making recommendations for the prevention of
work-related illnesses and injuries. (http://www.cdc.gov/niosh/homepage.html)
Obstructive Disease - Obstructive diseases are characterized by reduced air flow rates
making it more difficult to move air into and out of the lungs. These diseases often result
in a lowered FEV1 and FEV1 or FVC1%. Three of the most common diseases include
asthma, chronic bronchitis and emphysema.
OSHA - Occupational Safety and Health Administration - A government agency that
enforces laws and regulations in the workplace in regard to occupational safety and
health hazards.
PEF - Peak Expiratory Flow Rate - measured in liters/sec or liters/min. Also called FEF
max. or PEFR.
PIF - Peak Inspiratory Flow Rate - measured in liters/sec. Also called FIF max.
Restrictive Disease - These diseases are characterized by reduced lung volume or
impaired movement of the lungs. A lowered FVC with normal FEV1 and FEV1/FVC or
FEV% is often an indication of restrictive disease, although a poor patient effort is also a
common cause of lowered FVC. Restrictive disease includes gross obesity, lung fibrosis,
neuromuscular disease, or paralysis, as well as several occupational related diseases,
such as pneumoconiosis and “cotton-dust lung.”
Page 95

Glossary of Medical Terminology
P-495220-00 Rev. D 90
RR - Respiratory Rate or BPM (Breaths Per Minute)- The frequency of breaths during
an MVV maneuver. Also called the MVV Rate.
SVC - Slow Vital Capacity - The total amount of air that can be slowly exhaled from
full inspiration. Also called the Vital Capacity or VC.
Undetermined – An interpretation result generated by the Renaissance II when the FVC
and FEV1 are greater than 100% and FEV1% is less than 100% of the predicted values.
VC - Vital Capacity (Same as SVC).
Volume-Time Curve - A graphic printout of an FVC and SVC or MVV maneuver show-
ing volume vs. time.
Page 96

References
91 P-495220-00 Rev. D
References
1. Cherniack, R.M., Raber, M.B., Normal Standards for Ventilatory Function
using an Automated Wedge Spirometer, American Review of Respiratory
Disease, 1972, 106:38-46.
2. Crapo, Robert O., et al. Reference Spirometric Values Using Techniques and Equip-
ment that Meets ATS Recommendations, American Review of Respiratory Disease,
1981, 123: 659-674.
3. Hsu, Katharine., et al. Ventilatory Functions of Normal Children and Young Adults
-- Mexican-American, White, and Black, Journal of Pediatrics, 1979,
95:14-23.
4. Knudson, Ronald J., et al. Changes in the Normal Maximal Expiratory Flow-Vol-
ume Curve with Aging, American Review of Respiratory Disease, 1983,
127:725-734.
5. Knudson, Ronald J., et al. The Maximal Expiratory Flow-Volume Curve, American
Review of Respiratory Disease, 1976, 113:587-600.
6. Morris, James F., et al. Normal Values and Evaluation of Forced Expiratory Flow,
American Review of Respiratory Disease, 1975, 111:755-761.
7. Morris, James F., et al. Spirometric Standards for Healthy Non-Smoking Adults,
American Review of Respiratory Disease, 1971, 103: 57-67.
8. Polgar, P., Promadhat, V. Pulmonary Testing in Children, W.B. Saunders. Philadelphia, 1971, 100-153.
9. Wang, Xiaobin, D W. Dockery, ScD, David Wypij, PhD, Martha E. Fay, MPH, and
Benjamin G. Ferris, Jr. MD., Pulmonary Function Between 6 and 18 Years of Age,
Pediatric Pulmonology, 1993, 15:75-88.
10. AM Rev Respir. Dis. 1987, Standardization of Spirometry, 136:1285-98,1987
Update, ATS.
11. AM J Respir. Crit. Care Med. 1995, Standardization of Spirometry, 152:1107-
1136, 1994 Update, ATS.
12. American Thoracic Society, AM Rev Respir. Dis., Spirometry in the Lung Health
Study, 1991, 143:1215-1223.
13. American Thoracic Society, Lung Function testing: AM Rev Respir. Dis., Selection
of Reference Values and Interpretative Strategies, 144:1202-1218, 1991.
14. Morris, J.F. and Temple, W., Spirometric 'Lung Age' Estimation for Motivating
Smoking Cessation, Preventative Medicine, Volume 14, 1985, p. 655-662.
15. Higgins, MN, Keller J.B., AM Rev Respir. Dis., Risk of Chronic Obstructive
Pulmonary Disease,
1984, 130: 380-385.
16. Hankinson, John, Odencrantz, John and Fedan, Kathleen, Spirometric Reference
Values from a Sample of the General U. S. Population, (NHANES III) American
Journal of Respiratory and Critical Care Medicine, 1999, 159: 183-184.
17. Ferguson, G. T., et al. (NLHEP). Office Spirometry for Lung Health Assessment in
Adults, Chest, Volume 117, 2000, p. 1146-1161.
Page 97

Notes
P-495220-00 Rev. D 92
Notes
Page 98

Notes
93 P-495220-00 Rev. D
Page 99

Page 100

Tyco Healthcare Group LP
Nellcor Puritan Bennett Division
4280 Hacienda Drive
Pleasanton, CA 94588 USA
Toll Free: 1.800.635.5267
Rx ONLY
© 2003 Nellcor Puritan Bennett Inc. All rights reserved. P-495220-00 Rev. D 10/03
 Loading...
Loading...