
USER MANUAL
1900 Series
(Threaded Connections)
195405
shown
SAVE THESE INSTRUCTIONS
CAUTION
300 Held Drive Tel: (+001) 610-262-6090
Northampton, PA 18067 USA Fax: (+001) 610-262-6080
ISO 13485 Certied www.precisionmedical.com
Federal (USA) law restricts this device to
sale by or on the order of a physician.

CONTENTS
RECEIVING / INSPECTION ............................................................... 2
INTENDED USE ................................................................................. 2
READ ALL INSTRUCTIONS BEFORE USING .................................. 2
EXPLANATION OF ABBREVIATIONS .............................................. 2
SAFETY INFORMATION - WARNINGS AND CAUTIONS ................. 3
PRINCIPLES OF OPERATION .......................................................... 5
SPECIFICATIONS .............................................................................. 6
DIAGRAMS / COMPONENT DESCRIPTION ....................................7
INSTALLATION ..................................................................................8
OPERATING INSTRUCTIONS ........................................................... 9
MAINTENANCE / CLEANING .......................................................... 11
RETURNS ........................................................................................ 11
DISPOSAL INSTRUCTIONS ............................................................ 11
TROUBLESHOOTING .....................................................................12
REPLACEMENT PARTS .................................................................. 12
ACCESSORIES................................................................................13
LIMITED WARRANTY .....................................................................14
DECLARATION OF CONFORMITY ................................................. 15
1

RECEIVING / INSPECTION
Remove the Precision Medical, Inc. EasyPulse5 Oxygen Conserving
Regulator (Conserver) from the packaging and inspect for damage. If
there is any damage, DO NOT USE and contact your Equipment Provider.
INTENDED USE
To regulate high pressure cylinders that provide supplemental oxygen
to patients who may have difculty extracting oxygen from the air they
breathe. It is for patients who would normally receive the oxygen via a
nasal cannula. The device delivers 100% oxygen at ow settings. It is
intended to be used as an oxygen saving device that reduces the drying
of the airways.
READ ALL INSTRUCTIONS BEFORE USING
This manual instructs the user to install and operate the EasyPulse5
Oxygen Conserving Regulator (Conserver). This is provided for your
safety and to prevent damage to the Conserver. If you do not understand
this manual, DO NOT USE the Conserver and contact your Equipment
Provider.
DANGER
This product is not intended as a life-sustaining or life-supporting
device
.
EXPLANATION OF ABBREVIATIONS
kPa Kilopascal
psi Pounds Per Square Inch
l/min Liters Per Minute
b/min Breaths Per Minute
2

SAFETY INFORMATION - WARNINGS AND CAUTIONS
DANGER
WARNING
CAUTION
CAUTION
Indicates an imminently hazardous situation
which, if not avoided, will result in death or
serious injury.
Indicates a potentially hazardous situation
which, if not avoided, could result in death or
serious injury.
Indicates a potentially hazardous situation
which, if not avoided, may result in minor or
moderate injury.
Used without the safety alert symbol indicates
a potentially hazardous situation which, if not
avoided, may result in property damage.
CONSULT ACCOMPANYING DOCUMENTS
Symbol for “USE NO OIL”
Symbol for “NO SMOKING”
Symbol for “COVERING DEVICE WITH
GARMENTS WILL PRODUCE OXYGEN
ENRICHED ATMOSPHERE”
Symbol indicates the device complies with the
requirements of Directive 93/42/EEC concerning
medical devices and all applicable International
Standards. (On CE marked Devices ONLY)
3

WARNING
• ALWAYS conrm prescribed dose before administering to
patient and monitor on a frequent basis.
• Always follow standards for Medical Gas Products, and High
Pressure Oxygen Handling. In the United States, ANSI, CGA
and G-4 applies.
• Keep cylinder valve closed at all times when cylinder is not in use.
• NO OXYGEN is delivered when the pointer is aligned with “OFF”.
• This Conserver is NOT to be used by patients who breathe
through their mouths.
• DO NOT use if dirt or contaminants are present on or around
cylinder, valve, the Conserver or connecting devices.
• DO NOT use oils, greases, organic lubricants or any combustible
materials on or near the Conserver. Wash and dry hands properly
prior to use.
• DO NOT use a humidier with the Conserver.
• DO NOT allow cylinders to tip or fall. Secure gas cylinders
so they cannot fall. For optimum safety keep cylinder upright
whenever possible.
• DO NOT store cylinders near sources of heat or ame.
• DO NOT use while sleeping without consulting your Equipment
Provider.
• DO NOT smoke in an area where oxygen is being administered.
• DO NOT use near any type of ame or ammable/explosive
substances.
• Use only medical grade oxygen. In the United States, USP applies.
• The Conserver is equipped with a relief valve. If you hear a loud
hissing or popping sound coming from the Conserver, discontinue
use, close cylinder valve, and contact your Equipment Provider.
• The Conserver is designed to operate with a single lumen, adult
cannula with a maximum length of 7 feet (2.1 m).
• The cannula is for single patient use only.
4
CAUTION
• Only personnel instructed and trained in its use should operate
th
e Conserver
• Th
e Conserver
affect the results of an MRI.
• DO NOT autoclave.
• DO NOT gas sterilize with Ethylene Oxide.
• DO NOT clean with aromatic hydrocarbons.
• Store the Conserver in a clean area when not in use.
• Avoid dropping the
could fall and become damaged.
• Consistent with the recommendations of the medical community on
the use of conserving devices, it is recommended that the Conserver
be qualied on patients in the situations it will be used.
• The Conserver may not be able to detect all respiratory efforts of the
patient. (Shallow breathers may not be able to trigger the Conserver.)
• Operating the Conserver outside its range of operating
conditions may affect its accuracy and performance.
PRINCIPLES OF OPERATION
The Oxygen Conserver is designed to be used with high-pressure
oxygen systems. It consists of a cylinder connection, cylinder contents
gauge (if equipped), high-to-low pressure regulator, orice plate and a
conserving demand module. The regulator reduces the high pressure
of the cylinder to the working pressure of the orice plate. The orice
plate uses calibrated orices to deliver a selected ow to the conserving
demand module. The conserving demand module controls the pulse size
and timing to the patient. It supplies a pulse of oxygen at the beginning
of each breath. This reduces the oxygen demand on the system and
limits the drying of the airways. The ow is determined by setting the
ow control knob to the prescribed ow. The oxygen is supplied to the
patient through the cannula.
.
contains magnetic, ferrous material that may
Conserver
or placing it in a position where it
5
TECHNISCHE DATEN
Inlet Pressure Range: 300 - 3000 psi (2068 - 20684 kPa)
Pressure Gauge Accuracy: 3 - 2 - 3% of full scale
Dimensions: (Are approximate and may vary by model)
No Gauge Models
Weight: 10 oz (283 g)
Overall Length: 2.5 in (6.4 cm)
Width: 1.9 in (4.9 cm)
Height: 2.9 in (7.5 cm)
Pulse Settings: 1, 2, 3, 4, & 5 l/min Equivalents
Flow Settings: 2 l/min Continuous (195405)
1 through 6 l/min Continuous (195412)
Accuracy: Continuous Settings within ± 10%
Gauge Models
12.6 oz (357 g)
2.5 in (6.4 cm)
2.8 in (7.0 cm)
3.2 in (8.1 cm)
Accuracy: Pulse Settings: Within +/- 15% of the nominal
bolus value (at each breath rate)
Savings Ratio: Up to 5.7:1
Trigger Method: Inspiratory effort (Negative pressure from
PULSE ONLY
Breathing Frequency: Up to 35 b/min
patient inhalation)
Cannula Requirement: Maximum 7 foot (2.1 m) long standard adult
single lumen nasal cannula.
Operating Conditions:
Temperature: 35°F to 105°F (1.7°C to 40.6°C)
Altitude: Sea level to 10,000 ft (0 to 3,048 m)
Storage Conditions:
Temperature: -40°F to 140°F (-40°C to 60°C)
Maximum Humidity: 95% Noncondensing
Oxygen Cylinder Connection:
CGA 540 Valve
Ignition and Fault Tolerance: Meets ASTM G175-03
Specications are subject to change without prior notice. Manuals available on our Website; www.precisionmedical.com
6

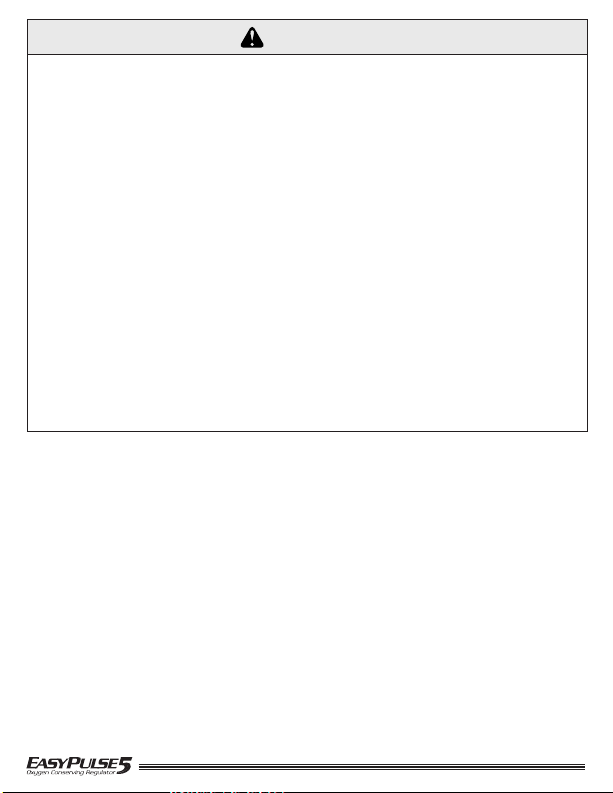
DIAGRAMS / COMPONENT DESCRIPTION
CAUTION
Missing or illegible labels must be replaced, contact Precision
Medical, Inc.
MODELS (3 versions shown)
CGA 540 Connection
195405/195412 (Shown) and 194504NG/195412NG
Cannula
Connection
Dial
Contents Gauge
(Gauge Models
ONLY)
19SE05/19SE12 (Shown) and 19SE05NG/19SE12NG
Cannula
Connection
Dial
Contents Gauge
(Gauge Models
ONLY)
DIN 477 #6 Connection (Swedish Standard)
(Labels are Green & White)
DIN 477 #9 Connection (German Standard)
19DE05/19DE12 and 19DE05NG/19DE12NG
(Labels are Black & White)
Indicator
Pointer
Green
Knob
Indicator
Pointer
Inlet Seal
(O-ring)
Inlet Seal
(O-ring)
White
Knob
7
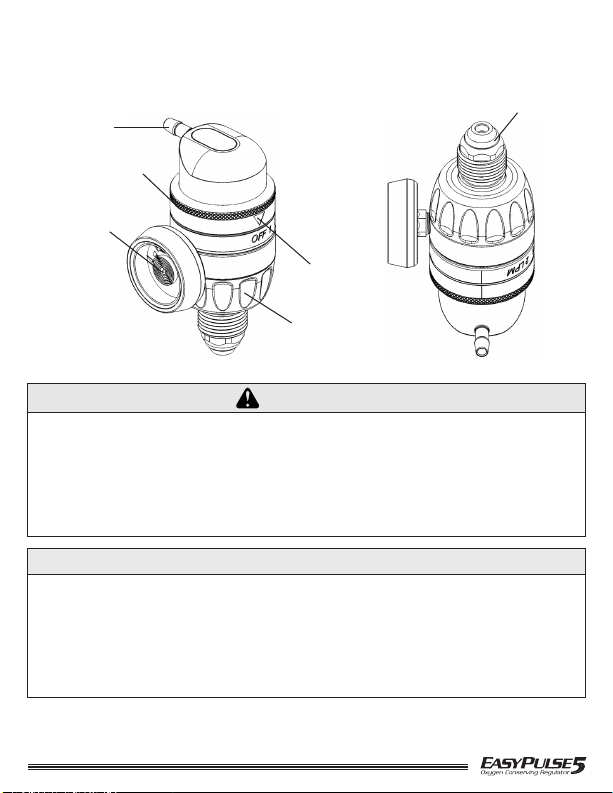
Bullnose Connection
British - 19GB05/19GB12 (Shown) and 19GB05NG/19GB12NG
Spanish - 19ES05/19SE12 and 19ES05NG/19SE12NG
Cannula
Connection
Contents Gauge
(Gauge Models
ONLY)
Dial
Indicator
Pointer
White
Knob
Inlet Seal
INSTALLATION
WARNING
• Read this User Manual before installing or operating the Oxygen
Conserving Regulator.
• Inspect the Conserver and cylinder valve to ensure they are free of
oils, greases or other contaminants.
• DO NOT direct ow of oxygen at any person, or ammable material
when cracking open the cylinder.
CAUTION
• Inspect the Oxygen Conserving Regulator for visual damage before
use, DO NOT USE if damaged.
• DO NOT use tools to tighten knob, this can lead to over tightening and
will cause damage to the Conserver.
• Be sure all connections are tight and leak free.
• DO NOT use liquid leak detector to test for leaks.
NOTE: For Operational Instructions on how to operate the cylinder
consult with your Equipment Provider.
(O-ring)
8

1. Position the cylinder so the oxygen cylinder valve outlet is pointing away
from the user and any other person (s).
2.
Before installing the Conserver, “crack” cylinder valve by opening the valve
slightly to remove any foreign particles and then close valve.
NOTE:
(For NO GAUGE (NG) Models ONLY) The Conserver should only
3. Be sure the Conserver is in the “OFF” position. Align “OFF” with the
indicating pointer .
4.
Be sure the high pressure inlet connection has a seal.
DO NOT USE without a seal. (See Product Diagram)
NOTE:
5. Attach the Conserver to the oxygen cylinder valve.
6. Hand tighten knob clockwise until tight.
be used on cylinders with a contents gauge.
For optimum safety, use only a Precision Medical, Inc.
Inlet Seal (Viton O-ring). (See Parts List)
The Inlet Seal supplied with the Conserver is reusable.
DO NOT USE any other type of seal.
OPERATING INSTRUCTIONS
1. Attach a standard adult single lumen oxygen nasal cannula, no longer
than 7 feet (2.1 m) to the Conserver’s outlet according to the cannula
manufacturer’s instructions.
WARNING
• DO NOT use pediatric, low ow nasal cannulas or oxygen masks
with the Conserver.
• DO NOT block the cannula connection or kink cannula tubing when the
Conserver is in use, this may damage the Conserver.
2. Place the cannula over your ears and position the prongs in your nose as
instructed by your Provider or cannula manufacturer.
OR
9

3.
Slowly open cylinder valve counterclockwise, until completely open.
4. Turn dial on the Conserver until indicating pointer is aligned with the
prescribed setting.
5. Breathe through your nose.
NOTE: •
• Because each patient’s breathing pattern is different and the envi
6. To remove the Conserver from cylinder:
When on a pulse setting, there is ow or a pulse only at the beginning of
each breath. If you do not feel the pulse at the beginning of each breath,
check the setting. If there is still no pulse, turn dial to an equivalent
“Continuous” setting.
varies, it may be difcult to feel some low setting pulses.
• Completely close oxygen cylinder valve.
• Turn the Conserver dial to any “Continuous” position.
• Wait for oxygen to stop owing from
Conserver
.
ronment
• Remove the Conserver from cylinder valve.
DANGER
NEVER attempt to remove the Conserver from a cylinder unless the
cylinder valve is closed.
WARNING
• When cylinder pressure is 500 psi (3447 kPa, 34.47 bar) and below, it
is recommended to change to a full oxygen cylinder.
• NO OXYGEN is delivered in between settings.
• To avoid injury to patient:
• A LWAYS conrm prescribed setting before administering to patient
and monitor ow on a frequent basis.
• Use only Precision Medical, Inc. Carry Bag designed for the
EasyPulse5 to prevent an oxygen enriched environment.
• DO NOT place the unit under clothing while in use.
• When the Conserver is in use a small amount of oxygen is vented. Wearing
the unit under clothing may saturate fabrics with oxygen and cause them
to burn rapidly if exposed to sparks or ame. It may take several hours
for oxygen levels in fabrics to return to normal.
• ALWAYS insert cylinder and the Conserver into bag, cylinder rst with
gauge facing mesh. Reference Accessory photo, (pg. 13).
WARNING
10

MAINTENANCE / CLEANING
1. Disconnect all connections before cleaning.
2. After each use, clean exterior of the Conserver with a cloth dampened
with mild detergent and water.
3. Wipe dry with a clean cloth.
4. Store the Conserver in a clean area free from grease, oil, and other
sources of contamination.
CAUTION
• DO NOT use cleaning solutions.
• DO NOT immerse the Conserver in any kind of liquid.
• DO NOT attempt to repair the EasyPulse5 Oxygen Conserving
Regulator.
• All repairs must be performed by Precision Medical, Inc.
RETURNS
Returned products require a Returned Goods Authorization (RGA)
number, contact Precision Medical, Inc. All returns must be packaged
in sealed containers to prevent damage. Precision Medical, Inc. will not
be responsible for goods damaged in transit. Refer to Precision Medical,
Inc. Return Policy available on the Internet, www.precisionmedical.com.
Manuals available on our Website; www.precisionmedical.com
DISPOSAL INSTRUCTIONS
This device and its packaging contain no hazardous materials. No
special precautions need to be taken when disposing the device and/
or its packaging.
Please Recycle
11

TROUBLESHOOTING
If the Conserver fails to function, consult the Troubleshooting Guide
below. If problem cannot be solved, consult your Provider.
TROUBLESHOOTING GUIDE
Problem Probable Cause Remedy
A. No ow 1. Cylinder valve closed
2. Regulator in “OFF”
position
3. Cylinder empty
4. Dial set between
settings
5. Conserver not sensing
breath
B. Leaking at
cylinder
connection
1. Missing or defective
Inlet Seal
2. Defective cylinder
valve
1. Turn on cylinder
2. Set to prescribed
setting
3. Replace cylinder
4. Set dial so indicator
points to a setting
5. a. Check position of
cannula in nose
b. Do not breathe
through mouth
1. Replace Inlet Seal
2. Contact your
Equipment Provider
REPLACEMENT PARTS
DESCRIPTION PART #
Cannula 504833
Inlet Seals (Viton O-rings)
For models: 195405 Serial # 100461or higher
195405NG Serial # 178367 or higher
For models: 19SE05, 19SE12, 19SE05NG & 19SE12NG
19DE05, 19DE12, 19DE05NG & 19DE12NG
19GB05, 19GB12, 19GB05NG & 19GB12NG
For models: 19ES05, 19SE12, 19ES05NG & 19SE12NG
505487
505214
505401
505214
505574
12

ACCESSORIES
DESCRIPTION PART #
Carry Bag M6 503920
Carry Bag M4/M6 504184
Carry Bag ML6/M9 504185
Model 195405NG Shown
Carry Bag with
shoulder strap
Model 195405 Shown
Conserver properly
installed in Carry Bag
Conserver properly
installed in Carry Bag
13

LIMITED WARRANTY
AND LIMITATION OF LIABILITY
Precision Medical, Inc. warrants that the EasyPulse5 Oxygen Conserving
Regulator (the Product) will be free of defects in workmanship and/or material
for the following periods:
Two (2) years from date of shipment.
Should any failure to conform to this warranty appear within the applicable period,
Precision Medical, Inc. shall, upon written notication thereof and substantiation
that the goods have been stored, installed, maintained and operated in accordance
with Precision Medical, Inc.’s instructions and standard industry practice, and that
no modications, substitutions, or alterations have been made to the goods, correct
such defect by suitable repair or replacement at its own expense.
ORAL STATEMENTS DO NOT CONSTITUTE WARRANTIES.
The representative of Precision Medical, Inc. or any retailers are not authorized
to make oral warranties about the merchandise described in this contract, and
any such statements shall not be relied upon and are not part of the contract for
sale. Thus, this writing is a nal, complete and exclusive statement of the terms
of that contract.
THIS WARRANTY IS EXCLUSIVE AND IS IN LIEU OF ANY WARRANTY OF
MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE OR OTHER
WARRANTY OF QUALITY, WHETHER EXPRESS OR IMPLIED.
Precision Medical, Inc. shall not under any circumstances be liable for special,
incidental or consequential damages including but not limited to lost prots, lost
sales, or injury to person or property. Correction of non-conformities as provided
above shall constitute fulllment of all liabilities of Precision Medical, Inc. whether
based on contract, negligence, strict tort or otherwise. Precision Medical, Inc.
reserves the right to discontinue manufacture of any product or change product
materials, designs, or specications without notice.
Precision Medical, Inc. reserves the right to correct clerical or typographical errors
without penalty.
14
DECLARATION OF CONFORMITY
Precision Medical, Inc.
300 Held Drive
Northampton, PA 18067, USA
Molenstraat 15
2513 BH, The Hague
The Netherlands
198705EN, 195405, 19DE05, 19ES05, 19ES05-EN,19GB05, 19SE05,
19SE05-ES, 198705NG, 195405NG, 19ES05NG, 19ES05NG-EN,
19GB05NG, 19SE05NG, 19SE05NG-NO, 19WM0501
EasyPulse 5+6 Oxygen Conserving Device Series;
198712EN, 195412EN, 19DE12, 19ES12, 19ES12-EN, 19GB12,
19GB12PC, 19SE12, 19SE12-ES, 198712NG, 195412NG,
19ES12NG, 19ES12NG-EN, 19GB12NG, 19SE12NG, 19SE12NG-NO
Classication: IIb
Classication criteria:
We hereby declare that an examination of the under mentioned production quality
assurance system has been carried out following the requirements of the UK
national legislation to which the undersigned is subjected, transposing Annex
II, 3 of the Directive 93/42/EEC and Directive 2007/47/EC on medical devices.
We certify that the production quality system conforms to the relevant provisions
of the aforementioned legislation, and the result entitles the organization to use
the CE 0473 marking on those products listed above.
Applied Standards: BS 341-3, DIN 477-1, EN 837-1, EN 1041, EN ISO 14971,
Notied Body:
Address: Davy Avenue Knowlhill Milton Keynes MK5 8NL, UK
Certication Registration No’s: 1126 CE Date of Expiry: 03 August 2017
Devices already manufactured: S/N traceability Device History Records
Validity of DOC: 04 August 2012 to Date of Expiry
Manufacture Representative: Quality Manager
Position: Quality Systems/ISO Representative
Date of Issue: 04 August 2012
Emergo Europe (European Ofce)
Phone: +31 (0) 70.345.8570 Fax: +31 (0) 70.346.7299
EasyPulse5 Oxygen Conserving Regulator
Clause 3.2 Rule 11 of Annex IX of MDD
EN ISO 15001, ISO 228-1, ISO 10524-1, ISO 10524-3,
ISO 15223-1, ISO 18779, SS 367615
AMTAC Certication Services Limited
Tell us how we are doing!
Visit us at www.precisionmedical.com
504429 Rev12 6/15 (?M) Printed in USA
 Loading...
Loading...