Page 1

LIFEPAK1000
®
OPERATING INSTRUCTIONS
DEFIBRILLATOR
Page 2

Page 3

LIFEPAK1000
®
OPERATING INSTRUCTIONS
DEFIBRILLATOR
Page 4

Important
!USA
!USA
LIFEPAK, LIFENET, and QUIK-COMBO are registered trademarks of Physi o-Control, Inc. ADAPTIV, CODE-STAT, cprMAX, REDI-PAK, and Shock
Advisory System are trademarks of Physio-Control, Inc. Microsoft and Windows are registered trademarks of Microsoft Corporation. Ambu is a
registered trademark of the Ambu Corporation. Specifications are subject to change without notice.
©2002–2008 Physio-Control, Inc. All rights reserved.
Publication Date: 10/2008
MIN 3205213-005
This instrument is to be used by authorized personnel only.
Rx Only
Device Tracking
The U.S. Food and Drug Administration requires defibrillator manufacturers and distribut ors to track the location of
their defibrillators. If the device is located somewhere other than the shipping address or the device has been sold,
donated, lost, stolen, exported, destroyed, permanently retired from use, or if the device was not obtained directly
from Physio-Control, please do one of the following: register the device at http://www.physio-control.com, call the
device tracking coordinator at 1.800.426.4448, or use one of the postage-paid address change cards located in the
back of this manual to update this vital tracking information
Responsibility for Information
It is the responsibility of our customers to ensure that the appropriate person(s) within their organization have
access t o this information, including cautions and warnings provided throughout this manual.
Page 5

TABLE OF CONTENTS 1
Preface
About Defibrillation ................................................................................................................................................. vi
Indications for Use ................................................................................................................................................... vi
Defibrillation..................................................................................................................................... .................. vi
ECG Monitoring................................................................................................................................................. vi
Operator Considerations ......................................................................................................... ............................ vii
About the LIFEPAK 1000 Defibrillator ............................................................................................................. vii
Defibrillator Features.................................................................................................................................... vii
Text Conventions ................................................................................................................................................... viii
1 Safety
Terms ......................................................................................................................................................................... 1-2
General Warnings and Cautions ........................................................................................................................1-2
Symbols ......................................................................................................................................................................1-3
2 Controls and Indicators
Controls and Indicators ........................................................... ........................................................................... 2-2
3 How to Use the LIFEPAK 1000 Defibrillator
Modes of Operation ........................................ .....................................................................................................3-2
Defibrillation Warnings and Cautions.....................................................................................................3-2
Defibrillation in AED Mode ..................................................................................................................................3-3
Basic Steps for Using the LIFEPAK 1000 Defibrillator......................................................................3-3
Special Situations for Electrode Placement........................................................................................3-4
Defibrillation in Manual Mode ............................................................................................................................3-5
Analysis..............................................................................................................................................................3-6
Troubleshooting Tips for Defibrillation .........................................................................................................3-6
LIFEPAK 1000 Defibrillator Operating Instructions iii
©2008 Physio-Control, Inc.
Page 6

ECG Monitoring (ECG Mode) .............................................................................................................................3-8
Troubleshooting Tips for ECG Monitoring............................................................................................3-9
4 Data Management
Managing Defibrillator Data ................................................................. .............................................................4 -2
Overview of Data Storage........................................................................................................................4-2
Data Stored by the LIFEPAK 1000 Defibrillator.................................................................................4-2
Overview of Connections for Transmitting Reports.......................................................................4-3
5 Caring for the LIFEPAK 1000 Defibrillator
Maintenance and Testing Schedule ................................................................................................................5-2
Self-Test Performance ............................................. ..........................................................................................5-2
Self-Tests..........................................................................................................................................................5-2
Auto Tests..................................................................................... ...................................................................5-2
Inspection ..................................................................................................................................................................5-3
Cleaning .....................................................................................................................................................................5-4
Battery Maintenance ............................................................................................................................................5-4
Electrode Storage ..................................................................................................................................................5-5
Service ......................................................................................................................................................................5-6
Product Recycling Information ................................................................. ........................................................5-6
Supplies, Accessories, and Training Tools ....................................................................................................5-7
Warranty Information .......................................... ..................................... ...........................................................5-7
A Specifications
B Shock Advisory System
C cprMAX™ Technology
D Changing Setup Options
E User’s Checklist
Index
iv LIFEPAK 1000 Defibrillator Operating Instructions
Page 7

PREFACE 1
This section provides information about defibrillation and an overview of the LIFEPAK® 1000 defibrillator.
About Defibrillation page vi
Indic ations for Use vi
Operator Considerat ions vii
About the LIFEPAK 1000 Defibrillator vii
Text Conventions viii
LIFEPAK 1000 Defibrillator Operating Instructions v
©2008 Physio-Control, Inc.
Page 8

Preface
ABOUT DEFIBRILLATION
Defibrillation is a recognized means of terminating certain potentially fatal arrhythmia s. A d irect current
defibrillator applies a brief, high-energy pulse o f electricity to the heart muscle. The Physio-Control
LIFEPAK
disposable defibrillation electrod es applied to the patient's chest.
Defibrillation is only one a s pect of the medical care required to resuscitate a p atient with a shockable ECG
rhythm. Depending on the situation, other measures may include:
• Cardiopulmonary r esuscitation (CPR)
• Supplemental oxygen
•Drug therapy
It is recognized that successful resuscitation is relat ed to the length of time be tween the onset of a heart
rhythm tha t does not circulate blood (ventricula r fibrillation, pulseless ventricular tachycardia) and
defibrillation. The American Heart Association has identified the following as critical links in the chain of
survival from sudden cardiac arre st (SCA).
•Early access
• Early CPR by first responders or bystanders
®
1000 defibrillator is an automated external defibrillator (AED) that delivers this energy through
• Early defibrillation
• Early advanced life support
The physiological s tate of the patient may affect the likelihood o f successful defibrillation. Thus, failure to
resuscitate a patient is not a reliable indicator of defibrillator performance. Often, patients will exhibit a
muscular re sp ons e (such as jumpin g or tw itchin g) durin g en ergy transfer. The absence of such a response
is not a reliable indicator of actual energy delivered or defibrillator performance.
INDICATIONS FOR USE
Defibrillation
Defibrillation is a recognize d means of terminating certain potentially fat al a rrhythmias, such as
ventricular fibrillation and symptomatic ventricular tachycardia.
The defibrillator is to be used in AED mode only on patients who are in cardiopulmonary arrest. The patient
must be unresponsive, not breathing normally, and showing no signs of circulation.
The defibrillator may be used with standard defibrillation pads only on adults and children who are 8 years
old or more or who weigh more than 25 kg (55 lbs). The defibrillator may be used on children who are less
than 8 years old or weigh less than 25 kg (55 lbs) with Infant/Child Reduced Energy Defibrillation
Electrodes.
ECG Monitoring
ECG monitoring is for use on conscious and unconsci ous patients of all ages for the purpose of ECG
rhythm recognition and heart rate monitoring.
vi LIFEPAK 1000 Defibrillator Operating Instructions
Page 9

Preface
OPERATOR CONSIDERATIONS
The LIFEPAK 1000 defibrillator requires operator interaction to defibrillate the patient.
The defibrillator is intended for use by personnel who are authorized by a physician or medical director
and have, at a minimum, the following skills and training.
•CPR training
• Defibrillator training equivalent to that recommended by the American Heart Association
• Training in the use of the LIFEPAK 1000 defibrillator
The LIFEPAK 1000 defibrillator is intended for use in hospital and out-of-h ospital environments.
Manual mode is intende d f or us e by personnel trained in ECG recognition who w ant t o us e the d efibrilla t or
to deliver a shock independent of AED mode. The operator has control over the charging and delivery of
shocks.
ECG mode provides a nondiagnostic ECG display and is intended for use by personnel trained in ECG
recognition to allow for rhythm and heart rate monitoring using standard ECG electrodes. When in ECG
mode, the defibrillator’s shock capability is disabled; however, the LIFEPAK 1000 defibrillator continues
to analyze the patient’s ECG for a potentially shockable rhythm.
ABOUT THE LIFEPAK 1000 DEFIBRILLATOR
The LIFEPAK 1000 defibrillator is a se miautomatic model that can be operated in either of three mod es:
AED mode, Manual mode , and ECG mode. The defibrillator uses the patented Physio-Control Shock
Advisory System™ (SAS) to analyze the patient's electrocardiographic (ECG) rhythm and prompts you
when it detects a shocka bl e rhythm and when it does not detect a shockable rhythm. Responder
interaction is required to provide therapy (defibrillation) to the patient.
Defibrillator Features
The following paragraphs introduce the LIFEPAK 1000 defibrillator features.
Heart Rhythm Analysis
The patented Physio-Control Shock Advisory System evaluates the patient’s heart rhythm.
ECG Display (optional)
This feature allows display of the ECG us ing the 3-wire (Lead II) cable and when using the defibrillator in
AED mode. This feature is also n ecessary to use the defibrillator in Manual mode.
Defibrillation Waveform
The defibrillation shock, using ADAPTIV™ Biphasic technology, is delivered in the form of a biphasic
truncated exponential (BTE) defibrillation waveform. LIFEPAK bip hasic d efibrillators me asure the patient’s
transthoracic impedance and automatically adjust the defibrillation waveform current, duration, and
voltage to meet the needs of the individual patient. Patient impedance is measured whenever
defibrillation electrodes are in contact with the patient.
LIFEPAK 1000 Defibrillator Operating Instructions vii
©2008 Physio-Control, Inc.
Page 10

Preface
cprMAX™ Technology
The cprMAX technology is designed to allow resuscitation protocols to maximize the amount of CPR
administered during treatment using the LIFEPAK 1000 defibrillator.
When used with the factory default settings enabled, the defibrillator allows AED protocols to be
consistent w i th the 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and
Emergency Cardiovascular Care and European Resuscitation Council Guidelines for Resuscitation.
Data Management
The LIFEPAK 1000 defibrillator digitally records patient data, including ECG rhythm and d e livered shocks.
Recorded data may be transferred from the defibrillator to a PC using an infrared connection. The PC
®
must have one of our LIFENET
Battery Options
products installed to collect and review the r e corded p atient data.
A nonrechargeable lithium manganese dioxide (Li/MnO2) battery provides power to the defibrillator. The
battery has indicators that a pproximate the remaining state of charge. To save battery life if the
defibrillator is accidentally turned on or left on, the defibrillator automatically turns off if it is not
connected to a patient and no buttons are pressed for 5 minutes.
Daily Self-Test
The defibrillator performs a daily self-test e v er y 24 hours an d every time you turn on the defibrillat or. This
feature tests the most important circuitry in the defibrillator to give the responder a high degree of
confidence that it is ready for use.
Readiness Display
The LIFEPAK 1000 defibrillator includes a readiness display. The OK symbol appears in the display if the
daily self-test is completed successfully. A battery symbol that approximates the remaining state of
charge is also visible. If the self-test detects that service is required, the
OK symbol disappears and the
service symbol appears.
TEXT CONVENTIONS
Throughout this manual, special text characters are used to indicat e labels, screen messages, and voice
prompts.
Operating contr ol labels:
Screen messages, and voice
prompts:
CAPITAL LETTERS such as ON/OFF and SHOCK.
CAPITAL ITALICIZED LETTERS such as PUSH ANALYZE and
CONNECT ELECTRODES
.
viii LIFEPAK 1000 Defibrillator Operating Instructions
Page 11
SAFETY 1
This section provides important information to help you operate the LIFEPAK 1000 defibrillator.
Familiarize yourself with all of these terms, warnings, and symbols.
Terms page 1-2
General Warnings and Cautions 1-2
Symbols 1-3
LIFEPAK 1000 Defibrillator Operating Instructions 1-1
©2008 Physio-Control, Inc.
Page 12

Safety
TERMS
The following terms are used either in this manual or on the LIFEPAK 1000 defibrillator.
Danger: Immediate hazards that will result in serious personal injury or dea th.
Warning: Hazards or unsafe practices that could result in serious personal injury or de ath.
Caution: Hazards or unsafe practices that could result in minor personal injury, product damage, or
property damage.
GENERAL WARNINGS AND CAUTIONS
The following section provides g eneral warning and caution statements. Other specific w arnings and
cautions are provided as needed in other sections of this m anual.
WARNINGS!
Shock hazard.
The defibrillator delivers up to 360 joules of electrical energy. Unless properly used as describ ed in these
operating instructions, this electrical energy may cause serious injury or death. Do not attempt to
operate this device unless thoroughly familiar with these operating instructions, and the function of all
controls, indicators, connections, and accessories.
Shock hazard.
Do not disassemble the defibrillator. It contains no responder-serviceable components and dangerous
high voltages may be present. Contact authorized service personnel.
Shock or fire hazard.
Do not immerse any portion of this device in water or other fluids. Avoid spilling any fluids on device or
accessories. Do not clean with ketones or other flammable agents. Do not autoclave or sterilize this
device or accessories unless otherwise specified.
Possible fire or explosion.
Do not use this device in the presence of flammable gases or anesthetics. Use care when operating this
device close to oxygen sources (such as bag-valve-mask devices or ventilator tubing). Turn off gas
source or move source away from patient during defibrillation.
Possible electrical interference with device performance.
Equipment operating in close proximity may emit strong electromagnetic or radio frequency
interference (RFI) which could affect the performance of this device. RFI may result in improper device
operation, distorted ECG, or failure to detect a shockable rhythm. Avoid operating the device nea r
cauterizers, diathermy equipment, cellular phones, or other portable and mobile RF communications
equipment. Do not rapidly key EMS radios on and off. Refer to “LIFEPAK 1000 De fibrillat or Electr omagne tic
Compliance Guidance” for recommended distances of equipment. Contact authorized service personnel
if assistance is required.
Possible electrical interference.
Using cables, electrodes, or accessories not specified for use with this device may result in increased
emissions or decreased resistance to electromagnetic interference which could affect the performance
of this device or of equipment in close proximity. Use only parts and accessories specified in these
operating instructions.
Possible electrical interference.
This defibrilla tor may cause electromagnetic interference (EMI) especially during charge and energy
transfers. EMI may affect the perf orm anc e of equipment operatin g in clos e proximity. Verify the effects
of defibrillator discharge on other equipment prior to using defibrillator in an emergency situation, if
possible.
1-2 LIFEPAK 1000 Defibrillator Operating Instructions
Page 13
Safety
WARNINGS ! (CONTINUED)
Possible device shutdown.
Always have access to a spare, fully-charged, properly maintained battery. Replace the batt er y when the
device display s a low battery warning.
Possible improper device performance.
Using other manufacturers’ cables, electrodes, or batteries may cause the device to perform improperly
and invalidates the safe ty agency certification and may invalidate the warranty. Use only the acc essories
specified in these operating instructions.
Safety risk and possible equipment damage.
Monitors, defibrillators, and their accessories (including ele ctrodes and cables) contain ferromagnetic
materials. As with all ferromagnetic equipment, these products must no t be used in the presence of the
high magnetic field created by a Magnetic Resonance Imaging (MRI) device. The high magnetic field
created by an MRI device will attract the equipment with a force sufficient to cause death or serious
personal injury to persons be tw een the equipment an d the MRI de vice. This ma gnetic a ttr a ction may als o
damage the equipment. Skin burns will also occur due to heating of electrically conductive materials,
such as patient leads and pulse oximeter sensors. Consult the MRI manufacturer for more information.
CAUTION!
Possible equipment damage.
This device may be damaged by mechanical or physical abuse such as immersion in wat er or dropping the
device. If the device has been abused, remove it from use and contact authorized service personnel.
SYMBOLS
The following symbols may be found in this manual or on various configurations of the LIFEPAK 1000
defibrillator and its accessories.
Defibrillation-protected. Type BF patient connection
Attention. Consult accompanying documents
Warning. High voltage
Type BF patient connection
Menu button
Battery status symbol
Service symbol
LIFEPAK 1000 Defibrillator Operating Instructions 1-3
©2008 Physio-Control, Inc.
Page 14
Safety
OK
!USA
YYYY
Symbol indicating self-test completed successfully
Use By date shown: yyyy-mm-dd or yyyy-mm
This end up
Fragile/breakable.
Handle with care.
Protect from water
Single use only
Mark of conformity to applicable European Directives
Canadian Standards Association certification for Canada and the United States
Cable Connector
For USA audiences only
Date of manufacture
Power On/Off
Shock button
Shock symbol
Symbol indicating location of battery compartment
Recommended storage temperature: 15° to 35°C (59° to 95°F). Storage at
extreme temperatures of -30° and 60°C (-22° and 140°F) is limited to seven
days. If storage at these temperatures exceeds one week, the electrode
shelf-life will be reduced.
1-4 LIFEPAK 1000 Defibrillator Operating Instructions
Page 15
Safety
LOT
YYWW
PN
MIN
,
CAT
REF
SN
Recommended shipping temperature: -20° to 50°C (-4° to 122°F).
Relative humidity range 5% to 95%
Do not place near an open flame
Do not crush, puncture, or disassemble battery
Nonrechargeable battery
Refer to instructions for disposal procedure
Rx Only or Rx Only
IP55
Do not dispose of this product in the unsorted municipal waste stream. Dispose
of this product according to local regulations. See
http://recycling.medtronic.com for instructions on disposing of this product.
Infant Child Reduced Energy Elec trodes are not compatibl e with QUIK-C OMBO
defibrillation and therapy cables. To use Infant/Child Electrodes, connect
Infant/Child electrodes directly to the AED.
Lot number (batch code). YY (year) and WW (week) of manufacture.
Manufacturer’s item number
Catalog number
Reorder number
Serial number
By prescription only
Enclosure ingress protection code per IEC 60529
LIFEPAK 1000 Defibrillator Operating Instructions 1-5
©2008 Physio-Control, Inc.
Page 16

Page 17
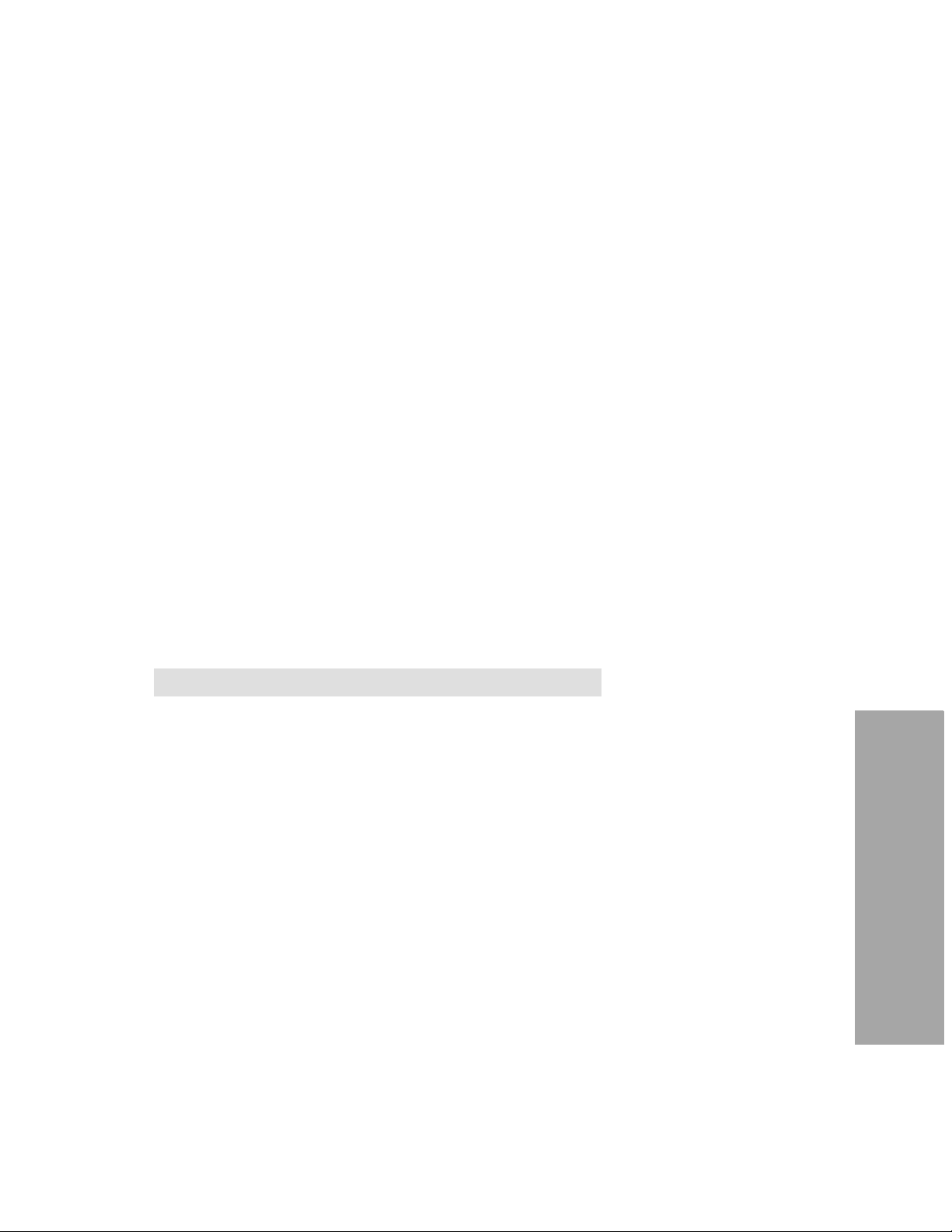
CONTROLS AND INDICATORS 2
This section provides a description of each of the LIFEPAK 1000 defibrillator primary controls and
indications.
Controls and Indicators page 2-2
LIFEPAK 1000 Defibrillator Operating Instructions 2-1
©2008 Physio-Control, Inc.
Page 18
Controls and Indicators
(Left sid e)
1
3
4
6
98
10
2
5
7
(Left sid e)
9
ON
CONTRO LS AND INDICATORS
This section introduces you to the controls and indicators on the LIFEPAK 1000 defibrillator.
Figure2-1 Controls and Indicator
Table 2-1 Controls and Indicators
Feature Description
1 Readiness displa y The re adiness display alerts you to the defibrillat or’s readiness status.
Three symbols ( , , ) allow you to determine whether the
defibrillator is ready for use or needs attention.
The following defines what each symbol represents and when/where
each appears.
The wrench indicator appears on the readiness display when a
condition exists that prevents or could prevent normal defibrillator
operation.
The OK symbol indicates that the defibrillator is ready for use.
This symbol is visible only when the defibrillator is off.
The battery symbol appears on the readiness display when the
defibrilla t or is o ff. When on e bar i s visible in the symbol, the b a tt er y is
low. If the symbol is blank, the battery is extremely low and the OK
symbol will not appear when the defibrillat or is off.
2 Speaker Provides audio voice prompts and tones.
3
Green
ON/OFF button turns the power on or off. The button is lit
whenever the defibrillator is on.
ON/OFF button
2-2 LIFEPAK 1000 Defibrillator Operating Instructions
Page 19

Controls and Indicators
Table 2-1 Controls and Indicators (Continued)
Feature Description
4
Pressing the SHOCK button (when flashing) delivers a shock to the
patient.
SHOCK button
5
Used to select operating modes (Manual or AED) and enter
information in Setup mode.
MENU button
6 Battery compartment Accommodates a single battery pak.
7
T wo softkeys work in c onjunc tion w ith the screen, providin g a way for
you to make selections while using the defibrillator.
Softkeys
The softkey functions vary, depending on the task you are
performing at the time. Their function is identified by the lab el above
them on the screen.
8 IrDA port Infrared Data Association. This port provides wireless
communications for transferring data from the defibrillator to a PC.
9 Screen Displays pertinent information for use during all modes of operation.
Figure 2-2 defines the information displayed on the screen.
10 Cable receptacle Allows direct connection to therapy electrodes (black), ECG cable
(green), Infant/Child electrod es (pink), and QUIK-COMBO™ therapy
electrodes (gr ay).
LIFEPAK 1000 Defibrillator Operating Instructions 2-3
©2008 Physio-Control, Inc.
Page 20
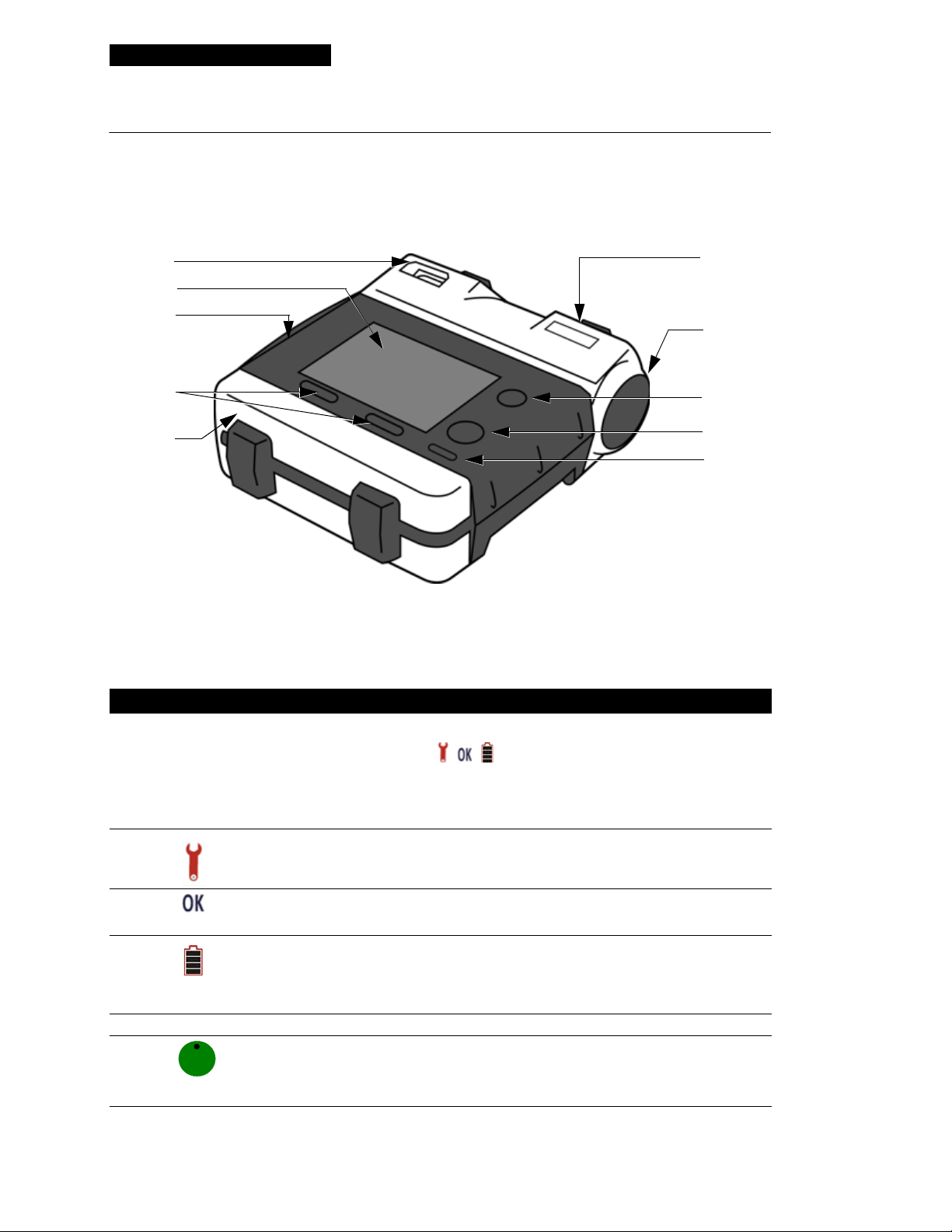
Controls and Indicators
Prompts
Heart rate
indicator
Message area
ECG
Softkey label
Battery status
Shock indicator
Elapsed time
Softkeys
YES NO
Enter Manual Mode?
Menu button
symbol
Figure 2-2 Defibrillator Screen
Heart rate indicator. The heart rate indicator displays hear t rates between 20 – 300 bpm. Indicator is
present in Manual mode or when the 3-wire ECG cable is used.
Battery status symbol. When the defibrillator is turned on, this symbol appears on the screen indicating
the relative level of charge . On e b ar indicates the batt ery is low. When the b attery is very low, the symbol
is blank and a
REPLACE BATTERY message appears on the screen.
ECG. The ECG appearing on the screen is a nondiagnostic ECG, obtained by means of the therapy
electrode s or the Lead II ECG ca ble. The presence of an ECG does not ensure that the patient has a pulse.
Softkey labels. These labels define the function that can be activated by pressing the softkey.
and
DISARM are function examples.
ANALYZE
2-4 LIFEPAK 1000 Defibrillator Operating Instructions
Page 21

HOW TO USE THE LIFEPAK 1000 DEFIBRILLATOR 3
This section provides an overview of information and instructions for using the LIFEPAK 1000 defibrillator.
Modes of Operation page 3-2
Defibrillation in AED Mode 3-3
Defibrillation in Manual Mode 3-5
Troubleshooting Tips for Defibrillation 3-6
ECG Monitoring (ECG Mode) 3-8
LIFEPAK 1000 Defibrillator Operating Instructions 3-1
©2008 Physio-Control, Inc.
Page 22

How to Use the LIFEPAK 1000 Defibrillator
MODES OF OPERATION
You can use the LIFEPAK 1000 defibrillator for:
• Automated external defibrillation (AED mode)
• Manual defibrillation therapy (Manual mode) (Requires ECG display option)
• ECG monitoring (ECG mode) (Requires ECG display option)
Defibrillation Warnings and Cautions
WARNINGS!
Shock hazard.
The defibrillator delivers up to 360 J of electrical energy. When discharging the de fibrillator, do not touch
the disposable therapy electrodes.
Shock hazard.
If a person is touching the patient, bed, or any conductive material in contact with the patient during
defibrillation, the delivered energy may b e partially discharge d through that person. Clear everyone away
from contact with the patient, bed, and other conductive material before discharging the defibrillator.
Possible skin burns.
During defibrillation, air pockets between the skin and therapy electrodes may cause patient skin burns.
Apply therapy electrodes so that entire electrode adheres to skin. Do no t reposition the electrodes once
applied. If the position must be changed, remove and replace with new electrodes.
Possible skin burns and ineffective energy delivery.
Therapy electrodes that are dried out or damaged may cause electrical arcing and patient skin burns
during defibrillation. Do not use therapy electrodes that have been removed from foil package for more
than 24 hours. Do not use electrodes beyond expiration date. Check that elec trode adhesive is intact and
undamaged. Replace ther apy electrodes after 50 shocks.
Possible interference with implanted electrical device.
De fibrillation m ay cause implanted de vic es t o m alfunction. Pla ce therapy electrode s away from implante d
devices if possible. Check implanted device function after defibrillation, if possible.
Possible misinterpretation of data.
Do no t analyze in a mo vin g v ehicle. Mo tion ar tifac t ma y a ff ect the ECG signal resulting in an inappropria t e
shock or no shock advised m e ssa ge. Motion detect ion m ay delay analysis. Stop vehicle and stand clear of
patient during analysis.
Possible misinterpretation of data.
Do not move the AED during analysis. Moving the AED during analysis may aff ec t the ECG signal resultin g
in an inappropriate shock or no shock advised decision. Do not touch the patient or the AED during
analysis.
CAUTION!
Possible equipment damage.
Before using this defibrillator, disconnect all equipment that is not defibrillator-protected from the
patient.
3-2 LIFEPAK 1000 Defibrillator Operating Instructions
Page 23
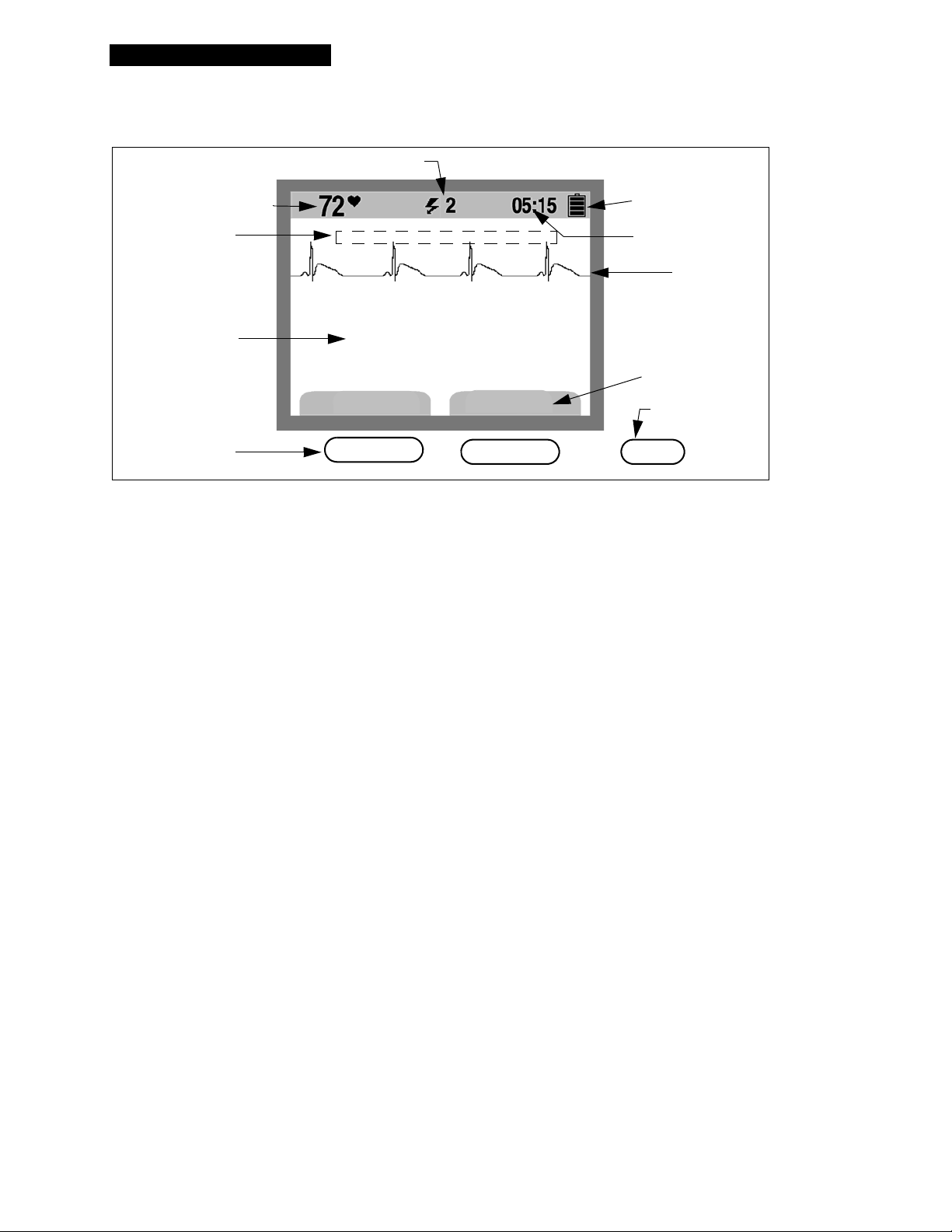
How to Use the LIFEPAK 1000 Defibrillator
Lateral
Anterior
WARNING!
Excessive Energy Delivery.
For children less than 8 years of age or 55 lbs (25 kg), use Infant/Child
Reduced Energy Defibrillation electrodes. Do not use Pediatric
QUIK-COMBO electrodes; these electrodes do not attenuate the energy
delivery by this defibrillator.
DEFIBRILLATION IN AED MODE
The LIFEPAK 1000 defibrillator uses the patented Physio-Control Shock Advisory System to evaluate the
patient’s heart rhythm. The LIFEPAK 1000 defibrillator has an optional feature that displays the ECG
wa veform and Heart Rate Indicator in AED mode. The operation in AED mode remains the same whether
or not the defibrillator displays the ECG waveform. When
with all of the AED messages and prompts. When
ECG DISPLAY is set to OFF, the messages and prompts
ECG DISPLAY is set to ON, the ECG appears
fill the screen.
Basic Steps for Using the LIFEPAK 1000 Defibrillator
1 Establish that the patient is in cardiopulmonary arrest (the patient
must be unre sponsive, not breathing normally and sho w ing no signs of
circulation).
2 Press
ON/OFF to turn on the defibrillator (the green LED illuminates).
Voice prompts will sound, guiding you through the rescue process.
3 Prepare the patient for therapy electrode placement.
• If possible, place the patient on a hard surface away from standing
water.
• Remove clothing from the patient's upper torso.
• Remove excessive hair from the electrode sites. If shaving is
necessary, avoid cutting the skin.
• Clean the skin an d dry it briskly with a towel or gauze.
• Do not apply alcohol, tincture of benzoin, or antiperspirant to the
skin.
4 Apply the ther ap y electr ode s t o the pa tient's che st. St artin g fr om one
end, pre ss the electrodes firmly onto the patient's skin, as shown.
5 Connect the electrodes to the defibrillator (if they are not already
connected).
6 Follow the screen messages and voice prompts provided by the
defibrillator.
LIFEPAK 1000 Defibrillator Operating Instructions 3-3
©2008 Physio-Control, Inc.
Page 24
How to Use the LIFEPAK 1000 Defibrillator
The following descriptions of voice prompts and messages are based on the default settings for AED
mode. Changing the setup options may result in different AED behavior.
CONNECT ELECTRODES
STAND CLEAR, ANALYZING
NOW, STAND CLEAR
PREPARING TO SHOCK
STAND CLEAR, PUSH SH OCK
BUTTON
Voice prompt and message when a patient has not been connected to
the defibrillator.
Voice prompt and message when a patient is connected to the
defibrillator.
Do not touch or move the patient, or therapy cables, during analysis.
ECG analysis requires 6–9 seconds.
Message displayed if the defibrillator detects a shockable rhythm.
The defibrillator charges to the joule setting for that shock number.
A rising tone and a chargin g bar on the screen indicate that the
defibrillator is charging.
Voice prompt and message when charging is complete.
The (shock) button flashes.
Clear everyone away from the patient, bed, or any equipment
connected to the patient.
Press the (shock) button to discharge the defibrillator.
The energy le v el f o r shocks depends on the energy protocol setup o pti on
and the analysis decisi on after shocks.
If the (shock) button is not pressed within 15 seconds, the defibrillator
disarms the shock button, and the
DISARMING... message appears on the
screen.
ENERGY DELIVERED
START CPR
Message displayed after each shock.
A message and countdown timer (min:sec format) appears for the CPR
time.
NO SHOCK ADVISED
V oic e pr ompt an d m essa g e whe n the de fibrilla t or d e t ects a nonsh ock able
rhythm. The defibrillator will not charge, and a shock cannot be delivered.
When a
NO SH OCK ADVISED pr ompt f ollows a shock and CPR, the energy
level will not increase for the next shock.
Special Situations for Electrode Placement
When placing electrodes on the patient, be aware of special situations:
Obese Patients or Patients with Large Breasts
Apply the electrodes to a flat area on the chest, if possible. If skin folds or breast tissue prevent good
adhesion, spread skin folds apart to create a flat surface.
Thin Patients
Follow the contour of the ribs and spaces when pressing the electrodes onto the torso. This limits air
space or gaps under the electrodes and promotes good skin c ontact.
Patients with Implanted Pacemakers
If possible, place defibrillation electrodes away from the internal pacemaker generator. Treat this patient
like any other patient requiring emerg e ncy care.
3-4 LIFEPAK 1000 Defibrillator Operating Instructions
Page 25

How to Use the LIFEPAK 1000 Defibrillator
ANTERIOR
POSTERIOR
Anterior
Posterior
Patients with Implanted Defibrillators
Apply the electrodes in the anter ior-lateral position. Treat this patient like any other patient requiring
emergency care.
Alternate Anterior-Posterior Electrode Position
The electrodes may be placed in an anterior-poste rior position as follows:
1 Place either the ♥ or + therapy electrode over the left precordium as shown in Figure 3-1. The upper
edg e of the electrode should be below the nipple. Avoid placement over the nipple, the diaphragm, or
the bony prominence of the sternum if possible.
2 Place the other electrode behind the heart in the infrascapular a rea as shown in Figure3-1. F or patient
comfort, place the cable connection away from the spine. Do not place the electrode over the bony
prominences of the spine or scapula.
Figure3-1 Anterior-Posterior Placement
DEFIBRILLATION IN MANUAL MODE
The LIFEPAK 1000 defibrillator provides a Manual mode to override th e AED features of the defibrillator.
Manual mode provides operator-initiated analysis, charge, shock, and disarm functions. This mode is
useful in a tier e d response system when a provider trained in manual defibrilla tion and auth orize d t o pla ce
the defibrillator in Manual mode takes over the scene from a BLS-AED trained provider.
To use Manual mode:
1 Press the Menu button.
2Select
3 If the displayed ECG rhythm appears shockable, press
4 Clear everyone away from the patient, bed, or any equipment connec ted to the patient.
5 When the charge is complete, press the flashing (shock) button to deliver energy to the patient.
6 After delivering a shock, the energy for each subsequent shock is automatically selected b as e d on the
Note: To remove an unwanted charge at any time, press
YES to enter Manual mode. The ECG trace and Heart Rate Indicator appear on the screen.
CHARGE to initiat e chargin g of the defibrillator.
The screen w ill indicate that the defibrillator is charging and a charge tone will sound.
energy level configured in Setup.
DISARM.
LIFEPAK 1000 Defibrillator Operating Instructions 3-5
©2008 Physio-Control, Inc.
Page 26

How to Use the LIFEPAK 1000 Defibrillator
Analysis
The LIFEPAK 1000 defibrillator can be set up to display an ANALYZE softkey when in Manual mode.
To initiate an analysis:
1 Confirm that the patient is unresponsive, not breathing, and without a pulse.
2 Press
ANALYZE.
3 If the rhythm analysis r e sul ts in a No Shock Advise d decision, the defibrillator remains in Manual mode
without further prompts.
4 If the rhythm analysis results in a Shock Advised decision, the defibrillator automatically begins
charging accompanied by a charge tone. If you determine tha t a sh ock is not warranted, press
DISARM.
5 When the charge is complete, clear everyone away from the patient, bed, or any equipment
connect ed to the patient.
6 Press the flashing (sho ck) button to deliver energy to the patient.
7 After delivering a shock, the defibrillator remains in Manual mode.
TROUBLESHOOTING TIPS FOR DEFIBRILLATION
This section ex pla ins problem conditions that you may encounter while using the defibrillator.
Table 3-1 Troubleshooting Tips for Defibrillation
Observation Possible Cause What To Do
Screen blank and ON
LED lit.
CONNECT ELECTRODES
voice prompt is heard.
CHECK CONNECTOR
AND ELECTRODES
prompt is heard.
Screen not functioning properly. • AED and therapy functions may still
operate. If needed for therapy, follow
voice prompts and continue to use
device to tr eat patient. If unable to use
voice prompts for any reason,
administer CPR if the patient is not
resp on din g, not breathing n orm ally, an d
showing no signs of circulation.
• Contact authorized service personnel.
Poor electrode-to-skin contact.
• Firmly press electrodes on patient’s
skin.
• Clean, shave, and dry the patient’s skin
prior to placing pads on skin.
Electrode pads are dry, damaged,
• Replace the electrode pads.
or have passed the expiration
date.
Electrode pads are not removed
from the liner.
• Remove the electrode pads from the
liner and apply them to the patient’s
chest
Connection to the defibrillator is
voice
inadequate.
• Check to be sure that the electrode
connector is completely inserted.
3-6 LIFEPAK 1000 Defibrillator Operating Instructions
Page 27

How to Use the LIFEPAK 1000 Defibrillator
Table 3-1 Troubleshooting Tips for Defibrillation (Continued)
Observation Possible Cause What To Do
Defibrillator cannot
deliver the requi red
shock.
Defibrillator battery power is low.
• Administer CPR if the patient is not
resp on din g, not brea thin g normally, an d
showing no signs of circulation.
• Check battery indicator. Replace
battery if needed.
Voic e prompts sound
faint or distorted.
Defibrillator battery power is low.
• Administer CPR if the patient is not
resp on din g, not brea thin g normally, an d
showing no signs of circulation.
• Check battery indicator. Replace
battery if needed.
MOTION DETECTED and
STOP MOTION voice
Patient movement because of
location.
• Move patient to stable location, if
possible.
prompts are heard.
Patient movement because of
• Check patient for normal breathing.
breathing.
CPR being performed during
• Stop CPR during analysis.
analysis.
Vehicle mot ion.
Electrical/radio frequency
interference.
• Stop vehicle during analysis, if possible.
• Move communication or other
suspected devices away from the
defibrillator when possible.
De fibrillator does not
deliver voice prompts or
beeping tone s after you
turn it on.
Speaker not functioning. • AED and therapy functions may still
operate. If needed for therapy, follo w
screen prompts and continue to use
device to treat patient. If unable to use
screen prompts for any reaso n,
administer CPR if the patient is not
resp on din g, not brea thin g normally, an d
showing no signs of circulation.
• Contact authorized service personnel.
Depleted battery.
• Administer CPR if the patient is not
resp on din g, not brea thin g normally, an d
showing no signs of circulation.
• Check battery indicator. Replace
battery if needed.
• Contact authorized service personnel.
The readiness display
is blank.
The defibrillator has been turned
on.
Operating temperature is too
low.
LCD not operating properly.
• Normal condition when the defibrillat or
is in use.
• Operate the defibrillator within the
specified temperature range.
• Contact authorized service personnel.
LIFEPAK 1000 Defibrillator Operating Instructions 3-7
©2008 Physio-Control, Inc.
Page 28

How to Use the LIFEPAK 1000 Defibrillator
AHA Labels IEC Labels
RA Right Arm R Right
LA Left Arm L Left
LL Left Leg F Foot
LA/LRA/R
LL/F
ECG MONITORING (ECG MODE)
WARNING!
Possible misinterpretation of ECG data.
The frequency response of the screen is intended only for basic ECG rhythm identification; it does not
provide the resolution required for pacemaker pulse visibility, accurate measurements, such as QRS
duration, and ST segment interpretation. For such purposes, use ECG monitors with an appropriate
frequency response.
Possible delay in therapy.
Do not attempt to connect a 3-wire ECG cable to a QUIK-COMBO therapy cable or any other AED. The
ECG cable is functional only with the LIFEPAK 1000 defibrillator.
The LIFEPAK 1000 defibrillator pr ovides nondiagnostic ECG display of the patient’s heart rhythm when the
ECG cable is connected and the electrodes are appl ied.
Note: You do not have to turn the defibrillator off befo re changing from therapy electrodes to the
ECG cable or vice versa.
To monitor a patient’s ECG:
1 Connect the ECG cable.
Note: The ECG cable uses the same receptacle used by the therapy electrodes.
2 Apply ECG electrodes to the patient’s chest as shown in Figure 3-2
Figure 3-2 Connecting the ECG Electrodes for ECG monitoring
After the ECG electrodes are connected, the defibrillator displays the patient’s heart rhythm and heart
rate in a lead II configuration. Lead II is the only lead available with this cable.
While in ECG mode, the defibrillator’s sh ock capability is disabled; however, the defibrillator continues to
evaluate the patient’s ECG for a potentially shockable rhythm. Remember that the presence of an ECG
rhythm does not ensure that the patient has a pulse.
If a shockable rhythm is detected, the defibrillator prompts
CONNECT THERAPY ELECTRODES.
1 Confirm the patient’s condition: Not responsive? Not breathing? No signs of circulation?
2 Remove the ECG cable and connect the therapy electrodes to the defibrillator.
3 Apply the therapy electrodes to the patient’s chest, keeping them at least 2.5 cm (one inch) away
from the ECG electrodes. If necessary, remove the ECG electrodes.
4 Follow the defibrillator’s voice and screen prompts.
3-8 LIFEPAK 1000 Defibrillator Operating Instructions
Page 29

How to Use the LIFEPAK 1000 Defibrillator
Troubleshooting Tips for ECG Monitoring
If problems occur while monitoring the ECG, check this list of observations for troubleshooting assistance.
Table 3-2 Troubleshooting Tips for ECG Monitoring
Observation Possible Cause What to Do
Screen blank and ON LED lit. Screen not functioning properly. • Contact authorized service
personnel.
• AED and ther ap y functions m a y still
operate. If needed for therapy,
continue to use device to treat
patient.
CONNECT ECG LEADS
message appears
Poor ECG signal quality. P oor electrode-to-skin contact. • Reposition cable and/or lead wires
Baseline wander
(low frequency/high
amplitude artifact).
One or more ECG electrodes are
disconnected.
• Confirm ECG electrod e
connections.
P oor electrode-to-skin contact. • Reposition cable and/or lead wires
to prevent electrodes from pulling
away from patient.
• Clean, shave, and dry the patient’s
skin as recommended on page 3-3.
•Replace electrodes.
• Change cable.
Broken ECG cable lead wire. • Check ECG cable continuity. If lead
wire is broken, replace ECG cable.
to prevent electrodes from pulling
away from patient. Secure cable
clasp to patient’s clothing.
• Clean, shave, and dry the patient’s
skin as recommended on page 3-3.
• Replace electrode(s).
Outdat ed, corroded, or dried-out
electrodes.
• Check date codes on electrode
packages.
• Use only silver/silver chloride
electrodes with Use By dates that
have not passed.
• Leave electrodes in sealed packet
until time of use.
Loose connection. • Check/reconnect cable
connections.
Dam ag e d c able or connect or/le ad
wire.
• Inspect ECG and therapy cables.
•Replace if damaged.
• Check cable with simulator and
replace if malfunction observed.
Noise because of radio frequency
interference (RFI).
• Check for equipment causing RFI
(such as a radio transmitter) and
relocate or turn off equipment
power.
Inadequate skin preparation.
Poor electrode-to-skin contact.
• Clean, shave, and dry the patient’s
skin as recommended on page 3-3.
•Replace electrodes.
LIFEPAK 1000 Defibrillator Operating Instructions 3-9
©2008 Physio-Control, Inc.
Page 30

How to Use the LIFEPAK 1000 Defibrillator
Table 3-2 Troubleshooting Tips for ECG Monitoring (Continued)
Observation Possible Cause What to Do
Fine baseline artifact (high
frequency/low amplitu de).
Inadequate skin preparation.
Isometric muscle tension in arms
or legs.
• Clean, shave, and dry the patient’s
skin as recommended on page 3-3.
• Replace electrodes.
• Confirm that limbs are resting on a
supportive surface.
• Check electrodes f or proper
adhesion.
3-10 LIFEPAK 1000 Defibrillator Operating Instructions
Page 31

DATA MANAGEMENT 4
This section introduces data management for the LIFEPAK 1000 defibrillator.
Managing Defibrillator Data page 4-2
LIFEPAK 1000 Defibrillator Operating Instructions 4-1
©2008 Physio-Control, Inc.
Page 32

Data Management
0
0
MANAGING DEFIBRILLATOR DATA
The LIFEPAK 1000 defibrillator provides an infrared method to transfer defibrillator data.
Overview of Data Storage
Every time you use the defibrillator, it digitally saves patient data that can be transferred to a PC. You can
provide patient data to aid in case review for quality control, traini ng, and research purposes. You should
become familiar with local requirements for reporting a use of the LIFEPAK 1000 defibrillator and for
providing use data. For assistance in retrieving data from the defibrillator, contact your local
Physio-Control sales representative or authorized service personnel.
Data Stored by the LIFEPAK 1000 Defibrillator
Whenever you turn on the defibrillator and connect it to a patient, it automatically stores data about the
patient. When this data is transferred to a data management system for review (for example,
CODE-STAT™ software), three patient reports are available: Event Log, Cont inuous ECG, and CODE
SUMMARY. Table 4-1 describes these reports.
Table 4-1 Patient Reports
Report Type Description
Event Log A chronological log of all events. An event is a condition noted by the defibrillator.
Event s are listed on page 4-3.
Continuous ECG Forty minutes of the patient’s ECG rhythm beginning when the patient is
connected to the defibrillator and ending when the defibrillator is turned off.
CODE SUMMARY Combine s the Event Log and a sampling of continuous ECG rhythms associated
with certain events, such as defibrillation.
The LIFEPAK 1000 defibrilla t or c an s t or e up t o two patient r ec or ds: one f or the cur r ent p a tient an d one for
the previous patient. When y ou us e the defibrillat or
, it is important t o transfer the pa tient data as soon as
possible af ter use. The Comple te Record for the current patient includes the Continuous ECG and Event
Log. If you treat a second patient, the first patient’s Continuous ECG is reformatted into a CODE
SUMMARY report. If you treat a third patient, all of the first patient’s data is deleted and the second
patient’s Continuous ECG is reform atted into a CODE SU MMARY Repo rt.
Table 4-2 Patient Records
Complete Record Summary Continuous ECG
Current Patient X X X
Previous Patient
X
If you turn the d efibrillator on and off without attaching electrodes to a patient, the defibrillator does not
create a new patient record and the patient records in the defibrillator are not altered.
The LIFEPAK 1000 defibrillator does not delete patient data afte r you transfer the data to a PC. The
defibrillator deletes previous patient data only when it is connected to a new patient or a simulator.
Test and Service Data
The LIFEPAK 1000 defibrillator stores a test log consisting of the most recent auto-tests, power cycles,
and battery re placements. The test log lists the test results and any errors detected. The test log data is
available only to authorized service personnel or to responders who are using the appropriate LIFENET
system product.
4-2 LIFEPAK 1000 Defibrillator Operating Instructions
Page 33

Data Management
Event and Test Log
Table 4-3 and Table 4-4 list the types of events that may be annotated on event and test log reports.
Table 4-3 Events
Events Events Events
Power On Shock X Abnormal Motion
Connect Electrode s No Shock Advised
Analysis Stopped
*
Patient Connected CPR Prompt Low Battery
AED Mode Stop CPR Prompt ECG Mode
Initial Rhythm
Analysis X
*
*
Check Patient
Charge Removed Out of Waveform Memory
*
Out of Event Memory
Shock Advised Manual Mode Power Off
Charge Complete Replace Bat tery
SHOCK X-XXXJ
*
These events include ECG samples in the Summary Report.
Table 4-4 Test Log Report
Test Log
*
Charg e Button Pressed
Reco very Time
*
Self Test Po wer On
Self Test Pass/Fa il
User Power On/Off
Battery Changed
Overview of Connections for Transmitting Reports
Patient, test, and service data can be transmitted from the LIFEPAK 1000 defibrillat or to a PC-compatible
computer equipped with CODE-STAT software, version 6.0 or later, a Physio-Control LIFENET system
product. LIFENET system products are compatible with Microsoft
Windows XP.
The LIFEPAK 1000 defibrillator (see Figure2-1) supports wireless, infrared communications for
transmitting data from the defibrillator to your computer. To receive the transmission, your computer
must have an operational IrDA port.
If your computer does not have an IrDA port, you can install an IrDA adapter to provide the needed
interface. Physio-Control recommends installing an IrDA adapter on all computers to ensure successful
communication connections and data transmissions.
IrDA adapters are available f or serial or USB computer por ts. Follow the inst allation and usag e ins truc tions
provided with the adapter, ensuring that the adapter mount (receiving end) is pos itioned on a stable
surface. Figure 4-1 provides guidelines to follow for positioning the defibrillator and the IrDA adapter
before initiating a transmission.
Note: The shaded cone in Figure4-1 represents the approximate parameters for positioning the
defibrillator’s IrDA port opposite the IrDA adapter. As the distance between the two increases, so
does the possible range for aligning them.
®
Windows 2000 Professional and
LIFEPAK 1000 Defibrillator Operating Instructions 4-3
©2008 Physio-Control, Inc.
Page 34

Data Management
IrDA
Adapter/
Compute
r
Distance: 1 meter (3.28 feet)
15°
Defibrillator
15°
Alignment Range: 30°
Figure4-1 IrDA Connections
You initiate and control transmission of device data at your computer using a LIFENET system product.
This includes initiating data download, selecting reports to be transmitted, and monitoring transmission
progress. More information about configuring your LIFENET system product and instructions for
transmi tting d e vic e da t a are provid ed i n the user s guide an d r eference c a r ds tha t a cc omp an y the LIFENE T
system product.
4-4 LIFEPAK 1000 Defibrillator Operating Instructions
Page 35

CARING FOR THE LIFEPAK 1000 DEFIBRILLATOR 5
This section expla ins how to help keep your LIFEPAK 1000 defibrillator in good working condition. Cared
for prop erly, the defibrillator is built to give you many years of service.
Maintenance and Testing Schedule page 5-2
Self-Test Performance 5-2
Inspection 5-3
Cleaning 5-4
Battery Maintenance 5-4
Electrode Storage 5-5
Service 5-6
Product Recycling Information 5-6
Supplies, Accessories, and Training Tools 5-7
Warranty Information 5-7
LIFEPAK 1000 Defibrillator Operating Instructions 5-1
©2008 Physio-Control, Inc.
Page 36

Caring for the LIFEPAK 1000 Defibrillator
MAINTENANCE AND TESTING SCHEDULE
Use the f ollowing schedule in conjunction with the internal quality assurance program of the hospital,
clinic, or emergency medical service where the defibrillator is used.
On a regular basis, you should do the following:
• Check the readiness display to determine the level of battery charge and that the OK symbol is visible.
• Check the Use By date on the therapy electrode packet.
• Check other emergency supplies that may be stored with the defibrillator.
If the OK symbol is not visible, the level of battery charge is low, or the electrode Use By date has passed,
the defibrillator needs attention. Replace the battery and the electrode packet, or call your authorized
service per sonnel.
When establishing your local inspection schedule, consider how often the defibrillator will be used and
how familiar the operators are with using a defibrilla tor. For example, if the defibrillator is used rarely,
weekly inspections are app ropriate. An inspection checklist is provided in Appendix E.
Table 5-1 Recommended Maintenance Schedule
Operation After Use As Required Weekly
Complete Operator’s Checklist (see Appendix E). X
Inspect defibrillator. X X
Clean defibrillator. X X
Check that all necessary supplies and accessories,
such as electrodes, are present.
XX
SELF-TEST PERFORMANCE
Whenever the LIFEPAK 1000 defibrillator is turn ed on after it has been off for at least 60 seconds, it takes
approximately 5seconds to complete a self-test and to indicate a low or replace battery condition.
Self-Tests
Each time you turn it on, the defibrillator performs internal self-tests to check that internal electrical
components and circuits work properly. The defibrillator stores the results of all user power on self-tests
in a test log. When the defibrillator is on and a problem requires immediate service, such as a
malfunctioning charging circuit, the defibrillator prompts
if needed for an emergency; otherwise, remove the defibrillator from active use and contact authorized
service personnel to correct the problem as soon as possible. The service symbol will remain visible until
the problem is corrected.
CALL SERVICE. Atte mpt to use the defibrillator
Auto Tests
The defibrillator performs automatic self-tests daily and monthly at 0300 (3:00 a.m.) if not in us e. During
the automatic self-test, the defibrillator turns itself on (ON/OFF LED illuminates) briefly and completes
the following tasks:
•Performs a self-test
• Stores the self-test results in the Test Log
• Turns itself off
5-2 LIFEPAK 1000 Defibrillator Operating Instructions
Page 37

Caring for the LIFEPAK 1000 Defibrillator
If the defibrillator detects a problem during an auto test that requires service, it displays the service
symbol. If the service symbol is visible, you should attempt to use the defibrillator, if needed, for a cardiac
emergency. However, you should contact authorize d s ervice pers onnel t o correct the problem as soon as
possible. The se rvice symbol will remain visible until the problem is corrected.
The automatic self-test is not performed if the defibrillator is already turned on at 0300 or if the battery
is not installed. If the defibrillator is turned on while a self-test is in progress, the test is halted; the
defibrillator will turn on normally.
INSPECTION
Routinely inspect all devices, accessories, and cables by following the ins tructions in Table 5-2.
Table 5-2 LIFEPAK 1000 Defibrillator Inspection
Instruction Inspect For Recommended Corrective Action
Examine the defibrillator
case, connector, battery
well, battery pins, and
accessories.
Foreign substances. Clean the device as described in
Table 5-3.
Damag e or cracks. Contact authorized service personn e l to
troubleshoot.
Battery pins bent or discolored. Contact authorized service personnel.
Expire d ba tt eries or d efibrilla tion
Replace.
electrodes.
Observe readiness display
OK symbol None needed.
Low or r epla ce ba tt ery in dica tion
Replace battery immediately.
displayed
Service symbol displayed Contact authorized service personnel.
Examine acces s ory cables. Foreign substances. Clean the cables as descr ibed in
Table 5-3.
Inspect for cracks, damag e,
Replace damaged or broken parts.
extreme wear, broken or bent
connectors and pins.
Confirm that conn ect ors engage
Replace damaged or broken parts.
securely.
LIFEPAK 1000 Defibrillator Operating Instructions 5-3
©2008 Physio-Control, Inc.
Page 38

Caring for the LIFEPAK 1000 Defibrillator
CLEANING
Clean the LIFEPAK 1000 defibrillator accessories as described in Table 5-3. Use only the cleaning agents
liste d in the table.
CAUTION!
Possible equipment damage.
Do not clean any part of the defibrillator or accessories with bleach, bleach dilution, or phenolic
compounds. Do not use abrasive or flammable cleaning agents. Do not steam, autoclave, or gas-sterilize
the defibrillator or accessories.
Table 5-3 Recommended Cleaning Methods
Items
Cleaning Practice Recommended Cleaning Agent
Defibrillator case, display,
crevices, and accessories
Clean with damp sponge or
cloth.
• Quaternary ammonium compounds
• Rubbing (isopropyl) alcohol
• Peroxide (peracetic acid) solutions
BATTERY MAINTENANCE
The LIFEPAK 1000 defibrillator is powered by the LIFEPAK 1000 nonrechargeable lithium manganese
dioxide batte ry pak.
Follow the guidel ines described in this section to help maximize battery life and performance. Use only
Physio-Control battery paks designed for use with the LIFEPAK 1000 defibrillator. Do not use any other
batteries.
WARNINGS!
Safety risk and possible equipment damage.
• Damaged batt eries may leak and cause personal injury or equipment damage. Handle damaged or
leaking batteries with extreme care.
• Do not c ar r y a b a tt er y pak where metal objects (such as ca r keys or paper clips) could short-circuit the
battery terminals. The resulting excessive current flow can cause extremely high temperatures and
may result in damage to the battery pak or cause fire or burns.
• Keep batteries away from children.
Possible defibrillator shutdown.
When the LIFEPAK 1000 defibrillator displays the REPLACE BATTERY message, replace the battery
immediately.
Possible loss of power during patient care.
Using an improperly maintained battery to power the defibrillator may cause power failure without
warning. Maintain batteries as described in these operating instructions.
Note: When a battery pak is removed from the defibrillator, battery and service symbols appear on
the readiness display. After replacing the battery pak, the device resets the readiness display.
The nonrechargeable battery pak never requires recharging. The approximate level of charge in the
battery appears on the readiness display when the defibrillator is off or on the screen when the
defibrillator is in use.
When optimally maintained, a new nonrechargeable battery pak can provide approximately 17 hours of
“on time” or 440 discharges at 200 joules. Just turning the defibrillator on (“on time”) uses up battery
5-4 LIFEPAK 1000 Defibrillator Operating Instructions
Page 39

Caring for the LIFEPAK 1000 Defibrillator
capacity. Each year, battery capacity decreases while the battery is in the defibrillator because of the
battery’s normal self-discharge rate and the energy used by the defibrillator auto tests. If installed in the
defibrilla tor and the defibrillator is not used, the battery pak has a standby life of five years.
A new nonrechargeable battery pak has a shelf life of five years if stored at the proper temperature. The
battery pak (s tored outside the defibrillator) self-discharges over time; therefore, when the battery is
eventually placed in the defibrillator, its useful life will be reduced depending on how long it “sat on the
shelf.”
To properly maintain nonrechargeable battery paks:
• Do not attempt to recharge.
• Do not expose to temperatures greater than those specified in Appendix A.
• Do not allow electrical connection between the battery contacts.
WARNING!
Possible explosion, fire, or noxious gas.
Attempting to recharge a nonrechargeable battery pak can cause an explosion or fire or release noxious
gas. Dispose of expired or depleted nonrechargeable battery paks as described in these operating
instructions.
CAUTION!
Possible battery damage.
Electrical connection between battery c ontacts can blow an internal fuse and permanently disable the
battery.
ELECTRODE STORAGE
For information about defibrillation electrode storage, refer to the electrode operat ing instructions.
LIFEPAK 1000 Defibrillator Operating Instructions 5-5
©2008 Physio-Control, Inc.
Page 40

Caring for the LIFEPAK 1000 Defibrillator
SERVICE
WARNING!
Shock hazard.
Do not disassemble the defibrillator. It contains no responder-serviceable components and dangerous
high voltages may be present. Contact authorized service personnel.
If the LIFEPAK 1000 defibrillator requires service as indicated by testing, troubleshooting, or the servi ce
symbol, contact authorized service personnel. In the USA, call
your local Physio-Control representative. When you call Physio-Control to request service, provide the
following information:
• Model number and part number
• Serial number
• Observation of the problem that led to the call
If the defibrillator must be shipped to a service center or to the factory, pack it in the original shipping
container. If this is no t possible, ship the defibrillator in protective packing to prevent shipping damage.
1.800.442.1142. Outside the USA, contact
PRODUCT RE CYCLING INFORMATION
All materials should be recycled according to national and local regulations. Contact your local
Physio-Control representative for assistance or refer to http://recycling.medtronic.com for instructions
on disposing of this product.
Preparing for Disposal of Nonrechargeable Batteries
Nonrechargeable batterie s should be fully discharged before disposal.
Before disposing of nonr echa rgeable batter y paks, cov er the b attery terminals with the plastic discha rger
cap provided with the new battery. Refer to the battery discharge instructions included with your new
battery.
Disposing of Nonrechargeable Batteries
Follow your national, regional, and local regulations for disposal. Contact a local Physio-Control
represent ative for more informat io n.
In the USA, Environmental Protection Agency and Department of Transportation re gulations allow
disposal of nonrechargeable batter ies with ordinary household waste provided that they are fully
discharged. Be sure to comply with any other loc al or regional regulations before disposal. For more
information or assistance, conta ct your local Physio-Control representative or call 1.800.442.1142.
Recycling the Defibrillator
Recycle the defibrillator at the end of its useful life. It should be clean and contaminant-free prior to
being recycled.
Recycling Disposable Electrodes
After disposable electrodes are used, follow your local clinical procedures for recycling.
Recycling Packaging
Pa ckaging should be recycled according to national and local regulations.
5-6 LIFEPAK 1000 Defibrillator Operating Instructions
Page 41

Caring for the LIFEPAK 1000 Defibrillator
SUPPLIES, ACCESSORIES, AND TRAINING TOOLS
Table 5-4 lists supplies, accessories, and training tools for the LIFEPAK 1000 defibrillator.
To order in the USA, call 1.800.442.1142. Outside the USA, contact your loc al Physio-Control
representative.
Table 5-4 Supplies, Accessories, and Training Tools
Item Description Catalog Number
QUIK-COMBO™ Electrodes with REDI-PAK™ preconnect system CAT. 11996-000017
Infant/Child Reduced Energy D efibrillation Electr od e s (not compatible w ith
QUIK-COMBO defibrillation cable)
Infant/Child Electrodes Star ter Kit (English, Dutch, French, German,
Spanish, Italian, Danish, Norwegian, Finnish, Swedish)
Infant/Child Electrodes Starter Kit (English, Hungarian, Polish, Brazilian
Portuguese, Iberian Portuguese, Spanish, Korean, Japanese, Mandarin
Chinese)
LIFEPAK 1000 nonrec hargeable lithium manganese dioxide battery pak CAT. 21300-006054
Carrying case CAT. 11260-000025
3-Wire Monitoring Cable CAT. 11111-000012
3-Wire Monitoring Cable (IEC) CAT. 11111-000013
Quick Reference Card CAT. 26500-002156
IrDA Adapter (attachment for a PC) CAT. 21300-0050 26
CODE-STAT Data Review Software CAT. 94404-000003
LIFENET DT Express Information Management System CAT. 21340-000095
CAT. 11101-000016
CAT. 11101-000017
CAT. 11101-000018
CAT. 21300-005027
WARRANTY INFORMATION
Refer to the product warranty statement included with your LIFEPAK 1000 defibrillator. For duplicate
copies, contact your local Physio-Control representative.
LIFEPAK 1000 Defibrillator Operating Instructions 5-7
©2008 Physio-Control, Inc.
Page 42

Page 43

APPENDIX A
SPECIFICATIONS A
LIFEPAK 1000 Defibrillator Operating Instructions
©2008 Physio-Control, Inc.
Page 44

Page 45

Specifications
R ated E ne r g y Outp u t
125
150
175
200
225
250
275
300
325
350
375
25 50 75 100 125 150 175
Patient Impeda nce (ohm s)
J
325 J *
300 J *
275 J *
Rated Energy Output
Energy (J)
Patient Impedance (Ω)
SPECIFICATIONS
All specificat ions a re at 20° C (68°F) unless otherwise stated.
Defibrillator
Waveform Biphasic Truncated Exponential with v o ltage and duration compensation for
patient impedance.
With Adult Pads:
Patient Impedance Range: 10 – 300 ohms
The following specifications apply from 25 t o 175 ohms.
Energy Accuracy:
10% of the energy setting into 50 ohms
15% of the rated energy output into 25 – 175 ohms
Rated energy outpu t is the nominal delivered energy based on the energy
setting and patient impedance, as defined in the following chart.
Energy (
* Energy setting selected
360 J *
250 J *
225 J *
200 J *
175 J *
150 J *
LIFEPAK 1000 Defibrillator Operating Instructions A-1
©2008 Physio-Control, Inc.
Page 46

Specifications
T1
T2
I1
I2
I3
I4
Waveshape and Measured Parameters:
Patient
Impedance (Ω)
25
50
75
100
125
150
175
I1 (A) I2 (A) I3 (A) I4 (A) T1 (ms) T2 (ms)
50.3 20.1 19.7 10.7 5.9 3.9
28.2 14.6 14.5 9.3 7.5 5.0
19.8 11.7 11.7 8.2 8.7 5.8
15.5 10.0 9.9 7.3 9.7 6.5
12.9 8.7 8.7 6.6 10.4 7.0
11.1 7.8 7.7 6.2 11.1 7.4
9.8 7.1 7.1 5.7 11.7 7.8
Note: Table values are nominal for a 200-joule shock.
A-2 LIFEPAK 1000 Defibrillator Operating Instructions
Page 47

Specifications
Rated Energy Output
20
30
40
50
60
70
80
90
25 50 75 100 125 150 175
Patient Impedance (ohms)
325 J *
300 J *
275 J *
225 J *
200 J *
150 J *
360 J *
Rated Energy Output
Patient Impedance (Ω)
Energy (J)
Waveform
(continued)
With Infant/Child pads:
The following specifications apply from 25 to 175 ohms.
Energy Accuracy (into 50 ohms):
Selected energy ÷ 4 +/- 15%; 86 joules +/- 15% maximum
Rated energy output is the nominal delivered energy based on the energy se ttin g
and patient impedance, as defined in the following chart.
250 J *
Energy (J
175 J *
* Energy setting selected
LIFEPAK 1000 Defibrillator Operating Instructions A-3
©2008 Physio-Control, Inc.
Page 48

Specifications
T1
T2
I1
I2
I3
I4
Waveshape and Measured Parameters:
Patient
Impedance (Ω)
I1 (A) I2 (A) I3 (A) I4 (A) T1 (ms) T2 (ms)
25 19.4 10.2 10.1 6.6 7.6 5.1
50 13.2 7.4 7.3 5.0 8.1 5.4
75 10.1 5.8 5.7 4.0 8.3 5.6
100 8.3 4.8 4.8 3.3 8.6 5.7
125 7.0 4.2 4.1 2.9 8.8 5.9
150 6.2 3.7 3.7 2.6 8.8 5.9
175 5.5 3.3 3.3 2.3 8.9 6.0
Note: Table values are nominal for a 50-joule shock (200 ÷ 4).
A-4 LIFEPAK 1000 Defibrillator Operating Instructions
Page 49

Specifications
Electrical Protection: Input protected against high voltage defibrillato r pulses per IEC60601-1.
See Figure A-1.
FigureA-1 Defibrillation–protected, Type BF Patient Co nnection
Safety Classification: In ternally powered equipment. IEC 60601-1
AED Mode
Shock Advisory
System (SAS):
ECG Analysis system tha t a dvis e s whe ther a shock is appr opria t e, mee ts
rhythm recognition criteria specified in DF80 and IEC 60601-2-4. In AED
mode, the device allows a shock only if SAS advises defibrillation.
Shock Ready Time Time to first shock (electrodes connected to a patient at power on and
initial rhythm finding is Shock Advised):
• Less than 25 sec to 200 joules
• Less than 30 sec to 360 joules
Energy Sequence: Multiple lev els, configurable from 150 to 360 joules
Shock-to-Shock cycle
Less than 25 seconds
time (200J to 300J):
Time for a 3-shock
Less t han 70 seconds
sequence
(200J/300J/360J):
Manual Mode
Energy Sequence Delivers energy at levels selected in Setup mode.
Charge Time Charge time:
• 200 joules in less than 7 sec (typical)
• 360 joules in less than 12 sec (typical)
ECG Mode
ECG Display Pro vides nondiagnostic ECG display of the patient’s heart rhythm.
Display
Size (Active viewing
area)
Display Type 320 dot x 240 dot LCD with backlight
Frequency Response 0.55 Hz to 21 Hz (-3 dB), nominal
Waveform Sweep
Speed
Wavefor m viewin g
time
Wavefor m Amplit ude 1 cm/mV, nominal
LIFEPAK 1000 Defibrillator Operating Instructions A-5
©2008 Physio-Control, Inc.
120 mm (4.7 in.) x 89 mm (3.5 in.)
25 mm/sec for ECG, nominal
Minimum 4 seconds
Display Range Differential: ±1.4 mV full scale, nominal
Page 50

Specifications
Heart Rate 20 to 300 BPM digital display.
Display "---" if heart rate is less than 20 BPM.
Heart symbol flashes for each QRS detection
Displayed ECG ECG information is received from therapy pads in anterior-lateral or
anterior-posterior positions, or from the 3-wire ECG cable in Lead II.
Controls
On/Off Controls device power
Shock Controls the delivery of defibrillation energy
Soft Keys Used durin g device setup and during patient use: Analyze, Charge,
Disarm
Menu button Used to access additional device features
Readiness Display
The readiness display shows device status
OK Indicator Indicates OK when the last self-test was completed successfully.
Battery Capacity
Segmented display showing battery capa city
Indicator
Service Indicator Service required when displayed
Environmental
Note: All performance specifications defined assume that the device has been stored
(two hours minimum) at the operating temperature prior to op eration.
Operating
Temperature
One-Hour Operating
Temperature
Storage Temperature With battery and electrodes, maximum exposure time limited to seven
Atmospheric Pressure 575 hPa to 1060 hPa, 4572 to -382 meters (15,000 feet to -1250 feet)
Relative Humidity 5% to 95% (noncondensing)
Dust/Water IEC 60529 IP55 with battery and REDI-PAK electrodes installed
Shock MIL-STD-810F, Method 516.5, Procedure 1, (40g peak, 15–23 msec pulse ,
Bump EN 1789 and IEC 60068-2-29, Test Eb: (1000 bumps, 15g, 6 ms, vertical
0° to 50°C (32° to 122°F)
From room temperature to temperature extreme, one-hour duration:
-20° to 60°C (-4° to 140°F)
days: -30° to 60°C (-22° to 140°F)
45 Hz crossover frequency)
direction)
Drop • 18-inch drop onto each surface, repeated 5 times each, 30 drops total
• EN 1789 0.75 meter drop onto each surface, 6 drops total
• MIL-STD-810F, 516.5 Procedure IV, 1 meter drop on each corner, edge,
and surface
Vibration MIL-STD-810F, Method 514.5, Category 20 Ground Vehicle: Random
vibration test, 1 hour per axis, 3.15g rms
EMC For EMC information, refer to the EMC tables
A-6 LIFEPAK 1000 Defibrillator Operating Instructions
Page 51

Specifications
Physical Characteristics
Weight 3.2 kg (7.1 lb) with nonrechargeable batt ery and REDI-PAK electrodes
Height 8.7 cm (3.4 in.)
Width 23.4 cm (9.2 in.)
Depth 27.7 cm (10.9 in.)
Data S torage
Memo ry Capacity • Dual pat ient st orage
• Minimum of 40 minutes of ECG for the current patient
• Summarized data stored for the previous patient
Repor t Types • Conti nuous ECG—Continuous patient ECG report
• Summary—Summary of critical resuscitat i on events and associated
ECG waveforms
• Event Log report—Report of time-stamped markers reflecting
operator and device activity
• Test Log report—Device self-test activity re port
Capacity Minimum 100 time-stamped event log entries
Data Review CODE-STAT 6.0 (minimum) or DATA TRANSFER Express 2.0 (minimum)
softwar e
Communications Infrared wireless transfer to a personal computer
Primary Ba ttery Pak
Type Lithium Manganese Dioxide (Li/MnO2), 12.0 V, 4.5 amp-hours
(nonrechargeable)
Capacity Typically will provide 440 200-joule discharges or 1030 minutes of
oper a tin g time w ith a new battery (370 200-joule shocks or 900 minute s
of operating time at 0°C (32°F)
Weight 0.45 kg (1.0 lb)
Shelf Life (prior to
installation)
Standby Life A new battery provid es device power for 5 years.
Low Battery Indicator At least 30 200-joule shocks or 75 minutes of operating time remain
After the battery is stored f or 5 years at 20°C to 30°C, the device will
provide 48 months of standby life.
when low battery is first indicated.
LIFEPAK 1000 Defibrillator Operating Instructions A-7
©2008 Physio-Control, Inc.
Page 52

Page 53

APPENDIX B
SHOCK ADVISORY SYSTEM B
LIFEPAK 1000 Defibrillator Operating Instructions
©2008 Physio-Control, Inc.
Page 54

Page 55

Shock Advisory System
OVERVIEW OF THE SHOCK ADVISORY SYSTEM
The Shock Advisory System (SAS) is an ECG analysis system built into the LIFEPAK 1000 defibrillat or that
advises the responder if it det ects a sh ockable or nonshockable rhythm. This system makes it possible for
individuals not traine d to interpret ECG rh ythms to prov ide potentially-lifesaving therapy to victims of
ventricular fibrillation or pulseless ventricular tachycardia. The SAS contains the following features:
• Electrode contact determination
• Automated interpretation of the ECG
• Responder control of sh ock therapy
• Motion detection
Electrode Contact Determination
The patient's transthoracic impedance is measured through the defibri llation electrodes. If the baseline
impedance is higher than a maximum limit, it is determined that the electrodes are not in sufficient
contact with the patient or not properly connected to the defibrillator. ECG analysis and shock delivery
are inhibited. The responder is advised to c onnect electrodes any time electrode contact is inadequate.
Automated Interpretation of the ECG
The Shock Advisory System is designed to recommend a shock if it detects the following:
•
Ventricular fibrillation — with a peak-to-peak amplitude of at least 0.08 mV
•
V entricul ar t a ch ycardia — defined as ha vin g a he a rt rate of at least 120 bea ts per minut e, QRS w idth of at
least 0.16 seconds, and no app arent P waves.
The SAS is designed to recommend no shock for ECG rhythms including pulseless electrical activity,
idioventricular rhythms, bradycardia, supraventricular tachycardias, and normal sinus rhythms.
ECG analysis is performed on consecutive 2.7 second segments of ECG. The analysis of two out of three
segments must agree before a decision (
The LIFEPAK 1000 defibrillator SAS performance for adult an d pediatric ECGs is summarized in the
LIFEPAK 1000 Shock Advisory System (SAS) Performance Rep ort on the LIFEPAK 1000 Product CD.
SHOCK ADVISED or NO SHOCK ADVISED) is made.
Control of Shock Therapy
The Shock Advisory System causes the AED to charge automatically when it detects the presence of a
shockable rhythm. When a sh ock able rhy thm is detected, the de fibrilla t or instructs the us er t o d eliver the
shock by pressing the shock button.
Motion Detection
The Shock Advisory System detects patient motion independent of ECG analysis. A motion detector is
designed into the LIFEPAK 1000 defibrillator
. Motion Detection can be configured to be ON or OFF.
LIFEPAK 1000 Defibrillator Operating Instructions B-1
©2008 Physio-Control, Inc.
Page 56

Shock Advisory System
A number of activities can create motion, including CPR, rescuer movement, patient movement, vehicle
mov ement, and some internal pacemakers. If variations in the transthora cic impedance signal exceed a
maximum limit, the Shock Advisory System determines that patient motion of some kind is present. If
motion is detected, the ECG analysis is inhibited. The operator is advised by a displayed message, a voice
prompt, and an audible alert. Aft e r 10 seconds, if motion is still present, the motion alert stops and the
analysis always proceeds to completion. This limits the delay in therapy in situations where it may not be
possible to st o p the motion. Howe v er, the rescuer should remove the source of motion whenever possible
to minimize the chance of artifact in the ECG.
There are two reasons why ECG analysis is inhibited when the motion alert occurs, and why the rescuer
should remove the source of the motion whenever possible:
• Such motion may cause artifact in the ECG signal. This artifact may occasionally cause the Shock
Advisory System to reach an incorrect decision.
• The motion may be caused by a responder's interventions. To reduce the risk of inadvertently shocking
a resp on d er, the motion alert prompts the resp on der to move away from the patient. This will stop the
motion and ECG analysis will proceed.
B-2 LIFEPAK 1000 Defibrillator Operating Instructions
Page 57

APPENDIX C
cprMAX™ TECHNOLOGY C
LIFEPAK 1000 Defibrillator Operating Instructions
©2008 Physio-Control, Inc.
Page 58

Page 59

cprMAX™ Technology
ABOUT cprMAX TECHNOLOGY
Physio-Control cprMAX t echnology is design e d t o allow resuscitation pr otocols to maximize the quantity
of CPR administered during treatment with an AED, consistent with the 2005 American Heart
Association Guidelines for Cardiopulmonary Resuscitation and Emergency Car diovascular Care
2
Guidelines) and the European Resuscitation Council Guidelines for Resuscitation 2005
(ERC Guidelines).
Setup options should be changed only under the direction of a physician knowledge able in
cardiopulmonary resuscitation who is familiar with the literature in this area.
The cprMAX technology includes the follo wing setup options:
•
INITIAL CPR. Prompts the user to perform an initial period of CPR. Applies only to immediately after
turning on the AED or after the first analysis.
•
PRESHOCK CPR Time . Prompts for CPR after a shockable ECG rhythm is detected, before the shock is
delivered. If
INITIAL CPR is set to OFF, then PRESHOCK CPR applies to all shock advised decisions
(including the first analysis).
•
CPR TIME 1 and 2. CPR time periods after shocks or no shock advised decisions respectively.
•
STACKED SHOCKS. Eliminates the analysis after each shock and inserts prompting for CPR after each
shock; when set to
•
PULSE CHECK. Indicates when, if ever, the device is to prompt for pulse checks.
OFF, eliminates the three-stack shock.
AED protocols are aligned w i th the AHA and ERC Guidelines when the setup options are set as follows:
1
(AHA
•Initial CPR:
OFF
•PreShock CPR Time: OFF
•CPR Times 1 and 2: 120 SECONDS
•Stacked Shocks: OFF
•Pulse Check: NEVER
The above options are the factory default settings for cprMAX technology. Your medical director
protocols should determine whether or not to change the options and should ensure that you receive
training.
AED OPERATION WITH CPRMAX TECHNOLOGY
The following paragraphs describe AED operation with cprMAX technology setup options.
Initial CPR
The INITIAL CPR option prompts the user to perform an initial period of CPR. The choices are: OFF,
ANALYZE FIRST and CPR FIRST. The factory d efault is OFF.
•The
OFF setting has no prompting for an initial CPR period.
•The
ANALYZE FIRST setting pr ompts f or analysis an d then CP R. If the analysis determines that a shock
is needed, the AED will prompt,
opportunity to end CPR early and proceed dire ctly to delivering a shock.
IF YOU WITNESSED THE ARREST, PUSH CANCEL, which provides the
1
2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care.
Circulation 2005;112 (Supplement IV).
2
European Resuscitation Council Guidelines for Resuscitation 2005. J. Resuscitation 2005; 67 (Supplement 1).
LIFEPAK 1000 Defibrillator Operating Instructions C-1
©2008 Physio-Control, Inc.
Page 60

cprMAX™ Technology
•The CPR FIRST setting prompts the user to perform CPR immedia tely after the defibrilla tor is powered
on. The AED will also prompt,
IF YOU WITNESSED THE ARREST, PUSH CANCEL, which provides the
opportunity to end CPR early and proceed directly to analysis.
Organizations that choose to implement this option should develop a protocol and provide training to
resp onders instructing them when to end the initial CPR interval early. Potential situations for instructing
responders to end CPR early include:
• The patient's collapse was witnessed by the responder.
• The responder a scerta ins that fewer than four or five minutes have elapsed since the patient's
collapse.
• The patient exhibits agonal breathing, an indicator of a short downtime.
• The responder ascertains that CPR of adequate quality and duration has already been provided before
att aching the AED electrodes.
Initial CPR Time
The INITIAL CPR TIME option applies when INITIAL CPR is set to ANALYZE FIRST or CPR FIRST. It sets the
CPR time for that CPR period. The tim e choices for
SECONDS. The default setting is 120 SECONDS.
INITIAL CPR TIME are: 15, 30, 45, 60, 90, 120, and 180
PreShock CPR Time
The PRE SHOCK CPR time option inserts prompting for CPR when a shockable ECG rhy thm is de tected and
during the time the AED is charging. It applies only when analysis results in
When
INITIAL CPR is set to OFF or CPR FIRST, PRESHOCK CPR time applies to the first and all subsequent
shocks. When
subsequent shocks. The choices for
INITIAL CPR is set to ANALYZE FIRST, PRESHOCK CPR time applies to the second and all
PRESHOCK CPR time are: OFF, 15, and 30 SECONDS. To prompt for
CPR only fo r the time the capacitor is charging, select the 15-seconds CPR interval. The
not enabled until charging and CPR time are completed. The default setting for
OFF.
Note: Although the SHOCK button is disabled during the PRESHOCK CP R interval, it becomes active
as soon as the
PRESHOCK CPR interval ends. To minimize the interval between the final chest
SHOCK ADVISED decisions.
SHOCK button is
PRESHOCK CPR time is
compression and shock delivery (while maintaining responder safety), protocols that select this
option should provide specific training and protocols to address the rapid transition from
CPR
to shock delivery.
PRESHOCK
Stacked Shocks
When set t o OFF, the STACKED SHOCKS option inserts pr omptin g f or CP R a ft er e ach (a sin gle) shock. This
eliminates the three-shock stack. CPR is prompted after the shock regardless of the ECG rhythm. The
CPR time following the shock is determined by the
SHOCKS
When this option is set to
optio n are ON or OFF. The default setting is OFF.
ON, the defibrillator follows the previously traditional stacked shock protocol
and delivers up to three consecutive shocks, as necessary, without interposed CPR.
CPR TIME 1 setting selected. Choices for the STACKED
Pulse Check
The PULSE CHECK option inserts prompting to check for a pulse or check the patient, depending on the
PULSE PROMPT setting. The choices for PULSE CHECK are: ALWAYS, AFTER EVERY NSA, AFTER SECOND
NSA
, and NEVER. The default setting is NEVER.
•The
ALWAYS option prompts for a pulse check after CPR TIME 1 and 2, after a NO SHOCK ADVISED
C-2 LIFEPAK 1000 Defibrillator Operating Instructions
Page 61

cprMAX™ Technology
decision, after a single SHOCK ADVISED decision with STACKED SHOCKS OFF, or aft er three
consecutive
•The
AFTER EVERY NSA option prompts for a pulse check after every NO SHOCK ADVISED decision.
•The
AFTER SECOND NSA op tion prompts for a pulse check after the second analysis if the second
analysis results in a
ADVISED
•The
NEVER option eliminates all PULSE CHECK prompts.
SHOCK ADVI SED decisions if STACKED SHOCKS is ON.
NO SHOCK ADVISED decision, re gardless of the first analysis decision (SHOCK
or NO SHOCK ADVISED).
LIFEPAK 1000 Defibrillator Operating Instructions C-3
©2008 Physio-Control, Inc.
Page 62

Page 63

APPENDIX D
CHANGING SETUP OPTIONS D
LIFEPAK 1000 Defibrillator Operating Instructions
©2008 Physio-Control, Inc.
Page 64

Page 65

Changing Setup Options
Setup
Increase
Decrease
CHANGING SETUP OPTIONS
Setup options allow you to define operating features for your defibrillator, such as CPR intervals. Setup
options are listed in tables beginning with Table D-1.
To enter Setup mode:
1 Ensure that no electrodes or cables are connected to the defibrillator.
2 Press and hold both softkeys and press the
Enter Setup Mode passcode
ON/OFF button. The Enter Setup Mode screen appears.
Press button to set
FigureD-1 En ter Setup Mode
3 Enter the Setup mode passcode. The factory default passcode is 0000—press the MENU button four
times to accept the default passcode. For info rmation on how to change the passcode, see page D-6.
Note : To exit Se tup mode, turn the d e fibrilla t or o ff. If you changed the s e tup o ptions, the chan g e s are
saved and will appear the next time you turn the defibrillator on. (Refer to Setup menu options that
follow.)
LIFEPAK 1000 Defibrillator Operating Instructions D-1
©2008 Physio-Control, Inc.
Page 66

Changing Setup Options
Labels
Softkeys
Menu button
General...
AED Mode...
Manual Mode...
Set Passcode...
Service Mode...
Setup
Set up general device options
Next Select
FigureD-2 Setup Mode Scre en
Setup Menu Options
All setup options for your defibrillator are grouped under these top-level headings.
• General
•AED Mode
• Manual Mode
• Set Passcode
•Service Mode
Use the so f tkeys to navigate and make selections on the Setup screen. The label on the screen and abo ve
each softkey identifies the current softkey function.
Press
NEXT to advance through the menu options.
When an option is highlighted, a Help message about the option appears at the top of the screen, as
show n in Table D-1.
Table D-1 Top-Level Setup Menu
Menu Item Help Message Options
GENERAL
AED MODE
MANUAL MODE Set up Manual mode defaults.
SET P ASSCODE
SERVICE MODE
To choose an option, highlight your choice on the screen and press
Set up general device options. See How to Enter and Delete Device
Information, page D-7.
Set up AED mode defaults.
Set passcode to enter Setup
mode.
View service options.
SELECT.
D-2 LIFEPAK 1000 Defibrillator Operating Instructions
Page 67

Changing Setup Options
Access the General Setup menu from Setup to view general purpose settings. The underlined bold
options in Table D-2 are the factory default settings.
Table D-2 General Setup Menu
Menu Item Help Message Options
DEVICE ID
DATE/TIME
AUDIO
DEVICE DATA
DELETE AFTER
SEND
PREVIOUS PAGE
Set the device ID. User selectable, 0-9, A-Z, up to 20 characters.
Default is
SERIAL NUMBER.
Set current date and time. Default is PACIF IC TIME.
Set audio parameters. See Table D-3.
Display device data.
Delete patient data after sending. ON, OFF.
Go back to previous page.
Access the audio options from Audio on the General Setup menu. The underlined bold options in
Table D-3 are the factory default settings.
Table D-3 General Setup Menu—Audio Setup Submenu
Menu Item Help Message Options
PROMPT VOLUME
Set volume for alarms, tones, and
MEDIUM, HIGH.
voice prompts.
SHOCK TONE
SERVICE ALERT
PREVIOUS PAGE
Enable shock tone. ON, OFF.
Enable the service alert tone. ON, OFF.
Go back to previous page.
Access the AED menu from the AED Mode option in Setup. The underlined bold options in
Table D-4 are the factory default settings.
Table D-4 AED Setup Menu
Menu Item Help Message Options
ENERGY
PROTOCOL
CPR
PULSE
Set the defibrillation energy
See Table D-5.
sequence.
Set CPR options for AED mode. See Table D-6.
Set pulse pr ompt o ptions for AED
See Table D-7.
mode.
ECG DISPLAY
Display ECG waveform in AED
ON, OFF.
mode.
AUTO ANALYZE
MOTION
DETECTION
PREVIOUS PAGE
LIFEPAK 1000 Defibrillator Operating Instructions D-3
©2008 Physio-Control, Inc.
Select analyze option. ON, AFTER FIRST SHOCK, OFF.
Alert when motion is detected. ON, OFF.
Go back to previous page.
Page 68

Changing Setup Options
Access Energy Protocols from the AED menu. The underl ined bold options in Table D-5 are the factory
default settings.
Table D-5 AED Setup Menu—Energy Protocols Submenu
Menu Item Help Message Options
ENERGY 1
ENERGY 2
Select energy level for shock 1.
Select energy equal to or greater
150, 175, 200, 225, 250, 275, 300, 325, 360
150, 175, 200, 225, 250, 275, 300, 325, 360 Joules.
*
joules.
than Energy 1.
ENERGY 3
Select energy equal to or greater
150, 175, 200, 225, 250, 275, 300, 325, 360 joules.
than Energy 2.
FLEXIBLE
PROTOCOL
STACKED SHOCKS
Repeat previous energy after NO
SHOCK ADVISED
SHOCK ADVISED
(only when NO
follows a shock).
Enable consecutive sh ocks
ON, OFF.
ON, OFF.
without CPR.
PREVIOUS PAGE
*
When selecting 360 joules for energy 1, consider AED use in children.
Go back to previous page.
Access CPR Setup from the AED menu. The underlined bold options in Table D-6 are the factory default
settings.
Table D-6 AED Setup Menu—CPR Submenu
Menu Item Help Message Options
CPR TIME 1
CPR TIME 2
INITIAL CPR
INITIAL CPR TIME
Set CPR interval af ter shocks. 15, 30, 45, 60, 90, 120, 180 seconds.
Set CPR interval after NO SHOCK
ADVISED
.
15, 30, 45, 60, 90, 120, 180 seconds.
Enable Initial CPR. OFF, ANALYZE FIRST, CPR FIRST.
Set CPR interval after first
15, 30, 45, 60, 90, 120, 180 seconds.
analysis.
PRESHOCK CPR
CPR PROMPT
Set CPR interval before SHOCK
ADVISED
decisions.
Enable extended CPR prompt:
OFF, 15, 30 seconds.
, OFF.
ON
PROVIDE RESCUE BREATHS AND
CHEST COMPRESSIONS
PREVIOUS PAGE
Go back to previous page.
D-4 LIFEPAK 1000 Defibrillator Operating Instructions
Page 69

Changing Setup Options
Access P ulse Setup from the AED menu. The un derlined bold options in Table D-7 are the factor y d efault
settings.
Table D-7 AED Setup Menu—Pulse Setup Submenu
Menu Item Help Message Options
PULSE CHECK
Enable pulse check prompt. NEVER: Never prompt for PULSE CHECK.
AFTER SECOND NSA: After every NSA except
for first analysis NSA result.
AFTER EVERY NSA: Only after NO SHOCK
ADVISED
.
ALWAYS: After e ver y st ack o f sh ocks an d e v er y
PULSE PROMPT
AED MONITORING
Select prompt for patient vital
signs.
Enable monitoring while in AED
NSA finding
CHECK PULSE, CHECK BREATHING, CHECK
CIRCULATION, OPEN AIRWAY
ON, OFF.
.
.
mode.
MONITORING
REPEAT
PREVIOUS PAGE
Select AED monitoring prompt
repeat time.
Go back to previous page.
OFF, 1, 2, 3, or 5 minutes.
Access the Manual menu from the Manual Mode option in Setup. The underlined bold options in
Table D-8 are the factory default settings.
Table D-8 Ma nual Setup Menu
Menu Item Help Message Options
MANUAL ACCESS
ANALYZE
Enable Manual mode access. ON, OFF.
Enable SAS analysis in Manual
mode. (An
ANALYZE softkey is
ON, OFF.
provided in Manual mode.)
PREVIOUS PAGE
Go back to previous page.
LIFEPAK 1000 Defibrillator Operating Instructions D-5
©2008 Physio-Control, Inc.
Page 70

Changing Setup Options
Setup / Set Passcode
Set Setup Mode passcode
Press button to set
Increase
Decrease
Access the Set Passcode screen, shown in Figure D-3, from the top-level Setup menu.
FigureD-3 Set Passcode Screen
Use the INCREASE and DECREASE softkeys and the MENU button to set the passcode. Be sure to record
the new passcode—the passcode is required each time yo u enter Setup mode.
Access Service mode, shown in Table D-9, from the top-level Setup menu.
Table D-9 Service Setup Menu
Menu Item Help Message Options
DEFIB CAL
PIXEL TEST
SERVICE LOG
SERVICE DATA
DEVICE LOG
SET P ASSCODE
Start defibrillator calibr ation.
Test display pixels.
Show service log.
Show d evice data.
Display device log.
Set Service mode access
passcode.
SETUP MODE
Go back to Setup mode.
D-6 LIFEPAK 1000 Defibrillator Operating Instructions
Page 71

Changing Setup Options
Softkeys
Menu button
How to Enter and Delete Device Information
FigureD-4 shows the Device ID screen used to enter device inf o rmation into the defibrillator.
FigureD-4 Device ID Screen
To enter device information:
1 Use the softkeys under the clockwise and counterclockwise arrows to navigate to the character or
number you want to enter.
Note: Pressing the clockwise arrow moves the cursor forward one space at a time; the
counterclockwise arrow moves it back one space at a time.
2 Press the
MENU button to choose the character. The character appears on the screen above the
alphabet area.
3 Continue adding characters to complete your entry.
4 When your completed entry is composed on the screen, select
END.
To delete device information:
1 Use the clockwise or counterclockwise arrows to navigate to the BACKSPACE option.
2 Navigate to the
the screen.
CLEAR option and press the MENU button again. The char acter no longer appears on
LIFEPAK 1000 Defibrillator Operating Instructions D-7
©2008 Physio-Control, Inc.
Page 72

Page 73

APPENDIX E
USER’S CHECKLIST E
LIFEPAK 1000 Defibrillator Operating Instructions
©2008 Physio-Control, Inc.
Page 74

Page 75

LIFEPAK® 1000 DEFIBRILLATOR
USER’S CHECKLIST
Unit Serial Number_________________________________________
Department/Location_____________________________________
Recommended
Instruction
1 Check readiness
display for:
WRENCH symbol Contact authorized service
OK symbol None.
Battery level Replace if low battery indicated.
Corrective Action
personnel.
Date
Initials
2Check Use By date
on electrode packet.
3 Check additional
supplies.
4 Check defibrillator
for:
Damage or cracks Contact authorized service
Foreign substances Clean the device.
5Comments:
Replace electrode packet if dat e
has passed.
Replenish as needed.
personnel.
Page 76

Page 77

INDEX
A
About
Defibrillation vi
, 3-3, 3-5
LIFEPAK 1000 defibrillato rvii
Accessories 5-7
AED mode vi
, 3-2, 3-3, A-5
AED Mode setup m enuD-3
AED operation C-1
Alternate anterior-poster ior
electrode position 3-5
Audio setup submenuD-3
Auto Tests 5-2
B
Battery viii, 5-4, 5-6, A-7
Battery maintenance 5-4
, 5-5
Battery Status Symbol 2-4
C
Caring for LIFEPAK 1000
defibrillator 5-1
Caution 1-2
Cautions
Cleaning 5-4
General caution 1-3
General thera py 3-2
Nonrechargeable battery5-5
Checklist, User’s E-3
Cleaning 5-4
CODE-STAT software 4-3
Controls and indicators 2-2
, A-6
CPR setup submenu D-4
cprMAX Technology viii
D
Danger 1-2
Data management viii
, 4-2
Event and Test Log 4-3
Test and service data 4-2
Data storage 4-2
Data transfer 4-3
, A-7
, 4-4
Defibrillation waveform vii, A-1
Defibrillator
Cleaning 5-4
Inspection 5-3
Recycling 5-6
Defibrillator data 4-2
Dele ting of patient data 4-2
Device ID screen D-7
Devi ce i nformation D-7
Disposing nonrechargeable
battery 5-6
E
ECG display (optional)vii, 2-4,
A-5
ECG electrodes, connecting 3-8
ECG monitoring/mode3-2
, 3-8,
A-5
Electrode Cont act
Determination B-1
Electrode placement,
defibrillation3-4
, 3-5
Electrodes storage 5-5
Energy Protocols submenu D-4
Entering
Device informationD-7
Setup modeD-1
Event and Test Log 4-3
F
Features
Battery viii
cprMAX Technology viii
Daily self-test viii
Data management viii
ECG display (optional)vii
Heart rhythm analysis vii
LIFEPAK 1000 defibrillator vii
Readiness display viii
G
Genera l setup menu D-3
LIFEPAK 1000 Defibrillator Operating Instructions Index-1
©2008 Physio-Control, Inc.
Page 78

Index
H
Heart Rate Indicator 2-4
Heart rhythm analysis vii
, B-1
I
Implanted pacemakers/
defibrillators 3-4
, 3-5
Indications for usevi
Inspection 5-3
IrDA adapter 4-3
IrDA connections 4-3
, 4-4
IrDA port 2-3
M
Maintenance and testing
schedule 5-2
Maintenance, Battery 5-4
Manual mode 3-2
, 3-5, A-5
Manual mode therapy 3-5
Manual setup menu D-5
Modes of operation 3-2
Monitoring in ECG mode 3-8
Motion Detected 3-7
Motion detection B-1
, B-2
N
No shock advised operating
scenarioC-1
O
Obese patients 3-4
Supplies 5-7
Symbols 1-3
T
Terms
Caution 1-2
Danger 1-2
Warning 1-2
Test and service data 4-2
Text conventions viii
Therapy in AED mode 3-3
Thin patients 3-4
Training tools 5-7
Transmitting reports 4-3
Troubleshooting
Defibrillation 3-6
During ECG monitoring 3-9
V
Voice prompts 3-4
W
Warnings
Battery maintenance 5-4
ECG monitoring3-8
General warnings 1-2
Nonrechargeable battery5-5
Pediatric patients
defibrillation3-3
Service and repair 5-6
Warranty information 5-7
, 5-5
P
Pacemakers, patients with
implanted3-4
Packaging recycling 5-6
Pediatric patients 3 -3
Physical Characteristics A-7
Placing electrode pads 3-3
, 3-5,
3-6
Product recycling 5-6
Pulse setup submenu D-5
R
Readiness Displayviii, 2-2, A-6
Recycling 5-6
S
Self-test viii, 5-2
Service 5-6
Setup options and menus D-2
Shock Advisory System vii
, B-1
Special situations for electrode
placement 3-4
Specifications A-1
Index-2 LIFEPAK 1000 Defibrillator Operating Instructions
Page 79

Page 80

Publication date: 10/2008
Physio-Control, Inc.
11811 Willows Road NE
Redmond, WA 98052 USA
Telephone: 425.867.4000
Toll Free (USA only): 800.442.1142
Fax: 425.867.4121
www.physio-control.com
Medtronic B.V.
Earl Bakkenstraat 10
6422 PJ Heerlen
The Netherlands
MIN 3205213-005
 Loading...
Loading...